Supplemental Digital Content is Available in the Text.
Key Words: beta-lactams, resistance, Gram-negative bacteria, pneumonia
Abstract
Background:
Antimicrobial resistance is a growing health concern worldwide. The objective of this study was to evaluate the effect of beta-lactam infusion on the emergence of bacterial resistance in patients with severe pneumonia in the intensive care unit.
Methods:
Adult intensive care patients receiving cefepime, meropenem, or piperacillin–tazobactam for severe pneumonia caused by Gram-negative bacteria were randomized to receive beta-lactams as an intermittent (30 minutes) or continuous (24 hours) infusion. Respiratory samples for culture and susceptibility testing, with minimum inhibitory concentrations (MIC), were collected once a week for up to 4 weeks. Beta-lactam plasma concentrations were measured and therapeutic drug monitoring was performed using Bayesian software as the standard of care.
Results:
The study was terminated early owing to slow enrollment. Thirty-five patients were enrolled in this study. Cefepime (n = 22) was the most commonly prescribed drug at randomization, followed by piperacillin (n = 8) and meropenem (n = 5). Nineteen patients were randomized into the continuous infusion arm and 16 into the intermittent infusion arm. Pseudomonas aeruginosa was the most common respiratory isolate (n = 19). Eighteen patients were included in the final analyses. No differences in bacterial resistance were observed between arms (P = 0.67). No significant differences in superinfection (P = 1), microbiological cure (P = 0.85), clinical cure at day 7 (P = 0.1), clinical cure at end of therapy (P = 0.56), mortality (P = 1), intensive care unit length of stay (P = 0.37), or hospital length of stay (P = 0.83) were observed. Achieving 100% ƒT > MIC (P = 0.04) and ƒT > 4 × MIC (P = 0.02) increased likelihood of clinical cure at day 7 of therapy.
Conclusions:
No differences in the emergence of bacterial resistance or clinical outcomes were observed between intermittent and continuous infusions. Pharmacokinetic/pharmacodynamic target attainment may be associated with a clinical cure on day 7.
BACKGROUND
Antimicrobial resistance puts millions of lives at risk.1 In the United States, antibiotic-resistant bacteria and fungi contribute to more than 35,000 deaths annually. The Centers for Disease Control and Prevention have specified that certain bacteria pose a greater threat because of their resistance pattern and high mortality rates, including carbapenem-resistant and extended-spectrum beta-lactamase-producing Enterobacteriaceae and multidrug-resistant Pseudomonas aeruginosa.2 Judicious use of antibiotics is warranted to minimize resistance, because bacteria are highly adaptable to antimicrobials through rapid replication, mutation, and exchange of genetic material within and between species.3 Because of the slow development of novel antibiotics, additional strategies to optimize treatment and minimize the development of bacterial resistance are warranted.
Pneumonia, including ventilator-associated pneumonia, is one of the most commonly encountered bacterial infections in intensive care units (ICUs) and is associated with prolonged mechanical ventilation, increased length of ICU stay, and a high mortality rate.4 Clinical trials have demonstrated that 10%–50% of patients with hospital- and ventilator-associated pneumonia because of P. aeruginosa treated with beta-lactams, developed resistance.5,6 In addition, early and adequate antibiotic pharmacodynamic target attainment has been demonstrated to significantly affect pneumonia outcomes, including the suppression of new bacterial resistance.7,8
Beta-lactams are commonly prescribed antibiotics in ICUs, and their bacterial killing is dependent on the amount of time that free drug concentrations remain above the minimum inhibitory concentration (MIC) of the bacteria.8 Package insert dosing may be inadequate to treat serious infections, such as pneumonia, especially in critically ill patients with high variability in pharmacokinetics.9 Currently, there are no published prospective clinical trials that have evaluated the impact of the beta-lactam infusion type with Bayesian-guided therapeutic drug monitoring (TDM) on the emergence of Gram-negative bacterial resistance in patients with severe pneumonia. Therefore, the primary objective of this study was to compare the incidence of Gram-negative bacterial resistance between participants receiving intermittent and continuous Bayesian-guided infusions of beta-lactams for severe pneumonia.
METHODS
This was a prospective, randomized, open-label study of participants admitted to the University of Florida (UF) Health Shands Hospital in Gainesville, FL, between September 2021 and February 2023. Participants were screened for the following inclusion criteria (revised in March of 2022 to improve the rate of enrollment, see Table S1, Supplemental Digital Content 1, http://links.lww.com/TDM/A689): age ≥18 years, admission to the ICU with severe pneumonia defined per IDSA/ATS 2019 definition,10 positive respiratory culture for Gram-negative bacteria, and received within the past 72 hours or will receive meropenem, cefepime, or piperacillin (in combination with tazobactam). Participants were excluded if they were pregnant, prisoners, had an allergy to beta-lactams in the study, received renal replacement therapy, had negative baseline respiratory cultures or baseline resistance to the study drugs, or were COVID-positive and were enrolled in other research trials. Participants randomized to the continuous infusion arm who were then started on renal replacement therapy were allowed to switch to the intermittent infusion arm. This study was reviewed and approved by the institutional review board at the University of Florida (IRB202101979). Participants or their families/designated health care surrogates provided written informed consent in accordance with the UF IRB policy.
The participants were randomized in a 1:1 ratio to receive beta-lactam as an intermittent (30 minutes) or a continuous (over 24 hours) infusion. Randomization was stratified according to the beta-lactam regimen. Table 1 shows the initial beta-lactam regimen based on creatinine clearance. The beta-lactam regimen and duration of therapy were determined by the treatment team. If the participants' therapy was changed to a nonstudy beta-lactam during the first 72 hours, they were withdrawn from the study. If it was changed to a nonstudy antibiotic after 72 hours, the patients remained enrolled. Participants who switched between beta-lactams in the study (ie, cefepime, meropenem, and piperacillin) would remain enrolled. Patients may have received concomitant antibiotics; however, beta-lactams were the drugs of interest.
TABLE 1.
Initial Beta-Lactam Doses per Infusion Arm and Creatinine Clearance
| Creatinine Clearance (mL/min) | Intermittent Infusion Arm | Continuous Infusion Arm |
| Cefepime | ||
| >60 | 2 g IV every 8 h | 6 g IV daily |
| 30–60 | 2 g IV every 12 h | 4 g IV daily |
| 10–30 | 2 g IV every 24 h | 2 g IV daily |
| <10 | 1 g IV every 24 h | 1 g IV daily |
| Meropenem | ||
| >50 | 2 g IV every 8 h | 6 g IV daily |
| 25–50 | 2 g IV every 12 h | 4 g IV daily |
| 10–25 | 1 g IV every 12 h | 2 g IV daily |
| <10 | 1 g IV every 24 h | 1 g IV daily |
| Piperacillin/tazobactam | ||
| >40 | 4.5 g IV every 6 h | 18 g IV daily |
| 20–40 | 3.375 g IV every 6 h | 13.5 g IV daily |
| <20 | 2.25 g IV every 6 h | 9 g IV daily |
According to the standard of care at the UF Health Shands, all participants had beta-lactam plasma concentrations measured for TDM.11 For the intermittent infusion arm, a peak was ordered 1 hour after the end of infusion and a trough was ordered before the next dose. For continuous infusion, 2 samples at least 3 hours apart were drawn at any time after the start of infusion. Total beta-lactam concentrations were quantified at the Infectious Disease Pharmacokinetics Laboratory at UF using validated liquid chromatography with tandem mass spectrometry assays. The detection was 2–100 mg/L, and the interday and intraday precision and accuracy were <10%. Pharmacokinetic/Pharmacodynamic (PK/PD) target attainment and beta-lactam dose recommendations were determined using BestDose software (LAPKB, University of Southern CA) and previously published models.12–14 Beta-lactam doses administered, beta-lactam concentrations, weight, age, and renal function were inputted into BestDose to determine therapy adjustments (to the same infusion arm) to achieve 100% ƒT >4 × MIC. All patients underwent TDM within the first day of randomization, and TDM was repeated if patients had an onset of acute kidney injury (per the KDIGO definition) or were at risk for neurotoxicity. Protein binding was assumed to be 20% for cefepime, 2% for meropenem, and 30% for piperacillin.15–17
Respiratory samples [sputum, endotracheal, or bronchoalveolar lavage (BAL)] were collected from participants and sent initially for pneumonia panel screening, culture, and susceptibility testing (with MICs) in the UF Health Shands lab. The FilmArray Pneumonia panel (BioFire Diagnostics, Salt Lake City, UT), a multiplex PCR-based diagnostic test, could also be performed initially. If positive, respiratory samples were collected once weekly for up to 4 weeks to track bacterial growth and MIC values. Bacteria were identified and had susceptibility tested using VITEK and Vitek II (bioMérieux, Durham, NC) or Etest. The Etest was used for the following bacteria (beta-lactams): Burkholderia cepacia complex (meropenem), Acinetobacter spp. (cefepime, meropenem, and piperacillin–tazobactam), and Gram-negative nonfermenters (cefepime, meropenem, and piperacillin–tazobactam).
New resistance was defined as new numeric increases (≥2-fold) in the bacterial MIC during the follow-up period compared with the baseline when starting beta-lactam therapy. Superinfection was defined as the growth of resistant Gram-negative bacteria during the follow-up period that was not observed in baseline cultures. Microbiological cure was defined as the lack of bacterial growth during the follow-up period, with no subsequent positive cultures from any site. Clinical cure was assessed by an infectious disease physician at 7 days and the end of therapy and was defined as the resolution of infection-related symptoms, including normalization of body temperature, white blood cell count, removal of mechanical ventilation and/or vasopressors if applicable, and noninitiation of a new antibiotic within 48 hours of stopping the study antibiotic. Adverse events monitored for included neurotoxicity, a decrease in platelets (<125 × 103 cells/mm3), a decrease in white blood cells (<2500 cells/mm3), increase in serum creatinine (≥1.3× baseline), and Clostridioides difficile colitis.
The primary objective of this study was to compare the incidence of Gram-negative bacterial resistance between participants receiving intermittent and continuous Bayesian-guided infusions of beta-lactams for severe pneumonia. Secondary objectives were to compare the outcomes of superinfection, microbiological cure, clinical cure, PK/PD target attainment, length of hospital and ICU stay, mortality rate, and incidence of adverse events.
Continuous data are presented as medians and interquartile ranges (IQR), whereas categorical data are presented as counts and percentages. Variables were compared using the Wilcoxon rank-sum or Fisher exact tests. Statistical significance was set at P < 0.05 significant. Statistical analyses were performed using JMP Pro v17 software (SAS Institute, Cary, NC).
RESULTS
In March 2023, the study was terminated because of slow enrollment. Between December 2021 and March 2023, 527 patients were screened. Eighteen participants were included in the final resistance and clinical outcome analysis and were enrolled in the study until 4 weeks (n = 3), hospital discharge (n = 10), death (n = 4), or were unenrolled because of the clinical need for continuous infusion (n = 1) (see Figure S1, Supplemental Digital Content 1, http://links.lww.com/TDM/A689). Seventeen (49%) participants were excluded from the final analysis, most commonly because of negative baseline cultures (n = 6) or switching to nonstudy antibiotics on the same day of enrollment (n = 4). The median (IQR) age was 68 (56–74) years, and 26 (74%) were males (Table 2). Common comorbidities included lung cancer (31%), chronic obstructive pulmonary disease (20%), and COVID coinfection (9%). At randomization, 22 (63%) patients were on cefepime, 8 (23%) on piperacillin/tazobactam, and 5 (14%) on meropenem. Nineteen (54%) participants were randomized to the continuous infusion arm and 16 (46%) to the intermittent infusion arm. The most commonly isolated bacteria were P. aeruginosa (n = 19), followed by Klebsiella pneumoniae (n = 4), Enterobacter cloacae (n = 3), and Klebsiella aerogenes (n = 3). Five participants in the intermittent infusion arm and 4 in the continuous infusion arm received continuous renal replacement therapy after enrollment. None of the patients underwent intermittent hemodialysis.
TABLE 2.
Participant Characteristics, n = 18, Median (IQR) or n (%)
| Demographics and Clinical Characteristics | ||
| Intermittent infusion (n = 13) | Continuous infusion (n = 5) | |
| Male | 8 (62) | 4 (80) |
| Age, yr | 69 (61–73) | 67 (49–74) |
| BMI, kg/m2 | 22.4 (19.2–30.7) | 31.6 (26.4–42.9) |
| SCr at randomization (mg/dL) | 0.67 (0.54–1.09) | 1.21 (0.66–2.16) |
| CrCl at randomization (mL/min) | 83.7 (69.4–105.4) | 92 (77.7–222.8) |
| CRRT | 5 (38) | 4 (80) |
| Comorbidities | ||
| Lung cancer | 3 (23) | 1 (20) |
| COPD | 2 (15) | 2 (40) |
| COVID-19 Coinfection | 1 (8) | 1 (20) |
| Beta-lactam at randomization | ||
| Cefepime | 7 (54) | 4 (80) |
| Meropenem | 2 (15) | 0 (0) |
| Piperacillin/tazobactam | 4 (31) | 1 (20) |
| Respiratory Isolates, n | ||
| Pseudomonas aeruginosa | 7 | 5 |
| Klebsiella pneumoniae | 0 | 1 |
| Klebsiella aerogenes | 1 | 1 |
| Enterobacter cloacae | 2 | 0 |
| Proteus mirabilis | 1 | 0 |
| Cronobacter sakazakii | 0 | 1 |
| Concomitant antibiotics for Gram-negative coverage | ||
| Aminoglycoside | 1 (7) | 0 (0) |
| Fluoroquinolone | 2 (15) | 0 (0) |
| Sulfamethoxazole–trimethoprim | 0 (0) | 1 (20) |
BMI, body mass index; SCr, serum creatinine; CrCl, creatinine clearance; CRRT, continuous renal replacement therapy; COPD, chronic obstructive pulmonary disease.
In the continuous infusion arm, the median MICs increased from 1 (1–4) mg/L in the initial culture to 6 (1–22) mg/L in the final culture (P = 0.2) (Fig. 1). For intermittent infusions, the median MICs increased from 3 (1–8) mg/L to 7 (0.25–16) mg/L from the initial to the final culture (P = 0.87). P. aeruginosa, median (IQR) MICs increased from 2.5 (0.25–8) mg/L to 16 (8–24) mg/L and from 4 (0.25–16) mg/L to 8 (0.25–256) mg/L in the continuous (P = 0.03)) and intermittent (P = 0.37) groups, respectively. Despite these trends, no significant differences in resistance development were identified between continuous or intermittent infusions when resistance was defined as any increase in MIC (P = 0.79) or an MIC increase of 2 tube dilutions (P = 0.67; Table 3). Sixty percent of the participants in the continuous arm and 69% of the patients in the intermittent arm received adjusted beta-lactam therapy after therapeutic drug monitoring. Forty-five percent had a dose decrease, 22% had a dose increase, and 33% had no change in therapy. Beta-lactam PK/PD target attainment was high in both infusion arms before TDM, with median free drug concentrations achieving 100% ƒT>MIC and 100% ƒT>4 × MIC for both infusion arms (Fig. 2). One hundred percent ƒT>MIC was correlated with clinical cure at day 7 (P = 0.04), but was not associated with microbiologic eradication (P = 0.81), clinical cure at end of study (P = 1), mortality (P = 1), ICU length of stay (P = 0.42), or hospital length of stay (P = 0.2). 100% ƒT>4 × MIC was associated with clinical cure at day 7 (P = 0.02), but not microbiologic eradication (P = 0.59), clinical cure at end of therapy (P = 0.26), mortality (P = 0.26), ICU length of stay (P = 0.84), or hospital length of stay (P = 0.38). In the intermittent infusion arm, 1 participant died of P. aeruginosa pneumonia and 1 died of lymphoma. In the continuous infusion arm, 1 participant died of cardiac arrest and 1 died of right ventricular failure from a submassive pulmonary embolism. None of the participants experienced adverse effects attributable to the study drugs.
FIGURE 1.
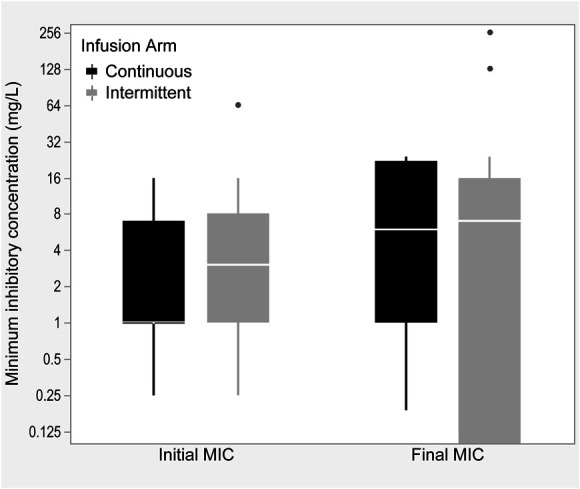
Boxplot for initial to final median MICs between intermittent and continuous infusion arms.
TABLE 3.
Study Outcomes, n = 18
| Gram-Negative Bacterial Resistance Emergence | |||
| Intermittent Infusion | Continuous Infusion | P | |
| Bacteria isolates, n | 18 | 8 | — |
| Any increase in MIC | 8 | 4 | 0.79 |
| 2× Increase in MIC | 6 | 2 | 0.67 |
| Secondary Outcomes, Median (IQR) or n (%) | |||
| Intermittent Arm (n = 13) | Continuous Arm (n = 5) | P | |
| Superinfection | 0 (0) | 0 (0) | 1.00 |
| Microbiologic cure | 4 (31) | 1 (20) | 0.85 |
| Clinical cure | |||
| At d 7 | 5 (38) | 0 (0) | 0.10 |
| At end of therapy | 8 (62) | 3 (60) | 0.56 |
| Mortality | 2 (15) | 2 (40) | 1.00 |
| Hospital length of stay, d | 18 (13–32) | 27 (12–32) | 0.83 |
| ICU Length of stay, d | 9 (6–17) | 23 (7–26) | 0.37 |
| PK/PD target attainment ƒT>MIC | 100 (94–100) | 100 (100–100) | 0.11 |
| PK/PD target attainment ƒT>4 × MIC | 100 (58–100) | 100 (50–100) | 0.68 |
MIC, minimum inhibitory concentration; ICU, intensive care unit; PK/PD, pharmacokinetic/pharmacodynamic.
FIGURE 2.
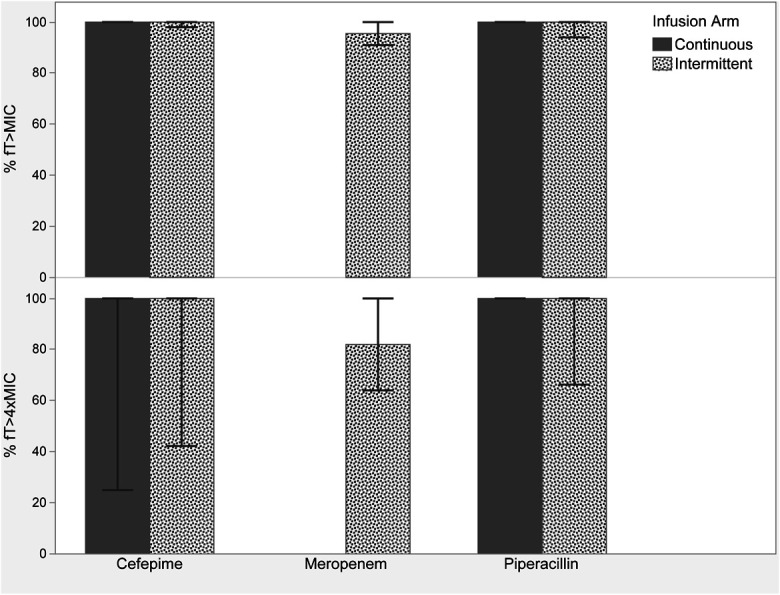
Median (IQR) beta-lactam target attainment of participants using the first plasma samples drawn (n = 18).
DISCUSSION
This study was conducted to determine the impact of continuous versus intermittent beta-lactam infusion on Gram-negative bacterial resistance in patients with severe pneumonia. Neither infusion arm showed an increased risk of bacterial resistance and there was no difference in the secondary clinical outcomes between the arms. 100% ƒT > MIC and 100% ƒT >4 × MIC were associated with clinical cure at day 7 of therapy, providing additional support for early beta-lactam target attainment. This study was underpowered because of poor enrollment and early termination.
Evidence regarding the effect of beta-lactam infusion on Gram-negative bacterial resistance in patients with pneumonia is limited. Prolonged infusions may lead to longer periods in which drug concentrations remain above the MIC, potentially decreasing bacterial regrowth between doses and minimizing the emergence of resistance. In a retrospective study by Dhaese et al, the emergence of antimicrobial resistance in adult ICU patients receiving piperacillin–tazobactam or meropenem as intermittent versus continuous infusions was compared in adult ICU patients. Resistant strains emerged in 24 of the 205 patients (12%); however, no differences in resistance emergence were observed between the infusion arms. P. aeruginosa was considered a significant predictor of the emergence of resistance. The authors proposed that further evidence is needed regarding the PK/PD index most associated with the suppression of bacterial resistance, because Cmin/MIC, area under the time concentration curve to MIC (AUC/MIC), and measures involving mutant prevention concentration have all been proposed as potential targets.18–21The authors also concluded that the emergence of resistance was not because of the mode of infusion, which is similar to the results of the present study and the findings of Yusuf et al.18,22 In addition, PK/PD target attainment in the present study was high, which may contribute to the finding that no differences in resistance emergence were observed between the infusion arms. Interestingly, Felton et al23 suggested that prolonged infusions may not be sufficient to limit the emergence of resistance in P. aeruginosa infections with high bacterial burdens, such as pneumonia. This proposal could help explain the results of Dhaese et al and those of the present study. However, other evidence suggests that there may be a benefit to prolonging the infusion. Sumi et al24 reported that prolonged beta-lactam infusions may suppress ESBL-producing bacteria. Sumi et al reported that the PK/PD index most likely to suppress the emergence of antibiotic resistance is Cmin/MIC ≥4.25 Although underpowered, in the present study, achieving 100% ƒT > MIC and 100% ƒT >4 × MIC was significantly associated with clinical cure at day 7 of therapy, supporting evidence that early beta-lactam target attainment is essential for improved patient outcomes.7,26,27
In contrast to the published literature, this study did not find differences in the secondary outcomes between the infusion arms. Rhodes et. performed a systematic review and meta-analysis of 18 studies on the effect of prolonged piperacillin–tazobactam infusions. Approximately 57% of the patients received prolonged infusions, which were associated with 1.46-fold lower odds of mortality, 1.77-fold higher odds of clinical cure, and 1.22-fold higher odds of microbiological cure.28 The mortality benefit of prolonged infusions has been further emphasized in other studies as well.29,30 A randomized, open-label, controlled trial of patients receiving meropenem as a continuous infusion versus a bolus (over 30 minutes) demonstrated a decrease in ICU stay and duration of treatment.31 Chan et al32 reported a decreased length of hospital stay with prolonged infusions. None of the participants experienced adverse effects from the study drugs, thus providing additional safety support for beta-lactams.
This study has several limitations. The primary limitation was the small enrollment size, which made the study underpowered. The inability to identify patients meeting the study criteria and the inability of critically ill patients to provide consent were the most common reasons for low enrollment. In addition, this study used MICs, which may only test 1 to 2 colonies with resistant subpopulations, potentially preventing detection.33 In addition, it is generally accepted that MICs vary with repeated assessments, potentially producing MIC values that differ by 200%.34 Finally, repeat-positive cultures may be because of colonization, especially in participants demonstrating a clinical cure before repeat cultures. To address these limitations, a multicenter study with a different study design is required. The proposed design for a future prospective study is a pragmatic, block randomized controlled trial that would automatically enroll all participants admitted to a specific ICU in a designated arm of the trial. However, even with this design, the percentage of candidates dropped may still be high, given the risk of baseline-negative or resistant cultures. Despite these limitations, this study had a strong design and followed previously published recommendations, including accounting for methodologic and biological variability of MIC, using continuous outcomes to improve study power, and performing therapeutic drug monitoring with Bayesian estimation to limit drug exposure as a confounder.35
CONCLUSION
No differences were observed in the emergence of bacterial resistance between the intermittent and continuous infusions. There were no differences in mortality, clinical cure on day 7 or at the end of therapy, adverse effects, microbiological eradication, length of stay, or mortality between the 2 groups. PK/PD target attainment was associated with clinical cure on day 7. Although underpowered, this finding supports previously published findings that early target attainment improves clinical outcomes in patients. Further investigations into the beta-lactam infusion type and the emergence of Gram-negative bacterial resistance are needed before providing recommendations for patient treatment, and a multicenter study may help improve study recruitment.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge the study participants and their families, in addition to the clinical staff at UF Health Shands Hospital in Gainesville, FL for helping make this study possible.
Footnotes
This work was funded by the Food and Drug Administration (Award ID: 75F40121C00157). Views expressed are solely the responsibility of the authors and do not necessarily represent the official views of the FDA.
The authors declare no conflict of interest.
The Institutional Review Board at the University of Florida (Ref # IRB202101979) reviewed and approved the study. Participants or their families/designated healthcare surrogates provided written informed consent in accordance with UF IRB policy.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.drug-monitoring.com).
N. Maranchick was the study coordinator, who performed data analysis and collection and wrote the manuscript. C. Trillo-Alvarez reviewed the trial candidates daily and facilitated the clinical aspects of the trial. V. Kariyawasam reviewed the candidates for study outcomes and facilitated data collection for study outcomes. V. Venugopalan reviewed the potential candidates daily, helped facilitate the clinical aspects of the trial, and assisted with data collection. A. Kwara secured the study funding and contributed to the study design. Kenneth Rand was involved in the study design. C.A. Peloquin was involved in securing the study funding, study design, and overseeing therapeutic drug monitoring of beta-lactams. M.H. Alshaer was involved in all aspects of the study, including securing funding, study design, data collection and analysis, and assistance with manuscript writing. All the authors reviewed the manuscript.
Contributor Information
Cesar Trillo-Alvarez, Email: cesar.trilloalvarez@medicine.ufl.edu.
Vidhu Kariyawasam, Email: vidhu.kariyawasam@medicine.ufl.edu.
Veena Venugopalan, Email: vvenugopalan@cop.ufl.edu.
Awewura Kwara, Email: awewura.kwara@medicine.ufl.edu.
Kenneth Rand, Email: kenneth.rand@medicine.ufl.edu.
Charles A. Peloquin, Email: peloquin@ufl.edu.
Mohammad H. Alshaer, Email: m.shaer@msn.com.
REFERENCES
- 1.World Health Organization. Antimicrobial resistance: global report on surveillance. 2014. Available at: https://www.who.int/publications/i/item/9789241564748. Accessed March 23, 2023.
- 2.Centers for Disease Control and Prevention. About Antimicrobial Resistance. 2022. https://www.cdc.gov/drugresistance/about.html [Google Scholar]
- 3.Fernandes P. Antibacterial discovery and development—the failure of success? Nat Biotechnol. 2006;24:1497–1503. [DOI] [PubMed] [Google Scholar]
- 4.Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46:888–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink MP, Snydman DR, Niederman MS, et al. Treatment of severe pneumonia in hospitalized patients: results of a multicenter, randomized, double-blind trial comparing intravenous ciprofloxacin with imipenem-cilastatin. The Severe Pneumonia Study Group. Antimicrob Agents Chemother. 1994;38:547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Réa-Neto A, Niederman M, Margareth Lobo S, et al. Efficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open-label, multicenter study. Curr Med Res Opin. 2008;24:2113–2126. [DOI] [PubMed] [Google Scholar]
- 7.Alshaer MH, Maranchick N, Bai C, et al. Using machine learning to define the impact of beta-lactam early and cumulative target attainment on outcomes in intensive care unit patients with hospital-acquired and ventilator-associated pneumonia. Antimicrob Agents Chemother. 2022;66:e0056322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Shaer MH, Neely MN, Liu J, et al. Population pharmacokinetics and target attainment of cefepime in critically ill patients and guidance for initial dosing. Antimicrob Agents Chemother. 2020;64:e007455-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 10.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venugopalan V, Hamza M, Santevecchi B, et al. Implementation of a β-lactam therapeutic drug monitoring program: experience from a large academic medical center. Am J Health-System Pharm. 2022;79:1586–1591. [DOI] [PubMed] [Google Scholar]
- 12.Felton TW, Roberts JA, Lodise TP, et al. Individualization of piperacillin dosing for critically ill patients: dosing software to optimize antimicrobial therapy. Antimicrob Agents Chemother. 2014;58:4094–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alshaer MH, Goutelle S, Santevecchi BA, et al. Cefepime precision dosing tool: from standard to precise dose using nonparametric population pharmacokinetics. Antimicrob Agents Chemother. 2022;66:e0204621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathew SK, Mathew BS, Neely MN, et al. A nonparametric pharmacokinetic approach to determine the optimal dosing regimen for 30-minute and 3-hour meropenem infusions in critically ill patients. Ther Drug Monit. 2016;38:593–599. [DOI] [PubMed] [Google Scholar]
- 15.Wong G, Briscoe S, Adnan S, et al. Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother. 2013;57:6165–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig WA. The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis. 1997;24(suppl 2):S266–S275. [DOI] [PubMed] [Google Scholar]
- 17.Adnan S, Paterson DL, Lipman J, et al. Pharmacokinetics of beta-lactam antibiotics in patients with intra-abdominal disease: a structured review. Surg Infections. 2012;13:9–17. [DOI] [PubMed] [Google Scholar]
- 18.Dhaese SAM, De Kezel M, Callant M, et al. Emergence of antimicrobial resistance to piperacillin/tazobactam or meropenem in the ICU: intermittent versus continuous infusion. A retrospective cohort study. J Crit Care. 2018;47:164–168. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Wang L, Zhang XJ, et al. Evaluation of meropenem regimens suppressing emergence of resistance in Acinetobacter baumannii with human simulated exposure in an in vitro intravenous-infusion hollow-fiber infection model. Antimicrob Agents Chemother. 2014;58:6773–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stearne LET, Goessens WHF, Mouton JW, et al. Effect of dosing and dosing frequency on the efficacy of ceftizoxime and the emergence of ceftizoxime resistance during the early development of murine abscesses caused by Bacteroides fragilis and Enterobacter cloacae mixed infection. Antimicrob Agents Chemother. 2007;51:3605–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam VH, Schilling AN, Neshat S, et al. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:4920–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yusuf E, Van Herendael B, Verbrugghe W, et al. Emergence of antimicrobial resistance to Pseudomonas aeruginosa in the intensive care unit: association with the duration of antibiotic exposure and mode of administration. Ann Intensive Care. 2017;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felton TW, Goodwin J, O'Connor L, et al. Impact of Bolus dosing versus continuous infusion of Piperacillin and Tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57:5811–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumi CD, Heffernan AJ, Naicker S, et al. Pharmacodynamic evaluation of intermittent versus extended and continuous infusions of piperacillin/tazobactam in a hollow-fibre infection model against Klebsiella pneumoniae. J Antimicrob Chemother. 2020;75:2633–2640. [DOI] [PubMed] [Google Scholar]
- 25.Sumi CD, Heffernan AJ, Lipman J, et al. What antibiotic exposures are required to suppress the emergence of resistance for gram-negative bacteria? A systematic review. Clin Pharmacokinet. 2019;58:1407–1443. [DOI] [PubMed] [Google Scholar]
- 26.Dulhunty JM, Roberts JA, Davis JS, et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Dis. 2013;56:236–244. [DOI] [PubMed] [Google Scholar]
- 27.Al-Shaer MH, Rubido E, Cherabuddi K, et al. Early therapeutic monitoring of β-lactams and associated therapy outcomes in critically ill patients. J Antimicrob Chemother. 2020;75:3644–3651. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes NJ, Liu J, O'Donnell JN, et al. Prolonged infusion piperacillin-tazobactam decreases mortality and improves outcomes in severely ill patients: results of a systematic review and meta-analysis. Crit Care Med. 2018;46:236–243. [DOI] [PubMed] [Google Scholar]
- 29.Falagas ME, Tansarli GS, Ikawa K, et al. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis. 2013;56:272–282. [DOI] [PubMed] [Google Scholar]
- 30.Yu Z, Pang X, Wu X, et al. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: a meta-analysis. PLoS One. 2018;13:e0201667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chytra I, Stepan M, Benes J, et al. Clinical and microbiological efficacy of continuous versus intermittent ap.plication of meropenem in critically ill patients: a randomized open-label controlled trial. Crit Care. 2012;16:R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan JD, Dellit TH, Lynch JB. Hospital length of stay among patients receiving intermittent versus prolonged piperacillin/tazobactam infusion in the intensive care units. J Intensive Care Med. 2018;33:134–141. [DOI] [PubMed] [Google Scholar]
- 33.Olofsson SK, Cars O. Optimizing drug exposure to minimize selection of antibiotic resistance. Clin Infect Dis. 2007;45(suppl 2):S129–S136. [DOI] [PubMed] [Google Scholar]
- 34.Doern GV, Brecher SM. The clinical predictive value (or lack thereof) of the results of in vitro antimicrobial susceptibility tests. J Clin Microbiol. 2011;49(9 suppl):S11–S14. [Google Scholar]
- 35.Märtson AG, Sturkenboom MGG, Stojanova J, et al. How to design a study to evaluate therapeutic drug monitoring in infectious diseases? Clin Microbiol Infect. 2020;26:1008–1016. [DOI] [PubMed] [Google Scholar]


