Abstract
Background:
Previously, The Joint United Nations Programme on HIV/AIDS estimated proportions of adult new HIV infections among key populations (KPs) in the last calendar year, globally and in 8 regions. We refined and updated these, for 2010 and 2022, using country-level trend models informed by national data.
Methods:
Infections among 15–49 year olds were estimated for sex workers (SWs), male clients of female SW, men who have sex with men (MSM), people who inject drugs (PWID), transgender women (TGW), and non-KP sex partners of these groups. Transmission models used were Goals (71 countries), AIDS Epidemic Model (13 Asian countries), Optima (9 European and Central Asian countries), and Thembisa (South Africa). Statistical Estimation and Projection Package fits were used for 15 countries. For 40 countries, new infections in 1 or more KPs were approximated from first-time diagnoses by the mode of transmission. Infection proportions among nonclient partners came from Goals, Optima, AIDS Epidemic Model, and Thembisa. For remaining countries and groups not represented in models, median proportions by KP were extrapolated from countries modeled within the same region.
Results:
Across 172 countries, estimated proportions of new adult infections in 2010 and 2022 were both 7.7% for SW, 11% and 20% for MSM, 0.72% and 1.1% for TGW, 6.8% and 8.0% for PWID, 12% and 10% for clients, and 5.3% and 8.2% for nonclient partners. In sub-Saharan Africa, proportions of new HIV infections decreased among SW, clients, and non-KP partners but increased for PWID; elsewhere these groups' 2010-to-2022 differences were opposite. For MSM and TGW, the proportions increased across all regions.
Conclusions:
KPs continue to have disproportionately high HIV incidence.
Key Words: key and vulnerable populations, HIV epidemiology, modeling, surveillance
INTRODUCTION
Unequal access to HIV-related prevention and treatment services for stigmatized and marginalized communities impedes the global HIV response. Estimates of new HIV infections among key populations (KPs) and their sexual partners are critical to monitor progress in the response among communities most often ignored by programs. However, incidence is difficult to measure, especially among marginalized and stigmatized populations, and is not measured nationally among KP.
Since 2016, The Joint United Nations Programme on HIV/AIDS (UNAIDS) has annually estimated proportions of new adult HIV infections among KPs globally and for each UNAIDS region. UNAIDS also published each KP's relative risk of acquiring HIV compared with adult men or women overall. Previously published proportions were point estimates, built on each year's national HIV estimates, without trends. Over the 2016–2021 rounds of estimates, proportions of new infections among KPs and their sex partners increased. However, because methods for calculating distributions of infections, including some input models' assumptions and parameters, were continuously updated based on evolving evidence, these changes did not indicate time trends, and their publication was annotated with the caveat that these should not be compared across rounds.1
Increasing incidence had been observed in some KP in some settings2,3; however, time trends in population distributions of new HIV infections have not been quantified systematically, except in select country models. In 2023, the authors refined the estimation of new infection distributions among KPs to include temporal comparisons covering the period since 2010, the baseline for targets set in the 2021 United Nations Political Declaration on HIV/AIDS. This article describes the methodology and results for years 2010 and 2022, building on the 2023 round of national HIV estimates (covering data and estimates through 2022) supplemented with UNAIDS-supported, peer-reviewed, dynamic models where appropriate.
METHODS
Multiple models and data sources have been used to estimate trends in new HIV infections among KP for different countries. Some countries employed multiple models; others had no modeled KP estimates. We combine results from available models using a hierarchy to select the best KP trend model for each country. This section describes the hierarchy of sources when more than one is available, methods used to combine and extrapolate model results to countries without a model, and aggregation to regional estimates.
We calculated numbers and proportions of new infections among adults (15–49 years throughout this analysis) in each KP. Incidence rates for each KP were calculated by dividing KP-specific numbers of new infections by the susceptible population. The susceptible population was defined as the group size estimate minus the number living with HIV. The incidence rate ratio (IRR) compares the risks of HIV acquisition among KPs relative to the overall adult population.
All countries that produced a national estimate using a Spectrum model during the 2023 round of UNAIDS-led country-derived HIV estimates were included. For countries not producing an estimate, UNAIDS created one with publicly available data; results for 172 countries with a population of at least 250,0004 were available (details in1 Annex on Methods).
New infections were estimated for the following KP, defined in UNAIDS Global AIDS Monitoring guidelines5:
Sex workers (SWs)
Gay men and other men who have sex with men (MSM)
Transgender women (TGW)
People who inject drugs (PWIDs).
Although UNAIDS provides guidance, countries use varying KP definitions.6 For TG people, most available data (99%) are for women, so all models' estimates refer to transgender women only.
For SW, not disaggregated by gender in Global AIDS Monitoring reporting, most data refer to women. From Estimates and Projections Package (EPP), AIDS Epidemic Model (AEM) and one Optima estimates for 2022 plus Cuba's case notifications averaged over 2020–2022, male SW averaged 11% (with a median of 0%) of new infections/diagnoses among female plus male SW; Goals, Thembisa, and 8 Optima models modeled only female sex workers (FSW). In this analysis, we refer to this group as SW, given that few sources included male SW.
Estimates were made for clients of FSW and sex partners of KP who themselves are not KP, for example, noninjecting sex partners of PWID and female partners of MSM.
New Infections Among KP: Country Estimates
Where available, new adult infections among each KP and for clients of FSW were retrieved from UNAIDS-supported, country-level, HIV trend estimation models, updated annually by national AIDS programs (Fig. 1).
FIGURE 1.
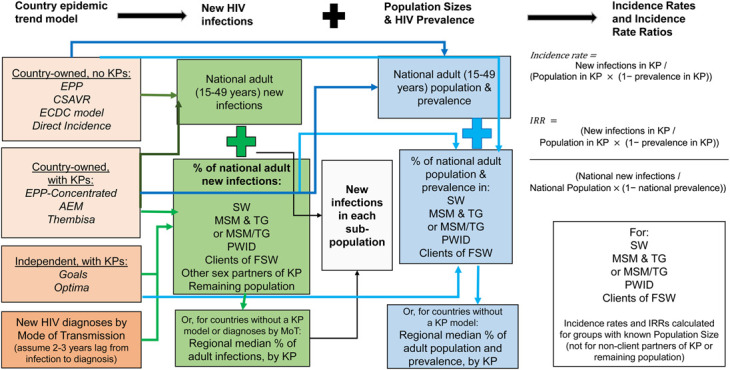
Conceptual overview of the data analysis method. All data and procedures apply to populations aged 15–49 years and are calculated for both 2010 and 2022 from the same source for any given country and population group. Dark green and dark blue arrows indicate information from country-owned models used for country-owned HIV estimates; light green and light blue arrows denote information flows from other sources.
Most national HIV estimates are generated within Spectrum. Spectrum incorporates several models to estimate incidence trends, appropriate to different epidemic and surveillance contexts. Countries with representative prevalence data use EPP, in either of two structural options: (1) Most countries in sub-Saharan Africa (SSA) fit EPP to historical surveillance data among pregnant women receiving antenatal care; for recent years, these are complemented with routine antenatal care–based HIV testing and national household serosurveys; (2) Other countries use an EPP model configured to match locally recognized and surveyed KP and other sentinel (antenatal care or other non-KP) populations. Thirteen Asian countries use the AIDS Epidemic Model7 (AEM) of transmission between KPs and other groups that also fits prevalence for each group. High-income countries and countries with strong HIV/AIDS case and death surveillance use models fitted to these surveillance data, without distinguishing KPs. South Africa uses a customized transmission model, Thembisa.8
We used estimates of trends in KP new HIV infections, prevalence, and population size available from EPP, AEM, and Thembisa models. For countries without KP-stratified trend estimates, results were sourced from 2 mechanistic dynamic transmission models:
Goals, calibrated by Avenir Health based on 2023 Spectrum models, for most high HIV-burden countries9;
Optima, calibrated by the Burnet Institute in collaboration with national HIV programs in eastern Europe and central Asia (EECA) countries to review and prioritize strategic HIV investments.10
Models are described in Supplemental Digital Content 1, http://links.lww.com/QAI/C175.
The hierarchy for data sources for SSA, Papua New Guinea, and Haiti was Goals for all countries, except for South Africa, modeled with Thembisa.
The hierarchy outside of SSA was
AEM for 13 countries in Asia;
Optima for 9 countries in EECA with a national model updated and calibrated in 2022 distinguishing SW, MSM/TGW, PWID, and clients11,12;
EPP, if it distinguished 2 or more locally relevant KPs;
Case-based surveillance of HIV diagnoses by the mode of transmission, judged to have reasonably complete modes of transmission for at least 2 groups, that is, without substantive underreporting of modes of transmission due to stigma (details in Supplemental Digital Content 1, http://links.lww.com/QAI/C175);
-
Goals for countries
○ without any of the above options (Bhutan, China, Djibouti, Russia, Montenegro, Yemen, Belize, Bhutan, Costa Rica, the Democratic People's Republic of Korea, Egypt, El Salvador, Lebanon, North Macedonia, Syria, Serbia, Uzbekistan);
○ with an EPP calibration with MSM representing less than 0.4% of adults (considered not to be plausible given WHO/UNAIDS recommended minimum estimated population size for MSM of 1.0% of adult men)13 (Morocco and Afghanistan);
○ with EPP implying an implausibly large decline in new infections among PWID that was incompatible with an independent national time trend analysis (Iran14);
○ where proportions of new infections among KP from EPP supplemented with regionally extrapolated proportions for missing groups (clients and nonclient sex partners) exceeded 100% of EPP-estimated national infections (Colombia, Nicaragua, Tunisia);
For PWID in Libya, new infections, population size, and prevalence in 2010 were taken from an independent model.15 The implied 26% of new adult infections, which aligns with diagnoses by the mode of transmission over 2008–2017,16 was applied for both years.
Proportions of new HIV infections or first-time HIV diagnoses in each KP, out of all new infections, or first-time diagnoses in the same year among men and women aged 15–49 years were extracted from the above sources. These proportions were applied to total national adult infections, 2010 and 2022, from each country's 2023 Spectrum estimate.17,18
Supplemental Digital Content 2, http://links.lww.com/QAI/C176 details sources used for each indicator and key assumptions, by KP, country, and year; Supplemental Digital Content 1, http://links.lww.com/QAI/C175 summarizes the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) checklist of information collated, used, and produced. All abbreviations are listed in Supplemental Digital Content 3, http://links.lww.com/QAI/C207.
New Infections: Nonclient Sexual Partners
To calculate numbers and proportions of new infections among nonclient, non-KP sexual partners of KP and among FSW clients, we used the following sources and definitions:
Goals countries and South Africa: Goals estimates for nonclient, stable male partners of FSW, stable female partners of MSM, male and female stable partners of PWID, and female stable partners of FSW clients.
AEM countries: AEM estimates for female partners of MSM and of TGW (separately) and male and female sex partners of PWID;
Optima countries: Optima estimates for nonclient male partners of FSW, female partners of MSM, male and female partners of PWID, and female partners of FSW clients.
In calculating HIV transmission to non-KP partners of KP, Goals accounts for the time-varying coverage and impact of antiretroviral treatment using coverage ratios in KPs relative to all adults based on surveys in SSA.19 Similarly, AEM models for some countries stratify ART coverage by KP, based on KP-specific ART coverage data (details in Supplemental Digital Content 1, http://links.lww.com/QAI/C175). For male partners of female KPs in SSA, Goals and Thembisa models account for coverage of medical male circumcision.
New Infections in KP, Clients and Partners: Extrapolation to Countries and Groups Without Data
Several countries had no qualifying model or diagnoses by the mode of transmission; other countries had a qualifying source but lacked 1 or more of the groups to be estimated. For those countries and groups, proportions of adult new infections in KP were inferred by applying a regional median proportion for each KP (relative to all adult men and women) that year, to the total new infections estimated in the national Spectrum model. Regional median proportions were calculated from values across countries with national estimates.
Regions were defined using UNAIDS classifications. Some results are reported for aggregated SSA, that is, East and southern Africa (ESA) and West and central Africa (WCA) regions and “non-SSA” regions.
In countries using AEM, which explicitly includes MSM, FSWs, clients of FSWs, and PWID in its population structure, any new infection estimate that equaled 0 was considered a valid and accurate estimate of zero. For EPP, by contrast, where inclusion of a group depended on data availability (requiring a minimum of 3 prevalence data points to estimate the trend), groups not represented in a given country model were estimated by extrapolating a regional proportion of new infections, assuming that they are part of the “remaining population” modeled in EPP, that is, without overlap with the KPs explicitly modeled in EPP.
New infections among TGW were available from 8 AEM and 9 EPP countries (Supplemental Digital Content 2, http://links.lww.com/QAI/C176). Canada, the United States, Australia, Singapore, and Cuba recorded TGW as a subpopulation in new HIV case diagnoses. Goals, Optima, and Thembisa had no TGW compartment, nor did most EPP models. For countries without estimates or diagnoses data for TGW, we assumed that these were (implicitly) incorporated with MSM (which historically occurred in surveys and surveillance systems informing models). We allocated these countries' estimated proportion of new infections among MSM between MSM and TGW using the median ratio between these two groups' new infections across countries with discrete estimates (EPP or AEM) or case diagnoses20–26 for TGW; the resulting allocation was 95% MSM and 5% TGW assumed from total MSM new infections.
Incidence Rate Ratios
As shown in Figure 1, the IRR is calculated as a KP-specific incidence rate relative to overall adult incidence. Incidence rates for each KP were calculated by dividing KP-specific numbers of new infections by the susceptible population. The susceptible population was defined as the group size estimate (see the next subsection) minus the number living with HIV. Numbers of KP members living with HIV were taken from the same national models used for new infections estimates and calculated by applying a regional median prevalence from available model estimates for other countries in the same region (Fig. 1).
Population Size Estimates
KP size estimates in 2010 and 2022 were taken from the same country models that were used above. These were nationally representative size estimates27 for AEM, Thembisa, and Optima, and medians of nationally representative size estimates (expressed as proportion of adults in a KP) across each region for Goals and countries without a model (details in Supplemental Digital Content 1, http://links.lww.com/QAI/C175).
For country models that estimated MSM but not TGW, MSM were split into MSM and TGW as 94% and 6%, respectively. This is based on the median ratio between the 2 populations' numbers across countries with a TGW population size estimate from an EPP or AEM model.27
HIV Prevalence
For countries without a national model that included KPs, numbers of people susceptible to HIV infection (the denominator for IRRs) were calculated as population size multiplied by 1 minus regional median HIV prevalence. UNAIDS Western and central Europe and North America (WCENA) region had only 1 country model (Serbia), which we supplemented with 2009–2019 prevalence data for FSW, MSM, TGW, and PWID from a systematic review,28 applying each country's time constant–pooled prevalence estimate to both 2010 and 2022. For WCENA countries not included in this review,28 we used regional medians. FSW clients in WCENA region were assigned Serbia's Goals-estimated prevalence.
RESULTS
New Infections by KPs: Regional Results
A country-specific source was most frequently available for MSM (149 countries), followed by PWID (137) and SW (111) (Table 1). Corresponding new infection numbers and proportions by data source and region are shown in Supplemental Digital Content 1, http://links.lww.com/QAI/C175.
TABLE 1.
Distribution of Countries Estimated, by Region and Source of KP Infection Estimates
| Region | Sex Workers | MSM | TGW | SUM, by Method Used for MSM | ||||||||||||||||||
| Goals | AEM | Optima | EPP-Conc | Thembisa | Diagnoses | Extrapolate | Goals | AEM | Optima | EPP-Conc | Thembisa | Diagnoses | Extrapolate | Goals | AEM | Optima | EPP-Conc | Thembisa | Diagnoses | Extrapolate | ||
| AP | 7 | 13 | — | 3 | — | 1 | 5 | 7 | 13 | — | 3 | — | 3 | 3 | — | 8 | — | 2 | — | 2 | 17 | 29 |
| CAR | 2 | — | — | 4 | — | 1 | 3 | 2 | — | — | 4 | — | 1 | 3 | — | — | — | 2 | — | 1 | 7 | 10 |
| LA | 4 | — | — | 7 | — | — | 6 | 4 | — | — | 7 | — | 1 | 5 | — | — | — | 5 | — | — | 12 | 17 |
| EECA | 4 | — | 9 | 1 | — | — | 2 | 4 | — | 9 | 1 | — | — | 2 | — | — | — | — | — | — | 16 | 16 |
| MENA | 9 | — | — | — | — | 10 | 9 | — | — | — | — | — | 10 | — | — | — | — | — | — | 19 | 19 | |
| ESA | 19 | — | — | — | 1 | — | — | 19 | — | — | — | 1 | — | — | — | — | — | — | — | — | 20 | 20 |
| WCA | 25 | — | — | — | — | — | — | 25 | — | — | — | — | — | — | — | — | — | — | — | — | 25 | 25 |
| WCENA | 1 | — | — | — | — | — | 35 | 1 | — | — | — | — | 35 | — | — | — | — | — | — | 8 | 28 | 36 |
| Global | 71 | 13 | 9 | 15 | 1 | 2 | 61 | 71 | 13 | 9 | 15 | 1 | 40 | 23 | — | 8 | — | 9 | — | 11 | 144 | 172 |
| PWID | Clients of FSW | Nonclient partners of KP | |||||||||||||||||||
| Goals | AEM | Optima | EPP-Conc | Mumtaz-GR et al 2018 (LBY) | Diagnoses | Extrapolate | Goals | AEM | Optima | EPP-Conc | Thembisa | Diagnoses | Extrapolate | Goals | AEM | Optima | EPP-Conc | Thembisa | Diagnoses | Extrapolate | |
| AP | 7 | 13 | — | 1 | — | 3 | 5 | 7 | 13 | — | — | — | — | 9 | 10 | 13 | — | — | — | — | 6 |
| CAR | 2 | — | — | — | — | 1 | 7 | 2 | — | — | — | — | — | 8 | 7 | — | — | — | — | — | 3 |
| LA | 4 | — | — | 1 | — | 1 | 11 | 4 | — | — | — | — | — | 13 | 10 | — | — | — | — | — | 7 |
| EECA | 4 | — | 9 | 1 | — | — | 2 | 4 | — | 9 | — | — | — | 3 | 5 | — | 9 | — | — | — | 2 |
| MENA | 9 | — | — | — | 1 | — | 9 | 9 | — | — | — | — | — | 10 | 9 | — | — | — | — | — | 10 |
| ESA | 20 | — | — | — | — | — | — | 19 | — | — | — | 1 | — | — | 20 | — | — | — | — | — | — |
| WCA | 25 | — | — | — | — | — | — | 25 | — | — | — | — | — | — | 25 | — | — | — | — | — | — |
| WCENA | 1 | — | — | — | — | 34 | 1 | 1 | — | — | — | — | — | 35 | 1 | — | — | — | — | — | 35 |
| Global | 72 | 13 | 9 | 3 | 1 | 39 | 35 | 71 | 13 | 9 | - | 1 | - | 78 | 87 | 13 | 9 | - | - | - | 63 |
Some countries had some KPs estimated from a model but other KPs from extrapolation or diagnoses; hence, results differ slightly when stratified by the source for FSWs, versus MSM, TGW, PWID, or clients. The source for PWID infection estimates in 1 country (Libya) in MENA region was provided elsewhere.15 For TGW, the allocation as a proportion of a modeled group, including MSM plus TGW for 83 countries, was listed in the “Extrapolate” category. Australia was the one country with a cohort measurement of incidence among (female) SW 21; this was grouped here under “diagnoses.” Thembisa (for South Africa) did not estimate PWID.
In 2022, an estimated 55% of adult (15–49 year) new HIV infections were among KP and their partners. Table 2 shows estimated new infections by KPs and region, as absolute numbers of new adult infections (Table 2a), proportions of overall adult new infections (Table 2b), and incidence rates (Table 2c). Across 172 countries, proportions of new infections in 2022 were 7.7% for SW, 20% for MSM, 1.1% for TGW, 8.0% for PWID, 10% for clients of FSW, and 8.2% for nonclient partners of any KP. In 2022, SW comprised relatively high proportions of new adult infections in EECA and WCA (15% in both). The highest proportions of new infections among clients of FSW were estimated for EECA (33%) and ESA (11%). For MSM, the proportion of new infections was highest in WCENA (59%) and the Middle East and North Africa (MENA) region (54%).
TABLE 2.
New Infections in Adults aged 15–49 Years, as (a) Numbers, (b) Proportions of Overall Adult (15–49 years) Infections, and (c) Rates per 100 Person-Years
| Region | 2010 | 2022 | ||||||||||||||
| (a) | SW | MSM | TGW | PWID | Clients of FSW | Non-client Partners of KP | Remaining Population | All 15-49 yr | SW | MSM | TGW | PWID | Clients of FSW | Nonclient Partners of KP | Remaining Population | All 15-49 yr |
| AP | 20,000 | 82,000 | 5320 | 49,000 | 23,000 | 16,000 | 99,000 | 290,000 | 17,000 | 110,000 | 5690 | 32,000 | 8260 | 30,000 | 55,000 | 260,000 |
| CAR | 1640 | 3080 | 380 | 100 | 1090 | 1410 | 7440 | 15,000 | 1170 | 2640 | 360 | 80 | 530 | 1300 | 6850 | 13,000 |
| LA | 3840 | 37,000 | 3220 | 3490 | 1350 | 8710 | 33,000 | 91,000 | 5460 | 44,000 | 3830 | 3050 | 650 | 8760 | 33,000 | 99,000 |
| EECA | 12,000 | 1670 | 110 | 45,000 | 23,000 | 11,000 | 7370 | 100,000 | 21,000 | 4080 | 210 | 40,000 | 48,000 | 24,000 | 8610 | 150,000 |
| MENA | 670 | 4080 | 260 | 1110 | 140 | 1200 | 1170 | 8630 | 970 | 7980 | 420 | 1320 | 110 | 1670 | 2380 | 15,000 |
| ESA | 43,000 | 20,000 | 1280 | 5050 | 130,000 | 25,000 | 660,000 | 890,000 | 21,000 | 12,000 | 630 | 2960 | 46,000 | 12,000 | 310,000 | 400,000 |
| WCA | 46,000 | 5710 | 360 | 5950 | 24,000 | 16,000 | 120,000 | 220,000 | 15,000 | 3970 | 210 | 3310 | 5760 | 6560 | 69,000 | 100,000 |
| WCENA | 1730 | 38,000 | 1200 | 4580 | 110 | 8690 | 12,000 | 66,000 | 1950 | 30,000 | 1130 | 4180 | 130 | 4940 | 8450 | 50,000 |
| Global | 130,000 | 190,000 | 12,000 | 110,000 | 200,000 | 89,000 | 940,000 | 16,80,000 | 84,000 | 210,000 | 12,000 | 87,000 | 110,000 | 89,000 | 490,000 | 10,90,000 |
| (b) | ||||||||||||||||
| AP | 6.7% | 28% | 1.8% | 17% | 7.9% | 5.5% | 33% | 100% | 6.6% | 42% | 2.2% | 12% | 3.2% | 12% | 21% | 100% |
| CAR | 11% | 20% | 2.5% | 0.6% | 7.2% | 9.3% | 49% | 100% | 9.0% | 20% | 2.8% | 0.60% | 4.1% | 10% | 53% | 100% |
| LA | 4.2% | 41% | 3.5% | 3.8% | 1.5% | 9.6% | 37% | 100% | 5.5% | 45% | 3.9% | 3.1% | 0.66% | 8.8% | 34% | 100% |
| EECA | 12% | 1.7% | 0.11% | 45% | 23% | 11% | 7.4% | 100% | 15% | 2.8% | 0.15% | 27% | 33% | 17% | 5.9% | 100% |
| MENA | 7.8% | 47% | 3.0% | 13% | 1.6% | 14% | 14% | 100% | 6.5% | 54% | 2.8% | 8.9% | 0.77% | 11% | 16% | 100% |
| ESA | 4.8% | 2.2% | 0.14% | 0.57% | 14.8% | 2.9% | 74% | 100% | 5.2% | 3.0% | 0.16% | 0.74% | 11% | 3% | 77% | 100% |
| WCA | 22% | 2.7% | 0.17% | 2.8% | 11.2% | 7.5% | 54% | 100% | 15% | 3.8% | 0.20% | 3.2% | 5.5% | 6% | 66% | 100% |
| WCENA | 2.6% | 58% | 1.8% | 6.9% | 0.17% | 13% | 18% | 100% | 3.9% | 59% | 2.2% | 8.3% | 0.25% | 10% | 17% | 100% |
| Global | 7.7% | 11% | 0.72% | 6.8% | 12% | 5.3% | 56% | 100% | 7.7% | 20% | 1.1% | 8.0% | 10% | 8.2% | 45% | 100% |
| (c) | ||||||||||||||||
| AP | 0.34 | 0.47 | 0.36 | 1.71 | 0.1 | 0.014 | 0.30 | 0.65 | 0.54 | 1.2 | 0.022 | 0.012 | ||||
| CAR | 0.56 | 0.77 | 1.85 | 0.4 | 0.20 | 0.078 | 0.36 | 0.68 | 1.9 | 0.27 | 0.069 | 0.063 | ||||
| LA | 0.26 | 1.03 | 1.4 | 1.4 | 0.03 | 0.031 | 0.25 | 1.2 | 1.7 | 1.1 | 0.011 | 0.031 | ||||
| EECA | 1.9 | 0.12 | 0.11 | 2.13 | 0.4 | 0.066 | 3.6 | 0.29 | 0.31 | 2.0 | 0.87 | 0.10 | ||||
| MENA | 0.09 | 0.42 | 0.38 | 0.54 | 0.004 | 0.004 | 0.11 | 0.68 | 0.70 | 0.54 | 0.003 | 0.006 | ||||
| ESA | 6.2 | 1.6 | 1.5 | 2.5 | 1.7 | 0.53 | 1.6 | 0.64 | 0.68 | 1.0 | 0.40 | 0.17 | ||||
| WCA | 3.3 | 0.55 | 0.50 | 1.1 | 0.44 | 0.10 | 0.66 | 0.24 | 0.26 | 0.42 | 0.068 | 0.035 | ||||
| WCENA | 0.10 | 0.34 | 0.04 | 0.20 | 0.001 | 0.014 | 0.08 | 0.24 | 0.08 | 0.11 | 0.001 | 0.011 | ||||
| Global | 0.97 | 0.52 | 0.52 | 0.25 | 0.27 | 0.047 | 0.53 | 0.55 | 0.43 | 0.85 | 0.13 | 0.028 |
“Remaining population” is defined as the difference between the sum of new infections among KP and their sex partners, and all new infections estimated for the region, based on UNAIDS 2023 national HIV estimates. All numbers and percentages were rounded to 2 significant digits, before or after the comma, except for values below 0.1, presented with 1 significant digit to reflect relatively large uncertainty.
Few new infections among TGW were estimated in all regions and both years, reflecting their small share of the population. Their contribution was largest in Latin America (LA, 3.9% in 2022), MENA and the Caribbean (CAR; both 2.8%). PWID comprised the highest proportions of new infections in EECA (27%) and Asia and the Pacific (AP, 12%).
Relatively large proportions of new infections occurred in the remaining populations in ESA (77%) and WCA (66%).
New Infections by KPs: 2010–2022 Differences
The distribution of new HIV infections shifted from 2010 to 2022. The proportion of new infections among MSM nearly doubled (from 11% to 20%; Table 2b) and rose among PWID (6.8%–8.0%). Infections among TGW increased from 0.72% to 1.1% (most notably so, in AP and WCENA). The proportional similarity in the 2010-to-2022 difference between MSM and TGW reflects the assumption that TGW covered 5% of MSM + TGW infections because they were not disaggregated in historical surveillance data.
The proportion of new infections was stable among SW (7.7% both years), whereas it decreased among clients of FSW (12%–10%) and in the remaining population (56%–45%).
From 2010 to 2022, overall estimated adult new HIV infections fell by 35% globally, specifically by 41% in women and 29% in men.17 The corresponding annual number of new infections increased by 11% and 3% among MSM and TGW, respectively, but declined by 24% among PWID and by 35% among SW (Fig. 2). Globally, new infections among clients of SW fell 47% (Fig. 2); it did not change for nonclient partners of KP.
FIGURE 2.
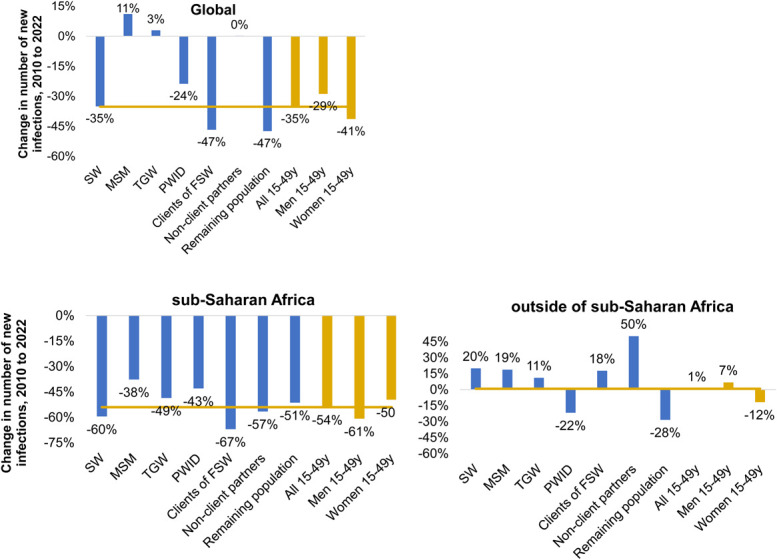
Proportional change, 2010 to 2022, in annual new adult infection numbers among KPs: (A) globally; (B) SSA; and (C) outside of SSA. Yellow lines indicate equality with the reduction in the overall 15- to 49-year-old population (as in the third but right most bar). Negative percentages indicate a decrease in new infections in 2022 compared to 2010; positive percentages a higher (increased) number.
Across regions and KPs, declines were larger (ie, 2022-to-2010 ratios were smaller) for absolute numbers of infections (Fig. 2) than for proportions (Table 3), reflecting that in most regions, the time trend in KP infections was in the same direction as the trend among adults overall.
TABLE 3.
Proportional Change (in %) in the Proportion of Adult New Infections, 2010 Compared with 2022, by KP and Region
| Region | SW | MSM | TGW | PWID | Clients of FSW | Nonclient Partners of KPs | Remaining Population |
| AP | −1.6 | 52 | 23 | −25 | −59 | 113 | −36 |
| CAR | −17 | 0.5 | 10 | −6 | −42 | 7.6 | 7.8 |
| LA | 30 | 10 | 8.9 | −20 | −56 | −8.0 | −8.6 |
| EECA | 19 | 66 | 37 | −39 | 43 | 50 | −20 |
| MENA | −16 | 14 | −6 | −31 | −52 | −19 | 18 |
| ESA | 7.2 | 33 | 10 | 29 | −24 | 0.1 | 3.0 |
| WCA | −32 | 43 | 18 | 14 | −51 | −17 | 23 |
| WCENA | 48 | 1.7 | 24 | 20 | 50 | −25 | −4.9 |
| Global | 0.3 | 72 | 59 | 18 | −18 | 55 | −19 |
Negative percentages indicate a lower (decreased) proportion in 2022 than 2010; positive percentages a higher (increased) proportion.
In SSA, annual adult new infections fell among all KP and among clients of FSW. Declines in annual numbers were proportionally less than the overall 54% decline among PWID, MSM, and TGW but proportionally more among SW, clients, and other partners.
Outside of SSA, overall adult new infection numbers increased by a relative 1% from 2010 to 2022 (decreasing in AP, CAR, and WCENA but increasing elsewhere; Table 2a and Fig. 2). Summed across the non-SSA regions, estimated annual new infections increased from 2010 to 2022 for SW, MSM, and TGW, FSW clients, and other partners of KP. Among the KP, annual new infection numbers decreased only for PWID and increased in all other groups, including among men in the remaining population (Table 2a and Fig. 2).
Trends in overall new adult and KP infections differed across regions: for MSM and TGW, proportions of infections increased in all regions albeit less so in CAR, LA, MENA, and WCENA than in ESA, WCA, AP, and especially EECA (Table 3). For SW, the annual number and proportion of adult infections decreased in WCA, AP, and CAR but increased in LA, EECA, and WCENA, whereas in MENA, the proportion decreased but the absolute number increased. For PWID, the proportion of adult infections decreased especially in AP, EECA, and MENA, but it increased in WCA, ESA, and WCENA. Clients experienced increasing proportions of adult new infections and infection numbers in EECA and WCENA but decreases in both metrics elsewhere.
Incidence Rate Ratios
IRRs were above 1 for all KPs, and all regions for both 2010 and 2022, except for clients of SWs (Table 4). In other words, incidence was higher among the KPs compared with that in the overall adult population.
TABLE 4.
IRRs in KPs Relative to Adult (15–49 years) Men and Women
| 2010 | 2022 | |||||||||||
| SW | MSM Incl. TG | MSM | TGW | PWID | Clients of FSW | SW | MSM Incl. TG | MSM | TGW | PWID | Clients of FSW | |
| AP | 31 | 16 | 16 | 13 | 70 | 4.7 | 23 | 41 | 42 | 19 | 42 | 3.9 |
| CAR | 7.7 | 10 | 11 | 16 | 4.1 | 2.8 | 5.9 | 13 | 16 | 18 | 3.8 | 1.2 |
| LA | 9.5 | 33 | 37 | 39 | 49 | 0.84 | 8.3 | 37 | 42 | 43 | 46 | 0.38 |
| EECA | 10 | 10 | 10 | 11 | 35 | 0.64 | 8.0 | 20 | 20 | 21 | 19 | 0.85 |
| MENA | 14 | 80 | 89 | 76 | 128 | 0.35 | 19 | 86 | 94 | 94 | 88 | 0.30 |
| ESA | 16 | 2.5 | 2.5 | 2.3 | 5.8 | 2.4 | 11 | 3.1 | 3.1 | 3.2 | 6.5 | 1.1 |
| WCA | 25 | 8 | 7.9 | 7.2 | 8.0 | 2.4 | 14 | 5 | 4.9 | 5.2 | 9.4 | 1.2 |
| WCENA | 11 | 16 | 21 | 4.0 | 18 | 0.08 | 7.4 | 17 | 19 | 18.0 | 6.2 | 0.11 |
| Global | 12 | 16 | 19 | 11 | 21 | 0.85 | 8.9 | 21 | 23 | 20 | 14 | 0.59 |
IRRs are reported as medians across countries. Population size estimates and KP prevalence that were combined with the infection estimates to calculate these IRRs are summarized in Supplemental Digital Content 1, http://links.lww.com/QAI/C175.
Globally, in 2022, IRRs for KPs relative to overall adult population ranged from 0.59 for FSW clients to 23 for MSM. The ratios decreased in all regions between 2010 and 2022 for SW except in MENA and for FSW clients except in EECA and WCENA. By contrast, ratios increased for MSM in all regions except WCA and WCENA and increased for TGW in all regions except WCA. For PWID, IRRs decreased in all non-SSA regions.
DISCUSSION
We estimated that MSM, TGW, and nonclient partners of KP made up larger proportions of adult new HIV infections in 2022 compared with 2010, although infection proportions were stable among SW and probably decreased among their clients. Results should be interpreted against a background of decreasing new adult infections overall (35% decline globally; Fig. 2) and median across countries 23% (Supplemental Digital Content 2, http://links.lww.com/QAI/C176), although the decline varied by region. Our analysis concurs with other research that suggests that in regions with expanding access to HIV services and declining overall incidence, such as SSA,1,18 HIV will concentrate in core groups including KP, whose relative risks for acquiring HIV will increase.29,30
These refined estimates have several strengths. First, where possible, they are based on national transmission-dynamic mathematical models, which explicitly capture transmission and turnover across groups. This enforces consistency in estimates over time, across population groups and across countries, and is explicit about overlap among groups. Second, where no model was available, alternative sources were systematic (eg, national case notifications by the mode of transmission) or a standardized extrapolation from regional patterns based on countries with an estimate or eligible data. The chosen hierarchy of sources assured that most inputs were derived with consistent methodology, comparable over the period and across countries, and used the highest-quality data and inputs available for each country. Finally, our analysis aggregated from country estimates instead of only regional levels. Although we did not present country-level estimates, this reflects a step toward KP estimates owned by countries.
Our estimates suggest lower proportions of new infections among KPs, with correspondingly lower IRRs, than published by UNAIDS between 2016 and 2022.1 This is principally explained by replacing pre-2010 modes-of-transmission studies data with up-to-date transmission-mechanistic models (details in Supplemental Digital Content 1, http://links.lww.com/QAI/C175).
Comparing these revised estimates with independent meta-analyses of cohort-based HIV incidence and of prevalence distributions in population-based studies and models, we note the following:
A meta-regression of longitudinal studies among MSM in SSA estimated a nonsignificant decrease in incidence rates among MSM over 2005–20203; the IRRs relative to all men (27–150) were larger than ours (median 2.5–7.9 between the 2 subregions and years). This partially reflects that our IRRs were expressed relative to men and women combined (with, in SSA, a higher overall incidence rate than men alone). Also, incidence studies in this meta-analysis may have oversampled higher-risk MSM;
Meta-analyses of FSW cohorts in SSA found declining incidence rates and stable IRRs over 2010–2020,31 and prevalence ratios of 1.5–1.8532,33 for clients of FSW relative to all men. Both are consistent with our results;
For FSWs and their partners in MENA, a 12-country modeling study estimated that FSW, their clients, and spouses of clients comprised 28% of adult infections in 2020,34 similar to our 23% and 19% for SW, FSW clients, and partners of FSW clients in 2010 and 2022, respectively;
Unlike for SW and MSM in SSA, for whom our infection estimates were supported by empirical meta-analyses,3,31 for PWID, data about incidence and its trends are scarce. A meta-analysis of data published in 2000–2022 found a 1.7 per 100 person-years global incidence,35 more than our 1.3 per 100 and 0.85 per 100 for 2010 and 2022, with most of this difference from MENA region. The review, based on limited temporal data, found no evidence of a shift over time. Lower incidence among PWID observed in high-income settings could reflect a larger and earlier impact of combined prevention and care compared with low- and middle-income settings.35,36
As a general caveat, annual infection metrics capture only part of the cumulative population-attributable fractions of new HIV infections among KP because onward transmission through and beyond current sex partners and networks is omitted. Transmission-dynamic models including those we used also show that because of the turnover from a KP (ie, when some individuals stop the defining behavior, eg, stop injecting drugs and other individuals start the behavior), new infections acquired by KPs accumulate as prevalent infections in general populations. Therefore, it is critical to consider the active modes of transmission as elucidated by this analysis to best design HIV prevention programs for KP and avert continued transmission.37–41
Limitations and Uncertainty
Proportions of new infections and IRRs varied considerably across countries and years, reflecting heterogeneity of national epidemics and temporal variations. Some variations reflect nonrepresentative and incomplete data, imperfect model assumptions (eg, on turnover), and suboptimal region-based extrapolations. An example is the high estimated proportion of adult infections among clients of FSW in Russia (estimated by Goals, fitted to one possibly unrepresentative prevalence measurement among FSW), which impacts the entire EECA region.
We did not statistically define uncertainty but presume it is substantial. Quantifying uncertainty in this multimethods synthesis is a future goal.
Model-specific limitations are outlined in Supplemental Digital Content 1, http://links.lww.com/QAI/C175. Additional uncertainties are enumerated below.
First, KP size estimates and turnover data are scarce. These drive modeled estimates of numbers of infections for a given prevalence. Data are scarce for TGW, and for MSM and PWID (Supplemental Digital Content 1, http://links.lww.com/QAI/C175). For SW and PWID, faster turnover implies more new infections for a given prevalence; some country models may have underestimated turnover and new infections42,43 (Supplemental Digital Content 1, http://links.lww.com/QAI/C175). Periodic data collection may not be comparable over time because of changes in sampling and inclusion criteria. For MSM, undersampling or missing younger, lower-risk, and hidden MSM from size estimations has been a particular challenge.13
Second, there are biases in group sizes and prevalence trends because of changes in how KP self-identify and how biobehavioral surveys categorize risks. This is especially true for TGW and MSM44 but also PWID in countries whose epidemics started among SW and MSM, with HIV surveillance among PWID started more recently.45 Fundamentally, most models and surveillance frameworks treat KP as if they are well-defined distinct populations. In reality, however, these populations are fluid and may overlap (eg, MSM who inject drugs), and turnover between the groups may occur not only in the general population (as assumed by all models) but also among KPs. The impact of these biases on KP infection estimates may vary: ignoring overlap among KPs would cause double counting and overestimation of incident and prevalent infections, whereas assuming no turnover among KP (as opposed to turnover from KP to the general population) could deflate (prevalent) infections among KP relative to the general population. Without expanded data collection (notably, size estimates) for these small double-risk KPs, these biases are hard to quantify. However, they likely do not substantively alter the results in terms of KP altogether—and thereby the programmatic needs.
Third, scarce comparable KP prevalence and incidence trend data from most countries leaves models undue freedom in fitting KP-specific trends. Some MSM surveys may oversample higher-risk men, whereas others oversample younger MSM with lower prevalence, potentially distorting the real prevalence in the MSM population at large or the time trend between successive surveys.46
Fourth, all compartmental models may simulate too many new infections in long-term heterosexual partnerships (as opposed to KPs) by assuming all low-risk individual pair with a new (low-risk) partner every year.47
Fifth, inferring recent infections in KPs from case diagnoses by the mode of transmission may not be valid. Case diagnoses in many settings underreported homosexuality and injection drug use as the mode of transmission.48 Until recently, TGW status was rarely recorded, and TG men often continue to be absent as a distinct group in reporting.
Finally, missing data for some regions required region-based extrapolations—notably for TGW, clients and other non-KP sex partners. Extrapolation is a common approach and UNAIDS regions broadly reflect interregional epidemic and health care patterns, except for some (lower-burden) countries. For example, New Zealand, Australia, Singapore, and Japan may fit better epidemiologically and programmatically with WCENA than Asia Pacific. Their KP infection estimates were largely based on national case diagnoses but resulting IRRs—combining diagnoses-based infections with regionally extrapolated group sizes—are less certain than for countries with KP populations and epidemics quantified within a national estimation framework.
Besides addressing the above limitations, future refinements may include systematic triangulation and reconciliation across epidemic models anchored in different data types, for example, prevalence versus case and death surveillance,49 within countries with multiple options. This could include case diagnoses by the mode of transmission for additional countries. Additional compartmental transmission models50–52 could be triangulated, and individual (agent)-based models could help refine and validate the representation of dynamic sexual networking effects38,53,54 not explicitly captured in compartmental transmission and statistical models.
In conclusion, despite large uncertainties in data inputs and modeled estimates, this multimethod analysis confirms that KPs account for considerable proportions of adult new HIV infections.37 There were probable increases from 2010 to 2022 in the proportion of new HIV infections among KPs in some regions; MSM and TGW outside of SSA experienced possible increases in both the proportions and absolute annual numbers of new HIV infections. This underscores the importance of offering and creating access to prevention, testing, and treatment services for these communities. The results also suggest that effective population-level services may be contributing to declining infections among some populations and regions, such as SW and their clients in SSA.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank for their thoughtful and constructive advice and comments, the following experts consulted during the estimation: Jeffrey Eaton (Imperial College London), Cari van Schalkwyk (South African Centre for Epidemiological Modelling and Analysis, Stellenbosch University), Anastasia Pharris (European Centers for Disease Prevention and Control), Avi Hakim (USA Centers for Disease Control and Prevention), James Hargreaves (London School of Hygiene and Tropical Medicine), Jerry Jacobson (independent consultant), Jesse Knight (University of Toronto), Kate Rucinski (John Hopkins University), Kelsey Case (independent consultant for UNAIDS Data for Impact), Ian Wanyeki (UNAIDS, Data for Impact), Ligia Kerr (Universidade Federal do Ceará), Mathieu Maheu-Giroux (McGill University), Monica Malta (University of Toronto), Phelister Abdalla (Network of Sex Work Professionals, Kenya), Sharmistha Mishra (Division of Infectious Diseases, Department of Medicine, University of Toronto), Stef Baral (Johns Hopkins University), Wolfgang Hladik (USA Centers for Disease Control and Prevention), Alex Whitlock (Division of Infectious Diseases, Department of Medicine, University of Toronto), Alfonso Silva-Santisteban (CIISSS Universidad Peruana Cayetano Heredia), Amrita Rao (Johns Hopkins University), Andreas Jahn (Malawi Ministry of Health & I-TECH Malawi), Andrew Scheibe (University of Pretoria), Ard van Sighem (Stichting HIV Monitoring), Bradley Mathers (WHO consultant), Carl Corcoran (US Centers for Disease Control and Prevention), Carlos Cáceres (Universidad H. Peruviana), Carrie Lyons (Johns Hopkins University), Ekow Wiah (Ghana Ministry of Health), Deepa Jahagirdar (Avenir Health), Guy Mahiane (Avenir Health), Irum Zaidi (PEPFAR, The U.S. President's Emergency Plan for AIDS Relief), Jack Stone (University of Bristol), James Stannah (McGill University), Joshua Salomon (Stanford University), Le Bao (Penn State University), Marie-Claude Boily (Imperial College London), Ray Shiraishi (US Centers for Disease Control and Prevention), Sidy Mokhtar Ndiaye (ENDA Santé), William Miller (USAID, US Agency for International Development), Wiwat Peerapatanapokin (East-West Center), John Ojo (Africa Centers for Disease Control), Kennedy Kipkoech Mutai (Bristol University), Maria Au (USAID, US Agency for International Development), Reshma Bhattacharjee (US Agency for International Development), Wade Ivy (US Centers for Disease Control and Prevention), Laura Porter (US Centers for Disease Control and Prevention).
Footnotes
O.S. was funded by UNAIDS. O.S. and R.S. were supported by the HIV Prevention Trial Network (HPTN) Modelling Centre, which is funded by the US National Institutes of Health (grant number UM1-AI068617) through the HPTN Statistical and Data Management Center and the MRC Centre for Global Infectious Disease Analysis (reference MR/R015600/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth and Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. P.V. acknowledges funding from the Wellcome Trust (WT 226619/Z/22/Z).
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Contributor Information
Keith Sabin, Email: sabink@unaids.org.
John Stover, Email: jstover@avenirhealth.org.
Tim Brown, Email: brownt@eastwestcenter.org.
Leigh F. Johnson, Email: leigh.johnson@uct.ac.za.
Rowan Martin-Hughes, Email: rowan.martin-hughes@burnet.edu.au.
Debra ten Brink, Email: debra.tenbrink@burnet.edu.au.
Yu Teng, Email: yteng@avenirhealth.org.
Oliver Stevens, Email: o.stevens@imperial.ac.uk.
Romain Silhol, Email: r.silhol@imperial.ac.uk.
Sonia Arias-Garcia, Email: ariasgarcias@unaids.org.
Joshua Kimani, Email: jkimani@csrtkenya.org.
Robert Glaubius, Email: rglaubius@avenirhealth.org.
Peter Vickerman, Email: peter.vickerman@bristol.ac.uk.
Mary Mahy, Email: mahym@unaids.org.
REFERENCES
- 1.UNAIDS. In Danger: UNAIDS Global AIDS Update 2022. Geneva, Switzerland: UNAIDS; 2022. [Google Scholar]
- 2.Patel EU, Solomon SS, Lucas GM, et al. Temporal change in population-level prevalence of detectable HIV viraemia and its association with HIV incidence in key populations in India: a serial cross-sectional study. Lancet HIV. 2021;8:e544–e553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stannah J, Soni N, Lam JKS, et al. Trends in HIV testing, the treatment cascade, and HIV incidence among men who have sex with men in Africa: a systematic review and meta-analysis. Lancet HIV. 2023;10:e528–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations Population Division. World Population Prospects 2022 Revision. New York, NY: United Nations Population Division; 2022. [Google Scholar]
- 5.UNAIDS. Indicators and Questions for Monitoring Progress on the 2021 Political Declaration on HIV and AIDS: Global AIDS Monitoring 2023. Geneva, Switzerland: UNAIDS; 2022. [Google Scholar]
- 6.Gall J, Sabin K, Frescura L, et al. Global trends of monitoring and data collection on the HIV response among key populations since the 2001 UN declaration of Commitment on HIV/AIDS. AIDS Behav. 2017;21(suppl 1):34–43. [DOI] [PubMed] [Google Scholar]
- 7.Brown T, Peerapatanapokin W. The Asian Epidemic Model: a process model for exploring HIV policy and programme alternatives in Asia. Sex Transm Infect. 2004;80(suppl 1):i19–i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson L, Dorrington R. Thembisa, Version 4.1: A Mathematical Model of HIV Transmission and Epidemic Spread in South Africa. Cape Town: Centre for Infectious Disease Epidemiology and Research, University of Cape Town; 2018; Available at: https://thembisa.org/downloads. [Google Scholar]
- 9.Stover J, Glaubius R, Teng Y, et al. Modeling the epidemiological impact of the UNAIDS 2025 targets to end AIDS as a public health threat by 2030. Plos Med. 2021;18:e1003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr CC, Stuart RM, Gray RT, et al. Optima: a model for HIV epidemic analysis, program prioritization, and resource optimization. J Acquir Immune Defic Syndr. 2015;69:365–376. [DOI] [PubMed] [Google Scholar]
- 11.Burnet Institute. Allocation of HIV Resources towards Maximizing the Funding in Selected Eastern European and Central Asian Countries: Findings from Optima HIV Modeling Analyses Across 12 Countries in Eastern Europe and Central Asia. Melbourne, Australia: Burnet Institute; 2023. [Google Scholar]
- 12.Burnet Institute. Technical Reports of HIV Applications. 2023; Available at: http://optimamodel.com/hiv/applications.html. Accessed July 07, 2023. [Google Scholar]
- 13.World Health Organization, UNAIDS. Recommended Population Size Estimates of Men Who Have Sex with Men. Geneva, Switzerland: World Health Organization, UNAIDS; 2020. [Google Scholar]
- 14.Sharifi H, Mirzazadeh A, Shokoohi M, et al. Estimation of HIV incidence and its trend in three key populations in Iran. PLoS One. 2018;13:e0207681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mumtaz GR, Awad SF, Feizzadeh A, et al. HIV incidence among people who inject drugs in the Middle East and North Africa: mathematical modelling analysis. J Int AIDS Soc. 2018;21:e25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daw MA, Daw AM, Sifennasr NEM, et al. Spatiotemporal analysis and epidemiological characterization of the human immunodeficiency virus (HIV) in Libya within a twenty five year period: 1993-2017. AIDS Res Ther. 2019;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UNAIDS. Global Data on HIV Epidemiology and Response; 2023. Available at: https://aidsinfo.unaids.org/. Accessed August 1, 2023. [Google Scholar]
- 18.UNAIDS. The Path that Ends AIDS: 2023 UNAIDS Global AIDS Update. Geneva, Switzerland: UNAIDS; 2023. [Google Scholar]
- 19.Stevens O Sabin K Arias Garcia S, et al. Population size, HIV prevalence, and antiretroviral therapy coverage among key populations in sub-Saharan Africa: collation and synthesis of survey data 2010-2023. medRxiv. 2022. doi: 10.1101/2022.07.27.22278071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuba Ministerio de Salud Publica Dirección Nacional de Epidemiología. Informe epidemiológico anual de Programa Nacional de Prevención y control de las ITS/VIH y hepatitis virales, Cuba, 2010-2012. Havana: Cuba Ministerio de Salud Publica Dirección Nacional de Epidemiología; 2023. [Google Scholar]
- 21.King J, McManus H, Kwon A, et al. HIV, Viral Hepatitis and Sexually Transmissible Infections in Australia: Annual Surveillance Report 2022. Sydney, Australia: The Kirby Institute, UNSW Sydney; 2022. [Google Scholar]
- 22.AIDS Bulletin—May 2022. ISSM 1170-2656 (Print) / ISSM 1178-2692 (Online).
- 23.Singapore Ministry of Health, National Center for Infectious Diseases. Update on the HIV/AIDS Situation in Singapore 2021 (June 2022); 2022. Available at: https://www.moh.gov.sg/resources-statistics/infectious-disease-statistics/hiv-stats/update-on-the-hiv-aids-situation-in-singapore-2021-(june-2022). Accessed August 1, 2023. [Google Scholar]
- 24.US Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report: Diagnoses of HIV Infection in the United States and Dependent Areas, 2020. US Centers for Disease Control and Prevention; 2022. [Google Scholar]
- 25.USA Centers for Disease Control and Prevention. HIV Surveillance Report 2021. Atlanta, GA; USA Centers for Disease Control and Prevention: 2023. [Google Scholar]
- 26.Public Health Agency of Canada. HIV in Canada, Surveillance Report to December 31, 2020. Ottawa: Public Health Agency of Canada. 2023. [Google Scholar]
- 27.Sabin K, Zhao J, Garcia Calleja JM, et al. Availability and quality of size estimations of female sex workers, men who have sex with men, people who inject drugs and transgender women in low- and middle-income countries. PLoS One. 2016;11:e0155150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stengaard AR, Combs L, Supervie V, et al. HIV seroprevalence in five key populations in Europe: a systematic literature review, 2009 to 2019. Eurosurveillance. 2021;26:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortblad KF, Baeten JM, Cherutich P, et al. The arc of HIV epidemics in sub-Saharan Africa: new challenges with concentrating epidemics in the era of 90-90-90. Curr Opin HIV AIDS. 2019;14:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garnett GP. Reductions in HIV incidence are likely to increase the importance of key population programmes for HIV control in sub-Saharan Africa. J Int AIDS Soc. 2021;24(suppl 3):e25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones HS, Anderson R, Cust H, et al. HIV Incidence among women engaging in sex work in sub-Saharan Africa: a systematic review and meta-analysis. medRxiv. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hodgins C, Stannah J, Kuchukhidze S, et al. Population sizes, HIV prevalence, and HIV prevention among men who paid for sex in sub-Saharan Africa (2000-2020): a meta-analysis of 87 population-based surveys. Plos Med. 2022;19:e1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xi M, Yiu KC, Srivatsav V, et al. HIV Prevalence Among Male Clients and Non-paying Male Sex Partners of Female Sex Workers in Sub-saharan Africa: A Systematic Review and Meta-Analysis. Paper presented at: International AIDS Conference 2022; Montreal.
- 34.Chemaitelly H, Ayoub HH, Omori R, et al. HIV incidence and impact of interventions among female sex workers and their clients in the Middle East and north Africa: a modelling study. Lancet HIV. 2022;9:e496–e505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Artenie A, Stone J, Fraser H, et al. Incidence of HIV and hepatitis C virus among people who inject drugs, and associations with age and sex or gender: a global systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8:533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aghaei AM, Gholami J, Sangchooli A, et al. Prevalence of injecting drug use and HIV, hepatitis B, and hepatitis C in people who inject drugs in the Eastern Mediterranean region: a systematic review and meta-analysis. Lancet Glob Health. 2023;11:e1225–e1237. [DOI] [PubMed] [Google Scholar]
- 37.Brown T, Peerapatanapokin W. Evolving HIV epidemics: the urgent need to refocus on populations with risk. Curr Opin HIV AIDS. 2019;14:337–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steen R, Hontelez JA, Veraart A, et al. Looking upstream to prevent HIV transmission: can interventions with sex workers alter the course of HIV epidemics in Africa as they did in Asia? AIDS. 2014;28:891–899. [DOI] [PubMed] [Google Scholar]
- 39.Mishra S, Pickles M, Blanchard JF, et al. Distinguishing sources of HIV transmission from the distribution of newly acquired HIV infections: why is it important for HIV prevention planning?. Sex Transm Infect. 2014;90:19–25. [DOI] [PubMed] [Google Scholar]
- 40.Mukandavire C, Walker J, Schwartz S, et al. Estimating the contribution of key populations towards the spread of HIV in Dakar, Senegal. J Int AIDS Soc. 2018;21(suppl 5):e25126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser H, Borquez A, Stone J, et al. Overlapping key populations and HIV transmission in Tijuana, Mexico: a modelling analysis of epidemic drivers. AIDS Behav. 2021;25:3814–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazito E, Cuchi P, Mahy M, et al. Analysis of duration of risk behaviour for key populations: a literature review. Sex Transm Infect. 2012;88(suppl 2):i24–i32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens O, Anderson R, Imai-Eaton JW. Age distributions and duration of risk behaviour for female sex workers, men who have sex with men, and people who inject drugs in sub-Saharan Africa. in preparation.
- 44.Sanders EJ, Jaffe H, Musyoki H, et al. Kenyan MSM: no longer a hidden population. AIDS. 2015;29suppl 3:S195–S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.United Nations Office on Drugs and Crime. World Drug Report 2023. Vienna; United Nations Office on Drugs and Crime; 2023. [Google Scholar]
- 46.Johnson LF, Mulongeni P, Marr A, et al. Age bias in survey sampling and implications for estimating HIV prevalence in men who have sex with men: insights from mathematical modelling. Epidemiol Infect. 2018;146:1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson LF, Geffen N. A comparison of two mathematical modeling frameworks for evaluating sexually Transmitted infection epidemiology. Sex Transm Dis. 2016;43:139–146. [DOI] [PubMed] [Google Scholar]
- 48.Dumchev K, Kornilova M, Kulchynska R, et al. Improved ascertainment of modes of HIV transmission in Ukraine indicates importance of drug injecting and homosexual risk. BMC Public Health. 2020;20:1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahiane SG, Eaton JW, Glaubius R, et al. Updates to Spectrum's case surveillance and vital registration tool for HIV estimates and projections. J Int AIDS Soc. 2021;24(suppl 5):e25777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maheu-Giroux M, Vesga JF, Diabaté S, et al. Changing dynamics of HIV transmission in Cote d'Ivoire: modeling who acquired and transmitted infections and estimating the impact of past HIV interventions (1976-2015). J Acquir Immune Defic Syndr. 2017;75:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stone J, Mukandavire C, Boily MC, et al. Estimating the contribution of key populations towards HIV transmission in South Africa. J Int AIDS Soc. 2021;24:e25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silhol R, Baral S, Bowring AL, et al. Quantifying the evolving contribution of HIV interventions and key populations to the HIV epidemic in yaoundé, Cameroon. J Acquir Immune Defic Syndr. 2021;86:396–405. [DOI] [PubMed] [Google Scholar]
- 53.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7:e629–e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagelkerke NJ, Arora P, Jha P, et al. The rise and fall of HIV in high-prevalence countries: a challenge for mathematical modeling. Plos Comput Biol. 2014;10:e1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


