Supplemental Digital Content is Available in the Text.
Key Words: Mozambique, antenatal, PMTCT, pediatric
Abstract
Background:
Routine health system data are central to monitoring HIV trends. In Mozambique, the reported number of women receiving antenatal care (ANC) and antiretroviral therapy for prevention of mother-to-child transmission (PMTCT) has exceeded the Spectrum-estimated number of pregnant women since 2017. In some provinces, reported HIV prevalence in pregnant women has declined faster than epidemiologically plausible. We hypothesized that these issues are linked and caused by programmatic overenumeration of HIV-negative pregnant women at ANC.
Methods:
We triangulated program-reported ANC client numbers with survey-based fertility estimates and facility birth data adjusted for the proportion of facility births. We used survey-reported ANC attendance to produce adjusted time series of HIV prevalence in pregnant women, adjusted for hypothesized program double counting. We calibrated the Spectrum HIV estimation models to adjusted HIV prevalence data to produce adjusted adult and pediatric HIV estimates.
Results:
ANC client numbers were not consistent with facility birth data or modeled population estimates indicating ANC data quality issues in all provinces. Adjusted provincial ANC HIV prevalence in 2021 was median 45% [interquartile range 35%–52% or 2.3 percentage points (interquartile range 2.5–3.5)] higher than reported HIV prevalence. In 2021, calibrating to adjusted antenatal HIV prevalence lowered PMTCT coverage to less than 100% in most provinces and increased the modeled number of new child infections by 35%. The adjusted results better reconciled adult and pediatric antiretroviral treatment coverage and antenatal HIV prevalence with regional fertility estimates.
Conclusions:
Adjusting HIV prevalence in pregnant women using nationally representative household survey data on ANC attendance produced estimates more consistent with surveillance data. The number of children living with HIV in Mozambique has been substantially underestimated because of biased routine ANC prevalence. Renewed focus on HIV surveillance among pregnant women would improve PMTCT coverage and pediatric HIV estimates.
INTRODUCTION
Mozambique has a generalized HIV epidemic with a 12.4% prevalence among adults aged 15–49 years in 2021.1 HIV incidence and prevalence remain high, and antiretroviral treatment (ART) coverage lags behind neighboring countries in Eastern and Southern Africa.2 Estimates of HIV prevalence and incidence trends are generated by fitting mathematical models to data on HIV prevalence from national household surveys, conducted every 5 years, and HIV prevalence among pregnant women attending antenatal clinics (ANC) informing prevalence trends during the presurvey, intersurvey, and postsurvey periods.3,4 Therefore, routinely collected data from ANC and ART programs are central to estimating population HIV incidence and prevalence in adults, which are used to derive estimates of mother-to-child transmission and the number of children living with HIV.
HIV prevalence measured among pregnant women attending ANC has been used to monitor HIV epidemic trends since the 1990s.5–11 In Mozambique, data collection began at a single facility in Maputo City in 1990 and expanded in the 1990s to biennial sentinel surveillance (ANC-SS) using unlinked anonymous testing of women at 36 sentinel ANC clinics.12 Incorporating ANC HIV prevalence into mathematical models estimating population HIV prevalence requires that HIV prevalence trends in pregnant women are relatable to population trends. Several systematic differences between HIV prevalence among pregnant women and population HIV prevalence are adjusted for in mathematical models, including different age distributions, selection for sexual activity, differential fertility by HIV and ART status, and changing age-specific fertility over time.6,13–17 In addition, health facilities selected for ANC-SS were often high-volume sites in urban areas with HIV prevalence 20%–40% higher than prevalence from nationally representative household surveys,14,15 requiring statistical adjustments to account for nonrepresentative sentinel site locations.
In 2015, WHO and UNAIDS recommended that countries move away from ANC-SS to use ANC routine testing data (ANC-RT) from all pregnant women nationwide to monitor HIV trends,18 which was possible after implementation of routine HIV testing of all pregnant women to identify women requiring antiretrovirals for prevention of mother-to-child HIV transmission (PMTCT). This transition increased the number of facilities included in ANC HIV prevalence calculation from 36 ANC sentinel sites to nearly 1,600 facilities providing routine ANC testing and, therefore, increased statistical precision of prevalence estimates, ensured geographic representativeness, and enabled subnational HIV estimation19,20. However, it required high-quality and consistent reporting of programmatic data, incurring additional workload on programs and clinic staff to report on all pregnant women across paper and digital clinic reporting systems.21–25 This could introduce nonsampling errors absent from sentinel surveys, including ANC “double counting” where multiple ANC visits from a single woman at one or more clinics are recorded as first visits of multiple women.19,26
Before the recently released INSIDA 2021 survey, the most recent nationally representative household HIV survey in Mozambique was in 2015, coinciding with the transition from ANC-SS to ANC-RT. Since the transition, several issues have persisted with key epidemic indicators calculated using routinely collected program data. These include the following: reported number of women seeking ANC exceeding modeled estimates for the total number of pregnant women; the reported number of women receiving ART for PMTCT exceeding the Spectrum-estimated number of HIV-positive pregnant women; ANC-RT HIV prevalence declining sharply, faster than could be plausibly caused by outmigration, mortality, incidence, or fertility declines among HIV-positive women; and population ART coverage rapidly approaching or exceeding the estimated number of people with HIV in several provinces.27 These discrepancies may reflect potential errors in a number of programmatic data inputs or interpretations thereof in the modeling process, including inaccurate population and demographic assumptions, duplicate reporting of patients, and transcription errors in data reporting between paper and electronic registers.28–31
Owing to the central position of antenatal clinical data within HIV estimation, the implausibly rapid declines in reported HIV prevalence among pregnant women, and larger-than-expected reported ANC clients and women receiving PMTCT services, we hypothesized that these issues are linked and stem from ANC data quality: (1) inflated reporting of HIV-negative pregnant women artificially reduces ANC-RT HIV prevalence; (2) calibrating to low ANC-RT HIV prevalence causes underestimation of total population HIV prevalence and number of PLHIV; and (3) underestimating the number of PLHIV leads to overestimation of PMTCT and ART coverage. We estimated the magnitude of reporting errors and triangulated ANC data using routinely collected programmatic birth data. We quantified the impact of ANC-RT on estimates of adult HIV prevalence and ART coverage and proposed HIV estimates using adjusted ANC data.
DATA AND METHODS
Our analysis consisted of 3 components: (1) assessing whether recent trends in routinely reported ANC clients and ANC-RT HIV prevalence were plausibly consistent with other data sources or likely inconsistent because of systematic reporting issues; (2) adjusting ANC-RT data for plausible bias mechanisms to adjust estimated HIV prevalence trends among pregnant women; and (3) analyzing the effect of calibrating to adjusted ANC-RT HIV prevalence on adult and pediatric HIV estimates in Mozambique.
Data
Routine ANC Data
Routine facility-aggregated antenatal care data for 2013–2022 were extracted from SISMA-DHIS2, the Mozambique electronic health information system. Data elements included the number of ANC clients, number of HIV-positive women who know their status, number of women tested for HIV at ANC, number of women tested HIV positive, and number of women already on ART at the first ANC visit. The number of HIV-positive pregnant women receiving PMTCT was extracted from SISMA-DHIS2.4
The number of live births at health facilities was extracted from SISMA-DHIS2 by district and month for 2017–2022. SISMA-DHIS2 does not link ANC visits with subsequent delivery. Women are recorded as entering the ANC cohort at their first visit and exiting the cohort 5 months later, regardless of their gestation at first ANC visit. To approximately align the recorded timing of first ANC visits and deliveries, month of birth was brought forward in time by 5 months.
Household Survey Data
Data from 6 nationally representative household surveys were used in this analysis: 2003 Demographic Health Survey (DHS),32 2009 Inquérito Nacional de Prevalência, Riscos Comportamentais e Informação sobre o HIV e SIDA em Moçambique (INSIDA),12 2011 DHS,33 2015 Inquérito de Indicadores de Imunização, Malária e HIV/SIDA em Moçambique (IMASIDA),34 2018 Malaria Indicator Survey (MIS),35 and 2021 INSIDA survey.1 Provincial estimates of HIV prevalence for ages 15–49 years were available from 2009 to 2015 surveys, and provincial ART coverage estimates for ages 15–49 years were available from 2015 surveys. Provincial estimates of HIV prevalence and ART coverage for ages 15–49 years were unavailable from the 2021 INSIDA survey. INSIDA 2021 survey of ages 15–49 years HIV prevalence estimates were approximated using provincial HIV prevalence estimates for ages older than 15 years and the 15–49:15+ years HIV prevalence ratio at the national level. INSIDA 2021 survey of ages 15–49 years ART coverage estimates were approximated using provincial viral load suppression (VLS) estimates for ages older than 15 years, the 15–49:15+ years VLS ratio at the national level, and the VLS:ART coverage ratio at the national level.1 The proportion of women attending ANC for their past pregnancy was available from 2007–2021, from 2011, 2015, and 2018. The proportion of women giving birth in a health facility was available for 2009–2011 and 2013–2015 from the 2011 and 2015 surveys, respectively. Total fertility rates (TFR) were calculated from 2003 and 2011 DHS, 2015 IMASIDA, and 2018 MIS surveys.
For the DHS and AIS surveys, individual-level survey data were extracted using the rdhs package in R.36 Point estimates, variance, and 95% confidence intervals were calculated using the survey package in R,37 accounting for the 2-stage survey design.
Provincial Modeled HIV Estimates With Spectrum
Spectrum is a demographic and HIV epidemic projection model that uses HIV incidence trends to simulate the HIV population over time by age, sex, CD4 cell count category, and ART status and calculates the epidemiologic and demographic impacts of HIV.4 Modeled estimates for number of births were extracted from provincial Spectrum HIV estimates files produced by the Mozambique Ministry of Health HIV Estimates Technical Working Group and shared by UNAIDS.4,38 Age-sex–stratified populations in the projection base year 1970 were manually adjusted to ensure the projected male and female populations at the provincial level in 2017 matched the 2017 Mozambique census results.39 National sex-age–stratified populations and TFR were extracted from the United Nations World Population Prospects 2022.40
Methods
Triangulating Reported ANC Clients With Birth Trends
To assess the plausibility of reported ANC data relative to population and demographic assumptions, we compared trends in the reported number of ANC clients with estimates of the number of births and fertility rates from multiple sources. First, we compared census population estimates with annual demographic estimates from Spectrum at the provincial level and from World Population Prospects 2022 at the national level.
Second, we compared monthly facility births with reported ANC clients in each province. Some births in Mozambique occur outside health facilities and are not recorded within the health information system. Household survey data about the proportion of women who give birth outside health facilities in each province were used to adjust reported facility births. This provided an alternative estimate of total births, comparable with modeled births, from which to calculate an alternative estimate of ANC coverage. To estimate the in-facility birth proportions by year in each province, we fit a mixed-effects logistic regression model with random slopes for each province to survey data spanning 2007–2015 and linearly projected annual estimates to 2022 (see Figure S1A, Supplemental Digital Content, http://links.lww.com/QAI/C150).
Adjusted ANC-RT HIV Prevalence Accounting for Potential Bias Mechanisms
The reported number of women receiving PMTCT exceeded the model-estimated number of HIV-positive pregnant women, and the reported HIV prevalence in pregnant women has declined implausibly rapidly in some provinces. Consequently, we hypothesized that inflated reporting of ANC clients occurs primarily in HIV-negative women, which would cause underestimation of HIV prevalence. We calculated the expected number of first ANC visits for 2014–2022 by multiplying the modeled number of births from Spectrum with survey-reported ANC attendance. Survey-reported ANC attendance was available for up to 2018 and was projected to 2022 using a mixed-effect logistic regression model with random slopes for each province (see Figure S1B, Supplemental Digital Content, http://links.lww.com/QAI/C150). The number of excess-reported ANC visits was calculated by subtracting the expected number of first ANC visits from the reported number of visits. We created an adjusted time series of ANC-RT HIV prevalence by subtracting excess ANC visits from the HIV prevalence denominator.
To quantify the potential consequences of these biases at the population level, we used the Estimates and Projection Package (EPP), an HIV epidemic model that calibrates to nationally representative household survey data and HIV prevalence data from ANC.3 Since 2017, EPP has incorporated ANC-RT HIV prevalence in incidence trends.20 EPP was calibrated in each province to 2009 and 2015 household survey data, ANC-SS data up to 2013, and 3 ANC-RT scenarios: (1) reported ANC-RT data as recorded in SISMA-DHIS2 for 2013–2022, (2) no ANC-RT data, and (3) adjusted ANC-RT data for 2014–2022 by subtracting excess ANC visits from the HIV prevalence denominator. INSIDA 2021 survey data were withheld from model calibration and used to validate model scenarios. Each scenario was used to create estimates of adult HIV prevalence, ART coverage, and HIV incidence, MTCT rate, PMTCT coverage, and new child HIV infections with the Spectrum model.
Finally, we conducted sensitivity analyses of the proportion of excess visits allocated to HIV-negative and HIV-positive women. Ten incremental scenarios assessed the impact of assigning excess ANC visits to only HIV-negative women (as assumed in the main results) up to proportional HIV prevalence (ie, in a province with 10% HIV prevalence, 10% of the excess ANC visits were presumed to be HIV-positive. This results in unchanged HIV prevalence derived from a smaller sample size).
Statistical analyses were conducted in R version 4.1.2. This study received ethical review and approval from the Imperial College London Research Governance and Integrity Team (ICREC #6324189).
RESULTS
Reported ANC-RT HIV Prevalence, ANC Coverage, and PMTCT Coverage
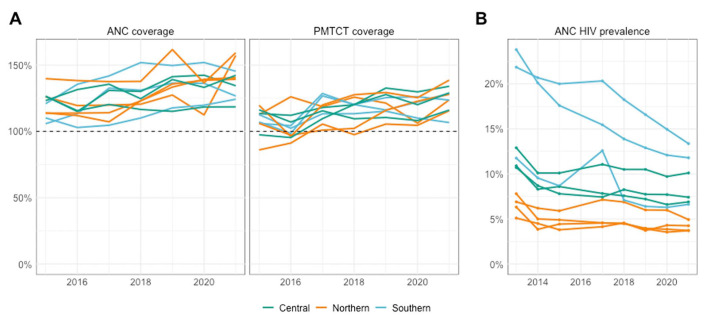
Using Spectrum-modeled birth estimates as denominators, program-derived ANC coverage exceeded 100% in all provinces in 2015 and has continued to rise, with a median provincial ANC coverage of 140% (IQR 129%–145%) in 2021 (Fig. 1A; see Figure S2, Supplemental Digital Content, http://links.lww.com/QAI/C151). Across provinces, program-derived ANC coverage was a median of 32 percentage points (%-pt) (IQR 21–38%-pt) higher than survey estimates of ANC attendance in 2015 and 32%-pt (IQR 26–41%-pt) higher in 2018. PMTCT coverage exceeded 100% in all provinces since 2017. In 2021, the median PMTCT coverage was 126% (IQR 118%–129%).
FIGURE 1.
A, Provincial estimates of ANC and PMTCT coverage from 2015 to 2021 colored by region. B, Provincial estimates of HIV prevalence in pregnant women from 2013 to 2021 colored by region.
From 2013 to 2014, ANC-RT HIV prevalence declined sharply in all provinces, and HIV prevalence from 2013 was excluded from further analyses. Between 2014 and 2021, ANC-RT HIV prevalence declined in Southern Mozambique (Maputo: 20.6%–13.4%; Gaza: 20.1%–11.8%; Inhambane: 9.6%–6.6%) while remaining relatively stable in provinces in Central Mozambique and Northern Mozambique (Fig. 1B).
Triangulation of Reported ANC and PMTCT Program Data With Births and Population
Spectrum estimates of total female population size and adult female age distribution aligned closely to World Population Prospects 2022 (WPP2022) estimates at the national level and with the 2017 Mozambique census at the provincial level (see Figure S3A–C, Supplemental Digital Content, http://links.lww.com/QAI/C152). Total fertility rate inputs to Spectrum were similar to nationally representative survey data and WPP2022 (see Figure S3D, Supplemental Digital Content, http://links.lww.com/QAI/C152). The observed consistency of population and fertility estimates indicated that ANC coverage exceeding 100% was unlikely to be caused by underestimation of the number of births in Spectrum.
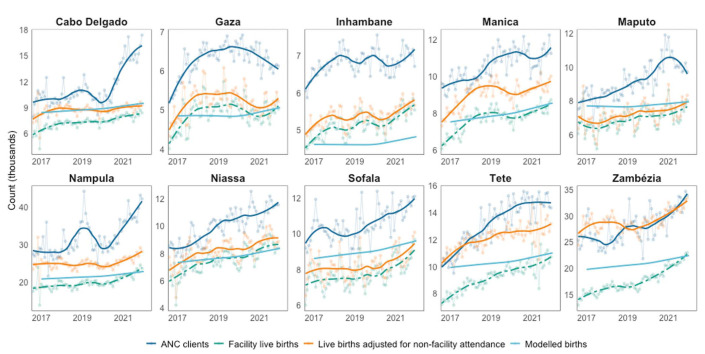
Monthly trends in health facility births aligned well with monthly ANC client trends in most provinces (Fig. 2). However, trends differed in Cabo Delgado in 2020–2022 when ANC clients increased from 10,000 women per month to 17,000 per month and in Nampula in 2019 when ANC clients increased from 28,000 per month to 50,000 per month, with no commensurate increase in facility births, and in Nampula from 2020 to 2021 when ANC clients increased by 51% while births only increased 24%.
FIGURE 2.
Comparison of reported antenatal clinic (ANC) clients [dark blue], raw [green dashed] and adjusted [orange solid] number of live births at health facilities (brought forward in time by 5 months to align approximately with the timing of first ANC visits), and modeled estimates for the number of births from Spectrum (light blue). Smoothed trends shown as bold lines, with raw data as transparent points and lines.
Adjusting facility live births for survey-reported birth location increased births in 2021 by a median of 12% across provinces (IQR 8%–20%), with larger adjustments in Northern and Central provinces where facility attendance was lower (see Figure 1A, Supplemental Digital Content, http://links.lww.com/QAI/C150). However, the reported number of ANC clients was substantially higher than the number of adjusted live births in all provinces except Zambézia, and consequently, ANC coverage calculated using program births or adjusted program births as denominators remained above 100% in most provinces.
Adjusted ANC-RT HIV Prevalence Among Pregnant Women
Across provinces, adjusted antenatal HIV prevalence was median 45% (IQR 35%–52%) higher than the reported HIV prevalence in 2021 or median 3.0 percentage points (IQR 2.5–3.5%-pt) higher. Adjustment had little impact on the rate of HIV prevalence decline. Reported HIV prevalence declined by median 10% (IQR 5%–20%) or 0.9%-pt (IQR 0.4–1.3%-pt) between 2014 and 2021 compared with adjusted HIV prevalence declined by median 9% (IQR 4%–15%) or 0.8%-pt (IQR 0.3–2.0%-pt).
Adult HIV Estimates Calibrated to Adjusted ANC-RT HIV Prevalence
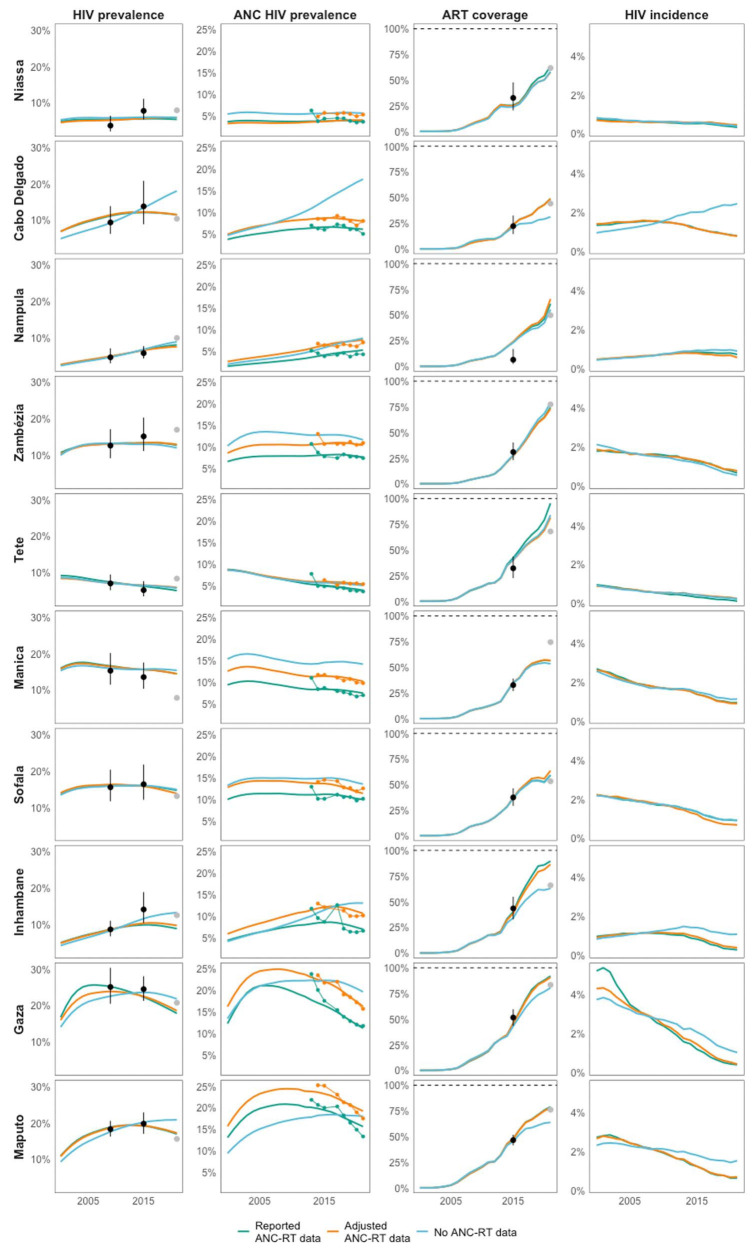
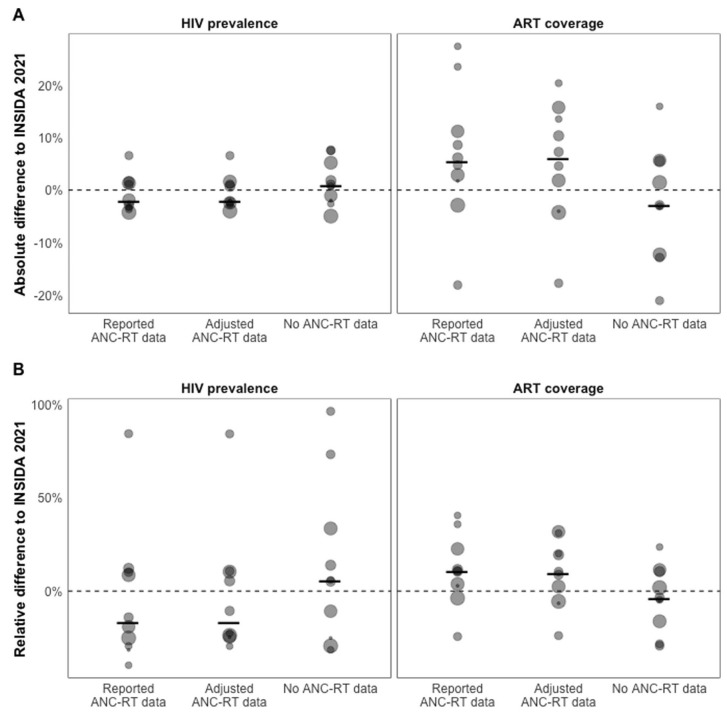
Figure 3 shows provincial estimates of HIV indicators when fitting the EPP and Spectrum models to reported, adjusted, and excluding ANC-RT HIV prevalence data. Estimates for HIV prevalence trends and ART coverage were similar for the reported and adjusted ANC-RT scenarios but differed in 4 of 10 provinces when ANC-RT data were excluded, and estimates were an extrapolation of the pre-2015 transmission rate. In those cases, HIV prevalence was higher and ART coverage lower when ANC-RT data were removed. Calibrating models to reported ANC-RT data produced 2021 HIV prevalence estimates closest to INSIDA 2021 data in 2 of 10 provinces and 1 of 10 provinces for ART coverage. Using adjusted ANC-RT data produced estimates closest to INSIDA prevalence and ART coverage in 4 of 10 provinces, and models without ANC-RT data were closest in 3 of 10 provinces for HIV prevalence and 5 of 10 provinces for ART coverage (Fig. 4; see Figure S4, Supplemental Digital Content, http://links.lww.com/QAI/C153).
FIGURE 3.
Estimates of adult HIV prevalence (left), HIV prevalence in pregnant women (middle left), adult ART coverage (middle right), and adult HIV incidence (right) in Cabo Delgado, Inhambane, Gaza, and Maputo. Models were calibrated to nationally representative household survey data and unadjusted ANC-RT HIV prevalence data (green points and line), adjusted ANC-RT data (orange points and line), and no ANC-RT data (blue points and line). Modeled indicator estimates are represented by lines without points. INSIDA 2009 and 2015 IMASIDA survey estimates of ART coverage and HIV prevalence used in calibration are represented by black points with uncertainty, and withheld INSIDA 2021 survey estimates are represented by gray points.
FIGURE 4.
Validation analysis against withheld 2021 INSIDA data by ANC-RT model scenario. A, Absolute difference and (B) relative difference between provincial INSIDA 2021 survey estimate and 2021 estimates of HIV prevalence and ART coverage by ANC-RT model scenario. Points are sized relative to the provincial HIV-positive population. The dotted line represents no difference between modeled estimate and INSIDA 2021 survey, and the solid black lines are the median difference by model scenario.
Across all provinces, calibrating to adjusted ANC-RT data compared with reported ANC-RT resulted in small changes to adult HIV prevalence (median +2%, IQR 0%–8%), HIV incidence (median +8%, IQR -4 to +34%), and ART coverage (median -1%, IQR -3 to 0%). However, estimates for HIV prevalence among pregnant women in 2021 were substantially higher (median +35%, IQR 25%–42%). Removing ANC-RT data increased 2021 adult HIV prevalence by median 12% (IQR 7%–23%) and HIV prevalence among pregnant women by median 52% (IQR 39%–84%).
While adult HIV estimates were minimally affected, model fit to adjusted ANC-RT data was much better than to reported data. The mean squared error (MSE) between observed and estimated HIV prevalence in pregnant women reduced from MSE = 1.59 when fitted to reported data to MSE = 0.81 when fitted to adjusted data, indicating that adjusted data were more consistent with underlying epidemic dynamics. Demographic and HIV disease progression dynamics in EPP constrain HIV prevalence and incidence trajectories. This can prevent estimated trends from fitting observed data when epidemiologically inconsistent.
Pediatric and Vertical Transmission Estimates Calibrated to ANC-RT Model Scenarios
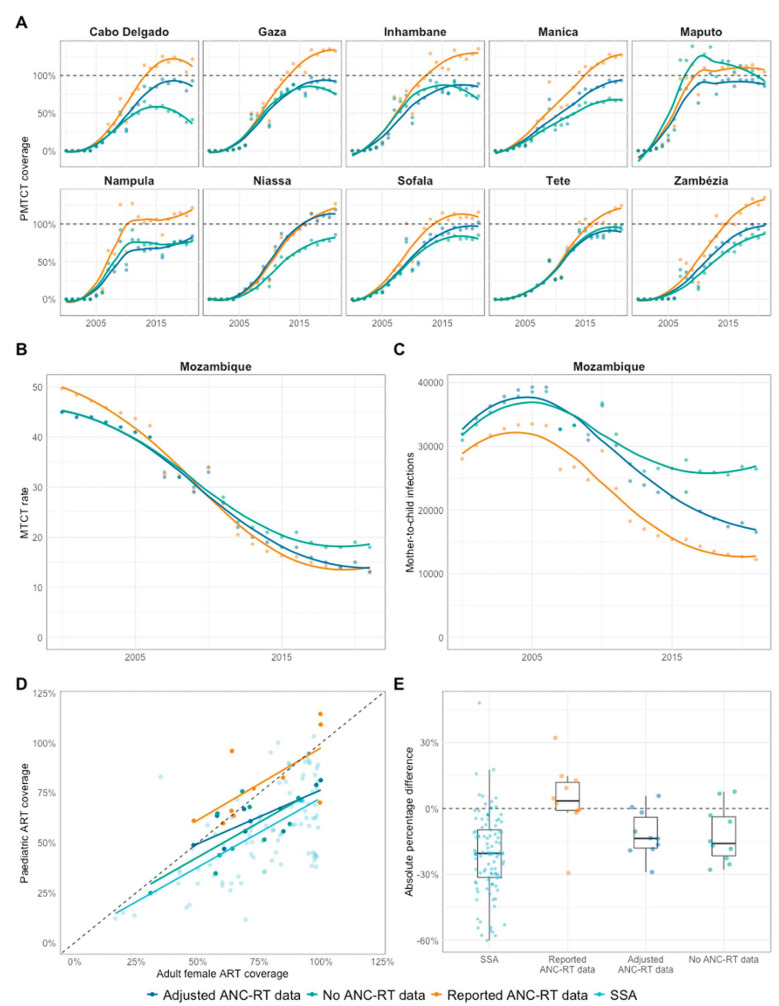
The higher HIV prevalence among pregnant women when calibrated to adjusted or no ANC-RT data resulted in lower and more plausible PMTCT coverage. In models calibrated to adjusted ANC-RT data, PMTCT coverage for 2021 was under 100% in all provinces except Niassa and Sofala (median 93%, IQR 90%–98%) and lower when removing ANC-RT data entirely (median 82%, IQR 73%–88%; Fig. 5A). This implied that higher ANC prevalence was more consistent with the reported number of HIV-positive women receiving PMTCT services.
FIGURE 5.
Vertical transmission and pediatric estimates by ANC-RT prevalence scenario. A, Prevention of mother-to-child transmission (PMTCT) coverage. B, Mother-to-child transmission (MTCT) rate. C, Vertical new child HIV infections. D and E, Comparison of Mozambique provincial adult female and pediatric ART coverage versus all other sub-Saharan African countries.
Lower PMTCT coverage when calibrating to adjusted ANC-RT data did not substantially change modeled rates of mother-to-child transmission in 2021 (MTCT; Fig. 5B) because the adjusted PMTCT coverage remained close to 100%. However, the larger estimated number of HIV-positive pregnant women resulted in a 43% increase in new mother-to-child infections in 2021, increasing from 12,400 to 17,700 (Fig. 5C). Removing ANC-RT data from model calibration increased 2021 MTCT rates to 18% (+36% increase or 5%-pt) and doubled the number of new mother-to-child infections to 26,400 (+112%).
Median provincial pediatric ART coverage in 2021 in models calibrated to reported ANC-RT was 74% (IQR 64%–93%). This declined to 62% (IQR 56%–67%) in models using adjusted ANC-RT and to 56% (IQR 44%–67%) in models without ANC-RT data (Fig. 5D). Sensitivity analysis of ANC excess allocation indicated that the smallest adjustment to ANC HIV prevalence that keeps all provincial PMTCT coverages under 100% would increase the number of new mother-to-child infections by 21% in 2021 (see Figure S5, Supplemental Digital Content, http://links.lww.com/QAI/C154).
The lower pediatric ART coverage with the adjusted ANC-RT prevalence was more consistent with the typical relationship between pediatric and adult female ART coverage. Across sub-Saharan Africa, adult female ART coverage is correlated with and is higher than pediatric ART coverage (median difference 20%, IQR 8%–31%, R2 = 0.39; Figs. 5D and 5E). However, with reported data in Mozambique, adult female ART coverage was lower than pediatric ART coverage (median difference −3%, IQR −11% to 1%). Adjusting or removing ANC-RT data lowered pediatric ART coverage compared with adult female ART coverage, aligning the Mozambique estimates with other SSA countries (adjusted data: 14%, IQR 4%–18%; no ANC-RT data: 16%, IQR 4%–22%).
The default Spectrum region-specific fertility rates by HIV status derived from a global analysis of survey data41 can be fit to ANC-RT data to produce “locally adjusted” fertility rates and internally consistent estimates of the number of HIV-positive pregnant women. If ANC-RT data are consistent with the global estimate of relative fertility in HIV-positive women, the fitted local adjustment factor (LAF) equals 0.93. The further the fitted LAF lies from 0.93, the larger the deviation from the best available estimate of relative fertility. Across all SSA Spectrum files, median-fitted LAF was 0.93 (IQR 0.93–1.1). In Mozambique, when calibrating to reported ANC-RT data, the LAF was 0.68 (IQR 0.56–0.79), the lowest in SSA. Calibrating to adjusted ANC-RT increased the LAF to 0.85 (IQR 0.7–1.07), reducing the fitted deviation from the global best estimate and aligning Mozambique with other SSA countries (see Figure S6, Supplemental Digital Content, http://links.lww.com/QAI/C155).
DISCUSSION
Our data triangulation supports the hypothesis that inconsistencies in Mozambique HIV estimates are due to routinely collected antenatal clinic data and that inclusion of these data in HIV estimates had important impacts on estimates for HIV prevalence, treatment coverage, new infections, and, especially, children living with HIV. Based on a hypothesized bias adjustment mechanism, we proposed a simple adjustment to ANC-RT prevalence data using nationally representative household survey data. Calibrating to adjusted ANC-RT prevalence data better reconciled survey-reported fertility rates, modeled birth estimates, and the number of women receiving PMTCT services. Adjusted estimates decreased PMTCT coverage to plausibly high levels below 100% in most provinces and increased mother-to-child HIV infections by 35% in 2021. These resulted in estimates for CLHIV more consistent with pediatric ART data and typical coverage patterns.
Routinely collected data are central to monitoring the HIV epidemic and tracking progress toward 95–95–95 targets, and their importance will continue to grow in the shift toward case-based surveillance in sub-Saharan Africa.18,28 This analysis demonstrates that routinely collected antenatal data are influential in HIV estimates in Mozambique, particularly in Southern Mozambique where HIV prevalence is highest. However, current routine data are presently unable to track the Mozambique HIV epidemic and led to substantial underestimation of the number of mother-to-child infections. Studies conducted around the transition from ANC-SS to ANC-RT, evaluating program quality and readiness, focused on HIV test fidelity, availability of routine testing, and nonresponse biases as recommended by the 2013 WHO guidelines.21,23,25,29 However, although some studies noted further nonsampling errors,25,29 they were unable to measure health system readiness for future programmatic scale-up, including the ability to track patients between health facilities or districts, quantify and record retesting, or assess data flows from paper notes at facility level through national electronic health systems.
Our hypothesized bias adjustment mechanism assumed that all excess ANC clients were HIV-negative across all years of data, producing a parallel upward shift in ANC-RT HIV prevalence estimates. Incorrect apportionment of excess ANC clients by HIV status would influence the magnitude of the shift, but the trend would remain unchanged. Although altering the level of the ANC-RT HIV prevalence trend had a large impact on HIV prevalence in pregnant women and pediatric estimates, the adult estimates were minimally affected. In Cabo Delgado, Inhambane, Gaza, and Maputo, where removing ANC-RT data altered adult HIV estimates, the presence of adjusted or reported ANC-RT data produced similar fits. This suggests that despite the long interval since the last household survey used in model calibration, the adult estimates are insensitive to the level of ANC-RT HIV prevalence data and that the HIV prevalence trend is more influential. This is because EPP is required to permit large sampling and nonsampling errors in ANC-RT HIV prevalence data so that epidemiologically implausible trends may be reconciled with HIV disease progression and mortality parameters.17,20,42 This impedes precise estimation of adult HIV incidence and HIV prevalence in pregnant women from routine data. Routine data quality assessments would capture evolving data quality issues over time to produce credible HIV prevalence trends and improve adult HIV estimates.
Data quality issues are not restricted to Mozambique. Seven other SSA countries reported ANC and/or PMTCT coverage over 100% in UNAIDS 2022 estimates38 (Malawi, Namibia, Zambia, Benin, Burkina Faso, Ghana, and Liberia). However, in most, the magnitude of discrepancy was smaller than that in Mozambique. Overall, discrepancies in these countries, which also have smaller populations and lower HIV prevalence than Mozambique, are unlikely to substantially affect regional estimates of the pediatric HIV epidemic. Implementing electronic medical registers and unique patient identifiers in antenatal settings will enable more detailed collation of retrospective data and efficient prospective oversight of data quality for HIV surveillance and program monitoring.28 However, because these will not be available in the short-term, establishing data quality assessments as a routine programmatic activity could both ensure high-quality data for tracking HIV epidemic trends in modeled estimates and ensuring accurate routine data for ANC and PMTCT program monitoring. To the extent that discrepancies arise through inaccurate recording of facility register data in aggregate reporting, retrospective data extraction from ANC registers at a representative selection of facilities could be conducted rapidly, at low cost, and may improve estimates of HIV prevalence in pregnant women and PMTCT coverage. The exercise could also record additional indicators lost in transitioning from sentinel surveys to routine aggregate reporting such as age, parity, HIV testing history, and other sociodemographic information that can be triangulated with household survey data and are useful for monitoring program equity. This will not address data issues, however, if inaccurate data have been recorded in registers. A retrospective register extraction conducted at 22 antenatal clinics in 2022 produced similar HIV prevalence estimates as calculated from the routinely reported aggregates. Planning is underway in Mozambique for further studies involving prospective data collection beginning at care entry.
We hypothesized that calibrating to adjusted ANC-RT data would increase adult HIV prevalence sufficiently to reduce implausibly high ART coverage in Gaza, Inhambane, and Tete from near, or exceeding, 100%. However, adjustment had minimal impact on adult estimates. During the COVID-19 pandemic, most patients on ART were transitioned to differentiated service delivery, picking up antiretroviral on a 3-month or 6-month basis instead of on a monthly basis to reduce loss to follow-up caused by service disruption.43,44 This has made measurement of loss to follow-up more challenging because patients engage with clinics less frequently and may underlie rapidly rising estimates of ART coverage. Routine data review should be extended to treatment programs and the impact of multimonth ART dispensing on estimates of program lost to follow-up and total numbers on treatment.
This analysis is subject to several limitations. We concluded that data quality issues existed within the number of ANC visits by triangulating with adjusted live facility births. This relied on extrapolating the proportion of women who gave birth in a facility over 7 years since the last available survey. If the proportion is lower than estimated, adjusted live facility births would be closer to the number of observed ANC visits. Second, we have assumed that the number of women receiving PMTCT services is unbiased. If PMTCT double counting is occurring, this analysis will have underestimated MTCT transmission and the number of new child infections.
CONCLUSIONS
Adjusting ANC-RT HIV prevalence trends using nationally representative household survey data on ANC attendance produced estimates that were more consistent with survey-reported fertility rates, modeled birth estimates, and the number of women receiving PMTCT services than raw data. We found that the number of children living with HIV in Mozambique has likely been substantially underestimated. Antenatal data quality improvements and renewed focus on HIV surveillance in pregnant women would strengthen routine reporting and guide adjustment to ANC-RT HIV prevalence and subsequent HIV estimates.
Footnotes
O. Stevens and J. W. Imai-Eaton are funded by UNAIDS, the Bill and Melinda Gates Foundation (OPP1190661), the National Institute of Allergy and Infectious Disease of the National Institutes of Health under award number R01AI152721, and the MRC Centre for Global Infectious Disease Analysis (reference MR/R015600/1), jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth and Development Office (FCDO), under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. J. McOwen is an employee of the US Centers for Disease Control and Prevention (US CDC). R. Glaubius was supported by Grants from UNAIDS (2022/12/1217200) and the Bill and Melinda Gates Foundation (OPP1191665)
The authors have no funding or conflicts of interest to disclose.
O. Stevens, M. Mahy, and J. W. Imai-Eaton conceptualized the study. O. Stevens analyzed the data and wrote the first draft of the manuscript. O. Tiberi provided data from SISMA-DHIS2. All authors contributed to interpretation of results and edited the manuscript for intellectual content. All authors read and approved the final version of the manuscript for submission.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Contributor Information
Makini Boothe, Email: boothem@unaids.org.
Orrin Tiberi, Email: oftiberi@gmail.com.
Mary Mahy, Email: mahym@unaids.org.
Patrick Walker, Email: patrick.walker06@imperial.ac.uk.
Robert Glaubius, Email: rglaubius@avenirhealth.org.
Jordan McOwen, Email: qfd9@cdc.gov.
Aleny Couto, Email: coutoaleny@gmail.com.
Morais Cunha, Email: moraiscunhajr@gmail.com.
Jeffrey W. Imai-Eaton, Email: jeffrey.eaton@imperial.ac.uk.
REFERENCES
- 1.Instituto Nacional de Saúde. INSIDA Factsheet; 2021. Available at: https://phia.icap.columbia.edu/mozambique-summary-sheet-en-pt-2021/. Accessed April 26, 2023. [Google Scholar]
- 2.UNAIDS Global. AIDS Update; 2021. Available at: https://www.unaids.org/en/resources/documents/2021/2021-global-aids-update. Accessed May 12, 2022. [Google Scholar]
- 3.Eaton JW, Brown T, Puckett R, et al. The estimation and projection package age-sex model and the r-hybrid model: new tools for estimating HIV incidence trends in sub-Saharan Africa. AIDS. 2019;33(suppl 3):S235–S244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stover J, Glaubius R, Mofenson L, et al. Updates to the Spectrum/AIM model for estimating key HIV indicators at national and subnational levels. AIDS. 2019;33(suppl 3):S227–S234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS and WHO. Guidelines for Conducting HIV Sentinel Serosurveys Among Pregnant Women and Other Groups. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2003. [Google Scholar]
- 6.Zaba B, Boerma T, White R. Monitoring the AIDS epidemic using HIV prevalence data among young women attending antenatal clinics: prospects and problems. AIDS. 2000;14:1633–1645. [DOI] [PubMed] [Google Scholar]
- 7.Zaba BW, Carpenter LM, Boerma JT, et al. Adjusting ante-natal clinic data for improved estimates of HIV prevalence among women in sub-Saharan Africa. AIDS. 2000;14:2741–2750. [DOI] [PubMed] [Google Scholar]
- 8.Walker N, Stanecki KA, Brown T, et al. Methods and procedures for estimating HIV/AIDS and its impact: the UNAIDS/WHO estimates for the end of 2001. AIDS. 2003;17:2215–2225. [DOI] [PubMed] [Google Scholar]
- 9.Ghys PD, Brown T, Grassly NC, et al. The UNAIDS estimation and projection package: a software package to estimate and project national HIV epidemics. Sex Transm Infect. 2004;80(suppl 1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Unlinked Anonymous Screening for the Public Health Surveillance of HIV Infections. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 1989. [Google Scholar]
- 11.Alkema L, Raftery AE, Clark SJ. Probabilistic projections of HIV prevalence using Bayesian melding. Ann Appl Stat. 2007;1:229–248. [Google Scholar]
- 12.Demographic Health Surveys. Key Findings National Survey on Prevalence, Behavioral Risks and Information About HIV and AIDS (2009 INSIDA) MOZAMBIQUE. Geneva, Switzerland: WHO; 2021. Available at: www.measuredhs.com. Accessed June 01, 2021. [Google Scholar]
- 13.Yeatman S, Eaton JW, Beckles Z, et al. Impact of ART on the fertility of HIV-positive women in sub-Saharan Africa. Trop Med Int Health. 2016;21:1071–1085. [DOI] [PubMed] [Google Scholar]
- 14.Marsh K, Mahy M, Salomon JA, et al. Assessing and adjusting for differences between HIV prevalence estimates derived from national population-based surveys and antenatal care surveillance, with applications for Spectrum 2013. AIDS. 2014;28:S497–S505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gouws E, Mishra V, Fowler TB. Comparison of adult HIV prevalence from national population-based surveys and antenatal clinic surveillance in countries with generalised epidemics: implications for calibrating surveillance data. Sex Transm Infect. 2008;84(suppl 1):i17–i23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton JW, Rehle TM, Jooste S, et al. Recent HIV prevalence trends among pregnant women and all women in sub-Saharan Africa: implications for HIV estimates. AIDS. 2014;28(suppl 1):S507–S514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eaton JW, Bao L. Accounting for nonsampling error in estimates of HIV epidemic trends from antenatal clinic sentinel surveillance. AIDS. 2017;31(suppl 1):S61–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Conducting HIV Surveillance Based on Routine Programme Data Among Pregnant Women Attending ANC. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 19.Sheng B, Eaton JW, Mahy M, et al. Comparison of HIV prevalence among antenatal clinic attendees estimated from routine testing and unlinked anonymous testing. Stat Biosci. 2020;12:279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng B, Marsh K, Slavkovic AB, et al. Statistical models for incorporating data from routine HIV testing of pregnant women at antenatal clinics into HIV/AIDS epidemic estimates. AIDS. 2017;31(suppl 1):S87–S94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young PW, Mahomed M, Horth RZ, et al. Routine data from prevention of mother-to-child transmission (PMTCT) HIV testing not yet ready for HIV surveillance in Mozambique: a retrospective analysis of matched test results. BMC Infect Dis. 2013;13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woldesenbet SA, Kufa T, Barron P, et al. Assessment of readiness to transition from antenatal HIV surveillance surveys to PMTCT programme data-based HIV surveillance in South Africa: the 2017 Antenatal Sentinel HIV Survey. Int J Infect Dis. 2020;91:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirengo M, Rutherford GW, Otieno-Nyunya B, et al. Evaluation of Kenya's readiness to transition from sentinel surveillance to routine HIV testing for antenatal clinic-based HIV surveillance. BMC Infect Dis. 2016;16:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mate KS, Bennett B, Mphatswe W, et al. Challenges for routine health system data management in a large public programme to prevent mother-to-child HIV transmission in South Africa. PLoS One. 2009;4:e5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gourlay A, Wringe A, Todd J, et al. Challenges with routine data sources for PMTCT programme monitoring in East Africa: insights from Tanzania. Glob Health Action. 2015;8:29987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maheu-Giroux M Jahn A Kalua T, et al. HIV surveillance based on routine testing data from antenatal clinics in Malawi (2011–2018): measuring and adjusting for bias from imperfect testing coverage. AIDS. 2019;33(suppl 3):S295–S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MISAU. UNAIDS Spectrum Files Retrieved From 2021 UNAIDS Global HIV Estimates. Geneva, Switzerland: WHO; 2022. [Google Scholar]
- 28.Harklerode R, Schwarcz S, Hargreaves J, et al. Feasibility of establishing HIV case-based surveillance to measure progress along the health sector cascade: situational assessments in Tanzania, South Africa, and Kenya. JMIR Public Health Surveill. 2017;3:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicol E, Dudley L, Bradshaw D. Assessing the quality of routine data for the prevention of mother-to-child transmission of HIV: an analytical observational study in two health districts with high HIV prevalence in South Africa. Int J Med Inform. 2016;95:60–70. [DOI] [PubMed] [Google Scholar]
- 30.Kripke K, Opuni M, Odoyo-June E, et al. Data triangulation to estimate age-specific coverage of voluntary medical male circumcision for HIV prevention in four Kenyan counties. PLoS One. 2018;13:e0209385. 10.1371/journal.pone.0209385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eaton JW, Dwyer-Lindgren L, Gutreuter S, et al. Naomi: a new modelling tool for estimating HIV epidemic indicators at the district level in sub-Saharan Africa. J Int AIDS Soc. 2021;24(suppl 5):e25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MISAU. Inquerito Demografico e de Saude 2003. MISAU ; 2003. Available at: https://dhsprogram.com/pubs/pdf/FR161/FR161.pdf
- 33.MISAU. Moçambique Inquérito Demográfi co e de Saúde. MISAU; 2011. Available at: https://dhsprogram.com/pubs/pdf/FR266/FR266.pdf [Google Scholar]
- 34.MISAU. Inquérito de Indicadores de Imunização, Malária e HIV/SIDA em Moçambique (IMASIDA) 2015; 2015. Available at: https://dhsprogram.com/publications/publication-ais12-ais-final-reports.cfm. Accessed May 11, 2021. [Google Scholar]
- 35.MISAU. Moçambique Inquérito Nacional sobre Indicadores de Malária (IIM) 2018; 2019. Available at: https://dhsprogram.com/publications/publication-mis33-mis-final-reports.cfm. Accessed May 19, 2021. [Google Scholar]
- 36.Watson OJ, FitzJohn R, Eaton JW. rdhs: an R package to interact with the demographic and health surveys (DHS) program datasets. Wellcome Open Res. 2019;4:103. [Google Scholar]
- 37.Lumley T. Package “Survey”—Analysis of Complex Survey Samples; 2022. Available at: http://r-survey.r-forge.r-project.org/survey/. Accessed November 02, 2022. [Google Scholar]
- 38.UNAIDS. Spectrum File Request—UNAIDS HIV Tools; 2022. Available at: https://hivtools.unaids.org/spectrum-file-request/. Accessed August 30, 2022. [Google Scholar]
- 39.IV RGPH 2017—Instituto Nacional de Estatistica; 2022. Available at: http://www.ine.gov.mz/iv-rgph-2017. Accessed July 21, 2022. [Google Scholar]
- 40.UN Population Division. World Population Prospects—Population Division—United Nations; 2020. Available: https://population.un.org/wpp/. Accessed April 03, 2020. [Google Scholar]
- 41.Chen WJ, Walker N. Fertility of HIV-infected women: insights from demographic and health surveys. Sex Transm Infect. 2010;86(suppl 4):ii22–ii27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown T, Bao L, Eaton JW, et al. Improvements in prevalence trend fitting and incidence estimation in EPP 2013. AIDS. 2014;28(suppl 4):S415–S425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MISAU. Modelos Diferenciados de Serviços em Moçambique Modelos Diferenciados de Serviços em Moçambique [in Portuguese]. Moçambique: CNCS; 2018. [Google Scholar]
- 44.World Health Organization. Updated Recommendations on Service Delivery for the Treatment and Care of People Living With HIV. Geneva, Switzerland: World Health Organization; 2021. [PubMed] [Google Scholar]