Abstract
The expression of lacZ has been analyzed and compared in a series of promoter cloning vectors by measuring the amount of lacZ mRNA by hybridization and the amount of β-galactosidase by standard enzymatic assay. Expression was driven by the promoter, Pspc, of the spc ribosomal protein operon. The vectors contained either the standard W205 trp-lac fusion with the trp operon transcription terminator, trpt, located in the lacZ leader sequence, or a deletion derivative that functionally inactivates trpt. In the presence of trpt, lacZ expression was temperature dependent so that increasing the growth temperature reduced the accumulation of lacZ mRNA and β-galactosidase activity. The frequency of transcript termination at trpt was estimated to be near zero at 20°C and at about 45% at 37°C. The amount of Pspc-derived lacZ mRNA and the amount of β-galactosidase produced per lacZ mRNA varied, depending on the mRNA 5′ leader sequence between Pspc and lacZ. These results demonstrate that the quantitative assessment of promoter activities with promoter cloning vectors requires careful analysis and interpretation. One particular construct without trpt did not seem to contain fortuitous transcription or translation signals generated at the fusion junction. In this strain, lacZ expression from Pspc was compared at the enzyme activity and mRNA levels with a previously constructed strain in which lacZ was linked to the tandem P1 and P2 promoters of the rrnB operon. At any given growth rate, the different activities of β-galactosidase in these two strains were found to reflect the same differences in their amounts of lacZ mRNA. Assuming that the promoter-lacZ fusions in these strains reflect the properties of the promoters in their normal chromosomal setting, it was possible to estimate the absolute transcription activity of Pspc and the relative translation efficiency of Pspc-lacZ mRNA at different growth rates. Transcription from the spc promoter was found to increase from about 10 transcripts per min at a growth rate of 1.0 doublings/h to a maximum plateau of about 23 transcripts per min at growth rates above 1.5 doublings/h. The translation frequency of lacZ mRNA expressed from Pspc was unaffected by growth rates.
To study gene regulation in bacteria and to define the basic properties of a promoter isolated from its normal control sites, the promoter of interest is often linked on a plasmid or phage vector to a reporter gene such as lacZ. The promoter activity in lacZ-based vectors is then assessed from measurements of β-galactosidase, an enzyme that is very stable and easy to assay. The amount of enzyme produced depends in part on the strength of the inserted promoter and in part on other factors, including the termination or antitermination properties of the transcribing RNA polymerase, the stability of lacZ mRNA, and the ability of the lacZ mRNA to compete with bulk mRNA for the initiation of translation. The latter factors may be affected by particular, unnatural sequence combinations at the junction of the operon fusion. In the work described below, we have attempted to identify and quantify some of these effects by measuring with various lacZ vectors the amounts of lacZ mRNA and of β-galactosidase produced from the promoter of the spc ribosomal protein operon.
Two widely used lac-based promoter cloning vectors, the phage λRS205 (3) and the plasmid pRS415 (33), were derived from the trp-lac fusion W205, isolated by Mitchell et al. (25). In these vectors, the W205 fusion is located downstream of the promoter cloning site and contains the end of trpA, followed by one of the two trp operon transcription terminators, trpt (29, 35, 36). Termination at trpt is independent of rho and occurs with an efficiency of about 37% in vivo (27) and of about 25% in vitro (36). The trpt element also serves as a pause or stop signal for 3′-to-5′ exonucleolytic degradation of mRNA (27). The second, rho-dependent transcription terminator, trpt′, is located 250 bp downstream of trpt in the normal trp operon (27). The trpt′ element is not present in the W205 fusion.
Expression from plasmid-cloned genes is difficult to quantify because of plasmid copy number effects; therefore, expression of the lacZ reporter gene is often studied after integration of the promoter-lac fusion into the chromosome, e.g., by using the λRS205 system. Lysogens constructed with these phage vectors carry a temperature-sensitive repressor to aid in preparing phage lysates for moving the operon fusion from one strain to another. Because of lysis induction at higher temperature, such strains are grown and analyzed at 30°C. In our laboratory, an alternative system to study gene expression was developed in which the plasmid-borne promoter-lacZ fusion is flanked by sequences of the Escherichia coli maltose (mal) genes. This allows for insertion of the plasmid-constructed promoter-lacZ fusions into the mal locus of the chromosome by a double recombination event (16, 38). These strains can be grown at any temperature.
We have observed that promoter-lacZ operon fusions derived from the original W205 trp-lac fusion exhibit temperature-sensitive expression of β-galactosidase activity. The temperature sensitivity in expression was seen with a number of promoters, including the P1 or P2 promoters from rrnB, the replication primer promoter of plasmid pBR322, and the promoter of the spc ribosomal protein operon. We show here that the temperature sensitivity in lacZ expression is caused by the presence of the trpt transcription terminator within the W205 trp-lac fusion. In addition, we show that new sequence combinations generated at the fusion junction can have other dramatic effects on lacZ expression and therefore may adversely affect the quantitation of promoter activity. The analysis allowed us to identify and characterize a particular Pspc-lacZ construct in which these anomalous effects were minimal or absent and in which the expression of lacZ appears to reflect the properties of Pspc in its natural chromosomal setting.
MATERIALS AND METHODS
Plasmids and strains.
The plasmids and bacterial strains used and details of their construction are presented in Tables 1 and 2. To delete trpt from plasmid pXZ09-B, a 2.3-kb fragment from this plasmid, spanning the region from the center of the trpt palindrome to a site beyond a unique SacI site within lacZ, was amplified by PCR. One of the primers added a BamHI site at the end bordering the trp terminator. After cleavage of the PCR product with BamHI and SacI, the resulting fragment was inserted between the BamHI and SacI sites of plasmid pXZ09-B, thereby deleting 50 bp of the trp region, including the end of trpA and half of the trpt terminator repeat (Fig. 1). The resulting new plasmid without a functional trpt transcription terminator is pSL03. The correct deletion was verified by DNA sequencing.
TABLE 1.
Plasmids used in this study
| Plasmid | Size (kb) | Genotype | Reference or construction |
|---|---|---|---|
| pXZ09 | 11.8 | bla malE′-(EcoRI-BamHI)-′trpA-trpt-lacZ-T1T2-kan-T1T2-′malKa | 38 |
| pXZ09-B | 11.8 | bla malE′-(EcoRI-BamHI)-′trpA-trpt-lacZ-T1T2-kan-T1T2-′malK | Same as for pXZ09, but two BamHI restriction sites flanking kan were removed by partial BamHI digestion, end filling, and religation |
| pRS415 | 10.8 | bla (T1)4-(EcoRI-BamHI)-lacZ-lacY-lacA-tetb | 33 |
| pSL415 | 10.9 | bla (T1)4-Pspc-lacZ-lacY-lacA-tet′ | 110 bp of spc promoter cloned into pRS415 |
| pSL02 | 11.9 | bla malE′-Pspc-′trpA-trpt-lacZ-T1T2-kan-T1T2-′malK | 110 bp of spc promoter cloned into pXZ09-B (see text and Fig. 1b) |
| pSL03 | 11.7 | bla malE′-(EcoRI-BamHI)-lacZ-T1T2-kan-T1T2-′malK | Same as for pXZ09-B, but 50 bp of trp sequences were deleted (see text) |
| pSL04 | 11.8 | bla malE′-Pspc-lacZ-T1T2-kan-T1T2-′malK | 110 bp of spc promoter cloned into pSL03 (see text and Fig. 1c) |
| pSL05 | 12.3 | bla malE′-Pspc-rplN-′trpA-trpt-lacZ-T1T2-kan-T1T2-′malK | 504 bp of Pspc-rplN cloned into pXZ09-B (see text) |
| pSL06 | 12.2 | bla malE′-PspcrplN-lacZ-T1T2-kan-T1T2-′malK | 504 bp of Pspc-rplN cloned into pSL03 (see text) |
T1T2 in the plasmid genotype refers to the two rrnB transcription terminators.
(T1)4 refers to four repeats of the rrnB transcription terminator T1.
TABLE 2.
Bacterial strains used in this study
| Strain | Genotype | Reference or construction |
|---|---|---|
| JC9387 | recB21 recC22 sbcB15 sbcC201 thr-1 ara-14 leuB6 lacY1 Δ(gpt-proA)62 tsx-33 galK2 hisG4 rfbD1 mgl-51 rpsL31 kdgK51 xyl-5 mtl-1 argE3 thi-1 | 34 |
| HB123 (same as RL331) | E. coli B/r A phe(Am) thr(Am) hsdR/M (K-12) | 31 |
| HB181 | E. coli B/r A malK+ Δ(argF-lacIOZYA) phe(Am) thr (Am) hsdR/M (K-12) | 38 |
| XZ231 | HB181 malB::malE′-rrnB UAR-P1-P2- box BAC-k′-lacZ-kan-′malK | 38 |
| SL102 | HB181 malE′-Pspc-′trpA-trpt-lacZ-T1T2-kan-T1T2-′malK | JC9387 was transformed with pSL02, selecting for Kmr; then kan was transduced with phage P1 into HB181 |
| SL103 | HB181 malE′-(EcoRI-BamHI)-lacZ-T1T2-kan-T1T2-′malK | Same as for SL102, but with pSL03 |
| SL104 | HB181 malE′-Pspc-lacZ-T1T2-kan-T1T2-′malK | Same as for SL102, but with pSL04 |
| SL105 | HB181 malE′-Pspc-rplN-′trpA-trpt-lacZ-T1T2-kan-T1T2-′malK | Same as for SL102, but with pSL05 |
| SL106 | HB181 malE′-Pspc-rplN-lacZ-T1T2-kan-T1T2-′malK | Same as for SL102, but with pSL06 |
FIG. 1.
Structure of the promoter cloning vector containing the W205 trp-lac reporter system and the mal insertion sequences. (a) Arrangement of genes in pSL03. Filled areas are the functional genes lacZ, kan, and bla, and the positions of the tandem rrnB transcription terminators, rrnB T1T2 are indicated. The malE′ and ′malK sequences are indicated by open boxes. The EcoRI-SmaI-BamHI sites are used for promoter insertion to drive expression of lacZ. The SacI site was used for construction of the trp deletion (see Materials and Methods). (b) Structure of the spc promoter-trp-lac fusion region on plasmid pSL02: the 110-bp fragment containing the spc ribosomal protein promoter from −51 to +59 relative to the transcription start site (+1) was inserted between the EcoRI and BamHI sites of plasmid pXZ09-B to give plasmid pSL02 (Table 1). This plasmid contains 75 nt of trp operon sequence (open box) that includes the rho-independent transcription terminator trpt. The coding region of lacZ begins at nt +160. The −10 and −35 elements of Pspc are indicated. (c) Structure of the spc promoter-trp-lac fusion region in plasmid pSL04. The same 110-bp fragment containing the spc promoter was cloned between the EcoRI and BamHI sites of plasmid pSL03 that lacks 50 bp of trp sequences to give plasmid pSL04. The deletion removes half of the trpt palindromic sequence. The coding region of lacZ begins at nt +110. (d) Structure of the spc promoter-trp-lac fusion region on plasmid pSL05: a 504-bp fragment from nt −51 to +453 (relative to the transcription start) containing the Pspc promoter and the rplN gene (from nt +73 to +441) was cloned between the EcoRI and the BamHI sites of plasmid pXZ09B to give plasmid pSL05. The coding region of lacZ begins at nt +554. (e) Structure of the spc promoter-trp-lac fusion region on plasmid pSL06: a 504-bp fragment from nt −51 to +453 (relative to the transcription start) containing the Pspc promoter and the rplN gene (from nt +73 to +441) was cloned between the EcoRI and BamHI sites of plasmid pSL03 to give plasmid pSL06. The coding region of lacZ begins at nt +504.
A 110-bp spc promoter fragment (from −51 to +59 relative to the transcription start; Fig. 1) and a 504-bp fragment, carrying the spc promoter and the coding sequence of r-protein L14 (rplN; from −51 to +453), were obtained by PCR with appropriate primers by using λdspc-1 DNA (12) as a template and adding EcoRI and BamHI restriction sites at the ends. After cleavage with EcoRI and BamHI, these fragments were inserted between the EcoRI and BamHI sites of pXZ09-B and pSL03 to yield pSL02, pSL04, pSL05, and pSL06 (Table 1).
All plasmids were constructed in duplicate with independently synthesized PCR products and checked for correct length of restriction fragments and enzyme activity. The promoter-lacZ fusions from these duplicate plasmids were first recombined into the mal genes of the recBC sbc strain JC9387 (Table 2) as described previously (16). The Pspc-lacZ-kan fusions were then transduced with phage P1 into the lac deletion strain HB181, selecting for kanamycin resistance (Kmr). The correct insertion was verified by streaking onto MacConkey maltose and MacConkey lactose plates (24). Before transduction, the HB181 recipient was positive for mal but lacked lac; after transduction the strains lacked mal but were positive for lac.
The absolute enzyme activities in bacteria transformed with plasmids pSL02 and pSL04 varied considerably from transformant to transformant, which probably resulted from variations in plasmid copy number. When the Pspc-lacZ fusions from different transformants (including plasmids with independently prepared PCR products) were recombined into the mal locus of the chromosome so that they were present as a single copy, there was no significant variation (less than 10%) in the β-galactosidase activity expressed from Pspc in replicate cultures and strains (Table 3).
TABLE 3.
β-galactosidase specific activities expressed from the spc promotera
| Strain | trpt | Promoter | rplN | Sp actb | No. of cultures tested |
|---|---|---|---|---|---|
| SL103 | − | − | − | 0.38 | 2 |
| SL102 | + | Pspc | − | 26 ± 2 | 14 |
| SL104 | − | Pspc | − | 83 ± 8 | 11 |
| SL105 | + | Pspc | + | 41 ± 3 | 2 |
| SL106 | − | Pspc | + | 82 ± 8 | 3 |
LB glucose medium at 37°C was used for these experiments. The presence (+) or absence (−) of features are indicated.
The units for specific activity are A420 per hour per OD600 unit (see Materials and Methods). Values given are averages and standard deviation.
As a background control, the host strain was separately transformed with vector plasmids without promoter inserts. In pXZ09-B and pSL03 transformants, a low level of β-galactosidase activity is expressed from an upstream tet promoter. After recombination of lacZ from these plasmids into the chromosomal mal locus, the tet promoter is excluded and the resulting strains showed a lacZ activity near zero.
Growth conditions.
Cultures were grown in Medium C (15) supplemented with either 0.2% (vol/vol) glycerol or 0.2% (wt/vol) glucose (with or without 0.8% Difco Casamino Acids plus 50 μg of tryptophan per ml), or they were grown in LB medium (24) with 0.2% glucose. Minimal media were supplemented with phenylalanine and threonine at 50 μg/ml. Experimental cultures were inoculated from overnight cultures in glycerol minimal medium by diluting at least 250-fold into minimal medium or 2,000-fold into amino acid-supplemented medium.
Growth was followed as the increase in turbidity at 600 nm with a 1-cm light path (i.e., the optical density at 600 nm [OD600]). Since the turbidity is not exactly proportional to the culture density, the observed values, after subtraction of the medium blank, were corrected for nonlinearity (4). The corrected OD values deviated by less than 1% from the average exponential curve, so that the accuracy of the average OD used for determination of the specific enzyme activity was about 1%. For measurements of mRNA decay (see Fig. 5), rifampin was used at a concentration of 300 μg/ml and was added when the culture had reached an OD600 of about 0.32.
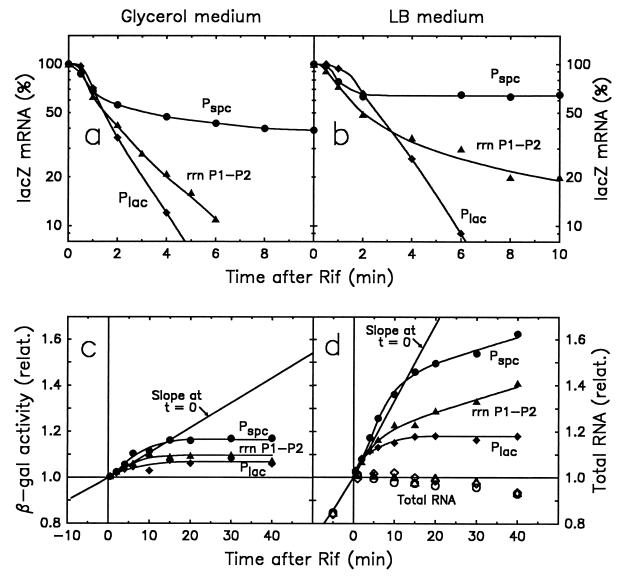
FIG. 5.
Residual accumulation of β-galactosidase and decay of lacZ mRNA in cultures treated with rifampin. Rifampin (300 μg/ml) was added to cultures of strains SL106 (•), XZ231 (▴), and HB123 (⧫) that were growing exponentially (OD600 = 0.32) in either glycerol minimal medium (panels a and c) or LB medium (panels b and d). The residual accumulation of β-galactosidase (panels c and d) and the levels of lacZ mRNA (panels c and d) were monitored. In addition, the accumulation of total RNA was determined (only in LB medium; panel d, open symbols). Due to the linear ordinate scale in panels c and d (no exponential growth in the presence of rifampin), the enzyme activity before zero time increases nonlinearly (i.e., exponentially) with time. The zero time slopes were calculated from the culture doubling times (63 min for glycerol minimal medium and 20.5 min for LB medium).
Determination of β-galactosidase specific activities.
For determination of β-galactosidase content, several 10- or 20-μl samples were taken from an exponential culture over a period of two or three generations, and β-galactosidase was assayed as described earlier (38). The volume of the final reaction mixture before the addition of an equal volume of stopping solution was 1.0 ml. The assays were incubated at 30°C, generally for 50 to 90 min. Similar assays were performed with media blanks. The β-galactosidase activity was determined as the increase in A420 per hour of assay time per sample volume. In the exponential cultures, the β-galactosidase activity always increased in parallel with the culture mass, and the observed points scattered by about 2% around the average exponential curve. The specific activity was calculated as β-galactosidase activity per OD600 unit of culture mass in the assay. One unit defined in this manner corresponds to 16.7 (1,000/60) Miller units (24). For a given culture, the specific activity was determined with an accuracy of about ±2%, but variations of about 10% were observed for cultures grown on different days.
RNA hybridization methods.
Total bacterial RNA was prepared with the glass fiber filter method (5, 7) by using commercial columns (RNAqueous; Ambion, Inc., Austin, Tex.). Samples (5 or 12.5 ml) of exponential culture were taken at an OD600 between 0.30 and 0.35 and added to a one-fifth volume of ethanol-phenol stopping solution (5% phenol in ethanol) at 22°C. The bacteria were pelleted by low-speed centrifugation (5 min at 5,000 rpm) and homogeneously resuspended in 0.3 ml of guanidinium thiocyanate lysis medium provided with the glass fiber column kit. After dilution with 0.3 ml of 64% (RNase-free) ethanol, the lysate was filtered through the RNAqueous column by centrifugation (15 s at 10,000 rpm at room temperature). The bound nucleic acids were washed with high-salt-concentration and ethanol-containing buffers provided with the columns. Essentially protein-free RNA was then eluted twice with 60 μl of 0.1 mM EDTA in diethyl pyrocarbonate (DEPC)-treated water. After the UV absorption spectra were measured at pH 12 in 10 mM NaOH, the preparations were diluted to an A260 of 10.0 with 0.1 mM EDTA in DEPC-treated water. For cultures grown in LB and glycerol minimal medium, the preparations yielded about 1.2 or 0.6, respectively, A260 U of RNA per OD600 U of culture mass, corresponding to about 150 or 75 μg, respectively, of RNA per 12.5-ml sample. The A280/A260 ratio was between 0.48 and 0.49, and the A235/A260 ratio was between 0.55 and 0.57. A sample of each preparation was subjected to agarose gel electrophoresis. The presence of the 23S and 16S rRNAs in ratios of greater than 1.5:1 suggested minimal RNA degradation.
To remove some residual DNA, 0.45 A260 U of RNA (45 μl) were treated for 1 h at 37°C with 10 U of RNase-free DNase I (Boehringer Mannheim) and 80 U of an RNase inhibitor (RNaseout; BRL Laboratories) in a total volume of 50 μl of reaction buffer with final concentrations of 10 mM MgCl2, 50 mM Tris-HCl (pH 7.9), 50 mM NaCl, and 1 mM dithiothreitol (DTT). After this treatment, the RNA was diluted with 1 mM DTT (in DEPC-treated water) to a total volume of 270 μl to give an A260 of 1.67, which corresponded to 50 μg of RNA/ml. It was found to be unnecessary to remove or inactivate the DNase or RNaseout enzymes. Again, a sample of the preparations was subjected to agarose gel electrophoresis to verify the removal of chromosomal DNA. Northern blot analysis with a lacZ probe showed several bands with molecular weights of greater than 3,000 nucleotides (23S rRNA marker) in addition to smaller RNAs. Aliquots (50 μl containing 2.5 μg of RNA) of the DNA-free RNA preparations were stored at −70°C. For the dot blot analysis (see below), 5 μl of these dilutions was added to 995 μl of 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), so that 100 μl of this dilution used in a single well of the dot blot apparatus contained 25 ng of total RNA.
For quantitation of lac-mRNA, a digoxigenin (DIG)-labeled probe complementary to the 5′ end of lacZ (308 nucleotides from −44 to +264, setting the beginning of the lacZ coding region to +1) was used. To prepare the probe, DIG-labeled dUTP (Boehringer Mannheim) at a 1/20 dilution in TTP was included in a PCR reaction with appropriate primers and linearized vector DNA as a template. Known amounts of bacterial RNA (between 12.5 and 50 ng), each in duplicate, were bound to a nylon membrane by slow vacuum filtration by using a dot blot manifold. The RNA was fixed to the membrane by heat treatment (40 min at 80°C under vacuum), followed by UV irradiation (900 J/m2). Hybridization and detection protocols used were those provided by Boehringer Mannheim. For the final detection by chemiluminescence, the membranes were exposed to X-ray film for 4, 8, and 16 min (in some cases also for 2 or 32 min). The chemiluminescent product formed in the solution surrounding the membrane binds tightly to the membrane; however, before this binding, the product may be moved from the site of its formation by convection, which causes a smearing and broadening of the signal on the membrane. To minimize this effect, the sealed plastic envelope was kept pressed flat, with essentially no liquid above and below the membrane during overnight incubation, i.e., until the amount of chemiluminescent product had reached its maximum steady-state concentration.
The photographic images were digitized by using either the ScanJet 4c (Hewlett-Packard Corp.) or Gel Print 2000i apparatus (BioPhotonics) and then analyzed with the ImageQuant program (Molecular Dynamics). The method yields relative values of darkness of the photographic images within defined areas (circles around hybridization “dots”). The values were plotted as both functions of exposure time and amount of RNA spotted. The values given in Fig. 2 and 3 and Table 4 represent the slopes from these plots in relative units and were obtained in the linear range of exposure time and RNA amounts spotted. As controls, RNA preparations from lac+ cultures grown in the presence and absence of IPTG (isopropyl-β-d-thiogalactopyranoside) inducer were analyzed with the Northern blot and dot blot methods. No detectable signals were obtained with RNA from uninduced cultures, corresponding to less than 1% of the signal from the induced culture. For the experiments presented in Fig. 2 to 5, eight RNA preparations were compared at a time, each represented by four samples (e.g., either 25 and 50 ng or 12.5 and 25 ng, each in duplicate) to give a total of 32 hybridization areas (“dots”) on one 5-by-10-cm nylon membrane. For background values, 45 additional circular areas between the dots (four areas surrounding each dot) were measured, averaged, and subtracted from the values obtained from the RNA-containing dot areas.
FIG. 2.
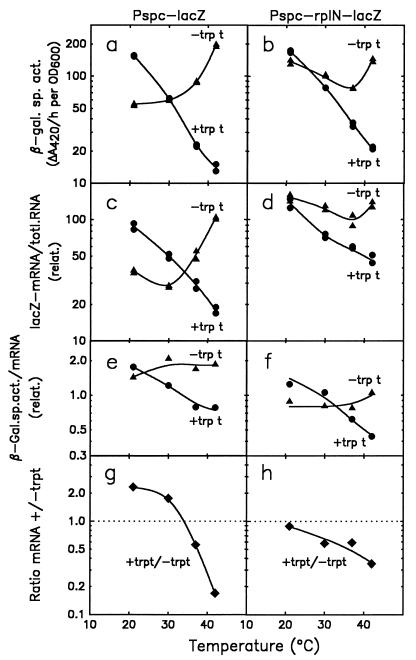
Temperature-dependent expression of lacZ from Pspc in the presence or absence of trpt and rplN. Four strains in which the Pspc promoter fusions were recombined into the mal locus of the chromosome were used: SL102 (Pspc-trpt-lacZ; left panels, •); SL104 (Pspc-lacZ; left panels, ▴); SL105 (Pspc-rplN-trpt-lacZ; right panels, •); and SL106 (Pspc-rplN-lacZ; right panels, ▴). Cultures were grown at different temperatures in LB medium supplemented with glucose, and β-galactosidase specific activities (panels a and b) and lacZ mRNA per total RNA (panels c and d) were measured. The ratio of β-galactosidase specific activity and lacZ mRNA per total RNA (panels e and f) is a measure for the translation efficiency of lacZ mRNA (from the data in panels a and c or in panels b and d, respectively). The ratios of the amounts of lacZ mRNA observed in the presence or absence of a functional trpt (panels g and h, ⧫) at temperatures between 20 and 42°C are illustrated.
FIG. 3.
Growth rate dependency of lacZ expression from Pspc. Four strains containing Pspc promoter fusions recombined into the mal locus were used: SL102 (Pspc-trpt-lacZ; left panels, •); SL104 (Pspc-lacZ; left panels, ▴); SL105 (Pspc-rplN-trpt-lacZ; right panels, •); and SL106 (Pspc-rplN-lacZ; right panels, ▴). The media used to give increasing growth rates were glycerol minimal, glucose minimal, glucose-amino acids, and LB medium supplemented with glucose. All cultures were grown at 37°C, and culture growth rates, β-galactosidase specific activities, and lacZ mRNA per total RNA were measured.
TABLE 4.
Expression of lacZ from the spc ribosomal protein promoter in different strains grown at 37°C in LB medium or glycerol minimal medium
| Medium and strain insert | β-Gal sp acta | lacZ/ mRNAb | β-Gal sp act/ mRNAc | Terminationd (fraction) |
|---|---|---|---|---|
| LB medium | ||||
| SL102 Pspc-trpt-lacZ | 23 ± 1 | 29 ± 2 | 0.79 | 0.43 |
| SL104 Pspc-lacZ | 89 ± 2 | 52 ± 1 | 1.71 | |
| SL105 Pspc-rplN-trpt-lacZ | 36 ± 2 | 57 ± 4 | 0.63 | 0.43 |
| SL106 Pspc-rplN-lacZ | 79 ± 1 | 100 ± 7e | 0.79 | |
| Glycerol minimal medium | ||||
| SL102 Pspc-trpt-lacZ | 25 ± 1 | 29 ± 2 | 0.86 | 0.74 |
| SL104 Pspc-lacZ | 298 ± 5 | 108 ± 10 | 2.75 | |
| SL105 Pspc-rplN-trpt-lacZ | 59 ± 1 | 52 ± 9 | 1.13 | 0.46 |
| SL106 Pspc-rplN-lacZ | 195 ± 2 | 97 ± 11 | 2.01 |
Measured as the increase in A420 per hour per OD600 unit of culture mass present in the β-galactosidase (β-Gal) assay. For each of the eight cultures (four strains in two media each), two enzyme assays were performed about one generation time apart before samples for RNA preparation were taken.
Amount of 5′-terminal lacZ mRNA in relative units per amount of total RNA. The standard deviation was obtained from four to five hybridization experiments (see Materials and Methods).
Ratio of average β-galactosidase specific activity to the average relative amount of lacZ mRNA per total RNA.
One minus the ratio of lacZ mRNA observed with trpt present to that observed without trpt.
The average hybridization values for strain SL106 grown in LB medium were set at 100 for normalization of all other hybridization values in this table.
RESULTS
Features of promoter cloning vectors pRS415, pXZ09, pXZ09-B, and pSL03.
Plasmids pRS415, pXZ09, pXZ09-B, and pSL03 (Table 1 and Fig. 1) are promoter cloning vectors, designed specifically to detect and measure promoter activities. The plasmid pRS415 (33) was derived from plasmid pBR322 and carries the W205 trp-lac fusion (25); its cloning site is located between four upstream rrnB transcription terminators and a downstream promoterless lacZ gene. The DNA section between the cloning site and the start codon of lacZ consists of 75 bp of trp sequence (including the distal 28 bp of trpA and the 47-bp trailer sequence containing the trp operon transcription terminator, trpt; refs. 28, 37) and the 17-bp lacZ leader sequence (including the wild-type ribosome binding site, but not the promoter and operator sequences). Without an insert, this plasmid expresses very low background β-galactosidase activity; with an insert containing a promoter, β-galactosidase activity is elevated, but the extent of elevation is temperature sensitive. That is, β-galactosidase activity is substantially reduced at higher temperatures; this effect has been observed with several different promoters and has been attributed to the presence of trpt in the cloning vector (see below).
Both pXZ09 and pXZ09-B carry the cloning site and the W205 trp-lacZ fusion of pRS415. In addition, these plasmids carry a kan gene and segments of the E. coli mal genes, which permits the recombinatorial insertion of operon fusions constructed on the plasmids into the mal locus of the chromosome (16). Plasmid pXZ09-B was obtained from pXZ09 (38) by removing two BamHI sites flanking the kan gene; the remaining BamHI site on pXZ09-B forms part of the multiple cloning site. The plasmid pSL03 (Fig. 1) was obtained from pXZ09-B by deleting 50 bp of trp sequences, beginning at BamHI of the cloning site and ending between the palindromic sequences that form the trp operon transcription terminator, trpt (37). By numerous criteria, this deletion appears to inactivate trpt and results in elevated expression of the downstream lacZ gene. Moreover, the temperature sensitivity of β-galactosidase expression was greatly reduced or eliminated by the deletional inactivation of trpt (see below).
Promoter constructs with and without trpt and with and without rplN.
To illustrate the effects of trpt and other features of operon fusions that might complicate the quantitation of promoter activity, two sets of promoter fusions were constructed by inserting DNA fragments containing the promoter for the spc ribosomal protein operon into pXZ09-B (with trpt) and into pSL03 (without trpt). In the first set, pSL02 and pSL04, the insert contained only the spc promoter (from nucleotides [nt] −51 to +59 relative to the transcription start site), whereas in the second set, pSL05 and pSL06, the insert contained the spc promoter and the first gene (rplN) of the spc operon (from nt −51 to +453; see Fig. 1). To eliminate plasmid copy number effects, the Pspc-lacZ fusions in pSL02 and pSL04 and the Pspc-rplN-lacZ fusions in pSL05 and pSL06 were recombined into the mal locus of the chromosome of the lacZ deletion strain HB181. With these chromosomal constructs (strains SL102, SL104, SL105, and SL106, respectively; Table 2), there was little variation in the amount of β-galactosidase specific activity in replicate constructs or replicate cultures (see standard deviations of enzyme activity in Table 3).
lacZ expression from Pspc at different temperatures.
Cultures of the four different strains, SL102 (Pspc-lacZ with trpt), SL104 (Pspc-lacZ without trpt), SL105 (Pspc-rplN-lacZ with trpt), and SL106 (Pspc-rplN-lacZ without trpt) were grown at four different temperatures between 20 and 42°C. The β-galactosidase specific activities and the relative amounts of lacZ mRNA for the cultures were determined. In the two strains carrying the trpt element upstream of lacZ, expression of both lacZ mRNA and β-galactosidase enzyme was temperature sensitive (Fig. 2a through d, circles). However, enzyme activity and mRNA were not strictly proportional, as seen from the nonparallel curves in the semilog plots used. This suggests that the amount of β-galactosidase produced per amount of lacZ mRNA varies with the temperature. This is best illustrated by visualizing the ratio of β-galactosidase specific activity to the amount of lacZ mRNA as a function of temperature (Fig. 2e and f, circles). The values in these curves decrease with increasing temperature. This suggests that, in the presence of sequences associated with trpt, lacZ mRNA translation is severalfold more efficient at 20°C than at 42°C.
Removal of the transcription terminator trpt from the respective promoter lacZ fusions altered both the β-galactosidase specific activity and the amount of lacZ mRNA produced (Fig. 2a through d, triangles). In this case, the response of the enzyme activity was nearly proportional to that of the mRNA, so that the ratio curves (Fig. 2e and f, triangles) are essentially flat. These results indicate that the presence of trpt at the spc-lac junction directly or indirectly influences translation initiation at the downstream lacZ ribosome binding site in a temperature-dependent manner. Removal of trpt abrogates this effect. It is also apparent that the efficiency of translation of lacZ is influenced by 5′ leader sequences in a temperature-independent manner. For example, the presence of the rplN sequence in the leader resulted in a twofold reduction in lacZ translation when the two fusions lacking a functional trpt are compared (Fig. 2e and f, triangles).
Effect of different temperatures on transcript termination at trpt.
To visualize the effect of trpt on transcript termination at different temperatures, the ratio of observed transcripts +trpt/−trpt has been plotted as a function of growth temperature (Fig. 2g and h; obtained from the data in Fig. 2c and d). With or without rplN sequences, this ratio decreased with increasing temperature. This suggests that the efficiency of termination at trpt increases with increasing temperature. The value of this ratio was expected to be maximal 1.0 when trpt is totally inactive, and this ratio should be less than unity when any portion of the transcripts terminate at trpt. When rplN was present upstream of lacZ, the results were consistent with this expectation (Fig. 2h): termination at trpt was about 10% efficient at 20°C but more than 60% efficient at 42°C. Surprisingly, however, when rplN was absent, the ratio was greater than unity at low temperatures (2.2 during growth at 20°C; Fig. 2g). In this case the amount of lacZ transcript was increased, rather than decreased, by the presence of trpt. This suggests that the particular spc-trp-lac fusion without both trpt and rplN generates a fortuitous signal at the fusion junction that reduces the accumulation of transcripts, particularly at lower temperatures (Fig. 2e). This signal, although undefined, could either cause transcript termination or reduce transcript stability. When rplN sequences are present between Pspc and the trp-lacZ fusion, indications of this additional signal were not present (Fig. 2h).
Effect of different growth rates on lacZ expression and termination at trpt.
Using the same four strains, the effects of both (i) the presence or absence of trpt and (ii) the presence or absence of rplN on lacZ expression (enzyme and mRNA) were determined during growth at 37°C in different nutritional media (Table 4; Fig. 3). At all growth rates of between 1.0 and 3.0 doublings/h, the deletion of trpt resulted in the expected increase in lacZ mRNA/total RNA and in β-galactosidase specific activity (e.g., compare strains SL104 and SL102 or strains SL106 and SL105 in Table 4 or Fig. 3). The simplest interpretation of this observation is that at all growth rates a fraction of transcripts initiated at Pspc is terminated at trpt when it is present. Assuming that only the transcript termination function of trpt is responsible for the reduction in the amount of lacZ mRNA, the fraction of transcripts terminated was estimated by comparing the amounts of lacZ mRNA (per total RNA) in the two isogenic (+trpt/−trpt) strain pairs (i.e., strains SL102 and SL104 and strains SL105 and SL106). With one exception, the efficiency of transcript termination at trpt was estimated to be between 43 and 46% in both fast- and slow-growing cultures (Table 4). The exception occurred in the SL102-SL104 pair in glycerol medium, where a higher value of 74% was found. This higher value apparently results from an exceptionally high accumulation of lacZ mRNA in the reference strain SL104 without trpt (compare hybridization data in Fig. 3c and d), which may be related to the abnormality described above for this strain and illustrated in Fig. 2g. Therefore, in the absence of other complicating factors, the transcript termination function of trpt is probably not growth rate dependent.
With increasing growth rate, the β-galactosidase specific activity was not strictly proportional to the amount of lacZ mRNA (Fig. 3). The main reason for this is that the specific activity represents enzyme activity per culture mass, whereas the hybridization data represent transcripts per amount of total RNA. The two reference units, culture mass and total RNA, change differently with respect to the exponential growth rate (see below).
Comparison of lacZ expression from different promoters.
Because of different sequences at the junction of the spc-trp-lac operon fusion, the β-galactosidase activities per amount of lacZ mRNA expressed from Pspc were different in the four strains examined (Table 4). The results in Fig. 2g and h suggest that lacZ expression in strain SL106 (deletion of trpt; inclusion of rplN upstream of lacZ) was less influenced by artificial or fortuitous transcription and translation signals at or near the fusion junction than was expression in the other three strains. To test this supposition, lacZ expression from Pspc in strain SL106 (Pspc-rplN-lacZ) was compared with lacZ expression from the P1-P2 tandem promoters of the rrnB operon by using strain XZ231 (rrnB P1-P2-lacZ [38]). The rRNA promoters were chosen because their absolute activity is known and their importance for the control of ribosome synthesis has been established.
Expression from the rRNA promoters increased with increasing growth rate at both enzyme and mRNA levels (Fig. 4a and b, circles), whereas expression from Pspc decreased at the enzyme level and was nearly constant at the mRNA level (Fig. 4a and b, triangles). Despite these differences, the amounts of β-galactosidase made per lacZ mRNA were essentially the same for both promoters (Fig. 4c, filled circles and triangles; the data are normalized for the different reference units as indicated in the figure legend). Thus, in these two strains, differences in β-galactosidase activity at a given growth rate were correctly reflected by differences in lacZ mRNA accumulation.
FIG. 4.
Comparison of lacZ expression from Pspc and from the P1 and P2 promoters of rrnB. Two strains, SL106 (Pspc-rplN-lacZ, ▴) and XZ231 (rrnB P1-P2-lacZ, •) were grown in four different media supporting growth rates of between 1.0 and 3.0 doublings/h (see legend to Fig. 3). Two samples were removed from each culture for measurement of β-galactosidase specific activity (panel a), and one sample was removed for preparation of total RNA. Each RNA preparation was used in two independent hybridization assays for determination of lacZ mRNA per total RNA (panel b). The β-galactosidase activity per mRNA (panel c, solid symbols) was obtained in relative units by first forming the quotient of the data in panels a and b and then dividing this quotient by the amount of RNA/OD600 (at growth rates of 0.97, 1.23, 2.20, and 2.90 doublings/h the RNA/OD600 values were 5.8 × 1016, 6.6 × 1016, 9.4 × 1016, and 10.7 × 1016 RNA nucleotides per OD U, respectively (4, 6). The division by these values corrects for the different reference units used for enzyme specific activity (OD600) and hybridization (total RNA). The rate of translation per lacZ mRNA (panel c, open symbols) was obtained by multiplying the data represented by the solid symbols by the growth rate (ln2/τ).
These values reflect the amount of β-galactosidase per lacZ mRNA; they may be multiplied with the rate of culture growth (ln2/τ, where τ is the culture doubling time) to obtain the relative rate of β-galactosidase accumulation per lacZ mRNA. This rate was approximately constant (Fig. 4c, open symbols). As anticipated, the translation per lacZ mRNA was independent of the promoter from which lacZ was expressed, i.e., rrnB P1-P2 or Pspc (Fig. 4c, circles and triangles, respectively).
Lifetime of lacZ mRNA.
To find the relative rate of lacZ mRNA synthesis expressed from Pspc and rrnB P1-P2, the decay of lacZ mRNA was determined by following the disappearance of the lacZ hybridization signal during growth in the presence of the antibiotic rifampin (Fig. 5a and b, circles and triangles, respectively). The hybridization probe, a 308-bp section that includes the 5′ end of the lacZ coding region, was the same as in the preceding experiments. For comparison, the decay of lacZ mRNA expressed from its natural promoter, Plac, was observed with the isogenic strain HB123 (Table 2) carrying a wild-type lac operon (Fig. 5, diamonds). To obtain information about the functional life of lacZ mRNA under these conditions, the residual accumulation of β-galactosidase during rifampin treatment was also measured (Fig. 5c and d). The cultures were grown in either glycerol minimal or LB medium (Fig. 5, left and right panels). In all three strains (i.e., SL106, XZ231, and HB123) the accumulation of total RNA (mainly rRNA) stopped immediately after the addition of rifampin (Fig. 5d, open symbols), indicating that the strains used were rifampin sensitive.
After the addition of rifampin to cultures grown in glycerol minimal medium, lacZ mRNA expressed from Pspc and rrnB P1-P2 decayed initially at about the same rate, corresponding to an average life of 1.8 min (Fig. 5a). However, for Pspc-derived mRNA, the decay slowed down after 1 min, leading to a plateau of apparently stable mRNA (i.e., of the 5′-terminal region of lacZ mRNA) at about 40% of the zero time (exponential growth) level (Fig. 5a). In LB medium, the initial decay rates appear to be slightly lower than in the minimal medium (about a 2.4-min average life), and the plateau of Pspc-derived mRNA was at about 64% of the zero time level (Fig. 5b). The decay of rrnB P1-P2-derived mRNA also slowed. In this rich medium, the stability of Pspc- and rrnB P1-P2-expressed lacZ mRNA was reflected in a continuing synthesis of β-galactosidase in the presence of rifampin at a rate corresponding to 15% of the enzyme synthesis rate observed immediately before the addition of rifampin (Fig. 5d). We cannot explain why lac mRNA from heterologous promoter constructs fails to decay completely in the presence of rifampin (see Discussion).
In control experiments, mRNA expressed from the lac operon promoter, Plac, decayed exponentially and apparently completely (Fig. 5a and b, diamonds). The decay rates for Plac-derived mRNA were identical to the initial decay rates observed for Pspc- and rrn P1-P2-derived mRNAs in the two media. We assume that these rates (ca. 1.8 min in glycerol medium and 2.4 min in LB medium) reflect the decay rate of lac mRNA during exponential growth.
In further control experiments (unpublished data), the decay of rplN and rplX mRNA (first and second genes in the spc operon) was measured in the strains HB123 and SL106 with appropriate probes. These mRNAs, when derived from the spc operon, decayed exponentially and identically in the two strains used (the same RNA preparations were used as for Fig. 5). This indicates that the spc promoter is not resistant to rifampin inhibition and that the construction of SL106 did not cause a special mutation that affects mRNA decay. Therefore, the stabilization of mRNA from the spc-lac fusion appears to be specific for the fusion mRNA. It might reflect some special properties of r-protein mRNAs with respect to the control of their decay rates and the absence of those control sites in the fusion mRNA (see Discussion).
Absolute activity of the spc promoter.
The activity of Pspc relative to the combined activity of the two rRNA promoters rrnB P1-P2 was obtained as the ratio of either the amounts of lacZ mRNA expressed from Pspc and rrnB P1-P2 (ratio of the two curves in Fig. 4b) or the corresponding β-galactosidase specific activities (ratio of the two curves in Fig. 4a). Either ratio decreased with increasing growth rate from about 1.5 at a growth rate of 1.0 doubling/h to about 0.3 at a growth rate of 3.0 doublings/h (Fig. 6a, circles and triangles, respectively). The results shown in Fig. 5 suggest that Pspc- and rrn P1-P2-derived lac mRNA decayed at essentially equal rates during exponential growth so that, for a given medium, the different amounts of Pspc- and rrnB P1-P2-derived lacZ mRNAs reflect the differences in their relative synthesis rates. The absolute activity of the rRNA promoters in rRNA transcripts per minute was determined previously (4, 6) and is illustrated in Fig. 6b (open symbols). The rRNA gene activity increases from about 3 to over 60 initiations/min in the range between 0.6 and 3.0 doublings/h. By multiplying the rRNA gene activity with the relative Pspc activity (relative to the rrnB P1-P2 activity), absolute Pspc activities were obtained (Fig. 6b, circles). According to these estimates, the Pspc activity increased from a value of about 10 transcripts/min at a growth rate of 1.0 doubling/h to a plateau of about 23 transcripts/min at growth rates of above 1.5 doublings/h.
FIG. 6.
Absolute activities of Pspc and rrnB P1-P2 promoters as a function of growth rate. The activity of Pspc relative to the activity of P1-P2 promoters of rrnB (panel a) was obtained from the ratios of β-galactosidase specific activities (•) or lacZ mRNA values (▴) by using the data presented in Fig. 4a and b, respectively. The absolute activities of the rrnB P1-P2 promoters (panel b, diamonds) were obtained from literature data that were based on measurements of stable RNA synthesis and of rRNA gene dosages (4, 6); the units are the transcripts per minute per rrn operon. The absolute activity of the spc promoter (panel b, solid symbols) was obtained by multiplying the absolute activity of the rRNA promoters with the activity of Pspc relative to the activity of the rrnB P1-P2 promoters shown in panel a.
DISCUSSION
Transcription termination and other effects of trpt.
The expression of β-galactosidase activity from promoter cloning vectors based on the classical W205 trp-lac fusion has been used in the past to study promoter activities under different physiological conditions. Generally, the promoter-lacZ fusions are integrated into the bacterial chromosome by using phage λ vectors (3), and the resulting lysogens are grown at 30°C because of the presence of a temperature-sensitive λ repressor. rrnB promoters have been studied by using the mal chromosomal integration system (16) at 37°C (38, 39), which is the standard temperature for physiological experiments with E. coli. However, when we tried to use this system to study the pBR322 replication primer promoter, we noticed that the β-galactosidase activity expressed from the primer promoter was temperature sensitive (unpublished observations). Subsequently this temperature sensitivity was confirmed with other promoters, including the rrnB P1 and P2 promoters and the ribosomal protein Pspc promoter (unpublished observations). Here we have traced the temperature sensitivity to the presence of the rho-independent trpt transcriptional terminator that is located immediately upstream of lacZ in the W205 trp-lac fusion (Fig. 1).
The frequency of transcription termination at trpt was estimated by comparing the amount of lacZ mRNA present in isogenic Pspc-rplN-lacZ fusion constructs with or without a functional trpt upstream of lacZ. At 37°C, transcript termination was estimated to be 43 to 46% and independent of the growth rate (Table 4). This value is similar to the previously reported value of 37% (27) based on galK expression from Plac with or without trpt. In that study, neither the growth temperature nor the growth medium were indicated. In another study also based on enzyme activity data, a higher termination efficiency of 83% at trpt was estimated at 30°C (17% readthrough [1]). Our comparison of strains SL106 (Pspc-rplN-lacZ) and SL105 (Pspc-rplN-trpt-lacZ) indicates that very little, if any, termination at trpt occurred at 20°C but that with increasing temperature the termination efficiency increased to about 60% at 42°C (Fig. 2h). An alternative interpretation of these results cannot be ruled out, namely, that trpt or the associated sequences that have been deleted in pSL03 (Fig. 1b) produces a temperature-dependent mRNA stability signal that causes the observed temperature effects.
A comparison of lacZ expression at the enzyme and mRNA levels in isogenic strain constructs with or without trpt and with or without an rplN sequence in the lacZ leader revealed a number of anomalous effects that remain uncharacterized. We suggest that these anomalies arise from artificial sequence combinations at the spc-trp-lac fusion junction and that they influence features such as termination and antitermination properties of transcribing RNA polymerase, mRNA stability, or translation of the lacZ cistron. These indirect effects defeat the purpose of promoter-lacZ fusions, i.e., to obtain information about the promoter activity. We therefore focused our attention on the Pspc fusion containing rplN and lacking trpt. In this strain (SL106), such anomalous effects appeared to be minimal if not completely absent.
Expression of β-galactosidase from Pspc and P1-P2rrnB.
In the strain SL106 (Pspc-rplN-lacZ without trpt), the β-galactosidase activities per amount of lacZ mRNA observed at different growth rates were the same as in a previously constructed strain XZ231 (Fig. 4c) in which the P1-P2 tandem promoters of the rRNA operon rrnB are linked to lacZ (38). The rrnB P1-P2-lacZ fusion includes trpt but, in addition, contains the antitermination elements of rRNA genes which are assumed to prevent or at least greatly reduce termination at trpt: the readthrough at trpt at 30°C has been reported to be fourfold increased, from 17 to 73%, by the presence of the rrnE antitermination sites (1). The rrnB P1-P2-lacZ fusion also includes 1,120 bp of phage λ DNA between the rrnB P1-P2 promoters and the trp-lacZ section. Insertion of this DNA “spacer” was necessary for the initial cloning of strong rRNA promoters on pBR322-derived plasmids (38). The λ DNA sequences inserted do not contain known promoters or ribosome binding sites. The observation that the differences in β-galactosidase synthesis in the strains SL106 and XZ231 correctly reflect the differences in the accumulation of 5′-terminal lacZ mRNA (Fig. 4c) suggests that fortuitous translation signals at the trp-lac fusion junction are either the same or absent for these two constructs. The anti-transcription termination sites of the rRNA promoters apparently do not affect the expression of β-galactosidase activity by suppressing polarity within lacZ. Furthermore, the initial decay rates of lacZ mRNA after the addition of rifampin were about the same in SL106 and XZ231 (Fig. 5a and b), suggesting that the different lacZ leaders in these two strains do not differently affect lacZ mRNA decay during exponential growth; at later times after rifampin addition lacZ mRNA decay was clearly different. For these reasons, the use of the rrn P1-P2-lacZ construct in XZ231 as a reference for comparison with the Pspc-rplN-lacZ fusion in SL106 appears to be justified, despite the differences in the leader regions.
The β-galactosidase specific activity expressed from rrnB P1-P2 in strain XZ231 increased with increasing growth rate (Fig. 4a, circles), as was expected in view of the increased ribosome synthesis at high growth rates and in agreement with previous reports (38). On the other hand, the β-galactosidase specific activity expressed from Pspc in strain SL106 decreased with increasing growth rate (Fig. 3b and Fig. 4a, triangles). A decreasing β-galactosidase expression from r-protein promoters with increasing growth rate is in contrast to the increasing amounts of r protein made per total protein (αr [8, 10, 32]). A similar decrease β-galactosidase specific activity with increasing growth rate has been observed previously with the promoter of another major r-protein operon, S10, linked to lacZ (19). As had been suggested in that study, it is possible that this discrepancy results from the omission on the fusion constructs of certain control sites located distally in these operons. These sites are thought to regulate the decay of r-protein mRNA via translational repression, translational coupling, and endonucleolytic cleavage followed by 3′-to-5′ exonucleolytic mRNA degradation (“retroregulation”; for a review, see reference 18). In the spc operon, such sites have been located downstream of rplN (22) and are expected to stabilize the mRNA when the production of 16S rRNA exceeds or equals the production of the S8 regulatory protein. Based on the arguments above, we suggest that, in the absence of fortuitous transcription and translation signals at the junction of the operon fusion, the β-galactosidase expression from r-protein promoters such as Pspc decreases with increasing growth rate, as seen in Fig. 4a (triangles). This is not in contradiction to an increasing transcriptional activity of Pspc (see below).
Other investigators have reported that the β-galactosidase specific activity expressed from Pspc is growth rate independent (2, 14, 26). In those studies the λRS205 system carrying trpt was used. The same result was obtained here with a similar construct carrying trpt and Pspc directly linked to lacZ (strain SL102). With this strain, we also observed that β-galactosidase specific activity was independent of the growth rate (Fig. 3a, circles). We suggest that those earlier results (2, 14, 26; Fig. 3a, circles) were influenced either by the presence of trpt or by the artificial sequence combinations generated at the fusion junction.
Transcriptional activity of Pspc.
The rate of spc mRNA synthesis has previously been measured per rate of total transcription (rspc/rt) by RNA pulse-labeling and with a hybridization probe that included spc mRNA (9). In that study, rspc/rt was found to decrease by about 20% (from 2.15 to 1.74%) when the growth rate increased threefold from 0.67 to 2.1 doublings/h. Here we observed the amount of Pspc-derived lacZ mRNA per amount of total RNA, which also decreased by about 20% in the range of growth rates studied (Fig. 4b, triangles). After the amounts of lacZ mRNA shown in Fig. 6b were converted into relative synthesis rates (by using the mRNA decay data of Fig. 5) and the different reference units were taken into account the decrease becomes somewhat greater than 20%. However, because of the differences in methods, growth media, and hybridization probes, these data sets are not strictly comparable and it is not clear whether the modest discrepancy between them is significant.
The rate of spc mRNA synthesis relative to the rate of total mRNA synthesis (rspc/rm) has been reported to increase with increasing growth rate similar to αr (13, 21). This has suggested that r-protein synthesis is primarily regulated at the transcriptional level, so that the translational regulation only provides a “fine-tuning” to accurately adjust r-protein synthesis to rRNA synthesis (6, 13, 21). This interpretation was based on the plausible but unproven assumption that the rates of translation and degradation of bulk mRNA change with growth rate in a way similar to that of translation and degradation of spc mRNA. However, this assumption may not be warranted, especially since the rate of spc mRNA degradation appears to be subject to a special regulation dependent on the synthesis of rRNA (22).
The absolute activity of Pspc was estimated above in transcripts initiated per minute per promoter by comparison with the known absolute activity of rRNA promoters. At low growth rates, the Pspc activity increased approximately in proportion to the rRNA promoter activity and then became constant above 1.5 doublings/h at about 23 transcripts/min (Fig. 6b). In view of the 1.1 kb of λ DNA spacer between the promoters and lacZ in the operon fusion on strain XZ231, it seems possible that a fraction of the transcripts originating at rrnB P1-P2 terminates before reaching lacZ. In that case transcription from rrnB P1-P2 in strain XZ231 would be underestimated, so that the Pspc activities in Fig. 6b would be overestimates. However, because of the transcription antitermination elements associated with the rRNA promoters, this may not be significant, so that the spc promoter activities in Fig. 6b should be essentially correct.
Decay of lacZ mRNA in the presence of rifampin.
An attempt was made to determine the average lifetime of Pspc- and rrnB P1-P2-derived lacZ mRNA sequences by using rifampin to inhibit transcription initiation. Surprisingly, in the presence of rifampin lacZ mRNA expressed from Pspc did not completely disappear (Fig. 5b), and in LB medium some residual β-galactosidase synthesis from Pspc and rrnB P1-P2 continued (Fig. 5d). A number of control experiments demonstrated that the rifampin used was fully active and that the bacterial strains were fully sensitive. First, the accumulation of stable RNA (rRNA and tRNA) ceased immediately after the addition of rifampin in all strains used (Fig. 5d). Second, lacZ mRNA derived from the lactose operon promoter in the isogenic strain HB123 decayed exponentially and completely in the presence of rifampin (Fig. 5a and b). Third, spc mRNA sequences derived from transcription of the spc operon in the spc-lac fusion strain decayed exponentially and completely in the presence of rifampin (data not shown). Finally, in the presence of rifampin, all bacterial cultures stopped growth immediately and none accumulated rifampin-resistant bacteria (data not shown). We therefore conclude that initiation of all RNA chains ceased in the presence of rifampin and that the incomplete or nonexponential decay of Pspc- and rrnB P1-P2-derived lacZ mRNA observed at later times was due to mRNA stabilization. The mechanism responsible for this stabilization of fusion mRNA is not known. In part, it might be caused by a crowding of mRNA with ribosomes when bulk mRNA gradually vanishes during rifampin treatment. Similar decreased rates of mRNA decay as a result of ribosome crowding have been reported (23, 28). Conversely, when translation of the mRNA was reduced, the rate of lacZ mRNA decay has been found to increase (17). Again, this indicates that increased translation can result in a decreased rate of mRNA decay.
Based on the preceding arguments, we assume that the initial decay rates in the presence of rifampin reflect the decay rates during balanced exponential growth. The initial decay kinetics of lacZ mRNA derived from Plac, Pspc, and rrnB P1-P2 for a given medium were virtually identical. For glycerol medium, the initial rate corresponded to an average lifetime of about 1.8 min and for LB medium it was about 2.4 min. Since in a given medium the decay rates for Pspc- and rrnB P1-P2-derived mRNAs were the same, we were able to estimate the absolute activity of Pspc from the observed accumulations of lacZ mRNA (see above).
Features of new cloning vector pSL03.
When a reporter system is used it does not seem prudent to include a transcription termination signal upstream of the reporter gene, particularly if the terminator activity is variable and affected by conditions such as temperature and growth media. For these reasons, we and other investigators (20) have removed trpt from W205-derived vectors. The presence of trpt in the W205 fusion might not have been apparent to all previous investigators; for example, when λRS205 was used as a cloning vector for Pspc by Miura et al. (26), they stated that the fusion W205 removes the transcription termination signal of the trp operon. Clearly, trpt′ was removed but trpt was not.
In addition to the higher expression values due to the absence of the transcription terminator, pSL03 has several other desirable features. (i) In contrast to phage λ-based vectors with a temperature-sensitive repressor, the mal-inserted constructs can be grown at any temperature. Although λ-based vectors with a temperature-independent repressor are available, the presence of the prophage may not always be desirable. (ii) In the absence of a cloned promoter, there is very little background β-galactosidase activity when the construct is integrated into the chromosome. (iii) The location of mal close to oriC on the E. coli chromosome produces a relatively constant gene dosage (11), in contrast to λatt near the middle of the E. coli replicon, which shows considerable changes in gene dosage at different growth rates (4). (iv) The orientation of the lacZ insertion into the chromosome at the mal locus is such that the directions of transcription and replication are aligned. This may be an advantage for active promoters since most operons with strong promoters are oriented in this manner.
A number of investigators (see, for example, reference 30) have also observed that in fusion constructs the translation of the reporter gene may be affected by fortuitous signals that arise at the junction of the fused operons. Linn and St. Pierre attempted to alleviate this problem by including in their vector an RNase III cleavage site upstream of lacZ so that all reporter gene mRNAs had the same 5′ terminus (20). However, it is not certain even with their vector that RNase III cleavage and RNA polymerase transcription termination-antitermination properties are completely independent of growth conditions and sequences at the fusion junction. Therefore, for accurate quantitation a careful analysis of fusion gene expression is necessary with any vector.
ACKNOWLEDGMENTS
This work was supported by grants from NIH and MRC.
REFERENCES
- 1.Albrechtsen B, Squires C L, Li S, Squires C. Antitermination of characterized transcriptional terminators by the Escherichia coli rrnG leader region. J Mol Biol. 1990;213:123–134. doi: 10.1016/S0022-2836(05)80125-1. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett M S, Gourse R L. Growth rate-dependent control of the rrnB P1 core promoter in Escherichia coli. J Bacteriol. 1994;176:5560–5564. doi: 10.1128/jb.176.17.5560-5564.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand K P, Postie K, Wray L V, Reznikoff W S. Construction of a single-copy promoter vector and its use in analysis of regulation of the transposon Tn10 tetracycline resistance determinant. J Bacteriol. 1984;158:910–919. doi: 10.1128/jb.158.3.910-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bipatnath M, Dennis P P, Bremer H. Initiation and velocity of chromosome replication in Escherichia coli B/r and K-12. J Bacteriol. 1998;180:265–273. doi: 10.1128/jb.180.2.265-273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M D, van der Noorda J. Rapid and simple method for purification of nucleic acids. J Clinical Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and Molecular Biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1553–1569. [Google Scholar]
- 7.Chen C W, Thomas C A. Recovery of DNA segments from agarose gels. Anal Biochem. 1979;101:339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- 8.Dennis P P. In vivo stability, maturation and relative differential synthesis rates of individual ribosomal proteins in Escherichia coli B/r. J Mol Biol. 1974;88:25–41. doi: 10.1016/0022-2836(74)90293-9. [DOI] [PubMed] [Google Scholar]
- 9.Dennis P P. Transcription patterns of adjacent segments on the chromosome of Escherichia coli containing genes coding for four 50 S ribosomal proteins and the β and β′ subunits of RNA polymerase. J Mol Biol. 1977;115:603–625. doi: 10.1016/0022-2836(77)90105-x. [DOI] [PubMed] [Google Scholar]
- 10.Dennis P, Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B/r. J Bacteriol. 1974;119:270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donachie W. Relationships between cell size and time of initiation of DNA replication. Nature. 1968;219:1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- 12.Fiandt M, Szybalski W, Blattner F R, Jaskunas S R, Lindahl L, Nomura M. Organization of ribosomal protein genes in Escherichia coli I. Physical structure of DNA from transducing λ phages carrying genes from the aroE-str region. J Mol Biol. 1976;106:817–835. doi: 10.1016/0022-2836(76)90267-9. [DOI] [PubMed] [Google Scholar]
- 13.Gausing K. Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and of ribosomal protein messenger RNA at different growth rates. J Mol Biol. 1977;115:335–354. doi: 10.1016/0022-2836(77)90158-9. [DOI] [PubMed] [Google Scholar]
- 14.Gourse R L, de Boer H A, Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986;44:197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- 15.Helmstetter C. Rate of DNA synthesis during the division cycle of Escherichia coli B/r. J Mol Biol. 1967;24:417–427. [Google Scholar]
- 16.Hernandez V J, Bremer H. Guanosine tetraphosphate (ppGpp) dependence of the growth rate control of rrnB P1 promoter activity in Escherichia coli. J Biol Chem. 1990;265:11605–11614. [PubMed] [Google Scholar]
- 17.Iost I, Dreyfus M. The stability of Escherichia coli lacZ mRNA depends upon the simultaneity of its synthesis and translation. EMBO J. 1995;14:3252–3261. doi: 10.1002/j.1460-2075.1995.tb07328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keener J, Nomura M. Regulation of ribosome synthesis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 1417–1431. [Google Scholar]
- 19.Lindahl L, Zengel J M. Autogenous control is not sufficient to ensure steady-state growth rate-dependent regulation of the S10 ribosomal protein operon of Escherichia coli. J Bacteriol. 1990;172:305–309. doi: 10.1128/jb.172.1.305-309.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linn T, St. Pierre R. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J Bacteriol. 1990;172:1077–1084. doi: 10.1128/jb.172.2.1077-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little R, Bremer H. Transcription of ribosomal component genes and lac in a relA+/relA pair of Escherichia coli strains. J Bacteriol. 1984;159:863–869. doi: 10.1128/jb.159.3.863-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattheakis L, Vu L, Sor F, Nomura M. Retroregulation of the synthesis of ribosomal proteins L14 and L24 by feedback repressor S8 in Escherichia coli. Proc Natl Acad Sci USA. 1989;86:448–452. doi: 10.1073/pnas.86.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick J R, Zengel J M, Lindahl L. Correlation of translation with the decay of lacZ mRNA in Escherichia coli. J Mol Biol. 1994;239:608–622. doi: 10.1006/jmbi.1994.1403. [DOI] [PubMed] [Google Scholar]
- 24.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 25.Mitchell D H, Reznikoff W S, Beckwith J R. Genetic fusions defining trp and lac operon regulatory elements. J Mol Biol. 1975;93:331–350. doi: 10.1016/0022-2836(75)90281-8. [DOI] [PubMed] [Google Scholar]
- 26.Miura A, Krueger J H, Itoh S, de Boer H A, Nomura M. Growth-rate-dependent regulation of ribosome synthesis in E. coli: expression of the lacZ and galK genes fused to ribosomal promoters. Cell. 1981;25:773–782. doi: 10.1016/0092-8674(81)90185-9. [DOI] [PubMed] [Google Scholar]
- 27.Mott J E, Galloway J L, Platt T. Maturation of Escherichia coli tryptophan operon mRNA: evidence for 3′ exonucleolytic processing after rho-dependent termination. EMBO J. 1985;4:1887–1891. doi: 10.1002/j.1460-2075.1985.tb03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pease A J, Wolf R E., Jr Determination of the growth rate-regulated steps in expression of the Escherichia coli K-12 gnd gene. J Bacteriol. 1994;176:115–122. doi: 10.1128/jb.176.1.115-122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981;24:10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- 30.Reznikoff W S, Michels C A, Terrance G C, Silverstone A E, Magasanik B. Inhibition of lacZ gene translation initiation in trp-lac fusion strains. J Bacteriol. 1974;117:1231–1239. doi: 10.1128/jb.117.3.1231-1239.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryals J, Little R, Bremer H. Temperature dependence of RNA synthesis parameters in Escherichia coli. J Bacteriol. 1982;151:879–887. doi: 10.1128/jb.151.2.879-887.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967;27:41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- 33.Simons R S, Houman F, Kleckner N. Improved single- and multicopy cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 34.Winans S C, Elledge S J, Krueger J H, Walker G C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985;161:1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu A M, Platt T. Nucleotide sequence at 3′ end of tryptophan operon in Escherichia coli. Proc Natl Acad Sci USA. 1978;75:5442–5446. doi: 10.1073/pnas.75.11.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu A M, Christie G E, Platt T. Tandem termination sites in the tryptophan operon of Escherichia coli. Proc Natl Acad Sci USA. 1981;78:2913–2917. doi: 10.1073/pnas.78.5.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanofsky C, Platt T, Crawford I P, Nichols B P, Christie G E, Horowitz H, van Cleemput M, Wu A M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981;9:6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Bremer H. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem. 1995;270:11181–11189. doi: 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Bremer H. Effects of Fis on ribosome synthesis and activity and on rRNA promoter activities in E. coli. J Mol Biol. 1996;259:27–40. doi: 10.1006/jmbi.1996.0299. [DOI] [PubMed] [Google Scholar]