Structured Abstract
INTRODUCTION:
MODEL-AD is creating and distributing novel mouse models with humanized, clinically relevant genetic risk factors to more accurately mimic LOAD than commonly used transgenic models.
METHODS:
We created the LOAD2 model by combining APOE4, Trem2*R47H, and humanized amyloid-beta. Mice aged up to 24 months were subjected to either a control diet or a high-fat/high-sugar diet (LOAD2+HFD) from two months of age. We assessed disease-relevant outcomes, including in vivo imaging, biomarkers, multi-omics, neuropathology, and behavior.
RESULTS:
By 18 months, LOAD2+HFD mice exhibited cortical neuron loss, elevated insoluble brain Aβ42, increased plasma NfL, and altered gene/protein expression related to lipid metabolism and synaptic function. In vivo imaging showed age-dependent reductions in brain region volume and neurovascular uncoupling. LOAD2+HFD mice also displayed deficits in acquiring touchscreen-based cognitive tasks.
DISCUSSION:
Collectively the comprehensive characterization of LOAD2+HFD mice reveal this model as important for preclinical studies that target features of LOAD independent of amyloid and tau.
Keywords: Alzheimer’s disease, genetics, high-fat diet, late-onset Alzheimer’s disease, LOAD, TREM2, APOE4, MODEL-AD
Background
Late-onset Alzheimer’s disease (LOAD2) is the most common form of dementia, caused by a combination of genetic and environmental factors1–3. Despite recent approval of anti-amyloid therapies such as Aduhelm®4 and Leqembi®5, additional therapeutic options are essential to prevent or slow cognitive decline in most cases of LOAD. To achieve this, preclinical models that more faithfully reproduce the complex features of LOAD are required to maximize translatability of preclinical studies to the clinic.
The MODEL-AD (Model Organism Development and Evaluation for Late-Onset Alzheimer’s disease) consortium is charged with creating and phenotyping new mouse models based on the genetics of LOAD6. The IU/JAX/PITT MODEL-AD Center has focused on creating models on the C57BL/6J (B6J) genetic background that incorporate the e4 allele of the apolipoprotein E gene (APOE4)7, the greatest genetic risk factor for LOAD. In addition, we created the e3 (neutral) allele (APOE3)7. These humanized APOE alleles allow for the unrestricted use and breeding that was not readily available with previous versions7. This allowed us to determine the effects of combining multiple genetic risk factors for LOAD. We generated and characterized B6J mice that were double homozygous for both APOE4 and the R47H variant in Trem2 (Triggering receptor expressed on myeloid cells 2). These mice were termed LOAD18. Although LOAD1 mice did not develop classic hallmarks of LOAD, such as amyloid pathology, neurodegeneration, and cognitive decline, they did show alterations in gene expression levels in the brain similar to those seen in LOAD patients, as well as changes in cerebrovascular blood flow and glucose uptake8.
We now present the comprehensive characterization of LOAD2 (B6J.APOE4.Trem2*R47H.hAβ triple homozygous), where the Aβ sequence of the mouse App gene of LOAD1 mice has been humanized8. Data support that the human Aβ sequence is more amyloidogenic than the mouse version and so we test the hypothesis that LOAD2 mice will develop features of LOAD that were absent in LOAD1 mice. In addition, we evaluate a high-fat diet/high-sugar diet (HFD), a common environmental stressor, that human and mouse studies show increases risk for LOAD9, 10. For instance, our previous study showed chronic consumption of a HFD exacerbated the genetic effects of LOAD1 mice carrying the Plcg2*M28L10. Here, using a combination of a cross-sectional and longitudinal design, cohorts of male and female LOAD1 and LOAD2 mice were fed either a control diet (CD) or HFD from 2 months of age and evaluated at 4, 12, 18 or 24 months of age. Data show that unlike LOAD1 mice, LOAD2 mice fed a HFD (LOAD2+HFD) resulted in age-related neurodegeneration, cognitive deficits, elevations in insoluble Aβ and LOAD-relevant imaging abnormalities, and increased neurofilament light chain (NfL) in the plasma. We propose LOAD2+HFD as a relevant mouse model for investigating therapeutic interventions independent of targeting tau and amyloid pathologies.
Methods
Creation of the humanized Aβ allele
The humanized Aβ allele was created by direct delivery of CRISPR-Cas9 reagents to mouse zygotes of the APOE4/Trem2*R47H model, (B6(SJL)-Apoetm1.1(APOE*4)Adiuj Trem2em1Adiuj/J or “LOAD1”, JAX #28709, https://www.jax.org/strain/028709) which was previously described8
Analysis of genomic DNA sequence surrounding the target region, using the Benchling (www.benchling.com) guide RNA design tool, identified a gRNA sequence (TTTGATGGCGGACTTCAAATC) with a suitable target endonuclease site in exon 14 of the mouse App locus. Streptococcus pyogenes Cas9 (SpCas9) V3 protein and gRNA were purchased as part of the Alt-R CRISPR-Cas9 system using the crRNA:tracrRNA duplex format as the gRNA species (IDT, USA). Alt-R CRISPR-Cas9 crRNAs (Product# 1072532, IDT, USA) were synthesized using the gRNA sequences specified in the DESIGN section and hybridized with the Alt-R tracrRNA (Product# 1072534, IDT, USA) as per manufacturer’s instructions. A single-stranded DNA repair construct (synthesized by Genscript) with the sequence 5’-CTGGGCTGACAAACATCAAGACGGAAGAGATCTCGGAAGTGAAGATGGATGCAGA ATTCCGACATGATTCAGGATATGAAGTCCATCATCAAAAACTGGTAGGCAAAAATA AACTGCCTCTCCCCGAGATTGCGTCTGGCCAGATGAAAT-3’ was used to introduce the G601R, F606Y, and R609H amino acid changes in the mouse App sequence (corresponding to G676R, F681Y and R864H in human APP) such that the Ab-42 region matches the human sequence (Figure 1A).
FIGURE 1:
Longitudinal metabolic and behavioral phenotyping of mice on high-fat diet.
LOAD1 (APOE4/Trem2*R47H) and LOAD2 (hAbeta/APOE4/Trem2*R47H) animal strains differ in the App allele with a humanized Abeta1–42 region (G601R, F606Y, R609H in the mouse gene, corresponding to amino acid positions 676, 681, 684 in the human APP locus) (A). Alignment of mouse (top; Uniprot ID P12023) and humanized (bottom; Uniprot ID P05067) amyloid precursor protein (APP) amino acid sequences. White letters denote non-homology. Red arrows indicate cleavage sites of processing enzymes. Yellow arrows denote sites of humanizing mutations in App allele (LOAD1, top, and LOAD2, bottom). (Cohort 1) Animals of an 18-month longitudinal cohort were assayed at 4-, 12-, and 18-months of age. Males and females, of LOAD1 and LOAD2 genotypes, fed either control diet (CD) or high-fat diet (HFD) beginning at 2-months of age were measured for body weight (B), fasted blood glucose (C), and frailty assay index score (D), as a measure of general animal health changes. Running wheel assay measured average animal activity time for three days and nights at the 18-month age timepoint (E). Spontaneous alternation behavioral assay was utilized to measure cognition longitudinally across ages at the 18-month age timepoint (F). (Three-way ANOVA [sex, genotype, diet effects]; *=p<0.05)
Founders were bred to the LOAD1 model and genotyped for the humanized Aβ locus by PCR using forward primer 5’-CAGTTTTTGCCTCCTTGTGG-3’ and reverse primer 5’-GGCTTCTGCTCAGCAAGAACTA-3’. A positive reaction was determined by the presence of a band of 362bp. The resulting strain (“LOAD2”) is available as JAX #30670, B6J.Cg-Apoetm1.1(APOE*4)Adiuj Appem1Adiuj Trem2em1Adiuj/J (https://www.jax.org/strain/030670; B6J.APOEE4/E4.Trem2R47H/ R47H.ApphAβ/ hAβ). The App allele alone is available as JAX #33013, B6J.Cg-Appem1Adiuj/J (https://www.jax.org/strain/033013).
Cohort generation and evaluation
To evaluate LOAD-relevant phenotypes, five cohorts of LOAD2 mice and controls were created at The Jackson Laboratory (JAX, cohort 1), Indiana University (IU, cohorts 2 and 3) and University of Pittsburgh (PITT, cohorts 4 and 5). Breeding, mouse husbandry, and assays common across sites were standardized as much as possible. Below, we provide brief details of each cohort and assays performed with full details included provided in Supplemental Methods.
Cohort 1 – The Jackson Laboratory
All procedures were approved by The Jackson Laboratory Institutional Animal Care and Use Committee (IACUC).
Experimental groups:
To create experimental groups, B6J.APOEE4/E4.Trem2R47H/R47H.ApphAβ/+ mice were intercrossed to create B6J.APOEE4/E4.Trem2R47H/R47H.ApphAβ/hAβ (LOAD2) and B6J.APOEE4/E4.Trem2R47H/R47H.App+/+ (LOAD1) control mice. In appreciation of sexual dimorphism observed in human aging and disease, four groups of male and female mice were established for a combination of longitudinal and cross-sectional phenotyping at 4-, 12-, 18-, and 24-months. The 18-months group was assessed for biometrics and plasma biomarkers at 4-, 8-, 12- and 18-months.
All mice were initially provided LabDiet® 5K52/5K67 (6% fat; control diet, CD). At 2 months of age, each experimental group was randomized into two groups, the control group, and the high-fat diet (HFD) group. The control groups continued on CD ad libitum, while the HFD groups were provided ResearchDiet® feed D12451i (45% high fat, 35% carbohydrates) ad libitum. Due to attrition between 18–24 months of age, the 24-month HFD cohort was not sufficiently powered and so not analyzed. For in vivo studies – at least 10 mice/sex/genotype/age/diet were evaluated. For post-mortem analyses, 6 mice/sex/genotype/age/diet were evaluated unless otherwise stated.
Phenotyping:
The cross-sectional phenotyping battery included in vivo frailty, behavioral phenotyping, and metabolic profiling and biomarker (e.g., Neurofilament light chain, NfL) analyses in the plasma. Postmortem brain tissue was examined for transcriptomic and proteomic analyses as well as neuropathological indications of disease (amyloid, neuronal cell loss, and glial activation). For full details see Supplemental Methods (Supplemental Figure 1).
Cohorts 2: Indiana University
All procedures were approved by the Indiana University Institutional Animal Care and Use Committee (IACUC). The same bedding, light cycle, and water conditions as The Jackson Laboratory were used at Indiana University.
Experimental groups:
LOAD2 mice were imported from JAX and bred at IU. LOAD2 mice were initially crossed to LOAD1 mice to create B6J.APOEE4/E4.Trem2R47H/R47H.ApphAβ/+ mice that were then intercrossed to create B6J.APOEE4/E4.Trem2R47H/R47H.ApphAβ/hAβ (LOAD2) mice. One group of at least 10 male and 10 female mice were established for longitudinal phenotyping at 4, 12, and 18 months. Similar to the JAX cohort, mice were initially provided CD before half the mice in each group were switched to HFD.
Phenotyping:
At 4, 12, and 18 months, mice underwent in vivo MR imaging (T2 weighted images) and blood draws for biomarker (e.g., Aβ species, cytokines) analyses. For full details see Supplemental Methods. At 18 months, tissues were collected as described for Cohort 1.
Cohort 3: Indiana University
All procedures were approved by the Indiana University Institutional Animal Care and Use Committee (IACUC). The same bedding, light cycle, and water conditions as The Jackson Laboratory were used at Indiana University.
Experimental groups:
Experimental groups of male and female LOAD2 mice on HFD or CD were established as described for Cohort 3 (n=12 mice/sex/genotype/age/diet). Three groups were established for cross-sectional analyses at 4, 12, and 18 months.
Phenotyping:
To evaluate neurovascular uncoupling, in vivo PET/CT imaging was performed on all mice measuring regional blood flow (via 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone, 64Cu-PTSM) and regional glycolytic metabolism (via 2-18F-2-deoxyglucose, 18F-FDG). Findings from in vivo PET/CT were confirmed using autoradiography. For full details see Supplemental Methods.
Cohort 4 and 5: University of Pittsburgh
All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC). Detailed mouse husbandry, diet restriction, and phenotyping methods are included in the Supplemental Methods.
Experimental groups:
Two experimental cohorts were evaluated for plasma biomarkers and cognitive testing. Breeding pairs of LOAD2 mice were imported from JAX and bred at the University of Pittsburgh to create Cohort 4. One group of n=18 male and n=18 female mice were established for longitudinal blood plasma collection followed by cognitive assessments using the touchscreen. Subjects were reared on normal control diet (CD) (LabDiet® 5P76) provided ad libitum until 2 months of age at which time n=13 male and n=14 female mice were randomly assigned to receive ad libitum HFD (LOAD2+HFD). For cohort 5, LOAD2 mice (B6J.APOEE4/E4.Trem2R47H/ R47H.ApphAβ/ hAβ ) were bred with C57BL/6J to provide littermate controls. The F1 offspring which were triple heterozygotes (B6J.APOEE4/+.Trem2R47H/+.ApphAβ/+) were then crossbred to produce F2 offspring including the LOAD2 triple homozygote mice (n=9/sex) and triple wildtype littermate controls (n=6/sex). All mice were initially reared on CD, with n=3/sex wildtype controls and n=6/sex LOAD2 switched to ad libitum HFD at 6–12 months of age. Prior to touchscreen testing, mice were individually housed and restricted to 80–85% of free-feeding body weight. Mice were weighed daily and provided a ration of the respective CD or HFD diets that maintained them at 80–85% restriction.
Phenotyping:
Blood plasma was evaluated longitudinally prior to the start of HFD, followed monthly for analysis of cytokines and every 3 months for analysis of Aβ species. To evaluate the effect of food restriction on plasma biomarker levels, brief 2-week periods of food restriction as described above were administered to Cohort 4 at 8–8.5 months of age and at 10–10.5 months of age. At 14 months of age, Cohort 4 was enrolled in Touchscreen cognitive testing and maintained continuously on dietary restriction until the conclusion of touchscreen testing (Figure 8A); while Cohort 5 began food restriction and touchscreen testing at 11–17 months of age (Supplemental Figure 7A). It is important to note that the present studies used a 10% sucrose solution for the reward which is a departure from the standard touchscreen protocols that use strawberry flavored milkshake-based rewards11. We intentionally chose to avoid milk-based rewards given that the constituents of milk and dairy products may contribute to attenuation of AD related pathologies including amyloid deposition and inflammation12–14. Notably 10% sucrose is a common and well-established reinforcer for mice in operant based tasks and therefore was a salient alternative as evidenced by the ability of all subjects to demonstrate consumption of the reward and acquire the touch-reward association during the initial phase of the task.
FIGURE 8:
Comprehensive validation of LOAD2 mouse model for preclinical drug testing.
As a confirmation and extension of initial characterization data of the LOAD2 mouse model conditioned on high-fat diet (HFD) to serve as a potential model for preclinical testing, independent cohorts were evaluated for disease trajectory of serial plasma biomarkers and cognitive testing. A) Illustration of timeline and procedures; B) plasma TNF-α (pg/mL); C) Plasma IL-6 (pg/mL); D) plasma IL-10 (pg/mL); E. Plasma IL-1β (pg/mL); F) plasma IFNy (pg/mL), G) plasma IL-5 (pg/mL); H) plasma KC-GRO (pg/mL); I) plasma IL-2 (pg/mL); J) plasma Aβ40 (pg/mL); K) plasma Aβ42 (pg/mL); L) calculated Aβ 42:40 ratio in plasma; M) Learning curves of aged (14+ month) LOAD2 mice ± HFD in comparison to age- and sex-matched WT controls during the acquisition phase of the touchscreen cognitive testing battery. Plasma cytokines and plasma Aβ were measured using MesoScale Discovery multiplex ELISA kits (in accordance with the manufacturer’s protocol.
Results
LOAD2 mice were created at JAX and distributed to IU and PITT for evaluation of LOAD relevant phenotypes using the IU/JAX/PITT MODEL-AD center pipeline that includes a combination of human-relevant in vivo and post-mortem assays. The primary goal of the phenotyping pipeline is to determine the utility of new LOAD models for preclinical testing. Five cohorts of LOAD2 and control mice fed either a HFD (high-fat diet) or CD (control diet) were evaluated at JAX (Cohort 1: biometrics, behavior, plasma biomarkers, neuropathology, transcriptomics, proteomics), IU (Cohort 2: MRI, plasma biomarkers and cytokines, biochemistry, neuropathology; Cohort 3: PET/CT, autoradiography), and PITT (Cohorts 4 and 5: longitudinal plasma biomarkers, Touchscreen cognitive testing) (Supplemental Figure 1). Unless otherwise stated, to evaluate LOAD2 phenotypes (on CD or HFD), LOAD1 mice were used as the control genotype to evaluate the effects of humanizing the Aβ sequence in the context of the APOE4 and Trem2*R47H risk alleles (LOAD1). LOAD1 and LOAD2 mice both express humanized APOE4 and Trem2*R47H risk alleles on a B6J background, however LOAD2 animals also express a humanized allele for App (Figure 1A).
LOAD2 mice show diet- and age-dependent neuronal cell loss and plasma NfL increases (cohort 1)
Cohort 1 comprised four age groups (4, 12, 18 and 24 months). The 4-, 12-, and 18-month groups included LOAD1 and LOAD2 mice on both HFD and CD. The 24-month group included only mice on CD. We first evaluated the 18-month group using in vivo assays following a longitudinal design. Longitudinal testing and sampling were performed at 4-, 12-, and 18-months of age. We observed significant, diet-driven increases in body weight with age (Figure 1B). All mice showed an increase in weight with age, but mice fed HFD showed pronounced weight gain until 18 months of age. Females on the HFD displayed significant weight gain from 4- to 12-months, but only made modest gains from 12- to 18- months. Males fed a HFD were more accelerated than females, and LOAD2 males were consistently heavier than LOAD1 males at all timepoints. However, fasted blood glucose measurements did not appear to be age-, diet-, or genotype-dependent, though slightly elevated levels were observed in LOAD2 mice fed HFD (LOAD2+HFD) (Figure 1C). As expected, age was a strong factor of increased frailty15 (Figure 1D). However, we did not see significant diet-related changes in frailty scores in females until 18 months of age and only in LOAD2 genotype animals. Males consistently displayed increased frailty driven by diet, but not genotype, as early as 8 months of age.
HFD reduced performance in open field assays compared to CD. Specifically total distance traveled decreased significantly only in LOAD2 animals (Supplemental Figure 2A), but performance was not affected by sex or genotype alone. Differences in total vertical activity were only observed in males, of both genotypes, to be decreased by HFD (Supplemental Figure 2B). Rotarod performance was decreased in a HFD-dependent manner only (Supplemental Figure 2C). LOAD2+HFD animals, however, particularly males, demonstrated a reduction in running wheel activity during the active period (dark cycle) compared to LOAD1 mice or those fed CD (Figure 1E). Hippocampal working memory in the spontaneous alternation assay as a measure of cognitive function was intact across all groups with all subjects performing >chance levels which is calculated as 22% in this assay (Figure 1F).
Brains from mice from the 18-month group were harvested (along with the 4-, 12-, and 24-month groups). One hemisphere (right) from each brain was prepared for transcriptomics and proteomics, while the other hemisphere (left) was evaluated for neuronal cell loss, microglia number, astrocyte reactivity and amyloid plaques in the cortex and hippocampus by immunofluorescence (Supplemental Figure 3). At 18 months of age, neuron counts in the cortex revealed a subtle but statistically significant decrease in NeuN+DAPI+ cells in female LOAD2+HFD compared to female LOAD2+CD and female LOAD1+HFD (Figure 2 A,B). No differences were observed in cortical IBA1+DAPI+ microglia number (Figure 2 C,D) across all groups, and a small but significant decrease in hippocampal astrocyte reactivity (assessed using GFAP+DAPI+) in male LOAD2+HFD mice compared to those fed a CD diet (Figure 2 E,F). ThioS staining revealed no evidence of amyloid plaques in any groups (Figure 2G). To determine whether neuronal cell changes in LOAD2+HFD was reflected by changes in the plasma, NFL, a clinically relevant biomarker16 was assessed. Plasma NfL levels were significantly increased in LOAD2 animals on both diets (Figure 2H). Similarly, we saw significant increase in LOAD1+HFD relative to LOAD1+CD.
FIGURE 2:
Neuropathological assessment of brain tissue.
Immunohistochemistry of brain tissue in the cortex and hippocampus from 18-month-old animals stained for cell markers to reveal genotype- and diet-driven differences in glial cell densities. Slices of brain hemispheres were stained with (A,B) NeuN (neurons) or (C,D) IBA1 (microglia) (representative cortical images shown from LOAD2 females with DAPI co-stain) and counted relative to area. Astrocytes (GFAP) quantitated in the hippocampus of LOAD2 females fed either CD or HFD (E-F). ThioS staining of brain tissue to visualize amyloid plaques (representative images shown from LOAD2 females) (G). Inlay: scaled image of 12mo B6J.APP-SAA hyper-amyloid positive controls. LISA testing for neurofilament light-chain (NfL) in plasma derived from terminal, peripheral blood samples at 18-months of age. Linear regression analyses were performed to identify effect of each factor on NfL levels (H). (NeuN=neuronal marker; ThioS=amyloid plaques; GFAP=astrocyte marker; IBA1=microglial marker. Scale bar equals 100μm.)
Differential analysis identifies strong transcriptional changes in female mice expressing humanized Aβ on high-fat high sugar diet (cohort 1)
To identify molecular effects of humanizing the Aβ peptide, we first performed pairwise differential analysis between LOAD2 and LOAD1 mice at all ages for both sexes. Differential expression analyses identified very few significantly differentially expressed genes (DEGs) (p < 0.05) at 4 and 18 months old LOAD2 mice compared to age and sex-matched LOAD1 mice (Supplementary Table A). At 12 months, there were 57 DEGs (38 upregulated, 19 downregulated; p < 0.05) in male LOAD2 mice, and 17 DEGs (3 upregulated, 14 downregulated; p < 0.05) in female LOAD2 mice (Supplementary Table A). KEGG functional enrichment analysis identified enrichment of “protein processing in ER” in upregulated DEGs in 12 months old LOAD2 male mice and “MAPK signaling pathway” in downregulated DEGs in 12 months old LOAD2 female mice (Supplementary Table B). At 24 months, there were only 5 significantly differentially upregulated genes (p < 0.05) in male LOAD2 mice, and 30 DEGs (12 upregulated, 18 downregulated; p < 0.05) in female LOAD2 mice (Supplementary Table A). Upregulated DEGs in male and female LOAD2 mice were enriched for the “motor proteins” KEGG pathways (Supplementary Table B).
Next, we performed differential analyses in 18-month-old LOAD2 and LOAD1 mice compared to age and sex-matched B6J control mice. In females, we observed only 9 significantly DEGs (3 upregulated, 6 downregulated) (p < 0.05) in LOAD2 mice on control diet (CD), while 2988 genes were significantly differentially expressed (1565 upregulated, 1423 downregulated) (p < 0.05) in LOAD2+HFD (Supplementary Table A). We observed 44 significantly DEGs (19 upregulated, 25 downregulated) (p < 0.05) in female LOAD1+CD, while 164 genes were significantly differentially expressed (117 upregulated, 47 downregulated) (p < 0.05) in female LOAD1+HFD (Supplementary Table A). In males, we observed a total of 7 and 23 significantly DEGs (p < 0.05) in LOAD1 and LOAD2 mice on CD, respectively, while 39 and 98 genes were significantly expressed (p < 0.05) in LOAD1 and LOAD2 mice on HFD, respectively (Supplementary Table A). Overall, we observed more differentially expressed genes in mice conditioned on HFD and this effect was more prominent in female mice expressing humanized Aβ.
Functional enrichment analyses of DEGs in female LOAD2+HFD identified enrichment of multiple KEGG pathways such as “glutamatergic synapse”, “dopaminergic synapse”, and “MAPK signaling pathway” in upregulated genes, while downregulated genes were enriched for KEGG pathways such as “lysosome”, “fatty acid metabolism”, “TCS cycle”, and “valine, leucine and isoleucine degradation”. Differentially upregulated genes in female LOAD1+HFD were enriched for “circadian entrainment”, while downregulated genes were enriched for “phagosome” KEGG pathway (Supplementary Table B). We did not observe enrichment for any KEGG pathways in DEGs in LOAD1 and LOAD2 mice on control diet.
Next, we assess the effect of HFD by performing differential analysis between mice fed the HFD with age, sex, and genotype-matched mice on CD. We observed 260 significantly DEGs (154 upregulated, 106 downregulated) (p < 0.05) in female LOAD2+HFD compared to female LOAD2+CD, while 45 significantly DEGs (9 upregulated, 36 downregulated) (p < 0.05) in male LOAD2+HFD compared to male LOAD2+CD (Supplementary Table A). In LOAD1 male mice, we observed a total of 12 DEGs (p < 0.05) on HFD compared to CD, while only 2 DEGs (p < 0.05) on HFD compared to CD in female LOAD1 mice (Supplementary Table A). Upregulated genes in female LOAD2 female mice on HFD compared to CD were enriched for KEGG pathways such as “glutamatergic synapse”, “dopaminergic synapse”, and “MAPK signaling pathway”, while downregulated genes in female LOAD2+HFD compared to CD were enriched for “motor proteins” pathway (Supplementary Table B).
Gene modules associated with AD pathology driven by age and high-fat high-sugar diet
Differential expression analyses identified subtle changes at the gene level and suggested pronounced effect of LOAD2 genotype by high-fat/high-sugar diet (HFD) in aged mice. To further ensure these signals, we performed a weighted gene co-expression network analysis (WGCNA) 17 on the brain transcriptome to identify gene expression changes in a system-level framework. WGCNA identified 30 distinct modules of co-expressed genes (Supplementary Table B2).
To understand the functional significance of these modules, we correlated each module eigengene with age, sex, diet, genotype, and measured behavioral assays such as cumulative frailty score, body weights, NfL, plasma cytokines, GFAP, Iba1 and NeuN counts (Supplemental Figure 4). Eighteen of these 30 modules were significantly correlated with age (p < 0.05) (turquoise, lightyellow, darkgreen, green, darkgrey, floralwhite, purple, brown4, red, skyblue3, lightcyan, orangred4, darkorange2, plum1, orange, lightcyan1, greenyellow, and blue). Six modules were significantly correlated with HFD (p < 0.05) (lightyellow, turquoise, brown4, darkgrey, darkred, orangered4). Seven modules were significantly correlated with sex (p < 0.05) (sienna3, greenyellow, orangered4, darkmagenta, skyblue3, red, bisque4). Two modules (greenyellow and sienna3) were significantly correlated with the Apoe4.Trem2*R47H allele combination in both LOAD1 and LOAD2 (p < 0.05), while five modules were significantly correlated with humanized Aβ specific to LOAD2 (p < 0.05) (brown, thistle3, greenyellow, blue, and orange) (Supplemental Figure 4).
We observed that HFD was strongly significantly correlated with the lightyellow module (r=0.45; p = 9 × 10−9), while age was strongly significantly correlated with both the turquoise (r=0.81; p = 2 × 10−35) and lightyellow modules (r=0.52; p = 1 × 10−11) (Supplemental Figure 4). To further elucidate the association of the lightyellow and turquoise modules with age, sex, and genotype-diet combinations, we performed linear regression analysis using module eigengene as the dependent variable. We determined that the lightyellow module was significantly positively correlated with LOAD1 and LOAD2 genotype with HFD (p < 0.05) and age (p < 0.001), while turquoise module was significantly positively correlated with age (p < 0.001) (Figure 3A). In summary, we observed age and genotype-by-diet effects on the lightyellow module, while the turquoise module is driven only by age. We also observed that the diet and age driven lightyellow module was significantly correlated (p < 0.05) with multiple assays such as NfL, frailty score, plasma cytokines (IL1β, IL10, IL5, IL6, KC-GRO) (Figure 3B, Supplemental Figure 4). In contrast, the age driven turquoise module was significantly correlated (p < 0.05) with effect of age on behavior and weakly correlated with a few plasma cytokines (IL2, IFN-γ) and inflammatory cell counts (Iba1 and GFAP counts) (Figure 3B, Supplemental Figure 4). The lightyellow module was uniquely driven by both age and diet and demonstrated strong positive associations with AD biomarkers such as NfL and multiple cytokines.
FIGURE 3:

A gene module associated with AD biomarkers is driven by age and high-fat high-sugar diet.
The lightyellow gene module was associated with advanced ages (p < 0.001) and both genotype on HFD (p < 0.05), while the turquoise module was associated with age only (p < 0.001) (A). Correlations between the turquoise and lightyellow module eigengenes. Lightyellow was significantly correlated with frailty score, body weight, NfL, and many plasma cytokines (IL-1β, IL-2, IL-12p70, IL-10, IL-5, IL-6, KC-GRO), while the age-driven turquoise module was correlated with behavioral assay (frailty score and body weights) and weakly correlated with a few plasma cytokines (IL-2, IFNγ) and inflammatory cell counts (IBA1 and GFAP counts) (B). Positive correlation coefficients are shown in blue and negative correlations in red, proportional to color intensity and circle size, with frames for significant correlations (FDR < 0.05). AD-related biological domain enrichment analysis in the age and HFD driven lightyellow module gene set using Fisher exact test, with the top six enriched GO terms within each enriched bidomain (C). Network of genes in each enriched biological domain and the lightyellow module (D).
We further assessed the enrichment of AD biological domains18 in gene modules. We found that genes in lightyellow modules were significant enriched for the Apoptosis (odds ratio=1.90, p = 2.11 × 10−4), Immune Response (odds ratio=1.63, p = 3.67 × 10−3), Lipid Metabolism (odds ratio=2.01, p = 3.64 × 10−6), Oxidative Stress (odds ratio=1.76, p = 3.32 × 10−2), and Vasculature (odds ratio=2.14, p = 1.14 × 10−4) AD biological domains (Figure 3C–D; Supplementary Table B3). We also identified GO-terms associated with these biological domains that are significantly enriched in lightyellow gene modules (Figure 3C–D, Supplementary Table B3). On the other hand, genes in the turquoise module were prominently enriched for the Immune Response biological domain (odds ratio=2.39, p = 1.8 × 10−49) (Supplementary Table B3). These data suggest that age is strongest risk factor for driving inflammatory changes, while diet effects in aged LOAD mice are associated with multiple AD endophenotypes such as lipid metabolism, immune response, and oxidative stress.
LOAD mice display proteomics changes characteristic of AD
Tandem mass tag proteomics was performed on hemibrains from LOAD1 and LOAD2 mice at 4, 12, and 18 months of age on control diet. LOAD1 and LOAD2 mice fed HFD were also assayed at 18 months, paired with B6J controls on CD. A total of 10,406 proteins were quantified across 106 samples. To focus on effects in aged mice, we assessed protein expression changes in at 18-month-old LOAD1 and LOAD2 mice on CD and HFD compared to age-matched B6J mice on CD by one-way ANOVA with post-hoc Tukey significance testing (Supplementary Table C). In LOAD1 mice on CD, we observed 1666 significantly differentially expressed proteins (838 upregulated, 828 downregulated) (padj < 0.05), while a total of 2590 proteins were significantly differentially expressed (1237 upregulated, 1353 downregulated) (padj < 0.05) in LOAD1+HFD compared to B6J mice on CD (Supplementary Table C). In LOAD2+CD, we observed a total of 1102 significantly differentially expressed proteins (535 upregulated, 567 downregulated) (padj < 0.05) while in LOAD2+HFD we observed 1839 significantly differentially expressed proteins (897 upregulated, 942 downregulated) (padj < 0.05). We therefore observed hundreds of differentially expressed proteins in both LOAD1 and LOAD2, with greater numbers for both strains on HFD.
To assess disease relevant aspects of mouse models, we performed a correlation analysis between mouse models and 44 human proteomics modules from a LOAD study of the dorsolateral prefrontal cortex19. These modules were functionally annotated and named based on protein enrichments and each was assessed for eigenprotein correlations to AD traits including neuropathological markers and cognitive outcomes19. Twelve modules were significantly correlated to one or more traits, referred to here as AD modules19. We compared protein expression changes in LOAD1 and LOAD2 mice relative to B6J mice at 18 months with changes observed in human AD subjects versus controls for each human protein module. This procedure allowed module-wide assessment of coordinated protein changes and therefore determined murine reproduction of each module that characterizes human LOAD.
LOAD1 and LOAD2 mice were significantly and positively correlated (p < 0.05) with multiple common human AD modules. These included M1_Synapse_Neuron, M3_Oligo_Myelination, and M12_Cytoskeleton (Figure 4A; Supplementary Table D). The M2_Mitochondria module exhibited significant positive correlation (p < 0.05) with all mouse models except LOAD2 mice on HFD, for which a positive correlation did not reach significance (p = 0.06) (Figure 4A; Supplementary Table D). Additional correlations reaching significance included LOAD1 mice on HFD and LOAD2 mice on both diets with M22_Post-Synaptic_Density and M38_Heat_Shock_Folding modules (Figure 4A; Supplementary Table D). LOAD2+HFD additionally showed significant positive correlations with M4_Synapse_Neuron, M7_MAPK_Metabolism, and M43_Ribonucleoprotein_Binding modules (Figure 4A; Supplementary Table D), whereas other mice (LOAD1+CD, LOAD1+HFD and LOAD2+CD) were positively correlated but below the significance threshold.
FIGURE 4:
LOAD mice exhibit proteomics changes similar to human LOAD.
Correlation coefficients between 18-month-old LOAD mouse models and 44 human proteomics co-expression modules [Johnson et al Ravi’s Ref 9] (A). Modules in bold face were significantly correlated to one or more AD traits. Circles corresponds to positive (blue) and negative (red) Pearson correlation coefficients for protein expression changes in LOAD mice (log fold change of LOAD strains versus B6J) and human disease (log fold change for cases versus controls). Color intensity and size of the circles are proportional to the Pearson correlation coefficient, with significant correlations (p < 0.05) framed. LOAD1 and LOAD2 mice were significantly and positively correlated (p < 0.05) with multiple human AD modules, primarily related to synaptic function. Five top enriched GO terms for proteins with common directional changes for 18-month-old LOAD2 mice on HFD and human AD cases (B). Protein module network with common directional changes for 18-month-old LOAD2 mice on HFD and human proteomics modules (C). Blue (red) nodes correspond to increased (reduced) protein abundance in both 18-month-old LOAD2 HFD mice compared to B6J mice and human AD cases versus controls.
Overall, we detected genetic effects from aged LOAD1 and LOAD2 mice that correlated with multiple human AD proteomics modules that were generally enhanced by exposure to HFD, especially in LOAD2 mice. LOAD2+HFD were positively correlated with ten of the 44 total protein modules, of which five modules (M1_Synapse_Neuron, M3_Oligo_Myelination, M4_Synapse_Neuron, M7_MAPK_Metabolism, and M22_Post-Synaptic_Density) were correlated with AD traits19. These modules frequently represented neuronal proteins, distinct from the immune and metabolic signatures observed in transcriptomic analyses.
To better understand the functions of proteins driving the significant positive correlations between LOAD2 mice on HFD and human AD, we isolated the proteins within each module with common directional changes (increased or decreased abundance) for LOAD2+HFD and human AD cases. We performed gene ontology enrichment analysis on these proteins. Proteins that showed mouse-human directional coherence in the M1_Synapse_Neuron module were enriched for biological functions including “synaptic vesicle cycle”, “vesicle-mediated transport in synapse”, and “synapse organization” (Figure 4B; Supplementary Table E). Proteins that showed directional coherence in the M4_Synapse_Neuron module were enriched for “synaptic vesicle cycle” and “exocytosis” biological functions (Figure 4B; Supplementary Table E). In the M22_Post-Synaptic_Density module, coherent mouse-human proteins were enriched for biological functions including “synapse organization”, “postsynapse organization”, and “dendrite development” (Figure 4B; Supplementary Table E). These functions represent the core neuronal processes recapitulated in LOAD2+HFD at the protein level.
Proteins in these synaptic AD protein modules mostly had reduced abundance in LOAD2.HFD mice (logFC < 0) compared to chow-fed B6J controls. This reduced expression of proteins associated with synapse/neuronal functions was similar to human AD cases (Figure 4C), although we note these mice did not exhibit frank neurodegeneration.
For non-synaptic modules, we found coherent proteins in the M3_Oligo_Myelination module were enriched for biological functions including “oligodendrocyte differentiation”, “myelination”, and “microtubule organization” (Figure 4B; Supplementary Table E). Proteins with directional coherence in the M7_MAPK_Metabolism module were enriched for biological functions such as “regulation of transforming growth factor beta production” and “carbohydrate metabolic process” (Figure 4B; Supplementary Table E). In the M28_Ribosome_Translation module, protein abundances mostly increased (logFC > 0) and were enriched for “ribosome biogenesis” and “cytoplasmic translation” (Figure 4B–C; Supplementary Table E). Finally, coherent proteins in the M38_Heat_Shock_Folding module were enriched for “protein folding” and “chaperone-mediated protein folding” biological functions (Figure 4B; Supplementary Table E). Proteins exhibiting directional coherence in the M3_Oligo_Myelination and M7_MAPK_Metabolism modules generally had greater abundances in LOAD2+HFD mice compared to B6J controls and human AD cases, corresponding to increased protein expression associated with ‘MAPK_metabolism’ and ‘Oligo myelination’ (Figure 4C).
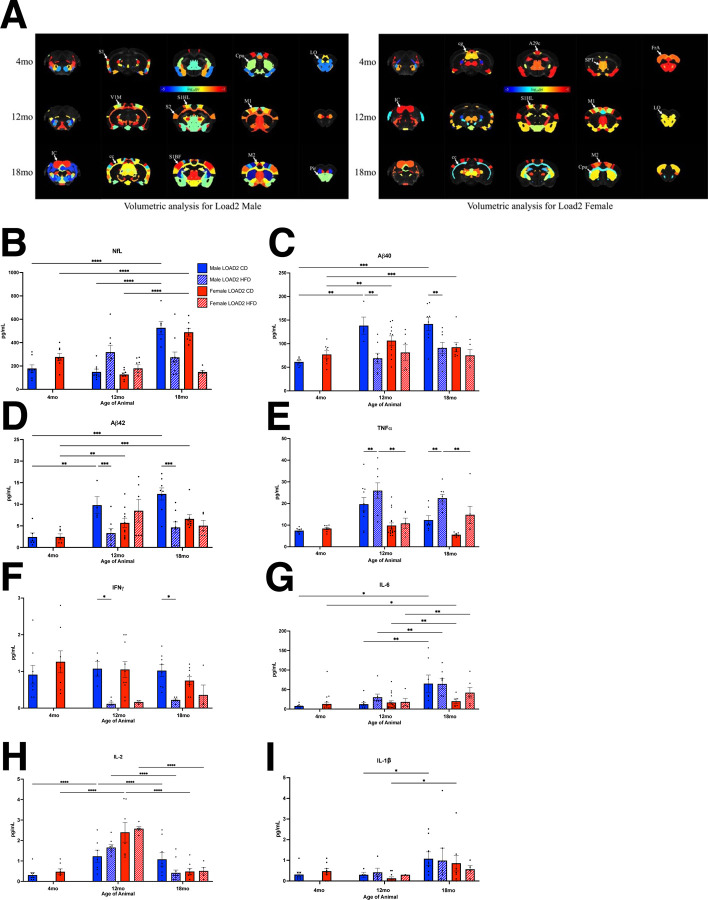
Longitudinal Volumetric Measurements reveals age-dependent changes in brain volume (cohort 2)
Longitudinal volumetric measurements were employed to elucidate age-dependent alterations in brain volume within Cohort 2. Building upon the findings derived from Cohort 1 at JAX, which encompassed indicators such as neuronal cell loss, increased levels of neurofilament light chain (NfL), and molecular manifestations of neuronal cell dysfunction (refer to Figures 1–4), Cohort 2 was established at Indiana University. This cohort consisted of male and female mice designated as LOAD1 and LOAD2, subjected to either a standard control diet (CD) or a high-fat diet (HFD). The primary objective at Indiana University was to assess brain volumes through in vivo magnetic resonance (MR) imaging and to conduct a comprehensive evaluation of LOAD2+HFD mice for plasma and brain biomarkers, with potential implications for preclinical testing.
In vivo MR imaging was conducted on LOAD2 CD and HFD-fed mice at 4, 12, and 18 months of age. The mean whole brain volumes for male LOAD2 CD and HFD mice were 478.56±18.63 and 459.56±6.41 mm^3 at 4 months, 464.53±15.16 and 468.40±9.83 mm^3 at 12 months, and 502.68±13.59 and 470.25±6.37 mm^3 at 18 months, respectively. Correspondingly, the mean whole brain volumes for female LOAD2 CD and HFD mice were 468.19±7.28 and 467.42±6.52 mm3 at 4 months, 479.84±12.60 and 483.48±8.20 mm3 at 12 months, and 507.29±11.46 mm3 and 487.37±11.86 mm3 at 18 months.
Statistical analysis revealed a significant reduction in brain volume was observed at 4 and 18 months in LOAD2+HFD male mice (4 months, p=0.0225 and 18 months, p=7.88e-6), while no significant difference was observed at 12 months (p=0.5134). For LOAD2 female mice on a HFD, a significant difference was observed at 18 months (p=0.0186), but no significant disparities at 4 months (p=0.812) or 12 months (p=0.4911).
Among the 165 brain labels analyzed, 45, 57, and 95 brain areas exhibited significant volumetric reductions at 4, 12, and 18 months, respectively, for male mice. Similarly, for female mice, 47, 67, and 51 brain areas displayed significant reductions at 4, 12, and 18 months. Volumetric statistical maps were generated for both male and female LOAD2 CD and HFD mice at the time points, and the significant areas were superimposed onto the T2-weighted template image. Notably, the analyses unveiled a progressive increase in the number of significant areas with advancing age of the mice (Figure 5A).
FIGURE 5.
High fat diet reduces brain volume in multiple brain regions and alters plasma biomarkers.
Volume statistics maps for LOAD2 male at 4 months, 12 months and 18 months. The significant brain areas were overlaid over gray scale subject template image. (The p-values were converted into logarithmic scale between range −5 to −1. S1, Primary Somatosensory Cortex; Cpu, striatum; LO, Lateral Orbital Cortex; V1M, Primary Visual Cortex, Monocular area; S2, Secondary Somatosensory Cortex; S1HL Primary Somatosensory Cortex, Hindlimb; S1BF Primary Somatosensory Cortex, Barrel Field; M1, Primary Motor Cortex; M2 Secondary Motor Cortex; IC Inferior Colliculus; cc Corpus Callosum; Pir, Piriform cortex. Volume statistics maps for Load2 female at 4 months, 12 months and 18 months. The significant brain areas were overlaid over gray scale subject template image. The p-values were converted into logarithmic scale between range −5 to −1. A29c, Cingulate Cortex; cg, Cingulum; SPT, Septum; FrA, Frontal Association Area; IC, Inferior Colliculus; S1HL, Primary Somatosensory Cortex, Hindlimb; LO, Lateral Orbital Cortex; M1 Primary Motor Cortex; M2 Secondary Motor Cortex; cc, Corpus Callosum; Cpu, Striatum) (A). At 12 months of age, NfL is increased in high fat diet males, but not in females (B). However, by 18 months of age, aging has a greater effect on NfL, with increases in NfL between 12 and 18 months in both males and female mice. Plasma Aβ40 is reduced in high fat diet males at 12 and 18 months of age but is unchanged in female mice at any timepoint (C). Aβ42 is not altered by a high fat diet (D). TNF-α is increased in male mice on a high fat diet at both 12 and 18 months of age (E). Females have increased TNF-α at 18 months of age, but it does not reach the level of significance. IFNγ is reduced at 12- and 18-month male mice on a high fat diet (F). IL-6 is increased in male mice between 12–18 months of age, but there is no effect of high fat diet (G). In females, IL-6 is not altered. IL-2 increases between 4–12 months in males and females regardless of diet (H). By 18 months, IL-2 is reduced in both males and females. IL-1β increases with age between 12–18 months regardless of diet (I). *p<0.05, **p<0.01, ***p<0.001, ****P<0.0001.
Plasma and Brain Cytokines Are Altered by HFD
We next used cohort 2 mice to identify cytokines in the plasma and brain that may be utilized as biomarkers for preclinical testing. Analysis of NfL and cytokines in the plasma revealed an increase in NfL at 12 months in HFD males (Figure 5B). By 18 months, however, the HFD effect was absent, but an increase in NfL was driven by age (Figure 5A). Aβ40 was lower in males fed a HFD at 12 and 18 months, but the same difference was not observed in females; Aβ42 levels were not significant at any time point, likely due to variability among groups, however, there was an overall trend for a reduction in Aβ42 in males fed a HFD at 12 and 18 months (Figure 5 C,D). In HFD animals, we found significant increases in TNFα (Figure 5E) in LOAD2+HFD males at 12 and 18 months, females were approaching a significant increase by 18 months, but did not reach significance (Figure 5F). Additional proinflammatory cytokines were examined (Figure 5 G–I), with reductions in IFNγ observed in HFD males at 12 and 18 months and age-related alteration in IL-6, IL-2 and IL1β. Interestingly, as a confirmation and extension of these data and to demonstrate cross-laboratory replicability, we also observed sustained TNFα levels in HFD mice from Cohorts 4 and 5 (University of Pittsburgh). LOAD2+HFD males from 2 months of age in Cohort 4 maintained significantly higher concentrations of TNFα in their plasma from 7 months of age onward compared to LOAD2 males fed CD, while females on HFD appeared to follow a similar trend as males but only reached significance at 8–8.5 and 15–18 months of age (Figure 8B).
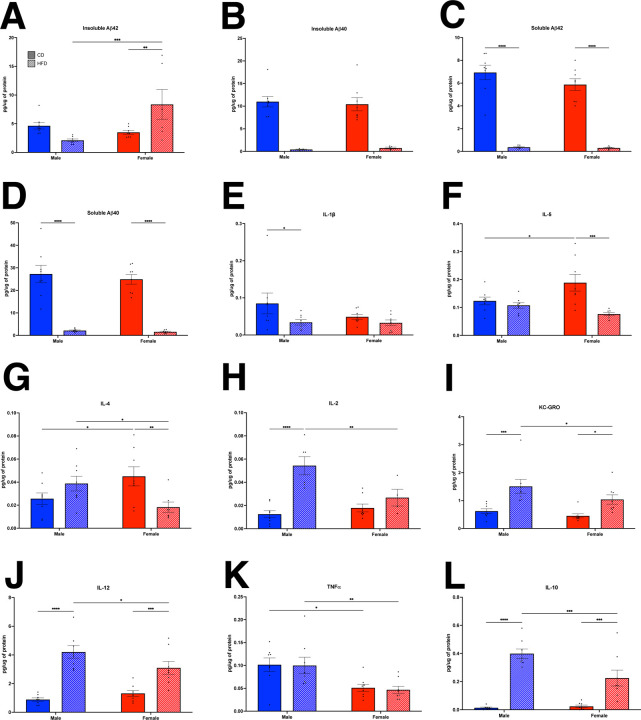
At 18 months of age, the brains from longitudinal cohort 2 were processed for biochemistry. In the brain, female LOAD2+HFD had increased insoluble Aβ42 (Figure 6A), but both males and females on a HFD had reduced insoluble Aβ40 and soluble Aβ40 and 42 (Figure 6 B–D). With regards to proinflammatory cytokines, HFD reduced IL-5 and IL-4 in females (Figure 6 F–G), but increased IL-2, KC-GRO, and IL-12 in both males and females (Figure 6 H–J). Interesting, in the brain (as compared to plasma), TNFα (Figure 6K) remained unchanged. Proinflammatory IL-10 was increased in males and females on a HFD (Figure 6L).
FIGURE 6:
Brain biomarkers in a longitudinal cohort of LOAD2 mice fed a high fat diet.
At 18 months, insoluble Aβ42 is increased in female mice on a high-fat diet (A), however, insoluble Aβ40 is decreased in both sexes (B). Soluble Aβ40 (C) and Aβ42 (D) are both reduced at 18 months in high fat diet animals of both sexes. IL-1β is reduced in males on a high fat diet (E) but remains unchanged in females. IL-5 (F) is unchanged in male mice, but is reduced in females on a HFD. IL-4 (G) is also reduced in females fed a HFD. IL-2 (H) is increased in males, but not females. KC-GRO (I) and IL-12 (J) are increased in both males and female mice on a high fat diet. TNF-α (K) remains unchanged on a high fat diet. IL-10 (L) was significantly increased in males and females on HFD. *p<0.05, **p<0.01, ***p<0.001, ****P<0.0001.
In vivo PET/CT Analysis of Regional Neurovascular Uncoupling (cohort 3)
Cohort 3 mice (female and male LOAD1 and LOAD2 mice on CD and HFD) were established at Indiana University (see Methods) to assess neurovascular uncoupling of 18F-FDG and 64Cu-PTSM, as measurements of cerebral glucose uptake and brain perfusion. Consistent with transcriptomics (Figure 3–4), along with blood and brain cytokines (Figures 5–6), the addition of HFD to 12 mo LOAD1 mice (relative to 12 mo LOAD on CD) resulted in a Type 1 neurovascular uncoupling of perfusion and glycolytic metabolism, which was sexually dimorphic in nature. Across multiple brain regions, denoted by region annotation (Figure 7A), female LOAD1 mice showed a significant reduction in glucose uptake, coupled with a hyperemia of the same brain regions, consistent with a cytokine driven diabetic phenotype. Statistical comparison revealed that dorsal-medial-ventral areas of the Auditory Cortex (AuDMV), Dysgranular Insular Cortex (DI), Lateral Orbital Cortex (LO), Primary Motor (M1) and Secondary Motor (M2) Cortex, Parietal Association Cortex (PtA), Retrosplenial Dysgranular Cortex (RSC), Primary Somatosenory Cortex (S1), and Thalmus (TH) were significantly different (p<0.05, unpaired t-test) relative to the control diet groups in female mice (Figure 7A, top panel). By comparison, regional uncoupling analysis of male LOAD1+HFD only showed significant changes (p<0.05, unpaired t-test) in the Dorsolateral Orbital Cortex (DLO), Primary Motor (M1) and Secondary Motor (M2) Cortices.
FIGURE 7:

Neurovascular Uncoupling of LOAD1 and LOAD2 mouse models.
The degree of neurovascular coordination in LOAD1 (A) and LOAD2 mouse models (B) conditioned on high-fat diet (HFD), we performed uncoupling analysis. (Left) Uncoupling analysis chart in male (blue) and female (red) mice at 12 months, with many brain regions showing significant decreases in metabolism with increases in perfusion. LOAD2 animals aged to 18 months (C) were similarly analyzed. (Upper Right) Female and (Lower Right) Male p-value males showing which regions were significantly different for perfusion, metabolism, and uncoupling.
Importantly, the addition of hAβ onto the LOAD1 background (yielding LOAD2), showed a Type 2 neurovascular uncoupling phenotype when placed on a HFD (relative to LOAD2 on a CD), which was only observed in the female cohort. Unlike LOAD1 mice, female LOAD2 mice on a HFD showed a significant increase in glucose uptake concomitant with regional reductions in tissue perfusion (Figure 7B) and aligned with the transcriptomic network changes (Figure 3B), and blood cytokine levels for TNFα, IL1β and IL6 (Figure 5). Statistical analysis of brain regions revealed that Corpus Callosum (CC), Entorhinal Cortex (ECT), LO, Medial Orbital Cortex (MO), Perirhinal Cortex (PRH), Prelimbic Cortex (PRL), and Ventral Orbital Cortex (VO) all were significantly different (p<0.05, unpaired t-test) from control diet groups (Figure 7A, top panel) in female LOAD2 mice. By contrast, male LOAD2+HFD showed different brain regions, which were uncoupled with treatment, with the AuDMV, Dorsintermed Entorhinal Cortex (DLIVEnt), ECT, PRH, RSC, Temporal Association Cortex (TeA), and Primary and Secondary Visual Cortex (V1V2).
To elucidate the role of aging on gene x environmental effect, we performed neurovascular uncoupling analysis on LOAD2 mice at 18 mo. Unlike LOAD1 and LOAD2 at 12 mos which showed a Type 1 and Type 2 uncoupling phenotype respectively, LOAD2 at 18 mos revealed a hypermetabolic and hyperemic phenotype in both sexes, which resulted in nearly all brain regions increasing in glucose uptake paired with increases in tissue perfusion (Figure 7C). This increase in both perfusion and metabolism at this age, and is consistent with the plasma cytokine data (Figure 8) that show a significant elevation in TNFα, IL-6 and IL-5, which are markers of cell activation and proliferation and are consist with clinical reports of prodromal conversion from healthy controls to MCI.
Evaluation of age-dependent plasma biomarkers and Touchscreen cognitive testing (Cohorts 4 and 5)
To further consider the relevance of the LOAD2 mice as a model for preclinical testing, a group of male and female LOAD2 mice fed a CD or HFD from 2 months of age (cohort 4) and a group of male and female LOAD2 and wildtype littermate controls (WT) fed CD or HFD from 6+ months of age (cohort 5) were established at the University of Pittsburgh. The goal of these cohorts was to track longitudinal biomarker measures for cytokines and Aβ throughout aging, in order to investigate the inflection point at which biomarkers revealed pathological consequences of environment x aging x gene effects, in order to identify a window for therapeutic intervention for future preclinical testing. In addition to biomarker analysis through aging, the cohort were also evaluated for cognitive function assessed by a touchscreen testing battery as well as evaluating the effects of food restriction necessary for touchscreen testing on cytokine levels (Figure 8 and Supplemental Figure 7).
Similar to cohort 2 at IU (Figure 5E), LOAD2 males fed HFD in cohort 4 had increased levels of plasma TNFα at 12 and 18 months of age relative to LOAD2 males fed CD (12 months, p=0.0001 and 18 months, p=0.0002). In fact, analysis of monthly plasma cytokines revealed increased TNFα as early as 4 months of age (p=0.0377) with consistent increases from 7 months of age onward in LOAD2+HFD males compared to LOAD2+CD males (p<0.05, Figure 8B). The effects of HFD on plasma TNFα was less robust in females, consistent with data from cohort 2 at IU. We observed an overall trend of higher concentrations of plasma TNFα in females fed HFD compared to females fed CD, but those levels were significantly different only at 8–8.5 months (p<0.05) and 15–18 months of age (p=0.0198 at 15 months and p=0.0068 at 18 months; Figure 8A). In addition to TNFα, we also measured 9 other cytokines, 7 of which were above the lower limits of detection (see Supplemental methods). Plasma levels of IL-6 (p=0.0022) and IL-10 (p=0.0375) were significantly higher in LOAD2+HFD males compared to LOAD2+CD males at 7 months of age, and levels of IL-10 (p=0.0025) but not IL-6 (p=0.0832) were also higher at 8 months of age in HFD males (Figure 8C, D) prior to the first trial of food restriction that preceded touchscreen testing. Interestingly, plasma levels of IL-5 were significantly higher in LOAD2 females, regardless of diet, compared to males at several time points (p<0.05, Figure 8G) while other plasma cytokines measured – IL-1β, IFNγ, KC-GRO and IL-2 (Figure 8E, F, H, and I) – did not show diet- or sex-related effects in this cohort. In cohort 5, the middle-aged (6–12 months old) start for HFD (Supplemental Figure 7A) produced a more modest effect on plasma TNFα with LOAD2+HFD males showing higher concentrations of plasma TNFα at 4 months on diet relative to WT males on CD (p=0.0241) and at 5 months on diet relative to LOAD2 and WT males on CD (p=0.0451 and p=0.0034), LOAD2+HFD females showing higher concentrations of TNFα relative to LOAD2+CD females only at 5 months on diet (p=0.0291), and WT males but not females on HFD showing higher concentrations of TNFα relative to WT+CD mice also only at 5 months on diet (p=0.0091 males and p=0.998 females, Supplemental Figure 7B). In all other cytokines measured – IL-6, IL-10, IL-1β, IFNγ, IL-5, KC-GRO and IL-2 (Supplemental Figure 7C–I) – there was no significant effect of diet on LOAD2 or WT males or females, though we did again observe a sex-effect in plasma IL-5 with concentrations being higher in LOAD2 females compared to LOAD2 males regardless of diet (p<0.01 at 2 months on diet and p<0.05 at 3 months on diet, Supplemental Figure 7G).
Concentrations of plasma Aβ40 and 42 were also altered by HFD in cohort 4 (Figure 8J–K) and by genotype but not diet or sex in cohort 5 (Supplemental Figure 7 J,K) consistent with the observation that HFD started earlier in life (cohort 4) produces a more robust phenotype than when initiated mid-life (cohort 5). Male LOAD2 mice +HFD beginning from 2 months of age had increased levels of plasma Aβ40 at 15 (p=0.01) and 18 months of age (p=0.0365) relative to LOAD2 +CD males while female LOAD2+HFD beginning from 2 months of age had increased plasma Aβ40 at 18 months (p<0.0001) relative to LOAD2+CD females (Figure 8J). As demonstrated in Fig S6A LOAD2 mice beginning HFD at 6+ months of age (cohort 5) failed to demonstrate increases in plasma Aβ40 relative to LOAD2+CD (Supplemental Figure 7J). Plasma Aβ42 was significantly increased in LOAD2+HFD females from cohort 4 compared to LOAD2+CD females at 18 months of age (p<0.0001, Figure 8K) and compared to age-matched WT+CD females (p=0.0319, Figure S6K).
While there was robust cross-cohort and cross-laboratory replicability for plasma cytokines irrespective of the different laboratory environments, we observed divergent results for plasma Aβ for cohort 2 (Figure 5C, D) versus cohorts 4 and 5 (Figure 8J, K and Supplemental Figure 7J, K). Two major factors may contribute to these differences beyond different laboratory environments: 1) plasma Aβ was analyzed from anesthetized subjects during terminal procedures for cohort 2 whereas cohorts 4 and 5 were longitudinally sampled in non-anesthetized mice; and 2) Cohorts 4 and 5 were subjected to periods of food restriction which was not a factor for cohort 2. It is well documented that variations in plasma Aβ are influenced by environmental factors including stress20. Despite the cross-lab variability for Aβ, irrespective of transient stressors, TNFα levels were sustained consistently across cohorts and align with transcriptomic and proteomic data which demonstrates the robustness of the LOAD2 x age x HFD model for preclinical studies of therapeutic interventions independent of amyloid.
We assessed the cognitive function of both cohort 4 and 5 using a touchscreen assay battery. All subjects demonstrated the ability to associate the response (nosespoke touch) with the presentation of a reward as measured by the ability to meet a priori criterion of 2 consecutive sessions of 30 rewards earned within 45 minutes. Interestingly, despite similar food restriction across cohorts, there was a significant effect of diet with either WT or LOAD2 mice regardless of HFD initiation (at 2 months or at 6+ months) failing to acquire the task as measured by % accuracy not exceeding chance levels (50%). LOAD2+HFD mice required a greater number of days to meet criteria relative to LOAD2 mice on control diet [One way ANOVA [F (3, 24) = 5.290; P=0.0061; Figure 8M]. Within sex analysis revealed a statistically significant increase in females reared on HFD (T-test; p<0.05) and a modest non-significant increase in males reared on HFD (T-test; p=0.38). Within control diet groups, there was no effect of genotype or sex (p>0.05). During the punish incorrect phase, 100% of CD mice, irrespective of genotype met criteria however only 27% of LOAD2+HFD male mice met a priori completion criteria for this task while 0% of female LOAD2+HFD mice met criteria. Analysis of accuracy levels during initial acquisition of the task prior to subjects advancing to the location discrimination phase (Training Days 1–18) revealed a statistically significant impairment in HFD treated subjects relative to genotype and age- and sex-matched non-HFD controls as measured by two-way repeated measures ANOVA: [F (5, 33) = 9.047; p<0.001; Figure 8M]. Analysis of WT and LOAD2 mice exposed to HFD at middle-age (6+ months age) revealed a less aggressive phenotype as indicated by lower plasma cytokine levels and lower plasma Aβ40 and Aβ42 when compared with plasma levels from mice exposed to HFD at 2 months of age (Figure 8). Supplemental Figure 7M illustrates performance of aged (18+ month) LOAD2 mice on CD (purple diamonds) and age- and sex-matched C57BL/6J (WT) mice on control diet (n=3–5 per genotype) on the LD task for both large (easy) and small (hard) separation conditions (mean ± s.e.m.). As expected, the increase in task difficulty was significant across genotypes as measured by increased number of trials required to reach criterion for easy relative to hard [F (1, 12) = 5.156; P=0.04]. While there was a modest increase in the number of trials to reach criterion in LOAD2 mice relative to WT the effect of genotype was not significant [F (1, 12) = 0.4134; P=0.53].
Discussion
Despite the recent approval of anti-amyloid therapies, there remains a need for improved therapies for Alzheimer’s disease. A key component of therapeutic testing is the development of mouse models that recapitulate the complexity of LOAD. Here we introduce LOAD2 mice – triple homozygous for APOE4, Trem2*R47H and hAβ. Although they lack amyloid and TAU pathologies, in combination with a HFD, LOAD2 mice show age-dependent development of key aspects of LOAD. Specifically, at 18 months of age, LOAD2+HFD, show loss of neurons in the cortex, increased insoluble Aβ42, brain region-specific volumetric changes, neurovascular uncoupling, and cognitive deficits, consistent with the prodromal stages of AD. Interestingly, these changes correlated with elevated plasma NfL and cytokine levels – clinically relevant biomarkers associated with LOAD and emphasize the robustness of modeling genetics x age x environment.
Neuronal cell number was evaluated by counting NeuN+DAPI+ cells in cortex and hippocampus and revealed a modest reduction in female LOAD2+HFD mice (Figure 2). Coupled with transcriptomic and proteomic signatures (Figures 3 and 4), neurovascular uncoupling (Figure 7), and cognitive deficits (Figure 8), these data suggest circuit dysfunction in LOAD2+HFD mice. Specific circuits are differentially susceptible to aging and/or AD and so neuronal cell loss may be a result of sporadic loss of neurons or loss of neurons within a specific circuity21–24. The mechanism by which neurons die in LOAD2+HFD mice is still to be determined. One recent study used a chimeric model system to show that human neurons in the mouse brains exposed to amyloid died by necroptosis25, although mouse neurons did not. Evaluating positive markers of programmed cell death (TUNEL, CASPASE3, MEG3) would provide insight into mechanisms driving reduced cortical neurons in female LOAD2+HFD mice. It is also expected that synaptic changes/loss would precede neuronal cell loss and further work is needed to determine this. Also, myelin integrity was not evaluated and may be disrupted in LOAD2 mice.
As observed in human AD19, we detected distinct proteomic and transcriptomic signatures in our LOAD mouse models. Transcriptomic signals tended to represent immune, vascular, and lipid metabolism (Figure 3), whereas proteomic signatures were focused in synaptic and myelination modules (Figure 4). The advent of proteomic technologies that commonly quantify around 10,000 proteins, such as the TMT approach used here, now enable deep characterization to reveal such differences. We also note that AD-relevant transcriptomic changes tended to be driven by age and diet (Figure 3A) while proteomic changes were primarily due to APOE4 and Trem2*R47H genetics and somewhat exacerbated by diet. These results demonstrate the importance of multi-omic analyses in fully characterizing causal factors (e.g., genetics, diet, age) and affected processes (e.g., synaptic, immunological) in translational research.
Data-driven analysis of transcriptomes through gene co-expression networks revealed two modules, denoted turquoise and lightyellow, that highlighted a molecular separation between normal age-related changes and changes related to AD-relevant biomarkers (Figure 3). While both modules were enriched for immune response, this enrichment was more significant in the turquoise (p = 1.8 × 10−49) than the lightyellow (p = 3.7 × 10−3) module (Supplementary Table B3). This suggests a more focused immune component in the lightyellow module, led by cytokine signaling (Figure 3C) and often co-annotated to lipid metabolism (Figure 3D). Furthermore, the lightyellow module was much more correlated with circulating cytokines and NfL, while the turquoise module was linked to IBA1 and GFAP markers (Figure 3B). These results suggest a signature for AD-related transcriptomic changes (the lightyellow module) that is distinct from usual brain aging (the turquoise module) and driven by diet in LOAD mice.
Though no deficits in hippocampal spatial working memory as measured by the spontaneous alternation task were observed in LOAD2+HFD mice (Figure 1), across two separate cohorts, aged mice reared on HFD either from 2 months of age or from 6+ months of age failed to meet acquisition criteria as measured by % correct responses less than 50% (chance levels) indicative of a learning impairment (Figure 8 and Supplemental Figure 7). The lack of ability to learn the task is unlikely to be explained by food motivation, as both HFD and CD groups were sufficiently restricted, and all groups independent of diet or genotype acquired initial touch-reward association. This indicates that failure of HFD mice to accurately perform the task is more likely a feature of impaired learning due to the combination of advanced age x environmental risk (e.g. HFD), than reward salience. Further studies may be required to investigate methods for enhancing motivation for rewards in mice reared on HFD which may include more aggressive water restriction protocols as HFD treated mice even in the presence of strawberry-milk shake reinforcer also have issues with touchscreen tasks (personal communication with Dr. Lisa Saksida, also see26). Regardless, for aged WT and LOAD2 mice on control diet, results from daily assessments on task acquisition reveal modest cognitive impairments in LOAD2 relative to WT. While modest, these cognitive deficits are further strengthened by proteomic analysis of LOAD2 mice revealing alterations in synaptic signaling. While we were not able to conduct additional cognitive tests in this advanced aged cohort due to attrition and eventual mortality, as subjects aged to 24 months by the conclusion of testing, these data indicate that translational touchscreen tests may be more sensitive for detecting more specific cognitive domains than traditional single day behavioral tests in mice that have been historically used for assessing cognition.
Commensurate with the cytokines and multi-omic (Figure 3B) associations, cerebral perfusion and metabolism via uncoupling analysis revealed a sexually dimorphic dysregulation with age and genotype (Figure 7,B). Importantly, the addition of a HFD in LOAD1 mice, resulted in Type 1 uncoupling (i.e. reductions in glycolysis and compensatory hyperemia), consistent with a cytokine27–30 driven down regulation of insulin receptors28, which have been shown to result in a reduction in neuronal glucose uptake via GLUT transporters29–31. Importantly, these data closely parallel the Type 2 diabetic phenotype32, 33 with reactive hyperemia via activation of eNOS34.
To further explore the impact of environment on gene and sex, analysis of neurovascular uncoupling was performed to assess the degree of regional metabolic dysregulation in LOAD2 mice. Unlike the base model, LOAD2+HFD at 12 mos resulted in a Type 2 neurovascular uncoupled phenotype (i.e. increased glycolysis and reduced perfusion) which was only observed in female mice. These data align with cytokines (i.e. TNFα, IL-2) and immunopathology changes (Figure 2, 5) at this age, and are consistent with previous reports of cytokine driven astrocytic proliferation and GLUT1 expression35. By contrast, LOAD2+HFD mice at 18 mos, resulted in whole brain increases in perfusion and metabolism. Importantly, these changes were observed in both sexes, and was consistent with reports of prodromal hyper-metabolism36–39and hyperemia40observed in clinical patients, suggesting that this model recapitulates the earliest manifestations of LOAD.
Collectively, data presented here suggest that LOAD2+HFD mice, particularly are presenting with early stages of LOAD by 18 months of age. Further work is needed to develop models that present a wider range of LOAD pathologies. We were unable to determine whether aging LOAD2+HFD longer would have enhanced their LOAD-relevant phenotypes. This is because LOAD2+HFD mice showed an increased incidence of tumors when aged beyond 18 months of age. Control mice, including WT mice, showed similar phenotypes, suggesting this resulted from chronic consumption of HFD (from 2 months). Interestingly, LOAD2+CD mice aged to 24 months or LOAD2+HFD after 12 months of age did not show as severe phenotypes as 18 months old LOAD2H+HFD from 2 months of age, highlighting the significance of environmental influence of chronic exposure to HFD. Several additional strategies are being tested to develop improved LOAD models. Alternative diets, e.g., a milder western diet, are being tested that may recapitulate LOAD2-relevant phenotypes of a HFD without the presence of age-related tumors and attrition. We are also evaluating additional genetic risk factors on the LOAD2 background that impact lipid metabolism (Abca7*A1527G), neuroinflammation (Plcg2*M28L) and vascular health/metabolism (Mthfr*677C>T). Perturbing these specific pathways may exacerbate the effects of APOE4, Trem2*R47H and hAβ in LOAD2. Tau pathology was not evaluated in LOAD2 mice and was not expected due to the lack of a humanized MAPT gene. Strains where the mouse Mapt gene has been replaced by human MAPT are now available and therefore, mouse models carrying combinations of hAβ, hMAPT, genetic risk factors (e.g., APOE4) are now being created and will be exposed to environmental risk factors such as HFD and toxic metals to evaluate their potential as preclinical models of AD.
In conclusion, the interaction of genetic risk and aging leads to a phenotype worsened by environmental factors, mirroring the risk for LOAD. This includes a pronounced neuroinflammation phenotype with cognitive impairment, unrelated to amyloid accumulation. Multi-omic analysis identified molecular signatures, such as synaptic signaling deficits, aligning with the observed cognitive impairment. The LOAD2 model, characterized by translational blood and imaging biomarkers, emerges as a crucial tool for preclinical drug testing in ADRD patients without prominent amyloid, potentially offering a more representative model of sporadic LOAD.
Supplementary Material
SUPPLEMENTAL FIGURE 1: Study design.
This was a multi-site, multi-cohort study. At each site, mice were provided a high fat diet or control diet starting at 2 months of age. At the Jackson Laboratory, the 18-month cohort only received a control diet. In cohort 1, four cohorts of mice were aged to 4, 12, 18, 24 months of age. Multiple ‘omics studies, behavior and cognition and neuropathology studies were completed. In cohort 2, at Indiana University, one cohort of mice were age to 18 months and plasma was collected at 4, 12 and 18 months for biomarker analysis. At each timepoint, mice had MRIs completed for volumetric analysis. Brains were collected at the termination of the study for neurodegeneration and neuroinflammation studies. In cohort 3 at Indiana University, two cohorts of mice were aged to 12 and 18 months and underwent PET/CT studies for two tracers, FDG and PTSM. In cohort 4 at University of Pittsburgh, mice were aged to 18 months, undergoing serial blood draws monthly and completing touch screen testing.
Supplemental Figure 2: Longitudinal behavioral phenotyping of mice on high-fat diet.
Males and females, of LOAD1 and LOAD2 genotypes, fed either control diet (CD) or high-fat diet (HFD) beginning at 2-months of age until 18-months of age were subjected to open field assay, measuring animal movements by way of total distance traveled (A) and total vertical activity (B) during 60 minute observation testing period. Rotarod assay measured the latency to fall times, over three consecutive trials, as a measure of motor coordination (C). (Three-way ANOVA [sex, genotype, diet effects]; *=p<0.05)
Supplemental Figure 3: Immunohistochemistry analysis. Representative, collated images of whole hemisphere, coronal sections displaying both cortical and hippocampal regions of interest, used for glia cell density measurements and neuropathological assessment of brain tissue from cohort 1 (JAX). Brains are from 12mo females from either LOAD2 (control diet) and B6J.APPSAA strains. NeuN=neuronal marker; ThioS=amyloid plaques; GFAP=astrocyte marker; IBA1=microglial marker. Scale bar equals 1,000μm (1mm).
Supplemental Figure 4: Mouse modules and trait relationships. Transcriptome module correlations and FDR values for module eigengenes and age, sex, diet, genotype, and measured behavioral assays, body weights, NfL, cytokine levels, and GFAP, IBA1, and NeuN cell counts.
Supplemental Figure 5: Neurodegeneration and gliosis in the cortex and subiculum. At 18 months old (Indiana University cohort 2), GFAP was used to label reactive astrocytes, which were increased in female mice on a high fat diet in the cortex. IBA1 was utilized to study microglia, which were increased in females on a high fat diet. Neurons (labeled with NeuN) were not changed in the cortex or subiculum.
Supplemental Figure 6: Touchscreen task acquisition is impaired in mice with genetic + environmental risk for AD. Initial touch-reward associations in LOAD2 mice + high-fat diet (HFD) in comparison to LOAD2 and WT mice maintained on a control diet (CD). Data are analyzed as number of days required for individuals to meet a priori task completion criteria.
Supplemental Figure 7: Evaluation of disease trajectory of LOAD2 mice conditioned on a High Fat Diet beginning mid-life at 6 months of age. In comparison to LOAD2 mice conditioned on HFD from 2 months of age, starting HFD after 6 months of age results in a milder phenotype (see Figure 8 for comparison). Chronic HFD exposure produces sustained TNF-α levels in male and female LOAD2 mice relative to WT or LOAD2 mice maintained on control diet (CD); consistent with HFD exposure from adolescence (see Figure 8). Other plasma biomarkers were not significantly elevated and sustained relative to mice on CD. A) Illustration of timeline and procedures; B) plasma TNF-α (pg/mL); C) Plasma IL-6 (pg/mL); D) plasma IL-10 (pg/mL); E. Plasma IL-1β (pg/mL); F) plasma IFNy (pg/mL), G) plasma IL-5 (pg/mL); H) plasma KC-GRO (pg/mL); I) plasma IL-2 (pg/mL); J) plasma Aβ40 (pg/mL) at 12–18 months of age; K) plasma Aβ42 (pg/mL) at 12–18 months of age. Plasma cytokines and plasma Aβ were measured using MesoScale Discovery multiplex ELISA kits in accordance with the manufacturer’s protocol. (*P<0.05 and **P<0.01 between diet groups; ^P<0.05 between sexes). One way ANOVA within sex was used to analyze Aβ40 and Aβ42 in 12–18-month aged mice across treatment groups. L) Touchscreen acquisition task data demonstrating impairment of HFD treatment independent of genotype in aged (18+month LOAD2 and WT mice). Both WT+HFD and LOAD2+HFD to fail to learn the task as measured by % accuracy less than chance levels (<50%) (n=3–5 per genotype/diet; combined sexes). Only CD treated WT and LOAD2 mice met acquisition criteria and advanced to assessments of pattern separation as measured by the spatial-location discrimination task. M.) Modest impairments in pattern separation of aged LOAD2 mice (18+ month) relative to age-matched WT (n=3–5 per genotype; sexes combined). Data for touchscreen are presented as mean ± sem.
Acknowledgments
The results published here are in whole or in part based on data obtained from the AD Knowledge Portal (https://adknowledgeportal.org). Study data were provided by the Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago. Data collection was supported through funding by NIA grants P30AG10161 (ROS), R01AG15819 (ROSMAP; genomics and RNAseq), R01AG17917 (MAP), R01AG36836 (RNAseq), the Illinois Department of Public Health (ROSMAP), and the Translational Genomics Research Institute (genomic). Additional phenotypic data can be requested at www.radc.rush.edu. Mount Sinai Brain Bank data were generated from postmortem brain tissue collected through the Mount Sinai VA Medical Center Brain Bank and were provided by Dr. Eric Schadt from Mount Sinai School of Medicine. The Mayo RNAseq study data was led by Nilüfer Ertekin-Taner, Mayo Clinic, Jacksonville, FL as part of the multi-PI U01 AG046139 (MPIs Golde, Ertekin-Taner, Younkin, Price). Samples were provided from the following sources: The Mayo Clinic Brain Bank. Data collection was supported through funding by NIA grants P50 AG016574, R01 AG032990, U01 AG046139, R01 AG018023, U01 AG006576, U01 AG006786, R01 AG025711, R01 AG017216, R01 AG003949, NINDS grant R01 NS080820, CurePSP Foundation, and support from Mayo Foundation. Study data includes samples collected through the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona. The Brain and Body Donation Program was supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium), and the Michael J. Fox Foundation for Parkinson’s Research.
The authors are grateful for the technical support of the IUSM Biomarker Core.
The authors would like the thank the Neurobehavioral Phenotyping core at Jackson Laboratory for their assistance and support during the in vivo testing of these animals.
The authors are grateful for the technical support of the University of Pittsburgh Preclinical Phenotyping Core facility as well as the following research staff: Gabi Little, Jason Hart, Aman Reddy, Umesh Nepali, Jenny Willis, and Amber Sanders
Funding Statement
The MODEL-AD Center was supported through funding by NIA grant U54AG054345. GH was supported by the Diana Davis Spencer Endowed Chair Research and GC was supported by the Bernard and Lusia Milch Endowed Chair. NW was supported by NINDS grant R01NS125020.
Footnotes
SUPPLEMENTARY TABLE A: Differentially expressed genes in mouse models.
Differentially expressed genes (DEGs) in mouse strains. Sheet1: DEGs observed in LOAD2 compared to age and sex-matched LOAD1 mice at all ages for both sexes. Sheet 2: DEGs observed in 18-month-old LOAD2 and LOAD1 mice on chow diet and HFD compared to age and sex-matched C57BL/6J mice on chow diet. Sheet 3: DEGs observed in HFD mice compared to sex and strain-matched mice on chow diet at 18 months.
SUPPLEMENTARY TABLE B: Enriched KEGG pathways in mouse models.
Sheet 1: enriched KEGG pathways for the significantly up and down-regulated genes in LOAD2 mouse strains compared to age and sex-matched LOAD1 mice at all ages for both sexes. Sheet 2: enriched KEGG pathways in the significantly up and down-regulated genes in 18-month-old LOAD2 and LOAD1 mice on chow diet and HFD compared to age and sex-matched C57BL/6J mice on chow diet. Sheet 3: enriched KEGG pathways in the significantly up and down-regulated genes in HFD mice compared to sex and strain-matched mice on chow diet at 18 months.
SUPPLEMENTARY TABLE B2: Mouse modules of co-expressed genes.
Genes in each of 30 mouse modules of co-expressed genes identified through WGCNA of mouse transcriptomics data.
SUPPLEMENTARY TABLE B3: Enrichment of AD biological domains in mouse modules.
Enrichment of AD biological domains and member Gene Ontology terms in each mouse module of co-expressed genes.
SUPPLEMENTARY TABLE C: Differentially expressed proteins in mouse models.
Differentially expressed proteins in 18-month-old LOAD1 and LOAD2 mice on chow and HFD compared to age-matched C57BL/6J mice on chow diet.
SUPPLEMENTARY TABLE D: Correlations between changes in mouse models and case-control changes in 44 human protein co-expression modules.
Pearson correlation coefficients and corresponding p-values for the correlation between protein expression changes in LOAD1 and LOAD2 mice relative to B6J mice at 18 months with changes observed in human AD subjects versus controls for each human protein module, as shown in Figure 4A.
SUPPLEMENTARY TABLE E: Enriched Gene Ontology terms in proteins driving the significant positive correlations between LOAD2 mice on HFD and human AD proteomics modules.
Enriched GO terms in proteins with common directional changes for 18-month-old LOAD2 mice on HFD and human AD cases, as shown in Figure 4.
Consent Statement
Due to the nature of this study, consent for human subjects was not needed.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability Statement
The MODEL-AD data sets are available via the AD Knowledge Portal (https://adknowledgeportal.org). The AD Knowledge Portal is a platform for accessing data, analyses, and tools generated by the Accelerating Medicines Partnership (AMP-AD) Target Discovery Program and other National Institute on Aging (NIA)-supported programs to enable open-science practices and accelerate translational learning. The data, analyses and tools are shared early in the research cycle without a publication embargo on secondary use. Data is available for general research use according to the following requirements for data access and data attribution (https://adknowledgeportal.org/DataAccess/Instructions). For access to content described in this manuscript see: https://doi.org/10.7303/syn53128146
References
- 1.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11(6):718–26. Epub 20150601. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Boyd RJ, Avramopoulos D, Jantzie LL, McCallion AS. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. J Neuroinflammation. 2022;19(1):223. Epub 20220908. doi: 10.1186/s12974-022-02584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solfrizzi V, Panza F, Frisardi V, Seripa D, Logroscino G, Imbimbo BP, Pilotto A. Diet and Alzheimer’s disease risk factors or prevention: the current evidence. Expert Rev Neurother. 2011;11(5):677–708. doi: 10.1586/ern.11.56. [DOI] [PubMed] [Google Scholar]
- 4.Biogen. FDA Approves Updated ADUHELM™ Prescribing Information to Emphasize Population Studied in Clinical Trials 2021. [cited 2023 11/21]. Available from: https://investors.biogen.com/news-releases/news-release-details/fda-approves-updated-aduhelmtm-prescribing-information-emphasize.
- 5.FDA. FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval 2023. [cited 2023 11/21]. Available from: https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval.
- 6.Oblak AL, Forner S, Territo PR, Sasner M, Carter GW, Howell GR, Sukoff-Rizzo SJ, Logsdon BA, Mangravite LM, Mortazavi A, Baglietto-Vargas D, Green KN, MacGregor GR, Wood MA, Tenner AJ, LaFerla FM, Lamb BT, and The M-A, Consortium. Model organism development and evaluation for late-onset Alzheimer’s disease: MODEL-AD. Alzheimers Dement (N Y). 2020;6(1):e12110. Epub 2020/12/08. doi: 10.1002/trc2.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley KE, Hewes AA, Garceau DT, Kotredes KP, Carter GW, Sasner M, Howell GR. The APOE (epsilon3/epsilon4) Genotype Drives Distinct Gene Signatures in the Cortex of Young Mice. Front Aging Neurosci. 2022;14:838436. Epub 20220316. doi: 10.3389/fnagi.2022.838436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotredes KP, Oblak AL, Pandey RS, Lin PB, Garceau D, Williams HM, Uyar A, O’Rourke R, O’Rourke S, Ingraham C, Bednarczyk D, Belanger M, Cope ZA, Foley KE, Logsdon BA, Mangravite LM, Sukoff Rizzo SJ, Territo PR, Carter GW, Sasner M, Lamb BT, Howell GR. Uncovering disease mechanisms in a novel mouse model expressing humanized APOEε4 and Trem2*R47H. Frontiers in Aging Neuroscience. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, Schneider J, Wilson RS. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60(2):194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 10.Oblak AL, Kotredes KP, Pandey RS, Reagan AM, Ingraham C, Perkins B, Lloyd C, Baker D, Lin PB, Soni DM, Tsai AP, Persohn SA, Bedwell AA, Eldridge K, Speedy R, Meyer JA, Peters JS, Figueiredo LL, Sasner M, Territo PR, Sukoff Rizzo SJ, Carter GW, Lamb BT, Howell GR. Plcg2(M28L) Interacts With High Fat/High Sugar Diet to Accelerate Alzheimer’s Disease-Relevant Phenotypes in Mice. Front Aging Neurosci. 2022;14:886575. Epub 20220624. doi: 10.3389/fnagi.2022.886575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukoff Rizzo SJ, Anderson LC, Green TL, McGarr T, Wells G, Winter SS. Assessing Healthspan and Lifespan Measures in Aging Mice: Optimization of Testing Protocols, Replicability, and Rater Reliability. Curr Protoc Mouse Biol. 2018;8(2):e45. doi: 10.1002/cpmo.45. [DOI] [PubMed] [Google Scholar]
- 12.Backstrom D, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L, Lenfeldt N. NfL as a biomarker for neurodegeneration and survival in Parkinson disease. Neurology. 2020;95(7):e827–e38. Epub 20200717. doi: 10.1212/WNL.0000000000010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. Epub 2008/12/31. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cary GA, Wiley JC, Gockley J, Keegan S, Heath L, R. R. Butler I, Mangravite LM, Logsdon BA, Longo FM, Levey A, Greenwood AK, Carter GW, The Emory-Sage SGCT-ADC. Genetic and Multi-omic Risk Assessment of Alzheimer’s Disease Implicates Core Associated Biological Domains. medRxiv. 2022:2022.12.15.22283478. doi: 10.1101/2022.12.15.22283478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson ECB, Carter EK, Dammer EB, Duong DM, Gerasimov ES, Liu Y, Liu J, Betarbet R, Ping L, Yin L, Serrano GE, Beach TG, Peng J, De Jager PL, Haroutunian V, Zhang B, Gaiteri C, Bennett DA, Gearing M, Wingo TS, Wingo AP, Lah JJ, Levey AI, Seyfried NT. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat Neurosci. 2022;25(2):213–25. Epub 20220203. doi: 10.1038/s41593-021-00999-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Csernansky JG. Effects of stress and stress hormones on amyloid-beta protein and plaque deposition. J Alzheimers Dis. 2009;18(2):459–69. doi: 10.3233/jad-2009-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, Saksida LM. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc. 2013;8(10):2006–21. Epub 20130919. doi: 10.1038/nprot.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ano Y, Nakayama H. Preventive Effects of Dairy Products on Dementia and the Underlying Mechanisms. Int J Mol Sci. 2018;19(7). Epub 20180630. doi: 10.3390/ijms19071927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Zhang ZH, Huang SL, Yue ZB, Yin XS, Feng ZQ, Zhang XG, Song GL. Whey protein powder with milk fat globule membrane attenuates Alzheimer’s disease pathology in 3xTg-AD mice by modulating neuroinflammation through the peroxisome proliferator-activated receptor gamma signaling pathway. J Dairy Sci. 2023;106(8):5253–65. Epub 20230704. doi: 10.3168/jds.2023-23254. [DOI] [PubMed] [Google Scholar]
- 20.Min LJ, Kobayashi Y, Mogi M, Tsukuda K, Yamada A, Yamauchi K, Abe F, Iwanami J, Xiao JZ, Horiuchi M. Administration of bovine casein-derived peptide prevents cognitive decline in Alzheimer disease model mice. PLoS One. 2017;12(2):e0171515. Epub 20170203. doi: 10.1371/journal.pone.0171515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milind N, Preuss C, Haber A, Ananda G, Mukherjee S, John C, Shapley S, Logsdon BA, Crane PK, Carter GW. Transcriptomic stratification of late-onset Alzheimer’s cases reveals novel genetic modifiers of disease pathology. PLoS Genet. 2020;16(6):e1008775. Epub 20200603. doi: 10.1371/journal.pgen.1008775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee S, Heath L, Preuss C, Jayadev S, Garden GA, Greenwood AK, Sieberts SK, De Jager PL, Ertekin-Taner N, Carter GW, Mangravite LM, Logsdon BA. Molecular estimation of neurodegeneration pseudotime in older brains. Nat Commun. 2020;11(1):5781. Epub 20201113. doi: 10.1038/s41467-020-19622-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naj AC, Schellenberg GD. Genomic variants, genes, and pathways of Alzheimer’s disease: An overview. Am J Med Genet B Neuropsychiatr Genet. 2017;174(1):5–26. doi: 10.1002/ajmg.b.32499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verheijen J, Sleegers K. Understanding Alzheimer Disease at the Interface between Genetics and Transcriptomics. Trends Genet. 2018;34(6):434–47. Epub 20180321. doi: 10.1016/j.tig.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Balusu S, Horre K, Thrupp N, Craessaerts K, Snellinx A, Serneels L, T’Syen D, Chrysidou I, Arranz AM, Sierksma A, Simren J, Karikari TK, Zetterberg H, Chen WT, Thal DR, Salta E, Fiers M, De Strooper B. MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease. Science. 2023;381(6663):1176–82. Epub 20230914. doi: 10.1126/science.abp9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urai AE, Aguillon-Rodriguez V, Laranjeira IC, Cazettes F, International Brain L, Mainen ZF, Churchland AK. Citric Acid Water as an Alternative to Water Restriction for High-Yield Mouse Behavior. eNeuro. 2021;8(1). Epub 20210211. doi: 10.1523/ENEURO.0230-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett DA. Mixed pathologies and neural reserve: Implications of complexity for Alzheimer disease drug discovery. PLoS Med. 2017;14(3):e1002256. Epub 20170314. doi: 10.1371/journal.pmed.1002256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985). 2006;100(1):328–35. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 29.Kalaria RN, Erkinjuntti T. Small vessel disease and subcortical vascular dementia. J Clin Neurol. 2006;2(1):1–11. Epub 20060320. doi: 10.3988/jcn.2006.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu CC, Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106–18. Epub 20130108. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–6. Epub 20120516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Abeta oligomers. J Clin Invest. 2012;122(4):1339–53. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 2014;10(1 Suppl):S76–83. doi: 10.1016/j.jalz.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102(31):10999–1004. Epub 20050725. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvadó G, Milà-Alomà M, Shekari M, Ashton NJ, Operto G, Falcon C, Cacciaglia R, Minguillon C, Fauria K, Niñerola-Baizán A, Perissinotti A, Benedet AL, Kollmorgen G, Suridjan I, Wild N, Molinuevo JL, Zetterberg H, Blennow K, Suárez-Calvet M, Gispert JD. Reactive astrogliosis is associated with higher cerebral glucose consumption in the early Alzheimer’s continuum. Eur J Nucl Med Mol Imaging. 2022;49(13):4567–79. Epub 20220718. doi: 10.1007/s00259-022-05897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashraf A, Fan Z, Brooks DJ, Edison P. Cortical hypermetabolism in MCI subjects: a compensatory mechanism? Eur J Nucl Med Mol Imaging. 2015;42(3):447–58. Epub 20140930. doi: 10.1007/s00259-014-2919-z. [DOI] [PubMed] [Google Scholar]
- 37.Rubinski A, Franzmeier N, Neitzel J, Ewers M. FDG-PET hypermetabolism is associated with higher tau-PET in mild cognitive impairment at low amyloid-PET levels. Alzheimers Res Ther. 2020;12(1):133. Epub 20201019. doi: 10.1186/s13195-020-00702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willette AA, Modanlo N, Kapogiannis D. Insulin resistance predicts medial temporal hypermetabolism in mild cognitive impairment conversion to Alzheimer disease. Diabetes. 2015;64(6):1933–40. Epub 20150109. doi: 10.2337/db14-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu L, Jin J, Xu Y, Zhu X. Aberrant Energy Metabolism in Alzheimer’s Disease. J Transl Int Med. 2022;10(3):197–206. Epub 20220924. doi: 10.2478/jtim-2022-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas KR, Osuna JR, Weigand AJ, Edmonds EC, Clark AL, Holmqvist S, Cota IH, Wierenga CE, Bondi MW, Bangen KJ. Regional hyperperfusion in older adults with objectively-defined subtle cognitive decline. J Cereb Blood Flow Metab. 2021;41(5):1001–12. Epub 20200702. doi: . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1: Study design.
This was a multi-site, multi-cohort study. At each site, mice were provided a high fat diet or control diet starting at 2 months of age. At the Jackson Laboratory, the 18-month cohort only received a control diet. In cohort 1, four cohorts of mice were aged to 4, 12, 18, 24 months of age. Multiple ‘omics studies, behavior and cognition and neuropathology studies were completed. In cohort 2, at Indiana University, one cohort of mice were age to 18 months and plasma was collected at 4, 12 and 18 months for biomarker analysis. At each timepoint, mice had MRIs completed for volumetric analysis. Brains were collected at the termination of the study for neurodegeneration and neuroinflammation studies. In cohort 3 at Indiana University, two cohorts of mice were aged to 12 and 18 months and underwent PET/CT studies for two tracers, FDG and PTSM. In cohort 4 at University of Pittsburgh, mice were aged to 18 months, undergoing serial blood draws monthly and completing touch screen testing.
Supplemental Figure 2: Longitudinal behavioral phenotyping of mice on high-fat diet.
Males and females, of LOAD1 and LOAD2 genotypes, fed either control diet (CD) or high-fat diet (HFD) beginning at 2-months of age until 18-months of age were subjected to open field assay, measuring animal movements by way of total distance traveled (A) and total vertical activity (B) during 60 minute observation testing period. Rotarod assay measured the latency to fall times, over three consecutive trials, as a measure of motor coordination (C). (Three-way ANOVA [sex, genotype, diet effects]; *=p<0.05)
Supplemental Figure 3: Immunohistochemistry analysis. Representative, collated images of whole hemisphere, coronal sections displaying both cortical and hippocampal regions of interest, used for glia cell density measurements and neuropathological assessment of brain tissue from cohort 1 (JAX). Brains are from 12mo females from either LOAD2 (control diet) and B6J.APPSAA strains. NeuN=neuronal marker; ThioS=amyloid plaques; GFAP=astrocyte marker; IBA1=microglial marker. Scale bar equals 1,000μm (1mm).
Supplemental Figure 4: Mouse modules and trait relationships. Transcriptome module correlations and FDR values for module eigengenes and age, sex, diet, genotype, and measured behavioral assays, body weights, NfL, cytokine levels, and GFAP, IBA1, and NeuN cell counts.
Supplemental Figure 5: Neurodegeneration and gliosis in the cortex and subiculum. At 18 months old (Indiana University cohort 2), GFAP was used to label reactive astrocytes, which were increased in female mice on a high fat diet in the cortex. IBA1 was utilized to study microglia, which were increased in females on a high fat diet. Neurons (labeled with NeuN) were not changed in the cortex or subiculum.
Supplemental Figure 6: Touchscreen task acquisition is impaired in mice with genetic + environmental risk for AD. Initial touch-reward associations in LOAD2 mice + high-fat diet (HFD) in comparison to LOAD2 and WT mice maintained on a control diet (CD). Data are analyzed as number of days required for individuals to meet a priori task completion criteria.
Supplemental Figure 7: Evaluation of disease trajectory of LOAD2 mice conditioned on a High Fat Diet beginning mid-life at 6 months of age. In comparison to LOAD2 mice conditioned on HFD from 2 months of age, starting HFD after 6 months of age results in a milder phenotype (see Figure 8 for comparison). Chronic HFD exposure produces sustained TNF-α levels in male and female LOAD2 mice relative to WT or LOAD2 mice maintained on control diet (CD); consistent with HFD exposure from adolescence (see Figure 8). Other plasma biomarkers were not significantly elevated and sustained relative to mice on CD. A) Illustration of timeline and procedures; B) plasma TNF-α (pg/mL); C) Plasma IL-6 (pg/mL); D) plasma IL-10 (pg/mL); E. Plasma IL-1β (pg/mL); F) plasma IFNy (pg/mL), G) plasma IL-5 (pg/mL); H) plasma KC-GRO (pg/mL); I) plasma IL-2 (pg/mL); J) plasma Aβ40 (pg/mL) at 12–18 months of age; K) plasma Aβ42 (pg/mL) at 12–18 months of age. Plasma cytokines and plasma Aβ were measured using MesoScale Discovery multiplex ELISA kits in accordance with the manufacturer’s protocol. (*P<0.05 and **P<0.01 between diet groups; ^P<0.05 between sexes). One way ANOVA within sex was used to analyze Aβ40 and Aβ42 in 12–18-month aged mice across treatment groups. L) Touchscreen acquisition task data demonstrating impairment of HFD treatment independent of genotype in aged (18+month LOAD2 and WT mice). Both WT+HFD and LOAD2+HFD to fail to learn the task as measured by % accuracy less than chance levels (<50%) (n=3–5 per genotype/diet; combined sexes). Only CD treated WT and LOAD2 mice met acquisition criteria and advanced to assessments of pattern separation as measured by the spatial-location discrimination task. M.) Modest impairments in pattern separation of aged LOAD2 mice (18+ month) relative to age-matched WT (n=3–5 per genotype; sexes combined). Data for touchscreen are presented as mean ± sem.
Data Availability Statement
The MODEL-AD data sets are available via the AD Knowledge Portal (https://adknowledgeportal.org). The AD Knowledge Portal is a platform for accessing data, analyses, and tools generated by the Accelerating Medicines Partnership (AMP-AD) Target Discovery Program and other National Institute on Aging (NIA)-supported programs to enable open-science practices and accelerate translational learning. The data, analyses and tools are shared early in the research cycle without a publication embargo on secondary use. Data is available for general research use according to the following requirements for data access and data attribution (https://adknowledgeportal.org/DataAccess/Instructions). For access to content described in this manuscript see: https://doi.org/10.7303/syn53128146