Abstract
The new epidemic serovar O139 of Vibrio cholerae has emerged from the pandemic serovar O1 biotype El Tor through the replacement of a 22-kbp DNA region by a 40-kbp O139-specific DNA fragment. This O139-specific DNA fragment contains an insertion sequence that was described previously (U. H. Stroeher, K. E. Jedani, B. K. Dredge, R. Morona, M. H. Brown, L. E. Karageorgos, J. M. Albert, and P. A. Manning, Proc. Natl. Acad. Sci. USA 92:10374–10378, 1995) and designated IS1358O139. We studied the distribution of the IS1358 element in strains from various serovars by Southern analysis. Its presence was detected in strains from serovars O1, O2, O22, O139, and O155 but not in strains from serovars O15, O39, and O141. Furthermore, IS1358 was present in multiple copies in strains from serovars O2, O22, and O155. We cloned and sequenced four copies of IS1358 from V. cholerae O22 and one copy from V. cholerae O155. A comparison of their nucleotide sequences with those of O1 and O139 showed that they were almost identical. We constructed a transposon consisting of a kanamycin resistance gene flanked by two directly oriented copies of IS1358 to study the functionality of this element. Transposition of this element from a nonmobilizable plasmid onto the conjugative plasmid pOX38-Gen was detected in an Escherichia coli recA donor at a frequency of 1.2 × 10−8. Sequence analysis revealed that IS1358 duplicates 10 bp at its insertion site.
A new epidemic serovar of Vibrio cholerae, designated O139, has recently emerged in India and Bangladesh, where it has been responsible for a large outbreak of cholera (2, 10, 31). The strain from this serovar was the first highly contagious non-O1 strain of V. cholerae ever described. It expresses most of the V. cholerae O1 virulence factors (1), and further genetic analyses have shown that it probably arose from the pandemic strain of V. cholerae O1 biotype El Tor (4, 18, 23, 41). However, in contrast to serovar O1 strains, and like most non-O1 strains, this strain was capsulated and the chemical composition of its lipopolysaccharide (LPS) was different from that of O1 strains (5, 6, 11, 12, 23, 40, 43). Genetic analysis of the region involved in O-antigen biosynthesis, formerly designated the rfb locus, has shown that a 22-kb DNA fragment present in O1 strains has been replaced in V. cholerae O139 by a 40-kb DNA fragment constituted by (i) seven genes, wbfA to -F and wzz, some of which are likely involved in the regulation of the O-antigen length (wzz = otnB) and in the capsule transport (wbtF = otnA) (6, 30, 36); (ii) a putative insertion sequence designated IS1358 (35); and (iii) 21 open reading frames (ORFs) thought to be involved in O-antigen and capsule biosynthesis (6, 11, 37).
We previously sequenced IS1358 from V. cholerae O139 strain MO45 (ATCC 51394) (GenBank accession no. U24571), which was identical to IS1358 from O139 strain AI1837 described by Stroeher et al. (35). These 1,326-bp-long insertion sequence (IS) elements have short, nearly perfect (16- or 17-bp) inverted repeats at their ends and encode a putative protein of 375 amino acid (aa) displaying 49% identity with the Hinc repeat (H-rpt)-associated protein of the RhsB and RhsE (rearrangement hot spot) elements found in Escherichia coli K-12 strains, 28% identity with the ISAS1 transposase of Aeromonas salmonicida (21), and 31% identity with the PGIS2 transposase of Porphyromonas gingivalis (42). A variant of IS1358 differing by 17 mutations has been described for O1 strains (35). Two of these mutations have generated in-frame stop codons in the IS1358 transposase gene, leading to the formation of three ORFs, designated rfbQ, rfbR, and rfbS.
The origin of the exogenous DNA in V. cholerae O139 is unknown, but this DNA could originate from a non-O1 strain of V. cholerae. Consistently, the wbfA to -F and wzz genes have been previously detected in V. cholerae strains from serovars O69 and O141 (6), and we have demonstrated that the genes wbfA to -B are present in strains from serovars O22, O141, and O155 (39). It has been therefore suggested that IS1358 might be involved in the chromosomal rearrangements that have led to the emergence of serovar O139 from serovar O1, although evidence for transposition of this element is still lacking.
In this work, we studied the distribution of IS1358 in V. cholerae strains from various serovars. We characterized several copies of this IS in O22 and O155 strains which possess O-antigen factors in common with strains from serovar O139 (34), and we demonstrated the functionality of one element originating in a strain from serovar O22.
MATERIALS AND METHODS
Bacterial strains, vectors, and culture media.
The V. cholerae strains used in this study are listed in Table 1. E. coli DH5α (22) and plasmids pUC18 (45) and pSU2718 (26) were used for cloning experiments. E. coli HB101 (8) and LC916 (9) and the conjugative plasmid pOX38-Gen (24) were used in the mating assay. DNA fragments to be sequenced were transfected into E. coli JM105 (45) by using bacteriophages M13mp18 and M13mp19 (29). All strains were cultured on tryptic soy (TS) broth or agar medium (Difco Laboratories, Detroit, Mich.), except for LC916, which was cultured on brain heart infusion broth or agar medium (Difco). The antibiotics and concentrations used for bacterial selection were as follows: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; rifampin, 100 μg/ml; gentamicin, 5 μg/ml; and streptomycin, 500 μg/ml.
TABLE 1.
V. cholerae strains used in this study
| Strain | Other designation | Serovar | Collection source |
|---|---|---|---|
| N18a | O1 | J.-M. Fournier | |
| N212 | O2 | J.-M. Fournier | |
| N226 | O15 | J.-M. Fournier | |
| N244 | 169-68 | O22 | T. Shimada |
| N217 | O39 | J.-M. Fournier | |
| N294 | 1861-79 | O69 | T. Shimada |
| N237 | MO45 (ATCC 51394) | O139 | Y. Takeda |
| N295 | 234-93 | O141 | T. Shimada |
| N296 | 490-93 | O155 | T. Shimada |
This strain was isolated during the first wave of the seventh pandemic in Peru in 1991.
Molecular cloning techniques.
Extraction of genomic DNA (17) and small-scale isolation of plasmid DNA (3) were done as described previously. Large-scale plasmid DNA preparations were purified on Qiagen columns in accordance with the manufacturer’s recommendations (Qiagen GmbH). Genomic or plasmid DNA was digested with the appropriate restriction endonuclease, and the resulting fragments were separated by electrophoresis on 0.8% agarose gels and transferred to positively charged nylon membranes (Boehringer, Mannheim, Germany). Prehybridization and hybridization under stringent conditions were carried out as described by the manufacturer (Boehringer). The probe used in this study was a 828-bp DNA fragment internal to IS1358 from strain MO45 of serovar O139 (IS1358O139) labeled by random priming with 11-dUTP-digoxigenin (Boehringer) or with [α-32P]dCTP. This fragment, designated rfbQRSO139, was amplified by PCR from V. cholerae O139 strain MO45 by using the primer set 5′-ACTGACGGATGGTGAA-3′ and 5′-TCACGTAAGGCTTTCAAGAA-3′. The PCR was performed as follows. Fifty nanograms of target DNA, 200 mM each deoxynucleoside triphosphate, 0.1 nmol of each primer, and 1 U of thermostable DNA polymerase (New England Biolabs, Beverly, Mass.) were mixed in the corresponding 1X polymerase buffer. Amplification involved 35 cycles, each consisting of (i) a denaturation step of 1 min at 94°C, (ii) an annealing step of 1 min at 55°C, and (iii) a polymerization step of 1 min 30 at 72°C. The resulting amplicon was purified from agarose gels by use of the Geneclean kit (Bio 101, La Jolla, Calif.) before labeling was performed.
Pulsed-field gel electrophoresis (PFGE).
Extraction of bacterial DNA from V. cholerae strains grown for 18 h at 37°C was performed as described previously (27). Total DNA was digested by SfiI (30 IU), and the resulting fragments were separated by electrophoresis on a 1.0% agarose gel (150 V for 28 h with total pulse times of 7 to 28 s) by use of a contour-clamped homogeneous-field electrophoresis apparatus (CHEF-DR II; Bio-Rad, Richmond, Calif.).
RNA isolation and dot blot analysis.
Total RNA was extracted from exponentially growing V. cholerae strains (10 ml of a bacterial culture with an optical density at 600 nm between 0.8 and 0.9) that had been cultured in TS broth as previously described (7). Equal amounts of RNA (10 μg) were then denatured for 15 min at 65°C in the presence of 7% formaldehyde. The sample volume was brought to 200 μl to facilitate the filling of the wells in the slot blot apparatus (Bio-Rad).
Sequencing of IS1358 from O22 and O155 strains.
There is no HincII restriction site in IS1358O139. Therefore, in order to clone related IS elements in V. cholerae O22, HincII-restricted DNA from strain N244 was separated by electrophoresis through an 0.8% agarose gel, and 1.4- to 3-kb DNA fragments were extracted from a low-melting-point agar gel and ligated with T4 DNA ligase into SmaI-digested pUC18 vector (Appligene, Illkirch, France). Recombinant plasmids were introduced into E. coli DH5α by transformation, and transformants were selected on TS agar containing ampicillin and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). White transformants resistant to ampicillin were screened for the presence of the IS1358-related sequences by colony blot hybridization (32) with the specific probe. Four clones gave positive signals, and restriction analysis (EcoRI or HindIII) of their plasmid content revealed that each recombinant molecule carried a different insert. These inserts were subsequently cloned into the replicative forms of M13 mp18 and M13 mp19 phages. The sequences of all four copies of IS1358 and their flanking regions were determined on both DNA strands by the dideoxynucleotide chain termination method (33) using modified T7 DNA polymerase (Sequenase version 2.0; Amersham, France) and primers derived from the known sequence of IS1358O139 (35) and from the sequence determined in this work. By using the same approach, we characterized one copy of IS1358 from V. cholerae O155 (strain N296).
Construction of an IS1358-based transposon.
The pairs of primers KpnQRS2 (5′-CGGGGTACCGACAGCTAAACGAGCAATGCAGGG-3′) and BamSRQ (5′-CGCGGATCCATTGATTTGAAAGCCTTGCCCGACA-3′) or BamQRS2 (5′-CGCGGATCCGACAGCTAAACGAGCAATGCAGGG) and PstSRQ (5′-AAACTGCAGATTGATTTGAAAGCCTTGCCCGACA-3′) were used to amplify a 1,420-bp DNA fragment containing IS1358O22-3 (copy 3 of IS1358 from V. cholerae O22) plus 19 and 76 bp of the upstream and downstream flanking regions, respectively (the polarity of the element being arbitrarily defined as the direction of transcription of the transposon-encoded transposase). These primers were designed to generate copies of IS1358O22-3 flanked by KpnI and BamHI or BamHI and PstI sites (underlined bases), which were designated IS1358O22-3R (right end) and IS1358O22-3L (left end), respectively. The PWO (Pyrococcus wosei) DNA polymerase (Boehringer) was used to minimize the misincorporation of nucleotides during PCR, and sequencing of one strand of the amplified IS1358O22-3L did not reveal any mutation. These two amplified ISs, after digestion with the appropriate enzymes, were mixed with a 1.5-kb BamHI fragment containing the kanamycin resistance gene aphA-3 (38) and with plasmid pSU2718 digested with KpnI and PstI and then treated with T4 DNA ligase, and the ligation products were introduced by transformation into E. coli DH5α. Restriction analysis (with EcoRI and HindIII) of the plasmid content of clones resistant to ampicillin and kanamycin revealed the presence in all eight clones studied of a pSU2718 derivative harboring IS1358O22-3R and IS1358O22-3L, in direct orientation, separated by the aphA-3 gene. This composite transposon constructed in vitro was designated Tn1358-Km. We also constructed pSU2718ΩKm, an IS-free pSU2718 derivative containing only the kanamycin resistance gene aphA-3.
Mating assay.
The transposition and cointegrate-forming properties of IS1358 and Tn1358-Km were studied in a mating assay as described previously (19). In this system, the mobility of a transposable element carried by a nontransferable and nonmobilizable plasmid to a self-transferable plasmid was revealed in a standard mating assay between the recA strains E. coli LC916, used as a donor, and E. coli HB101, used as a recipient. Plasmid pOX38-Gen, a conjugative F derivative which does not carry any known insertion elements except a small region of IS3, was used as a target molecule. The nonmobilizable plasmids pSU2718 and pUC18-Km, a pUC18 derivative in which the bla gene was replaced by the kanamycin resistance gene aphA-3, were used as transposon delivery vectors. Plasmids pUC18-KmΩIS1358 and pSU2718ΩTn1358-Km were used to detect the formation of cointegrates (pOX38-Gen::pUC18-KmΩIS1358 and pOX38-Gen::pSU2718ΩTn1358-Km), whereas only the latter replicon was used to characterize the direct transposition events (pOX38-Gen::Tn1358-Km). In these experiments, pUC18-Km and pSU2718ΩKm, a derivative of pSU2718 carrying the aphA-3 gene, were used to determine the background level of mobilization.
Nucleotide sequencing of the transposon target junctions in pOX38-Gen.
Genomic DNA of a transconjugant resulting from a direct transposition event was digested with TaqI and self-ligated. A PCR was then performed with the primer pair 5′-AGCCTTACGTGACGGTGATGTTCAT-3′ and 5′-GGTACTTTTCGTCCATTGCGCAG-3′ to characterize IS1358R::pOX38-Gen junction sequences. The amplified DNA fragment was then cloned into pUC18 and sequenced. Sequence analysis was performed to determine the exact insertion site of Tn1358-Km in pOX38-Gen. A second PCR was performed with primers 5′-GCGGCAAGTACGGCACTCAGACGG-3′ and 5′-CACCGCAGCCCTTATATATCAACGA-3′, and the resulting 293-bp fragment corresponding to the IS1358L::pOX38-Gen junction fragment was sequenced.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper will appear in the GenBank nucleotide sequence database under accession no. AF004381 to AF004383 and AF004385 for strain N244 of V. cholerae O22 and under accession no. AF004384 for strain N296 of V. cholerae O155.
RESULTS
Distribution of the IS1358 element in various serovars of V. cholerae.
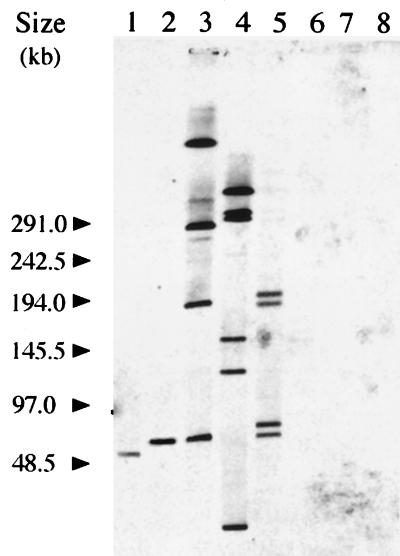
We studied, by Southern blot analysis, the distribution of the IS1358 element among a selection of wild-type strains from various serovars of V. cholerae. The SfiI-restricted chromosomal DNA fragments of strains from serovars O1, O2, O15, O22, O39, O139, O141, and O155 were separated by PFGE and hybridized under high-stringency conditions with the rfbQRSO139 probe. As illustrated in Fig. 1, hybridizing bands were detected only with DNA from strains from serovars O1, O2, O22, O139, and O155. Furthermore, IS1358 homologous sequences were present in multiple copies in strains from serovars O2, O22, and O155 whereas a single copy was found in strains from V. cholerae serovars O1 and O139. Interestingly, IS1358 was not detected in strains from serovars O15, O39, and O141. There is no SfiI site within the IS1358 element of V. cholerae O139, and we therefore estimated the IS copy number in strains from serovars O2, O22, and O155 as the number of bands hybridizing with the probe: four copies were detected in V. cholerae O2 strains, four copies were detected in V. cholerae O22 strains, and six copies were detected in V. cholerae O155 strains. This constitutes a rough estimation of the copy number since several copies might be present in the same band, a feature which would lead to an underestimation, and/or some copies might contain an internal SfiI site, which would lead to an overestimation. Multiple copies of IS1358 (four or more) were also found in strain N294 (serovar O69) (data not shown).
FIG. 1.
Southern blot analysis of V. cholerae genomic DNAs. SfiI-digested DNAs were separated by PFGE, transferred to a nylon membrane, and hybridized with a digoxigenin-labeled DNA probe specific for IS1358O139. Bacterial strains (serovars) were N18 (O1), MO45 (O139), N244 (O22), N296 (O155), N212 (O2), N295 (O141), N217 (O39), and N226 (O15) (lanes 1 to 8, respectively). Bacteriophage lambda concatemers were used as molecular size markers.
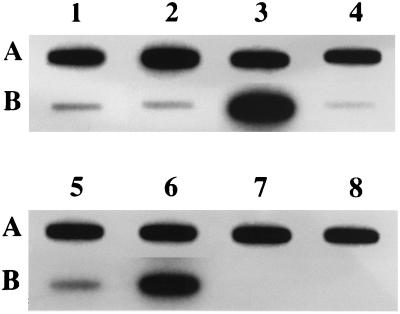
Transcriptional analysis of IS1358 elements in various V. cholerae strains.
A slot blot analysis was performed on RNAs extracted from exponentially growing cultures by using an rfbQRSO139-specific DNA probe. As shown in Fig. 2, transcripts corresponding to IS1358 were detected in all strains harboring this element. The intensity of the hybridizing dots was significantly higher in strains from two (O22 and O69) of the four serovars containing multiple copies of IS1358 (O2, O22, O69, and O155). In these experiments, strains from the IS1358-free serovars O15 and O141 were used as negative controls (Fig. 2). These results might suggest that in strains from serovars O22 and O69, at least one copy of IS1358 is transcribed from a strong chromosome-borne promoter.
FIG. 2.
Slot blot analysis of IS1358 transcription in various V. cholerae strains. Total RNAs (5 μg) were spotted onto a nylon membrane and hybridized with 32P-labeled DNA probes specific for IS1358O139 (B) or for V. cholerae 16S rDNA (A). Bacterial strains (serovars) were N18 (O1), MO45 (O139), N244 (O22), N296 (O155), N212 (O2), N294 (O69), N226 (O15), and N295 (O141) (lanes 1 to 8, respectively).
Sequence analysis of IS1358 elements from various V. cholerae serovars.
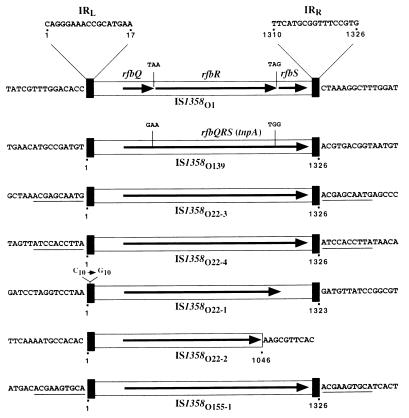
We cloned and sequenced four copies of IS1358 from V. cholerae O22 (designated IS1358O22-1, IS1358O22-2, IS1358O22-3, and IS1358O22-4) and one copy from O155 (designated IS1358O155-1). Sequence analysis revealed that IS1358O22-3, IS1358O22-4, and IS1358O155-1 were almost identical to IS1358O139. They had an identical size of 1,326 bp, displayed greater than 96% sequence identity, possessed identical 17-bp inverted repeats at their extremities, and, unlike IS1358O1, contained a single ORF coding for highly homologous 375-aa putative proteins (≥98% identity). This ORF was preceded by a putative ribosome binding site (GGAGC) located 6 bp upstream from the ATG start codon. Interestingly, IS1358O22-3, IS1358O22-4, and IS1358O155-1 were flanked by 10-bp direct repeats (Fig. 3). The 1,326-bp IS1358O22-1 is also highly homologous to IS1358O139 (97% identity), but its left inverted repeat (IRL) contained a C-to-G mutation at position 10. However, due to a mutation generating an in-frame stop codon, this IS coded for a 329-aa putative transposase truncated at its carboxylic moiety. IS1358O22-4 was a truncated form of the IS1358 that had lost 270 bp of the segment containing the 3′ moiety of the putative transposase gene and the inverted repeat designated the right inverted repeat (IRR) (Fig. 3). Sequence analysis revealed that (i) IS1358O1 is inserted into a noncoding region located between rfbO and rfbT of V. cholerae O1; (ii) IS1358O139 and IS1358O22-1 are inserted at the 3′ ends of the wzz gene of V. cholerae O139 and V. cholerae O22, respectively (16); and (iii) IS1358O22-4 is inserted at the 5′ extremity of a 59-bp element belonging to a novel class of integron recently described for the V. cholerae O1 genome (28). The sequences of the segments flanking IS1358O1, IS1358O139, IS1358O22-1, IS1358O22-2, IS1358O22-3, IS1358O22-4, and IS1358O155-1 were structurally unrelated.
FIG. 3.
Schematic comparison of IS1358 elements originating from various V. cholerae strains. IS1358O1, IS1358O139, and IS1358O155 originate from strain (serovar) N18 (O1), MO45 (O139), and N296 (O155), respectively; the four copies of IS1358O22 originate from strain N244 (O22). The prototype sequence IS1358O1 contains three ORFs, designated rfbQ, rfbR, and rfbS, which, due to point mutations, were fused in a single ORF designated rfbQRS (or tnpA) in IS1358O139, IS1358O22-3, IS1358O22-4, and IS1358O155-1. IS1358O22-1 contains a truncated tnpA gene due to the presence of an in-frame stop codon. IS1358O22-2 is a 270-bp deletion derivative devoid of the IRR. The six ISs of similar size display a high level of sequence identity (≥93%). Symbols: heavy black boxes represent the 17-bp IRL and IRR; horizontal arrows delineate the direction of transcription and extent of tnpA. The sequences of IRL and IRR and of the target sites are shown. The 10-bp sequences duplicated at the insertion sites of IS1358O22-3 and IS1358O22-4 are underlined.
IS1358 mediates direct transposition.
The ability of IS1358 to mediate cointegrate formation was studied in a mating assay by using an E. coli recA donor harboring either pOX38-Gen plus pUC18-KmΩIS1358 or pOX38-Gen plus pUC18-Km (Table 2). In these experiments, transfer of the Kmr determinant of pUC18-KmΩIS1358 and of pUC18-Km was detected at frequencies of 1.1 × 10−7 and of 2.3 × 10−7, respectively. The plasmid content of seven clones harboring pOX38-Gen::pUC18-KmΩIS1358 cointegrates originating from the same experiment and corresponding to all transconjugants obtained at the penultimate proficient dilution was digested with EcoRI and studied by Southern blot analysis with rfbQRSO139, the IS1358-specific DNA probe. This analysis revealed that all pOX38-Gen::pUC18-KmΩIS1358 cointegrates contained a single copy of the IS element (data not shown). These results suggest that the formation of cointegrates between pOX38-Gen and pUC18-KmΩIS1358 were not IS mediated and that IS1358 does not mediate cointegrate formation, at least in an E. coli genetic background. The fact that transfer of the IS-free vector pUC18-Km occurred at a frequency similar to that of pUC18-KmΩIS1358 is consistent with this proposal (Table 2).
TABLE 2.
Conjugative transfer of resistance determinants from E. coli LC916 to E. coli HB101
| Plasmid content of the donor | Antibiotics used for selectiona and concn (μg/ml) | Transfer frequencyb |
|---|---|---|
| pOX38-Gen + pUC18-KmΩIS1358 | SM, 500; GM, 5 | 0.8 |
| SM, 500; KM, 50 | 1.1 × 10−7 | |
| pOX38-Gen + pUC18-Km | SM, 500; GM, 5 | 0.8 |
| SM, 500; KM, 50 | 2.3 × 10−7 | |
| pOX38-Gen + pSU2718ΩTn1358-Km | SM, 500; GM, 5 | 0.6 |
| SM, 500; KM, 50 | 5.9 × 10−6 | |
| pOX38-Gen + pSU2718ΩKm | SM, 500; GM, 5 | 0.7 |
| SM, 500; KM, 50 | 7.8 × 10−7 |
SM, streptomycin; KM, kanamycin; GM, gentamicin.
Transfer frequencies were expressed as the number of transconjugants per donor CFU after mating.
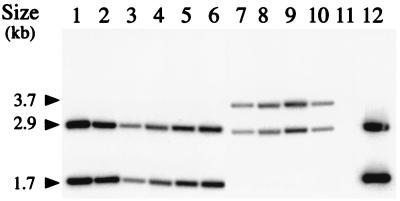
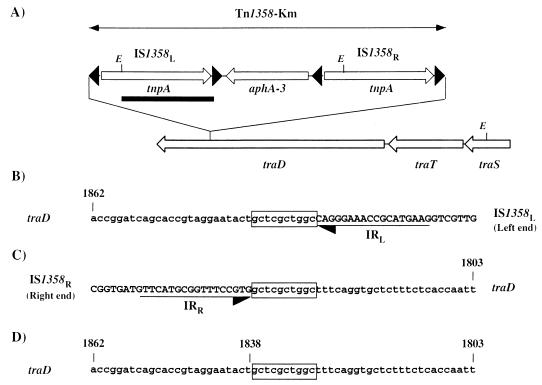
The transposon Tn1358-Km, in which the kanamycin resistance gene aphA-3 is flanked by two directly oriented copies of IS1358O22-3, was constructed to study the ability of this IS to mediate direct transposition. In mating experiments between LC916 donors harboring pOX38-Gen plus pSU2718ΩTn1358-Km and HB101 recipients, transfer of the Kmr determinant was detected at an average frequency of 5.9 × 10−6 (Table 2). The cotransfer of the chloramphenicol resistance marker of the vector pSU2718 was tested on transconjugants harboring Tn1358-Km. This analysis revealed that in the three mating experiments performed, the majority (≥80%) of the clones resistant to kanamycin were also resistant to chloramphenicol. We assume that these clones do not result from an IS-mediated cointegration event because the transfer of the IS-free vector pSU2718ΩKm was detected at a similar frequency of 7.8 × 10−7 (Table 2). Moreover, a Southern blot analysis revealed that the hybridization profile of the corresponding cointegrates obtained with the IS1358 probe is indistinguishable from that of pSU2718ΩTn1358-Km (Fig. 4 shows part of this analysis), thus suggesting that there is no IS duplication. The plasmid content of the four Kmr transconjugants harboring Tn1358-Km susceptible to chloramphenicol was studied by Southern blot analysis; the study revealed an indistinguishable hybridization pattern and the presence of two copies of IS1358, one of which was associated with a novel plasmid-transposon junction fragment (Fig. 5). We also demonstrate that the pOX38-Gen::Tn1358-Km molecules were devoid of sequence related to pSU2718 (data not shown). Taken together, these results demonstrate the transposition of Tn1358-Km at the same location in pOX38-Gen. Sequence analysis of the Tn1358-Km insertion site in pOX38-Gen revealed that transposition occurred within the traD gene and resulted in a 10-bp duplication of the target DNA (Fig. 5). Our inability to retransfer pOX38-Gen::Tn1358-Km from HB101 to LC916 (data not shown) is thus due to the insertional inactivation of the traD gene of pOX38-Gen with Tn1358-Km. It is noteworthy that the estimated size (3.7 kb) of the EcoRI IS1358R-traD junction fragment corresponded to that calculated from the nucleotide sequence (Fig. 5).
FIG. 4.
Southern blot analysis of the transposition behavior of IS1358O139 in E. coli. Genomic DNAs were digested with EcoRI, separated in an 0.8% agarose gel, transferred to a nylon membrane, and hybridized with a 32P-labeled DNA probe specific for IS1358O139. Bacterial strains were chloramphenicol-resistant HB101 transconjugants harboring pOX38-Gen::pSU2718ΩTn1358-Km (lanes 1 to 6), chloramphenicol-sensitive HB101 transconjugants harboring pOX38-Gen::pSU2718ΩTn1358-Km (lanes 7 to 10), LC916 donor harboring pOX38-Gen plus pSU2718ΩKm (lane 11), and LC916 donor harboring pOX38-Gen plus pSU2718ΩTn1358-Km (lane 12). The 1-kb ladder (Gibco-BRL) was used for molecular size markers.
FIG. 5.
Insertion of Tn1358-Km into pOX38-Gen. (A) The partial restriction map of the traD locus of pOX38-Gen::Tn1358-Km is shown. The open arrows indicate the directions of transcription of the genes tnpA, aphA-3, traD, traT, and traS. The closed arrowheads represent the 17-bp left IRs of IS1358. The black bar below IS1358L delineates the IS-specific DNA probe. E, EcoRI site. (B to D) The nucleotide sequences of the left (B) and right (C) pOX38-Gen::Tn1358-Km junction fragments and of the corresponding segment of the F traD gene (D) are indicated. The sequences of the traD gene and of IS1358 are indicated by lower- and uppercase letters, respectively. The horizontal arrows delineate the 17-bp IR of IS1358, and the sequence of the 10-bp duplicate motif at the insertion site is boxed. The coordinates refer to the first base of the traD gene (GenBank accession no. M29254).
DISCUSSION
In this work, we studied the distribution of the IS1358 element among a selection of wild-type V. cholerae strains from various serovars. This analysis revealed that sequences related to IS1358 were present in strains from serovars O1, O2, O22, O139, and O155 but not in strains from serovars O15, O39, and O141. These results suggest that the acquisition of the IS1358 element by V. cholerae is a relatively recent event which occurred after the bacterial speciation. Southern analysis revealed that multiple copies of this element were found in strains O2 (four or more copies), O22 (four or more copies), and O155 (six or more copies) whereas a single copy was detected in strains from serovars O1 and O139. The nucleotide sequences of IS1358O1 (25) and IS1358O139 (35) have been previously published. We therefore determined the nucleotide sequences of four copies of IS1358 from V. cholerae O22 (designated IS1358O22-1, IS1358O22-2, IS1358O22-3, and IS1358O22-4) and of one copy of IS1358 from V. cholerae O155 (IS1358O155-1) to carry out a detailed analysis of the sequence heterogeneity of this element in this species. Sequence analysis revealed that IS1358O22-3, IS1358O22-4, and IS1358O155-1 were almost identical to IS1358O139 and IS1358O1. These 1,326-bp elements displayed more than 96% nucleotide identity, possessed identical 17-bp inverted repeats at their extremities, and, with the exception of IS1358O1, contained a single ORF coding for highly homologous 375-aa putative proteins (≥98% of identity). This putative TnpA is homologous to the H-rpt-associated protein of RhsB and RhsE found in E. coli K-12 (49% identity) and to the H-rpt elements associated with loci that determine O-antigen biosynthesis genes in Salmonella enterica (44). It also displays 28% identity with the ISAS1 transposase of A. salmonicida (21) and 31% identity with the PGIS2 transposase of P. gingivalis (42). IS1358O22-1 is a 1,326-bp element containing a truncated TnpA due to the presence of an in-frame stop codon. IS1358O22-2 is a 270-bp deletion derivative of IS1358 which does not contain the right inverted repeat. Interestingly, IS1358O22-3, IS1358O22-4, and IS1358O155 were flanked by 10-bp direct repeats, a feature which might indicate that the corresponding IS elements are functional.
To study the functionality of IS1358, we constructed a compound transposon, designated Tn1358-Km, in which the kanamycin resistance gene aphA-3 was flanked by two directly oriented copies of IS1358O22-3. By using a mating assay in an E. coli genetic background, we demonstrated that IS1358O22-3 is able to mediate direct transposition but does not mediate the formation of cointegrates. Insertion of Tn1358-Km was obtained at a single locus of pOX38-Gen. Sequence analysis revealed that insertion occurred within the traD gene and resulted in a 10-bp duplication of the target DNA. The insertion-inactivation of a gene belonging to the transfer operon accounts for the inability of pOX38-Gen::Tn1358-Km to retransfer from HB101 donors to LC916 recipients. The transposition frequency of Tn1358-Km onto pOX38-Gen, determined by dividing the frequency of the conjugative transfer of the kanamycin resistance determinant from LC916 to HB101 (5.9 × 10−6) by (i) the transfer frequency of pOX38-Gen (6 × 10−1), (ii) the copy number of pSU2718 (20 copies per cell), (iii) the number of generations of the donor cell before selection (about eight generations), and (iv) the percentage of transconjugants originating from a direct transposition event (20%), was 1.2 × 10−8. This transposition frequency is comparable to those calculated for many other IS elements (20). Sequence analysis of IS1358 insertion sites associated with a 10-bp target duplication in V. cholerae O22 (IS1358O22-3 and IS1358O22-4) and O155 (IS1358O155-1) genomes and in the traD gene of pOX38-Gen did not reveal any obvious consensus motif for integration. Finally, it is important to note that the ability of IS1358 to translocate as a compound transposon might account for the fact that IS1358O1, IS1358O139, and IS1358O22-1 were not flanked by a 10-bp duplication (Fig. 3).
The new epidemic strain from serovar O139 of V. cholerae has probably emerged from the pandemic O1 biotype El Tor through a genetic rearrangement involving the horizontal transfer of exogenous O-antigen- and capsule-encoding genes of unknown origin. It has been reported that V. cholerae strains from serovars O22 and O155 possess O-antigen factors in common with V. cholerae serovar O139 strains (34). Furthermore, structural analysis of the LPS from V. cholerae serovars O22 and O139 have recently revealed that strains from these two serovars had almost the same O-antigen repeat unit (13–15). The presence of an IS element within these regions in both serovars O1 and O139 addresses the question of the role of IS1358 in the horizontal transfer of genes encoding O139 LPS biosynthesis and on the origin of the exogenous DNA. It is generally assumed that the cointegration pathway leads to large genome rearrangements whereas the direct transposition pathway results in the addition of small DNA fragments. Thus, if we assume that the transposition behavior of IS1358 is similar in E. coli, where it only mediates direct transposition, and in V. cholerae, it is unlikely that this element is directly implicated in the acquisition of novel O-antigen and capsule biosynthesis genes by V. cholerae O139.
ACKNOWLEDGMENTS
We thank J.-M. Fournier (Institut Pasteur, Paris), T. Shimada (Tokyo, Japan), and Y. Takeda (Kyoto, Japan) for the gift of V. cholerae strains. We are also very grateful to Eric Abachin for technical assistance with PFGE.
The work was supported by grants from MENESR, University Paris V, and INSERM.
REFERENCES
- 1.Albert M J. Epidemiology and molecular biology of Vibrio cholerae O139 Bengal. Indian J Med Res. 1996;104:14–27. [PubMed] [Google Scholar]
- 2.Albert M J, Siddique A K, Islam M, Faruque A S G, Ansaruzzaman M, Faruque S M, Sak R B. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 4.Berche P, Poyart C, Abachin E, Lelièvre H, Vandepitte J, Dodin A, Fournier J-M. The novel epidemic strain O139 is closely related to the pandemic strain O1 of Vibrio cholerae. J Infect Dis. 1994;170:701–704. doi: 10.1093/infdis/170.3.701. [DOI] [PubMed] [Google Scholar]
- 5.Bik E M, Bunschoten A E, Gouw R D, Mooi F R. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bik E M, Bunschoten A E, Willems R J L, Chang A C Y, Mooi F R. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol Microbiol. 1996;20:799–811. doi: 10.1111/j.1365-2958.1996.tb02518.x. [DOI] [PubMed] [Google Scholar]
- 7.Blazy B, Ullmann A. Two different mechanisms for urea action at the LAC and TNA operons in Escherichia coli. Mol Gen Genet. 1990;220:419–424. doi: 10.1007/BF00391748. [DOI] [PubMed] [Google Scholar]
- 8.Boyer H, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in E. coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 9.Chandler M, Galas D J. Cointegrate formation mediated by Tn9. II. Activity of IS1 is modulated by external DNA sequences. J Mol Biol. 1983;170:61–91. doi: 10.1016/s0022-2836(83)80227-7. [DOI] [PubMed] [Google Scholar]
- 10.Cholera Working Group ICDDRB. A large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet. 1993;342:387–390. [PubMed] [Google Scholar]
- 11.Comstock L E, Johnson J A, Michalski J M, Morris J G J, Kaper J B. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 12.Comstock L E, Maneval D J, Panigrahi P, Joseph A, Levine M M, Kaper J B, Morris J G J, Johnson J A. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect Immun. 1995;63:317–323. doi: 10.1128/iai.63.1.317-323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox A D, Brisson J-R, Thibault P, Perry M B. Structural analysis of the lipopolysaccharide from Vibrio cholerae serotype O22. Carbohydr Res. 1997;304:191–208. doi: 10.1016/s0008-6215(97)00207-3. [DOI] [PubMed] [Google Scholar]
- 14.Cox A D, Brisson J-R, Varma V, Perry M B. Structural analysis of the lipopolysaccharide from Vibrio cholerae O139. Carbohydr Res. 1996;290:43–58. doi: 10.1016/0008-6215(96)00135-8. [DOI] [PubMed] [Google Scholar]
- 15.Cox A D, Perry M B. Structural analysis of the O-antigen-core region of lipopolysaccharide from Vibrio cholerae O139. Carbohydr Res. 1996;290:59–65. doi: 10.1016/0008-6215(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 16.Dumontier S, Berche P. Vibrio cholerae O22 might be a putative source of exogenous DNA resulting in the emergence of the new strain of Vibrio cholerae O139. FEMS Microbiol Lett. 1998;164:91–98. doi: 10.1111/j.1574-6968.1998.tb13072.x. [DOI] [PubMed] [Google Scholar]
- 17.Ellington A. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1990. pp. 241–245. [Google Scholar]
- 18.Faruque A S, Mahalanabis D, Albert M J, Hoque S S. Studies of infection with Vibrio cholerae O139 synonym Bengal in family contacts of index cases. Trans R Soc Trop Med Hyg. 1994;88:439. doi: 10.1016/0035-9203(94)90423-5. [DOI] [PubMed] [Google Scholar]
- 19.Galas D J, Chandler M. Structure and stability of Tn9-mediated cointegrates, evidence for two pathways of transposition. J Mol Biol. 1982;154:245–272. doi: 10.1016/0022-2836(82)90063-8. [DOI] [PubMed] [Google Scholar]
- 20.Galas D J, Chandler M. Bacterial insertion sequences. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 109–162. [Google Scholar]
- 21.Gustafson C E, Chu S, Trust T J. Mutagenesis of the paracrystalline surface protein array of Aeromonas salmonicida by endogenous insertion elements. J Mol Biol. 1994;237:452–463. doi: 10.1006/jmbi.1994.1247. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 23.Johnson J A, Salles C A, Panigrahi P, Albert M J, Wright A C, Johnson R J, Morris J G J. Vibrio cholerae O139 synonym Bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect Immun. 1994;62:2108–2110. doi: 10.1128/iai.62.5.2108-2110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makris J C, Nordmann P L, Reznikoff W S. Mutational analysis of insertion sequence 50 (IS50) and transposon 5 (Tn5) ends. Proc Natl Acad Sci USA. 1988;85:2224–2228. doi: 10.1073/pnas.85.7.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning P A, Heuzenroeder M W, Yeadon J, Leavesley D I, Reeves P R, Rowley D. Molecular cloning and expression in Escherichia coli K-12 of the O antigens of the Inaba and Ogawa serotypes of the Vibrio cholerae O1 lipopolysaccharides and their potential for vaccine development. Infect Immun. 1986;53:272–277. doi: 10.1128/iai.53.2.272-277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez E, Bartolomé B, De la Cruz F. pACYC184-derived cloning vectors containing the multiple cloning site and lacZα reporter gene of pUC8/9 and pUC18/19 plasmids. Gene. 1988;68:159–162. doi: 10.1016/0378-1119(88)90608-7. [DOI] [PubMed] [Google Scholar]
- 27.Maslow J N, Slutsky A M, Arbeit R D. Application of pulsed-field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 28.Mazel D, Dychinco B, Webb V A, Davies J. A distinctive class of integron in the Vibrio cholerae Genome. Science. 1998;280:605–608. doi: 10.1126/science.280.5363.605. [DOI] [PubMed] [Google Scholar]
- 29.Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- 30.Mooi F R, Bik E M. The evolution of epidemic Vibrio cholerae strains. Trends Microbiol. 1997;5:161–165. doi: 10.1016/S0966-842X(96)10086-X. [DOI] [PubMed] [Google Scholar]
- 31.Ramamurthy T, Garg S, Sharma R, Bhattacharya S K, Nair G B, Shimada T, Takeda T, Karasawa T, Kurazono H, Pal A, Takeda Y. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet. 1993;341:703–704. doi: 10.1016/0140-6736(93)90480-5. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada T, Arakawa E, Itoh K, Nakazato T, Okitsu T, Yamai S, Kusum M, Nair G B, Takeda Y. Two strains of Vibrio cholerae non-O1 possessing somatic (O) antigen factors in common with V. cholerae serogroup O139 synonym “Bengal.”. Curr Microbiol. 1994;29:331–333. [Google Scholar]
- 35.Stroeher U H, Jedani K E, Dredge B K, Morona R, Brown M H, Karageorgos L E, Manning P A. Genetic rearrangements in the rfb regions of Vibrio cholerae O1 and O139. Proc Natl Acad Sci USA. 1995;92:10374–10378. doi: 10.1073/pnas.92.22.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stroeher U H, Manning P A. Vibrio cholerae serotype O139: swapping genes for surface polysaccharide biosynthesis. Trends Microbiol. 1997;5:178–180. doi: 10.1016/s0966-842x(97)85010-x. [DOI] [PubMed] [Google Scholar]
- 37.Stroeher U H, Parasivam G, Dredge B K, Manning P A. Novel Vibrio cholerae O139 genes involved in lipopolysaccharide biosynthesis. J Bacteriol. 1997;179:2740–2747. doi: 10.1128/jb.179.8.2740-2747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trieu-Cuot P, Courvalin P. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5"-aminoglycoside phosphotransferase type III. Gene. 1983;23:331–341. doi: 10.1016/0378-1119(83)90022-7. [DOI] [PubMed] [Google Scholar]
- 39.Vimont S, Dumontier S, Escuyer V, Berche P. The rfaD locus: a region of rearrangement in Vibrio cholerae O139. Gene. 1997;185:43–47. doi: 10.1016/s0378-1119(96)00625-7. [DOI] [PubMed] [Google Scholar]
- 40.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic: molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 42.Wang C-Y, Bond V C, Genco C A. Identification of a second endogenous Porphyromonas gingivalis insertion element. J Bacteriol. 1997;179:3808–3812. doi: 10.1128/jb.179.11.3808-3812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weintraub A, Widmalm G, Jansson P-E, Jansson M, Hultenby K, Albert M J. Vibrio cholerae O139 Bengal possesses a capsular polysaccharide which may confer increased virulence. Microb Pathog. 1994;16:235–241. doi: 10.1006/mpat.1994.1024. [DOI] [PubMed] [Google Scholar]
- 44.Xiang S-H, Hobbs M, Reeves P R. Molecular analysis of the rfb gene cluster of a group D2 Salmonella enterica strain: evidence for its origin from an insertion sequence-mediated recombination event between group E and D1 strains. J Bacteriol. 1994;176:4357–4365. doi: 10.1128/jb.176.14.4357-4365.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]