Abstract
Horizontal transposon transfer (HTT) plays an important role in the evolution of eukaryotic genomes, however the detailed evolutionary history and impact of most HTT events remain to be elucidated. To better understand the process of HTT in closely-related microbial eukaryotes, we studied Ty4 retrotransposon subfamily content and sequence evolution across the genus Saccharomyces using short- and long-read whole genome sequence data, including new PacBio genome assemblies for two S. mikatae strains. We find evidence for multiple independent HTT events introducing the Tsu4 subfamily into specific lineages of S. paradoxus, S. cerevisiae, S. eubayanus, S. kudriavzevii and the ancestor of the S. mikatae/S. jurei species pair. In both S. mikatae and S. kudriavzevii, we identified novel Ty4 clades that were independently generated through recombination between resident and horizontally-transferred subfamilies. Our results reveal that recurrent HTT and lineage-specific extinction events lead to a complex pattern of Ty4 subfamily content across the genus Saccharomyces. Moreover, our results demonstrate how HTT can lead to coexistence of related retrotransposon subfamilies in the same genome that can fuel evolution of new retrotransposon clades via recombination.
Keywords: genome evolution, horizontal transfer, recombination, retrotransposon, yeast
Introduction
Transposable elements (TEs) are mobile, repetitive DNA sequences that are found in nearly all eukaryotic genomes. Typically, TEs are vertically inherited from parents to offspring within a species (Wells and Feschotte, 2020). However, transmission of TEs between different species can also occur through the process of horizontal transposon transfer (HTT) (Daniels et al., 1990). With the widespread availability of whole genome sequencing data, HTT has been increasingly recognized as an important phenomenon in the evolution of eukaryotic genomes (Schaack et al., 2010; Wallau et al., 2012; Gilbert and Feschotte, 2018; Wallau et al., 2018).
HTT events can be identified using different sources of evolutionary evidence including phylogenetic incongruence between host genomes and TE sequences, unexpectedly high sequence similarity between TEs from divergent species, or the patchy distribution of a TE family across a set of closely-related lineages (Wallau et al., 2012; Peccoud et al., 2018). Based on these criteria, an increasing number of HTT events have been identified across the tree of life, with many examples in plants and animals (Dotto et al., 2015). While the number of HTT events detected in fungi is still relatively rare (Dobinson et al., 1993; Daboussi et al., 2002; Novikova et al., 2009; Amyotte et al., 2012; Sarilar et al., 2015), a growing number of HTT events have been identified among Saccharomyces yeast species (Liti et al., 2005; Carr et al., 2012; Bergman, 2018; Czaja et al., 2020; Bleykasten-Grosshans et al., 2021). As in other taxa, previous studies reporting HTT in yeast have typically focused on detecting their existence. However, the timing, geographic location, identity of donor/recipient lineages, and consequences of these HTT events remain unknown.
The Ty4 long-terminal repeat (LTR) retrotransposon family is a promising model to understand the process and impact of HTT in Saccharomyces yeasts. Ty4 elements – like other members of the Ty1/Copia superfamily – are composed of two overlapping ORFs (gag and pol) flanked by LTRs (Janetzky and Lehle, 1992; Stucka et al., 1992). The founding member of the Ty4 family was first identified in S. cerevisiae (Stucka et al., 1989) and defined the eponymous Ty4 subfamily. After the initial discovery of the Ty4 subfamily, Neuveglise et al. (2002) described a related subfamily called Tsu4 in the distant congener S. uvarum. More recently, Bergman (2018) reported the unexpected presence of Tsu4 sequences in S. paradoxus, S. cerevisiae, and S. mikatae and proposed these observations could be explained by HTT events from a donor related to S. uvarum or its sister species S. eubayanus. The strongest evidence for HTT involving the Tsu4 subfamily was found in S. paradoxus based on its patchy distribution among divergent S. paradoxus lineages, a high similarity between S. paradoxus Tsu4 elements and those from S. uvarum and S. eubayanus, and discordance between the phylogeny of Tsu4 elements and the host tree of Saccharomyces species. Bergman (2018) also reported evidence for a potential Tsu4 HTT event involving S. cerevisiae based on the presence of one Tsu4 full-length element (FLE) in a single strain (called 245) and its high sequence similarity with the Tsu4 sequences recently introduced into S. paradoxus. Subsequently, O’Donnell et al. (2023) confirmed the presence of Tsu4 sequences in S. cerevisiae in a different strain (called CQS), although it is currently unclear whether Tsu4 in strains 245 and CQS arose from the same or different HTT events. Likewise, Bergman (2018) provided limited evidence for a possible third Tsu4 HTT event in S. mikatae based on discordance between the phylogeny of Tsu4 elements and the accepted tree for the genus Saccharomyces (Borneman and Pretorius, 2015).
Despite these advances, several important aspects of how HTT events affect the evolution of the Ty4 family in Saccharomyces yeasts remain unresolved. First, the samples of S. cerevisiae and S. paradoxus genomes previously studied did not span the global diversity of these species. Thus the timing, geographic origin, and number of Tsu4 HTT events in these species is not fully understood. Second, previous inferences about the potential donor species for the Tsu4 HTT event into S. paradoxus were limited by the lack of high-quality whole genome assemblies (WGAs) for S. uvarum and S. eubayanus. For example, the inference that Tsu4 elements from S. eubayanus are most closely related to those transferred in S. paradoxus (Bergman, 2018) was made indirectly using genome data from the interspecific hybrid species S. pastorianus, which contains subgenomes from S. eubayanus and S. cerevisiae (Libkind et al., 2011; Baker et al., 2015; Okuno et al., 2016). Third, evidence for the putative Tsu4 HTT in S. mikatae was based on a single element from a highly fragmented draft WGA (Cliften et al., 2003), which are known to have incompletely reconstructed TE sequences (Myers et al., 2000). Finally, the single Ty4 family member identified in a draft genome for S. kudriavzevii (which is a key outgroup species to S. cerevisiae, S. paradoxus, and S. mikatae) was found to be in an intermediate phylogenetic position to both the Ty4 and Tsu4 subfamilies (Bergman, 2018). These results suggest that additional subfamilies in the Ty4 family remain to be discovered, or that recombination occurred between the Ty4 and Tsu4 lineages. Here we use large-scale short-read resequencing data and high-quality long-read WGAs from multiple species in the genus Saccharomyces to address the impact of HTT on the evolution of the Ty4 family. Our results support a complex model for Ty4 family evolution in yeast that is shaped by recurrent HTT events involving the Tsu4 subfamily, lineage-specific extinction events, and creation of new retrotransposon clades through recombination between pre-existing subfamilies that co-occur in the same species because of HTT.
Results and Discussion
To better understand the history and impact of HTT events involving the Ty4 family in Saccharomyces, we used four complementary genomic strategies. First, to more accurately infer the biogeographic distribution and ancestral states of the Ty4/Tsu4 subfamilies in S. paradoxus and S. cerevisiae, we investigated the presence/absence of Ty4/Tsu4 subfamily sequences in worldwide phylogenies for both species using unassembled short-read WGS datasets. For these analyses, we estimated copy number for LTRs and internal regions separately because recombination between LTRs within FLEs frequently excises internal sequences creating solo LTRs (Farabaugh and Fink, 1980). Doing this allows us to interpret the presence of internal sequences as evidence of recent activity, and the presence of LTRs as evidence of both recent and past activity in a strain. Second, we annotated Ty4/Tsu4 copies in a dataset of over 200 high-quality WGAs for all species in the Saccharomyces genus which allowed us to cross-validate Ty4/Tsu4 subfamily presence/absence data based on short-read WGS data, and to classify annotated copies at higher resolution into FLEs, truncated elements, and solo LTRs. We interpret the presence of FLEs in a WGA as evidence for recent activity in a strain, while truncated elements and solo LTRs represent past activity. Third, we generated phylogenetic networks and trees for internal coding regions of FLEs extracted from WGAs, which allowed us to directly investigate the molecular evolution of the Ty4 family across the entire Saccharomyces genus. Fourth, we generated strain-specific consensus sequences from unassembled short-read WGS datasets, which allowed us to study Tsu4 subfamily evolution among S. paradoxus, S. cerevisiae, S. eubayanus and S. uvarum using larger samples of strains that lack high-quality WGAs.
An ancestral Tsu4 HTT event occurred prior to radiation of indigenous American S. paradoxus lineages
Using short-read WGS datasets for 370 S. paradoxus strains, we reconstructed a maximum likelihood (ML) phylogenetic tree based on 713,556 SNPs that confirmed all major known lineages, sub-lineages, and their relationships (Figure 1A) (Naumov et al., 1997; Koufopanou et al., 2006; Kuehne et al., 2007; Liti et al., 2009; Leducq et al., 2016; Henault et al., 2019; Eberlein et al., 2019; He et al., 2022). Worldwide diversity in S. paradoxus splits into the two major known lineages: American and Eurasian. The American lineage includes several indigenous North American sub-lineages (SpB, SpC, SpC* and SpD), as well a lineage with a single strain from Hawaii. SpC* and SpD are hybrid lineages derived from crosses between SpB and SpC, and between SpB and SpC*, respectively (Leducq et al., 2016; Henault et al., 2019; Eberlein et al., 2019). The Hawaiian lineage has been reported to share similarity with either the SpB (Leducq et al., 2014) or SpC/SpC* lineages (He et al., 2022; Peris et al., 2023). Our analysis places the Hawaiian lineage as an outgroup to the SpC/SpC* lineages (circled number 3, Figure 1A). Importantly, we note that S. paradoxus lineage from S. America (circled number 2, Figure 1A) – which was formerly considered a distinct species called S. cariocanus (Naumov et al., 2000) – is contained within the North American SpB sub-lineage (Koufopanou et al., 2006; Liti et al., 2006, 2009; Hyma and Fay, 2013; Leducq et al., 2014; He et al., 2022). The Eurasian lineage includes sub-lineages indigenous to Europe, Far East Asia, and China, as well as a sub-lineage (SpA) composed of strains from North America that descend from a recent trans-oceanic migration event (Kuehne et al., 2007; Leducq et al., 2016).
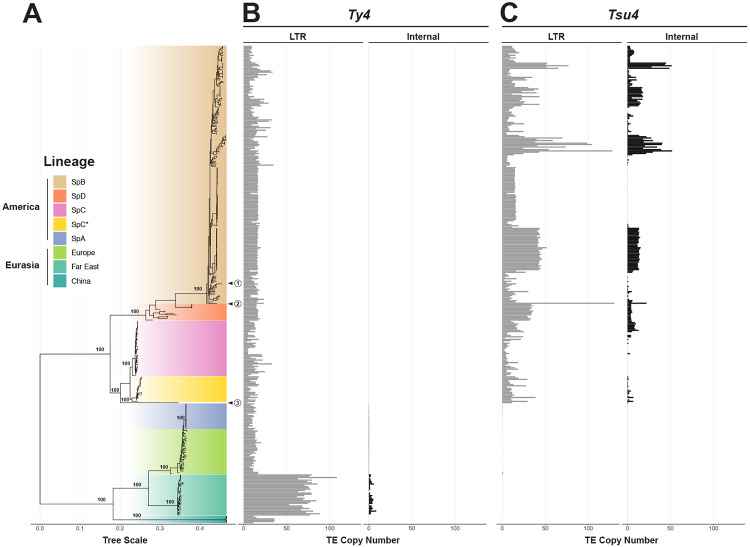
Figure 1: Host phylogeny of S. paradoxus annotated with estimated Ty4/Tsu4 copy number.
(A) Midpoint rooted ML phylogenetic tree of 370 S. paradoxus strains integrated from multiple public short-read WGS datasets (see Materials and Methods). Bootstrap support is annotated for key nodes. Major lineages and sub-lineages are annotated according to previously-reported population structure (Eberlein et al., 2019; He et al., 2022). The N. American strain DG1768 used in retromobility studies (Chen et al., 2022b) is found in the SpB sub-lineage and is indicated by the circled number 1. The S. American strain UFRJ50916 is found in the SpB sub-lineage and is indicated by the circled number 2. The Hawaiian strain UWOPS91-917.1 is found in its own sub-lineage indicated by the circled number 3. (B) Copy number estimates for the Ty4 subfamily. (C) Copy number estimates for the Tsu4 subfamily. In (B) and (C), gray bars represent copy number estimates for LTRs, whereas black bars represent estimated copy number for internal regions.
By mapping estimated Ty4/Tsu4 subfamily copy number onto the global phylogeny for S. paradoxus, we observe that Ty4 LTR sequences are present in all S. paradoxus strains from both the Americas and Eurasia (Figure 1B). Strains from the Far East sub-lineage show a significantly higher Ty4 LTR copy number in comparison to other sub-lineages. Ty4 internal regions are essentially absent across the species, except in the LTR-rich Far East sub-lineage (Figure 1B). In contrast, Tsu4 LTR sequences are only found in indigenous American strains and absent from strains with a Eurasian origin (Figure 1C). Tsu4 internal sequences are found in all indigenous American sub-lineages (SpB, SpC, SpC*, SpD, and Hawaii) with highly variable copy number (Figure 1C). Notably, we found no Tsu4 sequences in the Eurasian-derived North American SpA sub-lineage (see also (Henault et al., 2020)), which shows no evidence of admixture with indigenous American sub-lineages after secondary contact (Kuehne et al., 2007; Hyma and Fay, 2013; Leducq et al., 2014, 2016; He et al., 2022).
Analysis of Ty4/Tsu4 subfamily content in Ty4 sequences in a smaller dataset of 12 long-read WGAs that samples all major S. paradoxus lineages cross-validated results based on short-read WGS data. Ty4 solo LTRs were found in all strains but Ty4 FLEs were only found in the Far East strain N44 (Table S1). In contrast, Tsu4 solo LTRs are identified in the nine indigenous American S. paradoxus strains, and are absent from the other three strains with Eurasian origin (CBS432, N44, and LL2012_001) (Table S1). At least one Tsu4 FLE is identified in all indigenous American S. paradoxus strains with WGAs except for the SpB strain DG1768 that is commonly used in retromobility studies (Chen et al., 2022b) (circled number 1, Figure 1A). As previously reported (Bergman, 2018), Tsu4 copy number in the South American SpB strain UFRJ50916 is much higher than other S. paradoxus strains with WGAs.
These data indicate that the Ty4 subfamily was present in the most recent common ancestor (MRCA) of all S. paradoxus lineages prior to global dispersal, and therefore represents the ancestral subfamily in S. paradoxus. The Ty4 subfamily subsequently went extinct in most recognized S. paradoxus sub-lineages except for the Far East sub-lineage where it remains active. In contrast, the lack of Tsu4 sequences in Eurasian S. paradoxus and the Eurasian-derived SpA sub-lineage indicates this subfamily has never existed in Eurasia and therefore was not present in the MRCA of all S. paradoxus strains. Our results support the interpretation that a Tsu4 HTT event occurred in an ancestor of all indigenous American S. paradoxus sub-lineages after the divergence of American from Eurasian lineages. This HTT event most likely occurred in an ancestral lineage where the Ty4 subfamily had already gone extinct, thus explaining why Ty4 and Tsu4 FLEs have never been observed in the same S. paradoxus strain. Since this ancestral HTT event, Tsu4 has maintained activity in all indigenous American S. paradoxus sub-lineages. However, Tsu4 has secondarily gone extinct or expanded to very high copy-number in many strains in each American S. paradoxus sub-lineage. We note that this parsimonious scenario does not exclude the possibility of additional recent Tsu4 HTT events into indigenous American S. paradoxus lineages that are obscured by this initial ancestral HTT event.
Recent HTT has introduced Tsu4 into a small number of Central/South American S. cerevisiae strains
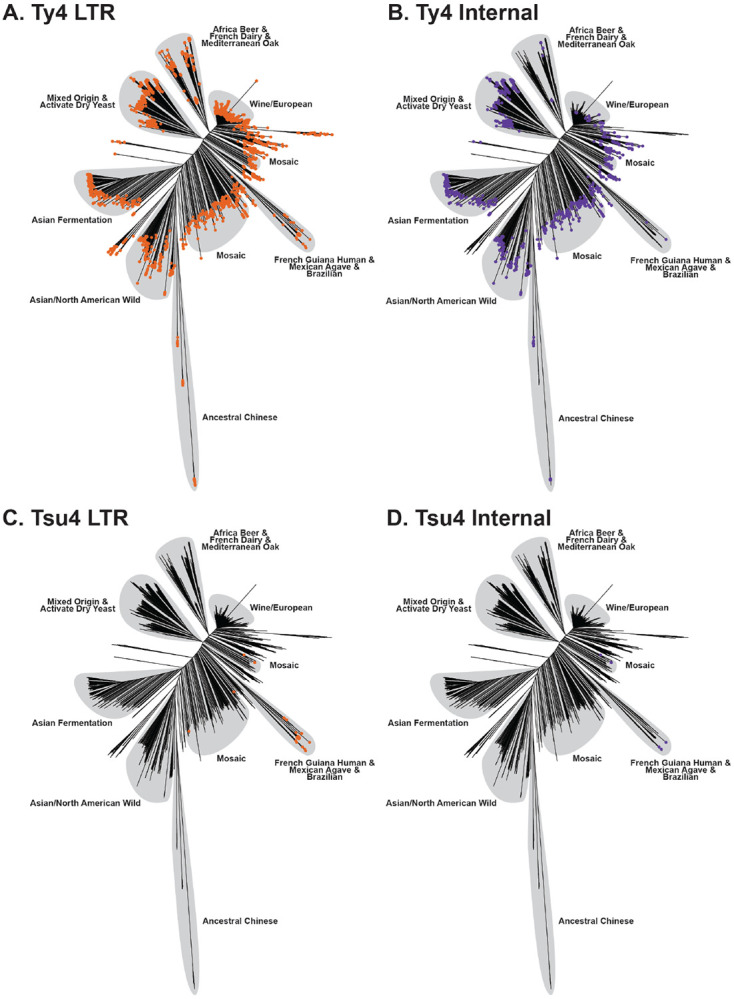
Using a similar short-read WGS-based approach as was used for S. paradoxus, we reconstructed a species-wide phylogeny for S. cerevisiae based on 2,787,577 genome-wide SNPs from 2,404 strains (Figure 2). In contrast to the ML approach used for S. paradoxus where admixture among lineages is rare, we followed Peter et al. (2018) in using a neighbor-joining (NJ) approach to generate the S. cerevisiae phylogeny which accommodate the well-established existence of admixed strains in this species (Liti et al., 2009; Peter et al., 2018). Despite using nearly twice as many strains, our phylogenetic tree of S. cerevisiae strains shows a similar topology as Peter et al. (2018), who identified a complex population structure including more than 26 distinct lineages plus many mosaic strains derived from admixture between these lineages. Strains in our integrated dataset that are not present in Peter et al. (2018) – such as those from Duan et al. (2018) and Barbosa et al. (2016) – cluster with known lineages previously characterized by Peter et al. (2018). For instance, “activated dry yeast” strains from Duan et al. (2018) cluster in the “mixed origin” lineage from Peter et al. (2018) (Figure 2).
Figure 2: Host phylogeny of S. cerevisiae annotated with the presence or absence of Ty4/Tsu4 subfamilies.
(A-D) NJ phylogenetic tree reconstructed using 2,787,577 genome-wide SNPs from 2,404 S. cerevisiae strains (see Materials and Methods). Major lineages are annotated with light gray shading based on previously-reported population structure (Peter et al., 2018; Duan et al., 2018). Colored dots indicate the presence of Ty4 (panels A and B) and Tsu4 (panels C and D) sequences for each S. cerevisiae strain. The presence of Ty4/Tsu4 subfamilies in a strain was inferred when copy number estimates were >1 for LTRs (annotated with orange dots in panel A and C) and >0.5 for internal regions (annotated with purple dots in panel B and D).
We then visualized the presence/absence of LTR and internal regions for Ty4/Tsu4 subfamilies on the phylogeny inferred for S. cerevisiae from short-read WGS data. This analysis revealed that Ty4 LTR and internal sequences are present in all S. cerevisiae lineages (Figure 2A,B). In contrast, Tsu4 LTR sequences are restricted to ~2% of strains surveyed (49/2,404) all of which are found in Central/South America (specifically French Guiana, Mexico, Brazil, and the French West Indies) (Figure 2C,D). Tsu4 sequences are completely absent from most other S. cerevisiae lineages, including the most ancestral Chinese lineages (Wang et al., 2012; Duan et al., 2018). We identified six S. cerevisiae strains that contain evidence of Tsu4 internal regions (245, AFQ and CDM from Mosaic lineage 2; CQS from the French Guiana lineage; UFMG-CM-Y641 and UFMG-CM-Y642 from the Brazil 3 lineage) (Marsit et al., 2015; Peter et al., 2018; Barbosa et al., 2016). Three of these S. cerevisiae strains (AFQ, CDM and CQS) also contain internal regions for Ty4.
Next, we analyzed Ty4/Tsu4 subfamily content in WGAs for a global sample of 183 S. cerevisiae strains (Table S1 and Figure S1), which confirmed results based on short-read WGS data. Ty4 subfamily sequences were found in all S. cerevisiae WGAs analyzed, while Tsu4 subfamily sequences were absent from the majority of S. cerevisiae WGAs. CQS is the only strain assembled using long-read data for which we identify FLEs for Tsu4 (n=9), confirming previous observations (O’Donnell et al., 2023). We also identified one full-length (245 and AFQ) or truncated (CDM) Tsu4 copy in short-read WGAs (Table S1) for three S. cerevisiae strains that we identified previously as containing Tsu4 internal regions in the short-read WGS scan. No publicly-available WGAs are available for the two Brazil 3 strains (UFMG-CM-Y641 and UFMG-CM-Y642, (Barbosa et al., 2016)) with evidence of Tsu4 internal regions based on WGS data. All three S. cerevisiae strains with Tsu4 FLEs in WGAs are geographically restricted to Central/South America. Importantly, we note that FLEs for both the Ty4 and Tsu4 subfamilies were identified in the long-read WGA for strain CQS.
The prevalence of the Ty4 subfamily in most S. cerevisiae lineages – including ancestral Chinese lineages (Wang et al., 2012; Duan et al., 2018) – indicates that the Ty4 subfamily was present in the MRCA of this species. However, despite being broadly active at the species level, the absence of Ty4 internal regions and FLEs in many strains indicates this subfamily has undergone many local extinction events (see also (Bleykasten-Grosshans et al., 2021)). In contrast, the absence of Tsu4 in most lineages (including ancestral Chinese lineages) strongly indicates that this subfamily was not present in the MRCA of S. cerevisiae. The small number of strains that do contain Tsu4 in S. cerevisiae do not form a single monophyletic group, which is consistent either with one HTT event followed by admixture among lineages, or multiple recent HTT events that have introduced Tsu4 into different lineages of S. cerevisiae in Central/South America. Finally, the observation of S. cerevisiae strains with FLEs for both Tsu4 and Ty4 subfamilies (i.e., CQS) demonstrates that FLEs from the Ty4 and Tsu4 subfamilies can co-exist in the same Saccharomyces strain.
Multiple HTT events have transferred Tsu4 into S. paradoxus and S. cerevisiae
The short-read WGS strategy used above allowed us to establish Ty4 as the ancestral subfamily in S. paradoxus and S. cerevisiae, and to identify at least one Tsu4 HTT event in both species. However, this approach cannot resolve how many Tsu4 HTT events occurred in either species, nor can it identify the potential donor lineages for these HTT events. To investigate whether the presence of Tsu4 in S. paradoxus and S. cerevisiae can be explained by one or more Tsu4 HTT event, and to identify the most likely donor lineage(s) for these HTT events, we analyzed the molecular evolution of all Ty4 family FLEs identified in a integrated dataset of 210 high-quality WGAs for all recognized species in the genus Saccharomyces. The majority of WGAs in this dataset were generated using PacBio or Oxford Nanopore Technology (ONT) long reads, including new high-quality WGAs for S. mikatae strains IFO 1815 and NBRC 10994 that we generated using PacBio long reads (Table S2). Phylogenetic analysis of our new S. mikatae WGAs confirmed the taxonomic placement of IFO1815 in the Asia A clade (Peris et al., 2023) and revealed that NBRC 10994 should be placed in a new clade (Asia C) (Figure S2). In addition, we also included WGAs based on short-read data for three S. cerevisiae strains with Tsu4 internal sequences (245, AFQ and CDM; see above) and for two strains of S. arboricola (H-6 and ZP960) that represent the best available WGAs for this species.
In total, we identified 247 FLEs for the Ty4 subfamily and 124 FLEs for the Tsu4 subfamily in this integrated dataset (Table S2, Figure S1). No FLEs for either subfamily were identified S. arboricola and S. kudriavzevii using our current query sequences. The absence of Ty4 family FLEs in S. arboricola may simply reflect the lack of high-quality WGAs for this species. However, the absence of Ty4 family FLEs in S. kudriavzevii is likely an artifact of divergence between our current query sequences and Ty4 family FLEs in this species. In S. kudriavzevii strain (IFO1802) we observed a high copy number of truncated Tsu4 elements (n=8) that, upon further inspection, revealed five nearly full-length elements that were highly similar to one another, dispersed throughout the IFO1802 genome, and overlapped full-length de novo LTRharvest predictions (Ellinghaus et al., 2008). We concluded that these five S. kudriavzevii elements represented FLEs from a novel branch in the Ty4 family and included them in our phylogenetic analysis of FLEs.
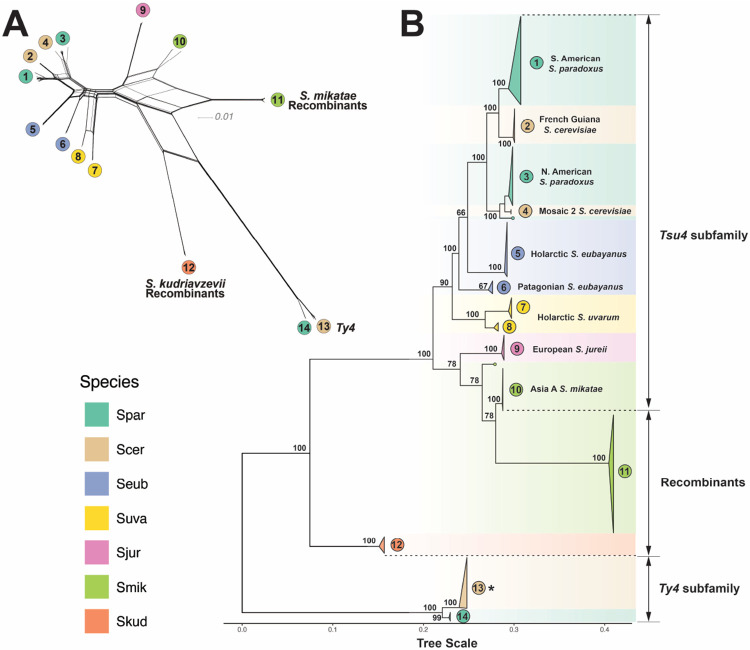
We next created a multiple sequence alignment and reconstructed phylogenetic networks and trees based on internal coding regions of all 376 FLEs in our integrated dataset (Figure 3A, B). We excluded LTR and untranslated sequences from this analysis, which exhibited poor alignment due to higher divergence in noncoding regions. This analysis identified 14 well-supported clades of FLEs each found in a single species, plus two branches with singleton FLEs from the Hawaiian S. paradoxus strain UWOPS91-917.1 and Asia C S. mikatae strain NBRC 10994, respectively. Two clades (Clades 11 and 12) with FLEs from either S. mikatae or S. kudriavzevii exhibit evidence of reticulation between the Ty4 and Tsu4 clades (Figure 3A), which we interpret as being caused by recombination between these subfamilies (see detailed analysis below). Exclusion of these clades eliminated the major signal for reticulation between the Ty4 and Tsu4 subfamilies in the phylogenetic network (Figure S3A) and increased bootstrap support for clades in S. jurei and S. mikatae, but did not alter the topological relationships of other clades in the ML tree (Figure S3B). The Ty4 subfamily is represented by only two clades with FLEs from only S. cerevisiae (Clade 13) or S. paradoxus (Clade 14), respectively. In contrast, the Tsu4 subfamily is represented by ten species-specific clades (1-10) with FLEs from all species except S. kudriavzevii (Figure 3A, S4, S5, S6). Two clades each of Tsu4 FLEs from S. paradoxus (Clades 1 and 3) and S. cerevisiae (Clades 2 and 4) together with the singleton FLE from S. paradoxus UWOPS91-917.1 form a monophyletic group. S. eubayanus is represented two Tsu4 FLEs clades from the Holarctic (Clade 5) and Patagonian (Clade 6) lineages, respectively. Tsu4 FLEs from S. uvarum form two clades (Clades 7 and 8) that are both found in a single strain from the Holarctic lineage. Tsu4 FLEs from European S. jurei (Clade 9) cluster with Tsu4 FLEs from the S. mikatae Asia A lineage (Clade 10) and the singleton branch from the S. mikatae Asia C lineage.
Figure 3: Phylogenetic network and tree of FLEs from the Ty4 family in Saccharomyces.
(A) Phylogenetic network for internal coding regions of Ty4/Tsu4 FLEs based on the NeighborNet algorithm. To simplify visualization, this network only includes Ty4 subfamily FLEs from WGAs reported in Yue et al. (2017). Lineages in the network are labeled according to monophyletic groups identified in panel B. (B) Midpoint rooted ML phylogeny of internal coding regions from Ty4/Tsu4 FLEs. Bootstrap support based on 100 replicates is shown for major nodes. The scale bar for branch lengths is in units of substitutions per site. All monophyletic groups are collapsed as triangles. Two singleton Tsu4 elements (f267 from Hawaiian S. paradoxus strain UWOPS91-917.1 and f256 from S. mikatae strain NBRC 10994) are denoted as dots at tips. Triangles, tip dots, and ranges are colored for each species. Vertical heights of triangles are proportional to the number of taxa. Horizontal widths of triangles are equal to the maximum branch length within the clade. Note that the monophyletic clade for the Ty4 subfamily from S. cerevisiae (annotated with an asterisk) is re-scaled to 5% of the real sample size both horizontally and vertically, due to the large number of Ty4 sequences (n=244) in S. cerevisiae genomes.
Previous analysis of Tsu4 HTT events in S. paradoxus and S. cerevisiae using a smaller dataset of FLEs (Bergman, 2018) suggested one primary HTT occurred in the ancestor of American S. paradoxus lineages followed by one secondary HTT from S. paradoxus into S. cerevisiae. This hypothesis predicts that Tsu4 FLEs from S. paradoxus and S. cerevisiae should form a single clade with S. cerevisiae FLEs forming a single sub-clade somewhere within the broader diversity of S. paradoxus FLEs. Two key features of the Tsu4 FLE phylogeny in our expanded dataset are inconsistent with this hypothesis (Figure 3). First, we observe two distinct clades of FLEs for both S. paradoxus and S. cerevisiae, each of whose closest sampled relatives are from a different species. Namely, S. cerevisiae Clade 2 (from the French Guiana strain CQS) clusters with S. American S. paradoxus Clade 1, while S. cerevisiae Clade 4 (from Mosaic 2 strains 245 and AFQ) clusters with the N. American S. paradoxus Clade 3. Second, the observed topology of Tsu4 FLEs in S. paradoxus does not strictly follow the host phylogeny for the SpB sub-lineage strain UFRJ50816. Specifically, Tsu4 FLEs from the S. American S. paradoxus strain UFRJ50816 form their own divergent Clade 1 instead of grouping as expected with Tsu4 FLEs from other SpB strains from N. America (MSH-604 and YPS138) in Clade 3 (Figure S4).
We interpret the phylogeny of Tsu4 FLEs in S. paradoxus and S. cerevisiae to be consistent with at least two Tsu4 HTT events into each species. In S. paradoxus, we infer one ancestral Tsu4 HTT event creating Clade 3 FLEs that are present in all SpB, SpC, SpC* and SpD strains (except UFRJ50816), and one recent HTT event creating Clade 1 FLEs present in the S. American sub-lineage containing UFRJ50816 (where Clade 3 FLEs had already gone extinct). Likewise, in S. cerevisiae, we infer one recent HTT into the French Guiana lineage creating Clade 2 FLEs, and another recent HTT into the Mosaic 2 lineage creating Clade 4 FLEs. These data suggest that when conditions are favorable for HTT events in Saccharomyces, they can occur more frequently than the most parsimonious interpretation based on presence/absence data would imply.
Tsu4 in S. paradoxus, S. cerevisiae, and Holarctic S. eubayanus were transferred from an unknown donor.
The collective monophyly of the Tsu4 clades found in S. paradoxus and S. cerevisiae suggests the Tsu4 HTT events in these species ultimately arose from a similar donor lineage, with distinct clades being formed at different times or in different geographic regions. Using indirect data from the hybrid species S. pastorianus that contains subgenomes from S. eubayanus and S. cerevisiae (Libkind et al., 2011; Baker et al., 2015; Okuno et al., 2016), Bergman (2018) concluded that the Holarctic lineage of S. eubayanus contains the most closely related Tsu4 sequences to those in S. paradoxus and S. cerevisiae. Our current analysis provides direct evidence for this conclusion, with Clade 5 FLEs from CDFM21L.1 in the the Holarctic S. eubayanus lineage clustering most closely with the common ancestor of all Tsu4 FLEs in S. paradoxus and S. cerevisiae (Figure 3). Taken at face value, this result implies that Holarctic S. eubayanus represents the most likely donor lineage for the multiple HTT events observed in S. paradoxus and S. cerevisiae.
However, several features of the Tsu4 FLE phylogeny suggest that the Holarctic S. eubayanus lineage is not the direct donor for the HTT events in S. paradoxus and S. cerevisiae (Figure 3). First, Tsu4 clades in S. paradoxus and S. cerevisiae are not nested within the diversity of FLEs from Holarctic S. eubayanus, but rather form a sister group separated by substantial divergence. Second, bootstrap support for the clustering of Holarctic S. eubayanus FLEs with the ancestor of FLEs from S. cerevisiae and S. paradoxus is relatively weak (>66%). The alternative clustering of FLEs from the Holarctic and Patagonia-B S. eubayanus lineages together would suggest that the donor into S. paradoxus and S. cerevisiae is from a currently-unsampled lineage of S. eubayanus, or a species closely related to S. eubayanus. Third, Tsu4 FLEs from Holarctic S. eubayanus are not nested with in the diversity of S. eubayanus Patagonian FLEs (Figure 3, S5), as is expected since Holarctic S. eubayanus is known to be a sub-lineage of the Patagonia-B lineage (Figure S7) (Peris et al., 2016). Discordance between the S. eubayanus Tsu4 and host strain phylogenies suggests the possibility of a previously-undetected Tsu4 HTT event into Holarctic S. eubayanus, which could lead to the false conclusion that the Holarctic S. eubayanus lineage is the most likely donor for HTT events into S. paradoxus and S. cerevisiae.
To test for a previously-undetected Tsu4 HTT event in the Holarctic S. eubayanus lineage, we developed a novel approach to study Tsu4 sequence evolution using strain-specific consensus sequences inferred from short-read WGS data. Importantly, this approach bypasses the limited number of WGAs available in S. eubayanus and other potential donor species and allows us to generalize results across larger samples of host strains and lineages. The premise behind this approach is based on the observation that Tsu4 FLEs typically cluster first within the same strain before clustering with FLEs from other strains (Figure 3). Thus, strain-specific Tsu4 consensus sequences should be a reasonable proxy for the common ancestor of elements within a strain, and can themselves be used for evolutionary inference across strains and species.
Using the short-read based WGS approach as above for S. paradoxus and S. cerevisiae, we first estimated Ty4/Tsu4 LTR and internal copy numbers in the context of host phylogenies for S. eubayanus (Figure S7) and S. uvarum (Figure S8), respectively. These results reveal that Tsu4 was present and Ty4 was absent in the ancestors of both S. eubayanus and S. uvarum, that Tsu4 is broadly active in both species, and that the Holarctic S. eubayanus lineage has the highest LTR copy number of Tsu4 in either species. We then computed consensus sequences for Tsu4 internal regions in all S. paradoxus, S. cerevisiae, S. eubayanus and S. uvarum strains with an estimated copy number of >0.75 and generated a ML tree of strain-specific sequences across the combined dataset of four species (Figure 4).
Figure 4: ML phylogeny of strain-specific consensus sequences for Tsu4 internal regions in all S. paradoxus, S. cerevisiae, S. eubayanus and S. uvarum.
Shown is the phylogeny reconstructed with 1,817 distinct alignment sites from 419 strain-specific consensus sequences. The consensus sequence is computed for each S. paradoxus, S. cerevisiae, S. eubayanus, and S. uvarum strain that has >0.75 depth and >0.9 breadth in its Tsu4 internal region. Tip points are colored by species. The phylogeny is midpoint rooted. Bootstrap supporting values are annotated for key nodes. Key clades are annotated with host lineage and/or clade numbers from the Tsu4 FLE phylogeny.
Key groupings in the strain-specific Tsu4 consensus tree (Figure 4) agreed with the phylogeny of Tsu4 FLEs based on a smaller number of strains above (Figure 3), cross-validating both approaches. Notably, Tsu4 consensus sequences for all N. American S. paradoxus strains (together with the two S. cerevisiae Brazil 3 strains, UFMG-CM-Y641 and UFMG-CM-Y642) form a large monophyletic group (corresponding to Clade 3 in the FLE tree) that clusters most closely with consensus sequences from Mosaic 2 S. cerevisiae strains (corresponding to Clade 4). Likewise, the consensus sequence for the S. American S. paradoxus strain UFRJ50916 (corresponding to Clade 1) clusters with the S. American S. cerevisiae strain CQS (corresponding to Clade 2). All seven Holarctic S. eubayanus strains form a monophyletic group (corresponding to Clade 5) that clusters with S. paradoxus and S. cerevisiae consensus sequences and is distinct from Tsu4 sequences found in all other S. eubayanus or S. uvarum strains.
Phylogenetic analysis of strain-specific consensus sequences also revealed two other Tsu4 HTT events that could not be detected in the FLE phylogeny because of limited samples of WGAs. The first involves the two S. cerevisiae Brazil 3 strains (UFMG-CM-Y641 and UFMG-CM-Y642). Based on the location of Brazil 3 samples in the consensus sequence tree (Figure 4) and the pattern of variation in the consensus sequences of all six S. cerevisiae strains that have Tsu4 (Figure S9), we conclude that Tsu4 sequences in the S. cerevisiae Brazil 3 lineage arose from a HTT event that is distinct from those detected using FLEs in the Mosaic 2 or French Guiana lineages. The second peviously-undetected Tsu4 HTT event involves the S. eubayanus Patagonia B strain yHAB565, whose Tsu4 consensus sequence is placed within the S. uvarum cluster. S. eubayanus yHAB565 is placed correctly in the Patagonia B lineage in our host strain tree (Figure S7), which rules out the possibility of sample mixups during sequencing or bioinformatic analysis and supports a recent HTT Tsu4 event from S. uvarum into S. eubayanus.
Taken together, our results suggest that the similarity between the Tsu4 clades in Holarctic S. eubayanus, S. paradoxus, and S. cerevisiae arose from parallel HTT events donated by a common but as-yet-unidentified Saccharomyces lineage. The substantial divergence between non-Holarctic S. eubayanus or S. uvarum and the clade comprised of Holarctic S. eubayanus, S. paradoxus and S. cerevisiae suggests that this unknown donor is either a currently-unsequenced lineage of S. eubayanus or S. uvarum (e.g., West China S. eubayanus (Bing et al., 2014)) or potentially an undiscovered species related to S. eubayanus and S. uvarum. Additionally, we show that our novel strain-specific consensus sequence approach complements analysis of FLEs from WGAs and can reveal previously undetected cases of Tsu4 HTT in the abundant WGS datasets that are available in multiple yeast species.
Tsu4 HTT fuels the evolution of recombinant clades in S. mikatae and S. kudriavzevii
Our phylogenetic network analysis of FLEs from the Ty4 family above revealed evidence of reticulation in S. mikatae Clade 11 and S. kudriavzevii Clade 12 (Figure 3A) that could be caused by recombination between the Ty4 and Tsu4 subfamilies (Huson and Bryant, 2006). In addition, the coexistence of FLEs for both Ty4 and Tsu4 in S. cerevisiae strain CQS indicates that conditions for recombination between Ty4 and Tsu4 subfamilies can occur in nature. To provide further evidence for recombination between the Ty4 and Tsu4 subfamilies, we first selected representative FLEs for “pure” Tsu4 (f32 from the S. uvarum Clade 8) and “pure” Ty4 (f49 from the S. cerevisiae Clade 13) from outgroup species not involved in the putative recombination events. We then plotted a sliding window of pairwise sequence divergence between these representative pure Ty4 and Tsu4 FLEs and a putatively “pure” S. mikatae Clade 10 FLE (f286 from IFO 1815) or a putatively “recombinant” S. mikatae Clade 11 FLE (f256 from IFO 1815) (Figure S10). This analysis revealed that that the 5’ internal region – including the complete gag gene and the first ~500bp of pol – shows lower levels of divergence between S. uvarum Tsu4 and pure S. mikatae Clade 10 (Figure S10A) than recombinant S. mikatae Clade 11 (Figure S10B). Conversely, the same 5’ internal region shows higher levels of divergence between S. cerevisiae Ty4 and pure S. mikatae Clade 10 (Figure S10D) than recombinant S. mikatae Clade 11 (Figure S10E). These data indicate that the 5’ internal segment in Clade 11 is derived from the Ty4 subfamily, while the rest of the Clade 11 internal region is derived from the Tsu4 subfamily.
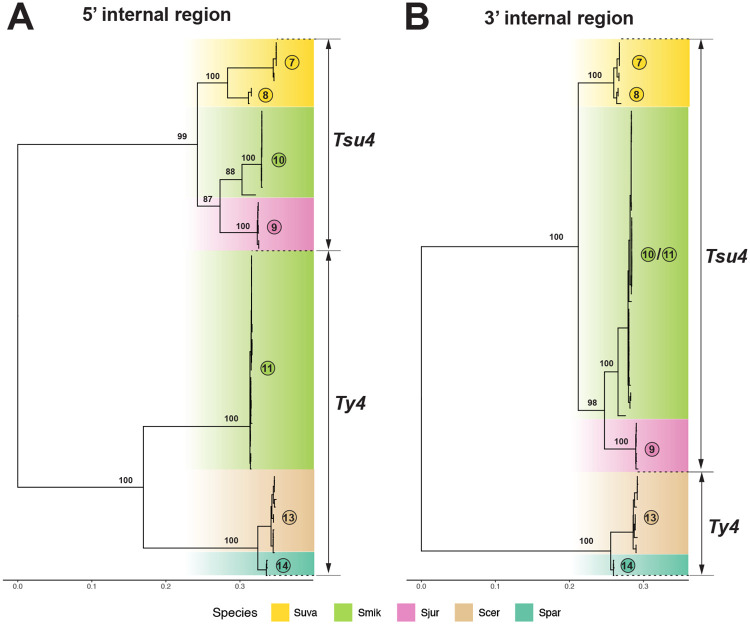
We then partitioned the multiple sequence alignment of Ty4 family FLE internal regions into 5’ and 3’ segments, and reconstructed ML phylogenies for both partitions from representative clades (Figure 5). A striking discordance can be observed in phylogenies reconstructed from 5’ and 3’ internal regions of Clade 11 sequences. In the 5’ partition, pure Clade 10 FLEs cluster with Tsu4 FLEs from S. jurei and S. uvarum, while recombinant Clade 11 FLEs cluster with strong support as a sister group to from S. paradoxus/S. cerevisiae in the Ty4 subfamily (Figure 5A). In contrast, in the tree reconstructed from the 3’ partition S. mikatae Clades 10 and 11 form a single monophyletic group that is closely related to Tsu4 sequences from S. jurei (Figure 5B).
Figure 5: ML phylogeny for 5’ and 3’ internal regions from Tsu4 FLEs in S. mikatae, S. jurei, and S. uvarum, plus representatives of Ty4 elements.
Panel (A) shows the ML phylogeny for 5’ internal region containing 459 distinct alignment sites; Panel (B) shows 3’ internal region containing 455 distinct alignment sites. Internal coding regions from 71 Ty4 and Tsu4 FLEs are included in both panels. Both trees are midpoint rooted, and visualized in the same tree scale which is shown in units of substitutions per site. Bootstrap supporting values are annotated for key nodes.
Based on these results and presence of Ty4 solo LTRs in WGAs from S. mikatae and S. jurei (Table S1), we propose the following scenario for the evolution of S. mikatae Clades 10 and 11. A divergent Ty4 subfamily was previously active in an ancestor of S. mikatae and S. jurei, which has subsequently gone extinct in both species but left Ty4 internal sequences that were retained in the S. mikatae genome for some period of time. A HTT event introduced the Tsu4 subfamily prior to the speciation of S. mikatae and S. jurei, which evolved into Clade 10 in S. mikatae and Clade 9 in S. jurei. This Tsu4 HTT event explains the discordance between the Ty4 family and host species phylogenies previously reported for S. mikatae Bergman (2018). The donor for this Tsu4 HTT into the ancestor of S. mikatae and S. jurei is unknown but related to the ancestor to all extant Tsu4 FLEs in S. eubayanus, S. uvarum, S. paradoxus and S. cerevisiae. Recombination of the 5’ internal region from the now-extinct Ty4 subfamily in S. mikatae onto a Clade 10-like pure Tsu4 FLE created the “recombinant” Tsu4 Clade 11. This model explains the lack of Ty4 FLEs in S. mikatae and S. jurei (Table S1), the coexistence of two highly divergent clades in S. mikatae genomes (Figure 3B), the long branch leading to Clade 11 in the FLE phylogeny (Figure 3B), and reticulation between Ty4 and Tsu4 subfamilies for Clade 11 in the phylogenetic network (Figure 3A).
We applied similar approaches to investigate whether reticulation in the phylogenetic network observed for S. kudriavzevii Clade 12 FLEs (Figure 3A) also is caused by recombination between Ty4 and Tsu4 subfamilies. Sliding window analysis of a representative Clade 12 FLE versus pure Tsu4 and Ty4 from outgroup species revealed an ~2kb segment starting at the beginning of Pol that shows very high similarity to Tsu4 (Figure S10C). Phylogenetic analysis of partitions corresponding to the “left,” “middle,” and “right” segments of FLE internal regions for representative clades reveals that the middle internal segment of Clade 12 is derived from the Tsu4 subfamily, while the left and right segments are divergent representatives of the Ty4 subfamily. Based on these results and presence of Ty4 LTRs in WGAs from S. kudriavzevii (Table S1), we propose that S. kudriavzevii ancestrally contained a divergent Ty4 subfamily which acquired a middle segment from a horizontally-transferred Tsu4 by recombination. The Tsu4 clade that was horizontally transferred into S. kudriavzevii and the original pure S. kudriavzevii Ty4 clade have both subsequently gone extinct, leaving the recombinant Clade 12 as the only extant representative of the Ty4 family currently identified in S. kudriavzevii . Based on clustering of the middle internal region (Figure 6B), the donor lineage for the Tsu4 HTT into S. kudriavzevii is related to the donor for Tsu4 HTT in S. mikatae and S. jurei. Together, the recombinant clades in S. mikatae and S. kudriavzevii support the conclusion that co-existence of Ty4 and Tsu4 subfamily sequences in the same genome mediated by HTT provides substrate for recombination to generate new retrotransposon clades in Saccharomyces.
Figure 6: ML phylogeny for partitioned internal regions from recombinants in S. kudriavzevii, pure Tsu4 FLEs in S. mikatae, S. jurei, and S. uvarum, plus representatives of Ty4 elements.
Panel (A) shows the ML phylogeny for left-side internal region based on 357 distinct alignment sites; Panel (B) for middle internal region based on 367 distinct alignment sites; Panel (C) for right-side internal region based on 423 distinct alignment sites. In all three panels, internal regions from 47 Ty4 and Tsu4 FLEs are included. All trees are midpoint rooted, and visualized in the same tree scale which is shown in units of substitutions per site. Bootstrap supporting values are annotated for key nodes.
Conclusions
Here we address open questions concerning the impact of HTT on the evolution of the Ty4 family in Saccharomyces by integrating large-scale short-read WGS data and high-quality long-read WGAs from multiple Saccharomyces species. We show that the previously detected Tsu4 HTT event in S. paradoxus (Bergman, 2018) occurred in the ancestor of all American lineages and report new evidence for a second recent Tsu4 HTT in the South American lineage of S. paradoxus. We also show that the previously reported presence of Tsu4 in S. cerevisiae (Bergman, 2018; O’Donnell et al., 2023) is explained by at least three independent recent HTT events into S. cerevisiae in Central/South America, at least one of which (into the French Guiana lineage) is also associated with HTT of another retrotransposon family (Ty1) and introgression of host genes from S. paradoxus (Peter et al., 2018; Bleykasten-Grosshans et al., 2021). We confirm that the Holarctic lineage of S. eubayanus contains Tsu4 elements that are most closely related to those in S. paradoxus and S. cerevisiae (Bergman, 2018) but conclude that this similarity is caused by an independent Tsu4 HTT into the Holarctic S. eubayanus lineage from an unidentified donor. Recurrent HTT of Tsu4 into different host species and lineages provides a mechanism to explain why this subfamily is more clade-rich than the Ty4 subfamily in the Ty4 family phylogeny.
Additionally, we investigate the putative Tsu4 HTT event reported for S. mikatae (Bergman, 2018) by generating new PacBio WGAs for two strains in this species (IFO 1815 and NBRC 10994), which revealed the presence of two active Ty4 family clades in S. mikatae. The first is a pure Tsu4 clade that clusters with FLEs from S. jurei, providing evidence for an ancestral Tsu4 HTT event prior to the divergence of S. mikatae and S. jurei that explains the discordance between Ty4 family and host species phylogenies for S. mikatae (Bergman, 2018). We also find a second recombinant clade in S. mikatae that shares similarity to the Ty4 and Tsu4 subfamilies in different parts of the internal region. The recombinant Clade 11 implies the co-existence of internal sequences for both subfamilies in the S. mikatae genome at some point in history. Likewise, we identify a novel clade in S. kudriavzevii that similarly exhibits recombination between the Ty4 and Tsu4 subfamilies, and explains the divergent position of S. kudriavzevii FLEs in the Ty4 family phylogeny (Bergman, 2018).
The discovery of novel Ty4 family clades in S. mikatae and S. kudriavzevii that were generated by recombination between resident (ancestral) and horizontally transferred (derived) retrotransposon subfamilies can be used to generalize prior results reported for the Ty1/Ty2 superfamily in S. cerevisiae (Jordan and McDonald, 1998; Czaja et al., 2020; Bleykasten-Grosshans et al., 2021). The canonical Ty1 subfamily found in S. cerevisiae evolved from an ancestral Ty1’ subfamily by independently acquiring segments from horizontally-transferred European S. paradoxus Ty1 and Ty2 elements by recombination (Jordan and McDonald, 1998; Czaja et al., 2020; Bleykasten-Grosshans et al., 2021). Similarly, the Ty101 subfamily evolved in S. cerevisiae through recombination between the ancestral Ty1’ subfamily and a horizontal transferred South American S. paradoxus Ty1 element (Bleykasten-Grosshans et al., 2021). Together these results suggest that recombination among divergent subfamilies that co-occur in the same species because of HTT may be a common mechanisms for the evolution of new of new retrotransposon lineages in Saccharomyces. In addition, Ty1c and Ty101 in S. cerevisiae and the recombinant Clade 11 in S. mikatae both retain a complete gag gene from the horizontally-transferred element. A truncated product from Ty1c gag has been shown to encode a copy-number dependent repressor of Ty1c transposition in S. cerevisiae and S. paradoxus (Saha et al., 2015; Cottee et al., 2021). Thus, determining the functional significance of recombinant elements with respect to fitness or transposition control mechanisms may be fruitful areas for future research.
Finally, the observation of multiple independent Tsu4 HTT events in both S. paradoxus and S. cerevisiae reinforces prior observations of parallel HTT events involving the Ty1 family in different lineages of S. cerevisiae (Czaja et al., 2020; Bleykasten-Grosshans et al., 2021). Together, these results suggest that parallel HTT events in different parts of a species range may be a common occurrence in Saccharomyces. If so, the number of HTT events in Saccharomyces species cannot be reliably inferred from simple presence/absence data within species, and reconstructing the complex history of HTT events will require high-resolution phylogenetic data from large samples of FLEs or strain-specific consensus sequences. Similarly, the observation of parallel HTT events on short timescales for multiple yeast TE families suggests that large-scale surveys that detect HTT among distantly related taxa may underestimate the frequency of HTT in eukaryotic genome evolution (Peccoud et al., 2017, 2018).
Materials and Methods
DNA preparation, PacBio sequencing and genome assembly of S. mikatae strains IFO 1815 and NBRC 10994
To prepare DNA for PacBio sequencing, single colonies of strain IFO 1815 (NCYC 2888) and NBRC 10994 was inoculated in 7 ml yeast extract-peptone-dextrose (YPD) liquid broth and cultured for ~24 hours at 30°C. DNA was isolated using the Wizard genomic DNA purification kit (Promega), and a PacBio library was prepared using the SMRTbell Express template prep kit (Pacific Biosciences), following the >15-kb size-selection protocol that includes Covaris g-TUBE shearing. PacBio sequencing was performed using the Sequel II instrument (sequencing kit v2.1). Whole genome assemblies were generated by performing Flye (v2.9) (Kolmogorov et al., 2019) with parameters “–pacbio-raw -g 12m.” Raw PacBio reads and genome assemblies of both S. mikatae strains have been submitted to NCBI under BioProject PRJNA934353.
Genome sequence datasets
We compiled public short-read paired-end WGS datasets of multiple Saccharomyces species to generate intraspecific phylogenies and survey Ty4/Tsu4 subfamily content within species. WGS datasets for each Saccharomyces species were cleaned and normalized using the following steps: (i) raw reads with identical BioSample accession, strain name and sequencing strategy (i.e., sequencing instrument and layout) were merged according to the metatable provided by NCBI SRA; (ii) for the NCBI BioSample accessions that have multiple records after merging, only the record with the highest sequencing depth was retained; and (iii) all samples with sequencing depth <10× were removed. After these quality control processes, the integrated WGS dataset used in this study includes 2,404 S. cerevisiae strains (Skelly et al., 2013; Bergstrom et al., 2014; Almeida et al., 2015; Marsit et al., 2015; Song et al., 2015; Strope et al., 2015; Barbosa et al., 2016; Drozdova et al., 2016; Gallone et al., 2016; Gayevskiy et al., 2016; Goncalves et al., 2016; Zhu et al., 2016; Coi et al., 2017; Istace et al., 2017; Kita et al., 2017; Maclean et al., 2017; Yue et al., 2017; Barbosa et al., 2018; Duan et al., 2018; Legras et al., 2018; Peter et al., 2018; Fay et al., 2019; Kang et al., 2019; Ramazzotti et al., 2019; Basile et al., 2021; Bigey et al., 2021; Han et al., 2021), 370 S. paradoxus strains (Bergstrom et al., 2014; Leducq et al., 2016; Xia et al., 2017; Eberlein et al., 2019; Koufopanou et al., 2020; He et al., 2022; Peris et al., 2023), 18 S. mikatae strains (Peris et al., 2023), 62 S. uvarum strains (Scannell et al., 2011; Almeida et al., 2014; Macias et al., 2021; Peris et al., 2023), and 292 S. eubayanus strains (Peris et al., 2016; Brouwers et al., 2019; Salazar et al., 2019; Langdon et al., 2020; Nespolo et al., 2020; Bergin et al., 2022; Mardones et al., 2022; Molinet et al., 2022; Peris et al., 2023).
We complied public high-quality WGAs of Saccharomyces species to identify Ty4/Tsu4 copies and extract FLEs for phylogenetic analysis. These WGAs were generated mostly with long-read sequencing data (PacBio or ONT), however we also included three WGAs generated with short-read sequencing data for S. cerevisiae that showed evidence of Tsu4 internal regions (strain 245 from (Marsit et al., 2015); strains AFQ and CDM from (Peter et al., 2018)) and two WGAs generated with short-read sequencing data for S. arboricola (strain H-6 from (Liti et al., 2013) and strain ZP960 from (Peris et al., 2023)) that represent the best available WGAs for this species. In total, we analyzed 183 S. cerevisiae WGAs (Marsit et al., 2015; Yue et al., 2017; Peter et al., 2018; O’Donnell et al., 2023), 12 S. paradoxus WGAs (Yue et al., 2017; Eberlein et al., 2019; Chen et al., 2022b), two S. mikatae WGAs (this study), two S. jurei WGAs (Naseeb et al., 2018), three S. kudriavzevii WGAs (Boonekamp et al., 2018; Salzberg et al., 2022), two S. arboricola WGAs (Liti et al., 2013; Peris et al., 2023), two S. uvarum WGAs (Chen et al., 2022a; Salzberg et al., 2022), and four S. eubayanus WGAs (Brickwedde et al., 2018; Brouwers et al., 2019; Mardones et al., 2022).
Ty4/Tsu4 copy number estimates
Copy number of LTRs and internal regions for the Ty4 and Tsu4 subfamilies were estimated by the coverage module of McClintock 2 (Chen et al., 2023) using public short-read WGS datasets compiled above. For this analysis, we used McClintock revision 7aa5298 with parameters “--keep_intermediate minimal,coverage -m coverage”. Reference genomes used for WGS based copy number were as follows: S. cerevisiae laboratory strain S288c (UCSC version sacCer2); S. paradoxus European strain CBS432 (GCA_002079055.1) (Yue et al., 2017); S. uvarum European strain CBS 7001 (GCA_019953615.1) (Chen et al., 2022a); S. eubayanus Patagonia strain FM1318 (GCA_001298625.1) (Baker et al., 2015). The Ty query library used for this analysis is the same as in Czaja et al. (2020). The edge-trimming option in the McClintock coverage module was disabled by specifying “omit_edges” as “False” in the configuration file “config/coverage/coverage.py”. To reduce the influence of variable coverage and computing resources, samples with original fold-coverage greater than 100× were down-sampled to 100× using seqtk (v1.3) (https://github.com/lh3/seqtk).
Phylogenetic analysis of host species
For each species, multi-sample variant calling was performed with BCFtools (v1.16, “bcftools mpileup -a 'FORMAT/DP' -Q 20 -q 20”; “bcftools call -f GQ,GP -mv – skip-variants indels”) (Li, 2011) using BAM files generated by McClintock 2 (Chen et al., 2023). Alignments with mapping quality less than 20, or bases with quality score less than 20, were removed. All indels were excluded from variant calling. Subsequently, the SNP matrix was filtered with BCFtools filter (v1.16) to discard sites with polymorphic probabilities under 99%; or genotypes with average supporting read depth less than 10×. Vcf2phylip (revision 0eb1b80) (https://github.com/edgardomortiz/vcf2phylip/tree/v2.0) was executed to create multi-sequence alignments from the filtered VCF file. For all species other than S. cerevisiae, maximum likelihood (ML) phylogenetic analysis was performed with RAxML (v8.2.12, “-f a -x 23333 -p 2333 –no-bfgs”) (Stamatakis, 2014) applying GTRGAMMA model and 100 times of bootstrap resampling. The host species trees were mid-point rooted and visualized in R (v4.2.3) using packages phytools (v1.5_1) (Revell, 2012) and ggtree (v3.6.0) (Yu et al., 2017), respectively. For S. cerevisiae, we followed the workflow in Peter et al. (2018): “snpgdsVCF2GDS” and “snpgdsDiss” from package SNPRelate (v1.32.0) (Zheng et al., 2012) were used to create the distance matrix from SNP data, and then function “bionj” from ape (v5.7_1) (Paradis et al., 2004) was used to reconstruct the neighbor joining (NJ) tree.
Annotation and sequence analysis of full-length elements from whole genome assemblies
Ty elements were annotated in WGAs using a RepeatMasker-based pipeline previously described in Czaja et al. (2020) updated to use RepeatMasker v4.0.9. Three S. cerevisiae Ty4 elements with secondary FLE insertions from other Ty families were excluded from the final dataset to prevent multi-sequence alignment artifacts. De novo LTR element prediction was performed using LTRharvest (“-seed 100 -minlenltr 100 -maxlenltr 1000 -mindistltr 1500 -maxdistltr 15000 -similar 80.0 -xdrop 5 -mat 2 -mis -2 -ins -3 -del -3 -mintsd 5 -maxtsd 5 -motif tgca -motifmis 0 -vic 60 -overlaps best”) followed by LTRdigest (PFAM models: PF00078, PF00665, PF01021, PF03732, PF07727, PF12384, PF13976) in GenomeTools 1.6.1 (Ellinghaus et al., 2008; Steinbiss et al., 2009; Gremme et al., 2013; Mistry et al., 2021). Multi-sequence alignments of annotated FLEs were generated using MAFFT (v7.508) with default parameters (Katoh and Standley, 2013). Sub-regions of FLEs in alignments were identified by aligning the “Tsu4p_nw” sequence from a public database of annotated canonical yeast transposons (https://github.com/bergmanlab/yeast-transposons) with the FLE dataset, then selecting sub-regions with seqkit subseq (v0.16.1) (Shen et al., 2016). ML phylogenetic trees were reconstructed using RAxML (v8.2.12, “-f a -x 23333 -p 2333 –no-bfgs”) (Stamatakis, 2014) with GTRGAMMA model and 100 bootstrap replicates. Phylogenetic network analysis was performed with SplitsTree4 (v4.15.1) (Huson and Bryant, 2006) applying the “Uncorrect_P” model and “NeighborNet” method. Pairwise sequence divergence was calculated based on Kimura’s 2-parameter substitution model with 50-bp sliding window size and 10-bp step size with R package spider (GitHub revision e93c5b4) (Brown et al., 2012) and phangorn (v2.11.1) (Schliep, 2011) in R (v4.2.3).
Phylogenetic analysis of Tsu4 strain-specific consensus sequences
Strain-specific consensus sequences were generated with BCFtools (v1.16, “bcftools mpileup -a 'FORMAT/DP' -Q 20 -q 20”; “bcftools call -f GQ,GP -mv–skip-variants indels; bcftools consensus”) (Li, 2011) using BAM files previously generated by McClintock 2 (Chen et al., 2023). The percentage of bases supported by mapped reads (i.e., breadth) was calculated with BEDtools (v2.30.0, “bedtools genomecov -d -split”) (Quinlan and Hall, 2010). To avoid generating consensus sequences that are biased towards the Tsu4 reference sequence, samples with normalized Tsu4 depth less than 0.75 (estimated by McClintock coverage module) or breadth less than 0.9 (estimated by BEDtools genomecov) were removed from consensus sequence analysis. A multi-sequence alignment of strain-specific consensus sequences was generated using MAFFT (v7.508) (Katoh and Standley, 2013) with default parameters. ML phylogenetic analysis was performed using RAxML (v8.2.12, “-f a -x 23333 -p 2333 –no-bfgs”) applying GTRGAMMA model and 100 bootstrap replicates (Stamatakis, 2014). The ML tree was mid-point rooted using R package phytools (v1.2_0) (Revell, 2012) and then visualized using ggtree (v3.6.0) (Yu et al., 2017).
Supplementary Material
Acknowledgments
We thank members of the Bergman and Garfinkel Labs for helpful discussion and comments during the project; the University of Georgia Genomics and Bioinformatics Core Facility (RRID:SCR_010994) for assistance with DNA extraction, PacBio library preparation and sequencing; and the Georgia Advanced Computing Resource Center for technical supporting and computational resources. This work was funded by the University of Georgia Research Foundation (CMB) and NIH grant R01GM124216 (DJG and CMB).
Footnotes
Conflicts of interest
N.A.
References
- Almeida P., Barbosa R., Zalar P., Imanishi Y., Shimizu K., Turchetti B., Legras J.-L., Serra M., Dequin S., Couloux A., Guy J., Bensasson D., Goncalves P., and Sampaio J. P., 2015. A population genomics insight into the Mediterranean origins of wine yeast domestication. Mol Ecol 24: 5412–5427. [DOI] [PubMed] [Google Scholar]
- Almeida P., Goncalves C., Teixeira S., Libkind D., Bontrager M., Masneuf-Pomarede I., Albertin W., Durrens P., Sherman D. J., Marullo P., Todd Hittinger C., Goncalves P., and Sampaio J. P., 2014. A Gondwanan imprint on global diversity and domestication of wine and cider yeast Saccharomyces uvarum. Nat Commun 5: 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyotte S. G., Tan X., Pennerman K., del Mar Jimenez-Gasco M., Klosterman S. J., Ma L.-J., Dobinson K. F., and Veronese P., 2012. Transposable elements in phytopathogenic Verticillium spp.: insights into genome evolution and inter- and intra-specific diversification. BMC Genomics 13: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker E., Wang B., Bellora N., Peris D., Hulfachor A. B., Koshalek J. A., Adams M., Libkind D., and Hittinger C. T., 2015. The genome sequence of Saccharomyces eubayanus and the domestication of lager-brewing yeasts. Mol Biol Evol 32: 2818–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa R., Almeida P., Safar S. V. B., Santos R. O., Morais P. B., Nielly-Thibault L., Leducq J.-B., Landry C. R., Goncalves P., Rosa C. A., and Sampaio J. P., 2016. Evidence of natural hybridization in Brazilian wild lineages of Saccharomyces cerevisiae. Genome Biol Evol 8: 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa R., Pontes A., Santos R. O., Montandon G. G., de Ponzzes-Gomes C. M., Morais P. B., Gonçalves P., Rosa C. A., and Sampaio J. P., 2018. Multiple rounds of artificial selection promote microbe secondary domestication—the case of cachaça yeasts. Genome Biol Evol 10: 1939–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile A., De Pascale F., Bianca F., Rossi A., Frizzarin M., De Bernardini N., Bosaro M., Baldisseri A., Antoniali P., Lopreiato R., Treu L., and Campanaro S., 2021. Large-scale sequencing and comparative analysis of oenological Saccharomyces cerevisiae strains supported by nanopore refinement of key genomes. Food Microbiol 97: 103753. [DOI] [PubMed] [Google Scholar]
- Bergin S. A., Allen S., Hession C., Ó Cinnéide E., Ryan A., Byrne K. P., Ó Cróinín T., Wolfe K. H., and Butler G., 2022. Identification of European isolates of the lager yeast parent Saccharomyces eubayanus. FEMS Yeast Research 22: foac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman C. M., 2018. Horizontal transfer and proliferation of Tsu4 in Saccharomyces paradoxus. Mob DNA 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom A., Simpson J. T., Salinas F., Barre B., Parts L., Zia A., Ba N., N A., Moses A. M., Louis E. J., Mustonen V., Warringer J., Durbin R., and Liti G., 2014. A high-definition view of functional genetic variation from natural yeast genomes. Mol Biol Evol 31: 872–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigey F., Segond D., Friedrich A., Guezenec S., Bourgais A., Huyghe L., Agier N., Nidelet T., and Sicard D., 2021. Evidence for two main domestication trajectories in Saccharomyces cerevisiae linked to distinct bread-making processes. Curr Biol 31: 722–732.e5. [DOI] [PubMed] [Google Scholar]
- Bing J., Han P.-J., Liu W.-Q., Wang Q.-M., and Bai F.-Y., 2014. Evidence for a Far East Asian origin of lager beer yeast. Curr Biol 24: R380–381. [DOI] [PubMed] [Google Scholar]
- Bleykasten-Grosshans C., Fabrizio R., Friedrich A., and Schacherer J., 2021. Species-wide transposable element repertoires retrace the evolutionary history of the Saccharomyces cerevisiae host. Mol Biol Evol 38: 4334–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonekamp F. J., Dashko S., van den Broek M., Gehrmann T., Daran J.-M., and Daran-Lapujade P., 2018. The genetic makeup and expression of the glycolytic and fermentative pathways are highly conserved within the Saccharomyces genus. Front Genet 9: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borneman A. R. and Pretorius I. S., 2015. Genomic insights into the Saccharomyces sensu stricto complex. Genetics 199: 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickwedde A., Brouwers N., van den Broek M., Gallego Murillo J. S., Fraiture J. L., Pronk J. T., and Daran J.-M. G., 2018. Structural, physiological and regulatory analysis of maltose transporter genes in Saccharomyces eubayanus CBS 12357T. Front Microbiol 9: 1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers N., Brickwedde A., Gorter de Vries A. R., van den Broek M., Weening S. M., van den Eijnden L., Diderich J. A., Bai F.-Y., Pronk J. T., and Daran J.-M. G., 2019. Himalayan Saccharomyces eubayanus genome sequences reveal genetic markers explaining heterotic maltotriose consumption by Saccharomyces pastorianus hybrids. Appl Environ Microbiol 85: e01516–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. D. J., Collins R. A., Boyer S., Lefort M.-C., Malumbres-Olarte J., Vink C. J., and Cruickshank R. H., 2012. Spider: An R package for the analysis of species identity and evolution, with particular reference to DNA barcoding. Mol Ecol Resour 12: 562–565. [DOI] [PubMed] [Google Scholar]
- Carr M., Bensasson D., and Bergman C. M., 2012. Evolutionary genomics of transposable elements in Saccharomyces cerevisiae. PLOS ONE 7: e50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Basting P. J., Han S., Garfinkel D. J., and Bergman C. M., 2023. Reproducible evaluation of transposable element detectors with McClintock 2 guides accurate inference of Ty insertion patterns in yeast. Mob DNA 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Garfinkel D. J., and Bergman C. M., 2022a. Long-read genome assembly of Saccharomyces uvarum strain CBS 7001. Microbiol Resour Announc 11: e00972–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., McQueary H., Hall D. W., Philippsen P., Garfinkel D. J., and Bergman C. M., 2022b. Genome assembly of the Ty1-less Saccharomyces paradoxus strain DG1768. Microbiol Resour Announc 11: e0086821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliften P., Sudarsanam P., Desikan A., Fulton L., Fulton B., Majors J., Waterston R., Cohen B. A., and Johnston M., 2003. Finding functional features in Saccharomyces genomes by phylogenetic footprinting. Science 301: 71–76. [DOI] [PubMed] [Google Scholar]
- Coi A. L., Bigey F., Mallet S., Marsit S., Zara G., Gladieux P., Galeote V., Budroni M., Dequin S., and Legras J. L., 2017. Genomic signatures of adaptation to wine biological ageing conditions in biofilm-forming flor yeasts. Mol Ecol 26: 2150–2166. [DOI] [PubMed] [Google Scholar]
- Cottee M. A., Beckwith S. L., Letham S. C., Kim S. J., Young G. R., Stoye J. P., Garfinkel D. J., and Taylor I. A., 2021. Structure of a Ty1 restriction factor reveals the molecular basis of transposition copy number control. Nat Commun 12: 5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja W., Bensasson D., Ahn H. W., Garfinkel D. J., and Bergman C. M., 2020. Evolution of Ty1 copy number control in yeast by horizontal transfer and recombination. PLOS Genetics 16: e1008632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daboussi M.-J., Daviere J.-M., Graziani S., and Langin T., 2002. Evolution of the Fot1 transposons in the genus Fusarium: discontinuous distribution and epigenetic inactivation. Mol Biol Evol 19: 510–520. [DOI] [PubMed] [Google Scholar]
- Daniels S. B., Peterson K. R., Strausbaugh L. D., Kidwell M. G., and Chovnick A., 1990. Evidence for horizontal transmission of the P transposable element between Drosophila species. Genetics 124: 339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobinson K. F., Harris R. E., and Hamer J. E., 1993. Grasshopper, a long terminal repeat (LTR) retroelement in the phytopathogenic fungus Magnaporthe grisea. Mol Plant Microbe Interact 6: 114–126. [DOI] [PubMed] [Google Scholar]
- Dotto B. R., Carvalho E. L., Silva A. F., Silva D., Fernando L., Pinto P. M., Ortiz M. F., and Wallau G. L., 2015. HTT-DB: Horizontally transferred transposable elements database. Bioinformatics 31: 2915–2917. [DOI] [PubMed] [Google Scholar]
- Drozdova P. B., Tarasov O. V., Matveenko A. G., Radchenko E. A., Sopova J. V., Polev D. E., Inge-Vechtomov S. G., and Dobrynin P. V., 2016. Genome sequencing and comparative analysis of Saccharomyces cerevisiae strains of the Peterhof genetic collection. PLOS ONE 11: e0154722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.-F., Han P.-J., Wang Q.-M., Liu W.-Q., Shi J.-Y., Li K., Zhang X.-L., and Bai F.-Y., 2018. The origin and adaptive evolution of domesticated populations of yeast from Far East Asia. Nat Commun 9: 2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlein C., Henault M., Fijarczyk A., Charron G., Bouvier M., Kohn L. M., Anderson J. B., and Landry C. R., 2019. Hybridization is a recurrent evolutionary stimulus in wild yeast speciation. Nat Commun 10: 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinghaus D., Kurtz S., and Willhoeft U., 2008. LTRharvest, an efficient and flexible software for de novo detection of LTR retrotransposons. BMC Bioinformatics 9: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J. and Fink G. R., 1980. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature 286: 352–356. [DOI] [PubMed] [Google Scholar]
- Fay J. C., Liu P., Ong G. T., Dunham M. J., Cromie G. A., Jeffery E. W., Ludlow C. L., and Dudley A. M., 2019. A polyploid admixed origin of beer yeasts derived from European and Asian wine populations. PLOS Biology 17: e3000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallone B., Steensels J., Prahl T., Soriaga L., Saels V., Herrera-Malaver B., Merlevede A., Roncoroni M., Voordeckers K., Miraglia L., Teiling C., Steffy B., Taylor M., Schwartz A., Richardson T., White C., Baele G., Maere S., and Verstrepen K., 2016. Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell 166: 1397–1410.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayevskiy V., Lee S., and Goddard M. R., 2016. European derived Saccharomyces cerevisiae colonisation of New Zealand vineyards aided by humans. FEMS Yeast Res 16: fow091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. and Feschotte C., 2018. Horizontal acquisition of transposable elements and viral sequences: patterns and consequences. Curr Opin Genetics Dev 49: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves M., Pontes A., Almeida P., Barbosa R., Serra M., Libkind D., Hutzler M., Goncalves P., and Sampaio J., 2016. Distinct domestication trajectories in top-fermenting beer yeasts and wine yeasts. Curr Biol 26: 2750–2761. [DOI] [PubMed] [Google Scholar]
- Gremme G., Steinbiss S., and Kurtz S., 2013. GenomeTools: a comprehensive software library for efficient processing of structured genome annotations. IEEE/ACM Transactions on Computational Biology and Bioinformatics 10: 645–656. [DOI] [PubMed] [Google Scholar]
- Han D.-Y., Han P.-J., Rumbold K., Koricha A. D., Duan S.-F., Song L., Shi J.-Y., Li K., Wang Q.-M., and Bai F.-Y., 2021. Adaptive gene content and allele distribution variations in the wild and domesticated populations of Saccharomyces cerevisiae. Front Microbiol 12: 631250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P.-Y., Shao X.-Q., Duan S.-F., Han D.-Y., Li K., Shi J.-Y., Zhang R.-P., Han P.-J., Wang Q.-M., and Bai F.-Y., 2022. Highly diverged lineages of Saccharomyces paradoxus in temperate to subtropical climate zones in China. Yeast 39: 69–82. [DOI] [PubMed] [Google Scholar]
- Henault M., Eberlein C., Charron G., Durand E., Nielly-Thibault L., Martin H., and Landry C. R., 2019. Yeast population genomics goes wild: The case of Saccharomyces paradoxus. In Population Genomics: Microorganisms, edited by Polz M. F. and Rajora O. P., pp. 207–230, Springer International Publishing, Cham. [Google Scholar]
- Henault M., Marsit S., Charron G., and Landry C. R., 2020. The effect of hybridization on transposable element accumulation in an undomesticated fungal species. eLife 9: e60474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D. H. and Bryant D., 2006. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23: 254–267. [DOI] [PubMed] [Google Scholar]
- Hyma K. E. and Fay J. C., 2013. Mixing of vineyard and oak-tree ecotypes of Saccharomyces cerevisiae in North American vineyards. Mol Ecol 22: 2917–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istace B., Friedrich A., d’Agata L., Faye S., Payen E., Beluche O., Caradec C., Davidas S., Cruaud C., Liti G., Lemainque A., Engelen S., Wincker P., Schacherer J., and Aury J.-M., 2017. De novo assembly and population genomic survey of natural yeast isolates with the Oxford Nanopore MinION sequencer. Gigascience 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetzky B. and Lehle L., 1992. Ty4, a new retrotransposon from Saccharomyces cerevisiae, flanked by tau-elements. J Biol Chem 267: 19798–19805. [PubMed] [Google Scholar]
- Jordan I. K. and McDonald J. F., 1998. Evidence for the role of recombination in the regulatory evolution of Saccharomyces cerevisiae Ty elements. Journal of Molecular Evolution 47: 14–20. [DOI] [PubMed] [Google Scholar]
- Kang K., Bergdahl B., Machado D., Dato L., Han T.-L., Li J., Villas-Boas S., Herrgard M. J., Forster J., and Panagiotou G., 2019. Linking genetic, metabolic, and phenotypic diversity among Saccharomyces cerevisiae strains using multiomics associations. GigaScience 8: giz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K. and Standley D.M., 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita R., Venkataram S., Zhou Y., and Fraser H. B., 2017. High-resolution mapping of cis-regulatory variation in budding yeast. Proc Natl Acad Sci USA 114: E10736–E10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmogorov M., Yuan J., Lin Y., and Pevzner P. A., 2019. Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 37: 540–546, Number: 5 Publisher: Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- Koufopanou V., Hughes J., Bell G., and Burt A., 2006. The spatial scale of genetic differentiation in a model organism: The wild yeast Saccharomyces paradoxus. Philos Trans R Soc Lond B Biol Sci 361: 1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufopanou V., Lomas S., Pronina O., Almeida P., Sampaio J. P., Mousseau T., Liti G., and Burt A., 2020. Population size, sex and purifying selection: comparative genomics of two sister taxa of the wild yeast saccharomyces paradoxus. Genome Biol Evol 12: 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehne H. A., Murphy H. A., Francis C., and Sniegowski P. D., 2007. Allopatric divergence, secondary contact, and genetic isolation in wild yeast populations. Curr Biol 17: 407–411. [DOI] [PubMed] [Google Scholar]
- Langdon Q. K., Peris D., Eizaguirre J. I., Opulente D. A., Buh K. V., Sylvester K., Jarzyna M., Rodríguez M. E., Lopes C. A., Libkind D., and Hittinger C. T., 2020. Postglacial migration shaped the genomic diversity and global distribution of the wild ancestor of lager-brewing hybrids. PLOS Genetics 16: e1008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leducq J.-B., Charron G., Samani P., Dubé A. K., Sylvester K., James B., Almeida P., Sampaio J. P., Hittinger C. T., Bell G., and Landry C. R., 2014. Local climatic adaptation in a widespread microorganism. Proceedings of the Royal Society B: Biological Sciences 281: 20132472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leducq J.-B., Nielly-Thibault L., Charron G., Eberlein C., Verta J.-P., Samani P., Sylvester K., Hittinger C. T., Bell G., and Landry C. R., 2016. Speciation driven by hybridization and chromosomal plasticity in a wild yeast. Nature Microbiology 1: 15003. [DOI] [PubMed] [Google Scholar]
- Legras J.-L., Galeote V., Bigey F., Camarasa C., Marsit S., Nidelet T., Sanchez I., Couloux A., Guy J., Franco-Duarte R., Marcet-Houben M., Gabaldon T., Schuller D., Sampaio J. P., Dequin S., and Wittkopp P., 2018. Adaptation of S. cerevisiae to fermented food environments reveals remarkable genome plasticity and the footprints of domestication. Mol Biol Evol 35: 1712–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., 2011. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27: 2987–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libkind D., Hittinger C. T., Valerio E., Goncalves C., Dover J., Johnston M., Goncalves P., and Sampaio J. P., 2011. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc Natl Acad Sci USA 108: 14539–14544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G., Ba A. N. N., Blythe M., Müller C. A., Bergström A., Cubillos F. A., Dafhnis-Calas F., Khoshraftar S., Malla S., Mehta N., Siow C. C., Warringer J., Moses A. M., Louis E. J., and Nieduszynski C. A., 2013. High quality de novo sequencing and assembly of the Saccharomyces arboricolus genome. BMC Genomics 14: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G., Barton D. B. H., and Louis E. J., 2006. Sequence diversity, reproductive isolation and species concepts in Saccharomyces. Genetics 174: 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G., Carter D. M., Moses A. M., Warringer J., Parts L., James S. A., Davey R. P., Roberts I. N., Burt A., Koufopanou V., Tsai I. J., Bergman C. M., Bensasson D., O’Kelly M. J. T., Oudenaarden A. v., Barton D. B. H., Bailes E., Nguyen A. N., Jones M., Quail M. A., Goodhead I., Sims S., Smith F., Blomberg A., Durbin R., and Louis E. J., 2009. Population genomics of domestic and wild yeasts. Nature 458: 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G., Peruffo A., James S. A., Roberts I. N., and Louis E. J., 2005. Inferences of evolutionary relationships from a population survey of LTR-retrotransposons and telomeric-associated sequences in the Saccharomyces sensu stricto complex. Yeast 22: 177–92. [DOI] [PubMed] [Google Scholar]
- Macias L. G., Flores M. G., Adam A. C., Rodriguez M. E., Querol A., Barrio E., Lopes C. A., and Perez-Torrado R., 2021. Convergent adaptation of Saccharomyces uvarum to sulfite, an antimicrobial preservative widely used in human-driven fermentations. PLOS Genet 17: e1009872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean C. J., Metzger B. P. H., Yang J.-R., Ho W.-C., Moyers B., and Zhang J., 2017. Deciphering the genic basis of yeast fitness variation by simultaneous forward and reverse genetics. Mol Biol Evol 34: 2486–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardones W., Villarroel C. A., Abarca V., Urbina K., Peña T. A., Molinet J., Nespolo R. F., and Cubillos F. A., 2022. Rapid selection response to ethanol in Saccharomyces eubayanus emulates the domestication process under brewing conditions. Microb Biotechnol 15: 967–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsit S., Mena A., Bigey F., Sauvage F.-X., Couloux A., Guy J., Legras J.-L., Barrio E., Dequin S., and Galeote V., 2015. Evolutionary advantage conferred by an eukaryote-to-eukaryote gene transfer event in wine yeasts. Mol Biol Evol 32: 1695–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry J., Chuguransky S., Williams L., Qureshi M., Salazar G., Sonnhammer E. L. L., Tosatto S. C. E., Paladin L., Raj S., Richardson L. J., Finn R. D., and Bateman A., 2021. Pfam: The protein families database in 2021. Nucleic Acids Res 49: D412–D419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinet J., Urbina K., Villegas C., Abarca V., Oporto C. I., Villarreal P., Villarroel C. A., Salinas F., Nespolo R. F., and Cubillos F. A., 2022. A Saccharomyces eubayanus haploid resource for research studies. Sci Rep 12: 5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E. W., Sutton G. G., Delcher A. L., Dew I. M., Fasulo D. P., Flanigan M. J., Kravitz S. A., Mobarry C. M., Reinert K. H., Remington K. A., Anson E. L., Bolanos R. A., Chou H. H., Jordan C. M., Halpern A. L., Lonardi S., Beasley E. M., Brandon R. C., Chen L., Dunn P. J., Lai Z., Liang Y., Nusskern D. R., Zhan M., Zhang Q., Zheng X., Rubin G. M., Adams M. D., and Venter J. C., 2000. A whole-genome assembly of Drosophila. Science 287: 2196–204. [DOI] [PubMed] [Google Scholar]
- Naseeb S., Alsammar H., Burgis T., Donaldson I., Knyazev N., Knight C., and Delneri D., 2018. Whole genome sequencing, de novo assembly and phenotypic profiling for the new budding yeast species Saccharomyces jurei. G3 8: 2967–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov G. I., James S. A., Naumova E. S., Louis E. J., and Roberts I. N., 2000. Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii and Saccharomyces mikatae. Int J Syst Evol Microbiol 50: 1931–1942. [DOI] [PubMed] [Google Scholar]
- Naumov G. I., Naumova E. S., and Sniegowski P. D., 1997. Differentiation of European and Far East Asian populations of Saccharomyces paradoxus by allozyme analysis. Int J Syst Bacteriol 47: 341–344. [DOI] [PubMed] [Google Scholar]
- Nespolo R. F., Villarroel C. A., Oporto C. I., Tapia S. M., Vega-Macaya F., Urbina K., Chiara M. D., Mozzachiodi S., Mikhalev E., Thompson D., Larrondo L. F., Saenz-Agudelo P., Liti G., and Cubillos F. A., 2020. An Out-of-Patagonia migration explains the worldwide diversity and distribution of Saccharomyces eubayanus lineages. PLOS Genetics 16: e1008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuveglise C., Feldmann H., Bon E., Gaillardin C., and Casaregola S., 2002. Genomic evolution of the long terminal repeat retrotransposons in hemiascomycetous yeasts. Genome Res 12: 930–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova O., Fet V., and Blinov A., 2009. Non-LTR retrotransposons in fungi. Funct Integr Genomics 9: 27–42. [DOI] [PubMed] [Google Scholar]
- Okuno M., Kajitani R., Ryusui R., Morimoto H., Kodama Y., and Itoh T., 2016. Next-generation sequencing analysis of lager brewing yeast strains reveals the evolutionary history of interspecies hybridization. DNA Res 23: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell S., Yue J.-X., Saada O. A., Agier N., Caradec C., Cokelaer T., De Chiara M., Delmas S., Dutreux F., Fournier T., Friedrich A., Kornobis E., Li J., Miao Z., Tattini L., Schacherer J., Liti G., and Fischer G., 2023. Telomere-to-telomere assemblies of 142 strains characterize the genome structural landscape in Saccharomyces cerevisiae. Nat Genet 55: 1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E., Claude J., and Strimmer K., 2004. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Peccoud J., Cordaux R., and Gilbert C., 2018. Analyzing horizontal transfer of transposable elements on a large scale: challenges and prospects. BioEssays 40: 1700177. [DOI] [PubMed] [Google Scholar]
- Peccoud J., Loiseau V., Cordaux R., and Gilbert C., 2017. Massive horizontal transfer of transposable elements in insects. Proc Natl Acad Sci USA 114: 4721–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris D., Langdon Q. K., Moriarty R. V., Sylvester K., Bontrager M., Charron G., Leducq J.-B., Landry C. R., Libkind D., and Hittinger C. T., 2016. Complex ancestries of lager-brewing hybrids were shaped by standing variation in the wild yeast Saccharomyces eubayanus. PLOS Genet 12: e1006155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris D., Ubbelohde E. J., Kuang M. C., Kominek J., Langdon Q. K., Adams M., Koshalek J. A., Hulfachor A. B., Opulente D. A., Hall D. J., Hyma K., Fay J. C., Leducq J.-B., Charron G., Landry C. R., Libkind D., Gonçalves C., Gonçalves P., Sampaio J. P., Wang Q.-M., Bai F.-Y., Wrobel R. L., and Hittinger C. T., 2023. Macroevolutionary diversity of traits and genomes in the model yeast genus Saccharomyces. Nat Commun 14: 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J., Chiara M. D., Friedrich A., Yue J.-X., Pflieger D., Bergstrom A., Sigwalt A., Barre B., Freel K., Llored A., Cruaud C., Labadie K., Aury J.-M., Istace B., Lebrigand K., Barbry P., Engelen S., Lemainque A., Wincker P., Liti G., and Schacherer J., 2018. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature 556: 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan A. R. and Hall I. M., 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramazzotti M., Stefanini I., Paola M. D., Filippo C. D., Rizzetto L., Berná L., Dapporto L., Rivero D., Tocci N., Weil T., Lenucci M. S., Lionetti P., and Cavalieri D., 2019. Population genomics reveals evolution and variation of Saccharomyces cerevisiae in the human and insects gut. Environ Microbiol 21: 50–71. [DOI] [PubMed] [Google Scholar]
- Revell L. J., 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3: 217–223. [Google Scholar]
- Saha A., Mitchell J. A., Nishida Y., Hildreth J. E., Ariberre J. A., Gilbert W. V., and Garfinkel D. J., 2015. A trans-dominant form of Gag restricts Ty1 retro-transposition and mediates copy number control. J Virol 89: 3922–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar A. N., Gorter de Vries A. R., van den Broek M., Brouwers N., de la Torre Cortes P., Kuijpers N. G. A., Daran J.-M. G., and Abeel T., 2019. Chromosome level assembly and comparative genome analysis confirm lager-brewing yeasts originated from a single hybridization. BMC Genomics 20: 916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg L. I., Martos A. A. R., Lombardi L., Jermiin L. S., Blanco A., Byrne K. P., and Wolfe K. H., 2022. A widespread inversion polymorphism conserved among Saccharomyces species is caused by recurrent homogenization of a sporulation gene family. PLOS Genetics 18: e1010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarilar V., Bleykasten-Grosshans C., and Neuveglise C., 2015. Evolutionary dynamics of hAT DNA transposon families in Saccharomycetaceae. Genome Biol Evol 7: 172–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell D. R., Zill O. A., Rokas A., Payen C., Dunham M. J., Eisen M. B., Rine J., Johnston M., and Hittinger C. T., 2011. The awesome power of yeast evolutionary genetics: New genome sequences and strain resources for the Saccharomyces sensu stricto genus. G3 1: 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaack S., Gilbert C., and Feschotte C., 2010. Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol Evol 25: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliep K. P., 2011. phangorn: phylogenetic analysis in R. Bioinformatics 27: 592–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Le S., Li Y., and Hu F., 2016. SeqKit: A cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLOS ONE 11: e0163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly D. A., Merrihew G. E., Riffle M., Connelly C. F., Kerr E. O., Johansson M., Jaschob D., Graczyk B., Shulman N. J., Wakefield J., Cooper S. J., Fields S., Noble W. S., Muller E. G. D., Davis T. N., Dunham M. J., MacCoss M. J., and Akey J. M., 2013. Integrative phenomics reveals insight into the structure of phenotypic diversity in budding yeast. Genome Res 23: 1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G., Dickins B. J. A., Demeter J., Engel S., Gallagher J., Choe K., Dunn B., Snyder M., and Cherry J. M., 2015. AGAPE (Automated Genome Analysis PipelinE) for pan-genome analysis of Saccharomyces cerevisiae. PLOS ONE 10: e0120671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A., 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbiss S., Willhoeft U., Gremme G., and Kurtz S., 2009. Fine-grained annotation and classification of de novo predicted LTR retrotransposons. Nucleic Acids Res 37: 7002–7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strope P. K., Skelly D. A., Kozmin S. G., Mahadevan G., Stone E. A., Magwene P. M., Dietrich F. S., and McCusker J. H., 2015. The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res 25: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucka R., Lochmäller H., and Feldmann H., 1989. Ty4, a novel low-copy number element in Saccharomyces cerevisiae: one copy is located in a cluster of Ty elements and tRNA genes. Nucleic Acids Res 17: 4993–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucka R., Schwarzlose C., Lochmüller H., Häcker U., and Feldmann H., 1992. Molecular analysis of the yeast Ty4 element: homology with Ty1, copia, and plant retrotransposons. Gene 122: 119–128. [DOI] [PubMed] [Google Scholar]
- Wallau G. L., Ortiz M. F., and Loreto E. L. S., 2012. Horizontal transposon transfer in eukarya: detection, bias, and perspectives. Genome Biol Evol 4: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallau G. L., Vieira C., and Loreto E. L. S., 2018. Genetic exchange in eukaryotes through horizontal transfer: connected by the mobilome. Mob DNA 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.-M., Liu W.-Q., Liti G., Wang S.-A., and Bai F.-Y., 2012. Surprisingly diverged populations of Saccharomyces cerevisiae in natural environments remote from human activity. Mol Ecol 21: 5404–5417. [DOI] [PubMed] [Google Scholar]
- Wells J. N. and Feschotte C., 2020. A field guide to eukaryotic transposable elements. Annu Rev Genet 54: 539–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Nielly-Thibault L., Charron G., Landry C. R., Kasimer D., Anderson J. B., and Kohn L. M., 2017. Population genomics reveals structure at the individual, host-tree scale and persistence of genotypic variants of the undomesticated yeast Saccharomyces paradoxus in a natural woodland. Mol Ecol 26: 995–1007. [DOI] [PubMed] [Google Scholar]
- Yu G., Smith D. K., Zhu H., Guan Y., and Lam T. T.-Y., 2017. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8: 28–36. [Google Scholar]
- Yue J.-X., Li J., Aigrain L., Hallin J., Persson K., Oliver K., Bergstrom A., Coupland P., Warringer J., Lagomarsino M. C., Fischer G., Durbin R., and Liti G., 2017. Contrasting evolutionary genome dynamics between domesticated and wild yeasts. Nat Genet 49: 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Levine D., Shen J., Gogarten S. M., Laurie C., and Weir B. S., 2012. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 28: 3326–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. O., Sherlock G., and Petrov D. A., 2016. Whole genome analysis of 132 clinical Saccharomyces cerevisiae strains reveals extensive ploidy variation. G3 6: 2421–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.