Summary
In vertebrate sexual development, two important steroid hormones, testosterone and estrogen, regulate the sex-specific development of many tissues. In contrast, invertebrates utilize a single steroid hormone, ecdysone, to regulate developmental timing in both sexes. However, here we show that in Drosophila melanogaster, sex-specific ecdysone (E) signaling controls important aspects of gonad sexual dimorphism. Rather than being regulated at the level of hormone production, hormone activity is regulated cell-autonomously through sex-specific hormone reception. Ecdysone receptor (EcR) expression is restricted to the developing ovary and is repressed in the testis at a time when ecdysone initiates ovary morphogenesis. Interestingly, EcR expression is regulated downstream of the sex determination factor Doublesex (Dsx), the founding member of the Dsx/Mab3 Related Transcription Factor (DMRT) family that regulates gonad development in all animals. E signaling is required for normal ovary development1,2, and ectopic activation of E signaling in the testis antagonized stem cell niche identity and feminized somatic support cells, which were transformed into follicle-like cells. This work demonstrates that invertebrates can also use steroid hormone signaling to control sex-specific development. Further, it may help explain recent work showing that vertebrate sexual development is surprisingly cell-autonomous. For example, chickens utilize testosterone and estrogen to control sex-specific development, but when they have a mixture of cells with male and female genotypes, the male cells develop as male and the female cells develop as female despite exposure to the same circulating hormones3. Sex-specific regulation of steroid hormone signaling may well underly such cell-autonomous sexual fate choices in vertebrates as it does in Drosophila.
Sexual dimorphism is a hallmark feature of metazoans and encompasses all discernible differences between males and females of the same species. Sex-specific gonad development is instructed by members of the Doublesex/Mab-3 Related Transcription Factor (DMRT) family in all animals for which their role has been characterized, including flies, nematodes, chickens, mice, and humans4. DMRT1-mutant male mice initiate testis development but the gonad then becomes feminized5,6, whereas in rabbits, loss of DMRT1 causes earlier sex reversal with ovary formation in XY embryos7. Humans that are XY, but also heterozygous for mutations in DMRT1, exhibit a range of phenotypes, including complete gonadal dysgenesis with sex reversal. Interestingly, these patients also have persistent Mullerian Ducts indicating an early problem in testis development (defects in Sertoli cells that produce anti-Mullerian Hormone)8.
The DMRT family was founded by the discovery of doublesex (dsx) in flies9. Drosophila sex determination leads to alternative splicing of dsx RNA and the production of either male or female isoforms of Dsx protein (DsxM or DsxF), which share the same DNA binding domain but have opposite effects on target gene transcription. dsx controls all known aspects of sexual dimorphism in the somatic gonad, including important components of the germline stem cell niche (the “hub” in males and terminal filaments (TFs) and cap cells in females), and the somatic cells that support gametogenesis (cyst stem cells and cyst cells in males, and escort cells, follicle stem cells and follicle cells in females)(reviewed by10). The timing of gonad development is also sexually dimorphic, with the testis being formed by the end of embryogenesis (24 hrs after fertilization, AF), while ovary development occurs several days later in the mid-third larval instar (L3) stage (4 days AF).
In mammals, the steroid hormones testosterone and estrogen control many aspects of sex-specific development in a variety of tissues. In contrast, invertebrates like Drosophila utilize the same steroid hormone, ecdysone (E), in both sexes to regulate developmental timing and adult female reproduction11. The ecdysone receptor (EcR) is a nuclear hormone receptor, similar to the mammalian testosterone and estrogen receptors, that is activated by 20-hydroxyecdysone (20E). EcR-dependent transcriptional activation requires a number of co-factors that are also conserved in mammals, including the RXR ortholog Ultraspiracle (Usp) and the SRC-3 homolog Taiman (Tai). EcR target gene activation has been implicated in the establishment of the female gonad niche during development1,2 although its role in regulating sex-specific gonadogenesis has not been examined.
dsx regulates ecdysone signaling in the gonad
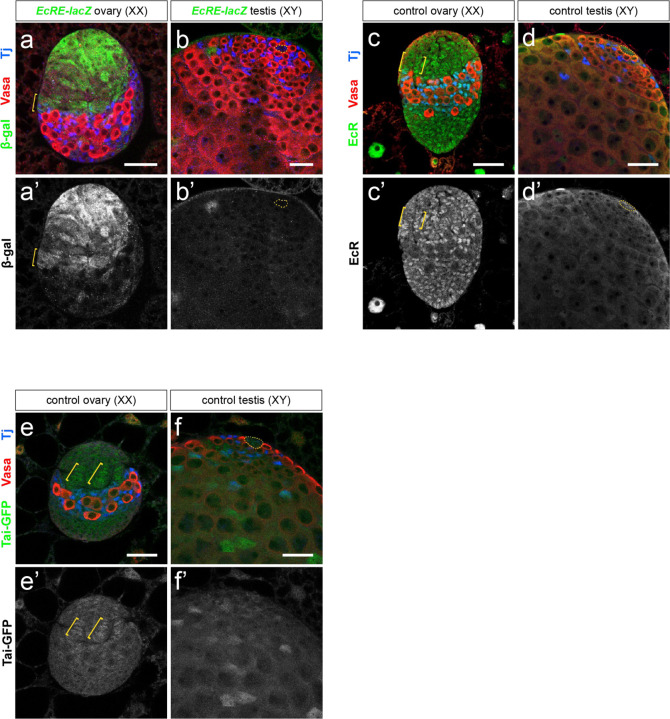
We examined transcriptional activation via EcR in developing gonads using a transgenic reporter containing EcR binding sites (EcRE-lacZ)12,13 and immunostaining for the EcR target Broad (Br)2. In late larval ovaries, EcRE-lacZ produced high levels of β-gal throughout the apical cap, including TF cells (Fig. 1a). Similarly, Br was expressed strongly in all somatic cells of the late larval ovary, including the developing TFs2 (Extended Data Fig. 1a). In the larval testis, however, little to no EcRE-lacZ activity or Br expression was detected, including in the testis niche (Fig. 1b outline, Extended Data Fig. 1b). However, Br was detected in the testis terminal epithelium cells (Extended Data Fig. 1c), indicating that the testis environment receives 20E and supports ecdysone signaling in the cells that can respond. The Z1 isoform of Br is the most ecdysone-responsive isoform in the developing ovary11 (Extended Data Fig. 1d), and Br-Z1 was consistently absent from the male stem cell niche (Extended Data Fig. 1e).
Figure 1. Ecdysone signaling activity is female-biased in the developing gonad.
(a-b) Beta-galactosidase expression (green) from a transgenic EcRE-lacZ25 reporter is higher in the ovarian somatic gonad (a) than in the testicular somatic gonad (b). (c-d) EcR protein expression is higher in the ovary (c) than in the testis (d). (e-f) Expression of Taiman-GFP from a genomic duplication is detected in several somatic cell types of an L3 ovary (e), including the apical cap, terminal filaments, and intermingled cells. In an L3 testis (d), Tai is detected at low levels in the hub and CySCs and at higher levels in differentiating cyst cells. Vasa (red) labels the germline, Traffic jam (Tj, blue) labels somatic cells. Scale bars = 25 μm. Yellow brackets indicate TF cells; yellow dotted outline indicates the hub.
In mammals, sex-biased hormone activity is regulated at the level of hormone production: females produce higher levels of estrogen, while males produce higher levels of testosterone. In contrast, we found that a female bias in hormone levels could not account for the observed dimorphism in ecdysone activity. Supplying exogenous 20E, or a membrane-permeable EcR agonist (chromafenozide), did not activate E signaling in testes but was able to induce premature EcR transcriptional activity in younger L3 ovaries (Extended Data Fig. 2a–f). Ecdysone is converted to its active form, 20E, by the P450 enzyme Shade27 (Shd). We found that depletion of shd from the ovary did not reproduce female niche defects that are seen upon blocking EcR activity2 (Extended Data Fig. 2g–h). We conclude that the female bias in E signaling is not due to sex-specific production or availability of 20E.
We previously conducted a collaborative genome-wide search for putative Dsx targets using genomic and computational approaches14. We identified numerous sequences across the EcR locus with high similarity to the Dsx consensus binding sequence, many of which were conserved in other Drosophila species and could be bound by Dsx based on DamID-seq14. We therefore explored whether sex differences in EcR expression might account for sexually dimorphic E signaling in the gonad. EcR was expressed at high levels in the female somatic gonad (Fig. 1c) and at very low levels in the larval testis (Fig. 1d). We also saw expression of EcR in the terminal epithelium of the testis (Extended Data Fig. 1h, k) which were the only cells of the testis that expressed the EcR target BrC (Extended Data Fig. 1c). An EcR co-factor, Taiman (Tai), that mediates ecdysone signaling in the adult ovary15 was expressed in the somatic gonad of both larval ovaries and testes (Fig. 1e–f).
To test whether EcR expression and activity are regulated downstream of dsx, we assessed EcR and Br levels in dsx-mutant gonads. We generated female animals heterozygous for the dsxD allele16, which cannot be spliced into the dsxF isoform. Thus, XX dsxD/+ animals express both DsxM and DsxF, which antagonize one another16. In the gonad, this leads to stochastic establishment of male or female niche structures (hubs or TFs)17. Importantly, all other aspects of the female sex determination pathway besides dsx are present in these animals, making this a good test of dsx function. We found that XX dsxD/+ gonads exhibited lower levels of EcR protein (Fig. 2a–d) and EcR mRNA (Extended Data Fig. 3a–c) compared with control ovaries. While Br-Z1 was predictably female-specific in control gonads (Fig. 2e–f), in dsxD/+ gonads Br-Z1 expression in the niche correlated with niche identity; dsxD/+ gonads with a hub expressed very low levels of Br-Z1 (Fig. 2g) while dsxD/+ gonads with TFs expressed higher levels of Br-Z1 (Fig. 2h), supporting the idea that high ecdysone signaling promotes female niche development.
Figure 2. EcR activity is regulated by Dsx in the developing somatic gonad.
(a-c) EcR protein levels are higher in an L3 ovary (a, green) than in an L3 testis (b). EcR expression is diminished in XX dsx-mutant gonads (dsxD/+, c-d). DAPI (blue) labels nuclei. (e-h) Br-Z1, an EcR target, is expressed in TFs of a control ovary (e) and is absent from hub cells in a control testis (f). Br-Z1 expression is high in an L3 XX dsxD/+ TF structure (g) and low in a hub structure (h). N-cad labels the gonad niche (TF or hub cells) (i-j) Expression of a 6.3-kb intronic EcR enhancer (EcR6kb-gfp) in larval gonads. Gonads shown in (i) and (j) contain two copies of enhancer construct (homozygous). EcR6kb-gfp is expressed throughout the somatic gonad of an LL3 ovary (h, arrowheads) and is expressed at very low levels in the hub (arrowhead) and CySCs of an L3 testis (j). (k-l) EcR6kb-gfp expression is greatly reduced in dsx-mutant (EcR6kb-gfp/+; dsxD/+) gonads (l) compared with control (EcR6kb-gfp/+; +) ovaries (k). Heterozygous expression of EcR6kb-gfp in a control testis is shown in (m). In k-m, DAPI (blue) labels nuclei and Tj (red) labels somatic cells. Endogenous GFP signal (without GFP antibody staining) is shown in green. In i-m, arrowheads indicate TF cells and arrows indicate the hub. Scale bars = 25 μm.
To evaluate whether dsx is a transcriptional regulator of EcR, we generated a transgenic reporter, EcR6kb-gfp, that expresses GFP under control of a 6.3-kb intronic enhancer element in the EcR locus and is expressed in the somatic gonad. EcR6kb-gfp was expressed in somatic cells of the larval ovary, while very low levels were observed in the larval testis (Fig. 2i, j). Interestingly, EcR6kb-gfp activity was lower in XX dsxD/+ gonads compared with control XX gonads (Fig. 2k–m; Extended Data Fig. 3d–f) indicating that the effect of dsx on EcR expression is transcriptional.
EcR antagonizes testis development
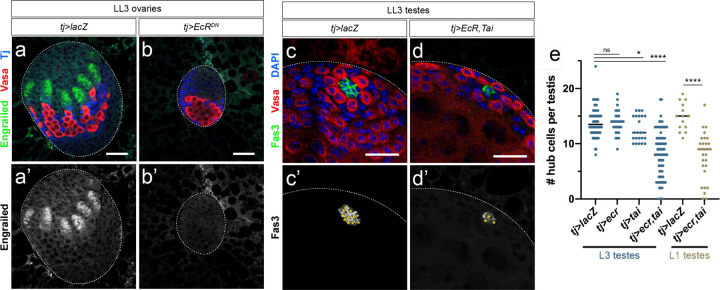
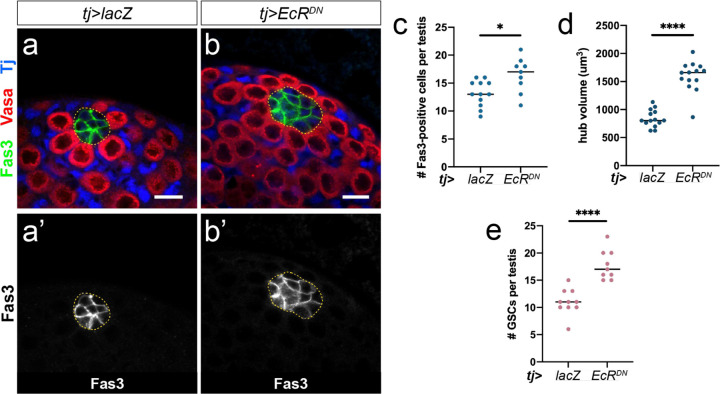
Since EcR activity was female-biased during gonad development, we investigated whether the requirement for EcR in gonad niche development is sexually dimorphic. EcR activation by 20E is required for development of the female gonad stem cell niche1,2. To investigate the role of EcR in gonad niche development, we blocked EcR activation in the somatic gonad by over-expressing a dominant-negative form of EcR-A18 (EcRDN) using the somatic gonad driver traffic jam (tj)-GAL4 (tj-GAL4; UAS-EcRDN, abbreviated as tj>EcRDN). EcR acts as a repressor in the absence of 20E19, which is required to prevent premature ovary development2, preventing the use of EcR null alleles for this experiment. However, expression of EcRDN specifically blocks the activator function of EcR18. As previously observed2, we found that EcRDN expression in the somatic gonad prevented the differentiation of female niche structures (TFs, Fig. 3a, b). In contrast, transcriptional activation via EcR was not required for establishment of the male gonad niche, as testes expressing EcRDN did not contain fewer hub cells than control testes. In fact, tj>EcRDN testes showed a slight increase in hub cell number and hub volume compared with controls (Extended Data Fig. 4).
Figure 3. EcR signaling is deleterious to male somatic gonad establishment.
(a) TFs are established in a control LL3 ovary (tj>lacZ). Engrailed (green) labels TF cells. (b) Blocking EcR activation in the somatic gonad (tj>EcRDN) prevents the establishment of terminal filaments in an L3 ovary. (c) In a control L3 testis, Fas3 (green) labels intact hub cells. (d) Activation of EcR by co-expressing EcR and Tai (tj>EcR,tai) in the male somatic gonad produces smaller hubs with visibly fewer cells. Fas3 (green) labels hub cells. (g) Quantification of hub cells per LL3 (blue dots) or L1 (yellow dots) testis in various genotypes. For statistical significance here and in subsequent figures, asterisks indicate statistical significance by Student’s t-test as follows: *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. Scale bars = 25 μm.
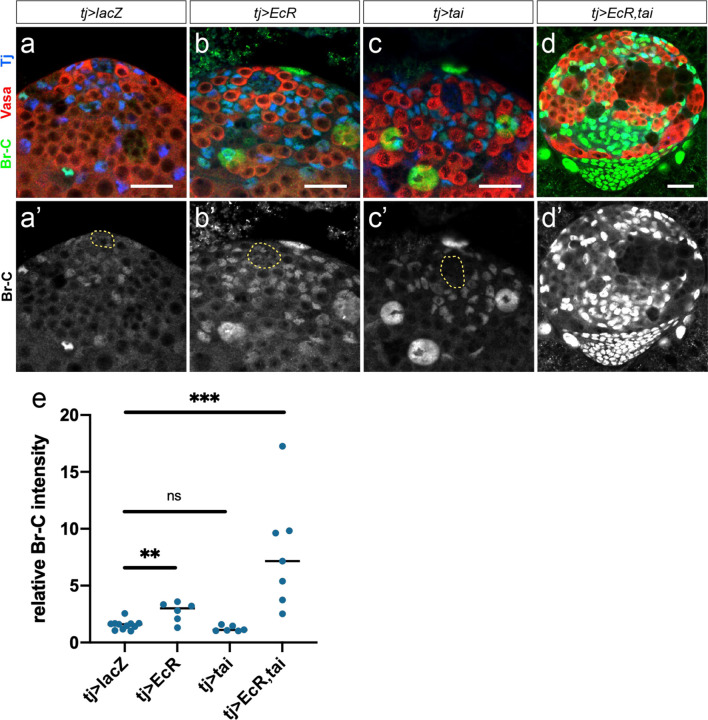
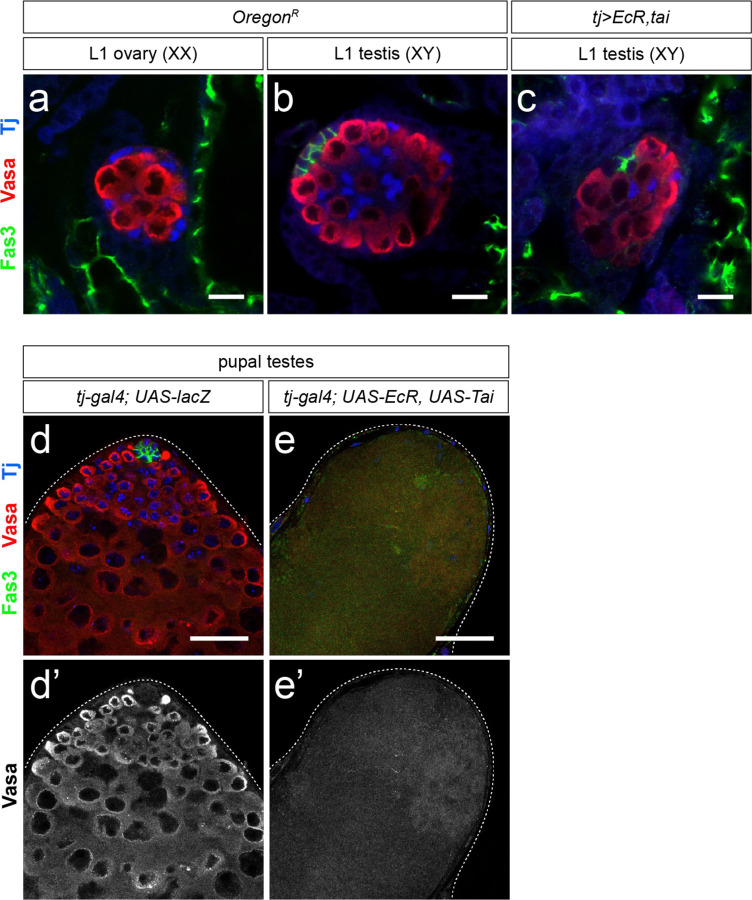
To test whether activating EcR in the developing testis is deleterious to male niche development, we first identified the rate-limiting components for E signaling in the larval testis. Over-expression of EcR alone was not sufficient to activate signaling, as determined by quantifying Br expression (Extended Data Fig. 5a–e). However, co-expression EcR along with its co-factor taiman (tj>EcR,tai) in the male somatic gonad led to a significant increase in Br expression (Extended Data Fig. 5a–e) indicating we could now ectopically activate E signaling in the testis. This led to a significant decrease in larval hub cell number compared with control testes (Fig. 3c–e) which was apparent as early as the L1 stage (24–48 hrs AF, Fig. 3e and Extended Data Fig. 6a–c). Hub cell loss continued beyond larval development, as some tj>EcR,tai pupal testes lacked hubs and, consistent with this, also lost the germline and somatic support cells dependent on the male niche (Extended Data Fig. 6d–e). While EcR activation caused a loss of male niche identity, we did not find a transformation to female niche identity, as occurs in loss of dsx function17. Expression of the female TF marker Engrailed increased in male somatic cells upon activation of E signaling (Extended Data Fig. 7) but no evidence of TF formation was observed.
EcR activity feminizes the adult testis
While activation of E signaling was not sufficient to sex-transform the gonad niche, we observed a striking male-to-female transformation of somatic support cells in the testis upon co-expression of EcR and Tai. Support cells in the adult testis include the cyst cells that surround each developing spermatogenic cyst and the cyst stem cells that produce them. In the ovary, somatic support cells include escort cells that nurture germ cells during the earliest stages of differentiation and epithelial follicle cells, made by follicle stem cells, that support maturing oocytes. The transcription factor Chronologically inappropriate morphogenesis (Chinmo) is normally expressed in hub cells and early cyst cells of L3 and adult testes, while in females it is expressed in germ cells but is absent from the somatic gonad at both stages20–22 (Fig. 4a, b and Extended Data Fig. 8a–b). Co-expression of EcR and Tai caused a loss of Chinmo expression in cyst cells but not hub cells (Fig. 4c). Interestingly, this was accompanied by a gain in expression of the follicle cell markers Fas3 and Castor (Fig. 4d–f), which also occurs upon Chinmo depletion from the testis21,22 (Fig. 4g). Repression of chinmo and activation of br by ecdysone signaling has also been observed in developing neuroblasts and in larval wing imaginal discs23,24 and has been proposed to regulate many aspects of development25. To investigate further the mutually repressive relationship between EcR and chinmo in the somatic gonad, we examined whether transcriptional activation via EcR was required for the male-to-female transformation observed upon loss of chinmo. Indeed, interfering with EcR activation by expressing EcRDN blocked the follicle cell-like transformation observed with loss of chinmo (Fig. 4g–h). Follicle-like cells were present in 92.3% of tj>chinmoRNAi testes, but they appeared in only 7.3% of tj>EcRDN; >chinmoRNAi testes (Fig. 4i). In addition, depletion of chinmo in the testis led to induction of the EcR target Br in the transformed follicle-like cells (Fig. 4j–l). These data are consistent with a model where EcR/Br and chinmo exhibit mutually repressive interactions in the somatic gonad.
Figure 4. EcR activation in the testis causes adult somatic sex transformation.
(a-c) Chinmo expression in adult gonads and with EcR activation in the testis. In an adult ovary (a), Chinmo protein (green) is absent from the female somatic gonad (arrowheads). Chinmo is expressed in early female germ cells as previously observed3. (b) In an adult testis, Chinmo is detectable in the hub and CySCs. (c) Chinmo expression is dramatically reduced upon EcR activation in the adult testis. (d-h) Expression of follicle cell markers Fas3 (red) and Castor (green) in adult gonads. Adult ovaries (d) show expression of Fas3 (red) and Castor (green) in early follicle cells, while in a control adult testis (e) Castor and Fas3 are absent from the cyst cell lineage. In adult testes, as seen in previous figures, Fas3 is expressed in hub cells. EcR activation in the testis (f) causes somatic feminization in the adult testis, characterized by the presence of Fas3- and Castor-positive somatic aggregates. Depletion of chinmo in the male somatic gonad (g) also causes somatic feminization as previously observed3. Blocking EcR activation by over-expressing EcRDN (h) suppresses somatic feminization in the absence of Chinmo. (i) Quantification of feminization rescue in tj>chinmoRNAi testes by blocking EcR activation. An abbreviated description of phenotypic categories: 0=no feminization or germline defects; 1=germline defects but no feminization; 2=mild feminization; 3=moderate feminization; 4=severe feminization; 5=acellular testes. Feminization and acellularity are indicated on the graph in blue. More complete descriptions and visual examples of each phenotypic category can be found in Extended Data Fig. 9. (j-l) Expression of Br-C (green) in the adult testis upon chinmo depletion. Br-C is expressed in follicle cells during mid-oogenesis in the adult ovary (j). Br-C is expressed at moderate levels in early cyst cells but is absent from differentiating cyst cells in an adult testis (k). In a tj>chinmoRNAi testis (l), Br-C is expressed in later cyst cells distal from the hub (hub and testis apex are out of frame in l and l’). Scale bars = 50 μm.
Discussion
Taken together, our data demonstrate that E signaling can be used to control sex-specific development in an invertebrate. This does not require sex-specific E production and, indeed, sufficient E is produced in both sexes to activate signaling in cells competent to respond to the hormone, such as the testis terminal epithelium (Extended Data Fig. 1a, c). We demonstrate that at the time that E signaling activates ovary development, E signaling is repressed in most somatic cells of the larval testis, including the hub and somatic support cells. Further, our work shows that this repression is necessary for proper testis development: activation of E signaling causes a loss of male niche (hub) identity and a transformation of adult somatic support cells toward a female follicle cell-like identity. We also show that repression of E signaling in the testis is due, in part, to dsx-dependent regulation of EcR expression. Therefore, invertebrates like Drosophila that utilize the steroid hormone E to regulate development in both sexes can also employ this same hormone to regulate sexual development via sex-specific regulation of its receptor.
We show that somatic gonad development and maintenance feature mutually exclusive expression of the transcription factors Chinmo and Br. Repression of chinmo by EcR and Br has been observed in other developmental contexts23–25, presenting this as a common regulatory mechanism. Thus, sex-specific EcR expression may well be used to control sexual dimorphism in other tissues such as the nervous system. It is notable that upon EcR hyperactivation in the adult testis, somatic cells appear transformed to a female follicle cell-like identity, whereas hub cells are lost but do not undergo a similar transformation toward a female niche identity. Male to female niche transformation is observed in dsx mutants17, indicating that there must be additional regulatory mechanisms downstream of dsx, in addition to EcR, that promote sex-specific niche development and maintenance. Candidates for this include fruitless, which encodes a transcription factor involved in hub maintenance during larval stages26, and bric a brac 1 and 2 (bab1/2), which are important for TF cell specification27,28. Interestingly, Fruitless, Bab1/2, Br, and Chinmo all encode transcription factors with BTB (Broad, Tramtrack, Bric a brac) domains that promote homotypic and heterotypic protein interactions. Further, they are all either predicted or known Dsx target genes1417,26,29. Thus, the regulation of these factors by Dsx and EcR, in addition to their potential physical associations, could allow for a particularly rich network of regulatory interactions controlling sexual dimorphism in the gonad and other tissues.
The autonomous regulation of ecdysone signaling we report in Drosophila gonads may provide a model for how cell-autonomous sexual identity is regulated in vertebrates. Birds, like mammals, utilize estrogen and testosterone to control development of sexually dimorphic characteristics. Indeed, manipulating the activity of aromatase, which converts testosterone to estrogen, can induce sex-reversal during chicken development30. However, in birds with a mixture of cells with male and female sex chromosome genotypes, the cells with a male genotype develop as male and the cells with a female genotype develop as female, despite the fact that these animals have a single circulating level of estrogen and testosterone3,31. Further, male chickens heterozygous for a non-functional allele of DMRT1 develop an ovary rather than testes, but all other tissues analyzed are phenotypically male32. These data clearly indicate that even when estrogen and testosterone are used to control sex-specific development, the responding tissues can cell-autonomously differ in how they respond to these hormones. The simplest explanation for this is that these tissues autonomously regulate their response to these steroid hormones according to their sex, similarly to how Drosophila cells regulate sex-specific response to ecdysone signaling in the gonads.
Methods
Fly stocks and husbandry
The following flies were used and are described in FlyBase: OregonR, dsx1, Df(3R)dsx3, dsxD, msl3-gfp, tj-gal4 (P{GawB}NP1624), EcRE-lacZ, UAS-lacZ, UAS-EcR.C, UAS-Tai, UAS-EcR-AW650A (referred to as UAS-EcRDN in text), UAS-chinmoRNAi (HMS00037) tai-GFP. EcR6kb-gfp enhancer line was generated in this study; see below for cloning details.
Antibodies
The following antibodies were used: rabbit anti-Vasa (1:10,000; gift of Ruth Lehmann, MIT/Whitehead Institute, Cambridge, MA, USA), guinea pig anti-Tj (1:1000), mouse anti-Fas3 (1:100; DSHB), rat anti-N-cadherin (1:20; DSHB), mouse anti-EcR (Ag10.2; 1:20; DSHB), mouse anti-EcR-A (15G1a; 1:20; DSHB), mouse anti-EcR-B1 (AD4.4; 1:20, DSHB), mouse anti-Broad-core (25E9.D7; 1:40; DSHB), mouse anti-Broad-Z1 (1:50; DSHB), mouse anti-β-galactosidase (40-1a; 1:40; DSHB), mouse anti-Engrailed (1:10; DSHB), rat anti-Chinmo (1:5,000; gift of N. Sokol, formerly Indiana University, Bloomington, IN), rabbit anti-Castor (gift of W. Odenwald, NIH, Bethesda, MD). Cross-adsorbed secondary antisera (Invitrogen) were raised in goat and diluted to 4 μg/mL for staining.
Larval hormone feeding experiments
Larval 20-hydroxyecdysone (20E) and chromafenozide (CF) feeding experiments were performed as follows. First, embryos were collected for 4 hours on apple juice agar petri dishes supplemented with regular yeast paste. 21 hours after the start of embryo collection, larvae that hatched prematurely were removed. First instar larvae that hatched over the following 4 hours were then transferred to a fresh apple juice plate supplemented with regular yeast paste. Newly ecdysed third instar larvae were transferred to a fresh apple juice plate supplemented with either control yeast paste (dry yeast mixed with H2O lacking hormone), yeast paste plus 20E (dry yeast mixed with 1 mM 20E in H2O), or yeast paste plus 1 mM CF (dry yeast mixed with 1 mM CF in H2O). Wandering third instar larvae were dissected 48 hours later for gonad staining and imaging.
Immunofluorescence
Testes and ovaries were dissected in PBS and fixed in 5% formaldehyde (diluted in PBS) for 20 minutes at room temperature (RT). Fixed tissue was washed twice for 10 minutes each in PBTx (PBS + 0.1% Triton X-100). Blocking was performed for 30 minutes or overnight in BBTx (PBS + 0.1% Triton X-100 + 1% BSA). Primary antibodies were diluted in BBTx and incubated overnight at 4° C. Primary antibodies were washed off twice for 10 minutes each in BBTx. Secondary antibodies were diluted in BBTx, incubated for 2–3 hours at RT in the dark, and washed off in PBTx. Samples were then incubated in 1 μg/mL DAPI for 10 minutes at RT in the dark, and finally washed twice in PBTx. HCR-FISH was performed according to manufacturer’s protocol (Molecular Instruments, Inc.) and EcR hybridization probes were generated commercially. Tissue mounting medium was supplemented with DABCO anti-fade reagent (company) prior to confocal analysis. Confocal images were captured using a Zeiss LSM 710 confocal microscope using 20x, 40x, or 63x objective lens.
ImageJ quantifications
For quantification of EcR6kb-gfp gonad expression shown in Fig. 2, total GFP intensity from a representative Z-slice was quantified for each gonad, then normalized to total DAPI intensity within the same area.
Generating EcR6kb-GFP constructs
Cloning was performed using a modified version of pStinger46 that replaces P-elements with attB site to facilitate ΦC31-mediated, site-directed genome integration. The MCS from pStinger (Barolo et al. 2000) was excised and ligated into pARE-GFPnls (see Chatterjee & Bohmann 2012 for vector map) to generate pStinger-attB. The hsp70 minimal promoter sequence was removed (since the 6.3kb putative enhancer contains a core promoter) to generate pStinger-attB. This vector was used as the starting point to generate EcR6kb-GFP. The 6.3-kb putative enhancer region was amplified from a BAC construct containing part of the EcR locus (BACR08A11) using the primers listed below, then digested with SphI/AgeI and ligated into pStinger-attB using the NEBuilder HiFi DNA cloning assembly kit (New England Biosciences, Inc.). Positive clones were screened by PCR and sequence integrity was verified using Sanger sequencing. Plasmid was injected into Drosophila embryos and stable lines were generated via BestGene, Inc.
Primers:
EcR6kb_sphI_ageI_fwd: tagtgctactgcatagca
EcR6kb+sph1_ageI_rev: ctatgcagccgccatata
Extended Data
Extended Data Figure 1. Expression of EcR and Br isoforms in the developing gonad.
(a-b) Expression of the EcR target Br-C in an LL3 ovary (a) and testis (b). Br-C expression in terminal epithelium cells of a larval testis is shown in (c). Vasa (green) labels the germline and Tj (blue) labels somatic cells. (d-e) Expression of Br-Z1 isoform in an LL3 ovary (d) and testis (e). Vasa (magenta) labels the germline. (f-k) Expression of EcR-A (f-h) and EcR-B2 isoforms in the LL3 ovary (f, i), testis (g, j) and testicular terminal epithelium cells (h, k). Vasa (green) labels the germline and Tj labels somatic cells. Scale bars = 25 μm.
Extended Data Figure 2.
(a-f) Expression of EcRE-lacZ reporter in L3 ovaries (a-c) or L3 testes (d-f) upon feeding with 10% DMSO (vehicle, a and d), 20-hydroxyecdysone (20E, b and e), or chromafenozide (CF, c and f). (g-h) Depletion of shd in the larval ovary does not visibly impair ovary development. Note that dcg-gal4 used in this experiment is expressed in both the ovary and surrounding fat body. Vasa (green) labels the germline, N-cad (red) labels TF cells, and Tj (blue) labels intermingled somatic cells. Scale bars = 25 μm.
Extended Data Figure 3.
(a-c) Detection of EcR mRNA (red) in LL3 gonads by HCR-FISH. A dsx-mutant (dsxD/+) gonad (c) shows lower EcR mRNA abundance than control ovaries (a). EcR mRNA in an LL3 testis is shown in (b). (d-f) Detection of GFP mRNA (red) in LL3 gonads by HCR-FISH. dsxD/+ gonad (f) contains higher GFP mRNA abundance than control ovaries (d). Scale bars = 25 μm.
Extended Data Figure 4.
(a-b) Neither hub specification nor hub maintenance are compromised by blocking EcR activity (tj>EcR-DN). Fas3 (green) labels hub cells, Vasa (red) labels the germline, and Tj (blue) labels somatic cells. (c-e) Quantification of hub cell number (c), hub volume (d), and germline stem cell (GSC) number (e) upon blocking EcR activation in the somatic gonad. Scale bars = 10μm.
Extended Data Figure 5.
(a-d) Br-C expression in LL3 control testes upon somatic over-expression of EcR (b), Tai (c), or EcR and Tai simultaneously (d). (e) Quantification of relative Br-C intensity from a-d. Scale bars = 25 μm.
Extended Data Figure 6.
(a-c) Representative images of early L1 (24-28h after egg lay, AEL) gonads, identified by the presence of a Fas3-positive hub (green). Ovaries lack a hub (a) while testes contain a Fas3-positive hub (green) by this stage (b). III staining. Co-expression of EcR and tai in the early somatic gonad (c) leads to a visibly smaller hub that contains significantly fewer hub cells than control L1 testes (see Fig. 3e for quantification). Vasa (red) labels the germline, Tj (blue) labels somatic gonadal precursor cells, which give rise to the somatic gonad in both sexes. (d-e) Pupal testes upon EcR/Tai co-expression. In a control pupal testis (d), the germline and somatic stem cells are maintained by the presence of a hub (Fas3, green) and thus differentiating germ cells (Vasa, red) are observed in the testis. In some pupal testes, following EcR/Tai co-expression in the somatic gonad (e), no Fas3-positive hub cells are present and the absence of Vasa and DAPI staining indicates that both germline and somatic cell lineages have been lost. DAPI-positive muscle sheath cells can be found at the periphery of the testis. Scale bars = 10 μm.
Extended Data Figure 7.
(a-c) Expression of the TF marker Engrailed (En) in LL3 gonads. En (green) labels TF cells in a control ovary (a) but is not detected in the niche of a control testis (b). En is not detected in hub cell nuclei upon EcR/Tai co-expression in the somatic gonad (c). En induction is observed in the cyst cell lineage upon EcR activation (c). In b and c, the hub is outlined with a yellow dotted line. Scale bars = 25 μm.
Extended Data Figure 8.
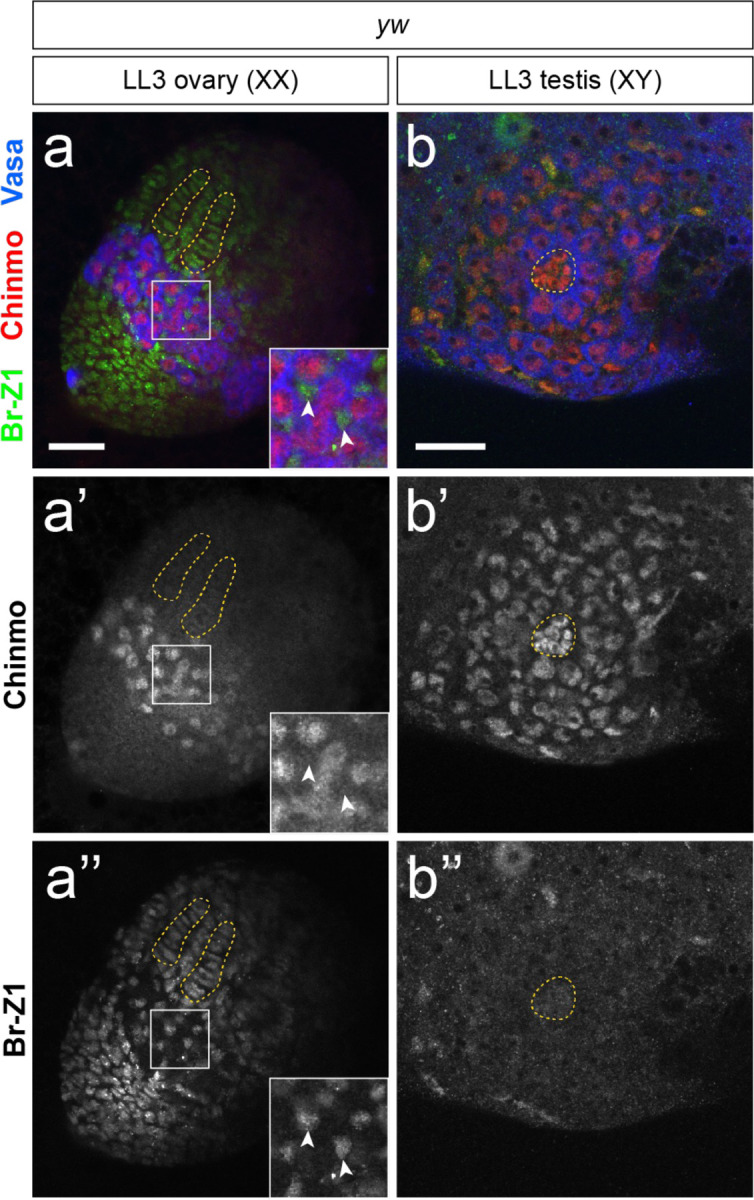
Mutually exclusive expression of Chinmo (red) and Br-Z1 (green) in somatic cells of LL3 gonads. In an LL3 ovary (a), Chinmo is absent from somatic cells (a’, inset arrowheads) while Br-Z1 is expressed broadly in the female somatic gonad. We note that Chinmo is expressed in female larval progenitor germ cells (PGCs) (a’). In an LL3 testis (b), Chinmo is expressed in the hub, CySCs/cyst cells, and GSCs/early germ cells. Br-Z1 is not detectable in the hub or early cyst cells. Vasa (blue) labels the germline. Scale bars = 25 μm.
Extended Data Figure 9.
Phenotypic category descriptions and representative images for rescue quantification in Figure 4i. (a) Category 0: no evidence of feminization (accumulation of Fas3/Castor-expressing somatic aggregates), early/late spermatogonia and large-nuclei spermatocytes present. (b) Category 1: No evidence of feminization, but germline defects seen (shortening of mitotic region and/or absence of large-nuclei spermatocytes). (c) Category 2: mild feminization (a few cells express Fas3 and/or Castor, but aggregates have not yet formed). (d) Category 3: moderate feminization (follicle-like aggregates present clearly expressing Fas3 and Castor, few germ cells are present and mostly seem arrested in spermatogonial stage). (e) Category 4: severe feminization (large follicle-like aggregates present, few/no germ cells remain). (f) Category 5: acellular. Vasa (blue) labels the germline. Scale bars = 50 μm.
Acknowledgments
We thank the Bloomington Drosophila Stock Center (Bloomington, IN, USA), the Vienna Drosophila Resource Center (Vienna, Austria), and the Developmental Systems Hybridoma Bank (Iowa City, IA, USA) for making available fly stocks and antibodies. We are also grateful to Van Doren Lab members and Dr. Shyama Nandakumar for project and manuscript feedback. This work was funded by NIH R01GM113001 (MVD).
References
- 1.Hodin J. & Riddiford L. M. The ecdysone receptor and ultraspiracle regulate the timing and progression of ovarian morphogenesis during Drosophila metamorphosis. Dev Genes Evol 208, 304–317 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Gancz D., Lengil T. & Gilboa L. Coordinated regulation of niche and stem cell precursors by hormonal signaling. PLoS Biol 9, e1001202 (2011). 10.1371/journal.pbio.1001202PBIOLOGY-D-11-01554 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao D. et al. Somatic sex identity is cell autonomous in the chicken. Nature 464, 237–242 (2010). 10.1038/nature08852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarkower D. & Murphy M. W. DMRT1: An Ancient Sexual Regulator Required for Human Gonadogenesis. Sex Dev 16, 112–125 (2022). 10.1159/000518272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raymond C. S., Murphy M. W., O’Sullivan M. G., Bardwell V. J. & Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes & development 14, 2587–2595 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matson C. K. et al. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476, 101–104 (2011). 10.1038/nature10239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dujardin E. et al. DMRT1 is a testis-determining gene in rabbits and is also essential for female fertility. Elife 12 (2023). 10.7554/eLife.89284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reyes A. P., Leon N. Y., Frost E. R. & Harley V. R. Genetic control of typical and atypical sex development. Nat Rev Urol 20, 434–451 (2023). 10.1038/s41585-023-00754-x [DOI] [PubMed] [Google Scholar]
- 9.Hildreth P. E. doublesex, a recessive gene that transforms both males and females of Drosophila into intersexes. Genetics 51, 659–678 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camara N., Whitworth C. & Van Doren M. The creation of sexual dimorphism in the Drosophila soma. Current topics in developmental biology 83, 65–107 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Morrow H. & Mirth C. K. Timing Drosophila development through steroid hormone action. Curr Opin Genet Dev 84, 102148 (2024). 10.1016/j.gde.2023.102148 [DOI] [PubMed] [Google Scholar]
- 12.Koelle M. R. et al. The Drosophila EcR gene encodes an ecdosyne receptor, a new member of the steroid receptor family. Cell 67, 59–77 (1991). [DOI] [PubMed] [Google Scholar]
- 13.Brennan C. A., Ashburner M. & Moses K. Ecdysone pathway is required for furrow progression in the developing Drosophila eye. Development 125, 2653–2664 (1998). 10.1242/dev.125.14.2653 [DOI] [PubMed] [Google Scholar]
- 14.Clough E. et al. Sex- and tissue-specific functions of Drosophila doublesex transcription factor target genes. Dev Cell 31, 761–773 (2014). 10.1016/j.devcel.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai J., Uehara Y., Montell D.J. Regulation of invasive behavior by Taiman, a Drosophila protein related to AIB1, a steroid coactivator amplified in breast cancer. Cell 103, 1047–1058 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Rolf Nöthiger M. L., Andersen Nils, Gerschwiler Pia, Grüter Armin, Keller Walther, Leist Christian, Roost Maja, Schmid Helen. Genetic and developmental analysis of the sex-determining gene ‘double sex’ (dsx) of Drosophila melanogaster. Genetical Research 50, 113–123 (1987). [Google Scholar]
- 17.Camara N., Whitworth C., Dove A. & Van Doren M. Doublesex controls specification and maintenance of the gonad stem cell niches in Drosophila. Development 146 (2019). 10.1242/dev.170001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherbas L., Hu X., Zhimulev I., Belyaeva E. & Cherbas P. EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development 130, 271–284 (2003). 10.1242/dev.00205 [DOI] [PubMed] [Google Scholar]
- 19.Dobens L., Rudolph K. & Berger E. M. Ecdysterone regulatory elements function as both transcriptional activators and repressors. Mol Cell Biol 11, 1846–1853 (1991). 10.1128/mcb.11.4.1846-1853.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaherty M. S. et al. chinmo is a functional effector of the JAK/STAT pathway that regulates eye development, tumor formation, and stem cell self-renewal in Drosophila. Dev Cell 18, 556–568 (2010). https://doi.org:S1534-5807(10)00104-8 [pii] 10.1016/j.devcel.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Q., Wawersik M. & Matunis E. L. The Jak-STAT target Chinmo prevents sex transformation of adult stem cells in the Drosophila testis niche. Dev Cell 31, 474–486 (2014). 10.1016/j.devcel.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grmai L., Hudry B., Miguel-Aliaga I. & Bach E. A. Chinmo prevents transformer alternative splicing to maintain male sex identity. PLoS genetics 14, e1007203 (2018). 10.1371/journal.pgen.1007203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syed M. H., Mark B. & Doe C. Q. Steroid hormone induction of temporal gene expression in Drosophila brain neuroblasts generates neuronal and glial diversity. Elife 6 (2017). 10.7554/eLife.26287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narbonne-Reveau K. & Maurange C. Developmental regulation of regenerative potential in Drosophila by ecdysone through a bistable loop of ZBTB transcription factors. PLoS Biol 17, e3000149 (2019). 10.1371/journal.pbio.3000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truman J. W. & Riddiford L. M. Chinmo is the larval member of the molecular trinity that directs Drosophila metamorphosis. Proceedings of the National Academy of Sciences of the United States of America 119, e2201071119 (2022). 10.1073/pnas.2201071119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H., Whitworth C., Pozmanter C., Neville M. C. & Van Doren M. Doublesex regulates fruitless expression to promote sexual dimorphism of the gonad stem cell niche. PLoS genetics 17, e1009468 (2021). 10.1371/journal.pgen.1009468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godt D. & Laski F. A. Mechanisms of cell rearrangement and cell recruitment in Drosophila ovary morphogenesis and the requirement of bric a brac. Development 121, 173–187 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Sahut-Barnola I., Godt D., Laski F. A. & Couderc J. L. Drosophila ovary morphogenesis: Analysis of terminal filament formation and identification of a gene required for this process. Developmental biology 170, 127–135 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Williams T. M. et al. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134, 610–623 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Estermann M. A., Major A. T. & Smith C. A. Genetic Regulation of Avian Testis Development. Genes (Basel) 12 (2021). 10.3390/genes12091459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agate R. J. et al. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proceedings of the National Academy of Sciences of the United States of America 100, 4873–4878 (2003). 10.1073/pnas.0636925100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ioannidis J. et al. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proceedings of the National Academy of Sciences of the United States of America 118 (2021). 10.1073/pnas.2020909118 [DOI] [PMC free article] [PubMed] [Google Scholar]