Abstract
Understanding how brain activity is related to animal behavior requires measuring multi-area interactions on multiple timescales. However, methods to perform chronic, simultaneous recordings of neural activity from many brain areas are lacking.
Here, we introduce a novel approach for independent chronic probe implantation that enables flexible, simultaneous interrogation of neural activity from many brain regions during head restrained or freely moving behavior. The approach enables repeated retrieval and reimplantation of probes. The chronic implantation approach can be combined with other modalities such as skull clearing for cortex wide access and optogenetics with optic fibers. Using this approach, we implanted 6 probes chronically in one hemisphere of the mouse brain.
The implant is lightweight, allows flexible targeting with different angles, and offers enhanced stability. Our approach broadens the applications of chronic recording while retaining its main advantages over acute recording (superior stability, longitudinal monitoring of activity and freely moving interrogations) and provides an appealing avenue to study processes not accessible by acute methods, such as the neural substrate of learning across multiple areas.
Introduction
Interactions between brain areas are critical for neural computations that drive a wide range of behaviors. Multi-electrode arrays allow recording from multiple areas simultaneously and, due to recent developments and the integration with CMOS technology, can now be deployed at scale. These advances have enabled cross-area recordings with high yield (e.g. (Allen et al., 2019; Durand et al., 2023; Jun et al., 2017; Steinmetz et al., 2021, 2019). Nonetheless, major challenges remain for monitoring neural activity chronically (over many days) with multiple probes. Here, we surmount these challenges with a novel approach to implanting and recovering multiple probes for chronic experiments.
Over the last few decades, the number of electrodes deployed to record neural activity has increased from less than a handful to several thousands per probe (Stevenson and Kording, 2011). These advances directly impact the number of neurons that can be recorded simultaneously and have enabled many studies that describe simultaneous population activity in multiple brain areas (de Vries et al., 2020; Steinmetz et al., 2019; Stringer et al., 2019; Wang et al., 2023). However, in most of these studies, the probes are introduced on the day of recording and retracted at the end of the session (commonly referred to as the “acute” recording configuration). This limits the ability to study activity as it changes during the time course of days to months, introduces delays that may compromise behavior performance, and cannot be used in freely moving behaviors. Existing approaches to record neuronal activity chronically in rodents are either prohibitively expensive or pose constraints on the areas that can be simultaneously measured. For example, one approach is to irreversibly cement probes to the skull (Krupic et al., 2018; Mimica et al., 2023; Okun et al., 2016; Steinmetz et al., 2021) which provides long and stable recordings, however the probes cannot be recovered. This limits the number of probes that experimenters are willing to implant at a time and makes these experiments only within reach of a select group of labs. Indeed, most labs still consider Neuropixels a precious resource and have a sufficiently limited supply that re-using probes is a necessity. Other approaches for chronic implantation secure the probes to a fixture, usually 3D-printed, that is attached to the skull (Bimbard et al., 2023; Jones, 2023; Juavinett et al., 2019; Luo et al., 2020; van Daal et al., 2021). There are a wide range of implant strategies, and existing fixtures often enable implanting 1–2 probes in mice and up to 4 in rats (Luo et al., 2020). Recoverability is possible and is reported for many of the designs; however, the reliability of the process remains largely uncharacterized (Bimbard et al., 2023; Jones, 2023; Juavinett et al., 2019; Luo et al., 2020; van Daal et al., 2021). In detail, Juavinett et al. 2019 report 4 explants out of 10 with 2 re-uses. Luo et al 2020 report 8 successful explants with 3 re-uses with 2 probes out of 22 insertions. Steinmetz et al. 2021 reports 4 reuses with 2 probes for periods of 2 weeks. Bimbard et al. 2023 reports no noticeable difference in single-unit yield across successful re-uses. It remains unclear, however, whether probes can be reused after being implanted for months. Further, when many probes are used in the same animal or when dealing with a limited supply of probes, implant recoverability becomes critical.
Several additional constraints limit the flexibility and adoption of published designs for chronic, multi-probe fixtures. First, in some cases, probes are attached to the same fixture and lowered together, as a unit, into the brain, as in (Bimbard et al., 2023; van Daal et al., 2021). This approach restricts the areas that can be targeted because it imposes a minimal distance between the probes (3mm in van Daal et al. 2021). Weight is the second critical consideration for multiprobe implants: animals can only carry a fraction of their body weight. Implants that weigh more than 15–20% of the animals’ weight are prohibitive. Third, fixtures are often composed of multiple parts that have complicated assembly protocols, e.g. (Jones, 2023; van Daal et al., 2021), making fixture assembly time consuming. Lastly, the duration of the surgery is a critical factor when implanting multiple probes. It is common that surgery times extend 4–6h for 2 probes. Some approaches offer improved flexibility, but at the cost of longer surgery times (Jones, 2023).
We set out to develop a novel fixture implant strategy that overcomes current limitations. Our design enables studying brain activity on long timescales, provides high targeting flexibility at reduced weight, affords improved re-use rates, and permits curtailed assembly and surgical times, using only a single mechanical structure. Using this approach, we were able to simultaneously record with 6 probes (24 shanks) from selected targets in one hemisphere of the mouse brain.
Results
Novel fixture for multiprobe implants and optimized surgical procedures.
Our approach takes advantage of commercially available Neuropixels probes (Jun et al., 2017; Steinmetz et al., 2021). We report results using 384 recording sites per probe; the approach is scalable to the higher channel devices currently under development. Neuropixels probes are currently available with a dovetail attached that we use to secure the probe in the fixture; we established procedures to secure the dovetail to the holder. We engineered a probe fixture (Fig. 1a) that can be 3D printed using stereolithography (SLA) technology, on a desktop printer. The use of a desktop printer (FormLabs, Form 3+) allows individual labs to extend/adapt the design with ease. The dovetail rails in the fixture require small tolerances that are at the limit of SLA technology so we provide detailed instructions to reliably manufacture holders (see Methods).
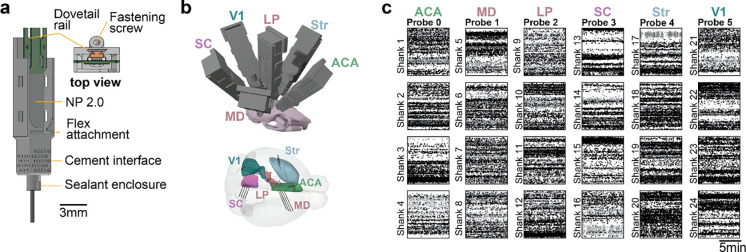
Figure 1. Novel fixture for chronic, large-scale electrophysiology.
– a) Neuropixels 2.0 probe in fixture and description of details. b) Simultaneous chronic 6 probe implant in the same hemisphere of a mouse targeting specific structures. The weight of the implant is 3.42g including the probes and all fixtures. c) Spike rasters for the 24 shanks implanted in (b). Color indicates spike amplitude (black is high amplitude); abscissa is time, ordinate is depth from the selected recording area in each shank.
The probe is housed within a fixture that forms the primary structure of the assembly (Fig. 1a). During stereotaxic implantation, the fixture is cemented to the skull, and a covering/cap is attached after implantation. At the end of the experiment (weeks to months later) the probe can be released from the fixture and recovered. Importantly, the fixture consists of a single 3D printed structure that forms the sole mechanical structure of the probe holder – the covering/cap offers protection and does not contribute to stabilizing the electrode. This design choice eliminates structural fastenings between 3D printed parts that can hinder stability, and are present in other approaches.
Multiple probes can be implanted using individual fixtures (at a weight-cost of 0.57g per probe - including the probe and flex cover); this allowed us to implant 6 probes in a single hemisphere of the mouse brain. After recovery from surgery, mice carry the implant with ease in the home cage (total weight of 3.42g including 6 probes, fixtures and coverings/caps – excluding 1–2g of cement). While the full, 6-probe configuration may impact behavior in freely moving experiments (more on this below), it is entirely feasible for head-restrained experiments in which the animal need not bear the weight of the headstages during the experiment. Using this approach, we were able to record 1176 single units (4030 multi-unit) across 6 target structures simultaneously (Fig. 1b and c - 1 mouse). Further, because probes have switchable sites (Jun et al., 2017), we can access cells in structures distributed along the insertion tracts across different recording days. Note that the probe recovery for this configuration requires further optimization; successful retrieval after two weeks was not achieved for the 2.0 probes that were at a high angle in this example mouse. Dummy probes in a similar configuration were successfully retrieved.
In addition to designing a novel fixture, it was essential to optimize the surgical procedures for multi-probe implants. Chronic surgical procedures are rarely standardized across labs, however certain principles, such as the use of viscous cement to seal the implant leaving the shank open to air, are relatively constant across published work (Bimbard et al., 2023; Jones, 2023; Juavinett et al., 2019; Luo et al., 2020; van Daal et al., 2021). When implanting a single probe, or multiple probes attached to the same fixture, surgeries are reported to take 4–5 hours(van Daal et al., 2021), which would render implanting multiple probes on independent fixtures impractical and time consuming. Long surgery times also introduce steep adoption curves for novice users. We therefore sought to simplify the procedure by encapsulating the probe in silicone adhesive so cement could be added ad-lib. We chose a low toxicity silicone adhesive (KwikSil, WPI) because it can be directly applied to craniotomies, it hardens quickly however allows the probe to slide though it without breaking, during explantation. To facilitate the application of KwikSil, we include a sealant enclosure (Fig. 1a) in the fixture design that ensures the sealant does not leak close to the base of the probe (where probe motion is could lead to breakage). With this design, the sealant offers protection to the probe shank(s) and it can be applied without a microscope. Importantly, because the shanks are completely covered, cement can then be applied ad-lib to secure the fixture in place. We include holes that act as interfaces to the cement but keep it away from the probe by surface tension (Fig. 1a). Optimizing the surgical procedures greatly reduced the time required to implant multiple probes to roughly 1h per probe when implanting 2 probes at a time.
Altogether, by creating a novel fixture and optimizing the surgical procedures we could implant multiple probes in a scalable fashion and target many distinct brain areas in a single hemisphere of the mouse brain. Importantly, the novel fixture allows retrieval of the probe for reuse in chronic or acute experiments.
Implant stability and comparison with a published dataset
We established a recoverable method for implanting electrodes that enables recording neural activity chronically. Ideally, the stability of measurements with our fixture would match that of cemented probes and the unit yield be on par with acute datasets, where the chances of loss of units due to inflammatory response are minimal.
First, we investigated recording stability in relation to brain motion. A major concern in electrophysiological experiments is that motion of the brain in relation to the probe causes drift of the recorded neurons during individual sessions. In our approach, covering the probe and craniotomy with silicone sealant could in principle reduce the amount of motion, leaving less room for the brain to move in the dorsal-ventral direction. We therefore set out to quantify the motion of the brain in relation to the probe by using the recorded voltage signals. We implanted a Neuropixels 2.0 alpha probe with 4 shanks, and recorded from 2 shanks in cortex and 2 shanks in thalamus. The recordings were done in a head restrained configuration, with the mouse allowed to run on a treadmill. We reasoned that a condition where the mouse is locomoting vigorously and the skull is fixed would be more likely to produce large artifacts than in a freely moving configuration. The probe might move together with the brain tissue in the anterior-posterior and medial-lateral directions but not in the dorsal-ventral direction. We therefore expect that most of the motion is along the probe axis so we quantified only this direction. We deployed DREDge (Windolf et al., 2023), a motion registration algorithm for electrophysiological data that has been extensively validated in similar recordings with imposed motion, to quantify motion on a single probe, with shanks in cortical and thalamic structures. We chose a superficial and a deep target because it is possible that motion affects different structures differently and wanted to investigate how stable the use of silicone adhesive is at reducing motion of tissue close to the craniotomy. Tissue motion, measured from voltage signals, at both cortical and thalamic sites was minimal (Fig. 2a – top cortical, bottom thalamic). When compared to the size of the electrodes, motion was less than the size of an electrode and close to the distance between sites (Fig. 2b).
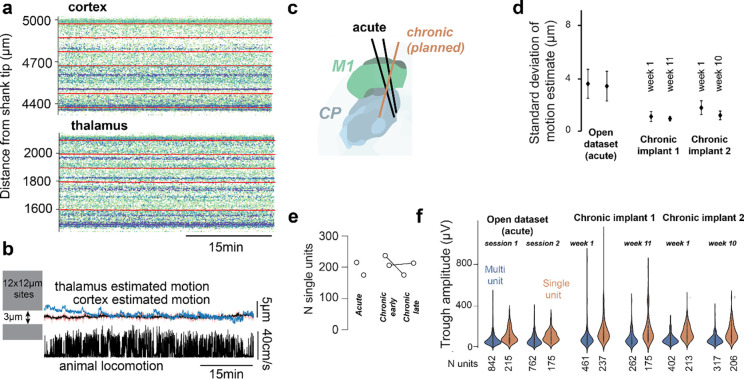
Figure 2. Negligible motion of the brain in relation to the shank and comparable unit yield in relation to published data.
– a) Spike amplitude raster for a shank with recording sites in cortex (top) and another shank with sites in thalamus (bottom). Red traces are motion estimates from DREDge at different depths. b) Estimated motion is smaller than the distance between sites in Neuropixels 2.0 probes (gray boxes denote the size of individual recording sites on a Neuropixels 2.0 probe). Animal locomotion in head restrained condition does not impact brain motion in relation to the probe. c) Planned chronic trajectories for comparison with available published datasets from the International Brain Laboratory that were acquired in the acute condition. d) motion in chronic is reduced in comparison to acute datasets. e) Unit yield is similar to recordings obtained with fresh craniotomies in an acute setting both in the first week of training and after 10–11 weeks. f) spike amplitudes are similar to the acute case and only show only a minor decay between early and late chronic timepoints.
In a separate set of animals implanted with Neuropixels 1.0, we compared motion within single sessions in our dataset with that in sessions recorded in the same brain areas (the primary motor cortex and striatum), during the same behavioral task, and using similar electrodes but collected in an acute setting (International Brain Laboratory et al., 2023b). This allowed us to compare early (first week of training) and late sessions (~2.5 months after) with published data. We compared the magnitude of motion of the brain in relation to the probe (Fig. 2d), the single unit yield (Fig. 2e) and the amplitude of single and multi-units (Fig. 2f). Importantly, we used the same sorting algorithm, without motion correction and the same criteria for single unit selection (<0.1 refractory period violations, <0.1 missed spikes, > 25μV amplitude).
Our chronic recordings had less tissue movement in relation to the probe than IBL acute recordings (Fig. 2d). This was statistically significant in comparison to both early and late chronic sessions (acute-early: p<1e-4, acute-late: p<1e-2, Mann–Whitney U test). Future work will quantify how this difference in the amplitude of brain motion in relation to the probe impacts unit stability.
We then set out to compare single unit yield. A major concern with chronic recordings is that since the craniotomies are not fresh, the single unit yield is reduced, reflecting recordings in unhealthy tissue. The single unit yield on our recordings was comparable if not higher than that of IBL recordings (Fig. 2e). Importantly, there was no noticeable difference between early and late chronic sessions.
The improved stability of our chronic device might come at the cost of worsened unit quality, e.g., a reduction in spike amplitudes due to inflammation. To test this, we compared spike amplitudes in our early and late chronic recordings with the IBL dataset. We found that spike amplitudes of single units are similar if not higher than IBL recordings made in the acute condition.
These results suggest that recordings using our approach are stable within single sessions and achieve similar yield to recordings obtained using standardized methods and protocols developed across laboratories (International Brain Laboratory et al., 2023a, 2023b).
Implant stability and unit yield across sessions
We developed this approach in part motivated by the need to track neural activity during long timescales such as during learning of perceptual decision-making task. Learning usually occurs over weeks to months depending on the difficulty of the task so we set out to quantify stability over long timescales. We first quantified the motion of the brain in relation to the probe, and then the single unit yield across months.
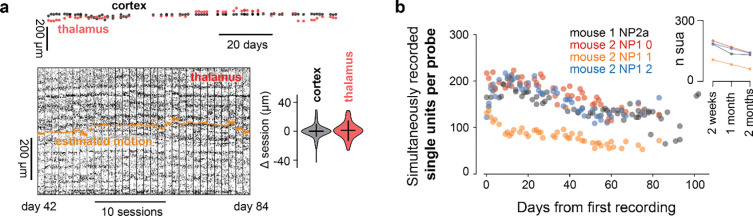
Using the same animal as in Fig. 2a we concatenated sessions acquired across 107 days (5 minutes for each session) and estimated the motion across the depth of cortex and thalamus, for two shanks. We found that inter-session movement depended on the brain region (Fig. 3a, right). The median shift between sessions was restricted to less than the size of one recording site (4.9±7.6 μm for thalamus and 1.6±8.2μm median ± std), suggesting that comparisons across sessions are not likely to be affected by motion of the tissue in relation to the probe. This magnitude of motion can be easily corrected with registration algorithms and is less than the motion observed within acute sessions, in the most extreme cases (Windolf et al., 2023).
Figure 3. Motion across sessions and long-term stability.
– a) Motion of the brain in relation to the probe for a cortical and thalamic recording across months for 107 days from the first recording (76 sessions are shown on top). Bottom: Activity raster over sessions for a thalamic shank (gray scale is spike amplitude). Absolute estimated motion (orange) is overlayed on the raster for 30 sessions. Right: Inter-session motion change for cortical and thalamic shanks. b) Single unit yield per probe for longitudinal recordings. Data are from 2 mice with implanted with a single NP2 (V1 and LP) alpha and another implanted with 3 NP1 (ACA, Str and VISAM). Mouse 1 data are in panel a.
The electrodes in chronic recordings can remain implanted for months and therefore can evoke inflammatory responses that in turn encapsulate the electrodes and impact single unit yield. This can be an issue if the implant is not stable, leading to a decay of single unit yield. For cemented probes, this can be less than a log unit over the time course of months (Steinmetz et al., 2021 – Fig 2E).
To measure the impact of our implant and optimized surgical procedures on single unit yield, we quantified single unit yield across months. Note that we use “single unit” term with caution here, we selected units based on a fixed criteria as opposed to manually inspecting units because of the large number of recorded sessions. We classified units as single unit when they had fewer than 10% of refractory period violations, less than 10% of the spikes missing (also known as amplitude cutoff threshold) and those that have an absolute trough amplitude above 25 μV.
Single unit yield after 2 months fluctuated between around 15 and 30% of the value in the initial 2 weeks, this represents a loss of approximately 0.5 single units per day, similar to cemented probes (Steinmetz et al., 2021). We note however that the decay is non-linear. The number of units increased in the first weeks after the implant, possibly reflecting recovery from the surgery, then decreased over the next 30 days, possibly reflecting a late immune response around the electrodes.
Probe extraction and reusability
A key requirement for scalable multi-probe recordings to be accessible to multiple laboratories is the re-usability of the implants. We therefore devised a mechanism to release the probe after an experiment and retrieve it unharmed. Importantly, our approach allows probes to be reused in acute experiments as well as re-implanted chronically because it does not permanently attach the probe to the fixture. In short, the probe fixture screw is removed, and the probe retracted through the rails. This mechanism allowed us to recover a majority of the implanted probes (several longitudinal studies still have implanted probes). The most common failure modes during fixture development were 1) using too much thread-glue and 2) too loose dovetail tolerance. We provide instructions to mitigate these issues in the methods section and are currently investigating ways of maximizing explant reliability.
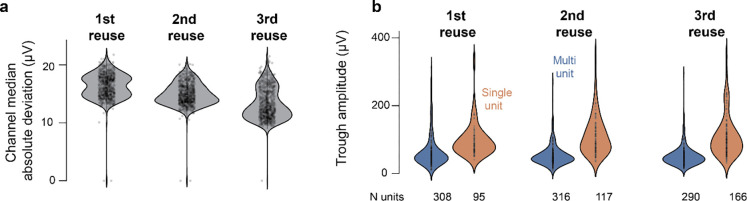
To assess the impact of probe reuse on our ability to isolate single units, we extracted 2 probes used in another experiment (implanted for 3 weeks, not shown because in other brain areas) and re-inserted them consistently with the same trajectories in 3 different animals. We waited at least 10 days before each explantation. Insertions targeted the same brain areas in the 2 hemispheres; data from the two hemispheres was pooled. Upon retraction, probes were placed in warm tergazime for at least 6h, DS-2025 for at least 2h and de-ionized water for 1–2h. First, we looked at the Median Absolute Deviation (MAD) for individual channels. If the recording sites are more noisy across re-insertions we expect MAD to increase. We did not observe an increase in MAD across re-insertions (Fig. 4a). Next, we looked at single and multi-unit yield across re-insertions of the same probes. Single and multi-unit yield were not qualitatively distinguishable across re-insertions.
Figure 4. Probes can be reused without impairing the ability to detect single units.
a) Median Absolute Deviation for channels after successive re-uses in the same brain area. b) Average trough amplitude of the electrode with highest spike for multi units and single units. Number of single units is similar across recordings.
Our experiments suggest that probes can be extracted and reused without impairing single unit yield. Thus, our approach enables large-scale interrogation of neural circuits on timescales relevant for studying how neural population activity evolves during learning.
Use in freely moving contexts
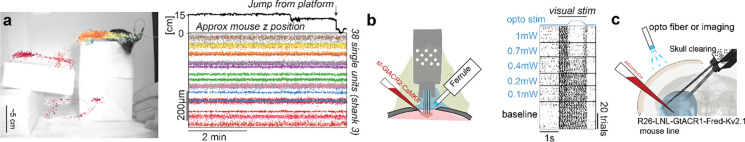
An advantage of chronic implants is that they can be used in freely moving animals. The reduction in weight not only allows more probes to be inserted simultaneously but invites use cases in freely moving contexts. We set out to test the severity of motion artifacts when mice were freely exploring an environment. We prepared an arena (Fig. 5a) that mice can explore in 3 dimensions. We wanted to test if there are artifacts when mice take a fall since this is one of the most severe tests of recording stability.
Figure 5. Freely moving use and implant flexibility enables combining with optogenetics.
a) Implants can be used in freely moving contexts. Recordings remain stable during natural exploration and a vertical drop of 15cm. b) Implant flexibility enables recording and stimulating neurons with optogenetics from the same craniotomy. c) The implant also allows experimental approaches that were previously restricted to acute settings such as inserting probes chronically from the opposing hemisphere and stimulating cortical neurons through the intact cleared skull.
We allowed a mouse to explore an environment and recorded video to extract the animal position while recording neural activity. An underestimate of the z position (not corrected for perspective; Fig.5a) was extracted using pose estimation software (Mathis et al., 2018). We observed no artifacts due to animal motion in single unit activity (sorted without motion correction – Fig.5b). Importantly, this was true even when the mouse took a fall of 15cm suggesting that the approach can be used to track cells even in highly dynamic environments prone to motion of the brain in relation to the probe.
Furthermore, we designed a series of protective caps for the Neuropixels 1.0 and 2.0 fixtures that enclose the headstage that can be used in freely moving contexts.
Discussion
We developed a method to implant and recover multiple probes chronically in targeted locations of the mouse brain. We report simultaneous 6 probe recordings from the same hemisphere of the mouse brain in head-restrained contexts. Importantly, we devised a strategy to independently set probe angles which both allows more probes to be inserted and enables experiments previously impossible in chronic settings such as combining with optogenetics in the same craniotomy (Fig.5b) or inserting probes chronically from the opposing hemisphere and stimulating/recording from the cortex through the cleared skull using optical methods.
Comparison to other solutions
Our fixture tripled the number of probes simultaneously implanted in comparison to the state-of-the-art work in mouse (Bimbard et al., 2023; Jones, 2023; van Daal et al., 2021). Moreover, our new method for encapsulating the probes reduced the implant time to 1h per probe, a ~3-fold improvement relative to (4h) published work (van Daal et al., 2021). Importantly, this was accomplished using standard stereotaxic surgery equipment, future work will optimize the surgery equipment to further reduce surgery time. Published alternatives that implant probes independently either have been designed for larger rodents (Luo et al., 2020) and are therefore much heavier than our solution; or require multiple parts implanted in succession rendering them, to our knowledge, impractical for implanting more than 2 probes in mice.
Caveats
First, we observed in some cases where implants were left for extended periods of time, the recovered probes had acquired a wee bend. These small but mysterious deviations could be due to the electrodes remaining in the brain for a long time or because of the use of kwik-sil to cover the shank. Ongoing work will quantify the impact of this on targeting of future insertions and find ways to mitigate the issue. Second, although our approach allows feasible implantation of up to 6 Neuropixels probes chronically in head fixed animals, implantation of this number in freely moving animals could pose some challenges. Specifically, because each pair of probes has a bespoke cable, implantation of 6 probes would require 3 cables which could be cumbersome for freely moving animals. Possible solutions include using a commutator to manage the cables, and the development of a headstage that can accommodate more than two probes.
Future directions
We see two natural extensions of this work. First, an appealing future direction for our approach would be to modify it for use in larger animals, such as rats or marmosets. The performance of different chronic fixtures has not been thoroughly tested in those animals, with some notable exceptions (Bimbard et al., 2023; Luo et al., 2020; van Daal et al., 2021). Implants in larger animals would benefit from a shield surrounding the implant (as in Luo), and could even be machined in metal, allowing easy autoclaving in advance of surgery. Second, additional modifications could further lighten the total weight of the implant. For example, the dovetail on the probes could be shortened to save weight, provided that the extraction is not impacted. Another way to reduce weight is to shorten the height of the fixture, the current height is set to allow comfortable extraction. However, it seems feasible to, instead, create an external aiding mechanism for extraction. This would allow the probe encasing to be shorter and therefore lighter; effectively reducing the momentum acting over the head of the animal.
Lastly, our approach rests on the miniaturization and probe integration developments done by others (Jun et al., 2017; Steinmetz et al., 2021), a field that is under active development. Developments in probe technology will further open novel avenues to record at scale. Our method offers a flexible approach to maximize accessibility, reuse and extend the range of applications enabled by current probe technology.
Methods
Fixture fabrication
Design files and instructions are available in https://github.com/spkware/chronic_holder. The fixtures were printed using a Form 3+ resin printer (Formlabs). We selected the GreyPro resin for manufacturing, due to its optimal density, rigidity, and impact resistance properties. When setting up a print, we first used Inventor (Autodesk) to export .stl files from the original .ipt CAD files. These files were then loaded into PreForm (Formlabs) where printing orientations and support structures were defined. Fixtures were printed vertically and with the dovetail side facing the mixer side of the printer (multiple orientations were tested during prototyping and this was the most reliable). At the conclusion of 3D printing, parts were removed from the printing plate and washed for 15–20 minutes in isopropanol using the Form Wash tank (Formlabs). After washing, parts were allowed to dry for at least 4 hours. Parts were then UV-cured for 15 minutes at 80 degrees Celsius using the Form Cure (Formlabs). After curing, the support structures were carefully removed with tweezers and iris scissors. The screw-hole of the probe chassis was then hand-tapped with an M1 thread. Multiple dovetail tolerance values were printed, in the same platform, until the correct value was found. That is, when the probe could slide until 3mm from the bottom of the fixture with ease and tweezers were required to drive the probe to the bottom.
Probe assembly and preparation
After probe fixture fabrication, the implant assemblies were prepared for surgery. We first used a dummy probe (or a non-functional real probe) to test the dovetail slide mechanism. This is done because the dovetail requires high dimensional accuracy and there can be dimensional variability across printing/washing rounds. When the dummy probe was confirmed to be properly held by the dovetail, we then inserted a real probe. Once the probe was in place, we secured it with an M1 screw. Loctite was applied to the screw threads only for the final turns of the screw. We discourage dipping the screw in loctite before screwing because loctite can flow into the dovetail mechanism, making removal more difficult. It is important to not overtighten or under tighten the screw, 1/4 to 1/3 turn once the initial resistance of hitting the dovetail occurs is sufficient to hold the probe in place for > 100 days. Before assembling the probe, we soldered a silver wire (bare 0.01”, 782500, A-M Systems) to the ground and reference. We tested different configurations, in our hands connecting the ground and reference of the probe to a ground screw (M1) touching the dura was preferred to using internal reference. The silver wires from all probes were connected when using multiple probes and a single wire connected to the ground screw.
Probe sharpening
Neuropixels probes were sharpened with an EG-45 Microgrinder (Narishige) before being inserted in the holder. We only sharpened probes for the first use. Probes were held at a 25-degree angle. Importantly, the probe was placed in the holder so that the backside of the shank (the side of the shank without electrical contacts) was facing the grinder. Then, the disk speed was set to 7 rotations per second. The probe was then lowered onto the spinning disk until there was a noticeable bend in the shank. Probes were left to sharpen for 5 minutes before being raised and observed under a stereoscope.
Implantation surgery
Animal experiments followed NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the University of California Los Angeles. We report results from implants in 9 male and 1 female mice (20–32g at time of surgery of different genetic backgrounds; C57BL6, FezF-CreER, stGtACR1xFezF or B6129SF1).
For some mice, headbar and Neuropixels implantation were performed within the same surgery. For other mice, these procedures were performed as separate surgeries, separated by a few weeks or months during which the mice were trained on behavioral tasks. For headbar implantation, the dorsal surface of the skull was first cleared of skin and periosteum. A thin layer of cyanoacrylate (VetBond, World Precision Instruments) was applied to the edges of the skull and allowed to dry. The skull was then scored with a scalpel or dental drill to ensure optimal adhesion. After ensuring the skull was properly aligned within the stereotax, craniotomy locations were marked by making a small etch in the skull with a dental drill. A titanium headbar was then affixed to the back of the skull with a small amount of glue (Zap-a-gap). The headbar and skull were then covered with Metabond, taking care to avoid covering the marked craniotomy locations. After the Metabond dried, the craniotomies for the probes and grounding screw were drilled. Once exposed, the craniotomies were kept continuously moist with saline.
The implants were held using the 3D-printed stereotax holder and positioned using a motorized micro-manipulator (Neurostar) and/or a manual stereotaxic manipulator (Stoelting) (in the case of multi-probe implants, we positioned, inserted, and cemented two probes at a time). After positioning the shanks at the surface of the brain, avoiding blood vessels, probes were inserted at slow speed (~5 μm/s). The dura was left intact (when probes had difficulty penetrating the dura, repeated light poking of the dura with the probe tip eventually allowed insertion). Once the desired depth was reached, the craniotomy was dried and sealed with Dowsil 3−4680 (Dow Corning, Midland, MI). After the Dowsil 3–4680 dried, the craniotomy and shanks were sealed with Kwil-Sil (World Precision Instruments), completely enclosing the shanks, craniotomy, and the sealant enclosure in the fixture (Fig. 1a). We are currently investigating whether an alternative silicone sealant (e.g., Dowsil 3–4680) might be a preferable alternative to Kwik-Sil for high-angle, 2.0 Neuropixels probes that are implanted for longer than 1 week (see Results). The fixture was then secured to the skull with Metabond (C&B); mixed in a cold dish with 2 scoops to 4 drops of liquid and 1 of catalyst. After the Metabond dried, the stereotaxic arm was removed. This process was repeated until all probes were implanted. If a grounding screw was used, we connected it to the silver wire with silver epoxy. After all probes were implanted, additional layers of black Orthojet (Lang Dental) were applied to fully cover the fixture cement interface (Fig 1a). The mouse was then removed from the stereotax, and the 3D printed caps were secured to the fixtures. After surgical recovery, mice were treated with meloxicam and enrofloxacin for three days, then acclimated to head-restraint if required.
Head-restrained recordings
Mice were habituated to head-restraint over a period of several days, where mice were head-restrained for increasingly long durations. After habituation, mice were trained to perform auditory or visual decision making tasks. Animal behavior was captured with 3 video cameras (Chamaeleon3, FLIR). During the performance of these tasks, Neuropixels data was simultaneously acquired using SpikeGLX (https://github.com/billkarsh/SpikeGLX).
Freely-moving recordings
Mice were placed atop a platform and allowed to freely roam a playground. The position of the animal was recorded with a camera (Chamaeleon3, FLIR). Animal posture was extracted using DeepLabCut (Mathis et al., 2018) and depth estimated without correcting for the camera perspective. The posture labels were used to infer the location of the mouse in the arena. Neuropixels data was simultaneously acquired using SpikeGLX.
Spike-sorting
Data were first preprocessed with a 300–12000Hz 3 pole butterworth bandpass filter, followed by ADC phase shift correction and common median subtraction. Sessions were spike-sorted with Kilosort2.5 (Pachitariu et al., 2023; Steinmetz et al., 2021) using default parameters. To compute motion estimates using DREDGe, we disabled Kilosort2.5 inbuilt motion correction. All other sessions were run with motion correction enabled.
Sorting jobs were run on a local GPU node and the Hoffman2 Shared Cluster provided by UCLA Institute for Digital Research and Education’s Research Technology Group. Sorting jobs were automatically generated and executed by custom Python packages (https://github.com/jcouto/labdata-tools and https://github.com/spkware/spks).
Probe recovery
To perform probe recovery, the mouse was first anesthetized with isoflurane or ketamine. The 3D printed caps were removed from the fixture, exposing the Neuropixels probe. We then cut the grounding wire ~1cm away from the PCB and freed the flex cable from the tabs that secured it. We then loosened the fastening screw and slowly retracted the probe by pulling gently on the flex cable. The dovetail fastening mechanism ensures that the shanks stay parallel to their insertion trajectory and do not break. Once the shanks were fully outside the brain and could be visualized, we carefully removed the probe from the fixture. Detailed step-by-step instructions are provided on the GitHub repository.
Probe washing
After probe recovery, probes were washed in warm (<37deg) Tergazyme for 12 hours, followed by fresh DowSil-DS2025 for 2–8 hours to remove any layer of KwikSil that remained attached to the shanks. Finally, the probes were soaked in distilled water for 1–2 hours. Shanks were inspected under a stereo microscope; the procedure was repeated if the shank was not clean.
Acknowledgements
We thank all members of the Churchland Lab for helpful discussions and common resources. UCLA DLAM for animal husbandry and colony maintenance, Anup Khanal for invaluable support with animals and tissue clearing, Laura DeNardo for advice and equipment for tissue clearing, Thrish Chari for used test probes at the start of the project, Federico Jimka and Daniel Aharoni for advice and support with 3d printing at the start of the project. This work used computational and storage services associated with the Hoffman2 Shared Cluster provided by UCLA Institute for Digital Research and Education’s Research Technology Group. This work was supported by an NSF-NCS collaborative award (2219946) and by NIH U19NS123716.
References
- Allen WE, Chen MZ, Pichamoorthy N, Tien RH, Pachitariu M, Luo L, Deisseroth K. 2019. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364:eaav3932. doi: 10.1126/science.aav3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimbard C, Takacs F, Fabre JMJ, Melin MD, O’Neill N, Robacha M, Street JS, Beest EHV, Churchland AK, Harris KD, Kullmann DM, Lignani G, Carandini M, Coen P. 2023. Reusable, flexible, and lightweight chronic implants for Neuropixels probes. doi: 10.1101/2023.08.03.551752 [DOI] [Google Scholar]
- de Vries SEJ, Lecoq JA, Buice MA, Groblewski PA, Ocker GK, Oliver M, Feng D, Cain N, Ledochowitsch P, Millman D, Roll K, Garrett M, Keenan T, Kuan L, Mihalas S, Olsen S, Thompson C, Wakeman W, Waters J, Williams D, Barber C, Berbesque N, Blanchard B, Bowles N, Caldejon SD, Casal L, Cho A, Cross S, Dang C, Dolbeare T, Edwards M, Galbraith J, Gaudreault N, Gilbert TL, Griffin F, Hargrave P, Howard R, Huang L, Jewell S, Keller N, Knoblich U, Larkin JD, Larsen R, Lau C, Lee E, Lee F, Leon A, Li L, Long F, Luviano J, Mace K, Nguyen T, Perkins J, Robertson M, Seid S, Shea-Brown E, Shi J, Sjoquist N, Slaughterbeck C, Sullivan D, Valenza R, White C, Williford A, Witten DM, Zhuang J, Zeng H, Farrell C, Ng L, Bernard A, Phillips JW, Reid RC, Koch C. 2020. A large-scale standardized physiological survey reveals functional organization of the mouse visual cortex. Nat Neurosci 23:138–151. doi: 10.1038/s41593-019-0550-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Heller GR, Ramirez TK, Luviano JA, Williford A, Sullivan DT, Cahoon AJ, Farrell C, Groblewski PA, Bennett C, Siegle JH, Olsen SR. 2023. Acute head-fixed recordings in awake mice with multiple Neuropixels probes. Nat Protoc 18:424–457. doi: 10.1038/s41596-022-00768-6 [DOI] [PubMed] [Google Scholar]
- International Brain Laboratory, Banga K, Benson J, Bhagat J, Biderman D, Birman D, Bonacchi N, Bruijns SA, Campbell RA, Carandini M, Chapuis GA, Churchland AK, Davatolhagh MF, Lee HD, Faulkner M, Gerçek B, Hu F, Huntenburg J, Hurwitz C, Khanal A, Krasniak C, Meijer GT, Miska NJ, Mohammadi Z, Noel J-P, Paninski L, Pan-Vazquez A, Roth N, Schartner M, Socha K, Steinmetz NA, Svoboda K, Taheri M, Urai AE, Wells M, West SJ, Whiteway MR, Winter O, Witten IB. 2023a. Reproducibility of in-vivo electrophysiological measurements in mice. doi: 10.1101/2022.05.09.491042 [DOI] [Google Scholar]
- International Brain Laboratory, Benson B, Benson J, Birman D, Bonacchi N, Carandini M, Catarino JA, Chapuis GA, Churchland AK, Dan Y, Dayan P, DeWitt EE, Engel TA, Fabbri M, Faulkner M, Fiete IR, Findling C, Freitas-Silva L, Gerçek B, Harris KD, Häusser M, Hofer SB, Hu F, Hubert F, Huntenburg JM, Khanal A, Krasniak C, Langdon C, Lau PYP, Mainen ZF, Meijer GT, Miska NJ, Mrsic-Flogel TD, Noel J-P, Nylund K, Pan-Vazquez A, Pouget A, Rossant C, Roth N, Schaeffer R, Schartner M, Shi Y, Socha KZ, Steinmetz NA, Svoboda K, Urai AE, Wells MJ, West SJ, Whiteway MR, Winter O, Witten IB. 2023b. A Brain-Wide Map of Neural Activity during Complex Behaviour. doi: 10.1101/2023.07.04.547681 [DOI] [Google Scholar]
- Jones EAA. 2023. Chronic Recoverable Neuropixels in Mice. [Google Scholar]
- Juavinett AL, Bekheet G, Churchland AK. 2019. Chronically implanted Neuropixels probes enable high-yield recordings in freely moving mice. eLife 8:e47188. doi: 10.7554/eLife.47188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, Lee AK, Anastassiou CA, Andrei A, Aydın Ç, Barbic M, Blanche TJ, Bonin V, Couto J, Dutta B, Gratiy SL, Gutnisky DA, Häusser M, Karsh B, Ledochowitsch P, Lopez CM, Mitelut C, Musa S, Okun M, Pachitariu M, Putzeys J, Rich PD, Rossant C, Sun W, Svoboda K, Carandini M, Harris KD, Koch C, O’Keefe J, Harris TD. 2017. Fully integrated silicon probes for high-density recording of neural activity. Nature 551:232–236. doi: 10.1038/nature24636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupic J, Bauza M, Burton S, O’Keefe J. 2018. Local transformations of the hippocampal cognitive map. Science 359:1143–1146. doi: 10.1126/science.aao4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo TZ, Bondy AG, Gupta D, Elliott VA, Kopec CD, Brody CD. 2020. An approach for long-term, multi-probe Neuropixels recordings in unrestrained rats. eLife 9:e59716. doi: 10.7554/eLife.59716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, Bethge M. 2018. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci 21:1281–1289. doi: 10.1038/s41593-018-0209-y [DOI] [PubMed] [Google Scholar]
- Mimica B, Tombaz T, Battistin C, Fuglstad JG, Dunn BA, Whitlock JR. 2023. Behavioral decomposition reveals rich encoding structure employed across neocortex in rats. Nat Commun 14:3947. doi: 10.1038/s41467-023-39520-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun M, Lak A, Carandini M, Harris KD. 2016. Long Term Recordings with Immobile Silicon Probes in the Mouse Cortex. PLOS ONE 11:e0151180. doi: 10.1371/journal.pone.0151180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachitariu M, Sridhar S, Stringer C. 2023. Solving the spike sorting problem with Kilosort. doi: 10.1101/2023.01.07.523036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Aydin C, Lebedeva A, Okun M, Pachitariu M, Bauza M, Beau M, Bhagat J, Böhm C, Broux M, Chen S, Colonell J, Gardner RJ, Karsh B, Kloosterman F, Kostadinov D, Mora-Lopez C, O’Callaghan J, Park J, Putzeys J, Sauerbrei B, van Daal RJJ, Vollan AZ, Wang S, Welkenhuysen M, Ye Z, Dudman JT, Dutta B, Hantman AW, Harris KD, Lee AK, Moser EI, O’Keefe J, Renart A, Svoboda K, Häusser M, Haesler S, Carandini M, Harris TD. 2021. Neuropixels 2.0: A miniaturized high-density probe for stable, long-term brain recordings. Science 372:eabf4588. doi: 10.1126/science.abf4588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz NA, Zatka-Haas P, Carandini M, Harris KD. 2019. Distributed coding of choice, action and engagement across the mouse brain. Nature 576:266–273. doi: 10.1038/s41586-019-1787-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson IH, Kording KP. 2011. How advances in neural recording affect data analysis. Nat Neurosci 14:139–142. doi: 10.1038/nn.2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD. 2019. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364:eaav7893. doi: 10.1126/science.aav7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Daal RJJ, Aydin Ç, Michon F, Aarts AAA, Kraft M, Kloosterman F, Haesler S. 2021. Implantation of Neuropixels probes for chronic recording of neuronal activity in freely behaving mice and rats. Nat Protoc 16:3322–3347. doi: 10.1038/s41596-021-00539-9 [DOI] [PubMed] [Google Scholar]
- Wang ZA, Chen S, Liu Y, Liu D, Svoboda K, Li N, Druckmann S. 2023. Not everything, not everywhere, not all at once: a study of brain-wide encoding of movement. doi: 10.1101/2023.06.08.544257 [DOI] [Google Scholar]
- Windolf C, Yu H, Paulk AC, Meszéna D, Muñoz W, Boussard J, Hardstone R, Caprara I, Jamali M, Kfir Y, Xu D, Chung JE, Sellers KK, Ye Z, Shaker J, Lebedeva A, Raghavan M, Trautmann E, Melin M, Couto J, Garcia S, Coughlin B, Horváth C, Fiáth R, Ulbert I, Movshon JA, Shadlen MN, Churchland MM, Churchland AK, Steinmetz NA, Chang EF, Schweitzer JS, Williams ZM, Cash SS, Paninski L, Varol E. 2023. DREDge: robust motion correction for high-density extracellular recordings across species. doi: 10.1101/2023.10.24.563768 [DOI] [Google Scholar]