Abstract
The nucleoid-associated protein H-NS is a major component of the chromosome-protein complex, and it is known to influence the regulation of many genes in Escherichia coli. Its role in gene regulation is manifested by the increased expression of several gene products in hns mutant strains. Here we report findings showing that H-NS and the largely homologous protein StpA play a positive role in the expression of genes in the maltose regulon. In studies with hns mutant strains and derivatives also deficient in the stpA gene, we found that expression of the LamB porin was decreased. Our results showed that the amounts of both LamB protein and lamB mRNA were greatly reduced in hns and hns-stpA mutant strains. The same results were obtained when we monitored the amount of transcription from the malEFG operon. The lamB gene is situated in the malKlamBmalM operon, which forms a divergent operon complex together with the malEFG operon. The activation of these genes depends on the action of the maltose regulon activator MalT and the global activator cyclic AMP receptor protein. Using a malT-lacZ translational fusion and antiserum raised against MalT to measure the expression of MalT, we detected reduced MalT expression in hns and hns-stpA mutant strains in comparison with the wild-type strain. Our results suggest that the H-NS and StpA proteins stimulate MalT translation and hence play a positive role in the control of the maltose regulon.
The nucleoid-associated protein H-NS is known to bind in a relatively nonspecific manner to double-stranded DNA, with some preference for curved sequences, and it has also been suggested to cause compaction of the chromosome (14, 23, 33, 34, 40, 45). Mutations in the gene coding for H-NS, hns, result in elevated expression of several other gene products, and it appears that H-NS may cause silencing of many different operons (reviewed in reference 2). Furthermore, two-dimensional gel electrophoretic studies have suggested that the expression of some genes is stimulated by the H-NS protein (21, 44). For example, in the case of the expression of flagellin genes, there seems to be stimulation, since the lack of a functional H-NS protein abolishes the motility of Escherichia coli (5). However, the molecular basis or mechanism by which H-NS affects motility has not yet been revealed.
In addition to the hns gene in E. coli, a gene denoted stpA encodes an H-NS-like protein. The StpA protein has 58% amino acid identity with H-NS, and the gene was found as a multicopy suppressor of the Td− phenotype of a splicing-defective phage T4 td intron mutant (42, 43). Although a mutation in the stpA gene seems to affect the expression of only a few proteins, the number of proteins affected in an hns-stpA double mutant is greater than that affected in an hns mutant (44). Furthermore, the expression of StpA is increased in hns mutant E. coli, and recent studies have established that StpA is able to function as a molecular backup for H-NS and thereby affect the expression of some genes (32, 44).
In preliminary studies with hns mutant E. coli, we observed effects on the expression of the LamB protein (9). The gene lamB, which encodes the LamB protein, is part of the maltose regulon, which consists of at least 10 genes and which has been extensively studied (7, 30). The LamB porin works as a diffusion pore for maltodextrins (18) but is not necessary when maltose is present at high concentrations (35). It is also known as the bacteriophage lambda receptor (25). Additionally, the LamB protein can mediate the binding of basement membrane laminin by E. coli in vitro (37).
The expression of the malKlamBmalM operon and that of the divergent malEFG operon are directly controlled at the transcriptional level by the cyclic AMP (cAMP)-cAMP receptor protein (CRP) complex and the MalT protein, the activator of the maltose regulon (27, 30). In this study, we investigated the involvement of the H-NS and StpA proteins in the regulation of the malKlamBmalM and malEFG operons. We also examined their role in the regulation of the malT gene, which encodes MalT and which is controlled by cAMP-CRP (24). Our results suggest positive roles for both the H-NS and the StpA proteins at the level of MalT and lamB or malE expression.
MATERIALS AND METHODS
Bacterial strains.
The strains and plasmids used in this study are shown in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| BSN1 | M182 hns::Cmr | 16 |
| DK2179 | MC4100 φ42-1 lamB-lacZ | 31 |
| BDG100 | MC4100 φ42-1 lamB-lacZ hns::Cmr | This study |
| JGJ100 | MC4100 φ42-1 lamB-lacZ stpA60::Kmr | This study |
| JGJ101 | MC4100 φ42-1 lamB-lacZ hns::CmrstpA60::Kmr | This study |
| BSN5 | MC1061 stpA60::Kmr | 32 |
| pop2481 | MC4100 malT′-lac′ZYA | 24 |
| BEU603 | Δhns trp::Tn10 | 32 |
| JGJ102 | pop2481 trp::Tn10 | This study |
| JGJ103 | pop2481 trp::Tn10 Δhns | This study |
| JGJ104 | pop2481 stpA60::Kmr | This study |
| JGJ105 | pop2481 trp::Tn10 stpA60::Kmr | This study |
| JGJ106 | pop2481 trp::Tn10 Δhns stpA60::Kmr | This study |
| JGJ107 | MC4100 mlc | This study |
| JGJ108 | JGJ107 trp::Tn10 | This study |
| JGJ109 | JGJ107 trp::Tn10 Δhns | This study |
| JGJ110 | JGJ107 trp::Tn10 stpA60::Kmr | This study |
| JGJ111 | JGJ107 trp::Tn10 Δhns stpA60::Kmr | This study |
| MC4100 | rpoS+ Δ(argF-lac) | 8 |
| BSN26 | MC4100 trp::Tn10 | This study |
| BSN27 | MC4100 trp::Tn10 Δhns | This study |
| BSN28 | MC4100 trp::Tn10 stpA60::Kmr | This study |
| BSN29 | MC4100 trp::Tn10 Δhns stpA60::Kmr | This study |
| BSN19 | MC4100 stpA60::Kmr | This study |
| Plasmids | ||
| pHA7* | crp* gene on pBR332 | 36 |
| pBR322 | Cloning vector | 6 |
| pJGJ101 | ′mlc′ fragment cloned into pCH257 | This study |
| pCH257 | Cloning vector | 15 |
Growth media and culture conditions.
The different strains were grown aerobically in Luria-Bertani (LB) media (4) at 30 or 37°C with vigorous shaking (220 rpm). Growth was monitored by measuring Klett units on a Klett-Summerson colorimeter, where 50 Klett units approximately corresponds to an optical density at 600 nm of 0.4. When necessary, antibiotics were used at the following concentrations: carbenicillin, 50 μg/ml; chloramphenicol, 10 μg/ml; kanamycin, 25 or 50 μg/ml; and tetracycline, 7.5 μg/ml. Plates containing 40 μg of the chromogenic β-glucoside 5-bromo-4-chloro-3-indolyl β-d-glucopyranoside (Sigma) per ml were used to monitor the Bgl phenotype.
Genetic techniques.
Standard recombinant procedures were performed essentially as described by Sambrook et al. (29). Generalized bacteriophage P1 transduction was done as described by Willets et al. (39). BDG100 (hns::Cmr) was derived from DK2179 (MC4100 carrying the φ42-1 lamB-lacZ fusion and referred to as pop3186 in reference 31). DK2179 was transduced with P1 grown on BSN1 (hns::Cmr), and transductants were selected for growth on chloramphenicol plates. JGJ100 (stpA60::Kmr) was derived from DK2179. DK2179 was transduced with P1 grown on BSN5 (stpA60::Kmr). JGJ101 (hns::Cmr stpA60::Kmr) was derived from BDG100 transduced with P1 grown on BSN5 (stpA60::Kmr). Both JGJ100 and JGJ101 were isolated by selection for kanamycin resistance. JGJ102 (trp::Tn10) and JGJ103 (Δhns trp::Tn10) were derived from pop2481 (MC4100 carrying a malT′-lac′ZYA fusion [24]). pop2481 was transduced with P1 grown on BEU603 (Δhns trp::Tn10; the Δhns allele used here was formally denoted drdX [19]), and transductants were selected for growth on tetracycline plates. The strain demonstrating a negative Bgl phenotype (hns+) was named JGJ102, and the strain demonstrating a positive Bgl phenotype (hns) was named JGJ103. JGJ104 (stpA60::Kmr) was derived from pop2481 transduced with P1 grown on BSN5 (stpA60::Kmr). Transductants were selected on kanamycin plates and thereafter transduced with P1 grown on BEU603 (Δhns trp::Tn10). Transductants were selected for growth on tetracycline plates. The strain with a negative Bgl phenotype (hns+) was named JGJ105, and the strain with a positive Bgl phenotype (hns) was named JGJ106. BSN26 and BSN27 were derived from MC4100. MC4100 was transduced with P1 grown on BEU603 (Δhns trp::Tn10), and transductants were isolated by selection for tetracycline resistance. The strain with a negative Bgl phenotype (hns+) was named BSN26, and the strain with a positive Bgl phenotype (hns) was named BSN27. To construct BSN28 and BSN29, we transduced MC4100 with P1 grown on BSN5 (stpA60::Kmr), and a kanamycin-resistant clone (stpA) was isolated and named BSN19. BSN19 was subsequently transduced with P1 grown on BEU603 (Δhns trp::Tn10). Transductants were isolated by selection for tetracycline resistance. The strain with a negative Bgl phenotype was named BSN28, and the strain with a positive Bgl phenotype was named BSN29.
For construction of an Mlc-deficient derivative, a 320-bp fragment containing DNA from positions +6 to +317 (as measured from the A in ATG) of the Mlc-encoding gene was PCR amplified with Pfu polymerase (Stratagene) by use of primer mlc-4 (5′-GCTCTAGAGCTGAAAACCAGCCTGGGC-′3 [underlining indicates an additional XbaI site]) and primer mlc-5 (5′-CTGCTCAGATCGCGCAGAGC-′3). The obtained fragment was digested with the XbaI enzyme and inserted into an XbaI-EcoRV-digested preparation of plasmid vector pCH257 (15). Plasmid pCH257 is a suicide vector. The resulting plasmid, pJGJ101, was integrated into the chromosome of BSN26 by a single recombination event between the 312-bp long mlc sequence in our construct and the corresponding region of mlc on the chromosome. The resulting strain was denoted JGJ107. JGJ108 (trp::Tn10), JGJ109 (Δhns trp::Tn10), JGJ110 (trp::Tn10 stpA60::Kmr), and JGJ111 (Δhns trp::Tn10 stpA60::Kmr) were derived from BSN26, BSN27, BSN28, and BSN29, respectively. BSN26, BSN27, BSN28, and BSN29 were transduced with P1 grown on JGJ107 (mlc), and transductants were isolated by selection for chloramphenicol resistance.
SDS-PAGE and immunoblot analysis.
For determination of the LamB and MalT protein contents in cells, samples were taken at 50 Klett units from cultures that had been growing exponentially for several generations. The cells were pelleted by centrifugation and resuspended in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer. The different samples were subjected to SDS-PAGE (the polyacrylamide concentrations were 10% for LamB detection and 8% for MalT detection) and thereafter blotted onto a 0.2-μm-pore-size polyvinylidene difluoride transfer membrane (Bio-Rad) by use of a semidry blotting apparatus. Polyclonal rabbit antiserum raised against either LamB (37) or MalT was used as the primary antibody. Subsequently, the treatment of the membrane essentially followed the Vistra Fluorescence Western blotting kit protocol, with the exception that we incubated the membrane with an alkaline phosphatase-linked anti-rabbit antibody (Dakopatt) after the membrane had been incubated with the respective primary antibody. Furthermore, the membrane was developed with AttoPhos substrate (JBL Scientific Inc.) and scanned with the STORM system (Molecular Dynamics). The amounts of the different proteins were quantified by use of the ImageQuant program (Molecular Dynamics). All data represent the average from at least two independent experiments, and the means + (maximum−minimum)/2 are shown in the plotted graphs.
β-Galactosidase assay.
For the β-galactosidase assay, all samples were taken at 50 Klett units. The β-galactosidase reactions were assayed essentially as described by Miller (22), with the exception that we used chloroform and 0.002% SDS to disrupt the bacteria. All data represent the average from assays performed in duplicate in three independent experiments, and the means + (maximum−minimum)/2 are shown in the plotted graphs.
RNA extraction and Northern blot analysis.
At 50 Klett units, RNA was extracted from bacterial cultures grown at both 30 and 37°C by the hot phenol method as described by von Gabain et al. (38). Twenty micrograms of each RNA sample was loaded onto the gel. RNA blotting and hybridization were performed essentially as described by Arnqvist et al. (1). The probes used for analyzing the expression of the lamB and malE mRNAs were the following [γ-32P]ATP kinase-labelled oligonucleotides: for lamB, 5′-GTCTGGAAACACTGTTGTTCACCGCCGCTACCTGTCCAAC-3′; for malE, 5′GGCGAGAGCCGAGGCGGAAAACATCATCGTCGTTAATGCGGATAATGCGAGG-3′. Washing of the membrane was performed with a Hybaid oven in accordance with the manufacturer’s protocol. Bands were visualized and quantified by use of a PhosphorImager (Molecular Dynamics) and the ImageQuant program. The amount of labelled mRNA after hybridization was quantified and divided by the value for the wild type grown at either 30 or 37°C in the absence of maltose (arbitrarily set to 1.0).
RESULTS
The H-NS and StpA proteins are required for full expression of the LamB protein.
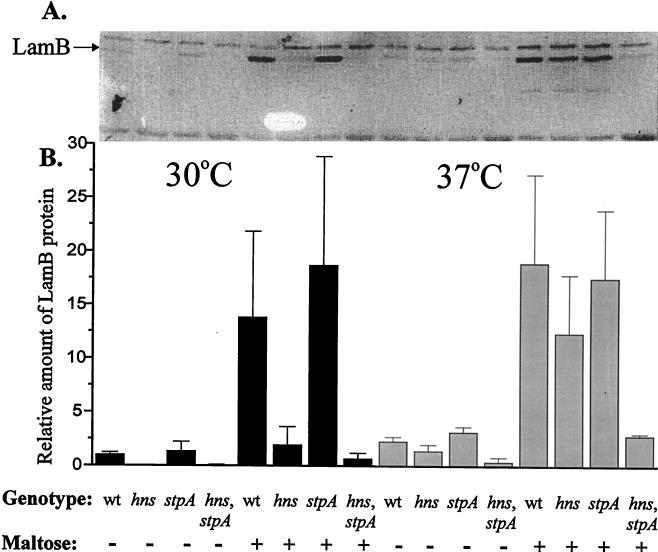
To monitor the possible role of the H-NS and StpA proteins in LamB expression, we constructed strains with nonfunctional hns and/or stpA genes. LamB expression was measured in the middle of the logarithmic growth phase, and the amount of LamB was determined by Western blotting with a polyclonal antiserum raised against LamB (see Materials and Methods). We performed the experiments at both 30 and 37°C, since it has been shown that the expression of StpA is temperature controlled (17, 32). The maltose regulon was induced by the addition of 0.2% maltose. In cultures supplemented with maltose, the levels of LamB protein were reduced to 14% at 30°C and 65% at 37°C in an hns mutant strain (BSN27) in comparison with the wild-type strain (BSN26) (Fig. 1). The same effect was observed for cultures grown in the absence of maltose. However, we could not detect any differences in induced or noninduced LamB levels in an stpA mutant strain (BSN28) at either 30 or 37°C (Fig. 1). Interestingly, in cultures supplemented with maltose, the levels of LamB protein were reduced to approximately 5% at 30°C and 15% at 37°C in an hns-stpA double-mutant strain (BSN29) in comparison with the wild-type strain (Fig. 1). When the corresponding measurements were made for cultures grown in the absence of maltose, similar patterns were obtained.
FIG. 1.
(A) Quantitative determination of LamB protein content by Western blotting of wild-type (wt) (BSN26), hns (BSN27), stpA (BSN28), and hns-stpA (BSN29) strains. The cells were grown in Luria-Bertani medium with (+) or without (−) 0.2% maltose at either 30 or 37°C. At 50 Klett units, the cells were harvested and then treated as described in Materials and Methods. (B) The relative amounts of LamB protein were obtained by dividing the values for the mutant strains by the value for the wild-type strain grown in the absence of maltose at 30°C (arbitrarily set to 1.0).
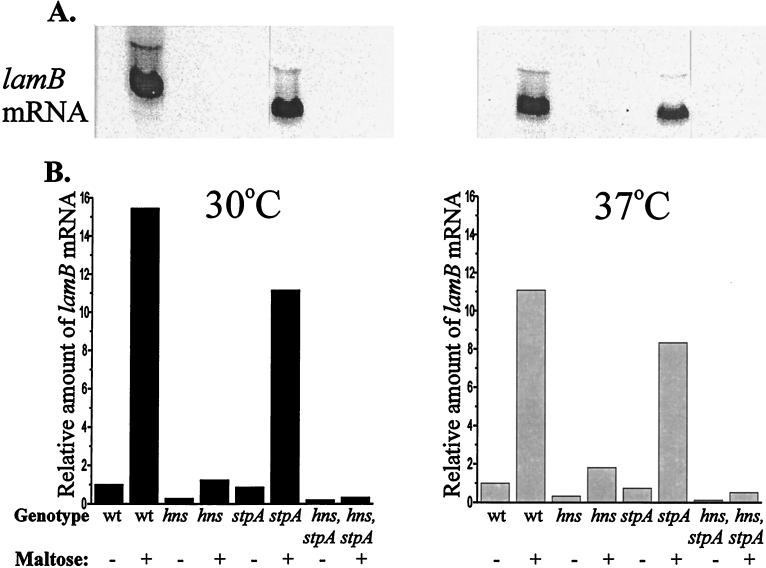
In order to determine if the effects exerted by the H-NS and StpA proteins occurred at the translational or the transcriptional level, we isolated total RNA and performed Northern blot analysis with a probe complementary to the 5′ end of the transcribed lamB gene (see Materials and Methods). The data are presented in Fig. 2, and the results were in good agreement with the results obtained from the Western blot experiment described above. We found a reduction in the amount of maltose-induced lamB mRNA at 37°C from both the hns and the hns-stpA strains, BSN27 and BSN29, respectively (Fig. 2). The reduction was somewhat larger than that observed when the amount of LamB protein was analyzed by the Western blot technique in the corresponding experiment (Fig. 1). The level of lamB mRNA in the stpA mutant strain (BSN28) was slightly lower than that in the wild-type strain. Taken together, the results of the Western and Northern blot analyses strongly suggest that the effects of H-NS and StpA on the regulation of LamB expression are exerted at the transcriptional level.
FIG. 2.
(A) Northern blot analysis of lamB transcripts at 50 Klett units for wild-type (wt) (BSN26), hns (BSN27), stpA (BSN28), and hns-stpA (BSN29) strains grown with (+) or without (−) 0.2% maltose at 30 and 37°C. The samples were treated as described in Materials and Methods. (B) The relative amounts of lamB mRNA were obtained by dividing the values for the mutant strains by the value for the wild-type strain grown in the absence of maltose at either 30 or 37°C (arbitrarily set to 1.0).
Effect of hns and stpA mutations on the expression of the malE operon.
Activation of both the malEFG promoter and the divergent malKlamBmalM promoter requires the formation of an activation complex in the intercistronic region by the MalT protein and the cAMP-CRP complex (26, 28). We therefore studied if the H-NS and/or StpA proteins also could influence expression from the malEFG operon. By reprobing the Northern filters from Fig. 2 with a probe complementary to the 5′ end of the malE gene, we could detect and quantitate differences in the levels of malE mRNA (Fig. 3). As was observed with lamB mRNA, a large reduction in the amount of malE mRNA was detected in the hns strain (BSN27) and the hns-stpA double-mutant strain (BSN29) in comparison with the wild-type strain (BSN26) at 30°C. The effect was less pronounced after growth at 37°C in cultures supplemented with maltose. The low level of malE mRNA expressed in the absence of maltose was reduced in a similar fashion in the hns and hns-stpA double-mutant strains at both 30 and 37°C. As with lamB mRNA, we did observe slightly lower levels of malE mRNA in the stpA mutant strain (BSN28) than in the wild-type strain.
FIG. 3.
(A) Northern blot analysis of malE transcripts at 50 Klett units for wild-type (wt) (BSN26), hns (BSN27), stpA (BSN28), and hns-stpA (BSN29) strains grown with (+) or without (−) 0.2% maltose at 30 and 37°C. The samples were treated as described in Materials and Methods. (B) The relative amounts of malE mRNA were obtained by dividing the values for the mutant strains by the value for the wild-type strain grown in the absence of maltose at either 30 or 37°C (arbitrarily set to 1.0).
MalT expression is reduced in hns and hns-stpA mutant strains.
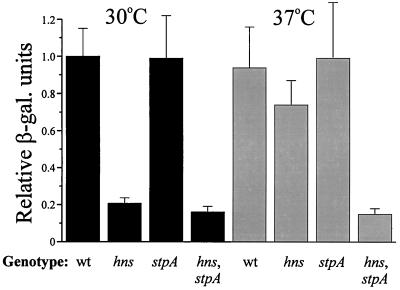
The MalT protein is a transcriptional activator essential for the expression of all the genes in the maltose regulon, except the malT gene itself. We therefore examined if the level of the MalT protein was altered in H-NS- and/or StpA-deficient strains. As a reporter system to measure MalT expression, we used a chromosomal malT-lacZ gene fusion (24). In order to study if the temperature effect seen at the level of lamB and malE expression also was manifested at the level of MalT expression, β-galactosidase assays were performed at both 30 and 37°C. At 30°C, we observed that the expression of the malT-lacZ fusion was reduced to approximately 20% in the hns (JGJ103) and hns-stpA (JGJ106) strains in comparison with the wild-type strain (JGJ102) (Fig. 4). This pattern was essentially repeated at 37°C, except that the level of β-galactosidase was only reduced to 78% in the hns single-mutant strain in comparison with the wild-type strain. We could not detect any significant differences in the expression of the malT-lacZ fusion between the stpA mutant strain (JGJ105) and the wild-type strain at either 30 or 37°C.
FIG. 4.
Measurement of MalT expression with a malT-lacZ translational fusion. Wild-type (wt) (JGJ102), hns (JGJ103), stpA (JGJ105), and hns-stpA (JGJ106) strains were grown in Luria-Bertani medium at either 30 or 37°C and harvested at 50 Klett units. β-Galactosidase (β-gal.) activity was monitored as described in Materials and Methods. The relative expression was plotted, and the values for the mutant strains were divided by the value for the wild-type strain grown at 30°C (arbitrarily set to 1.0). All data are the average of three independent experiments.
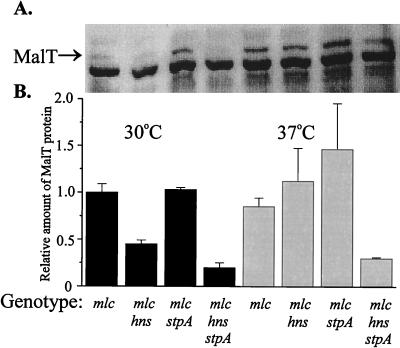
Recently, Decker et al. (10) discovered that the expression of the malT gene was repressed by a protein named Mlc (making large colonies [20]). A direct interaction was demonstrated between the Mlc protein and the malT leader region (from positions +1 to +23), and there was an approximately threefold-higher level of expression of malT in an mlc mutant derivative than in the wild type (10). To assess if the decreased expression of malT seen in hns and hns-stpA strains was due to the increased expression of the Mlc repressor protein, we constructed an mlc mutant derivative (JGJ107; see Materials and Methods for details) and introduced the mutation into strains with appropriate genetic backgrounds, generating an mlc mutant strain (JGJ108), an mlc-hns mutant strain (JGJ109), an mlc-stpA mutant strain (JGJ110), and an mlc-hns-stpA mutant strain (JGJ111). As shown in Fig. 5, the level of the MalT protein was reduced in the mlc-hns (30°C) and mlc-hns-stpA (30 and 37°C) strains to approximately the same levels as those shown above for the hns and hns-stpA strains with the mlc locus intact (Fig. 4). The results ruled out the possibility of an indirect effect via Mlc repression of malT transcription as the reason for the effects of H-NS and StpA found. Furthermore, a malT-lacZ translational fusion with a deletion in the upstream region (extending from position −123 in malTp to position −21 in malPp [24]) gave results similar to those obtained with the construct having the transcriptional regulatory region intact (Fig. 4 and data not shown). Attempts to directly monitor and quantify the mRNA of the malT locus were unsuccessful, presumably because of its very low abundance and/or low stability. However, we learned from M. Heyde (19a) that tests with a malT-lacZ transcriptional fusion indicated that malT transcription was not affected by H-NS. Using the same operon fusion, we obtained similar results with derivatives showing H-NS and/or StpA deficiency (data not shown). The effect on malT expression therefore appeared to be exerted at the level of translation.
FIG. 5.
(A) Determination of MalT content in different genetic backgrounds by Western blotting. mlc (JGJ108), mlc-hns (JGJ109), mlc-stpA (JGJ110), and mlc-hns-stpA (JGJ111) strains were grown in Luria-Bertani medium at either 30 or 37°C. The cells were harvested at 50 Klett units and then treated as described in Materials and Methods. (B) Plot of the relative levels of MalT protein detected in mlc mutant strains. The different values were divided by the value for the mlc strain grown at 30°C (arbitrarily set to 1.0).
We also performed Western blot experiments (see Materials and Methods) by using a polyclonal MalT antiserum and extracts from different strains (i.e., wild type, BSN26; hns, BSN27; stpA, BSN28; and hns-stpA, BSN29). The bacteria were grown at both 30 and 37°C. The results obtained (data not shown) confirmed the findings obtained with the malT-lacZ fusion, and we conclude that full MalT expression requires the presence of H-NS.
The expression of the LamB protein in hns and hns-stpA strains can be restored by CRP* at 37°C but not at 30°C.
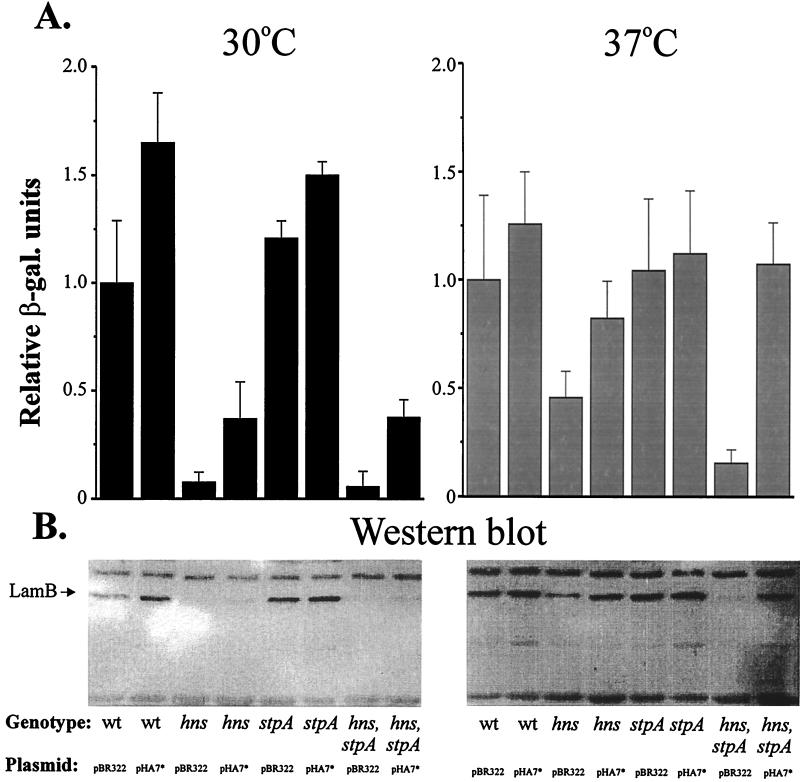
We recently observed that the cellular level of CRP might be somewhat reduced in hns and hns-stpA mutant strains (unpublished data). With this in mind, we needed to consider the possibility that the decreased amount of LamB protein in hns and hns-stpA mutant strains was caused by a reduced level of CRP protein and/or a reduced level or activity of the adenylate cyclase protein. Therefore, we performed experiments in which the level of active CRP in the strains was restored by the addition of a plasmid (pHA7*) containing the crp* gene under the control of the bla promoter. The CRP* protein contains an amino acid substitution (G141D), making it independent of cAMP for its activity (36). We monitored the expression of LamB by using a lamB-lacZ translational fusion (31), and the strains were grown with 0.2% maltose in the growth medium at 30 or 37°C. The results are shown in Fig. 6A. The presence of CRP* led to the restoration of LamB expression at 37°C in hns and hns-stpA mutant strains; the expression levels were similar to that in a wild-type strain. At 30°C, a partial plasmid suppression effect (five- to sevenfold) was detected when the hns (BDG100) and hns-stpA (JGJ101) strains were supplied with CRP* (pHA7*), but it was notable that the level of LamB expression at that temperature in these strains only reached about 40% the level in the wild-type strain. We could not detect any significant differences in the level of LamB expression in an stpA strain (JGJ100) transformed with pHA7* in comparison with a wild-type strain under either of the conditions tested (30 or 37°C with 0.2% maltose).
FIG. 6.
(A) Determination of LamB levels with a lamB-lacZ fusion in strains containing crp*. Wild-type (wt) (DK2179), hns (BDG100), stpA (JGJ100), and hns-stpA (JGJ101) strains were transformed with either pBR322 or pHA7* (crp*). The cells were grown in Luria-Bertani medium containing 0.2% maltose at either 30 or 37°C and harvested at 50 Klett units. β-Galactosidase (β-gal.) activity was monitored as described in Materials and Methods. The different values were divided by the value for the wild-type strain with pBR322 grown at either 30 or 37°C (arbitrarily set to 1.0). All data are the average of three independent experiments. The relative value of 1.0 corresponds to 1,837 Miller units at 30°C and 1,200 Miller units at 37°C. (B) Determination of LamB protein content by Western blotting of wild-type (BSN26), hns (BSN27), stpA (BSN28), and hns-stpA (BSN29) strains transformed with either pBR322 or pHA7*. The cells were grown in Luria-Bertani medium supplemented with 0.2% maltose at either 30 or 37°C. At 50 Klett units, the cells were harvested and then treated as described in Materials and Methods.
We also performed Western blot experiments with the LamB antiserum (see Materials and Methods) and whole-cell extracts from cultures of strains carrying pHA7* or the vector and grown at both 30 and 37°C in the presence of 0.2% maltose (Fig. 6B). The results essentially confirmed the expression patterns seen with the lamB-lacZ fusion (Fig. 6A) at both temperatures. Altogether, these data suggest that a reduced level of cAMP-CRP in hns and hns-stpA strains may be one of the reasons why malE and lamB expression is reduced in hns and hns-stpA strains.
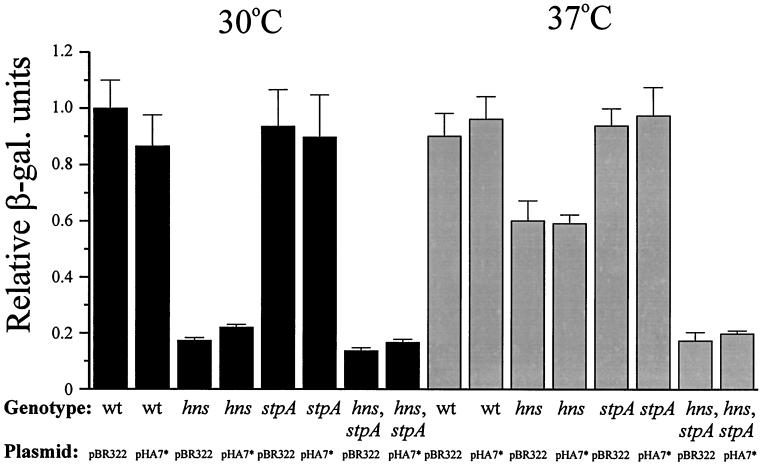
The expression of the MalT protein is not restored by CRP* in hns and hns-stpA mutant strains.
Since the malT promoter is controlled by the cAMP-CRP complex, we considered the possibility that the decreased expression of MalT observed in hns and hns-stpA strains was in part also due to a reduced level of cAMP-CRP. To test this hypothesis, we added pHA7* to the malT-lacZ fusion strains to see if excess active CRP levels would restore the levels of MalT expression. We did not detect any appreciably increased levels of MalT expression as a result of the pHA7* plasmid being present in the strains at either 30 or 37°C (Fig. 7). These results suggest that in the wild-type and mutant strains, the concentration of cAMP-CRP was high enough to saturate the CRP binding site of the malT promoter and hence that the level of cAMP-CRP was not the limiting factor causing decreased amounts of MalT in the hns strain (JGJ103) at 30°C and the hns-stpA strain (JGJ106) at 30 and 37°C. We conclude that the low levels of MalT detected in these strains were due to the absence of H-NS, which appeared to play a positive role in malT gene expression at the level of translation.
FIG. 7.
β-Galactosidase (β-gal.) activity from a malT-lacZ translational fusion in the presence of crp*. Wild-type (wt) (JGJ102), hns (JGJ103), stpA (JGJ105), and hns-stpA (JGJ106) strains were transformed with either pBR322 or pHA7* (crp*). The cells were grown in Luria-Bertani medium at either 30 or 37°C and harvested at 50 Klett units. β-Galactosidase activity was monitored as described in Materials and Methods. The different values were divided by the value for the wild-type strain with pBR322 grown at 30°C (arbitrarily set to 1.0). All data are the average of three independent experiments.
DISCUSSION
The present data show that the H-NS and StpA proteins play a positive role in the expression of some genes of the maltose regulon. In the absence of both H-NS and StpA, the expression of the divergently oriented malKlamBmalM and malEFG operons was almost completely abolished. One major effect was shown to be mediated at the level of the regulation of expression of the MalT protein, which is the main regulator of the maltose regulon. The absence of the H-NS and StpA proteins resulted in a reduction in the levels of MalT to about 20% the normal level. The fact that CRP* overproduction did not suppress this effect excluded the possibility that it was due solely to a decrease in the level of active CRP protein, the activator of the malT promoter, observed in hns and hns-stpA strains (unpublished data). Altogether, these results indicate that H-NS and StpA positively control malT expression independently of their effects on CRP level.
It appears that in most cases, H-NS influences gene expression at the transcriptional level, and it was a feasible hypothesis that the observed effect in the case of malT was due to facilitated activation of transcription. Expression from the malT gene is dependent on correct positioning of the cAMP-CRP complex with respect to the RNA polymerase and requires correct DNA topology (11). The H-NS and StpA proteins may stabilize a favorable DNA conformation that contributes to direct contact between the cAMP-CRP complex and the RNA polymerase and favors the formation of a productive initiation complex (13). Note that the expression of MalT in an hns-stpA strain was not totally abolished; there was still residual 20% expression. This result argued for a mechanism in which the H-NS and StpA proteins are not absolutely required components in the activation process. Rather, they may work as architectural components, presumably through alteration of the DNA conformation.
Alternatively, H-NS and StpA may act at the translational level or act indirectly through negative control of a gene encoding a repressor of the expression of the malT gene. A recent report (10) showed that the transcription of malT is negatively controlled by the mlc gene product and that a loss of Mlc function leads to a threefold increase in malT expression. At present, it is not known if expression of the mlc gene is affected by H-NS and/or StpA, but evidently Mlc is subject to autoregulation and represses its own expression (10). The autoregulation presumably keeps the Mlc level rather constant; this idea argues against the hypothesis that the effect of H-NS deficiency is mediated via Mlc. A direct test with mlc mutant derivatives confirmed that the H-NS effect was not dependent on Mlc (Fig. 5). Our results from preliminary studies with a malT-lacZ transcriptional fusion furthermore suggested that the effect may not be exerted primarily at the transcriptional level but that the H-NS and StpA proteins are important for malT translation. The exact mode of action of H-NS and StpA in malT translation remains to be elucidated.
An involvement of H-NS in posttranscriptional regulation was seen earlier, as H-NS was implicated in the quantitative control of the RpoS sigma factor (3, 41). In that case, translation of the rpoS mRNA appeared to increase in the absence of H-NS, suggesting the hypothesis that H-NS may act by repressing translational induction of rpoS (3). The results of the present study with the maltose regulon imply that H-NS may stimulate translation and add a novel aspect to the physiological role of H-NS and StpA.
The observed severe decrease in malEFG and malKlamBmalM transcription as well as LamB levels in hns and hns-stpA mutant strains was most likely caused by the combined and additive effects of the lower levels of both MalT and CRP which characterized these strains (this work and unpublished data).
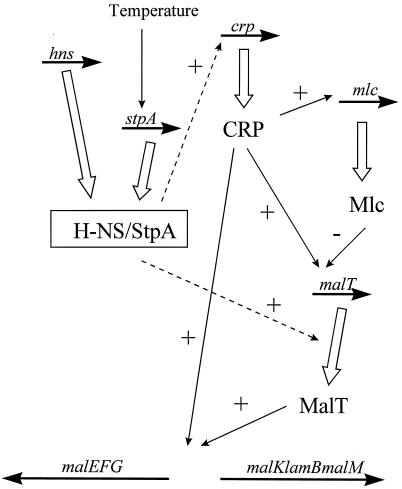
We also noted a temperature effect. For example, the lack of the H-NS protein resulted in lower expression of the MalT protein at 30°C than at 37°C. This observation was consistent with the finding that CRP* overproduction did not restore lamB expression by complementing for the lack of H-NS and StpA at 30°C. Our results are the first example which show that the H-NS protein may play a positive role concomitant with thermoregulated activity. In several other cases of thermoregulated gene expression, the H-NS protein (and presumably also the StpA protein) has been found to play a negative role at the transcriptional level (reviewed in, e.g., references 2 and 12). Interestingly, the expression of MalT was greatly reduced in an hns-stpA strain at both 30 and 37°C. This result, together with the result obtained with the hns strain, suggests that the StpA protein is able to substitute and function as a molecular backup for the activating role of the H-NS protein at 37°C but not at 30°C. This idea is consistent with the finding that the level of the StpA protein was lower at 26°C than at 37°C (32). This finding may suggest a possible thermal regulation role for StpA, and it remains to be elucidated if and how such a role may affect gene expression under different physiological conditions. A summary showing schematically how and where H-NS and StpA may influence gene expression in the maltose regulon is presented in Fig. 8.
FIG. 8.
Schematic summary of how the H-NS and StpA proteins influence gene expression in the maltose regulon. The relevant genes are indicated above horizontal arrows. Gene products and their mode of action are indicated by thick open and thin solid vertical or diagonal arrows, respectively. Broken arrows show where H-NS and StpA influence gene expression. +, stimulatory; −, inhibitory.
It is becoming increasingly evident that nucleoid-associated proteins such as H-NS may play important roles as participants in transcriptional regulatory mechanisms and as modulators and/or architectural components. The present work extends the scope of the mechanisms in which H-NS and StpA are involved and establishes that their roles can be the stimulation of the expression of certain gene products. These findings will prompt further analysis of how H-NS and StpA interact at malT and affect the expression of the maltose regulon. This analysis should yield new information about how these proteins work and about their role in bacterial cell physiology and gene regulation.
ACKNOWLEDGMENTS
We thank T. J. Silhavy for strain DK2179, H. Aiba for plasmid pHA7*, and M. Heyde for supplying the malT-lacZ transcriptional fusion. We also thank Berit Sondén for valuable suggestions.
This work was supported by grants from the Swedish Natural Science Research Council, the Swedish Medical Research Council, and the Göran Gustafsson Foundation for Research in Natural Sciences and Medicine.
REFERENCES
- 1.Arnqvist A, Olsén A, Pfeifer J, Russel D G, Normark S. The Crl protein activates cryptic genes for curli formation and fibronectin binding in Escherichia coli HB101. Mol Microbiol. 1992;6:2443–2452. doi: 10.1111/j.1365-2958.1992.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 2.Atlung T, Ingmer H. H-NS: a modulator of environmentally regulated gene expression. Mol Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- 3.Barth M, Marschall C, Muffler A, Fischer D, Hengge-Aronis R. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of ςs and many ςs-dependent genes in Escherichia coli. J Bacteriol. 1995;177:3455–3464. doi: 10.1128/jb.177.12.3455-3464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertin P, Terao E, Hee Lee E, Lejeune P, Colson C, Danchin A, Collatz E. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J Bacteriol. 1994;176:5537–5540. doi: 10.1128/jb.176.17.5537-5540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 7.Boos W, Shuman H. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev. 1998;62:204–229. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 9.Dagberg B. Regulation of virulence-associated genes in Escherichia coli: involvement of the histone-like protein H-NS. Umeå University medical dissertations, new series no. 375. Umeå, Sweden: Umeå University; 1993. [Google Scholar]
- 10.Decker K, Plumbridge J, Boos W. Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol Microbiol. 1998;27:381–390. doi: 10.1046/j.1365-2958.1998.00694.x. [DOI] [PubMed] [Google Scholar]
- 11.Déthiollaz S, Eichenberger P, Gieselmann J. Influence of DNA geometry on transcriptional activation in Escherichia coli. EMBO J. 1996;15:5449–5458. [PMC free article] [PubMed] [Google Scholar]
- 12.Dorman C J. Genetics of bacterial virulence. Oxford, England: Blackwell Scientific Publications; 1994. [Google Scholar]
- 13.Eichenberger P, Déthiollaz S, Buc H, Gieselmann J. Structural kinetics of transcription activation at the malT promoter of Escherichia coli by UV-laser footprinting. Proc Natl Acad Sci USA. 1997;94:9022–9027. doi: 10.1073/pnas.94.17.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falconi M, Gualtieri M T, La Teana A, Losso M A, Pon C L. Proteins from the prokaryotic nucleoid: primary and quaternary structure of the 15-kD Escherichia coli DNA binding protein H-NS. Mol Microbiol. 1988;2:323–329. doi: 10.1111/j.1365-2958.1988.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg Å J, Pavitt G D, Higgins C F. Use of transcriptional fusions to monitor gene expression: a cautionary tale. J Bacteriol. 1994;176:2128–2132. doi: 10.1128/jb.176.7.2128-2132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forsman K, Sondén B, Göransson M, Uhlin B E. Antirepression function in Escherichia coli for the cAMP-cAMP receptor protein transcriptional activator. Proc Natl Acad Sci USA. 1992;89:9880–9884. doi: 10.1073/pnas.89.20.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Free A, Dorman C J. The Escherichia coli stpA gene is transiently expressed during growth in rich medium and is induced in minimal medium and by stress conditions. J Bacteriol. 1997;179:909–918. doi: 10.1128/jb.179.3.909-918.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freundlieb S, Ehmann U, Boos W. Facilitated diffusion of p-nitrophenyl-alpha-d-maltohexaoside through the outer membrane of Escherichia coli. Characterization of LamB as a specific and saturable channel for maltooligosaccharides. J Biol Chem. 1988;263:314–320. [PubMed] [Google Scholar]
- 19.Göransson M, Sondén B, Nilsson P, Dagberg B, Forsman K, Emanuelsson K, Uhlin B E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990;344:682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- 19a.Heyde, M. Personal communication.
- 20.Hosono K, Kakuda H, Ichihara S. Decreasing accumulation of acetate in a rich medium by Escherichia coli on introduction of genes on a multicopy plasmid. Biosci Biotechnol Biochem. 1995;59:256–261. doi: 10.1271/bbb.59.256. [DOI] [PubMed] [Google Scholar]
- 21.Laurent-Winter C, Ngo S, Danchin A, Bertin P. Role of Escherichia coli histone-like nucleoid-structuring protein in bacterial metabolism and stress response: identification of targets by two-dimensional electrophoresis. Eur J Biochem. 1997;244:767–773. doi: 10.1111/j.1432-1033.1997.00767.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 23.Owen-Hughes T A, Pavitt G D, Santos D S, Sidebotham J M, Hulton C S J, Hinton J C D, Higgins C F. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell. 1992;71:255–265. doi: 10.1016/0092-8674(92)90354-f. [DOI] [PubMed] [Google Scholar]
- 24.Raibaud O, Vidal-Ingigliardi D, Kolb A. Genetic studies on the promoter of malT, the gene that encodes the activator of the Escherichia coli maltose regulon. Res Microbiol. 1991;142:937–942. doi: 10.1016/0923-2508(91)90003-s. [DOI] [PubMed] [Google Scholar]
- 25.Randall-Hazelbauer L, Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973;116:1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richet E, Vidal-Ingigliardi D, Raibaud O. A new mechanism for coactivation of transcription initiation: repositioning of an activator triggered by the binding of a second activator. Cell. 1991;66:1185–1195. doi: 10.1016/0092-8674(91)90041-v. [DOI] [PubMed] [Google Scholar]
- 27.Richet E, Raibaud O. Supercoiling is essential for the formation and stability of the initiation complex at the divergent malEp and malKp promoters. J Mol Biol. 1991;218:529–542. doi: 10.1016/0022-2836(91)90699-7. [DOI] [PubMed] [Google Scholar]
- 28.Richet E. On the role of the multiple regulatory elements involved in the activation of the Escherichia coli malEp promoter. J Mol Biol. 1996;264:852–862. doi: 10.1006/jmbi.1996.0682. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Schwartz M. The maltose regulon. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1482–1502. [Google Scholar]
- 31.Silhavy T J, Shuman H A, Beckwith J, Schwartz M. Use of gene fusions to study outer membrane protein localization in Escherichia coli. Proc Natl Acad Sci USA. 1977;74:5411–5415. doi: 10.1073/pnas.74.12.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sondén B, Uhlin B E. Coordinated and differential expression of histone-like proteins in Escherichia coli: regulation and function of the H-NS analog StpA. EMBO J. 1996;15:4970–4980. [PMC free article] [PubMed] [Google Scholar]
- 33.Spassky A, Rimsky S, Garreau H, Buc H. H1a, an E. coli DNA-binding protein which accumulates in stationary phase, strongly compacts DNA in vitro. Nucleic Acids Res. 1984;12:5321–5340. doi: 10.1093/nar/12.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spurio R, Dürrenberger M, Falconi M, La Teana A, Pon C L, Gualerzi C O. Lethal overproduction of the Escherichia coli nucleoid protein H-NS: ultramicroscopic and molecular autopsy. Mol Gen Genet. 1992;231:201–211. doi: 10.1007/BF00279792. [DOI] [PubMed] [Google Scholar]
- 35.Szmelcman S, Schwartz M, Silhavy T J, Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976;65:13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- 36.Tagami H, Inada T, Kunimura T, Aiba H. Glucose lowers CRP* levels resulting in repression of the lac operon in cells lacking cAMP. Mol Microbiol. 1995;17:251–258. doi: 10.1111/j.1365-2958.1995.mmi_17020251.x. [DOI] [PubMed] [Google Scholar]
- 37.Valkonen K H, Veijola J, Dagberg B, Uhlin B E. Binding of basement-membrane laminin by Escherichia coli. Mol Microbiol. 1991;5:2133–2141. doi: 10.1111/j.1365-2958.1991.tb02143.x. [DOI] [PubMed] [Google Scholar]
- 38.von Gabain A, Belasco J G, Schottel J L, Chang A C Y, Cohen S N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segment of transcripts. Proc Natl Acad Sci USA. 1983;80:653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willets N S, Clark A J, Low B. Genetic locations of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969;97:244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada H, Muramatsu S, Mizuno T. An Escherichia coli protein that preferentially binds to sharply curved DNA. J Biochem (Tokyo) 1990;108:420–425. doi: 10.1093/oxfordjournals.jbchem.a123216. [DOI] [PubMed] [Google Scholar]
- 41.Yamashino T, Ueguchi C, Mizuno T. Quantitative control of the stationary phase-specific sigma factor, ςs, in Escherichia coli: involvement of the nucleoid protein H-NS. EMBO J. 1995;14:594–602. doi: 10.1002/j.1460-2075.1995.tb07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang A, Belfort M. Nucleotide sequence of a newly-identified Escherichia coli gene, stpA, encoding an H-NS-like protein. Nucleic Acids Res. 1992;20:6735. doi: 10.1093/nar/20.24.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang A, Derbyshire V, Galloway Salvo J L, Belfort M. Escherichia coli protein StpA stimulates self-splicing by promoting RNA assembly in vitro. RNA. 1995;1:783–793. [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang A, Rimsky S, Reaban M E, Buc H, Belfort M. Escherichia coli protein analogs StpA and H-NS: regulatory loops, similar and disparate effects on nucleic acid dynamics. EMBO J. 1996;15:1340–1349. [PMC free article] [PubMed] [Google Scholar]
- 45.Zuber F, Kotlarz D, Rimsky S, Buc H. Modulated expression of promoters containing upstream curved DNA sequences by the Escherichia coli nucleoid protein H-NS. Mol Microbiol. 1994;12:231–240. doi: 10.1111/j.1365-2958.1994.tb01012.x. [DOI] [PubMed] [Google Scholar]