Abstract
Hidradenitis suppurativa is a chronic inflammatory disease which affects apocrine glands and hair follicles of the skin, primarily in the axillary and groin regions. This condition can be highly debilitating, causing painful lesions and a negative psychological impact on patients. While medical and minimally invasive treatments are available, surgical intervention may be necessary for severe cases. In cases involving axillary defects, the use of local flaps such as the parascapular flap is a viable option. In this case report, we present a 34-year-old woman who presented to our clinic with a history of recurrent abscesses and cutaneous infections in the axillary region. After thorough evaluation, we chose to use the parascapular flap for reconstruction. The parascapular flap is a one-stage procedure that allows for extensive resection of the axillary area without resulting in contractions or retractions over the long term. Additionally, this technique allows for preservation of the axilla’s original shape with minimal donor site morbidity.
Keywords: Hidradenitis suppurativa, Parascapular flap, Reconstructive surgery
Introduction
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic inflammatory disease that primarily affects the apocrine glands and hair follicles of the skin in the axillary, mammary, groin, and perianal regions. The characteristic lesion consists of painful subcutaneous nodules that progress to abscesses, resulting in fistula formation and scarring and fibrosis in moderate to severe stages. This condition has a negative psychological impact on patients, underscoring the need for effective and definitive treatment options [1].
This condition can be classified based on the number of abscesses, diffuse disease, and the presence of sinus tracts and scarring. The most widely used classification system was proposed by Hurley [2] and has evolved to more prognostic staging systems such as the European Hidradenitis Suppurativa Foundation, the initial HS Severity Score (HS4), and the more recent International HS4 (IHS4) [3]. The latter gives points to nodules, abscesses, tunnels with the use of the next formula:
HS is so classified as mild (3 points or less), moderate (4–10 points), or severe (11 points or more). Conservative treatment options for HS include antibiotics, retinoids, corticosteroids, radiation, and laser therapy, but these procedures are associated with high rates of recurrence. Only radical debridement offers a potential cure [4, 5]. In the past, secondary healing and abscess drainage were utilized for the treatment of HS. However, due to the associated morbidity and complications, novel and safer approaches have replaced these methods. Primary closure, skin grafts, and different flaps are effective alternatives for reconstructing the area after lesion resection [6–8].
Local flaps harvested from the back provide high-quality skin, but their thickness can negatively impact functional and aesthetic results, particularly in defects located in the axillary region [9]. First described in 1982, the parascapular flap is a fasciocutaneous flap based on the descending branch of the circumflex scapular artery, a subscapular branch. It can be used as a pedicled or free flap, as it has a safe and reliable pedicle. Located in the lateral portion of the scapula, it provides excellent coverage for extensive defects, and the donor area can be closed primarily [10]. Here, we present a surgical technique for reconstructing axillary defects in a patient with a severe case of HS using a parascapular flap based on its perforators.
Anatomy of the Parascapular Flap
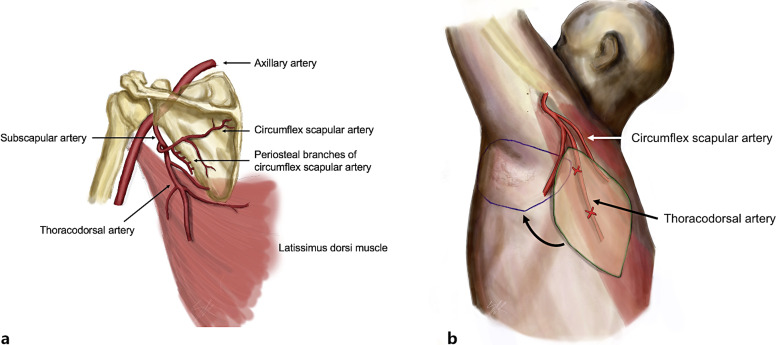
The main pedicle for the latissimus dorsi muscle is the thoracodorsal artery, which arises from the subscapular artery along with the circumflex scapular artery (on which scapular and parascapular flaps are based). After giving off the serratus muscle arterial branch, the thoracodorsal vessels divide into two branches: the lateral or descending branch and the horizontal or medial branch, which diverge at angle of 45°. The lateral branch follows a 2–3-cm intramuscular course and divides into two or three skin perforator arteries. The most proximal vessel reaches the subcutaneous tissue at a point located 2 or 3 cm posterior to the lateral edge of the muscle and 8 cm below the posterior axillary fold. The second perforator artery is located 1–2 cm below the previous one and is usually smaller in diameter. All these perforator arteries give off numerous muscular branches before penetrating the fascia to supply the overlying skin and subcutaneous fat layers [11–13]. A scheme of the flap can be seen in Figure 1.
Fig. 1.
Anatomy of the parascapular flap. a Vascular anatomy. b Parascapular flap design based on the TDAP (green) and its recipient site (blue).
Surgical Technique
The location of the perforator is typically identified using a portable bidirectional Doppler. The patient is placed in a lateral decubitus position with the arm abducted 90° over the head. A thorough and complete extensive resection is performed, ensuring the inclusion of all the hair follicles and hair-bearing area to minimize recurrence rates. Once the resection is complete, the parascapular flap is harvested. Using the portable Doppler, the thoracodorsal perforating artery is identified and marked in the superior-external scapular area, and the distance to the center of the ipsilateral axilla is measured, which determines the size of the flap. The flap is designed to allow enough mobility for a 180° rotation or direct advancement toward the axilla.
The preferred perforator is the first one arising from the descending branch of the thoracodorsal trunk. To locate the perforator, an incision is made along the anterior border of the latissimus dorsi. Once the perforator is identified, the skin is incised inferior to the pedicle, and the anterior and inferior incisions are completed to facilitate intramuscular dissection prior to harvesting the contralateral border of the flap, elevating it from distal to proximal. Dissection is carried out in a suprafascial plane with direct visualization of the perforator under magnification. The perforator dissection is continued until the junction of the descending and horizontal branches of the thoracodorsal artery is reached, with careful approach near the teres major muscle as the pedicle is usually located immediately superior to the superior border of the muscle. Once the pedicle is located, a superior skin incision is made. Dissection beyond this point, into the quadrangular space, is recommended if the flap is intended to be used as a free flap. However, if used as a pedicled flap, the arc of rotation is sufficient to cover the recipient site comfortably. Hemostasis must be meticulous, and compression and torsion of the pedicle must be avoided. The resultant arc of rotation is well tolerated by the vascular pedicle. The donor site can be closed primarily, and a suction drain is usually recommended.
Case Report
We present a case of a 34-year-old female with no significant medical history, who presented to our clinic with a history of abscesses and cutaneous infections bilaterally in her axillary regions. She had been previously diagnosed with HS by her dermatologist and had undergone various treatments, including topical and systemic antibiotics, corticosteroids, and adalimumab, due to progression to stage III HS according to Hurley’s classification, or severe HS according to IHS4. The patient expressed dissatisfaction with her current situation and sought a definitive solution from our team. After multidisciplinary assessment (including a dermatologist, pathologist, and plastic surgeon), the plan of action was to perform radical debridement of the affected region and cover the defect with a locoregional flap.
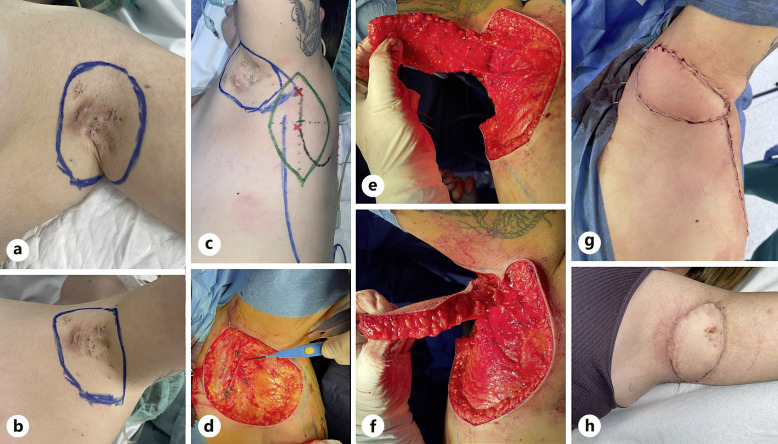
The patient received general anesthesia, and a complete en-bloc resection of the axillary region was performed, ensuring that no major hair-bearing area was left. The procedure was carried out as previously described, with particular care taken to avoid torsion and compression of the pedicle (Fig. 2). In this case, tunneling of the flap was not necessary and the arc of rotation based on perforators was sufficient. The flap showed no signs of complications. The donor site required a suction drain for 2 days.
Fig. 2.
Surgical procedure. a, b Design of the affected area to be resected. c Design of the propeller flap (green line) with location of the perforator vessels (red x) over the lateral border of the scapula (black line). d Affected area is resected, showing the defect to be covered and the perforator vessels. e Parascapular flap elevated on two perforator vessels. f Parascapular flap rotated to the anterior area. g Final reconstruction with the parascapular flap in its definitive location, properly vascularized. h Reconstruction after removal of the surgical stitches (2 weeks postoperatively).
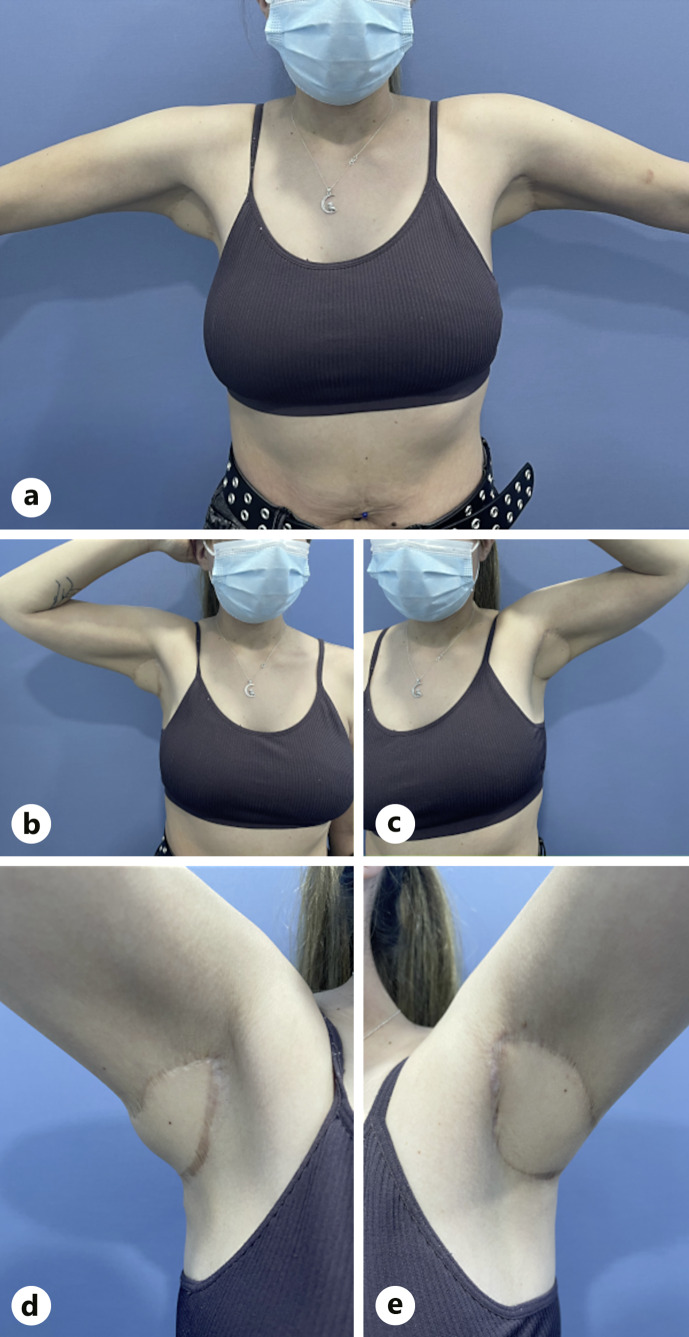
Ten months later, the patient remained asymptomatic in her left axilla but had worsening symptoms in her right axilla, leading to the performance of the same procedure in that region. At 18 months post-surgery, the patient reported high satisfaction, and no recurrence was observed (Fig. 3). Furthermore, arm mobility and abduction were not compromised. Adalimumab was discontinued due to disease control and no visible recurrence in other locations.
Fig. 3.
Clinical result at the end of follow-up. a Anterior view, 80° abduction. b Right axilla 90° abduction and external rotation. c Left axilla 90° abduction and external rotation. d Left axilla, closer inspection, 130° abduction. e Right axilla, closer inspection, 120° abduction.
Refer to the online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000533387) for a more detailed timeline (online suppl. Table 1). The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material.
Discussion
HS is primarily caused by follicular occlusion, with secondary involvement of the apocrine glands. It is a chronic disorder of multifactorial etiology, associated with obesity, chemical irritants, tight clothing, hyperandrogenism, as well as an important genetic and hormonal component [2, 14]. Surgical treatments typically include drainage, excision and healing by secondary intention, CO2 laser excision, wide local excision, and reconstruction with split-skin grafting or local/distant flaps. Recurrence of the disease can be reduced by wide local excision of all the hair-bearing skin, with or without posterior reconstruction [7, 15], offering a potential cure for HS [16]. Among the surgical options for this pathology after resection, we have chosen primary closure, secondary intention healing, skin grafts, and local flaps, to analyze the pros and limitations in Table 1.
Table 1.
Pros and limitations of different surgical techniques after resection of the affected skin area
| Technique | Pros | Limitations |
|---|---|---|
| Primary closure [17, 18] | Small defects that can be sutured primarily | Not suited for large defects |
| Natural creases that can hide the scar | Risk of wound dehiscence if high tension | |
| Following resting skin tension lines | Conspicuous scarring | |
| Rapid healing | Highest recurrence rates | |
| Low morbidity | ||
| Secondary intention healing [19, 20] | Simplicity | Prolonged healing period and wound management |
| Reduced invasiveness | Scar hypertrophy | |
| Wound shrinkage | Risk of deformities and contractures | |
| Acceptable cosmetic results | Need for a revision surgery | |
| Low recurrence rates | Conspicuous scarring | |
| Used traditionally after the deroofing technique | May need VAC therapy | |
| Skin graft [21, 22] | Good choice for large wounds not suitable for primary closure | Donor site morbidity |
| Donor site contracture | ||
| Low recurrence rates with split-thickness skin grafting | Wound management | |
| May need VAC therapy for better graft take | ||
| Local flaps [18] | Allows for large and extensive resections | Large areas |
| Low to moderate recurrence rates | ||
| Patient satisfaction | Graft failure | |
| Primary closure of the donor area | ||
| Tensionless | Tension at the donor site\ | |
| Acceptable cosmetic results |
Despite the variety of techniques available for managing axillary skin defects, split-thickness skin grafting remains the most used surgical procedure, which can lead to problems with scar contraction. In our opinion, the best reconstruction option is the immediate coverage of the defect with a fasciocutaneous transposition flap. Using the parascapular flap, the donor site can be closed primarily [5–7]. This technique avoids the use of traditional muscle flaps, which can fill the defect but potentially limit the range of adduction on the arm by its muscle bulk, causing limited movement. It provides good coverage with smooth skin and excellent elastic properties, allowing for the reconstruction of axillary defects with satisfactory aesthetic outcomes [23].
Another noteworthy aspect is that this flap can also be harvested as a propeller flap. Propeller flaps, as defined by the 2009 Tokyo meeting, are island flaps that reach their recipient site through axial rotation [24]. They are classified based on the type of pedicle as subcutaneous (random subcutaneous pedicle), perforator pedicled (skeletonized perforator pedicle), and supercharged (anastomosis of a superficial vein or an extra artery to increase vascularization). A propeller flap allows for a direct closure of the donor site with minimal morbidity and scarring and enables a one-stage procedure. Propeller flaps based on the thoracodorsal artery perforator (TDAP) have also been used for partial breast reconstruction [25]. In this case, the proximity of the recipient site allowed for a 90° rotation of the flap, with no coloration or temperature changes neither intraoperatively nor postoperatively, suggesting spasm or vascular insufficiency.
In the case of HS, propeller TDAP flaps have been used with good results. These flaps have not only improved function and aesthetics but also increased the quality of life of the patient, which is commonly measured using the Dermatology Quality of Life Index [15]. Small series have shown significant improvements of more than 20 points to less than 4 points [26, 27]. Other randomly rotated flaps have also demonstrated successful resolution of the problems associated with HS, albeit with some limitations of shoulder mobility [16].
In addition to the TDAP flap, other upper limb flaps have demonstrated better outcomes compared to excision and split-thickness skin grafts, although surgical time was longer. One of these flaps is the posterior arm flap [28]. For other locations of HS, such as gluteal and perineal disease, superior and inferior gluteal artery perforator flaps have been utilized. These flaps have long pedicles and a wide arc of rotation, allowing for early mobilization and good aesthetic results [29].
The parascapular flap procedure offers several advantages over other surgical procedures. It is a one-stage procedure and does not require additional interventions. The skin used for reconstruction has a similar texture, color, and thinness to that of the axilla because it is non-hair-bearing skin in the majority of cases. The parascapular flap allows for extensive resection of the axillary area without contractions or retractions on the long term, while maintaining the axilla’s original shape. Additionally, it offers advantages in terms of pliability to the defect. Donor site morbidity is minimal and well tolerated in most cases [30], as seen in our case, allowing for a good range of motion, with minimal or no restriction and a satisfactory aesthetic result. The type of flap used in our patient also had aesthetic consideration, as cited by other groups who have used it [31].
In conclusion, the parascapular flap based on its perforators is a good alternative for reconstructing the axilla after radical excision of hair-bearing skin in severe stage III HS cases. It provides good aesthetic and functional outcomes, along with a relatively straightforward approach and technique.
Statement of Ethics
Ethical approval is not required for this study in accordance with local or national guidelines. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding resources were obtained.
Author Contributions
Data acquisition: S.K., R.A.-C., H.A., and A.D.-M.; analysis and interpretation of data: S.K., S.A.-M., M.T., and A.D.-M.; drafting: S.K., N.B., and A.D.-M.; critical review: N.B., R.A.-C., S.A.-M., M.T., M.F.-F., and A.D.-M.
Funding Statement
No funding resources were obtained.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
References
- 1.Plastic surgical management of hidradenitis suppurativa. United States; 2021. [DOI] [PubMed]
- 2. Hurley HJ. Hidradenitis Suppurativa. Roenigk & Roenigk’s dermatologic surgery: principles and practice. 2nd ed. New York, NY: CRC press; 1996. p. 623–45. [Google Scholar]
- 3. Zouboulis CC, Tzellos T, Kyrgidis A, Jemec GBE, Bechara FG, Giamarellos-Bourboulis EJ, et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401–9. [DOI] [PubMed] [Google Scholar]
- 4. Slade DEM, Powell BW, Mortimer PS. Hidradenitis suppurativa: pathogenesis and management. Br J Plast Surg. 2003;56(5):451–61. [DOI] [PubMed] [Google Scholar]
- 5. Soldin MG, Tulley P, Kaplan H, Hudson DA, Grobbelaar AO. Chronic axillary hidradenitis--the efficacy of wide excision and flap coverage. Br J Plast Surg. 2000;53(5):434–6. [DOI] [PubMed] [Google Scholar]
- 6. Kagan RJ, Yakuboff KP, Warner P, Warden GD. Surgical treatment of hidradenitis suppurativa: a 10-year experience. Surgery. 2005;138(4):734–40; discussion 740-741. [DOI] [PubMed] [Google Scholar]
- 7. Altmann S, Fansa H, Schneider W. Axillary hidradenitis suppurativa: a further option for surgical treatment. J Cutan Med Surg. 2004;8(1):6–10. [DOI] [PubMed] [Google Scholar]
- 8. Rompel R, Petres J. Long-term results of wide surgical excision in 106 patients with hidradenitis suppurativa. Dermatol Surg. 2000;26(7):638–43. [DOI] [PubMed] [Google Scholar]
- 9. Busnardo FF, Coltro PS, Olivan MV, Busnardo APV, Ferreira MC. The thoracodorsal artery perforator flap in the treatment of axillary hidradenitis suppurativa: effect on preservation of arm abduction. Plast Reconstr Surg. 2011;128(4):949–53. [DOI] [PubMed] [Google Scholar]
- 10. Nassif TM, Vidal L, Bovet JL, Baudet J. The parascapular flap: a new cutaneous microsurgical free flap. Plast Reconstr Surg. 1982;69(4):591–600. [DOI] [PubMed] [Google Scholar]
- 11. Ortiz CL, Mendoza MM, Sempere LN, Sanz JS, Torres AN, Barraquer EL. Versatility of the pedicled thoracodorsal artery perforator (TDAP) flap in soft tissue reconstruction. Ann Plast Surg. 2007;58(3):315–20. [DOI] [PubMed] [Google Scholar]
- 12. Heitmann C, Guerra A, Metzinger SW, Levin LS, Allen RJ. The thoracodorsal artery perforator flap: anatomic basis and clinical application. Ann Plast Surg. 2003;51(1):23–9. [DOI] [PubMed] [Google Scholar]
- 13. Thomas BP, Geddes CR, Tang M, Williams J, Morris SF. The vascular basis of the thoracodorsal artery perforator flap. Plast Reconstr Surg. 2005;116(3):818–22. [DOI] [PubMed] [Google Scholar]
- 14. Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol. 1996;35(2 Pt 1):191–4. [DOI] [PubMed] [Google Scholar]
- 15. Chawla S, Toale C, Morris M, Tobin A, Kavanagh D. Surgical management of hidradenitis suppurativa. A Narrative Rev. 2022;15(1). [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Y, Ngaage LM, Ge S, Rada EM, Silverman RP, Rasko YM. Reconstruction for axillary hidradenitis suppurativa using one-stage local tissue rearrangement: a retrospective analysis of 53 cases. Int Wound J. 2020;17(3):701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandal A, Watson J. Experience with different treatment modules in hidradenitis suppuritiva: A study of 106 cases. Surgeon. 2005;3(1):23–6. [DOI] [PubMed] [Google Scholar]
- 18. Mehdizadeh A, Hazen PG, Bechara FG, Zwingerman N, Moazenzadeh M, Bashash M, et al. Recurrence of hidradenitis suppurativa after surgical management: A systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(5):S70–7. [DOI] [PubMed] [Google Scholar]
- 19. Bieniek A, Matusiak Ł, Chlebicka I, Szepietowski JC. Secondary intention healing in skin surgery: our own experience and expanded indications in hidradenitis suppurativa, rhinophyma and non-melanoma skin cancers: Secondary intention healing in skin surgery. J Eur Acad Dermatol Venereol. 2013;27(8):1015–21. [DOI] [PubMed] [Google Scholar]
- 20. Van Rappard DC, Mooij JE, Mekkes JR. Mild to moderate hidradenitis suppurativa treated with local excision and primary closure: HS treated with local excision and primary closure. J Eur Acad Dermatol Venereol. 2012;26(7):898–902. [DOI] [PubMed] [Google Scholar]
- 21. Sugio Y, Tomita K, Hosokawa K. Reconstruction after Excision of Hidradenitis Suppurativa: Are Skin Grafts Better than Flaps? Plast Reconstr Surg Glob Open. 2016;4(11):e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alharbi M, Perignon D, Assaf N, Qassemyar Q, Elsamad Y, Sinna R. Application of the inner arm perforator flap in the management of axillary hidradenitis suppurativa. Ann Chir Plast Esthet. 2014;59(1):29–34. [DOI] [PubMed] [Google Scholar]
- 23. Amarante J, Reis J, Santa Comba A, Malheiro E. A new approach in axillary hidradenitis treatment: the scapular island flap. Aesthet Plast Surg. 1996;20(5):443–6. [DOI] [PubMed] [Google Scholar]
- 24. D’Arpa S, Toia F, Pirrello R, Moschella F, Cordova A. Propeller flaps: a review of indications, technique, and results. BioMed Res Int. 2014;2014:986829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomsen JB, Rindom MB, Rancati A, Angrigiani C. Thoracodorsal artery flaps for breast reconstruction – the variants and its approach. Arch Plast Surg. 2021;48(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elgohary H, Nawar AM, Zidan A, Shoulah AA, Younes MT, Hamed AM. Outcome of pedicled thoracodorsal artery perforator flap in the surgical treatment of stage II and III hidradenitis suppurativa of axilla. Ann Plast Surg. 2018;81(6):688–93. [DOI] [PubMed] [Google Scholar]
- 27. Marchesi A, Amendola F, Garieri P, Steinberger Z, Vaienti L. Wide local excisions and pedicled perforator flaps in hidradenitis suppurativa: a study of quality of life. Ann Plast Surg. 2021;86(2):201–5. [DOI] [PubMed] [Google Scholar]
- 28. Tereshenko V, Schweizer R, Waldner M, Kim BS, Giovanoli P, Klein HJ. Outcome comparison of different reconstructive approaches for axillary defects secondary to radical excision of hidradenitis suppurativa. Dermatology. 2022;238(5):851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Unal C, Yirmibesoglu OA, Ozdemir J, Hasdemir M. Superior and inferior gluteal artery perforator flaps in reconstruction of gluteal and perianal/perineal hidradenitis suppurativa lesions. Microsurgery. 2011;31(7):539–44. [DOI] [PubMed] [Google Scholar]
- 30. Ortiz CL, Castillo VL, Pilarte FS, Barraguer EL. Experience using the thoracodorsal artery perforator flap in axillary hidradentitis suppurativa cases. Aesthet Plast Surg. 2010;34(6):785–92. [DOI] [PubMed] [Google Scholar]
- 31. Elboraey MA, Alali AB, Alkandari QA. Immediate and delayed reconstruction after excision of axillary hidradenitis suppurativa using a propeller flap. Plast Reconstr Surg Glob Open. 2019;7(8):e2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material. Further inquiries can be directed to the corresponding author.