Abstract
Introduction
Li-Fraumeni syndrome (LFS) is a rare autosomal dominant disorder brought on by pathogenic mutations in the TP53 tumor suppressor gene. LFS is characterized by a high lifetime risk of developing various cancers at a relatively young age.
Case Presentation
We are presenting a 48-year-old male with a diagnosis of LFS that was confirmed by a genetic test triggered by the patient’s son’s diagnosis of LFS and leukemia. The patient’s main symptoms were abdominal pain and weight loss. The patient was diagnosed with two synchronous primary tumors: first, a metastatic gastric invasive adenocarcinoma that is microsatellite instability (MSI) -high; and second, a low grade (G1) (non-function) well-differentiated pancreatic neuroendocrine tumor. These cancers are not the usual type associated with LFS. After eight cycles of chemo-immunotherapy in the form of FOLFOX-Nivolumab, our radiological assessment showed significant response in the metastatic gastric adenocarcinoma and stable disease in pancreatic neuroendocrine tumor. The patient remains on single agent Nivolumab and has had stable disease for the last 12 months.
Conclusion
Gastric cancer and neuroendocrine tumors are not usually associated with LFS. This case illustrates a rare clinical presentation of multiple malignancies in LFS patients.
Keywords: Li-Fraumeni syndrome, Gastric adenocarcinoma, Pancreatic neuroendocrine tumor, Case report
Introduction
Li-Fraumeni syndrome (LFS) is a rare, autosomal dominant genetic condition that places carriers at increased risk for developing cancer. LFS can result in the development of multiple primary tumors. Further, LFS can afflict several members of the same family, including children and young adults [1].
The pathogenic gene for LFS results from germline mutations in the TP53 tumor suppressor gene [2]. By the age of 70, the cumulative cancer incidence among those with LFS is approximately 100% [3]. Sarcomas, premenopausal breast cancer, brain tumors, and adrenocortical carcinoma are most associated with LFS. Other cancers associated with LFS include leukemia, lymphoma, and nephroblastoma [4]. Here, we present a rare case of a young patient with LFS recently diagnosed with two synchronous primary tumors that are not usually associated with LFS.
Case Report
A 48-year-old male diagnosed recently with LFS as a result of genetic testing he underwent following the patient’s son’s diagnosis of LFS and leukemia earlier in the year. The patient also has one young daughter who is well and does not have LFS. The patient’s sister died at age of 14 years old following a diagnosis of a brain tumor. His father died at age of 50 years after diagnosis of hematological malignancy. It is assumed the patient’s sister and father had LFS, although there was no formal diagnosis (see Fig. 1).
Fig. 1.
LFS case family pedigree.
The patient presented to emergency room with a 3-month history of abdominal pain in the epigastric region associated with lower back pain. He had history of weight loss of approximately 20 pounds over a few weeks.
Imaging showed a pancreatic head mass with distal common bile duct dilatation, a gastric mass with wall thickening, left adrenal nodule, and necrotic para-aortic lymphadenopathy, deemed highly suspicious for malignancy involving potentially more than one primary. CT spine, CT head, and bone scan revealed no obvious evidence of metastasis. He underwent gastroscopy and endoscopic ultrasound which revealed a large gastric cardia mass with involvement of the esophagus and extension along the lesser curvature, which was biopsied, as well as a cystic lesion within the pancreas head with soft tissue component, which was biopsied as well.
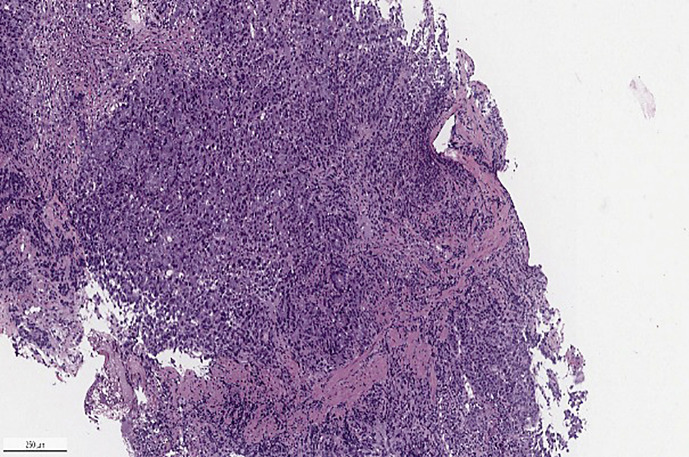
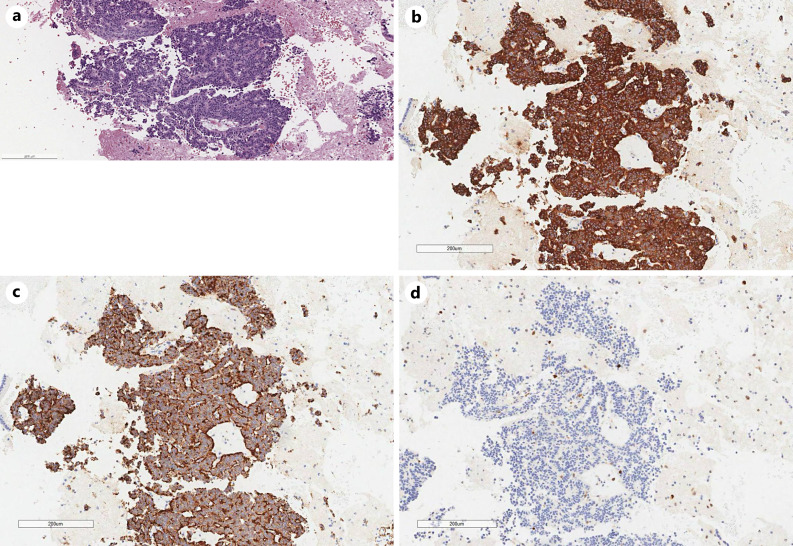
Pathology of gastric biopsy showed a poorly differentiated invasive adenocarcinoma, CK 8/18 positive, P40 negative, p53 overexpressed, MMR deficient with loss of nuclear expression of MLH1 and PMS2 via immunohistochemistry (see Fig. 2, 3a–e), MLH promoter hypermethylated, estimated CPS = 5 and HER 2 neu negative. Pathology of the pancreatic head lesion showed a well-differentiated neuroendocrine tumor, positive for synaptophysin, chromogranin, pancytokeratin, Ki 67 < 1% without necrosis or mitotic activity (see Fig. 4a–d).
Fig. 2.
Photomicrograph of gastric mass biopsy, invasive poorly differentiated adenocarcinoma (Hematoxylin & Eosin, H&E, ×100).
Fig. 3.
a Photomicrograph of gastric mass, immunohistochemical (IHC) testing for mismatch repair (MMR) proteins. MLH1 showing loss of nuclear expression in adenocarcinoma (IHC, ×40). b Photomicrograph of gastric mass, immunohistochemical testing for mismatch repair (MMR) proteins. PMS2 showing loss of nuclear expression in adenocarcinoma (IHC, ×40). c Photomicrograph of gastric mass, immunohistochemical testing for mismatch repair (MMR) proteins. MSH2 with intact nuclear expression (IHC, ×40). d Photomicrograph of gastric mass, immunohistochemical testing for mismatch repair (MMR) proteins. MSH6 with intact nuclear expression (IHC,×40). e Photomicrograph of gastric mass, immunohistochemical staining for p53 (IHC, ×40).
Fig. 4.
a Photomicrograph of pancreatic lesion biopsy, well-differentiated neuroendocrine tumor (H&E, ×100). b Photomicrograph of pancreatic lesion biopsy, immunohistochemical staining for synaptophysin (IHC, ×100). c Photomicrograph of pancreatic lesion biopsy, immunohistochemical staining for Chromogranin (IHC, ×100). d Photomicrograph of pancreatic lesion biopsy, immunohistochemical staining for KI67 (IHC, ×100).
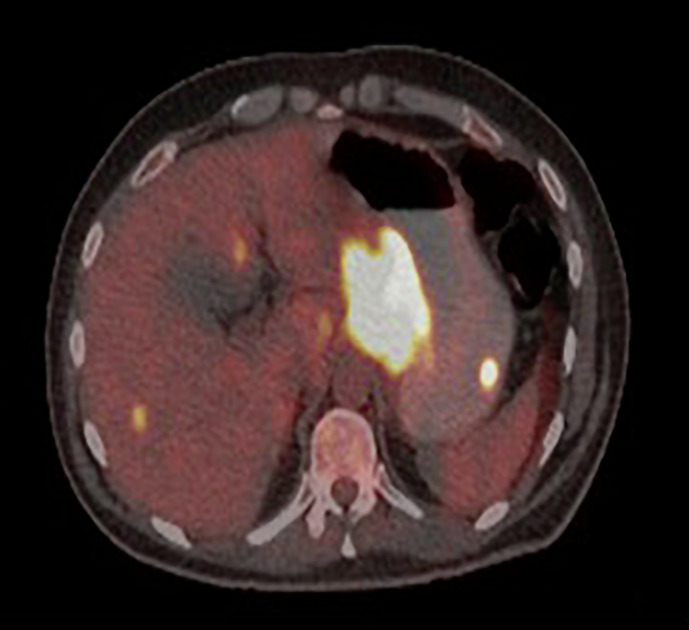
NM PET scan showed hypermetabolic mass involving gastric cardia, fundus, and lesser curvature. Hypermetabolic pancreatic head mass. Foci of FDG avid lesions in the liver highly suspicious for hypermetabolic metastasis (see Fig. 5).
Fig. 5.
NM PET scan showed hypermetabolic mass involving gastric cardia, fundus, and lesser curvature. Hypermetabolic pancreatic head mass with central necrosis hypermetabolic celiac, gastrohepatic, para-aortic lymph nodes, and peritoneal metastases. Foci of FDG avid lesions in the liver highly suspicious for hypermetabolic metastasis.
Gallium 68 scan showed somatostatin avid receptor rich tumor in the pancreatic head. Malignant process in the gastric cardia demonstrates relatively low somatostatin receptor expression, in keeping with the known diagnosis of invasive adenocarcinoma. Likewise, the lymphadenopathy seen in the subcarinal region and upper abdomen showed similar characteristics, favoring metastatic disease from the gastric adenocarcinoma, rather than the pancreatic neuroendocrine tumor. The previously seen liver foci on FDG PET scan are not identified with confidence, favoring adenocarcinoma origin as well.
We selected a treatment regimen directed toward the metastatic MSI-H gastric cancer. The patient started on chemotherapy-immunotherapy in the form of FOLFOX + Nivolumab every 2 weeks. He was compliant with the treatment plan. After eight cycles of treatment, CT scan follow-up showed significant response (more than 50%) regression in metastatic gastric cancer and stable disease in pancreatic neuroendocrine tumor. The patient tolerated the treatment well. Regular assessment in oncology clinic prior/post each cycle of treatment showed no adverse events.
Discussion
Frederick Pei Li and Joseph F. Fraumeni, Jr. discovered and described LFS in 1969. LFS is a typical hereditary tumor susceptibility syndrome with autosomal dominant inheritance [3]. LFS has been clinically linked to numerous cancers, and those who have it frequently develop malignancies at a young age [5]. It is now known that LFS is caused by a germline variant in the tumor suppressor gene TP53 on chromosome 17 [6]. Around 70–80% of families with LFS have this germline pathogenic variant [2]. It is crucial to diagnose LFS and to determine whether the patient has the TP53 variant. The results of a large-sample clinical research by Bougeard et al. [7] included 1,730 individuals with LFS in France; showed that the average age of first tumor was 24.9 years old, and 41% of the patients were unambiguously recognized as tumors before the age of 18. Osteosarcoma, adrenocortical carcinoma (ACC), CNS neoplasm, and soft tissue sarcoma have incidence rates of 30%, 27%, 26%, and 23% respectively in patients with LFS.
In this case, the patient did not have any of cancers that are usually associated with LFS. Instead, the patient was synchronously diagnosed with two primary cancers: first, a gastric cancer with MSI-High status and a second, a well-differentiated pancreatic neuroendocrine tumor. We were able to detect and distinguish between these primary malignancies because we performed a number of biopsies on this patient.
In our case, MLH-1 and PMS2 loss expression (MSI-High) was discovered in the primary gastric cancer, and the second primary PNET was identified as low grade. Our radiological assessment after eight cycles of chemo-immunotherapy in form of (FOLFOX + Nivolumab) showed response in metastatic gastric cancer and stable disease in pancreatic neuroendocrine tumor without significant deterioration or complications. The patient remains on single agent Nivolumab and continues to have stable disease after 1 year of treatment.
This case illustrates the importance of taking multiple biopsies in patients with LFS. All cancer patients, but particularly those with LFS should have biopsies taken of as many tumors as feasible where multiple primary cancers are suspected. Results from these multiple biopsies may aid in the identification of prospective therapeutic targets and may aid in planning systemic therapy treatment which will likely improve the prognosis for patients.
Conclusion
MSI-H gastric cancer and pancreatic neuroendocrine tumor are not usually associated with LFS. Further study is recommended to understand the incidence of unusual malignancies that develop in patients with LFS.
The CARE Checklist has been completed by the authors for this case report, attached as supplementary material (for all online suppl. material, see https://doi.org/10.1159/000535099).
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
This study protocol was reviewed and the need for approval was waived by the Ottawa Hospital Research Institute Research Ethics Board.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received for this study.
Author Contributions
Writing: Essa Al Mansor; editing: Timothy Asmis and Anna Adamiak; analysis: Timothy Asmis.
Funding Statement
No funding was received for this study.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Hisada M, Garber JE, Fung CY, Fraumeni JF, Li FP. REPORTS multiple primary cancers in families with Li-fraumeni syndrome. 1998. [Online]. Available from: https://academic.oup.com/jnci/article/90/8/606/987603. [DOI] [PubMed] [Google Scholar]
- 2. Foulkes WD, Polak P. Li-fraumeni syndrome in the cancer genomics era. J Natl Cancer Inst. 2021;113(12):1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer. 2016;122(23):3673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamihara J, Rana HQ, Garber JE. Germline TP53 mutations and the changing landscape of Li-Fraumeni syndrome. Hum Mutat. 2014;35(6):654–62. [DOI] [PubMed] [Google Scholar]
- 5. Kratz CP, Villani A, Nichols KE, Schiffman J, Malkin D. Cancer surveillance for individuals with Li-Fraumeni syndrome. Eur J Hum Genet. 2020;28(11):1481–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, et al. Integrated analysis of TP53 gene and pathway alterations in the cancer genome atlas. Cell Rep. 2019;28(11):3010–1384.e5. [DOI] [PubMed] [Google Scholar]
- 7. Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, Belotti M, et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol. 2015;33(21):2345–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.