Abstract
Immune checkpoint inhibitors (ICIs)-based combinations have improved survival outcomes of advanced renal cell carcinoma (RCC) patients and are currently recommended as first-line treatment options. Rheumatoid arthritis (RA) is a systemic autoimmune disease (AD) of unknown etiology characterized by a chronic inflammatory process involving joints and extra-articular organs. Patients with AD are usually excluded from large randomized clinical trials investigating immunotherapeutic drugs. Therefore, little is known about clinical outcomes of patients with a history of RA treated with ICIs in real-world practice. In the present study, we report the clinical outcome of an advanced RCC patient with a history of RA treated with pembrolizumab in combination with axitinib. The patient experienced serious immune-related adverse events (irAEs) and achieved pathological complete response following only one ICI administration. Our case report shows that ICI-based combinations can be administered efficaciously in advanced RCC patients with a history of AD. However, a close monitoring of these patients is required, given the risk of irAEs and clinical exacerbations of symptoms associated with the preexisting AD. Moreover, prospective clinical data are needed to assess the hypothesis of a correlation between the onset of irAEs and AD flares and responses and survival outcomes to ICIs.
Keywords: Renal cell carcinoma, Rheumatoid arthritis, Immunotherapy, Immune-related adverse events, Pathological complete response
Introduction
Renal cell carcinoma (RCC) accounts for approximately 400,000 diagnoses and 175,000 deaths each year [1]. Based on the pivotal role of the immune system in RCC development and progression [2], immunotherapy-based combinations have become a standard of care in the management of advanced RCC across all International Metastatic RCC Database Consortium (IMDC) prognostic subgroups. Both combinations of dual immune checkpoint inhibitors (ICIs) [3] and ICI plus antiangiogenic tyrosine kinase inhibitor (TKI) [4–6] have been shown to improve overall survival (OS) and are currently recommended as first-line treatment options for metastatic RCC [7, 8]. However, since special subgroups of patients are usually excluded from clinical trials, little is published on ICI use in patients with a history of autoimmune disease (AD).
Rheumatoid arthritis (RA) is defined as a multifactorial systemic autoimmune pathology of unknown etiology associated with a chronic inflammatory process which can damage both joints and extra-articular organs, including the heart, kidney, lung, digestive system, eye, skin, and nervous system [9]. The autoreactive recruitment of T cells and B cells, the production of inflammatory cytokines and autoantibodies, and the abnormal activation of synovial fibroblasts play a key role in the inflammatory milieu of RA, leading to the articular and extra-articular clinical manifestations of the disease [10]. Due to the exclusion criteria of large randomized clinical trials, the clinical outcome of RA patients receiving ICIs is largely unknown.
In the present study, we report the clinical outcome of an advanced RCC patient with a history of RA treated with the anti-programmed death 1 (PD-1) agent pembrolizumab in combination with the antiangiogenic TKI axitinib. The patient experienced serious immune-related adverse events (irAEs), which might be interpreted as a clinical exacerbation of the preexisting AD, and achieved pathological complete response (pCR) following only one ICI administration.
Case Report
We present the case of a 69-year-old woman with a history of RA treated with methotrexate 15 mg a week and prednisone 5 mg OD since 2005. In June 2022, a significant increase in C-reactive protein (CRP) serum levels was reported despite no clinical signs of active arthritis neither symptoms, signs or laboratory examinations suggesting infectious disease. Therefore, the patient underwent a computed tomography (CT) scan of the thorax, abdomen, and pelvis. The radiological examination showed a 11 × 8.5 × 10.5 cm exophytic mass with a heterogeneous structure, lobular profile, and contrast enhancement originating from the middle/lower third of the right kidney (Fig. 1a1, a2). Posteriorly, the lesion was in contact with the back muscles and the psoas muscle. Focal lack of a surgical plane of cleavage with the back muscles was observed. The mass was also in contact with the right wall of the inferior cava vein, with the posteromedial wall of the ascending colon, and with the posteromedial side of the right hepatic lobe. A thin surgical plane of cleavage with these organs was observed. Several nodules both in the right and in the left lung were also described (Fig. 1a3).
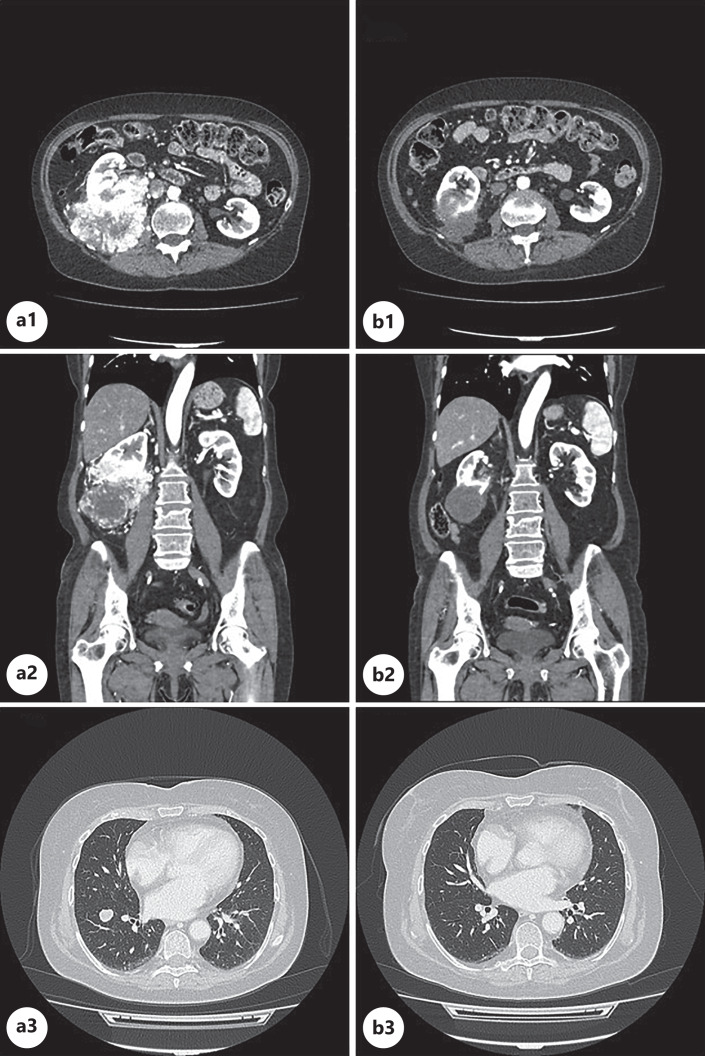
Fig. 1.
Axial (a1, b1, a3, b3) and coronal (a2, b2) images from CT scan examinations performed by the patient in June 2022 (a1, a2, a3) and in October 2022 (b1, b2, b3). Baseline CT scan showed a 11 × 8.5 × 10.5 cm exophytic mass with contrast enhancement originating from the middle/lower third of the right kidney. Focal lack of a surgical plane of cleavage with the back muscles was observed (a1, a2). Several nodules in the right and in the left lung were described (a3). The CT scan performed 3 months after the beginning of the oncological treatment showed a significant size reduction and the lack of contrast enhancement of the renal mass (b1, b2) and the vanishing of the lung metastases (b3). CT, computed tomography.
Since an accurate diagnostic assessment was needed, the patient was admitted to the Medical Oncology Unit of the ASST Spedali Civili of Brescia (Italy). At the hospital admission, the patient’s performance status according to the Eastern Cooperative Oncology Group scale was 0. Despite mild macroematuria, biochemical examinations showed no anemia, neutrophils and platelets counts were within normal limits, while the serum calcium level was 12.5 mg/dL. Due to persistent mild hypercalcemia despite treatment with hydrating solutions, diuretics, and corticosteroids, a single dose of zoledronic acid 4 mg was administered. Treatment with methotrexate was discontinued, and corticosteroid dosage was tapered until discontinuation. Meanwhile, an ultrasound-guided percutaneous biopsy of the renal mass was performed, revealing a PAX8+, CAIX+, and cathepsin K− clear cell RCC (Fig. 2).
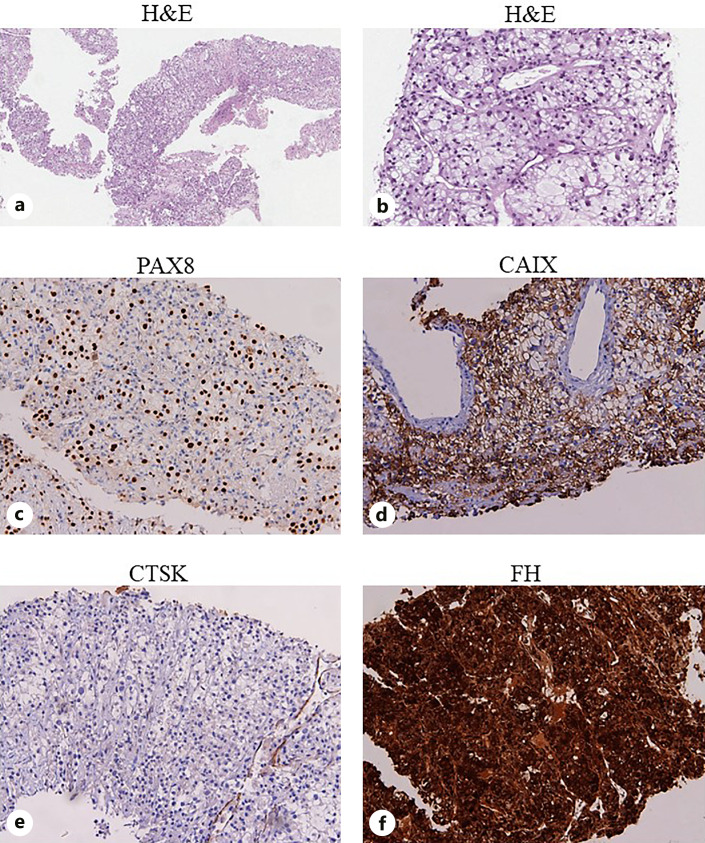
Fig. 2.
Sections are from renal mass biopsy and stained as labeled. The renal biopsy revealed tumor cells with abundant and vacuolated cytoplasm and large nuclei with variably prominent nucleoli (a, b). Tumor cells were positive to PAX8 (c) and CAIX (d) immunohistochemical staining and negative for CTSK (e). FH expression was preserved (f). Based on the morphological and immunohistochemical features, the patient was diagnosed with a clear cell RCC. H&N, hematoxylin and eosin; CAIX, carbonic anhydrase IX; CTSK, cathepsin K; FH, fumarate hydratase.
Therefore, first-line systemic treatment for advanced RCC was needed. The IMDC risk score was considered to be intermediate due to hypercalcemia and start of systemic treatment within 1 year of disease diagnosis. Moreover, RA had been clinically stable for several years. Hence, 1 week following hospital discharge, the patient was started on combination therapy with pembrolizumab 200 mg every 21 days and axitinib 5 mg bis in die (BID) after being offered counseling on the possibility of irAEs, including RA flares.
However, few days following the first anti-PD-1 administration and the start of TKI treatment, the patient presented with diffuse myalgias, hyperpyrexia, and a significant increase in CRP serum levels, while no signs of active arthritis were described. The patient was admitted to the Medical Oncology Unit, axitinib was held, and corticosteroid therapy with prednisone 25 mg was introduced. After pain relief and CRP serum levels decrease, corticosteroid dosage was tapered, axitinib was reintroduced, and the patient was discharged. However, few days later, a new hospital admission was required due to fatigue, severe and diffuse arthralgia, and myalgia, limiting self-care activities and dysphagia for liquids. Creatine kinase serum levels were 2914 mU/L, classified as grade 4 according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Neurological examination and electroneuromyography showed signs of polyneuropathy and muscular damage, while no sign of neuromuscular junction disease was revealed. Axitinib was held. Based on current international guideline recommendations for the management of irAEs [11, 12], methylprednisolone 2 mg/kg was introduced, leading to pain relief and a decrease in CRP and creatine kinase serum levels. Therefore, corticosteroid dosage was tapered. Whether the described irAEs could be interpreted as a flare of RA was not clear, given the atypical clinical presentation with predominant muscular involvement. However, due to the severity of the aforementioned irAEs, immunotherapy was permanently discontinued after a single administration. Instead, TKI monotherapy was reinstated, but in the following weeks, axitinib dosage was decreased to 3 mg BID due to persistent grade 2 hepatic toxicity.
In October, about 3 months after the beginning of oncological therapy, a CT scan was performed in order to assess treatment response. A significant size reduction of the renal lesion was described, the longest diameter of the mass now measuring 4.6 cm (Fig. 1b1, b2). No contrast enhancement was observed. Even though a slight contact with the psoas muscle was still present, there was no sign of infiltration of the back muscles and of the cava vein. The CT scan also revealed the vanishing of the lung metastases (Fig. 1b3). Given the good objective response to systemic treatment and the lack of metastatic disease, the patient was considered eligible for radical resection by the multidisciplinary team, the renal mass being deemed surgically resectable. After axitinib dose interruption 7 days before surgery, a radical right nephrectomy with laparotomic access was performed. The histopathological examination described the renal tumor as a grayish-brownish mass, which mainly consisted of coagulative necrosis foci with cholesterin, apoptotic bodies, and neutrophils surrounded by histiocytes. No viable tumor cells were present, classifying this as pCR (Fig. 3).
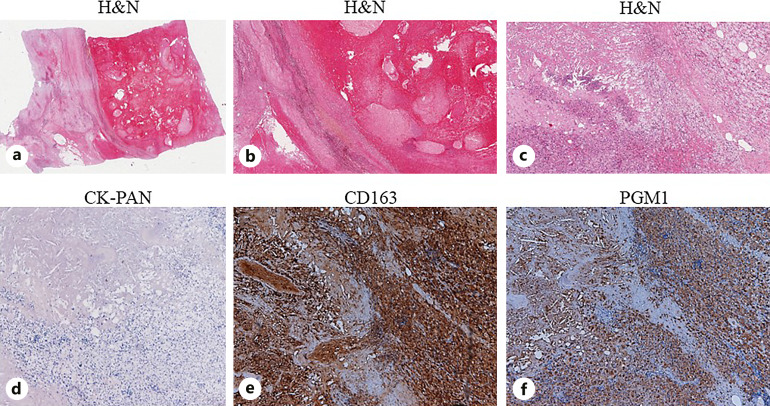
Fig. 3.
Sections are from surgical specimen and stained as labeled. The histopathological examination described coagulative necrosis foci with cholesterin, apoptotic bodies, and neutrophils surrounded by histiocytes (a–c). CK-PAN immunohistochemical staining was negative as no viable tumor cells were present (d). Histiocytes were positive to CD163 (e) and PGM1 (f) immunohistochemical staining. H&N, hematoxylin and eosin; CK-PAN, pan-cytokeratin.
The patient did well postoperatively, and no wound complications related to TKI treatment were observed. Following hospital discharge, axitinib 3 mg BID was reinstated and planned to be continued. As mentioned above, pembrolizumab was permanently discontinued, given the serious grade of the irAEs, and the patient is being closely monitored for RA and for potential relapses of AD.
Discussion
ICI-based combinations are nowadays the preferred treatment option for advanced RCC, with rates of objective responses ranging from 39% to 71% and rates of complete responses ranging from 6% to 16% [3–6, 13, 14]. Moreover, a correlation between depth of response and survival outcomes was observed in large randomized phase 3 trials [4, 15]. However, the diagnosis of objective and complete responses is usually based on imaging, and, without pathological examination of tissue from prospective clinical trials, the rate of pCR is unknown. To our knowledge, only few cases of pCR on specimens from resected RCC after ICI-based combinations are described in the literature [16–26] (Table 1). However, since surgical resection of residual disease is rarely performed, the true rate of pCR to ICI-based treatments may be underestimated.
Table 1.
Cases of pCR to ICI-based combinations described in the literature
| Age, sex; reference | Histology | Disease stage | Disease sites | IMDC risk score | Treatment | Line of treatment | Previous treatments | Months from ICI start and surgery |
|---|---|---|---|---|---|---|---|---|
| 67, male; Shimizu 2022 [16] | Clear cell | IV | Right kidney, IVC thrombus, lymph nodes | Intermediate | Pembrolizumab + axitinib | 1st | None | >7 |
| 68, male; Neuzil 2022 [17] | Poorly differentiated with sarcomatoid features | IV | Right kidney, lymph nodes | n/a | Pembrolizumab + axitinib | 1st | None | >6 |
| 68, female; Tucker 2020 [18] | Clear cell | IV | Left kidney, adrenal gland, lymph nodes, lung, liver | Intermediate | Pembrolizumab + axitinib | 1st | None | >11 |
| 64, female; Tucker 2020 [18] | Clear cell | IV | Right kidney, IVC thrombus, bone, lung, liver | Poor | Nivolumab | 2nd | Cabozantinib + denosumab | >10 |
| 79, female; Hara 2021 [19] | n/a | III | Right kidney, IVC thrombus | n/a | Avelumab + axitinib | 1st | None | >6 |
| 58, female; Labbate 2019 [20] | Clear cell | III | Left kidney, IVC | Poor | Nivolumab + ipilimumab | 1st | None | >4 |
| 60, male; Studentova 2022 [21] | Clear cell | IV | Left kidney, bone | Intermediate | Nivolumab + ipilimumab | 1st | None | >8 |
| 66, male; Peak 2020 [22] | Poorly differentiated | IV | Left kidney, lymph nodes | Intermediate | Nivolumab + ipilimumab | 1st | None | >11 |
| 57, female; Bhat 2019 [23] | Clear cell | IV | Right kidney, IVC thrombus, liver | n/a | Nivolumab + cabozantinib | 2nd | Pazopanib | >9 |
| 60, male; Billon 2019 [24] | Clear cell | IV | Left kidney, lung, pleura, bone | n/a | Nivolumab | 2nd | Sunitinib | >24 |
| 52, male; Shirotake 2019 [25] | Clear cell | IV | Left kidney, lymph nodes, lung, brain | n/a | Nivolumab | 4th | Pazopanib + SRS, everolimus, axitinib | >5 |
| 65, male; Woldu 2018 [26] | Clear cell | IV | Left kidney, renal vein thrombus, lung | n/a | Nivolumab | 2nd | Sunitinib | n/a |
IMDC, International Metastatic RCC Database Consortium; ICI, immune checkpoint inhibitor; IVC, inferior vena cava; n/a, not available; SRS, stereotactic radiosurgery.
Following the relevant objective response revealed by CT scan examination, our patient was submitted to surgery after multidisciplinary discussion. The decision was also based on clinical data showing that complete surgical resection, if possible, is associated with promising survival outcomes in metastatic RCC [27–32]. Moreover, as reported in retrospective series of patients [28, 30, 31, 33], cytoreductive nephrectomy following ICI-based systemic therapy is safe in high-volume centers with experienced surgeons. Results of the CARMENA trial showed that upfront cytoreductive nephrectomy should not be considered as the standard of care in most intermediate-poor risk patients with synchronous metastatic RCC requiring systemic treatment [34]. However, delayed cytoreductive nephrectomy still plays a role in the management of metastatic RCC, and it is recommended for selected patients by current international guidelines [7, 35]. Of note, as careful patient selection is of utmost importance, the ideal candidates and the best timing for this approach remain uncertain.
There are anecdotal reports of withdrawal of systemic therapy in advanced RCC patients who had a complete response to ICI-based combinations and were rendered disease-free by delayed cytoreductive nephrectomy [29, 32, 33]. However, given the absence of prospective data confirming that the discontinuation of systemic treatment after nephrectomy is safe in the metastatic setting, even in the presence of a pCR, axitinib monotherapy was resumed after surgery. Instead, pembrolizumab was permanently discontinued due to the serious grade of irAEs experienced following a single ICI administration. Moreover, retrospective data from a cohort of metastatic RCC patients show that immunotherapy seems to maintain efficacy even after early interruption due to severe irAEs [36]. The decision was discussed within the multidisciplinary team and with the patient, the risk-benefit ratio being judged favorable.
The major issue of our case report is the safety of cancer immunotherapy administration in a patient with a history of RA and the possible correlation between AD flare and the exceptional response to ICI. Clinical trials examining immunotherapy-based combinations in metastatic RCC and immunotherapeutic drugs across all cancer types excluded patients with baseline AD, out of fear of an increased rate or grade of irAEs or disease flare. Hence, optimal management of these patients remains unclear. Evidence about the efficacy and safety of ICIs in these patients is limited and mostly comes from subgroup analyses, case reports of larger clinical trials, and small retrospective cohorts. The available evidence indicates that anti-PD-1 agents can usually be given safely and retain activity in patients with a history of AD.
In retrospective cohorts of melanoma [37, 38] and urological cancer [39] patients with preexisting AD treated with ICIs, immunotherapy was shown to induce relatively frequent irAEs. However, flares of the AD were usually mild and occurred more often in patients with active symptoms or those requiring immunosuppressants at treatment start. Moreover, they were often easily manageable with oral steroids or steroid-sparing agents, and they rarely led to discontinuation of therapy. Though flares of preexisting AD were common, the rate of other irAEs appeared similar to data coming from clinical trial populations. Similar results were described in a recent meta-analysis [40] showing that cancer patients with AD are more likely to develop irAEs in the same system involved by their AD. However, the frequency of irAEs involving other organs and systems is similar to what is observed in cancer patients without AD. When it comes to patients with RA receiving ICIs, evidence suggests that approximately 50% of these patients experience flares or have irAEs [41, 42].
As regarding immunotherapy activity, one hypothesis is that ICIs may produce greater responses in patients with AD, especially those not on immune suppression, due to a propensity for immune stimulation [43, 44]. However, the aforementioned retrospective cohorts failed to show greater response rates or improved survival in cancer patients with preexisting AD receiving immunotherapeutic drugs [37–39], though suggesting that immunosuppressants may negatively influence these outcomes [37]. As for the use of corticosteroids in the management of AD flares and irAEs, Tison et al. found that the survival of patients with AD was significantly shortened when corticosteroids were administered [45]. However, results from other studies failed to confirm this correlation [46, 47].
When it comes to the association between irAE and ICI efficacy, a physiological correlation appears to be quite logical [48], based on the hypothesis that the greater the activation of PD-1 positive T cells, the greater the immunotherapy-related toxicities and treatment responses. IrAEs were associated with progression-free survival and OS in many retrospective studies, especially in lung cancer and melanoma [49–57]. Besides, a relationship between ICI toxicity and patients’ survival was reported in a published trial-based meta-analysis [58]. However, Amoroso et al. [59] recently conducted a systematic review and meta-analysis of randomized trials of experimental ICIs in several cancer types [59]. The authors showed that the irAE rate should not be regarded as a valid surrogate for OS. As for randomized trials evaluating ICIs in RCC, an exploratory post hoc analysis of the CheckMate 9ER trial did not reveal a correlation between the incidence of AEs and depth of response to nivolumab and cabozantinib combination [60]. Therefore, the general consensus is that irAEs are not required to benefit from cancer immunotherapy [61] since their impact on long-term survival outcomes remains unknown.
Conclusion
ICI-based combinations can be administered efficaciously in advanced RCC patients with a history of AD. However, the stimulation of immune response may lead to clinically significant irAEs and to the relapse of the preexisting AD. Therefore, it is important to balance risks and benefits, and a close monitoring of these patients is required. The management of irAEs is challenging and often requires high-dose corticosteroids, whose impact on the efficacy of ICIs is still uncertain. Moreover, though the correlation between the onset of irAEs or AD flares and the response to anti-PD-1 may appear quite logical, prospective clinical data are needed to confirm this hypothesis. Since data about pCR to immunotherapy-based treatments in advanced RCC are scant, a longer follow-up of our patient is needed to assess if a long-term response was achieved.
Acknowledgments
We acknowledge the Medical Oncology Unit, the Urology Unit, and the Pathology Unit of ASST Spedali Civili, Brescia, Università degli Studi di Brescia, that played a crucial role in the management of this clinical case and in the writing and editing of this case report.
Statement of Ethics
Written informed consent was obtained from the patient for the publication of the details of the medical case and any accompanying images. No study protocol requiring an Ethics Committee approval is presented within this article. This retrospective review of a single patient data did not require ethical approval in accordance with local/national guidelines.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Sources
There is no funding to disclose for this study.
Author Contributions
MarBer: conceptualization, manuscript writing, and iconographic support. A.D.V.: conceptualization and manuscript writing. F.V.: conceptualization and manuscript editing. I.C., MarBuf, T.Z., and N.R.S.: manuscript editing. E.M. and S.F.: iconographic support and manuscript editing. A.B.: conceptualization, tutoring, and manuscript editing. All the authors contributed to the article and approved the submitted version.
Funding Statement
There is no funding to disclose for this study.
Data Availability Statement
All relevant data are contained within the article. The original contributions presented in the study are included in the article/figures/tables. Further inquiries can be directed to the corresponding author. The CARE Checklist has been completed by the authors for this case report, attached as supplementary material (for all online suppl. material, see https://doi.org/10.1159/000535460).
Supplementary Material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, et al. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169(4):736–49.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–73. [DOI] [PubMed] [Google Scholar]
- 5. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–300. [DOI] [PubMed] [Google Scholar]
- 7. Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, et al. European association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82(4):399–410. [DOI] [PubMed] [Google Scholar]
- 8. Motzer R, Jonasch E, Agarwal N, Alva A, Baine M, Beckermann K, et al. Kidney cancer, version 3.2022, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(1):67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Radu AF, Bungau SG. Management of rheumatoid arthritis: an overview. Cells. 2021;10(11):2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferraccioli G, Gremese E. Pathogenetic, clinical and pharmaco-economic assessment in rheumatoid arthritis (RA). Intern Emerg Med. 2011;6(Suppl 1):11–5. [DOI] [PubMed] [Google Scholar]
- 11. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(12):1217–38. [DOI] [PubMed] [Google Scholar]
- 12. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–126. [DOI] [PubMed] [Google Scholar]
- 13. Albiges L, Gurney H, Atduev V, Suarez C, Climent MA, Pook D, et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2023;24(8):881–91. [DOI] [PubMed] [Google Scholar]
- 14. Stellato M, Buti S, Maruzzo M, Bersanelli M, Pierantoni F, De Giorgi U, et al. Pembrolizumab plus axitinib for metastatic papillary and chromophobe renal cell carcinoma: NEMESIA (non clear MEtaStatic renal cell carcinoma pembrolizumab axitinib) study, a subgroup analysis of I-RARE observational study (Meet-URO 23a). Int J Mol Sci. 2023;24(2):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grünwald V, et al. Survival by depth of response and efficacy by international metastatic renal cell carcinoma Database Consortium subgroup with lenvatinib plus pembrolizumab versus sunitinib in advanced renal cell carcinoma: analysis of the phase 3 randomized CLEAR study. Eur Urol Oncol. 2023;6(4):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimizu K, Tamada S, Matsuoka Y, Go I, Okumura S, Ogawa M, et al. Pathologic complete response with pembrolizumab plus axitinib in metastatic renal cell carcinoma. Int Cancer Conf J. 2022;11(3):205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neuzil K, Gessner K, Hedgepeth J, Wobker SE, Wallen EM, Morgan KP, et al. Complete pathologic response to pembrolizumab and axitinib in a patient with sarcomatoid RCC and ocrelizumab-treated multiple sclerosis. Urology. 2022;164:50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tucker MD, Beckermann KE, Gordetsky JB, Giannico GA, Davis NB, Rini BI. Complete pathologic responses with immunotherapy in metastatic renal cell carcinoma: case reports. Front Oncol. 2020;10:609235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hara T, Terakawa T, Hyodo T, Jinbo N, Nakano Y, Fujisawa M. Pathological complete response of renal cell carcinoma with vena cava tumor thrombus to neoadjuvant TKI/IO combination therapy. Urol Case Rep. 2021;39:101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Labbate C, Hatogai K, Werntz R, Stadler WM, Steinberg GD, Eggener S, et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer. 2019;7(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Studentova H, Zemankova A, Spisarova M, Skanderova D, Tudos Z, Melichar B, et al. A pathological complete response to the combination of ipilimumab and nivolumab in a patient with metastatic renal cell carcinoma. Med Kaunas. 2022;58(3):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peak TC, Fenu EM, Rothberg MB, Thomas CY, Davis RL, Levine EA. Pathologic complete response to neoadjuvant nivolumab/ipilimumab in a patient with metastatic renal cell carcinoma. Case Rep Urol. 2020;2020:8846135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhat A, Nahar B, Venkatramani V, Banerjee I, Kryvenko ON, Parekh DJ. Metastatic renal cell carcinoma with level IV thrombus: contemporary management with complete response to neoadjuvant targeted therapy. Case Rep Urol. 2019;2019:7102504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Billon E, Walz J, Brunelle S, Thomassin J, Salem N, Guerin M, et al. Vitiligo adverse event observed in a patient with durable complete response after nivolumab for metastatic renal cell carcinoma. Front Oncol. 2019;9:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shirotake S, Kaneko G, Nagata K, Oyama M, Nishimoto K. Histological complete response with nivolumab for renal cell carcinoma with multiple metastases: a case report. Mol Clin Oncol. 2019;10(2):244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Woldu SL, Brugarolas J, Kapur P, Margulis V. What is the role of nephrectomy following complete response to checkpoint inhibitors? Urol Case Rep. 2018;18:60–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Das A, Shapiro DD, Craig JK, Abel EJ. Understanding and integrating cytoreductive nephrectomy with immune checkpoint inhibitors in the management of metastatic RCC. Nat Rev Urol. 2023;20(11):654–68. [DOI] [PubMed] [Google Scholar]
- 28. Fransen van de Putte EE, van den Brink L, Mansour MA, van der Mijn JC, Wilgenhof S, van Thienen JV, et al. Indications and outcomes for deferred cytoreductive nephrectomy following immune checkpoint inhibitor combination therapy: can systemic therapy be withdrawn in patients with No evidence of disease? Eur Urol Open Sci. 2023;55:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pignot G, Thiery-Vuillemin A, Albigès L, Walz J, Lang H, Balssa L, et al. Oncological outcomes of delayed nephrectomy after optimal response to immune checkpoint inhibitors for metastatic renal cell carcinoma. Eur Urol Oncol. 2022;5(5):577–84. [DOI] [PubMed] [Google Scholar]
- 30. Shirotake S, Miyama YU, Baba Y, Tajima H, Okada Y, Nakazawa K, et al. Impact of cytoreductive nephrectomy following nivolumab plus ipilimumab therapy for patients with advanced renal cell carcinoma. Anticancer Res. 2022;42(5):2727–35. [DOI] [PubMed] [Google Scholar]
- 31. Singla N, Hutchinson RC, Ghandour RA, Freifeld Y, Fang D, Sagalowsky AI, et al. Improved survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy era: an analysis of the National Cancer Database. Urol Oncol. 2020;38(6):604.e9–604.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gross EE, Li M, Yin M, Orcutt D, Hussey D, Trott E, et al. A multicenter study assessing survival in patients with metastatic renal cell carcinoma receiving immune checkpoint inhibitor therapy with and without cytoreductive nephrectomy. Urol Oncol. 2023;41(1):51.e25–51.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singla N, Elias R, Ghandour RA, Freifeld Y, Bowman IA, Rapoport L, et al. Pathologic response and surgical outcomes in patients undergoing nephrectomy following receipt of immune checkpoint inhibitors for renal cell carcinoma. Urol Oncol. 2019;37(12):924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Méjean A, Ravaud A, Thezenas S, Chevreau C, Bensalah K, Geoffrois L, et al. Sunitinib alone or after nephrectomy for patients with metastatic renal cell carcinoma: is there still a role for cytoreductive nephrectomy? Eur Urol. 2021;80(4):417–24. [DOI] [PubMed] [Google Scholar]
- 35. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(5):706–20. [DOI] [PubMed] [Google Scholar]
- 36. Stellato M, Procopio G, De Giorgi U, Maruzzo M, Bimbatti D, Mennitto A, et al. Clinical outcome of renal cancer patients who early interrupted immunotherapy due to serious immune-related adverse events. Meet-Uro 13 trial on behalf of the MeetUro investigators. J Transl Med. 2021;19(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–76. [DOI] [PubMed] [Google Scholar]
- 38. Brown LJ, Weppler A, Bhave P, Allayous C, Patrinely JR Jr, Ott P, et al. Combination anti-PD1 and ipilimumab therapy in patients with advanced melanoma and pre-existing autoimmune disorders. J Immunother Cancer. 2021;9(5):e002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martinez Chanza N, Xie W, Issa M, Dzimitrowicz H, Tripathi A, Beuselinck B, et al. Safety and efficacy of immune checkpoint inhibitors in advanced urological cancers with pre-existing autoimmune disorders: a retrospective international multicenter study. J Immunother Cancer. 2020;8(1):e000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cai Q, Huo GW, Zhu FY, Yue P, Yuan DQ, Chen P. Safety and efficacy of immune checkpoint inhibitors in advanced cancer patients with autoimmune disease: a meta-analysis. Hum Vaccin Immunother. 2022;18(7):2145102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2(2):234–40. [DOI] [PubMed] [Google Scholar]
- 42. Lee B, Wong A, Kee D, Neeson P, Shackleton M, McArthur G, et al. The use of ipilimumab in patients with rheumatoid arthritis and metastatic melanoma. Ann Oncol. 2016;27(6):1174–7. [DOI] [PubMed] [Google Scholar]
- 43. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tison A, Quéré G, Misery L, Funck-Brentano E, Danlos FX, Routier E, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 2019;71(12):2100–11. [DOI] [PubMed] [Google Scholar]
- 46. Fountzilas E, Lampaki S, Koliou GA, Koumarianou A, Levva S, Vagionas A, et al. Real-world safety and efficacy data of immunotherapy in patients with cancer and autoimmune disease: the experience of the Hellenic Cooperative Oncology Group. Cancer Immunol Immunother. 2022;71(2):327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers. 2020;12(3):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17(6):610–6. [DOI] [PubMed] [Google Scholar]
- 49. Toi Y, Sugawara S, Kawashima Y, Aiba T, Kawana S, Saito R, et al. Association of immune-related adverse events with clinical benefit in patients with advanced non-small-cell lung cancer treated with nivolumab. Oncologist. 2018;23(11):1358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rzepecki AK, Cheng H, McLellan BN. Cutaneous toxicity as a predictive biomarker for clinical outcome in patients receiving anticancer therapy. J Am Acad Dermatol. 2018;79(3):545–55. [DOI] [PubMed] [Google Scholar]
- 51. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–85. [DOI] [PubMed] [Google Scholar]
- 52. Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151(11):1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakamura Y, Tanaka R, Asami Y, Teramoto Y, Imamura T, Sato S, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44(2):117–22. [DOI] [PubMed] [Google Scholar]
- 54. Hasan Ali O, Diem S, Markert E, Jochum W, Kerl K, French LE, et al. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology. 2016;5(11):e1231292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 2018;4(3):374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21–7. [DOI] [PubMed] [Google Scholar]
- 57. Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–4. [DOI] [PubMed] [Google Scholar]
- 58. Petrelli F, Grizzi G, Ghidini M, Ghidini A, Ratti M, Panni S, et al. Immune-related adverse events and survival in solid tumors treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Immunother. 2020;43(1):1–7. [DOI] [PubMed] [Google Scholar]
- 59. Amoroso V, Gallo F, Alberti A, Paloschi D, Ferrari Bravo W, Esposito A, et al. Immune-related adverse events as potential surrogates of immune checkpoint inhibitors' efficacy: a systematic review and meta-analysis of randomized studies. ESMO Open. 2023;8(2):100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suárez C, Burotto M, Powles T, Bourlon MT, Shah AY, Tomita Y, et al. Association between depth of response (DepOR) and clinical outcomes: exploratory analysis in patients with previously untreated advanced renal cell carcinoma (aRCC) in CheckMate 9ER. 2022 ASCO Annual Meeting, J.o.C. Oncology, Editor. 2022. [Google Scholar]
- 61. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are contained within the article. The original contributions presented in the study are included in the article/figures/tables. Further inquiries can be directed to the corresponding author. The CARE Checklist has been completed by the authors for this case report, attached as supplementary material (for all online suppl. material, see https://doi.org/10.1159/000535460).