Abstract
Background:
Performance monitoring entails rapid error detection to maintain task performance. Impaired performance monitoring is a candidate pathophysiological process in psychotic disorders, which may explain the broader deficit in executive function and its known associations with negative symptoms and poor functioning. The current study models cross-sectional pathways bridging neurophysiological measures of performance monitoring with executive function, symptoms, and functioning.
Methods:
Data were from the 20-year assessment of the Suffolk County Mental Health Project. Individuals with psychotic disorders (N=181) were originally recruited from inpatient psychiatric facilities. Data were also collected from a geographically- and demographically-matched group with no psychosis history (N=242). Neural measures were the error-related negativity (ERN) and error positivity (Pe). Structural equation modeling tested mediation pathways.
Results:
Blunted ERN and Pe in the clinical cohort related to impaired executive function (r=.26-.35), negative symptom severity (r=.17-.25), and poor real-world functioning (r=.17-.19). Associations with executive function were consistent across groups. Multiple potential pathways were identified in the clinical cohort: reduced ERN to inexpressivity was mediated by executive function (β=.10); reduced Pe to global functioning was mediated by executive function and avolition (β=.10).
Conclusions:
This supports a transdiagnostic model of psychotic disorders by which poor performance monitoring contributes to impaired executive function, which contributes to negative symptoms and poor real-world functioning. If supported by future longitudinal research, these pathways could inform the development of targeted interventions to address cognitive and functional deficits that are central to psychotic disorders.
Keywords: schizophrenia, psychosis, ERP, ERN, executive function, cognition
Introduction
A core feature of schizophrenia is widespread cognitive impairment (Reichenberg & Harvey, 2007), which strongly predicts poor functional outcomes (Bowie & Harvey, 2006; Bowie et al., 2008; Bowie, Reichenberg, Patterson, Heaton, & Harvey, 2006; Green, 1996; Green, Kern, Braff, & Mintz, 2000; Strassnig et al., 2015), in part through negative symptom severity (Ventura, Hellemann, Thames, Koellner, & Nuechterlein, 2009). Effective treatments for cognition and negative symptoms, however, are lacking (Buchanan et al., 2005; Firth et al., 2017; Fusar-Poli et al., 2015; Insel, 2010; Revell, Neill, Harte, Khan, & Drake, 2015; Wykes, Huddy, Cellard, McGurk, & Czobor, 2011). Cognitive impairment in psychotic illness is also transdiagnostic, with the same profile of impairment across psychotic disorders (Hill et al., 2013; Reichenberg et al., 2009). Here, we extend models of cognitive impairment, negative symptomatology, and functional outcomes to incorporate candidate neurophysiological measures that are applicable across the spectrum of psychotic disorders.
We focus on the cognitive domain of executive function because it is among the most impaired in psychotic disorders (Reichenberg et al., 2009) and predicts everyday functioning: Whereas general cognitive ability predicts activities of daily living and vocational functioning (Strassnig et al., 2015), executive function also plays a role in interpersonal behavior (Bowie et al., 2008). Thus, both global functioning and domain-specific functioning are of interest. We focus on performance monitoring because it is a proposed mechanism of impaired executive function in psychotic illness (Kerns, Nuechterlein, Braver, & Barch, 2008) and is a promising treatment target (Reinhart, Zhu, Park, & Woodman, 2015). Performance monitoring involves the rapid detection of behavioral errors, signaling the need for increased cognitive control to maintain task performance (Holroyd, Yeung, Coles, & Cohen, 2005). Performance monitoring is most active in situations where stimulus-response rules are well-learned and response certainty is high (Holroyd & Coles, 2002) and is distinguishable from other aspects of executive function that are impaired in psychotic disorders (Kerns et al., 2008). Thus, impaired performance monitoring is a candidate pathophysiological process of psychosis that may partially account for the broader deficit in executive function and its associated illness features. We test this possibility using structural equation modeling, examining mediated effects of performance monitoring on behavioral measures of executive function, clinical ratings of symptoms, and everyday functioning within a heterogeneous sample of individuals with psychotic disorders and never-psychotic adults.
Performance monitoring was assessed using event-related potentials (ERPs). Studies distinguish between two functionally and anatomically distinct neural measures: the error-related negativity (ERN) and error positivity (Pe) (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, Meyer, & Donchin, 1993). The ERN occurs within 100 ms following error commission on speeded tasks and captures automatic error detection, whereas the Pe occurs from 300–500 ms and relates to conscious error recognition (Hajcak, McDonald, & Simons, 2003; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001). The ERN is generated by the dorsal anterior cingulate cortex and motor cortex (Debener et al., 2005; Iannaccone et al., 2015; Mathalon, Whitfield, & Ford, 2003), whereas the Pe has a distinct source, possibly in the posterior cingulate cortex (O’Connell et al., 2007; Vocat, Pourtois, & Vuilleumier, 2008). Numerous studies have shown that the ERN is reduced among individuals with schizophrenia (Alain, McNeely, He, Christensen, & West, 2002; Bates, Kiehl, Laurens, & Liddle, 2002; Horan, Foti, Hajcak, Wynn, & Green, 2012; Kansal, Patriciu, & Kiang, 2014; Kim et al., 2006; Kopp & Rist, 1999; Llerena, Wynn, Hajcak, Green, & Horan, 2016; Mathalon et al., 2002; Morris, Yee, & Nuechterlein, 2006). It is also reduced in high-risk populations (Laurens et al., 2010; Perez et al., 2012) and unaffected siblings (Simmonite et al., 2012), suggesting a neural endophenotype of risk for psychosis and associated cognitive impairment. There is evidence of a reduced Pe in schizophrenia (Foti, Kotov, Bromet, & Hajcak, 2012; Foti et al., 2016; Kansal et al., 2014; Perez et al., 2012), although also see (Alain et al., 2002; Horan et al., 2012; Kim et al., 2006; Mathalon et al., 2002; Morris et al., 2006). The ERN is reduced in other psychotic disorders (Foti et al., 2012; Minzenberg, Gomes, Yoon, Swaab, & Carter, 2014), whereas a reduced Pe is relatively specific to schizophrenia (Foti et al., 2012; Foti et al., 2016). While correlations with symptoms are equivocal (Horan et al., 2012; Kansal et al., 2014; Kim et al., 2006; Llerena et al., 2016; Mathalon et al., 2002), there is some evidence that performance monitoring deficits relate to greater negative symptom severity (Bates et al., 2002; Foti et al., 2012; Foti et al., 2016; Reinhart et al., 2015) and occupational functioning (Foti et al., 2012), which is consistent with the neurocognitive models described above (Ventura et al., 2009).
Evidence has primarily come from bivariate correlations between neural measures of performance monitoring and clinical characteristics; pathway models explaining the nature of these relationships are lacking. As an example, a recent study of impaired auditory processing in schizophrenia utilized structural equation modeling to test a bottom-up model whereby abnormal ERPs contribute to cognitive impairment and clinical outcomes (Thomas et al., 2017). Auditory ERPs related to global functioning through general cognitive ability and the negative symptom dimension of avolition. Such pathway models may be clinical useful insofar as they lay the groundwork for targeted interventions on neural deficits, enabling estimates of which outcomes are most likely to be affected by improvement in neural functioning and the likely magnitude of those effects.
Here, we followed the same conceptual approach to test a bottom-up model whereby neural deficits in performance monitoring contribute to impaired executive function, which in turn contributes to negative symptom severity and everyday functioning. Pathways were examined among an epidemiologic cohort with psychotic disorders (N=181), including schizophrenia, mood disorders with psychotic features, and substance-induced psychosis, as well as never-psychotic adults (N=242). The ERN/Pe are high-priority neural measures for clinical translation to improve executive function in psychotic disorders (Kerns et al., 2008), and the current study adds to this foundation. Previous reports from a subsample of this cohort (92 cases) explored bivariate links between symptoms, everyday functioning, and course of illness across a battery of ERPs, including performance monitoring (Foti et al., 2012; Foti, Kotov, & Hajcak, 2013; Foti et al., 2016; Kotov et al., 2016), attention (Perlman et al., 2015), semantic processing (Jackson et al., 2014), and emotional reactivity (Culbreth, Foti, Barch, Hajcak, & Kotov, 2018). The current analyses are the first to use the full cohort and model pathways from ERPs to cognition and associated illness features, with the following aims:
(1). Is performance monitoring associated with cognitive impairment across the psychosis spectrum?
Performance monitoring is a key sub-process of impaired executive function in schizophrenia (Kerns et al., 2008), yet only one study has tested whether performance monitoring is associated with independent, behavioral assessments of cognitive ability, with equivocal results (Kim et al., 2006). As a first step toward establishing pathways from performance monitoring to functioning, we tested whether reduced ERN and Pe correlated with poor performance on a range of neuropsychological tests. We expected ERN and Pe to relate most strongly to tests of executive functioning, above and beyond general cognitive ability. Given that cognitive impairment (Reichenberg et al., 2009) and performance monitoring deficits (Foti et al., 2012) are most severe in schizophrenia, we also tested whether neural-cognitive links are observed across the psychosis spectrum (Sheffield et al., 2017). If so, this would support the modeling of trandsiagnostic pathways from neural impairment to functional outcomes. On the other hand, if neural-cognitive links are moderated by diagnostic status, it would indicate that different pathway models are called for in these subgroups.
(2). How does performance monitoring relate to symptom dimensions?
Cognitive impairment strongly relates to negative symptom severity (Ventura et al., 2009), including its two sub-dimensions of avolition (i.e., avolition, apathy, anhedonia, asociality) and inexpressivity (i.e., alogia, flat affect) (Blanchard & Cohen, 2006; Kotov et al., 2016; Kring, Gur, Blanchard, Horan, & Reise, 2013; Strauss et al., 2013). Avolition and inexpressivity have distinct relationships with brain functioning (Shaffer et al., 2015) and everyday outcomes (Harvey, Khan, & Keefe, 2017). Greater construct precision will enhance the ability to model pathways from neural measures to functioning (Thomas et al., 2017). Preliminary findings from a subsample of this cohort suggest that ERN primarily relates to inexpressivity and Pe to avolition (Foti et al., 2016), and we tested associations with these sub-dimensions among the full cohort here. Although we focus primarily on negative symptoms, correlations with positive and disorganized symptoms were included to demonstrate specificity. This clinical cohort contains 20 years of archival data, enabling tests of replicability with retrospective illness characteristics.
(3). What pathways link performance monitoring to cognition, symptom severity, and functioning?
Based on models that negative symptoms mediate links between cognition and functioning (Ventura et al., 2009), we tested an extended model linking performance monitoring to cognition, then negative symptom severity, then functioning. Included were measures of global (Thomas et al., 2017; Ventura et al., 2009) and domain-specific real-world functioning (Bowie et al., 2008). We expected effects of ERN and Pe on negative symptoms would be mediated by poor executive function and not concurrent deficits in attention or general cognitive ability. We expected that reduced ERN and Pe would relate to poor everyday functioning through a pathway of impaired executive function and then negative symptom severity (Foti et al., 2016).
Methods
Participants
The clinical cohort was from the Suffolk County Mental Health Project (Bromet et al., 2011; Bromet et al., 1992), an epidemiologic study of first-admission psychosis. Participants were recruited from the 12 inpatient psychiatric facilities of Suffolk County, NY, from 1989–1995. Eligibility criteria were the presence of psychosis, age 15–60, and ability to provide consent. Follow-up assessments were conducted 6 months later and again at years 2, 4, 10, and 20. Master’s-level interviewers administered the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer, Gibbon, & Williams, 2001). Primary DSM-IV diagnoses were formulated at year 20 by consensus of three or more psychiatrists using all available longitudinal information. Analyses focused on year 20 measures; archival measures were considered when probing the temporal stability of correlations with year-20 ERPs.
At year 20, ERP data were available from 181 individuals with psychotic disorders (out of 222, see Supplement): 93 with schizophrenia or schizoaffective disorder, 50 with bipolar disorder, 16 with major depression, 9 with substance-induced psychosis, and 13 with psychotic disorders not otherwise specified. ERN/Pe data have been published as part of longitudinal analyses (Foti et al., 2016) and phenotypic models of psychosis (Kotov et al., 2016). Based on previous findings (Foti et al., 2012; Foti et al., 2013; Foti et al., 2016) and to maximize power, two subgroups were created for diagnostic comparisons: schizophrenia spectrum (schizophrenia, schizoaffective disorder) and other psychotic disorders (bipolar disorder, major depression, substance-induced, and not otherwise specified psychotic disorders).
Contemporaneous with the year 20 assessment and using the same laboratory equipment, data were collected from never-psychotic (NP) adults. Data were available from 242 (out of 254). The NP group was recruited using random digit dialing from zip codes where the clinical group currently resided and was matched on age and gender. Exclusion criteria were lifetime psychosis (assessed by the SCID) or lifetime psychiatric hospitalization.
Written informed consent was obtained at each session. This research was approved annually by the Committee on Research Involving Human Subjects at Stony Brook University. Participants received financial compensation for their time.
Measures
Performance monitoring.
ERP procedures are described elsewhere (Foti et al., 2012; Foti et al., 2013; Foti et al., 2016) and in the Supplementary Material. An arrow flankers task was used (Eriksen & Eriksen, 1974). An array of arrows was presented on each trial, and participants pressed a button indicating the orientation of the center arrow. The electroencephalograph was processed using standard procedures including re-referencing, filtering, segmentation, ocular correction, artifact rejection, and baseline correction. ERP waveforms were averaged separately for correct and error trials (clinical cohort: M=22.45 error trials averaged, SD=16.71, Range=2–90; NP: M=19.89, SD=12.72, Range=2–69; see Supplementary Material). Error minus correct waveforms were calculated for the ERN (0–100 ms at Cz) and Pe (300–500 ms at Pz).
Cognition.
Cognitive functioning was assessed at year 20 using three tests: Trail Making, Stroop Color-Word, Letter-Number Sequencing, and Vocabulary (Lezak, Howieson, & Loring, 2004; Spreen & Strauss, 1998; Wechsler, 1981). A composite score of executive function was calculated as the mean of standardized scores on Trail Making B, Stroop, and Letter-Number Sequencing. For comparison, we considered Trail Making A as a measure of simple attention/processing speed, and the Vocabulary subtest of the Wechsler Adult Intelligence Scale—Revised as a measure of general cognitive ability (Canivez & Watkins, 2010; Silverstein, 1982).
Symptoms and functioning.
Symptoms of psychosis in the month preceding each assessment wave were rated by master’s-level interviewers using the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1983b) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983a). Sub-dimensions were scored as factor-analytically derived subscales (Kotov et al., 2016). SAPS was scored as reality distortion (hallucinations, delusions) and disorganized (bizarre behavior, thought disorder), and SANS as inexpressivity (affective flattening, alogia) and avolition (apathy, anhedonia, asociality) (Blanchard & Cohen, 2006; Kring et al., 2013; Strauss et al., 2013).
Functioning was assessed using social, role, and global measures, which have differential associations with cognition (Bowie et al., 2008) and negative symptoms (Harvey et al., 2017; Kotov et al., 2016). Past-month social functioning was calculated from the Quality of Life Scale (QLS) (Heinrichs, Hanlon, & Carpenter, 1984) as the sum of social activity, sociosexual relations, and relationships with friends (Kotov et al., 2016; Velthorst et al., 2017). Past-month role functioning was assessed on the QLS as the degree of impairment in expected role. The Social and Occupational Functioning Assessment scale (SOFAS) was rated by psychiatrist consensus as participants’ global functioning in the past year, based on all available information (Goldman, Skodol, & Lave, 1992). Symptoms and functioning were rated at each assessment wave.
Statistical Analysis
Characteristics of the clinical and NP groups were compared using t-tests and chi-square tests. Associations with ERPs in the clinical cohort were assessed using bivariate correlations and adjusting for multiple comparisons by setting a false discovery rate of q=.05 (Benjamini & Hochberg, 1995). Where significant concurrent associations were observed, we examined their emergence and stability by calculating correlations between 20-year ERP variables and clinical variables from previous assessments. Analyses were performed using IBM SPSS Statistics (Version 24.0).
Moderation models tested the generalizability of ERP-cognition correlations across the psychosis spectrum (schizophrenia, other psychosis, never psychotic) (Sheffield et al., 2017), and mediation models tested how cognitive variables accounted for ERP-symptom relationships; analyses were calculated using PROCESS (Hayes, 2012). Results informed the full mediation model (i.e., structural equation model) by which ERPs influence cognition, symptoms, and functioning, estimated in Mplus (Version 7) with bootstrapped standard errors (Muthén & Muthén, 1998–2012).
Results
Characteristics of the clinical and NP groups are in Tables 1 and S1. Groups were similar with regard to gender. The clinical cohort was slightly younger and included a larger proportion of non-white participants. As expected, antipsychotic medications were more common among the clinical cohort, symptom scores were higher, performance on cognitive tests was poorer, and everyday functioning was worse. ERP contrasts (error versus correct) were significant in both the clinical cohort (ERN: d= −.15, p<.05; Pe: d=1.19, p<.001) and NP group (ERN: d= −.79, p<.001; Pe: d=1.52, p<.005), indicating error-related brain activity (Figure S1). ERP difference scores were used for subsequent analyses.
Table 1.
Sample Characteristics
| Variable | Psychotic Disorders (n = 181) | Never Psychotic (n = 242) | Group Comparison | ||
|---|---|---|---|---|---|
|
| |||||
| n | % | n | % | p-value | |
|
| |||||
| Gender | .29 | ||||
| Male | 111 | 61.3 | 136 | 56.2 | |
| Female | 70 | 38.7 | 106 | 43.8 | |
| Race | |||||
| White | 143 | 79.0 | 216 | 89.3 | .004 |
| Other | 38 | 21.0 | 26 | 10.7 | |
| Antipsychotic Medication | 103 | 56.9 | 4 | 1.7 | <.001 |
|
| |||||
| M | SD | M | SD | p | |
|
| |||||
| Age (years) | 47.82 | 8.75 | 50.58 | 9.07 | .002 |
| Symptoms | |||||
| Reality Distortion | 4.62 | 8.41 | .08 | .51 | <.001 |
| Disorganized | 4.75 | 6.50 | .91 | 2.37 | <.001 |
| Inexpressivity | 6.83 | 9.33 | .85 | 2.63 | <.001 |
| Avolition | 13.35 | 9.31 | 2.93 | 3.79 | |
| Executive Function | |||||
| Trail Making (B) | 105.32 | 46.49 | 71.26 | 33.82 | <.001 |
| Stroop | 88.56 | 22.12 | 100.82 | 15.97 | <.001 |
| Letter-Number | 8.75 | 3.19 | 10.77 | 2.79 | <.001 |
| Composite | −.43 | .93 | .28 | .67 | <.001 |
| Simple Attention | |||||
| Trail Making (A) | 38.40 | 16.08 | 28.47 | 9.90 | <.001 |
| General Cognitive Ability | |||||
| Vocabulary | 19.89 | 5.54 | 22.41 | 4.00 | <.001 |
| Everyday Functioning | |||||
| Role Functioning | 3.17 | 1.77 | 5.49 | .83 | <.001 |
| Social Functioning | 10.12 | 4.61 | 14.14 | 2.78 | <.001 |
| Global Functioning | 48.85 | 17.15 | 74.98 | 11.87 | <.001 |
| Accuracy (%) | 91.34 | 6.30 | 93.26 | 4.12 | <.001 |
| Total Correct Responses | 296.88 | 27.29 | 306.68 | 14.13 | <.001 |
| Total Error Responses | 26.23 | 18.58 | 22.02 | 13.48 | <.01 |
| Reaction Time (ms) | 556.88 | 115.77 | 491.21 | 76.29 | <.001 |
| ERN difference (μV) | −1.03 | 6.79 | −5.50 | 7.17 | <.001 |
| Pe difference (μV) | 7.41 | 6.72 | 8.48 | 6.39 | .10 |
Is performance monitoring impaired across psychotic disorders?
As expected, participants were slower and less accurate on congruent versus incongruent trials, and these flanker effects were consistent cross groups (Table S2). We examined ERPs across schizophrenia, other psychosis, and NP groups (Figure 1).1 There were main effects of group on the ERN (F(2,420)=22.43, p<.001) and Pe (F(2,420)=4.21, p<.05); effects remained significant adjusting for age and gender (ERN: F(2,418)=23.06, p<.001; Pe: F(2,418)=5.04, p<.01). The ERN was blunted in schizophrenia versus NP (M=5.28, SE=1.04, p<.001) and other psychosis versus NP (M=3.62, SE=.88, p<.001); the schizophrenia and other psychosis groups did not differ (M=1.67, SE=1.04, p=.11). The Pe was blunted in schizophrenia versus both NP (M=−2.18, SE=.79, p<.01) and other psychosis (M=−2.29, SE=.97, p<.05), which did not differ (M=.11, SE=.81, p=.90). Thus, reduced ERN was common across psychotic disorders, whereas reduced Pe was relatively specific to schizophrenia.2
Figure 1.
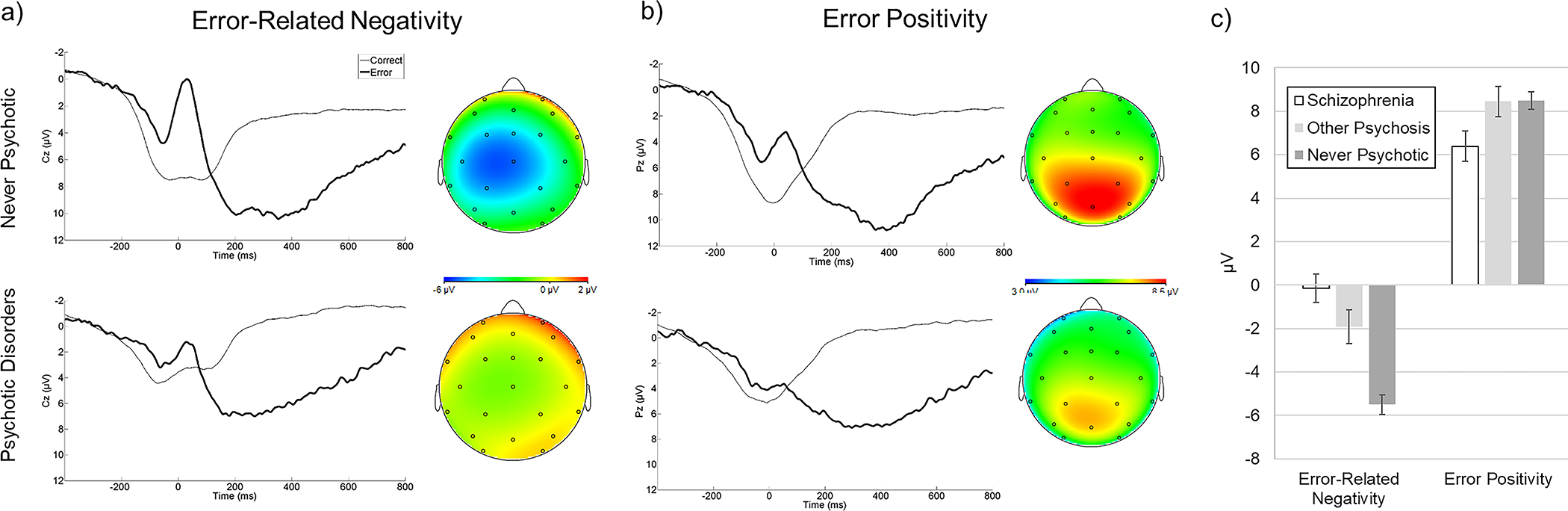
ERP data. (a) Averaged waveforms for the error-related negativity, presented separately for never-psychotic adults (top) and individuals with psychotic disorders (bottom). Scalp topographies are the error minus correct difference from 0–100 ms. (b) Averaged waveforms for the error positivity. Scalp topographies are the difference from 300–500 ms. (c) Group means and standard errors of neural measures of performance monitoring across the psychosis spectrum.
What illness characteristics are associated with impaired performance monitoring?
Concurrent associations with illness characteristics were examined among the clinical cohort (Table 2). ERPs were associated with independent indicators of executive function (Stroop, Letter-Number, Trail Making B), attention/processing speed (Trail Making A), and general cognitive ability (Vocabulary; also Table S3). Because the three executive function tests were strongly correlated (r’s=.58-.62, p’s<.001), we also considered the composite score. Reduced ERN and Pe were associated with worse composite executive function.
Table 2.
Cross-Sectional Associations of performance monitoring with Clinical and Neuropsychological Measures among Individuals with Psychotic Disorders
| Variable | ERN | Pe |
|---|---|---|
|
| ||
| Symptoms | ||
| Reality Distortion | .01 | −.03 |
| Disorganized | −.01 | −.13 |
| Inexpressivity | .25a | −.10 |
| Avolition | .15 | −.17a |
| Executive Function | ||
| Trail Making (B) | −.20a | .29a |
| Stroop Color Word | −.39a | .17 |
| Letter-Number | −.32a | .21a |
| Composite | −.35a | .26a |
| Simple Attention | ||
| Trail Making (A) | −.27a | .19a |
| General Cognitive Ability | ||
| Vocabulary | −.28a | .16 |
| Everyday Functioning | ||
| Role functioning | −.17a | .18a |
| Social Functioning | −.09 | .12 |
| Global Functioning | −.19a | .18a |
Note: N=181. The ERN difference score is a negative-going ERP, so positive correlation coefficients indicate a direct association, and vice versa.
p<.03 (critical value adjusting for false discovery rate).
ERPs were correlated with negative symptom: blunted ERN with inexpressivity and Pe with avolition; neither ERP was associated with psychotic or disorganized symptoms. Greater ERN and Pe amplitudes were associated with better role functioning and global functioning, but not social functioning. Subsequent analyses focused on SOFAS as a summary index of functioning.
We evaluated the replicability of associations by testing correlations of 20-year ERPs with retrospective clinical characteristics (Tables S4 and S5). The ERN-inexpressivity link emerged 6 months following first hospitalization, the Pe-avolition link at 2 years, and the Pe-SOFAS link at 6 months. While the magnitude of associations fluctuated across assessments, there was no evidence of crossover between illness characteristics: converse associations (ERN-avolition, ERN-SOFAS, Pe-inexpressivity) were not significant at previous assessments.
Does performance monitoring relate to cognition across the psychosis spectrum?
The generalizability of ERP associations with composite executive function was tested by including group (schizophrenia, other psychosis, NP) as a moderator. We evaluated two contrasts: schizophrenia versus NP, and schizophrenia versus other psychosis. Slopes and scatterplots are in Figure 2. Reduced ERN (β= −.29, p<.001) and Pe (β=.18, p<.001) predicted worse executive function. Relationships were not moderated by group, and contrasts were not significant (p’s>.05). Neurophysiological measures of performance monitoring are associated with independent measures of executive function across the psychosis spectrum.
Figure 2.
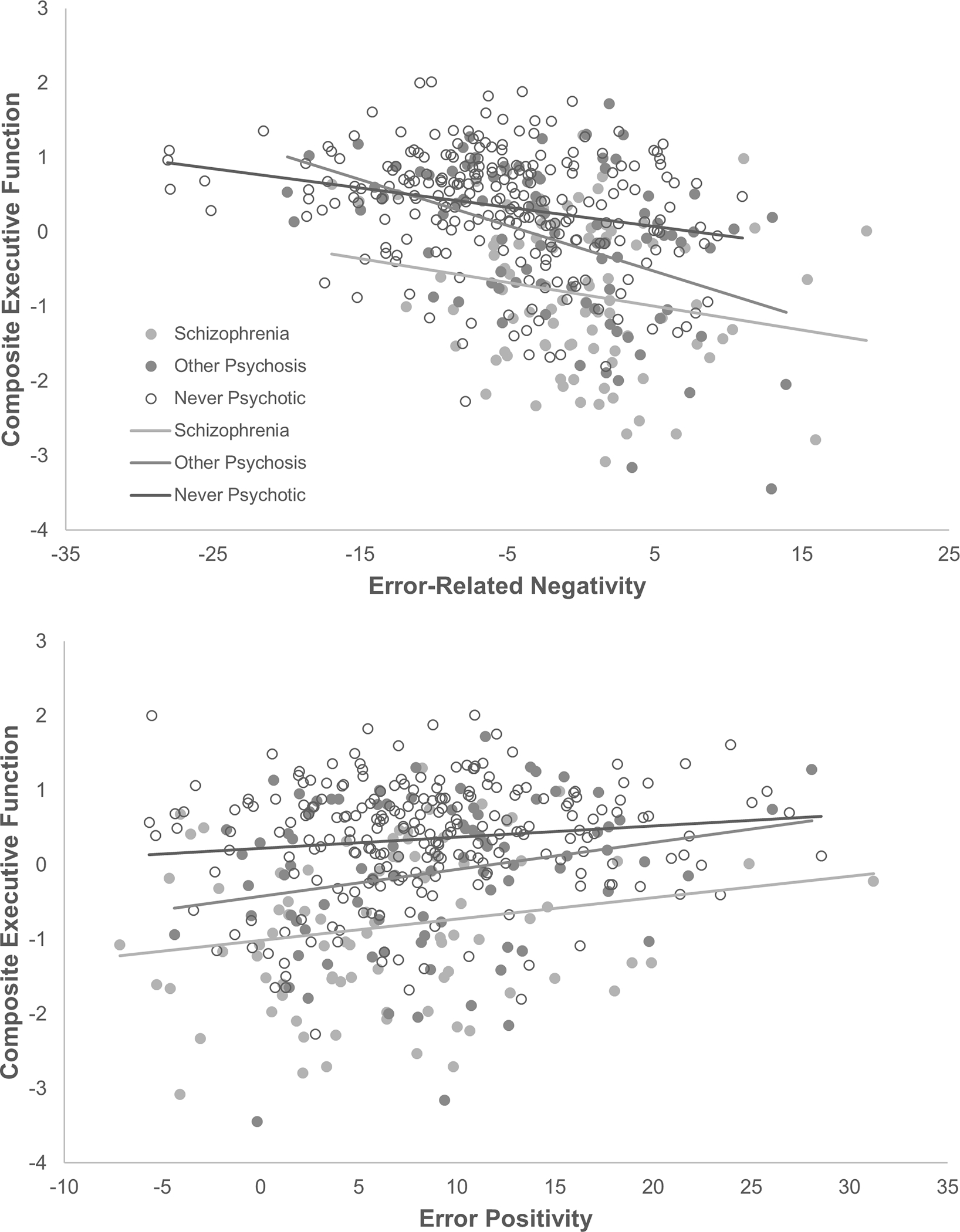
Relationships between neural measures of performance monitoring and independent measures of executive function across the psychosis spectrum. Outcome is composite executive function score, combining the Stroop, Letter-Number Sequencing, and Trail Making (B) tests.
What are the pathways by which performance monitoring affects symptoms and functioning?
Pathway analyses form ERPs to negative symptoms and global functioning were conducted within the clinical cohort. First, we tested how cognition mediated ERP-symptom relationships, with three parallel mediators: composite executive function, attention/processing speed, and general cognitive ability. The ERN-inexpressivity link was fully mediated (direct effect of .15, p=.11) by composite executive function (standardized indirect effect of .12, p<.05), but not attention/processing speed or general cognitive ability (p’s>.05). The Pe-avolition link was fully mediated (direct effect of −.11, p=.14) by composite executive function (indirect effect of −.08, p<.05) and attention/processing speed (indirect effect of −.03, p<.05), but not general cognitive ability (p>.05). Mediation effects of cognitive variables were not moderated by diagnosis (schizophrenia versus other psychosis; p’s>.05). Thus, executive function and attention/processing speed were retained for pathway analyses.
The full mediation model connected error-related ERPs, performance-based cognitive variables, negative symptom sub-domains, and global functioning within the clinical cohort (Figure 3). Based on the results above, pathways were specified whereby ERN affects global functioning through impaired cognition and inexpressivity, and Pe acts through avolition. Among the cognitive variables, composite executive function and attention/processing speed were included as simultaneous mediators of symptoms and functioning. All paths were significant except for attention to inexpressivity (p=.08), avolition (p=.07), and global functioning (p=.57), as well as Pe to avolition (p=.53). Indirect paths of executive function were significant: ERN→executive function→inexpressivity, β=.10, p<.05; and Pe→executive function→avolition, β= −.12, p<.01. The ERN→inexpressivity relationship reflected partial mediation (direct path of β=.13, p<.05). Finally, we tested double mediation from Pe to global functioning, with cognition and avolition as mediators. The path was significant: Pe→executive function→avolition→SOFAS, β=.10, p<.01.
Figure 3.
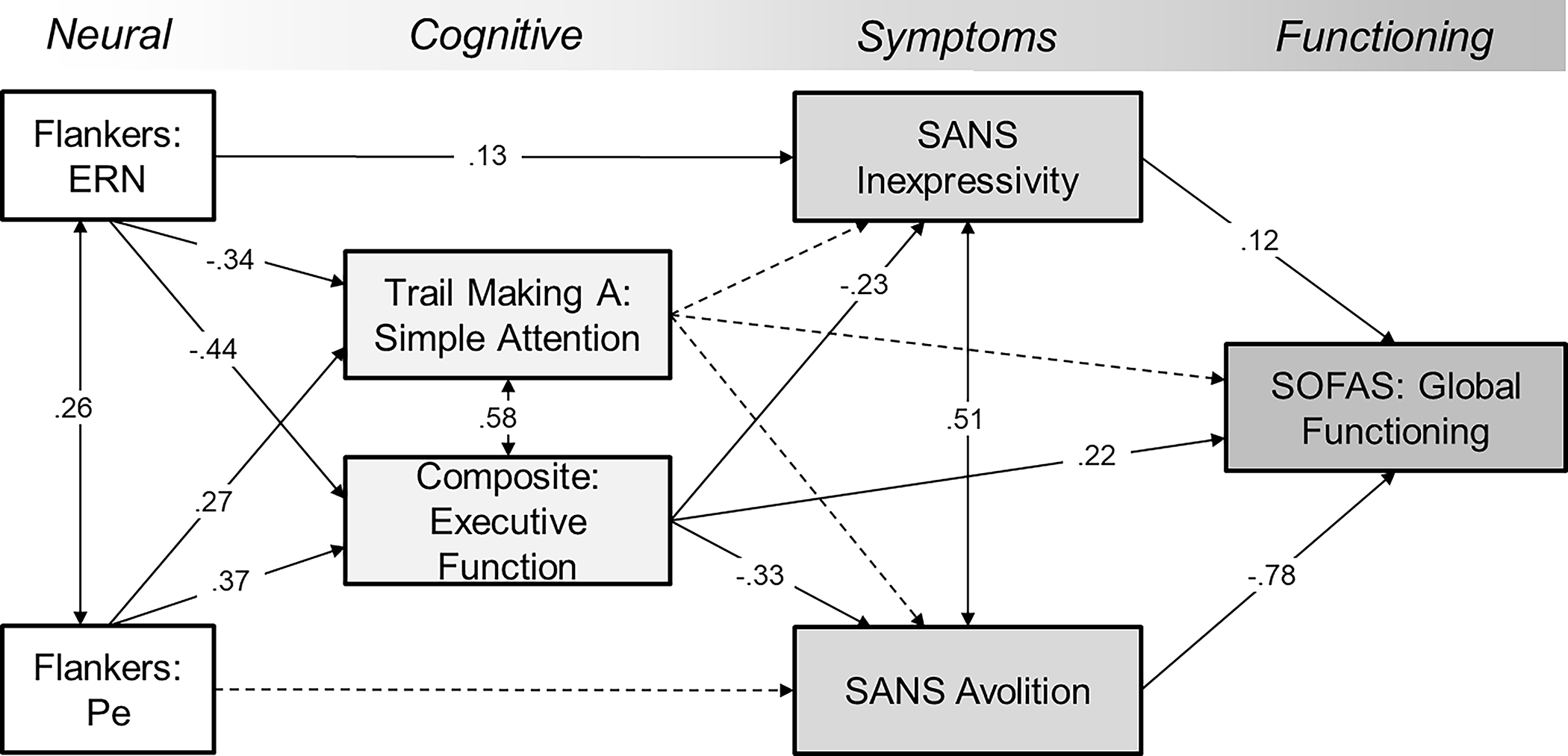
Structral equation model of performance monitoring, cognitive impairment, negative symptoms, and everyday functioning among individuals with psychotic disorders (N=181). All variables are observed (not latent constructs). Executive Function is the composite of Trail Making (B), Letter-Number, and Stroop; Inexpressivity and Avolition are SANS subscales. Global Functioning is the highest in the past year. Coefficients are completely standardized. All paths with solid lines are statistically significant (p’s<.05).
Discussion
This study is the first to show that the ERN and Pe relate to performance-based measures of executive function, assessed separately, and that these ERP-cognition links are consistent across individuals with psychotic disorders and never-psychotic adults. Impaired performance monitoring related to concurrent negative symptom severity and functioning among individuals with psychotic disorders. There was also partial evidence of selectivity observed across two decades: ERN related most consistently to inexpressivity (but not avolition or functioning), and Pe to avolition and global functioning (but not inexpressivity). Finally, path models indicated that neural deficits in performance monitoring may contribute to negative symptom severity and functional impairment in psychotic disorders primarily through impairment in executive function.
Structural equation modeling identified candidate pathways linking neural measures with cognitive deficits and illness features: reduced ERN to inexpressivity was partially mediated by executive function; reduced Pe to global functioning was mediated by executive function, then avolition. These cross-sectional patterns are consistent with a model in which performance monitoring deficits—in combination with other illness processes—contribute to cognitive impairment, which in turn contributes to negative symptoms and everyday functioning. Paths from cognitive impairment to negative symptoms to everyday functioning have been documented before (Bowie et al., 2006; Harvey et al., 2017; Strassnig et al., 2015), and this expanded model incorporates neurophysiological deficits. These results can inform proposed mechanisms of negative symptoms: inexpressivity may arise in part from reduced cognitive capacity (Cohen, McGovern, Dinzeo, & Covington, 2014), and avolition from deficits in maintaining contextual information to accurately predict outcomes, assign value, and compute effort (Gold et al., 2013; Kring & Barch, 2014). Therefore, the ERN-inexpressivity path may relate to diminished cognitive capacity in psychotic disorders, whereas the Pe-avolition-functioning path may instead relate to context maintenance.
These results have implications for future studies to facilitate outcome-specific interventions. Impaired executive function is an important treatment target in psychosis (Buchanan et al., 2005; Insel, 2010), and outcomes have been unsatisfactory to date (Firth et al., 2017; Revell et al., 2015; Wykes et al., 2011). The ERN is partially modifiable with antipsychotic medications (Bates, Liddle, Kiehl, & Ngan, 2004; Schneider et al., 2013), although these do not target performance monitoring directly. One study used direct current stimulation to improve performance monitoring and increase ERN amplitude in schizophrenia (Reinhart et al., 2015). While downstream effects on cognition and illness features were not tested, parameter estimates from the current sample enable the following predictions: a 1 SD improvement in ERN amplitude would produce improvement of .35 in executive function and .25 in inexpressivity. Likewise, a 1 SD improvement in Pe amplitude would produce improvement of .26 in executive function, .17 in avolition, and .18 in global functioning—approximately 3 SOFAS points. While inferences of causality are premature given the cross-sectional nature of these analysis, one possible implication is that ERN and Pe may be upstream contributors to an etiologic pathway that flows from neural abnormalities to cognitive deficits, negative symptoms, and functional impairment. Understanding of this pathway is valuable insofar as it contributes to broader efforts to explicate etiology of psychotic disorders, and identify actionable targets for treatment of cognitive impairment and negative symptoms.
Perspectives on the psychological processes reflected by error-related ERPs may help contextualize the links with illness features. ERN modulation has been interpreted with respect to reinforcement learning (Holroyd & Coles, 2002), conflict monitoring (Yeung, Botvinick, & Cohen, 2004), and preconscious error detection (Nieuwenhuis et al., 2001). Individual differences in ERN amplitude have been proposed to reflect trait differences in defensive motivation and action preparation (Weinberg, Riesel, & Hajcak, 2012). The ERN-inexpressivity link suggests that, in psychotic disorders, symptoms of inexpressivity may reflect impairment in the continuous self-monitoring and adaptation of expressive behavior in response to environmental demands. The Pe, on the other hand, is thought to reflect conscious error awareness (Nieuwenhuis et al., 2001) and decision confidence (Boldt & Yeung, 2015). The Pe-avolition link may capture a shared deficit in conscious maintenance of goals to facilitate decision-making.
With regard to functioning, the bivariate correlations suggest that the Pe-functioning link reflects role rather than social functioning, as measured here. Other work has shown that executive function uniquely predicts real-world interpersonal behavior (Bowie et al., 2008). It is notable that the extension of the Pe-avolition link to global functioning is to be expected given that avolition and global functioning are strongly related constructs, partly due to definitional overlap. Future work should clarify pathways from each ERP to domain-specific functioning. While independent ERN and Pe pathways were observed, this should be viewed with caution in light of their differential psychometric properties (Foti et al., 2013).
A strength of this study is the test of neural-cognitive links spanning the psychosis spectrum. The ERN and Pe related to performance-based measures of executive function across schizophrenia, other psychotic disorders, and never-psychotic groups. This is broadly consistent with neuroimaging evidence that functional connectivity is a generalizable mechanism of cognitive impairment across psychotic disorders and controls (Sheffield et al., 2017). The current report complements another recent study modeling pathways from early auditory ERPs to functioning specifically in schizophrenia (Thomas et al., 2017). Here, we characterized a pathway from performance monitoring to clinical outcomes that may be broadly relevant across the psychosis spectrum.
The current results have several limitations. Pathway analyses were cross-sectional, precluding inferences of causality. While consistent with a model in which performance monitoring has downstream consequences for negative symptoms and functioning, this requires direct testing in longitudinal data. Indeed, there is evidence that performance monitoring prospectively predicts future negative symptoms (Foti et al., 2016), and the ERN is modifiable through targeted intervention (Reinhart et al., 2015). Data were drawn from the 20-year assessment, and the path models are most applicable to middle and late-phase psychosis. Other work has found reduced ERN and Pe in early-phase schizophrenia (Perez et al., 2012), warranting extension of the current pathways to earlier illness phases. The executive function tasks were somewhat constrained, although there is evidence of their ecological validity in schizophrenia (Bowie et al., 2008; Zayat, Rempfer, Gajewski, & Brown, 2011). Finally, a majority of the clinical cohort was prescribed antipsychotic medication. Pathways were independent of medication status, however, and as an epidemiological study this cohort is reasonably representative of individuals with psychotic illness in clinical settings.
The current study sheds light on performance monitoring deficits in psychotic disorders by conducting the first evaluation bridging neurophysiological dysfunction with cognitive impairment, symptom presentation, and everyday functioning across the psychosis spectrum. The ERN and Pe relate to impaired executive function in psychotic disorders, as well as downstream associations with illness features. Identifying these pathways may ultimately lead to the development of personalized treatments based on neurophysiological functioning.
Supplementary Material
Acknowledgments
We gratefully acknowledge the participants and mental health community of Suffolk County for contributing their time and energy to this project. We are indebted to dedicated efforts of study coordinators, interviewers for their careful assessments, and to the psychiatrists who derived the consensus diagnoses. Special thanks to Janet Lavelle for her many contributions to the study.
Financial Support:
This work was supported by the National Institutes of Health (MH44801 to EB and MH110434 to RK) and Stony Brook University (Clinical Research Scholar Award).
Footnotes
Conflicts of Interest
PH has received consulting fees or travel reimbursements from Alkermes, Boehringer Ingelheim, Intra-Cellular Therapies, Jazz Pharma, Minerva Pharma, Otsuka America, Roche Pharma, Sanofi Pharma, Sunovion Pharma, Takeda Pharma, and Teva during the past year; he receives royalties from the Brief Assessment of Cognition in Schizophrenia; he is chief scientific officer of i-Function, Inc; he has a research grant from Takeda and from the Stanley Medical Research Foundation. DM has served as a consultant for Aptinyx, Boehringer-Ingelheim Pharmaceuticals, Cadent Therapeutics, and Greenwich Biosciences. All other authors report no biomedical financial interests or potential conflicts of interest.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Participants in the schizophrenia group were more likely to be male than in the other psychosis group (χ2(1)=4.26, p<.05). The two diagnostic groups did not significantly differ by race or age (p’s>.20).
To test for unique effects of trial type, we calculated two logistic regression models predicting group membership from error and correct trials entered as simultaneous predictors. Case status (i.e., clinical cohort versus NP) was uniquely predicted by a reduced (less negative) ERN on error trials (OR=1.06, p<.001) and an increased (more negative) correct-related negativity on correct trials (OR=.87, p<.001). SZ diagnosis (versus OP) was predicted by a reduced Pe on error trials only (OR=.95, p<.05) and not by correct trials (OR=1.03, p=.41).
References
- Alain C, McNeely HE, He Y, Christensen BK, & West R (2002). Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cerebral Cortex, 12(8), 840–846. doi: 10.1093/cercor/12.8.840 [DOI] [PubMed] [Google Scholar]
- Andreasen NC (1983a). Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa. [Google Scholar]
- Andreasen NC (1983b). Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa. [Google Scholar]
- Bates AT, Kiehl KA, Laurens KR, & Liddle PF (2002). Error-related negativity and correct response negativity in schizophrenia. Clinical Neurophysiology, 113(9), 1454–1463. doi:S1388245702001542 [DOI] [PubMed] [Google Scholar]
- Bates AT, Liddle PF, Kiehl KA, & Ngan ET (2004). State dependent changes in error monitoring in schizophrenia. Journal of Psychiatric Research, 38(3), 347–356. doi: 10.1016/j.jpsychires.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B, 57(1), 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Blanchard JJ, & Cohen AS (2006). The structure of negative symptoms within schizophrenia: implications for assessment. Schizophrenia Bulletin, 32(2), 238–245. doi: 10.1093/schbul/sbj013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt A, & Yeung N (2015). Shared neural markers of decision confidence and error detection. J Neurosci, 35(8), 3478–3484. doi: 10.1523/JNEUROSCI.0797-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, & Harvey PD (2006). Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatric Disease and Treatment, 2(4), 531–536. doi: 10.2147/nedt.2006.2.4.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, & Harvey PD (2008). Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biological Psychiatry, 63(5), 505–511. doi: 10.1016/j.biopsych.2007.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, & Harvey PD (2006). Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. American Journal of Psychiatry, 163(3), 418–425. doi: 10.1176/appi.ajp.163.3.418 [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, & Chang SW (2011). Diagnostic shifts during the decade following first admission for psychosis. American Journal of Psychiatry, 168(11), 1186–1194. doi: 10.1176/appi.ajp.2011.11010048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet EJ, Schwartz JE, Fennig S, Geller L, Jandorf L, Kovasznay B, . . . et al. (1992). The epidemiology of psychosis: the Suffolk County Mental Health Project. Schizophrenia Bulletin, 18(2), 243–255. doi: 10.1093/schbul/18.2.243 [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, . . . Marder SR (2005). A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophrenia Bulletin, 31(1), 5–19. doi: 10.1093/schbul/sbi020 [DOI] [PubMed] [Google Scholar]
- Canivez GL, & Watkins MW (2010). Investigation of the factor structure of the Wechsler Adult Intelligence Scale--Fourth Edition (WAIS-IV): exploratory and higher order factor analyses. Psychological Assessment, 22(4), 827–836. doi: 10.1037/a0020429 [DOI] [PubMed] [Google Scholar]
- Cohen AS, McGovern JE, Dinzeo TJ, & Covington MA (2014). Speech deficits in serious mental illness: a cognitive resource issue? Schizophrenia Research, 160(1–3), 173–179. doi: 10.1016/j.schres.2014.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreth AJ, Foti D, Barch DM, Hajcak G, & Kotov R (2018). Electrocortical Responses to Emotional Stimuli in Psychotic Disorders: Comparing Schizophrenia Spectrum Disorders and Affective Psychosis. Frontiers in Psychiatry, 9(586). doi: 10.3389/fpsyt.2018.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, & Engel AK (2005). Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. Journal of Neuroscience, 25(50), 11730–11737. doi: 10.1523/JNEUROSCI.3286-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, & Eriksen CW (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. doi: 10.3758/BF03203267 [DOI] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, & Blanke L (1991). Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology, 78(6), 447–455. doi: 10.1016/0013-4694(91)90062-9 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2001). Structured Clinical Interview for DSM-IV-TR Axis I Disorders--Patient Edition (SCID-I/P 2/2001 Revision). New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Firth J, Stubbs B, Rosenbaum S, Vancampfort D, Malchow B, Schuch F, . . . Yung AR (2017). Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis. Schizophrenia Bulletin, 43(3), 546–556. doi: 10.1093/schbul/sbw115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Kotov R, Bromet EJ, & Hajcak G (2012). Beyond the broken error-related negativity: Functional and diagnostic correlates of error processing in psychosis. Biological Psychiatry, 71(10), 864–872. doi: 10.1016/j.biopsych.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Kotov R, & Hajcak G (2013). Psychometric considerations in using error-related brain activity as a biomarker in psychotic disorders. Journal of Abnormal Psychology, 122(2), 520–531. doi: 10.1037/a0032618 [DOI] [PubMed] [Google Scholar]
- Foti D, Perlman G, Hajcak G, Mohanty A, Jackson F, & Kotov R (2016). Impaired error processing in late-phase psychosis: Four-year stability and relationships with negative symptoms. Schizophrenia Research, 176(2–3), 520–526. doi: 10.1016/j.schres.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, & McGuire P (2015). Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials. Schizophrenia Bulletin, 41(4), 892–899. doi: 10.1093/schbul/sbu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, & Donchin E (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385. doi: 10.1111/j.1467-9280.1993.tb00586.x [DOI] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, & Frank MJ (2013). Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biological Psychiatry, 74(2), 130–136. doi: 10.1016/j.biopsych.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman HH, Skodol AE, & Lave TR (1992). Revising axis V for DSM-IV: a review of measures of social functioning. American Journal of Psychiatry, 149(9), 1148–1156. doi: 10.1176/ajp.149.9.1148 [DOI] [PubMed] [Google Scholar]
- Green MF (1996). What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry, 153(3), 321–330. doi: 10.1176/ajp.153.3.321 [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, & Mintz J (2000). Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin, 26(1), 119–136. doi: 10.1093/oxfordjournals.schbul.a033430 [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, & Simons RF (2003). To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology, 40(6), 895–903. doi: 10.1111/1469-8986.00107 [DOI] [PubMed] [Google Scholar]
- Harvey PD, Khan A, & Keefe RSE (2017). Using the Positive and Negative Syndrome Scale (PANSS) to Define Different Domains of Negative Symptoms: Prediction of Everyday Functioning by Impairments in Emotional Expression and Emotional Experience. Innovations in Clinical Neuroscience, 14(11–12), 18–22. [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2012). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper]. Retrieved from http://www.afhayes.com/public/process2012.pdf
- Heinrichs DW, Hanlon TE, & Carpenter WT Jr. (1984). The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin, 10(3), 388–398. doi: 10.1093/schbul/10.3.388 [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, . . . Sweeney JA (2013). Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. American Journal of Psychiatry, 170(11), 1275–1284. doi: 10.1176/appi.ajp.2013.12101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, & Coles MG (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. doi: 10.1037/0033-295X.109.4.679 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N, Coles MG, & Cohen JD (2005). A mechanism for error detection in speeded response time tasks. Journal of Experimental Psychology: General, 134(2), 163–191. doi: 10.1037/0096-3445.134.2.163 [DOI] [PubMed] [Google Scholar]
- Horan WP, Foti D, Hajcak G, Wynn JK, & Green MF (2012). Impaired neural response to internal but not external feedback in schizophrenia. Psychological Medicine, 42(8), 1637–1647. doi: 10.1017/S0033291711002819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone R, Hauser TU, Staempfli P, Walitza S, Brandeis D, & Brem S (2015). Conflict monitoring and error processing: new insights from simultaneous EEG-fMRI. NeuroImage, 105, 395–407. doi: 10.1016/j.neuroimage.2014.10.028 [DOI] [PubMed] [Google Scholar]
- Insel TR (2010). Rethinking schizophrenia. Nature, 468(7321), 187–193. doi: 10.1038/nature09552 [DOI] [PubMed] [Google Scholar]
- Jackson F, Foti D, Kotov R, Perlman G, Mathalon DH, & Proudfit GH (2014). An incongruent reality: the N400 in relation to psychosis and recovery. Schizophrenia Research, 160(1–3), 208–215. doi: 10.1016/j.schres.2014.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansal V, Patriciu I, & Kiang M (2014). Illness insight and neurophysiological error-processing deficits in schizophrenia. Schizophrenia Research, 156(1), 122–127. doi: 10.1016/j.schres.2014.03.023 [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, & Barch DM (2008). Executive functioning component mechanisms and schizophrenia. Biological Psychiatry, 64(1), 26–33. doi: 10.1016/j.biopsych.2008.04.027 [DOI] [PubMed] [Google Scholar]
- Kim MS, Kang SS, Shin KS, Yoo SY, Kim YY, & Kwon JS (2006). Neuropsychological correlates of error negativity and positivity in schizophrenia patients. Psychiatry and Clinical Neurosciences, 60(3), 303–311. doi: 10.1111/j.1440-1819.2006.01506.x [DOI] [PubMed] [Google Scholar]
- Kopp B, & Rist F (1999). An event-related brain potential substrate of disturbed response monitoring in paranoid schizophrenic patients. Journal of Abnormal Psychology, 108(2), 337–346. doi: 10.1037/0021-843X.108.2.337 [DOI] [PubMed] [Google Scholar]
- Kotov R, Foti D, Li K, Bromet EJ, Hajcak G, & Ruggero CJ (2016). Validating dimensions of psychosis symptomatology: Neural correlates and 20-year outcomes. Journal of Abnormal Psychology, 125(8), 1103–1119. doi: 10.1037/abn0000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, & Barch DM (2014). The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. European Neuropsychopharmacology, 24(5), 725–736. doi: 10.1016/j.euroneuro.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Gur RE, Blanchard JJ, Horan WP, & Reise SP (2013). The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. American Journal of Psychiatry, 170(2), 165–172. doi: 10.1176/appi.ajp.2012.12010109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens KR, Hodgins S, Mould GL, West SA, Schoenberg PL, Murray RM, & Taylor EA (2010). Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biological Psychiatry, 67(3), 238–245. doi: 10.1016/j.biopsych.2009.07.030 [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson D. b., & Loring DW (2004). Neuropsychological Assessment (4th ed.). New York, NY: Oxford University Press. [Google Scholar]
- Llerena K, Wynn JK, Hajcak G, Green MF, & Horan WP (2016). Patterns and reliability of EEG during error monitoring for internal versus external feedback in schizophrenia. International Journal of Psychophysiology, 105, 39–46. doi: 10.1016/j.ijpsycho.2016.04.012 [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, & Ford JM (2002). Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. Journal of Abnormal Psychology, 111(1), 22–41. doi: 10.1037/0021-843X.111.1.22 [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, & Ford JM (2003). Anatomy of an error: ERP and fMRI. Biological Psychology, 64(1–2), 119–141. doi: 10.1016/S0301-0511(03)00105-4 [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Gomes GC, Yoon JH, Swaab TY, & Carter CS (2014). Disrupted action monitoring in recent-onset psychosis patients with schizophrenia and bipolar disorder. Psychiatry Research, 221(1), 114–121. doi: 10.1016/j.pscychresns.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Yee CM, & Nuechterlein KH (2006). Electrophysiological analysis of error monitoring in schizophrenia. Journal of Abnormal Psychology, 115(2), 239–250. doi: 10.1037/0021-843X.115.2.239 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2012). Mplus User’s Guide (7th ed.). Los Angeles, CA: Muthén & Muthén [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, & Kok A (2001). Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology, 38(5), 752–760. [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, . . . Foxe JJ (2007). The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. European Journal of Neuroscience, 25(8), 2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x [DOI] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Woods SW, McGlashan TH, Srihari VH, . . . Mathalon DH (2012). Error monitoring dysfunction across the illness course of schizophrenia. Journal of Abnormal Psychology, 121(2), 372–387. doi: 10.1037/a0025487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Foti D, Jackson F, Kotov R, Constantino E, & Hajcak G (2015). Clinical significance of auditory target P300 subcomponents in psychosis: Differential diagnosis, symptom profiles, and course. Schizophrenia Research, 165(2–3), 145–151. doi: 10.1016/j.schres.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, & Harvey PD (2007). Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychological Bulletin, 133(5), 833–858. doi: 10.1037/0033-2909.133.5.833 [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK, & Bromet E (2009). Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophrenia Bulletin, 35(5), 1022–1029. doi: 10.1093/schbul/sbn044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RM, Zhu J, Park S, & Woodman GF (2015). Medial-Frontal Stimulation Enhances Learning in Schizophrenia by Restoring Prediction Error Signaling. Journal of Neuroscience, 35(35), 12232–12240. doi: 10.1523/JNEUROSCI.1717-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell ER, Neill JC, Harte M, Khan Z, & Drake RJ (2015). A systematic review and meta-analysis of cognitive remediation in early schizophrenia. Schizophrenia Research, 168(1–2), 213–222. doi: 10.1016/j.schres.2015.08.017 [DOI] [PubMed] [Google Scholar]
- Schneider S, Bahmer TJ, Metzger FG, Reif A, Polak T, Pfuhlmann B, . . . Ehlis AC (2013). Quetiapine and flupentixol differentially improve anterior cingulate cortex function in schizophrenia patients: an event-related potential study. International Journal of Neuropsychopharmacology, 16(9), 1911–1925. doi: 10.1017/S1461145713000540 [DOI] [PubMed] [Google Scholar]
- Shaffer JJ, Peterson MJ, McMahon MA, Bizzell J, Calhoun V, van Erp TG, . . . Manoach DS (2015). Neural Correlates of Schizophrenia Negative Symptoms: Distinct Subtypes Impact Dissociable Brain Circuits. Molecular Neuropsychiatry, 1(4), 191–200. doi: 10.1159/000440979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, . . . Barch DM (2017). Transdiagnostic Associations Between Functional Brain Network Integrity and Cognition. JAMA Psychiatry, 74(6), 605–613. doi: 10.1001/jamapsychiatry.2017.0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AB (1982). Factor structure of the Wechsler Adult Intelligence Scale--Revised. Journal of Consulting and Clinical Psychology, 50(5), 661–664. doi: 10.1037/0022-006X.50.5.661 [DOI] [PubMed] [Google Scholar]
- Simmonite M, Bates AT, Groom MJ, Jackson GM, Hollis C, & Liddle PF (2012). Error processing-associated event-related potentials in schizophrenia and unaffected siblings. International Journal of Psychophysiology, 84(1), 74–79. doi: 10.1016/j.ijpsycho.2012.01.012 [DOI] [PubMed] [Google Scholar]
- Spreen O, & Strauss E (1998). A Compendium of Neuropsychological Tests (2nd ed.). New York, NY: Oxford University Press. [Google Scholar]
- Strassnig MT, Raykov T, O’Gorman C, Bowie CR, Sabbag S, Durand D, . . . Harvey PD (2015). Determinants of different aspects of everyday outcome in schizophrenia: The roles of negative symptoms, cognition, and functional capacity. Schizophrenia Research, 165(1), 76–82. doi: 10.1016/j.schres.2015.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Horan WP, Kirkpatrick B, Fischer BA, Keller WR, Miski P, . . . Carpenter WT Jr. (2013). Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. Journal of Psychiatric Research, 47(6), 783–790. doi: 10.1016/j.jpsychires.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, . . . Light GA (2017). Modeling Deficits From Early Auditory Information Processing to Psychosocial Functioning in Schizophrenia. JAMA Psychiatry, 74(1), 37–46. doi: 10.1001/jamapsychiatry.2016.2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthorst E, Fett AJ, Reichenberg A, Perlman G, van Os J, Bromet EJ, & Kotov R (2017). The 20-Year Longitudinal Trajectories of Social Functioning in Individuals With Psychotic Disorders. American Journal of Psychiatry, 174(11), 1075–1085. doi: 10.1176/appi.ajp.2016.15111419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Hellemann GS, Thames AD, Koellner V, & Nuechterlein KH (2009). Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophrenia Research, 113(2–3), 189–199. doi: 10.1016/j.schres.2009.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocat R, Pourtois G, & Vuilleumier P (2008). Unavoidable errors: a spatio-temporal analysis of time-course and neural sources of evoked potentials associated with error processing in a speeded task. Neuropsychologia, 46(10), 2545–2555. doi: 10.1016/j.neuropsychologia.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). Wechler Adult Intelligence Scale--Revised Manual. New York, NY: The Psychological Corporation. [Google Scholar]
- Weinberg A, Riesel A, & Hajcak G (2012). Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion, 1–17. doi: 10.1007/s11031-011-9269-y [DOI] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, & Czobor P (2011). A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. American Journal of Psychiatry, 168(5), 472–485. doi: 10.1176/appi.ajp.2010.10060855 [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, & Cohen JD (2004). The neural basis of error detection: conflict monitoring and the error-related negativity. Psychological Review, 111(4), 931–959. doi: 10.1037/0033-295X.111.4.939 [DOI] [PubMed] [Google Scholar]
- Zayat E, Rempfer M, Gajewski B, & Brown CE (2011). Patterns of association between performance in a natural environment and measures of executive function in people with schizophrenia. Psychiatry Research, 187(1–2), 1–5. doi: 10.1016/j.psychres.2010.11.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


