Abstract
Proteus mirabilis swarming behavior is characterized by the development of concentric rings of growth that are formed as cyclic events of swarmer cell differentiation, swarming migration, and cellular differentiation are repeated during colony translocation across a surface. This cycle produces the bull’s-eye colony often associated with cultures of P. mirabilis. How the cells communicate with one another to coordinate these perfectly synchronized rings is presently unknown. We report here the identification of a genetic locus that, when mutated, results in a precocious swarming phenotype. These mutants are defective in the temporal control of swarming migration and start swarming ca. 60 min sooner than wild-type cells. Unlike the wild type, precocious swarming mutants are also constitutive swarmer cells and swarm on minimal agar medium. The defects were found to be localized to a 5.4-kb locus on the P. mirabilis genome encoding RsbA (regulator of swarming behavior) and the P. mirabilis homologs to RcsB and RcsC. RsbA is homologous to membrane sensor histidine kinases of the two-component family of regulatory proteins, suggesting that RsbA may function as a sensor of environmental conditions required to initiate swarming migration. Introduction of a rsbA mutation back into the wild type via allelic-exchange mutagenesis reconstructed the precocious swarming phenotype, which could be complemented in trans by a plasmid-borne copy of rsbA. Overexpression of RsbA in wild-type cells resulted in precocious swarming, suggesting that RsbA may have both positive and negative functions in regulating swarming migration. A possible model to describe the role of RsbA in swarming migration is discussed.
Proteus mirabilis is a dimorphic, motile gram-negative bacterium associated with urinary-tract infections (UTIs) in patients with complicated urinary tracts, i.e., individuals with functional or anatomic abnormalities or with chronic instrumentation (41). It is believed that the ability of P. mirabilis to colonize the surfaces of catheters and the urinary tract may be aided by the characteristic first described over a century ago and presently referred to as swarmer cell differentiation and behavior. The process of swarmer cell differentiation, swarming migration, and swarming behavior may be divided into four separate phases: (i) the induction of swarmer cell differentiation, (ii) the lag period prior to onset of swarming behavior (motility), (iii) active motile swarming migration (or translocation), and (iv) consolidation, a phase in which migration stops and cell morphology returns to an undifferentiated (vegetative) swimmer cell. Since the topics of swarmer cell differentiation and swarming behavior have been reviewed recently (9–11), the reader is referred to these publications for more detail.
P. mirabilis swarmer cell differentiation is induced by contact with a surface or viscous medium and is mediated through a torque-sensing flagellar dynamometer (2, 12). Paradoxically, individual swarmer cells by themselves do not have the ability to swarm. Rather, swarming behavior is cyclic in nature and is the result of a coordinated, multicellular effort of groups of differentiated swarmer cells functioning through cell-cell interactions (9, 53). The cycles that demarcate the phases of differentiation, lag, migration, and consolidation are repeated to produce the classic “bull’s-eye” colony morphology typically associated with P. mirabilis. Interestingly, swarming migration does not occur immediately upon swarmer cell differentiation, a process that occurs 30 to 45 min postinoculation. Rather, swarming motility is seen only after ca. 3 h (at 37°C) of incubation, long after differentiation and division have produced a visible colony of swarmer cells. Thus, the differentiated cell is only one part of the process, which also requires a lag period prior to the commencement of swarming migration.
The presence of a lag period prior to swarming behavior and the coordination of the swarming colony to form bull’s-eye patterns suggests that some form of cell-cell interaction and communication occurs to control these processes. How do bacteria communicate with one another within the same population? Populations of many bacteria exhibit attributes or abilities that extend beyond the individual cells (23, 26, 38, 43). To coordinate activity within a population, bacteria communicate among themselves by producing extracellular signal compounds that, when present during appropriate conditions and in sufficient quantities, trigger specific responses. “Quorum sensing” describes one mechanism that acts in response to population density. It relies on the accumulation of small extracellular signaling molecules to modulate the transcription of target genes and operons. Quorum-sensing mechanisms have been found in numerous gram-negative bacteria, a topic that has been extensively reviewed in recent years (26, 48).
In this communication, we describe a gene that, when mutated, decreases the length of the lag phase prior to swarming migration, which is shown to be a cell density-dependent event. In the characterization of this gene, referred to as rsbA, for regulator of swarming behavior, we demonstrate that it encodes a sensory protein with similarity to the histidine kinases of the large two-component regulatory superfamily of bacterial response regulators. RsbA appears to function to coordinate the initiation of P. mirabilis swarming migration, which may be crucial to pathogenicity during UTI.
MATERIALS AND METHODS
Strains, plasmids, oligonucleotides, and media.
The strains, plasmids, and oligonucleotides used in this study are listed in Table 1. P. mirabilis BB2000 is wild type for swimming and swarming behaviors. Escherichia coli and P. mirabilis strains were grown as previously described (13, 14).
TABLE 1.
Strains, plasmids, and oligonucleotides used
| Strain, plasmid, or oligonucleotide | Relevant characteristic(s)a or sequence | Derivation or description | Source and/or reference |
|---|---|---|---|
| Strains | |||
| E. coli K-12 | |||
| DH5α | F− φ80d lacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17(rk− mk+) deoR thi-1 supE44λ− gyrA96 relA1 | Laboratory stock (42) | |
| SM10 (λpir) | Rec− RP4-2Tc::Mu λpir | C600 | 21, 45 (Invitrogen) |
| INVαF′ | F′ endA1 recA1 hsdR17(rk− mk+) supE44 gyrA96 relA1 φ80d lacZΔM15 Δ(lacZYA-argF)U169 deoR thi-1 λ− | ||
| P. mirabilis | |||
| BB2000 | Wild type; Rfr Tcr | Spontaneous from PRM1 | 14 |
| BB2231 | swr2231::Tn5-Cm; Cmr Rfr; precocious swarming | Mini-Tn5–Cm insertion in BB2000 | This study |
| BB2232 | swr2232::Tn5-Cm; Cmr Rfr; precocious swarming | Mini-Tn5–Cm insertion in BB2000 | This study |
| BB2233 | swr2233::Tn5-Cm; Cmr Rfr; precociouis swarming | Mini-Tn5–Cm insertion in BB2000 | This study |
| BB2234 | swr2234::Tn5-Cm; Cmr Rfr; precocious swarming | Mini-Tn5–Cm insertion in BB2000 | This study |
| BB2235 | swr2235::Tn5-Cm; Cmr Rfr; precocious swarming | Mini-Tn5–Cm insertion in BB2000 | This study |
| BB2236 | swr2236::Tn5-Cm; Cmr Rfr; precocious swarming | Mini-Tn5–Cm insertion in BB2000 | This study |
| MM100 | ′rsbA::cam::rsbA; precocious swarming | cam gene from pUT–mini-Tn5–Cm inserted at the HpaI site in BB2000 rsbA gene; merodiploid | This study |
| Plasmids | |||
| Vectors | |||
| pBluescriptII | Apr | pBluescriptII SK(+) and KS(−) | Stratagene |
| pCR2.1 | Apr Kmr | PCR TA cloning vector | Invitrogen |
| pGP704 | AproriR6K | Conjugatable suicide vector | 40 |
| pUC-Cam | Apr Cmr | pUC18 with pUT–mini-Tn5–Cm chloramphenicol resistance gene inserted at the HindIII site | This study |
| pMM106 | Apr Cmr | pBluescriptII SK(+) with SalI clone from BB2231 | This study |
| pMM301 | Apr Kmr | pCR2.1 with 1.96-kb PCR product, rsbA from nt 1117 to 3076 | This study |
| pMM303 | Apr Cmr | Derived from pMM301; cam gene inserted at HpaI site in rsbA fragment (nt 1117 to 3076) | This study |
| pMM305 | Apr | 5.4-kb EcoRI fragment in pBluescriptII SK(+) containing rsbA, rcsB, and portion of rcsC | This study |
| pMM309 | Apr Kmr | pCR2.1 TA cloning vector containing PCR amplicon with intact rsbA (nt 234 to 3076) oriented behind lac promoter | This study |
| pMM313 | Apr Cmr | Derived from pMM303; EcoRI insert containing ′rsbA::cam::rsbA in pGP704 | This study |
| Oligodeoxyribonucleotides | |||
| rsbA1F | 5′-TCGATTTCAGTGTTTGGCCAT-3′ | PCR primer for cloning P. mirabilis rsbA (paired with rcsB1R) | This study |
| rcsB1R | 5′-CCGAGCTTCATCATGGCTG-3′ | PCR primer for cloning P. mirabilis rsbA (paired with rsbA1F) | This study |
| i1b | 5′-GTGCTTAGTGCATCTAACGCTTGAG-3′ | IPCR primer for cloning the DNA flanking mini-Tn5 insertions (paired with i2b) | This study |
| i2b | 5′-CATCCGCATTAAAATTTAGCGAGGGC-3′ | IPCR primer for cloning the DNA flanking mini-Tn5 insertions (paired with i1b) | This study |
| yojF11-28 | 5′-CATCGCAGGAATACCCTA-3′ | PCR primer for cloning rsbA forward, corresponding to nt 1117 to 1134 (paired with yojR1970-1953) | This study |
| yojR1970-1953 | 5′-AAGATTTACGAATGCCGA-3′ | PCR primer for cloning rsbA reverse, corresponding to nt 3059 to 3076 (paired with yojF11-28) | This study |
| yojF854-871 | 5′-TTTATTTAATGATGGCCC-3′ | PCR primer for finding Tn5::2232; corresponding to nt 1960 to 1977 (paired with yojR1879-1862) | This study |
| yojR1879-1862 | 5′-AACTGTCGGATCCATTTA-3′ | PCR primer for finding Tn5::2232; corresponding to nt 2985 to 2968 (paired with yojF854-871) | This study |
| yojF1870-1886 | 5′-TCCGACAGTTGCTGTAG-3′ | PCR primer for finding Tn5::2232; corresponding to nt 2976 to 2992 (paired with yojR3379-3363) | This study |
| yojR3379-3363 | 5′-ACCGATCATGATGAATG-3′ | PCR primer for finding Tn5::2232; corresponding to nt 4485 to 4469 (paired with yojF1870-1886) | This study |
| rsbF371-387 | 5′-AACGGCCATTGATAACG-3′ | PCR primer for finding Tn5::2232; corresponding to nt 1477 to 1493 (paired with rsbR1185-1167) | This study |
| rsbR1185-1167 | 5′-TACGGAAATGAGAGCCAA-3 | PCR primer for finding Tn5::2232; corresponding to nt 2291 to 2273 | This study |
| rsbAR234 | 5′-TGATGGTTGTTTAGCCAA-3′ | PCR primer used to amplify intact rsbA for pMM309, corresponding to nt 234 to 251 (used in conjunction with yojR1970-1953) | This study |
| rcsCF158 | 5′-ATGGAACGCTCTCTACAAAAT-3′ | PCR primer for finding Tn5::2234 and Tn5::2236; corresponding to nt 5371 to 5391 | This study |
| rcsC3′F | 5′-TTTTCCGAAGAGATTAACCGCTC-3′ | PCR primer for finding Tn5::2234, and Tn5::2236; corresponding to nt 3892 to 3912 |
Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Rfr, rifampin resistance; Tcr, tetracycline resistance.
Swarming behavior assay.
P. mirabilis was grown overnight (14 to 16 h) at 37°C in L (Luria) broth with shaking. Following incubation, the cells were pelleted by centrifugation (5,000 × g for 5 min) and the supernatant was decanted. The cell pellet was resuspended in 1× phosphate-buffered saline (PBS; 20 mM sodium phosphate, 100 mM NaCl [pH 7.5]), and the cells were pelleted a second time by centrifugation. The resulting pellet was once again resuspended in 1× PBS, and the cell density was adjusted to 5 × 108 cells per ml (optical density at 600 nm = 0.4, as calibrated by a standard curve).
For swarming-behavior assays, 25 ml of sterilized L agar was dispensed to each petri dish and allowed to gel. The plates were air dried at room temperature for 24 to 36 h and then at 42°C for 30 min and were held at 37°C (usually less than 30 min) prior to use. In a warm room held at 37°C and 35% humidity, a 5-μl aliquot of PBS-washed P. mirabilis cells (ca. 2.5 × 106 cells) was dispensed to the center of a dried L-agar plate by using a micropipette. After adsorption of the droplet into the agar matrix (ca. 2 min), the plate was inverted and the incubation was started. All observations and manipulations of the swarming cultures after this point were done within the 37°C warm room to maintain a constant 37°C temperature and minimize unwanted temperature effects.
Swarming motility was observed every 15 to 30 min by using a stereo dissection microscope and microcalipers to determine the distances the cells moved over each time point. Swarmer cell differentiation, i.e., the overproduction of flagella, cellular elongation, and polyploidy, was also examined microscopically as described by Belas et al. (13).
Swimming motility was assessed by both Mot agar analysis and light microscopy as previously described (13), and chemotaxis was measured by the procedures described by Burkart et al. (18).
Measurement of urease, hemolysin, and protease activities.
Urease activity was measured by using urea agar medium (Difco Laboratories, Detroit, Mich.). After 6 to 8 h of incubation at 37°C, a zone of pink color, indicating a change in pH resulting from urea breakdown, was observed around each positive colony. The presence of hemolytic activity was detected as a zone of greenish discoloration surrounding positive colonies on trypticase soy agar with 5% sheep erythrocytes (TSA II; Becton Dickinson Microbiology Systems, Cockeysville, Md.) after overnight incubation at 37°C. Protease activity was measured by using the azocasein assay as described by Wassif et al. (52).
Tn5 mutagenesis.
P. mirabilis precocious mutants were identified from a bank composed of 212 swarming null mutants (Swr−) and crippled mutants (Swrcr; strains that produce aberrant swarming behavior) previously produced (13, 14) through mini-Tn5–Cm (21) mutagenesis.
DNA manipulation.
Standard methods were used for the manipulation of DNA (6, 42, 44) unless specified otherwise.
PCR DNA amplification.
PCR was performed with 2.5 U of recombinant Taq polymerase (AmpliTaq; Perkin-Elmer Cetus, Norwalk, Conn.) and an MJ Research DNA engine thermocycler. The primers used for cloning a fragment containing rsbA and rcsB and to check Tn5 insertions were oligonucleotides rsbA1F and rcsB1R.
IPCR.
Inverse PCR (IPCR) (49) was performed for 30 cycles by using oligonucleotides i1b and i2b, corresponding to nucleotide sequences at the I end of mini-Tn5 (21), as primers and HhaI-digested chromosomal DNA as the template. IPCR was performed as described by Han et al. (34).
Cloning of PCR products.
PCR DNA amplicons were cloned by using plasmid pCR2.1 and the TA Cloning Kit (Invitrogen Corporation; Carlsbad Calif.) according to the recommendations of the manufacturer.
Nucleotide sequencing and analysis.
Double-stranded DNA was used as a template for nucleotide sequencing by using the recommended procedures of the Prism Ready Reaction Dye Deoxy Termination Kit (Applied Biosystems) in conjunction with Taq polymerase and a model 373A DNA sequencer (Applied Biosystems). The Genetics Computer Group suite of computer programs (22) was used to analyze the DNA sequence, while the deduced amino acid sequences were analyzed with the BLAST family of programs (3, 4, 29, 54).
Southern blot analysis.
Southern blot analysis of chromosomal DNA bound to a nylon membrane (Hybond−N+; Amersham) was performed by using the DIG High Prime labeling kit according to the recommendations of the manufacturer (Boehringer Mannheim).
Construction of precocious swarming mutants.
rsbA mutants were constructed by allelic-exchange mutagenesis using the suicide plasmid pGP704 (40) and the wild-type strain BB2000. In brief, the cloned gene on pGP704 was modified in vitro by recombinant DNA techniques to incorporate the insertion of a chloramphenicol resistance element from pUT mini-Tn5–Cm (21). The recombinant plasmid was then conjugally transferred to BB2000 as previously described (14), where failure of plasmid replication and allelic exchange between the mutant plasmid-borne gene and the wild-type chromosomal copy resulted in gene replacement and consequent mutagenesis.
A precocious swarming mutant was constructed by PCR amplification of a region in rsbA from nucleotide (nt) 1117 to nt 3076 (numbered as in AF071215) by using wild-type genomic DNA as a template and yojF11-28 and yojR1970-1953 as primers, followed by cloning of the amplicon to pCR2.1. This produced plasmid pMM301. The mini-Tn5–Cm cam gene was purified after HindIII digestion of pUT–mini-Tn5–Cm and fragment separation by using 1.0% agarose in 1× TAE (Tris-acetate-EDTA) buffer. The Klenow fragment of DNA polymerase I was used to fill in the ends of the cam gene to construct a blunt-ended DNA fragment, which was ligated into the HpaI site in rsbA (nt 2121) to produce pMM303. The ′rsbA::cam::rsbA fragment was then transferred to pGP704 by digestion with EcoRI, ligation of the fragments, and transformation of E. coli SM10 (λpir) with selection for both Apr and Cmr. The construction was confirmed by restriction site mapping of the recombinant plasmid DNA, and a positive clone, referred to as pMM313, was chosen for further studies. Plasmid pMM313 was conjugally transferred into P. mirabilis by filter mating, resulting in MM100 (′rsbA::cam::rsbA) as described by Belas et al. (14). Following mating, P. mirabilis transconjugants were selected by plating on LSW− (L swarm minus) agar (14) containing chloramphenicol. Antibiotic-resistant colonies from each mating were then screened for plasmid carriage (Apr) and precocious swarming. The resulting precocious swarming strains were then assayed for swimming motility, swarming motility and behavior, and swarmer cell differentiation. The nature of the mutation in rsbA was confirmed through Southern blot analysis of the region from rsbA through rcsC.
Overexpression of RsbA and complementation of precocious swarming mutants.
The effects of overexpressing RsbA in a wild-type background were tested by transferring pMM309 into BB2000 via electroporation. The resulting Apr Kmr colonies were then assayed for precocious swarming and other phenotypic characteristics as previously described. Plasmid pMM309 was also electroporated into precocious swarming mutant MM100 (Table 1) for complementation studies, with selection for Kmr transformants and screening of precocious swarming as before.
Materials and reagents.
All reagents were of the highest purity available. Components of bacteriological media were purchased from Difco. Restriction endonucleases and DNA modifying enzymes were obtained from either New England Biolabs, Boehringer Mannheim Biochemicals, or Promega and were used according to the supplier’s recommendations.
Nucleotide sequence accession number.
The nucleotide sequence of 5,458 bp encoding rsbA, rcsB, and the 3′ portion of rcsC has been submitted to the DDBJ/EMBL/GenBank databases under accession no. AF071215.
RESULTS
Precocious mutants have defects in the temporal control of swarming migration.
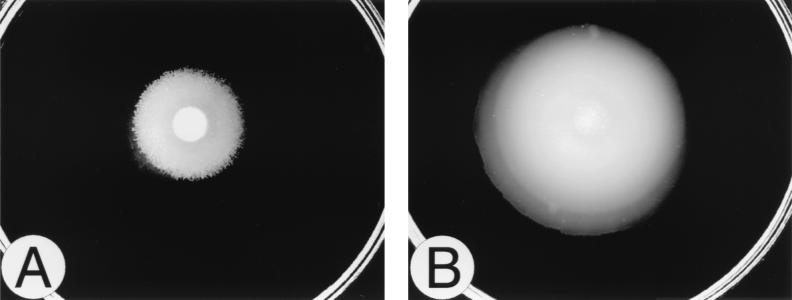
Six mini-Tn5–Cm (21) insertion mutants of P. mirabilis BB2000 were identified as possessing a unique, non-wild-type swarming pattern and behavior. These mutants, BB2231 through BB2236 (Table 1), were not swarming null mutants but instead, when incubated on an L-agar plate, showed a more progressive translocation across the agar surface compared to the wild-type control (Fig. 1). This behavior resulted in a colony that moved out farther than the wild type over a given time. We refer to this unusual swarming phenotype as “precocious swarming.”
FIG. 1.
Swarming behavior of the precocious phenotype. The swarming behaviors of the wild-type parent (BB2000) (A) and a Tn5-generated precocious mutant (BB2235) (B) were compared. Cells were grown overnight in L broth and washed in 1 × PBS, and a 5-μl spot of inoculum was added to the center of the agar. The plates were incubated at 37°C and observed after 4 h. Precocious swarming behavior is characterized by a decreased initial lag period prior to swarming onset, resulting in a larger swarming colony compared to that of the parent.
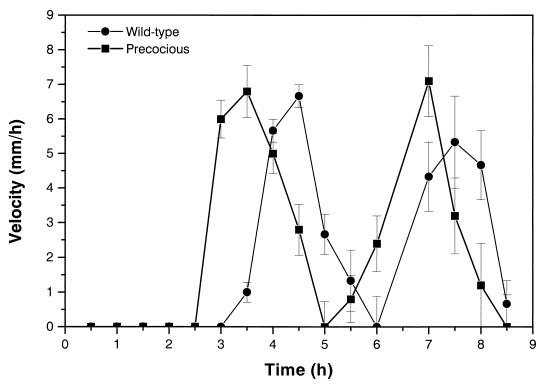
We analyzed swarming behavior and motility by following the swarm fronts of the precocious mutants and wild-type cells by the method of Gygi et al. (33) through two cycles of swarming migration. The interval migration velocities of a representative precocious mutant (BB2235) and the wild type are shown in Fig. 2. The most conspicuous difference between the wild-type cells and the precocious swarming mutants is the difference in time spent during the initial lag phase. Wild-type cells typically spend 3 to 3.25 h (at 37°C) in the initial lag phase prior to the onset of swarming migration, while precocious mutants spend ca. 30 to 60 min less in this phase.
FIG. 2.
Swarming velocity of precocious mutants during cyclic phases of swarmer cell differentiation and behavior. The swarming behaviors of a precocious swarming mutant (BB2235) and wild-type cells were characterized through two cycles of migration and consolidation by measuring the translocation distance at the swarming-colony edge at 30-min intervals according to the procedure of Gygi et al. (33). Comparison of velocity between wild-type and precocious swarming cells indicates that there are no defects in speed but that the precocious mutants start swarming much sooner (at least 30 min) than the wild type. Error bars, standard errors of the means (n = 5).
The velocity of both the wild-type cells and the precocious mutants varied during the course of one cycle of swarming behavior, with no differences observed between the wild-type cells and the precocious swarming mutants. Swarming translocation speed was observed to increase during the first 30 min after initiation of migration to peak at ca. 7 mm/h, and then to decrease until the cell mass stopped and consolidated. One complete cycle required 2.5 h on L agar at 37°C. Unlike Cps− (capsular polysaccharide [CPS]-defective) mutants, which have defects in a sugar transferase required for lipopolysaccharide (LPS) core modification (33), the rate of change and the swarming velocity of precocious mutants are identical to those of wild-type cells (Fig. 2). Therefore, this mutation does not appear to affect flagellar rotation. By spending less time in the initial lag period (and perhaps less time in consolidation), the precocious mutants thus translocate farther in a given time than do the wild-type cells.
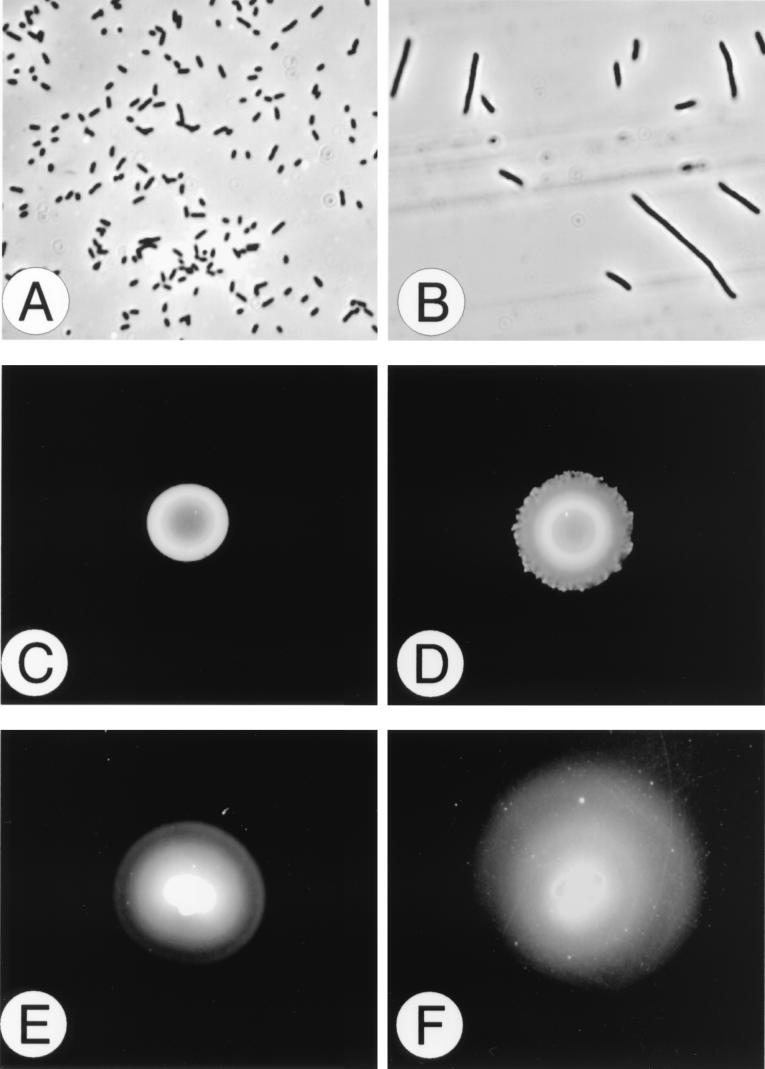
The mutation resulting in precocious swarming extends to other phenotypes, as shown in Fig. 3. For example, the precocious mutants are constitutive for swarmer cell differentiation (Fig. 3A and B) and swarm, albeit slowly, on minimal glycerol agar (Fig. 3D), whereas wild-type P. mirabilis is unable to initiate swarming behavior on minimal medium (Fig. 3C). Precocious swarming mutants also swarm over LSW− agar, a medium that phenotypically prevents swarming of wild-type P. mirabilis and all other known P. mirabilis mutants (13, 16). Other differences between wild-type cells and precocious swarming mutants were also observed, especially in their swimming motility and chemotactic behavior (Fig. 3E and F). The alteration in chemotactic ring formation may be a function of a constitutive swarmer cell phenotype, since we and others (2) have noted that swarmer cells have limited ability to initiate tumbles and instead swim in long, straight paths that may give rise to the less-defined rings observed in Fig. 3F. Precocious swarming mutants were also examined for expression of virulence factors associated with P. mirabilis pathogenicity, such as expression of flagellin (flaA), ZapA immunoglobulin A (IgA)-degrading metalloprotease activity, and the production of urease and hemolysin. The expression of each of these virulence factors was elevated in precocious mutants. For example, the activity of the ZapA IgA-degrading metalloprotease enzyme was 141% that of the wild-type as measured by the azocasein assay (52).
FIG. 3.
Phenotype of precocious mutants. In addition to an early onset of swarming behavior, precocious mutants show other phenotypic differences from the wild type, including retention of the differentiated swarmer cell morphology when grown under noninducing conditions, swarming movement on minimal agar medium, and non-wild-type chemotaxis patterns. The upper panels compare the cell morphology of the wild-type cells (A) and the precocious swarming mutant BB2235 (B) after 8 h of growth in L broth at 37°C. Precocious mutants possess the characteristics of swarmer cells (elongated, polyploid, nonseptate cells with numerous flagella) when they are in liquid medium (a noninducing environment), while the wild-type cells remain undifferentiated swimmer cells. The wild type does not swarm on minimal glycerol medium (C), but after 48 h of incubation at 37°C, the precocious mutants show signs of swarming behavior (D). The lower panels compare the chemotactic behavior (a swimmer cell phenotype) in relation to ribose. The wild-type cells (E) form a tighter and more discrete series of chemotactic rings than the precocious mutants (F). This difference may be due to a higher percentage of swarmer cells in the precocious mutant population compared to the wild type when P. mirabilis is grown in the semisolid Mot agar.
The precocious phenotype is the result of mutations in a locus encoding RsbA, a member of the two-component family of sensory proteins.
Identification of the P. mirabilis DNA flanking the Tn5 insertion sites in the six precocious mutants was accomplished by two methods. First, an IPCR amplification (34, 49) of HhaI-digested and ligated genomic DNA isolated from each precocious mutant was performed with oligonucleotide primers (Table 1) homologous to two regions within the I end of the transposon (21). The second approach involved a more conventional digestion and cloning of the DNA flanking the transposons using either EcoRI, HindIII, or SalI restriction endonuclease digestion of genomic DNA. The nucleotide sequence was obtained from each of the cloned DNA fragments flanking the transposon, open reading frames (ORFs) were identified, and computer-based homology searches were conducted in order to obtain insights into the nature of the mutated gene.
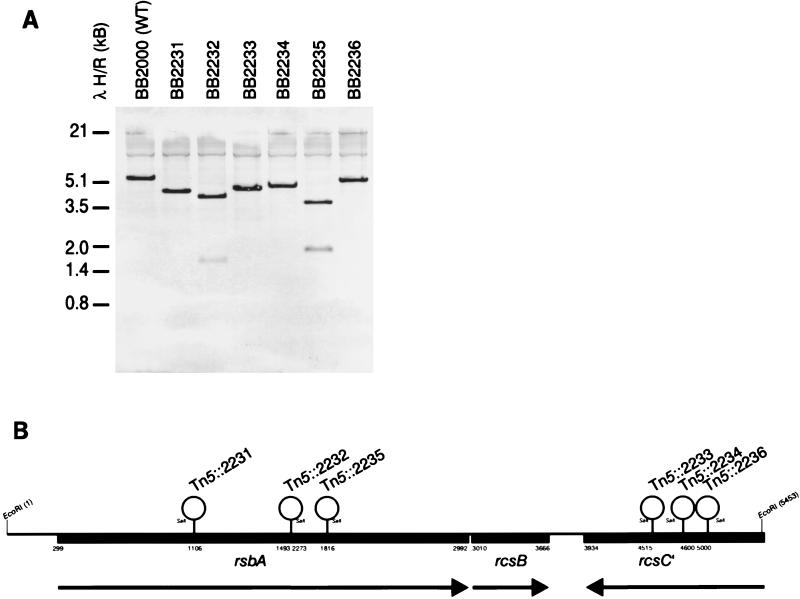
Initially, this approach successfully located the Tn5 insertion site in the precocious mutants BB2231, BB2235, and BB2233, defining a locus of ca. 3.4 kb on the wild-type chromosome. Subsequent Southern blot analysis of EcoRI-digested genomic DNA isolated from wild-type and precocious strains (Fig. 4A), as well as PCR-based analysis of the same DNA, revealed that all six of the Tn5 insertions giving rise to the precocious swarming phenotype were located within a 5,458-bp EcoRI fragment of the P. mirabilis genome (Fig. 4B). The nucleotide sequence of 5,458 bp encoding the three ORFs (rsbA, rcsB, and the 3′ portion of rcsC) has been submitted to the DDBJ/EMBL/GenBank databases under accession no. AF071215.
FIG. 4.
Location of the Tn5 insertion on the P. mirabilis chromosome. The insertion point of the mini-Tn5–Cm transposon in each of the six precocious mutants (B) was determined by using either IPCR cloning and subsequent nucleotide sequence analysis (BB2235), cloning of Cmr fragments from precocious mutant chromosomal DNA (BB2231 and BB2233), Southern blot analysis (A) with probes to sites within the region, or PCR amplification of segments of the region adjacent to the Tn5 insertions (16). The oligonucleotides used to locate each transposon insertion point are listed in Table 1. The transposon insertions clustered in two loci. Precocious mutants BB2231, BB2232, and BB2235 were found to have Tn5 insertions in rsbA, while BB2233, BB2234, and BB2236 were located in the 3′ end of rcsC. The nucleotide sequence of 5,458 bp encoding rsbA, rcsB, and the 3′ portion of rcsC has been submitted to the DDBJ/EMBL/GenBank databases under accession no. AF071215.
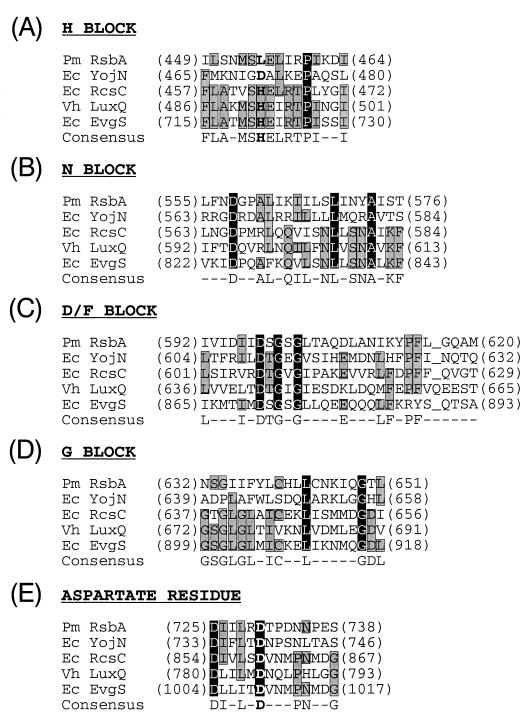
The Tn5 insertions were found to be in two of three linked ORFs on the P. mirabilis genome in a region that is highly homologous to the E. coli yojN-rcsB-rcsC region (17). In E. coli, RcsC and RcsB are members of a two-component regulatory circuit controlling capsular synthesis (46), where RcsC is a histidine kinase and RcsB is its cognate response regulator (30). Upstream of rcsB and transcribed in the same direction as rcsB is an ORF referred to as yojN (GenBank accession no. AE000310) that has not been assigned a function in E. coli, although YojN has motif similarity to other bacterial sensory proteins (17). Since three of the Tn5 insertions mapped to the P. mirabilis ORF homologous to E. coli yojN (Fig. 4B), we have chosen to call this gene rsbA, for regulator of swarming behavior.
Figure 4B shows a map of the rsbA, rcsB, and rcsC region. Analysis of the deduced amino acid sequence of RsbA indicates that it may be localized in the inner membrane as a transmembrane protein. RsbA contains a putative N-terminal signal sequence stretching from M1 through a possible cleavage site between A31 and Y32, and it contains two membrane-spanning domains, one between L15 and F35 and one between I299 and I319 (37). Both RsbA and YojN have strong motif similarity to many other sensory proteins that are members of the two-component family of proteins (1, 36, 47). Computer-assisted homology searches using BLASTP (4) of protein sequences stored in the GenBank/EMBL database indicate that RsbA is homologous to E. coli YojN (29% identity, 50% similarity), E. coli RcsC (19% identity, 39% similarity), Vibrio harveyi LuxQ (22% identity, 41% similarity), and E. coli EvgS (22% identity, 41% similarity). Specifically, as shown in Fig. 5, domains, such as the N and D/F blocks and the phospho-aspartate residue, that are associated with histidine kinases (36) are maintained within RsbA. Not conserved, however, are the sequences of the H and G blocks. Both RsbA and YojN are conspicuously lacking a His in the H block that is required for autophosphorylation of histidine kinases. Therefore, while RsbA may function as a sensory protein, it is unlikely to function as a typical histidine kinase.
FIG. 5.
Alignment of RsbA domains with those of related bacterial sensor proteins. Underlined spaces indicate gaps introduced to optimize alignments. Amino acids that are shared among all five proteins are shown in white on a solid background, and those shared by 3 or 4 of the proteins are shaded. Boldface letters indicate alignment of the conserved histidine and aspartate residues. BLASTP analysis P values for the overall alignments of these four proteins with RsbA were 1 × 10−125 for E. coli YojN (29% identity, 50% similarity), 1 × 10−18 for E. coli ResC (19% identity, 39% similarity), 6 × 10−17 for V. harveyi LuxQ (22% identity, 41% similarity), and 3 × 10−12 for E. coli EvgS (22% identity, 41% similarity).
The three remaining precocious mutants, BB2233, BB2234, and BB2236, all possessed Tn5 insertions in the 3′ region of the P. mirabilis homolog to E. coli rcsC (Fig. 4B). The migration phenotype of these mutants is identical to that of precocious swarming mutants found to have defects in RsbA. It is unlikely that the insertion of a transposon in the P. mirabilis rcsC gene would affect transcription of either rsbA or rcsB, since this gene is transcribed in the opposite direction and possesses a possible transcriptional terminator (nt 3659 to 3678) 3′ of its stop codon. For the remainder of this report, we will focus on the function of RsbA in producing the precocious swarming phenotype. However, the location of transposon insertions upstream of rcsB and in rcsC suggests that there may be some component of CPS biosynthesis or RcsC histidine kinase activity necessary for the proper timing of the onset of swarming behavior. Possible roles for RcsB and RcsC in the initiation of swarming behavior are described in the Discussion.
Construction and analysis of a mutation in rsbA through allelic-exchange mutagenesis.
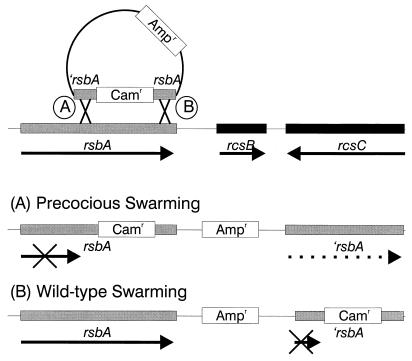
To demonstrate the role of RsbA in producing the precocious phenotype, we sought to construct a mutation in rsbA through allelic-exchange mutagenesis using a selectable chloramphenicol resistance gene cassette to disrupt a cloned copy of rsbA (′rsbA::cam::rsbA). As diagrammed in Fig. 6, a portion of rsbA from nt 1117 to nt 3076 (Fig. 4B) was cloned into the oriR6K suicide plasmid vector pGP704 (40). The 3.5-kb chloramphenicol resistance gene (cam) from pUT–mini-Tn5–Cm (21) was inserted at the HpaI site (nt 2121) in rsbA, and this plasmid was conjugally mated into wild-type P. mirabilis, with selection for chloramphenicol resistance (Cmr), and screening of the resulting 532 Cmr colonies for loss of the plasmid (Aps) and acquisition of the precocious swarming phenotype. From this mutagenesis no mutants were found that were concomitantly Aps and precocious swarming. Instead, 12 Apr colonies were observed to be precocious swarmers. The allelic-exchange mutagenesis was repeated twice more, and a total of 1,360 Cmr transconjugants were screened for the precocious swarming phenotype; as previously observed, no Aps strains were found that also possessed the precocious swarming phenotype. A total of 31 Apr precocious swarming mutants were obtained from the three rounds of allelic-exchange mutagenesis. Analysis of 12 of the wild-type swarming Cmr Apr strains indicated that the plasmid vector was replicating in the cytoplasm, an event that occurs in ca. 35% of the transconjugants, based on previous reports (14).
FIG. 6.
Molecular analysis of the rsbA mutation producing the precocious phenotype. Based on analysis of Tn5::2235, a truncated rsbA gene (nt 1117 to 3076) containing a Cmr gene cassette (′rsbA::cam::rsbA) inserted at the HpaI site (nt 2121) was used to construct a precocious swarming mutant via allelic-exchange mutagenesis. Selection for chloramphenicol-resistant colonies was followed by a screen for plasmid carriage (Apr or Aps) and the precocious swarming phenotype. Two types of mutations were observed. The first (A) involved a single crossover (Campbell integration) that resulted in a merodiploid containing a cam gene inserted in the whole rsbA gene, the plasmid, and a truncated copy of rsbA. These mutants had the precocious swarming phenotype. The second type of single crossover (B) resulted in a wild-type copy of rsbA, the plasmid, and the truncated rsbA plus cam gene insert. These colonies were wild type for swarming.
The retention of the Apr phenotype in precocious mutants produced by allelic-exchange mutagenesis indicated the possibility that the plasmid had not been lost as expected. This was confirmed by Southern blot probing with plasmid DNA probes and through PCR amplification using oligonucleotide primers homologous to plasmid sequences. In each of these precocious mutants, the plasmid had integrated along with the truncated rsbA gene through a single-crossover event. Mapping data revealed that two types of single-crossover integration events had occurred (Fig. 6A and B). The first type of event (Fig. 6A) resulted when the rsbA region upstream of the HpaI-inserted cam gene recombined with the genomic copy of rsbA. The result of this single crossover was a partial duplication of rsbA where a complete copy of rsbA along with the cam cassette insertion was followed by the integrated plasmid, which was then followed by a second, but truncated, copy of rsbA (Fig. 6A). When the single-crossover junction occurred after the inserted cam cassette, the resulting mutant was wild type for swarming (Fig. 6B). Northern blot hybridizations of mRNA obtained from the wild type and each of the single-crossover mutant types indicate that expression of rsbA is regulated concomitantly with swarmer cell differentiation in wild-type cells and in single-crossover mutants showing wild-type swarming but is constitutively expressed in the single-crossover precocious mutants (16). Thus, the single-crossover precocious swarming phenotype may be the result of a constitutively expressed truncated RsbA protein.
At no time did we find double-crossover (Aps) mutants. This result is unusual, since this method has been highly successful for the allelic-exchange mutagenesis of other P. mirabilis genes (8, 15). This may have been due in part to the production of a lethal, rsbA null phenotype. Despite the failure to produce a null mutant, the data show that mutations which produce a truncated RsbA protein result in a precocious swarming phenotype, thus confirming the importance of RsbA in the onset of swarming migration.
Initiation of P. mirabilis swarming migration is a density-dependent process.
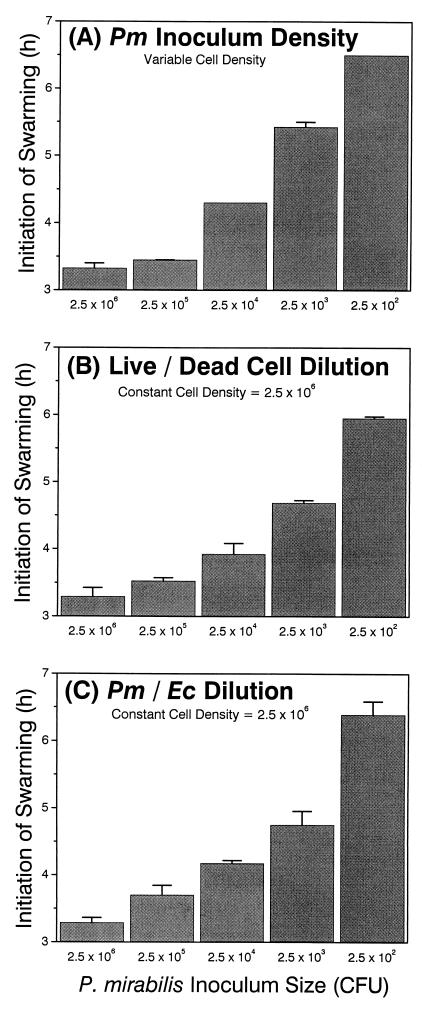
Since RsbA has homology with other known sensory proteins, particularly V. harveyi LuxQ (a cell population density sensor), one possible hypothesis for RsbA function is that it acts as a cell density sensor that triggers swarming migration. To test this, we first determined if the initiation of P. mirabilis swarming behavior is density dependent. In a series of experiments the results of which are shown in Fig. 7, we tested whether the initiation of swarming behavior was correlated with population density (Fig. 7A), whether swarming initiation requires living cells (Fig. 7B), and if cells other than P. mirabilis affect swarming initiation (Fig. 7C). In these experiments, the initial inoculum concentration was varied in 10-fold increments from 2 × 106 to 2 × 102 cells (CFU) delivered in 5-μl-aliquot droplets to the L-agar surface. We observed that the onset time of P. mirabilis swarming behavior is dependent on the population density, such that the time to swarming migration increased as the cell density decreased. These data also demonstrate that swarming behavior requires living cells, because when the ratio of living to UV-killed P. mirabilis was varied (Fig. 7B), with the final density kept constant at 2 × 106, the time to swarming initiation increased with decreasing numbers of living cells. The time of onset of swarming is dependent on some component of the physiology of P. mirabilis, because when different ratios of P. mirabilis and E. coli cells were mixed together while the total density was held constant (at 2.5 × 106 cells), the time of swarming behavior onset was responsive only to P. mirabilis cell density (and was independent of E. coli density), just as had been observed when only P. mirabilis was used. Thus, the onset of swarming behavior requires a specific population density of living P. mirabilis cells.
FIG. 7.
Initiation of P. mirabilis swarming behavior is density dependent. (A) Swarming onset compared to the density of the inoculum. (B) Swarming onset when a constant density of cells is maintained but the ratio of living to UV-killed cells varies. (C) Swarming onset when the ratio of P. mirabilis to E. coli cells changes but the total cell density remains constant. The horizontal axis shows the number of P. mirabilis cells in the inoculum as CFU. Error bars, standard errors of the means (n = 4).
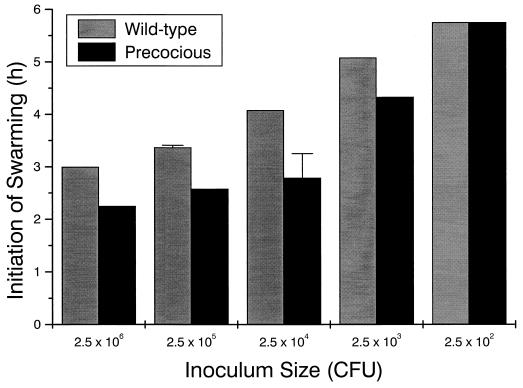
If RsbA is a population density sensor, defects in RsbA should alter the ability of the cells to detect changes in the population size and may change the time of initiation of migration. To test this, we compared the timing of swarming initiation of wild-type cells to that of MM100 (precocious swarming). The results, shown in Fig. 8, indicate that, while MM100 does indeed initiate swarming behavior sooner than wild-type cells, the phenomenon is still density dependent. However, precocious swarming strains require ca. 100-fold fewer cells to commence migration (2 × 106 wild-type cells versus 2 × 104 MM100 to begin swarming at 3 h). These data suggest that precocious swarming is the result of defects in the density-sensing mechanism; although precocious mutants are still capable of detecting density, they appear to be hyperresponsive to this unknown cue. Since the precocious mutants most likely produce a truncated form of RsbA, it is feasible that the deleted portion of the protein is required for a regulatory function governing this response (see the Discussion).
FIG. 8.
Precocious swarming mutants retain density dependence. The density-dependent initiation of swarming behavior in wild-type and precocious swarming cells was compared. The initial inoculum concentration was varied in 10-fold increments from 2 × 106 to 2 × 102 cells (CFU) delivered in 5-μl-aliquot droplets to the L-agar surface. The onset time of swarming behavior was then recorded. Error bars, standard errors of the mean (n = 4).
Overexpression of RsbA in wild-type cells produces a precocious swarming phenotype.
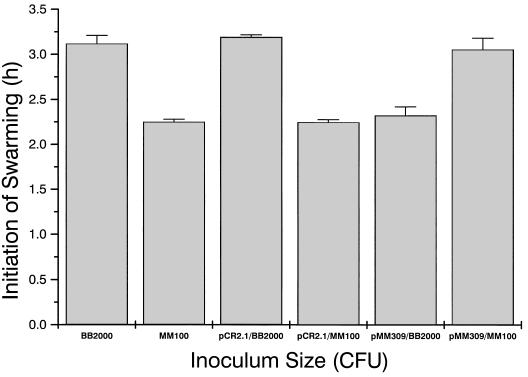
One possible role for RsbA in the initiation of swarming could be as an affector of swarming differentiation and motility. To test this, the effects of overexpression of RsbA on swarmer cell differentiation and behavior were examined by placing a copy of the intact rsbA gene and its cognate ribosome binding site downstream from the lac promoter on pCR2.1 (Kmr Apr; Stratagene). As shown in Fig. 9, when RsbA is overexpressed on a multicopy plasmid (pMM309), P. mirabilis initiates swarming behavior ca. 1 h earlier than the parental strain by itself (mean time of swarming onset, 135 min versus 192 min). Plasmid-only controls have no effect on the time of swarming. Wild-type cells overexpressing RsbA also swarmed on LSW− agar medium, as well as on minimal glycerol (or glucose) agar medium, and expressed a constitutive swarmer cell phenotype indistinguishable from that observed with precocious swarming mutants (16). Thus, overexpression of RsbA in a wild-type background produces a precocious swarming phenotype.
FIG. 9.
Overexpression of RsbA and complementation of precocious mutants. pMM309, a multicopy plasmid (pCR2.1) with an intact rsbA gene, was introduced into wild-type cells and precocious mutant MM100. Swarming behavior was then observed by measuring the time of initiation of swarming migration on L agar. A 5-μl aliquot containing 2 × 106 cells was used as an inoculum. Error bars, standard errors of the means (n = 4).
Complementation of precocious mutants by rsbA in trans results in wild-type initiation of swarming migration.
The data from overexpression of rsbA in a wild-type background suggest that one function of RsbA may be as a positive regulator of swarming, such that increased levels of the protein reduce the lag phase before swarming and result in an early, precocious swarming phenotype. In contrast are the data from precocious mutants such as MM100 (truncation of rsbA) suggesting that the N-terminal portion of the protein may have a negative regulatory function. Such dual functionality has been observed in proteins such as LuxR (19, 35), the regulator of bioluminescence in Vibro fischeri. If this is true, then the overexpression of RsbA provided in trans to a precocious swarming mutant with a truncated copy of rsbA should restore the wild-type swarming behavior. To test this, MM100 was transformed with the same multicopy plasmid clone of rsbA (pMM309) that was used previously. As shown in Fig. 9, overexpression of rsbA leads to a partial restoration and complementation of the swarming phenotype (192 min for the wild type, 144 min for the precocious swarming mutant, and 186 min when MM100 was complemented in trans by an rsbA-containing plasmid). Similar results were obtained when either BB2231, BB2232, or BB2235 was complemented by pMM309. These data suggest that loss of the N-terminal section of RsbA may remove a negative regulatory domain from the protein, creating the precocious swarming behavior.
DISCUSSION
Numerous genes required for flagellum synthesis and function have been associated with aspects of P. mirabilis swarmer cell differentiation and (to a limited extent) swarming behavior. For example, mutations in the FlhDC central regulator of flagellum synthesis and in a negative regulator, FlhA, have been described that directly affect swarmer cell differentiation and produce swarming null mutants through direct effects on the function of the flagellum (28, 31). Other genes associated with flagellar synthesis and function have also been shown to directly affect differentiation and to produce a nonswarming colony as a consequence of lack of flagella (8, 15, 32). An additional layer of regulation may also be exerted through Lrp (leucine-responsive regulatory protein), which functions as a global regulator and which, when mutated, also produces a nonswarming colony.
The regulatory genes flhDC and lrp thus result in swarming null mutants and affect swarming behavior or migration only as a consequence of their impact on flagellar synthesis. One gene that has been observed to affect swarming behavior is cmfA (colony migration factor), associated with the assembly of CPS (33). Unlike RsbA, the effect of CmfA appears to indirectly affect the cyclic swarming migration through defects in LPS, and CmfA− mutants generate closely spaced terraces during cyclic swarming behavior (33). In marked contrast to RsbA− precocious mutants, which show changes in the temporal control of the swarming cycle, CmfA− mutants are impaired in the spatial regulation of the cycle, and their translocation velocity is drastically reduced (33). This suggests that CmfA has a “housekeeping” function in the assembly of LPS and CPS and that it most likely is a type II molecule linked to phospholipid (33). The loss of the Cmf CPS thus results in the loss of a lubricant, with the indirect consequence of an increase in friction of the cellular mass as it moves over the surface; this increase in friction reduces overall translocation velocity and generates the tightly clustered consolidation zones observed.
We have focused our attention on a unique group of mutants that we refer to as precocious swarming mutants. These behavioral mutants, originally described by us as “superswarmers” because they moved out farther than the wild type, in fact do not move any faster than wild-type cells (Fig. 2). RsbA− mutants are also constitutive for swarmer cell differentiation and migrate on minimal agar medium, two phenotypes not associated with CmfA− or other behavioral mutations. As indicated above, precocious mutants also show no differences in swarming velocity, unlike either flhDC or cmfA mutants, further distinguishing this group of mutants as unique.
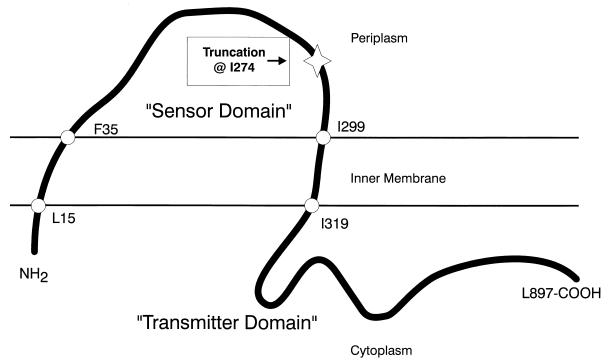
The precocious swarming phenotype produced from either Tn5 insertions or allelic-exchange mutagenesis of rsbA is not the consequence of a nonsense mutation; rather, our data suggest that a truncation of rsbA is the possible cause of precocious swarming (Fig. 10). Specifically, in the construction of MM100 we used a segment of rsbA with the 5′ end of the gene deleted, which removes the N-terminal 273 amino acid residues of RsbA (nt 299 to 1117) to produce a truncated protein that begins at I274 (nt 1118). MM100 is the consequence of a single crossover (Campbell integration) that results in a duplication of portions of rsbA and insertion of the pGP704 plasmid into the chromosome. A truncated RsbA has not been identified, but preliminary Northern blot data indicate that the rsbA transcript is constitutively expressed in precocious strains, and DNA probes to the 3′ end of rsbA hybridize to the mRNA from precocious mutants, while probes to the 5′ end of rsbA do not (16). Although preliminary, the mRNA hybridization data support the data obtained from Southern blots summarized in Fig. 6 and suggest that a truncated rsbA transcript may be produced by precocious mutants. As shown in Fig. 10, we hypothesize that truncating RsbA in this manner removes the N-terminal domain of the protein, which may function as a sensory domain, as has been observed in other sensory proteins in the two-component family (36).
FIG. 10.
Membrane topology of native RsbA and of the mutant RsbA producing precocious swarming. RsbA has amino acid motifs indicative of membrane spanning sensory proteins. Computer-assisted motif searches (37) suggest a possible topology for RsbA that separates an N-terminal regulatory domain from a C-terminal transmitter domain. This model also predicts that the mutation in MM100 removes the N-terminal portion of the protein, thus eliminating the regulatory functions while retaining the transmitter domain.
What may be the molecular mechanism by which RsbA functions? Although the direct answer to this question is not known, it may be informative to compare RsbA to homologous sensory proteins. YojN currently has no assigned function, but RcsC, LuxQ, and EvgS are known histidine kinases that sense environmental conditions. Although the signal sensed by RcsC remains obscure, the environmental stimuli sensed by LuxQ and EvgS have been reported. LuxQ functions along with LuxP in the second density-sensing system of V. harveyi, where it controls bioluminescence. EvgS expression is regulated by low temperature, MgSO4, and nicotinic acid, factors known to control the virulence of Bordetella pertussis via BvgS, which is reported to be functionally similar to EvgS (50, 51).
The data demonstrate that RsbA is involved in aspects of P. mirabilis swarming migration and behavior, and perhaps has functions similar to those of LuxQ or EvgS. Testable hypotheses about the molecular function of RsbA may be developed from these observations. First, RsbA likely serves as a membrane sensor that controls swarmer cell migration by monitoring a key aspect of the environment around the cell. The amino acid sequence similarity between RsbA and LuxQ suggests that RsbA may be important in quorum sensing. If this is true, RsbA mutants should show changes in the density-dependent lag period prior to migration. Indeed, a precocious swarming mutant of RsbA requires 100-fold fewer cells to start swarming than the wild type (Fig. 8), but the process is still density dependent. This result may be due to the nature of the mutation, which presumably generated a truncated RsbA protein that retains density-sensing ability and density-dependent response but is partially relieved of a negative control that prevents migration. We are currently testing this hypothesis by constructing specific deletion mutants of RsbA and measuring the onset of swarming migration.
Although it lacks the H block His residue (Fig. 5), RsbA does have homology to many of the functional domains associated with sensor histidine kinase and contains the aspartate residue required for phosphoryl transfer to an unknown response regulator protein. How might RsbA function lacking the autophosphoryl histidine? It is curious that Tn5 insertions in the P. mirabilis homolog to rcsC also produce a precocious phenotype. Analysis of the nucleotide sequence of this locus suggests that rcsC is transcribed in the opposite orientation from rsbA; thus, Tn5 insertions in rcsC are unlikely to affect rsbA expression via polar effects. One possibility is that some type of cooperativity or interaction between RsbA and RcsC is required in order to provide kinase activity. Alternatively, perhaps the mutations in rsbA have polar effects on rcsB, so that defects in RcsB and RcsC affect swarming through an unknown mechanism similar to the effects of RcsB-RcsC seen in Salmonella typhi virulence genes (5). We are pursuing this idea through the construction of specific mutations in rcsB and rcsC. Interestingly, none of the precocious mutants demonstrates major defects in CPS as detected in colony morphology; precocious cells are neither rough nor mucoid in appearance. Also, complementation and overexpression of RsbA do not produce overt changes in the colony that can be linked to changes in CPS. However, in view of this possibility and the observed effects of mutations of cmfA (33), we are exploring the regulation of P. mirabilis capsule synthesis in more detail.
The data in Fig. 7 are a strong indication that the onset of swarming migration is a cell density-dependent phenomenon. This onset requires a threshold population density of live P. mirabilis cells, as evidenced by the fact that substitution with live E. coli does not produce a similar response. What molecule is sensed in the density-dependent control of swarming migration initiation? Quorum sensing mediated through a homoserine lactone (HSL) autoinducer identified as N-butanoyl-l-homoserine lactone (BHL) has been described in Serratia liquefaciens, where it has been shown to be involved in the initiation of swarming motility (24, 25). This suggests that an HSL autoinducer molecule is required for control of swarming migration in this organism. However, this may be atypical of swarming migration in general. For example, Vibrio parahaemolyticus swarms on minimal medium, while S. liquefaciens, like P. mirabilis, does not. It has been suggested by McCarter (39) that the ability to swarm over minimal medium may thus be correlated with dependence on an autoinducer in control of swarming migration. Since P. mirabilis does not swarm on minimal medium, it might be expected to use HSL autoinducers to control this migration. We have attempted to test this in cross-feeding experiments with P. mirabilis and various strains of V. harveyi, V. fischeri, and Agrobacterium tumefaciens that are defective in autoinducer synthesis (26, 27). The results from such experiments failed to prove the existence of a P. mirabilis HSL autoinducer. We have also used standard autoinducer purification techniques that include organic extractions with either ethyl acetate or chloroform and again have not been able to isolate an autoinducer activity from P. mirabilis cultures or to concentrate an activity that produces precocious swarming migration (16). Therefore, these data suggest that P. mirabilis may use an alternate form of density-dependent quorum sensing that does not rely on the “classical” HSL molecule, and no direct correlation can be made between the requirement for HSL autoinducer in swarming migration initiation and the inability to swarm over minimal media.
What is the purpose of RsbA in the survival of P. mirabilis? As a working model, we hypothesize that RsbA may function in a manner similar to that of B. pertussis BvgS (20) and that it controls two distinct phases of the P. mirabilis life cycle during UTI. Coexisting with the swimmer-to-swarmer cell transition, the two phases divide swarmer cell life into two modes of existence. In the first mode, the cells are nonmotile, weakly differentiated swarmer cells, and they express adhesive fimbriae, such as Mrp fimbriae (7, 55). This phase we call the adhesive mode, and it corresponds to the lag period seen prior to swarming migration and to the consolidation phase demarcating cycles of migration. This model assumes that there are times during the adhesive mode when the cells come under localized attack from host immune molecules, principally IgA. The cells sense this, perhaps through the proteolysis of host IgA molecules by the ZapA IgA-degrading metalloprotease (52), and respond to this signal by initiating swarming migration (the motile mode). This movement would move the cells away from an area of active host immune response to other sites suitable for temporary colonization. Thus, RsbA would function as a sensor of the host defense response signal and would regulate the mode of existence, either adhesive or motile, of the colonizing swarmer cell. We are in the process of examining the virulence of precocious swarming mutants, using a mouse model of ascending urinary tract pathogenesis.
ACKNOWLEDGMENTS
This work was supported by National Science Foundation grant MCB-9514803 to R.B. R.S. was a recipient of a National Science Foundation Research Enrichment for Undergraduates Internship.
We are especially grateful to Michael Silverman, who encouraged us to look at superswarming mutants and whose insight and thoughtful discussions have been most stimulating and helpful. We thank S. Gottesman, T. Silhavy, and J. S. Parkinson for general discussions regarding the rsbA locus, histidine kinases, and P. mirabilis swarming. Thanks are also due to E. P. Greenberg and P. Dunlap for discussions on HSL autoinducer detection and LuxR and to B. Bassler for discussions on AI-2. We also express our appreciation to A. Bertinuson for her initial observations and measurements of the swarming motility of precocious mutants, to K. Walker for mRNA and protease measurements, and to K. Walker and C. Murphy for critical reading of the manuscript.
REFERENCES
- 1.Alex L A, Simon M I. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994;10:133–138. doi: 10.1016/0168-9525(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 2.Allison C, Lai H C, Gygi D, Hughes C. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol Microbiol. 1993;8:53–60. doi: 10.1111/j.1365-2958.1993.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 3.Altschul S, Gish W, Miller W, Myers E, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 4.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey P, Popoff M. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. doi: 10.1046/j.1365-2958.1998.00976.x. [DOI] [PubMed] [Google Scholar]
- 6.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and John Wiley & Sons, Inc; 1987. [Google Scholar]
- 7.Bahrani F K, Mobley H L. Proteus mirabilis MR/P fimbrial operon: genetic organization, nucleotide sequence, and conditions for expression. J Bacteriol. 1994;176:3412–3419. doi: 10.1128/jb.176.11.3412-3419.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belas R. Expression of multiple flagellin-encoding genes of Proteus mirabilis. J Bacteriol. 1994;176:7169–7181. doi: 10.1128/jb.176.23.7169-7181.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belas R. Proteus mirabilis and other swarming bacteria. In: Shapiro J, Dworkin M, editors. Bacteria as multicellular organisms. New York, N.Y: Oxford University Press; 1997. pp. 183–219. [Google Scholar]
- 10.Belas R. Proteus mirabilis swarmer cell differentiation and urinary tract infection. In: Mobley H, Warren J, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: American Society for Microbiology; 1996. pp. 271–298. [Google Scholar]
- 11.Belas R. Sensing, response and adaptation to surfaces: swarmer cell differentiation and behavior. In: Fletcher M, editor. Molecular and ecological diversity of bacterial adhesion. New York, N.Y: Wiley-Liss, Inc.; 1996. pp. 281–331. [Google Scholar]
- 12.Belas R. The swarming phenomenon of Proteus mirabilis. ASM News. 1992;58:15–22. [Google Scholar]
- 13.Belas R, Erskine D, Flaherty D. Proteus mirabilis mutants defective in swarmer cell differentiation and multicellular behavior. J Bacteriol. 1991;173:6279–6288. doi: 10.1128/jb.173.19.6279-6288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belas R, Erskine D, Flaherty D. Transposon mutagenesis in Proteus mirabilis. J Bacteriol. 1991;173:6289–6293. doi: 10.1128/jb.173.19.6289-6293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belas R, Goldman M, Ashliman K. Genetic analysis of Proteus mirabilis mutants defective in swarmer cell elongation. J Bacteriol. 1995;177:823–828. doi: 10.1128/jb.177.3.823-828.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belas, R., and M. Melch. 1998. Unpublished data.
- 17.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 18.Burkart M, Toguchi A, Harshey R. The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:2568–2573. doi: 10.1073/pnas.95.5.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi S H, Greenberg E P. The C-terminal region of the Vibrio fischeri LuxR protein contains an inducer-independent lux gene activating domain. Proc Natl Acad Sci USA. 1991;88:11115–11119. doi: 10.1073/pnas.88.24.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotter P A, Miller J F. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol Microbiol. 1997;24:671–685. doi: 10.1046/j.1365-2958.1997.3821741.x. [DOI] [PubMed] [Google Scholar]
- 21.De Lorenzo M, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eberl L, Christiansen G, Molin S, Givskov M. Differentiation of Serratia liquefaciens into swarm cells is controlled by the expression of the flhD master oepron. J Bacteriol. 1996;178:554–559. doi: 10.1128/jb.178.2.554-559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eberl L, Winson M, Sternberg C, Stewart G, Christiansen G, Chhabra S, Bycroft B, Williams P, Molin S, Givshov M. Involvement of N-acyl-L-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol Microbiol. 1996;20:127–136. doi: 10.1111/j.1365-2958.1996.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 26.Fuqua C, Winans S, Greenberg E. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 27.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furness R, Fraser G, Hay N, Hughes C. Negative feedback from a Proteus class II flagellum export defect to the flhDC master operon controlling cell division and flagellum assembly. J Bacteriol. 1997;179:5585–5588. doi: 10.1128/jb.179.17.5585-5588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gish W, States D. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 30.Gottesman S. Regulation of capsule synthesis: modification of the two-component paradigm by an accessory unstable regulator. In: Hoch J, Silhavy T, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 253–262. [Google Scholar]
- 31.Gygi D, Bailey M, Allison C, Hughes C. Requirement for FlhA in flagella assembly and swarm-cell differentiation by Proteus mirabilis. Mol Microbiol. 1995;15:761–769. doi: 10.1111/j.1365-2958.1995.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 32.Gygi D, Fraser G, Dufour A, Hughes C. A motile but non-swarming mutant of Proteus mirabilis lacks FlgN, a facilitator of flagella filament assembly. Mol Microbiol. 1997;25:597–604. doi: 10.1046/j.1365-2958.1997.5021862.x. [DOI] [PubMed] [Google Scholar]
- 33.Gygi D, Rahman M, Lai H-C, Carlson R, Guard-Petter J, Hughes C. A cell-surface polysaccharide that facilitates rapid population migration by differentiated swarm cells of Proteus mirabilis. Mol Microbiol. 1995;17:1167–1175. doi: 10.1111/j.1365-2958.1995.mmi_17061167.x. [DOI] [PubMed] [Google Scholar]
- 34.Han N, Whitlock J, Progulski-Fox A. The hemagglutinin gene A (hagA) of Porphyromonas gingivalis 381 contains four large, contiguous, direct repeats. Infect Immun. 1996;64:4000–4007. doi: 10.1128/iai.64.10.4000-4007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanzelka B L, Greenberg E P. Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J Bacteriol. 1995;177:815–817. doi: 10.1128/jb.177.3.815-817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoch J, Silhavy T. Two-component signal transduction. Washington, D.C: ASM, Press; 1995. [Google Scholar]
- 37.Hofmann K, Stoffel W. TMbase—a database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;347:166. [Google Scholar]
- 38.Kaiser D. Bacteria also vote. Science. 1996;272:1598. doi: 10.1126/science.272.5268.1598. [DOI] [PubMed] [Google Scholar]
- 39.McCarter L. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J Bacteriol. 1998;180:3166–3173. doi: 10.1128/jb.180.12.3166-3173.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mobley H. Virulence of Proteus mirabilis. In: Mobley H, Warren J, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: American Society for Microbiology; 1996. pp. 245–269. [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Shapiro J. The significances of bacterial colony patterns. Bioessays. 1995;17:597–607. doi: 10.1002/bies.950170706. [DOI] [PubMed] [Google Scholar]
- 44.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1984. [Google Scholar]
- 45.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1982;1:784–791. [Google Scholar]
- 46.Stout V, Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990;172:659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swanson R V, Alex L A, Simon M I. Histidine and aspartate phosphorylation: two-component systems and the limits of homology. Trends Biochem Sci. 1994;19:485–490. doi: 10.1016/0968-0004(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 48.Swift S, Throup J, Williams P, Salmond G, Stewart G. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 49.Triglia T, Peterson M, Kemp D. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 1988;16:8186. doi: 10.1093/nar/16.16.8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Utsumi R, Katayama S, Ikeda M, Igaki S, Nakagawa H, Miwa A, Taniguchi M, Noda M. Cloning and sequence analysis of the evgAS genes involved in signal transduction of Escherichia coli K-12. Nucleic Acids Symp Ser. 1992;27:149–150. [PubMed] [Google Scholar]
- 51.Utsumi R, Katayama S, Taniguchi M, Horie T, Ikeda M, Igaki S, Nakagawa H, Miwa A, Tanabe H, Noda M. Newly identified genes involved in the signal transduction of Escherichia coli K-12. Gene. 1994;140:73–77. doi: 10.1016/0378-1119(94)90733-1. [DOI] [PubMed] [Google Scholar]
- 52.Wassif C, Cheek D, Belas R. Molecular analysis of a metalloprotease from Proteus mirabilis. J Bacteriol. 1995;177:5790–5798. doi: 10.1128/jb.177.20.5790-5798.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams F D, Schwarzhoff R H. Nature of the swarming phenomenon in Proteus. Annu Rev Microbiol. 1978;32:101–122. doi: 10.1146/annurev.mi.32.100178.000533. [DOI] [PubMed] [Google Scholar]
- 54.Worley K, Wiese B, Smith R. BEAUTY: an enhanced BLAST-based search tool that integrates multiple biological information resources into sequence similarity search results. Genome Res. 1995;5:173–184. doi: 10.1101/gr.5.2.173. [DOI] [PubMed] [Google Scholar]
- 55.Zhao H, Li X, Johnson D E, Blomfield I, Mobley H L. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol Microbiol. 1997;23:1009–1019. doi: 10.1046/j.1365-2958.1997.2791645.x. [DOI] [PubMed] [Google Scholar]