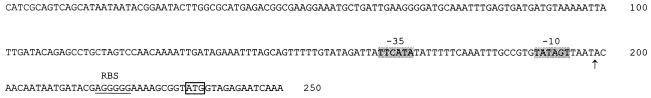Abstract
A gene (htrA) coding for a stress-inducible HtrA-like protein from Lactobacillus helveticus CNRZ32 was cloned, sequenced, and characterized. The deduced amino acid sequence of the gene exhibited 30% identity with the HtrA protein from Escherichia coli; the putative catalytic triad and a PDZ domain that characterize the HtrA family of known bacterial serine proteases were also found in the sequence. Expression of the L. helveticus htrA gene in a variety of stress conditions was analyzed at the transcriptional level. The strongest induction, resulting in over an eightfold increase in the htrA transcription level, was found in growing CNRZ32 cells exposed to 4% (wt/vol) NaCl. Enhanced htrA mRNA expression was also seen in CNRZ32 cells after exposure to puromycin, ethanol, or heat. The reporter gene gusA was integrated in the Lactobacillus chromosome downstream of the htrA promoter by a double-crossover event which also interrupted the wild-type gene. The expression of gusA in the stress conditions tested was similar to that of htrA itself. In addition, the presence of an intact htrA gene facilitated growth under heat stress but not under salt stress.
In their natural environments, bacteria spend most of their life in a starving or nongrowing state because of various growth-limiting conditions (14). To face starvation and other stresses, bacteria are able to rapidly and transiently express a characteristic set of proteins in order to survive and protect the cells from fatal damage (12, 20). Stress-inducible proteins can be divided into two main groups: specific stress proteins and general stress proteins (14).
The HtrA protein of Escherichia coli is located at the periplasmic side of the inner membrane (28) and is a member of the stress-inducible rpoE regulon, which responds to misfolded proteins in the extracellular compartment (23). In addition to the E. coli HtrA (DegP/Do) (15, 26, 30), in the last few years several other HtrA homologs have been identified in a variety of bacteria as well as some eukaryotes (21, 22, 34). With only a few exceptions, the same putative proteolytic active site can be found in all the HtrA homologs identified (21). The members of the growing HtrA family also commonly possess a PDZ domain (10, 21) and are characterized as trypsin-like serine proteases (16, 17). Degradation of abnormal proteins in the periplasm has been suggested to be the main physiological role of HtrA in E. coli, but regulatory functions have also been reported for this protein (21). In Bacillus subtilis, the deduced protein products of the genes yyxA 6 [accession no. P39668]) and ykdA (accession no. AJ002571) have recently been reported as HtrA-like proteins, but no HtrA homologs from lactobacilli have yet been described.
Lactobacillus helveticus is a lactic acid bacterium widely used as a starter in the manufacturing of Swiss-type cheeses and other fermented dairy products (11). During the manufacturing and ripening of these cheeses, the lactic acid bacteria starters are exposed to a variety of stresses, as the temperature is elevated in the cheese cooking process and the addition of NaCl increases osmolarity.
In this study, we describe the cloning, DNA sequence, and expression of a stress-regulated htrA-like gene of L. helveticus CNRZ32. The effects of different stresses on the htrA expression were analyzed at the transcriptional level.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
L. helveticus CNRZ32 and E. coli DH5αF′ (Gibco BRL) were propagated in MRS broth (Difco) and Luria broth (Difco), respectively. When plasmid pUC19 was present, ampicillin (50 μg/ml) was supplied to the growth medium. For plasmid pSA3, tetracycline (12.5 μg/ml), chloramphenicol (100 μg/ml), or erythromycin (4 μg/ml) was used. L. helveticus cells first grown to the exponential phase (optical density at 600 nm [OD600] of 0.8) at 37°C were studied in the following stress conditions: for heat shock, the growth temperature of the cells was shifted from 37 to 52°C; for salt stress, NaCl was added to a final concentration of 4% (wt/vol); for puromycin stress, 100 μg of puromycin per ml was supplied to the growth medium; for ethanol stress, cells were exposed to 5% (wt/vol) ethanol; and for oxidative stress, H2O2 was added to a final concentration of 0.005% (wt/vol).
Screening of an L. helveticus genome library.
An L. helveticus genomic library was established in λgt10 by using the λ DNA in vitro packaging and cDNA rapid cloning module (λgt10; Amersham) (31). The library was screened by DNA hybridization, using an internal 1.2-kb fragment of the L. delbrueckii subsp. bulgaricus CNRZ397 pepI gene as a probe (1). The probe was labeled with digoxigenin-dUTP (Boehringer Mannheim).
DNA isolation and cloning methods.
Plasmid DNAs of E. coli clones were isolated by alkaline lysis, using Wizard Minipreps (Promega) or FlexiPrep (Pharmacia) kits. Other standard DNA methods were performed as specified in reference 24. E. coli and L. helveticus strains were transformed by electroporation (3) using a Gene Pulser (Bio-Rad Laboratories).
DNA syntheses.
Oligonucleotides were synthesized with a model 392 Applied Biosystems DNA/RNA synthesizer and purified by ethanol precipitation. DNA was amplified by PCR as recommended by the manufacturer of Dynazyme DNA polymerase (Finnzymes).
DNA sequencing and sequence analysis.
DNA sequencing was performed on an A.L.F. DNA sequencer according to the manual for the AutoRead sequencing kit (Pharmacia). Both DNA strands were sequenced by using pUC19-specific primers and sequence-specific oligonucleotides for primer walking. DNA sequence data were assembled and analyzed with the PC/GENE set of programs (release 14.0; IntelliGenetics). The BLAST program was used for searching homologous protein sequences at the National Center for Biotechnology Information, and alignment studies were performed on the ExPASy server, using the SIM program. The comparison matrix used in the alignments was PAM400 (gap open penalty, 12; gap extension penalty, 4).
Construction of a gusA expression cassette.
A 0.9-kb SalI-BamHI fragment from the upstream region of htrA, including the promoter and the 12 first nucleotides downstream of ATG, was ligated to the second codon of a promoterless gusA reporter gene with flanking BamHI sites. A transcription terminator (a hairpin with a free energy of −24.9 kcal mol−1) from the slpA gene of L. brevis (33) was fused to the 3′ end of gusA at the BamHI site. A 0.85-kb HindIII-SalI fragment, carrying the 3′ end of htrA, was also ligated to the hairpin at the cleavage site for HindIII. The cassette was cloned into the shuttle vector pSA3 (7) at the SalI site, thus disrupting the gene coding for tetracycline resistance. The pSA3::gusA construct was transformed into E. coli DH5αF′ cells, and plasmid DNA was isolated by alkaline lysis (FlexiPrep; Pharmacia).
Integration of pSA3::gusA into the L. helveticus CNRZ32 chromosome.
Isolated plasmid pSA3::gusA DNA was introduced into L. helveticus CNRZ32 by electroporation (3) and cultured anaerobically for 72 h on MRS agar with 4 μg of erythromycin per ml (MRSE). Transformants were propagated for 30 generations in MRSE broth at 37°C, further cultured overnight at 45°C (1% inoculation), and plated on MRSE agar at 45°C.
Gene replacement at the chromosomal 5′ htrA locus.
For the integration and curing of plasmid pSA3 and a double-crossover event (4), transformed L. helveticus colonies were picked from the MRSE agar at 45°C and grown at 37°C in MRS broth for 78 generations. The cells were plated and incubated on MRS agar for 48 h. In the screening for erythromycin-sensitive colonies, randomly chosen colonies were transferred to both MRS and MRSE plates. One colony of three erythromycin-sensitive colonies found was shown by PCR to contain the expected integrant (data not shown). As a consequence of the second crossover event, the reporter gene gusA was fused next to the htrA promoter on the chromosome of L. helveticus CNRZ32, generating the new strain GRL56.
Transcription analyses.
Total RNA was isolated with RNeasy Midi kit according to instructions provided by Qiagen. For removing chromosomal DNA, the samples were treated with 45 U of DNase, phenol-chloroform extracted, and ethanol precipitated. RNA gel electrophoresis and Northern blotting were performed as described by Hames and Higgins (13). Total RNA isolated from wild-type L. helveticus CNRZ32 cells was hybridized with a 1.2-kb digoxigenin-labeled htrA-specific DNA probe, and total RNA isolated from GRL56 was hybridized with a 1.8-kb digoxigenin-labeled gusA-specific DNA probe. A DIG luminescent detection kit (Boehringer Mannheim) was used for hybrid detection. The level of transcripts was quantitated with a laser densitometer, and the specific induction ratios were calculated by dividing the signals from RNA of stressed cells by the signals from the control RNA. Primer extension was performed with total RNA in an A.L.F. DNA sequencer essentially as described earlier (19, 32), using the oligonucleotide 5′-TGGCACTCTTTTCTGAAACC-3′ with a fluorescein label as the primer.
Nucleotide sequence accession number.
The nucleotide sequence described in this paper has been deposited in the EMBL sequence data bank under accession no. AJ005672.
RESULTS
Cloning and sequencing of the L. helveticus htrA gene.
Screening of the λgt10-based genomic library of L. helveticus 53/7 by plaque hybridization, with an internal 1.2-kb fragment of the L. delbrueckii subsp. bulgaricus CNRZ397 pepI gene as the probe, gave the hybridization-positive clone gt10/cl30 of 6.5 kb (31). The clone was isolated and further subcloned, and the DNA was sequenced. A 1,239-bp open reading frame (ORF), encoding a gene product showing homology with heat shock proteins, was found in the same gene locus as the pepI operon in the sequence analysis of two of the subclones. Primers with flanking restriction sites for BamHI and HindIII were designed up- and downstream of the ORF, followed by PCR with isolated chromosomal DNA from L. helveticus CNRZ32 as the template. The CNRZ32 PCR product was cloned into plasmid pUC19 (Pharmacia Biotech) and sequenced. L. helveticus CNRZ32 was chosen for further characterization of the htrA gene, since chromosomal modifications are more difficult to perform with strain 53/7. A search of protein databases with the BLAST program and alignment studies with the SIM program revealed significant homology (29.3% identity) with the HtrA/DegP protein from E. coli (16, 30). Other proteins showing high similarity with the CNRZ32 protein were Sphtra from Streptococcus pneumoniae (42.3% identity), YkdA from B. subtilis (38.7% identity), and YyxA from B. subtilis (37.6% identity).
The L. helveticus ORF encodes a protein with a calculated molecular mass of 42,647 Da. A putative Shine-Dalgarno sequence AGGGGG (29) was identified 9 nucleotides upstream of the ATG. Regions with reasonable homology to consensus −35 and −10 regions of bacterial promoters (TTCATAN20TATAGT) were also identified upstream of the start codon (Fig. 1). An inverted repeat structure (ΔG of −18.8 kcal mol−1) was found 24 nucleotides downstream of the stop codon; this may be the transcriptional terminator of the gene.
FIG. 1.
Promoter region of the htrA gene from L. helveticus. The predicted −35 and −10 regions of the putative promoter are shadowed. RBS refers to the presumed ribosome binding site. The transcription start site of htrA, determined by primer extension, is marked with an arrow. The start codon is boxed.
The N-terminal amino acid sequence of the ORF revealed a strong hydrophobic region preceded by positively charged amino acids. However, a consensus sequence for the cleavage site of the signal peptidases could not be found downstream of the hydrophobic region.
The same active-site catalytic triad as previously noted in the HtrA/DegP family of bacterial serine proteases (2, 17, 21) was also present (Fig. 2). Furthermore, the substrate binding domain PDZ (10, 22) was found at the C-terminal end of the amino acid sequence (data not shown). Due to its homology to the HtrA protein family, the L. helveticus CNRZ32 gene product analyzed was designated the L. helveticus HtrA.
FIG. 2.
Alignment of regions spanning the putative active-site catalytic triad with presumed members of the HtrA/DegP family of serine proteases. The numbers represent sequence gaps, and the putative catalytic triad residues (H, His; D, Asp; S, Ser) are boxed. The most conserved regions are shadowed. Accession numbers (Ac.) are taken from the GenBank, EMBL and SwissProt databases.
Analysis of htrA transcription.
The size of the htrA transcript was determined by Northern blotting, using a 1.2-kb digoxigenin-labeled htrA-specific probe. Under heat shock conditions, the probe detected an 1.4-kb transcript, confirming that the gene coding for the HtrA like protein is a monocistronic transcriptional unit. Mapping of the 5′ end (data not shown) revealed that the transcription start site is located 32 nucleotides upstream of the ATG codon (Fig. 1).
Regulation of htrA gene expression.
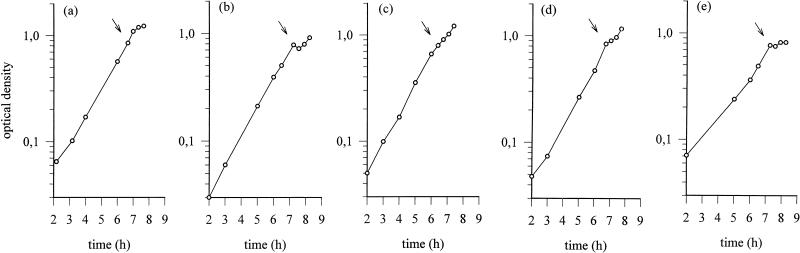
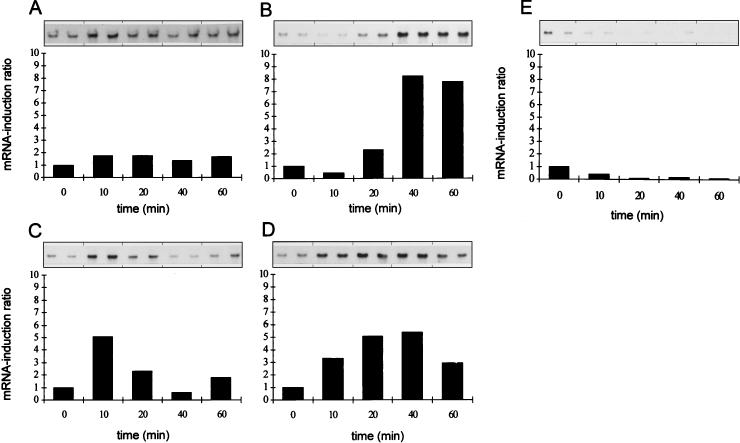
It is known that the HtrA protease from E. coli is a heat shock protein whose synthesis is induced both by various stress factors and by protein misfolding in general (9, 17, 21). To examine the possibility that the HtrA homolog from L. helveticus CNRZ32 could also be stress induced, we exposed exponentially growing L. helveticus CNRZ32 cells (OD600 of 0.8) to different stress conditions. The precise conditions chosen on the basis of their reduction of the bacterial growth rate (Fig. 3). Total RNAs isolated from CNRZ32 cells before and after stress were blotted on a nylon membrane and hybridized with a digoxigenin-labeled htrA-specific DNA probe (Fig. 4). The strongest induction, resulting in over an eightfold increase in the level of htrA transcription, was found in cells exposed to 4% (wt/vol) NaCl (Fig. 4B). Exposure of growing CNRZ32 cells to ethanol (5%, wt/vol) (Fig. 4C) or puromycin (100 μg/ml) (Fig. 4D) resulted in about a fivefold induction. The induction in cells exposed to a temperature upshift from 37 to 52°C was rapid but resulted in only a doubling of the amount of htrA mRNA (Fig. 4A). However, oxidative stress did not affect htrA transcription (Fig. 4E). As a control, total RNA isolated from heat-stressed wild-type L. helveticus CNRZ32 cells was hybridized with a digoxigenin-labeled ldh-specific DNA probe (25). Heat shock did not affect the level of ldh transcripts (data not shown).
FIG. 3.
Growth of L. helveticus CNRZ32 before and after stress. The cells were grown to an OD600 of 0.8 and exposed to heat shock (a) and to salt (b), ethanol (c), puromycin (d), and oxidative (e) stress. The time of initiation of the stress is indicated by an arrow.
FIG. 4.
Northern blotting analyses of the htrA transcripts under different stress conditions. Transcription was induced by heat shock (A), and by salt (B), ethanol (C), puromycin (D), and oxidative (E) stress. Samples (10 μg of total RNA per each lane from parallel cultures) were taken before (0) and 10 min, 20 min, 40 min, and 1 h after initiation of the stress. The mRNA induction ratio was calculated by dividing the signals from RNA of stressed cells by the signals from RNA of the control (0).
To elucidate the function and essentiality of the htrA gene, we replaced the 5′ end of htrA with the gusA reporter gene as described in Materials and Methods (4). The fusion of the gusA reporter gene was downstream of the stress-inducible htrA promoter and also disrupted the htrA gene in the resulting strain GRL56. When total RNA from GRL56 cells exposed to heat and salt stress as described in Materials and Methods were analyzed, the induction of gusA transcripts was found to be similar to the induction of htrA transcripts in the wild-type L. helveticus CNRZ32 cells (data not shown).
Bacterial growth analysis.
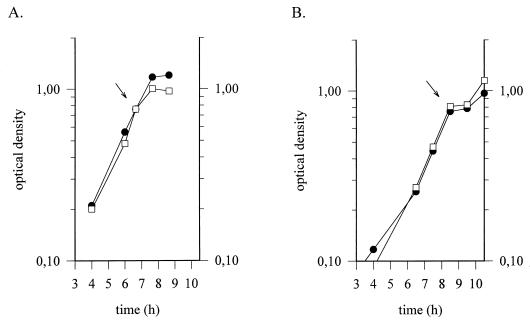
To examine the possibility that the HtrA protein from L. helveticus CNRZ32 is essential for growth in unfavorable environments, growth experiments with wild-type L. helveticus CNRZ32 cells and GRL56 cells with the interrupted htrA gene were performed (Fig. 5). The growth rate declined after heat shock (52°C), and the growth of both wild-type CNRZ32 cells and GRL56 cells ceased after 1 h of the provocation. However, the cell density of CNRZ32 was clearly higher within 1 h after the heat shock than that of GRL56 cells (Fig. 5A). This difference was not noticeable in cells exposed to temperature upshift from 37 to 48°C, although induction of htrA transcripts could be demonstrated (data not shown). After salt stress (4% NaCl), no distinctive difference in the growth profiles of the wild-type and mutant strains could be found (Fig. 5B).
FIG. 5.
Comparison of growth of wild-type and htrA mutant strains of L. helveticus under stress. Wild-type L. helveticus CNRZ32 (•) and mutant GRL56 (□) cells grown at 37°C were subjected to heat shock (A) and salt stress (B). The time of initiation of the stress is indicated by an arrow.
DISCUSSION
In this work, we have identified and characterized a gene, htrA, coding for a putative member of the HtrA/DegP family of serine proteases (2, 16, 21). The htrA gene is located at the pepI locus (31) on the chromosome of L. helveticus. The L. helveticus HtrA protein carries a putative catalytic domain containing a triad of His, Ser, and Asp residues (Fig. 2). The domain is characteristic for trypsin-like serine proteases (16, 21), and mutating two of the catalytic triad residues has been shown to lead to a loss of protease activity in E. coli (27). We also found a putative PDZ domain at the C terminus of HtrA which is probably involved in protein-protein interactions (9, 22). Pallen and Wren (21) suggested that the recognition of target protein is carried out by the PDZ domain and that the recognition of the sites for cleavage is carried out by the catalytic domain (21). HtrA homologs from gram-positive bacteria generally have one PDZ domain, whereas other HtrA homologs mostly have two (21). The deduced protein sequence of the htrA gene from L. helveticus revealed an apparent identity with those from other gram-positive bacteria, described as serine proteases and/or putative members of the HtrA/DegP family (21). Secondary structure predictions from L. helveticus HtrA indicate a strong preference for β structure, as has also been experimentally found for E. coli HtrA (28).
Expression of htrA was induced at the transcriptional level as a response to environmental changes. The specific mRNA induction was not directly correlated to the decline of the bacterial growth rate after the stress given but rather was characteristic of the stress condition chosen (Fig. 3 and 4). The amount of htrA transcripts quite unexpectedly only doubled as the response to the heat shock, possibly due to the slow temperature upshift rate in the growth medium used. However, the induction ratio varied between experiments, being occasionally much higher. Interestingly, the heat shock response in L. helveticus cells exposed to a less severe heat shock (temperature upshift from 37 to 48°C) exhibited an induction pattern quite distinct from that of the cells exposed to 52°C. At 48°C a twofold induction level was also obtained, but the amount of htrA transcripts rapidly declined between 10 and 20 min after heat shock and remained at a very low level thereafter (data not shown). This may suggest an involvement of a repressor in the regulation of the htrA gene. The lower temperature would allow the repressor to refold, thus regaining its binding ability to the putative operator sequences. However, no sequence homologous to the inverted repeat structure (CIRCE) typical of genes encoding heat shock proteins in gram-positive bacteria (5, 14) was found upstream of the htrA gene.
A clear gusA-specific mRNA induction was seen in GRL56 cells exposed to salt stress. The expression level and profile of gusA mRNA in GRL56 cells (which are mutant for htrA) were similar to those of htrA mRNA in the wild-type cells, indicating that the htrA structural gene in L. helveticus does not participate in the regulation of its own expression and can thus be successfully replaced. Furthermore, the htrA gene product from L. helveticus CNRZ32 appeared to facilitate growth at 52°C, whereas growth in salt stress conditions was not affected by the deletion of htrA (Fig. 5). HtrA from E. coli is thought to be essential for growth at elevated temperatures (15), but nonessential functions for the HtrA homologs of Helicobacter pylori, E. coli (HhoA and HhoB), and Campylobacter jejuni have also been reported (21). Preliminary Southern blotting analyses (data not shown) suggest that the htrA gene in L. helveticus is present in only one copy (data not shown).
L. helveticus GRL56 showed no β-glucuronidase activity as a response to the heat and salt stresses tested, possibly due to the instability of the enzyme under such conditions. β-Glucuronidase is thought to be rapidly degraded and/or inactivated under heat and salt stress (8).
On the basis of the amino acid sequence, we propose that HtrA from L. helveticus is located at the outer surface of the plasma membrane. Without further experimental evidence, it is difficult to predict whether the N-terminal region functions as a cleavable signal sequence (without an apparent cleavage site) or as a membrane anchor sequence. Interestingly, the closest homologs of HtrA, i.e., S. pneumoniae Sphtra and B. subtilis YyxA, appear to possess similar N-terminal sequences lacking an obvious leader peptide cleavage site. On the other hand, sequence analysis of YkdA from B. subtilis suggests an intracellular location. If the N-terminal sequences of these three proteins indeed function as membrane anchor domains, it represents a somewhat unusual way to locate proteins on the outer surface of the cytoplasmic membrane in gram-positive bacteria, which commonly use the lipoprotein type of attachment.
The regulation of HtrA in E. coli has recently been described as a complex network of signal transduction pathways (18). The alternative sigma factor RpoE, the anti-sigma factor RseA, the two-component regulatory system CpxRA, and two phosphoprotein phosphatases, PrpA and PrpB, are all components of the network (18). Homologs to these components exist also in several other gram-negative bacterial species, indicating similar regulatory systems (21). However, it is still unclear how expression of htrA-like genes from gram-positive bacteria is regulated and if there are conserved regulatory pathways at all. Indeed, SigB-like promoter sequences, typical for general stress-inducible genes from gram-positive bacteria (14), could not be detected upstream of the htrA gene in L. helveticus. So far, no alternative stress-inducible sigma factor has been reported for lactobacilli. The exact role of the HtrA protein and the regulation of its expression in L. helveticus remain to be elucidated.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Agriculture and Forestry of Finland.
We are grateful to Anneli Virta for the running of the A.L.F. sequencer and to Jaana Jalava and Jouni Nukkala for technical assistance. Ilkka Palva is acknowledged for helpful discussions.
REFERENCES
- 1.Atlan D, Gilbert C, Blanc B, Portalier R. Cloning, sequencing and characterization of the pepIP gene encoding a proline iminopeptidase from Lactobacillus delbrueckii subsp. bulgaricus CNRZ397. Microbiology. 1994;140:527–535. doi: 10.1099/00221287-140-3-527. [DOI] [PubMed] [Google Scholar]
- 2.Bass S, Gu Q, Christen A. Multicopy suppressors of prc mutant Escherichia coli include two HtrA (DegP) protease homologs (HhoAB), DskA, and a truncated RlpA. J Bacteriol. 1996;178:1154–1161. doi: 10.1128/jb.178.4.1154-1161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhowmik T, Steele J L. Development of an electroporation procedure for gene disruption in Lactobacillus helveticus CNRZ32. J Gen Microbiol. 1993;139:1433–1439. [Google Scholar]
- 4.Bhowmik T, Fernández L, Steele J L. Gene replacement in Lactobacillus helveticus. J Bacteriol. 1993;175:6341–6344. doi: 10.1128/jb.175.19.6341-6344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broadbent J R, Oberg C J, Wei L. Characterization of the Lactobacillus helveticus groESL operon. Res Microbiol. 1998;149:243–253. doi: 10.1016/s0923-2508(98)80300-8. [DOI] [PubMed] [Google Scholar]
- 6.Calogero S, Gardan R, Glaser P, Schweizer J, Rapoport G, Debarbouille M. RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J Bacteriol. 1994;176:1234–1241. doi: 10.1128/jb.176.5.1234-1241.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dao M L, Ferretti J J. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl Environ Microbiol. 1985;49:115–119. doi: 10.1128/aem.49.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deuerling E, Paeslack B, Schumann W. The ftsH gene of Bacillus subtilis is transiently induced after osmotic and temperature upshift. J Bacteriol. 1995;177:4105–4112. doi: 10.1128/jb.177.14.4105-4112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson J W, Gross C A. Identification of the ςE subunit of Escherichia coli RNA polymerase: a second alternate ς factor involved in high temperature gene expression. Genes Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- 10.Fanning A S, Anderson J M. Protein-protein interactions: PDZ domain networks. Curr Biol. 1996;6:1385–1388. doi: 10.1016/s0960-9822(96)00737-3. [DOI] [PubMed] [Google Scholar]
- 11.Gilliand S E, editor. Lactic acid bacteria as starter cultures for foods. Boca Raton, Fla: CRC Press Inc.; 1985. [Google Scholar]
- 12.Gottesman S. Bacterial regulation: global regulatory networks. Annu Rev Genet. 1984;18:415–451. doi: 10.1146/annurev.ge.18.120184.002215. [DOI] [PubMed] [Google Scholar]
- 13.Hames B, Higgins S. Nucleic acid hybridization: a practical approach. Oxford, England: IRL Press; 1985. [Google Scholar]
- 14.Hecker M, Schumann W, Völker U. Heat-shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 15.Lipinska B, Sharma S, Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988;16:10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipinska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989;171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipinska B, Zylics M, Georgopoulos C. The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol. 1990;172:1791–1797. doi: 10.1128/jb.172.4.1791-1797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Missiakas D, Raina S. Protein misfolding in the cell envelope of Escherichia coli: new signaling pathways. Trends Biochem Sci. 1997;22:59–63. doi: 10.1016/s0968-0004(96)10072-4. [DOI] [PubMed] [Google Scholar]
- 19.Myöhänen S, Wahlfors J. Automated fluorescent primer extension. BioTechniques. 1993;14:16–17. [PubMed] [Google Scholar]
- 20.Neidhardt F C, VanBogelen R A. Heat shock response. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 1334–1345. [Google Scholar]
- 21.Pallen M J, Wren B W. Micro review: the HtrA family of serine proteases. Mol Microbiol. 1997;26:209–221. doi: 10.1046/j.1365-2958.1997.5601928.x. [DOI] [PubMed] [Google Scholar]
- 22.Ponting C P. Evidence for PDZ domains in bacteria, yeast and plants. Protein Sci. 1997;6:464–468. doi: 10.1002/pro.5560060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the sigma E (sigma 24) heat shock sigma factor of Escherichia coli. EMBO J. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Savijoki K, Palva A. Molecular genetic characterization of the l-lactate dehydrogenase gene (ldhL) of Lactobacillus helveticus and biochemical characterization of the enzyme. Appl Environ Microbiol. 1997;63:2850–2856. doi: 10.1128/aem.63.7.2850-2856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seol J H, Woo S K, Jung E M, Yoo S J, Lee C S, Kim K, Tanaka K, Ichihara A, Ha D B, Chung C H. Protease Do is essential for survival of Escherichia coli at high temperatures: its identity with the htrA gene product. Biochem Biophys Res Commun. 1991;176:730–736. doi: 10.1016/s0006-291x(05)80245-1. [DOI] [PubMed] [Google Scholar]
- 27.Skórko-Glonec J, Wawrzynow A, Krzewski K, Kurpierz K, Lipinska B. Site-directed mutagenesis of the HtrA (DegP) serine protease, whose proteolytic activity is indispensable for Escherichia coli survival at elevated temperatures. Gene. 1995;163:47–52. doi: 10.1016/0378-1119(95)00406-v. [DOI] [PubMed] [Google Scholar]
- 28.Skórko-Glonec J, Lipinska B, Krzewski K, Zolese G, Bertoli E, Tanfani F. HtrA heat shock protease interacts with phospholipid membranes and undergoes conformational changes. J Biol Chem. 1997;272:8974–8982. doi: 10.1074/jbc.272.14.8974. [DOI] [PubMed] [Google Scholar]
- 29.Shine J, Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1974;254:34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- 30.Strauch K, Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci USA. 1988;85:1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varmanen P, Rantanen T, Palva A. An operon from Lactobacillus helveticus composed of a proline iminopeptidase gene (pepI) and two genes coding for putative members of the ABC transporter family of proteins. Microbiology. 1996;142:1–10. doi: 10.1099/13500872-142-12-3459. [DOI] [PubMed] [Google Scholar]
- 32.Vesanto E, Peltoniemi K, Purtsi T, Steele J, Palva A. Molecular characterization, over-expression and purification of a novel dipeptidase from Lactobacillus helveticus. Appl Microbiol Biotechnol. 1996;45:638–645. doi: 10.1007/s002530050741. [DOI] [PubMed] [Google Scholar]
- 33.Vidgrén G, Palva I, Pakkanen R, Lounatmaa K, Palva A. S-layer protein gene of Lactobacillus brevis: cloning by polymerase chain reaction and determination of the nucleotide sequence. J Bacteriol. 1992;174:7419–7427. doi: 10.1128/jb.174.22.7419-7427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zumbrunn J, Trueb B. Primary structure of a putative serine protease specific for IGF-binding proteins. FEBS Lett. 1996;398:187–192. doi: 10.1016/s0014-5793(96)01229-x. [DOI] [PubMed] [Google Scholar]