Abstract
Adults who experience an acquired brain injury often experience disorders of consciousness, physical difficulties, and maladaptive behaviours. Multimodal sensory therapy may benefit brain injured patients, however the extent this therapy can facilitate rehabilitation is not well understood. This systematic review aimed to synthesize multimodal sensory therapy research for adults affected by acquired brain injury. PRISMA guidelines were followed and searches for work published up until July 2021 were undertaken in 5 databases, finding 1054 articles. 43 articles were included in the study. Results describe 29 studies related to coma following an acquired brain injury and 14 to no coma studies (mostly stroke). Multimodal sensory therapy was mostly used as a coma arousal technique following traumatic brain injury, finding positive effects. Multimodal sensory therapy was less applied in stroke, no coma rehabilitation, where most studies found improvement in somatosensory sensation and motor control in an affected limb. In several no coma studies, effects were maintained after several months. The most common senses stimulated in coma studies were audio (N = 30), tactile (N = 28), visual (N = 26), olfactory (N = 22), and gustatory (N = 17), while the most common senses stimulated in stroke, no coma studies were proprioception (N = 7), tactile (N = 8), and stereognosis (N = 4). Multimodal sensory therapy can be beneficial for patients, especially those in a minimally conscious state or attempting physical rehabilitation following stroke. Negative findings are infrequent in the current literature base. Multimodal sensory therapy appears to be a low-risk intervention with positive outcomes.
Keywords: Sensory environment, Stroke, Brain injury, Sensory stimulation, Systematic review, Coma
Introduction
Following an acquired brain injury (ABI), as a result of motor vehicle accident, fall, assault or a cerebrovascular event, adults can initially experience disorders of consciousness (i.e., coma), physical difficulties in movement and kinaesthesia, and subsequent maladaptive behaviours such as agitation, aggression or apathy (Deiva et al., 2017). These outcomes are difficult to manage and costly to treat, and may involve a combined approach of psychological, physical or chemical restraint in the most extreme cases (Frasca et al., 2013). In other neuropsychiatric populations a growing body of research indicates that environmental enrichment interventions can promote neuroplasticity and positively impact on disorders of consciousness and subsequent behavioural, cognitive, and social functioning of individuals, such as increased attentional focus (Nithianantharajah & Hannan, 2006; Simpson & Kelly, 2011), increased activity in rehabilitation units (Janssen et al., 2014), and reduced agitation (Fava & Strauss, 2010; Frasca et al., 2013; Kaplan et al., 2006, 2007; McKee et al., 2007). Despite the potential impact of this approach on the rehabilitation of people with ABI, the nature and extent of environmental enrichment therapy and how it could best facilitate positive behavioural adaptation and general rehabilitation is not yet well understood (Li et al., 2020; Pinto et al., 2020).
Environmental Enrichment and Clinical Application
There is increasing focus on how manipulation of the external environment can influence rehabilitation and recovery following serious injury or illness. Environmental enrichment is the practice of providing external enhancements to a setting that are both complex and novel, thereby increasing environmental engagement and stimulation on behalf of the user (McDonald et al., 2018; Nithianantharajah & Hannan, 2006). The experimental approach, often using rodents, is to encourage exploration, and physical and social activity by enhancing the size of the living space and increasing the quantity of novel objects of various shapes (Benaroya-Milshtein et al., 2004; Zebunke et al., 2013). Benefits of environmental enrichment on sensorimotor and cognitive outcomes are wide ranging (Nithianantharajah & Hannan, 2006; Simpson & Kelly, 2011) and animal studies have reported cerebral changes at a morphological and molecular level (Alwis & Rajan, 2014; Mesa-Gresa et al., 2013; Rosenzweig et al., 1978; Sozda et al., 2010).
At a cellular level, environmental enrichment affects neuronal functioning through a range of interactions, leading to positive changes in sensorimotor and cognitive behaviour, making environmental enrichment an ideal treatment approach for ABI such as traumatic brain injury (TBI) (Alwis & Rajan, 2014). TBI fundamentally alters neuronal functioning in the sensory cortices (Ding et al., 2011; Hall & Lifshitz, 2010) and approximately 60% of patients display sensory deficits (Carey, 1995), which Alwis and Rajan (2014) argue contributes to persistent cognitive deficits typically found in patients. Thus, one obvious area for environmental intervention in TBI relates to enrichment of the sensory cortex – this can be achieved through targeted sensory stimulation therapy, aided by environment design input (Gardner et al., 2000). Outcomes from environmental enrichment in rats have been improved by the addition of sensory stimulation (Maegele et al., 2005). For example, in animal studies, the most common sensory stimulation in environmental enrichment is through auditory stimuli (Alwis & Rajan, 2014) and findings include enhanced synaptic transmission in the auditory cortex (Percaccio et al., 2005, 2007).
Sensory Stimulation
Humans engage in at least five sensory experiences, namely touch, taste, smell, sight, and hearing, though there are other sensory modalities that do not receive as much attention (Gardner et al., 2000; Stillman, 2002). Sensory stimulation may occur through environmental design such as is done in environmental enrichment. However, it may also involve direct stimulation of any sensory modality (Karma & Rawat, 2006). Sensory stimulation can be unimodal or multimodal, however, contemporary neuroscience research suggests that sensory modalities more effectively operate in concert with each other (i.e., multimodal) as part of a ‘whole of brain’ response, as opposed to in a unimodal process (e.g., Baier et al., 2006). Indeed, it would seem multimodal approaches to sensory stimulation are more effective than unimodal (Pinto et al., 2020; Zuo et al., 2021).
Multimodal Sensory Stimulation
Studying sensorimotor recovery following an ABI as a unimodal construct is not justified as the idea of ‘modality-specific’ cortices is no longer prevalent. As Shimojo and Shams (2001) stated, “interaction between modalities is the rule as opposed to the exception in brain function” (pg. 508); the brains cross-modal cortical processing plays a substantial role in day-to-day adaptive behaviour (Shimojo & Shams, 2001). A variety of evidence supports the notion of multi-modularity (Shimojo & Shams, 2001). For example, following an ABI, improved sensorimotor functioning appears to reflect behavioural compensation from unimpaired alternate modalities rather than functional recovery from impaired brain regions (Jadavji et al., 2006; Rose et al., 1993). Furthermore, visual dependence displayed by stroke patients does not mean other sensory modalities are neglected as stroke patients also rely on visual, proprioceptive and vestibular information for posture control (Bonan et al., 2016). Indeed, environmental enrichment is limited when tasks involved are unimodal (Rose, 1988) and Zuo et al. (2021) found that family centred sensory stimulation was more effective when multimodal sensory approaches are taken. Generally, multimodal sensory approaches are economical, simple, stimulate a number of senses (Park, 2016), and are commonly delivered by nurses or therapists (Zuo et al., 2021). For example, Megha et al. (2013) describe a multimodal sensory approach that included speaking to the patient and reading (auditory), displaying photographs (visual), presenting favourite aromas (olfactory), and applying different materials to the patient’s arm (touch).
Yet, despite the reasoning for the multimodal sensory stimulation approach, the research literature relating to multimodal sensory stimulation and its relationship to human ABI rehabilitation and environmental design is limited. Previous systematic and scoping reviews shed some light on the evidence. However, these reviews used limited search terms (Li et al., 2020; Padua et al., 2019), limited databases (Cameron et al., 2020; Li et al., 2020; Padua et al., 2019), included unimodal sense therapies (Li et al., 2020; Padua et al., 2019) or found them prevalent in their search results (Pinto et al., 2020), focused on the delivery by family members (Zuo et al., 2021), did not divide outcomes into senses stimulated (Cameron et al., 2020), or focused on methodological characteristics (Pinto et al., 2020).
This systematic review aims to synthesize research evidence relating to multimodal sensory interventions for adults affected by ABI. This review builds on previous reviews by excluding unimodal studies, expanding the search strategy, and extracting data based on injury, actual senses stimulated, and outcomes reported. The specific research question was (a) What is the influence of multimodal sensory therapy on cognitive, physical or behavioural functioning on adults affected by ABI?
Method
Search Strategy and Selection Criteria
In accordance with PRISMA guidelines (Moher et al., 2009), a systematic review was undertaken of the published research literature relating to multimodal sensory interventions for people with ABI up to and inclusive of July 2021. The databases CINAHL, PubMed, ProQuest, PsychInfo, and Web of Science were used. Search strategy included terms around sensory, brain injury, and therapy; these can be found in Appendix 1. Inclusion criteria were adult populations (aged over 18 years) receiving multimodal sensory stimulation in a rehabilitation or treatment context. Peer-reviewed and English language studies with any publication date were included. Exclusion criteria were participants with co-diagnosis of autism or intellectual disability and dementia populations. The review was registered with the Centre for Reviews and Dissemination (UK) in October 2016 and PROSPERO International prospective register of systematic reviews in 2016 (Zeeman et al., 2016).
Data Extraction and Quality Assessment
Initial title and abstract checks were completed by a single reviewer. Two researchers then reviewed the full-text showing overall agreement of 92%. Cohen’s K suggested substantial agreement between the two researchers, ĸ = .643 (95% CI, 430–.856). Discrepancies around final inclusion were resolved on agreement from all authors. The following data was extracted from the selected studies: author, year of publication, country, study design, sample, treatment conditions, sensory modalities tested, intervention description, outcome measures, and main results. Quality of studies and risk of bias were assessed using the McMaster quantitative rating scale (Law et al., 1998). The rating form is comprised of 8 overarching criteria containing descriptive and yes–no questions for respondents to answer. 15 yes–no response questions were rated in the present review where a “yes” response was designated with a 1 and a “no” or “not addressed-unclear” response with a 0. McMaster’s quantitative rating scale divides bias into 3 main areas, (a) sample biases, (b) measurement biases, and (c) intervention biases and this is recorded qualitatively. Two researchers independently rated studies. For moderation, a sub-sample of studies were cross-checked and any areas of uncertainty where rectified.
To evaluate the quality of included studies further, studies will also be categorised into international guidelines on evidence level (I, II, III, IV, V).
Results
Screening and Study Selection
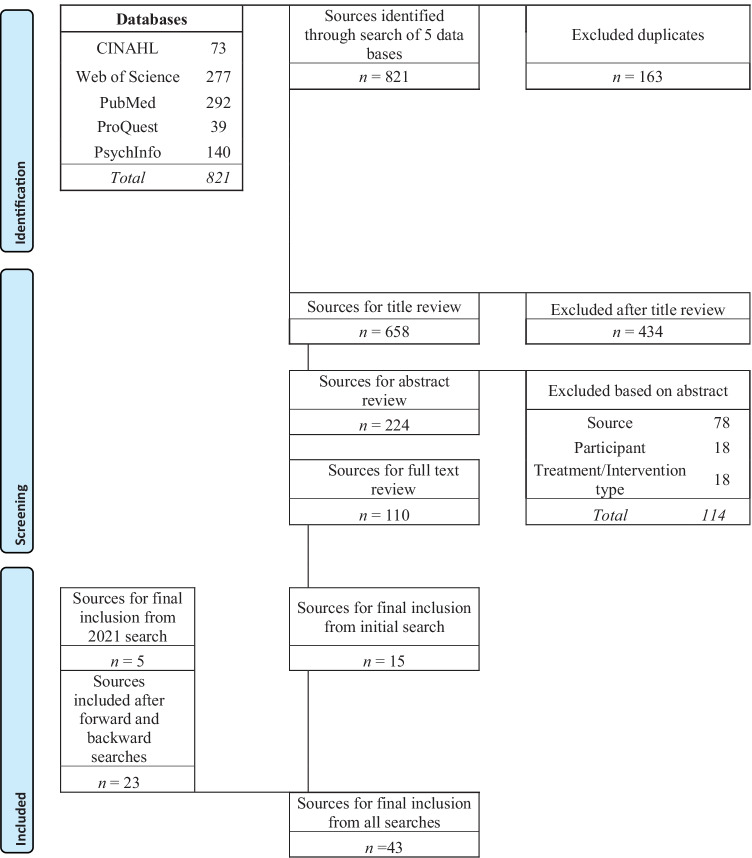
A total of 821 articles were found in June 2017 with 15 included and 23 included through forward and backward searching. A second search was carried out in July 2021 finding 233 additional papers with 5 finally included. The number of articles at final inclusion totalled 43. Papers were screened in the following steps, (a) duplicate search, (b) title review, (c) abstract review, (d) full text review and, (e) backward and forward searches. See Fig. 1 for detailed flow chart.
Fig. 1.
Screening and study selection process
Level of Evidence
Study selection included 11 randomized-control trials, 5 quasi-experimental prospectively controlled study, 5 pre-post test or retrospective control group studies, 1 case controlled study, 3 case studies, and 18 observational studies without a control group.
The number and types of studies at each level of evidence can be seen in Table 1 below.
Table 1.
Number and types of studies at each evidence level
| Levels of Evidence - Effectiveness | Number of Sources | |
|---|---|---|
| Level 1 – Experimental Designs | Level 1.a – Systematic review of Randomized Controlled Trials (RCTs) | |
| Level 1.b – Systematic review of RCTs and other study designs | ||
| Level 1.c – RCT | 11 | |
| Level 1.d – Pseudo-RCTs | ||
| Level 2 – Quasi-experimental Designs | Level 2.a – Systematic review of quasi-experimental studies | |
| Level 2.b – Systematic review of quasi-experimental and other lower study designs | ||
| Level 2.c – Quasi-experimental prospectively controlled study | 5 | |
| Level 2.d – Pre-test – post-test or historic/retrospective control group study | 5 | |
| Level 3 – Observational – Analytic Designs | Level 3.a – Systematic review of comparable cohort studies | |
| Level 3.b – Systematic review of comparable cohort and other lower study designs | ||
| Level 3.c – Cohort study with control group | ||
| Level 3.d – Case – controlled study | 1 | |
| Level 3.e – Observational study without a control group | 19* | |
| Level 4 – Observational – Descriptive Studies | Level 4.a – Systematic review of descriptive studies | |
| Level 4.b – Cross-sectional study | ||
| Level 4.c – Case series | ||
| Level 4.d – Case study | 3 | |
| Level 5 – Expert Opinion and Bench Research | Level 5.a – Systematic review of expert opinion | |
| Level 5.b – Expert consensus | ||
| Level 5.c – Bench research/ single expert opinion | ||
| Total | 44 (43 total sources) |
*One source reported a study and a pilot study in the same publication, both have been rated on the JBI
Methodological Quality
The methodological quality assessment of each article is provided in Table 2. The range of quality appraisal scores was 7–15 (out of 15) and the average was 12.27.
Table 2.
Quantitative studies review form
| Study | Purpose clearly stated | Relevant literature reviewed | Sample described in detail | Sample size justified | Informed consent obtained | Reliable outcome measure | Valid outcome measure | Intervention described in detail | Contamination avoided | Cointervention avoided | Statistical significance reported | Appropriate analysis method | Clinical importance reported? | Drop-outs reported | Conclusion appropriate | Total /15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 11 |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| 4 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| 5 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 9 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| 7 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 13 |
| 8 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 10 |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| 10 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 7 |
| 11 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 13 |
| 12 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 8 |
| 13 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| 14 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 12 |
| 15 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 13 |
| 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 14 |
| 17 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| 18 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 12 |
| 19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 12 |
| 20 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 13 |
| 21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| 22 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 7 |
| 23 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 10 |
| 24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 14 |
| 25 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 13 |
| 26 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 14 |
| 27 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| 28 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 10 |
| 29 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 14 |
| 30 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
| 31 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| 32 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 11 |
| 33 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 10 |
| 34 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 14 |
| 35 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 12 |
| 36 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 11 |
| 37 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 |
| 38 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
| 39 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 10 |
| 40 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 10 |
| 41 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 10 |
| 42 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 10 |
| 43 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 |
For each criterion: 1 = criterion met; 0 = criterion not met or unclear if met
1. Abbasi et al. (2009); 2. Attwell et al. (2019); 3. Bonan et al. (2016); 4. Byl et al. (2008); 5. Canedo et al. (2002); 6. Carey et al. (1993); 7. Cheng et al. (2018); 8. Deiva et al. (2017); 9. de Diego et al. (2013); 10. de Jersey (1979); 11. Di Stefano et al. (2012); 12. Doman et al. (1993); 13. Gomez et al. (2016); 14. Hall et al. (1992); 15. Heine et al. (2017); 16. Helliwell (2009); 17. Dogru Huzmeli et al. (2017); 18. Johnson et al. (1993); 19. Kaewsriwong et al. (2015); 20. Kater (1989); 21. Keller et al. (2007); 22. Grüner and Terhaag (2000); 23. Lynch et al. (2007); 24. Mandeep (2012); 25. Megha et al. (2013); 26. Mitchell et al. (1990); 27. Moattari et al. (2016); 28. Noda et al. (2004); 29. Oh and Seo (2003); 30. Pierce et al. (1990); 31. Poza et al. (2013); 32. Rader et al. (1989); 33. Sargolzaei et al. (2017); 34. Sedghi and Ghaljeh (2020); 35. Smania et al. (2003); 36. Talbot and Whitaker (1994); 37. Urbenjaphol et al. (2009); 38. Wijnen et al. (2006); 39. Wilson et al. (1991); 40. Wilson et al. (1993); 41. Wilson et al. (1996); 42. Wood et al. (1993); 43. Yekutiel and Guttman (1993)
Risk of Bias
Identified and potential risks of bias in the main areas of sampling, measurement and treatment for each study can be found in Appendix 2. Biases were scored on which direction they would skew the results (i.e., would they favour treatment/experimental hypothesis or control/null hypothesis). Some common risks of bias were unavoidable and existed across almost all studies. This included sampling biases, where recruitment was largely on a volunteer basis and with family involvement; measurement biases, which centred around raters being non-blinded or the rater was unknown (for studies in a hospital blinding measurement would require more resources); and treatment biases, regarding co-intervention, due to the severe medical needs of patients, and a lack of consistency in who the treating therapist was, and what the treatment time was throughout the day. An attention bias, where people are aware of the study so perform better or give favourable responses, was likely present in many studies. However, this was also largely unavoidable given the presence of families and their involvement in many studies as delivering the intervention or rating the outcome. Finally, case studies were not usually clear on how they recruited or if they excluded patients, which may have created a reporting bias. Altogether, this resulted in a small amount of bias favouring the intervention or experimental hypothesis in a large majority of coma studies (19/28; 76%) and just over half no coma studies (7/15; 54%). Bias was found to be negligible in 6/28 (21%) coma studies and 7/15 (54%) no coma studies with no studies biasing the control group or null hypothesis.
Almost all studies had at least one bias where there was not enough information to judge the direction of bias (e.g., it was often unknown who collected data, if they were blinded, and if the same person delivered the treatment across the study.) but in only 4/43 studies was it not possible to give an overall judgement of direction.
Study Characteristics
11 studies were from Europe, 7 were from Asia, 6 were from the UK, 6 were from the U.S.A, 4 were from Australia, 2 were from Canada, 5 were from the Middle East, 1 was from Turkey and 1 was cross-cultural (China and Italy).
The studies were heterogenous in terms of the assessment criteria and outcome measures, although there was commonality in coma studies that primarily measured improvement on the Glasgow Coma Scale (GCS), and to a lesser extent the Rancho Los Amigos Level of Cognitive Function Scale (RLA) and the Western Neuro Sensory Stimulation Profile (WNSSP). Other measures included the Functional Impairment Measure (FIM), Mini-Mental State Exam (MMSE), Semmes–Weinstein, Wessex Head Injury Matrix (WHIM), Glasgow Outcome Scale (GOS), posture, Fugl Meyer Assessment (FMA), Rivermead Assessment of Somatosensory Performance (RASP), Coma Recovery Scale (CRS) and Coma Recovery Scaled-Revised (CRS-R), Richmond Agitation and Sedation Scale (RASS), Sensory Modality Assessment and Rehabilitation Technique (SMART), texture discrimination, sensory assessments and various neurological or behavioural measures such as eye opening or tracking, electroencephalogram (EEG), fMRI, or heart rate. Table 3 presents the main characteristics and results of articles.
Table 3.
Main Characteristics and Results of Included Studies
| First Author (year), Country | Study Design | Outcome Measurement | Positive (+) and/or Negative (-) Findings | Statistically Significant Results Reported | Sensory Modalities Tested | Intervention/Test duration |
|---|---|---|---|---|---|---|
| Coma Studies (n = 29) | ||||||
| Abbasi et al. (2009), Iran | RCT | GCS | + | Yes | A,T,Affect | Daily, 15 min for 6 days |
| Attwell et al. (2019), Switzerland | Prospective Cross over | CRS-R | + | Yes | A, V, T, Ph | 2 × 25 min session Indoor & Outdoor - randomized |
| Canedo et al. (2002), U.S.A | Case study | GCS, RLA | + | No | A,V,G,T | < 3 months |
| Cheng et al. (2018), China/Italy | Single-case; ABAB Withdrawal | CRS-R | -/+ | Yes | A, T, O, V, G | 6 × 20 min over 4 weeks, A Phase = Rehabilitation |
| Deiva et al. (2017), India | Pre-Post | CRS-R, GCS | ± | Yes | A, V, O, T | 2 weeks |
| Di Stefano et al. (2012), Italy | Pre-Post | WHIM | + | Yes | A,V,O,T | Daily, 5 weeks |
| Doman et al. (1993), U.S.A | Pre-Post | GOS | + | No | A,V,O,G,T | Hourly, 6 days/week > 4 months |
| Hall et al. (1992), Canada | Pre-Post | WNSSP, GCS, RLA, Rader Scale | + | No | A,V,O,G,T | Once daily, 30 min, weekdays for two weeks. 2 or 3 rotations per participant |
| Johnson et al. (1993), UK | RCT | GCS, GSR, HR | -/+ | Yes | A,V,O,G,T | 1 h daily |
| Kaewsriwong et al. (2015), Thailand | Case study | GCS, RLA, SMART | + | No | A,V,O,G, T,K | Daily, < 6 months |
| Kater (1989), U.S.A | Pre-Post | GCS, EII, Cog Assess | + | Yes | A,V,O,G,K,C | 2 × day 45 min for 6 days over 1–3 months |
| Keller et al. (2007), Germany | Pre-Post | EEG | + | No | A,T | Insufficient detail |
| Grüner and Terhaag (2000), Germany | Pre-Post | GOS, Neurological condition | + | No | A,V,O,G,T,P | 2, 1 h sessions daily for around 9 days. 10 min rest 10 min stimulation pattern |
| Megha et al. (2013), India | RCT | GCS, WNSSP, eye tracking, arousal | + | Yes | A,V,O, G, T, | Group A: 5 × day 20 min for 5 weekdays over 2 weeks; Group B: 2 × day 50 min for 5 weekdays over 2 weeks |
| Mandeep (2012), India | RCT | GCS, CRS | + | Yes | A,V,T,K | Each sense stimulated twice a day (approx. 30 s each) for 2 weeks |
| Mitchell et al. (1990), UK | Pre-Post | GCS | + | No | A,V,O, G, T, | 2 × day over 4 weeks |
| Moattari et al. (2016), Iran | RCT | GCS, RLA, WNSSP | + | Yes | A,V,O,T | 2 × day over 7 days |
| Noda et al. (2004), Japan | Pre-Post | PVS | + | Yes | A,T,K | 5 min × 3 over 30 min |
| Oh and Seo (2003), Republic of Korea | Observational | GCS | + | No | A,V,O,G,T,Ph | 2 × day over 7 days, 4 weeks over 4 months |
| Pierce et al. (1990), Australia | Pre-Post | GCS | - | No | A,V,T | 8 h daily, until patient accepted for conventional therapy |
| Rader et al. (1989), U.S.A | Observational | Eye opening | + | No | A,V,O,G, T, | 30-45 min |
| Sargolzaei et al. (2017), Iran | RCT | SMART | -/+ | Yes | A,V, O, G, T, Ph | 14 weeks x 90 min daily |
| Talbot and Whitaker, (1994), Canada | Pre-Post | GCS, DRS, LCFS, CNCS, Freeman Questionnaire | + | No | A,V,O,T,P | Insufficient detail; 4 intervention phases, participants received at least 2; Some indication intervention lasted for 24 months |
| Urbenjaphol et al. (2009), Thailand | RCT | GCS, SMART | ± | Yes | A,V,O,G,T | 30 min every 2 h daily over 2 weeks |
| Wijnen et al. (2006), The Netherlands | Pre-Post |
WNSSP, DRS, HR GOS-E, |
+ | Yes | A,V,O,T | 15 min |
| Wilson et al. (1991), UK | Pre-Post | Eye opening, Body movement | ± | Yes | A,V,O,G,T | 10 min daily |
| Wilson et al. (1993), UK | Observational | Eye opening, Body movement | ± | Yes | A,V,O,G,T | Daily over 15 days, < > 3 months |
| Wilson et al. (1996), UK | Cohort | Eye opening, Body movement, Activity Engagement, Vocalisation | + | Yes | A,V,O,G,T | 3-week blocks, 2 × daily; 1–8 sets of treatment blocks needed |
| Wood et al. (1993), U.S.A | Pre-Post | GCS, SRH, RLA | + | Yes | A,V,O,T | 5 s stimulus, 10 s break; 5 trials per modality. Unclear how long each session took |
| No Coma Studies (n = 14) | ||||||
| Gomez et al. (2016), Spain | Pre-Post | EEG | ± | Yes | A,V | 18 min |
| de Diego et al. (2013), Spain | RCT | FMA, SIS-16, MAL, ST | + | Yes | P, T | 16 one hr sessions over 8 weeks |
| Poza et al. (2013), Spain | Pre-Post | EEG | + | Yes | V,A | 18 min |
| Smania et al. (2003), Italy | Observational | Task performance | + | Yes | V,T,W,P,S | Insufficient detail – 30 training sessions completed |
| Carey et al. (1993), Australia | Pre-Post | Texture discrimination | + | No | T, P | 10 sessions, 15–30 min |
| Yekutiel and Guttman (1993), Israel | Pre-Post | Sensory training | + | Yes | T, P, S | 45 min over 6 weeks |
| de Jersey (1979), Australia | Pre-Post | Sensory Assessments | -/+ | No | T, Temp, Pressure | 1–2 min |
| Heine et al. (2017), France | Pre-Post | CRS-R | ± | Yes | A,O,N | 4 sessions 20 min over 4 weeks |
| Bonan et al. (2016), France | Pre-Post | Posture: Optokinetic and galvanic vestibular stimulation | + | Yes | V,P,K | 15 s rest, 35 s right side stimulation, 3 min rest, 35 s left side stimulation |
| Byl et al. (2008), U.S.A | RCT | FIM, FMC, Strength | ± | Yes | K, S, G | 12–72 h up to 6 weeks |
| Lynch et al. (2007), Australia | RCT | Semmes–Weinstein | -/+ | Yes | T, Temp | 10 × 30 min over 2 weeks |
| Helliwell (2009), UK | Case study | RASP, FIM | - | No | T, P, S | Weekly over 3 weeks |
| Dogru Huzmeli et al. (2017), Turkey | RCT | MMSE, TIS | - | Yes | T, P | 10 × 45 min over 2 weeks |
| Sedghi and Ghaljeh (2020), Iran | Pre-Post | RASS | -/+ | Yes | A, T | 7 × 10 min over 1 week, stimulation measured pre stimulation and 30 min post |
Sample Characteristics
The sample sizes ranged from 1 to 233 participants. 28 studies related to coma following ABI (mostly TBI) and 1 to disorder of consciousness following stroke; these have been grouped together and labelled ‘coma studies.’ An additional 3 studies related to TBI no coma and 10 to stroke no coma; these have been grouped together and labelled ‘no coma’ studies. There were no studies identified relating to MSST for adults with multiple sclerosis, cerebral palsy, or spinal cord injury. There were no studies identified relating to use of MSST in people who were in post-traumatic amnesia (PTA) or post-PTA and medically stable following acquired or traumatic brain injury. For more details on sample characteristics see Table 4.
Table 4.
Participant Characteristics in Included Studies
| Coma studies | N | Patient Population | Sex (% male) | Mean Age | Time since injury | Ethnicity | Affected side (% right) |
|---|---|---|---|---|---|---|---|
| Abbasi et al. (2009) | 50 (25 in intervention) | TBI | 88% | 30.4 | Newly admitted | Iranian | N/A |
| Attwell et al. (2019) | 15 | TBI | 73% | 52.6 | 25.2 days | Swiss | N/A |
| Canedo et al. (2002) | 2 | TBI (mixed) | 50% | 24 & 45 | 3 months | Caucasian & African American | Bilateral |
| Cheng et al. (2018) | 29 | TBI (mixed) | 65% | 48 | less than a year to 10 years | Chinese or Italian | N/A |
| Deiva et al. (2017) | 30 (15 intervention) | TBI | 77% | N/A | 72 h | Southern Indian | N/A |
| Di Stefano et al. (2012) | 11 | TBI/Stroke/Anoxic BI | N/A | 30.75 | 5.75 months | Italian - Unknown | N/A |
| Doman et al. (1993) | 233 | TBI | 67% | N/A | 6 months average (1–12 months) | N/A | N/A |
| Hall et al. (1992) | 6 | TBI | 83% | 37.5 | 15.8 days | N/A | 17% |
| Johnson et al. (1993) | 14 (7 intervention) | TBI | 100% | 27.7 | < 48 h | N/A | N/A |
| Kaewsriwong et al. (2015) | 2 | TBI | 50% | 27 and 19 | N/A | N/A | 0 |
| Kater (1989) | 30 (15 intervention) | TBI | 60% | 28 | < 2 weeks | N/A | N/A |
| Keller et al. (2007) | 18 | TBI/Hypoxia | 61% | 39.3 | 18 months | Germany - unknown | N/A |
| Grüner and Terhaag (2000) | 89 or 16 | BI | N/A | 43.6 | < 48 h | Germany - unknown | N/A |
| Megha et al. (2013) | 30 | TBI | N/A | 39.3 | 7.7 days | Indian | N/A |
| Mandeep (2012) | 30 (15 in intervention) | TBI | N/A | N/A | N/A | Indian | N/A |
| Mitchell et al. (1990) | 24 (12 intervention) | TBI | 83% | 22.3 | 7.9 days | UK | 25% |
| Moattari et al. (2016) | 60 (20 in each of two intervention groups) | TBI | 82% | 37 | 4 days | Iranian | N/A |
| Noda et al. (2004) | 26 | TBI/Stroke | 66% | 38.5 | N/A | Japan | |
| Oh et al. (2003) | 5 | TBI | 100% | 50.2 | < 3 months | South Korean | N/A |
| Pierce et al. (1990) | 31 | TBI | 67% | 24 | N/A | N/A | N/A |
| Rader et al. (1989) | 6 | TBI | 66% | 3 patients 18–19 yrs + 3 40 -55 | 15.5 months | N/A | 0 |
| Sargolzaei et al. (2017) | 80 | Stroke | 45% | 66.2 | N/A | Iranian | 37.50% |
| Talbot and Whitaker (1994) | 8 | BI | 71% | N/A | N/A | N/A | N/A |
| Urbenjaphol et al. (2009) | 40 (20 intervention) | TBI | 70% | 33.4 | 6.8 days | Thai | 15% |
| Wijnen et al. (2006) | 16 | TBI | 61.10% | 21.5 | 2.3 months | Dutch - Unknown | N/A |
| Wilson et al. (1991) | 3 | TBI | 100% | 15, 36, &41 | UK - Unknown | ||
| Wilson et al. (1993) | 7 | TBI | 7 single case studies - all appear to be male | UK - Unknown | |||
| Wilson et al. (1996) | 24 | TBI/Hypoxia/Other | N/A | 28.75 | 16.2 months | UK - Unknown | N/A |
| Wood et al. (1993) | 15 | TBI/SAH | 40% | 34.6 | N/A | N/A | N/A |
| No Coma Studies | |||||||
| Gomez et al. (2016) | 36 | TBI/CP | 72% | 41 | > 8 years | N/A | 19% |
| de Diego et al. (2013) | 21 (12 in experimental) | Stroke | N/A | 61.9 | 44.7 months | N/A | N/A |
| Poza et al. (2013) | 36 (18 finally included) | TBI | 61% | 38.4 | Majority more than 8 years | Spain - Unknown | 22% |
| Smania et al. (2003) | 3 | Stroke | 50% | 51.8 | N/A | N/A | 75% |
| Carey et al. (1993) | 8 | Stroke | 87% | 49.8 | 12.8 weeks | N/A | 25% |
| Yekutiel and Guttman (1993) | 39 | Stroke | 65% | 64 | 6.2 years | Israel - Unknown | 50% |
| de Jersey et al. (1979) | 20 | Stroke | 55% | N/A | N/A | N/A | 25% |
| Heine et al. (2017) | 13 | TBI/Stroke | 69% | 44.5 | 30 months | French - Unknown | N/A |
| Bonan et al. (2016) | 35 | Stroke | 62% | 54.1 | 3 months | N/A | 48% |
| Byl et al. (2008) | 45 | Stroke | 62% | 62 | 2.4 | Caucasian | 57% |
| Lynch et al. (2007) | 21 (10 in intervention) | Stroke | 70.00% | 61 | 48.7 days | N/A | 50% |
| Helliwell (2009) | 1 | Stroke | 0 | 78 | 21 days | N/A | 0 |
| Dogru Huzmeli et al. (2017) | 26 (13 in intervention) | Stroke | 69% | 53 | 40.23 months | Turkey - Unknown | 92% |
| Sedghi and Galjeh (2020) | 80 (40 intervention) | TBI | 60% | 40.2 | N/A | Iranian | N/A |
Intervention Findings
Information on the main characteristics and outcomes of studies can be found in Table 3. Multimodal sensory exposure was mostly implemented as a coma arousal technique following very severe ABI (commonly TBI). Most coma studies reported only positive changes in level of consciousness (N = 21) with six reporting mixed results and one reporting no significant changes. One study reported lower oscillatory waves as measured by EEG and interpreted this as a state of relaxation (Poza et al., 2013). One study reported higher activation in the right middle frontal gyrus, right superior temporal gyrus and bilateral ventro-anterior thalamic nucleus when using fMRI during treatment (Cheng et al., 2018) but urge caution of strong interpretation of these findings given a sample of 3. The most common senses stimulated in coma studies were audio (N = 30), tactile (N = 28), visual (N = 26), olfactory (N = 22), and gustatory (N = 17). Treatment doses varied, and included frequent as well as less frequent exposure over day, weekly and monthly periods. One study concluded that more frequent, less intense exposure is better (Megha et al., 2013).
Secondary use of multimodal sensory stimulation was in stroke, or TBI no coma, rehabilitation. Most studies found improvement in somatosensory sensation and motor control in an affected limb (Carey et al., 1993; de Diego et al., 2013; de Jersey, 1979; Dogru Huzmeli et al., 2017; Smania et al., 2003; Yekutiel & Guttman, 1993). Training was only required for 2 – 8 weeks, although high intensity promoted better outcomes in sensory discrimination and strength (Byl et al., 2008). In several studies, effects were maintained after several months (Carey et al., 1993; Smania et al., 2003). One study reported positive findings in posture and balance (Bonan et al., 2016). Contrastingly, one study found balance was not significantly different than an active control (Dogru Huzmeli et al., 2017). A single person case study found positive effects on proprioception, but without this affecting motor recovery (Helliwell, 2009), and Lynch et al. (2007) found no differences between experimental group and a control group (all of whom improved in posture, gait, or assisted walking). In contrast to coma studies, audio was never the sensory stimulation method used in post-stroke, no-coma rehabilitation studies. The most common senses stimulated were proprioception (N = 7), tactile (N = 8), and stereognosis (N = 4).
Discussion
This systematic review aimed to synthesise research evidence relating to multimodal sensory interventions for adults affected by ABI, asking the question, what is the influence of multimodal sensory therapy on cognitive, physical or behavioural functioning on adults affected by ABI? This review finds that multimodal sensory stimulation may be a facilitator of arousal in minimally conscious or comatose states following severe TBI; a finding reported in previous reviews (Li et al., 2020). This is a promising finding given that behavioural responses during a minimally conscious state are associated with emergence from this state (Wilson et al., 1996). There is also some evidence that following stroke (no coma), participants presented with enhanced sensations and motor control in affected limbs following multimodal sensory stimulation. Therefore, intervention with patients with different levels of consciousness appears to have different requirements, which led to the exclusion of coma patients on previous reviews (Pinto et al., 2020).
The results of this review suggest practice has been to use more, and a wider variety of senses, for patients in a coma and minimally conscious states, and less but more targeted senses for those recovering from a stroke (no coma). In minimally conscious states it is common for a minimum of four senses to be targeted to improve level of consciousness. Likely due to more targeted treatment needs, stroke patients were commonly stimulated with two or three senses related to effective movement and orientation in a physical environment, such as proprioception, balance, posture and touch. This difference reflects the patients underlying condition. The specific senses stimulated in stroke reflect the focus in stroke rehabilitation of addressing mobility and activities of daily living (Stein et al., 2021). The higher number of senses in minimally conscious states reflects the contemporary neurosciences ‘whole of brain’ approach (e.g., Baier et al., 2006). For example, synchronized communication across several brain regions, of sufficient complexity, is needed to maintain consciousness (Alnes et al., 2021; Deco et al., 2015).
There is little research exploring dosage, a finding consistent with past research (Pinto et al., 2020). Guidance may be found in previous related environmental enrichment research which suggests shorter periods of exposure have limited effect, therefore there is a threshold of exposure needed before benefits are seen (de Witt et al., 2011). The current findings suggest that high frequency stimulation targeting physical movement may promote better outcomes in no coma stroke patients, and that more frequent but less intense stimulation may be beneficial for patients in a coma aiming to improve conscious state. Indeed, following their review of the literature on sensory stimulation for people in a coma after an ABI, Padilla and Domina (2016) also suggest frequent stimulation is more effective. It has also been suggested that stimulation must start early (Padilla & Domina, 2016; Zuo et al., 2021). However, these conclusions are based on limited research and require more investigation before they can be meaningfully suggested.
Related to dosage is the notion of personalisation of sensory stimulation. Preferred music had a greater effect than neutral music on patient’s responsiveness (Heine et al., 2017) and sensory stimulation was improved when delivered by families rather than by clinical staff (Moattari et al., 2016; Sedghi & Ghaljeh, 2020). Cheng et al. (2018) and Sargolzaei et al. (2017) even concluded a priori that multimodal sensory stimulation was better delivered by family members and chose this as part of their intervention group. It is likely that personalised sensory stimulation therapy arouses increased affective responses. For example, music elicits a greater emotional response (Moattari et al., 2016) and music that a patient prefers may result in stronger emotions than neutral music. In essence, personalised approaches may result in more intense, emotion eliciting dosages that may encourage stronger cortical responses. This outcome is supported by research exploring unimodal therapies (e.g., Sullivan et al., 2018; Tavangar et al., 2015; Zuo et al., 2021). For example, a recent review found family-centred sensory stimulation for comatose patients following a TBI was more effective than clinician implemented or routine care (Zuo et al., 2021).
One recent study compared outdoor multimodal sensory stimulation with indoor (Attwell et al., 2019). The outdoor therapy was embedded in a natural setting and the authors found this was more effective than indoor settings. This finding is not surprising, given the differing effect of a green environment on brain activity (Norwood et al., 2019) and the weight of research finding positive effects of green environments on cognitive functions (e.g., Bratman et al., 2012; Kuo et al., 2019). Research suggests the positive effects of natural settings are also stronger in multimodal sensory green environments rather than unisensory. For example, a recent study found natural olfactory stimuli may be more important than natural visual stimuli for stress reduction, leading the authors to conclude that urban planners should consider multimodal sensory stimuli in greenspaces, where current practice prioritises visual stimuli (Hedblom et al., 2019). Another study found that during green exercise, the occlusion of individual sensory stimuli resulted in lower mood than a full sensory experience (Wooller et al., 2015). Indeed, a recent review of studies exploring the effects of simulated nature on human health and cognitive functioning concluded multimodal sensory stimulations were a prime opportunity for research (Browning et al., 2021). If it is practical to complete multimodal sensory stimulation in green environments, then this is recommended. Future research can now explore the specific effects of dosage, specific natural stimuli, and frequency of exposure. In line with the aforementioned, this will likely be more effective if completed by family members and if stimuli can be personalised for the patient.
The overall findings suggest multimodal sensory stimulation can be beneficial for patients, especially those in a minimally conscious state or attempting physical rehabilitation following stroke. Evidence is not strong enough for a recommendation of wide-spread uptake in clinical practice. The research base is limited making it difficult to establish best practice for developing and administering multimodal sensory stimulation. And although negative findings are infrequent in the current literature base, it is unknown if this reflects a publication bias of significant findings. However, from available publications risks appear to be minimal and positive effects common. The evidence base so far suggests future research would be worthwhile.
Limitations
The small number of studies included in this review makes findings less conclusive. Included studies are generally quite old with only 14 of 38 (36%) occurring in the last decade; more studies took place prior to 2000 (15 of 43). Comparison between studies (including meta-analysis) was made less plausible, and is less reliable, due to the heterogeneity of methods used including senses stimulated, outcome measures used, and dosage (including duration, frequency, and intensity). For example, more than 20 different outcome measures are reported here, from just 25 coma studies. And although it seems the more senses stimulated the better for coma arousal, it is not possible to draw firm conclusions on which sensory modalities are more important; almost all coma studies used auditory, visual, and olfactory as a minimum. Across all studies about 70% of the population are male and for coma studies are aged around 30, for non-coma the average age is much higher at about 53. This homogeneity means results can’t necessarily be applied to populations outside these demographic characteristics. On the other hand, heterogeneity in the state of consciousness between participants at the acute stage, and recovery level at the start and end of the intervention makes comparison and conclusions on when and how long to implement an intervention difficult.
The level of evidence included varies significantly, with the largest number of studies not including a control group, which makes it difficult to compare the reported positive effects to other treatments, or spontaneous recovery. Further, the efficacy of interventions is harder to establish as only 3 identified studies reported effect sizes. Future research should report an effect size.
It is also acknowledged that access to green space for sensory stimulation will not always be practical for many reasons including location of care, stage of recovery etc. Research could explore how to facilitate this access and suggestions include modification of hospital and home internal environments, use of technology such as VR, and increased accessibility to outdoor environments.
Future Directions
The current paper describes the process for multimodal sensory stimulation as found in academic literature. It is presumed this reflects the practice at the hospitals and rehabilitation units involved. However, it may not fully represent the process in current clinical practice. Further research may shed light on how multimodal sensory stimulation is used in clinical practice, outside of a research design.
Padilla and Domina (2016) conducted a review in 2016 focusing on coma studies and found positive results. The current review finds multimodal sensory stimulation and coma studies in the last five years have not been prevalent; only seven extra papers were found, and most papers since 2016 reported here are no coma studies. Given the promise of multimodal sensory stimulation for increasing arousal during coma further research on this is suggested.
Dosage, including how intense and frequently to administer stimuli, is a priority, especially for patients in a minimally conscious state where evidence for positive changes is consistent but dosage inconsistent. Currently, frequent, small doses of personally relevant stimuli appear to be the most effective approach.
Given the positive findings reported in studies included in this review, it may be worth exploring the use of multimodal sensory stimulation in other injuries and conditions such as PTA, multiple sclerosis, cerebral palsy, or spinal cord injury.
Only one study was identified that explored the effects of injury on behaviours such as agitation, aggression or apathy. This study explored levels of agitation in patients with decreased consciousness after a TBI. Over seven days, an experimental group received auditory and tactile sensory stimulation delivered by a family member, and a control group received routine care. They found no difference between groups in days one to five, but on days six and seven the experimental group experienced significantly lower levels of agitation (Sedghi & Ghaljeh, 2020). Given this positive finding, and the positive effects of multimodal sensory stimulation on these behaviours in other conditions, this would be an interesting and potentially fruitful course of research.
Conclusion
This review finds studies have been completed in coma and stroke patients. Coma studies measured outcomes in level of consciousness and stroke studies measures motor control and sensorimotor sensations. Multimodal sensory stimulation was adapted so that senses stimulated were appropriate for the outcomes targeted and positive changes are mostly reported. Multimodal sensory stimulation may work better when personalised and made pertinent for the patient; it appears to be a low-risk intervention with positive outcomes.
Acknowledgements
A preliminary version of this review protocol was presented at the Arts and Health Conference, Sydney, October 2016
Appendix 1. Full Final Search Strategy
The search strategy was developed through a combination of studying past reviews and including other terms of interest. A research librarian assisted in further development of these terms including trialling them for effectiveness.
| Database | Limits | Search Terms |
|---|---|---|
| Web of Science | Topic, academic | ("multi sens*" OR bisens* OR unisens* OR "single sense" OR "sens* stimulation" OR "snoezelen" OR "sens* design*" OR "multi modal sens*" OR "unimodal sens*" OR "bimodal sens*") AND (neurolog* OR "traumatic brain injur*" OR "TBI" OR "acquired brain injur*" OR "ABI" OR "head injur*" OR "brain injur*" OR stroke OR "viral encephal*") AND (relax* OR recove* OR rehabilit* OR intervention OR treatment OR therap*) |
| CINAHL | Abstract, academic | |
| ProQuest | Abstract, academic | |
| PsychInfo | Abstract | |
| PubMed | All text |
Appendix 2. Risk of Bias for Included Studies
| First Author (year) | Sample Bias | Measurement Bias | Intervention Bias | Overall Direction | ||
|---|---|---|---|---|---|---|
| Coma studies (n = 28) | ||||||
| Abbasi et al. (2009) |
(=) Random assignment to treatment or control; No significant difference between control and treatment in level of consciousness (+) Demographics between groups similar, but intervention group significantly more likely to be married (?) Unknown how patients were recruited |
(=) Raters were blinded and independent | (=) Nurses were blind to condition (care as usual) | = | ||
| Attwell et al. (2019) | (+) Recruitment on a volunteer basis (presumably family/carers) | (=) Raters were blinded and independent; Behaviour grid based on CRS-R |
(=) Same tasks indoor as outdoor; Sessions delivered on same day by same therapist; Protocol order randomized (+) Tasks adapted to patients’ abilities |
+ | ||
| Canedo et al. (2002) | (+) Selected case studies | (?) Measurements deemed not useful were discontinued |
(+) Co-intervention probable (?) Unknown if same person administered all treatments |
+ | ||
| Deiva et al. (2017) |
(=) No significant difference between control and treatment in level of consciousness (?) Demographic differences between the groups unknown |
(+) Unknown who collected data – presumably the research team | (=) Unknown if same person administered all treatments – it appears they may have | = | ||
| Di Stefano et al. (2012) | (+) Recruitment on a volunteer basis (presumably family/carers) |
(=) Same rater each week; Single case ABCBA design (+) Raters were not blinded |
(=) Sessions delivered at same times of day (?) Unknown if same person administered all treatments |
+ | ||
| Doman et al. (1993) | (+) Recruitment on a volunteer basis (presumably family/carers) and not random; Control group selected by not wishing to take part in treatment. May have been biased against treatment, been in worst condition, and might not have had same support as treatment group | (?) Unknown who collected data or if they were blinded |
(+) Attention bias (?) Unknown if same person administered all treatments |
++ | ||
| Hall et al. (1992) | (+) Recruitment on a volunteer basis (presumably family/carers) |
(=) Single case ABAB design (+) Raters were not blinded (therapists were also raters) |
(=) Visits of family equal between conditions; Consistency of therapists (+) Probable co-intervention (?) Environmental control not possible |
++ | ||
| Johnson et al. (1993) | (=) Random assignment to treatment or control; Patients similar in age and GCS score at baseline |
(=) Some data collection techniques relatively objective (+) Raters were not blinded (therapists were also raters) |
(=) Treatment delivered at same time each day (+) Differences in biochemicals and skin conductance within and between groups |
+ | ||
| Kaewsriwong et al. (2015) | (+) Selected case studies – unclear exactly who was excluded; Recruitment on a volunteer basis (family/carers) | (=) Formal training provided to rater’s team for consistency; Same rater each week |
(=) Formal training provided to treatment team for consistency (+) Co-intervention not avoided (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments |
+ | ||
| Kater (1989) | (=) Demographics between groups not significantly different | (?) Unknown who collected data or if they were blinded | (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments | ? | ||
| Keller et al. (2007) | (+) Recruitment on a volunteer basis (presumably family/carers) | (?) Unknown who collected data or if they were blinded |
(+) Co-intervention not avoided; Possible attention bias (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments |
+ | ||
| Grüner and Terhaag (2000) |
(+) Recruitment on a volunteer basis (presumably by family/carers); No control group or phase (?) Unknown how patients were recruited or selected for the study |
(+) One measure introduced after study start (?) Unknown who collected data or if they were blinded |
(-) Contamination not avoided (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments |
+ | ||
| Megha et al. (2013) |
(=) Random assignment to study group; Demographics between groups not significantly different (+) Recruitment on a volunteer basis (family/carers) |
(?) Unknown who collected data or if they were blinded |
(-) Contamination not avoided (?) Unknown if treatment took part at same times and days |
= | ||
| Mandeep (2012) |
(=) Random assignment to treatment or control (+) Recruitment on a volunteer basis (family/carers) |
(?) Unknown who collected data or if they were blinded | (?) Unknown if treatment took part at same times; Unknown if same person administered all treatments | ? | ||
| Mitchell et al. (1990) |
(=) Matched controls; Demographics between groups not significantly different (+) Recruitment on a volunteer basis (family/carers) |
(=) Patients families were trained to rate behaviour; inter-rater reliability confirmed (+) Research was not blinded |
(?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments | = | ||
| Moattari et al. (2016) | (=) Random assignment to study group; Demographics and level of consciousness between groups not significantly different | (=) Raters were blinded; Ratings took place at same time each day | (=) Treatment delivered at same time each day; it appears treatment was delivered by same family member/nurse each time | = | ||
| Noda et al. (2004) | (+) No control group or phase | (?) Unknown who collected data or if they were blinded |
(+) Probable attention bias; Probable co-intervention (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments |
+ | ||
| Oh and Seo (2003) | (+) Recruitment on a volunteer basis (family/carers) | (=) Single case ABAB design; Rater was blinded to intervention and inter-rater reliability obtained |
(+) Probable attention bias; Probable co-intervention (=) Treatment delivered at same time each day in the same order by same researcher |
+ | ||
| Pierce et al. (1990) |
(=) No significant differences between control and treatment in demographics (+) Treatment group was on average younger than control |
(=) Rater was independent from study and appears to be blinded to intervention (+) Final outcome status not blinded or independent |
(=) Treatment delivered at same time each day ( +) Unknown if same person delivered treatment each day within individuals; Co-intervention for treatment group (?) Potential differences in timing and environments between groups |
++ | ||
| Rader et al. (1989) | (?) Unknown how patients were recruited | (?) Unknown who collected data or if they were blinded |
(=) Treatment delivered at same time each day (+) Unknown if same person delivered treatment each day; Attention bias; Co-intervention not avoided |
++ | ||
| Sargolzaei et al. (2017) | (=) No significant differences between control and treatment in demographics or level of consciousness |
(+) Research was not blinded; Modalities of outcome measure matched the treatment provided (?) Unknown if same person administered all assessments – it appears not |
(+) Possible attention bias (?) Unknown if same person administered all treatments – it appears not |
+ | ||
| Talbot and Whitaker, (1994) | (+) Recruitment on a volunteer basis (family/carers) through a relevant association; Families had to agree to be actively present in process |
(=) Final evaluation by blinded rater (-) Lowest score for functioning always used |
(=) Formal training provided to treatment team for consistency (+) Co-intervention not avoided |
+ | ||
| Urbenjaphol et al. (2009) |
(=) Random assignment to treatment or control (+) Recruitment on a volunteer basis (family/carers) |
(=) Raters were blinded; Ratings took place at same time each day (?) Unknown if same person administered all assessments |
(=) Treatment delivered at same time each day (-) Contamination not avoided (?) Unknown if same person administered all treatments |
= | ||
| Wijnen et al. (2006) | (+) Recruitment on a volunteer basis (presumably by family/carers); No control group or phase |
(=) Rating always took part at same time of day by the same person (+) Research was not blinded |
(-) Contamination not avoided (?) Unknown if same person administered all treatments |
+ | ||
| Wilson et al. (1991) | (+) Selected case studies – unclear exactly who was excluded; Recruitment on a volunteer basis (presumably by family/carers) | (+) Unknown who collected data – presumably the research team; Raters unlikely blinded |
(-) Contamination not avoided (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments |
++ | ||
| Wilson et al. (1993) | (+) Selected case studies – unclear exactly who was excluded; Recruitment on a volunteer basis (presumably by family/carers) | (+) Unknown who collected data – presumably the research team; Raters unlikely blinded |
(-) Contamination not avoided (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments |
++ | ||
| Wilson et al. (1996) | (+) Selected case studies – unclear exactly who was excluded; Recruitment on a volunteer basis (presumably by family/carers) | (+) Unknown who collected data – presumably the research team; Raters unlikely blinded |
(-) Contamination not avoided (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments |
++ | ||
| Wood et al. (1993) |
(+) No control group or phase (?) Unknown how patients were recruited |
(+) Raters were not blinded (?) Baseline measures conducted by different therapists |
(-) Contamination not avoided (?) Unknown if treatment took part at same times and days; Unknown if same person administered all treatments |
? | ||
| Post-Coma Studies (n = 15) | ||||||
| Gomez et al. (2016) |
(=) Demographics between groups not significantly different (+) Recruitment on a volunteer basis |
(+) Raters were not blinded | (?) Unknown if same person administered all treatments | = | ||
| de Diego et al. (2013) |
(=) Random assignment to treatment or control (+) Recruitment on a volunteer basis; Intervention group had less time since lesion occurred |
(=) Raters were blinded and independent; Data analyst was blinded and independent |
(+) Some therapy for treatment group conducted at home; Attention bias (?) Unknown if treatment/control group actually completed rehabilitation at home |
++ | ||
| Poza et al. (2013) |
(=) Demographics between groups not significantly different (+) Recruitment on a volunteer basis |
(=) Data collection techniques relatively objective |
(=) Some control of contamination stated (?) Unknown if same person administered all treatments; Unknown if treatment took part at same times and days |
= | ||
| Smania et al. (2003) | (+) Recruitment on a volunteer basis; No control group or phase | (+) Unknown who collected data – presumably the research team | (?) Unknown if same person administered all treatments; Unknown if treatment took part at same times and days | ++ | ||
| Carey et al. (1993) | (+) Selected case studies – unclear exactly who was excluded; Recruitment on a volunteer basis |
(+) Unknown who collected data – presumably the research team (?) Outliers removed (unclear which direction they lay) |
(?) Unknown if same person administered all treatments | ++ | ||
| Yekutiel and Guttman (1993) |
(=) Demographics between groups not significantly different (+) Recruitment on a volunteer basis |
(=) It appears the same researcher administered all assessments; Different researcher from treatment delivery rated outcomes (+) Raters were not blinded |
(=) The same researcher administered all treatments (?) Unclear if control group were active (potentially creating an attention bias for treatment group), or where they were (potentially creating a site of treatment bias as treatment group were at home) |
+ | ||
| de Jersey et al. (1979) |
(+) No control group or phase (?) Patients were not randomly selected |
(=) It appears the same researcher administered all assessments (+) Raters were not blinded |
(?) Unknown if same person administered all treatments | + | ||
| Heine et al. (2017) |
(+) Recruitment on a volunteer basis (presumably family/carers); No control group or phase (?) Unknown how patients were recruited |
(=) Stimuli order random; Raters were blinded; Inter-rater reliability calculated between raters (high agreement) |
(+) Possible co-intervention (?) Unknown if same person administered all treatments |
= | ||
| Bonan et al. (2016) |
(=) Demographics between groups not significantly different (+) Recruitment on a volunteer basis (?) Unknown how patients were selected |
(=) Data collection techniques relatively objective (+) Unknown who collected data – presumably the research team |
(?) Unknown if same person administered all treatments | = | ||
| Byl et al. (2008) |
(=) Demographics between groups not significantly different; Some differences between groups at baseline but not statistically significant (+) Recruitment on a volunteer basis by participants who could/would commit to significant involvement and time |
(=) Raters were blinded and same rater was used across all studies | (?) Unknown if same person administered all treatments | = | ||
| Lynch et al. (2007) |
(=) Random assignment to treatment or control (+) Recruitment on a volunteer basis |
(=) Raters were blinded; Inter-rater reliability calculated between raters (high agreement) (+) Unclear if same researcher delivered control (relaxation) activities |
(=) Both groups received standard physical training (co-intervention) | = | ||
| Helliwell (2009) | (+) Recruitment on a volunteer basis; Majority of potential participants excluded due to inadequate communication or cognition resulting in individual case study | (=) Independent rater compared with researcher ratings |
(=) Sessions delivered on same day by same therapist (+) Co-intervention |
+ | ||
| Dogru Huzmeli et al. (2017) |
(=) Demographics between groups not significantly different (?) Unclear how participants were assigned to control or treatment |
(+) Unknown who collected data – presumably the research team | (?) Unclear if treatment took part at same times and days; Unknown if same person administered all treatments | ? | ||
| Sedghi and Ghaljeh (2020) |
(=) Random assignment to treatment or control (+) Volunteered by family members who took part |
(+) Unknown who collected data – presumably the research team | (?) Unknown if same person administered all treatments; Unknown if contamination occurred | + | ||
| Cheng et al. (2018) | (+) Volunteered by family members who took part | (=) Same rater each week; Raters were blinded and independent; Single case ABAB design |
(+) Possible attention bias (-) Contamination not avoided (?) Unknown if same person administered all treatments |
= | ||
+ favours treatment group/hypothesis; - favours control group/null hypothesis; = no or negligible effect of bias; ? direction unknown; more than one of + or – signifies a greater effect
Author Contribution
Michael Norwood: Validation; Formal analysis; Investigation; Data Curation; Writing - Original Draft; Writing - Review & Editing; Supervision. Ali Lakhani: Conceptualization; Methodology; Formal analysis; Data Curation; Writing - Original Draft; Writing - Review & Editing. David Watling: Methodology; Validation; Formal analysis; Investigation; Data Curation. Chelsea Marsh: Formal analysis; Investigation; Writing - Review & Editing. Heidi Zeeman: Conceptualization; Methodology; Data Curation; Writing - Original Draft; Supervision; Project administration.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No external funding providing – salary from Griffith University funded researcher time.
Availability of Data and Material
No original data collected – material such as data extraction tables available on request.
Code Availability
Not applicable.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi M, Mohammadi E, Sheaykh Rezayi A. Effect of a regular family visiting program as an affective, auditory, and tactile stimulation on the consciousness level of comatose patients with a head injury. Japan Journal of Nursing Science. 2009;6(1):21–26. doi: 10.1111/j.1742-7924.2009.00117.x. [DOI] [PubMed] [Google Scholar]
- Alnes SL, De Lucia M, Rossetti AO, Tzovara A. Complementary roles of neural synchrony and complexity for indexing consciousness and chances of surviving in acute coma. NeuroImage. 2021;245:118638. doi: 10.1016/j.neuroimage.2021.118638. [DOI] [PubMed] [Google Scholar]
- Alwis DS, Rajan R. Environmental enrichment and the sensory brain: the role of enrichment in remediating brain injury. Frontiers in Systems Neuroscience. 2014;8:156–156. doi: 10.3389/fnsys.2014.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell C, Jöhr J, Pincherle A, Pignat JM, Kaufmann N, Knebel JF, Diserens K. Neurosensory stimulation outdoors enhances cognition recovery in cognitive motor dissociation: a prospective crossover study. NeuroRehabilitation. 2019;44(4):545–554. doi: 10.3233/NRE-192692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier B, Kleinschmidt A, Muller NG. Cross-modal processing in early visual and auditory cortices depends on expected statistical relationship of multisensory information. The Journal of Neuroscience. 2006;26(47):12260–12265. doi: 10.1523/JNEUROSCI.1457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. The European Journal of Neuroscience. 2004;20(5):1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- Bonan IV, Leblong E, Leplaideur S, Laviolle B, Ponche ST, Yelnik AP. The effect of optokinetic and galvanic vestibular stimulations in reducing post-stroke postural asymmetry. Clinical Neurophysiology. 2016;127(1):842–847. doi: 10.1016/j.clinph.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Bratman GN, Hamilton JP, Daily GC. The impacts of nature experience on human cognitive function and mental health: Nature experience, cognitive function, and mental health. Annals of the New York Academy of Sciences. 2012;1249(1):118–136. doi: 10.1111/j.1749-6632.2011.06400.x. [DOI] [PubMed] [Google Scholar]
- Browning MHEM, Saeidi-Rizi F, McAnirlin O, Yoon H, Pei Y. The role of methodological choices in the effects of experimental exposure to simulated natural landscapes on human health and cognitive performance: a systematic review. Environment and Behavior. 2021;53(7):687–731. doi: 10.1177/0013916520906481. [DOI] [Google Scholar]
- Byl NN, Pitsch EA, Abrams GM. Functional outcomes can vary by dose: Learning-based sensorimotor training for patients stable poststroke. Neurorehabilitation and Neural Repair. 2008;22(5):494–504. doi: 10.1177/1545968308317431. [DOI] [PubMed] [Google Scholar]
- Cameron A, Burns P, Garner A, Lau S, Dixon R, Pascoe C, Szafraniec M. Making sense of multi-sensory environments: a scoping review. International Journal of Disability, Development, and Education. 2020;67(6):630–656. doi: 10.1080/1034912X.2019.1634247. [DOI] [Google Scholar]
- Canedo A, Grix MC, Nicoletti J. An analysis of assessment instruments for the minimally responsive patient (MRP): Clinical observations. Brain Injury. 2002;16(5):453–461. doi: 10.1080/02699050110119853. [DOI] [PubMed] [Google Scholar]
- Carey, L. M. (1995). Somatosensory loss after stroke. Critical Reviews™ in Physical and Rehabilitation Medicine, 7(1). 10.1615/CritRevPhysRehabilMed.v7.i1.40
- Carey LM, Matyas TA, Oke LE. Sensory loss in stroke patients: Effective training of tactile and proprioceptive discrimination. Archives of Physical Medicine and Rehabilitation. 1993;74(6):602–611. doi: 10.1016/0003-9993(93)90158-7. [DOI] [PubMed] [Google Scholar]
- Cheng L, Cortese D, Monti MM, Wang F, Riganello F, Arcuri F, Di H, Schnakers C. Do sensory stimulation programs have an impact on consciousness recovery? Frontiers in Neurology. 2018;9:826–826. doi: 10.3389/fneur.2018.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Tononi G, Boly M, Kringelbach ML. Rethinking segregation and integration: Contributions of whole-brain modelling. Nature Reviews Neuroscience. 2015;16(7):430–439. doi: 10.1038/nrn3963. [DOI] [PubMed] [Google Scholar]
- Deiva K, Vijayalakshmi S, Suseela T. Effectiveness of coma stimulation therapy on traumatic brain injury patients in a government hospital setup –a feasibility study. Asian Journal of Nursing Education and Research. 2017;7(4):569–572. doi: 10.5958/2349-2996.2017.00110.0. [DOI] [Google Scholar]
- de Diego C, Puig S, Navarro X. A sensorimotor stimulation program for rehabilitation of chronic stroke patients. Restorative Neurology and Neuroscience. 2013;31(4):361–371. doi: 10.3233/RNN-120250. [DOI] [PubMed] [Google Scholar]
- de Jersey, M. C. (1979). Report on a sensory programme for patients with sensory deficits. Australian Journal of Physiotherapy, 25(4), 165–170. 10.1016/s0004-9514(14)61039-4 [DOI] [PubMed]
- de Witt BW, Ehrenberg KM, McAloon RL, Panos AH, Shaw KE, Raghavan PV, Skidmore ER, Kline AE. Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabilitation and Neural Repair. 2011;25(4):343–350. doi: 10.1177/1545968310390520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Wang Q, Lo EH, Stanley GB. Cortical excitation and inhibition following focal traumatic brain injury. The Journal of Neuroscience. 2011;31(40):14085–14094. doi: 10.1523/JNEUROSCI.3572-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano C, Cortesi A, Masotti S, Simoncini L, Piperno R. Increased behavioural responsiveness with complex stimulation in VS and MCS: Preliminary results. Brain Injury. 2012;26(10):1250–1256. doi: 10.3109/02699052.2012.667588. [DOI] [PubMed] [Google Scholar]
- Dogru Huzmeli E, Yildirim SA, Kilinc M. Effect of sensory training of the posterior thigh on trunk control and upper extremity functions in stroke patients. Neurological Sciences. 2017;38(4):651–657. doi: 10.1007/s10072-017-2822-z. [DOI] [PubMed] [Google Scholar]
- Doman G, Wilkinson R, Dimancescu MD, Pelligra R. The effect of intense multi-sensory stimulation on coma arousal and recovery. Neuropsychological Rehabilitation. 1993;3(2):203–212. doi: 10.1080/09602019308401436. [DOI] [Google Scholar]
- Fava L, Strauss K. Multi-sensory rooms: Comparing effects of the Snoezelen and the Stimulus Preference environment on the behavior of adults with profound mental retardation. Research in Developmental Disabilities. 2010;31(1):160–171. doi: 10.1016/j.ridd.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Frasca D, Tomaszczyk J, McFadyen BJ, Green RE. Traumatic brain injury and post-acute decline: What role does environmental enrichment play? A scoping review. Frontiers in Human Neuroscience. 2013;7(2013):31–31. doi: 10.3389/fnhum.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EP, Martin JH, Jessell TM. The bodily senses. In: Kandel ER, Schwarz JH, Jessell TM, editors. Principles of neural science. 4. McGraw-Hill; 2000. pp. 430–450. [Google Scholar]
- Gomez C, Poza J, Gutierrez MT, Prada E, Mendoza N, Hornero R. Characterization of EEG patterns in brain-injured subjects and controls after a Snoezelen® intervention. Computer Methods and Programs in Biomedicine. 2016;136:1–9. doi: 10.1016/j.cmpb.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Grüner ML, Terhaag D. Multimodal early onset stimulation (MEOS) in rehabilitation after brain injury. Brain Injury. 2000;14(6):585–594. doi: 10.1080/026990500120484. [DOI] [PubMed] [Google Scholar]
- Hall ME, MacDonald S, Young GC. The effectiveness of directed multisensory stimulation versus non-directed stimulation in comatose CHI patients: Pilot study of a single subject design. Brain Injury. 1992;6(5):435–445. doi: 10.3109/02699059209008139. [DOI] [PubMed] [Google Scholar]
- Hall KD, Lifshitz J. Diffuse traumatic brain injury initially attenuates and later expands activation of the rat somatosensory whisker circuit concomitant with neuroplastic responses. Brain Research. 2010;1323:161–173. doi: 10.1016/j.brainres.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine L, Tillmann B, Hauet M, Juliat A, Dubois A, Laureys S, Kandel M, Plailly J, Luaute J, Perrin F. Effects of preference and sensory modality on behavioural reaction in patients with disorders of consciousness. Brain Injury. 2017;31(10):1307–1311. doi: 10.1080/02699052.2017.1306108. [DOI] [PubMed] [Google Scholar]
- Hedblom M, Gunnarsson B, Iravani B, Knez I, Schaefer M, Thorsson P, Lundström JN. Reduction of physiological stress by urban green space in a multisensory virtual experiment. Scientific Reports. 2019;9(1):1–12. doi: 10.1038/s41598-019-46099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell S. Does the use of a sensory re-education programme improve the somatosensory and motor function of the upper limb in subacute stroke? A single case experimental design. British Journal of Occupational Therapy. 2009;72(12):551–558. doi: 10.4276/030802209x12601857794853. [DOI] [Google Scholar]
- Jadavji NM, Kolb B, Metz GA. Enriched environment improves motor function in intact and unilateral dopamine-depleted rats. Neuroscience. 2006;140(4):1127–1138. doi: 10.1016/j.neuroscience.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Janssen H, Ada L, Bernhardt J, McElduff P, Pollack M, Nilsson M, Spratt NJ. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: a pilot non-randomized controlled trial. Disability and Rehabilitation. 2014;36(3):255–262. doi: 10.3109/09638288.2013.788218. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Roethig-Johnston K, Richards D. Biochemical and physiological parameters of recovery in acute severe head injury: Responses to multisensory stimulation. Brain Injury. 1993;7(6):491–499. doi: 10.3109/02699059309008176. [DOI] [PubMed] [Google Scholar]
- Kaewsriwong, S., Sukonthasarn, A., Wangsrikhun, S., & Chanprasit, C. (2015). Sensory stimulation process and cognitive function among persons with traumatic brain injury: a case study. Pacific Rim International Journal of Nursing Research, 19(1), 45–57. Available from: https://he02.tci-thaijo.org/index.php/PRIJNR/article/view/1898
- Kaplan H, Clopton M, Kaplan M, Messbauer L, McPherson K. Snoezelen multi-sensory environments: Task engagement and generalization. Research in Developmental Disabilities. 2006;27:443–455. doi: 10.1016/j.ridd.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Kaplan M, Clopton M, Khazanova O, Kitaichik T. The effects of snoezelen use in adult developmental delay. Developmental Disabilities Special Interest Section Quarterly / American Occupational Therapy Association. 2007;30(4):1. [Google Scholar]
- Karma D, Rawat AK. Effect of stimulation in coma. Indian Pediatrics. 2006;43(10):856–860. [PubMed] [Google Scholar]
- Kater KM. Response of head-injured patients to sensory stimulation. Western Journal of Nursing Research. 1989;11(1):20–33. doi: 10.1177/019394598901100103. [DOI] [PubMed] [Google Scholar]
- Keller I, Hülsdunk A, Müller F. The influence of acoustic and tactile stimulation on vegetative parameters and EEG in persistent vegetative state. Functional Neurology. 2007;22(3):159–163. [PubMed] [Google Scholar]
- Kuo M, Barnes M, Jordan C. Do experiences with nature promote learning? Converging evidence of a cause-and-effect relationship. Frontiers in Psychology. 2019;10:305–305. doi: 10.3389/fpsyg.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law, M., Stewart, D., Letts, L., Pollock, N., Bosch, J., & Westmorland, M. (1998). Guidelines for critical review of qualitative studies. McMaster University Occupational Therapy Evidence-Based Practice Research Group, 1–9.
- Li J, Cheng Q, Liu FK, Huang Z, Feng SS. Sensory stimulation to improve arousal in comatose patients after traumatic brain injury: a systematic review of the literature. Neurological Sciences. 2020;41(9):2367–2376. doi: 10.1007/s10072-020-04410-9. [DOI] [PubMed] [Google Scholar]
- Lynch EA, Hillier SL, Stiller K, Campanella RR, Fisher PH. Sensory retraining of the lower limb after acute stroke: a randomized controlled pilot trial. Archives of Physical Medicine and Rehabilitation. 2007;88(9):1101–1107. doi: 10.1016/j.apmr.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Maegele M, Lippert-Gruener M, Ester-Bode T, Garbe J, Bouillon B, Neugebauer E, Klug N, Lefering R, Neiss WF, Angelov DN. Multimodal early onset stimulation combined with enriched environment is associated with reduced CNS lesion volume and enhanced reversal of neuromotor dysfunction after traumatic brain injury in rats. The European Journal of Neuroscience. 2005;21(9):2406–2418. doi: 10.1111/j.1460-9568.2005.04070.x. [DOI] [PubMed] [Google Scholar]
- Mandeep PK. Effectiveness of early intervention of coma arousal therapy in traumatic head injury patients. International Journal of Head and Neck Surgery. 2012;3(3):137–142. doi: 10.5005/jp-journals-10001-1114. [DOI] [Google Scholar]
- McDonald MW, Hayward KS, Rosbergen ICM, Jeffers MS, Corbett D. Is environmental enrichment ready for clinical application in human post-stroke rehabilitation? Frontiers in Behavioral Neuroscience. 2018;12:135–135. doi: 10.3389/fnbeh.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harris GT, Rice ME, Silk L. Effects of a snoezelen room on the behavior of three autistic clients. Research in Developmental Disabilities. 2007;28(3):304–316. doi: 10.1016/j.ridd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Megha M, Harpreet S, Nayeem Z. Effect of frequency of multimodal coma stimulation on the consciousness levels of traumatic brain injury comatose patients. Brain Injury. 2013;27(5):570–577. doi: 10.3109/02699052.2013.767937. [DOI] [PubMed] [Google Scholar]
- Mesa-Gresa P, Pérez-Martinez A, Redolat R. Environmental enrichment improves novel object recognition and enhances agonistic behavior in male mice: Environmental enrichment. Aggressive Behavior. 2013;39(4):269–279. doi: 10.1002/ab.21481. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Bradley VA, Welch JL, Britton PG. Coma arousal procedure: a therapeutic intervention in the treatment of head injury. Brain Injury. 1990;4(3):273–279. doi: 10.3109/02699059009026177. [DOI] [PubMed] [Google Scholar]
- Moattari M, Alizadeh Shirazi F, Sharifi N, Zareh N. Effects of a sensory stimulation by nurses and families on level of cognitive function, and basic cognitive sensory recovery of comatose patients with severe traumatic brain injury: a randomized control trial. Trauma Monthly. 2016;21(4):e23531. doi: 10.5812/traumamon.23531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Reprint—Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Physical Therapy. 2009;89(9):873–880. doi: 10.1093/ptj/89.9.873. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature Reviews Neuroscience. 2006;7(9):697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Noda R, Maeda Y, Yoshino A. Therapeutic time window for musicokinetic therapy in a persistent vegetative state after severe brain damage. Brain Injury. 2004;18(5):509–515. doi: 10.1080/02699050310001645810. [DOI] [PubMed] [Google Scholar]
- Norwood, M. F., Lakhani, A., Maujean, A., Zeeman, H., Creux, O., & Kendall, E. (2019). Brain activity, underlying mood and the environment: A systematic review. Journal of Environmental Psychology, 65, 101321. 10.1016/j.jenvp.2019.101321
- Oh H, Seo W. Sensory stimulation programme to improve recovery in comatose patients: Sensory stimulation and recovery from coma. Journal of Clinical Nursing. 2003;12(3):394–404. doi: 10.1046/j.1365-2702.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- Padilla, R., & Domina, A. (2016). Effectiveness of sensory stimulation to improve arousal and alertness of people in a coma or persistent vegetative state after traumatic brain injury: a systematic review. The American Journal of Occupational Therapy, 70(3), 7003180030p1–7003180030p8. 10.5014/ajot.2016.021022 [DOI] [PubMed]
- Padua L, Cuccagna C, Pazzaglia C. Novel sensory paradigms for neuromodulation in disorders of consciousness in traumatic brain injury. Current Opinion in Neurology. 2019;32(6):844–849. doi: 10.1097/WCO.0000000000000747. [DOI] [PubMed] [Google Scholar]
- Park S. Effectiveness of direct and non-direct auditory stimulation on coma arousal after traumatic brain injury. International Journal of Nursing Practice. 2016;22(4):391–396. doi: 10.1111/ijn.12448. [DOI] [PubMed] [Google Scholar]
- Percaccio CR, Engineer ND, Pruette AL, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment increases paired-pulse depression in rat auditory cortex. Journal of Neurophysiology. 2005;94(5):3590–3600. doi: 10.1152/jn.00433.2005. [DOI] [PubMed] [Google Scholar]
- Percaccio CR, Pruette AL, Mistry ST, Chen YH, Kilgard MP. Sensory experience determines enrichment-induced plasticity in rat auditory cortex. Brain Research. 2007;1174(1):76–91. doi: 10.1016/j.brainres.2007.07.062. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Lyle DM, Quine S, Evans NJ, Morris J, Fearnside MR. The effectiveness of coma arousal intervention. Brain Injury. 1990;4(2):191–197. doi: 10.3109/02699059009026165. [DOI] [PubMed] [Google Scholar]
- Pinto, J. O., Dores, A. R., Peixoto, B., Geraldo, A., & Barbosa, F. (2020). Systematic review of sensory stimulation programs in the rehabilitation of acquired brain injury. European Psychologist. Advance online publication. 10.1027/1016-9040/a000421
- Poza J, Gomez C, Gutierrez MT, Mendoza N, Hornero R. Effects of a multi-sensory environment on brain-injured patients: Assessment of spectral patterns. Medical Engineering and Physics. 2013;35(3):365–375. doi: 10.1016/j.medengphy.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Rader MA, Alston JB, Ellis DW. Sensory stimulation of severely brain-injured patients. Brain Injury. 1989;3(2):141–147. doi: 10.3109/02699058909004545. [DOI] [PubMed] [Google Scholar]
- Rose FD. Environmental enrichment and recovery of function following brain damage in the rat. Medical Science Research. 1988;16(6):257–263. [Google Scholar]
- Rose FD, Al-Khamees K, Davey MJ, Attree EA. Environmental enrichment following brain damage: an aid to recovery or compensation? Behavioural Brain Research. 1993;56(1):93–100. doi: 10.1016/0166-4328(93)90025-L. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Research. 1978;153(3):563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- Sargolzaei, K., Fallah, M. S., Aghebati, N., Esmaily, H., & Farzadfard, M. T. (2017). Effect of a structured sensory stimulation program on the sensory function of patients with stroke-induced disorder of consciousness. Evidence Based Care: Quarterly Journal of Mashhad School of Nursing and Midwifery, 7(2), 7–16. 10.22038/ebcj.2017.23807.1505
- Sedghi T, Ghaljeh M. Effect of auditory and tactile stimulation by family members on the level of consciousness in comatose patients: a quasi-experimental study. Hayāt. 2020;26(4):357–370. [Google Scholar]
- Shimojo S, Shams L. Sensory modalities are not separate modalities: Plasticity and interactions. Current Opinion in Neurobiology. 2001;11(4):505–509. doi: 10.1016/S0959-4388(00)00241-5. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats—behavioural and neurochemical aspects. Behavioural Brain Research. 2011;222(1):246–264. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Smania N, Montagnana B, Faccioli S, Fiaschi A, Aglioti SM. Rehabilitation of somatic sensation and related deficit of motor control in patients with pure sensory stroke. Archives of Physical Medicine and Rehabilitation. 2003;84(11):1692–1702. doi: 10.1053/S0003-9993(03)00277-6. [DOI] [PubMed] [Google Scholar]
- Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. Journal of Neurotrauma. 2010;27(6):1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J, Katz DI, Black Schaffer RM, Cramer SC, Deutsch AF, Harvey RL, America Heart Association/American Stroke Association Clinical performance measures for stroke rehabilitation: Performance measures from the American Heart Association/American Stroke Association. Stroke. 2021;52(10):e675–e700. doi: 10.1161/STR.0000000000000388. [DOI] [PubMed] [Google Scholar]
- Stillman BC. Making sense of proprioception: The meaning of proprioception, kinaesthesia and related terms. Physiotherapy. 2002;88(11):667–676. doi: 10.1016/S0031-9406(05)60109-5. [DOI] [Google Scholar]
- Sullivan EG, Guernon A, Blabas B, Herrold AA, Pape TLB. Familiar auditory sensory training in chronic traumatic brain injury: a case study. Disability and Rehabilitation. 2018;40(8):945–951. doi: 10.1080/09638288.2016.1277403. [DOI] [PubMed] [Google Scholar]
- Talbot LR, Whitaker HA. Brain-injured persons in an altered state of consciousness: Measures and intervention strategies. Brain Injury. 1994;8(8):689–699. doi: 10.3109/02699059409151023. [DOI] [PubMed] [Google Scholar]
- Tavangar H, Shahriary-Kalantary M, Salimi T, Jarahzadeh M, Sarebanhassanabadi M. Effect of family members’ voice on level of consciousness of comatose patients admitted to the intensive care unit: a single-blind randomized controlled trial. Advanced Biomedical Research. 2015;4:106. doi: 10.4103/2277-9175.157806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbenjaphol P, Jitpanya C, Khaoropthum S. Effects of the sensory stimulation program on recovery in unconscious patients with traumatic brain injury. The Journal of Neuroscience Nursing. 2009;41(3):E10–E16. doi: 10.1097/JNN.0b013e3181a23e94. [DOI] [Google Scholar]
- Wijnen VJ, Heutink M, van Boxtel GJ, Eilander HJ, de Gelder B. Autonomic reactivity to sensory stimulation is related to consciousness level after severe traumatic brain injury. Clinical Neurophysiology. 2006;117(8):1794–1807. doi: 10.1016/j.clinph.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Wilson SL, Powell GE, Elliott K, Thwaites H. Sensory stimulation in prolonged coma: Four single case studies. Brain Injury. 1991;5(4):393–400. doi: 10.3109/02699059109008112. [DOI] [PubMed] [Google Scholar]
- Wilson SL, Powell GE, Elliot K, Thwaites H. Evaluation of sensory stimulation as a treatment for prolonged coma—seven single experimental case studies. Neuropsychological Rehabilitation. 1993;3(2):191–201. doi: 10.1080/09602019308401435. [DOI] [Google Scholar]
- Wilson SL, Powell GE, Brock D, Thwaites H. Vegetative state and responses to sensory stimulation: an analysis of 24 cases. Brain Injury. 1996;10(11):807–818. doi: 10.1080/026990596123927. [DOI] [PubMed] [Google Scholar]
- Wood RL, Winkowski T, Miller J. Sensory regulation as a method to promote recovery in patients with altered states of consciousness. Neuropsychological Rehabilitation. 1993;3(2):177–190. doi: 10.1080/09602019308401434. [DOI] [Google Scholar]
- Wooller J-J, Barton J, Gladwell VF, Micklewright D. Occlusion of sight, sound and smell during Green Exercise influences mood, perceived exertion and heart rate. International Journal of Environmental Health Research. 2015;26(3):267–280. doi: 10.1080/09603123.2015.1109068. [DOI] [PubMed] [Google Scholar]
- Yekutiel M, Guttman E. A controlled trial of the retraining of the sensory function of the hand in stroke patients. Journal of Neurology, Neurosurgery and Psychiatry. 1993;56(3):241–244. doi: 10.1136/jnnp.56.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebunke M, Puppe B, Langbein J. Effects of cognitive enrichment on behavioural and physiological reactions of pigs. Physiology & Behavior. 2013;118:70–79. doi: 10.1016/j.physbeh.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Zeeman, H., Watling, D., Bishara, J., & Lakhani, A. (2016). Multi-sensory stimulation therapy (MSST) for adult head trauma rehabilitation: A systematic review. PROSPERO2016. Available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016048484
- Zuo J, Tao Y, Liu M, Feng L, Yang Y, Liao L. The effect of family-centered sensory and affective stimulation on comatose patients with traumatic brain injury: a systematic review and meta-analysis. International Journal of Nursing Studies. 2021;115:103846. doi: 10.1016/j.ijnurstu.2020.103846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data collected – material such as data extraction tables available on request.
Not applicable.