Abstract
Tanzania adopted a Dolutegravir (DTG)-based regimen as first-line treatment in 2019 following the World Health Organization recommendation. Data on the DTG safety profile from sub-Saharan Africa including Tanzania are limited. We investigated the incidence of DTG-related adverse events (AEs) and associated factors among people living with HIV (PLHIV) initiated on a DTG regimen. A prospective cohort study was conducted from 25 Care and Treatment Clinics in mainland Tanzania. PLHIV aged 12 years and above who were initiated on a DTG-based regimen were actively followed up for three months. The Cox regression model was used to determine the predictors of occurrence of AEs over time. A p-value of 0.05 was considered statistically significant. From January 2020 to June 2022, a cohort of 935 participants who were both newly diagnosed and ART-experienced who transitioned to a DTG-based regimen was enrolled. Out of 935 participants, 59 (6.3%) reported a total of 62 AEs. The most frequently experienced AE was skin itching and rashes (15/62; 24.2%). DTG-associated neuropsychiatric AEs were less common and included headache (6 [9.6%]) and sleep disturbances (3 [4.8%]). The overall incidence of occurrence of the first AEs was 96.7 per 1000 person-months [95% C.I: 74.4–125.7] with the highest incidence observed among the elderly (≥ 60 years). Individuals on WHO HIV Clinical Stage 2 had a 2.7 significantly higher risk of developing AEs (adjusted hazard ratio = 2.73, 95% CI = 1.46–5.12, p = 0.017). We report a low incidence of grade I (mild) and grade II (moderate) DTG-associated AEs suggesting that the regimen is generally safe in the population. Continued monitoring of DTG safety in the population is recommended.
Subject terms: HIV infections, Viral infection
Introduction
Globally, an estimated 39 million people were living with HIV/AIDS in 20221. Like in many sub-Saharan African countries, HIV is still a major public health problem in Tanzania, with a prevalence of 4.7%, corresponding to 1.7 million people living with HIV/AIDS (PLHIV) as of 20212. Since the introduction of Antiretroviral therapy (ART) in Tanzania in the early 2000s, over 1.5 million PLHIV have been initiated on ART, and 83% have attained viral load suppression and adequate immunity2. Consequently, this has reduced HIV-related complications, improved health, prolonged survival, and reduced risk of HIV transmission3. However, ARTs have toxicities and adverse reactions, which may lead to attrition of patients from care4–6. The incidence of ARTs-related adverse drug reactions (ADR) has been reported to range from 11 to 35.9% in both developed and developing countries7.
In 2014, the Joint United Nations Programme launched the 90-90-90 AIDS program which was later updated to 95-95-95 in 2020, targeting to diagnose 95% of all HIV-positive individuals, provide ART for 95% of those diagnosed and achieve viral suppression for 95% of those treated by 20308. As of 2021, Tanzania has made significant progress towards achieving the 95-95-95 targets as indicated by the increased ART coverage to 86%2,9. Nevertheless, the achievement of these targets is affected by several factors including drug resistance and ADRs related to some ART drugs.
DTG is a second-generation integrase-strand transfer inhibitor (INSTI) with higher efficacy, excellent tolerability, infrequent drug-drug interactions and mild adverse events2,10. Due to its safety profile and fewer chances of developing resistance, the World Health Organization (WHO) recommended a DTG-based regimen to be the first line of ART to stimulate progress towards achieving the 95-95-95 targets11,12. Tanzania adopted a DTG-based regimen as a first and second-line regimen in 2019.
Studies done in real-world settings reported DTG-associated neuropsychiatric adverse reactions and increased risk of neural tube defects among infants13–18. These AEs may negatively impact ART adherence leading to discontinuation, therapeutic failure, and viral resistance interrupting the achievement of 95-95-95 AIDS targets19–21. Data on the DTG safety profile from sub-Saharan African countries are limited7,9,22. A large study conducted in six Eastern-southern African countries including Tanzania, reported that a DTG based regimen was safe with mild AEs among children and adolescents23. Similarly, mild to moderate AEs were reported among children and adolescents attending CTC in the Mbeya region, mainland Tanzania24. While adults comprise the majority of PLHIV, data on DTG safety profiles among adults from Tanzania are not available to the best of our knowledge. The present study reports the incidence of AEs and risk factors among adolescents and adults PLHIV initiated on a DTG based regimen from 11 regions of mainland Tanzania.
Results
Baseline characteristics of study participants
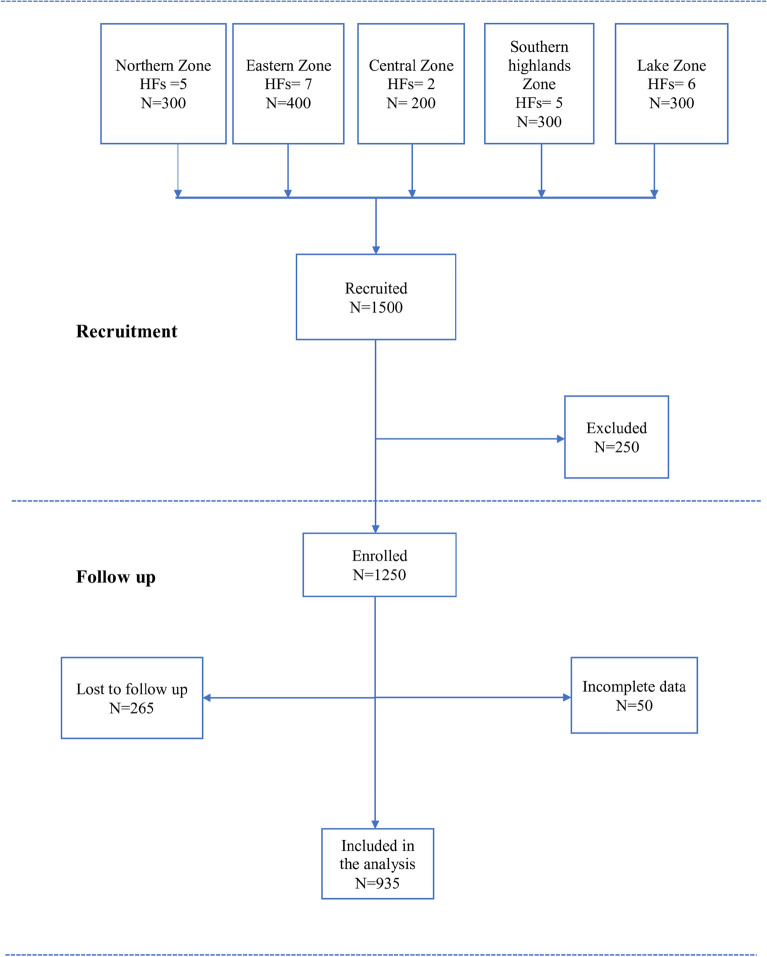
Figure 1 indicates the number of individuals recruited from each administrative zone and the corresponding total number of those followed up to 90 days. Among 1500 individuals recruited, 250 participants were excluded at enrollment due to age (< 12 years) or serious illness that needed hospitalization. From January 2020 to June 2022, a total of 935 participants were followed up for 90 days (Fig. 1).
Figure 1.
Study flow chart. HFs = Health facilities.
The majority of participants were females (591) (63.2%). More than eighty per cent were aged between 25 and 59 years (763/935; 81.6%), with the median age (IQR) of the study population being 35 (28, 44) years. At baseline, the majority of participants had a body weight of more than 40 kg (882/935; 94.3%), and about two-thirds of them were categorized as WHO HIV clinical stage I (628/935; 67.2%). Participants with a CD4 cell count of ≥ 500 cells/mm3 accounted for the majority (436/935; 46.6%). Table 1 presents the baseline characteristics of the study population.
Table 1.
Baseline characteristics of study participants.
| Variable | Frequency (n) | Percent (%) |
|---|---|---|
| Age group (years) | ||
| 12–19 | 26 | 2.8 |
| 20–24 | 101 | 10.8 |
| 25–59 | 763 | 81.6 |
| > 60 | 45 | 4.8 |
| Median age in years (IQR) | 35 (28, 44) | |
| Sex | ||
| Male | 344 | 36.8 |
| Female | 591 | 63.2 |
| Marital status | ||
| Single | 249 | 26.6 |
| Cohabiting | 38 | 4.1 |
| Married | 457 | 48.9 |
| Divorced | 98 | 10.5 |
| Widow/separated | 93 | 9.9 |
| Education level | ||
| No formal education | 90 | 9.6 |
| Primary education | 610 | 65.2 |
| Secondary education | 200 | 21.4 |
| College and University | 35 | 3.7 |
| Body weight (kg) | ||
| 25–40 | 53 | 5.7 |
| > 40 | 882 | 94.3 |
| Median weight in Kg (IQR) | 57.00 (50.00, 63.40) | |
| Absolute CD4 count (cells/mm3) | ||
| < 50 | 3 | 0.3 |
| 50– < 200 | 196 | 21 |
| 200– < 500 | 718 | 32.1 |
| ≥ 500 | 436 | 46.6 |
| Median CD4 count in cells/ mm3 (IQR) | 401 (230, 585) | |
| WHO HIV Clinical stages | ||
| Stage I | 628 | 67.2 |
| Stage II | 173 | 18.5 |
| Stage III | 98 | 10.5 |
| Stage IV | 36 | 3.9 |
Reported adverse events (AEs)
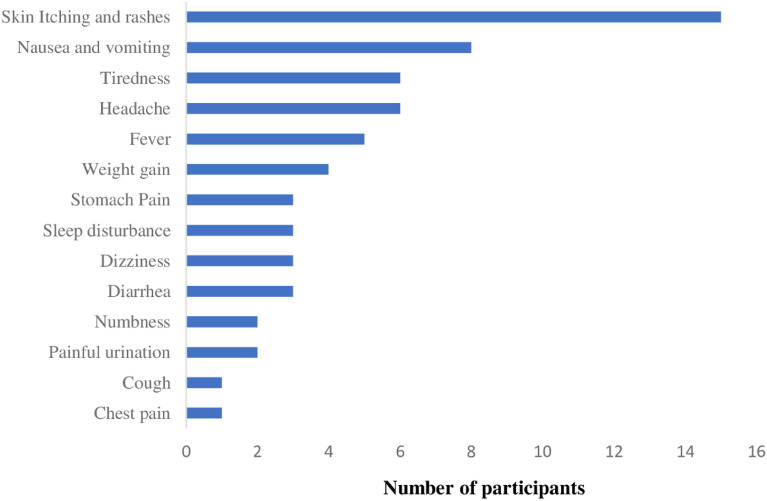
Out of 935 participants, 59 (6.3%) reported experiencing at least one AE with a total of 62 AEs (Fig. 2). Three participants (0.3%) reported experiencing two AEs at the same encounter. The most frequently reported AEs were skin itching and rashes (15) (1.6%) followed by nausea and vomiting (8) (0.9%); tiredness (6) (0.6%); headache (6) (0.6%) and fever (5) (0.5%). The least reported AEs were numbness (2) (0.2%); painful urination (2) (0.2%); cough (1) (0.1%) and chest pain (1) (0.1%) (Fig. 2). All the reported AEs were mild (grade I) to moderate (grade II) according to the internationally regognised Common Terminology Criteria for Adverse Events (CTCAE).
Figure 2.
Adverse events reported within 90 days following initiation of DTG based-regimen.
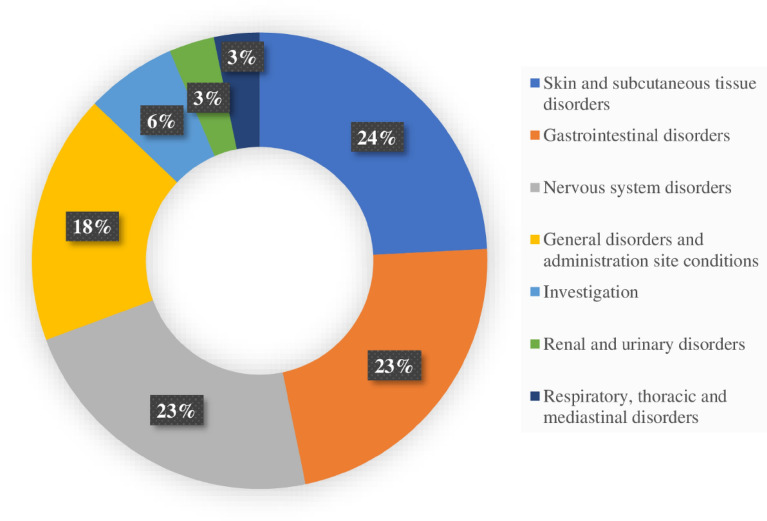
The reported AEs fell within 7 out of 27 MedDRA System Organ Classes (SOC). Skin and Subcutaneous tissue disorders accounted for the majority (24%) followed by gastrointestinal disorders (23%), nervous system disorders (23%), general disorders and administrative site conditions (18%) (Fig. 3).
Figure 3.
System Organ Classification (SOC) of the reported adverse events within 90 days following initiation of TLD.
Incidence rates of adverse events
A total of 56 AEs were analyzed during 578.97 person-months of follow-up. The overall incidence of occurrence of the first AEs was 96.7 per 1000 person-months (pm) [95% C.I 74.4–125.7]. Figure 4 shows the Kaplan–Meier plot for the time to events. The median time to the first AE with a 95% confidence interval was 87.9 (47.4 to 128.4) days (Fig. 4).
Figure 4.
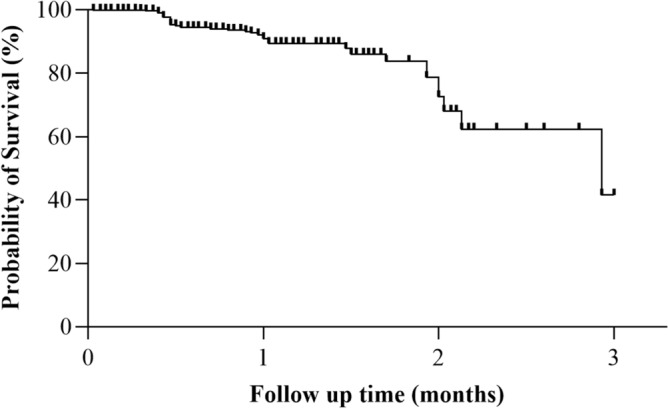
Kaplan–Meier plot for the time to Adverse Events.
The highest incidence was observed among the elderly aged 60 years and above, individuals in WHO HIV Clinical Stage 2, individuals who were widowed/separated and individuals with a secondary level of education (Table 2).
Table 2.
Incidence rate of occurrence of AEs across the population characteristics (N = 887). CI confidence interval.
| Characteristics | Person-months (pm) | Event | AE incident rate per 1000 pm (95% CI) |
|---|---|---|---|
| Overall | 578.97 | 56 | 96.7 (74.4–125.7) |
| Sex | |||
| Male | 205.5 | 20 | 97.3 (62.8–150.9) |
| Female | 370.5 | 36 | 97.2 (70.1–134.7) |
| Age group (Years) | |||
| 12–19 | 18.4 | 2 | 108.9 (27.2–435.4) |
| 20–24 | 62.1 | 9 | 144.9 (75.4–278.5) |
| 25–59 | 467.5 | 41 | 87.7 (64.6–119.1) |
| ≥ 60 | 26.4 | 4 | 151.5 (56.9–403.7) |
| Weight categories (In Kg) | |||
| 21–40 | 31.3 | 3 | 95.7 (30.9–296.9) |
| Above 40 | 504.4 | 50 | 99.1 (75.56–130.8) |
| Marital status | |||
| Single | 154.8 | 17 | 109.8 (68.3–176.7) |
| Cohabiting | 26.8 | 4 | 149.4 (56.1–398.2) |
| Married | 272.8 | 19 | 69.7 (44.4–109.2) |
| Divorced | 55.8 | 4 | 71.6 (26.9–190.9) |
| Widow/Separated | 56.9 | 9 | 158.1 (82.2–303.8) |
| Education level | |||
| None | 52.1 | 2 | 38.4 (9.6–153.5) |
| Primary | 382.8 | 39 | 101.9 (74.4–139.4) |
| Secondary | 121.3 | 13 | 107.1 (62.2–184.5) |
| College and above | 20.2 | 2 | 38.4 (9.6–153.5) |
| Baseline WHO stage | |||
| Stage 1 | 380.3 | 27 | 71.0 (48.7–103.5) |
| Stage 2 | 102.6 | 18 | 175.4 (110.5–278.5) |
| Stage 3 | 67.9 | 8 | 117.9 (59.0–235.7) |
| Stage 4 | 23.0 | 3 | 130.4 (42.1–404.4) |
| CD4 count at baseline (Cells/mm3)* | |||
| < 50 | 9.5 | 0 | – |
| 50–< 200 | 78.4 | 9 | 114.7 (59.7–220.5) |
| 200– < 500 | 194.9 | 22 | 112.9 (74.3–171,4) |
| ≥ 500 | 10.6 | 0 | – |
Risk factors for incidence rates of reported AEs
In the univariable analysis, the WHO HIV clinical stage was the significant predictor of reported AEs. After adjusting for potential confounders, WHO HIV clinical stage II, was the only independent risk factor for developing AEs within 90 days after initiating TLD (adjusted hazard ratio (aHR) = 2.73, 95% CI = 1.46–5.12) (Table 3).
Table 3.
Predictors of incidence rates of reported AEs. cHR crude Hazard ratio, aHR adjusted Hazard ratio, CI confidence interval.
| Characteristics | Univariate Cox proportional hazards model | Multivariate Cox proportional hazards model | ||
|---|---|---|---|---|
| cHR (95% CI) | P-value | aHR (95% CI) | P-value | |
| Sex | ||||
| Male | 1.06 (0.61–1.84) | 0.83 | ||
| Female | 1 | |||
| Age group (Years) | ||||
| 12–19 | 0.97 (0.23–4.14) | 0.58 | ||
| 20–24 | 1.65 (0.80–3.39) | |||
| 25–59 | 1 | |||
| ≥ 60 | 1.45 (0.51–4.13) | |||
| Weight categories (Kg) | ||||
| 20–40 | 1 | 0.92 | ||
| Above 40 | 0.95 (0.29–3.05) | |||
| Marital status | ||||
| Single | 1.56 (0.81–2.99) | 0.31 | 1.51 (0.78–2.93) | 0.20 |
| Cohabiting | 2.02 (0.68–5.95) | 2.00 (0.67–6.02) | ||
| Married | 1 | 1 | ||
| Widowed/Separated | 2.18 (0.98–4.84) | 2.43 (1.08–5.45) | ||
| Divorced | 1.04 (0.35–3.07) | 0.89 (0.30–2.68) | ||
| Education level | ||||
| None | 1 | 0.42 | 1 | 0.43 |
| Primary | 2.78 (0.67–11.59) | 3.19 (0.72–14.11) | ||
| Secondary | 2.85 (0.64–12.68) | 3.65 (0.76–17.59) | ||
| College and above | 2.95 (0.41–21.21) | 3.97 (0.54–29.49) | ||
| Baseline WHO HIV stage | ||||
| Stage 1 | 1 | 0.042 | 1 | 0.017 |
| Stage 2 | 2.47 (1.36–4.50) | 2.73 (1.46–5.12) | ||
| Stage 3 | 1.52 (0.69–3.37) | 1.46 (0.62–3.43) | ||
| Stage 4 | 1.40 (0.41–4.76) | 2.11 (0.60–7.43) | ||
Significant are in value [bold].
Discussion
The major findings of this prospective study include a relatively low incidence of reported AEs suggesting that a DTG-based regimen is generally safe in the population; geriatrics and participants with WHO HIV clinical stage 2 were more likely to report AEs.
The proportion of participants who experienced at least one AE in this study was 6.3%. Our finding is relatively lower compared to that reported in other studies conducted among patients who also initiated DTG-based regimens in Brazil (10%), Kenya (18%)25 and Uganda (33%)26. Our study had a relatively larger sample size than these previous studies which might partly explain the observed differences, albeit other predisposing factors might also explain. The reported AEs were mild and included skin itching and rashes, nausea and vomiting, tiredness, diarrhoea, fever and stomach pain corroborating with several other studies26. Neuropsychiatric AEs were reported as one of the reasons for discontinuation of DTG-based regimens in previous studies12,27. However, in our present study, few individuals reported neuropsychiatric AEs which were mild and tolerable including headache, sleep disturbances and dizziness suggesting that AEs associated with treatment discontinuation may be less likely. The reason for the observed differences could be due to the design of the studies, or other predisposing factors across the different populations. Similar to our study, a previous study done in Switzerland reported a low proportion of neuropsychiatric AEs28.
DTG is also known to affect the level of cellular insulin interfering with lipid metabolism, resulting in obesity among patients26. In this study, few individuals had increased weight within three months of follow-up. Our study revealed that most reported AEs were classified as disorders affecting skin and subcutaneous tissues, gastrointestinal system, nervous system, metabolism and nutrition, respiratory and thoracic system according to MedDRA. Similar findings were reported from Brazil, and Zambia29,30. Several other studies are also in tandem with our findings31–35.
In our study, the overall incidence of occurrence of the first AE was 96.7 per 1000 person-months. This incidence rate is higher compared to 20–60 per 1000 person-months of ADRs among DTG users reported in previous studies36–40. However, our finding is lower than other studies conducted in South Africa and Congo26,41,42. The higher incidence observed in other studies might be due to longer follow-up times as compared to the present study. Furthermore, our study reports a lower median time for the first ADR (87.9 days) than a study in Brazil, which had a median time of occurrence of 210.6 days25.
The incidence rates of AEs were high in elderly participants aged 60 years and above and those who had a baseline WHO HIV clinical stage 2. The observed wide confidence intervals in the analysis may be explained by the few number of events observed. Nevertheless, this finding should be interpreted with caution as it may suggest little knowledge of the effect requiring further research. On further analysis, individuals on WHO HIV clinical stage 2 at baseline had significantly 2.7 higher AEs reported than those on stage 1. This finding is consistent with the findings from studies done in Brazil and Ethiopia25,29,30. Contrary to our finding, a study from Uganda reported that individuals who have initiated DTG-based regimens on lower stages 1, 2 and 3 were more likely to report AEs than those in stage 4. Individuals with advanced AIDS disease and elders may be more immune-compromised than those on stage 1, and younger, therefore, susceptible to developing AEs.
Our study had some limitations, which included a short period of follow-up which was 90 days. This might cause an underestimation of the actual effects of the DTG-based regimen. Nevertheless, our findings were similar to other studies with both longer and shorter follow-up times36–40,43, suggesting that the safety profiles of DTG-based regimens may not differ significantly with different times of follow-up. On the flip side, the strength of our study is the prospective design whereby AEs were reported as they occurred in real-world situations as well as involving many (n = 25) CTCs in 11 regions of the country.
We recommend continued monitoring of the safety of the DTG-based regimen in the population, particularly in elders and those with advanced HIV disease. Further studies should focus on the long-term effects of DTG on the liver, kidney and other systemic organs.
Materials and methods
Ethical statement
Ethical clearance was obtained from the National Institute for Medical Research (BA. 126/329/01A/85) and the study was performed according to relevant regulations and guidelines. Permission to conduct the study was obtained from the relevant region, districts and health facility authorities. Written informed consent and assent were obtained from all eligible participants. All data were encoded and treated confidentially, following data security principles and good clinical practices.
Study design, population and setting
This was a prospective cohort study which involved PLHIV who were initiated on DTG based- regimen. The study included DTG naïve patients (newly ART initiated as well as clients who transitioned from another ART regimen to DTG). This study included individuals aged 12 years and above, newly diagnosed with HIV/AIDS and those initiated on or transitioned to Tenofovir, Lamivudine and Dolutegravir (TLD) during the study period. The study excluded seriously ill and hospitalized individuals. The study was conducted in 25 Care and Treatment Clinics (CTCs) from 11 regions of Tanzania's mainland. The regions were purposefully selected to represent administrative zones of the country, namely; the eastern zone (Morogoro, Dar es Salaam and Pwani), northern zone (Arusha and Tanga), lake zone (Shinyanga, Geita and Mwanza), central zone (Dodoma) and southern highlands zone (Iringa and Songwe). The CTCs were purposively selected by considering the number of PLHIV who were about to be initiated/transitioned to a DTG-based regimen. The CTCs with a relatively large number of PLHIV who were about to be initiated/transitioned to DTG-based regimen in each region were purposefully included. The list of CTCs and the corresponding number of individuals recruited is shown in Table 4.
Table 4.
List of health facilities and the number of recruited individuals.
| S/N | Health facilities | Individuals recruited | Region | Administrative zone |
|---|---|---|---|---|
| 1 | Kahama district hospital | 50 | Shinyanga | Lake zone |
| 2 | Shinyanga regional referral hospital | 50 | Shinyanga | |
| 3 | Geita regional referral hospital | 50 | Geita | |
| 4 | Katoro health center | 50 | Geita | |
| 5 | Sekou-Toure regional referral hospital | 50 | Mwanza | |
| 6 | Nyamagana hospital—district hospital | 50 | Mwanza | |
| 7 | Mwananyamala regional referral hospital | 60 | Dar es Salaam | Eastern Zone |
| 8 | Amana regional referral hospital | 60 | Dar es Salaam | |
| 9 | Mnazi Mmoja health center | 50 | Dar es Salaam | |
| 10 | Temeke regional referral hospital | 60 | Dar es Salaam | |
| 11 | Mbagala Rangi Tatu health center | 50 | Dar es Salaam | |
| 12 | Kilosa district hospital | 60 | Morogoro | |
| 13 | Bagamoyo district hospital | 60 | Pwani | |
| 14 | Mafinga district hospital | 60 | Iringa | Southern Highlands Zone |
| 15 | Iringa regional referral hospital | 60 | Iringa | |
| 16 | Ipamba designated hospital | 60 | Iringa | |
| 17 | Vwawa hospital | 60 | Songwe | |
| 18 | Tunduma town hospital | 60 | Songwe | |
| 19 | Dodoma regional referral hospital | 100 | Dodoma | Central Zone |
| 20 | Makole health center | 100 | Dodoma | |
| 21 | Ngamiani health center | 60 | Tanga | Northern Zone |
| 22 | Muheza DD hospital | 60 | Tanga | |
| 23 | Tanga regional referral hospital | 60 | Tanga | |
| 24 | St. Elizabeth council designated hospital | 60 | Arusha | |
| 25 | Mount Meru regional referral hospital | 60 | Arusha | |
| Total individuals recruited | 1500 |
Data collection
Data were collected electronically using structured questionnaires through the Open Data Kit (ODK®). Prior to data collection, healthcare workers from selected CTCs were recruited as research assistants and were trained on essential components of the study, including a thorough orientation of the tools (i.e., informed consent/assent and study questionnaires). The tool was pre-tested in the field, validated and finalized.
Baseline information collected were socio-demographic characteristics and clinical profiles of participants. The socio-demographic characteristics including sex, age, body weight, marital status and educational level were collected during recruitment; whereas clinical profile (WHO HIV clinical stage, presence of any comorbidities, CD4 cell count and treatment history) were abstracted from the client’s medical records. The adverse events experienced by study participants anytime within 90 days of TLD initiation were also collected at the CTCs during scheduled visits. In all participants (both initiated and transitioned to DTG-based regimen), the visits followed the routine standard of care, at day 14, day 30 and day 90 since drug initiation.
Study outcomes
The primary endpoint was the time to occurrence of any AE after initiation of DTG-based regimen. At enrollment, a thorough assessment of any presenting symptoms was done for all individuals. The reported AE was only considered DTG-based regimen associated if it was not reported at enrollment before drug initiation. Other secondary study outcomes were the types and severity of AEs. AEs were graded according to the internationally regognized Common Terminology Criteria for Adverse Events (CTCAE) version 4. According to these criteria, AEs are graded as Mild (Grade 1: asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated); Moderate (Grade 2: minimal symptoms; local or non-invasive intervention indicated; limiting age-appropriate instrumental activities of daily living) or Severe (Grade 3: medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living)44. The AEs were further classified according to the Medical Dictionary for Regulatory Activities (MedDRA) which is a clinically validated international medical terminology dictionary-thesaurus used by regulatory authorities for safety information.
Statistics and analysis
Data analysis was done using STATA 15 (College Station, TX). Categorical variables were summarized using frequencies with proportions and median with interquartile range (IQR) were used to summarize continuous variables. Univariable and multivariable Cox regression were used to investigate risk factors for the occurrence of AEs. Variables of clinical significance or with p-value ≤ 0.2 were added to the multivariable model. P-values < 0.05 were considered statistically significant.
Acknowledgements
We would like to thank all study participants who volunteered to participate in this study from all the CTCs in the country. We also convey our sincere thanks to all healthcare workers from all the facilities for their tireless cooperation during the study.
Author contributions
A.F., Y.H.M., K.M., D.M., and E.M. designed the study. Data management, statistical analyses and results interpretation was conducted by A.F., Y.H.M., K.M., D.M., E.M., J.L., A.S., S.M., E.B., G.G., D.R., A.N., E.M., R.H.M., P.P.K., C.M., C.C.M. E.M.M. and O.M.S.M. A.F., Y.H.M. E.M.M. and RHM drafted the manuscript, which was critically revised by all authors. All authors (A.F., Y.H.M., K.M., D.M., E.M., J.L., A.S., S.M., E.B., G.G., D.R., A.N., E.M., R.H.M., P.P.K., C.M., C.C.M. E.M.M. and O.M.S.M.) read and approved the final manuscript.
Funding
This study funded by the Global fund and the World Health Organization. The funder had no role in study design, data collection and analysis, decision to publish or preparation of this manuscript.
Data availability
All data generated or analyzed during this study are included in this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS. Global HIV & AIDS statistics-Fact sheet. Available at https://www.unaids.org/en/resources/fact-sheet. Accessed on 18/09/2023.
- 2.UNAIDS. Country progress report - United Republic of Tanzania (2020). Available from: https://www.unaids.org/sites/default/files/country/documents/TZA_2020_countryreport.pdf. Accessed on 18/4/2023.
- 3.Palella FJ, Jr, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 1998;338:853–860. doi: 10.1056/nejm199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Agu KA, Oparah AC. Adverse drug reactions to antiretroviral therapy: Results from spontaneous reporting system in Nigeria. Perspect. Clin. Res. 2013;4:117–124. doi: 10.4103/2229-3485.111784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gudina EK, et al. Magnitude of antiretroviral drug toxicity in adult HIV Patients in Ethiopia: A cohort study at seven teaching hospitals. Ethiop. J. Health Sci. 2017;27:39–52. doi: 10.4314/ejhs.v27i1.5s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weldegebreal F, Mitiku H, Teklemariam Z. Magnitude of adverse drug reaction and associated factors among HIV-infected adults on antiretroviral therapy in Hiwot Fana specialized university hospital, eastern Ethiopia. Pan. Afr. Med. J. 2016;24:255. doi: 10.11604/pamj.2016.24.255.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Severe P, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UN. UNAIDS Issues New Fast-Track Strategy to End AIDS by 2030. Available at https://www.pedaids.org/2014/11/20/unaids-issues-new-fast-track-strategy-to-end-aids-by2030/#:~:text=Only%20if%20these%20targets%20are,treatment%20with%20suppressed%20viral%20loads.
- 9.CDC. Tanzania HIV Impact Survey: Tracking Progress and Informing Action (2022). Available from: https://www.cdc.gov/globalhealth/stories/2023/tanzania-hiv-impact-surve.html. Accessed on September 15, 2023.
- 10.Bonfanti P, et al. Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group. J. Acquir. Immune Defic. Syndr. 2000;23:236–245. doi: 10.1097/00126334-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. A practical handbook on the pharmacovigilance of antiretroviral medicines. World Health Organization (2009). Available at http://apps.who.int/iris/bitstream/handle/10665/44236/9789241547949_eng.pdf;jsessionid=1CD2FCB7DC9347A7EFBEBB3F45FECEF3?sequence=1. Accessed on 18/4/2023. .
- 12.World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens (2019). Available at https://www.who.int/publications/i/item/WHO-CDS-HIV-19.15. Accessed on 18/4/2023.
- 13.Hoffmann C, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18:56–63. doi: 10.1111/hiv.12468. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization (2018). Available at https://apps.who.int/iris/handle/10665/277395. Accessed on 18/4/2023.
- 15.Wadesango, L., Wiafe, E., Padayachee, N., Mensah, K. & Bangalee, V. Dolutegravir Reported Adverse Drug Reactions: A Systematic Review Protocol. (2021).
- 16.Lamorde M, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV. 2020;7:e461–e462. doi: 10.1016/s2352-3018(20)30042-4. [DOI] [PubMed] [Google Scholar]
- 17.Bonfanti P, et al. Discontinuation of treatment and adverse events in an Italian cohort of patients on dolutegravir. Aids. 2017;31:455–457. doi: 10.1097/qad.0000000000001351. [DOI] [PubMed] [Google Scholar]
- 18.de Boer MG, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. Aids. 2016;30:2831–2834. doi: 10.1097/qad.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 19.Ndashimye E, Arts EJ. The urgent need for more potent antiretroviral therapy in low-income countries to achieve UNAIDS 90–90-90 and complete eradication of AIDS by 2030. Infect Dis Poverty. 2019;8:63. doi: 10.1186/s40249-019-0573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bavaro DF, et al. Pretreatment HIV drug resistance and treatment failure in non-Italian HIV-1-infected patients enrolled in ARCA. Antivir. Ther. 2020;25:61–71. doi: 10.3851/imp3349. [DOI] [PubMed] [Google Scholar]
- 21.Shet A, et al. Influence of adverse drug reactions on treatment success: prospective cohort analysis of HIV-infected individuals initiating first-line antiretroviral therapy in India. PLoS One. 2014;9:e91028. doi: 10.1371/journal.pone.0091028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eluwa GI, et al. Adverse drug reactions to antiretroviral therapy (ARVs): incidence, type and risk factors in Nigeria. BMC Clin. Pharmacol. 2012;12:7. doi: 10.1186/1472-6904-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacha JM, Dlamini S, Anabwani F, Gwimile J, Kanywa JB, Farirai J, Bvumbwe M, Tsotako M, Steffy T, Nguyen D, Mendez-Reyes JE, Elyanu P, Haq H. Realizing the promise of dolutegravir in effectively treating children and adolescents living with HIV in real-world settings in 6 Countries in Eastern and Southern Africa. Pediatr. Infect. Dis. J. 2023;42(7):576–581. doi: 10.1097/INF.0000000000003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutagonda RF, Mlyuka HJ, Maganda BA, Kamuhabwa AAR. Adherence, Effectiveness and Safety of Dolutegravir Based Antiretroviral Regimens among HIV Infected Children and Adolescents in Tanzania. J Int Assoc Provid AIDS Care. (2022). [DOI] [PMC free article] [PubMed]
- 25.Abutika, R. A. Burden of Neuropsychiatric Adverse Effects and Changes in Weight Among Hiv Infected Patients Switched From an Efavirenz Based to a Dolutegravir Based First Line Regimen at the Kenyatta National Hospital, University of Nairobi, (2020).
- 26.Namulindwa A, Wasswa JH, Muyindike W, Tamukong R, Oloro J. Prevalence and factors associated with adverse drug events among patients on dolutegravir-based regimen at the Immune Suppression Syndrome Clinic of Mbarara Regional Referral Hospital, Uganda: a mixed design study. AIDS Res. Ther. 2022;19:18. doi: 10.1186/s12981-022-00442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuzin L, et al. Integrase strand transfer inhibitors and neuropsychiatric adverse events in a large prospective cohort. J. Antimicrob. Chemother. 2019;74:754–760. doi: 10.1093/jac/dky497. [DOI] [PubMed] [Google Scholar]
- 28.Elzi L, et al. Adverse events of raltegravir and dolutegravir. Aids. 2017;31:1853–1858. doi: 10.1097/qad.0000000000001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendes, J. C., das Graças Braga, M., Reis, A. M. M. & Silveira, M. R. Incidence and factors associated with adverse drug reactions in a cohort of individuals starting dolutegravir or efavirenz. Res. Soci. Develop.11, e0811426250-e0811426250 (2022).
- 30.Chilambe M, Kalungia AC, Mangani A, Munkombwe Z. An analysis of pharmacovigilance case reports of adverse drug events attributable to dolutegravirbased antiretroviral treatment for HIV in Zambia. Med. J. Zambia. 2019;46:305–313. doi: 10.55320/mjz.46.4.602. [DOI] [Google Scholar]
- 31.Wohl DA, et al. A randomized, double-blind comparison of single-tablet regimen elvitegravir/cobicistat/emtricitabine/tenofovir DF versus single-tablet regimen efavirenz/emtricitabine/tenofovir DF for initial treatment of HIV-1 infection: analysis of week 144 results. J. AIDS. 2014;65:e118–e120. doi: 10.1097/QAI.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 32.Kolakowska A, Maresca AF, Collins IJ, Cailhol J. Update on adverse effects of HIV integrase inhibitors. Curr. Treat. Opt. Infect. Dis. 2019;11:372–387. doi: 10.1007/s40506-019-00203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cahn P, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382:700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 34.Wohl DA, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6:e355–e363. doi: 10.1016/S2352-3018(19)30077-3. [DOI] [PubMed] [Google Scholar]
- 35.Sax PE, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet. 2017;390:2073–2082. doi: 10.1016/S0140-6736(17)32340-1. [DOI] [PubMed] [Google Scholar]
- 36.Correa A, et al. Dolutegravir: Virologic response and tolerability of initial antiretroviral regimens for adults living with HIV. PLoS One. 2020;15:e0238052. doi: 10.1371/journal.pone.0238052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walmsley SL, et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. New Engl. J. of Med. 2013;369:1807–1818. doi: 10.1056/NEJMoa1215541. [DOI] [PubMed] [Google Scholar]
- 38.Clotet B, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383:2222–2231. doi: 10.1016/S0140-6736(14)60084-2. [DOI] [PubMed] [Google Scholar]
- 39.Todd S, et al. Early clinical experience of dolutegravir in an HIV cohort in a larger teaching hospital. Intern. J. STD & AIDS. 2017;28:1074–1081. doi: 10.1177/0956462416688127. [DOI] [PubMed] [Google Scholar]
- 40.Taramasso, L., De Vito, A., Ricci, E.D., Orofino, G., Squillace, N., Menzaghi, B., Molteni, C., Gulminetti, R., De Socio, G.V., Pellicanò, G.F., Sarchi, E., Celesia, B.M., Calza, L., Rusconi, S., Valsecchi, L., Martinelli, C.V., Cascio, A., Maggi, P., Vichi, F., Angioni, G., Guadagnino, G., Cenderello, G., Dentone, C., Bandera, A., Falasca, K., Bonfanti, P., Di Biagio, A., Madeddu, G.; Behalf of the CISAI Study Group. Durability of Dolutegravir-Based Regimens: A 5-Year Prospective Observational Study. AIDS Patient Care STDS 35(9):342–353 (2021). [DOI] [PubMed]
- 41.Tatz, G. Adverse reactions to dolutegravir reported to the National HIV & TB Health Care Worker Hotline in South Africa. Depression1, 100.
- 42.Nabitaka VM, et al. High acceptability and viral suppression of patients on Dolutegravir-based first-line regimens in pilot sites in Uganda: a mixed-methods prospective cohort study. PloS one. 2020;15:e0232419. doi: 10.1371/journal.pone.0232419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Wang Y, Li E, Yan D, Yu J, Zhu M, Shi J, Zheng L. Neuropsychiatric adverse events following antiretroviral therapy in people living with HIV: A real-world study of dynamic trends and risk factors in Hangzhou, China. Infect Drug Resist. 2023;2(16):5007–5019. doi: 10.2147/IDR.S419308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Institutes of Health, US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.03. Bethesda, MD. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 7 December (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this manuscript.