Abstract
Probiotics have gained a significant attention as a promising way to improve gut health and overall well-being. The increasing recognition of the potential health advantages associated with functional food products, leading to a specific emphasis on co-encapsulating probiotic bacteria and bioactive compounds within a unified matrix. To further explore this concept, a meta-analysis was performed to assess the effects of probiotics encapsulated in nanoparticles. A comprehensive meta-analysis was conducted, encompassing 10 papers published from 2017 to 2022, focusing on the encapsulation of probiotics within nanoparticles and their viability in various gastrointestinal conditions. The selection of these papers was based on their direct relevance to the research topic. Random-effect models were used to aggregate study-specific risk estimates. In the majority of studies, it was observed that nano-encapsulated nanoparticles showed improved viability over time compared to their free state counterparts. At various time intervals, the odds ratios (OR) with 95% confidence intervals (CI) were estimated using fixed and random effect models. At 0 min, the OR (95%CI) was 2.79 (2.79; 2.80) and 2.38 (2.14; 2.64) for. At 30 and 60 min observation was at similar rate of 2.23 (2.23; 2.24) and 2.05 (1.73; 2.43). However, at 90 min it was 1.39 (1.39; 1.39) and 1.66 (1.29; 2.14) and at 120 min 2.41 (2.41; 2.42) and 2.03 (1.63; 2.52). Overall evaluation of encapsulation revealed an improvement in probiotic bacterial viability in simulated the gastrointestinal environments.
Subject terms: Biotechnology, Nanoscience and technology
Introduction
Probiotics are nonpathogenic bacteria that are naturally derived from sources such as dairy foods. The global probiotics market is experiencing rapid expansion, and there is a growing momentum in research efforts to convert probiotics into medicinal adjuvants. Accordingly, the global probiotics market rose every year. The medical usage of probiotics has a lengthy history, grounded in the concept that oral or topical probiotic treatment has the potential to replace damaged human microbiota. Additionally, they have been linked to a variety of favorable outcomes through aiding digestion1,2, increasing nutrient absorption3,4, improve metabolism (including lactose intolerance5, calcium absorption6), and strengthening the immune system7. It also has a regulatory function in the body, such as boosting biological defense systems, reducing particular diseases, managing mental and physical illnesses, and slowing the aging process8. Ensuring the adequate quantity and targeted release of probiotics is crucial for their effective delivery to the large intestine. This is because free probiotics are susceptible to destruction due to the harsh conditions present in the upper gastrointestinal tract (GIT) of humans. These conditions include antimicrobial lysozyme in the oral cavity9 acidic environment in the gut10 bile salts and digestive enzymes in the small intestine11, as well as other factors like osmotic pressure and oxidative stress across the gastrointestinal tract.
Presently, microbial strains need to fulfill specific criteria to be recognized as potential probiotics. In accordance with the guidelines proposed by the FAO/WHO, each probiotic strain must undergo accurate identification and undergo various in vitro assays to evaluate their functional properties12. Despite the availability of numerous commercial probiotics in the market, there continues to be an ongoing need for new probiotic strains that exhibit improved characteristics compared to current ones. In recent years, several bacterial species including Lactobacillus spp. have emerged as potential probiotics. Lactobacilli are a type of Gram-positive bacteria that are catalase-negative13. They are naturally present in the oral cavity, intestine, and female vaginal tract. They play a crucial function in controlling the growth of undesirable microorganisms, making them natural bio preservatives14. Lactobacilli have been designated as 'generally recognized as safe' (GRAS) by the United States Food and Drug Administration (USFDA) and 'qualified presumption of safety' (QPS) by the European Food Safety Authority (EFSA), allowing them to be used in food preparation15. Their significant economic importance has led to extensive research, resulting in a comprehensive understanding of their genomics and relationships with humans concerning both health and disease. Lactobacillus species are great candidates for probiotics due to these properties. The link between Lactobacilli and humans is mutually beneficial. Lactobacillus species aid the host in the digestion of specific dietary components and provide protection against infections16. Furthermore, continuing research is examining novel approaches to optimizing probiotic delivery, functionality, and monitoring through the use of new technologies such as nanotechnology17.
Nanotechnology contributes to various domains within the realms of science and technology. To get the beneficial effects, nanotechnology emerging as an effective alternative to traditional therapies. The convergence of probiotic science, with the realm of nanotechnology gives rise to a novel field called “nanoprobiotics”18. This evolving field employs a specific strategy to address certain limitations associated with the utilization of probiotics in food and therapeutic applications. It involves encapsulating probiotics and other bioactive components within protective shells of nanoparticles, which act as physical barriers. This technique aims to enhance the viability and bioavailability of probiotics by safeguarding them during storage and transit. This meta-analysis and systematic review present a comprehensive overview of recent nano-formulation approaches aimed at optimizing the delivery of probiotics, and formulation technologies utilized in the field to improve the efficacy and viability of nanoparticle encapsulated probiotics.
Materials and methods
Study design and search strategy
This study followed the guidelines set forth in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement in both its design and reporting19 Various Medline search engines, including PubMed, Google Scholar, Science Direct, Scopus and Web of Science were utilized to conduct a comprehensive review of the literature from 2016 to 2022, without language restrictions. Different MeSH terms (“probiotics” [MeSH Terms] OR “probiotics” [All Fields]) AND “Nanoparticle encapsulation” [MeSH Terms] OR “Nanoparticle encapsulation” [All Fields] AND “encapsulation techniques”) OR “probiotics” [MESH Terms] AND “Nano formulations” [All Fields] OR “probiotics” (MeSH Terms] AND “Nano formulations” [All Fields] OR “Lactobacillus” [MeSH Terms] AND “Nano formulations” [All Fields]) searched on different Medline databases and additional searches were conducted using known probiotic types, referencing author names, meeting abstracts, and the reference lists of included literature. Furthermore, a systematic search was conducted by reviewing the bibliographies of all publications obtained.
Inclusion and exclusion criteria
We searched for the articles for the meta-analysis, the studies that were included met the following criteria: (1) articles should be from 2017 to 2022 (2) articles had to be published in peer-reviewed journals, (3) the probiotic bacteria used had to be Lactobacillus spp., (4) the articles needed to mention nanoparticle encapsulation materials and techniques, (5) only probiotics that met the standard criteria (living microbe, adequate dose, and demonstrated efficacy for optimal health effect) were considered, and (6) studies reporting survivability/viability outcomes in colony-forming units (CFU).
Whereas the following criteria were applied to exclude the articles (1) conference, abstracts, perspectives, review articles, and meta-analysis (2) study protocols and articles lacking full text or not published in English (3) articles that only mentioned microencapsulation but not nanoparticle-based encapsulation, and (4) studies that did not provide the necessary data.
Data extraction
The data related to different types of nanoparticles, probiotics, and their encapsulation were collected and organized in a single sheet using Microsoft Office Excel® (2013). Prior to the complete extraction, a pre-test was conducted. The extracted data consisted of authors' names, publication years (2017–2022), microorganism species, encapsulating nanomaterials, encapsulating techniques, characteristics (with emphasis on particle size and morphological characteristics), viability, and encapsulation yield. The data underwent careful examination, and information specifically related to Nanocarrier-Mediated Probiotic Delivery was visualized in a table and forest plot, including the relevant citations, using Mendeley (version 1.19.8).
Statistical analysis
The statistical analysis and generation of forest plots for pooled summary estimates were conducted using the meta or metafor package in R software20. The summary estimates were derived from pooled data of forest plots representing different time points after encapsulation of probiotics of the same type of probiotic bacteria (i.e., Lactobacillus spp.) and measuring the common outcome of probiotic bacteria survivability in the nanoparticles. 95% confidence intervals (CI) and odds ratios (OR) for both fixed-effect and random-effects models were calculated. The I2 and τ2 statistics were used to evaluate the heterogeneity of the data. All meta-analyses used random-effects models, and the results were displayed in forest plots. Furthermore, the statistical significance was validated by the p-value (p < 0.05).
Publication bias
The assessment of publication bias is a vital step in ensuring the robustness and validity of our meta-analysis of the effects of probiotics encapsulated in nanoparticles. To evaluate the potential impact of publication bias on our findings, we employed several methods recommended in the field. We visually inspected risk of bias graph to detect asymmetry, a potential indicator of publication bias. By employing this comprehensive approaches, we aimed to account for any potential bias and ensure that our meta-analysis provides an unbiased synthesis of the available evidence on the efficacy of probiotics encapsulated in nanoparticles.
Result
Study selection and characteristics
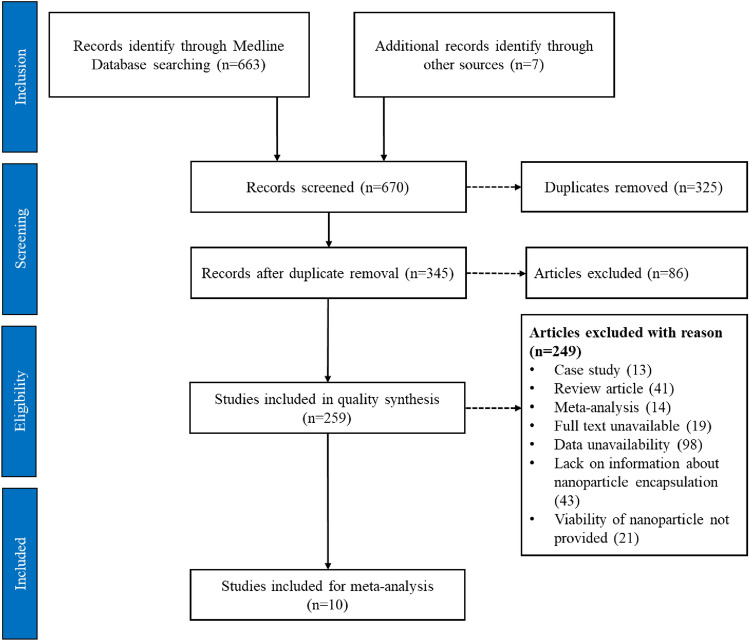
A total of 670 papers published between 2017 and 2022 were categorized using Medline databases. After removing duplicates, 345 studies underwent initial screening based on their title and abstract. From these, 86 articles were excluded due to irrelevance or redundancy, leaving 259 articles for further examination. Subsequently, 249 articles were excluded for various reasons: 13 were case studies, 41 were review articles, and 14 were meta-analyses. Additionally, 19 articles lacked full-text availability, and 98 articles lacked essential data or statistics. Furthermore, 43 articles did not provide information about nanoparticle encapsulation, and 21 articles did not report nanoparticle viability within the specified time frame. Ultimately, this meta-analysis and systematic review incorporated a total of 10 studies. The study selection process adhered to the PRISMA flow diagram, depicted in Fig. 1.
Figure 1.
Visual Representation of the Study Selection Process using PRISMA Guidelines.
Meta-analysis
Risk of bias
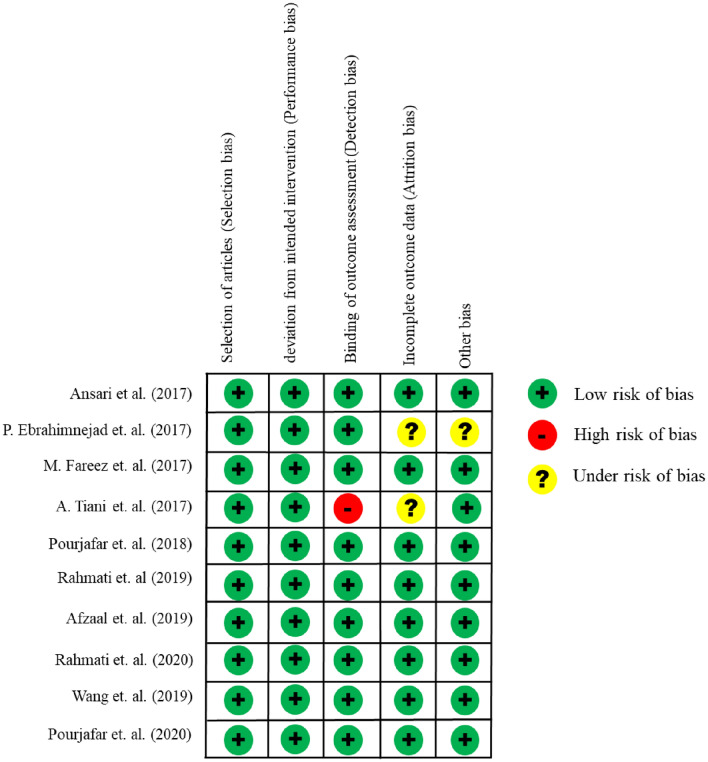
Figure 2 presents a comprehensive assessment of the overall risk and individual biases in each included study. All researchers conducted evaluations to determine the likelihood of bias, and the assessment findings demonstrated remarkable consistency across all investigations. On the basis of the outcomes displayed in Figure 2, it is clear that the study was conducted by by Tiani et al.21 exhibited a high risk of bias Furthermore, Ebrahimnejad et al.22 found a potential risk of bias in their investigation. This robust and uniform approach strengthens the reliability of the research paper's results, ensuring a high level of confidence in the reported biases and their impact on the study outcomes.
Figure 2.
Risk of bias graph for the selected studies.
Literature data search and data mining
The meta-analysis process involved the selection of ten peer-reviewed research articles21–30 published between 2017 and 2021. These articles provided data on various aspects, including the study and publication year, probiotic species, encapsulating nanomaterials, encapsulating techniques, particle size, morphological characteristics, viability at different time intervals (0 min, 30 min, 60 min, 90 min, and 120 min), and encapsulation yield as illustrated in Table 1.
Table 1.
Summary of subgroup analysis results.
| Study (year) | Probiotic species | Encapsulating nanomaterials | Encapsulating technique | Characteristics of microcapsules | Viability (CFU/ml) | Encapsulation yield | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Particle size | Morphological characteristics | State | 0 min | 30 min | 60 min | 90 min | 120 min | |||||
| Ansari et al. (2017)24 | Lactobacillus acidophilus | Eudragit S100 and chitosan | Extrusion | 100 nm |
- 123.66 ± 41.73 μm in diameter - Sphere in shape - Polydispersity index was 0.410 |
Free | 9.78 | 7.27 | 5.53 | 4.56 | 3.0 | - Survivability increased |
| Encapsulated | 9.52 | 8.17 | 7.54 | 6.6 | 5.02 | |||||||
| Ebrahimnejad et al. (2017)22 | Lactobacillus acidophilus | Chitosan | Ionic gelation | 120 to 338 nm | - Spherical, distinct and regular shape of particle | Free | 3.3 | 3.26 | 3.2 | 3.11 | 3.09 |
- Bacterial survival in simulated gastric and intestinal environments improves - Concentration of chitosan determine size of particles |
| Encapsulated | 3.2 | 3.27 | 3.25 | 3.23 | 3.23 | |||||||
| M. Fareez et al. (2017)25 | Lactobacillus plantarum | Alginate | Extrusion-dripping | 1299 to 1341 μm |
- Beads appeared ‘crumpled’ and irregular in shape - Polydispersity index was 0.05 |
Free | 10.4 | 9.4 | 7.5 | 3.6 | 3.15 |
- Probiotic bacteria show improved viability under simulated gastric and intestinal conditions - Concentration doesn’t determine size of particles |
| Encapsulated | 10.6 | 8.2 | 8.1 | 8.05 | 7.5 | |||||||
| A. Tiani et al. (2017)21 | Lactobacillus plantarum | Sodium alginate | Extrusion | 324 and 327 μm | - Irregularly shaped, 150–350 μm in diameter | Free | 9.86 | 8.64 | 8.52 | 8.51 | 8.35 | - Alginate micro beads maintained cell viability during refrigerated storage and enhanced resistance to simulated gastric and intestinal conditions |
| Encapsulated | 10.1 | 8.69 | 9.2 | 9.27 | 9.26 | |||||||
| Pourjafar et al. (2018)26 | Lactobacillus acidophilus | Eudragit S100 | Extrusion | 100 nm | – | Free | 10.5 | 8.0 | 6.1 | 5.4 | 8.35 | - Probiotic bacteria survive in adverse conditions |
| Encapsulated | 11.1 | 9.1 | 8.3 | 6.5 | 8.35 | |||||||
| Rahmati et. al (2019)29 | Lactobacillus casei | Eudragit S100 |
Homogenization/Supercritical antisolvent - technique |
100 to 170 nm/120 nm | - 70–180 μm in diameter | Free | 6.0 | 4.4 | 6.1 | – | 5.3 |
- Organoleptic attributes like texture, flavor, and aroma is improved - Function in gastric conditions improved |
| Encapsulated | 6.9 | 3.9 | 5.9 | – | 3.6 | |||||||
| Afzaal et al. (2019)30 | Lactobacillus casei | Calcium alginate and whey protein | Extrusion | – | - 716–727 μm in diameter | Free | 10.79 | 4.4 | 8.1 | 6.8 | 5.48 | - Function is improved under simulated gastrointestinal conditions |
| Encapsulated | 10.72 | 9.8 | 9 | 6.3 | 7.65 | |||||||
| Rahmati et al. (2020)28 | Lactobacillus acidophilus |
Alginate chitosan, - Eudragit S100 |
Homogenization/Supercritical antisolvent - technique |
110–170 nm/122 nm |
- Polydispersity index was 0.460 - 80–180 μm in diameter |
Free | 6.4 | 6.4 | 5.0 | – | 4.3 | - Viability increased |
| Encapsulated | 6.8 | 6.8 | 6.1 | – | 5.6 | |||||||
| Wang et al. (2019)23 | Lactobacillus pentosus | Chitosan and sodium phytate | Layer-by-layer | – | - Zeta potential of coated nanoparticle is + 39.9 mV | Free | 8.5 | 8.4 | 8.6 | 8.4 | 7.9 | - Higher survival rates in simulated gastrointestinal fluid and bile salts |
| Encapsulated | 8.6 | 8.5 | 8.6 | 8.5 | 8 | |||||||
| Pourjafar et al. (2020)27 | Lactobacillus acidophilus | Eudragit S100 and chitosan |
Homogenization/Supercritical antisolvent - technique |
100–150 nm/100 nm |
- Polydispersity index was 0.410 - beads were sphere-shaped with - a mean diameter about 1 mm |
Free | 5.3 | 3.3 | 7.8 | 1.3 | 1.1 | - Survivability increased |
| Encapsulated | 8.7 | 2.9 | 5.1 | 4.9 | 8 | |||||||
Lactobacillus acidophilus
Five articles22,24,26–28 investigated the encapsulation of Lactobacillus acidophilus, and the majority of them employed Eudragit S100 nanomaterial in combination with chitosan24,26,27,29. However, one article by Ebrahimnejad et al.22 used chitosan alone as the encapsulating nanomaterial The encapsulation techniques varied among the studies, with Ansari et al24 and Pourjafar et al26 employing the extrusion method, Rahmati et al29 and Pourjafar et al27 using a different technique, and Ebrahimnejad et al22 utilizing the Ionic gelation method The average nanoparticle size across all the articles was observed to be 100 nm (Table 1).
Lactobacillus casei
In two separate studies29,30, Lactobacillus casei was the subject of investigation. Afzaal et al30 employed the extrusion technique for encapsulation, while Rahmati et al29 used Eudragit S100 nanomaterial with two distinct encapsulation techniques: homogenization and supercritical antisolvent The resulting nanoparticle sizes showed variability, with Rahmati et al29 achieving sizes ranging from 100 to 170 nm and 120 nm (as shown in Table 1) The encapsulation of Lactobacillus casei using different methods and nanomaterials demonstrates the versatility of the approach, allowing for control over the size of the nanoparticles.
Lactobacillus plantarum
Fareez et al25 and Tiani et al21 conducted separate studies involving Lactobacillus plantarum Both groups utilized alginate as the encapsulating material, with the extrusion technique employed in both cases However, notable differences in the size of the resulting nanoparticles were observed Fareez et al25 achieved nanoparticle sizes ranging from 1299 to 1341 μm, whereas Tiani et al21 obtained sizes of 324 and 327 μm Importantly, the alginate microbeads were found to effectively preserve the viability of the encapsulated cells during refrigerated storage. Additionally, these microbeads exhibited enhanced resistance to simulated gastric and intestinal conditions, suggesting their potential as protective carriers for Lactobacillus plantarum (Table 1).
Lactobacillus pentosus
In a study carried out by Wang et al23 Lactobacillus pentosus was encapsulated using chitosan and sodium phytate through a layer-by-layer approach. While the specific size of the nanoparticles was not mentioned in the article, the encapsulated nanoparticles exhibited higher survival rates when subjected to simulated gastrointestinal fluid and bile salts, as indicated in Table 1.
Analysis of nanoparticle encapsulated probiotic efficiency
In this study, a total of ten research studies21–30 were selected based on their suitability for quantitative analysis. The objective was to investigate the flexural strength data for different fraction weights of encapsulated probiotics in nanoparticles To analyze the data, five meta-analysis were conducted, and forest plots were constructed to visualize the results.
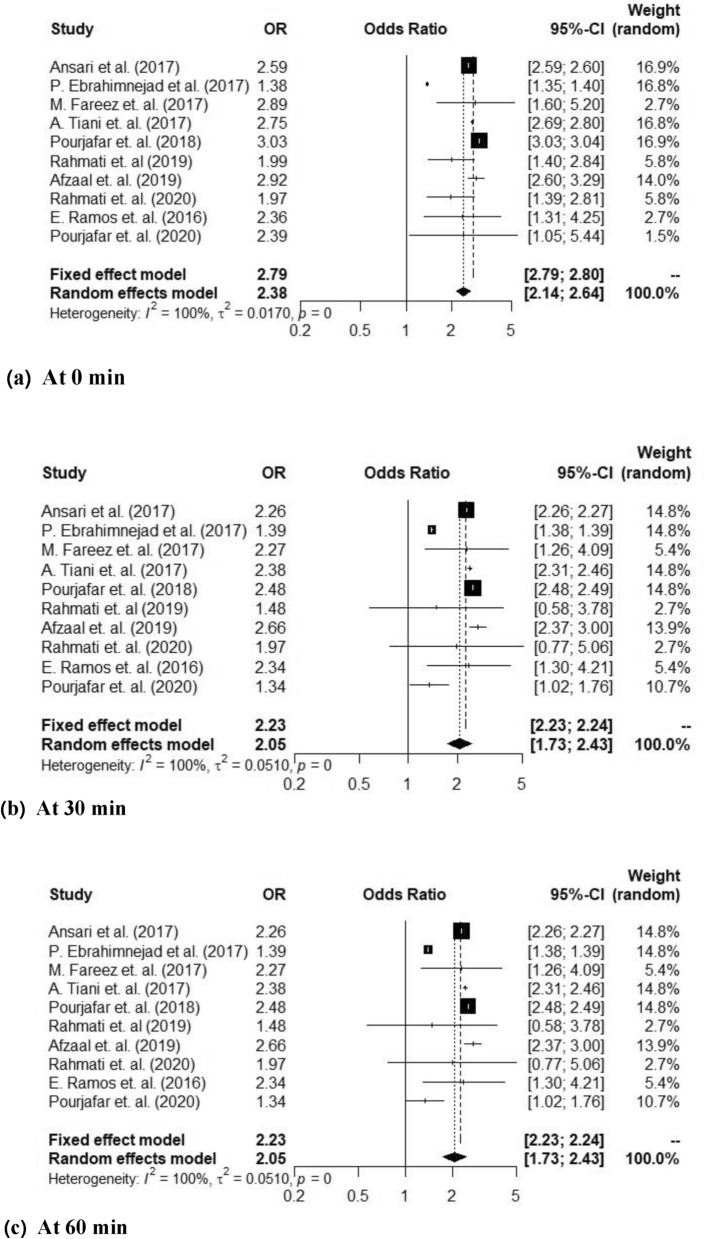
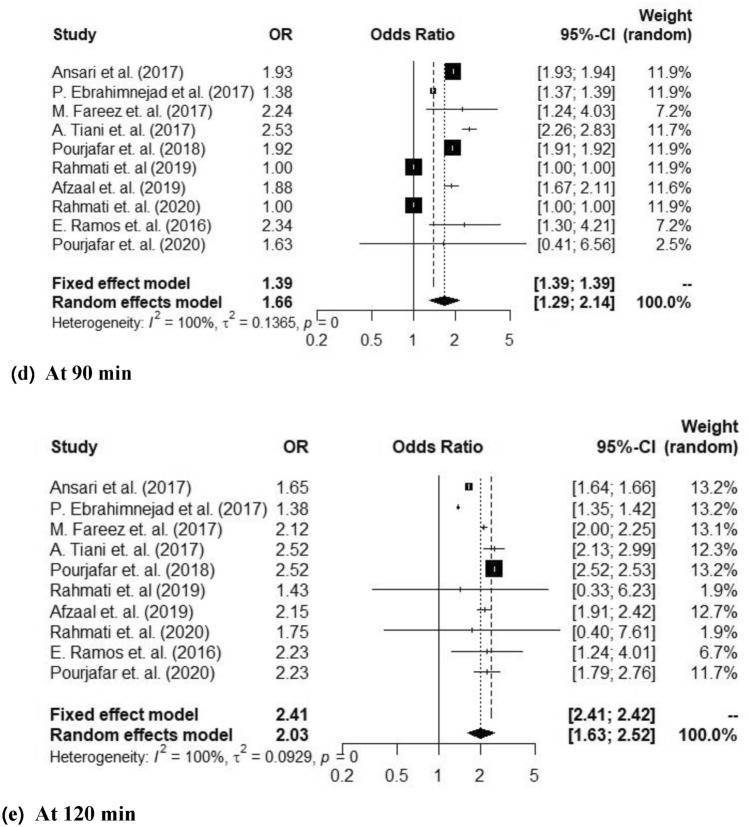
In Fig. 3a, the forest plot presents the results for the 0-min time point following lactobacillus spp. encapsulation, showing a 95% confidence interval (CI) of (2.79; 2.80) for the fixed effect model and (2.14; 2.64) for the random effect model. Notably, all included studies demonstrated a high level of heterogeneity with an inconsistency test (I2) of 100% and a study variance (τ2) for random-effects across studies of 0.0.0170. The odds ratio (OR) values were 2.79 for the fixed effect model and 2.38 for the random effect model, and the p-value of 0 indicates significant statistical significance at 0 min after encapsulation. Moving to Fig. 3b, representing the 30-min time point after encapsulation, the analysis yielded a 95% CI of (0.23; 2.24) for the fixed effect model and (1.73; 2.24) for the random effect model, with OR values of 2.23 and 2.05, respectively The included studies exhibited a low level of heterogeneity (I2) of 100% and a study variance (τ2) of 0.01510, with a p-value of 0 indicating strong statistical significance at 30 min. Similarly, Fig. 3c focused on the 60-min time point, revealing a 95% CI of (2.23; 2.24) for the fixed effect model and (1.73; 2.43) for the random effect model, with OR values of 2.23 and 2.05, and a low level of heterogeneity (I2) and study variance (τ2) of 0, and the p-value was 0.97 Figure 3d displayed the forest plot for the 90-min time point after encapsulation, with a 95% CI of (1.39; 1.39) for the fixed effect model and (1.29; 2.14) for the random effect model, OR values of 1.39 and 1.66, and a low level of heterogeneity (I2) of 100% and study variance (τ2) of 0.1365, while the p-value was 0 indicating statistical significance at 90 min Finally, Fig. 3e illustrated the forest plot for the 120-min time point after encapsulation, revealing a 95% CI of (2.41; 2.42) for the fixed effect model and (1.63; 2.52) for the random effect model, with OR values of 2.41 and 1.78, and a low level of heterogeneity (I2) of 2.41 and study variance (τ2) of 2.03, while the p-value was 0 indicating statistical significance at 120 min.
Figure 3.
Forest plot of the viability of nanoparticle encapsulated probiotic lactobacillus spp showing the odd ratio (OR), 95% confidence intervals (CI) and weight (random) at different time intervals.
Global market share of probiotics
The global food probiotics market recorded a significant milestone in 2022, reaching a value of US$ 60.5 billion. Forecasts indicate that this market is poised for further growth, with an estimated value of US$ 100.1 billion projected by 2030. This predicted growth implies a compound annual growth rate (CAGR) of 6.5% from 2023 to 2030. Notably, the Regulatory Affairs Professionals Society revealed that the total probiotic market surpassed US$ 48 billion in 2021, underlining its substantial presence. Among the geographical regions, North America stands out as the fastest-growing market in the food probiotics industry31. As consumers become increasingly aware of the potential health benefits associated with probiotic consumption, the market continues to expand, driven by factors like increased demand for functional foods and dietary supplements. The promising trajectory of the food probiotics market underscores its significance in the global food industry and its potential to provide opportunities for businesses operating in this sector. In terms of revenue, Lactobacillus held about 65% market share of the worldwide probiotics market in 2021, followed by Streptococcus and Bifidobacterium, in that order32. Due to the rising demand for non-pharmacological treatments to lower the cost of production for human probiotic applications, the market for Lactobacilli-based probiotics the estimated value of probiotics was worth more than USD 1.8 billion in 202233.
Discussion
This systematic review and meta-analysis provide the most in-depth look to date regarding the utilization of encapsulated probiotic viability efficacy. We have evaluated a total of 10 research papers related to probiotic encapsulation with nanoparticles and their efficacy that were published in the period between 2017 and 2022. A designed follow-up probiotics risk group assessment was performed at a variety of nanoparticles that is used as an encapsulating material for probiotic bacteria Lactobacillus spp. The forest plots provide an in-depth analysis of the data, showing that the I2 value of 100% shows that all observed variance is caused by heterogeneity rather than chance. In a random-effects meta-analysis model, the numbers also show the estimated between-study variance. Its range, 0.0929–0.02296, indicates that effect sizes among the research included in the study varied. According to this number, there is some variation in the impact sizes among studies, but the level of heterogeneity is not particularly high. The results of the current study concur that the statistically significant difference should be p 0.05, however this follow-up investigation with the forest plot shows the p-value is 0, which suggests that the observed data is statistically significant.
Despite the current limitations, the growing significance of new technologies and advancements in research on the impact of probiotics and postbiotics on human health based on the microbiota will undoubtedly be pivotal in crafting personalized treatments for prevalent diseases. The encapsulation of probiotics in nanoparticle provides numerous benefits for probiotics, such as enhancing their survival by shielding them from the severe circumstances of the gastrointestinal environment as well as external factors like oxygen, temperature, and light during storage and handling34. Additionally, it facilitates the precise and regulated release of the encapsulated materials at the appropriate concentrations within the digestive tract and makes it possible to incorporate probiotics at a range of concentration, ranging from lower to higher concentrations. These advantages underscore the potential of encapsulation as a significant approach, ranging from low to high levels. These advantages highlight the potential of encapsulation as a valuable technique for improving the functionality and effectiveness of probiotic products. However, until now, there have no standard use of nanoparticle encapsulated drug in humans were registered. Additionally the limited of research in this area offers researchers ample opportunities to explore and develop functional food products. By incorporating bioactive compounds and probiotics as co-encapsulation materials, researchers can create products that offer multiple functionalities and improve the delivery of active ingredients in the human gut, this presents a promising avenue for the development of innovative food formulations that provide enhanced health benefits and targeted effects on the human body35. The main drawbacks of adding natural food antimicrobials directly to food products could be eliminated by encapsulating them using various techniques.
Currently, probiotics are categorized and regulated differently by various regulatory agencies worldwide, including biologics, drugs, foods, and nutritional supplements. Consequently, each regulatory category has its own set of guidelines. To ensure the integrity, security, durability, and efficacy of probiotic formulations throughout the whole production, handling, storage chain, and post-marketing surveillance, it is crucial to establish an effective regulatory framework and harmonize guidelines. However, the lack of proper standardization parameters for probiotics poses a significant challenge in establishing the credibility of their health-promoting functions36. Different organizations in worldwide are working in order to build guidelines, policies and regulations. Several international organizations such as Food and Agriculture Organization (FAO)/ World health organization (WHO) established protocols for the assessment of probiotics in food products12,16. International dairy federation, initiated the formulation of protocols to assess distinct functional and safety attributes outlined in the FAO criteria for assessing probiotics in food37. Codex standard for fermented milk (CODEX STAN 243-2003), outlines the minimum quantities of characterizing and extra labeled microorganisms in yoghurt, acidophilus milk, kefir, kumis, and other fermented milks, in addition to other composition requirements38. International scientific association for probiotics and prebiotics, investigate the validation of techniques and the establishment of laboratory facilities for the analysis of microbiological content in probiotic products39 and World Gastroenterology Organization (WGO) focus on determining the genus, species, and strain of each probiotic present in a product, as well as the viability and survival rate of the probiotic strains throughout the product's shelf life40 as depicts in Table 2.
Table 2.
Worldwide regulation and policies for probiotics products.
| Organization | Role | References |
|---|---|---|
| Food and Agriculture Organization (FAO)/ World health organization (WHO) | Developed guidelines for evaluating probiotics in food | 12,16 |
| International dairy federation | Has begun developing procedures for determining specific functional and safety features indicated in the FAO recommendations for the evaluation of probiotics in food | 37 |
| Codex standard for fermented milk (CODEX STAN 243–2003) | This standard specifies minimum numbers of characterizing and extra labelled microorganisms in yoghurt, acidophilus milk, kefir, kumis, and other fermented milks, among other composition requirements | 38 |
| International scientific association for probiotics and prebiotics | The Industry Advisory Committee and the Board of Directors will explore technique validation and the development of laboratory locations to analyze probiotic product microbiological content | 39 |
| World Gastroenterology Organization (WGO) | It focus on the genus, species, and strain of each probiotic in a product, as well as the amount of viable cells of each probiotics strain that will survive till the end of the shelf-life | 40 |
Conclusion
With the ongoing expansion of the probiotic sector, an increasing number of individuals are recognizing the benefits that probiotics offer to human health. Probiotics play a crucial role in sustaining digestive health and addressing dysbiosis in intestinal flora. Moreover, they serve as preventive and therapeutic measures against various diseases such as obesity, colitis, colorectal cancer, and metabolic disorders. Consequently, the global probiotic market experiences continuous annual growth. Additionally, there are many benefits to entrapping probiotics in a nano system, including maintaining probiotic stability, delivering a barrier to protect them from damage, isolating bacteria from their environment, providing a carrier with a high probiotics load, allowing controlled and continuous probiotics release etc. In conclusion, it can be said that nano-encapsulation offers a promising outlook for incorporating live probiotic bacteria into foods and ensuring their survival during simulated gastric and intestinal processes. These findings provide valuable insights into the efficacy of encapsulated probiotics at different time intervals and support the need for further research in this area (Supplementary Information).
Supplementary Information
Author contributions
All authors contributed to writing-original draft preparation, and editing.
Funding
This research was funded by VtR Inc-CGU (SCRPD1L0221); DOXABIO-CGU (SCRPD1K0131), and CGU grant (UZRPD1L0011, UZRPD1M0081).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ramendra Pati Pandey and Gunjan.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-50972-x.
References
- 1.Bekiaridou A, Karlafti E, Oikonomou IM, Ioannidis A, Papavramidis TS. Probiotics and their effect on surgical wound healing: A systematic review and new insights into the role of nanotechnology. Nutrients. 2021;13:4265. doi: 10.3390/NU13124265/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott BM, Gutiérrez-Vázquez C, Sanmarco LM, da Silva Pereira JA, Li Z, Plasencia A, Hewson P, Cox LM, O’Brien M, Chen SK, Moraes-Vieira PM, Chang BSW, Peisajovich SG, Quintana FJ. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Med. 2021;27:1212–1222. doi: 10.1038/s41591-021-01390-x. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan, P.O., Bindels, L.B., Saulnier, D.M., Reid, G., Nova, E., Holmgren, K., O’Toole, P.W., Bunn, J., Delzenne, N., & Scott, K.P. Can prebiotics and probiotics improve therapeutic outcomes for undernourished individuals? Gut Microbes5, 74–82. 10.4161/GMIC.27252 (2013). [DOI] [PMC free article] [PubMed]
- 4.Barkhidarian B, Roldos L, Iskandar MM, Saedisomeolia A, Kubow S. Systematic review probiotic supplementation and micronutrient status in healthy subjects: A systematic review of clinical trials. Nutrients. 2021;13:3001. doi: 10.3390/NU13093001/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayichew T, Belete A, Alebachew T, Tsehaye H, Berhanu H, Minwuyelet A. Bacterial probiotics their importances and limitations: A review. J. Nutr. Health Sci. 2017;4(2):202. doi: 10.15744/2393-9060.4.202. [DOI] [Google Scholar]
- 6.Dubey MR, Patel VP. Probiotics: A promising tool for calcium absorption. Open Nutr. J. 2018;12:59–69. doi: 10.2174/1874288201812010059. [DOI] [Google Scholar]
- 7.Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo JC. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells. 2023;12:184. doi: 10.3390/CELLS12010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020;19:1–22. doi: 10.1186/S12934-020-01426-W/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Gao S, Yun S, Zhang M, Peng L, Li Y, Zhou Y. Microencapsulating alginate-based polymers for probiotics delivery systems and their application. Pharm. 2022;15:644. doi: 10.3390/PH15050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassani L, Gomez-Zavaglia A, Simal-Gandara J. Technological strategies ensuring the safe arrival of beneficial microorganisms to the gut: From food processing and storage to their passage through the gastrointestinal tract. Food Res. Int. 2020;129:108852. doi: 10.1016/J.FOODRES.2019.108852. [DOI] [PubMed] [Google Scholar]
- 11.Feng K, et al. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020;310:125977. doi: 10.1016/J.FOODCHEM.2019.125977. [DOI] [PubMed] [Google Scholar]
- 12.Probiotics in food Health and nutritional properties and guidelines for evaluation FAO FOOD AND NUTRITION PAPER
- 13.Peng C, Sun W, Dong X, Zhao L, Hao J. Isolation, identification and utilization of lactic acid bacteria from silage in a warm and humid climate area. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-92034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawaz E. Isolation and identification of probiotic lactic acid bacteria from curd and in vitro evaluation of its growth inhibition activities against pathogenic bacteria. Afr. J. Microbiol. Res. 2014;8:1419–1425. doi: 10.5897/AJMR2014.6639. [DOI] [Google Scholar]
- 15.Dempsey E, Corr SC. Lactobacillus spp. for gastrointestinal health: Current and future perspectives. Front. Immunol. 2022;13:840245. doi: 10.3389/FIMMU.2022.840245/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi A, Ebrahimi M, Kharazmi MS, Jafari SM. Role of nanomaterials in improving the functionality of probiotics; integration of nanotechnology onto micro-structured platforms. Food Biosci. 2023;53:102843. doi: 10.1016/J.FBIO.2023.102843. [DOI] [Google Scholar]
- 18.Durazzo A, Nazhand A, Lucarini M, Atanasov AG, Souto EB, Novellino E, Capasso R, Santini A. An updated overview on nanonutraceuticals: Focus on nanoprebiotics and nanoprobiotics. Int. J. Mol. Sci. 2020;21:2285. doi: 10.3390/IJMS21072285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/BMJ.N71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R: The R Project for Statistical Computing, https://www.r-project.org/
- 21.Tiani KA, Yeung TW, McClements DJ, Sela DA. Extending viability of Lactobacillus plantarum and Lactobacillus johnsonii by microencapsulation in alginate microgels. Int. J. Food Sci. Nutr. 2018;69:155–164. doi: 10.1080/09637486.2017.1343285. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimnejad P, Khavarpour M, Khalili S. Survival of Lactobacillus acidophilus as probiotic bacteria using chitosan nanoparticles. Int. J. Eng. Trans. A Basics. 2017;30:456–463. doi: 10.5829/idosi.ije.2017.30.04a.01. [DOI] [Google Scholar]
- 23.Wang M, Yang J, Li M, Wang Y, Wu H, Xiong L, Sun Q. Enhanced viability of layer-by-layer encapsulated Lactobacillus pentosus using chitosan and sodium phytate. Food Chem. 2019;285:260–265. doi: 10.1016/j.foodchem.2019.01.162. [DOI] [PubMed] [Google Scholar]
- 24.Ansari F, Pourjafar H, Jodat V, Sahebi J, Ataei A. Effect of Eudragit S100 nanoparticles and alginate chitosan encapsulation on the viability of Lactobacillus acidophilus and Lactobacillus rhamnosus. AMB Express. 2017 doi: 10.1186/s13568-017-0442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fareez IM, Lim SM, Zulkefli NAA, Mishra RK, Ramasamy K. Cellulose derivatives enhanced stability of alginate-based beads loaded with Lactobacillus plantarum LAB12 against Low pH, high temperature and prolonged storage. Probiotics Antimicrob. Proteins. 2018;10:543–557. doi: 10.1007/s12602-017-9284-8. [DOI] [PubMed] [Google Scholar]
- 26.Pourjafar H, Noori N, Gandomi H, Basti AA, Ansari F. Stability and efficiency of double-coated beads containing lactobacillus acidophilus obtained from the calcium alginate-chitosan and Eudragit S100 nanoparticles microencapsulation. Int. J. Probiotics Prebiotics. 2018;13:77–84. [Google Scholar]
- 27.Pourjafar H, Noori N, Gandomi H, Basti AA, Ansari F. Viability of microencapsulated and non-microencapsulated Lactobacilli in a commercial beverage. Biotechnol. Rep. 2020 doi: 10.1016/j.btre.2020.e00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahmati F. Microencapsulation of Lactobacillus acidophilus and Lactobacillus plantarum in Eudragit S100 and alginate chitosan under gastrointestinal and normal conditions. Appl. Nanosci. 2020;10:391–399. doi: 10.1007/s13204-019-01174-3. [DOI] [Google Scholar]
- 29.Rahmati F. Impact of microencapsulation on two probiotic strains in alginate chitosan and Eudragit S100 under gastrointestinal and normal conditions. Open Biotechnol. J. 2019;13:59–67. doi: 10.2174/1874070701913010059. [DOI] [Google Scholar]
- 30.Afzaal M, Khan AU, Saeed F, Arshad MS, Khan MA, Saeed M, Maan AA, Khan MK, Ismail Z, Ahmed A, Tufail T, Ateeq H, Anjum FM. Survival and stability of free and encapsulated probiotic bacteria under simulated gastrointestinal conditions and in ice cream. Food Sci. Nutr. 2020;8:1649–1656. doi: 10.1002/fsn3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Global Food Probiotics Market—2023-2030, https://www.marketresearch.com/DataM-Intelligence-4Market-Research-LLP-v4207/Global-Food-Probiotics-34371221/.
- 32.Probiotics Market Size, Trends and Forecast to 2030, https://www.coherentmarketinsights.com/market-insight/probiotics-market-3988.
- 33.Probiotics Market Size Statistics, Global Report 2023-2032, https://www.gminsights.com/industry-analysis/probiotics-market.
- 34.Razavi S, Janfaza S, Tasnim N, Gibson DL, Hoorfar M. Nanomaterial-based encapsulation for controlled gastrointestinal delivery of viable probiotic bacteria. Nanoscale Adv. 2021;3:2699. doi: 10.1039/D0NA00952K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misra S, Pandey P, Mishra HN. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021;109:340–351. doi: 10.1016/J.TIFS.2021.01.039. [DOI] [Google Scholar]
- 36.Hoffmann DE, et al. Probiotics: Achieving a better regulatory fit. Food Drug Law J. 2014;69:237. [PMC free article] [PubMed] [Google Scholar]
- 37.IDF - Global Dairy Expertise Since 1903, https://fil-idf.org/.
- 38.Milk and Milk Products Second edition. (2011)
- 39.ISAPP - International Scientific Association for Probiotics and Prebiotics, https://isappscience.org/.
- 40.World Gastroenterology Organisation, https://www.worldgastroenterology.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.