Abstract
Essential thrombocythemia is a risk factor for thrombosis and hemorrhage. During the perioperative period of cardiac surgery, the risk of thrombosis and hemorrhage increases. Coronavirus disease 2019 (COVID-19) is also associated with thrombosis. We present the case of a 69-year-old man with essential thrombocythemia complicated by COVID-19 who developed a left ventricular thrombus. We performed thrombectomy, but the patient developed recurrent left ventricular thrombus 8 days after surgery. Emergency redo thrombectomy was performed followed by aggressive blood-thinning therapy. The postoperative course was complicated by cardiac tamponade requiring surgical drainage 8 days after the second surgery. The patient was discharged home 25 days after the second operation without any complications.
Learning objective
Left ventricular thrombus is a rare but fatal complication associated with essential thrombocythemia. COVID-19 has also been reported to cause coagulopathy. This case suggested that after surgery for left ventricular thrombus complicated by multiple risk factors including essential thrombocythemia and COVID-19, aggressive blood-thinning therapy with combination of anticoagulation, antiplatelet, and metabolic antagonist may help prevent recurrent thrombosis.
Keywords: Left ventricular thrombus, Essential thrombocythemia, COVID-19
Introduction
Essential thrombocythemia is a clonal stem cell disorder characterized by chronically high platelet count (>450 × 109/L) and is associated with mutations in one of the three genes, JAK2, CALR, or C-MPL [1]. The reported incidence rates of arterial and venous thrombus in patients with essential thrombocythemia are 16 % and 5.5 %, respectively [2], and the formation of left ventricular thrombus has also been reported [3]. Anticoagulation with heparin and then transition to an oral anticoagulant is recommended for thrombus with essential thrombocythemia [1].
Coronavirus disease 2019 (COVID-19) caused by infection with severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) is also associated with thromboembolism. In a large study of hospitalized COVID-19 patients (n = 3334) by Bilaloglu et al., the incidence of venous thromboembolism, pulmonary embolism, and arterial thromboembolism was 6.2 %, 3.2 %, and 11.1 %, respectively [4]. Another case report has documented the occurrence of left ventricular thrombosis related to COVID-19 [5]. COVID-19 patients at high risk of thrombosis are also treated with heparin from a prophylactic perspective, but the effect on arterial thrombosis is unknown [5].
In this report, we present a case of left ventricular thrombus due to essential thrombocythemia complicated by COVID-19. The patient developed recurrent left ventricular thrombus after surgery, requiring a redo thrombectomy.
Case report
A 69-year-old man was diagnosed with essential thrombocythemia by genetic testing for JAK2 mutation, and oral aspirin and hydroxycarbamide were commenced. Echocardiography at that time did not show any intracardiac thrombus, and the left ventricular ejection fraction (LVEF) was 41.5 % with apical akinesis. Six months later, the patient presented with right foot pain preceded by lethargy. Contrast-enhanced computed tomography (CT) revealed a thrombus in the right popliteal artery and the left ventricular apex (Fig. 1a). The severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) antigen level was 1.9 pg/mL and polymerase chain reaction test was positive at 26 cycles. His past medical history included a history of percutaneous coronary intervention (PCI) seven years before and two histories of cerebral infarction one year before and seven months before. At the time of PCI, the proximal left anterior descending artery lesion was obstructed and drug-eluting stent was placed. Echocardiography at that time showed LVEF was 38.1 % with apical severe hypokinesis. Electrocardiogram (ECG) showed findings of V1-4 ST segment elevation. At the time of the second stroke, ECG showed abnormal Q waves in V1 and V2. This time, preoperative echocardiography showed that the LVEF was 35 % with apical akinesis, and there was a 3-cm thrombus at the left ventricular apex. His blood platelet count was 1128 × 109/L and his body weight was 70 kg.
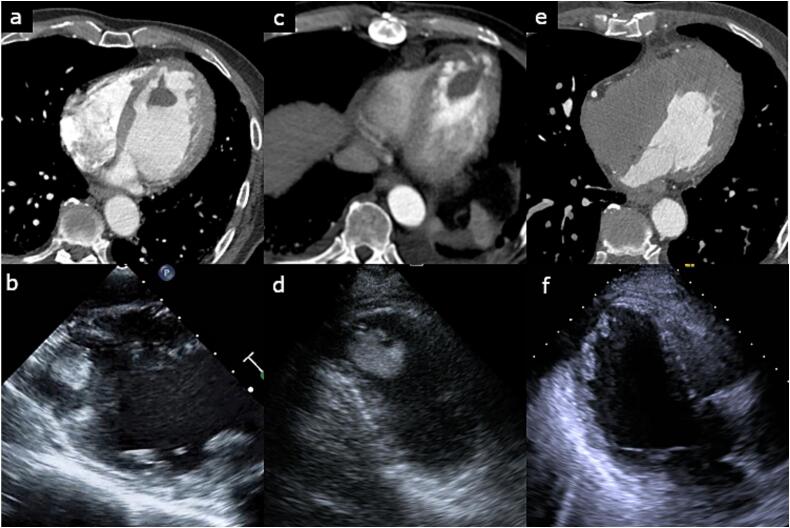
Fig. 1.
Computed tomography image and echocardiography showing a left ventricular thrombus.
(a, b) First visit, (c, d) before second operation, (e, f) after second operation.
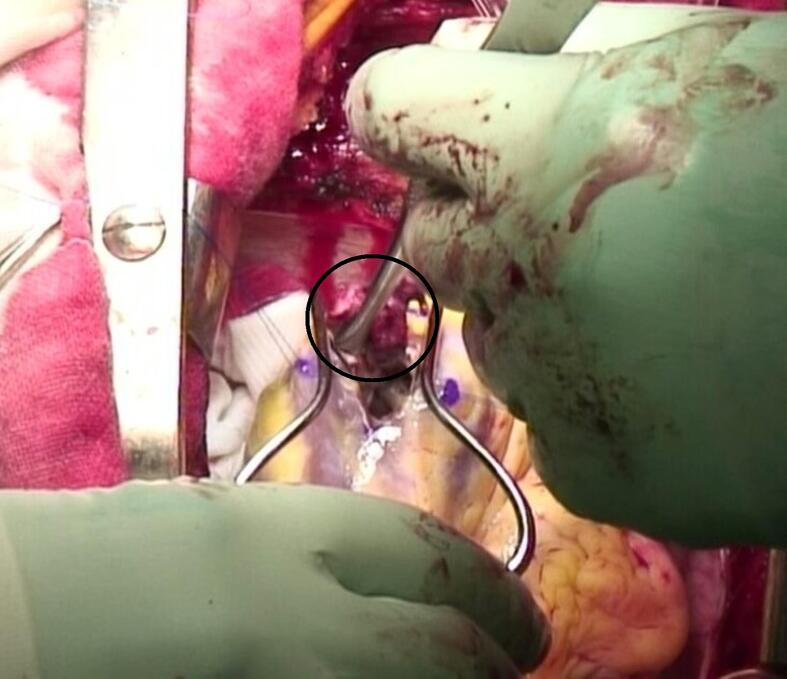
Emergency thrombectomy was performed for the right popliteal artery thrombus using a Fogarty catheter while the cardiopulmonary bypass (CPB) machine was prepared. Transesophageal echocardiography also showed a spherical thrombus with a smooth surface (Fig. 1b). Subsequently, thrombectomy was performed from the left ventricular apex using CPB. A total of 31,000 U of intravenous heparin was administered for CPB installation, but the activated coagulation time (ACT) was 350 s. Thereafter, 10,000 U of intravenous heparin and 1 mg of intravenous argatroban hydrate were administered and the ACT increased to 466 s. ACT was maintained over 400 s during the operation with the addition of 75 mg of nafamostat into the CPB circuit. A solid thrombus was successfully removed via left ventriculotomy without blood transfusion (Fig. 2). The thrombus was about 3 cm in size, smooth-surfaced, spherical, firm, and relatively fresh. Thus, it could be removed without resistance. After surgery, intravenous heparin infusion and oral warfarin were commenced on days 1 and 2, respectively. With the goal of an activated partial thromboplastin time (APTT) of 45 s or longer, heparin was started at 8000 units/day and increased to 12,000 and 15,000 units every 24 h, but the APTT was not prolonged enough. Therefore, the interval of dose increase was shortened to 18,000, 20,000, 25,000, and 30,000 units every 4 to 12 h, and the APTT was prolonged to 80.7 s on day 5. The dose was reduced to 27,000 units and the dosage of warfarin was increased while maintaining APTT over 45 s. On day 6, heparin was discontinued as the prothrombin time-international normalized ratio (PT-INR) reached above 1.5, and the PT-INR exceeded 2 on day 8. Contrast-enhanced CT and echocardiography performed on day 8 revealed a recurrent left ventricular thrombus (Fig. 1c, d). Therefore, we performed an emergency thrombectomy.
Fig. 2.
Intraoperative photograph showing a solid thrombus being removed from the left ventricle during the first operation.
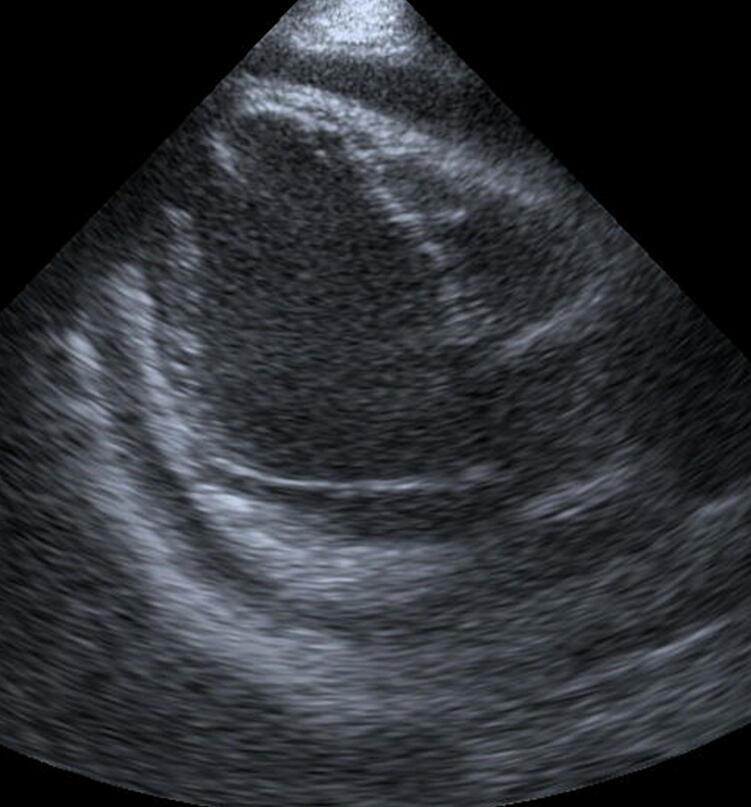
At the time of the second operation, his PT-INR was 2.08. The ACT exceeded 400 s after intravenous administration of 30,000 U of heparin and 1 mg of argatroban hydrate. A fresh and fragile thrombus was removed through a left ventriculotomy. Fresh frozen plasma (FFP) (720 mL) was used after discontinuation of CPB. Postoperatively, intravenous heparin infusion was started 3 h after the patient entered the intensive care unit. The activated partial thromboplastin time was 137.7 s on the next day. Oral warfarin, aspirin, and hydroxycarbamide were commenced on the next day after the operation. Eight days after the second operation, the patient developed cardiac tamponade and underwent surgical drainage (Fig. 3). It was thought that the effect of early intensified anticoagulation to prevent recurrence of thrombus led to cardiac tamponade. Contrast-enhanced CT performed for evaluation of the coronary arteries 19 days after the second operation revealed a low-density thin layer inside the left ventricular apex (Fig. 1e). However, echocardiography did not show any overt thrombus (Fig. 1f). The patient was discharged home after 6 days.
Fig. 3.
Echocardiography showing a significant amount of pericardial effusion 8 days after the second operation.
Discussion
Essential thrombocythemia is a rare myeloproliferative disorder characterized by an overproduction of platelets and it is associated with the risk of thrombosis and hemorrhage [1]; age > 60 years, history of thrombosis, and platelet count > 1500 × 109/L are risk factors in these patients [6]. Cardiac operation on patients with essential thrombocythemia is associated with a high risk of thrombosis and hemorrhage. According to Gurrieri et al., the platelet count in these patients should be maintained at <800 × 109/L in order to reduce the risk of thrombotic or hemorrhagic complications in the perioperative period [7]. A case report described a patient with essential thrombocythemia who required approximately three times the amount of heparin, as well as antithrombin III for carrying out CPB [8]. They also reported no recurrence of thrombus using aspirin and warfarin from day 5 [8]. In this present case, the platelet count was high, and we used argatroban and nafamostat in addition to more heparin than usual to maintain intraoperative anticoagulation. Heparin and warfarin were used for postoperative management, but recurrence of thrombus was observed. Platelet suppression with aspirin and hydroxycarbamide and strict management with heparin and warfarin were necessary.
COVID-19 infection is also associated with thromboembolism. An association between COVID-19 infection and essential thrombocythemia related to thrombosis has also been reported. Among the classic myeloproliferative neoplasms, essential thrombocythemia is reported to be associated with a high risk of thrombosis in patients with COVID-19 infection, with a 16.7 % reported incidence of thrombotic complications [9].
Left ventricular thrombosis occurs due to the interplay of three factors: stasis attributable to reduced ventricular function, endocardial injury, and inflammation/hypercoagulability [10]. It typically occurs in the setting of ischemic or dilated cardiomyopathy and left ventricular aneurysm [10]. In this present case, hypercoagulability due to essential thrombocythemia, inflammation due to COVID-19 infection, and decreased left ventricular contractility due to previous coronary disease were the presumed causative factors for thrombosis.
The patient suffered recurrence of left ventricular thrombus despite routine anticoagulation therapy with heparin and warfarin. Therefore, after the second operation, we started anticoagulation with heparin and warfarin immediately and used aspirin and anticancer drugs from the next day to prevent recurrence. However, the patient developed cardiac tamponade which required surgical drainage. Effective and safe anticoagulation after cardiac surgery is difficult in patients with multiple risk factors for thrombosis.
In summary, left ventricular thrombus in a patient with essential thrombocythemia complicated by COVID-19 and reduced left ventricular function was treated by emergency thrombectomy. Routine postoperative anticoagulation therapy resulted in recurrent left ventricular thrombus which required redo thrombectomy. Aggressive postoperative blood-thinning therapy resulted in cardiac tamponade which required surgical drainage. Meticulous management is essential to maintain the balance between the safety and efficacy of anticoagulation in patients with various thrombotic risk factors.
Consent statement
Written informed consent was obtained from the patients for publication of this case report, including accompanying images.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Kaushansky K. In: Williams hematology. 10th ed. Kaushansky K., editor. McGraw Hill; New York: 2021. Essential thrombocythemia; pp. 1377–1387. [Google Scholar]
- 2.Gangat N., Wolanskyj A.P., Schwager S.M., Hanson C.A., Tefferi A. Leukocytosis at diagnosis and the risk of subsequent thrombosis in patients with low-risk essential thrombocythemia and polycythemia vera. Cancer. 2009;115:5740–5745. doi: 10.1002/cncr.24664. [DOI] [PubMed] [Google Scholar]
- 3.Taruya A., Hatada A., Nishimura Y., Uchita S., Toguchi K., Honda K., et al. Left ventricular ball-like thrombus after acute myocardial infarction with essential thrombocythemia. J Cardiol Cases. 2014;10:1–3. doi: 10.1016/j.jccase.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City Health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh G., Attique H.B., Gadela N.V., Mapara K., Manickaratnam S. COVID-19 related arterial coagulopathy. Cureus. 2020;12 doi: 10.7759/cureus.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggeri M., Finazzi G., Tosetto A., Riva S., Rodeghiero F., Barbui T. No treatment for low-risk thrombocythaemia: results from a prospective study. Br J Haematol. 1998;103:772–777. doi: 10.1046/j.1365-2141.1998.01021.x. [DOI] [PubMed] [Google Scholar]
- 7.Gurrieri C., Smith B.B., Nuttall G.A., Pruthi R.K., Said S.M., Smith M.M. Essential thrombocythemia and cardiac surgery: a case series and review of the literature. Ann Thorac Surg. 2018;106:482–490. doi: 10.1016/j.athoracsur.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi M., Oota E., Soeda T., Masumo K., Tomita Y., Kato T., et al. Emergency cardiac surgery and heparin resistance in a patient with essential thrombocythemia. JA Clin Rep. 2016;2:35. doi: 10.1186/s40981-016-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbui T., Stefano V.D., Alvarez-Larran A.A., Iurlo A., Masciulli A., Carobbio A., et al. Among classic myeloproliferative neoplasms, essential thrombocythemia is associated with the greatest risk of venous thromboembolism during COVID-19. Blood Cancer J. 2021;11:21. doi: 10.1038/s41408-021-00417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine G.N., McEvoy J.W., Fang J.C., Ibeh C., McCarthy C.P., Misra A., et al. Management of patients at risk for and with left ventricular thrombus: a scientific statement from the American Heart Association. Circulation. 2022;146:e205–e223. doi: 10.1161/CIR.0000000000001092. [DOI] [PubMed] [Google Scholar]