Abstract
We report the case of a 16-year-old female patient with protein-losing enteropathy that was suspected to be caused by thoracic duct congestion associated with postural compression of right subclavian vein. Non-contrast magnetic resonance lymphangiography showed that the thoracic duct connected to the right-sided venous angle of the right subclavian vein which was obstructed when her right arm was lifted. In this case, comprehensive screening of the lymphatics using non-contrast magnetic resonance lymphangiography, which is a minimally invasive tool with high spatial resolution, was helpful for the recognition of the specific pathophysiology.
Learning objective
Lymphatic disorders associated with congenital heart disease can be fatal. The morphology and dysfunction of the lymphatic system are complicated, and when added to the complex hemodynamics inherent to congenital heart disease, the pathophysiology is more difficult to understand. To understand the complexity of the lymphatic disease, it is necessary to learn a systematic diagnostic process of lymphatic disorders. In the present case, it is beneficial to know the usefulness of non-contrast magnetic resonance lymphangiography to screen overall lymphatics.
Keywords: Congenital heart disease, Lymphatic disorder, Magnetic resonance lymphangiography, Protein-losing enteropathy
Introduction
The anatomy of the lymphatic pathway and the pathophysiology of lymphatic disorders are varied and complex. Therefore, the mechanism of lymphatic disorders is not always fully understood. This is especially true in patients with congenital heart disease (CHD). Although diagnostic imaging has been developed in recent years, comprehensive screening methods of the lymphatics in these entities have not been fully established.
In this case, protein-losing gastroenteropathy (PLE) is suspected to be caused by thoracic duct (TD) congestion associated with postural compression of subclavian vein. The pathophysiology was complex and non-contrast magnetic resonance (MR) lymphangiography was useful to assess the overall morphology of the lymphatic system. This is a case in which a comprehensive evaluation led to elucidating the cause of complex pathophysiology.
Case report
The patient was a 16-year-old female, who had experienced intermittent severe generalized edema for 3 years. When the edema was exacerbated, blood examination showed hypoproteinemia, serum albumin of 1.9 g/dL, and immunoglobulin (Ig) G of 179 mg/dL. Although there were no symptoms specific to PLE, such as diarrhea or ascites, the diagnosis of PLE was established by a significant increase in alpha-1 antitrypsin clearance and functional imaging with Tc-99m human serum albumin (Tc-99m HSA). The edema spontaneously improved only with rest without additional medications, and albumin and IgG level improved to 2.7 g/dL and 364 mg/dL respectively. However, PLE relapsed twice in 2 years that required intensive treatments with steroids and heparin.
After birth, she was diagnosed with 22q11.2 deletion, interrupted aortic arch type B, and ventricular septal defect (VSD). She underwent aortic arch repair and VSD closure via a midline thoracotomy using cardio-pulmonary bypass at 16 days of age. No lymphatic disorders such as chylothorax occurred, and she was discharged from the hospital with good progress. She had outpatient follow-up after discharge. In addition, she has mental retardation related to a chromosomal abnormality.
When she was 16 years old, she was admitted to our hospital for a medical check-up. The body weight and height were 46 kg and 162 cm. She has a slender figure with sloped shoulders and poor posture. The patient was clinically asymptomatic. Chest X-ray cardiothoracic ratio was 47 % with no pulmonary congestion and no pleural effusion. Echocardiography showed normal left and right ventricular function and no significant semilunar and atrioventricular valve insufficiency.
According to our present routine practice, as an initial screening for a lymphatic disorder, non-contrast MR lymphangiography and lymphatic scintigraphy were performed to evaluate the morphology and function of the lymphatic system with the approval of the local IRB, Showa University Hospital. Non-contrast MR lymphangiography was performed with a 1.5 T MR system (SIGNA® HDxt ver.16, GE Healthcare, Waukesha, WI, USA). Heavily T2-weighted MR lymphangiography was performed using a respiratory-navigated and cardiac-gated three-dimensional fast recovery fast spin-echo sequence (3D FRFSE) with the following parameters: TR/TE, 2500/350–1000 ms; slice thickness, 2.0 mm; field of view, 360 mm × 360 mm; matrix size, 288 × 192; and flip angle (FA), 140°. It showed that the TD ascended the left side of the spine and connected to the right-sided venous angle of the right subclavian vein (RSCV), an uncommon pattern (Fig. 1a). The common left venous angle was not readily visible. Lymphatic scintigraphy showed congestion of the Tc-99mTc tracer in the right arm.
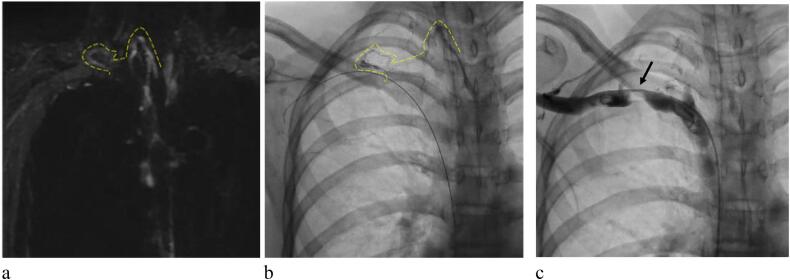
Fig. 1.
(a) Non-contrast magnetic resonance lymphangiography. (b) Lipiodol lymphangiography. (c) Angiography of the right subclavian vein (RSCV). (a) The thoracic duct (TD) connected to the right-side venous angle with high reproducibility to visualize TD (dotted line). (b) The TD connected to the narrowing part of the RSCV (dotted line). (c) There was a tight obstruction of the RSCV (black arrow).
Cardiac catheterization showed that the central venous pressure was 8 mmHg (Table 1), which was not high enough to explain the development of PLE. Interestingly, when her right arm was lifted, a tight obstruction was recognized at the proximal RSCV. The distal pressure increased to 24 mmHg, and the pressure gradient through the obstruction was 14 mmHg, while the distal pressure decreased to 8 mmHg with no pressure gradient when her right arm was down. The left subclavian vein pressure did not change regardless of the position of the left arm.
Table 1.
The result of cardiac catheterization. Right subclavian vein pressure was increased when the right arm was lifted.
| Site | Pressure (mmHg) |
|---|---|
| CVP | 8 |
| RSCV arm elevation | 24 |
| RSCV arm down | 8 |
| LSCV arm elevation | 9 |
| LSCV arm down | 8 |
| Pressure gradient | |
| RSCV-SVC arm elevation | 14 |
| RSCV-SVC arm down | 0 |
CVP, central venous pressure; RSCV, right subclavian vein; LSCV, left subclavian vein; SVC, superior vena cava.
A lipiodol lymphangiography confirmed that the TD connects to right venous angle, which is the obstructed part of the RSCV (Fig. 1b, c) (Video 1).
On physical examination, a positive Adson's sign was confirmed. Therefore, recurrent PLE was strongly suspected to be caused by TD congestion, which is probably associated with right thoracic outlet syndrome (TOS) with postural compression of the RSCV. Then, conservative treatment with shoulder rehabilitation for TOS was started. The progression of PLE might promote surgical options for TOS.
We instructed the patient not to keep her right arm elevated. We also encouraged family members to help adjust the position if she keeps her right arm up while she sleeps. And she goes to the hospital once a month to continue shoulder rehabilitation. The serum albumin is maintained at 3.5 g/dL, and the intermittent severe edema has not been recurrent.
Discussion
It has been suggested that most severe cases result from morphological and functional disorders of the lymphatic system: central lymphatic flow disorder (CLFD) or pulmonary lymphatic perfusion syndrome (PLPS) [1]. Hsu et al. suggested that there are nine types of TD pathways; TD drainage into the left venous angle is the most common [2]. Johnson et al. showed that the most common type of TD pathway occurs in only 40 %–60 % of patients, and there are diverse patterns [3]. Patients with CHD are supposed to have more variations in lymphatic pathways and anatomy.
Dynamic contrast-enhanced MR lymphangiography has become the gold standard of lymphatic imaging. This imaging provides dynamic monitoring of the lymphatic flow with high spatial and temporal resolution [4]. However, it has not become common as a screening method because of contrast medium exposure, the learning curve for lymph node puncture, longer acquisition time, and requirement for special facilities (such as an integrated X-ray and MR imaging suite with detachable table). Meanwhile, non-contrast MR lymphangiography has a short acquisition time, approximately 10 min, and requires a commonly used MR setting. Furthermore, the procedure does not require contrast medium (or lymph node puncture).
Therefore, we perform non-contrast MR lymphangiography as an initial screening process, to assess the overall anatomy of the lymphatic system. We additionally perform lipiodol lymphangiography or dynamic contrast-enhanced MR lymphangiography if a detailed evaluation is required.
In our patient, non-contrast MR lymphangiography showed the overall morphology of the TD — an unusual variant. Combined with venography, we strongly suspected that the development of PLE was associated with TD congestion because of the obstruction of the RSCV associated with TOS. The key to the diagnosis was to detect the uncommon right-sided TD by the initial screening using non-contrast MR lymphangiography. Therefore, we believe that comprehensive screening using non-contrast MR lymphangiography is helpful in the diagnostic process of lymphatic disorders.
As our case does not have several typical symptoms of TOS, the diagnosis of TOS at this moment is not completely definitive. Generally, TOS has a variety of symptoms, and its pathogenesis has not been fully established. Ferrante et al. [5] classified TOS into five categories depending on etiology and pathology. Previous reports showed that venous vascular TOS causes acute swelling of upper extremity, cyanosis, pain, and venous distention due to venous obstruction and thrombus formation [6,7]. Melloni et al. [8] reported a patient with TOS who developed transient cervical and mediastinal lymphedema after sleeping with her arms elevated and abducted. The author considered that the symptoms resulted from the temporary obstruction of the TD at the junction of the subclavian vein. Tallroth et al. [9] also reported a case of steatorrhea because of TOS. The authors concluded that the TD was obstructed at the thoracic outlet. We believe that the pathophysiology in those cases was similar to that in our patient. Additionally, in our case, the lack of subjective symptoms was probably due to the underestimation because of her mental retardation.
There is a history of thoracotomy, and although no lymphatic disorder occurs until adolescence, it is undeniable that surgical invasion affects the patient's condition. And her body shape and sloped shoulders may be risk factors that could have caused TOS. PLE is not currently recurrent by conservative treatment. Assuming that the postural change contributed to her good progress, similar to the previous report [8], the symptoms may result from the intermittent obstruction of the TD at the junction of the subclavian vein. However, it remains unclear whether conservative treatment is effective in improving PLE in terms of improving congestion in the TD. The efficiency might be insufficient, so we need to consider surgical options for TOS if it recurs.
Conclusions
We report a patient with PLE that is suspected to be caused by TD congestion associated with postural compression of subclavian vein. Comprehensive evaluation is crucial for the diagnosis of pathophysiology of the lymphatic system in CHD. Non-contrast MR lymphangiography was helpful for the recognition of the specific pathophysiology.
Funding
No grants or other financial support were used in the preparation of this case report.
Informed consent
Informed consent was obtained from the patient for the publication of this case report.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Savla J.J., Itkin M., Rossano J.W., Dori Y. Post-operative chylothorax in patients with congenital heart disease. J Am Coll Cardiol. 2017;69:2410–2422. doi: 10.1016/j.jacc.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Hsu M.C., Itkin M. Lymphatic anatomy. Tech Vasc Interv Radiol. 2016;19:247–254. doi: 10.1053/j.tvir.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Johnson O.W., Chick J.F.B., Chauhan N.R., Fairchild A.H., Fan C.M., Stecker M.S., et al. The thoracic duct: clinical importance, anatomic variation, imaging, and embolization. Eur Radiol. 2016;26:2482–2493. doi: 10.1007/s00330-015-4112-6. [DOI] [PubMed] [Google Scholar]
- 4.Pimpalwar S., Chinnadurai P., Chau A., Pereyra M., Ashton D., Masand P., et al. Dynamic contrast enhanced magnetic resonance lymphangiography: categorization of imaging findings and correlation with patient management. Eur J Radiol. 2018;101:129–135. doi: 10.1016/j.ejrad.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Ferrante M.A. The thoracic outlet syndromes. Muscle Nerve. 2012;45:780–795. doi: 10.1002/mus.23235. [DOI] [PubMed] [Google Scholar]
- 6.Habibollahi P., Zhang D., Kolber M.K., Pillai A.K. Venous thoracic outlet syndrome. Cardiovasc Diagn Ther. 2021;11:1150–1158. doi: 10.21037/cdt-20-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders R.J., Hammond S.L., Rao N.M. Diagnosis of thoracic outlet syndrome. J Vasc Surg. 2007;46:601–604. doi: 10.1016/j.jvs.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 8.Melloni G., Giovanardi M., De Gaspari A., Zannini P. Transient thoracic duct obstruction in a patient with thoracic outlet syndrome. Eur J Cardiothorac Surg. 2006;30:674. doi: 10.1016/j.ejcts.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Tallroth K., Telaranta T., Salmenkivi K. Thoracic duct obstruction associated with the thoracic outlet syndrome. Acta Chir Scand. 1983;149:797–800. [PubMed] [Google Scholar]