Abstract
Sirtuins (Sirts) are a family of nicotinamide adenine dinucleotide-dependent protein deacetylases that share diverse cellular functions. Increasing evidence shows that Sirts play a critical role in podocyte injury, which is a major determinant of proteinuria-associated renal disease. Membranous nephropathy (MN) is a typical glomerular disease in which podocyte damage mediates proteinuria development. In this study we investigated the molecular mechanisms underlying the regulatory roles of Sirt in podocyte injury in MN patients, rats with cationic bovine serum albumin (CBSA)-induced MN and zymosan activation serum (ZAS)-stimulated podocytes. Compared with healthy controls, MN patients showed significant reduction in intrarenal Sirt1 and Sirt6 protein expression. In CBSA-induced MN rats, significant reduction in intrarenal Sirt1, Sirt3 and Sirt6 protein expression was observed. However, only significant decrease in Sirt6 protein expression was found in ZAS-stimulated podocytes. MN patients showed significantly upregulated protein expression of Wnt1 and β-catenin and renin-angiotensin system (RAS) components in glomeruli. CBSA-induced MN rats exhibited significantly upregulated protein expression of intrarenal Wnt1 and β-catenin and their downstream gene products as well as RAS components. Similar results were observed in ZAS-stimulated podocytes. In ZAS-stimulated podocytes, treatment with a specific Sirt6 activator UBCS039 preserved the protein expression of podocin, nephrin and podocalyxin, accompanied by significant inhibition of the protein expression of β-catenin and its downstream gene products, including Snail1 and Twist; treatment with a β-catenin inhibitor ICG-001 significantly preserved the expression of podocyte-specific proteins and inhibited the upregulation of downstream β-catenin gene products accompanied by significant suppression of the protein expression of RAS components. Thus, we demonstrate that Sirt6 ameliorates podocyte injury by blocking RAS signalling via the Wnt1/β-catenin pathway. Sirt6 is a specific therapeutic target for the treatment of podocyte damage-associated renal disease.
Keywords: Sirtuins, podocyte injury, membranous nephropathy, renin-angiotensin system, Wnt1/β-catenin pathway, UBCS039
Introduction
Sirtuins (Sirts), which are mammalian silent information regulator 2 orthologues, are an evolutionarily conserved family of nicotinamide adenine dinucleotide-dependent protein deacetylases that share diverse cellular functions [1]. Mammals express seven Sirts (Sirt1 to Sirt7) that are localised in different subcellular compartments [2]. Sirt1, Sirt2 and Sirt3 are associated with early kidney development [3]. In renal disease, the most widely reported Sirt is Sirt1, which exerts cytoprotective effects by suppressing inflammation, apoptosis and fibrosis together with Sirt3 [2, 4]. Li et al. showed that Sirt1 ameliorated renal fibrosis by inhibiting hypoxia-inducible factor 2α expression in mice and cultured NRK-49F cells [5]. Jung et al. reported that the decrease in Sirt2 expression inhibited cisplatin-induced phosphorylation of p38 and C-Jun N-terminal kinase, while overexpression of Sirt2 further increased the phosphorylation of p38 and C-Jun N-terminal kinase in kidney tissues and proximal tubular cells [6]. Sirt3, which is a key metabolic sensor that modulates adenosine triphosphate production and the mitochondrial adaptive response to stress, was shown to be the second most important regulator. Feng et al. demonstrated that Sirt3 deficiency exacerbated angiotensin-II-induced renal fibrosis [7]. Shi et al. reported that Sirt4 overexpression protected against diabetic kidney disease (DKD) by suppressing podocyte apoptosis [8]. Among the nuclear Sirts, Sirt6 can deacetylate lysines 9 and 56 in histone H3 to preserve genome stability and telomere function. Liu et al. reported that Sirt6 expression was decreased in DKD and adriamycin-induced nephropathy in mice, and Sirt6 deficiency deteriorated podocyte injury and proteinuria [9]. In addition, Li et al. reported that Sirt6 overexpression protected against cisplatin-induced renal injury by increasing Sirt6 binding to the promoters of extracellular signal-regulated kinase 1/2, deacetylating histone 3 lysine 9 and suppressing extracellular signal-regulated kinase 1/2 expression [10]. These findings suggest that changes in Sirt expression are involved in podocyte injury and contribute to proteinuria-associated renal disease. Substantial advances have suggested that podocyte impairment is a major determinant in multiple renal diseases, including membranous nephropathy (MN), DKD, membranous glomerulonephritis (MGN), immunoglobulin A nephropathy (IgAN) and focal segmental glomerulosclerosis (FSGS) [11].
MN is an immune-mediated glomerular disease in adults. Major damage to the podocyte results in cellular simplification and breakdown of the glomerular filtration barrier, leading to complement activation and proteinuria development [12]. Proteinuria development is the result of the formation of a membrane attack complex, which is assembled from complement components to form C5b–9 [12]. The membrane attack complex causes sublethal podocyte injury, subsequently leading to cytoskeletal rearrangement in podocytes and glomerulosclerosis, which are associated with a variety of underlying mechanisms, including the dysregulation of molecules such as the renin-angiotensin system (RAS), reactive oxygen species and metabolites from the host and the gut microbiota [13, 14] and the activation of pathways such as the Wnt1/β-catenin pathway [13]. However, no report has demonstrated whether changes in Sirt expression are involved in podocyte injury in patients with MN.
Wnt/β-catenin signalling is a conserved developmental pathway that modulates tissue development and homeostasis. The Wnt/β-catenin pathway is activated in various podocyte damage-associated renal diseases, such as DKD, polycystic kidney disease, FSGS, lupus nephritis and chronic allograft nephropathy [15, 16]. However, few studies have reported hyperactivation of the Wnt/β-catenin pathway in MN. Two studies demonstrated significantly increased intrarenal Wnt4 mRNA expression in patients with MN and β-catenin protein expression in rats with passive Heymann nephritis [16, 17]. An in vitro experiment demonstrated a hyperactive Wnt/β-catenin pathway in zymosan activation serum (ZAS)-induced podocytes [18]. A recent study showed significantly increased mRNA levels of Wnt3 and β-catenin in human podocytes treated with MN plasma [19]. These findings suggest that a hyperactive Wnt/β-catenin pathway is involved in MN. A recent study showed that significantly upregulated Sirt6 protein expression in renal fibrosis interacted with β-catenin and then was recruited to the promoters of β-catenin target to deacetylate lysine 56 in histone H3 and negatively regulate the β-catenin pathway [20]. Blocking Sirt6 deacetylase activity exacerbated renal fibrosis, revealing the antifibrotic effect of Sirt6 on renal fibrosis via epigenetic regulation of the β-catenin pathway [20].
Previous publications indicated that intrarenal RAS was activated by the upregulation of multiple RAS genes, including angiotensinogen (AGT), renin, angiotensin-converting enzyme (ACE) and angiotensin II type 1 receptor (AT1R), after renal damage [15]. Therefore, the recommended first-line therapy for chronic proteinuric nephropathy is angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), which slow but may not halt disease progression and may not be completely effective to the same degree in all patients [21]. Previous bioinformatics results showed that promoter regions of all RAS genes included putative T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF)-binding sites, and β-catenin triggered the binding of LEF-1 to these sites in kidney tubular cells [15]. Overexpression of β-catenin or different Wnt ligands induced the expression of all RAS genes, which was consistent with the reduction in the expression of multiple RAS genes, proteinuria and renal damage after treatment with the β-catenin inhibitor ICG-001 [15].
To date, no study has reported whether Sirts are expressed in MN and further affect the Wnt1/β-catenin pathway and RAS signalling in injured podocytes, which are involved in proteinuria-associated renal disease. In this study, we hypothesised that changes in Sirt expression could ameliorate podocyte injury by blocking RAS signalling by inhibiting the Wnt/β-catenin pathway. To test this hypothesis, we investigated the regulatory effect of Sirts on the Wnt/β-catenin pathway and RAS signalling in patients with MN, rats with cationic bovine serum albumin (CBSA)-induced MN and ZAS-stimulated podocytes.
Materials and methods
Chemicals, antibodies and reagents
Bovine serum albumin and zymosan A were purchased from Sigma‒Aldrich Co. (Saint Louis, MO, USA). UBCS039 and ICG-001 were purchased from Topscience Co., Ltd. (Shanghai, China). Primary antibodies against active β-catenin (1:800, 4270S) and plasminogen activator inhibitor-1 (PAI-1, 1:800, 27535S) were purchased from Cell Signaling Technology (Danvers, MA, USA). Primary antibodies against synaptopodin (1:1000, sc-21537), podocalyxin (1:300, sc-23903), Wnt1 (1:800, sc-514531), snail1 (1:500, sc-271977), AGT (1:200, sc-7419) and ACE (1:800, sc-23908) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Primary antibodies against podocin (1:1000, ab50339), nephrin (1:250, ab58968), Sirt1 (1 µg/mL, ab110304), Sirt3 (1:1000, ab217319), Sirt4 (3 µg/mL, ab10140), Sirt6 (2 µg/mL, ab62739), β-catenin (ab32572, 1:5000), Twist (1:250, ab50887), matrix metallopeptidase 7 (MMP-7, 1:300, ab5706), fibroblast-specific protein 1 (FSP1, 1:800, ab197896) and AT1R (1:800, ab124505) were purchased from Abcam Company (Cambridge, MA, USA). Primary antibodies against α-tubulin (1:2000, 11224-1-AP) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:50,000, 60004-1-Ig) were purchased from Proteintech Group (Wuhan, China). 4',6-Diamidino-2-phenylindole (DAPI) staining solution was purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China).
Participants and sample collection
This was a prospective and case–control study. MN patients were recruited from Shaanxi Traditional Chinese Medicine Hospital and Baoji Central Hospital between January 2016 and December 2020. Patients who were diagnosed with MN by renal biopsy and/or detection of positive anti-phospholipase A2 receptor (PLA2R) with nephrotic syndrome were included in this study, while MN patients with secondary to infectious or other systemic autoimmune diseases were excluded from the study [22]. Each specimen, including thirty kidney tissues from kidney biopsies of patients or donors, was collected to determine the protein expression of Sirts. Control samples were obtained from healthy transplant donors. This study was approved by the Ethics Committee (No. SXSY-235610). All patients provided informed consent before entering the study. Serum samples were collected after overnight fasting for biochemical analysis.
CBSA preparation
Bovine serum albumin is used as a substrate to prepare charge-modified CBSA. CBSA was prepared as described previously [23].
Animal experiment
Sixteen Sprague‒Dawley male rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), with initial body weights of 180–220 g. After a 7-day adaptation period, the rats were randomly divided into two groups (n = 8): healthy control and CBSA-induced MN groups. MN rats were administered multiple subcutaneous injections of a mixed solution (1.0 mg CBSA, 0.5 mL phosphate buffered saline and 0.5 mL incomplete Freund’s adjuvant) on Day 1 for pre-autoimmunisation. CBSA solution (2.0 mg/mL) was prepared by dissolving CBSA in phosphate buffered saline. After 7 days, the rats were administered CBSA via tail vein (16 mg/kg) every other day for 2 weeks. Subsequently, the rats were administered CBSA via tail vein (25 mg/kg) every other day for 4 weeks. Albuminuria levels were detected within 24 h to ensure successful production of the MN model. The controls were treated with normal saline. Urine was collected for 24 h during week 6. The rats were anaesthetised by an intraperitoneal injection of 10% urethane. Blood and kidneys were collected for further analysis. All experiments were approved by the Committee of Experimental Animal Administration of the University (20200713-06).
Zymosan activation serum (ZAS) preparation and cell treatment
Mouse podocyte cell (MPC) culture was described in our previous publication [23]. C5b-9 was prepared by using healthy human serum as a complement source and treated with zymosan A according to a previous study [18]. Podocytes were treated with ZAS (10%) for 4 h in the presence or absence of UBCS039 (100 µM) or ICG-001 (10 µM). After 4 h of treatment, the cells were collected for subsequent experiments.
Serum and urine biochemical indices and complement 3 (C3) levels
Serum levels of total protein, albumin, creatinine, urea, uric acid, total cholesterol (TC), triglyceride (TG) and low-density lipoprotein cholesterol (LDL-C) were measured using a Backman AU680 automatic analyser. The levels of urinary total protein (UTP), albumin and creatinine in urine were measured using a Roche Cobas C501 Chemical Analyser. The urinary protein/creatinine (P/C) ratio was calculated. Serum C3 levels were measured based on our previous publication [23].
Kidney histopathological analysis
For histological analysis, kidney tissues from participants and rats were fixed with 4% paraformaldehyde for 24–48 h and then embedded in paraffin wax. The paraffin blocks were cut into 5 µm sections and mounted on glass slides. Kidney tissue sections were subjected to haematoxylin-eosin (H&E) and periodic acid-silver methenamine (PASM) staining according to previous methods [23–25]. The degree of kidney injury was assessed blindly by two pathologists under a light microscope.
Transmission electron microscope analysis
A Hitachi H-600 transmission electron microscope (Tokyo, Japan) was used in this study. Transmission electron microscopy was performed as described in our previous publication [23].
Immunohistochemistry and immunofluorescence
Immunohistochemical and immunofluorescent analyses were described in our previous publication [23]. The ratio of the positively stained area to the total area was analysed by using Image-Pro Plus 6.0 (Bethesda, MD, USA). Immunofluorescence analysis was performed by using Leica Microsystems CMS GmbH (Munich, Germany).
Western blot analysis
Western blot analysis was performed as previously described [26, 27]. The bands were visualised by using Western quick horseradish peroxidase chemiluminescent substrate. The band levels were normalised to GAPDH or α-tubulin. The relative intensities were quantified by ImageJ software.
Statistical analysis
Statistical analysis was performed by GraphPad Prism software (Version 6.0, San Diego, CA, USA). The number of replicates was 6–8 per group for each dataset, and the results are expressed as the mean ± SEM. A two-tailed unpaired Student’s t test was used for comparisons between two groups, and statistically significant differences among more than two groups were analysed using one-way analysis of variance followed by Dunnett’s post hoc tests. P < 0.05 was considered to indicate a significant difference.
Results
Clinical data of MN patients and controls
As shown in Table 1, serum levels of total protein and albumin, as well as the estimated glomerular filtration rate (eGFR), were significantly decreased, while the urine protein/creatinine ratio was significantly increased in patients with MN (Table 1), indicating glomerular injury. Moreover, the levels of total cholesterol, triglycerides and low-density lipoprotein in serum were significantly increased in patients with MN (Table 1). In addition, white blood cell counts were significantly increased, while red blood cell counts and haemoglobin levels were significantly decreased in patients with MN, indicating that anaemia occurred (Table 1). There were no significant differences in the levels of serum creatinine, urea, uric acid and high-density lipoprotein cholesterol, as well as age and body weight, between the two groups (Table 1).
Table 1.
Demographic and clinical baseline characteristics of healthy controls and MN patients.
| Demographics | Control group | MN group | P value |
|---|---|---|---|
| Gender (male %) | 108 (54%) | 77 (57.9%) | – |
| Age (year) | 56.6 ± 1.0 | 54.9 ± 1.0 | 0.256 |
| Body weight (kg) | 68.8 ± 0.8 | 65.7 ± 0.9 | 0.074 |
| Diabetes (%) | N/A | 29 (21.8%) | – |
| Hypertension (%) | N/A | 48 (36.1) | – |
| Laboratory values | |||
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 104.3 ± 0.9 | 83.8 ± 2.3 | <0.0001 |
| Serum total protein (g/L) | 74.1 ± 0.3 | 45.4 ± 0.69 | <0.0001 |
| Serum albumin (g/L) | 48.1 ± 0.3 | 24.5 ± 0.5 | <0.0001 |
| Serum creatinine (μmol/L) | 69.6 ± 1.0 | 65.7 ± 2.4 | 0.088 |
| Serum urea (mmol/L) | 5.44 ± 0.30 | 5.49 ± 0.27 | 0.984 |
| Serum uric acid (μmol/L) | 335.7 ± 6.8 | 342.6 ± 8.3 | 0.521 |
| Serum total cholesterol (mmol/L) | 4.63 ± 0.09 | 6.15 ± 0.19 | <0.0001 |
| Serum triglycerides (mmol/L) | 1.88 ± 0.11 | 2.72 ± 0.19 | <0.0001 |
| Serum low-density lipoprotein cholesterol (mmol/L) | 2.96 ± 0.07 | 4.71 ± 0.17 | <0.0001 |
| Serum high-density lipoprotein cholesterol (mmol/L) | 1.51 ± 0.05 | 1.63 ± 0.07 | 0.142 |
| 24-h urinary protein (g/24 h) | 0.07 ± 0.01 | 5.26 ± 0.24 | <0.0001 |
| Urine protein/creatinine ratio | 0.15 ± 0.01 | 4.76 ± 0.37 | <0.0001 |
| White blood cells (109/L) | 6.06 ± 0.10 | 6.61 ± 0.25 | 0.021 |
| Red blood cells (1012/L) | 4.56 ± 0.04 | 4.13 ± 0.05 | <0.0001 |
| Hemoglobin (g/L) | 138.3 ± 1.2 | 126.5 ± 1.6 | <0.0001 |
| Platelets (109/L) | 209.5 ± 3.4 | 213.3 ± 5.1 | 0.523 |
N/A not available.
Sirt impairment, a hyperactive Wnt1/β-catenin pathway and RAS overexpression in patients with MN
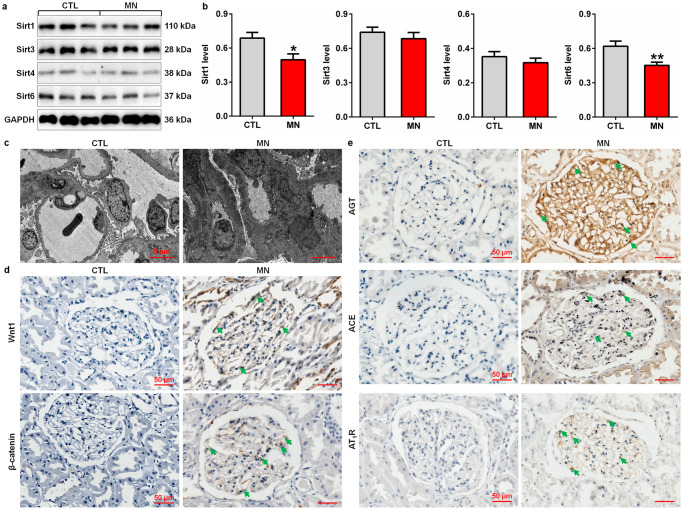
The protein expression of intrarenal Sirt1, Sirt3, Sirt4 and Sirt6 was determined. The protein expression of intrarenal Sirt1 and Sirt6 was significantly decreased in patients with MN compared with controls (Fig. 1a, b), and the reduced expression of Sirts in damaged podocytes that was confirmed by podocyte foot process effacement and increased electron-dense deposits in glomeruli (Fig. 1c). Compared with controls, patients with MN showed significantly upregulated Wnt1 and β-catenin protein expression in glomeruli (Fig. 1d). A previous study demonstrated that the hyperactive Wnt/β-catenin pathway mediated the expression of all RAS genes [28]. Our results indicated that patients with MN showed significantly upregulated protein expression of RAS components, including AGT, ACE and AT1R, in glomeruli (Fig. 1e), which led to elevated blood pressure. Collectively, these findings suggested that impairment of Sirt expression, hyperactivation of the Wnt1/β-catenin pathway and RAS signalling were involved in proteinuria-associated renal disease.
Fig. 1. Intrarenal Sirt deficiency, a hyperactive Wnt1/β-catenin pathway and RAS overexpression in MN patients.
a Protein expression levels of intrarenal Sirt1, Sirt3, Sirt4 and Sirt6 in controls and patients with MN. b Quantitative analysis of the protein expression levels of intrarenal Sirt1, Sirt3, Sirt4 and Sirt6 in controls and patients with MN. *P < 0.05, **P < 0.01 compared with CTL. c Electron microscopy images of kidney tissues from controls and patients with MN. d Immunohistochemical analysis of intrarenal Wnt1 and β-catenin expression in controls and patients with MN. e Immunohistochemical analysis of intrarenal AGT, ACE and AT1R expression in controls and patients with MN. Arrows indicate protein expression.
Sirt6 deficiency in CBSA-induced MN rats
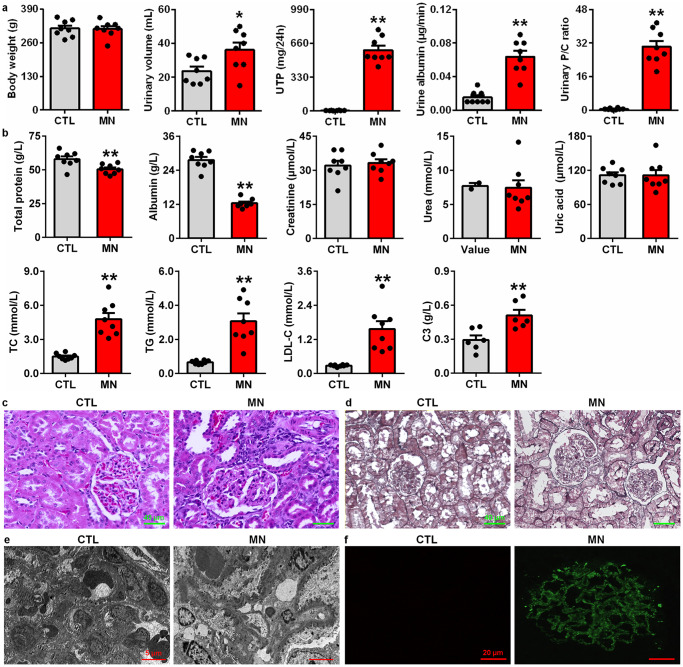
To explore the underlying molecular mechanism of MN, we first examined whether Sirt expression, the Wnt1/β-catenin pathway and RAS signalling were activated in CBSA-induced MN rats. Compared with control rats, CBSA-induced rats exhibited significant increases in urine volume, UTP, urine albumin excretion rate and the urinary P/C ratio and slight changes in body weight (Fig. 2a). CBSA-induced rats showed significantly decreased total protein and albumin levels in serum and significantly increased TC, TG and LDL-C levels compared with control rats. In addition, CBSA-induced rats did not show significantly increased serum levels of creatinine, urea and uric acid compared with control rats (Fig. 2b). Notably, significantly increased serum C3 levels were observed in CBSA-induced MN rats compared with control rats (Fig. 2b).
Fig. 2. Renal function decline and renal injury in CBSA-induced rats.
a Body weight, urine volume, UTP, urine albumin excretion rate and the urinary protein/creatinine ratio in control and MN rats. b Levels of total protein, albumin, creatinine, urea, uric acid, TC, TG, LDL-C and C3 in control and MN rats. c Images of H&E staining of kidney tissues in control and MN rats. d Images of PASM staining of the kidney tissues in control and MN rats. e Electron microscopy images of the kidney tissues of control and MN rats. f Images of IgG4 immunofluorescence staining of the kidney tissues of control and MN rats. *P < 0.05, **P < 0.01 compared with CTL rats.
As expected, H&E staining showed slightly enlarged glomeruli and severe glomerular damage in the kidney tissues of CBSA-induced rats (Fig. 2c). PASM staining showed a thickened glomerular basement membrane and capillary walls in glomeruli (Fig. 2d). Electron microscopy showed diffuse and patchy subepithelial electron-dense deposit distribution, glomerular basement membrane thickening and podocyte foot process effacement in glomeruli (Fig. 2e). Immunofluorescence analysis showed that IgG4 expression showed a granular pattern of deposition along the glomerular basement membrane in the glomeruli of CBSA-induced rats (Fig. 2f).
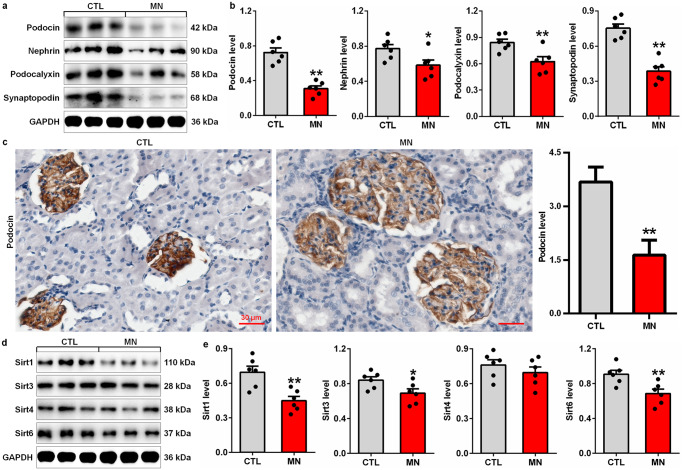
Compared with control rats, CBSA-induced rats had significantly decreased expression of podocyte-specific proteins, including podocin, nephrin, podocalyxin and synaptopodin, in glomeruli (Fig. 3a, b). Protein expression of intrarenal Sirt1, Sirt3, Sirt4 and Sirt6 was also determined in control and CBSA-induced rats. The protein expression of Sirt1, Sirt3 and Sirt6 was significantly decreased in CBSA-induced rats compared with control rats (Fig. 3c, d), which was consistent with the significant decrease in the protein expression of Sirt1 and Sirt6 in patients with MN. Collectively, these findings suggested impaired Sirt6 expression in CBSA-induced rats.
Fig. 3. Podocyte injury and downregulated protein expression of Sirts in CBSA-induced rats.
a Protein expression levels of intrarenal podocin, nephrin, podocalyxin and synaptopodin in control and CBSA-induced MN rats. b Quantitative analysis of the protein expression of intrarenal podocin, nephrin, podocalyxin and synaptopodin in control and CBSA-induced MN rats. c Immunohistochemical analysis of intrarenal podocin expression in control and CBSA-induced MN rats. d Protein expression levels of intrarenal Sirt1, Sirt3, Sirt4 and Sirt6 in control and CBSA-induced MN rats. e Quantitative analysis of the protein expression levels of intrarenal Sirt1, Sirt3, Sirt4 and Sirt6 in control and CBSA-induced MN rats. *P < 0.05, **P < 0.01 compared with CTL rats.
Activation of the intrarenal Wnt1/β-catenin pathway and RAS signalling in CBSA-induced MN rats
A previous study suggested that all RAS genes were downstream targets of the Wnt/β-catenin pathway [28]. We examined whether the Wnt1/β-catenin pathway was activated in CBSA-induced rats. Immunohistochemical analysis showed upregulated intrarenal Wnt1 protein expression (Fig. 4a), which was accompanied by increased intrarenal β-catenin protein expression in CBSA-induced rats (Fig. 4b). Similarly, Western blotting showed the upregulation of intrarenal Wnt1 and β-catenin protein expression in CBSA-induced rats (Fig. 4c, d), which was accompanied by the upregulation of β-catenin downstream gene products, including Snail1, Twist, MMP-7, PAI-1 and FSP1 (Fig. 4c, d). These results suggested activation of the Wnt1/β-catenin pathway in MN.
Fig. 4. Activation of the Wnt1/β-catenin pathway and RAS signalling in CBSA-induced rats.
a Immunohistochemical analysis of intrarenal Wnt1 expression in control and CBSA-induced MN rats. b Immunohistochemical analysis of intrarenal β-catenin expression in control and CBSA-induced MN rats. c Protein expression levels of intrarenal Wnt1, active β-catenin and β-catenin and the downstream gene products, including Snail1, Twist, MMP-7, PAI-1 and FSP1, in control and CBSA-induced MN rats. d Quantitative analysis of the protein expression of intrarenal Wnt1, active β-catenin, β-catenin Snail1, Twist, MMP-7, PAI-1 and FSP1 in control and CBSA-induced MN rats. e Immunohistochemical analysis of intrarenal ACE expression in control and CBSA-induced MN rats. f Immunohistochemical analysis of intrarenal AT1R expression in control and CBSA-induced MN rats. g Protein expression levels of intrarenal AGT, ACE and AT1R in control and CBSA-induced MN rats. h Quantitative analysis of the protein expression of intrarenal AGT, ACE and AT1R in control and CBSA-induced MN rats. *P < 0.05, **P < 0.01 compared with CTL rats.
We next examined whether RAS signalling was activated in CBSA-induced rats. As shown in Fig. 4e, f, the protein expression of intrarenal ACE and AT1R was significantly increased in CBSA-induced rats compared with control rats. In line with our Western blot results, the protein expression of intrarenal AGT, ACE and AT1R was significantly upregulated in CBSA-induced rats (Fig. 4g, h). These results suggested the activation of RAS signalling in rats. Overall, these findings suggested that activation of RAS signalling might be associated with the Wnt1/β-catenin pathway in CBSA-induced rats.
Sirt6 deficiency exacerbates podocyte injury in ZAS-stimulated podocytes
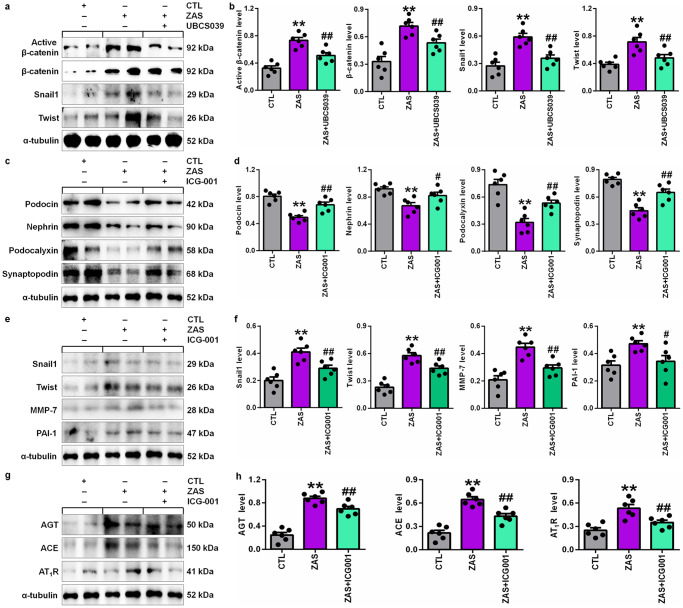
To further reveal the underlying molecular mechanism of MN, we examined Sirt6 deficiency in injured podocytes. Compared with the control group, ZAS-stimulated podocytes showed significant decreases in the protein expression of podocin, nephrin, podocalyxin and synaptopodin (Fig. 5a, b). The protein expression of Sirt1, Sirt3, Sirt4 and Sirt6 was also determined. Only Sirt6 protein expression showed a significant decrease in ZAS-stimulated podocytes compared with controls (Fig. 5c, d), which was consistent with patients with MN and CBSA-induced rats. However, treatment with UBCS039, a specific Sirt6 activator, preserved Sirt6 protein expression in ZAS-stimulated podocytes (Fig. 5e, f). Furthermore, UBCS039 treatment preserved the protein expression of podocin, nephrin and podocalyxin in ZAS-stimulated podocytes (Fig. 5e, f). Therefore, in vivo and in vitro experiments suggested the downregulation of Sirt6 protein expression in MN, indicating that Sirt6 deficiency was associated with podocyte injury.
Fig. 5. Sirt6 deficiency exacerbated podocyte injury in ZAS-stimulated podocytes.
a Protein expression levels of podocin, nephrin, podocalyxin and synaptopodin in control and ZAS-stimulated podocytes. b Quantitative analysis of the protein expression of podocin, nephrin, podocalyxin and synaptopodin in control and ZAS-stimulated podocytes. c Protein expression levels of intrarenal Sirt1, Sirt3, Sirt4 and Sirt6 in control and ZAS-stimulated podocytes. d Quantitative analysis of the protein expression levels of intrarenal Sirt1, Sirt3, Sirt4 and Sirt6 in control and ZAS-stimulated podocytes. e Protein expression levels of Sirt6, podocin, nephrin and podocalyxin in ZAS-stimulated podocytes treated with UBCS039. f Quantitative analysis of the protein expression levels of Sirt6, podocin, nephrin and podocalyxin in ZAS-stimulated podocytes treated with UBCS039. *P < 0.05, **P < 0.01 compared with CTL; ##P < 0.01 compared with ZAS-stimulated podocytes.
RAS signalling was mediated through the Wnt1/β-catenin pathway in ZAS-stimulated podocytes
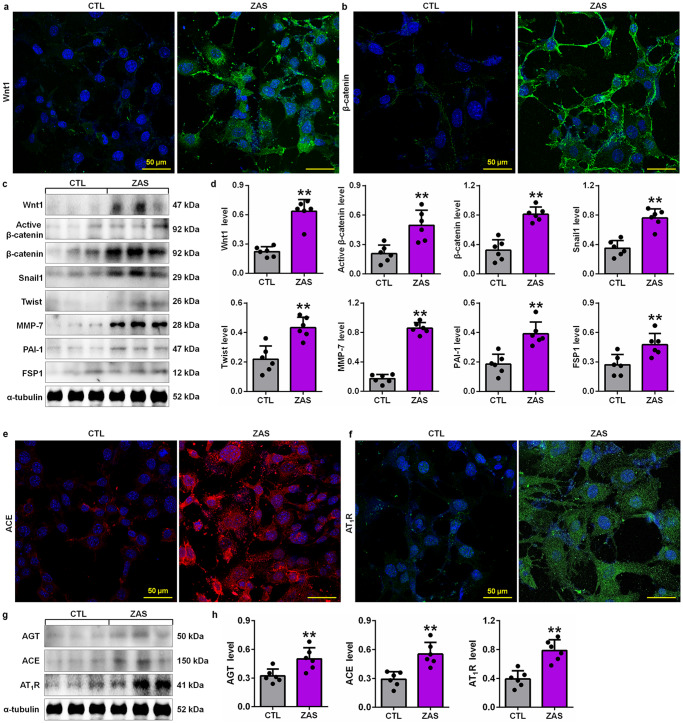
We further examined whether the Wnt1/β-catenin pathway was activated in ZAS-stimulated podocytes. Immunofluorescence staining showed significantly increased intrarenal Wnt1 protein expression in ZAS-stimulated podocytes (Fig. 6a), which was accompanied by upregulated β-catenin protein expression (Fig. 6b). Similarly, Western blotting showed upregulated protein expression of Wnt1 and β-catenin in ZAS-stimulated podocytes (Fig. 6c, d), which was accompanied by the upregulation of downstream β-catenin gene products, including Snail1, Twist, MMP-7, PAI-1 and FSP1, in ZAS-stimulated podocytes (Fig. 6c, d). These data were consistent with the results of CBSA-induced rats, suggesting activation of the Wnt1/β-catenin pathway in MN.
Fig. 6. Activation of the Wnt1/β-catenin pathway and RAS signalling in ZAS-stimulated podocytes.
a Images of Wnt1 immunofluorescence staining in control and ZAS-stimulated podocytes. b Images of β-catenin immunofluorescence staining in control and ZAS-stimulated podocytes. c Protein expression levels of Wnt1, active β-catenin and β-catenin and its downstream gene products, including Snail1, Twist, MMP-7, PAI-1 and FSP1, in control and ZAS-stimulated podocytes. d Quantitative analysis of the protein expression of Wnt1, active β-catenin, β-catenin Snail1, Twist, MMP-7, PAI-1 and FSP1 in control and ZAS-stimulated podocytes. e Images of ACE immunofluorescence staining in control and ZAS-stimulated podocytes. f Images of AT1R immunofluorescence staining in control and ZAS-stimulated podocytes. g Protein expression levels of AGT, ACE and AT1R in control and ZAS-stimulated podocytes. h Quantitative analysis of the protein expression of AGT, ACE and AT1R in control and ZAS-stimulated podocytes. **P < 0.01 compared with CTL rats.
We next examined whether RAS signalling was activated in podocytes. As shown in Fig. 6e, f, the levels of ACE and AT1R were significantly increased in ZAS-stimulated podocytes, which was consistent with the protein expression of significantly upregulated AGT, ACE and AT1R (Fig. 6g, h), indicating activation of RAS signalling in podocytes. Taken together, these findings might demonstrate that activation of RAS signalling might be associated with the Wnt1/β-catenin pathway in MN.
Sirt6 ameliorated podocyte injury by blocking RAS signalling via the Wnt1/β-catenin pathway
Finally, we investigated whether Sirt6 ameliorated podocyte injury by inhibiting the activation of RAS signalling via the Wnt1/β-catenin pathway. We first determined the effect of the decrease in Sirt6 protein expression on the Wnt1/β-catenin pathway. UBCS039 treatment significantly inhibited the protein expression of active β-catenin and β-catenin, as well as the downstream gene products, including Snail1 and Twist, in ZAS-stimulated podocytes (Fig. 7a, b), indicating that enhancing Sirt6 expression inhibited activation of the Wnt1/β-catenin pathway in injured podocytes. ICG-001, which is a small-molecule inhibitor, inhibited β-catenin-induced gene transcription in a cyclic adenosine monophosphate response element-binding protein-dependent fashion [15]. As shown in Fig. 7c, d, treatment with ICG-001 significantly preserved the protein expression of podocin, nephrin, podocalyxin and synaptopodin in ZAS-stimulated podocytes. Our results also showed that treatment with ICG-001 significantly inhibited the protein expression of downstream β-catenin gene products, including Snail1, Twist, MMP-7 and PAI-1 (Fig. 7e, f). We next examined whether inhibiting β-catenin protein expression could affect RAS signalling in ZAS-stimulated podocytes. As shown in Fig. 7g, h, treatment with ICG-001 significantly inhibited the protein expression of AGT, ACE and AT1R in ZAS-stimulated podocytes. Collectively, these data suggested that Sirt6 ameliorated podocyte injury by blocking RAS signalling via the Wnt1/β-catenin pathway (Fig. 8). Therefore, Sirt6 might be a specific therapeutic target for MN.
Fig. 7. Sirt6 reduced podocyte injury by blocking RAS signalling via the Wnt1/β-catenin pathway.
a Protein expression levels of active β-catenin, β-catenin, Snail1 and Twist in ZAS-stimulated podocytes treated with UBCS039. b Quantitative analysis of the protein expression levels of active β-catenin, β-catenin, Snail1 and Twist in ZAS-stimulated podocytes treated with UBCS039. c Protein expression levels of podocin, nephrin, podocalyxin and synaptopodin in ZAS-stimulated podocytes treated with ICG-001. d Quantitative analysis of the protein expression of podocin, nephrin, podocalyxin and synaptopodin in ZAS-stimulated podocytes treated with ICG-001. e Protein expression levels of Snail1, Twist, MMP-7 and PAI-1 in ZAS-stimulated podocytes treated with ICG-001. f Quantitative analysis of the protein expression of Snail1, Twist, MMP-7 and PAI-1 in ZAS-stimulated podocytes treated with ICG-001. g Protein expression levels of AGT, ACE and AT1R in ZAS-stimulated podocytes treated with ICG-001. h Quantitative analysis of the protein expression of AGT, ACE and AT1R in ZAS-stimulated podocytes treated with ICG-001. **P < 0.01 compared with CTL; #P < 0.05, ##P < 0.01 compared with ZAS-stimulated podocytes.
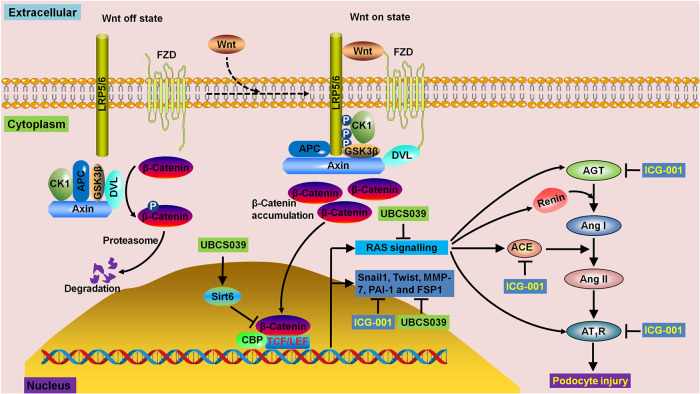
Fig. 8. Summary of the underlying molecular mechanism of Sirt6 deficiency, the hyperactive Wnt1/β-catenin pathway and RAS overexpression in podocyte injury.
Sirt6 deficiency, a hyperactive Wnt1/β-catenin pathway and RAS overexpression are observed in patients with MN, CBSA-injected rats and ZAS-stimulated podocytes. UBCS039 treatment significantly inhibits the protein expression of β-catenin and its downstream gene products, including Snail1 and Twist, in ZAS-stimulated podocytes. Treatment with ICG-001 significantly inhibits the protein expression of downstream β-catenin gene products, including Snail1, Twist, MMP-7 and PAI-1, which is accompanied by significantly downregulated protein expression of AGT, ACE and AT1R in ZAS-stimulated podocytes. Podocyte injury is also ameliorated by treatment with UBCS039 and ICG-001.
Discussion
Proteinuria development often occurs in the early stages of primary glomerular diseases such as MN, DKD, MGN and IgAN. Podocyte injury and/or dysfunction are major causes of proteinuria development and lead to glomerulosclerosis and end-stage renal disease. The result presented in this study suggests that change in Sirt6 protein expression is a critical modulator of the pathogenesis of podocyte dysfunction and proteinuria development. Our findings first demonstrated that intrarenal Sirt6 deficiency occurred in patients with MN, indicating that proteinuria development and podocyte injury were associated with Sirt6 impairment. Our study further showed that the downregulation of Sirt6 protein expression occurred in ZAS-stimulated podocytes. Several previous publications have shown the downregulation of Sirt6 protein expression in renal biopsies from patients with podocyte damage-associated renal disease [9, 29, 30]. A seminal publication highlighted the downregulation of Sirt6 protein expression in podocytes from patients with DKD, MGN, IgAN, FSGS and diabetes [9]. In addition, Fan et al. reported that Sirt6 protein expression was downregulated in the glomeruli of patients with DKD and hypertensive nephropathy [29, 30]. This research group also demonstrated a decrease in Sirt6 gene expression in the glomeruli of patients with hypertensive nephropathy [31].
Border et al. first established a novel MN model in rabbits immunised with CBSA [32]. Our findings first demonstrated that intrarenal Sirt6 deficiency occurred in CBSA-induced rats, which was consistent with the downregulation of intrarenal Sirt6 protein expression in patients with MN. Recently, a number of studies have revealed the downregulation of Sirt6 protein expression in the glomeruli of mice or rats with diabetes, DKD and adriamycin-induced nephropathy [9, 30, 33]. In addition, the downregulation of Sirt6 protein expression was observed in the glomeruli of angiotensin II-infused mice [29, 31, 34]. Podocyte-specific deletion of Sirt6 exacerbated podocyte injury and proteinuria in these mouse models [9, 30, 31, 33, 34]. Consistent with this conclusion was the finding that Sirt6 overexpression alleviated renal injury by promoting M2 macrophage transformation in DKD rats [33].
Finally, our findings demonstrated the downregulation of Sirt6 protein expression in ZAS-stimulated podocytes. Recently, in vitro experiments have shown the downregulation of Sirt6 protein expression in podocytes treated with high glucose, adriamycin, angiotensin II or advanced glycation end-products [9, 29, 30]. Sirt6 deletion exacerbates angiotensin II-induced podocyte injury [29, 31, 34], whereas Sirt6 overexpression ameliorates angiotensin II- or high glucose-induced podocyte injury [29–31, 34]. Similarly, our findings first demonstrated that treatment with UBCS039, a specific Sirt6 agonist, preserved the protein expression of Sirt6 and podocyte-specific markers, including podocin, nephrin and podocalyxin, in ZAS-stimulated podocytes. Therefore, the current work and that of others suggest that the downregulation of Sirt6 protein expression in podocytopathies contributes to proteinuria-associated renal disease. Collectively, these findings suggested that the deacetylase Sirt6 showed a cytoprotective effect on podocyte injury. Therefore, Sirt6 might represent a promising target for developing novel therapeutic strategies to treat patients with proteinuria-associated renal disease.
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are recommended first-line therapies for chronic proteinuric nephropathy. Earlier studies have demonstrated that RAS signalling is a downstream target of the Wnt/β-catenin pathway [15]. Cai et al. reported that sirt6 deficiency enhanced the expression of downstream target proteins of β-catenin signalling and that sirt6 interacted with β-catenin and bound to the promoters of β-catenin target genes in renal proximal tubular epithelial cells treated with transforming growth factor-β [20]. To determine the underlying molecular mechanism of Sirt6 against podocyte injury, we further examined the Wnt/β-catenin pathway and RAS signalling. Our findings showed activation of the intrarenal Wnt/β-catenin pathway and RAS signalling in patients with MN, CBSA-induced MN rats and ZAS-stimulated podocytes.
Studies have demonstrated that the Wnt/β-catenin pathway is involved in a variety of diseases, such as ageing, cancer and renal disease [35–37]. Our present study first showed significantly increased protein expression of intrarenal Wnt1 and β-catenin in patients with MN. Kiewisz and colleagues demonstrated that the mRNA level of Wnt4 was significantly increased in the kidney tissues of patients with MN compared with those with lupus nephritis or IgAN [16]. Therefore, the current work and that of others suggests that activation of the Wnt/β-catenin pathway occurs in patients with MN. Our study showed significantly increased protein expression of intrarenal Wnt1 and β-catenin and its downstream gene products, including Snail1, Twist, MMP-7, PAI-1 and FSP1, in CBSA-induced rats (Fig. 8). Gao and colleagues demonstrated significantly increased intrarenal β-catenin protein expression in passive Heymann nephritis rats [17]. These results suggest that activation of the Wnt/β-catenin pathway might occur in animal models of MN. Our current study further demonstrated significantly increased protein expression of Wnt1 and β-catenin and its downstream gene products in ZAS-stimulated podocytes. Several in vitro experiments demonstrated the upregulated mRNA and protein expression of Wnt and β-catenin in podocytes [18, 19]. A recent study showed significantly increased mRNA levels of Wnt3 and β-catenin in human podocytes treated with MN plasma [19]. Dong and colleagues demonstrated significantly increased protein expression of β-catenin and phosphorylated β-catenin, while treatment with Dickkopf-1, a Wnt pathway inhibitor, could significantly decrease the protein expression of β-catenin and phosphorylated β-catenin in ZAS-stimulated podocytes [18]. Treatment with ICG-001 downregulated the protein expression of Snail1, Twist, MMP-7, PAI-1 and FSP1 in ZAS-stimulated podocytes. In addition, our previous studies demonstrated that renal injury was ameliorated by targeting the Wnt1/β-catenin cascade pathway with natural products [38–40]. These findings suggest that MN can be ameliorated by improving the Wnt1/β-catenin pathway.
Another important finding showed an increase in the protein expression of intrarenal RAS components, including AGT, ACE and AT1R, in patients with MN. Studies have demonstrated that circulating RAS plays a key role in arterial pressure control and sodium homeostasis regulation [41, 42]. In contrast, a tissue-specific RAS is present in several organs that are independent of circulating RAS. Among organ-specific RAS, intrarenal RAS plays an important role in oxidative stress and inflammation, sodium reabsorption and renal fibrosis and is one of the most important contributors to the pathophysiology in patients and animal models of renal disease [43].
AGT, the sole precursor of angiotensin peptides, is the only substrate for renin in the RAS [44]. Serum AGT cannot be freely filtered via the glomerular basement membrane in healthy subjects. A few studies demonstrated that urinary AGT levels indicated the association of intrarenal RAS activity and chronic kidney disease severity rather than a nonspecific consequence of proteinuria-associated DKD and IgAN [45, 46]. In addition, Jang et al. demonstrated that urinary AGT was mainly produced by filtered AGT in the systemic circulation in patients with nephrotic-associated proteinuria, which only occurred in patients with DKD or minimal change disease but not in patients with MN [47]. In contrast, urinary AGT is primarily generated locally, which indicates intrarenal RAS activation [48]. Intrarenal RAS activation was observed in patients with MN, and increased in situ production of angiotensin II and ACE was associated with disease severity [49]. Tang et al. demonstrated that urinary AGT levels were significantly increased in patients with MN compared with healthy controls [50]. Further results showed that urinary AGT levels were negatively correlated with serum albumin and eGFR and positively correlated with 24 h proteinuria in patients with MN [50]. Similarly, Ohashi et al. demonstrated that urinary AGT levels in patients with MN were significantly increased compared with those in IgAN patients [51]. However, urinary AGT/albumin ratios were the same in patients with IgAN and MN. Multiple linear regression analysis showed that urinary AGT/albumin ratios had a significant positive association with IgAN after adjustments [51]. We hypothesised that these differences may be associated with several mechanisms. First, the origin of urinary AGT is different in the different subtypes of glomerulonephritis. Urinary AGT is correlated with serum AGT in patients with minimal change disease but not in patients with MN, which indicates that urinary AGT is primarily filtered from serum in nephrotic minimal change disease but generated locally in MN [47]. Second, the different types of urinary AGT might be triggered by different pathogenesis between MN and minimal change disease. RAS activation is likely an outcome of primary damage in the kidney. However, studies suggest that MN is an autoimmune disease caused by circulating autoantibodies against corresponding receptors on podocytes, such as PLA2R [12]. However, minimal change disease is caused by dysfunctional T cells [52]. Furthermore, clinical evidence has shown that ACEIs and ARBs might be effective in patients with MN but not in patients with minimal change disease.
Our present study showed increased ACE protein expression in patients with MN and CBSA-induced rats. It has been reported that significantly increased blood pressure was observed and renal ACE activity was increased threefold in uninephrectomized passive Heymann nephritis rats, while increases in blood pressure were lowered and ACE activity was significantly inhibited after ACEI treatment [53]. Regarding renal function and proteinuria reduction, Dikow et al. suggested that patients with MN who were treated without immunosuppressants but with determinate to ensure optimal blood pressure control and full RAS blockade showed similar outcomes compared with patients treated with additional immunosuppressants [54]. Treatment with ICG-001 downregulated the protein expression of AGT, ACE and AT1R in ZAS-stimulated podocytes. In addition, our previous studies demonstrated that renal injury was ameliorated by targeting the RAS/Wnt1/β-catenin cascade pathway [23, 55, 56]. Overall, improving the Wnt1/β-catenin/RAS signalling axis might be an effective therapeutic strategy for the treatment of MN (Fig. 8).
UBCS039 treatment inhibited the protein expression of β-catenin and its downstream gene products, including Snail1 and Twist, in ZAS-stimulated podocytes, indicating that enhancing Sirt6 expression inhibited the hyperactive Wnt1/β-catenin pathway in injured podocytes. Treatment with ICG-001 inhibited the protein expression of AGT, ACE and AT1R in ZAS-stimulated podocytes. Collectively, these data suggest that Sirt6 ameliorated podocyte injury by blocking RAS signalling via the Wnt1/β-catenin pathway. Therefore, Sirt6 might be a specific therapeutic target for the treatment of podocyte damage-associated renal disease.
Conclusions
This study showed intrarenal Sirt6 impairment, a hyperactive Wnt1/β-catenin pathway and RAS overexpression in patients with MN and CBSA-induced rats, as well as ZAS-stimulated podocytes. The present study first demonstrated that Sirt6 protected against podocyte injury by blocking RAS by inhibiting the Wnt1/β-catenin pathway. Sirt6 might be a promising therapeutic target for the treatment of podocyte damage-associated renal disease.
Acknowledgements
This study was supported by the National Key Research & Development Program of China (No. 2019YFC1709405), the National Natural Science Foundation of China (No. 82274192, 82174366, 82074002, 82274079), the Shaanxi Key Research & Development Program (No. 2023-ZDLSF-26) and the Inheritance and Innovation of Traditional Chinese Medicine & Key Scientific Research on the Development of “Qin Medicine” of Shaanxi Administration of Traditional Chinese Medicine (No. 2021-03-22-005).
Author contributions
YYZ conceived and designed the experiments. HM, YNW and YYZ conducted the experiments and analysed the data. HM and YYZ performed the statistical analysis. XYY and WS collected the clinical samples. YYZ wrote the initial draft of the paper. LZ, SGZ and FL revised the paper. All authors have critically revised the paper and approved the final version.
Competing interests
The authors declare no competing interests.
Contributor Information
Xiao-yong Yu, Email: gub70725@126.com.
Fei Liu, Email: liufei_2359@163.com.
Ying-yong Zhao, Email: zhaoyybr@163.com.
References
- 1.Morigi M, Perico L, Benigni A. Sirtuins in renal health and disease. J Am Soc Nephrol. 2018;29:1799–809. doi: 10.1681/ASN.2017111218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi W, Hu C, Zhao D, Li X. SIRT1-SIRT7 in diabetic kidney disease: biological functions and molecular mechanisms. Front Endocrinol (Lausanne) 2022;13:801303. doi: 10.3389/fendo.2022.801303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu T, Yang L, Mao H, Ma F, Wang Y, Li S, et al. Sirtuins as novel pharmacological targets in podocyte injury and related glomerular diseases. Biomed Pharmacother. 2022;155:113620. doi: 10.1016/j.biopha.2022.113620. [DOI] [PubMed] [Google Scholar]
- 4.Zhong Y, Lee K, He JC. SIRT1 is a potential drug target for treatment of diabetic kidney disease. Front Endocrinol (Lausanne) 2018;9:624. doi: 10.3389/fendo.2018.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P, Liu Y, Qin X, Chen K, Wang R, Yuan L, et al. SIRT1 attenuates renal fibrosis by repressing HIF-2α. Cell Death Discov. 2021;7:59. doi: 10.1038/s41420-021-00443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung YJ, Park W, Kang KP, Kim W. SIRT2 is involved in cisplatin-induced acute kidney injury through regulation of mitogen-activated protein kinase phosphatase-1. Nephrol Dial Transpl. 2020;35:1145–56. doi: 10.1093/ndt/gfaa042. [DOI] [PubMed] [Google Scholar]
- 7.Feng X, Su H, He X, Chen JX, Zeng H. SIRT3 deficiency sensitizes angiotensin-II-induced renal fibrosis. Cells. 2020;9:2510. doi: 10.3390/cells9112510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi JX, Wang QJ, Li H, Huang Q. SIRT4 overexpression protects against diabetic nephropathy by inhibiting podocyte apoptosis. Exp Ther Med. 2017;13:342–48. doi: 10.3892/etm.2016.3938. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Liu M, Liang K, Zhen J, Zhou M, Wang X, Wang Z, et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun. 2017;8:413. doi: 10.1038/s41467-017-00498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Xu K, Zhang N, Amador G, Wang Y, Zhao S, et al. Overexpressed SIRT6 attenuates cisplatin-induced acute kidney injury by inhibiting ERK1/2 signaling. Kidney Int. 2018;93:881–92. doi: 10.1016/j.kint.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Liu Y. Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015;11:535–45. doi: 10.1038/nrneph.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YN, Feng HY, Nie X, Zhang YM, Zou L, Li X, et al. Recent advances in clinical diagnosis and pharmacotherapy options of membranous nephropathy. Front Pharmacol. 2022;13:907108. doi: 10.3389/fphar.2022.907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao H, Zhang YM, Yu XY, Zou L, Zhao YY. Membranous nephropathy: systems biology-based novel mechanism and traditional Chinese medicine therapy. Front Pharmacol. 2022;13:969930. doi: 10.3389/fphar.2022.969930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao YY. Recent advances of gut microbiota in chronic kidney disease patients. Explor Med. 2022;3:260–74. doi: 10.37349/emed.2022.00090. [DOI] [Google Scholar]
- 15.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol. 2015;26:107–20. doi: 10.1681/ASN.2014010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiewisz J, Skowronska A, Winiarska A, Pawlowska A, Kiezun J, Rozicka A, et al. Wnt4 expression in primary and secondary kidney diseases: dependence on staging. Kidney Blood Press Res. 2019;44:200–10. doi: 10.1159/000498989. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Dai H, Zhang N, Jiang H, Zhang Z, Feng Z, et al. The ameliorative effect of Mahuang fuzi and shenzhuo decoction on membranous nephropathy of rodent model is associated with autophagy and Wnt/β-catenin pathway. Front Pharmacol. 2022;13:820130. doi: 10.3389/fphar.2022.820130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Z, Dai H, Gao Y, Feng Z, Liu W, Liu F, et al. Inhibition of the Wnt/β-catenin signaling pathway reduces autophagy levels in complement treated podocytes. Exp Ther Med. 2021;22:737. doi: 10.3892/etm.2021.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao S, Cui Z, Zhao MH. Complement C3a and C3a receptor activation mediates podocyte injuries in the mechanism of primary membranous nephropathy. J Am Soc Nephrol. 2022;33:1742–56. doi: 10.1681/ASN.2021101384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai J, Liu Z, Huang X, Shu S, Hu X, Zheng M, et al. The deacetylase sirtuin 6 protects against kidney fibrosis by epigenetically blocking β-catenin target gene expression. Kidney Int. 2020;97:106–18. doi: 10.1016/j.kint.2019.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322:1294–304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seitz-Polski B, Dahan K, Debiec H, Rousseau A, Andreani M, Zaghrini C, et al. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14:1173–82. doi: 10.2215/CJN.11791018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YN, Miao H, Hua MR, Yang JZ, Pei M, Yu HX, et al. Moshen granule ameliorates membranous nephropathy by blocking intrarenal renin-angiotensin system signalling via the Wnt1/β-catenin pathway. Phytomedicine. 2023;114:154763. doi: 10.1016/j.phymed.2023.154763. [DOI] [PubMed] [Google Scholar]
- 24.Miao H, Cao G, Wu XQ, Chen YY, Chen DQ, Chen L, et al. Identification of endogenous 1-aminopyrene as a novel mediator of progressive chronic kidney disease via aryl hydrocarbon receptor activation. Br J Pharmacol. 2020;177:3415–35. doi: 10.1111/bph.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao H, Wu XQ, Wang YN, Chen DQ, Chen L, Vaziri ND, et al. 1-Hydroxypyrene mediates renal fibrosis through aryl hydrocarbon receptor signalling pathway. Br J Pharmacol. 2022;179:103–24. doi: 10.1111/bph.15705. [DOI] [PubMed] [Google Scholar]
- 26.Cao G, Miao H, Wang YN, Chen DQ, Wu XQ, Chen L, et al. Intrarenal 1-methoxypyrene, an aryl hydrocarbon receptor agonist, mediates progressive tubulointerstitial fibrosis in mice. Acta Pharmacol Sin. 2022;43:2929–45. doi: 10.1038/s41401-022-00914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo LP, Suo P, Ren LL, Liu HJ, Zhang Y, Zhao YY. Shenkang injection and its three anthraquinones ameliorates renal fibrosis by simultaneous targeting IƙB/NF-ƙB and Keap1/Nrf2 signaling pathways. Front Pharmacol. 2021;12:800522. doi: 10.3389/fphar.2021.800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo Y, Liu Y. New insights into the role and mechanism of Wnt/β-catenin signalling in kidney fibrosis. Nephrol (Carlton) 2018;23:38–43. doi: 10.1111/nep.13472. [DOI] [PubMed] [Google Scholar]
- 29.Fan Y, Cheng J, Yang Q, Feng J, Hu J, Ren Z, et al. Sirt6-mediated Nrf2/HO-1 activation alleviates angiotensin II-induced DNA DSBs and apoptosis in podocytes. Food Funct. 2021;12:7867–82. doi: 10.1039/D0FO03467C. [DOI] [PubMed] [Google Scholar]
- 30.Fan Y, Yang Q, Yang Y, Gao Z, Ma Y, Zhang L, et al. Sirt6 suppresses high glucose-induced mitochondrial dysfunction and apoptosis in podocytes through AMPK activation. Int J Biol Sci. 2019;15:701–13. doi: 10.7150/ijbs.29323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q, Hu J, Yang Y, Chen Z, Feng J, Zhu Z, et al. Sirt6 deficiency aggravates angiotensin II-induced cholesterol accumulation and injury in podocytes. Theranostics. 2020;10:7465–79. doi: 10.7150/thno.45003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Border WA, Ward HJ, Kamil ES, Cohen AH. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J Clin Invest. 1982;69:451–61. doi: 10.1172/JCI110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji L, Chen Y, Wang H, Zhang W, He L, Wu J, et al. Overexpression of Sirt6 promotes M2 macrophage transformation, alleviating renal injury in diabetic nephropathy. Int J Oncol. 2019;55:103–15. doi: 10.3892/ijo.2019.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Liang W, Hu J, Zhu Z, Feng J, Ma Y, et al. Sirt6 deficiency contributes to mitochondrial fission and oxidative damage in podocytes via ROCK1-Drp1 signalling pathway. Cell Prolif. 2022;55:e13296. doi: 10.1111/cpr.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu HH, Cao G, Wu XQ, Vaziri ND, Zhao YY. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev. 2020;60:101063. doi: 10.1016/j.arr.2020.101063. [DOI] [PubMed] [Google Scholar]
- 36.Garg M, Maurya N. Wnt/β-catenin signaling in urothelial carcinoma of bladder. World J Nephrol. 2019;8:83–94. doi: 10.5527/wjn.v8.i5.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li SS, Sun Q, Hua MR, Suo P, Chen JR, Yu XY, et al. Targeting the Wnt/β-catenin signaling pathway as a potential therapeutic strategy in renal tubulointerstitial fibrosis. Front Pharmacol. 2021;12:719880. doi: 10.3389/fphar.2021.719880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao H, Wu XQ, Zhang DD, Wang YN, Guo Y, Li P, et al. Deciphering the cellular mechanisms underlying fibrosis-associated diseases and therapeutic avenues. Pharmacol Res. 2021;163:105316. doi: 10.1016/j.phrs.2020.105316. [DOI] [PubMed] [Google Scholar]
- 39.Chen DQ, Wu XQ, Chen L, Hu HH, Wang YN, Zhao YY. Poricoic acid A as a modulator of TPH-1 expression inhibits renal fibrosis via modulating protein stability of β-catenin and β-catenin-mediated transcription. Ther Adv Chronic Dis. 2020;11:2040622320962648. doi: 10.1177/2040622320962648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu D, Chen L, Zhao H, Vaziri ND, Ma SC, Zhao YY. Small molecules from natural products targeting the Wnt/β-catenin pathway as a therapeutic strategy. Biomed Pharmacother. 2019;117:108990. doi: 10.1016/j.biopha.2019.108990. [DOI] [PubMed] [Google Scholar]
- 41.Bhullar SK, Shah AK, Dhalla NS. Role of angiotensin II in the development of subcellular remodeling in heart failure. Explor Med. 2021;2:352–71. doi: 10.37349/emed.2021.00054. [DOI] [Google Scholar]
- 42.Rossi GP, Lenzini L, Caroccia B, Rossitto G, Seccia TM. Angiotensin peptides in the regulation of adrenal cortical function. Explor Med. 2021;2:294–304.
- 43.Yang T, Xu C. Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J Am Soc Nephrol. 2017;28:1040–9. doi: 10.1681/ASN.2016070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lalouel JM, Rohrwasser A, Terreros D, Morgan T, Ward K. Angiotensinogen in essential hypertension: from genetics to nephrology. J Am Soc Nephrol. 2001;12:606–15. doi: 10.1681/ASN.V123606. [DOI] [PubMed] [Google Scholar]
- 45.Satirapoj B, Siritaweesuk N, Supasyndh O. Urinary angiotensinogen as a potential biomarker of diabetic nephropathy. Clin Kidney J. 2014;7:354–60. doi: 10.1093/ckj/sfu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, et al. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transpl. 2011;26:170–7. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang HR, Jeon J, Park JH, Lee JE, Huh W, Oh HY, et al. Clinical relevance of urinary angiotensinogen and renin as potential biomarkers in patients with overt proteinuria. Transl Res. 2014;164:400–10. doi: 10.1016/j.trsl.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Urushihara M, Kondo S, Kagami S, Kobori H. Urinary angiotensinogen accurately reflects intrarenal renin-angiotensin system activity. Am J Nephrol. 2010;31:318–25. doi: 10.1159/000286037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mezzano SA, Aros CA, Droguett A, Burgos ME, Ardiles LG, Flores CA, et al. Renal angiotensin II up-regulation and myofibroblast activation in human membranous nephropathy. Kidney Int Suppl. 2003:86:S39–45. [DOI] [PubMed]
- 50.Tang Z, Wang Y, Tao L, Guo Y, Zheng Y, Zheng D. The elevated levels of urinary angiotensinogen are correlated with the severity of idiopathic membranous nephropathy. BMC Nephrol. 2018;19:357. doi: 10.1186/s12882-018-1165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohashi N, Aoki T, Matsuyama T, Ishigaki S, Isobe S, Katahashi N, et al. The urinary angiotensinogen to urinary albumin ratio reflects whether the renin-angiotensin system in the kidney is activated due to filtration of plasma angiotensinogen through the damaged glomeruli or the production of angiotensinogen in the proximal tubules. Intern Med. 2020;59:357–64. doi: 10.2169/internalmedicine.3624-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertelli R, Bonanni A, Di Donato A, Cioni M, Ravani P, Ghiggeri GM. Regulatory T cells and minimal change nephropathy: in the midst of a complex network. Clin Exp Immunol. 2016;183:166–74. doi: 10.1111/cei.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zoja C, Corna D, Camozzi D, Cattaneo D, Rottoli D, Batani C, et al. How to fully protect the kidney in a severe model of progressive nephropathy: a multidrug approach. J Am Soc Nephrol. 2002;13:2898–908. doi: 10.1097/01.ASN.0000034912.55186.EC. [DOI] [PubMed] [Google Scholar]
- 54.Dikow R, Quentmeier P, Schwenger V, Waldherr R, Andrassy K, Ritz E, et al. Optimal blood pressure control versus additional immunosuppressive therapy in idiopathic membranous nephropathy - a retrospective analysis. Clin Nephrol. 2009;72:366–72. doi: 10.5414/CNP72366. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Chen DQ, Chen L, Cao G, Zhao H, Liu D, et al. Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br J Pharmacol. 2018;175:2689–708. doi: 10.1111/bph.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang YN, Liu HJ, Ren LL, Suo P, Zou L, Zhang YM, et al. Shenkang injection improves chronic kidney disease by inhibiting multiple renin-angiotensin system genes by blocking the Wnt/β-catenin signalling pathway. Front Pharmacol. 2022;13:964370. doi: 10.3389/fphar.2022.964370. [DOI] [PMC free article] [PubMed] [Google Scholar]