Abstract
Introduction:
Intra-articular (IA) calcium crystal deposition is common in knee osteoarthritis (OA), but of unclear significance. It is possible that low-grade, crystal-related inflammation may contribute to knee pain. We examined the longitudinal relation of CT-detected IA mineralization to the development of knee pain.
Methods:
We used data from the NIH-funded longitudinal Multicenter Osteoarthritis (MOST) Study. Participants had knee radiographs and bilateral knee CTs at baseline, and pain assessments every eight months for two years. CT images were scored using the Boston University Calcium Knee Score (BUCKS). We longitudinally examined the relation of CT-detected IA mineralization to the risk of frequent knee pain (FKP), intermittent or constant knee pain worsening, and pain severity worsening using generalized linear mixed-effects models.
Results:
We included 2093 participants (mean age 61 years, 57% female, mean BMI 28.8 kg/m2). Overall, 10.2% of knees had IA mineralization. The presence of any IA mineralization in the cartilage was associated with 2.0 times higher odds of having FKP (95% CI 1.38-2.78) and 1.86 times more frequent intermittent or constant pain (95% CI 1.20-2.78), with similar results seen for the presence of any IA mineralization in the meniscus or joint capsule. A higher burden of IA mineralization anywhere within the knee was associated with a higher odds of all pain outcomes (ORs ranged from 2.14-2.21).
Conclusion:
CT-detected IA mineralization was associated with risk of having more frequent, persistent, and worsening knee pain over two years. Targeting IA mineralization may have therapeutic potential for pain improvement in knee OA.
INTRODUCTION
Pain and its fluctuation in knee osteoarthritis (OA) are well-recognized, though remain poorly understood. The frequency and severity of pain typically increases with worsening disease severity [1], and the specific patterns of pain may vary depending on the stage of the disease [2]. Patients with knee OA often report pain flares, but the triggers for such flares are not well characterized, and studies have primarily focused on biomechanical risk factors [3,4].
Intra-articular (IA) crystal deposition is common in knee OA [5,6], yet its clinical significance remains unclear. Crystals that can deposit intra-articularly in OA include calcium pyrophosphate (CPP) and basic calcium phosphate (BCP); each of these crystals can be associated with painful clinical conditions characterized often by acute flares. One hypothesis is that the presence of crystals in the joint may contribute to variable degrees of inflammation [7] and therefore potentially contribute to pain symptoms in OA [8]. Whether these crystals may also contribute to subacute pain fluctuation in knee OA is not known. Chondrocalcinosis on radiographs, reflecting calcium crystal deposition, have been associated with OA disease severity [9,10], although studies of the relationship between IA mineralization and OA structural progression have shown mixed results [11-14]. It is yet to be resolved as to whether IA mineralization in OA contributes to joint symptoms such as pain.
Clarification of the role of IA mineralization in knee OA pain symptoms is important given the large unmet need for therapies that reduce and control pain in symptomatic knee OA. In gout and CPPD, biologics targeting interleukin (IL)-1β have been successful in reducing pain and inflammation [15]. While studies of anti-IL-1β therapies in knee OA have been largely negative with regard to primary pain outcomes [16-18], a recent exploratory analysis of a large trial investigating the use of canakinumab in individuals with atherosclerotic cardiovascular disease demonstrated reductions in total knee and hip replacements over a median follow-up of 3.7 years [19]. This finding supports the hypothesis that there may be a subset of individuals with knee OA in whom directly targeting inflammation, potentially related to IA crystal deposition resulting in mineralization, might be helpful. Whether this may be the case for other clinical outcomes in OA remains to be elucidated.
In the majority of prior studies, chondrocalcinosis has been characterized on radiographs, which have low sensitivity due to their two-dimensional projectional nature [10,20,21]. This may have contributed to conflicting findings from earlier cohort studies that attempted to evaluate radiographically-detected chondrocalcinosis and its relationship with pain and inflammation [11,13,22]. Further, prior studies have not assessed the impact of tissue- or location-specific effects of IA mineralization or the burden of mineralization on clinically relevant outcomes. In the current study, we had the opportunity to use CT to overcome the limitation of poor detection on radiographs for the identification, localization, and quantification of IA mineralization [23,24]. We used longitudinal data from a well-characterized prospective cohort to examine the relation of IA mineralization to the development of knee pain over time.
METHODS
Data source and study sample
The Multicenter Osteoarthritis (MOST) Study is a NIH-funded longitudinal study of community-dwelling adults. An original, existing cohort between the ages of 50–79 years who had or were at risk of developing knee OA at baseline was recruited from Birmingham, Alabama and Iowa City, Iowa. Details of the cohort have been published elsewhere [25]. A new cohort was recruited at the 12th year of this study, who were age 45-69 years with Kellgren-Lawrence (KL) grade ≤2 in both knees, and either no knee pain or at most only mild intermittent knee pain.
The 12th year visit served as the baseline for this analysis as this was the first study visit at which CTs were obtained. All participants answered pain questionnaires and underwent knee radiographs, and bilateral knee CTs at baseline, and were assessed for pain outcomes (details below) every eight months for three additional study visits. The study was approved by the institutional review boards at the University of Iowa, University of Alabama at Birmingham, University of California at San Francisco, and Boston University Medical Center.
Intra-articular mineralization
A musculoskeletal radiologist (MJ) scored multiplanar CT images (axial images with coronal and sagittal 2D reformats) using the Boston University Calcium Knee Score (BUCKS) [26]. Both knees were imaged and scored. Mineralization in each of the Whole-Organ Magnetic Resonance Imaging Score (WORMS)-defined subregions of cartilage and menisci [27] was scored on a 0-3 scale. Ligament and joint capsule mineralization were scored as present or absent. A second musculoskeletal radiologist (AG) read 50 knees for inter-reader reliability. The intra-reader reliability ranged from 0.92 for ligaments to 1.0 for joint capsule. The inter-reader reliability ranged from 0.94 for cartilage and ligaments, to 1.0 for joint capsule.
The exposures of interest for this study were: 1) mineralization anywhere in the joint (which included the cartilage, meniscus, and joint capsule); 2) mineralization anywhere in the hyaline cartilage; 3) mineralization anywhere in the meniscus; 4) mineralization anywhere in the capsule; and 5) burden of mineralization anywhere in the joint (cartilage, meniscus, capsule) defined based upon the number of BUCKS subregions involved. The cut-points were determined based upon distribution of number of regions affected (0 [referent], 1, 2-5, and >5 subregions).
Pain outcome measures
The three pain outcomes for this study were: 1) frequent knee pain (FKP); 2) intermittent or constant pain; 3) pain worsening (each to be defined in further detail below). These were assessed at the baseline visit (12th year overall in the parent study) and every eight months, for a total of four assessments over two years.
We defined FKP as a response of yes to the question, “During the past 30 days, have you had pain, aching, or stiffness in your knee on most days?” during the in-person study visits at baseline and at two years, and during telephone interviews at 8 and 16 months. We identified the presence of FKP at each of the two in-person visits and the two telephone assessments to perform a repeated measures analysis.
Intermittent and constant pain was assessed at each time-point using the Intermittent and Constant OA Pain (ICOAP) instrument [28]. The ICOAP is an 11-item measure consisting of items for each of two subscales, Intermittent and Constant. The ICOAP was obtained in a knee-specific manner, inquiring about symptoms over the prior seven days. ICOAP pain patterns were defined as follows: 1) no intermittent or constant pain; 2) intermittent pain only (of at least “mild” severity and with a frequency of at least “sometimes”); 3) constant pain only (of at least “mild” severity); and 4) a combination of constant and intermittent pain, as defined above.
Pain severity was measured at each time-point using a knee-specific Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Likert version pain subscale (0-20 range). Higher scores on the WOMAC indicate greater pain.
Other covariates
Potential confounders defined a priori included age, sex, race, site, body mass index (BMI), and KL grade. Bilateral weight-bearing fixed-flexion posteroanterior radiographs of the knee were obtained at each study visit. Radiographic severity in the tibiofemoral joint was graded by two experienced readers blinded to clinical data according to KL criteria (0–4). The inter-rater reliability weighted kappa for the KL grade was 0.80.
Radiographic knee OA (ROA) was defined as KL ≥ 2. Disagreements on the presence/absence of radiographic OA at any timepoint between readers were adjudicated by a third reader along with the first two readers to reach consensus. Although effusion-synovitis and Hoffa-synovitis are related to inflammation and possibly to IA mineralization, we did not include them as confounders in our models as they are potential intermediates in the causal pathway between IA mineralization and knee pain.
Analyses
We examined the presence of any IA mineralization (i.e., anywhere in the joint in the cartilage, meniscus, or capsule), any cartilage mineralization, any meniscal mineralization, any capsular mineralization, and the burden of mineralization (based upon the number of affected subregions) to the risk of FKP, intermittent or constant pain worsening, or more severe pain. We used generalized linear mixed-effect models, with random-effects for persons and for study visits to account for the correlation due to knees within subjects and repeated pain assessments over time in each knee, to estimate the odds of pain outcomes due to mineralization. For the burden of mineralization, we performed tests for linear trend.
In a sensitivity analysis of the repeated measures WOMAC pain analysis, we employed a mixed-effects Tobit regression [29] to account for scale truncation at 0, with random-effects to account for the correlation due to knees within subjects and the repeated WOMAC assessments over time in each knee.
We also performed sensitivity analyses restricted to those free of the FKP at baseline to estimate risk of incident FKP by limiting the analytic sample those who were free of FKP at the baseline (12th year) study visit. Similarly, we evaluated the risk of developing more frequent intermittent knee pain or constant pain among those with no constant pain or only infrequent intermittent pain (occurring no more than “sometimes” based on the ICOAP) at baseline.
All analyses were adjusted for age, sex, BMI, race, site, and KL grade and were performed using SAS 9.3 (SAS Institute, Gary, North Carolina, USA).
RESULTS
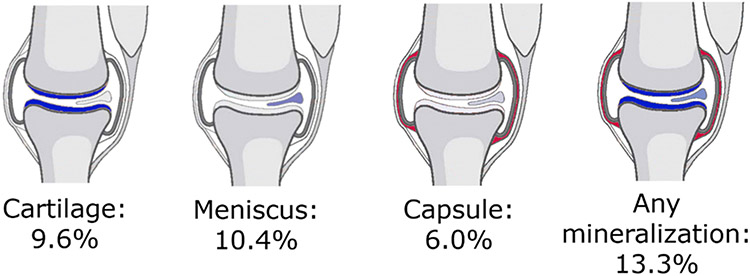
A total of 2093 participants (4168 knees) were included. Overall, participants had a mean age of 61 years, 57% were female, and the mean BMI was 28.8 kg/m2 (Table 1). On a person-level, the prevalence of CT-detected mineralization anywhere in the joint (i.e., in any tissue – cartilage, meniscus, capsule) was 13.3%. Mineralization in the cartilage, meniscus, and capsule was 9.6%, 10.4%, and 6.0%, respectively (Figure 1). Unilateral IA mineralization anywhere in the knee was present in 130 (6.2%) participants. Overall, 10.2% of knees had any IA mineralization (present in the cartilage, meniscus, and/or capsule) on CT, while on radiograph, the prevalence was 6.0%. Any IA mineralization in the cartilage was present in 7.3% of knees, while IA mineralization in the meniscus was present in 8.3% and in the joint capsule in 4.6%.
Table 1.
Participant characteristics
| Overall (n= 2093 participants; n= 4183 knees) |
Any IA Mineralization Present (n= 208 participants; n= 426 knees) |
Any IA Mineralization Absent (n= 1884; participants n=3740 knees) |
|
|---|---|---|---|
| Age, years (mean, SD) | 61.2 ± 9.7 | 69.1 ± 10.2 | 60.4 ± 9.2 |
| Female sex, % | 56.7 | 53.4 | 57.1 |
| White race, % | 80.1 | 87.0 | 81.8 |
| BMI, kg/m2 (mean, SD) | 28.8 ± 5.2 | 29.0 ± 5.3 | 28.8 ± 5.2 |
| Radiographic knee OA, % | 35.9 | 60.2 | 32.2 |
| Pain measures at baseline for this analysis (12th year study visit) | |||
| Frequent knee pain, % | 24.5 | 24.9 | 19.6 |
| ICOAP intermittent pain subscore [0-100] (mean, SD) | 9.7 ± 15.6 | 9.1 ± 15.2 | 9.7 ± 15.6 |
| ICOAP constant pain subscore [0–100] (mean, SD) | 1.9 ± 9.3 | 2.2 ± 9.7 | 1.9 ± 9.1 |
| ICOAP total [0-100] (mean, SD) | 6.2 ± 10.8 | 6.0 ± 10.4 | 6.1 ± 10.7 |
| WOMAC pain score [0-20] (mean, SD) | 2.0 ± 2.8 | 2.4 ± 3.1 | 1.9 ± 2.7 |
Abbreviations: IA, intra-articulra; BMI, body mass index; SD, standard deviation; ICOAP, intermittent and constant osteoarthritis pain; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index
Figure 1. Person-level prevalence of intra-articular mineralization in either knee, by location.
Any mineralization includes mineralization in the cartilage, meniscus, and/or joint capsule.
The prevalence of any mineralization in either the cartilage or meniscus was 13.1% at the person level.
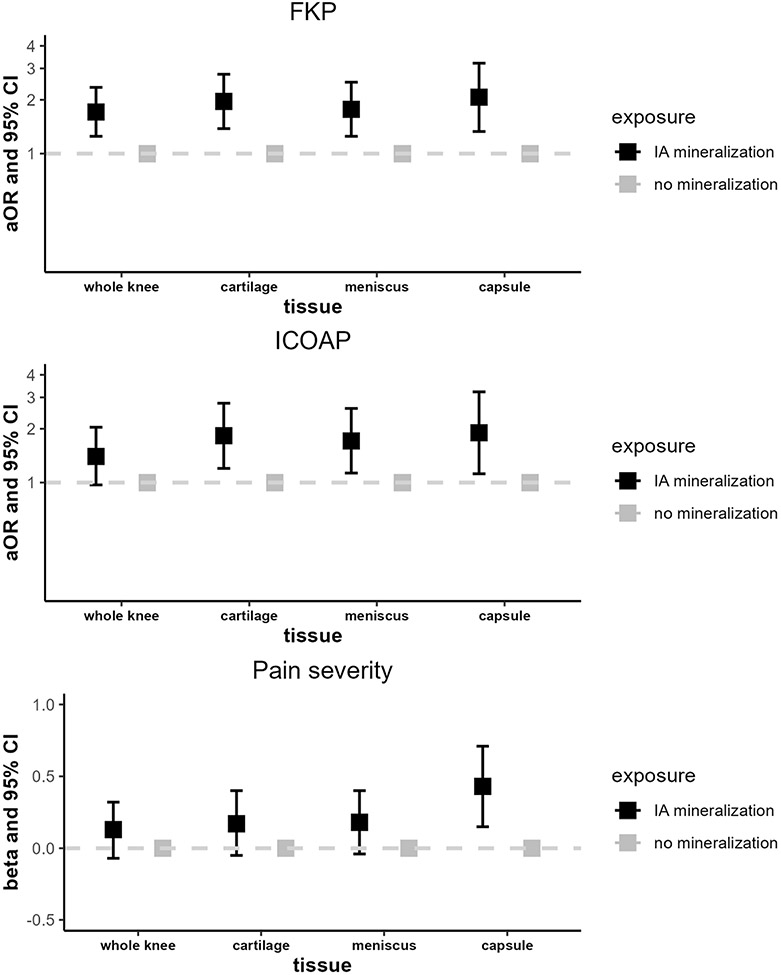
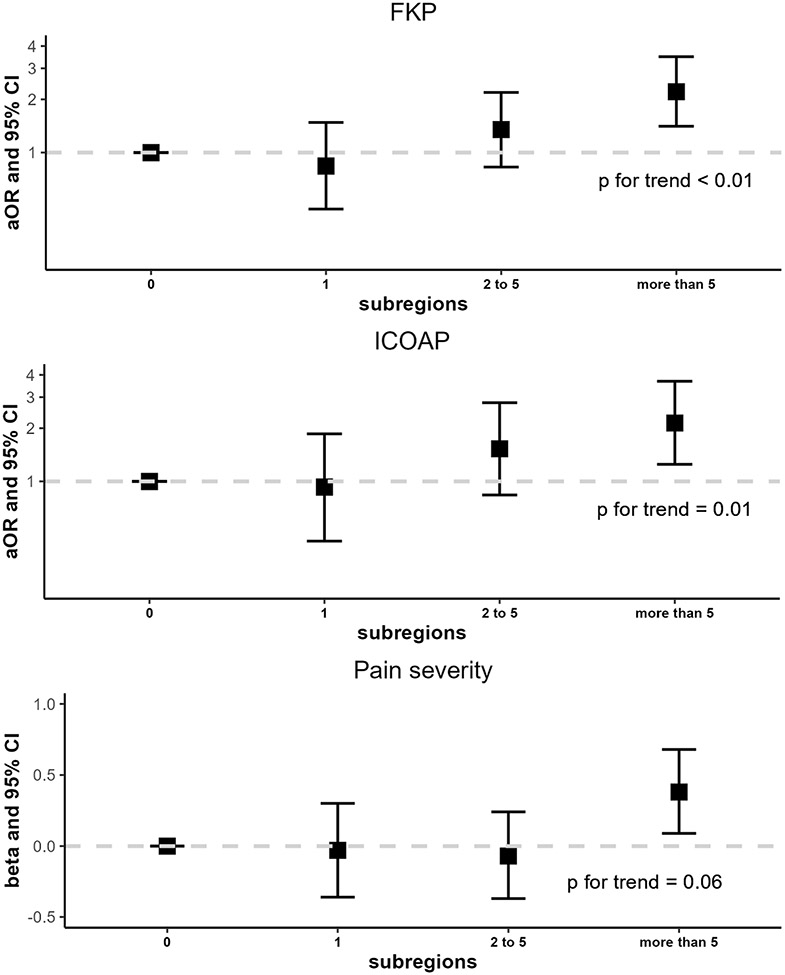
IA mineralization anywhere in the joint was significantly associated with the odds of FKP (OR 1.71, 95% CI 1.25-2.35), but not the other pain outcomes (Figure 2; Supplemental Table 1). Cartilage mineralization increased the odds of FKP and frequent intermittent/constant pain by 96% and 83%, respectively, compared with no cartilage mineralization (95% CI 1.38-2.78 and 1.20-2.78, respectively). However, IA mineralization in the cartilage was not significantly associated with pain severity (β 0.17, 95% CI −0.05–0.40). The findings for meniscal mineralization were similar (FKP OR 1.77, 95% CI 1.25-2.51; frequent intermittent/constant pain OR 1.71, 95% CI 1.13-2.60; pain severity β 0.18, 95% CI −0.04–0.40). Capsular mineralization was associated with a 2.07-fold increase in the odds of FKP (95% CI 1.33-3.21), 1.90-fold increase in the odds of intermittent/constant pain (95% CI 1.12-3.22) and 0.43-point higher difference in the average WOMAC pain score (95% CI 0.15 –0.71). A higher burden of mineralization was significantly associated with higher risk of having FKP, frequent intermittent or constant pain, and greater pain severity (Figure 3). Linear tests for trend for the burden of mineralization were statistically significant for the outcomes of FKP and frequent intermittent/constant pain (p<0.01 and p=0.01, respectively).
Figure 2. Longitudinal relation of intra-articular mineralization to knee pain outcomes of frequent knee pain, intermittent/constant pain, and pain severity.
Abbreviations: FKP, frequent knee pain; ICOAP, intermittent and constant osteoarthritis pain.
Analyses were adjusted for age, sex, race, site, body mass index, and KL grade.
Any mineralization includes mineralization in the cartilage, meniscus, and/or joint capsule.
Figure 3. Longitudinal relation of intra-articular mineralization burden to knee pain outcomes of frequent knee pain, intermittent/constant pain, and pain severity.
Abbreviations: FKP, frequent knee pain; ICOAP, intermittent and constant osteoarthritis pain.
Analyses were adjusted for age, sex, race, site, body mass index, and KL grade.
Mineralization burden was defined by the presence of mineralization anywhere in the joint (cartilage, meniscus, capsule) and the number of BUCKS subregions involved. This was categorized as mineralization in 0 [referent], 1, 2-5, and >5 subregions.
When the relation of IA mineralization to WOMAC pain worsening was analyzed using Tobit regression, mineralization in the cartilage, meniscus, and capsule, were all associated with more severe pain to a similar degree (Supplemental Table 2). The linear test for trend was also statistically significant (p=0.02) for a higher burden of mineralization.
Among those free of FKP at baseline, the presence of IA mineralization in the cartilage, meniscus, or capsule, anywhere in the joint, and in >5 subregions were all significantly associated with the risk of developing FKP, similar to our main results (Supplemental Table 3). However, among those free of constant/severe pain at baseline, only the presence of mineralization in >5 subregions was significantly associated with the risk of developing more frequent intermittent or constant pain (Supplemental Table 3).
DISCUSSION
CT-detected IA mineralization was associated with an increased risk of having frequent knee pain, more intermittent or constant knee pain, and greater knee pain severity over two years. There were subtle differences in the magnitude of risk depending on the tissue type with IA mineralization (i.e., cartilage, meniscus, or capsule), though it was unclear if this implies that localization may have a specific clinical relevance to the development of pain. This first report of the longitudinal association of CT-based IA mineralization with changes in knee pain highlights the important role that crystal deposition plays in the pain experience in knee OA. These findings also suggest that IA mineralization and/or the presumed inflammation associated with such crystal-related mineralization may be an attractive target for the development of future therapies in a subset of individuals with knee OA.
Chondrocalcinosis, the radiographic presence of calcium deposition in articular cartilage, is associated with OA [6] and with higher levels of knee pain [22]. The hypothesis that IA mineralization contributes to the development of knee pain through inflammation has yet to be confirmed. In a study using data from the Osteoarthritis Initiative (OAI), baseline radiographic chondrocalcinosis was significantly associated with pain, as ascertained with three different measures, both cross-sectionally and at four years of follow-up [22]. However, radiographic chondrocalcinosis was not associated with synovitis detected on MRI, which is thought to be a potential mediator in the relationship between mineralization and pain. In contrast, a French cohort study of older individuals with symptomatic knee and/or hip OA did not find chondrocalcinosis on knee radiographs to be significantly associated with worsening pain or function as measured by the minimal clinically relevant change in WOMAC subscore over five years of follow-up [13].
These prior studies were limited by their use of conventional radiographs, which have a lower sensitivity for identifying chondrocalcinosis [9,10], leading to under-detection and misclassification. CT has been demonstrated in a pilot study to be a feasible modality for the identification of IA mineralization in knees with OA with greater sensitivity than radiograph [23], particularly given the two-dimensional nature of radiographs. Thus, use of CT enables improved ascertainment of IA mineralization, thereby overcoming limitations of relying on radiographs to study impact on OA outcomes.
Our current study’s findings add to the growing understanding regarding the connection between IA mineralization and pain in knee OA. In vitro studies and animal models have connected the crystal-induced activation of the NLRP3 inflammasome with the subsequent production of pro-inflammatory cytokines, and have suggested that targeting this pathway can reduce pain in knee OA [7,8,30]. Unfortunately, a few small clinical trials of biologics addressing crystal-induced inflammation as a target for the management of OA pain have had disappointing results [16-18]. These trials may have been limited by their small size and lack of enrichment for specific OA phenotypes that may benefit from targeting particular inflammatory cytokines.
It is possible that the presence of crystals in specific anatomic sites within the joint may have differential effects on pain. While the cartilage is aneural and avascular, the joint capsule and meniscus are both richly innervated with nociceptors [31,32]. Nonetheless, in our study, we did not note an obvious difference in the relation of IA mineralization in various joint tissues to the risk of our pain outcomes. The slight differences in magnitudes of effect likely reflect differences in performance characteristics of the various instruments. Furthermore, the presence of IA mineralization in the joint capsule, which has not been previously well-studied, may be a reflection of overall burden of IA mineralization within the joint, rather than providing insights into potential differential location-specific effects.
Interpretation of these results should take into account limitations of our study. Although we included participants without OA as well as those with early OA to try to discern potential contributions to pain at earlier stages before other pathology may contribute to pain, it is not known at what stages mineralization may be important, and our results may not be generalizable to other samples. We were not able to capture pain fluctuations that may occur in between study visits, nor were we able to study the incident development of IA mineralization. We used three different pain outcomes since how IA mineralization may affect the pain experience in OA is not clear. Nonetheless, we did not have measures that directly capture the concept of pain fluctuation. Finally, we were not able to assess specific crystal types (e.g., CPP versus BCP) [33] in the current study for their potentially different contributions to OA-related knee pain. Further work is needed to differentiate the effects of specific crystal types on knee OA outcomes.
Strengths of our study include the use of the more sensitive modality of CT to assess IA mineralization, inclusion of participants with early OA, longitudinal follow-up over two years of a large sample, and the use of validated measures of pain to assess frequency, severity, and pain patterns. In addition to improved sensitivity, CT also allowed for the visualization and quantification of mineralization in different joint tissues, including the meniscus.
CONCLUSIONS
IA knee mineralization was associated with an increased risk of developing frequent knee pain, more intermittent or constant pain, and greater knee pain severity over two years. These findings implicate calcium crystal deposition in changing pain patterns over time in knee OA. These insights also raise the potential for developing and testing therapies directed towards crystal deposition and downstream mediators to improve knee OA symptoms.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the Boston Musculoskeletal Clinical Research Collaboratory Research ACCELERATOR group for their insightful comments on this manuscript.
Funding
This work was supported by NIH P30 AR 072571.
Tuhina Neogi was supported by NIH K24 AR070892
S. Reza Jafarzadeh was supported by NIH R03 AG060272
The MOST Study was supported by U01-AG-18820, U01-AG-18832, U01-AG-18947, and U01-AG-19079.
Footnotes
Disclosures
Liew – Research grant from Pfizer, unrelated to this work
Jarraya – None
Guermazi – Shareholder of BICL, LLC; consultant to Pfizer, AstraZeneca, MerckSerono, Regeneron, TissueGene and Novartis.
Lynch - None
Wang – None
Miller – None
Jafarzadeh - None
Nevitt - None
Torner - None
Lewis - None
Felson – None
TN –Novartis, Pfizer/Lilly, Regeneron
REFERENCES
- 1.Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L, et al. Understanding the pain experience in hip and knee osteoarthritis - an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008;16:415–22. [DOI] [PubMed] [Google Scholar]
- 2.Carlesso LC, Hawker GA, Torner J, Lewis CE, Nevitt M, Neogi T. Association of intermittent and constant knee pain patterns with knee pain severity, radiographic knee osteoarthritis duration and severity. Arthritis Care Res 2020;0–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makovey J, Metcalf B, Zhang Y, Chen JS, Bennell K, March L, et al. Web-Based Study of Risk Factors for Pain Exacerbation in Osteoarthritis of the Knee (SPARK-Web): Design and Rationale. JMIR Res Protoc 2015;4:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas MJ, Rathod-mistry T, Harper S, Parry EL, Neogi T, Peat G, et al. Acute Flares of Knee Osteoarthritis (the ACT-FLARE Study): Protocol for a Web-Based Case-Crossover Study in Community-Dwelling Adults. JMIR Res Protoc 2019;8:e13428. doi: 10.2196/13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schumacher HR. Crystals, inflammation, and osteoarthritis. American Journal of Medicine 1987;83:11–6. [DOI] [PubMed] [Google Scholar]
- 6.Ea HK, Nguyen C, Bazin D, Bianchi A, Guicheux J, Reboul P, et al. Articular cartilage calcification in osteoarthritis: Insights into crystal-induced stress. Arthritis Rheum 2011;63:10–8. [DOI] [PubMed] [Google Scholar]
- 7.Denoble AE, Huffman KM, Stabler TV, Kelly SJ, Hershfield MS, McDaniel GE, et al. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc Natl Acad Sci 2011;108:2088–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres R, Macdonald L, Croll SD, Reinhardt J, Dore A, Stevens S, et al. Hyperalgesia, synovitis and multiple biomarkers of inflammation are suppressed by interleukin 1 inhibition in a novel animal model of gouty arthritis. Ann Rheum Dis 2009;68:1602–8. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuyama H, Healey RM, Terkeltaub RA, Coutts RD, Amiel D. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthritis Cartilage 2007;15:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum 2009;60:2694–703. [DOI] [PubMed] [Google Scholar]
- 11.Neogi T, Nevitt M, Niu J, LaValley MP, Hunter DJ, Terkeltaub R, et al. Lack of association between chondrocalcinosis and increased risk of cartilage loss in knees with osteoarthritis: Results of two prospective longitudinal magnetic resonance imaging studies. Arthritis Rheum 2006;54:1822–8. [DOI] [PubMed] [Google Scholar]
- 12.Muehleman C, Li J, Aigner T, Rappoport L, Mattson E, Hirschmugl C, et al. Association between crystals and cartilage degeneration in the ankle. J Rheumatol 2008;35:1108–17. [PMC free article] [PubMed] [Google Scholar]
- 13.Latourte A, Rat AC, Ngueyon Sime W, Ea HK, Bardin T, Mazières B, et al. Chondrocalcinosis of the knee and the risk of osteoarthritis progression: Data from the Knee and Hip Osteoarthritis Long-term Assessment cohort. Arthritis Rheum 2020;72:726–32. [DOI] [PubMed] [Google Scholar]
- 14.Foreman SC, Gersing AS, von Schacky CE, Joseph GB, Neumann J, Lane NE, et al. Chondrocalcinosis is associated with increased knee joint degeneration over 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2020;28:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cipolletta E, Di Matteo A, Scanu A, Isidori M, Di Battista J, Punzi L, et al. Biologics in the treatment of calcium pyrophosphate deposition disease: systematic literature review. Clinical Experimental Rheumatology 2020;38:1001–7. [PubMed] [Google Scholar]
- 16.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res 2009;61:344–52. [DOI] [PubMed] [Google Scholar]
- 17.Fleischmann RM, Bliddal H, Blanco FJ, Schnitzer TJ, Peterfy C, Chen S, et al. A Phase II Trial of Lutikizumab, an Anti–Interleukin-1α/β Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis. Arthritis Rheum 2019;71:1056–69. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SB, Proudman S, Kivitz AJ, Burch FX, Donohue JP, Burstein D, et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res Ther 2011;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schieker M, Conaghan PG, Mindeholm L, Praestgaard J, Solomon DH, Scotti C, et al. Effects of interleukin-1β inhibition on incident hip and knee replacement: Exploratory analyses from a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2020;173:509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derfus BA, Kurian JB, Butler JJ, Daft LJ, Carrera GF, Ryan LM, et al. The high prevalence of pathologic calcium crystals in pre-operative knees. J Rheumatol 2002;29:570–4. [PubMed] [Google Scholar]
- 21.Sirotti S, Becce F, Sconfienza LM, Terslev L, Naredo E, Zufferey P, et al. Reliability and diagnostic accuracy of radiography for the diagnosis of calcium pyrophosphate deposition: performance of the novel definitions developed by an international multidisciplinary working group. Arthritis Rheumatol 2023;75:630–638. [DOI] [PubMed] [Google Scholar]
- 22.Han BK, Kim W, Niu J, Basnyat S, Barshay V, Gaughan JP, et al. Chondrocalcinosis in knee joints is associated with pain but not with synovitis: Data from the Osteoarthritis Initiative. Arthritis Care Res 2017;69:1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misra D, Guermazi A, Sieren JP, Lynch J, Torner J, Neogi T, et al. CT imaging for evaluation of calcium crystal deposition in the knee: Initial experience from the Multicenter Osteoarthritis (MOST) study. Osteoarthritis Cartilage 2015;23:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarraya M, Guermazi A, Liew J, Tolstykh I, Lynch J, Aliabadi P. Prevalence of Intra-articular Mineralization on Knee Computed Tomography: The Multicenter Osteoarthritis Study. Osteoarthritis Cartilage 2023; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Englund M, Felson DT, Guermazi A, Roemer FW, Wang K, Crema MD, et al. Risk factors for medial meniscal pathology on knee MRI in older US adults: A multicentre prospective cohort study. Ann Rheum Dis 2011;70:1733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guermazi A, Jarraya M, Lynch JA, Felson DT, Clancy M, Nevitt M, et al. Reliability of a new scoring system for intraarticular mineralization of the knee: Boston University Calcium Knee Score (BUCKS). Osteoarthritis Cartilage 2020;28:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12:177–90. [DOI] [PubMed] [Google Scholar]
- 28.Hawker GA, Davis AM, French MR, Cibere J, Jordan JM, March L, et al. Development and preliminary psychometric testing of a new OA pain measure - an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008;16:409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pullenayegum EM, Tarride JE, Xie F, Goeree R, Gerstein HC, O’Reilly D. Analysis of health utility data when some subjects attain the upper bound of 1: Are tobit and CLAD models appropriate? Value in Health 2010;13:487–94. [DOI] [PubMed] [Google Scholar]
- 30.Ramonda R, Oliviero F, Galozzi P, Frallonardo P, Lorenzin M, Ortolan A, et al. Molecular mechanisms of pain in crystal-induced arthritis. Best Pract Res Clin Rheumatol 2015;29:98–110. [DOI] [PubMed] [Google Scholar]
- 31.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Rheumatic Disease Clinics of North America 2008;34:623–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salaffi F, Ciapetti A, Carotti M. The sources of pain in osteoarthritis: A pathophysiological review. Reumatism. 2014;66:57–71. [DOI] [PubMed] [Google Scholar]
- 33.Tedeschi SK, Becce F, Pascart T, Guermazi A, Budzik J, Dalbeth N, et al. Imaging features of calcium pyrophosphate deposition (CPPD) disease: consensus definitions from an international multidisciplinary working group. Arthritis Care Res 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.