Abstract
Objective:
The Emotion Dysregulation Inventory (EDI) was designed and validated to quantify emotion dysregulation (ED) in ages 6+. The purpose of this study was to adapt the EDI for use in young children (EDI-YC).
Method:
Caregivers of 2139 young children (ages 2–5) completed 48 candidate EDI-YC items. Factor and item response theory (IRT) analyses were conducted separately for clinical (neurodevelopmental disabilities; N = 1369) and general population (N = 768) samples. The best performing items across both samples were selected. Computerized adaptive testing simulations were utilized to develop a short-form version. Concurrent calibrations and convergent/criterion validity analyses were performed.
Results:
The final calibrated item banks included 22 items – 15 items for Reactivity, characterized by rapidly escalating, intense, and labile negative affect, and difficulty downregulating that affect, and 7 items for Dysphoria, characterized primarily by poor up regulation of positive emotion, as well an item each on sadness and unease. The final items did not show differential item functioning based on age, sex, developmental status, or clinical status. IRT co-calibration of the EDI-YC Reactivity with psychometrically robust measures of anger/irritability and self-regulation demonstrated its superiority in assessing emotion dysregulation in as few as seven items. EDI-YC validity was supported by expert review and its association with related constructs (e.g., anxiety, depression, aggression, temper loss).
Conclusion:
The EDI-YC captures a broad range of emotion dysregulation severity with a high degree of precision in early childhood. It is suitable for use in all children ages 2–5, regardless of developmental concerns, and would be an ideal broadband screener for emotional/behavioral problems during well-child checks and to support early childhood irritability and emotion regulation research.
Keywords: autism spectrum disorder, emotion dysregulation, irritability, early childhood, Patient-Reported Outcomes Measurement Information System (PROMIS®)
INTRODUCTION
[The term autistic will be used throughout the manuscript in alignment with preference for identity-first language expressed by many autistic adults. The term neurodevelopmental disabilities (NDD) will refer to both autistic participants and those with other neurodevelopmental disabilities or developmental concerns.]
Emotion dysregulation (ED), or difficulty modifying arousal and emotional state in the service of one’s goals, is a transdiagnostic process with relevance to young children1–3. Early ED has been characterized in the context of studies of temperamental features such as effortful control and affective intensity4,5. Research has linked emotional temperament in the first few years of life to increased rates of internalizing and externalizing disorders in middle childhood and adolescence, which provides a rationale for studying ED at a young age as a precursor to psychopathology.”6,7
Recent literature has demonstrated that children with developmental delays or neurodevelopmental disabilities (NDD), particularly autism spectrum disorder, have an elevated likelihood of clinically impairing ED8–12. By age 4, over 50% of autistic children had temper tantrums and over 50% had a mood disorder documented in their health or education records based on a recent prevalence study.6 The majority of autistic children have at least one behavioral/psychiatric co-occurring condition.8 Moreover, ED in autistic youth has been linked to higher likelihood of psychiatric service use.13,14 Interestingly, there are distinct differences in ED between autistic and neurotypical children by the toddler and preschool years, which includes greater reactivity and less effective regulation.9,12
Screening for ED during the toddler and preschool years would facilitate early identification of risk for emotional and behavioral difficulties, prior to a clinically significant, diagnosable psychiatric disorder9,12,15–17. Importantly, ED is an appropriate target for early intervention based on preliminary studies18,19. One of the greatest challenges, however, in characterizing early ED is the lack of suitable measures. Parent report is necessary given that children cannot self-report. Moreover, early childhood is unique because emotion regulation skills are still developing, and some degree of dysregulation is normative (e.g., most preschoolers have tantrums)15,16. Thus, measures developed for older children are not appropriate for use in early childhood given marked differences in parent scaffolding and normative expectations for ED21–23. Also, measures for older samples may have more demanding indicators of emotion regulation and have questions that require children to express how they feel in order for the parent to choose ratings13. Measures must also have norms for young children and be psychometrically sensitive across the full range of ED.
The Patient-Reported Outcomes Measurement Information System - (PROMIS®) is an National Institutes of Health (NIH) initiative (http://www.nihpromis.org)24,25 that developed a robust methodology for creating brief, efficient, and valid measures of physical, mental, and social health. PROMIS was expanded recently to include an early childhood battery (PROMIS-EC)26,27. This work represents a significant advance in available measurement options for early childhood. Nonetheless, available evidence suggests that measures developed for the general (non-NDD) population are often not as psychometrically sound in autistic youth13,28,29, which is a critical barrier given the elevated rates of ED in those with NDD8–11. This issue motivated the development of the Emotion Dysregulation Inventory (EDI)13,30,31 using PROMIS methods, and the EDI is one of the few measures validated in both autistic and general populations. Interestingly, the EDI was developed originally to fill a gap in options for autistic individuals, but it outperformed existing measures in the general population, perhaps because it was constructed to capture the wide range of ED in an observable fashion31.
The original conceptual model for the EDI included both the experience and regulation of emotion because these concepts tend to converge when measured by questionnaire32, and both are related to uncontrolled or dysregulated emotion30. The final scale structure was based on factor analysis and yielded two scales: Reactivity – tapping rapidly escalating, intense, and labile negative affect (i.e., heightened reactivity) as well as difficulty downregulating that affect (i.e., poor regulation) – and Dysphoria – capturing sadness, unease, and poor upregulation of positive emotion.
The EDI was originally developed and validated for ages 6–17 (full details here13,31; adult norms are currently being generated and an EDI self-report for ages 11+ is being tested). Therefore, we created a young child version of the EDI (for 2- to 5-year-olds; EDI-YC) to account for ED behaviors and developmental needs specific to early childhood. As described in the EDI-YC development paper23, an item pool, including original EDI items as well as new and modified items, was developed. Cognitive interviewing was completed with 10 parents of autistic young children to refine the item bank and ensure construct validity and content coverage.
The primary objective of the present study was to establish the final items and psychometric characteristics of the EDI-YC for 2- to 5-year-old NDD children (“clinical sample”) and non-NDD children (“general sample”), including evaluating item properties, traditional validity analyses, and concurrent IRT calibration to determine how much additional information the EDI-YC provides in comparison to other measures. Like the original EDI, analyses were conducted separately for clinical and general samples to enable the evaluation of psychometric properties within each group and to allow for generation of expected norms from the general sample. Following PROMIS guidelines, we: 1) determined the factor structure of the EDI-YC; 2) identified the most sensitive and psychometrically robust items; 3) ensured items were not biased within the clinical or general populations based on sex, age, or developmental status; and 4) examined the validity of the EDI-YC by assessing the convergence between the EDI-YC and related measures.
METHOD
Overview
The item development process is described in detail in Day, Northrup, and Mazefsky23. The initial item pool for the EDI-YC included final items from the original EDI as well as some items included in the preliminary EDI item pool, which were generated based on a comprehensive literature review and development of a conceptual model. Additional items specific to early childhood were created based on an updated literature search and the first co-authors’ (Day, Mazefsky) clinical expertise. The item pool was tested with 10 parents of autistic children (ages 2–5) to assess their understanding and decision-making related to items and response options. Information from these interviews, as well as input from a panel of experts in early childhood, autism, and ED, were used to derive the final pool of 48 candidate items included for psychometric analysis and calibration.
Participants
Our sampling strategy was designed to accrue participants in two groups: the “clinical” sample which included NDD young children and the “general” sample which included non-NDD young children. Parents were asked to indicate if their child had any developmental concerns, delays, or diagnosed disorders. If yes, children were included in the clinical sample. If no, children were included in the general sample. Children in the clinical sample were predominantly autistic (n = 983, 71.8%). Approximately 2/3 of the remaining clinical sample did not have autism, but parents endorsed developmental concerns about their child. Of these, approximately 1/3 reported that their child had a specific genetic disorders or syndrome (e.g., Down’s Syndrome, Rett Syndrome) and the remainder reported unknown or other etiologies. All participants were between the ages of 2 and 5.
Children in the clinical sample, N = 1369, were obtained from: 1) the Simons Powering Autism Research (SPARK) registry (n = 811)33; 2) local recruitment through 14 pediatrics practices in urban, suburban, and rural locations that are part of Pediatric PittNet at the University of Pittsburgh; 25 early intervention (EI) programs across Pennsylvania, Ohio, West Virginia, and New York; and over 100 preschools and community daycare programs in this same region (n = 338); and 3) baseline assessments from two NIH-funded intervention studies for parents of autistic and non-autistic NDD children (n = 220; R15HD091726 [PI: Neece] and R01HD093667 [PIs: McIntyre, Neece]. Children in the general sample, N = 768, were also recruited from the second and third sources, with 715 children through local pediatric, EI, preschool, and community sources and 53 children who served as a comparison group for one of the NIH-funded intervention studies (R15HD091726).
Measures.
Selected measures were chosen based on their common use in early childhood and acceptable to strong psychometrics for young children (all Cronbach’s alpha ranged from 0.64 to 0.97)17,34. One exception is the PROMIS Early Childhood battery, which was under development at the time of data collection; it was utilized due to its similar development process to the EDI and high dissemination likelihood. Its psychometrics have since been published and they were strong as anticipated35,36.
Emotion Dysregulation Inventory – Young Child.
The EDI-YC item pool for psychometric analysis consisted of 48 items23. Items were rated on a five-point scale of problem severity over the past 7 days: Not at all = 0, Mild = 1, Moderate = 2, Severe = 3, Very Severe = 4. The final questionnaires and scoring information, including theta look-up tables, may be requested for use at no cost at www.reaact.pitt.edu.
PROMIS - Early Childhood.
The emotion-focused scales from the PROMIS EC Version 1.0 battery for children ages 1–526,35,36 were used for the present study: Anger/Irritability, Anxiety, Depressive Symptoms, and Self-Control – Self-Regulation. Items were rated on a five-point Likert scale from 1 = Never, 2= Almost Never, 3 = Sometimes, 4 = Almost Always, 5 = Always.
Multidimensional Assessment Preschool Behavior.
The MAPS (formerly called the Multidimensional Assessment of Preschool Disruptive Behavior) is a multidimensional measure of disruptive behavior17,37. The Temper Loss and Aggression scales of the Preschool Age version were used for the present study. [Unlike the Temper Loss scale, IRT scoring was not available for the Aggression scale at the time this paper was completed. For the present study, we used a sum of raw scores on the Aggression scale for analyses.] The parent rated each item on a six-point rating scale based on the frequency of the behavior over the past month: Never = 0, Rarely = 1, Some = 2, Most = 3, Daily = 4, Many Times Each Day = 5.
Family Life Impairment Scale.
The FLIS is a parent-report measure assessing impairment within the family due to the child’s developmental, behavioral, or emotional difficulties34. The Family Impairment, Childcare Impairment, and Parent Impairment scales were used for the present study. The parent rated each item on a three-point scale: Not True = 0, Somewhat True = 1, Very True = 2.
Procedures
All participants were first determined to meet the inclusion criterion regarding age. All participants then completed the EDI-YC. Families recruited via SPARK and local sources also completed the PROMIS EC measures, MAP-DB, and FLIS. Parents from R15HD091726 and R01HD093667 completed the EDI-YC at the baseline appointment as well as at the conclusion of the intervention (outcome data to be reported in a future publication). Data was collected online for SPARK and local recruitment. Data was collected on paper for the R15HD091726 and R01HD093667 studies. In addition, these studies included some Spanish-speaking only participants (n = 60) who completed the EDI-YC in Spanish after translation and back translation.
Psychometric Analysis
All analyses were performed separately for the clinical and general population samples. Decisions on factor structure and item selection were informed by examining both groups; if there was substantial disagreement (which was rare), results from the clinical group were prioritized.
Factor analysis.
Similar to the original EDI, we did not expect that all 48 initial items would represent a single construct. Our goal was to identify the most robust latent constructs for young children and to ensure unidimensionality within each factor, which was necessary for subsequent item response theory (IRT) analyses. We hypothesized (a) that a two-factor structure, similar to the original EDI13,31, would emerge and (b) that the clinical and general samples would have similar factor structures. Each sample was split to allow subsamples for exploratory factor analysis (EFA; n for clinical = 659, n for general = 387) and for confirmatory factor analysis (CFA; n for clinical = 710, n = 381 for general). Both EFA and CFA were performed using MPlus 6.2 with promax rotation38. Factor loadings, scree plots, and eigenvalues were evaluated to derive the best fitting models.
IRT analysis.
Similar to the original EDI, we used a two-parameter graded response model (GRM)39, which has a slope parameter and n-1 threshold parameters for each item where n is the number of response categories. The slope parameter is a measure of item discrimination or how well an item differentiates between high and low severity. Larger slope parameters indicate greater differentiation. We also examined threshold parameters to examine item difficulty, which reflects the ease or difficulty of endorsing different response options for an item. Each threshold parameter within an item differentiates two item responses; for example, the first threshold parameter differentiates between responding “not at all” versus “mild” for EDI-YC items. Items were calibrated with the two-parameter GRM using IRTPRO 3.1. The convergence criterion was set to 0.00001 for EM cycles, and the number of cycles was set to 100.
Differential item functioning (DIF) analysis.
DIF reveals characteristics that have an effect on measurement independent of the level of severity of the construct being assessed; that is, an item flagged for DIF indicates it is more/less difficult or more/less discriminating for subgroups displaying a particular characteristic. We conducted uniform and non-uniform DIF analyses for age (2–3 years vs. 4–5 years), sex, clinical status (clinical vs. general sample), and developmental status (in the clinical sample only: autistic vs. non-autistic NDD). Two DIF procedures were used: the IRT likelihood ratio method40 and an ordinal logistic regression procedure41. Items with significant DIF, p < .01, for both methods were reviewed for further consideration.
Local dependency.
We also examined local dependency (LD) of items, i.e., residual correlations, in the IRT models using LD X2.
Short form item selection.
Computerized adaptive testing (CAT) simulations were conducted to select items for a static short form. The simulations were performed using the Firestar program42. The minimum number of items to be administered was set to 7 and the maximum number of items to be administered was the total for the full item bank.
Concurrent calibrations with PROMIS EC.
Concurrent calibrations provide estimated item parameters for multiple measures using the same latent trait scale. For the present analyses, we co-calibrated the PROMIS EC scales using the newly derived metric from the EDI-YC items to compare the test information curves for the measures.
Convergent and criterion validity.
We correlated theta scores from the EDI-YC factors with selected PROMIS EC, FLIS, and MAPS subscales.
RESULTS
Sample Characteristics
Table 1 reports the demographic characteristics of the clinical and general population samples separately to mirror analyses, which were conducted separately by group.
Table 1:
Demographic Characteristics
| Clinical Sample (N = 1369) | General Sample (N = 768) | |
|---|---|---|
| Age (months mean, SD) | 53.0 (13.20) | 46.8 (13.23) |
| Sex (%, n) | ||
| Male | 72.0 (987) | 46.1 (354) |
| Female | 26.5 (363) | 51.4 (395) |
| Race %, (n) | ||
| Asian | 4.3 (59) | 5.7 (44) |
| Black | 12.2 (167) | 16.5 (127) |
| Native American | 1.8 (24) | 1.6 (12) |
| Native Hawaiian | 1.2 (17) | 1.7 (13) |
| White | 76.6 (1048) | 81.0 (622) |
| Other | 2.6 (36) | 1.3 (10) |
| Ethnicity %, (n) | ||
| Hispanic | 21.5 (295) | 8.1 (62) |
| Non-Hispanic | 76.9 (1053) | 89.5 (687) |
| Income - less than $81k (median) %, (n) | 52.4 (718) | 46.1 (354) |
| Primary Caregiver Education - Bachelor’s Degree or Higher %, (n) | 45.6 (624) | 60.7 (466) |
Note: Means and SDs reported for continuous data. Percentages and n reported for categorical data.
Factor Structure
Using EFA, 1- through 10-factor solutions were generated, and the results were similar for the clinical and general population samples. Based on visualization of the scree plots, magnitudes of eigenvalues, clinical interpretation, and the factor structure of the original EDI, a 2-factor solution emerged as the most meaningful. Factor 1 (F1) was analogous to the Reactivity scale on the original EDI; it was characterized by items capturing rapidly escalating, intense, and labile negative affect as well as difficulty downregulating that affect, i.e., strong reactions and trouble calming down. Factor 2 (F2) was analogous to the Dysphoria scale on the original EDI; it included items that reflected poor upregulation of positive affect, sadness, and unease. Fourteen items were dropped due to factor loadings less than 0.45, cross-loadings between factors, or clinical judgment regarding content validity. A second round of EFA was conducted with the remaining 34 items. Factor loadings were again similar across the clinical and general samples, with the exception that 4 items did not load clearly on either factor (> 0.45) for the general sample; those items loaded on the Reactivity scale for the clinical sample. The correlation between the two factors was 0.57 for the clinical sample and 0.60 for the general sample. Final EFA loadings are presented in Table S1, available online.
Single-factor CFA on the reduced item pool was conducted to confirm unidimensionality within each factor, using the second subsample (n for clinical = 710, n = 381 for general). The same 25 items for F1 and the same 9 items for F2 were used in both samples. For F1, all factor loadings were greater than 0.69 for the clinical sample and 0.63 for general sample. For F2, all factor loadings were greater than 0.55 for the clinical sample and 0.62 for the general sample. Fit indices were strong (clinical: CFI = 0.96, TLI = 0.96, SRMR = 0.05, RMSEA = 0.08; general: CFI = 0.97, TLI = 0.97, SRMR = 0.06, RMSEA = 0.06). The correlation between the two factors was 0.66 for the clinical sample and 0.74 for the general sample. Final CFA loadings are presented in Table S2, available online.
IRT Analyses
All IRT analyses were conducted separately for clinical and general samples, except for DIF which varied based on the comparison as noted below. Items from F1 and F2 were calibrated separately. Seven items with the lowest item information curves and discrimination parameters were removed from F1, leaving a total of 18 items. One item was removed from F2 on the same basis, leaving a total of 8 items.
DIF Analyses
No items were identified for DIF by age or sex in the combined samples, or for developmental status in the clinical sample (autistic vs. non-autistic NDD). For clinical status (clinical vs. general), 3 items were flagged for F1 and 3 items for F2. Within F1, 2 of the flagged items were due to location DIF. Given expected differences in severity in the clinical vs. general population samples, we retained these items. One item (“seems to be in a rage”) was flagged for discrimination DIF and was eliminated. All 3 flagged items for F2 were related to location DIF and were retained.
Local dependency
Two additional items from F1 and one item from F2 were eliminated due to local dependency.
Final Item Banks
The final calibrated item banks included 22 items – 15 items for F1: Reactivity and 7 items for F2: Dysphoria. Nine items for Reactivity and five items for Dysphoria overlap with final items on the original EDI. The correlations between the two final EDI-YC factors were 0.58 for the clinical sample and 0.51 for the general sample. The final items and their IRT parameters are displayed in Table 2. Item discrimination values (α) were generally larger for F1 than F2, indicating greater differentiation of ED severity for F1. Test information curves (TICs) and plots of corresponding standard errors for both factors in both samples are depicted in Figure 1. Information values of 10 correspond to classical test theory reliability of 0.9. Using this standard, the effective range of measurement for F1 is −2 to +2.5 SDs in the clinical sample and −1 to +3 SDs in the general sample. For F2, the effective range of measurement is shifted to the right, +0.5 to +3 SDs for the clinical sample and +1 to +3 SDs for the general sample. Thus, F2 offers less information about average to below average levels of dysphoria and is more informative for moderate to severe levels.
Table 2:
Item Parameter Estimates for F1 and F2
| Reactivity | Clinical Sample | General Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Stem | a | b1 | b2 | b3 | b4 | a | b1 | b2 | b3 | b4 |
| a EDI19 | Has extreme or intense emotional reactions | 3.61 | −0.96 | −0.19 | 0.58 | 1.39 | 2.70 | −0.17 | 0.93 | 1.86 | 2.93 |
| a EDI46 | Reactions usually are more severe than the situation calls for | 3.58 | −0.88 | 0.02 | 0.76 | 1.60 | 2.89 | −0.06 | 1.08 | 1.99 | 3.03 |
| a EDI11 | Has strong emotional reactions | 3.40 | −1.54 | −0.53 | 0.42 | 1.36 | 2.41 | −1.08 | 0.36 | 1.72 | 3.04 |
| a EDI90 | Reactions are so intense that he/she has to be pulled aside to calm down or removed from an activity or place | 3.21 | −0.82 | −0.04 | 0.66 | 1.43 | 2.73 | 0.13 | 1.20 | 1.99 | 2.72 |
| a EDI34 | Emotions go from 0 to 100 instantly | 3.15 | −0.81 | 0.12 | 0.89 | 1.54 | 2.69 | 0.27 | 1.46 | 2.12 | 3.05 |
| a EDI21 | Hard to calm him/her down when he/she is mad or upset | 3.14 | −1.28 | −0.22 | 0.63 | 1.58 | 2.64 | −0.24 | 0.99 | 2.05 | 2.73 |
| a EDI36 | Has trouble calming him/herself down | 3.08 | −1.16 | −0.03 | 0.85 | 1.64 | 2.72 | −0.11 | 1.28 | 2.23 | 3.25 |
| EDI37 | Emotions change quickly | 3.03 | −0.99 | 0.05 | 0.95 | 1.83 | 2.59 | −0.21 | 1.31 | 2.29 | 3.18 |
| EDI8 | Frustrates easily | 2.78 | −1.72 | −0.52 | 0.47 | 1.45 | 2.22 | −0.82 | 0.72 | 1.98 | 3.08 |
| EDI53 | Difficult to distract if he/she is frustrated or upset | 2.72 | −1.08 | −0.01 | 0.79 | 1.67 | 2.47 | 0.03 | 1.29 | 2.15 | 3.04 |
| EDI3 | Has explosive outbursts | 2.65 | −1.09 | −0.11 | 0.89 | 1.82 | 2.10 | −0.30 | 1.14 | 2.20 | 3.46 |
| EDI89 | Breaks down (for example, crying, screaming) if told he/she can’t do or have something | 2.62 | −1.64 | −0.46 | 0.44 | 1.31 | 1.80 | −0.96 | 0.68 | 1.94 | 3.01 |
| EDI4 | Cries or stays angry for 5 minutes or longer | 2.34 | −1.11 | −0.03 | 0.95 | 1.87 | 2.08 | −0.35 | 0.96 | 2.17 | 3.52 |
| EDI85 | Easily triggered/upset (you have to walk on eggshells or be cautious around him/her) | 2.29 | −0.11 | 0.73 | 1.52 | 2.38 | 2.22 | 0.87 | 1.96 | 2.90 | 3.86 |
| EDI71 | Becomes frustrated when requests are not immediately met | 2.23 | −1.72 | −0.29 | 0.53 | 1.52 | 1.75 | −0.77 | 1.07 | 2.32 | 3.55 |
| Dysphoria | Clinical Sample | General Sample | |||||||||
| Item | Stem | a | b1 | b2 | b3 | b4 | a | b1 | b2 | b3 | b4 |
| EDI69 | Does not appear to enjoy pleasurable activities (for example, does not smile, laugh, jump up and down) | 3.62 | 0.93 | 1.69 | 2.33 | 3.13 | 5.01 | 1.60 | 2.06 | 2.45 | 2.92 |
| EDI31 | Does not seem to enjoy anything | 3.21 | 0.65 | 1.49 | 2.31 | 3.09 | 3.97 | 1.42 | 2.15 | 2.57 | 3.16 |
| EDI43 | Very little makes him/her happy | 2.94 | 0.55 | 1.46 | 2.20 | 2.67 | 2.98 | 1.29 | 2.13 | 2.64 | 3.18 |
| EDI63 | Seems sad or unhappy | 2.35 | 0.24 | 1.48 | 2.61 | 3.45 | 2.22 | 0.76 | 2.30 | 2.80 | 3.39 |
| EDI64 | Appears uneasy throughout the day | 2.25 | 0.27 | 1.34 | 2.26 | 3.20 | 2.77 | 1.36 | 2.21 | 2.97 | 3.38 |
| EDI57 | Not responsive to praise or good things happening | 1.86 | 0.19 | 1.29 | 2.31 | 3.43 | 2.76 | 1.26 | 2.11 | 2.69 | 3.60 |
| EDI95 | Shows minimal emotional expression even in situations that are exciting or upsetting | 1.83 | 0.49 | 1.38 | 2.38 | 3.09 | 3.38 | 1.45 | 2.13 | 2.59 | 3.20 |
Note: Column a displays the slope parameter (how well the item discriminates between respondents with low or high reactivity), listed from highest to lowest based on the clinical sample’s values. Columns b1-b4 display threshold values for individual responses (low threshold values indicate that the item is sensitive to low severity levels and high threshold values indicate that the item is sensitive to high severity levels).
denotes short form items.
Figure 1:
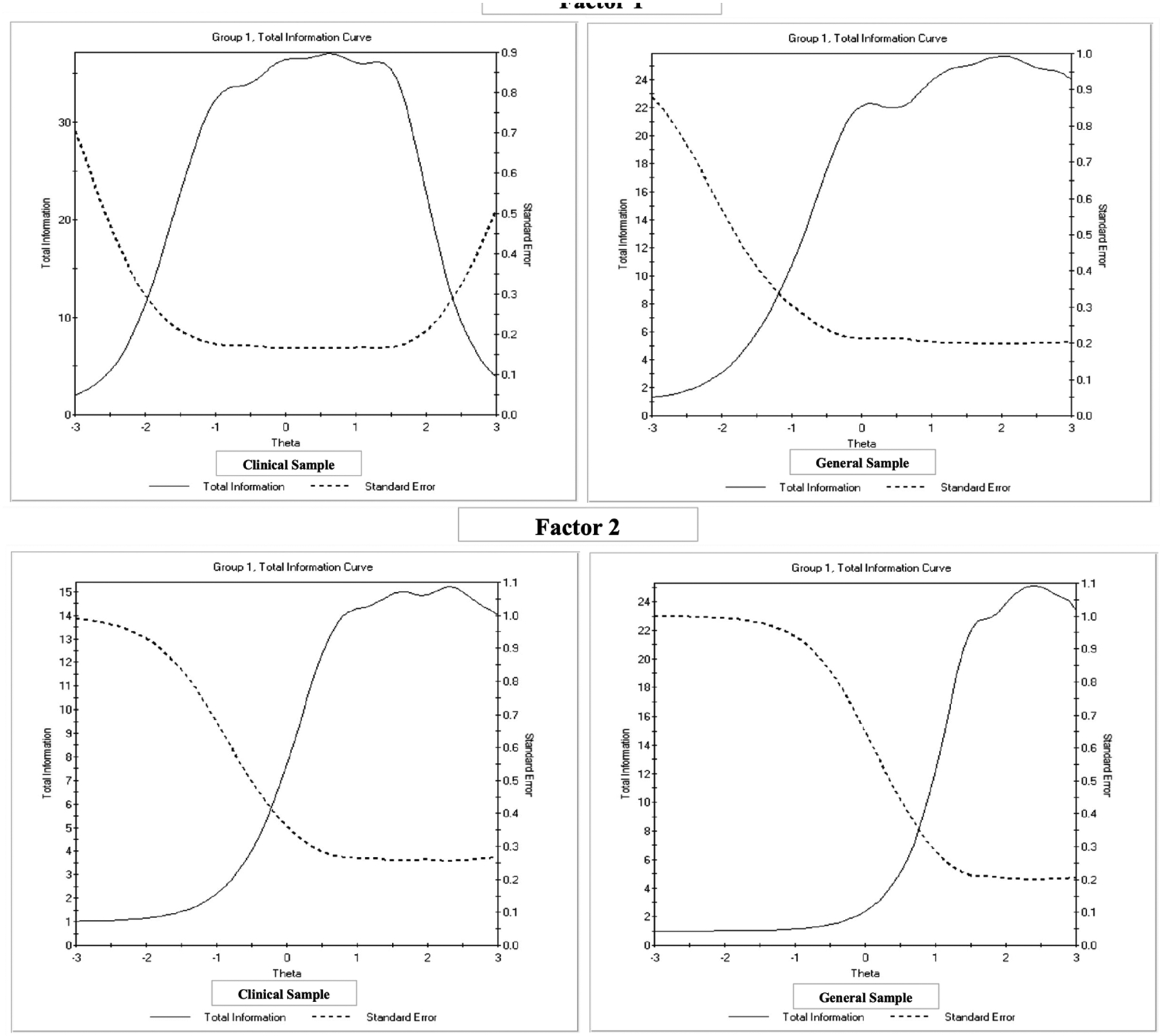
Total Test Information Curves for Factor 1 (Reactivity) and Factor 2 (Dysphoria)
Consistent with the original EDI, scoring tables were created to convert raw scores to theta scores and t-scores for both the clinical and general samples (look-up tables are provided with the EDI). IRT-calibrated scores are reported as theta, with a mean of 0 and SD of 1, whereas t-scores (common in psychological testing) are reported with a mean of 50 and SD of 10. Clinically significant thresholds equal to one standard deviation above the general sample mean were generated.
A static short form was also created for the Reactivity scale (F1). We rank ordered items based on the following criteria: (1) discrimination parameters, (2) results from simulations of computerized adaptive testing (CAT), i.e., the percentage of times an item would have been selected for CAT administration using our current data from both the clinical and general samples, (3) expected information under a normal distribution (M = 0, SD = 1), and (4) expected information under a normal distribution with a larger SD, i.e., 1.543. Seven items were selected for the short form based on those criteria, and they are identified in Table 2. The correlations between Ɵ scores for the short form and the full Reactivity bank were 0.98 for the clinical sample and 0.97 for the general sample.
Concurrent Calibrations with PROMIS EC
Similar to the original EDI, we compared the performance of the EDI-YC Reactivity item bank (primary scale) and its short form to previously validated measures of ED – PROMIS EC Anger/Irritability and PROMIS EC Self-Control: Self-Regulation. Figure 2 displays the test information curves for these measures when they are co-calibrated using the EDI-YC metric. The TICs document that the EDI-YC and EDI-YC SF provide more test information than either PROMIS EC scale for both the clinical and general samples. The full EDI-YC Reactivity item bank provided the most test information, in large part because it had the largest number of items.
Figure 2:
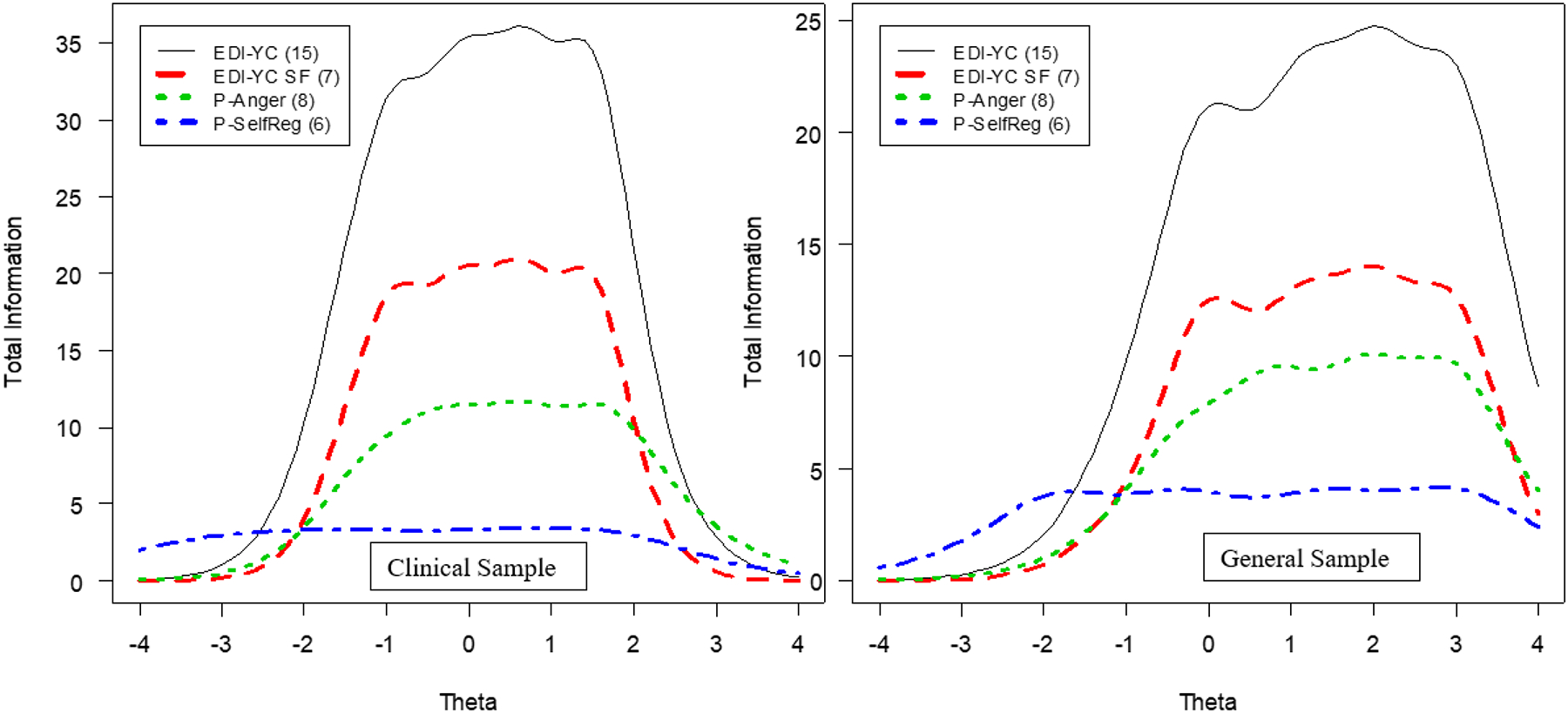
Total Test Information Curves for Factor 1 (Reactivity) With Related Measures
Note: EDI-YC = Emotion Dysregulation Inventory – Young Child; EDI-YC SF= Emotion Dysregulation Inventory – Young Child Short Form; P-Anger = Patient-Reported Outcome Measurement Information System - Early Child Anger/Irritability; P-SelfReg = Patient-Reported Outcome Measurement Information System - Early Child Self-Control – Self-Regulation.
**p < .01.
Convergent and criterion validity
Initial evidence for construct validity was documented in the EDI-YC development paper (see 23). Correlations between the EDI-YC scales and well-known measures assessing related constructs demonstrated expected patterns, as summarized in Table 3. All correlations with the EDI-YC scales were moderate to large; were related to more symptoms of ED, temper loss, depression, and anxiety; and were comparable in the clinical and general samples. The only exceptions were the FLIS Family Impairment and Childcare Impairment scales in the general sample, which were in the small range. However, the FLIS does not measure impairment related exclusively to ED, and its range was truncated in the general sample due to a left skewed distribution.
Table 3:
Correlations between Emotion Dysregulation Inventory - Young Child Theta Scores and Related Measures
| Clinical | n | EDI-YC Reactivity | EDI-YC Reactivity SF | EDI-YC Dysphoria | PROMIS EC Anxiety | PROMIS EC Depressive Symptoms | FLIS-Family Impairment | FLIS-Childcare Impairment | FLIS-Parent Impairment | MAPS Temper Loss | MAPS Aggression |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EDI-YC Reactivity | 1149 | 1 | .98** | .58** | .49** | .51** | .42** | .44** | .40** | .844** | .509** |
| EDI-YC Reactivity SF | 1149 | .98** | 1 | .54** | .47** | .48** | .41** | .43** | .38** | .811** | .471** |
| EDI-YC Dysphoria | 1149 | .58** | .54** | 1 | .56** | .72** | .39** | .38** | .40** | .546** | .400** |
| PROMIS EC Anxiety | 1082 | .49** | .47** | .56** | 1 | .66** | .28** | .32** | .38** | .501** | .344** |
| PROMIS EC Depressive Symptoms | 1081 | .51** | .48** | .72** | .66** | 1 | .38** | .36** | .41** | .543** | .351** |
| FLIS-Family Impairment | 1076 | .42** | .41** | .39** | .28** | .38** | 1 | .67** | .52** | .405** | .330** |
| FLIS-Childcare Impairment | 796 | .44** | .43** | .38** | .32** | .36** | .67** | 1 | .49** | .422** | .290** |
| FLIS-Parent Impairment | 858 | .40** | .38** | .40** | .38** | .41** | .52** | .49** | 1 | .385** | .254** |
| MAPS Temper Loss | 1108 | .84** | .81** | .55** | .50** | .54** | .41** | .42** | .39** | 1 | .628** |
| MAPS Aggression | 1070 | .51** | .47** | .40** | .34** | .35** | .33** | .29** | .25** | .628** | 1 |
| General | n | EDI-YC Reactivity | EDI-YC Reactivity SF | EDI-YC Dysphoria | PROMIS EC Anxiety | PROMIS EC Depressive Symptoms | FLIS-Family Impairment | FLIS-Childcare Impairment | FLIS-Parent Impairment | MAPS Temper Loss | MAPS Aggression |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EDI-YC Reactivity | 715 | 1 | .97** | .51** | .46** | .50** | .25** | .23** | .33** | .727** | .409** |
| EDI-YC Reactivity SF | 715 | .97** | 1 | .49** | .44** | .50** | .25** | .24** | .31** | .683** | .386** |
| EDI-YC Dysphoria | 715 | .51** | .49** | 1 | .49** | .61** | .35** | .28** | .29** | .386** | .346** |
| PROMIS EC Anxiety | 689 | .46** | .44** | .49** | 1 | .66** | .30** | .29** | .33** | .441** | .324** |
| PROMIS EC Depressive Symptoms | 687 | .50** | .50** | .61** | .66** | 1 | .39** | .38** | .37** | .520** | .422** |
| FLIS-Family Impairment | 689 | .25** | .25** | .35** | .30** | .39** | 1 | .59** | .39** | .278** | .345** |
| FLIS-Childcare Impairment | 689 | .23** | .24** | .28** | .29** | .38** | .59** | 1 | .35** | .240** | .273** |
| FLIS-Parent Impairment | 688 | .33** | .31** | .29** | .33** | .37** | .39** | .35** | 1 | .366** | .297** |
| MAPS Temper Loss | 692 | .73** | .68** | .39** | .44** | .52** | .28** | .24** | .37** | 1 | .541** |
| MAPS Aggression | 691 | .41** | .39** | .35** | .32** | .42** | .35** | .27** | .30** | .541** | 1 |
Note: Scores for the MAPS Temper Loss scale were based on IRT calibrations and scores for the MAPS Aggression scale were a sum of raw score. EDI-YC = Emotion Dysregulation Inventory – Young Child; FLIS = Family Life Impairment Scale; MAPS = Multidimensional Assessment Preschool Behavior; PROMIS EC = Patient-Reported Outcome Measurement Information System - Early Child
p < .01
DISCUSSION
The EDI-YC was adapted from the EDI to assess emotion dysregulation in early childhood. It was developed and tested in over 2000 young children ages 2–5 years with and without neurodevelopmental disabilities. The item bank was constructed and refined through a systematic process23 and psychometric analysis, which resulted in a 15-item bank and a 7-item short form for Reactivity and a 7-item bank for Dysphoria. The EDI-YC measures a broad range of ED severity with a high degree of precision and was not biased by age, sex, or developmental status. These results were particularly true for the Reactivity scale, which had larger item discrimination parameters than the Dysphoria scale. Both the full EDI-YC Reactivity item bank and the Reactivity short-form provided more information and greater precision than the PROMIS EC Anger/Irritability scale and the PROMIS Self-Control – Self-Regulation scale, evidence of the psychometric advantages of the Reactivity measures.
The two final EDI-YC scales mirror the original EDI constructs13,31. Reactivity is characterized by items capturing intense, rapidly escalating negative emotions and difficulty downregulating strong emotions once aroused. Dysphoria predominantly captures poorly upregulated positive emotion, with one item each tapping unease and sadness. Consistent with our prior work with older youth samples13,31,44 and a large body of work supporting the transdiagnostic nature of emotion dysregulation1–3, Reactivity was moderately correlated with every other indicator of mental health that was measured. Although Reactivity typically has greater salience for parents, i.e., most parents identify aspects of reactivity as the presenting problem when seeking psychiatric services45,46, there is a need to understand atypical upregulation of positive emotion and internalized forms of emotion dysregulation in early childhood as well. Our results indicated that while Dysphoria was most strongly correlated with depression (r = .72), it was also highly correlated with anxiety, temper loss, and reactivity (correlations hovering around ~.50), suggesting that, like Reactivity, Dysphoria also has transdiagnostic relevance. Additional research is needed to better understand how Reactivity and Dysphoria, their combination, or association with other factors, may lead to particular types of later mental health difficulties.
There is some precedent for emotion research to consider nuanced models that further differentiate aspects of emotion dysregulation. For example, temperament models, which are often applied to early childhood, attempt to distinguish affective intensity from aspects of emotional and behavioral regulation (e.g., effortful control)4,5 and irritability has been considered in terms of distinct tonic (persistently angry or grumpy mood) and phasic (temper tantrums or outbursts) components. Interestingly, the only study to date that has examined the factor structure of irritability in early childhood found that tonic and phasic irritability were not as distinct in 3-year-olds when compared with older children47. The EDI Reactivity results similarly placed various aspects of dysregulated negative emotion (i.e., initial intensity, outbursts, difficulty calming down or regulating) into a single factor. This may offer a more parsimonious way to conceptualize early ED and supports brief and efficient quantification of ED for clinical practice. Nonetheless, ED is certainly not a unitary phenomenon and future research could determine whether trajectories of ED or treatment response vary if quantified using the EDI or more nuanced models. Further, it is plausible that biological aspects of ED may be more separable than parent report of observable indicators of ED, which is worthy of additional study.
One of the strengths of the EDI-YC is that it was developed with consideration for both normative and atypical development. Item selection was informed by performance in both young children with neurodevelopmental delays/disabilities (i.e., clinical sample) and children from the general population without developmental concerns. Thus, the EDI-YC is the first measure of ED in early childhood that was validated in NDDs, including autistic young children who are often excluded from measure development samples. Moreover, online data collection allowed the EDI-YC to be validated in a large, heterogenous sample regarding development and diagnosis (N = 1369 and 768 for the clinical and general samples, respectively). Subsequently, we feel confident in recommending the EDI-YC for use as a measure of ED in all children ages 2–5 who may or may not have developmental concerns.
Annually, 13–20% of children and adolescents experience symptoms of a mental health disorder, including behavioral issues48. As such, the American Academy of Pediatrics recommends beginning routine screening in middle childhood; for example, they recently updated their recommendations to indicate that depression screening should start at age 1049. Given prevalence estimates from the CDC indicating that 3.8% of 3- to 5-year-olds have behavioral problems50 and burgeoning evidence that depression can onset in preschool18,51,52, earlier screening of emotion dysregulation may be justified. Importantly, a review completed in support of the 2019–2021 American Academy of Child and Adolescent Psychiatry Presidential Initiative concluded that emotional outbursts are often “chronic and disabling,” further emphasizing the significance of monitoring of ED, and reactivity in particular.3 Brief screenings for ED would be a good candidate for Level 1 screening, given that ED is characteristic of many behavioral difficulties and psychiatric disorders6,7,17,19. Such screening could complement existing pediatric screening that includes measures such as the Ages and Stages Questionnaires for developmental delays and the Modified Checklist for Autism in Toddlers for autism screening. Identifying elevated ED (Reactivity or Dysphoria) would provide an opportunity to potentially intervene before mental health and behavior worsens. Screening in autistic young children has been noted as particularly important53, given the high rates of co-occurring disorders in autism54. The EDI-YC would be ideal given its brevity and its validation in young children with and without neurodevelopmental disabilities. The lack of DIF by age in this sample (ages 2-3 versus 3–5) supported a single EDI-YC for ages 2- to 5-years old, which supports ease of administration. Given the same factor structure and the overlap of a sufficient number of items in the EDI-YC and the original EDI, the two forms can be linked to allow for longitudinal assessment of ED.
The original EDI has been widely disseminated and used in both research and clinical settings for universal screening, treatment monitoring, and clinical trials in more than 40 countries31 (see www.reaact.pitt.edu for more details on where it is being used). Similar to the EDI, the EDI-YC has promise for use in clinical trials that include young children where ED is a target13,55. Its precision is advantageous for measurement of small changes, and evidence of its test-retest reliability and change sensitivity is forthcoming. Additionally, the EDI-YC could be used to further refine the phenotypes of early-onset behavioral, psychiatric, and neurodevelopmental disorders, a potential advantage for both biological and clinical research.
Several aspects of the EDI-YC should be considered when using the measure and interpreting findings. First, the majority of the data were collected via parent report. Although additional data to support diagnoses and assessment of ED was not available, a recent study of the SPARK sample found that parent-reported autism diagnoses were confirmed in 98.8% of cases56, providing evidence that this method is effective as well as efficient for collecting large samples needed for psychometric analysis. Second, regarding diagnoses, the clinical sample predominantly included autistic young children. Although we did not find differences in item performance for the autistic vs. non-autistic NDD groups, the non-autistic NDD group was smaller, and we did not have the power to explore different types of NDD (e.g., unspecified developmental concerns, Down’s Syndrome, Fragile X Syndrome). Also, regarding sample composition, it is important to note that approximately 75% of the sample was White. Future work in these areas is indicated. Finally, as described above, the clinical understanding and significance of Dysphoria in young children requires more research. Additional research exploring item severity thresholds and distributions of Reactivity alongside other models of early disruptive behavior (such as the MAPS17,37) would enhance our understanding of emotional development and the dimensional spectrum of irritability.
In sum, the EDI-YC is a brief, valid, and sensitive measure of ED in children ages 2–5. This adaptation of the original EDI extends the measurement of ED down to toddlerhood. The EDI-YC is unique in that it was designed for use in young children regardless of developmental concerns or diagnosis. Thus, the EDI-YC may be a promising candidate to add to universal screening during the early childhood years to monitor for ED, allowing for earlier intervention with emotional and behavioral challenges that can lead to more prominent disorders.
Supplementary Material
Acknowledgments
This research was funded by the National Institute of Health grant R01HD079512. It was also supported by National Institute of Health grants R15HD091726, R01HD093677, and T32MH016804. Approved researchers can obtain the SPARK population dataset described in this study by applying at https://base.sfari.org. The complete dataset will also be made publicly available through the NIMH data repository.
The authors are grateful to the families who participated in this research. The authors are grateful to the families in SPARK, the SPARK clinical sites, and SPARK staff. The authors appreciate obtaining access to recruit participants through SPARK research match on SFARI Base. The authors are grateful for Dr. Neece and Laura Lee McIntyre, PhD, of the University of Oregon, for integration of the EDI-YC into their clinical trials to support additional data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented as a poster at the International Society for Autism Research Annual Meeting; May 11–14, 2022; Austin, Texas.
Disclosure: Drs. Day, Mazefsky, Yu, Neece, and Pilkonis and Ms. Zeglen have reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Taylor N. Day, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania..
Carla A. Mazefsky, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania..
Lan Yu, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania..
Katharine N. Zeglen, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania..
Cameron L. Neece, Loma Linda University, Loma Linda, California..
Paul A. Pilkonis, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania..
REFERENCES
- 1.Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, ed. Handbook of Emotion Regulation. Guilford Press; 2007:3–24. [Google Scholar]
- 2.Vogel AC, Tillman R, El-Sayed NM, et al. Trajectory of emotion dysregulation in positive and negative affect across childhood predicts adolescent emotion dysregulation and overall functioning. Dev Psychopathol. 2021;33(5):1722–1733. doi: 10.1017/S0954579421000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson GA, Singh MK, Amaya-Jackson L, et al. Narrative Review: Impairing Emotional Outbursts: What They Are and What We Should Do About Them. J Am Acad Child Adolesc Psychiatry. Published online March 28, 2022. doi: 10.1016/J.JAAC.2022.03.014 [DOI] [PubMed] [Google Scholar]
- 4.Rothbart MK. Temperament, Development, and Personality. Curr Dir Psychol Sci. 2007;16(4):207–212. doi: 10.1111/j.1467-8721.2007.00505.x [DOI] [Google Scholar]
- 5.MacNeill LA, Pérez‐Edgar K. Temperament and Emotion. In: The Encyclopedia of Child and Adolescent Development. Wiley; 2020:1–12. doi: 10.1002/9781119171492.wecad180 [DOI] [Google Scholar]
- 6.Keenan K Emotion Dysregulation as a Risk Factor for Child Psychopathology. Clin Psychol Sci Pract. 2000;7(4):418–434. doi: 10.1093/CLIPSY.7.4.418 [DOI] [Google Scholar]
- 7.Cole PM, Ashana Ramsook K, Ram N. Emotion dysregulation as a dynamic process. Dev Psychopathol. 2019;31(3):1191–1201. doi: 10.1017/S0954579419000695 [DOI] [PubMed] [Google Scholar]
- 8.Soke GN, Maenner MJ, Christensen D, Kurzius-Spencer M, Schieve LA. Prevalence of Co-occurring Medical and Behavioral Conditions/Symptoms Among 4- and 8-Year-Old Children with Autism Spectrum Disorder in Selected Areas of the United States in 2010. J Autism Dev Disord. 2018;0. doi: 10.1007/s10803-018-3521-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day TN, Mazefsky CA, Wetherby AM. Characterizing difficulties with emotion regulation in toddlers with autism spectrum disorder. Res Autism Spectr Disord. 2022;96:101992. doi: 10.1016/j.rasd.2022.101992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conner CM, Golt J, Righi G, Shaffer R, Siegel M, Mazefsky CA. A Comparative Study of Suicidality and Its Association with Emotion Regulation Impairment in Large ASD and US Census-Matched Samples. J Autism Dev Disord. 2020;50(10):3545–3560. doi: 10.1007/s10803-020-04370-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norona AN, Baker BL. The effects of early positive parenting and developmental delay status on child emotion dysregulation. J Intellect Disabil Res. 2017;61(2):130–143. doi: 10.1111/JIR.12287 [DOI] [PubMed] [Google Scholar]
- 12.Nuske HJ, Hedley D, Woollacott A, Thomson P, Macari SL, Dissanayake C. Developmental delays in emotion regulation strategies in preschoolers with autism. Autism Res. 2017;10(11):1808–1822. doi: 10.1002/aur.1827 [DOI] [PubMed] [Google Scholar]
- 13.Mazefsky CA, Yu L, White SW, Siegel M, Pilkonis PA. The emotion dysregulation inventory: Psychometric properties and item response theory calibration in an autism spectrum disorder sample. Autism Res. 2018;11(6):928–941. doi: 10.1002/aur.1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazefsky CA, Conner CM, Breitenfeldt K, et al. Evidence Base Update for Questionnaires of Emotion Regulation and Reactivity for Children and Adolescents. J Clin Child Adolesc Psychol. 2021;50(6):683–707. doi: 10.1080/15374416.2021.1955372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glascoe FP. Screening for developmental and behavioral problems. Ment Retard Dev Disabil Res Rev. 2005;11(3):173–179. doi: 10.1002/MRDD.20068 [DOI] [PubMed] [Google Scholar]
- 16.Weitzman C, Wegner L, Blum NJ, et al. Promoting Optimal Development: Screening for Behavioral and Emotional Problems. Pediatrics. 2015;135(2):384–395. doi: 10.1542/PEDS.2014-3716 [DOI] [PubMed] [Google Scholar]
- 17.Wakschlag LS, Choi SW, Carter AS, et al. Defining the developmental parameters of temper loss in early childhood: implications for developmental psychopathology. J Child Psychol Psychiatry. 2012;53(11):1099–1108. doi: 10.1111/J.1469-7610.2012.02595.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luby J, Lenze S, Tillman R. A novel early intervention for preschool depression: findings from a pilot randomized controlled trial. J Child Psychol Psychiatry. 2012;53(3):313–322. doi: 10.1111/J.1469-7610.2011.02483.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waxmonsky JG, Baweja R, Bansal PS, Waschbusch DA. A Review of the Evidence Base for Psychosocial Interventions for the Treatment of Emotion Dysregulation in Children and Adolescents. Child Adolesc Psychiatr Clin N Am. 2021;30(3):573–594. doi: 10.1016/j.chc.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 20.Cole PM, Michel MK, Teti LOD. The Development of Emotion Regulation and Dysregulation: A Clinical Perspective. Soc Res Child Dev. 1994;59(2):73–100. [PubMed] [Google Scholar]
- 21.Kopp CB. Regulation of distress and negative emotions: A developmental view. Dev Psychol. 1989;25(3):343–354. doi: 10.1037/0012-1649.25.3.343 [DOI] [Google Scholar]
- 22.Thompson RA, Goodman M. Development of emotion regulation: More than meets the eye. Emot Regul Psychopathol A transdiagnostic approach to Etiol Treat. Published online 2010:38–58. [Google Scholar]
- 23.Day TN, Northrup JB, Mazefsky CA. A PROMIS®ing New Measure for Quantifying Emotion Dysregulation in Toddlers and Preschoolers: Development of the Emotion Dysregulation Inventory-Young Child. J Autism Dev Disord. Published online April 11, 2022:1–13. doi: 10.1007/S10803-022-05536-9/TABLES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 SUPPL. 1). doi: 10.1097/01.MLR.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Blackwell CK, Wakschlag LS. Bringing PROMIS to Early Childhood: Introduction and Qualitative Methods for the Development of Early Childhood Parent Report Instruments. J Pediatr Psychol. 2022;47(5):500–509. doi: 10.1093/JPEPSY/JSAC027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackwell CK, Wakschlag L, Krogh-Jespersen S, et al. Pragmatic Health Assessment in Early Childhood: The PROMIS® of Developmentally Based Measurement for Pediatric Psychology. J Pediatr Psychol. 2020;45(3):311–318. doi: 10.1093/JPEPSY/JSZ094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White SW, Lerner MD, McLeod BD, et al. Anxiety in Youth With and Without Autism Spectrum Disorder: Examination of Factorial Equivalence. Behav Ther. 2015;46(1):40–53. doi: 10.1016/j.beth.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe SJ, Hewitt K, Baraskewich J, Cassidy S, McMorris CA. Suicidality Among Children and Youth With and Without Autism Spectrum Disorder: A Systematic Review of Existing Risk Assessment Tools. J Autism Dev Disord. 2020;50(10):3462–3476. doi: 10.1007/S10803-020-04394-7/TABLES/5 [DOI] [PubMed] [Google Scholar]
- 30.Mazefsky CA, Day TN, Siegel M, White SW, Yu L, Pilkonis PA. Development of the Emotion Dysregulation Inventory: A PROMIS®ing Method for Creating Sensitive and Unbiased Questionnaires for Autism Spectrum Disorder. J Autism Dev Disord. 2018;48(11):3736–3746. doi: 10.1007/s10803-016-2907-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazefsky CA, Yu L, Pilkonis PA. Psychometric Properties of the Emotion Dysregulation Inventory in a Nationally Representative Sample of Youth. J Clin Child Adolesc Psychol. Published online January 7, 2020:1–13. doi: 10.1080/15374416.2019.1703710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelkowitz RL, Cole DA. Measures of emotion reactivity and emotion regulation: Convergent and discriminant validity. Pers Individ Dif. 2016;102:123–132. doi: 10.1016/j.paid.2016.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feliciano P, Daniels AM, Green Snyder LA, et al. SPARK: A US Cohort of 50,000 Families to Accelerate Autism Research. Neuron. 2018;97(3):488–493. doi: 10.1016/J.NEURON.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mian ND, Soto TW, Briggs-Gowan MJ, Carter AS. The Family Life Impairment Scale: Factor Structure and Clinical Utility with Young Children. 10.1080/1537441620181458313. 2018;47(sup1):S530–S541. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackwell CK, Kallen MA, Lai J, Bevans KB, Wakschlag LS, Cella D. Measuring PROMIS Well-Being in Early Childhood. J Pediatr Psychol. 2022;47(April):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherlock P, Blackwell CK, Kallen MA, et al. Measuring PROMIS®Emotional Distress in Early Childhood. J Pediatr Psychol. 2022;47(5):547–558. doi: 10.1093/jpepsy/jsac029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakschlag LS, Briggs-Gowan MJ, Choi SW, et al. Advancing a Multidimensional, Developmental Spectrum Approach to Preschool Disruptive Behavior. J Am Acad Child Adolesc Psychiatry. 2014;53(1):82–96.e3. doi: 10.1016/J.JAAC.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthen LK, Muthen B. Mplus stastistical software program. www.statmodel.com
- 39.Samejima F Estimation of latent ability using a response pattern of graded scores. Psychom Monogr Suppl. 34AD;4(2). Accessed August 29, 2022. https://psycnet.apa.org/record/1972-04809-001 [Google Scholar]
- 40.Thissen D, Steinberg L, Howard W. Detection of differential item functioning using the parameters of item response models. In: Holland PW, Wainer H, eds. Differential Item Functioning. Lawrence Erlbaum Associates, Inc.; 1993:67–113. doi: 10.1075/Z.62.13KOK [DOI] [Google Scholar]
- 41.Zumbo BD. A Handbook on the Theory and Methods of Differential Item Functioning (DIF): Logistic Regression Modeling as a Unitary Framework for Binary and Likert-Type (Ordinal) Item Scores. Directorate of Human Resources Research and Evaluation, Department of National Defense; 1999. [Google Scholar]
- 42.Choi SW. Firestar: Computerized adaptive testing simulation program for polytomous item response theory models. Appl Psychol Meas. 2009;33(8):644–645. doi: 10.1177/0146621608329892 [DOI] [Google Scholar]
- 43.Choi SW, Reise SP, Pilkonis PA, Hays RD, Cella D. Efficiency of static and computer adaptive short forms compared to full-length measures of depressive symptoms. Qual Life Res. 2010;19(1):125–136. doi: 10.1007/S11136-009-9560-5/TABLES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conner CM, Golt J, Shaffer R, Righi G, Siegel M, Mazefsky CA. Emotion Dysregulation is Substantially Elevated in Autism Compared to the General Population: Impact on Psychiatric Services. Autism Res. 2021;14(1):169–181. doi: 10.1002/AUR.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsch E, Davis K, Cao Z, Roy AK. Understanding Phasic Irritability: Anger and Distress in Children’s Temper Outbursts. Child Psychiatry Hum Dev. 2022;53(2):317–329. doi: 10.1007/S10578-021-01126-5/FIGURES/3 [DOI] [PubMed] [Google Scholar]
- 46.Carlson GA, Dyson M. Diagnostic implications of informant disagreement about rage outbursts: bipolar disorder or another condition? Isr J Psychiatry Relat Sci. 2012;49(1):44–51. Accessed August 24, 2022. https://europepmc.org/article/med/22652928 [PubMed] [Google Scholar]
- 47.Silver J, Bufferd SJ, Dougherty LR, Goldstein BL, Carlson GA, Klein DN. Is the distinction between tonic and phasic irritability meaningful in 3-year-old children? Eur Child Adolesc Psychiatry. 2022;1:1–9. doi: 10.1007/S00787-022-01995-8/TABLES/4 [DOI] [PubMed] [Google Scholar]
- 48.Foy JM, Green CM, Earls MF. Mental health competencies for pediatric practice. Pediatrics. 2019;144(5). doi: 10.1542/PEDS.2019-2757/38256 [DOI] [PubMed] [Google Scholar]
- 49.Zuckerbrot RA, Cheung A, Jensen PS, Stein REK, Laraque D. Guidelines for adolescent depression in primary care (GLAD-PC): Part I. Practice preparation, identification, assessment, and initial management. Pediatrics. 2018;141(3). doi: 10.1542/PEDS.2017-4081/37626 [DOI] [PubMed] [Google Scholar]
- 50.Bitsko RH, Claussen AH, Lichstein J, et al. Mental Health Surveillance Among Children — United States, 2013–2019. MMWR Suppl. 2022;71(2):1. doi: 10.15585/MMWR.SU7102A1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luby JL, Gaffrey MS, Tillman R, April LM, Belden AC. Trajectories of preschool disorders to full DSM depression at school age and early adolescence: Continuity of preschool depression. Am J Psychiatry. 2014;171(7):768–776. doi: 10.1176/APPI.AJP.2014.13091198/ASSET/IMAGES/LARGE/768F2.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whalen DJ, Sylvester CM, Luby JL. Depression and Anxiety in Preschoolers: A Review of the Past 7 Years. Child Adolesc Psychiatr Clin N Am. 2017;26(3):503–522. doi: 10.1016/j.chc.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandler S, Howlin P, Simonoff E, et al. Emotional and behavioural problems in young children with autism spectrum disorder. Dev Med Child Neurol. 2016;58(2):202–208. doi: 10.1111/dmcn.12830 [DOI] [PubMed] [Google Scholar]
- 54.White SW, Maddox BB, Mazefsky CA, eds. The Oxford Handbook of Autism and Co-Occurring Psychiatric Conditions. Oxford University Press; 2020. [Google Scholar]
- 55.Lerner MD, White SW, McPartland JC. Mechanisms of change in psychosocial interventions for autism spectrum disorders. Dialogues Clin Neurosci. 2012;14(3):307–318. doi: 10.1161/01.RES.0000109414.78907.F9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fombonne E, Coppola L, Mastel S, O’Roak BJ. Validation of Autism Diagnosis and Clinical Data in the SPARK Cohort. J Autism Dev Disord. 2021;52(8):3383–3398. doi: 10.1007/S10803-021-05218-Y/TABLES/3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


