Abstract
Endovascular therapy (EVT) is effective in the treatment of large vascular occlusive stroke. However, many factors are associated with the outcomes of acute ischemic stroke (AIS) after EVT. This study aimed to identify the main factors related to the prognosis of AIS patients after EVT. We analyzed the clinical data of AIS patients in the neurology department of our medical center from June 2017 to August 2021 following treatment with EVT. The data included the patients’ blood pressure upon admission, blood glucose concentration, National Institutes of Health Stroke Scale (NIHSS) score, 90-day modified Rankin scale (mRs) score follow-up data, and time from LKN to the successful groin puncture (GP). A good outcome was defined as a 90-day mRs score of 0–2, and a poor outcome was defined as a 90-day mRs score of 3–6. A total of 144 patients were included in the study. Admission, smoking, and LKN-to-GP time, NIHSS score of 6–12 was found to be relevant to the prognosis. The results of multivariate analysis showed that prognosis was significantly influenced by baseline NIHSS (odds ratio = 3.02; 95% confidence interval, 2.878–4.252; P = 0.001), LKN-to-GP time (odds ratio = 2.17; 95% confidence interval, 1.341–2.625; P = 0.003), and time stratification (6–12 h) (odds ratio = 4.22; 95% confidence interval, 2.519–5.561; P = 0.001). Our study indicated that smoking, baseline NIHSS score, and LKN-to-GP time were the risk factors for a poor outcome in stroke patients following an EVT. Quitting smoking and shortening LKN time to GP should improve the outcome of AIS after EVT.
Subject terms: Neuroscience, Neurology
Introduction
Stroke is one of the main causes of death among the elderly people. In addition, the mortality and disability rates after stroke are 115/100,000 (95% confidence interval, 96–133) and 2.77% (Estonia) to − 0.23% (Romania), respectively, which results in serious consequences for the patient’s family and reduces the patient’s quality of life1. Endovascular therapy (EVT) is highly efficacious for acute ischemic stroke (AIS) due to large-vessel occlusion when performed within 24 h after the onset in selected cases2. Recent advances in AIS, which is caused by large-vessel occlusion and treated with EVT, have reduced the severity of disability, morbidity, and mortality rates of stroke3. In our medical center, the ratio of patients with AIS treated with EVT was 52% (154/302). The mortality of AIS patients treated with EVT was approximately 10% (16/154), which was lower than that of AIS patients who only received medical treatment (12.8%, 19/148, P < 0.05). About 6–7% of AIS patients were eligible to receive EVT4. With the increasing number of AIS patients who receive EVT, the benefits of EVT are also increasing. A previous study showed that clinical outcomes of EVT patients with failed reperfusion did not differ significantly from the outcomes of patients treated with best medical management; moreover, not all patients with large-vessel cerebral infarction can benefit from EVT5. In this study, we retrospectively analyzed the data of patients from our center who had undergone EVT—which is now indicated in patients within 24 h from their last known normal (LKN), provided that they meet specific clinical and imaging criteria —We want to analyze the factors associated with prognosis. This study aimed to identify the main factors that promote poor prognosis in AIS patients after EVT.
Materials and methods
Study population and parameter definitions
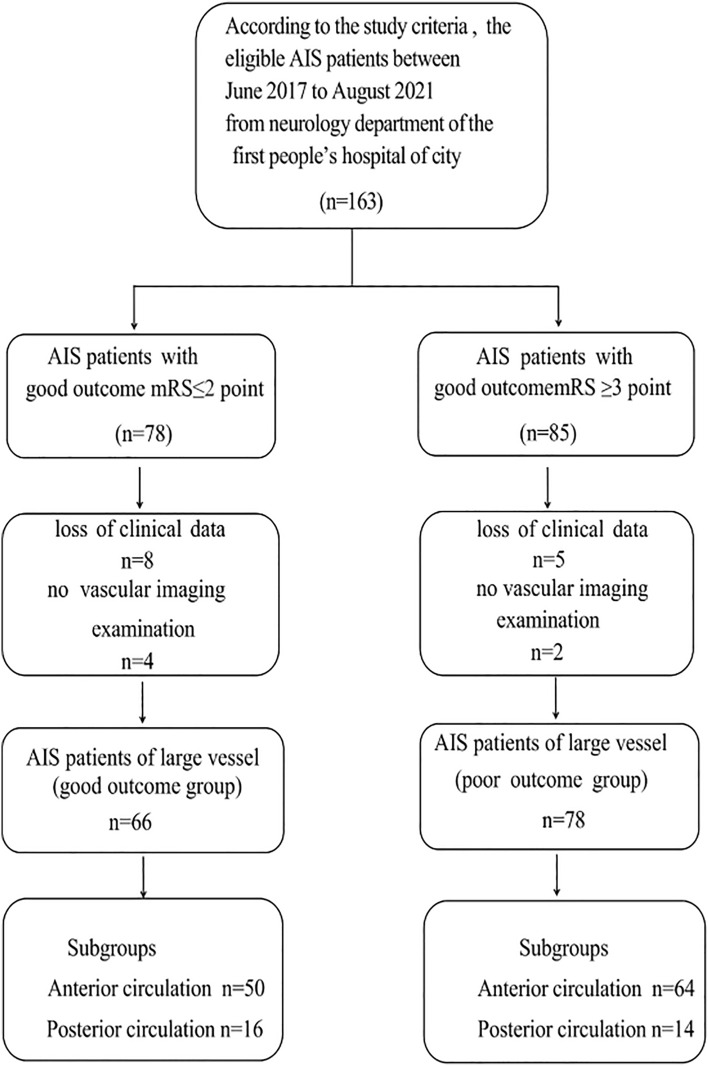
All 163 patients treated with EVT at our academic stroke center from June 2017 to August 2021 were recruited into the study. 19 patients were eliminated for the lack of clinical and imaging examination data, and 144 stroke patients (including anterior and posterior circulation large-artery occlusions) were finally included (Fig. 1). The time of disease onset was within 24 h. The patients met specific clinical and imaging criteria6. Each patient was followed up after 90 days. According to the time from stroke onset to visit time (0–3, 3–6, 6–12, > 12 h, unknown), the patients were stratified into five groups (Table 1). The patients underwent several examinations, including digital subtraction angiography (DSA), noncontrast computed tomography (CT), CT angiography (CTA), and CT perfusion. Those who went to hospital late underwent noncontrast CT7,8 and magnetic resonance angiography (MRA). The image checking, selection, and evaluation were conducted by two experienced radiologists.
Figure 1.
The flowchart of recruitment and selection process.
Table 1.
The clinical baseline data of 144 stroke patients.
| Item | Overall (n = 144) | Good Outcome (n = 66) | Poor Outcome (n = 78) | P value |
|---|---|---|---|---|
| Age | 65.41 ± 11.45(yrs) | 67.72 ± 10.42(yrs) | 63.65 ± 11.94(yrs) | 0.19 |
| Gender (male/female) | 80/64 | 38/28 | 42/36 | 0.23 |
| Hypertension | 61/144 | 23/66 | 38/78 | 0.15 |
| Anterior Circulation | 114 | 50/114 | 64/114 | 0.31 |
| Posterior Circulation | 30 | 16/30 | 14/30 | 0.18 |
| Dyslipidemia | 8/144 | 3/66 | 5/78 | 0.20 |
| Atrial Fibrillation | 60/144 | 27/66 | 33/78 | 0.25 |
| Diabetes mellitus | 13/144 | 5/66 | 8/78 | 0.19 |
| Glucose(mmonl/L) | 9.2 ± 2.31 | 8.3 ± 2.32 | 10.3 ± 3.04 | 0.11 |
| LDL (mmonl/L) | 4.2 ± 1.17 | 3.8 ± 1.43 | 4.5 ± 2.56 | 0.17 |
| INR | 1.1 ± 0.45 | 0.9 ± 0.46 | 1.2 ± 0.87 | 0.15 |
| Systolic blood pressure(mmHg) | 161 ± 35 | 167 ± 34 | 155 ± 52 | 0.23 |
Good Outcome: modified Rankin scale (mRs)≦2, Poor Outcome: mRS≧3, INR international normalized ratio; LDL low density lipoprotei.
The inclusion criteria were as follows: (1) patients were at least 18 years old; (2) the diagnosis of AIS due to large-vessel obstruction (LVO) was based on clinical presentation and imaging examinations, including MRA, CTA, or conventional DSA. In CTA, MRA, and DSA, patients with AIS presented with blockage of large cerebral blood vessels; (3) patients were admitted to neurology wards; (4) patients were conscious, cooperative, and able to provide all necessary information. Patients were excluded if they were diagnosed without imaging examinations; they had venous vascular disease, head trauma, acute intracranial infection, renal or hepatic failure, acute myocardial infarction, chronic heart failure, or hematological malignancy; or they were unwilling to cooperate or unable to provide reliable information.
Data collection
The collected data included the patients’ age, gender, LKN-to-GP time, neurological deficit (National Institutes of Health Stroke Scale [NIHSS]), laboratory test results, common risk factors for stroke (hypertension, diabetes mellitus, atrial fibrillation, and smoking history), symptomatic intracerebral hemorrhage (sICH) within 24 h after EVT, and time stratification (time since stroke onset). NIHSS was obtained by depending on calculating by the investigators of this study, and laboratory test data collected before undergoing EVT. While a number of scores could be used in the evaluation of stroke severity, the mRs scale—which represents the severity of functional impairment—is generally used in stroke research; mRS score takes values of 1, 2, 3, 4, 5, or 6, where a higher score indicates more serious impairment. A favorable prognosis was defined as an mRs score of 0–2, and a score ≥ 3 was defined as a poor outcome at 90 days after AIS.
Statistical analysis
The data were analyzed using SPSS software (IBM SPSS Statistics for Windows, version 22.0, New York, U.S.A.). Continuous variables were shown as mean ± SD, or the median (interquartile range [IQR]). Student’s t-test was carried out on the data that conformed to a normal distribution (Shapiro–Wilk test, P ≥ 0.05). If the data were collected as rates, the χ2 test was used. Multivariate binary logistic regression analysis for the predictors of favorable clinical prognosis was performed for all variables that were significant at the P < 0.05 level in the univariate analyses. Discrete numeric variables were depicted by medians (IQR). Age, gender, and other factors were adjusted for in the multivariate binary logistic regression analysis. A value of P < 0.05 was considered statistically significant.
Ethical approval
This study was approved by the Ethical Committee of First People’s Hospital of Xian Yang city in Sha’anXi province. All patients signed an informed consent approved by the review board. Research involving human participants, human material, or human data, must have been performed in accordance with the Declaration of Helsinki. All procedures carried out in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee. The ethical approval ID of the study (No:20230201).
Results
A total of 144 patients were analyzed in our study. 66 patients had mRs scores of 0–2, and 78 patients had mRs scores ≥ 3 (Fig. 2). The mean age was 64.4 ± 12.6 years, namely 67.7 ± 10.4 years in the favorable-prognosis group and 63.6 ± 11.9 years in the poor-prognosis group (P = 0.19). In our study, the anterior/posterior circulation ratio of the two groups was not significantly different (P > 0.05, Table 1). The admission NIHSS scores and other baseline clinical data are displayed in Table 1. There were no significant intergroup differences in the following factors: hypertension history (23/66 vs. 38/78, P = 0.15), dyslipidemia (3/66 vs. 5/78, P = 0.20), diabetes mellitus (5/66 vs. 8/78, P = 0.19), atrial fibrillation (27/66 vs. 33/78, P = 0.25), glucose concentration (8.3 ± 2.32 vs. 10.3 ± 3.04, P = 0.11), low-density lipoprotein–cholesterol (LDL) concentration (3.8 ± 1.43 vs. 4.5 ± 2.56, P = 0.17), International Normalized Ratio (INR: 1.0 ± 0.46 vs. 1.2 ± 0.87, P = 0.15), and systolic blood pressure (mm Hg) (167 ± 34 vs. 155 ± 52, P = 0.23) (Table 1). Smokers had a poorer outcome than nonsmokers (19/66 vs. 36/78, P = 0.005). The baseline NIHSS score was lower in the patients with a favorable prognosis than in those with a poor prognosis (10.2 vs. 14.23, P = 0.019). The LKN-to-GP time was shorter in the favorable-outcome group than in the poor-outcome group (350 ± 71 vs. 570 ± 92 min, P = 0.003). According to the stratification of the onset time, the number of patients with a poor prognosis was higher than other groups (6–12 h, stratification of onset to visit time). The prognosis of stroke patients with sICH (PH2) after stroke was often poor (Table 2).
Figure 2.
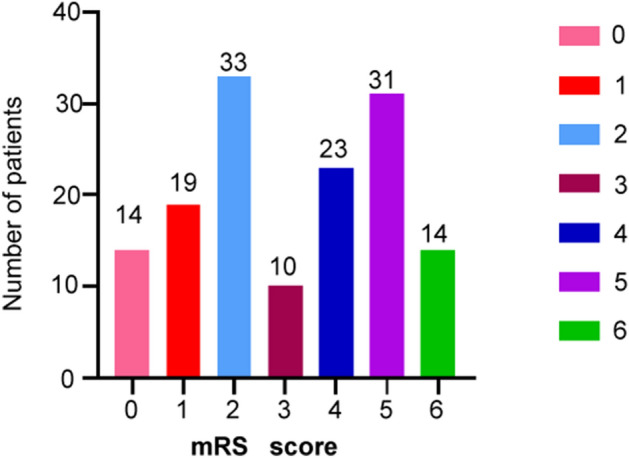
The mRS score information of 144 stroke patients (0–2 means a good outcome, and ≥ 3 means a poor outcome).
Table 2.
The clinical data of 144 stroke patients after endovascular treatment.
| Item | Overall (n = 144) | Good Outcome (n = 66) | Poor Outcome (n = 78) | P value |
|---|---|---|---|---|
| Smoking | 55/144 | 19/66 | 36/78 | 0.005 |
| Baseline NIHSS | 12.56 [10.04, 16.32] | 10.2 [7.95, 13.26] | 14.23 [10.81, 18.21] | 0.019 |
| LKN to grion puncture (mins) | 406.13 [296.47,499.53] | 350.34 [241.73,427.41] | 570.19 [404.83,724.14] | 0.003 |
| Time stratification (h) | ||||
| 0–3 | 15/144 | 5 | 10 | 0.155 |
| 3–6 | 54/144 | 22 | 32 | 0.120 |
| 6–12 | 36/144 | 24 | 12 | 0.005 |
| > 12 | 10/144 | 4 | 6 | 0.232 |
| Unknown | 29/144 | 11 | 18 | 0.271 |
| NIHSS stratification | ||||
| < 10 | 39/144 | 25 | 14 | 0.002 |
| 10–20 | 78/144 | 34 | 43 | 0.115 |
| > 20 | 27/144 | 5 | 22 | 0.002 |
| Safety (sICH) | ||||
| PH-1 | 5/144 | 3 | 2 | 0.112 |
| PH-2 | 9/144 | 2 | 7 | 0.009 |
INR international normalized ratio, LDL low density lipoprotein, LKN last known normal, Time stratification Stratification of onset to visit time; Modified Thrombolysis in Cerebral Infarction, NIHSS National Institutes of Health Stroke Scale, sICH symptomatic intracranial hemorrhage, and PH parenchymal hematoma.
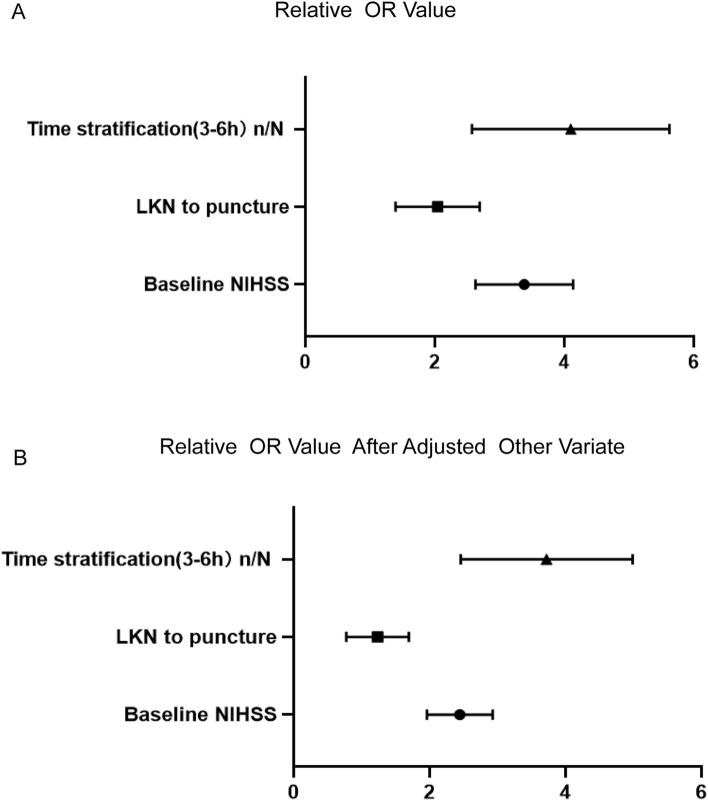
Baseline NIHSS, LKN-to-GP time and time stratification (6–12 h), age, gender, arterial hypertension, diabetes mellitus, smoking, random blood glucose, and LDL were included into the regression model. Baseline NIHSS, LKN-to-GP time, and time stratification (6–12 h) were closely associated with the outcome. After adjusting for the covariates, baseline NIHSS (OR = 2.57, P = 0.023), LKN-to-GP time (OR = 1.17, P = 0.035), and time stratification (6–12 h) (OR = 3.819, P = 0.015) were still closely associated with the outcome (Fig. 3). The data are displayed in Table 3.
Figure 3.
The odds Ratio (OR) of smoking, baseline NIHSS and LKN time to puncture as in the poor outcome group were significantly higher than good outcome group (A, B).
Table 3.
Multivariable binary logistic regression analyses outcome according to status in stroke patients treated with endovascular treatment.
| Items | B | Wald | P value Unadjusted [OR, 95% CI] | P value Adjusted [OR, 95% CI] |
|---|---|---|---|---|
| Baseline NIHSS | −1.45 | 1.533 |
0.001 3.02 (2.878–4.252) |
0.023 2.57 (1.912–2.853) |
| LKN to puncture (minutes) | 1.307 | 2.621 |
0.003 2.17 (1.341–2.625) |
0.035 1.17 (0.812–1.723) |
| Time stratification(3–6 h)n/N | 2.508 | 23.01 |
0.001 4.22 (2.519–5.561) |
0.015 3.819 (2.412–4.937) |
NIHSS National Institutes of Health Stroke Scale, LKN last known normal, OR Odds ratio, CI Confidence interval.
Discussion
EVT is effective in the treatment of large vascular stroke. However, the prognosis of stroke is affected by many factors9. A previous study has shown that EVT in patients with vertebrobasilar artery occlusion may improve the long-term prognosis10, and a higher level of cerebral edema is associated with a poor prognosis in AIS patients eligible for EVT11. A previous study has indicated that near complete or complete revascularization (TICI 2c/3) is associated with higher rates of functional outcomes after EVT12. In our research, the baseline NIHSS score, LKN-to-puncture time, smoking, time stratification, and PH2 showed a clear correlation with the outcome in the patients with AIS after receiving EVT. Stroke is prevalent worldwide and has a high morbidity rate. Many scales are used to measure the severity and outcome of stroke. The NIHSS is a quantifiable scale that is used to evaluate the severity of stroke13. The patients who had a low baseline NIHSS score often had more favorable clinical outcomes than the patients with a high baseline NIHSS score. A previous study, which analyzed 484 stroke patients, showed that the recanalization in patients with an NIHSS score of 5 (IQR, 4–7) (thrombolysis in cerebral infarction, TICI 2b–3) was achieved in 26 (78.7%) patients14, and the patients all had favorable clinical outcomes. A low baseline NIHSS score was independently predictive of a favorable outcome in patients with posterior-circulation (odds ratio [OR] 1.547; 95% confidence interval [CI], 1.232–1.941) and anterior-circulation (OR, 1.279; 95% CI, 1.188–1.376) stroke. The baseline NIHSS score for a favorable functional outcome in anterior-circulation stroke was 4 (IQR, 3–7), compared with 3 (IQR, 1–5) for posterior-circulation stroke15.
Time was related to the clinical outcomes. A previous study showed that the outcomes could be enhanced by reducing the door-to-GP time16. Our study suggests that any effort to improve the time from LKN to GP time will result in a positive outcome. A study by Sun et al. showed that when the logistic regression model was adjusted for age, NIHSS, hypertension, diabetes mellitus, reperfusion status, and symptomatic hemorrhage, the preprocedural time frame from LKN to GP was directly associated with 90-day positive outcomes (OR, 0.996; 95% CI, 0.993–0.998; P < 0.001)17. Another study also showed that shorter LKN-to-GP time improved the outcome, particularly in those with good ASPECTS (CT stroke score system) presenting within 6 h. Strategies to decrease reperfusion times should be investigated, particularly early on and in patients with good ASPECTS18. Our study is consistent with the previously reported studies.
Smoking is a major risk factor for many diseases, including stroke, especially among young and middle-aged stroke patients. Our study also showed that the percentage of smokers among the patients with poor outcomes was higher than that among the patients with favorable outcomes19. A previous study showed a strong dose–response relationship between cigarettes smoked daily and ischemic stroke among young men. The study was a population-based case–control study of risk factors for ischemic stroke in men aged 15–49 years. The OR for the current-smoking group compared with the never-smoking group was 1.88. Although complete smoking cessation is the goal, even smoking fewer cigarettes may reduce the risk of ischemic stroke in young men20. The relationship between smoking and stroke is controversial. According to some studies, smoking in stroke patients could be beneficial after EVT. However, these data should not be misunderstood as a benefit of smoking. Another study reported that smoking was not associated with a good functional prognosis (mRS ≤ 2) at 3 months in AIS patients who were treated with intravenous thrombolysis21. Due to the controversial effects of cigarette smoking on cardiovascular health, smoking cessation is still recommended for stroke prevention22.
The sooner a stroke patient is treated, the better the prognosis. As shown in a multicenter randomized clinical trial of endovascular treatment for AIS in the Netherlands, patients treated for an occlusion of the intracerebral internal carotid artery or the middle cerebral artery within a 6-h window from the LKN time benefit from EVT23. A previous study showed that EVT could benefit patients and provided evidence of reversible cerebral ischemia in the time window of 6–24 h. The previous study findings suggested that in AIS patients, EVT should be carried out based on the mode of presentation or the time of presentation within the 6–24-h time window13.
sICH is the most serious complication after IVT and EVT. Using a combination of the predictors that were available both before and at the end of bridging therapy and direct thrombectomy may provide indications for the early identification of patients who are candidates for a more intensive postprocedural management. In those patients who are at a high risk of sICH, the monitoring and treatment of hypertension and hyperglycemia should be intensified. Patients who are at a low risk of sICH should be transferred back to the referring hospital quickly in a sedative state24. A previous study found no difference in the rates of sICH between primarily utilized stent retriever devices and non-stent retriever devices, and the incidence of sICH in care-dependent stroke patients was similar to that in care-independent patients25. Our data suggested that the incidence of sICH was higher than previously reported (10% vs. 6%)26, and the type PH2 occurred more often in the poor-outcome group than in the favorable-outcome group.
There are two biases or limitations in the current work. First, many factors influence the stroke outcomes, and our study did not consider all factors related to stroke. Second, the stroke outcomes also correlate with early rehabilitation, but our study did not include the early rehabilitation factor.
Conclusions
Smoking, baseline NIHSS score, and LKN-to-GP time were the risk factors for a poor outcome of stroke patients following an EVT. Quitting smoking and shortening LKN-to-GP time should improve the outcome of AIS after EVT.
Acknowledgements
Thank all the researchers participated in this work.
Author contributions
Y.W. acquired the data, analyzed he results, and wrote the main manuscript text, X.Y. and Y.K. guided the process, interpreted the results, L.Y., W.C. and G.F. revised the article. All authors read and approved the manuscript.
Funding
This study was supported by the science foundation of first people’s hospital of Xianyan city (No: 20170312).
Data availability
Requests for access to the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Department of Neurology, first people’s hospital of Xianyan city. The contacting person is the corresponding author and E-mail is concocon@163.com.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained an error in Figure 2 labelling, where number of patients was incorrect.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/9/2024
A Correction to this paper has been published: 10.1038/s41598-024-59017-3
Contributor Information
Yugang Wang, Email: conconcon@163.com.
Xingyun Yuan, Email: yuanxingyun0305@163.com.
References
- 1.Wafa HA, Wolfe CDA, Emmett E, Roth GA, Johnson CO, Wang Y. Burden of stroke in Europe: Thirty-year projections of incidence, prevalence, deaths, and disability-adjusted life years. Stroke. 2020;51:2418–2427. doi: 10.1161/STROKEAHA.120.029606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katan M, Luft A. Global burden of stroke. Semin. Neurol. 2018;38(2):208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 3.Ganesh A, Kashani N, Ospel JM, Wilson AT, Foss MM, Saposnik G, et al. Endovascular therapy or alteplase in patients with comorbidities: Insights from unmask evt. Can. J. Neurol. Sci. 2021;48:77–86. doi: 10.1017/cjn.2020.158. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues FB, Neves JB, Caldeira D, et al. Endovascular treatment versus medical care alone for ischaemic stroke: Systematic review and meta-analysis. BMJ. 2016;353:i1754. doi: 10.1136/bmj.i1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ospel JM, Hill MD, Demchuk A, et al. Clinical impact of EVT with failed reperfusion in patients with acute ischemic stroke: Results from the ESCAPE and ESCAPE-NA1 trials. Neuroradiology. 2021;63:1883–1889. doi: 10.1007/s00234-021-02723-w. [DOI] [PubMed] [Google Scholar]
- 6.Quandt F, Flottmann F, Madai VI, et al. Machine learning-based identification of target groups for thrombectomy in acute stroke. Transl. Stroke Res. 2023;14:311–321. doi: 10.1007/s12975-022-01040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai SM, Starr M, Molyneaux BJ, et al. Acute ischemic stroke with vessel occlusion-prevalence and thrombectomy eligibility at a comprehensive stroke center. J. Stroke Cerebrovasc. Dis. 2019;28(11):104315. doi: 10.1016/j.jstrokecerebrovasdis.2019.104315. [DOI] [PubMed] [Google Scholar]
- 8.Heiss WD, Zaro-Weber O. Extension of therapeutic window in ischemic stroke by selective mismatch imaging. Int. J. Stroke. 2019;14(4):351–358. doi: 10.1177/1747493019840936. [DOI] [PubMed] [Google Scholar]
- 9.Mohammaden MH, Haussen DC, Perry da Camara C, et al. Hyperdense vessel sign as a potential guide for the choice of stent retriever versus contact aspiration as first-line thrombectomy strategy. J. Neurointerv. Surg. 2021;13(7):599–604. doi: 10.1136/neurintsurg-2020-016005. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z-X, Lin J, Han Y, et al. Prognostic factors for acute vertebrobasilar artery occlusion-reperfusion: A multicenter retrospective cohort study. Int. J. Surg. 2023;109:2303–2311. doi: 10.1097/JS9.0000000000000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z-X, Li Y-K, Li S-Z. A dynamic nomogram for 3-month prognosis for acute ischemic stroke patients after endovascular therapy: A pooled analysis in Southern China. Front. Aging Neurosci. 2021;13:796434. doi: 10.3389/fnagi.2021.796434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghozy S, Kacimi SEO, Azzam AY. Successful mechanical thrombectomy in acute ischemic stroke: Revascularization grade and functional independence. Rev. J. Neurointerv. Surg. 2022;14(8):779–782. doi: 10.1136/neurintsurg-2021-018436. [DOI] [PubMed] [Google Scholar]
- 13.Grassl N, Baumann S, Kruska M, et al. Acute ischemic stroke and elevated troponin: Diagnostic work-up and therapeutic consequences. Dtsch. Med. Wochenschr. 2021;146(8):534–541. doi: 10.1055/a-1308-7490. [DOI] [PubMed] [Google Scholar]
- 14.Kazi SA, Majid S. Stroke outcome prediction using admission NIHSS in anterior and posterior circulation stroke. J. Ayub. Med. Coll. Abbottabad. 2021;33(2):274–278. [PubMed] [Google Scholar]
- 15.Pfaff J, Herweh C, Pham M, et al. Mechanical thrombectomy in patients with acute ischemic stroke and lower NIHSS scores: Recanalization rates, periprocedural complications, and clinical outcome. Am. J. Neuroradiol. 2016;37(11):2066–2071. doi: 10.3174/ajnr.A4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun C-HJ, Ribo M, Goyal M, et al. Door-to-puncture: A practical metric for capturing and enhancing system processes associated with endovascular stroke care, preliminary results from the rapid reperfusion registry. J Am. Heart Assoc. 2014;3:e000859. doi: 10.1161/JAHA.114.000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun CH, Ribo M, Goyal M, et al. Door-to-puncture: A practical metric for capturing and enhancing system processes associated with endovascular stroke care, preliminary results from the rapid reperfusion registry. J. Am. Heart Assoc. 2014;3(2):e000859. doi: 10.1161/jaha.114.000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder T, Agarwal S, Huang J, et al. Stroke treatment delay limits outcome after mechanical thrombectomy: Stratification by arrival time and ASPECTS. J. Neuroimaging. 2020;30(5):625–630. doi: 10.1111/jon.12729. [DOI] [PubMed] [Google Scholar]
- 19.Esenwa C, Gutierrez J. Secondary stroke prevention: Challenges and solutions. Vasc. Health Risk Manag. 2015;11:437–450. doi: 10.2147/VHRM.S63791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markidan J, Cole JW, Cronin CA, et al. Smoking and risk of ischemic stroke in young men. Stroke. 2018;49(5):1276–1278. doi: 10.1161/STROKEAHA.117.018859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang P, Guo ZN, Sun X, et al. Meta-analysis of the smoker's paradox in acute ischemic stroke patients receiving intravenous thrombolysis or endovascular treatment. Nicotine Tob. Res. 2019;21(9):1181–1188. doi: 10.1093/ntr/ntz094. [DOI] [PubMed] [Google Scholar]
- 22.von Martial R, Gralla J, Mordasini P, et al. Impact of smoking on stroke outcome after endovascular treatment. PLoS ONE. 2018;13(5):e0194652. doi: 10.1371/journal.pone.0194652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonsen CZ, Leslie-Mazwi TM, Thomalla G. Which imaging approach should be used for stroke of unknown time of onset? Stroke. 2021;52(1):373–380. doi: 10.1161/STROKEAHA.120.032020. [DOI] [PubMed] [Google Scholar]
- 24.Jovin TG, Nogueira RG, Lansberg MG, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): A systematic review and individual patient data meta-analysis. Lancet. 2022;399(10321):249–258. doi: 10.1016/S0140-6736(21)01341-6. [DOI] [PubMed] [Google Scholar]
- 25.Oesch L, Arnold M, Bernasconi C, Kaesmacher J, Fischer U, Mosimann PJ, et al. Impact of pre-stroke dependency on outcome after endovascular therapy in acute ischemic stroke. J. Neurol. 2021;268:541–548. doi: 10.1007/s00415-020-10172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Steen WR, van der Ende NAM, van Kranendonk KR, et al. Timing of symptomatic intracranial hemorrhage after endovascular stroke treatment. Eur. Stroke J. 2022;7(4):393–401. doi: 10.1177/23969873221112279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for access to the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Department of Neurology, first people’s hospital of Xianyan city. The contacting person is the corresponding author and E-mail is concocon@163.com.