Summary
Background
Despite significant progress in malaria control over the past twenty years, malaria remains a leading cause of child morbidity and mortality in Tropical Africa. As most patients do not consult any health facility much uncertainty persists about the true burden of the disease and the range of individual differences in susceptibility to malaria.
Methods
Over a 25-years period, from 1990 to 2015, the inhabitants of Dielmo village, Senegal, an area of intense malaria transmission, have been monitored daily for their presence in the village and the occurrence of diseases. In case of fever thick blood films were systematically examined through microscopy for malaria parasites and patients received prompt diagnosis and treatment.
Findings
We analysed data collected in 111 children and young adults monitored for at least 10 years (mean 17.3 years, maximum 25 years) enrolled either at birth (95 persons) or during the two first years of life. A total of 11,599 episodes of fever were documented, including 5268 malaria attacks. The maximum number of malaria attacks in a single person was 112. Three other persons suffered one hundred or more malaria attacks during follow-up. The minimum number of malaria attacks in a single person was 11. The mean numbers of malaria attacks in children reaching their 4th, 7th, and 10th birthdays were 23.0, 37.7, and 43.6 attacks since birth, respectively. Sixteen children (14.4%) suffered ten or more malaria attacks each year at ages 1–3 years, and six children (5.4%) each year at age 4–6 years.
Interpretation
Long-term close monitoring shows that in highly endemic areas the malaria burden is higher than expected. Susceptibility to the disease may vary up to 10-fold, and for most children childhood is an endless history of malaria fever episodes. No other parasitic, bacterial or viral infection in human populations has such an impact on health.
Funding
The Pasteur Institutes of Dakar and Paris, the Institut de Recherche pour le Développement, and the French Ministry of Cooperation provided funding.
Keywords: Malaria, Africa, Morbidity, Epidemiology, Immunology, Cohort study, Longitudinal study, Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, Children, Infants, Senegal
Research in context.
Evidence before this study
The prevalence of clinical malaria in tropical Africa has been well documented in many dispensaries and other health structures. However, few of the patients come to the attention of any formal health system. This prevent to have a clear idea of what is the real burden of malaria.
Added value of this study
A cohort of 111 African villagers living in an area of intense and perennial malaria transmission was enrolled at birth and monitored daily at home for an average period of 17.3 year for malaria and other causes of fever. We documented 11,599 episodes of fever, including 5268 malaria attacks. The mean number of malaria attacks per person was 47.5 ± 22.1 (median: 46 attacks). Four children (3.6%) suffered one hundred malaria attacks or more, with a maximum of 112 attacks, and 44 children (40%) from 50 to 91 malaria attacks. During the first years of life, for many children malaria was an endless history of fever episodes, with 10 attacks or more each year. Most of them would have been undiagnosed in absence of our surveillance system.
Implications of all the available evidence
It was unexpected that the burden of malaria could reach such enormous levels in a human population. This suggest that current WHO estimates of malaria morbidity in Africa could be largely underestimated. Scaling up malaria control and prevention interventions will require increased domestic and international commitment and funding.
Introduction
Funding for malaria control and elimination in the world increased dramatically during the period 2000–2009, allowing a significant reduction of the malaria burden in the WHO African Region during the period 2010–2015 compared to the previous decade.1,2 However, no further progress was observed in most African countries after 2015 and malaria morbidity and mortality remain very high, with an estimated 247 million cases and 619,000 deaths in 2021.1 In rural areas of sub-Saharan Africa, most villagers receive each year dozens or hundreds of bites of malaria infected mosquitoes and first malaria infections are contracted during infancy.3, 4, 5 After three to five months of age, when maternally-transmitted antibodies decrease, malaria infections give high fever and may cause death by cerebral or multi-systems malaria.6, 7, 8 A high risk of death during malaria attacks is observed until age 4 years when malaria transmission is intense and perennial, and until age 5–9 years when malaria transmission is low to moderate and seasonal.8, 9, 10 The prevalence of clinical malaria in sub-Saharan Africa has been well documented in many dispensaries and other health structures. However, few of the patients come to the attention of any formal health system and little longitudinal data is available at the community level. This prevent accurate estimates of the real burden of malaria.
In 1990, we implemented a longitudinal study of malaria and the determinants of the disease in a rural African community, Dielmo village, Senegal.11 Until 2008, when long-lasting insecticidal nets (LLINs) were deployed, our only intervention was to provide prompt and effective diagnostic and treatment for clinical malaria attacks and other causes of fever.11,12 Here we describe and analyse the longitudinal data collected in persons enrolled in the project at birth or during their first months of life who were actively monitored from 10 to 25 years after enrolment. Our objective was to evaluate the natural history and distribution of malaria attacks overall and at the individual level, as well as explore the association of the attacks with some epidemiological, genetical and immunological factors.
Methods
Study area and participants
Dielmo village (13°45′N, 16°25′W), Senegal, is located on the marshy bank of a permanent stream (the Nema) in an area of Sudan-type savanna, 280 km south-east of Dakar and about 15 km north of the Gambian border, an area where malaria was holoendemic with year-round transmission. Most of the houses are built in the traditional style with mud walls and thatched roofs. The inhabitants of the village are settled agricultural workers. A detail description of the study area and the origin of the project was reported previously.11,12
The project was initially approved by the Ministry of Health of Senegal and the assembled village population. Approval was then renewed on a yearly basis with written informed consent from individuals enrolled in the project and parents or guardians of children. National Ethics Committee of Senegal and ad-hoc committees of the Ministry of Health, the Pasteur Institutes (Dakar and Paris), and the Institut de Recherche pour le Développement (IRD, formerly ORSTOM) did audits regularly.11,12
Procedures
We visited all households daily, and recorded nominative information 6 days a week (i.e., excluding Sunday) at home on the presence or absence in the village of every individual we had enrolled, their location when absent, and the presence of fever or other symptoms. We systematically recorded body temperature at home either daily (except Sunday) or three times a week (every second day) in children younger than 5 years of age, and in older children and adults in cases of history of fever or fever-related symptoms (hot body, asthenia, headache, vomiting, diarrhoea, abdominal pain, cough). In case of suspected or confirmed fever, we did blood testing by finger prick at home or at the dispensary, we systematically measured malaria parasite density through blood slide microscopy (200 oil-immersion fields on a thick smear, approximately 0.5 μl of blood examined), and we provided detailed medical examination, prompt diagnosis and specific treatment for malaria and other diseases. The dispensary created for our project within the village was open 24 h a day, 7 days a week, to allow both active and passive case detection.
We successively used four first-line drugs regimens for treatment of malaria attacks: oral quinine (June 1990–December 1994), chloroquine, with sulfadoxine-pyrimethamine for second-line treatment in case of clinical failure (January 1995–October 2003), sulfadoxine-pyrimethamine plus amodiaquine (November 2003–May 2006), and artesunate plus amodiaquine (from June 2006 onwards).12 Antimalarial drugs were systematically given to young children in case of fever associated with a parasite: leukocyte ratio (PLR) equal or higher to 200%.13 When parasitaemia was lower, the physician or nurse resident in Dielmo decided the requirement for antimalarial treatment, taking into account all the patient’s data. For adults and older children permanently living in the village, clinical malaria attacks often lasted only a few hours,14 and thus in most cases (apart from pregnant women) only symptomatic treatment was given to reduce selection for drug-resistance in malaria parasites. From 2011 onwards, treatment policy was modified to maximise efforts to limit malaria transmission, and artesunate plus amodiaquine was systematically given to all patients with fever associated with malaria parasites independently of age and parasite density.
At the beginning of the project, 49% of the villagers used traditional mosquito nets, which were untreated, and this proportion remained almost unchanged until July, 2008, when we offered LLINs (PermaNet 2.0, Lausanne, Switzerland) to all villagers. There were no insecticide treated nets in Dielmo before July 2008, and the LLINs of all villagers were renewed in July 2011, then in August 2014, after we documented rebounds in malaria morbidity. We measured malaria prevalence and density for each Plasmodium species at least quarterly from 1990 onwards in all villagers enrolled in the project, but monthly in children aged from six months to 9 years, and twice a month in infants aged 1–5 months. Blood was taken using a finger prick and we examined 200 oil-immersion fields. We applied similar procedures when examining thick blood films from patients.
We counted separately two episodes of fever (including history of fever and allegation of hot body) and malaria attacks if they occurred 14 days or more apart. We attributed fever to malaria when parasite density was higher than an age-dependent threshold calculated for each Plasmodium species during the corresponding period according to methods described elsewhere.15,16 For infants aged less than six months we attributed fever episodes to malaria when the onset of fever corresponded to the onset of patent parasitaemia and/or to peaks of high parasitaemia.14
To investigate factors associated to the occurrence of malaria attacks, we included in data analysis a series of variables including age, sex, distance from the river, bed net use, family ties, and genetic information (ethnic group, Glucose-6-phosphate dehydrogenase (G6PD) deficiency status, ABO blood type, hemoglobin type).17 For a subgroup of 31 children born during the first years of the project we also studied immune responses at ages 3, 5, 7, and 10 years using a multiplex bead-based serological assay for a panel of 11 antigens (DBL1-Pf13, PfMSP1-19, PfAMA1, PfCSP, DBL3X, GLURP, SALSA, LSA1-41, LSA1-J, LSA3, gSG6).18
Statistical analysis
In the present study we restricted data analysis to children fitting the following criteria: (i) to be born between December 1988 and July 2002, i.e., children enrolled in the study either at birth or when aged less than 2 years, and aged at least 6-year-old when LLINS were deployed in Dielmo, (ii) to be monitored during at least 10 years after enrollment, either continuously or with only short absences from Dielmo (maximum 20% of days of absence during the minimum 10 years period of follow-up). Since malaria pre-elimination was achieved in Dielmo in 2012,12 with a single limited rebound in 2014 and an almost total elimination of malaria from 2015 onwards,19 only data collected up to December 31st, 2015, are analyzed in this paper. A total of 111 children fitted these criteria. The environmental, clinical and biological parameters investigated and data analysis methods were previously described.11,12,15, 16, 17, 18 We used a Stata 13 software. In brief the association between two categorical variables was tested using Fisher’s exact test. The means of two groups were compared using the student’s t-test if the variable was normally distributed and using Mann–Whitney U test otherwise. Three or more groups were compared using analysis of variance if the variable was normally distributed and using Kruskal–Wallis analysis of variance on rank otherwise. The strengths of association between two normal continuous variables and two ordinal variables were measured using the Spearman’s rank correlation coefficient. A p-value of 5% or lower was considered statistically significant and confidence intervals were computed at 95% level.
Epidemiological background
Due to the Nema River, breeding sites for anopheline mosquitoes were present year round and malaria transmission was perennial.12 The EIR fluctuated markedly according to years, but it always remained very high from 1990 to 2007, where it averaged 267 infected bites per person per year, with a maximum of 384 during the period 1998–2002, then decreased to 155 in 2008 when LLINs were deployed at mid-year and reached low levels during the most recent periods, averaging 71 infected bites per person per year during the period 2009–2011, then decreased from 7.6 infected bites per person per year in 2012 to 2.8 in 2015.12,19 For the whole population of Dielmo village, the parasite rate in children 0–14 years, which was 87% the first year of the study, decreased to 60–77% during the period 1991–2004, then to 30–40% during the period 2005–2007 when the first line treatment of malaria attacks switched from chloroquine to sulfadoxine-pyrimethamine plus amodiaquine then to artesunate plus amodiaquine, and finally the parasite rate decreased to 12% in 2008 and to less than 5% from 2009 onwards after the deployment of LLINs.12 It was 0.3%, 0.2% and 0.5% in 2012, 2013 and 2014, respectively, and nil in 2015. Malaria morbidity in children 0–14 years also decreased markedly during follow-up, from 3.8 attacks per child per year for the period 1990–2003, to 1.4 attacks per child per year for the period 2004–2008, and 0.15 attacks per child per year for the period 2009–2014.12,19
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the reports. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Results
Malaria attacks in the study cohort
We monitored a total of 111 children, 57 males and 54 females, enrolled either at birth (95 children), or during the second trimester of life (one child), the second semester of life (four children), or the second year of life (11 children, including 9 children enrolled before 18 months). The mean duration of follow-up was 17.3 years, with a minimum of 10 years and a maximum of 25.6 years. Children enrolled more than three months after birth were in 7 cases children born in the village before the beginning of the study, and in 9 cases children from mothers who migrated to the village during the study. The total number of person-days of follow-up was 699,045 (Supplementary Information 1).
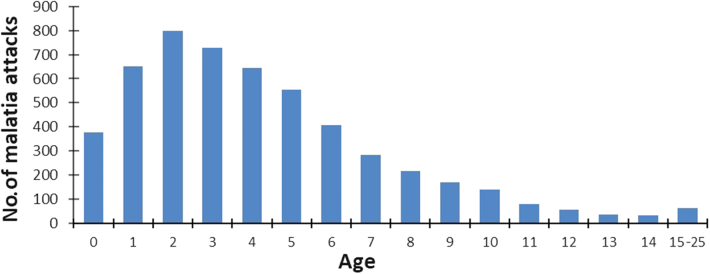
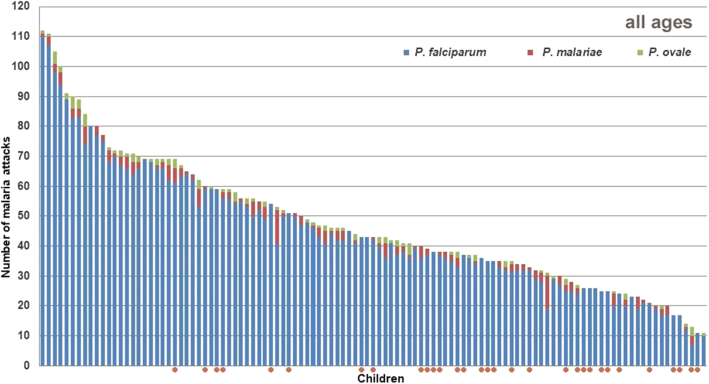
A total of 11,599 episodes of illness were documented between June 1990 and December 2015, including 4954 Plasmodium falciparum, 211 Plasmodium malariae, and 103 Plasmodium ovale clinical attacks. The mean number of malaria attacks per person was 47.5 ± 22.1 (median: 46 attacks). Most malaria attacks occurred during infancy and early childhood, with a maximum during the third year of life (Fig. 1). Severe hyper parasitaemia (PLR > 5000%) was observed during 8 P. falciparum attacks among 7 children, all aged between 6 months and 2.8 years. Four children (3.6%), three males and one female, suffered at least one hundred malaria attacks during follow-up, 112, 111, 105 and 100 malaria attacks, respectively (Fig. 2). Supplementary Information 1 shows, for each child, the number of days of effective follow-up each year, and Supplementary Information 2 the age distribution of P. falciparum, P. malariae, and P. ovale malaria attacks.
Fig. 1.
Age distribution of malaria attacks recorded in the study cohort.
Fig. 2.
Distribution of 111 children monitored during at least 10 years after birth, maximum 25 years, according to the number of P. falciparum, P. malariae and P. ovale malaria attacks they experienced during the study. Red circles show children with less than 5500 days (15 years) of effective monitoring. Dielmo, June 1990–December 2015.
The boy who suffered the maximum number of malaria attacks during follow-up was enrolled at birth in July 1991 and was monitored during 24.6 years, with 8883 days of effective monitoring. He suffered a total of 110 P. falciparum, one P. malariae and one P. ovale attacks. Six P. falciparum attacks occurred during infancy, the first one at 86 days of age, the other attacks during infancy at ages 3, 6, 7, 10, and 11 months. The following years, he suffered 36, 29, 22, 15, and 2 attacks at ages 1–3, 4–6, 7–9, 10–14, and 15–19 years, respectively. A single P. ovale attack occurred at 7 months, and a single P. malariae attack at 8 years. He also suffered 77 non-malaria episodes of illness during follow-up.
The minimum number of malaria attacks was observed in a boy born in November 1990 and monitored during 25 years, with 8525 days of effective monitoring. He suffered 10 P. falciparum and one P. ovale clinical attacks, that occurred for P. falciparum at ages 3, 4, 7, 8, 9, 11, 12, and 14 months, and 2 and 5 years, and for P. ovale at 15 months. He also suffered 116 non-malaria episodes of illness.
Among the 95 children enrolled at birth, 55 (58%) presented their first malaria infection before the age of 3 months (including one P. malariae infection), 79 (83%) before the age of 6 months, 85 (90%) before their first birthday (including one P. ovale infection), and all before 22 months. The mean numbers of P. falciparum clinical attacks per infants were 1.5 attacks before 6 months (1.6 for all malaria species) and 2.2 attacks between 6 and 11 months (2.4 for all malaria species). There was one case of congenital malaria and the minimum age for a P. falciparum infection and attack was 15 days. Up to 9 malaria attacks during infancy were documented in two children, all with P. falciparum.
The mean numbers of P. falciparum clinical attacks per person per age period were 18.8 between age 1 and 3 years (19.5 for all Plasmodium species), 13.7 attacks between age 4 and 6 years (14.7 for all species), 5.5 attacks between age 7 and 9 years (6.0 for all species), 2.9 attacks between age 10 and 14 years (3.2 for all species), and 0.5 attacks between age 15 and 19 years (Supplementary Information 2). Sixteen children (14.4%) suffered 30 malaria attacks or more between age 1 and 3 years, i.e., a mean of 10 or more malaria attacks per year, 6 children (5.4%) between age 4 and 6 years, and one child between age 7 and 9 years. At least one P. malariae attack was observed in 80 children (72%), with a maximum of 11 attacks in two children, and at least one P. ovale attack was observed in 61 children (55%), with a maximum of 4 attacks in four children. It is at age 4–6 years that the incidence of P. malariae and P. ovale attacks were highest (Supplementary Information 2).
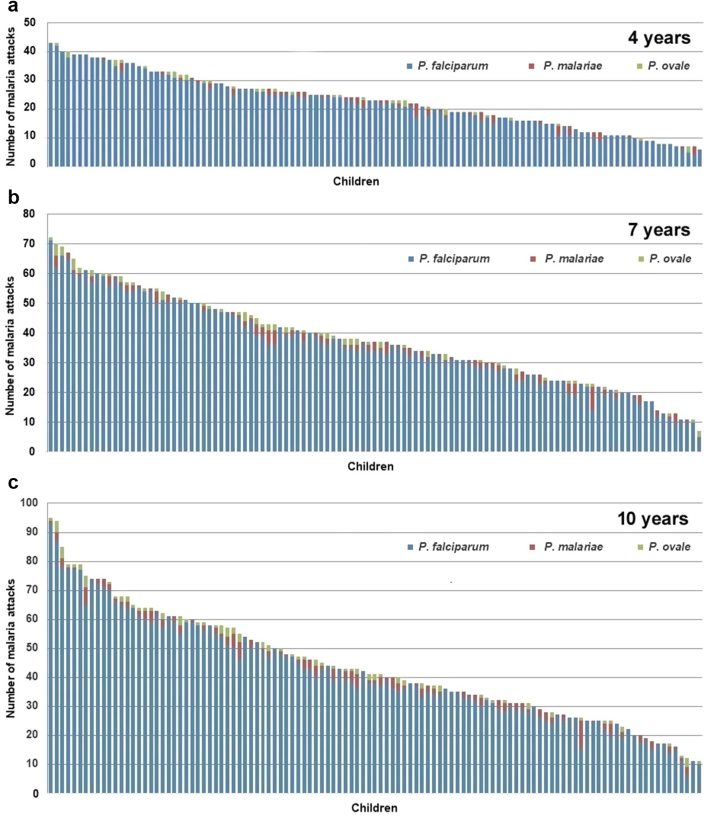
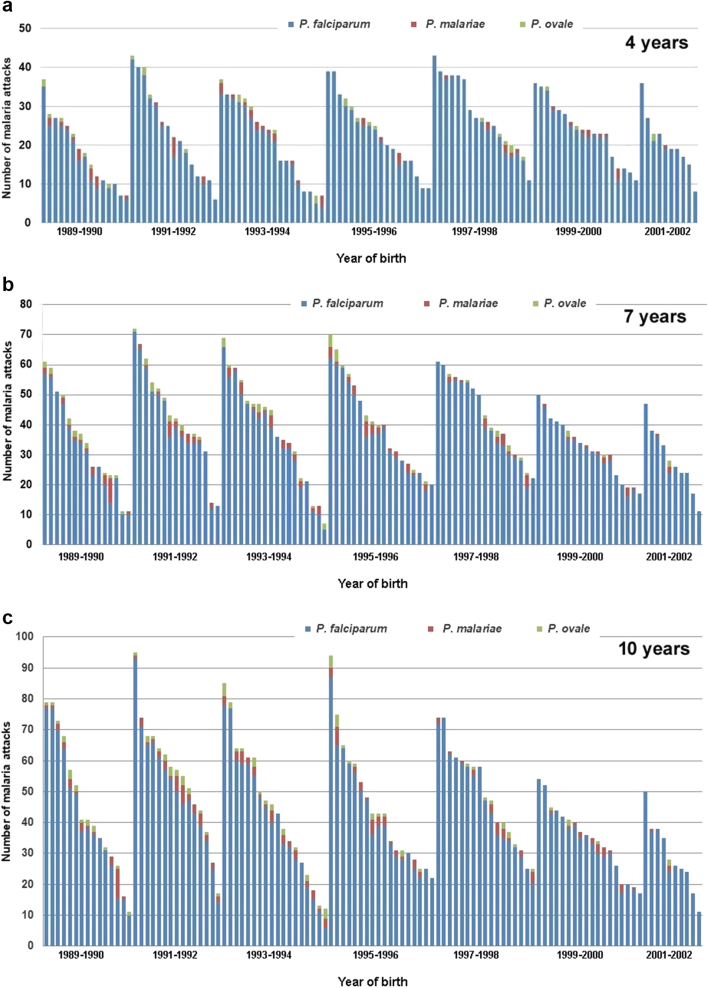
The distribution of malaria attacks in children when reaching their 4th, 7th, and 10th birthday is shown in Fig. 3. The mean number of malaria attacks per child since birth was 23.0 (±9.5) at the 4th birthday (median: 23), 37.7 (±15.1) at the 7th birthday (median: 37), and 43.6 (±19.2) attacks at the 10th birthday (median: 41). The maximum numbers of malaria attacks since birth when reaching the 4th, 7th and 10th birthdays were 43, 72 and 95 attacks, respectively, and were observed in the child that suffered 112 attacks during the study. Minimum numbers of attacks when reaching the 4th, 7th, and 10th birthdays were 6, 7 and 11 attacks since birth, respectively, all in children closely monitored and aged at least 10 years when combination therapy and LLINS were introduced in Dielmo. As shown in Fig. 3, the distributions of the numbers of malaria attacks in children at the 4th, 7th and 10th birthdays decreased almost linearly between the maximum and minimum values.
Fig. 3.
Distribution of 111 children according to the number of P. falciparum, P. malariae and P. ovale malaria attacks they cumulated when reaching their 4th birthday (a), 7th birthday (b), and 10th birthday (c).
Although most children that totalized a high number of attacks when aged 10 years had already suffered a high number of attacks when reaching 4 and 7 years, exceptions were not rare and in particular the number of attacks occurring during the first year of life was poorly predictive of the future number of attacks during childhood: among the 25 children who suffered 6 or more malaria attacks during infancy, only 6 (24%) were in the group of 25 children totalizing 60 or more malaria attacks at 10 years (Supplementary Information 2). The age of first infection had no impact on the number of malaria attacks during childhood: children infected before 3 months of age totalized 46.6 (±18.2) attacks at 10 years compared to 41.7 (±20.3) for those infected between 4 and 21 months (Supplementary Information 4, n.s. by Spearman’s rank correlation test).
Relationships between the number of malaria attacks and some variables
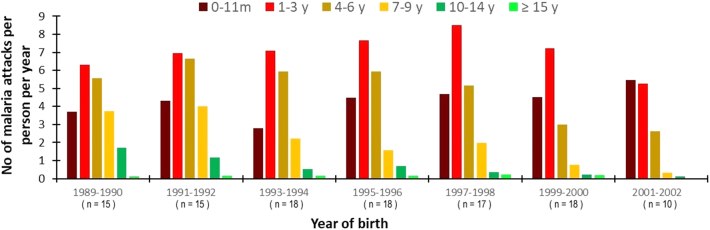
The age distribution and incidence density of malaria attacks according to the year of birth is shown in Fig. 4. For the youngest age groups the incidence density of malaria attacks remained very high during the whole study. Maximum incidence in children aged 1–3 years was observed for those born in 1997–1998, i.e., those exposed to the very high levels of malaria transmission of the period 1998–2002. For older children a progressive decline in the incidence of malaria attacks occurred during the study. Attacks became rare after the 7th birthday for children born from 1999 onwards (Supplementary Information 3). Among the 28 children born from 1999 to 2002, i.e., the youngest children when the first line treatment of malaria attacks was switched from chloroquine to combination therapy then when LLINs were deployed, none was among the 30 children of the cohort of 111 children that totalized the highest number of malaria attacks when reaching 10 years of age, and 12 (43%) were among the 30 children that totalized the lowest number of malaria attacks. The distribution of cumulated malaria attacks experienced by children born in the same year when reaching their 4th, 7th and 10th birthday are shown in Fig. 5. According to the year of birth, ratios of minimum and maximum numbers of malaria attacks in individual children ranged from 3.3 to 7.2 fold at 4 years (mean 4.8), from 2.8 to 9.9 fold at 7 years (mean: 4.9), and from 3.0 to 7.2 fold at 10 years (mean: 5.0).
Fig. 4.
Distribution of the incidence density of malaria attacks by age-group according to the year of birth. Dielmo, 111 children, June 1990–December 2015.
Fig. 5.
Distribution of 111 children according to year of birth and the number of P. falciparum, P. malariae and P. ovale malaria attacks they cumulated when reaching their 4th birthday (a), 7th birthday (b), and 10th birthday (c).
High numbers of malaria attacks were observed both in males and in females. There were 8 boys and 8 girls among the 16 children that had the highest numbers of attacks during the whole follow-up, and also 8 boys and 8 girls among the 16 children that had the lowest numbers of attacks. The mean number of attacks was 44.8 (±20.4) in boys and 41.9 (±17.0) in girls at age 10 years, and 48.9 (±23.3) in boys and 45.9 (±20.8) in girls during the whole follow-up (n.s. by Mann–Whitney U test). The age and parity of the mother had no effect on the number of malaria attacks during infancy and childhood. In particular, the mean number of malaria attacks during infancy was similar in infants born from primiparous (3.8) and multiparous women (3.8).
None of the genetic traits examined (ethnic group, family ties, haemoglobin type, ABO group, rhesus type, G6PD deficiency status) explained the differences observed between children in the numbers of malaria attacks (Supplementary Information 2, n.s. by Mann–Whitney U test or Kruskal–Wallis test). The sickle cell trait was present in 7 of the 111 children of the study (6%), including 4 and 1 of the 30 children with the lowest and highest numbers of malaria attacks, respectively.
The use of non-impregnated mosquito nets during infancy and childhood had no impact on the number of malaria attacks (Supplementary Information 4, n.s. by Mann–Whitney U test). When reaching 4 years, children never using nets during infancy and early childhood (n = 37) experienced on average 23.9 ± 10.3 malaria attacks since birth, compared to 21.5 ± 8.8 malaria attacks for those using nets at least intermittently (n = 56), and 21.6 ± 9.6 for those always using good quality nets (n = 10). When reaching 7 years, children never using nets during infancy and childhood (n = 22) experienced on average 39.5 ± 17.8 attacks since birth, compared to 34.8 ± 14.5 attacks since birth for children always using nets at least intermittently (n = 42). The type of housing of children, with (n = 61) or without open space between walls and roof (n = 50), had no impact on the mean number of malaria attacks, either when reaching 4 years of age (23.9 ± 10.6 attacks vs 21.8 ± 8.9 attacks) or when reaching 10 years (43.5 ± 19.2 attacks vs 43.3 ± 18.5 attacks) (Supplementary Information 4, n.s. by Mann–Whitney U test). The distance from the river to the houses ranged from 50 m to 480 m and had no impact on the number of malaria attacks (n.s. by Spearman’s rank correlation test).
Supplementary Information 5 shows levels of antibodies at ages 3, 5, 7 and 10 years for P. falciparum DBL1-Pf13, PfMSP1-19, PfAMA1, PfCSP, DBL3X, GLURP, SALSA, LSA1-41, LSA1-J, and LSA3 antigens, and gSG6 A. gambiae saliva antigen, in a subgroup of 31 children born between 1990 and 1995, i.e., the first years of the project, and the numbers of malaria attacks in these children at ages 1–3, 4–6, 7–9, and 10–14 years. A strong negative association was observed in children aged 4–6 years between high antibodies levels to LSA1-41 and GLURP and low numbers of P. falciparum attacks (r = −0.54 and r = −0.49, respectively, p = 0.002 and p = 0.005, respectively, by Spearman rank test) (Supplementary Information 6). At age 7–9 years, LSA1-41 and GLURP antigens remained strongly associated with a low number of P. falciparum attacks (r = −0.47 and r = −0.58, respectively, p = 0.007 and p = 0.001, respectively, by Spearman rank test).
Discussion
Based on the close monitoring of a village population during 25 years, this study provides unique data on individual medical histories from birth to adulthood among persons living in an area of rural Africa where malaria is highly endemic. The idea that malaria was a huge burden for African populations emerged in the 1950’s, and only a limited number of studies were available in 1974 when WHO provided a first estimate of malaria mortality in tropical Africa, indicating that malaria was probably directly responsible for about one million deaths annually in infants and children.20, 21, 22 The knowledge that African children exposed to high levels of transmission can suffer several malaria attacks each year associated to peaks of high parasitaemia was first documented in 1958 in Liberia,23 but it is only in the 1980’s and 1990’s that parasite density measurement was adopted as standard method for evaluating malaria morbidity in highly endemic areas, allowing precise assessments of the incidence of malaria attacks among populations where malaria parasites are also present in asymptomatic persons and fortuitously associated to other causes of fever.13,22,24 Because of the rarity of longitudinal epidemiological studies, the dramatic clinical impact of the emergence of high levels of chloroquine resistance in Africa in the 1980’s and 1990’s has long been underestimated, and changes in drug treatment policies in most countries of tropical Africa were initiated only after 2004.10,25
It was unexpected that the burden of malaria could reach such enormous levels. Four children out of 111 (3.6%) in our cohort suffered one hundred malaria attacks or more, and 44 children (40%) from 50 to 91 malaria attacks. During the first years of life, for many children malaria was an endless history of fever episodes: for 15 children (13.5%) from 30 to 39 P. falciparum malaria attacks occurred within a 3-year period when they were aged from 1 to 3 years, and for 6 children (5.4%) from 30 to 35 P. falciparum malaria attacks also occurred within a 3-year period when they were aged from 4 to 6 years. For these children with a very high number of malaria attacks, their medical histories from six months to three or six years of age were similar, with almost always the same repeated sequences: high fever with high P. falciparum parasitemia (PLR 200–2000% or more), malaria treatment given the same day or the day after, total disappearance of fever and malaria parasites (at microscopy) within a few days, new episode of fever with again high P. falciparum parasitemia occurring within 3–5 weeks after the previous one, immediate treatment and rapid disappearance of fever and malaria parasites, then new malaria attack within 3–5 weeks, and again treatment and cure of malaria parasites. During their first years of age, asymptomatic malaria infections were almost never documented in these children, probably due to the immediate treatment of attacks that prevented the development of partial antiparasite and anti-disease immunities in a context of high exposure to reinfections, even if part of the attacks that we recorded could correspond to clinical recrudescences, at least during the chloroquine treatment period where drug resistance increased rapidly.26
For a majority of children, malaria attacks become rare after 6 years of age, suggesting that partial immunity has been achieved,27,28 but for 20 children (18%) from 10 to 30 P. falciparum attacks also occurred when they were aged from 7 to 9 years. Due to their short duration, even in absence of specific treatment,14 most attacks would have been undiagnosed in absence of our surveillance system based on the daily monitoring of children. This is reminiscent of what was observed in a cohort of 55 Kenyan children under surveillance for malaria from the age of 5–15 years, with one child experiencing up to 32 episodes of clinical malaria.29 The very high number of malaria attacks documented in children in longitudinal studies based on a close monitoring both in Senegal and Kenya suggest that current WHO estimates of malaria morbidity in the WHO African region—241 million cases in 20211—could be largely underestimated.
Although all children lived in the same small village with very similar living conditions and exposition to malaria vectors, there has been dramatic differences in the number of malaria attacks they presented during the study, with up to a 10-fold difference between the number of attacks recorded in two boys born a few months apart and closely monitored during two decades and half until 2015. Interestingly, when looking at the distribution of the number of attacks in children when reaching 4, 7 and 10 years in our study, the curve was almost linear, preventing sub-groups of high-, low- and medium-risk children from being defined in any other way than arbitrarily. These differences between children were not explained by any of the potential environmental and genetic factors investigated. Regarding the immune responses investigated, high antibodies levels against LSA1-41 and GLURP were strongly associated with low numbers of P. falciparum attacks after the age of 3 years, suggesting a protective role of these antibodies, either by blocking parasite development at the liver stage, and/or by controlling parasite density. In a 10-year study in Kenya, children aged 5–15 years reaching a plateau in the number of P. falciparum attacks had better responses to PfAMA1 and MSP3 antigens than children who continued to experience malaria attacks.29 No association was found here for PfAMA1 antibodies. Responses to MSP3 antigens, that were not studied here in our multiplex assay but were previously investigated in Dielmo and other settings, were consistently associated with partial clinical protection against P. falciparum malaria in these studies.30,31
No other parasitic, bacterial or viral disease in humans is known to represent a burden similar to malaria in African or other populations in the world. Progress in malaria control in Senegal, and elsewhere in Africa, which has been enabled by the use of more effective antimalarial drugs, the widespread use of insecticide-treated nets and the increasing use of rapid diagnostic tests, show that this burden is not inevitable even when populations of anopheles vectors remain abundant.1 However, only limited progress in the fight against malaria have been made so far in many rural areas of tropical Africa, suggesting that for many children, childhood continues to be an endless history of malaria fever episodes. Scaling up malaria control and prevention interventions will require increased domestic and international commitment and funding.
Contributors
JFT, PD, CR, and OMP designed the study. JFT, ND, FDS, JF, HB, AB, CB, AT, SD, ANW, CR, CL, and CS conducted field work. JFT, ND, FDB, IVW, MGB, MN, ATB, RP, CR, PD, CR, OMP, and CS conducted laboratory work. JFT, ND, FDS, JF, HB, AT, CR, CL, and CS contributed to data management. RD, CT, CR, and CL contributed to statistical analysis. JFT analysed the data and wrote the paper with the assistance of ND, CR, and OMP. All authors had full access to all the data in the study and approved the final manuscript.
Data sharing statement
Most data of this study are available in Supplementary Information 1–6. Additional data may be obtained by contacting the corresponding author.
Declaration of interests
We declare that we have no conflict of interest.
Acknowledgements
We thank the villagers of Dielmo for their continuous support to the project. We are grateful to all those who participated in field and laboratory work at different stages of the project. We are indebted to all those who provided support and encouragements, and all those who every year contributed to convince our institutions, donors and audits, to let us add another 1 year to the project. We thank the French Ministry of Cooperation and our institutions for providing funding. This study is still going on in 2023, confirming the almost total elimination of malaria in Dielmo from 2015 onwards and focusing on the other causes of diseases in this population.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102379.
Appendix A. Supplementary data
References
- 1.World Health Organization . WHO; Geneva: 2022. World malaria report 2022; p. 293. [Google Scholar]
- 2.Bhatt S., Weiss D.J., Cameron E., et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay S.I., Rogers D.J., Toomer J.F., Snow R.W. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans R Soc Trop Med. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald G. The analysis of malaria parasite rates in infants. Trop Dis Bull. 1950;47:915–938. [PubMed] [Google Scholar]
- 5.Dobbs K.R., Dent A.E. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology. 2016;143:129–138. doi: 10.1017/S0031182015001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyd M.F. WB Saunders Co; Philadelphia and London: 1949. Malariology. A comprehensive survey of all aspects of this group of diseases from a global standpoint. [Google Scholar]
- 7.Snow R.W., Nahlen B., Palmer A., et al. Risk of severe malaria among African infants: direct evidence of clinical protection during early infancy. J Infect Dis. 1998;177:819–822. doi: 10.1086/517818. [DOI] [PubMed] [Google Scholar]
- 8.Schellenberg D., Menendez C., Kahigwa E., et al. African children with malaria in an area of intense Plasmodium falciparum transmission: features on admission to the hospital and risk factors for death. Am J Trop Med Hyg. 1999;61:431–438. doi: 10.4269/ajtmh.1999.61.431. [DOI] [PubMed] [Google Scholar]
- 9.Carneiro I., Roca-Feltrer A., Griffin J.T., et al. Age patterns of malaria vary with severity, transmission intensity and seasonality in sub-Saharan Africa: a systematic review and pooled analysis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trape J.F., Pison G., Preziosi M.P., et al. Impact of chloroquine resistance on malaria mortality. C R Acad Sci III. 1998;321:689–697. doi: 10.1016/s0764-4469(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 11.Trape J.F., Rogier C., Konate L., et al. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–137. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]
- 12.Trape J.F., Tall A., Sokhna C., et al. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis. 2014;14:476–488. doi: 10.1016/S1473-3099(14)70712-1. [DOI] [PubMed] [Google Scholar]
- 13.Trape J.F., Peelman P., Morault-Peelman B. Criteria for diagnosing clinical malaria among a semi-immune population exposed to intense and perennial transmission. Trans R Soc Trop Med. 1985;79:435–442. doi: 10.1016/0035-9203(85)90054-9. [DOI] [PubMed] [Google Scholar]
- 14.Rogier C., Ly A.B., Tall A., et al. Plasmodium falciparum clinical malaria in Dielmo, a holoendemic area in Senegal: no influence of acquired immunity on initial symptomatology and severity of malaria attacks. Am J Trop Med Hyg. 1999;60:410–420. doi: 10.4269/ajtmh.1999.60.410. [DOI] [PubMed] [Google Scholar]
- 15.Roucher C., Rogier C., Dieye-Ba F., et al. Changing malaria epidemiology and diagnostic criteria for Plasmodium falciparum clinical malaria. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0046188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roucher C., Rogier C., Sokhna C., et al. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0087169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loucoubar C., Grange L., Paul R., et al. High number of previous Plasmodium falciparum clinical episodes increases risk of future episodes in a sub-group of individuals. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perraut R., Varela M.L., Loucoubar C., et al. Serological signatures of declining exposure following intensification of integrated malaria control in two rural Senegalese communities. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wotodjo A.N., Doucoure S., Diagne N., et al. The impact of renewing long-lasting insecticide-treated nets in the event of malaria resurgence: lessons from 10 years of net use in Dielmo, Senegal. Am J Trop Med Hyg. 2021;104:255–262. doi: 10.4269/ajtmh.20-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . World Health Organization Technical Report Series, no 537; Geneva: 1974. Malaria control in countries where time-limited eradication is impracticable at present; p. 66. [PubMed] [Google Scholar]
- 21.Najera J.A. Malaria control: achievements, problems and strategies. Parassitologia. 2001;43:1–89. [PubMed] [Google Scholar]
- 22.Snow R.W. Sixty years trying to define the malaria burden in Africa: have we made any progress? BMC Med. 2014;12:227. doi: 10.1186/s12916-014-0227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller M.J. Observations on the natural history of malaria in the semi-resistant West African. Trans R Soc Trop Med Hyg. 1958;52:152–168. doi: 10.1016/0035-9203(58)90036-1. [DOI] [PubMed] [Google Scholar]
- 24.Schellenberg J.R., Smith T., Alonso P.L., et al. What is clinical malaria? Finding case definitions for field research in highly endemic areas. Parasitol Today. 1994;10:439–442. doi: 10.1016/0169-4758(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 25.Attaran A., Barnes K.I., Curtis C., et al. WHO, the global fund, and medical malpractice in malaria treatment. Lancet. 2004;363:237–240. doi: 10.1016/S0140-6736(03)15330-5. [DOI] [PubMed] [Google Scholar]
- 26.Noranate N., Durand R., Tall A., et al. Rapid dissemination of Plasmodium falciparum resistance under strictly controlled anti-malaria use. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doolan D.L., Dobaño C., Baird J.K. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langhorne J.L., Ndungu F.M., Sponaas A.M., Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–731. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 29.Addy W.G., Bediako Y., Ndungu F.M., et al. 10-year longitudinal study of malaria in children: insights into acquisition and maintenance of naturally acquired immunity. Wellcome Open Res. 2022;6:79. doi: 10.12688/wellcomeopenres.16562.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roussilhon C., Oeuvray C., Muller-Graf C., et al. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 2007;4:1791–1803. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowkes F.J., Richards J.S., Simpson J.A., Beeson J.G. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.