Highlights
-
•
Monitored flavor substances changes during storage.
-
•
Analyzed the contribution of volatile compounds to tea quality.
-
•
The substances changes beyond oxidation effect tea quality.
Keywords: Storage duration, Flavor substances, Head space-solid phase micro extraction gas chromatography mass spectrometry (HS-SPME-GC–MS), Gas chromatography ion mobility spectrometry (GC-IMS), Relative odor activity value (ROAV)
Abstract
To study the effect of storage (for 0, 3, 6, and 12 months) on the flavor of green tea (GT), we monitored the volatile organic compounds (VOCs) in GT through gas chromatography (GC) combined with ion mobility spectrometry and headspace solid-phase micro extraction, GC–MS (mass spectrometry). Then, relative odor activity value (ROAV) was applied to analyze the aroma contribution of the VOCs. During storage, the polyphenol and caffeine contents gradually decreased from 22.38 % to 18.51 % and from 4.37 % to 3.74 %, respectively, and the total soluble sugar first increased and then decreased (from 4.89 % to 7.16 % and then 5.02 %). Although the total free amino acid contents showed a fluctuating trend, the content of cysteamine increased gradually. The contents of VOCs with positive contribution to GT aroma, including linalool, geraniol, nonanal, and 6-methyl-5-hepten-2-one, decreased. They also contributed less in the ROAV after storage. The ROAVs of nonanal, linalool, and geraniol decreased from 3.37 to 0.79, from 100 to 38.21, and from 2.98 to 1.8, respectively, after 12 months of storage. Principal component analysis can be used to identify the samples with different storage durations based on these data. Given the increase in amount of cysteamine and decrease in that of linalool oxide, oxidation may be not the only factor responsible for tea quality in storage.
1. Introduction
Tea is one of the top beverages in the world (Yang, Wang, & Sheridan, 2018), and it can be divided to green tea (GT), black tea, oolong tea, dark tea, white tea, and yellow tea depending on the manufacturing processes. Each tea has its own unique flavor and odors (Wang et al., 2018). GT is the most consumed tea in China because of its attractive sensory qualities, such as freshness, sweetness, nut-like aroma, or roasted and umami taste of tea infusions (Feng et al., 2019, Rong et al., 2023, Wang et al., 2022). Fuliang GT has long history since Han dynasty, with Geographical Indications of Agricultural Products in China. Also, it is one of the representative GT varieties in tea trade for Jiangxi Province.
Fresh GT has a higher commercial value than long-term-stored ones because of its better sensory flavor. However, the chemical dynamic changes in GT during storage remain unclear (Dai et al., 2020, Xu et al., 2023). In presented work, Fuliang green tea was used to study the effect of storage on flavor substances. Tea flavor, including aroma and taste, changes in response to chemicals (Joshi & Gulati, 2015). Volatile organic compounds (VOCs) are found in very low contents in fresh leaves. Although VOCs represent only 0.01 % to 0.05 % of the total mass of dried tea, over 600 kinds are very important for tea aroma (Yang, Baldermann, & Watanabe, 2013).
The taste of tea also plays an important role in the evaluation of tea quality, and in addition to aroma, it is connected to the composition and content of non-VOCs (Zhou et al., 2021). The nonvolatile substances that constitute tea taste mainly include caffeine, tea polyphenols, sugar, amino acids, and other substances. Caffeine, amino acids, and tea polyphenols have the greatest effect on tea quality (Guo, Ho, Schwab, & Wan, 2021). Although some reports focused on other GTs (Liu et al., 2018, Xu and Wang, 2020), few publications have centered on Fuliang GT, one of the most important varieties.
Gas chromatography–mass spectrometry (GC–MS) can apply in food flavor analysis (Wang et al., 2020, Li et al., 2022) and quality testing (Gu, Zhang, Wang, Wang, & Du, 2021). Combined with headspace solid-phase microextraction (HS-SPME) (Yu, He, Ding, & Chang, 2010), GC–MS is widely applied in the separation and identification of volatiles in food with good repeatability, sensitivity, and selectivity (Joshi and Gulati, 2015, Joshi et al., 2015, Lopez et al., 2015).
Gas chromatography–ion mobility spectrometry (GC-IMS), and can analyze the test results quickly, simply, intuitively, and accurately (Vautz, Franzke, Zampolli, Elmi, & Liedtke, 2018). It has been also used in food flavor analysis (Wang et al., 2020) and quality inspection (Gu et al., 2021). GC–IMS coupled with HS–SPME–GC–MS can monitor chemical changes esp. volatile compounds comprehensively and accurately (Xu et al., 2023).
Here, a flavor substances changes in Fuliang GT during storage were researched comprehensively by GC–IMS coupled with HS–SPME–GC–MS and analyzed using relative odor activity values (ROAV) for the first time. This study provides novel insights into the flavor-substance relation in Fuliang GT, which is helpful in guiding tea storage of GT.
2. Materials and methods
2.1. Reagents and materials
Fuliang GT was made by tea masters in the Yellow Mountain region (Fuliang, China) with over 30 years of tea making experience, in accordance with the traditional method of processing fresh leaves into tea (Xu et al., 2022), and stored at room temperature. The teas were collected for 3 days at 0 (GT-0), 3 (GT-3), 6 (GT-6), and 12 (GT-12) months and promptly stored at −20 °C pending further analysis.
2.2. Chemicals
l-Theanine (purity ≥ 98.5 %), glucose (purity ≥ 98 %), caffeine (purity ≥ 98 %), methanol, sodium carbonate, 1 mol forinol, potassium dihydrogen phosphate (KH2PO4), disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O), hydrated ninhydrin, stannous chloride (SnCl2·2H2O), anthrone concentrated sulfuric acid, basic lead acetate, and hydrochloric acid (all reagents and compounds were of analytical grade) were purchased from Nanchang Lusi Biotechnology Co., Ltd.
2.3. Conventional taste components analysis
The total free amino acids were quantified in accordance with GB/T 8314–2013 (National standard of China: Determination of total free amino acids in tea with theanine as the standard). For tea polyphenols, we referred to GB/T 8313–2018 (National standard of China: Determination of tea polyphenols and catechins in tea). In accordance with the anthrone-sulfuric acid method from the work of Guo et al., (Guo, Song, Ho, & Wan, 2018), glucose was used as the standard for total sugars. We referred to GB/T 8312–2013 (National standard of China: Determination of caffeine content) for the determination of caffeine content.
2.4. Free amino acid analysis
The preparation of tea sample was conducted following the work of Qu et al., (Qu et al., 2019) with slight modifications. A total of 0.6 g tea powder was placed in a conical flask and added with 85 mL boiling water. The tea powder was simmered in boiling water for 30 min and strained to a constant volume of 100 mL. A total of 10 mL tea liquid was added with 10 mL sulfosalicylic acid (4 %). Before the subsequent analysis, the liquid was centrifuged at 3000 rpm for 30 min, and the supernatant was filtered through a 0.22 µm nylon membrane.
The contents of free amino acids were determined using an automatic amino acid analyzer system (Sykam S-433D, Germany). Cation-exchange high-performance chromatography (LCA K07 column, 4.6 mm × 150 mm, Sykam, Germany, with a resin of 7 µm ion exchange) was applied to separate amino acids, and the column was followed by derivatization with ninhydrin. The loading volume was 50 µL, and the detector wavelengths set at 570 and 440 nm corresponded to channels 1 and 2, respectively. Free amino acids were determined through comparison of the retention time and reference amino acid spectrum. Quantitative analysis was carried out using an external standard curve.
2.5. GC-IMS analysis
The GC-IMS described by Yang et al., (Yang et al., 2021) was used to analyze Fuliang GT but with slight modifications. Specifically, a GT sample (1.0 g) was placed in the headspace sample bottle, and the bottle was incubated at 80 °C for 15 min. Then, the product was injected into a 500 µL headspace bottle using an injection syringe (splitless) and was heated to 85 °C. The MXT-5 capillary column (15 mm × 0.53 mm, 1 µm; 60 °C) was used as the chromatographic column to separate the volatile components, and it was coupled to IMS at 45 °C. The carrier gas (nitrogen, purity ≥ 99.99 %) was allowed to flow for 2 min at the flow rate of 2 mL/min. This rate was increased gradually to 100 mL/min within 18 min. The drift gas was nitrogen gas, which was allowed to flow at a rate of 150 mL/min. The retention index (RI) was calculated under the same chromatographic conditions for all volatiles. A C4–C9 n-ketone (Sinopharm Chemical, Beijing, China) was used as an external reference. The identification of volatile compounds was based on comparing RI and the drift time with the GC-IMS library.
Instrumental analysis from different angles was performed using various software, including Laboratory Analytical Viewer (LAV version 2.0.0, G.A.S., Dortmund, Germany), Gallery Plot, Reporter, and GC-IMS Library Search.
2.6. HS-SPME-GC–MS analysis
Each GT sample (1.0 g) was placed in 20 mL SPME vials, mixed with 10 mL ultrapure water at 80 °C, and equilibrated for 20 min. Then, the SPME fiber (2 cm) was inserted into the equilibrated sample vial and soaked in a constant-temperature water bath at 80 °C for 40 min. Then, the SPME fiber was placed at the GC separation-free injection port at 250 °C for 5 min for thermal desorption for further analysis.
The GC–MS conditions were modified based on the procedure by Zhang et al., (Zhang et al., 2021), and an Agilent GC–MS system (Model 7890B-7000D, Agilent, USA) with a capillary column (DB-35MS, Agilent, USA) was applied in the analysis of volatiles compounds. The temperature was initially held at 50 °C for 2 min, then increased to 90 °C at the rate of 4 °C/min, and held for 3 min, then to 150 °C at a rate of 3 °C/min and held for 5 min; finally to 250 °C at a rate of 5 °C/min heated and held for 5 min. The carrier gas was helium, which allowed to flow through at a flow rate of 1.0 mL/min through a separation-free GC inlet. Fragmentation was carried out in electronic impact mode (70 eV and 230 °C) and at 150 °C for quadrupole. Full-scan mode acquisition was performed at a large-scale acquisition range of 35–550 m/z.
The chromatograms were obtained and analyzed by determining the internal retention time, standard area, spectra, and base peaks, and each peak was detected with reference to the characteristic mass spectra of each compound listed by the National Institute of Standards and Technology. The retention time of each compound was calibrated using n-alkanes C7-C30 (Sigma-Aldrich, St. Louis, MO, USA) as external references.
The relative content of each VOC in the samples was quantified through area normalization. A compound was regarded to be present in the sample when its similarity index was higher than 80.
2.7. ROAV analysis
In this study, the obtained VOC concentrations were expressed as relative content (%); we determined the ROAV to calculate the specific contribution of compounds to the aroma of GT (Zhu, Chen, Chen, Chen, & Deng, 2020):
| (1) |
where Ci represents the content of an aroma compound in sample (%), Ti denotes the aroma threshold of a compound in water (µg/kg); Cmax and Tmax are the relative content and aroma threshold of the compound that contributed the most to the sample flavor, respectively. Evidently, the ROAV of each compound is ≤ 100, and the contribution of a component to the overall flavor of the sample is positively correlated with its ROAV. The components with ROAV ≥ 1 were regarded as crucial aroma compounds in the samples, and those with 0.1 ≤ ROAV < 1 had a great modifying effect on the aroma.
2.8. Statistical analysis
Microsoft Excel 2010 was used for data entry and sorting, and Origin 2018 and GraphPad Prism 8 software were used for mapping analysis.
3. Result and discussion
3.1. Taste components during storage of GT
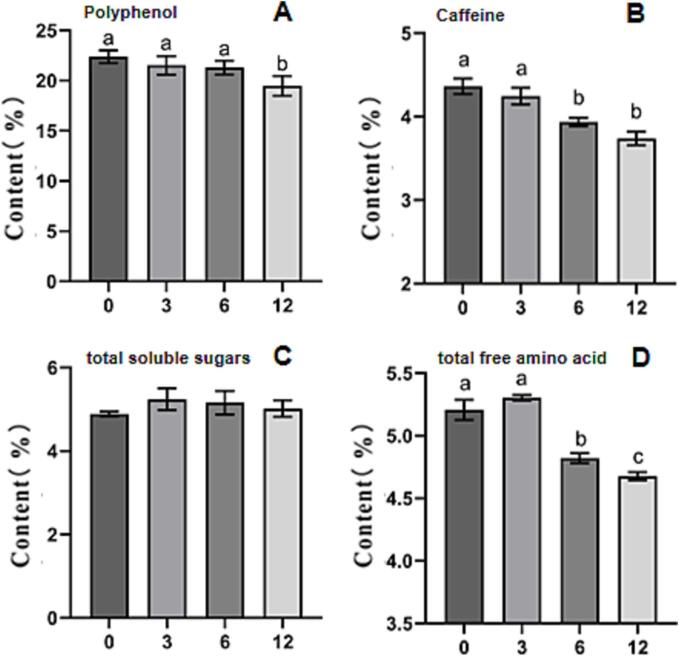
As show in Fig. 1A, the content of polyphenol gradually decreased during storage. In GT-12, the content decreases to 18.51 % from 22.38 % of GT-0, with a decrease of 18.10 %. Polyphenol is closely related to the formation of tea quality characteristics (Qu et al., 2019). During storage, it can be joined with caffeine by H-bonds, and polymerization and oxidation can occur between polyphenols (Sun et al., 2018). However, there was no significant change in polyphenols during the first six months.
Fig. 1.
Changes of polyphenol, caffeine, total soluble sugars and free amino acid content in green tea samples during storage (A) Polyphenol; (B) Caffeine; (C) Polysaccharide; (D) Total free amino acid. Columns label ‘a’,‘b’, and‘c’ indicated significant difference (p < 0.05) The X-axis (0, 3, 6, 12) represents the storage time (0, 3, 6, 12 months) of green tea.
Caffeine is one of the important taste substances in tea, and it contributes to the bitterness of tea broth (Guo et al., 2021). Its content in the GTs gradually decreased during storage. The caffeine content of GT-0 was 4.37 %, and it decreased to 3.74 % in GT-12 (Fig. 1B). The highest caffeine content can be observed in new tea, and therefore, in new teas, the bitterness of caffeine may dominate (Yu, Yeo, Low, & Zhou, 2014).
Sugars are the main flavor compound affecting the sweetness and freshness of tea broth (Yamaguchi & Ninomiya, 2000). During storage, the contents of total soluble sugars in GT increased first and then decreased gradually. The sugar content in GT-0 was 4.89 %; it increased to 7.16 % and then decreased to 5.02 %, which is slightly higher than that of GT-0 (Fig. 1C).
Free amino acids are flavoring substances that result in a fresh and brisk taste in GT (Qu et al., 2019). The contents of total free amino acids in GT fluctuated, that is, they exhibited a small increase followed by a large decrease, during storage (Fig. 1D). During storage, some soluble proteins were hydrolyzed and formed free amino acids, which caused the increase in free amino acid contents. Later, the amino acids were hydrolyzed, oxidized, and showed decreased amounts (Ning et al., 2016).
3.2. Free amino acids during storage of GT
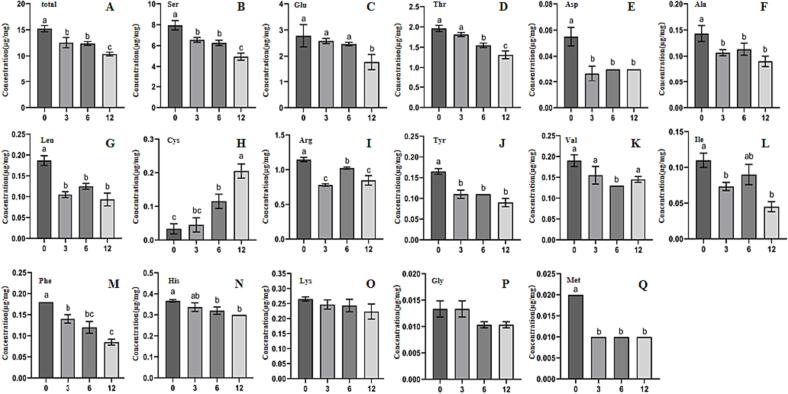
In addition to eight essential amino acids, sixteen free amino acids were identified and quantified (Fig. 2). Based on the content change, free amino acids can be classified into three groups during storage. The contents of the first group, including Ser, Glu, Thr, Tyr, Phe, His, Lys, and Gly, gradually decreased. The second group, which consisted of Asp, Ala, Leu, Arg, Val, and Ile, showed fluctuating content changes that eventually decreased. The contents of the third group, which contained Cys only, gradually increased. Some amino acids (Asp, Glu, etc.) that play important roles in taste and aroma were oxidized, which debased the tea quality (Kaneko, Kumazawa, Masuda, Henze, & Hofmann, 2006). Given its strong reducibility, the increase in the content of Cys indicates that oxidation may be not the only factor responsible for the deterioration of tea quality.
Fig. 2.
Amount changes of free amino acid in green tea samples during storage. (A) Total; (B) Ser (Serine); (C) Glu (Glutamine); (D) Thr (Threonine);(E)Asp (Asparagine); (F) Ala (Alanine); (G) Leu (Leucine); (H) Cys (Cysteine); (I) Arg (Arginine); (J) Tyr (Tyrosine); (K) Val (Valine); (L) Ile (Isoleucine); (M) Phe (Phenylalanine); (N) His (Histidine); (O) Lys (Lysine); (P) Gly (Glycine); (Q) Met (Methionine). Columns label ‘a’,‘b’, and‘c’indicated significant difference (p < 0.05) with each other The numbers under X-axis (0,3,6,12) represent the storage time (0, 3, 6, 12 months) of green tea.
3.3. GC-IMS topographic plots of volatile components
The VOCs of teas under different storage times were analyzed via GC-IMS, which takes the advantages of the high separation efficiency and fast response of GC and the high sensitivity of IMS (Vautz et al., 2018).
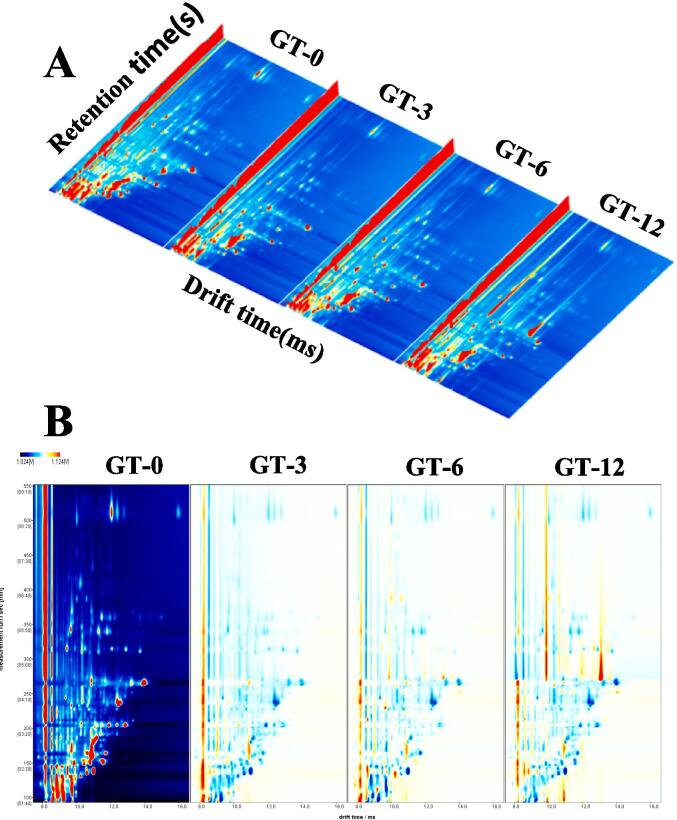
Fig. 3A shows the three-dimensional topo graphy of the volatile components of four samples. Although similarities were observed in the volatile components, some peaks were notably different. We obtained an overhead view to for the clear observation of the variations in volatile composition (Fig. 3B). GT-0 fresh tea was used as the reference, and the spectra of GT-3, GT-6, and GT-12 were obtained after subtracting the reference value. Blue and red colors indicate lower and higher amounts of volatile components in the sample than that in the reference, respectively. The presence of more blue spots suggested that volatile components were present in lower concentrations in the sample compared with the reference, and the spots in yellow indicated that of higher concentration. The result reveal that the contents of volatile components decreased first and then increased slowly during storage, and all of them showed decreased amounts compared with those in fresh GT.
Fig. 3.
GC-IMS topographic plots of tea samples: (A)3D-topographic plot and (B) differentiation plot of volatile compounds. GT-0(3, 6, 12) represents green tea stored for 0(3, 6, 12) months. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Variation of volatile compounds by GC-IMS
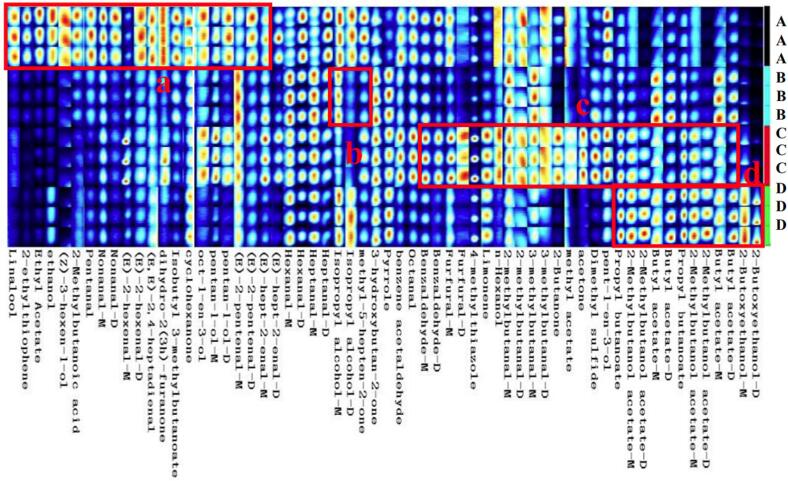
To show the variation in VOCs, we adopted the Gallery Plot analysis as a fingerprinting technology (Vautz et al., 2018). During storage, 65 volatile components were detected, and 56 known volatile components were identified qualitatively. The volatile components consisted of 24 aldehydes, 12 alcohols, 5 ketones, 9 esters, 1 alkene, 1 acid, and 4 others (Fig. 4 and Table S1). These VOCs all had C2–C13 carbon chains, and each single compound can generate multiple spots or signals (dimer or monomer forms) depending on the content and characteristics (Rodriguez-Maecker et al., 2017).
Fig. 4.
GC-IMS Topographic plots of gallery plots. A: GT-0; B: GT-3; C: GT-6; D: GT-12. GT-0(3, 6, 12) represents green tea stored for 0(3, 6, 12) months. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fingerprint analysis indicated that the levels of VOCs in the four GT samples were highly variable in the gallery pot area. As shown in Fig. 4, each VOC was placed in one column, and the samples were in a line. GT-0 contained more pentanal, nonanal, hexanal, heptanal, (E)-2-pentenal, (E)-2-hexenal, linalool, and ethyl acetate (box a), whose contents gradually decreased or disappeared during storage. GT-3 showed high levels of heptanal-M and hexanal-M (box b), and GT-6 exhibited high contents of benzene acetaldehyde, benzaldehyde, (E)-2-hexenal, n-hexanol, limonene, and dimethyl sulfide (box c). These VOCs presented a fluctuating trend that presented an increase followed by a decrease during storage. GT-12 had high concentrations of propyl butyrate, butyl acetate, acetic acid-2-methylbutyl, and 2-butoxyethanol (box d), which showed an increasing trend during storage.
During storage, the VOCs in tea samples decreased to the lowest level in GT-3 and then slowly increased. However, the amounts of VOCs were still slightly lower than those in GT-0 after 12 months of storage. The main changes in VOCs during the storage occurred on aldehydes, alcohols, and ketones, which were the main functional groups in the GT samples, accounting for over 72 % of the total VOCs. Ketones and aldehydes may play a crucial role in the unique aroma of tea (Li et al., 2019), which is consistent with our current findings.
Aldehydes, as the most abundant and diverse compounds detected, stabilized in early storage, and their contents decreased substantially in late storage. After 12 months, the content of GT-0 decreased by one third from 33.65 % to 22.73 %. Aldehydes may help in differentiating GT because they were present in low levels in GT-12 and high levels in samples GT-0, GT-3, and GT-6. The amounts of pentanal, nonanal, hexanal, heptanal, (E)-2-pentenal, and (E)-2- hexenal gradually decreased, and those of 2-methylbutanal, 3-methylbutanal, and (E)-hept-2-enal level increased first and then dropped during storage. Heptanal and nonanal were the most abundant aldehydes in all GT samples, and both have green flavor and can contribute to distinguishing the characteristic aromas of different GTs based on their content differences. Hexanal can be produced by oxidation of hexanol during storage. However, the contents of hexanol and hexanal both decreased in the present study.
Alcohols are generally thought to have a fruity and flowery flavor. During storage, these compounds showed a fluctuating trend of showing a small decrease followed by a large increase. After 12 months, the alcohol content of GT-0 nearly doubled from 18.99 % to 33.86 %. As the material with the largest increase, 2-butoxyethanol increased by a factor of 10 from 0.93 % to 3.29 % to 9.30 %–12.31 %. This compound may help to differentiate diverse GTs due to the variation in its levels. The content of linalool, the most important VOC in the aroma of GT decreased to about 0.23 % of the total aroma in GT-6, which was approximately 72 % lower than that of GT-0.
As the second most abundant category in VOCs, the content of ketones increased first and then decreased during storage. Their content reached a maximum in GT-6 (33.72 % of the VOCs) and then decreased again to 24.42 % in GT-12. However, this value was still higher compared with that in GT-0 (20.63 %). The content of, 3-hydroxy-2-butanone, as the most abundant ketone, increased substantially in the early stages of storage and then stabilized, and different kinds of GTs can be distinguished based on the changes in its content levels. The amount of 6-methyl-5-hepten-2-one, a compound found in many GTs, decreased before increasing and then decreasing, with a final decrease observed in GT-12.
3.5. Variation in volatile components according to GC–MS
The flavor differences in various GTs were revealed via VOC analysis through the combination of GC–MS and HS-SPME. Fig. S1A and S1B show the proportion and types of VOCs in each sample. All 64 volatile components were detected, with 37, 37, 44, and 41 volatile components identified in GT-0, GT-3, GT-6, and GT-12, respectively. Most of these organic molecules had carbon chains between C8 and C15 (Table S2). During storage, a variety of alcohols content were observed highest, followed by those of aldehydes, ketones, esters, and alkenes.
GC-IMS and GC–MS were used to quantify alcohols, ketones, and aldehydes sensitively, and the contents of VOCs in GT varied greatly during storage. Alcohols were the main aroma components of GT-0, and they accounted for more than 55 % of all VOCs. The amounts of aldehydes, esters, and ketones were close to each other and ranged from 6.78 % to 8.28 %. The contents of alkenes and alkanes were 4.83 % and 4.01 %, respectively. The variation trends of different VOCs varied. During storage, the amounts of alcohol and aldehydes gradually decreased, but those of alkenes gradually increased. In addition, the ketone levels initially dropped and then increased, and the ketone level was the highest in GT-0 and lowest in GT-6. The ester level increased first, dropped, and then increased again, and the highest content was found in GT-12.
The VOCs that gradually decreased or disappeared during storage comprised geraniol, linalool and its oxides, caffeine, phenylethanol, nonanal, dehydrolinalool, 3-hexenyl hexanoate, and heptadecanol. These substances mainly have clear, woody, floral, and fruity aromas, which contribute considerably to the aroma of tea. The substances detected only in GT-0 included 2-bromo-octadecanal, Z-8-methyl-9-tetradecenoic acid, E-13-octadecenoic acid, methoxy-phenyl-oxime, and [(octyloxy) methyl]-benzene. Methoxy-phenyl-oxime, which is reported to be found only in oolong tea, was found in the GT sample. However, to date, its aroma types have not been identified in the literature (Li et al., 2022).
The amount contents of alcohols gradually decreased, and the number of their kinds increased during storage. The alcohol content decreased from 55.24 % (GT-0) to 28.68 % (GT-12). Linalool and geraniol were the most abundant alcohols, and decreased the most in terms of contents. These compounds have clear and floral aromas, which contribute to the aroma of GT, and are the material basis of floral and fruity aroma of teas. However, linalool was oxidized, and its level decreased and disappeared during storage. The kinds of alcohols increased in number, with chrysanthemyl alcohol, 1-eicosanol, and α-terpineol detected in GT-6. Thus, alcohols may accumulate during storage and may serve as markers to distinguish fresh tea from stored tea.
The content of aldehydes, as another important aroma compounds in tea, gradually decreased during storage. In GT-12, the aldehyde content decreased to 2.26 % compared with that in GT-0 (7.59 %). Hexanal and nonanal were most abundant aldehydes found in all GTs, and their contents gradually decreased or disappeared during storage. The amount of (E, E)-2,4-nonadienal gradually increased. The contents of heptanal and citral first increased and then decreased or disappeared. The highest contents of both compounds were observed in GT-3. Notably, we identified diverse derivatives of hexanal and nonanal, such as 9-acetoxy nonanal and cis-9-hexadecenal, which were both found in GT-12.
Ketones are commonly believed to have tallow, burnt, and floral aromas that gradually enhance with the growth of carbon chain (De Girolamo, Lattanzio, Schena, Visconti, & Pascale, 2016). The VOCs with floral and fruity aromas, such as β-ionone, gradually increased in content during storage, and this result may be due to a fermentation process similar to the oxidation of black tea under storage conditions. Song Tingting (Song, 2010) showed that β-ionone gradually increased with the storage time of GT. We observed 2-dodecanone and 2-octanone, which can be produced by the enzymatic activity of microorganisms on lipids or amino acids, during storage (Wang et al., 2018).
Eleven esters were identified in the four samples, and they were dominated by linalyl acetate and methyl salicylate. Methyl salicylate, with the aroma of wintergreen oil and mint, is a vital VOC that contributes to the characteristic flavor of tea (Li et al., 2020). Alkenes were the second richest group of VOCs in the four GTs, and their content increased rapidly and then changed slightly during storage. d-Limonene had the highest content and the same changing trend as alkenes. Trans-calamenene was initially found in GT-3, and its content gradually increased. Dimethyl sulfide, as a substance in new tea, provides a new tea aroma. Its content gradually decreased until it disappeared during storage. On the other hand, 3-ethyl-1H-pyrrole and 2,5-dimethyl-pyrazine were both produced in the late storage period. Their nutty and roasted taste had a negative effect on the flavor of GT.
3.6. Clustering analysis of volatile components
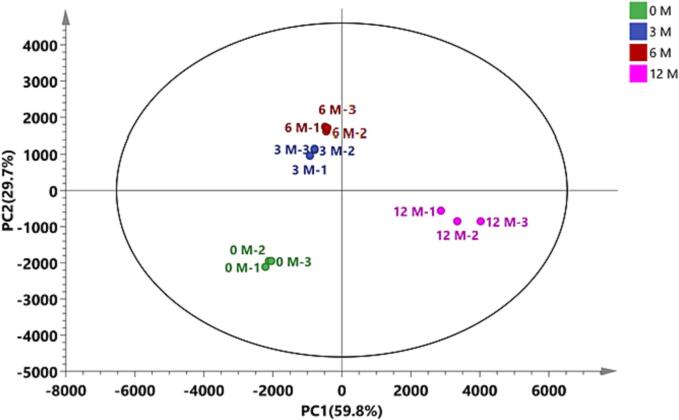
Principal component analysis (PCA) was applied to reveal the similarities and differences in the VOCs identified via GC-IMS among all the GT samples. Data on VOCs in samples were collected and classified on the basis of similar information (Xu, Song, Li, & Wan, 2012), and their clustering was analyzed using a specific software. Fig. 5 shows the results.
Fig. 5.
K-means clustering analysis of green tea during storage 0、3、6、12 M means that the storage time of tea is 0、3、6、12 months. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Differently colored dots represent the classification of volatile components in each tea sample with various storage durations. According to the distances observed among the dots, the VOCs in the stored samples changed greatly. The points representing GT-3 and GT-6 samples showed a close distance, which indicates that the VOCs of GT were stable in the period of 3–6 months. After 12 months of storage, more changes in the VOCs. Based on PCA results, the GTs with different storage times can be differentiated.
3.7. Analysis of key aroma substances
Large amounts of VOCs in food have been determined, but only a few, that is, key aroma compounds, contribute substantially to the food flavor (Zhu, Niu, & Xiao, 2021). ROAV was proposed to estimate the contributions of each compound to food flavor (Y F Zhu et al., 2020). In the four samples, 10 key VOCs (ROAV ≥ 0.1) were found (Table 1), and they were further discussed as two groups.
Table 1.
The ROAV values of main VOCs.
| No. | Volatile compounds | Odor description* | Threshold (μg/L) |
ROAV |
Trends | |||
|---|---|---|---|---|---|---|---|---|
| 0 M | 3 M | 6 M | 12 M | |||||
| 1 | Dimethyl sulfide | Fresh tea, fresh, slightly | 0.30 | 3.88 | 2.11 | 0.39 | <0.01 | ↓ |
| 2 | Hexanal | Grassy, green, fatty, fresh | 4.50 | 0.81 | 0.82 | 0.39 | <0.01 | |
| 3 | Heptanal | Green, grassy, oily | 2.80 | 0.21 | 0.55 | 0.32 | 0.29 | |
| 4 | (E,E)-2,4-Nonadienal | Fatty and green | 0.06 | <0.01 | 0.97 | 3.52 | 15.69 | ↑ |
| 5 | Nonanal | Floral, green, fatty, lemon-like | 1.10 | 3.37 | 1.81 | 1.16 | 0.79 | ↓ |
| 6 | Linalool | Floral, sweet, grape-like | 0.22 | 100.00 | 75.12 | 38.61 | 38.21 | ↓ |
| 7 | Geraniol | Rose-like, honey-like, sweet | 7.50 | 2.98 | 2.53 | 1.99 | 1.80 | ↓ |
| 8 | Jasmone | Flowery | 7.00 | 1.39 | 1.23 | 0.46 | 0.63 | |
| 9 | β-Ionone | Floral, woody, sweet, fruity | 8.40 | 0.11 | 0.21 | 0.32 | 0.39 | ↑ |
| 10 | d-Cadinene | dry wood, spicy, burnt | 1.50 | 1.90 | 1.95 | 2.20 | 2.36 | ↑ |
* Odor descriptions were from FEMA database. 0、3、6、12 M means that the storage time of tea is 0、3、6、12 months.
The first group included seven VOCs with ROAV ≥ 1 and are vital aroma compounds that affected sample flavor. As an important aroma substance in many excellent tea products, such as Jingshan tea (Flaig, Qi, Wei, Yang, & Schieberle, 2020) and Xihu Longjing tea (Zhu et al., 2016), linalool easily affects the aroma characteristics of tea. Linalool results from terpenes during drying (Yang et al., 2021). Dimethyl sulfide has the aroma of fresh tea, and geraniol, with its rose, sweet, honey aroma, is another important alcohol. Both of their ROAVs decreased during storage. Similar to (E,E)-2,4-nonadienal, it is responsible for fatty and green odors, found in unfresh tea and tea with sun-baked flavor for photic oxidation, and has a negative effect on the flavor of GT (Xu, Mo, Yan, & Zhu, 2007). Its ROAV was less than 0.01 in GT-0 and increased gradually during storage. d-Cadinene with dry wood, spicy, and burnt odors may have also an adverse effect on the aroma of GT, and its ROAV gradually increased during storage.
In the second group (0.1 ≤ ROAV < 1), the VOCs, including hexanal, heptanal, and β-ionone, exhibited a modification effect on the overall aroma of GTs. The ROAVs of hexanal and heptanal increased and then decreased during storage. A preview study suggested that the oxidation of the oleic acid precursors and palmitoleic can produce hexanal and heptanal, respectively, which contribute to grassy and green odors in tea infusions (Ho, Zheng, & Li, 2015). β-Ionone has various odors, including floral, woody, sweet, and fruity, and its ROAV increased gradually during storage.
In the study, we observed that the content of a strong reductant, cysteamine, increased continuously, and the contents of linalool and its oxide decreased during storage. In addition, a number of VOCs showed a fluctuating change during storage. These results indicate that oxidation is not a linear process in storage. Other processes may affect the quality of GT during storage, regardless of being accompanied with oxidation or not.
4. Conclusion
The nonvolatile and volatile compositions of GT were quantified and analyzed after 12 months of storage. During storage, most compounds, especially VOCs, were oxidized, but some, such as Cys, were not. These findings indicate that oxidation is not the only factor responsible for the deterioration of tea quality. As observed in HS-SPME-GC–MS and GC-IMS, the changes in substances can be quantified to reflect the storage duration, and the samples with different storage times can be identified through PCA. With the use of ROAV, the key aroma substances in Fuliang GT were determined. Focusing on the main taste substances and important VOCs would help in improving the storage of fresh GT.
CRediT authorship contribution statement
Jiyuan Xu: Writing – original draft, Formal analysis, Data curation. Ying Zhang: Writing – original draft, Visualization, Validation, Methodology, Data curation. Changbao Hu: Writing – review & editing, Visualization, Validation, Data curation. Bo Yu: Writing – review & editing, Resources, Methodology, Investigation. Cuixiang Wan: Validation, Methodology, Data curation, Conceptualization. Bin Chen: Resources, Data curation. Lirong Lu: Validation, Resources, Data curation. Liren Yuan: Resources, Conceptualization. Zhihua Wu: . Hongbing Chen: Supervision, Resources, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangxi Province [No. 20212ACB205013]; and Research Program of State Key Laboratory of Food Science and Technology, Nanchang University [SKLF-ZZB-202130].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.101047.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Dai W., Lou N., Xie D., Hu Z., Song H., Lu M.…Lin Z. N-ethyl-2-pyrrolidinone-substituted flavan-3-ols with anti-inflammatory activity in lipopolysaccharide-stimulated macrophages are storage-related marker compounds for green tea. Journal of Agricultural and Food Chemistry. 2020;68(43):12164–12172. doi: 10.1021/acs.jafc.0c03952. [DOI] [PubMed] [Google Scholar]
- De Girolamo A., Lattanzio V., Schena R., Visconti A., Pascale M. Effect of alkaline cooking of maize on the content of fumonisins B1 and B2 and their hydrolysed forms. Food Chemistry. 2016;192:1083–1089. doi: 10.1016/j.foodchem.2015.07.059. [DOI] [PubMed] [Google Scholar]
- Feng Z., Li Y.F., Li M., Wang Y.J., Zhang L., Wan X.C. Tea aroma formation from six model manufacturing processes. Food Chemistry. 2019;285:347–354. doi: 10.1016/j.foodchem.2019.01.174. [DOI] [PubMed] [Google Scholar]
- Flaig M., Qi S., Wei G.D., Yang X.G., Schieberle P. Characterization of the key odorants in a high-grade Chinese green tea beverage (Camellia sinensis; Jingshan cha) by means of the sensomics approach and elucidation of odorant changes in tea leaves caused by the tea manufacturing process. Journal of Agricultural and Food Chemistry. 2020;68(18):5168–5179. doi: 10.1021/acs.jafc.0c01300. [DOI] [PubMed] [Google Scholar]
- Gu S., Zhang J., Wang J., Wang X.Y., Du D.D. Recent development of HS-GC-IMS technology in rapid and non-destructive detection of quality and contamination in agri-food products. TrAC Trends in Analytical Chemistry. 2021;116435 doi: 10.1016/j.trac.2021.116435. [DOI] [Google Scholar]
- Guo X.Y., Ho C.T., Schwab W., Wan X.C. Effect of the roasting degree on flavor quality of large-leaf yellow tea. Food Chemistry. 2021;347 doi: 10.1016/j.foodchem.2021.129016. [DOI] [PubMed] [Google Scholar]
- Guo X., Song C., Ho C.T., Wan X. Contribution of l-theanine to the formation of 2,5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chemistry. 2018;263:18–28. doi: 10.1016/j.foodchem.2018.04.117. [DOI] [PubMed] [Google Scholar]
- Ho C.T., Zheng X., Li S.M. Tea aroma formation. Food science and human wellness. 2015;4(1):9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- Joshi R., Gulati A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chemistry. 2015;167:290–298. doi: 10.1016/j.foodchem.2014.06.112. [DOI] [PubMed] [Google Scholar]
- Joshi R., Rana A., Gulati A. Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chemistry. 2015;176:357–366. doi: 10.1016/j.foodchem.2014.12.067. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Kumazawa K., Masuda H., Henze A., Hofmann T. Molecular and sensory studies on the umami taste of Japanese green tea. Journal of Agricultural and Food Chemistry. 2006;54(7):2688–2694. doi: 10.1021/jf0525232. [DOI] [PubMed] [Google Scholar]
- Li Q., Li Y.D., Luo Y., Xiao L.Z., Wang K.B., Huang J.N., Liu Z.H. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. Lwt-Food Science And Technology. 2020;127 doi: 10.1016/J.Lwt.2020.109355. [DOI] [Google Scholar]
- Li Y.C., Ran W., He C., Zhou J.T., Chen Y.Q., Yu Z., Ni D.J. Effects of different tea tree varieties on the color, aroma, and taste of Chinese Enshi green tea. Food Chemistry: X. 2022;14 doi: 10.1016/J.Fochx.2022.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.Y., Xiao Y., Zhong K., Bai J.R., Wu Y.P., Zhang J.Q., Gao H. Characteristics and chemical compositions of Pingwu Fuzhuan brick-tea, a distinctive post-fermentation tea in Sichuan province of China. International Journal Of Food Properties. 2019;22(1):878–889. doi: 10.1080/10942912.2019.1614951. [DOI] [Google Scholar]
- Liu Y., Ge L., Wang Y.X. Principal component analysis of volatile compounds in different grades of Lu Mountain Clouds-Mist tea from three regions. Food Science. 2018;39(10):206–214. doi: 10.7506/spkx1002-6630-201810032. [DOI] [Google Scholar]
- Lopez P., van Sisseren M., De Marco S., Jekel A., Nijs M., Mol H.G. A straightforward method to determine flavouring substances in food by GC–MS. Food Chemistry. 2015;174:407–416. doi: 10.1016/j.foodchem.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Ning J.M., Ding D., Song Y.S., Zhang Z.Z., Luo X., Wan X.C. Chemical constituents analysis of white tea of different qualities and different storage times. European Food Research And Technology. 2016;242(12):2093–2104. doi: 10.1007/s00217-016-2706-0. [DOI] [Google Scholar]
- Qu F.F., Zhu X.J., Ai Z.Y., Ai Y.J., Qiu F.F., Ni D.J. Effect of different drying methods on the sensory quality and chemical components of black tea. Lwt-Food Science And Technology. 2019;99:112–118. doi: 10.1016/j.lwt.2018.09.036. [DOI] [Google Scholar]
- Rodriguez-Maecker R., Vyhmeister E., Meisen S., Rosales Martinez A., Kuklya A., Telgheder U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Analytical And Bioanalytical Chemistry. 2017;409(28):6595–6603. doi: 10.1007/s00216-017-0613-2. [DOI] [PubMed] [Google Scholar]
- Rong Y., Xie J., Yuan H., Wang L., Liu F., Deng Y.…Yang Y. Characterization of volatile metabolites in Pu-erh teas with different storage years by combining GC-E-Nose, GC–MS, and GC-IMS. Food Chemistry: X. 2023;30(18) doi: 10.1016/j.fochx.2023.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T. Zhejiang University, Hangzhou, China; Thesis of Mater degree: 2010. The study on the change of green tes's quality during storage. [Google Scholar]
- Sun B., Wang W., He Z., Zhang M., Kong F., Sain M., Ni Y. Improvement of Stability of Tea Polyphenols: A Review. Current Pharmaceutical Design. 2018;24(29):3410–3423. doi: 10.2174/1381612824666180810160321. [DOI] [PubMed] [Google Scholar]
- Vautz W., Franzke J., Zampolli S., Elmi I., Liedtke S. On the potential of ion mobility spectrometry coupled to GC pre-separation–A tutorial. Analytica chimica acta. 2018;1024:52–64. doi: 10.1016/j.aca.2018.02.052. [DOI] [PubMed] [Google Scholar]
- Wang S., Chen H., Sun B. Recent progress in food flavor analysis using gas chromatography–ion mobility spectrometry (GC–IMS) Food Chemistry. 2020;315 doi: 10.1016/j.foodchem.2019.126158. [DOI] [PubMed] [Google Scholar]
- Wang H.J., Ouyang W., Yu Y.Y., Wang J.J., Yuan H.B., Hua J.J., Jiang Y.W. Analysis of non-volatile and volatile metabolites reveals the influence of second-drying heat transfer methods on green tea quality. Food Chemistry: X. 2022;14 doi: 10.1016/J.Fochx.2022.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Zhi P., Thompson H.J., Ling T.J., Ho C.T., Li D.X., Wan X.C. Impact of six typical processing methods on the chemical composition of tea leaves using a single Camellia sinensis cultivar, Longjing 43. Journal of Agricultural and Food Chemistry. 2018;67(19):5423–5436. doi: 10.1021/acs.jafc.8b05140. [DOI] [PubMed] [Google Scholar]
- Xu, C., & Wang, Y. (2020). Discrimination of three famous teas in Jiangxi using gas chromatography-mass spectrometry combined with chemometrics. Food Science, 41(20), 141-150. https://doi.org/1002-6630-20190925-309.
- Xu J., Chen B., Lu L.R., Yuan L.R., Li Z.Q., Wu Z.H., Chen H.B. Quality and chemical composition changes of black tea and green tea processed from Fuliang Castanopsis sinensis summer tea. Journal of Food Safety and Quality. 2022;13(5):1604–1610. doi: 10.19812/j.cnki.jfsq11-5956/ts.2022.05.038. [DOI] [Google Scholar]
- Xu X., Mo H.Z., Yan M.C., Zhu Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick-tea by gas chromatography/mass spectrophotometry. Journal of the Science of Food and Agriculture. 2007;87(8):1502–1504. doi: 10.1002/jsfa.2874. [DOI] [Google Scholar]
- Xu W., Song Q., Li D., Wan X. Discrimination of the production season of Chinese green tea by chemical analysis in combination with supervised pattern recognition. Journal of Agricultural and Food Chemistry. 2012;60(28):7064–7070. doi: 10.1021/jf301340z. [DOI] [PubMed] [Google Scholar]
- Xu J.Y., Zhang Y., Yan F., Tang Y., Yu B., Chen B.…Chen H.B. Monitoring Changes in the volatile compounds of tea made from summer tea leaves by GC-IMS and HS-SPME-GC-MS. Foods. 2023;12(1) doi: 10.3390/foods12010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Zhao Y., Lv Y., Bouphun T., Jia W., Liao S.…Zou Y. Variations in microbial diversity and chemical components of raw dark tea under different relative humidity storage conditions. Food Chemistry: X. 2023;30(19) doi: 10.1016/j.fochx.2023.100863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Ninomiya K. Umami and food palatability. Journal of Nutrition. 2000;130(4S Suppl):921S–926S. doi: 10.1093/jn/130.4.921S. [DOI] [PubMed] [Google Scholar]
- Yang Z., Baldermann S., Watanabe N. Recent studies of the volatile compounds in tea. Food Research International. 2013;53(2):585–599. doi: 10.1016/j.foodres.2013.02.011. [DOI] [Google Scholar]
- Yang Y., Chen J.Y., Jiang Y.W., Qian M.C., Deng Y.L., Xie J.L.…Yuan H.B. Aroma dynamic characteristics during the drying process of green tea by gas phase electronic nose and gas chromatography-ion mobility spectrometry. Lwt. 2021;154 doi: 10.1016/j.lwt.2021.112691. [DOI] [Google Scholar]
- Yang C.S., Wang H., Sheridan Z.P. Studies on prevention of obesity, metabolic syndrome, diabetes, cardiovascular diseases and cancer by tea. Journal of Food and Drug Analysis. 2018;26(1):1–13. doi: 10.1016/j.jfda.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.H., He X.X., Ding Y., Chang H.S. Research progress on solid phase micro-extraction determination of volatile components in foods. Cereals & Oils. 2010;7 doi: 10.3390/polym12020292. [DOI] [Google Scholar]
- Yu P.G., Yeo A.S.L., Low M.Y., Zhou W.B. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chemistry. 2014;155:9–16. doi: 10.1016/j.foodchem.2014.01.046. [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhang C., Zhuang H.N., Liu Y., Feng T., Nie B., Lucarini M. Characterization of volatile component changes in peas under different treatments by GC-IMS and GC-MS. Journal of Food Quality. 2021;2021:1–13. doi: 10.1155/2021/6533083. [DOI] [Google Scholar]
- Zhou F., Li Y.L., Zhang X., Wang K.B., Huang J.A., Liu Z.H., Zhu M.Z. Polyphenols from Fu brick tea reduce obesity via modulation of gut microbiota and gut microbiota-related intestinal oxidative stress and barrier function. Journal of Agricultural and Food Chemistry. 2021;69(48):14530–14543. doi: 10.1021/acs.jafc.1c04553. [DOI] [PubMed] [Google Scholar]
- Zhu Y.F., Chen J., Chen X.J., Chen D.Z., Deng S.G. Use of relative odor activity value (ROAV) to link aroma profiles to volatile compounds: Application to fresh and dried eel (Muraenesox cinereus) International Journal Of Food Properties. 2020;23(1):2257–2270. doi: 10.1080/10942912.2020.1856133. [DOI] [Google Scholar]
- Zhu Y., Lv H.P., Dai W.D., Guo L., Tan J.F., Zhang Y.…Lin Z. Separation of aroma components in Xihu Longjing tea using simultaneous distillation extraction with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Separation and Purification Technology. 2016;164:146–154. doi: 10.1016/j.seppur.2016.03.028. [DOI] [Google Scholar]
- Zhu J., Niu Y., Xiao Z. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC x GC-qMS) Food Chemistry. 2021;339 doi: 10.1016/j.foodchem.2020.128136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.