Abstract
The use of post-consumer recycled (PCR) polymers in food contact materials (FCMs) can facilitate achieving a circular economy by reducing environmental waste and landfill accumulation. This study aimed to identify potentially harmful substances, including non-intentionally added substances (NIAS) and unapproved intentionally added substances (IAS), in polyolefin samples from material recovery facilities using gas-chromatography mass-spectrometry. Selected phthalates and bisphenols were quantified by targeted gas-chromatography tandem mass-spectrometry. The analysis detected 9 compounds in virgin polymers and 52 different compounds including alcohols, hydrocarbons, phenols in virgin and hydrocarbons, aromatic, phthalates, organic acids, per- and polyfluoroalkyl substances (PFAS) in PCR polymers. The Cramer classification system was used to assesses the Threshold of Toxicological Concern associated with the detected compounds. The PCR sample showed a slightly higher proportion of Cramer Class III compounds (48.08 %) than the virgin sample (44.44 %), indicating higher toxicity potential. Quantification detected bisphenols only in PCR material including BPA (2.88 ± 0.53 μg/g), BPS (5.12 ± 0.003 μg/g), BPF (3.42 ± 0.01 μg/g), and BADGE (4.638 μg/g). Phthalate concentrations were higher in PCR than virgin samples, with the highest levels detected as DIDP, at 6.18 ± 0.31 μg/g for PCR and 6.04 ± 0.02 for virgin. This study provides critical understanding of the safety and potential risks associated with using PCR polyolefins from different sources in food contact applications.
Keywords: Food contact materials (FCMs), (non) intentionally added substances, Endocrine modulating chemicals, Cramer classification, Post-consumer plastic recycling
1. Introduction
Plastic is undeniable in almost all areas of our life. The demand for plastics is ever growing because of their low density, versatility, and durable properties. In 2018, the global plastic production was estimated to be 359 million tons. At the same time, the United States generated 35.7 million tons of plastics, which accounted for approximately 12.2 % of the total Municipal Solid Waste (MSW) generated in the country [1,2]. World plastic production in 2020 was estimated at 367 million tons, of which 55 million tons were produced in European Union (EU) [3]. Packaging is one of the most important sectors for their application at almost 40 % of the total plastic demand. According to the Environmental Protection Agency (EPA) 14.5 million tons of plastic container and packaging were produced in 2018 in USA [4].
Polyolefins (polypropylene, polyethylene: high-density polyethylene, low-density polyethylene, linear low-density polyethylene, medium density polyethylene) materials have great importance in food packaging industry because of its mechanical strength and good thermal stability. The properties of high melting point, good heat seal ability, and low price has made polypropylene (PP) a popular food packaging material. For example, PP is used in cold-chain, heat-treated, microwaveable food products [5]. High-density polyethylene (HDPE) and low-density polyethylene (LDPE) have high demand in the packaging sector with varying applications such as rigid packaging and flexible film production, respectively. Apart from their advantageous applications, it is hard to deny that plastic packaging is an enormous source of waste generation and responsible for 70 % of the total plastics municipal solid waste (MSW) stream [6]. In 2018, 14.53 million tons of containers and packaging were manufactured in the USA, among which 1.98 million tons was recycled and 10.09 million tons were dumped into Landfill [4]. Since plastic waste is ever increasing, it becomes crucial to ensure their recyclability and reduce the amount that enters landfills daily. In 2020, the EPA announced the ‘U.S. first National Recycling Goal’ to increase the U.S. recycling rate 50 % by 2030 [7,8].
Post-consumer recycled (PCR) plastics have several applications ranging from food packaging to industrial products. However, PCR usage as raw material in direct food packaging/food contact materials (FCMs) has some concerns regarding chemical safety. For example, PCR collected from households (curbside) can be highly contaminated [9], and may have odor, discoloration problems. The contaminants may come from mishandling during domestic use (e.g., exposure to heat, sun, and environment), additives and their degradation products, printing inks, coatings materials, organic waste, etc. [10]. Determining decontamination levels in PCR during the sortation at the material recovery facilities (MRF) is challenging and is the main cause of the downcycling to lower grade products: e.g., construction, pipe, car parts etc. [9]. Therefore, identifying potential migrants and their Threshold of Toxicological Concern from different polyolefin sources is critical to understand the potential to use each source as FCMs.
These contaminants are often the source of the non-intentionally added substances (NIAS), intentionally added substances (IAS), or restricted substances (RS) in FCMs. NIAS are not added into the polymer to impart technical benefits. They are often byproducts from the degradation process, manufacturing process, or sorbed during domestic uses from consumer abuse [11]. According to commission regulation (EU) No October 2011 [12], compounds with molecular weights <1000 Da are considered NIAS and substances >1000 Da are exempt from NIAS consideration due to the assumption of low migration potential (Geueke, 2018). IAS are part of the raw material in packaging production which imparts and intended function such as facilitating manufacturing or altering mechanical properties. Examples of IAS are antioxidants, lubricants, surfactants, UV stabilizers, etc. [13]. A restricted substance refers to a substance or chemical that is regulated by a government or regulatory agency, and whose use or presence is limited or prohibited in certain products, materials, or applications due to concerns about its potential risks to human health [14]. It is important to identify and quantify these components before the authorization of PCR as FCM as these substances have potential to migrate into the food matrix and cause potential health risk [15].
New advanced technologies are arising that are effective in removing contaminants from PCR materials e.g., de-inking of plastic films using surfactants or selective dissolution [16], treatments with supercritical carbon dioxide, hot air, etc. [6], which may increase the utilization potential of PCR materials. The EU's Single-Use Plastics Directive mandates that plastic bottles made of polyethylene terephthalate (PET) contain a minimum percentage of recycled plastic [17]. Washington State's plastics law requires producers to use a minimum amount of PCR plastic in their plastic product. Beginning January 1, 2023, plastic trash bags must contain at least 10 % recycled content and adhere to labeling requirements. Additionally, plastic beverage bottles (excluding dairy and 187 mL wine bottles) must use a minimum of 15 % recycled content [18]. CFR Title 21, Part 177 sets specifications for food contact plastics, including requirements for migration testing of substances from the plastic material to food or food simulants.
The Food And Drug Administration (FDA) provides guidance to industry through its publication titled "Guidance for Industry: Use of Recycled Plastics in Food Packaging (Chemistry Considerations)", which outlines considerations for the safe use of recycled plastics in food packaging, including the need for appropriate testing to ensure compliance with regulations [19,20]. If the industries are confident to use PCR as FCM without any obligation, they can seek a ‘Letter of No Objection’ [21] stating that the FDA has reviewed the recycling process and has no objection to materials recycled with the process be in direct food contact in certain conditions. However, it is necessary to have broad knowledge about the composition of contaminants in PCR material to be used in further production. The presence of NIAS, IAS, and RS in PCR polyolefins needs additional understanding prior to widespread use as FCMs. The scientific community has been struggling to develop new methods and resources, such as advanced analytical techniques and databases, to aid in the identification and evaluation of NIAS in FCMs [22].
A common analytical approach for NIAS, IAS detection in food packaging material is Gas Chromatography-Mass Spectrometry (GC-MS) due to the high reproducibility, accuracy and extensive spectral libraries to support identification [14,23]. Extraction of the sample is the prerequisite of any analyte detection by GC-MS and ideally analyte concentration for lower detection limits. GC-MS analysis requires extensive manual analysis of the acquired data to identify the unknown substances from the total ion chromatogram [24] with the NIST mass spectral library [25].
The present study aims to extract and identify NIAS, IAS, and RS in Virgin and PCR packaging materials from different sources understand the chemical safety of recycled polyolefins for use as FCM. Here, we aim to analyze the samples by solvent extraction technique followed by gas chromatography, mass spectrometry (GC/MS), (GC–QqQ- GC-MS/MS), and unknown detection by NIST Mass Spectral Library.
2. Materials and methods
A total of 29 different polyolefin samples (6 virgin, 23 P CR) collected from commercial suppliers and material recovery facilities listed in Table 1. Di-ethyl phthalate (DEP), di-isobutyl phthalate (DIBP), di-butyl phthalate (DBP), Dipentyl phthalate (DPENP), dihexyl phthalate (DHEXP), di-cyclohexyl phthalate (DCHP), di-(2-ethylhexyl) phthalate (DEHP), Diisononyl phthalate (DINP), diisodecyl phthalate (DIDP), Bisphenol-F (BPF), Bisphenol-A (BPA), Bisphenol-B (BPB), Bisphenol-S (BPS), Bisphenol A diglycidyl ether (BADGE) and benzophenone standards were purchased from Sigma-Aldrich (St. Louis, MO, USA), with purity higher than 99 %. Acetone (Fisher Scientific Inc., Fair Lawn, NJ, USA), xylene (Fisher Scientific, HanoverPark, IL, USA) were HPLC grade. The water used was purified using a Milli-Q gradient A10 system (Billerica, MA, USA).
Table 1.
Sample identification.
| Sample No. | Sample code and detail |
|---|---|
| 1 (Virgin) | LDPE (Low density polyethylene) |
| 2 (Virgin) | HDPE (High density polyethylene) |
| 3 (Virgin) | LLDPE (Linear low-density polyethylene) |
| 4 (Virgin) | HDPE (High density polyethylene) |
| 5 (Virgin) | MDPE (Medium-density polyethylene) |
| 6 (Virgin) | LLDPE (Linear low-density polyethylene) |
| 7 (PCR) | – |
| 8 (PCR) | LDPE (Low-density polyethylene)-Film |
| 9 (PCR) | CDPE (Carbon doped polyethylene)-Washed |
| 10 (PCR) | PE (Polyethylene) |
| 11 (PCR) | PPR HDPE (Polypropylene Random Copolymer, High density polyethylene) |
| 12 (PCR) | PE (Polyethylene) |
| 13 (PCR) | LLDPE (Linear low-density polyethylene) |
| 14 (PCR) | PPR PP (Polypropylene Random Copolymer)-Clean |
| 15 (PCR) | PP (Polypropylene)-Unwashed |
| 16 (PCR) | PP (Polypropylene)-Unwashed |
| 17 (PCR) | PE/PP (Polyethylene/Polypropylene) |
| 18 (PCR) | LDPE (Low-density polyethylene)-Film |
| 19 (PCR) | PE (Polyethylene) |
| 20 (PCR) | LLDPE (Linear low-density polyethylene) |
| 21 (PCR) | LLDPE (Linear low-density polyethylene) |
| 22 (PCR) | – |
| 23 (PCR) | PE/PP (Polyethylene/Polypropylene) |
| 24 (PCR) | PE (Polyethylene) |
| 25 (PCR) | PP (Polypropylene) |
| 26 (PCR) | LDPE (Low-density polyethylene) |
| 27 (PCR) | LDPE (Low-density polyethylene) |
| 28 (PCR) | PE (Polyethylene) |
| 29 (PCR) | LLDPE (Linear low-density polyethylene) |
2.1. Sample extraction and preparation
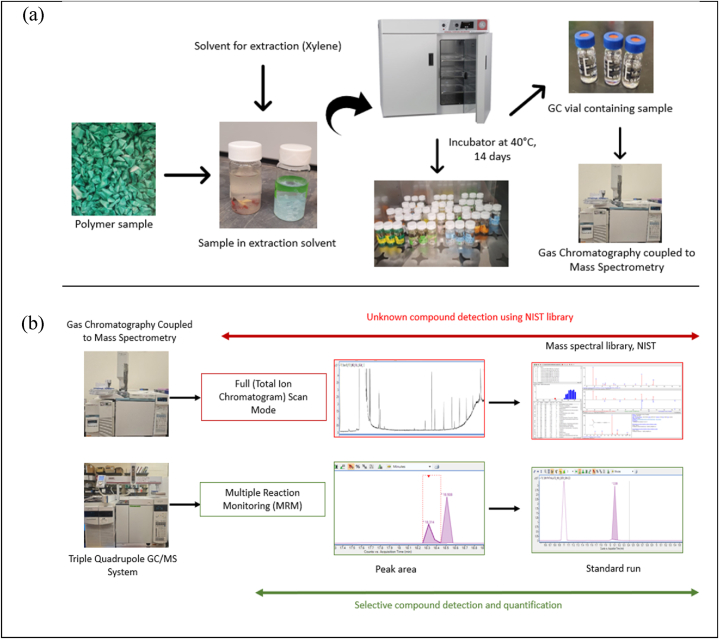
All the materials (spatula, scissors, glass materials) in direct contact with the sample were washed with detergent (Alconox, Inc, New York, USA), deionized water and acetone (HPLC grade) then dried in the oven at 150 °C and covered with aluminum foil until use to avoid cross-contamination. The use of plastic materials for extraction and material handling was strictly avoided. The samples were shredded into small pieces (2–5 mm). 2.0 g of each sample were weighed using a Mettler Toledo Model AB104 scale into individual 20 mL glass scintillation vials with foil liners. 15 mL of ACS grade xylene ≥98 % was added as extraction solvent to each scintillation vial containing the pre-weighted sample (Fig. 1 (a)). All the vials were placed into the VWR® General Purpose Digital Incubators, USA, at 40 °C for 14 days. According to the FDA guidance document, the food contact notification (FCN) test is conducted to perform migration studies in food contact substance (FCS) for room temperature application at 40 °C for 10 days [20]. The authors used xylene instead of the traditional food simulants at 40 °C for 14 days to explore more extreme conditions to ensure the extraction of the highest substances out of the PCR polymer as a worst-case scenario. Xylene is a strong solvent for polyolefins and will swell the polymer to increase extraction of additives and contaminants in the PCR plastic [26]. This work was completed per the guidance of our industrial advisory board (Polymer and Food Protection Consortium [27]) and the request of industry professionals in order to understand the potential presence of a variety of chemicals in the PCR materials.
Fig. 1.
Experimental methodology: (a) Sample extraction procedure (b) detection and quantification of compounds in polyolefin plastics.
After the extraction was completed, the solvent was filtered through a 0.22-μm, Nylon membrane filter (China, Thermo scientific) and analyzed by gas chromatographic mass spectrometry. To avoid cross-contamination, the glass syringe was cleaned using extraction solvent five times before proceeding to the next sample. The process blank contained only extraction solvent to determine any contribution of the process and filter to measurable chemicals. Samples were extracted in three repeated measures. The developed analytical strategy included solvent extraction of polymer, GC-MS analysis, data processing, and unknown compound identification, is illustrated in Fig. 1(b).
2.2. Standard run
All standards were prepared in xylene. Stock solutions of standards as cocktail at a concentration of 100 mg/L were prepared and stored at 4 °C. The standards solution for calibration curve was prepared by serial dilution with a concentration range of 0.04–25 mg/L from stock solution (Appendix II). Benzophenone at 10 mg/L concentration was added as internal standard. The survey scans confirmed that any residual benzophenone in the extractions did not interfere with the internal standard. After preparing the calibration curve and adding the internal standard to the sample, the area of the target analyte is divided by the area of the internal standard. BSTFA (N,O-bis (trimethylsilyl)trifluoroacetamide) was used as the derivatization agent for bisphenols. 50 μL of BSTFA and 100 μL of acetone to accelerate the reaction rate were added to each sample containing GC-vials at exact volume. One microliter of the standards and samples were injected into the chromatography system and analyzed by Agilent MassHunter Qualitative software.
2.3. Experimental design and analysis
2.3.1. Unknown compound qualitative analysis
A total of 90 samples including blanks were analyzed via three repeated measures using the same method. Aliquots of pure solvent were subjected to the same extraction protocol as the samples and analyzed by GC-MSD for unknown compound identification analysis as process blank. Volatile organic compounds (VOCs) in GC vials were qualified by using a full scan mode with splitless detection in HP 6890 series GC system equipped with an auto-sampler and HP Agilent 5973 mass selective detector. Each aliquot was injected onto a 30-m-long fused silica capillary column (i.d. DB-5 ms - 250 μm × 0.25 μm film thickness), Agilent Technologies, Santa Clara, CA, USA. The GC oven temperature was programmed at: 40 °C for 5 min, then increased by 7 °C/min up to 220 °C, then increased to 300 °C with a hold time of 3 min. The total run time for each analysis was 45 min. The carrier gas was helium at 13 mL/min. The mass spectra were obtained with a mass selective detector under electron impact ionization at a voltage of 70 eV and a transfer line temperature of 300 °C. The MS was operated in full scan mode scanning over 40–500 m/z. Data were collected and evaluated by Agilent MassHunter Qualitative software. Compound identification was carried out by comparison of the measured spectrum with known mass spectral library (National Institute of Standards and Technology (NIST) mass spectral library version 2021). A threshold of reverse fit >600 and forward fit >600 from the NIST library was utilized for the identification of unknowns. To interpret the similarity values, NIST has established guidelines for determining the quality of the match. A value of 900 or greater is considered an excellent match, a value of 800–900 is a good match, and a value of 700–800 is a fair match and value less than 600 is considered a poor match [28].
2.3.2. Analyte quantification analysis
Quantification of the phthalates and bisphenols were performed using a gas chromatograph combined with a tandem mass spectrometer and an auto-sampler (GC–QqQ- GC-MS/MS; Agilent 7000, Triple Quad, GC 7890A) with an Agilent DB-5MS column (30 m × 250 μm x 0.25 μm). Helium (99.999 %) was used as quench gas at 2.25 mL/min and N2 as collision gas at 1.5 mL/min. The injection mode was pulsing splitless. GC oven temperature was initially set at 40 °C raising to 120 °C hold for 1 min, then 300 °C at 7.5 °C/min. The transfer line temperature was 300 °C, Source heater for both MS1 and MS2 was 230 °C. Mass detector was operated in the electronic ionization mode (EI) with the ionization energy of 70 eV. Multiple reaction monitoring (MRM) mode was selected to enhance analytical sensitivity and selectivity (Table 2 for detail). The solvent delay time was 3 min. To identify the presence of the 9 phthalates and 5 bisphenols the stock standards in cocktail at 100 μg/mL was prepared and run in GC-QqQ to confirm their retention time and precursor ion. The standards for calibration curve and the samples were analyzed with the same method mentioned above.
Table 2.
Transition detail in MRM method in GC-QqQ.
| Analyte name | CAS | Precursor ion | Product ion | Collision Energy |
|---|---|---|---|---|
| Di-ethyl phthalate | 84-66-2 | 149 | 65 | 20 |
| Diisobutyl phthalate | 84-69-5 | 149 | 65 | 25 |
| Di-butyl phthalate | 84-74-2 | 149 | 65 | 25 |
| Dipentyl phthalate | 131-18-0 | 149 | 65 | 25 |
| Dihexyl phthalate | 84-75-3 | 149 | 65 | 20 |
| Di-cyclohexyl phthalate | 84-61-7 | 149 | 65 | 20 |
| Di-(2-ethylhexyl) phthalate | 117-81-7 | 149 | 93 | 20 |
| Diisononyl phthalate | 28553-12-0 | 293.3 | 149 | 4 |
| Diisodecyl phthalate | 26761-40-0 | 307 | 149 | 5 |
| Bisphenol-F | 620-92-8 | 344 | 179.2 | 20 |
| Bisphenol-A | 80-05-7 | 357 | 191.1 | 20 |
| Bisphenol-B | 77-40-7 | 357 | 191.1 | 20 |
| Bisphenol-S | 80-09-1 | 394 | 229 | 20 |
| Bisphenol A diglycidyl ether | 1675-54-3 | 325 | 141 | 20 |
| Benzophenone | 119-61-9 | 181.8 | 105.1 | 25 |
2.4. Quality assurance
The instrument capabilities were estimated by determining both the LOD and limit of quantification (LOQ). The LOD and LOQ were determined for method validation by GC-QqQ analysis using the standard deviation (SD) of the response and the slope of the calibration curve. The LOD was calculated as 3.3 SD/slope (μg/g) and the LOQ was 10 SD/slope (μg/g) [29].
3. Result and discussion
3.1. Screening of polyolefin materials
During mechanical recycling of post-consumer plastic waste, the material is collected from households and businesses, comprising a mixture of both food and non-food application waste products. This mixed waste stream is then transported to a MRF for sorting and further processing. During sorting, the primary focus is to remove paper, metal, and sort the types of plastics (by resin number or using technology), depending on the MRF-specific responsibilities and capabilities. However, plastic waste derived from both food and non-food applications is not differentiated and there is potential for small concentrations of non-food grade materials to be co-mingled as contamination in single source reclamation streams. Consequently, additives originating from non-food applications can be blended into the PCR materials intended to be used in food contact applications. As a result, the European Food Safety Authority (EFSA) generally allows the feedstocks from recycled plastic to contain up to 5 % of incidental non-food application plastic waste [30]. This blending can pose a significant challenge for FCM applications [31] and understanding NIAS and IAS from both food and non-food applications is critical for FCM materials.
Some comingling of FCM and non-FCMs is unavoidable and presents limited risk at very low concentrations. Hence, the FDA requires the use of food grade materials with strict source control of the materials for a recycler/reprocessor to obtain a ‘Letter of No Objection’. The risk of comingling is mitigated through the surrogate challenge testing as part of the FDA industry guidance protocol [32] where representative chemical contaminants (polar volatile, non-polar volatile, polar semivolatile, non-polar semivolatile, and a metal salt) are intentionally contaminated into a representative feedstock. The intentionally contaminated materials are then recycled to understand the ability for the process to remove unapproved additives and potential contaminants. Given this context, it is critical to understand the presence of NIAS and IAS chemicals from both food grade and non-food grade feedstocks to understand of the potential constituents present in PCR plastic.
The GC-MS screening analysis of the studied polyolefins materials provided chromatograms with several peaks. Any peaks detected in the process blank were subtracted from sample chromatograms (Appendix III). The NIST mass spectral search identified 61 different compounds in virgin and PCR polyolefin samples grouped as hydrocarbons, aldehydes, alcohols, phthalates, carboxylic acids, per-and polyfluoroalkyl substances (PFAS), ketones, and amines. Overall, 9 substances were detected in the virgin PE samples, while 52 components were detected in the recycled polyolefin samples. The detected compounds list, their CAS number, chemical class, tentative application, and Cramer classification are displayed in Table 3 and Appendix 1. Most of the compounds detected can be attributed to degradation products, process additives, or food additives in the samples. Process additives are used to improve the manufacturing, usability, appearance in plastic polymers [33]. In Europe, around 600 substances are authorized as additives and polymer production aids for plastic FCMs [34]. Title 21 of the U.S. Code of Federal Regulations (21CFR) specifies various types of indirect food additives that are utilized in food-contact articles. These additives comprise adhesives and elements of coatings (Part 175), paper and paperboard components (Part 176), polymers (Part 177), as well as adjuvants and production aids (Part 178). The priority based assessment of food additive (PAFA) evaluates 3300 direct and 3200 indirect food additives' regulatory information in the Code of Federal Regulations used in the US food industry [35].
Table 3.
Cramer classification of compounds in Virgin and PCR polymer samples.
| Sample | # Class I Cramer No. of compounds (% in total compounds detected) |
# Class II Cramer No. of compounds (% in total compounds detected) | # Class III Cramer No. of compounds (% in total compounds detected) | # Unconfirmed Class No. of compounds (% in total compound detected) |
|---|---|---|---|---|
| Virgin (N = 6) | 2 (22.22 %) | 1 (11.11 %) | 4 (44.44 %) | 2 (22.22 %) |
| PCR (N = 23) | 25 (48.08 %) | 1 (1.92 %) | 25 (48.08 %) | 1 (1.92 %) |
*Percentage was calculated as follows: percentage of particular group = (number of particular group in compound/total number of compound detected in sample) x 100 %.
The occurrence rate of phthalate-based molecules (e.g., dicyclohexyl phthalate, di (2-ethyl-hexyl) terephthalate (DEHT), terephthalic acid, di(2-methoxyethyl) ester, isophthalic acid, di(2-isopropylphenyl)) ester was 7.69 % and per- and polyfluoroalkyl substances (PFAS) was 3.84 % detected in the PCR materials. It can be hypothesized that phthalates and PFAS were processing aids in the recovered materials [36] and after their service life they retain in PCR material. PFAS is utilized to create fluoropolymer coatings, additives, and products that resist heat, oil, stains, grease, and water [37,38] and are often used as a polymer processing aid in blown film polyolefin applications. PFAS has a significant role in the food packaging industry [39]. A study by Bečanová et al. found PFAAs in recycled consumer products and household items (paper boards, plastics materials) at a concentration of up to 78 ng/g [40]. There are few studies on PFAS in recycled polymers and more research is needed to better understand the levels and potential risks of PFAS contamination in recycled polymers. It is important to conduct further studies to characterize and monitor the levels of PFAS in recycled polymer to mitigate any potential risks to human health and the environment. A similar result from the study of [41] was found where phthalates were detected in PCR polymer (HDPE, PET) [41].
When there is no intentional use, external contamination from other polymer types during recycling can be a source of phthalate contamination, considered as NIAS. In virgin and PCR polymers samples, common compounds detected were 4,6-Octadiyn-3-one, 2,4-Di-tert-butylphenol, 3-Methylbenzyl alcohol, 2-Phenethyl-β-phenylpropionate, and 1, 3 cyclopentadiene 5 (1- methylethylidene). Most of these compounds are the degradation products from antioxidants and stabilizers for example, 2,4-Di-tert-butylphenol is the major degradation product of phenolic antioxidant Irgafos used in UV irradiation protection [42]. According to Hirata-Koizum et al., the toxicity of 2,4-Di-tert-butylphenol (DTBP) is higher than Irgafos [43]. DTBP has shown to have chronic effect on human and animals [44]. 2-Phenethyl-β-phenylpropionate is a minimum risk pesticide (bittle trap) and According to EPA, this compound has shown at or near zero risk for human [45]. As many of these compounds are processing aids, it can remain in the final product even in the virgin material from plastic manufacturing. The presence of certain flavoring additives in a virgin sample cannot necessarily indicate their intentional use; they can be from external contamination [6,46,47].
Hydrocarbon compound groups were detected in the majority of PCR samples (23.08 %) such as decane, tridecane, tetradecane, hexadecane, octadecane compared to virgin samples (11.11 %). One possible hypothesis is that polymer chains may undergo scission during the recycling process, resulting in the generation of shorter chain hydrocarbons [48]. Hydrocarbons are generally associated with negative health impact (skin irritation, eye irritation, and respiratory syndromes) [49]. Based on this perspective, it could be assumed that recycled materials might pose a higher risk of containing more harmful/toxic substances resulting from the recycling process or previous service life if not removed. Polycyclic aromatic hydrocarbon (PAH) for example naphthalene, which is genotoxic (damage DNA) and carcinogenic to health [50,51] and 2,5-bis(1,1-dimethylethyl)-phenol were found in one PCR sample (sample 17-PP/PE unwashed sample) but not in virgin sample or other PCR samples. Since naphthalene has applications as a secondary plasticizer in polymer manufacturing and dyes, it is reasonable to assume that these compounds were intentionally added to the polymer. Naphthalene was found in the unwashed PP PCR sample (sample 16). However, the degradation products resulting from the breakdown of naphthalene can be considered as non-intentionally added substances (NIAS).
Most amines were found in virgin samples (11.11 %) compared to PCR (5.77 %) while amides were only detected in some of the PCR samples (5.77 %) (Sample 9, Sample 21, Sample 22, Sample 25). Caprolactam amide (Sample 21, Sample 22) was only detected in PCR polymers. This compound can be used as a slip agent in polyethylene and other polyolefins polymers [52]. Another study found caprolactam in baby bottle plastic packaging (Polyethylene terephthalate- PET/aluminum- Al/polyethylene-PE) from a migration test and attributed it to an oligomer of monomers adipic acid [22]. Caprolactam is also a key raw material used in the production of nylon [53]. However, during the recycling process, nylon waste may inadvertently get mixed with the polyolefin waste, which can affect the composition of the resulting PCR polymer. Therefore, it is important to ensure proper sorting and processing of the recycled material to minimize contamination and maintain the desired composition of the PCR polymer. The detection of methadone N-oxide in a PCR sample suggests that it may have been formed as a result of the degradation of initial reactants or additives used in the production of the food packaging material. As our samples were not sorted for food-grade and non-food-grade polyolefins prior to recycling, there is a chance that the resulting PCR polymer may contain impurities from both material grades. These compounds, although not intentionally added to the packaging, are regarded as contaminants, and their presence in food packaging materials may pose a risk to human health [54].
Additional detected compounds included bumetrizole, 2,6-bis(1,1-dimethylethyl)-4-methylphenyl, palmitic acid, and propanoic acid found in the PCR samples which are often used as processing aids and additives to slow down the oxidation process of the polymer (Lau and Wong, 2000). The bumetrizole is an approved stabilizer (manufacturing, packaging, processing) in polyolefins by the FDA [54,55], palmitic acid (naturally occurring fatty acid), stearic acid [56], 2,2-dimethyl- propionic acid, 2,6-bis(1,1-dimethylethyl)-4-methylpheny (butylated hydroxytoluene-BHT) are food additives and polymer processing aids and generally recognized as safe (GRAS) by the FDA [57,58].
Nickel tetracarbonyl is a polymer processing aid detected in one PCR sample (Sample 23). According to the literature, nickel tetracarbonyl can be sourced from various factors such as the degradation of phosphite antioxidant or antioxidant hostastat FA 38 during recycling, or from external contamination. This compound is considered toxic to aquatic environments [54]. Although some of the compounds found in the PCR sample could not be traced back to a specific source according to the literature, their presence may indicate an ineffective cleaning process during recycling or unknown degradation products. This raises concern regarding the risks of FCMs that these compounds may pose to consumers, depending on compound diffusion coefficients and use conditions. PCR samples are heterogeneous, with different parts of the same plastic material having varying levels of contamination or impurities. As a result, the composition of the PCR polymer can differ between samples of the same material due to variability.
Cramer classification is a method to predict the toxicity of chemicals based on their structural features, used by the Toxtree software (v3.1). This method can provide an initial assessment of its potential toxicity and help identify any structural features that may contribute to its toxicity level into one of three classes (I, II, or III) based on their molecular structure and functional groups. Class I (low toxicity, structurally similar to naturally occurring compounds). Class II (moderate toxicity, often synthetic chemicals that contain functional groups that may be reactive or potentially harmful to living organisms). Class III (high toxicity, may contain structural features that are known to be toxic or mutagenic) [59].
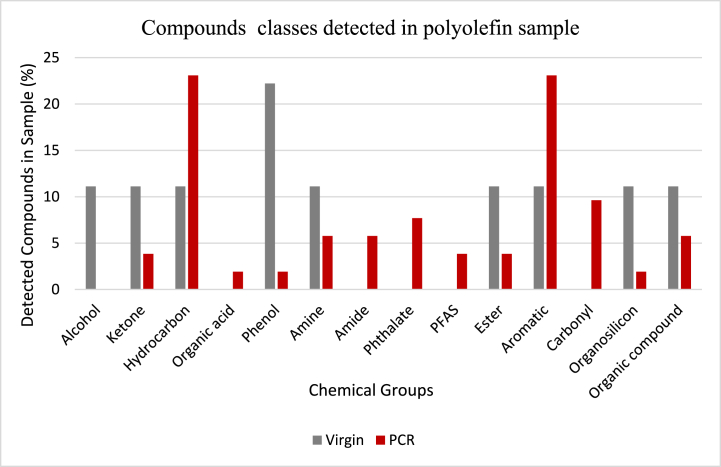
The detected compounds were also classified by their major functional groups. Fig. 2 indicates that phenol has the highest composition (22.22 %) in virgin materials, followed by hydrocarbons, alcohols, and aromatic compounds, all at 11.11 %. Meanwhile, hydrocarbons and aromatics have the highest composition in PCR materials, both at 23.08 %, with notable amounts of phthalates (7.69 %) and PFAS (3.84 %) also present. The composition of compounds in materials is crucial to consider for a variety of reasons. For example, the presence of phthalates and PFAS in plastic materials has been linked to health issues such as cancer and endocrine modulation. Therefore, the higher concentration of phthalates in PCR materials compared to virgin materials may pose a risk in certain applications. The composition of organic compounds in PCR materials can vary depending on their source and processing method, which can affect their suitability for different applications.
Fig. 2.
Detected compound groups in polyolefin samples.
3.2. Quantification of phthalates and bisphenols in PCR polyolefin sample
Targeted phthalates were detected and quantified in the PCR and virgin samples. Bisphenols were not detected in virgin samples but only in PCR samples (Table 4). The PCR sample was determined to contain four bisphenols’ compounds: BPA (2.88 ± 0.53 μg/g), BPS (5.12 ± 0.003 μg/g), BPF (3.42 ± 0.01 μg/g), and BADGE (4.638 μg/g) respectively. BADGE was only detected in one PCR PE (Sample 10) sample. The analytes detected in the number of samples are reported in Table 4. Phthalates were detected in both virgin and PCR polymer, but their concentrations were different. For example, DBP, DPENP, DHEXP, DCHP, DEHP, DINP, DIDP were 1.56 ± 0.01 μg/g, 3.33 ± 0.0003 μg/g, 2.26 ± 0.003 μg/g, 2.01 ± 0.006 μg/g, 2.51 ± 0.01 μg/g 4.08 ± 1.08 μg/g 6.04 ± 0.02 μg/g respectively in virgin samples and 1.94 ± 0.54 μg/g, 3.33 ± 0.001 μg/g, 2.40 ± 0.22 μg/g, 4.56 ± 4.05 μg/g, 2.99 ± 0.73 μg/g, 3.46 ± 0.04 μg/g, 6.18 ± 0.31 μg/g respectively in PCR samples. DEP was only detected at 1.27 μg/g in the PE PCR sample (Sample 24).
Table 4.
Quantified result of phthalate and bisphenols in the polyolefin sample.
| Compound | CAS | Concentration range in Virgin polymer (μg/g) | Concentration range in PCR polymer (μg/g) | Instrument LOD (μg/mL) | Instrument LOQ (μg/mL) | Method LOD (μg/g) | Method LOQ (μg/g) |
|---|---|---|---|---|---|---|---|
| DEP | 84-66-2 | <MLOD | 1.27 (N = 1) | 0.169 | 0.514 | 1.27 | 3.85 |
| DIBP | 84-69-5 | <MLOD | <MLOD | 0.22 | 0.67 | 1.66 | 5.05 |
| DBP | 84-74-2 | 1.56 ± 0.01 (N = 6) | 1.94 ± 0.54 (N = 18) | 0.04 | 0.10 | 0.25 | 0.78 |
| DPENP | 131-18-0 | 3.33 ± 0.0003 (N = 6) | 3.33 ± 0.001 (N = 16) | 0.16 | 0.51 | 1.26 | 3.82 |
| DHEXP | 84-75-3 | 2.26 ± 0.003 (N = 6) | 2.40 ± 0.22 (N = 21) | 0.16 | 0.50 | 1.24 | 3.77 |
| DCHP | 84-61-7 | 2.01 ± 0.006 (N = 3) | 4.56 ± 4.05 (N = 6) | 0.08 | 0.24 | 0.61 | 1.85 |
| DEHP | 117-81-7 | 2.51 ± 0.01 (N = 6) | 2.99 ± 0.73 (N = 21) | 0.13 | 0.41 | 1.00 | 3.03 |
| DINP | 28553-12-0 | 4.08 ± 1.08 (N = 5) | 3.46 ± 0.04 (N = 19) | 0.27 | 0.83 | 2.07 | 6.27 |
| DIDP | 26761-40-0 | 6.04 ± 0.02 (N = 6) | 6.18 ± 0.31 (N = 20) | 0.18 | 0.56 | 1.38 | 4.20 |
| BPF | 620-92-8 | <MLOD | 3.42 ± 0.01 (N = 3) | 0.06 | 0.21 | 0.51 | 1.56 |
| BPA | 80-05-7 | <MLOD | 2.88 ± 0.53 (N = 10) | 0.04 | 0.14 | 0.35 | 1.06 |
| BPB | 77-40-7 | <MLOD | <MLOD | 0.22 | 0.66 | 1.65 | 5.00 |
| BPS | 80-09-1 | <MLOD | 5.12 ± 0.003 (N = 2) | 0.18 | 0.56 | 1.39 | 4.22 |
| BADGE | 1675-54-3 | <MLOD | 4.638 (N = 1) | 0.23 | 0.71 | 1.76 | 5.33 |
*LOD: Limit of detection.
*LOQ: Limit of quantification.
*N In no. of sample detected.
According to the Toxics in Packaging Clearinghouse (TPCH), the limit for ortho-phthalates in packaging component is combinedly 100 μg/g (parts per million) [60]. In addition, the limit for DEHP in children's toys and childcare articles is 0.1 % by weight. All of the phthalates detected in the virgin and PCR polymer samples were below the TPCH limit. For some phthalates, such as DEP (not detected in virgin samples) and DIBP were not detected in both virgin and PCR samples. Based on the information in Table 4, DEHP and DHEXP were the phthalates that were found in the highest number of PCR samples, with 21 out of a total of 23 PCR samples. On the other hand, DIBP and BPB were not detected in any of the samples. BPF, BPA, BPS, and BADGE were also detected in PCR samples, with BPA being detected in the highest number of PCR samples (10) while BPF was found in 3 PCR samples; BPA was detected in 10 PCR samples, BPS in 2 PCR samples, and BADGE in 1 PCR sample. DEHP and DHEXP are plasticizers commonly used in PVC plastics, medical devices, and building materials, while BPA is a component of some plastics, such as polycarbonate plastics and epoxy resins, which are used in food packaging. These chemicals can be found in recycled plastics due to the mixture of different plastics used in these products [61,62]. This suggests that the recycling process and comingled sources could potentially introduce some of these compounds.
It is important to consider the potential health and environmental impacts of these compounds and to monitor their concentrations in both virgin and PCR polymer. The FDA has set guidelines for manufacturers to test and ensure the safety of recycled plastics used in food packaging [20]. Similarly, California has established regulations under the California Green Chemistry Initiative that require manufacturers to disclose information on the chemical composition of products, including recycled materials (Green-Chemistry, 2008). Developing a restricted substance list can be helpful for producers to understand high priority substances and regulate their products accordingly. While such a list is not yet in place (expect from some private companies), its development could provide further guidance to manufacturers on the use of recycled materials.
Firstly, virgin polymer is made from new raw materials, whereas PCR polymer had a previous service life that may have been exposed to a wider variety of chemicals or abuse conditions during their previous use. For example, if the recycled materials were previously used in food packaging, they may have been exposed to food contact materials that contain some of the detected compounds in the identification screening experiments [63]. Secondly, the recycling process itself may introduce or concentrate certain compounds in the recycled polymer. For example, certain plastic additives or contaminants may be more likely to accumulate in the recycled polymer due to their increased concentration in the recycling stream.
It is recommended by the EFSA that, the levels of phthalates and bisphenols in food should be kept as low as reasonably achievable (ALARA) to minimize exposure due to their known toxicity [14]. The specific migration limits (SMLs) for phthalates utilized in food contact materials (FCMs) in the European Union, as per Directive 2007/19/EC, are 0.3 mg/kg and 1.5 mg/kg for DBP and DEHP, respectively [64]. The SMLs limit for BPA established by the European Union is 0.6 mg/kg of food [65]. According to the Consumer Product Safety Improvement Act of 2008, Congress has banned the use of three phthalates, including DBP, DEHP, and BBP, in children's toys if the concentration is higher than 0.1%. The United States Environmental Protection Agency (EPA) has recommended the oral Reference Dose (RfD) for DBP to be 0.1 mg/kg/day and for DEHP to be 0.02 mg/kg/day based on animal studies [66]. Overall, the differences in the concentrations of these compounds between virgin and PCR polymer highlight the importance of considering the potential impacts of the recycling process on the composition and safety of the resulting material.
4. Conclusion
This study emphasizes the importance of understanding the presence of non-intentionally added substances (NIAS) in recycled polyolefin materials with intended use as Food Contact Materials to safeguard public and environmental health. It also highlights the necessity of monitoring (NIAS) and aligning them with regulations to limit the use of harmful additives in plastic manufacturing and enhance the safe recycling and disposal of plastic waste.
In the present study, a GC-MS full scan coupled to NIST spectral library analysis of 6 virgin and 23 post-consumer recycled (PCR) polyolefin materials detected several peaks after extraction in xylene. Xylene was selected over the traditional food simulations to increase the extraction of additives and potential contaminants as a worst-case scenario. There were 9 compounds in virgin and 52 compounds in post-consumer recycled polyolefin samples detected. Some detected compounds do not have detailed information about their sources in the literature. Utilization of recycled polymers manufactured prior to legislation that limits or bans highly scrutinized chemicals such as phthalates, bisphenols, and perfluorinated substances increases the potential of accumulation in the supply chain. Therefore, it is critical to monitor and understand the chemical composition of virgin and PCR materials for compliance and consumer/environmental safety.
The detected compounds in the xylene extract were classified as hydrocarbons, phenols, alcohols, phthalates, organic acids, per- and polyfluoroalkyl substances (PFAS), ketones, amides, and amines. Phthalates and (PFAS) were detected in the recycled materials but not in the virgin materials from survey analysis. Functional group analysis revealed that phenol has the highest proportion in virgin materials, while hydrocarbons, aromatics, phthalates, and PFAS are notable components of PCR materials used in packaging. Some of the compounds detected were degradation products or processing aids mainly in PCR samples that could remain in the final product. The presence of potentially harmful compounds in polyolefin materials can have various impacts on human health and the environment. For example, phthalates and PFAS have been linked to adverse health effects such as endocrine disruption/modulation, developmental and reproductive toxicity, and cancer. PFAS are also persistent in the environment and can accumulate in living organisms, including humans.
The detection of hydrocarbons, polycyclic aromatic hydrocarbons (PAHs), and other toxic substances in recycled polyolefins also raises various concerns about the potential for exposure to these compounds during the use and disposal of plastic products. The quantification study determined that both virgin and PCR polymer samples contained detectable levels of phthalates, although they were generally in higher concentrations in PCR samples, however, both virgin and PCR polymer samples were compliant with the TPCH threshold limit. Bisphenols were only detected in PCR samples, suggesting that the recycling process or consumer abuse could introduce these compounds. The Cramer classification showed a higher proportion of Cramer Class III was determined in the PCR polymer suggesting there may be some concern for toxicity associated with the use of recycled plastics; however, diffusion coefficients and conditions of use must be considered to understand exposure risk.
This study highlights the necessity to understand and identify chemicals in post-consumer polymers and the need for continued monitoring of these compounds for specific applications, such as food packaging. Overall, the presence of these compounds in polyolefins emphasizes the need for better understanding of the potential risks associated with the production, use, and disposal of plastic products. It also underscores the importance of implementing effective regulatory measures to reduce the use of potentially harmful additives in plastic manufacturing and to ensure the safe recycling and disposal of plastic waste. Further research is necessary to combine chemical and toxicological data of the identified compounds to determine the origin (impurities, byproducts, etc.) and compliance of these compounds. Additionally, understanding migration kinetics of these chemicals from the different sources of post-consumer plastics is critical to understand exposure risk under various conditions of use as FCMs.
CRediT authorship contribution statement
Khairun Tumu: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Keith Vorst: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Greg Curtzwiler: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Greg Curtzwiler reports financial support was provided by Institute for the Advancement of Food and Nutrition Sciences. Greg Curtzwiler reports financial support was provided by Polymer and Food Protection Consortium. Greg Curtzwiler reports a relationship with Ideopak, LLC that includes: equity or stocks. Keith Vorst reports a relationship with Ideopak, LLC that includes: equity or stocks.
Acknowledgements
This work was supported in part by the Polymer and Food Protection Consortium (PFPC) at Iowa State University, Agriculture and Home Economics Experiment Station HATCH Project 04202, and the Institute for the Advancement of Food and Nutrition Sciences (IAFNS) through a grant from the Food Packaging Safety and Sustainability Committee. IAFNS is a nonprofit science organization that pools funding from industry collaborators and advances science through the in-kind and financial contributions from public and private sector participants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23620.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.EPA Plastics: material-specific data. 2022. https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data
- 2.Leal Filho W., Salvia A.L., Bonoli A., Saari U.A., Voronova V., Klõga M., Kumbhar S.S., Olszewski K., De Quevedo D.M., Barbir J. An assessment of attitudes towards plastics and bioplastics in Europe. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142732. [DOI] [PubMed] [Google Scholar]
- 3.PlasticsEurope Plastics - the Facts. 2021. https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf
- 4.EPA Containers and packaging: product-specific data. 2018. https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/containers-and-packaging-product-specific
- 5.Novák I., Popelka A., Špitalský Z., Krupa I., Tavman S. In: Polyolefin Compounds and Materials: Fundamentals and Industrial Applications. Al-Ali AlMa'adeed M., Krupa I., editors. Springer International Publishing; 2016. Polyolefin in packaging and food industry; pp. 181–199. [DOI] [Google Scholar]
- 6.Horodytska O., Cabanes A., Fullana A. Non-intentionally added substances (NIAS) in recycled plastics. Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126373. [DOI] [PubMed] [Google Scholar]
- 7.EPA U.S. National recycling goal. 2020. https://www.epa.gov/recyclingstrategy/us-national-recycling-goal
- 8.FDA, 21 CFR 170.39 Threshold of regulation for substances used in food-contact articles (Accessed July 7, 2022). https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=TOR.
- 9.Roosen M., Harinck L., Ügdüler S., De Somer T., Hucks A.-G., Belé T.G.A., Buettner A., Ragaert K., Van Geem K.M., Dumoulin A., De Meester S. Deodorization of post-consumer plastic waste fractions: a comparison of different washing media. Sci. Total Environ. 2022;812 doi: 10.1016/j.scitotenv.2021.152467. 2022/03/15/ [DOI] [PubMed] [Google Scholar]
- 10.Su Q.-Z., Vera P., Nerín C., Lin Q.-B., Zhong H.-N. Safety concerns of recycling postconsumer polyolefins for food contact uses: regarding (semi-) volatile migrants untargetedly screened. Resour. Conserv. Recycl. 2021;167 [Google Scholar]
- 11.Nerin C., Alfaro P., Aznar M., Domeño C. The challenge of identifying non-intentionally added substances from food packaging materials: a review. Anal. Chim. Acta. 2013;775:14–24. doi: 10.1016/j.aca.2013.02.028. 2013/05/02/ [DOI] [PubMed] [Google Scholar]
- 12.EU Union Guidelines on Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. 2014. https://ec.europa.eu/food/system/files/2016-10/cs_fcm_plastic-guidance_201110_en.pdf
- 13.Peters R.J.B., Groeneveld I., Sanchez P.L., Gebbink W., Gersen A., de Nijs M., van Leeuwen S.P.J. Review of analytical approaches for the identification of non-intentionally added substances in paper and board food contact materials. Trends Food Sci. Technol. 2019;85:44–54. doi: 10.1016/j.tifs.2018.12.010. 2019/03/01/ [DOI] [Google Scholar]
- 14.Tumu K., Vorst K., Curtzwiler G. Endocrine modulating chemicals in food packaging: A review of phthalates and bisphenols. Comprehensive Reviews in Food Science and Food Safety. 2023;22:1337–1359. doi: 10.1111/1541-4337.13113. [DOI] [PubMed] [Google Scholar]
- 15.Geueke B., Dossier-Non-intentionally added substances (NIAS). Food Packag. Forum, Geueke, B. (2018). Accessed June 21, 2022. https://dtsc.ca.gov/dtsc-website-archive/green-chemistry/.
- 16.Sánchez-Rivera K.L., Munguía-López A.d.C., Zhou P., Cecon V.S., Yu J., Nelson K., Miller D., Grey S., Xu Z., Bar-Ziv E., Vorst K.L., Curtzwiler G.W., Van Lehn R.C., Zavala V.M., Huber G.W. Recycling of a post-industrial printed multilayer plastic film containing polyurethane inks by solvent-targeted recovery and precipitation. Resour. Conserv. Recycl. 2023;197 doi: 10.1016/j.resconrec.2023.107086. 2023/10/01/ [DOI] [Google Scholar]
- 17.Kahlert S., Bening C.R. Why pledges alone will not get plastics recycled: comparing recyclate production and anticipated demand. Resour. Conserv. Recycl. 2022;181 [Google Scholar]
- 18.Washington-State-Department-of-Ecology Recycled content minimums. 2023. https://ecology.wa.gov/Waste-Toxics/Reducing-recycling-waste/Waste-reduction-programs/Plastics/2021-plastic-pollution-laws/Recycled-content-minimums
- 19.CFR-21-177 . 2023. Indirect Food Additives: Polymers.https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-177 [Google Scholar]
- 20.FDA Guidance for industry: preparation of premarket submissions for food contact substances. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-preparation-premarket-submissions-food-contact-substances-chemistry#iid3a
- 21.FDA Recently published submissions on recycled plastics in food packaging. 2020. https://www.fda.gov/food/recycled-plastics-food-packaging/recently-published-submissions-recycled-plastics-food-packaging
- 22.Kato L.S., Conte-Junior C.A. Safety of plastic food packaging: the challenges about non-intentionally added substances (NIAS) discovery, identification and risk assessment. Polymers. 2021;13(13):2077. doi: 10.3390/polym13132077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley E., Coulier L. An investigation into the reaction and breakdown products from starting substances used to produce food contact plastics. CSL. 2007:1–629. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85081015679&partnerID=40&md5=1bd9d10f215b4410c08a6b56f6bdbc01 [Google Scholar]
- 24.Canellas E., Vera P., Nerín C. UPLC–ESI-Q-TOF-MSE and GC–MS identification and quantification of non-intentionally added substances coming from biodegradable food packaging. Anal. Bioanal. Chem. 2015;407(22):6781–6790. doi: 10.1007/s00216-015-8848-2. [DOI] [PubMed] [Google Scholar]
- 25.Miralles P., Yusà V., Pineda A., Coscollà C. A fast and automated strategy for the identification and risk assessment of unknown substances (IAS/NIAS) in plastic food contact materials by GC-Q-Orbitrap HRMS: recycled LDPE as a proof-of-concept. Toxics. 2021;9(11):283. doi: 10.3390/toxics9110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FDA, Substances for use as basic components of single and repeated use food contact surfaces, CFR - Code of Federal Regulations Title 21 (2023).Accessed May 3, 2022. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-177/subpart-B?toc=1.
- 27.PFPC. Polymer and Food Protection Consortium. Iowa State University. https://pfpc.cals.iastate.edu/.
- 28.NIST NIST standard reference database 1A. 2014. https://www.nist.gov/system/files/documents/srd/NIST1aVer22Man.pdf
- 29.Şengül Ü. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2016;24(1):56–62. doi: 10.1016/j.jfda.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Insights I. FDA and EFSA food grade recycled resins are required to reach global industry targets. 2021. https://interplasinsights.com/plastic-industry-insights/fda-and-efsa-food-grade-recycled-resins-are-required-to-reac/
- 31.Kol R., Roosen M., Ügdüler S., Van Geem K.M., Ragaert K., Achilias D.S., De Meester S. IntechOpen; 2021. Recent Advances in Pre-treatment of Plastic Packaging Waste. [Google Scholar]
- 32.Fda U. 2021. Use of Recycled Plastics in Food Packaging (Chemistry Considerations): Guidance for Industry. Preprint at. [Google Scholar]
- 33.Li H., Aguirre-Villegas H.A., Allen R.D., Bai X., Benson C.H., Beckham G.T., Bradshaw S.L., Brown J.L., Brown R.C., Cecon V.S., Curley J.B., Curtzwiler G.W., Dong S., Gaddameedi S., García J.E., Hermans I., Kim M.S., Ma J., Mark L.O., Mavrikakis M., Olafasakin O.O., Osswald T.A., Papanikolaou K.G., Radhakrishnan H., Sanchez Castillo, Sánchez-Rivera K.L., Tumu K.N., Van Lehn, Vorst K.L., Wright M.M., Wu J., Zavala V.M., Zhou P., Huber G.W. Expanding plastics recycling technologies: chemical aspects, technology status and challenges [10.1039/D2GC02588D] Green Chemistry. 2022;24(23):8899–9002. doi: 10.1039/D2GC02588D. [DOI] [Google Scholar]
- 34.Matthews C., Moran F., Jaiswal A.K. A review on European Union's strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2021;283 [Google Scholar]
- 35.FDA . 2023. Food Ingredient & Packaging Terms.https://www.fda.gov/food/food-ingredients-packaging/food-ingredient-packaging-terms#:∼:text=Indirect%20food%20additives%20mentioned%20in,production%20aids%20(Part%20178) [Google Scholar]
- 36.Naspolini N.F., Machado P.P., Moreira J.C., Asmus C.I.R.F., Meyer A. vol. 37. Cadernos de Saúde Pública; 2021. (Maternal Consumption of Ultra-processed Foods and Newborn Exposure to Perfluoroalkyl Substances (PFAS)). [DOI] [PubMed] [Google Scholar]
- 37.Curtzwiler G.W., Silva P., Hall A., Ivey A., Vorst K. Significance of perfluoroalkyl substances (PFAS) in food packaging. Integrated Environ. Assess. Manag. 2021;17(1):7–12. doi: 10.1002/ieam.4346. [DOI] [PubMed] [Google Scholar]
- 38.Monge Brenes A.L., Curtzwiler G., Dixon P., Harrata K., Talbert J., Vorst K. PFOA and PFOS levels in microwave paper packaging between 2005 and 2018. Food Addit. Contam. B. 2019:1–10. doi: 10.1080/19393210.2019.1592238. [DOI] [PubMed] [Google Scholar]
- 39.CDC . Centers for Disease Control and Prevention; 2022. Per- and Polyfluorinated Substances (Pfas) Factsheet.https://www.cdc.gov/biomonitoring/PFAS_FactSheet.html [Google Scholar]
- 40.Bečanová J., Melymuk L., Vojta Š., Komprdová K., Klánová J. Screening for perfluoroalkyl acids in consumer products, building materials and wastes. Chemosphere. 2016;164:322–329. doi: 10.1016/j.chemosphere.2016.08.112. 2016/12/01/ [DOI] [PubMed] [Google Scholar]
- 41.Dutra C., Freire M.T.d.A., Nerín C., Bentayeb K., Rodriguez-Lafuente A., Aznar M., Reyes F.G. Migration of residual nonvolatile and inorganic compounds from recycled post-consumer PET and HDPE. J. Braz. Chem. Soc. 2014;25:686–696. [Google Scholar]
- 42.Yang Y., Hu C., Zhong H., Chen X., Chen R., Yam K.L. Effects of ultraviolet (UV) on degradation of irgafos 168 and migration of its degradation products from polypropylene films. J. Agric. Food Chem. 2016;64(41):7866–7873. doi: 10.1021/acs.jafc.6b03018. [DOI] [PubMed] [Google Scholar]
- 43.Hirata‐Koizumi M., Hamamura M., Furukawa H., Fukuda N., Ito Y., Wako Y., Yamashita K., Takahashi M., Kamata E., Ema M. Elevated susceptibility of newborn as compared with young rats to 2‐tert‐butylphenol and 2, 4‐di‐tert‐butylphenol toxicity. Congenital. Anom. 2005;45(4):146–153. doi: 10.1111/j.1741-4520.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z.-h., Yin H., Dang Z. Do estrogenic compounds in drinking water migrating from plastic pipe distribution system pose adverse effects to human? An analysis of scientific literature. Environ. Sci. Pollut. Control Ser. 2017;24:2126–2134. doi: 10.1007/s11356-016-8032-z. [DOI] [PubMed] [Google Scholar]
- 45.Baker B.P., Grant J.A. 2018. 2-Phenethyl Propionate Profile.https://ecommons.cornell.edu/bitstream/handle/1813/56134/PeP-MRP-NYSIPM.pdf?sequence=1 [Google Scholar]
- 46.Aigotti R., Giannone N., Asteggiano A., Mecarelli E., Dal Bello F., Medana C. Release of selected non-intentionally added substances (NIAS) from PET food contact materials: a new online SPE-UHPLC-MS/MS multiresidue method. Separations. 2022;9(8):188. [Google Scholar]
- 47.Rung C., Welle F., Gruner A., Springer A., Steinmetz Z., Munoz K. Identification and evaluation of (non-) intentionally added substances in post-consumer recyclates and their toxicological classification. Recycling. 2023;8(1):24. [Google Scholar]
- 48.Vollmer I., Jenks M.J., Roelands M.C., White R.J., van Harmelen T., de Wild P., van Der Laan G.P., Meirer F., Keurentjes J.T., Weckhuysen B.M. Beyond mechanical recycling: giving new life to plastic waste. Angew. Chem. Int. Ed. 2020;59(36):15402–15423. doi: 10.1002/anie.201915651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bayati M., Vu D.C., Vo P.H., Rogers E., Park J., Ho T.L., Davis A.N., Gulseven Z., Carlo G., Palermo F. Health risk assessment of volatile organic compounds at daycare facilities. Indoor Air. 2021;31(4):977–988. doi: 10.1111/ina.12801. [DOI] [PubMed] [Google Scholar]
- 50.El-Masri H. Agency for Toxic Substances and Disease Registry; 2005. Toxicological Profile for Naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene. [Google Scholar]
- 51.Souza R.H., Cardoso M.d.G., Machado A.M.R., Santiago W.D., Pedroso M.P., Brandão R.M., Oliveira R.E., Barbosa R.B., Alvarenga G.F., Caetano A.R. Polycyclic aromatic hydrocarbons in cachaças packed in bottles of polyethylene terephthalate. J. Food Sci. 2022;87(4):1906–1915. doi: 10.1111/1750-3841.16095. [DOI] [PubMed] [Google Scholar]
- 52.García Ibarra V., Rodríguez Bernaldo de Quirós A., Paseiro Losada P., Sendón R. Identification of intentionally and non-intentionally added substances in plastic packaging materials and their migration into food products. Anal. Bioanal. Chem. 2018;410(16):3789–3803. doi: 10.1007/s00216-018-1058-y. [DOI] [PubMed] [Google Scholar]
- 53.Yan C., Fraga-Dubreuil J., Garcia-Verdugo E., Hamley P.A., Poliakoff M., Pearson I., Coote A.S. The continuous synthesis of ε-caprolactam from 6-aminocapronitrile in high-temperature water. Green Chem. 2008;10(1):98–103. [Google Scholar]
- 54.Lahimer M.C., Ayed N., Horriche J., Belgaied S. Characterization of plastic packaging additives: food contact, stability and toxicity. Arab. J. Chem. 2017;10:S1938–S1954. [Google Scholar]
- 55.NCATS Bumetrizole. National center for advancing translational sciences (NCATS) 2023. https://drugs.ncats.io/drug/7ZF18Q354W
- 56.Alin J., Hakkarainen M. Microwave heating causes rapid degradation of antioxidants in polypropylene packaging, leading to greatly increased specific migration to food simulants as shown by ESI-MS and GC-MS. J. Agric. Food Chem. 2011;59(10):5418–5427. doi: 10.1021/jf1048639. [DOI] [PubMed] [Google Scholar]
- 57.FDA GRAS notice for cetylated fatty acids. 2021. https://www.fda.gov/media/161881/download
- 58.Sheu C., Cain K., Rushbrook C., Jorgenson T., Generoso W. Tests for mutagenic effects of ammoniated glycyrrhizin, butylated hydroxytoluene, and gum Arabic in rodent germ cells. Environ. Mutagen. 1986;8(3):357–367. doi: 10.1002/em.2860080305. [DOI] [PubMed] [Google Scholar]
- 59.Patlewicz G., Jeliazkova N., Safford R., Worth A., Aleksiev B. An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ. Res. 2008;19(5–6):495–524. doi: 10.1080/10629360802083871. [DOI] [PubMed] [Google Scholar]
- 60.TPCH Model toxics in packaging legislation. 2021. https://toxicsinpackaging.org/model-legislation/model/
- 61.Cheon Y.-P. Di-(2-ethylhexyl) phthalate (DEHP) and uterine histological characteristics. Development & reproduction. 2020;24(1):1. doi: 10.12717/DR.2020.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le H.H., Carlson E.M., Chua J.P., Belcher S.M. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicology letters. 2008;176(2):149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welle F. Twenty years of PET bottle to bottle recycling—an overview. Resour. Conserv. Recycl. 2011;55(11):865–875. [Google Scholar]
- 64.Lawson-Wood K. Quantification of phthalate leaching from food contact materials by gc/ms. 2016. https://resources.perkinelmer.com/lab-solutions/resources/docs/app_quantification-of-phthalate-leaching-from-food-contact-materials-by-gcms_013011_01.pdf
- 65.Valeria Ceballos-Luna, David Chávez-Flores, Ibiza Martínez-Serrano, Beatriz A. Rocha-Gutierrez, Myrna C. Nevárez-Rodríguez, Blanca G. Beltrán “Bisphenol and Phthalate Migration Test from Mexican Meat Packaging Using HPLC-DAD Technique”. Journal of Chemistry. 2022;2022:10. doi: 10.1155/2022/2688236. Article ID 2688236. [DOI] [Google Scholar]
- 66.EPA Draft proposed approach for cumulative risk assessment of high-priority phthalates and a manufacturer-requested phthalate under the toxic substances control act. 2023. https://www.epa.gov/system/files/documents/2023-02/Draft%20Phthalate%20CRA%20Approach.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.