Abstract
Microgravity, in space travel and prolonged bed rest conditions, induces cardiovascular deconditioning along with skeletal muscle mass loss and weakness. The findings of microgravity research may also aid in the understanding and treatment of human health conditions on Earth such as muscle atrophy, and cardiovascular diseases. Due to the paucity of biomarkers and the unknown underlying mechanisms of cardiovascular and skeletal muscle deconditioning in these environments, there are insufficient diagnostic and preventative measures. In this study, we employed hindlimb unloading (HU) mouse model, which mimics astronauts in space and bedridden patients, to first evaluate cardiovascular and skeletal muscle function, followed by proteomics and metabolomics LC-MS/MS-based analysis using serum samples. Three weeks of unloading caused changes in the function of the cardiovascular system in c57/Bl6 mice, as seen by a decrease in mean arterial pressure and heart weight. Unloading for three weeks also changed skeletal muscle function, causing a loss in grip strength in HU mice and atrophy of skeletal muscle indicated by a reduction in muscle mass. These modifications were partially reversed by a two-week recovery period of reloading condition, emphasizing the significance of the recovery process. Proteomics analysis revealed 12 dysregulated proteins among the groups, such as phospholipid transfer protein, Carbonic anhydrase 3, Parvalbumin alpha, Major urinary protein 20 (Mup20), Thrombospondin-1, and Apolipoprotein C-IV. On the other hand, metabolomics analysis showed altered metabolites among the groups such as inosine, hypoxanthine, xanthosine, sphinganine, l-valine, 3,4-Dihydroxyphenylglycol, and l-Glutamic acid. The joint data analysis revealed that HU conditions mainly impacted pathways such as ABC transporters, complement and coagulation cascades, nitrogen metabolism, and purine metabolism. Overall, our results indicate that microgravity environment induces significant alterations in the function, proteins, and metabolites of these mice. These observations suggest the potential utilization of these proteins and metabolites as novel biomarkers for assessing and mitigating cardiovascular and skeletal muscle deconditioning associated with such conditions.
Keywords: Hindlimb unloading, Cardiovascular, Skeletal muscle, Deconditioning, Metabolomic, Proteomic, Biomarkers
1. Introduction
Human space travel has resulted in a plethora of discoveries and technologies that have profoundly transformed our way of life and revolutionized various aspects of our daily activities. A growing number of nations are becoming interested in space as part of future global exploration [1]. Yet, the weightlessness or "microgravity" environment to which astronauts are subjected has major deleterious impacts on general health status, particularly on the cardiovascular system and skeletal muscle structure and function. However, the exact mechanisms of these deleterious effects are not well known. Microgravity research has benefited not only in space but also on Earth as it gives insight into the mechanisms involved in various pathological conditions brought on by microgravity mimic in prolonged bed rest. Studies have shown that even a mere one week of physical inactivity or bed rest can significantly reduce skeletal muscle mass and strength. It is worth noting that nearly one-third of all inpatients stay in the hospital for this length of time [[2], [3], [4]]. Likewise, astronauts encounter similar challenges to patients undergoing prolonged bed rest due to the effects of microgravity in space. These effects include cardiovascular deconditioning, cephalad fluid shift, decreased left ventricular mass, decreased ventricular stroke volume, and hypotension [5,6]. Upon their return to Earth, the consequences of these physiological changes become even more apparent among astronauts. They experience difficulties in maintaining an upright position, a condition known as orthostatic intolerance, higher heart rate, and palpitations, in addition to decreased physical performance [5]. One of the main factors contributing to the decline in physical performance is the loss of skeletal muscle mass and strength, commonly referred to as "skeletal muscle deconditioning." This phenomenon occurs in microgravity environments experienced by astronauts and during prolonged bed rest [7,8]. Inadequate recovery from cardiovascular and skeletal muscle abnormalities and deconditioning can impair performance and cause irreversible abnormalities, leading to physiological exhaustion along with reduced adaptation to environmental changes. Recovering from these conditions has been neglected due to the lack of studies concerning the recovery process. According to the World Health Organization (WHO), cardiovascular diseases CVDs were responsible for 17.9 million deaths globally in 2019 hence, emphasizing CVDs, which are the main cause of death and morbidity both globally and locally, is of high importance [9,10]. Discovering novel biological markers (biomarkers) is critical for better understanding the molecular mechanisms underlying human diseases and evaluating disease prognosis [11]. Omics studies, such as genomics, transcriptomics, proteomics, and metabolomics, when linked to clinical data, provide structural and functional information, proposes causal molecular mechanisms, and can then be used to identify novel biomarkers and improve disease diagnosis, prognosis, prevention, and treatment [12]. Serum samples are considered the most essential diagnostic type of specimen due to their low invasiveness, high degree of stability, and careful description of an individual's physiological condition [13]. Because proteins carry out a wide range of biological functions, and metabolites are typically intermediates or byproducts of several processes, the human metabolome and proteome are considered essential to gain insights into the pathophysiology of a disease and/or condition, as well as the creation of energy and maintaining organs system homeostasis [14]. However, the changes in cardiovascular and skeletal muscle function that occur in conditions such as microgravity and prolonged bed rest are poorly understood, in part because the causes of these changes are unknown. Hence, there is a lack of biomarkers associated with these conditions. Our hypothesis postulates that particular proteins and metabolites are linked to detrimental changes in skeletal muscles and the cardiovascular system induced by microgravity during space flights and prolonged bed rest. These specific biomarkers could serve as diagnostic, therapeutic, and prognostic indicators for both space flights and extended periods of bed rest. Notably, recent research in a bed-rest model study has demonstrated changes in serum Transthyretin, Haptoglobin-related protein, and thyroid hormone-binding protein, indicating their potential use as markers for muscle atrophy [15]. Moreover, in the context of heart rate variability during long space missions, specific proteins undergo alterations. These include Plasma serine protease inhibitor, Protein-72kDa type IV collagenase, and Fibulin-1 [16]. This information advances our understanding of the physiological changes occurring in response to extended space missions, offering valuable insights for the development of diagnostic tools and therapeutic interventions. The results presented here complement earlier studies in this emerging field and indicate an unprecedented panel of biomarkers associated with these conditions.
2. Methods
2.1. Experimental design
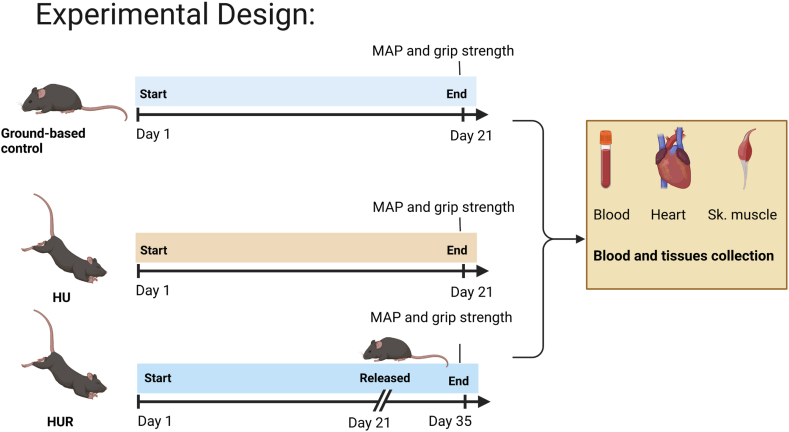
In this study, C57/Bl6 male mice at 10 weeks of age were used as the experimental subjects. The animals were randomly divided into control and experimental groups and housed in controlled environments (20 ± 1 °C, with light/dark cycles of 12 h each), with access to food (normal chow diet for mice) and water. HU mice were suspended separately in specially prepared cages using strings at an angle of 30° and checked daily to ensure the procedure was performed effectively, as described previously [17]. In addition to the control group, the experimental groups were subdivided into 2 groups (total n = 4–10 mice/group). The first group was HU mice, that was unloaded for 20 days and sacrificed at day 21, second group is the HU-recovery (HUR) group which was unloaded for 20 days, followed by 2 weeks of loading, and releasing the mice on the ground and sacrificed on day 35. Ground-based control mice were sacrificed on day 21. Before the sacrifice, blood pressure and muscle strength of the mice were measured. On the day of sacrifice, blood samples were collected using the cardiac puncture method, ensuring humane procedures under anesthesia followed by animal sacrifice and collection of tissues. Fig. 1 illustrates the experimental design.
Fig. 1.
Experimental design of the study. Mice randomly divided into three groups: 1) Ground-based controls 2) HU: Hindlimb Unloading, and 3) HUR: Hindlimb Unloading Recovery. Grip strength and blood pressure were taken before the day of sacrifice, blood was collected from the mice. After sacrifice tissues were collected.
2.2. Blood pressure and pulse measurements with a tail cuff
Using Hatteras Systems, MC4000 Blood Pressure Analysis System we measured mean systolic pressure, mean diastolic pressure, mean arterial pressure (MAP), and heart rate from the tail of mice in the three groups. In brief, mice were restrained and positioned on a specimen tray using mouse holders with magnetic bases, then the mice's tail was placed into the tail cuff and sensory assembly to obtain blood pressure readings. As described elsewhere [18], three days before the experiment (before day 1), mice were trained to the measuring system, and measurements were obtained on the day of sacrifice. Supplementary fig. 1A shows the MC4000 Blood Pressure Analysis System.
2.3. Grip strength measurement
As previously described [17], peak force was measured (in Newton) using Digital Grip Strength meter with a Hind Limb Pull Bar Assembly (Columbus Instruments, Columbus, OH). In brief, mice were permitted to hold the grip meter's metal grids using their paws and then gradually dragged backward in a horizontal direction to the point they could no longer hold the grip. The peak grip strength measured after 5 trials was recorded with 1 min resting period between each trial. Supplementary fig. 1B shows the grip strength meter.
2.4. Proteins and metabolites extraction and precipitation
Proteins and metabolites fractions were extracted from the sample as described previously [19]. Briefly, the serum samples were treated with 400 μL of methanol and 300 μL of chloroform before being centrifuged for 5 min at 4 C, 13000 rpm. Three phases emerged: The top phase includes metabolites, the intermediate phase contains protein, and the bottom phase contains additional metabolites. The top phase of the metabolites was stored in a fresh Eppendorf tube at −20 C. After adding 300 μL methanol to the middle and lower phases, they were combined and centrifuged for 1 min at 13000 rpm. The supernatant was transferred to the metabolite tube, which was subsequently stored at - 80 °C for longer. The extracted protein is the pellet that remains after centrifugation. After allowing the protein to air dry for 10–15 min, 300 μL denaturation buffer was added to thoroughly dissolve the pellet without particles. The extracted protein concentrations were determined using the Bradford assay, and the plate absorbance was measured at 570 nm using a biotek plate reader.
The extracted protein samples were processed as follows: Reduction buffer (1 M dithiothreitol (DTT) in 50 mM ammonium bicarbonate) was added to the sample to achieve a final concentration of 1 mM DTT and incubated at room temperature for 1 h. Samples were treated with alkylation buffer (550 mM iodoacetamide (IAA) in 50 mM ammonium bicarbonate) to a final concentration of 5.5 mM IAA and incubated for 1 h at room temperature in the dark. The pH was tested and, if necessary, adjusted to 8.0. After that, the sample was diluted four times with 20 mM ammonium bicarbonate. 1 g of sequencing grade modified trypsin (Promega) was added per 50 g protein and incubated at room temperature for 3 h. Another 1 g of trypsin was added per 50 g of sample protein and incubated at room temperature overnight. The treated samples were transferred to small glass tubes the next day and fully dried using the vacuum evaporator equipment.
2.5. Liquid chromatography Tandem mass spectrometry (LC–MS/MS)
Metabolites were detected using an Elute UHPLC and a Q-TOF Mass Spectrometer (Bruker, Bremen, Germany) as described before [19]. Using reversed-phase chromatography, the Elute HPG 1300 pumps, Elute Autosampler (Bruker, Bremen, Germany), and Hamilton® Intensity Solo 2C18 column (100 mm 2.1 mm, 1.8 m beads) were used. For separation, 0.1 % FA in LC grade water (solvent A) and 0.1 % FA in ACN (solvent B) were utilized. Each metabolite and protein extract was analyzed twice (duplicate).
The column was maintained at 35 °C, and each sample was injected twice with a 10 μL injection volume. The sample was eluted across a 30-min gradient, beginning with 1 % ACN for 2 min and ramping up to 99 % ACN in 15 min. Following that, 99 % ACN was held for 3 min before being re-equilibrated to 1 % ACN for 10 min. The flow rate was 0.25 mL/min for 20 min, 0.35 mL/min for 8.3 min, and 0.25 mL/min for 1.7 min.
In proteomics analysis, LC-MS/MS analysis (Bruker Daltonics) was performed using a nano elute (Bruker Daltonics) linked to a quadrupole-time-of-flight mass spectrometer (Q-TOF) with a CaptiveSpray ion source (Bruker Daltonics).
2.6. Proteomics data processing
MaxQuant version 1.6.17.0 was used to identify proteins and peptides in proteomics raw data files using the Andromeda search engine and the Uniprot reference proteome for Mice (December 05, 2022). The default parameter settings were used for the MS/MS database search, with carbamidomethylation of cysteine residues set as a fixed modification and acetylation of protein N-termini and methionine oxidation set as variable modifications. Peptide spectral matching (PSMs) were filtered using a 0.05 P-value.
2.7. Metabolomics data processing
MetaboScape® 4.0 (Bruker Daltonics) was used to process and statistically analyze raw metabolomics data.
2.8. Statistical analysis
GraphPad Prism v8.0 (GraphPad Software, Inc. CA, USA) was used for statistical analysis. For statistical tests, p-value <0.05 was considered statistically significant using the one-way ANOVA test. Post hoc Tukey's test was used for one-way ANOVA.
3. Results
3.1. Functional abnormalities of the cardiovascular and skeletal muscle in the HU model
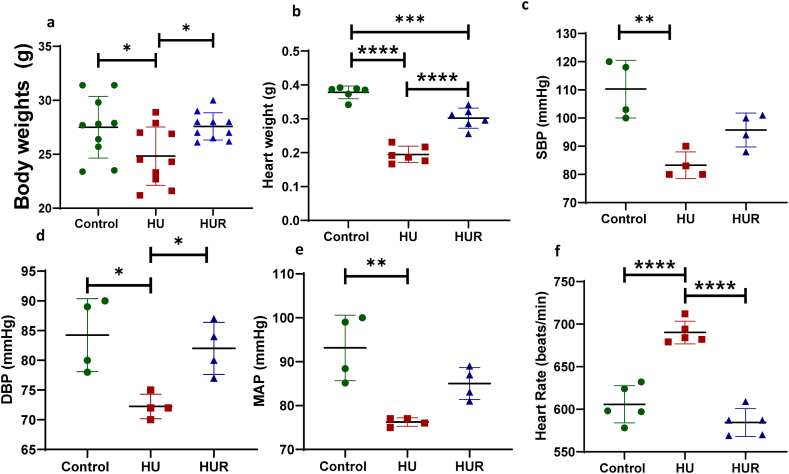
We initially characterized the HU model by measuring body, heart, and gastrocnemius muscle weights. The HU mice showed a significant decrease in body weight compared to both ground-based control and recovery mice (p < 0.0001, p < 0.0001) (Fig. 2a). Moreover, HU mice showed a significant decrease in heart weight compared to ground-based control mice (p < 0.0001). However, the recovery group showed a significant increase in heart weight compared to HU mice (p < 0.0001), yet the heart weight of HUR mice showed a significant decrease compared to ground-based control (p = 0.0003) (Fig. 2b). Next, we evaluated the function of the cardiovascular function by measuring systolic pressure, diastolic pressure, mean arterial pressure (MAP), and heart rate. The systolic blood pressure (SBP) was significantly decreased in HU mice (83 ± 4.7 mmHg), compared to ground-based controls (110 ± 10 mmHg, p = 0.001), but it didn't show significant difference compared to HUR group (98 ± 3.7 mmHg, p = 0.057) (Fig. 2c). Diastolic blood pressure (DBP) also showed the same pattern where HU mice showed decrease in diastolic pressure (72 ± 2.6 mmHg) compared to ground-based controls and HUR groups (84 ± 6.1, 83 ± 3.5 mmHg, p = 0.011, p = 0.021 respectively) (Fig. 2d). Similarly, HU mice showed a significant decrease in MAP (75.8 ± 2.2 mmHg) compared to the control (100.2 ± 12.4, p = 0.005), while HUR mice didn't show a significant change in MAP compared to HU mice (86.3 ± 3, p = 0.28) (Fig. 2e). In contrast, the heart rate of HU mice increased 693 ± 13.7 beat/min compared to ground-based controls and HUR (588 ± 20, 612 ± 17.9 beat/min, p = 0.0004, p = 0.0001 respectively) (Fig. 2f).
Fig. 2.
Body, and heart weight, and cardiovascular functional measurements. Fig. 2A Shows body weight, and Fig. 2B shows heart weight of ground-based control, HU, and HUR mice. Using MC4000 Blood Pressure Analysis System we measured C the systolic blood pressure (SBP), D the diastolic blood pressure (DBP), and E the mean arterial pressure (MAP) of ground-based control, HU, and HUR mice. Data is expressed as mean ± SD (n = 4–10 per group), one-way analysis of variance. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
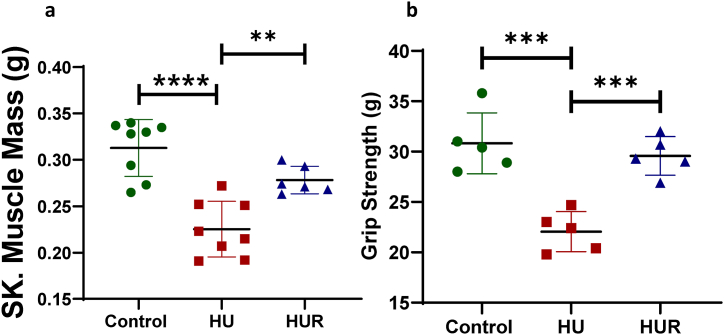
Next we measured the weight of gastrocnemius muscle in different groups, HU mice showed a significant decrease compared to ground-based controls (p < 0.0001), however HUR mice showed a significant increase in the weight of gastrocnemius muscle compared to HU (p = 0.0051) (Fig. 3a). We also evaluated the function of the skeletal muscle by using Digital Grip Strength meter. The grip strength of HU mice showed a significant decrease compared to ground-based controls (p = 0.0002). While HUR mice showed a significant increase in grip strength compared to HU mice (p = 0.0008) (Fig. 3b).
Fig. 3.
Skeletal muscle weight and functional measurements. Fig. 3A shows gastrocnemius muscle weight, and Fig. 3B shows the grip strength using grip strength meter of ground-based control, HU, and HUR mice. Data is expressed as mean ± SD (n = 5–8 per group), one-way analysis of variance. **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.2. Proteomics analysis
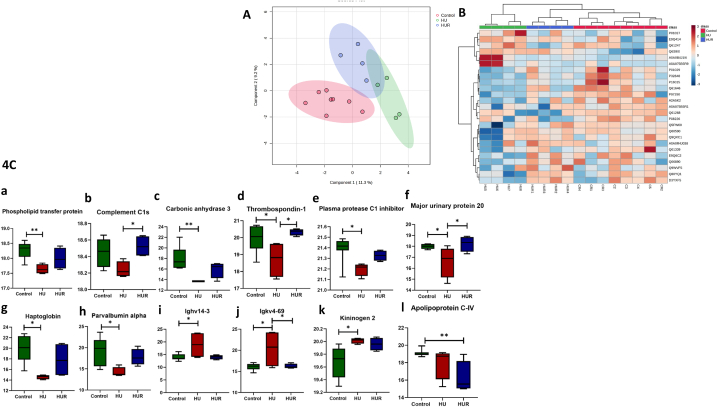
To get an understanding of the possible mechanisms underlying the deconditioning that occurs in microgravity, we performed a discovery proteomics analysis combined with an untargeted metabolomics analysis using LC–MS/MS. We identified 60,759 spectra which yielded 2385 non-redundant peptides (refer to supplementary material). After filtering out reverse hits and potential contaminants, 243 proteins were confidently identified with at least one unique and two total peptides per protein from these peptides for downstream analysis by utilizing label-free quantification (LFQ). Fig. 4A shows sparse Partial Least Squares Discriminant Analysis (sPLS-DA), which clearly demonstrates three distinct proteome groups that cluster separately. This prompted us to do additional statistical analysis (one-way ANOVA) to determine the difference between deregulated proteins. Fig. 4B shows the hierarchical clustering of significantly differentially abundant proteins among the three different groups of mice (Fig. 4C and Table 1). Proteins indicated in Fig. 4C, such as Phospholipid transfer protein (a), Complement C1s (b), Carbonic anhydrase 3 (c), Thrombospondin-1 (d), Plasma protease C1 inhibitor (e), Major urinary protein 20 (f), Haptoglobin (g), and Parvalbumin alpha (h) present a similar pattern where there is decrease abundance of the protein in HU mice compared to control mice, while HUR group present similar protein levels to that observed in control. On the other hand in the same Fig. 4C such as, Immunoglobulin heavy variable V14-3 (Ighv14-3) (i), and immunoglobulin kappa variable 4–69 (Igkv4-69) (j) showed the opposite pattern, where HU group showed a significant increase in the expression of these proteins while HUR group showed a decrease compared to HU group. Proteins, also in Fig. 4C such as Kininogen 2 (k), and Apolipoprotein C-IV (l) did not follow any of these two patterns, where kininogen 2 protein showed a significant increase in the HU group compared to the control group. In contrast, HUR group did not show change compared to HU mice. In Fig. 4C, Apolipoprotein C-IV (l) showed a decrease in HU mice compared to control group, but HUR did not show change compared to HU mice, and a significant decrease compared to the control group.
Fig. 4.
Proteomic characterization of serum proteins. Fig. 4A shows Sparse Partial Least Squares Discriminant Analysis (sPLS-DA), Fig. 4B shows the hierarchical clustering of significantly differentially abundant proteins, and Fig. 4C shows significant proteins in ground-based controls, HU and HUR mice. (n = 4 per group). *p < 0.05, **p < 0.01.
Table 1.
Summary of proteins dysregulated among the groups. *Compared to the control.
| Protein ID | Description | Direction* | Possible Association with CVDs or skeletal muscle atrophy. |
|---|---|---|---|
| A0A075B5R9 | Detecting foreign antigens and triggering immune responses. | HU: Increased HUR: Not changed |
NA |
| A0A0B4J1I6 | immune response | HU: Increased HUR: Not changed |
NA |
| A2A5K2 | lipid binding | HU: Decreased HUR: Decreased |
In LDL receptor-deficient mice, macrophage phospholipid transfer Protein deficiency induces atherosclerosis, showing that it has an atheroprotective role [26]. |
| E9Q6C2 | complement activation, classical pathway | HU: Decreased HUR: Not changed |
The complement system may defend against atherosclerosis by attaching to apoptotic cells and cell debris and clearing them from plaques [65]. |
| P16015 | carbonate dehydratase activity | HU: Decreased HUR: Decreased |
Cardiac muscle is protected from oxidative damage by antioxidative activity of carbonic acid III [66]. |
| P32848 | Played a role in relaxing following contraction. | HU: Decreased HUR: Decreased |
Parvalbumin alpha is an "atrogene" that is downregulated in the majority of muscular atrophy diseases [47]. |
| P97290 | play vital role in regulating key physiological processes such as complement activation, blood coagulation, and fibrinolysis. | HU: Decreased HUR: Decreased |
critical functions in regulating vascular permeability and suppressing inflammation [67]. |
| Q5FW60 | mating pheromone activity | HU: Decreased | NA |
| HUR: Not changed | |||
| Q61268 | engage in the metabolism of lipoproteins. | HU: Decreased HUR: Decreased |
Significantly upregulated in serum of AAD patients compared to AMI patients [15] |
| Q61646 | hemoglobin binding, antioxidant activity | HU: Decreased HUR: Decreased |
Haptoglobin genotype (Hp2-2) has been linked to atherosclerosis in ischemic stroke patients [68]. |
| Q6S9I0 | protease binding | HU: Increased HUR: Increased |
The kallikrein-kinin system regulates blood pressure and maintains vascular smooth muscle tone [69]. |
| Q80YQ1 | fibrinogen binding | HU: Decreased HUR: Not changed |
In ApoE-deficient mice, thrombospondin-1 deficiency has been found to increase the inflammatory changes required for plaque generation [28]. |
3.3. Metabolomics analysis
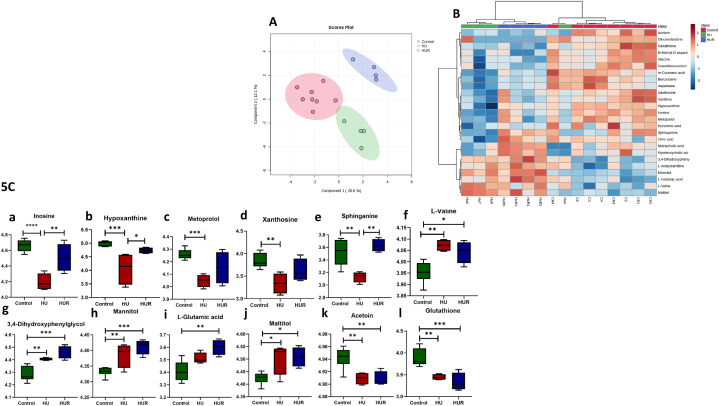
This study employs untargeted LC–MS/MS-based metabolomics analysis to compare the metabolome of HU mice to those in ground-based controls and recovery group. Each metabolite extract was analyzed in duplicate by LC-QTOF-MS from 16 serum samples. After data processing (mining), only compounds registered in HMDB 4.0 and significant values with P-value. Fig. 5A shows sPLS-DA that focuses on the differences between the groups. Fig. 5B shows the hierarchical clustering of the significant metabolites between the groups. Fig. 5C shows the metabolites significant among the groups and Table 2 summarizes significantly different metabolites among the groups. Metabolites in Fig. 5C such as inosine (a), hypoxanthine (b), metoprolol (c), xanthosine (d), and sphinganine (e) have a similar pattern where there is a decrease concentration of the metabolite in HU mice compared to control mice, while HUR group shows an increase in concentration compared in HU group. On the other hand, in Fig. 5C such as l-valine (f) showed the opposite pattern. Metabolites also in Fig. 5C such as 3,4-Dihydroxyphenylglycol (g), Mannitol (h), l-Glutamic acid (i), and Maltitol (j) have a similar pattern where there is an increase of the metabolites concentration in HU mice compared to the control group, while HUR shows a further increase compared to HU group. Lastly, Acetoin (k), and Glutathione (l) showed similar pattern where there is decrease of the metabolite's concentration in HU and HUR mice compared to control group.
Fig. 5.
Metabolomic characterization of serum metabolites. Fig. 5A shows Sparse Partial Least Squares Discriminant Analysis (sPLS-DA), Fig. 5B shows the hierarchical clustering of the significantly differentially abundant metabolites, and Fig. 5C shows significant metabolites in ground-based controls, HU and HUR mice (n = 4 per group). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Table 2.
Summary of significantly different metabolites among the groups. *Compared to the control.
| Metabolite | HMDB ID# | p.log10 | FDR | Direction* | HMDB link |
|---|---|---|---|---|---|
| Inosine | 0000195 | 4.2227 | 0.00737 | HU: Decreased HUR: Decreased |
https://hmdb.ca/metabolites/HMDB0000195 |
| 3,4-Dihydroxyphenylglycol | 0000318 | 3.7431 | 0.00918 | HU: Increased HUR: Increased |
https://hmdb.ca/metabolites/HMDB0000318 |
| Mannitol | 0000765 | 3.5441 | 0.00918 | HU: Increased HUR: Increased |
https://hmdb.ca/metabolites/HMDB0000765 |
| Glutathione | 0000125 | 3.5248 | 0.00918 | HU: Decreased HUR:Decreased |
https://hmdb.ca/metabolites/HMDB0000125 |
| Hypoxanthine | 0000157 | 3.1138 | 0.01801 | HU: Decreased HUR: Decreased |
https://hmdb.ca/metabolites/HMDB0000157 |
| l-Valine | 0000883 | 3.0073 | 0.01801 | HU: Increased HUR: Increased |
https://hmdb.ca/metabolites/HMDB0000883 |
| Metoprolol | 0001932 | 2.9241 | 0.01801 | HU: Decreased HUR: Decreased |
https://hmdb.ca/metabolites/HMDB0001932 |
| l-Glutamic acid | 000148 | 2.9017 | 0.01801 | HU: Increased HUR: Increased |
https://hmdb.ca/metabolites/HMDB0000148 |
| Acetoin | 0003243 | 2.8802 | 0.01801 | HU: Decreased HUR: Decreased |
https://hmdb.ca/metabolites/HMDB0003243 |
| Sphinganine | 0000269 | 2.7342 | 0.02268 | HU: Decreased HUR: Not changed |
https://hmdb.ca/metabolites/HMDB0000269 |
| Maltitol | 0002928 | 2.3847 | 0.04611 | HU: Increased HUR: Increased |
https://hmdb.ca/metabolites/HMDB0002928 |
| Xanthosine | 0000299 | 2.3384 | 0.04703 | HU: Decreased HUR: Decreased |
https://hmdb.ca/metabolites/HMDB0000299 |
3.4. Multi-omics integrated analysis
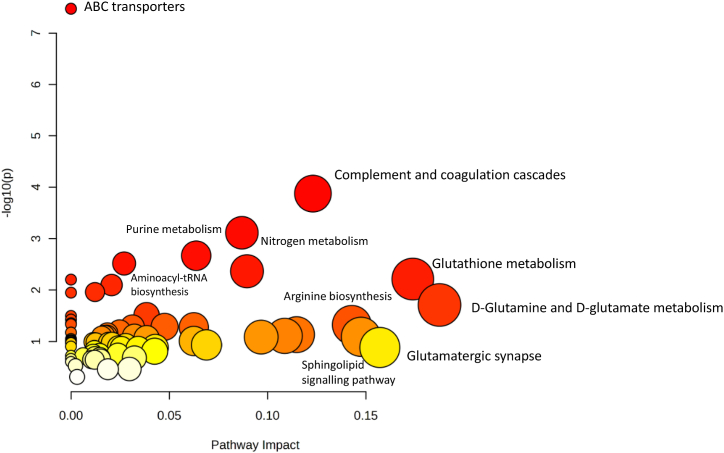
To integrate the information obtained through proteomics and metabolomics approaches, we utilized the MetaboAnalyst (https://www.metaboanalyst.ca/) option Joint-Pathways Analysis (all pathways). We used the entry IDs of 12 proteins, and 12 metabolites that were found to be significantly changed. Pathways such as ABC transporters, complement and coagulation cascades, nitrogen metabolism, purine metabolism, glutathione metabolism, aminoacyl-tRNA biosynthesis were found, as shown in Fig. 6.
Fig. 6.
Analysis of joint pathway enrichment. Using MetaboAnalyst joint-pathway enrichment, pathways enriched for dysregulated proteins and metabolites (p 0.05). The Joint-Pathways Analysis findings are represented by -log10(P) values on the y axis. The data point sizes are related to their x values, and the colours are related to their y values. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The gravitational forces of the Earth are critical to human health, in particular during space travel and prolonged bed rest. Astronauts in space are subjected to prolonged microgravity, which causes physiological changes such as immune dysfunction, dysbiosis, muscle atrophy, fluid shift, and cardiovascular deconditioning [20]. In our study, HU mice exhibited heart atrophy and decreased MAP, along with skeletal muscle atrophy and decreased muscle strength, comparable to what was observed due to microgravity in space travel and prolonged bed rest [21]. However, during the recovery phase in the HUR group, these changes were largely reversed.
Changes in cardiovascular systems are among the major or critical adaptations to a microgravity environment and prolonged bed rest resulting in cardiac remodeling. Astronauts suffer from hypovolemia as well as a lack of orthostatic pressure and a decrease in arterial pressures, resulting in decrease of the cardiac workload and, eventually, the development of cardiac atrophy [20]. Zhong et al. reported that in simulated microgravity, HU rodent model suspended for 28 days showed reduced left ventricle ejection fraction (LV-EF) and LV-FS (fractional shortening) with a reduction in heart weight, indicating a decline in heart function. Similarly, astronauts showed a 9.1 % decrease in LV mass, cardiac atrophy after 10 days of spaceflight that returned to normal after the third day of recovery [[22], [23], [24]]. The effect of simulated microgravity on the heart weight was reversed after two weeks of recovery or reloading the HU mice on the ground. However, although MAP of the HUR group showed a trend toward increase compared to HU mice it's more difficult to restore MAP to the normal state in two weeks of reloading only, perhaps due to the blood pressure measurement variability that required a larger number of mice. Proteomics data revealed 12 significant proteins among the groups, some of these proteins have a role in atherosclerosis. Phospholipid transfer protein (Fig. 4C (a)) is one of these proteins, and it is responsible for the transfer of lipid molecules like sphingomyelin and phosphatidylcholine (PC) to high-density lipoproteins (HDL) [25]. Studies have reported various roles for this protein in the cardiovascular system, immunology, and inflammation [25]. According to one of these research, Macrophage Phospholipid transfer protein deficiency causes atherosclerosis in LDL receptor-deficient mice, indicating that it has an atheroprotective function which is a critical risk factor in microgravity environment [26,27]. Thrombospondin-1 is a multi-modular glycoprotein with physiological and pathological effects on vascular responsiveness, inflammation, wound healing, apoptosis, and angiogenesis. Furthermore, Thrombospondin-1 deficiency has been shown to accelerate the inflammatory alterations essential for plaque growth in ApoE-deficient mice [28]. Under microgravity conditions, increased inflammation and plaque formation are significant factors that promote atherosclerosis which might explain the decrease in HU mice compared to ground-based controls. HUR on the other hand, showed an increase compared to HU mice indicating a recovery process in this group [27,28]. Moreover, although most of proteins altered during microgravity were recovered in two weeks period, interestingly two proteins did not follow the same pattern. One of these proteins is kininogen 2, kininogen deficiency has been shown in previous studies to protect against stroke and other thromboembolic diseases [29]. The prolonged overexpression of kininogen 2 in HUR mice may imply a risk for thromboembolic disorders in these mice, as well as the possible use of this marker as a target for thrombotic diseases. This further indicate the nature of these proteins necessitates a longer recovery period than two weeks or/and this continuous increased in the HUR group could lead to other consequences linked to cardiovascular health.
Apolipoprotein C is a family of four proteins that regulate triglyceride levels and cholesterol levels in plasma (APOC- I, APOC- II, APOC-III, and APOC IV) [30]. Although apolipoproteins C–I, C-II, and C-III have been associated to a higher risk of venous thromboembolism, little is known about apolipoprotein C-IV, and more research is needed to determine the role it plays in the cardiovascular system [31].
Purine metabolism has been shown in studies to disrupt the normal energy flow of the heart, which may be a crucial metabolic modification in the course of cardiovascular diseases [32,33]. Purine metabolism involves inosine, hypoxanthine, and xanthosine. Inosine, a stable analog of adenosine, is currently being investigated for its potential use as a medication for the treatment of cardiovascular diseases such as atherosclerosis by activating endothelial nitric oxide synthase (eNOS) and decreasing inflammation pathways in aortic tissues [34]. Although the direct effect of endogenous inosine on the cardiovascular system has not been investigated, Akkermansia muciniphila (A. muciniphila), which is a common resident in the gastrointestinal tract of humans and responsible for producing the metabolite inosine, has been shown to protect against atherosclerosis [[35], [36], [37]]. Interestingly, A. muciniphila was not observed in HU mice but was found in control mice in a recent study [38], which explains the drop in inosine levels in HU mice, suggesting that this microbe and its metabolite are beneficial and have a protective role [38]. Purine and pyrimidine metabolism diseases result from cardiac energy abnormalities, which in turn affect DNA and protein synthesis. In acute myocardial infarction (AMI), xanthosine levels were found to be lower, as was uric acid, which is metabolized by xanthosine and xanthine as a byproduct of purine metabolism [39]. Furthermore, HU mice showed a significant decrease in xanthosine compared to ground-based controls, whereas the HUR group showed a trend toward an increase. HU mice showed an increase of 3,4-Dihydroxyphenylglycol, while HUR showed a further increase compared to ground-based controls. Norepinephrine is converted to dihydroxyphenylglycol via monoamine-oxidases after re-uptake from the synaptic cleft. Studies have shown that astronauts with less orthostatic tolerance had significantly higher dihydroxyphenylglycol plasma concentrations as well as a reduced norepinephrine response along with lower standing systemic vascular resistance [40]. Norepinephrine has a blood pressure-raising effect that HU mice may require [41]. In line with this, the sphingolipids signaling pathway was one of the functional enrich in the joint pathway analysis (Fig. 6), indeed, they are an essential type of lipid that is crucial for the cardiovascular system. Sphingosine, an amino alcohol, is a highly bioactive molecule that participates in several biological processes, including cell-cell interaction, cell proliferation, differentiation, and apoptosis. However, further studies are needed to fully comprehend the role of sphingolipids in cardiovascular disease [42,43].
Moreover, in a microgravity environment, several alterations in the structure, function, and proteomic profile of skeletal muscle have been reported [44,45]. In our study, HU mice showed a reduction in gastrocnemius muscle weight, as well as a loss in grip strength when compared to ground-based controls, indicating both muscular atrophy and a change in skeletal muscle function. However, compared to HU mice, the HUR group exhibited a substantial increase in gastrocnemius muscle mass and grip strength, indicating the possibility of repairing skeletal muscle mass and function in this condition. A recent study made by Oliveira et al. showed no significant change in gastrocnemius muscle mass in rat models that were unloaded for two weeks, followed by two weeks of recovery. These controversial findings might be explained by the fact that they used aged rats (26 months old), and while muscle mass recovery following disuse-induced atrophy is possible at early ages, it is often hindered in old age muscles from older rodents [46]. Interestingly, HU mice showed a drop in parvalbumin, which is referred to as an "atrogene" and is commonly shown to be downregulated in skeletal muscle atrophy, whereas HUR demonstrated some reverse to this decline, showing that two weeks is adequate to minimize skeletal muscle atrophy [47]. Hypoxanthine is another purine derivative that plays a role in skeletal muscle mass, as studies show that treatment with xanthine oxidase inhibitors like allopurinol, which is responsible for the breakdown of hypoxanthine to xanthine to uric acid, reduces disuse muscle atrophy, primarily by reducing ROS generation [48]. When HU mice were compared to ground-based controls, hypoxanthine levels decreased; however, the HUR group reversed this reduction. Studies have shown that following exercise, there is a rise in plasma hypoxanthine levels, indicating that net adenine nucleotide breakdown has occurred, explaining the increase in hypoxanthine levels in the HUR group compared to the HU group [49]. Branched-chain amino acids (BCAA) such as leucine and valine are frequently found in high concentrations among those at risk for CVD, and they are responsible for a significant increase in skeletal muscle wet weight by increasing protein synthesis and decreasing degradation [50,51]. Despite the positive effects of BCAA, Bajotto et al. demonstrated that BCAA only supplementation during unloading does not prevent protein breakdown which can further explain that although HU mice showed increase in levels of l-valine compared to ground-based controls, and HUR group showed a slight decrease compared to HU and a significant increase compared to ground-based controls, it was not enough to prevent and/or decrease the muscle loss that happened [52]. l-Glutamic acid showed a similar pattern, and studies have demonstrated that leucine, either alone or in combination with glutamic acid, is advantageous for the growth of muscles. Additionally, supplementing with arginine and glutamic acid lowers the mRNA levels of genes in pig skeletal muscle that are involved in the degradation of proteins [53]. Further studies are needed to investigate the exact role of different BCAAs and glutamic acid in muscle atrophy and cardiovascular system.
It has been well documented that the immune system is dysregulated in space, specifically the humoral response, with changes in the immunoglobulin heavy variable chain indicating that space affects the humoral response and B-cell selection, which explains the increase in Ighv14-3 and Igkv4-69 in HU mice [54,55]. The immunoglobulin heavy chain gene encodes the heavy chain of antibodies, which is responsible for recognizing foreign antigens and initiating immunological responses. Moreover, HU mice also showed a decrease in both C1 and plasma protease C1 inhibitor, indicating that activation of C1, which leads to covalently binding of plasma protease C1 inhibitor activated C1r and C1s, which removes C1r and C1s from the complex [56]. Overall, the findings demonstrate dysregulation of four immune-related proteins, demonstrating that simulated microgravity alters the immune system and that recovery from this condition can restore regular immune function.
The microgravity environment may cause many abnormalities, including changes in reproductive function, musculoskeletal system, and oxidative damage in mitochondria [[57], [58], [59]]. HU mice showed a reduction in carbonic anhydrase 3, an enzyme discovered to have antioxidant properties and can catalyze the hydration of carbon dioxide to bicarbonate and proton [60]. There was a drop in serum glutathione levels in HU and HUR mice, which suggest an elevation of oxidative stress in these mice because Joint-Pathways Analysis showed glutathione is a significant antioxidant that is responsible for eliminating ROS and hence preventing CVDs and skeletal muscle atrophy [[61], [62], [63]].
Of interest, the proteomics data indicate that one of the major urinary protein 2 was significantly decreased in HU animals and reversed in the HUR group to comparable levels found in the control group, or higher, although not significant. This protein has been identified as a male pheromone that increases female attraction to male urine smell and generates a strong learnt attraction to an individual male's airborne urinary Odor [64]. Additionally, MUP20 promotes male aggressive behavior that occurs through vomeronasal organ and the main olfactory epithelium. It is plausible that HU primary goal as an individual is to endure unfavorable adverse conditions, which obviously compromises any type of social interaction, including sexual competitiveness and opposite sexual attraction. This explains why MUP20 levels in HU mice are reduced, whereas MUP20 levels in HUR are restored, close to that observed in control, as HUR resume their supposedly natural lives, including their inherent sexual attraction.
Altogether, these observed changes in proteins and metabolites in HU and HUR groups are involved in cardiovascular diseases such as atherosclerosis, skeletal muscle atrophy, changes in the immune system, alterations in reproductive function, and oxidative stress. Moreover, Joint-Pathways Analysis showed pathways such as ABC transporters, complement and coagulation cascades, nitrogen metabolism, purine metabolism, glutathione metabolism, aminoacyl-tRNA biosynthesis were found. Our findings provide a descriptive insight into specific changes in proteins and metabolites during unloaded and recovery periods in a robust microgravity mouse model. As a result of our findings, it's expected that numerous studies can develop diagnostic and therapeutic approaches for space flights and prolonged bed rest that incorporate these biomarkers and their broader physiological and clinical implications.
Our findings suggest that various proteins and metabolites may be responsible for changes in cardiovascular and skeletal muscle in situations such as microgravity, and that they might be employed as biomarkers to monitor the function of both cardiovascular and skeletal muscle and overall astronauts’ health. Moreover, future research should investigate the beneficial effect of decreased metabolites in HU mice, in an attempt to compensate these metabolites and whether it will change the cardiovascular and skeletal muscle function and structure in simulated microgravity environment.
5. Limitation and future direction
We have undertaken a descriptive study, and additional functional testing of the modified proteins and metabolites is essential. Furthermore, a thorough investigation into their direct effects on the cardiovascular system and skeletal muscle is warranted in future studies.
Funding source
This project is partialy supported by Cardiovascular Diseases Research Group and Space Medicine Research Group Operational Grants, University of Sharjah (UOS). Zeinab Ibrahim is supported by College of Graduate Studies and Scientific Research, UOS. Ruqaiyyah Siddiqui and Naveed Ahmed Khan are supported by the Air Force Office of Scientific Research (AFOSR), USA Grant number: FA9550-23-1-0711.
Data Availability Statement
Proteomics mass spectrometry data have been submitted to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD042753. Metabolomics data are available in Metabolomics Workbench under the research ID PR001669 (DOI: 10.21228/M8ZQ5Q).
CRediT authorship contribution statement
Zeinab Ibrahim: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Naveed A. Khan: Writing – review & editing, Supervision, Formal analysis, Conceptualization. Rizwan Qaisar: Writing – review & editing, Software, Resources, Methodology, Formal analysis. Mohamed A. Saleh: Writing – review & editing, Software, Methodology, Formal analysis. Ruqaiyyah Siddiqui: Writing – review & editing, Methodology, Investigation, Formal analysis. Hamza M. Al-Hroub: Writing – review & editing, Methodology, Data curation. Alexander D. Giddey: Writing – review & editing, Investigation, Formal analysis. Mohammad Harb Semreen: Software, Resources, Investigation. Nelson C. Soares: Writing – original draft, Validation, Supervision, Formal analysis, Conceptualization. Adel B. Elmoselhi: Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23592.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- [1.Jemison M., Olabisi R. Biomaterials for human space exploration: a review of their untapped potential. Acta Biomater. 2021;128:77–99. doi: 10.1016/j.actbio.2021.04.033. [DOI] [PubMed] [Google Scholar]
- 2.Dirks M.L., Wall B.T., van de Valk B., Holloway T.M., Holloway G.P., Chabowski A., Goossens G.H., van Loon L.J. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes. 2016;65(10):2862–2875. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 3.Wall B.T., van Loon L.J. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013;71(4):195–208. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- 4.Baek H., Cho M., Kim S., Hwang H., Song M., Yoo S. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonutto G., di Prampero P.E. Cardiovascular deconditioning in microgravity: some possible countermeasures. Eur. J. Appl. Physiol. 2003;90(3–4):283–291. doi: 10.1007/s00421-003-0884-5. [DOI] [PubMed] [Google Scholar]
- 6.Vernice N.A., Meydan C., Afshinnekoo E., Mason C.E. Long-term spaceflight and the cardiovascular system. Precis Clin Med. 2020;3(4):284–291. doi: 10.1093/pcmedi/pbaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jollet M., Nay K., Chopard A., Bareille M.P., Beck A., Ollendorff V., Vernus B., Bonnieu A., Mariadassou M., Rue O., Derbre F., Goustard B., Koechlin-Ramonatxo C. Does physical inactivity induce significant changes in human gut microbiota? New answers using the dry immersion hypoactivity model. Nutrients. 2021;13(11) doi: 10.3390/nu13113865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fovet T., Guilhot C., Stevens L., Montel V., Delobel P., Roumanille R., Sempore M.Y., Freyssenet D., Py G., Brioche T., Chopard A. Early deconditioning of human skeletal muscles and the effects of a thigh cuff countermeasure. Int. J. Mol. Sci. 2021;22(21) doi: 10.3390/ijms222112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardiovascular Diseases (CVDs) 2021. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds June 11. [Google Scholar]
- 10.Shehab A., Bakir S., Sabbour H., Elnour A.A., Mahmeed W.A., Salam A.M., Kholy D.E. Prevalence of cardiovascular risk factors and 10-years risk for coronary heart disease in the United Arab Emirates. Curr. Diabetes Rev. 2023;19(3):38–48. doi: 10.2174/1573399818666220421113607. [DOI] [PubMed] [Google Scholar]
- 11.Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1(2):182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlachavas E.I., Bohn J., Uckert F., Nurnberg S. A detailed catalogue of multi-omics methodologies for identification of putative biomarkers and causal molecular networks in translational cancer research. Int. J. Mol. Sci. 2021;22(6) doi: 10.3390/ijms22062822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muqaku B., Eisinger M., Meier S.M., Tahir A., Pukrop T., Haferkamp S., Slany A., Reichle A., Gerner C. Multi-omics analysis of serum samples demonstrates reprogramming of organ functions via systemic calcium mobilization and platelet activation in metastatic melanoma. Mol. Cell. Proteomics. 2017;16(1):86–99. doi: 10.1074/mcp.M116.063313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L., Surapaneni A., Rhee E.P., Yu B., Boerwinkle E., Coresh J., Grams M.E., Schlosser P. Integrated proteomic and metabolomic modules identified as biomarkers of mortality in the atherosclerosis risk in communities study and the african American study of kidney disease and hypertension. Hum. Genom. 2022;16(1):53. doi: 10.1186/s40246-022-00425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murgia M., Brocca L., Monti E., Franchi M.V., Zwiebel M., Steigerwald S., Giacomello E., Sartori R., Zampieri S., Capovilla G., Gasparini M., Biolo G., Sandri M., Mann M., Narici M.V. Plasma proteome profiling of healthy subjects undergoing bed rest reveals unloading-dependent changes linked to muscle atrophy. J Cachexia Sarcopenia Muscle. 2023;14(1):439–451. doi: 10.1002/jcsm.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastushkova L.K., Rusanov V.B., Goncharova A.G., Nosovskiy A.M., Luchitskaya E.S., Kashirina D.N., Kononikhin A.S., Kussmaul A.R., Yakhya Y.D., Larina I.M., Nikolaev E.N. Blood plasma proteins associated with heart rate variability in cosmonauts who have completed long-duration space missions. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.760875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan A.A., Gul M.T., Karim A., Ranade A., Azeem M., Ibrahim Z., Ramachandran G., Nair V.A., Ahmad F., Elmoselhi A., Qaisar R. Mitigating sarcoplasmic reticulum stress limits disuse-induced muscle loss in hindlimb unloaded mice. NPJ Microgravity. 2022;8(1):24. doi: 10.1038/s41526-022-00211-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isenberg J.S., Qin Y., Maxhimer J.B., Sipes J.M., Despres D., Schnermann J., Frazier W.A., Roberts D.D. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28(2):110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zenati R.A., Giddey A.D., Al-Hroub H.M., Hagyousif Y.A., El-Huneidi W., Bustanji Y., Abu-Gharbieh E., Alqudah M.A.Y., Shara M., Abuhelwa A.Y., Soares N.C., Semreen M.H. Evaluation of two simultaneous metabolomic and proteomic extraction protocols assessed by ultra-high-performance liquid chromatography tandem mass spectrometry. Int. J. Mol. Sci. 2023;24(2) doi: 10.3390/ijms24021354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baran R., Marchal S., Garcia Campos S., Rehnberg E., Tabury K., Baselet B., Wehland M., Grimm D., Baatout S. The cardiovascular system in space: focus on in vivo and in vitro studies. Biomedicines. 2021;10(1) doi: 10.3390/biomedicines10010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrew N.G., Blaber P. Da Xu Prolonged unloading of the cardiovascular system during bedrest and spaceflight weakens neural coupling between blood pressure and heart rate. Acta Astronaut. 2022;195(0094–5765):567–573. [Google Scholar]
- 22.Perhonen M.A., Franco F., Lane L.D., Buckey J.C., Blomqvist C.G., Zerwekh J.E., Peshock R.M., Weatherall P.T., Levine B.D. Cardiac atrophy after bed rest and spaceflight. J. Appl. Physiol. 2001;91(2):645–653. doi: 10.1152/jappl.2001.91.2.645. 1985. [DOI] [PubMed] [Google Scholar]
- 23.Zhong G., Li Y., Li H., Sun W., Cao D., Li J., Zhao D., Song J., Jin X., Song H., Yuan X., Wu X., Li Q., Xu Q., Kan G., Cao H., Ling S., Li Y. Simulated microgravity and recovery-induced remodeling of the left and right ventricle. Front. Physiol. 2016;7:274. doi: 10.3389/fphys.2016.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers R.L., Martin D.S., Meck J.V., Coleman T.G. Mechanism of spaceflight-induced changes in left ventricular mass. Am. J. Cardiol. 2005;95(9):1128–1130. doi: 10.1016/j.amjcard.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Albers J.J., Vuletic S., Cheung M.C. Role of plasma phospholipid transfer protein in lipid and lipoprotein metabolism. Biochim. Biophys. Acta. 2012;1821(3):345–357. doi: 10.1016/j.bbalip.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valenta D.T., Ogier N., Bradshaw G., Black A.S., Bonnet D.J., Lagrost L., Curtiss L.K., Desrumaux C.M. Atheroprotective potential of macrophage-derived phospholipid transfer protein in low-density lipoprotein receptor-deficient mice is overcome by apolipoprotein AI overexpression. Arterioscler. Thromb. Vasc. Biol. 2006;26(7):1572–1578. doi: 10.1161/01.ATV.0000225700.43836.ae. [DOI] [PubMed] [Google Scholar]
- 27.Sucosky P., Kalaiarasan V.V., Quasebarth G.B., Strack P., Shar J.A. Atherogenic potential of microgravity hemodynamics in the carotid bifurcation: a numerical investigation. NPJ Microgravity. 2022;8(1):39. doi: 10.1038/s41526-022-00223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kale A., Rogers N.M., Ghimire K. Thrombospondin-1 CD47 signalling: from mechanisms to medicine. Int. J. Mol. Sci. 2021;22(8) doi: 10.3390/ijms22084062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langhauser F., Gob E., Kraft P., Geis C., Schmitt J., Brede M., Gobel K., Helluy X., Pham M., Bendszus M., Jakob P., Stoll G., Meuth S.G., Nieswandt B., McCrae K.R., Kleinschnitz C. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood-brain barrier damage, and inflammation. Blood. 2012;120(19):4082–4092. doi: 10.1182/blood-2012-06-440057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu C.C., Kanter J.E., Kothari V., Bornfeldt K.E. Quartet of APOCs and the different roles they play in diabetes. Arterioscler. Thromb. Vasc. Biol. 2023;43(7):1124–1133. doi: 10.1161/ATVBAHA.122.318290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orsi F.A., Lijfering W.M., Van der Laarse A., Ruhaak L.R., Rosendaal F.R., Cannegieter S.C., Cobbaert C. Association of apolipoproteins C-I, C-II, C-III and E with coagulation markers and venous thromboembolism risk. Clin. Epidemiol. 2019;11:625–633. doi: 10.2147/CLEP.S196266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G., Zou R., Liu L., Wang Z., Zou Z., Tan S., Xu W., Fan X. A circular network of purine metabolism as coregulators of dilated cardiomyopathy. J. Transl. Med. 2022;20(1):532. doi: 10.1186/s12967-022-03739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin N., Yu M., Du X., Wu Z., Zhai C., Pan H., Gu J., Xie B. Identification of potential serum biomarkers for congenital heart disease children with pulmonary arterial hypertension by metabonomics. BMC Cardiovasc. Disord. 2023;23(1):167. doi: 10.1186/s12872-023-03171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lima G.F., Lopes R.O., Mendes A.B.A., Brazao S.C., Autran L.J., Motta N.A.V., Brito F.C.F. Inosine, an endogenous purine nucleoside, avoids early stages of atherosclerosis development associated to eNOS activation and p38 MAPK/NF-kB inhibition in rats. Eur. J. Pharmacol. 2020;882 doi: 10.1016/j.ejphar.2020.173289. [DOI] [PubMed] [Google Scholar]
- 35.Kroemer G., Zitvogel L. Inosine: novel microbiota-derived immunostimulatory metabolite. Cell Res. 2020;30(11):942–943. doi: 10.1038/s41422-020-00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwaza R W.R., Dubourg G., Raoult D., muciniphila Lagier J-C Akkermansia. The state of the art, 18 years after its first discovery. Frontline Gastroenterol. 2022;1 – 2022 [Google Scholar]
- 37.Li J., Lin S., Vanhoutte P.M., Woo C.W., Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in apoe-/- mice. Circulation. 2016;133(24):2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 38.Shama S., Qaisar R., Khan N.A., Tauseef I., Siddiqui R. The role of 4-phenylbutyric acid in gut microbial dysbiosis in a mouse model of simulated microgravity. Life. 2022;12(9) doi: 10.3390/life12091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rong W., Li J., Wang L., Luo S., Liang T., Qian X., Zhang X., Zhou Q., Zhu Y., Zhu Q. Investigation of the protective mechanism of leonurine against acute myocardial ischemia by an integrated metabolomics and network pharmacology strategy. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.969553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan J., Limper U., Tank J. Cardiovascular autonomic nervous system responses and orthostatic intolerance in astronauts and their relevance in daily medicine. Neurol. Sci. 2022;43(5):3039–3051. doi: 10.1007/s10072-022-05963-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foulon P., De Backer D. The hemodynamic effects of norepinephrine: far more than an increase in blood pressure. Ann. Transl. Med. 2018;6(Suppl 1):S25. doi: 10.21037/atm.2018.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brogden G., Husein D.M., Steinberg P., Naim H.Y. Isolation and quantification of sphingosine and sphinganine from rat serum revealed gender differences. Biomolecules. 2019;9(9) doi: 10.3390/biom9090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borodzicz-Jazdzyk S., Jazdzyk P., Lysik W., Cudnoch-Jedrzejewska A., Czarzasta K. Sphingolipid metabolism and signaling in cardiovascular diseases. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.915961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitts R.H., Riley D.R., Widrick J.J. Functional and structural adaptations of skeletal muscle to microgravity. J. Exp. Biol. 2001;204(Pt 18):3201–3208. doi: 10.1242/jeb.204.18.3201. [DOI] [PubMed] [Google Scholar]
- 45.Murgia M., Ciciliot S., Nagaraj N., Reggiani C., Schiaffino S., Franchi M.V., Pisot R., Simunic B., Toniolo L., Blaauw B., Sandri M., Biolo G., Fluck M., Narici M.V., Mann M. Signatures of muscle disuse in spaceflight and bed rest revealed by single muscle fiber proteomics. PNAS Nexus. 2022;1(3):pgac086. doi: 10.1093/pnasnexus/pgac086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveira J.R.S., Mohamed J.S., Myers M.J., Brooks M.J., Alway S.E. Effects of hindlimb suspension and reloading on gastrocnemius and soleus muscle mass and function in geriatric mice. Exp. Gerontol. 2019;115:19–31. doi: 10.1016/j.exger.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butera G., Vecellio Reane D., Canato M., Pietrangelo L., Boncompagni S., Protasi F., Rizzuto R., Reggiani C., Raffaello A. Parvalbumin affects skeletal muscle trophism through modulation of mitochondrial calcium uptake. Cell Rep. 2021;35(5) doi: 10.1016/j.celrep.2021.109087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller S.G., Hafen P.S., Brault J.J. Increased adenine nucleotide degradation in skeletal muscle atrophy. Int. J. Mol. Sci. 2019;21(1) doi: 10.3390/ijms21010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ketai L.H., Simon R.H., Kreit J.W., Grum C.M. Plasma hypoxanthine and exercise. Am. Rev. Respir. Dis. 1987;136(1):98–101. doi: 10.1164/ajrccm/136.1.98. [DOI] [PubMed] [Google Scholar]
- 50.Grajeda-Iglesias C., Aviram M. Specific amino acids affect cardiovascular diseases and atherogenesis via protection against macrophage foam cell formation: review article. Rambam Maimonides Med J. 2018;9(3) doi: 10.5041/RMMJ.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eley H.L., Russell S.T., Tisdale M.J. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem. J. 2007;407(1):113–120. doi: 10.1042/BJ20070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bajotto G., Sato Y., Kitaura Y., Shimomura Y. Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur. J. Appl. Physiol. 2011;111(8):1815–1828. doi: 10.1007/s00421-010-1825-8. [DOI] [PubMed] [Google Scholar]
- 53.Kamei Y., Hatazawa Y., Uchitomi R., Yoshimura R., Miura S. Regulation of skeletal muscle function by amino acids. Nutrients. 2020;12(1) doi: 10.3390/nu12010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boxio R., Dournon C., Frippiat J.P. Effects of a long-term spaceflight on immunoglobulin heavy chains of the urodele amphibian Pleurodeles waltl. J. Appl. Physiol. 2005;98(3):905–910. doi: 10.1152/japplphysiol.00957.2004. 1985. [DOI] [PubMed] [Google Scholar]
- 55.Bascove M., Huin-Schohn C., Gueguinou N., Tschirhart E., Frippiat J.P. Spaceflight-associated changes in immunoglobulin VH gene expression in the amphibian Pleurodeles waltl. Faseb. J. 2009;23(5):1607–1615. doi: 10.1096/fj.08-121327. [DOI] [PubMed] [Google Scholar]
- 56.John T.W.D.C., Atkinson P., Mold Carolyn, Kulkarni Hrishikesh, Hourcade Dennis, Wu Xiaobo. The Human Complement System: Basic Concepts and Clinical Relevance, Clinical Immunology. fifth ed. Elsevier; 2019. pp. 299–317.e1. 9780702068966. [Google Scholar]
- 57.Mishra B., Luderer U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019;15(12):713–730. doi: 10.1038/s41574-019-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juhl O.J.t., Buettmann E.G., Friedman M.A., DeNapoli R.C., Hoppock G.A., Donahue H.J. Update on the effects of microgravity on the musculoskeletal system. NPJ Microgravity. 2021;7(1):28. doi: 10.1038/s41526-021-00158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen H.P., Tran P.H., Kim K.S., Yang S.G. The effects of real and simulated microgravity on cellular mitochondrial function. NPJ Microgravity. 2021;7(1):44. doi: 10.1038/s41526-021-00171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Fiore A., Monti D.M., Scaloni A., De Simone G., Monti S.M. Protective role of carbonic anhydrases III and VII in cellular defense mechanisms upon redox unbalance. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/2018306. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon D.H., Cha H.J., Lee H., Hong S.H., Park C., Park S.H., Kim G.Y., Kim S., Kim H.S., Hwang H.J., Choi Y.H. Protective effect of glutathione against oxidative stress-induced cytotoxicity in RAW 264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants. 2019;8(4) doi: 10.3390/antiox8040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matuz-Mares D., Riveros-Rosas H., Vilchis-Landeros M.M., Vazquez-Meza H. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants. 2021;10(8) doi: 10.3390/antiox10081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J., Lu M., Ahn Y., Cao K., Pinkus C.A., Stansfield J.C., Wu Z., Zhang B.B. CHAC1 inactivation is effective to preserve muscle glutathione but is insufficient to protect against muscle wasting in cachexia. PLoS One. 2023;18(4) doi: 10.1371/journal.pone.0283806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts S.A., Simpson D.M., Armstrong S.D., Davidson A.J., Robertson D.H., McLean L., Beynon R.J., Hurst J.L. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male's odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Speidl W.S., Kastl S.P., Huber K., Wojta J. Complement in atherosclerosis: friend or foe? J. Thromb. Haemostasis. 2011;9(3):428–440. doi: 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 66.Feng H.Z., Jin J.P. Transgenic expression of carbonic anhydrase III in cardiac muscle demonstrates a mechanism to tolerate acidosis. Am J Physiol Cell Physiol. 2019;317(5):C922–C931. doi: 10.1152/ajpcell.00130.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis A.E., 3rd, Mejia P., Lu F. Biological activities of C1 inhibitor. Mol. Immunol. 2008;45(16):4057–4063. doi: 10.1016/j.molimm.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merkler A., Sertic J., Bazina Martinovic A., Kriz T., Milicic I., Simic M., Caban D., Ljubic H., Markeljevic J., Simicevic L., Kastelan S., Pecin I., Reiner Z. Haptoglobin genotype 2-2 associated with atherosclerosis in patients with ischemic stroke. Gene. 2020;752 doi: 10.1016/j.gene.2020.144786. [DOI] [PubMed] [Google Scholar]
- 69.Rhaleb N.E., Yang X.P., Carretero O.A. The kallikrein-kinin system as a regulator of cardiovascular and renal function. Compr. Physiol. 2011;1(2):971–993. doi: 10.1002/cphy.c100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Proteomics mass spectrometry data have been submitted to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD042753. Metabolomics data are available in Metabolomics Workbench under the research ID PR001669 (DOI: 10.21228/M8ZQ5Q).