Abstract
Mucosal leishmaniosis (ML) is a rare manifestation of leishmaniosis, usually caused by Leishmania brasiliensis in northeast Brazil and by Leishmania infantum and Leishmania donovani in the Mediterranean Europe and Africa. We present the case of a 66-year-old man living between Belgium and Congo, presenting with dysphonia for several months.
Imaging work-up with PET-CE, CT scan, and MRI of the tongue, larynx, and esophagus reflected inflammatory and granulomatous tissue, confirmed at the biopsy. The histological examination confirmed the presence of inflammatory granulomatous tissue with Donovan bodies in the tongue, larynx, and esophageal specimens, in keeping with multifocal ML.
In conclusion, inflammatory and granulomatous mucosal lesions in individuals leaving or traveling in endemic areas should prompt suspect ML. Imaging can facilitate the appropriate histological and biological examination and nonivasively confirm the response to antiparasitic treatment on follow-up.
Keywords: Mucosal leishmaniosis, Imaging of leishmaniosis, Tongue leishmaniosis, Laryngeal leishmaniosis, Esophageal leishmaniosis, Donovan bodies
Introduction
Mucosal leishmaniosis (ML) is a rare clinical variant of tegumentary leishmaniosis in Mediterranean Europe [1]. ML is characterized by mucosal destruction, with mucous membrane involvement of the nose, oral cavity, pharynx, or larynx. The symptomatology of the disease depends on the localization of the lesion and may include nasal obstruction, swallowing difficulties, mucosal bleeding, and hoarseness [2].
Most ML cases are observed in Latin America and are due to Leishmania brasiliensis [2]; however, there have been few reports of ML with L infantum and L donovani which are typical of the Mediterranean basin, India, and sub-Saharan Africa [1].
The few described cases of ML merely provide a description of clinical findings in the case of superficial lesions or after fibroscopy in the case of deep lesions [3].
In the present case, we report an unusual multifocal manifestation of ML with localizations on the tongue, larynx, and esophagus. We focus on CT and MRI results and try to establish a relation with anatomopathologic findings.
Case report
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its amendments or comparable ethical standards and were approved by our Institutional Review Board (IRB).
A 66 years old man, living between Belgium and Congo, presented to our outpatient clinic with dysphonia for several months. He had a past medical history of malaria and a social history of smoking and alcohol abuse. The patient had a CT scan of the neck in another institution (not shown) reporting 2 suspected neoplastic lesions, one at the level of the anterior third of the tongue and another on the left vocal cord.
On clinical examination, we identified an infiltration of the anterior third of the tongue and Reinke edema with keratosis of the anterior third of the left vocal cord.
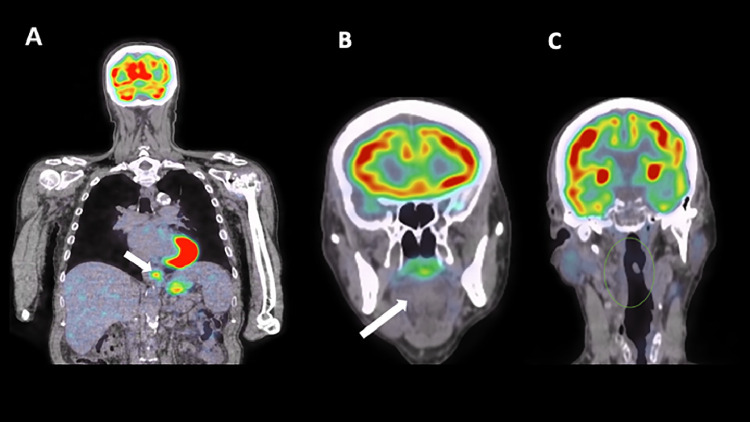
The 18-FDG PET performed thereafter showed increased uptake and parietal thickening of the distal esophagus (Fig. 1A) and no uptake of the tongue (white arrow, Fig. 1B) and neck area (green circle, Fig. 1C).
Fig. 1.
18-FDG PET/CT shows focal increased uptake of the distal esophagus (A, white arrow) and no abnormal uptake of the tongue (B, white arrow) and neck area (C, green circle).
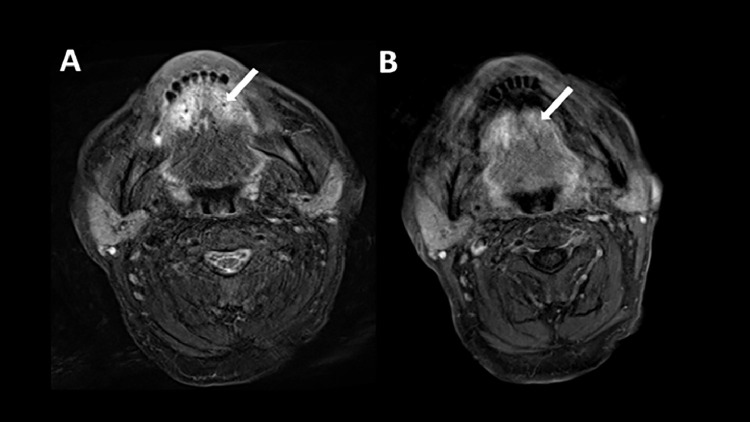
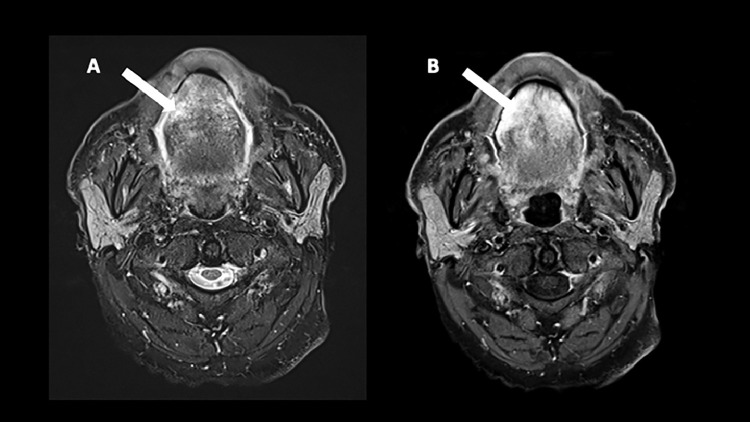
An MRI was performed a few days later showing mild T2 hyperintensity of a thickened anterior third of the tongue (white arrow, Fig. 2A) with mild enhancement (white arrow, Fig. 2B).
Fig. 2.
The first MRI realized a few days after the PET-CT shows on axial T2W (image A) a mild hyperintensity of a thickened anterior third of the tongue (A, white arrow) and a mild enhancement on the T1W fat-saturated postgadolinium (B, white arrow).
The patient underwent a biopsy of the tongue and of the anterior third of the left vocal cord, showing nonspecific granulation tissue with polymorph infiltrate, after which he refused an oesophagoscopy and went back to Congo.
Two years later the patient presented again with complaints of dysphonia and even severe weight loss of 20 kg. Clinical examination showed a persistent infiltration covered by keratosis on the anterior third of the tongue as well as persistent Reinke edema on both edema of vocal cords.
A gastroscopy was subsequently performed showing inflammation with ulcerations of the distal esophagus. A biopsy of the ulcered esophageal mucosa was taken at that moment.
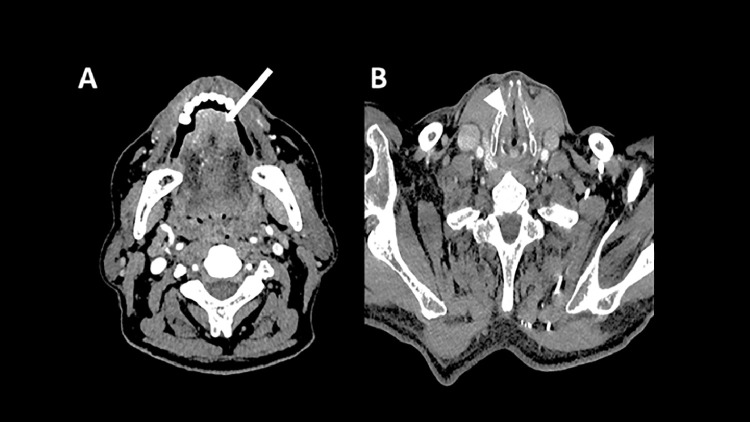
The CT scan performed thereafter showed moderate enhancement and thickening of the anterior tongue (white arrow, Fig. 3A) and mild nonenhancing thickening of the true vocal cords (arrowhead, Fig. 3B).
Fig. 3.
The CT scan showed a moderate enhancement and thickening of the anterior tongue (A, white arrow) and mild nonenhancing thickening of the true vocal cords (B, arrowhead).
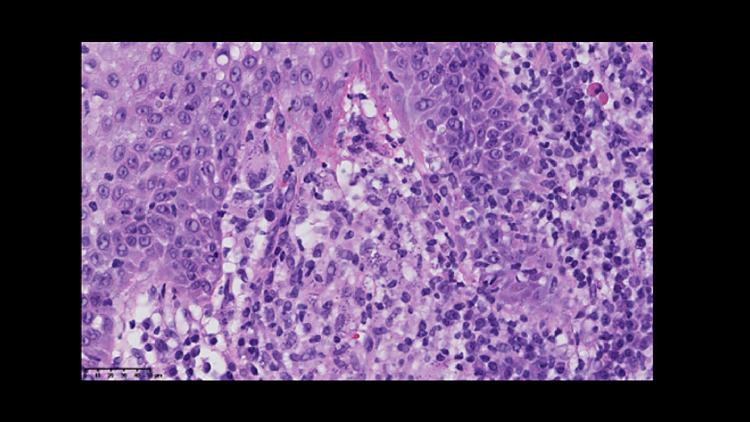
The Patient subsequently underwent a second biopsy of the anterior tongue and left vocal cord showing abundant inflammatory infiltrates with lymphoplasmacytic cells, macrophages, and Langerhans cells. Similar findings were observed in the specimen from the distal esophagus at the time of the gastroscopy. These conclusions prompted the revision of the previous biopsies, confirming the presence of Donovan bodies on the initial anatomopathological study (Fig. 4, tongue specimen), in agreement with the diagnosis of leishmaniosis.
Fig. 4.
The histopathological examination (magnification to 40x) of the tongue specimen shows abundant inflammatory infiltrates with lymphoplasmacytic cells, macrophages, and Langerhans cells with the presence of Donovan bodies, in agreement with the diagnosis of Leishmaniosis.
The follow-up MRI performed after 5 months of treatment with Amphotericine B (Ambisome) showed a decrease in the T2 signal and enhancement of the tongue (white arrows, Figs. 5A and B).
Fig. 5.
Axial images on the second MRI realized after 5 months of treatment show a decreased T2 hyperintensity (A, white arrow) and hyperenhancement on T1W fat-saturated postgadolinium (B, white arrow).
Discussion
Leishmaniosis is an infectious disease caused by different Leishmania species that are transmitted through phlebotomic sandflies [4]. Both L infantum and L donovani affect predominantly immunocompromised patients and cause visceral leishmaniosis and cutaneous leishmaniosis, whereas mucosal involvement in the absence of VL and CL is rarely seen [5].
ML was first described in 1912, in 2 patients showing lesions on the labial and nasal mucosa. Since then, several cases have been reported, all related to a Leishmania infantum infection [1]. The travel history to Congo reported by our patient suggests that the infection might be caused by both L infantum and L donovani species.
Leishmania is transmitted by a sandfly bite, reaching the mucosal tissue by lymphatic and bloodstream after being inoculated in a more accessible site.
Isolated laryngeal leishmaniosis has been related with L donovani and L infantum infection. Only some strains belonging to L donovani or L infantum complex are able to adapt themselves to electively live in the laryngeal tissue. In this case, mucosal lesions reflect the capability of macrophages to confine the disease [2].
The diagnosis of leishmaniosis is established by visualizing the Leishmania species amastigotes in tissue or by growing promastigotes in culture with the Giemsa stain revealing the characteristic kinetoblast of leishmania. PCR-band analysis is also emerging as the method of choice for diagnosis and identification [4].
Our case report describes for the first time imaging findings of leishmaniosisn in ML. After carefully reviewing the images of the 3 localizations we conclude that the lesions show:
-
(i)
At the lingual level, a mucosal tissue thickening associated with mild, fuzzy enhancement, without evidence of a focal, heterotopic localization, reflecting inflammatory granulomatous tissue, confirmed at the biopsy.
-
(ii)
At the laryngeal level, both CT and MRI identified discrete edema of the true vocal cords, which is also an unspecific sign of inflammatory disease.
-
(iii)
At the esophageal level, the parietal thickening of the esophagus first described on CT scan was, similar to the other locations, nonspecific in nature.
Altogether that means that the described imaging findings are nonspecific, therefore completion by biopsy and biological examination remains imperative and should prompt appropriate histological and biological examination with the search for Donovan's bodies. Nonspecific imaging findings might also have a role in post-treatment follow-up of ML.
Conclusions
In conclusion, nonspecific imaging findings compatible with inflammatory and granulomatous lesions, in individuals with compatible clinical history, should prompt to consider the diagnostic hypothesis of ML, which needs to be confirmed by biopsy.
Patient consent
This study was approved by our Institutional Review Board after written informed consent was obtained by the patient.
Footnotes
Acknowledgments: The authors received no financial support for the research, authorship, and publication of this article.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Aliaga L, Cobo F, Mediavilla JD, Bravo J, Osuna A, Amador JM, et al. Localized mucosal leishmaniasis due to Leishmania (Leishmania) infantum: clinical and microbiologic findings in 31 patients. Medicine (Baltimore) 2003;82(3):147. doi: 10.1097/01.md.0000076009.64510.b8. [DOI] [PubMed] [Google Scholar]
- 2.Cincurá C, de Lima CMF, Machado PRL, Oliveira-Filho J, Glesby MJ, Lessa MM, et al. Mucosal leishmaniasis: a retrospective study of 327 cases from an endemic area of Leishmania (Viannia) braziliensis. Am J Trop Med Hyg. 2017;97(3):761–766. doi: 10.4269/ajtmh.16-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH) Clin Infect Dis. 2016;63(12) doi: 10.1093/cid/ciw670. [DOI] [PubMed] [Google Scholar]
- 4.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet North Am Ed. 2018;392(10151):951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 5.Cocuzza S, Strazzulla A, Pinzone MR, Cosentino S, Serra A, Caltabiano R, et al. Isolated laryngeal leishmaniasis in immunocompetent patients: an underdiagnosed disease. Case Rep Infect Dis. 2013;2013:165409. doi: 10.1155/2013/165409. [DOI] [PMC free article] [PubMed] [Google Scholar]