Highlights
-
•
Significant decreases in temporal muscle dimensions observed in OHCA survivors.
-
•
The median daily rate for temporal muscle area atrophy was 2.0% per day.
-
•
No correlation found between temporal muscle atrophy and 30-day survival.
-
•
Clinical factors did not show a significant link with temporal muscle atrophy.
Keywords: Cardiac arrest, Computed tomography, Muscle atrophy, Post-cardiac arrest syndrome, Temporal muscle
Abstract
Objective
This study investigates temporal muscle atrophy in out-of-hospital cardiac arrest patients post-resuscitation, seeking associations with neurological outcomes and factors associated with atrophy.
Methods
Using data from six Japanese intensive care units, adult patients’ post-resuscitation who underwent head computed tomography scans on admission and two to five days post-admission were assessed. Temporal muscle area, thickness, and density were quantified from a single cross-sectional image. Patients were categorized into 'atrophy' or 'no atrophy' groups based on median daily temporal muscle atrophy rates. The primary outcome was changes in temporal muscle dimensions between admission and follow-up two to five days later. Secondary outcomes included assessing the impact of temporal muscle atrophy on 30-day survival, as well as identifying any clinical factors associated with temporal muscle atrophy.
Results
A total of 185 patients were analyzed. Measurements at follow-up revealed significant decreases in temporal muscle area (214 vs. 191 mm2, p < 0.001), thickness (4.9 vs. 4.7 mm, p < 0.001), and density (46 vs. 44 HU, p < 0.001) compared to those at admission. The median daily rate for temporal muscle area atrophy was 2.0% per day. There was no significant association between temporal muscle atrophy and 30-day survival (hazard ratios, 0.71; 95% CI, 0.41–1.23, p = 0.231). Multivariable logistic regression found no clinical factors significantly associated with temporal muscle atrophy.
Conclusions
Temporal muscle atrophy in post-resuscitation patients occurs rapidly at 2.0% per day. However, there was no significant association with 30-day mortality or any identified clinical factors. Further investigation into its long-term functional implications is warranted.
Background
Muscle wasting is frequently observed in critically ill patients due to critical illness myopathy and/or polyneuropathy.1 It is recognized as one of the pivotal domains of post-intensive care syndrome (PICS), which persistently interfering with patients’ quality of life.2 While the majority of PICS studies to date have focused on critically ill patients other than those after cardiac arrest,3, 4 a recent study of long-term out-of-hospital cardiac arrest (OHCA) survivors revealed that nearly half of them experienced PICS.5
Muscle atrophy in critically ill patients can occur in any part of the body, including the extremities, diaphragm and respiratory muscles.6 The thickness or volume of the temporal muscle (TM), as measured by computed tomography (CT) scan, is increasingly recognized as an indicator of muscle function and nutritional status in stroke patients.7 Notably, head CT scan used to diagnose or monitor stroke provide an objective and quantitative evaluation of the TM’s status. Decrease in temporal muscle volume following a subarachnoid hemorrhage have been associated with neurological prognosis upon hospital discharge. Although the direct relationship between TM atrophy and poor neurological outcomes has yet to be clearly elucidated, it is believed that several interrelated factors may play a role, including the severity of the stroke leading to greater immobilization, potential malnutrition or a catabolic state, and an extended recovery period characterized by physical decline.8, 9 Although a direct link between elevated cytokine levels following cardiac arrest and muscle atrophy has not been conclusively demonstrated, it is reasonable to speculate that such a connection may exist. This speculation is supported by the evidence that high levels of inflammatory cytokines are associated with increased mortality and poorer neurological outcomes post-cardiac arrest,10 combined with the knowledge that systemic inflammation is associated with muscle atrophy in critically ill patients.11
Remarkably, muscle wasting in critically ill patients manifests at an early stage.12 However, to our knowledge, whether muscle atrophy occurs soon after intensive care unit (ICU) admission in OHCA survivors, the factors associated with this muscle atrophy, and its related outcomes are little understood. Early identification of patients at risk for muscle atrophy may facilitate targeted care delivery. We hypothesized that OHCA survivors would experience atrophy of TM at an early stage and TM atrophy would be associated with neurological outcomes in OHCA patients, similar to that experienced by stroke patients. Accordingly, this study aims to investigate temporal muscle atrophy as evaluated by head CT scans during post-resuscitation care, and to determine its associated characteristics and relevant clinical outcomes among OHCA survivors.
Methods
Study design and population
We conducted this multicenter retrospective cohort study across six hospitals in Japan. The characteristics of the participating six institutions are summarized in Additional file 1. All adult patients admitted to the intensive care unit (ICU) at any of the six hospitals between January 2007 and December 2019 following OHCA were considered eligible for inclusion in the study. Patients younger than 18 years old and those who died within 48 hours of ICU admission were excluded. Additionally, patients without a head CT examination upon ICU admission or within five days of ICU admission for follow-up imaging, as well as those lacking CT evaluation at both time points, were further excluded. Patients with low-quality CT scans (e.g., with artifacts or where the temporal muscle was outside the scanned area), or those with a medical history of neurosurgery, were excluded due to the potential for insufficient measurement of bilateral temporal muscles. The study was conducted in line with the Declaration of Helsinki and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations. Patient consent was waived due to the retrospective analysis of preexisting data. This study was approved by the Ethics Committee of Okayama University Hospital (K 2208-034), as well as all other investigational sites.
Post-resuscitation care and CT evaluation
Post-resuscitation care, including decisions on providing therapeutic temperature management, its duration, target temperature, and the use of neuromuscular blocking agents (NBA) if therapeutic temperature management was administered, as well as the timing of mobilization, was at the discretion of the participating institution or attending physician. A follow-up head CT scan was performed as a neurological prognostication based on the judgement of the participating institution or attending physician. These post-resuscitation care practices were primarily in accordance with the guidelines at the time.13, 14 Details of the local protocols for post-resuscitation care and CT evaluation are provided in Additional file 1.
Temporal muscle measurement
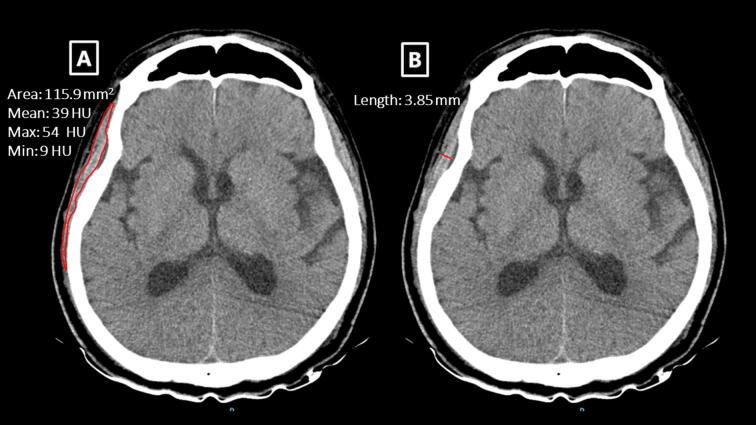
All head CT examinations were performed using 64 channel scanners. CT scans settings were as follows: field of view, 4 × 4–32 × 32 cm; tube voltage, 100–140 kV; and tube current, 150–500 mA. All images were scanned or reconstructed parallel to the orbito-meatal line, with a slice thickness between 3 and 5 mm. TM area, TM thickness, and TM density were manually measured using previously described methods.15, 16 Briefly, a cross-sectional image of the TM at the level of the orbital roof and the Sylvian fissure was chosen for quantifying TM characteristics. At this level, TM thickness was measured perpendicularly to the TM’s long axis. In contrast, TM area and TM density were determined by manually outlining the TM (Additional file 2). TM density, expressed as the mean Hounsfield unit (HU) values from the outlined area, was generated by the CT software at each institution (Additional file 1). The mean values provided by an acute care physician at each institution were derived from the averages of the right and left side measurements for each patient. We defined the thresholds for low TM area as 193 mm2 for women and 333 mm2 for man, respectively.16 The threshold for low attenuation in the TM was defined as 30 HU.17
Group definitions and outcomes
To determine the rate of TM atrophy, we analyzed the TM area on CT scans taken at ICU admission and a subsequent scan two to five days later. The rate of TM atrophy was calculated from the change in TM area over this period, adjusted to a per-day basis considering the time elapsed between scans. Based on this calculation, participants were categorized into an 'atrophy' group, for those with a daily rate of atrophy at or above the median of 2.0%, and a 'no atrophy' group, for those below this median.
The primary outcome was the atrophic changes in TM, as observed via head CT scans, between ICU admission and follow-up (i.e., two to five days after ICU admission). Secondary outcomes assessed the effects of TM atrophy on 30-day survival rates and neurological outcomes. Additionally, we identified clinical variables associated with TM atrophy. A favorable neurological outcome was defined as cerebral performance category (CPC) scores of 1 or 2.18
Data collection
Demographic data, details regarding cardiac arrest, post-ICU admission treatment, and outcome data were extracted from patients’ medical records. Demographics included age, sex, and body mass index (BMI). The CPC level before the cardiac arrest was obtained from family members or other caregivers. Information on the cardiac arrest included bystander witness status, bystander initiation of cardiopulmonary resuscitation by bystanders, the initially documented rhythm, and the etiology of arrest (either cardiac or non-cardiac). Laboratory data upon arrival, such as pH, lactate, and albumin levels, were collected, as was the sequential organ failure assessment (SOFA) score on the first ICU day.19 Treatment details included the use of sedative agents, analgesic agents, and NBA; administration of insulin, total calorie and protein intake; and whether physical therapy was initiated up to the day of the follow-up CT examination. Additionally, data regarding the provision of therapeutic temperature management and the use of veno-arterial extracorporeal membrane oxygenation were also retrieved.
Statistical analyses
Continuous variables are expressed as median with interquartile ranges (IQRs) while categorical variables are shown as counts with percentages. For comparing TM values (i.e., TM thickness, area, and density) between the baseline and follow-up imaging, we used the paired-sample Student's t-test or Wilcoxon's rank-sum test, as appropriate. Mann-Whitney U test was applied for continuous variables, and Fisher’s exact test was employed for categorical variables to determine differences between the groups.
The 30-day cumulative incidence of all-cause mortality was examined using the Kaplan-Meier method, and the log-rank test was used to compare differences between the atrophy group and the no atrophy group. A Cox proportional hazards model was then fit to estimate the hazard ratios (HRs) and 95% CIs for 30-day survival, controlling for the independent effect of muscle atrophy and potential confounders including age, sex, atrophic change in TM defined as above (yes vs. no), initial cardiac rhythm (shockable vs. non-shockable), witnessed collapse, and lactate levels on arrival.20 To further explore the impact of muscle atrophy on 30-day survival, we replaced the definition of temporal muscle atrophic changes (initially defined by temporal muscle area) with temporal muscle thickness (for model 2) and density (for model 3). Temporal muscle atrophy is defined by the median rate of decrease in temporal muscle thickness or density per day. In a supplementary analysis, the impact of TM atrophy on 30-day survival and the proportion of favorable neurological outcomes were compared between the highest and lowest quartiles of the daily TM area atrophy rate. Due to the limited sample size, this analysis was conducted as an unadjusted crude comparison.
Next, we created multivariable logistic regression models to identify variables associated with TM atrophy. Selection of these variables was based on prior studies or clinical reasoning and included age, BMI, SOFA score on the first ICU day, use of sedative agents, NBA use, calorie and protein intake by the day of the follow-up CT, and insulin use.12, 21, 22 The results were expressed as odds ratios (ORs) and their 95% confidence intervals (CIs).
All tests were two-tailed with p < 0.05 being considered statistically significant. All the analyses were performed using Stata SE version 17 statistical software (Stata-Corp LP, College Station, TX, USA).
Results
During the 12-year study period, a total of 185 patients were included in the analysis, as detailed in Fig. 1. The patients’ demographic and clinical characteristics are summarized in Table 1. Overall, the median age was 67 years (IQR, 56–75), 124 (67%) patients were man, and 61 (33%) patients were women. Nearly half of the patients were of cardiac origin. The median lactate and pH levels upon arrival were 8.4 mmol/L (IQR, 5.7–11.3) and 6.98 (IQR, 6.89–7.17), respectively.
Fig. 1.
Flow diagram of the study population. OHCA: out-of-hospital cardiac arrest, ICU: intensive care unit, CT: computed tomography.
Table 1.
Demographics and characteristics of the participants.
| Characteristics | All (n = 185) | Atrophy group (n = 93) | No atrophy group (n = 92) | p-value |
|---|---|---|---|---|
| Day of follow-up CT– median [IQR], day | 4 [3,4] | 3 [3,4] | 4 [3–5] | 0.029 |
| Male sex, n (%) | 124 (67.0) | 61 (65.5) | 63 (68.4) | 0.676 |
| Age – median [IQR], y | 67 [56–75] | 65 [53–75] | 67 [59–74] | 0.490 |
| BMI– median [IQR], kg/m2 | 21.5 [19.4–24.1] | 21.8 [19.7–24.2] | 21.3 [19.0–23.9] | 0.411 |
| CPC before the onset, n (%) | 0.726 | |||
| CPC1 | 159 (85.9) | 79 (85.0) | 80 (87.0) | |
| CPC2 | 17 (9.2) | 10(10.7) | 7 (7.6) | |
| CPC3 | 9 (4.9) | 4 (4.3) | 5 (5.4) | |
| Charlson comorbidity index – median [IQR] | 1 [0–1] | 1 [0–1] | 1 [0–1] | 0.582 |
| Dementia | 14 (7.5) | 8 (8.6) | 6 (6.5) | 0.593 |
| Cerebrovascular disease, n (%) | 21 (11.3) | 10 (10.7) | 11 (11.9) | 0.796 |
| Chronic heart failure, n (%) | 22 (11.8) | 11 (11.8) | 11 (11.9) | 0.978 |
| Chronic kidney disease, n (%) | 19 (10.2) | 12 (12.9) | 7 (7.6) | 0.236 |
| Diabetes, n (%) | 36 (19.4) | 15 (16.1) | 21 (22.8) | 0.250 |
| Malignancy, n (%) | 21 (11.3) | 9 (9.6) | 12 (13.0) | 0.471 |
| Initial rhythm, n (%) | ||||
| VF/VT | 76 (41.1) | 37 (39.8) | 39 (42.4) | 0.719 |
| PEA/Asystole | 109 (58.9) | 56 (60.2) | 53 (57.6) | 0.719 |
| Cardiac etiology, n (%)a | 84 (48.0) | 43 (48.1) | 41 (47.6) | 0.932 |
| Witnessed collapse, n (%)b | 114 (62.6) | 59 (64.1) | 55 (61.1) | 0.674 |
| Bystander CPR, n (%)c | 83 (46.1) | 46 (50.5) | 37 (41.5) | 0.227 |
| Adrenaline doses post-arrival– median [IQR], mg | 1 [0–2] | 1 [0–2] | 1 [0–2] | 0.920 |
| Laboratory findings | ||||
| Albumin – median [IQR], g/dl | 3.6 [3.1–3.9] | 3.5 [3.0–3.9] | 3.6 [3.2–3.9] | 0.519 |
| pH – median [IQR] | 6.98 [6.89–7.17] | 7.02 [6.90–7.22] | 6.97 [6.88–7.14] | 0.120 |
| Lactate – median [IQR], mmol/l | 8.4 [5.7–11.3] | 8.5 [5.9–11.5] | 8.2 [5.6–11.0] | 0.625 |
| SOFA score on the first day– median [IQR] | 10 [8–11] | 10 [7–11] | 10 [8–11] | 0.625 |
| Post-resuscitation care | ||||
| ECMO, n (%) | 20 (10.8) | 12 (12.9) | 8 (8.7) | 0.357 |
| TTM, n (%) | 144 (78.2) | 67 (72.8) | 77 (83.7) | 0.074 |
| Sedative agent, n (%) | 146 (78.9) | 67 (72.0) | 79 (85.8) | 0.021 |
| Analgesic agent, n (%) | 141 (76.2) | 70 (75.2) | 71 (77.1) | 0.761 |
| Neuromuscular blocking agent, n (%) | 94 (50.8) | 46 (49.4) | 48 (52.1) | 0.712 |
| Nutrition by day of follow-up CT | ||||
| Calorie – median [IQR], kcal | 0 [0–300] | 0 [0–600] | 0 [0–0] | 0.028 |
| Protein – median [IQR], g | 0 [0–12] | 0 [0–21] | 0 [0–0] | 0.039 |
| Insulin use by day of follow-up CT, n (%)d | 105 (57.0) | 48 (52.1) | 57 (61.9) | 0.180 |
| Physical therapy by day of follow-up CT, n (%) | 11 (5.9) | 3 (3.2) | 8 (8.7) | 0.116 |
CT: computed tomography, IQR: interquartile range, BMI: body mass index, CPC: Cerebral Performance Category, VF/VT: ventricular fibrillation/ventricular tachycardia, PEA: pulseless electrical activity, CPR: cardiopulmonary resuscitation, SOFA: Sequential Organ Failure Assessment, ECMO: extracorporeal membrane oxygenation, TTM: target temperature management, TM: temporal muscle.
Of 185, 4 and 6 patients were missing in the atrophy and the no atrophy group, respectively.
Of 185, 1 and 2 patients were missing in the atrophy and the no atrophy group, respectively.
Of 185, 2 and 3 patients were missing in the atrophy and the no atrophy group, respectively.
Of 185, 1 patient was missing in the atrophy group.
Patients’ characteristics between the groups
Patients’ characteristics were compared between the atrophy group, with a daily rate of TM atrophy of 2.0% or more, and the no atrophy group, with a rate of less than 2.0% (Table 1). The timing of follow-up head CT scans was earlier in the atrophy group (3 vs. 4 days, p = 0.029). Age, sex proportions, BMI, and cardiac arrest status were similar between the two groups. As for severity and post-resuscitation care, there were no differences in lactate or pH levels upon arrival, SOFA scores on the first day, and proportion of patients who received therapeutic temperature management, NBA, and insulin between the two groups. The only exceptions were that the atrophy group used fewer sedative agents and received a higher nutrition dose.
CT evaluation of temporal muscle dimensions by group
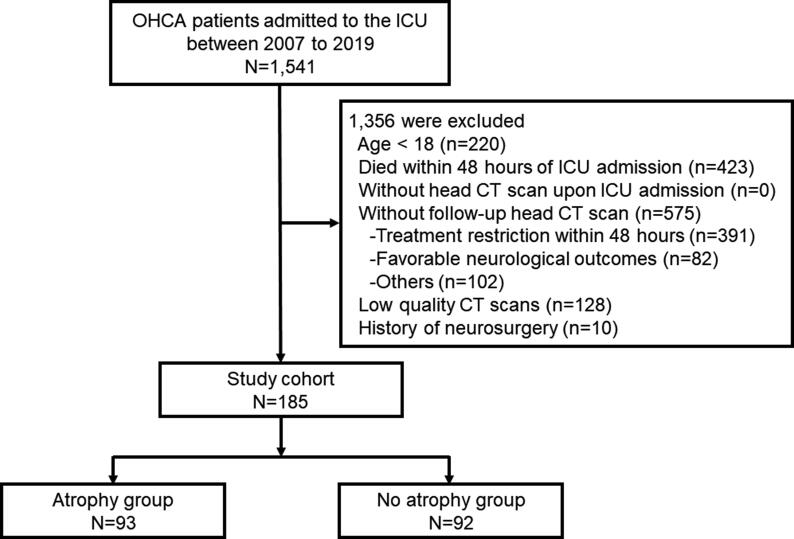
The TM area (214 vs. 191 mm2, p < 0.001), TM thickness (4.9 vs. 4.7 mm, p < 0.001), and TM density (46 vs. 44 HU, p < 0.001) upon follow-up examination at a median of four days post-ICU admission showed a significant decrease compared to the measurements upon admission (Fig. 2).
Fig. 2.
Comparison of TM values via CT scans between ICU admission and follow-up. A: TM area, B: TM thickness, C: TM density TM: temporal muscle, CT: computed tomography, ICU: intensive care unit. Box plot indicates median, interquartile range, and lower and upper adjacent values.
TM area, thickness, and density did not differ significantly between the groups at both admission and follow-up timepoints (Table 2). As expected, given our criteria for group classification, a significant daily rate of atrophy in TM area was observed in the atrophy group compared to the no atrophy group (5.7% vs. −0.2%, p < 0.001). Similarly, the rate of TM thickness decline per day was significantly higher in the atrophy group compared to the no atrophy group (2.5% vs. 0.7%, p < 0.001). However, the daily rate of TM density decline was not significantly different between the groups (1.7% vs. 1.6%, p = 0.942).
Table 2.
Temporal muscle dimensions evaluated by CT.
| Parameters | All (n = 185) | Atrophy group (n = 93) | No atrophy group (n = 92) | p-value |
|---|---|---|---|---|
| TM area atrophy rate – median [IQR], % | 7.6 [−1.0 to 19.7] | 19.7 [13.2–28.7] | −1.0 [−10.0 to 4.1] | <0.001 |
| TM area atrophy rate – median [IQR], % per day | 2.0 [−0.2 to 5.7] | 5.7 [3.5–8.7] | −0.2 [−2.7 to 1.0] | <0.001 |
| TM area on admission – median [IQR], mm2 | 214 [136–290] | 234 [177–298] | 189 [121–275] | 0.072 |
| TM area on follow-up – median [IQR], mm2 | 191 [131–270] | 188 [131–239] | 196 [128–316] | 0.054 |
| TM thickness decline rate – median [IQR], % per day | 1.3 [−0.7 to 3.6] | 2.5 [0.3–5.3] | 0.7 [−2.1 to 2.4] | <0.001 |
| TM thickness on admission – median [IQR], mm | 4.9 [3.8–6.0] | 5.0 [3.9–6.2] | 4.8 [3.8–5.8] | 0.538 |
| TM thickness on follow-up – median [IQR], mm | 4.7 [3.5–5.7] | 4.4 [3.3–5.6] | 4.9 [3.6–6.1] | 0.110 |
| TM density decline rate – median [IQR], % per day | 1.6 [−0.7 to 4.5] | 1.7 [−0.7 to 4.5] | 1.6 [−0.6 to 4.4] | 0.942 |
| TM density on admission – median [IQR], HU | 46 [41–52] | 47 [40–52] | 46 [41–52] | 0.822 |
| TM density on follow-up – median [IQR], HU | 44 [38–48] | 44 [36–48] | 44 [39–48] | 0.814 |
| Low TM area on admission, n (%)a | 54 (29.1) | 31 (33.3) | 23 (25.0) | 0.213 |
| Low attenuation in the TM on admission, n (%)b | 11 (5.9) | 8 (8.6) | 3 (3.2) | 0.125 |
CT: computed tomography, IQR: interquartile range, TM: temporal muscle, HU: Hounsfield unit.
Low TM area was defined as 193 mm2 or lower for women and 333 mm2 or lower for men, respectively.
Low attenuation in the TM was defined as 30 HU or lower.
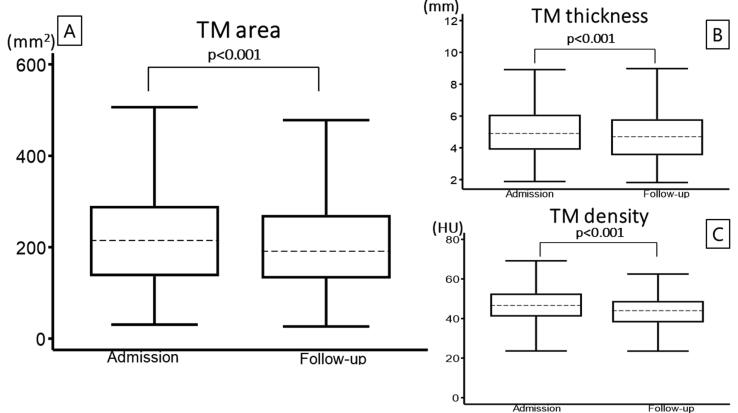
Outcomes
In univariate analysis, there were no differences in 30-day survival between the groups. However, the atrophy group demonstrated more favorable neurological outcomes at 30 days compared to the no atrophy group (Table 3). As shown by the Kaplan-Meier curve, there were no difference in 30-day survival between the groups (Fig. 3). In multivariable Cox regression model, TM atrophy was not associated with 30-day survival (HR, 0.71; 95% CI, 0.41–1.23, p = 0.231) (Table 4). Further exploratory analysis indicated that neither the change in TM thickness nor density was associated with 30-day survival (Additional file 3). In the supplementary analysis, no differences in 30-day survival or favorable neurological outcomes were observed between the highest (median atrophy rate of 6.8%) and lowest (median atrophy rate of −0.3%) quartiles of the daily TM area atrophy rate (Additional file 4).
Table 3.
Crude analysis of the relationship between temporal muscle atrophy and patient outcomes.
| All (n = 185) | Atrophy (n = 93) | No atrophy (n = 92) | Unadjusted OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Outcomes | |||||
| Survival at 30 days, n (%) | 125 (67.5) | 67 (67.5) | 58 (63.0) | 1.53 (0.81–2.80) | 0.192 |
| Favorable neurological outcomes at 30 days, n (%) | 25 (13.5) | 18 (19.3) | 7 (7.6) | 2.91 (1.15–7.36) | 0.024 |
CI: confidence interval, OR: odds ratio.
Fig. 3.
Comparison of 30-day survival between the atrophy group and no atrophy group using the log-rank test.
Table 4.
Cox proportional hazard regression analysis for 30-day survival.
| Variables | HR (95% CI) | p-value |
|---|---|---|
| Model 1 | ||
| Temporal muscle atrophy | 0.71 (0.41–1.23) | 0.231 |
| Age | 0.98 (0.96–0.99) | 0.048 |
| Male | 1.17 (0.65–2.10) | 0.597 |
| Witnessed collapse | 0.93 (0.53–1.63) | 0.807 |
| Initiated shockable rhythm | 2.42 (1.24–4.71) | 0.009 |
| Lactate levels | 1.01 (0.95–1.07) | 0.691 |
Variables for the outcomes in the cox proportional hazard regression analysis included age, sex, initial rhythm, witness status, lactate levels, and the presence or absence of temporal muscle atrophy.
HR: hazard ratio, CI: confidence intervals.
Factors associated with TM atrophy
Multivariable logistic regression analysis revealed that no factors were associated with TM atrophy (Table 5).
Table 5.
Factors associated with temporal muscle atrophy.
| Adjusted OR (95% CI) | p-value | |
|---|---|---|
| Factor | ||
| Age | 0.98 (0.96–1.01) | 0.250 |
| BMI | 1.05 (0.97–1.12) | 0.172 |
| NBA use | 1.62 (0.73–3.61) | 0.230 |
| SOFA score on the first ICU day | 1.01 (0.89–1.15) | 0.820 |
| Insulin use | 0.70 (0.34–1.46) | 0.348 |
| Use of sedative agents | 0.44 (0.16–1.20) | 0.113 |
| Calorie dose by the day of follow-up CT | 1.00 (0.99–1.00) | 0.925 |
| Protein dose by the day of follow-up CT | 1.01 (0.98–1.04) | 0.301 |
Variables in multivariable logistic regression analysis for temporal muscle atrophy: Age, BMI, NBA use, SOFA score on the first ICU day, insulin use, use of sedative agents, calorie and protein intake by follow-up CT day.
OR, odds ratio; CI, confidence intervals; BMI, body mass index; NBA, neuromuscular blocking agent; SOFA, Sequential Organ Failure Assessment, ICU, intensive care unit; CT computed tomography.
Discussion
In this multicenter retrospective study of 185 patients with OHCA who received post-resuscitation care in the ICU, we observed rapid TM atrophy. Indeed, measures of TM area, thickness, and density—obtained through qualitative analysis of head CT scans—showed a significant decrease after ICU admission, with a median atrophy rate of 2.0% per day for the TM area. However, TM atrophy was not associated with 30-day mortality in our cohort, and we were unable to identify clinical factors associated with this atrophy.
Our study for the first time examined the changes in TM status by head CT scans in patients after OHCA in the ICU. Earlier studies have indicated that muscle mass in critically ill patients decreases over a span of seven to 10 days.12, 23 A previous study estimated the daily atrophy rate to be 1.3–3.0% for lower limbs and 0.7–2.4% for upper limbs.6 In a more recent meta-analysis, Fazzini et al. demonstrated that muscle mass of rectus femoris decreased by approximately 2% daily in critically ill patients.24 Our results of a TM atrophy rate of 2.0% per day are consistent with these findings. Importantly, prior work suggested that TM atrophy in neurocritical care patients was well correlated with that of rectus femoris.25 Collectively, our observations of atrophic changes in TM could mirror muscle wasting in OHCA survivors.
Contrary to our hypothesis, our results revealed no significant relationship between TM atrophy and 30-day mortality. While we do not have a clear explanation for this, a short-term outcome characterized by higher mortality may have made it challenging to discern a difference. Moreover, OHCA survivors may present a different set of challenges compared to stroke patients. The immediate post-cardiac arrest phase involves a multitude of systemic disturbances, such as reperfusion injury, inflammatory response, and oxidative stress.26 These could outweigh the potential effects of muscle atrophy on survival. Notably, our findings that the atrophy group exhibited more favorable neurological outcomes at 30 days suggest that atrophy may not necessarily be an adverse outcome but rather a sign of a different recovery trajectory. However, these results should be interpreted with caution, as it stems from a univariate analysis. Furthermore, the impact of pre-admission “sarcopenia” on muscle atrophy observed post-admission warrants consideration, particularly in light of the observed trend toward a larger TM area on admission in the atrophy group compared to the no atrophy group. Patients with prior muscle loss may exhibit different atrophy patterns compared to those with “healthier” muscle mass, which could influence our findings.
An early head CT after return of spontaneous circulation (ROSC) can help determine the cause of cardiac arrest.27 Of note, a recent study reported that a delayed head CT scan taken around 3–4 days after ROSC demonstrated better prognostic value compared with initial examination.28 While conducting repetitive CT examinations solely to evaluate muscle wasting would not be practical; however, two-time points head CT scans (i.e., an initial assessment and neurological prognostication several days later) in patients with OHCA admitted to the ICU would tell us whether muscle wasting occurs by identifying atrophic changes in the TM.
Previous studies have identified various risk factors for muscle wasting in the ICU, including sepsis, multiple organ failure, the use of NBA, and prolonged mechanical ventilation.12, 21, 29 Regarding preventive measures, intensive insulin therapy was shown to effectively reduce the incidence of critical illness polyneuropathy/myopathy.29 In our study, we were not able to identify the specific factors responsible for TM atrophy. A more comprehensive investigation is required to identify OHCA survivors who are at risk for muscle atrophy.
Our study has several limitations. First, the participants in this study were restricted to those who underwent a head CT examination upon admission and for subsequent follow-up imaging. This approach excludes a significant subset of patients: those who died shortly after admission and those who did not undergo CT scanning due to rapid clinical improvement or at the discretion of the attending physician. Particularly, the latter decision can significantly affect our findings. This selection criteria introduce a notable bias, thus limiting the generalizability of our findings. Second, while the atrophy rate was calculated on a daily basis, the timing of the second CT examination varied among patients (ranging from two to five days after admission), which could affect the consistency of the atrophy rate calculation. Third, changes in clinical practice during the study period could introduce confounding variables that we did not account for, potentially affecting the results. Forth, the regression models were restricted due to relatively small sample size and a lack of diverse cofounders. A prospective cohort study with more robust inclusion criteria would solve this issue. Fifth, we did not collect long-term outcomes. The effects of early TM atrophy post-OHCA on long-term functional outcomes warrant further investigation. Previous studies noted that TM thickness was inversely associated with the severity of dysphagia or cognitive decline.30, 31 Moreover, the mid- to long-term trajectory of TM changes and their correlation with functional outcomes should be explored further. Lastly, while HU measurements are standardized and calibrated against water and air, which allows for comparability across different CT scanners, slight variations in absolute HU values may still occur between machines.32
Despite these limitations, our findings add to the growing body of knowledge, highlighting the frequent occurrence of muscle atrophy during the initial days of ICU stay in post-OHCA patients. Regular head CT scans following OHCA can offer valuable insights into the occurrence of TM wasting in a quantitative and objective manner. Future research should aim to minimize selection bias and include a baseline assessment of sarcopenia to better understand its influence on post-admission muscle atrophy while focusing on understanding the long-term implications of TM atrophy and devising strategies to prevent or mitigate this event.
Conclusions
In our multicenter retrospective analysis of post-resuscitation OHCA patients in the ICU, we found rapid TM atrophy, with a median atrophy rate of 2.0% per day in TM area. Despite this, TM atrophy did not correlate with 30-day mortality, and no clinical factors linked to this atrophy were identified. Further prospective studies are needed to understand its long-term functional outcomes.
Funding
This study was funded by the Okayama Medical Foundation. The Okayama Medical Foundation was not involved in the study design, data collection, analysis, interpretation of the data, and did not have final approval of the manuscript for submission.
CRediT authorship contribution statement
Takashi Hongo: Writing – original draft, Visualization, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Hiromichi Naito: Writing – review & editing, Validation, Supervision, Investigation, Conceptualization. Keibun Liu: Writing – review & editing, Validation, Methodology, Investigation, Conceptualization. Yuya Murakami: Investigation, Conceptualization. Satoshi Nozaki: Validation, Conceptualization. Hiroki Maeyama: Investigation, Conceptualization. Ayaka Matsuoka: Investigation, Conceptualization. Hisashi Dote: Investigation, Conceptualization. Kazumasa Inaba: Investigation, Conceptualization. Satoshi Miike: Validation, Conceptualization. Shigeki Fujitani: Investigation, Conceptualization. Tomohiro Hiraoka: Investigation, Conceptualization. Takafumi Obara: Investigation, Conceptualization. Tsuyoshi Nojima: Investigation, Conceptualization. Atsunori Nakao: Writing – review & editing, Supervision, Conceptualization. Tetsuya Yumoto: .
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Koya Yamashita for collecting data.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.resplu.2023.100527.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
Supplementary file 2.
References
- 1.Latronico N., Bolton C.F. Critical illness polyneuropathy and myopathy: A major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 2.Rousseau A.F., Prescott H.C., Brett S.J., et al. Long-term outcomes after critical illness: recent insights. Crit Care. 2021;25:1–7. doi: 10.1186/s13054-021-03535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maley J.H., Brewster I., Mayoral I., et al. Resilience in survivors of critical illness in the context of the survivors’ experience and recovery. Ann Am Thorac Soc. 2016;13:1351–1360. doi: 10.1513/AnnalsATS.201511-782OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marra A., Pandharipande P.P., Girard T.D., et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018;46:1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent A., Beck K., Thommen E., et al. Post-intensive care syndrome in out-of-hospital cardiac arrest patients: A prospective observational cohort study. PLoS One. 2022;17:1–17. doi: 10.1371/journal.pone.0276011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakanishi N., Takashima T., Oto J. Muscle atrophy in critically ill patients: A review of its cause, evaluation, and prevention. J Med Investig. 2020;67:1–10. doi: 10.2152/jmi.67.1. [DOI] [PubMed] [Google Scholar]
- 7.Katsuki M., Kakizawa Y., Nishikawa A., et al. Temporal muscle and stroke-a narrative review on current meaning and clinical applications of temporal muscle thickness, area, and volume. Nutrients. 2022;14:687. doi: 10.3390/nu14030687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsuki M., Yamamoto Y., Uchiyama T., Wada N., Kakizawa Y. Clinical characteristics of aneurysmal subarachnoid hemorrhage in the elderly over 75; would temporal muscle be a potential prognostic factor as an indicator of sarcopenia? Clin Neurol Neurosurg. 2019;186 doi: 10.1016/j.clineuro.2019.105535. [DOI] [PubMed] [Google Scholar]
- 9.Onodera H., Mogamiya T., Matsushima S., et al. High protein intake after subarachnoid hemorrhage improves oral intake and temporal muscle volume. Clin Nutr. 2021;40:4187–4191. doi: 10.1016/j.clnu.2021.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Jou C., Shah R., Figueroa A., Patel J.K. The role of inflammatory cytokines in cardiac arrest. J Intensive Care Med. 2020;35:219–224. doi: 10.1177/0885066618817518. [DOI] [PubMed] [Google Scholar]
- 11.Witteveen E., Wieske L., van der Poll T., et al. Increased early systemic inflammation in ICU-acquired weakness; a prospective observational cohort study. Crit Care Med. 2017;45:972–979. doi: 10.1097/CCM.0000000000002408. [DOI] [PubMed] [Google Scholar]
- 12.Puthucheary Z.A., Rawal J., McPhail M., et al. Acute skeletal muscle wasting in critical illness. Jama. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 13.Nolan J.P., Soar J., Zideman D.A., et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation. 2010;81:1219–1276. doi: 10.1016/j.resuscitation.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Nolan J.P., Soar J., Cariou A., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015. Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Katsuki M., Suzuki Y., Kunitoki K., et al. Temporal Muscle as an Indicator of Sarcopenia is Independently Associated with Hunt and Kosnik Grade on Admission and the Modified Rankin Scale Score at 6 Months of Patients with Subarachnoid Hemorrhage Treated by Endovascular Coiling. World Neurosurg. 2020;137:e526–e534. doi: 10.1016/j.wneu.2020.02.033. [DOI] [PubMed] [Google Scholar]
- 16.Lee B., Bae Y.J., Jeong W.J., Kim H., Choi B.S., Kim J.H. Temporalis muscle thickness as an indicator of sarcopenia predicts progression-free survival in head and neck squamous cell carcinoma. Sci Rep. 2021;11:1–10. doi: 10.1038/s41598-021-99201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aubrey J., Esfandiari N., Baracos V.E., et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210:489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perkins G.D., Jacobs I.G., Nadkarni V.M., et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update of the Utstein resuscitation registry templates for out-of-hospital cardiac arrest: A statement for healthcare professionals from a task force of the international liaison committee. Circulation. 2015;132:1286–1300. doi: 10.1161/CIR.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 19.Vincent J.L., Moreno R., Takala J., et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Nishikimi M., Ogura T., Nishida K., et al. External validation of a risk classification at the emergency department of post-cardiac arrest syndrome patients undergoing targeted temperature management. Resuscitation. 2019;140:135–141. doi: 10.1016/J.RESUSCITATION.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Price D.R., Mikkelsen M.E., Umscheid C.A., Armstrong E.J. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44:2070–2078. doi: 10.1097/CCM.0000000000001839. [DOI] [PubMed] [Google Scholar]
- 22.Zorowitz R.D. ICU-acquired weakness: a rehabilitation perspective of diagnosis, treatment, and functional management. Chest. 2016;150:966–971. doi: 10.1016/j.chest.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 23.McNelly A.S., Bear D.E., Connolly B.A., et al. Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest. 2020;158:183–194. doi: 10.1016/j.chest.2020.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Peres L.M., Luis-Silva F., Menegueti M.G., et al. Comparison of ultrasound with computed tomography to measure skeletal muscle mass in critically ill patients: A prospective study protocol. Medicine (Baltimore) 2022;101:e31921. doi: 10.1097/MD.0000000000031921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maskos A., Schmidbauer M.L., Kunst S., et al. Diagnostic utility of temporal muscle thickness as a monitoring tool for muscle wasting in neurocritical care. Nutrients. 2022;14:4498. doi: 10.3390/nu14214498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumar R.W., Nolan J.P., Adrie C., et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication a consensus statement from the International Liaison Committee on Resuscitation. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 27.Nolan J.P., Sandroni C., Böttiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47:369–421. doi: 10.1007/s00134-021-06368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.In Y.N., Lee I.H., Park J.S., et al. Delayed head CT in out-of-hospital cardiac arrest survivors: Does this improve predictive performance of neurological outcome? Resuscitation. 2022;172:1–8. doi: 10.1016/j.resuscitation.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Goligher E.C., Dres M., Fan E., et al. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 30.Sakai K., Katayama M., Nakajima J., et al. Temporal muscle thickness is associated with the severity of dysphagia in patients with acute stroke. Arch Gerontol Geriatr. 2021;96 doi: 10.1016/j.archger.2021.104439. [DOI] [PubMed] [Google Scholar]
- 31.Cho J., Park M., Moon W.J., Han S.H., Moon Y. Sarcopenia in patients with dementia: correlation of temporalis muscle thickness with appendicular muscle mass. Neurol Sci. 2022;43:3089–3095. doi: 10.1007/s10072-021-05728-8. [DOI] [PubMed] [Google Scholar]
- 32.Oh J.H., Choi S.P., Wee J.H., Park J.H. Inter-scanner variability in Hounsfield unit measured by CT of the brain and effect on gray-to-white matter ratio. Am J Emerg Med. 2019;37:680–684. doi: 10.1016/j.ajem.2018.07.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.