Abstract
Introduction
One of the causes of congenital hearing loss are infections suffered by the mother during pregnancy. The objective of this study was to investigate the effects on hearing in newborns to SARS-CoV-2 seropositive mothers during pregnancy. We also studied the hearing impact in the first year of life of the newborns to investigate whether neonatal infection produced a risk of infantile sensorineural hearing loss.
Material and methods
All children born in our center whose mother had been infected with SARS-CoV-2 positive COVID were included and were audiologically studied at two and a half months and at one year of life. All infants were evaluated by brainstem evoked response audiometry (BERA) and auditory steady-state responses (ASSR).
Results
The range of the latencies for BERA founded were inside the desired ranges of normality both at two and a half months and at one year of life No significant differences by sex and ears were found in the BERA performed (p > 0,05). The mean ASSR values were found to be significantly below 30 dB in all frequencies studied both at two and a half months, and at one year of life (p < 0,05).
Conclusion
There is no association between COVID-19 infection during pregnancy and neonatal hearing loss. Further studies are needed to clarify this field since it is still unclear whether pregnant women infected with SARS-CoV-2 can produce hearing alterations in their newborns according to the current evidence in the literature.
Keywords: COVID-19, SARS-CoV-2, Hearing loss
1. Introduction
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Since the first outbreak that took place in Wuhan, the COVID-19 pandemic continues to date; despite 11 billion doses of vaccines, we find more than 500 million confirmed cases and more than 6 million deaths worldwide [2].
SARS-CoV-2 infection mainly presents symptoms such as fever, cough, sore throat, headache, myalgia, diarrhea, and dyspnea. It predominantly affects the respiratory system of the human body, but can also cause damage to the central nervous, cardiovascular, gastrointestinal, hepatobiliary and renal systems [3]. In addition, COVID-19 causes alterations in the otolaryngology area such as anosmia, taste alterations and sudden deafness [4,5].
Some infections during pregnancy can affect the fetus and cause abnormalities. Sensorineural hearing impairment is one of the most serious complications of intrauterine exposure to certain pathogens such as toxoplasma, cytomegalovirus, herpes, syphilis, measles, rubella, varicella or zika [[6], [7], [8], [9], [10]]. Thus, infection timing is also an important, the sooner the mother becomes infected, the higher the risk of developing an abnormality; being the first trimester the one with the highest risk to develop an abnormality.
COVID-19 has been compared to a TORCH infection as it demonstrates a similar incidence [11,12]. Both COVID-19 and TORCH infections show higher infection rates in the third trimester [13]. Hematogenous spread of SARS-CoV-2 to the central nervous system is via angiotensin-converting enzyme 2 (ACE2) receptors [[14], [15], [16]]. Viruses can directly damage inner ear structures, (hair cells, support cells or the organ of Corti) or cause induction of immune-mediated damage [16,17].
SARS-CoV-2 virus can infect both the central and peripheral nervous system, resulting in neurological disorders [14,18,19]. Middle ear, Eustachian tube and cochlea tissue susceptibility to SARS-CoV-2 have been confirmed in several papers [[20], [21], [22]].
The aim of this study was to investigate the possible effects on the hearing of newborns to mothers with gestational SARS-CoV-2 infection during pregnancy. In addition, the potential audiological impact in the first year of life of the newborns was also studied.
The secondary objective was to analyze whether there is some evidence of abnormality to contemplate that COVID-19 should be included as a risk factor in the universal screening for neonatal hearing loss, include the diagnosis of this virus in the Spanish Commission for the Early Detection of Hearing Loss (CODEPEH) recommendations [18] (Table 1).
Table 1.
Risk factors of late-onset hearing loss according to CODEPEH.
| First and second degree family history of hearing loss |
|---|
| Craniofacial malformations |
| Low birth weight (under 1500 g) |
| Maternal infections during pregnancy (toxoplasma, syphilis, rubella, cytomegalovirus, herpes, HIV, zika) |
| Severe hyperbilirubinemia (over 20 mg/dl) |
| Ototoxic drugs |
| Uncontrolled pregnancy |
| Bacterial meningitis |
| Head injury |
| Perinatal hypoxia |
| Mechanical ventilation |
| ICU admission for more than 5 days |
| Features or alterations corresponding to syndromes usually associated with hypoacusis - Neurological pathology and neurodegenerative disorders - Waardenburg syndrome - Retinitis pigmentosa - Prolonged Q-T interval - Osteogenesis imperfecta - Mucopolysaccharidosis |
The primary hypothesis was that newborns to mothers with COVID-19 do not differ significantly from normal clinical data. The secondary hypothesis derived was that it is not necessary to add COVID-19 infection in pregnancy as a risk factor for childhood hearing loss since the values obtained are not significantly different from normal. The secondary objective was researched by making successive auditory studies until one year of newborns life and comparing with normal children's data.
2. Materials and methods
A single-center prospective analytical study was made from May 2020 to May 2021. All children born in the hospital whose mother had been infected with SARS-CoV-2 positive COVID were included as our single study cohort and were audiologically studied at two and a half months and at one year of life. Newborns to coronavirus-positive mothers with other risk factors for late hearing loss or multiple births were excluded to avoid bias. The babies who were not full term from week 38–41 were excluded, as well as children who associates pathology in the middle ear such otitis media with effusion diagnosed by otoscopy, in order to not to interfere in the interpretation of the potentials due to their delay in the latencies.
Functional hearing measurements were performed on all newborns whose mothers were positive for SARS-CoV2 infection during gestation from May 2020 to May 2021.
COVID-19 infection was diagnosed by COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) test obtained from nasopharyngeal swab.
Due to the emergence of SARS-CoV-2 and the lack of knowledge of hearing impairment in newborns born to mothers who passed COVID-19, these children will be followed up in the same way as if they were a risk factor for the development of late-onset hearing loss.
Our study dependent variables were brainstem evoked response audiometry (BERA) and auditory steady-state responses (ASSR) with INTERACOUSTISC equipment model ECLIPSE serial number 862985. All infants were evaluated with both tests and were always performed by same qualified team in the same room. The performance of these tests is not invasive, the direct collaboration of the patient is not necessary, but it is preferable for the patient to be relaxed and asleep.
BERA allows us to obtain an objective idea of hearing, the intensity of the hearing loss and the location of the lesion after recording the electrical activity of the brain following a click-type stimulus. Wave I, III and V at 30, 40, 60 dB intensity in both ears and their latencies were recorded and compared between the first BERA at two and a half months and the second BERA at one year of life.
ASSR are brain response obtained by repetitive acoustic stimulus, allowing us to establish the auditory threshold objectively and specifically at all frequencies of the audiogram. It can establish a reliable audiometry in the whole frequency range, not only in the high frequencies. The threshold in the normal range was considered above 30 dB at frequencies 500, 1,000, 2000 and 4000 Hz. A comparison between the auditory thresholds in the first ASSR at two and a half months with the ASSR at one year of life was made and analyzed.
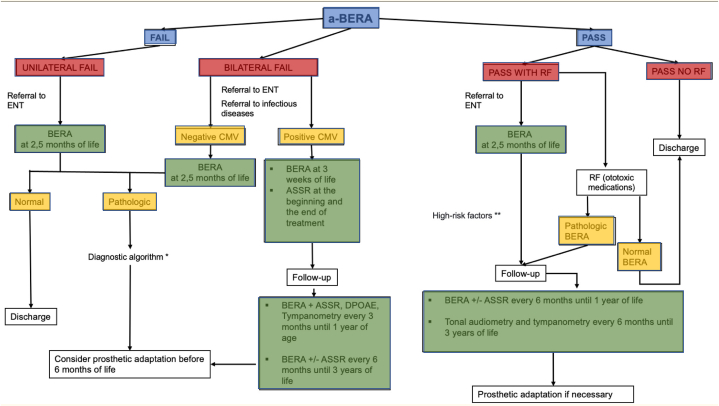
The University Hospital of Torrejón is a 300-bed center, the number of births in a year is 1792 on average. Screening tests are performed on all newborns according to the protocol described in Fig. 1. All children born in the first 48 h of life by performing automatic brainstem evoked response audiometry (a-BERA) in the pediatric room prior to discharge home. All children who pass the test and have no risk factors for late hearing loss are monitored by their pediatrician. All children who do not pass the test will have a-BERA repeated at 15 days of life and only those who do not pass the test again will have a urine sample taken for cytomegalovirus detection and an appointment in ENT office for diagnostic brainstem evoked response audiometry (d-BERA) at two and a half months of life. In addition, all children who pass the automatic potentials but have risk factors for late-onset hearing loss, will also undergo d-BERA at two and a half months (Fig. 1).
Fig. 1.
Universal hearing screening protocol. ASSR: auditory steady-state responses; a-BERA: automatic brainstem evoked response audiometry; CMV: Citomegalovirus; DPOAE: Distorsion products of otoacoustic emission; ENT: Ear nose and throat doctor; RF: Risk Factors. * Tympanometry, Stapedial reflex, Distortion products of Otoacoustic Emission (DPOAE), Genetic study, computerized tomography (CT) and nuclear magnetic resonance (NMR) with sedation, referral to early Hearing Impairment Care Center.** See risk factor in table 1
The ethics committee of hospital university of Torrejon with reference CE83/2022 approved the study and informed consent was obtained from all parents of children participating in the study. Confidentiality and data protection were maintained throughout the study.
Statistical analyses on the data collected were carried out using R software (version 4.2.3.) BERA data were summarized for quantitative variables by mean ± standard deviation and PEEE data by median (and interquartile range). The approximation to the normal distribution was studied using the Shapiro-Wilk test and Lilliefors (Kolmogorov-Smirnov) test. The difference of central measures by sex was estimated using Mann Whitney test and by ear it was estimated using paired Wilcoxon test. A two-sided p-value of 0.05 was considered statistically significant.
It also was estimated the size effect of that differences by Wilcoxon R. The calculated statistical power was 0,1.
In order to evaluate the normality of the BERA's latencies, it was compared by Wilcoxon test for one sample the medians of each condition (Wave, dB, Ear and Moment) with the average of the maximum and minimum values of each normality intervals criterion. Confidence intervals obtained were compared with the clinical reference normality intervals. Finally, the normality of the ASSR obtained values was studied comparing the values of each condition (Hz, Ear and Moment) with the criterion 30 dB by Wilcoxon test for one sample. Differences between the sexes and between the two ears were also studied.
3. Results
Initially, 116 patients were included but we have found difficulties in following up until the first year of life by 19,5 % of families who throughout the study have refused to continue with the study due to the lack of data on possible hearing damage in the literature and to see an evolutionary development within the normality of the acquisition of physiological items in children.
From the initial sample studied we also excluded the only case of neonatal hypoacusis that we found with a V wave at 60 dB bilaterally and delayed latencies in the first BERA at two and a half months because it was associated with a cleft lip malformation.
All newborns underwent nasopharyngeal COVID-19 test at birth. Only 4 infants had a positive swab in the absence of COVID-19 symptomatology.
Finally, a sample of 90 newborns was studied between May 2020 and May 2021, among which we found a sex distribution of 38 boys (42,2 %) and 52 girls (57,7 %). Representing 5,02 % of all newborns born in our hospital in this time interval. The null hypothesis of normal distribution was rejected (p < 0.05) for almost all observations, so non-parametric tests were applied.
3.1. BERA/PEACT
3.1.1. Descriptive analyses
We analyzed the latency of wave I, III, V of the BERA performed at two and a half months and at one year of life, finding a tendency to progressively increase the latencies until the appearance of wave V, always in the normal range.
In the first BERA, wave I at 60 dB is found at an average of 2,4s ± 0,4 in the right ear and 2,3 s ± 0,4 in the left ear and wave III at 4,8 ± 0,4 and 4,7 ± 0,4 respectively. The V wave is studied at different intensities we found a mean of 6,8s ± 0,4 at 60 dB, 7,3s ± 0,7 at 40 dB and 7,9s ± 0,4 at 30 dB in the right ear and 6,7s ± 0,4 at 60 dB, 7,3s ± 0,8 at 40 dB and 7,8s ± 0,5 at 30 dB in the left ear.
In the second BERA, the data are similar to the first ones and wave I at 60 dB is found at a mean of 2,4s ± 0,4 in the right ear and 2,3 s ± 0,4 in the left ear and wave III at 4,7 ± 0,7 and 4,6 ± 0,7 respectively. The V wave is studied at different intensities, we found an average of 6,8 ± 0,4 at 60 dB, 7,2s ± 1,2 at 40 dB and 7,6s ±,3 at 30 dB in right ear and 6,6s ± 0,4 at 60 dB, 7,3s ± 0,9 at 40 dB and 7,7s ± 1,0 at 30 dB in left ear (Table 2).
Table 2.
PEACT comparison with normality averages and intervals.
| WAVE | Hz | Ear | Median | Average normality criterion value | p1 | Estimated 95 % CI | Normality interval | |
|---|---|---|---|---|---|---|---|---|
|
PEACT 1 |
V | 30 dB | LE | 7,84 | 8,05 | <0,05 | 7,69- 7,96 | 6,91-9,19 |
| RE | 7,87 | <0,05 | 7,73- 7,99 | |||||
| 40 dB | LE | 7,34 | 7,45 | <0,05 | 7,23-7,49 | 6,61-8,29 | ||
| RE | 7,4 | 0,33 | 7,27- 7,53 | |||||
| 60 dB | LE | 6,7 | 6,74 | 0,23 | 6,59- 6,80 | 5,92-7,56 | ||
| RE | 6,8 | 0,22 | 6,69-6,90 | |||||
| III | 60 dB | LE | 4,66 | 4,71 | 0,103 | 4,56-4,76 | 3,71-5,71 | |
| RE | 2,33 | 0,225 | 4,66- 4,83 | |||||
| I | 60 dB | LE | 2,23 | 2,54 | <0,05 | 2,16- 2,33 | 1,64-3,44 | |
| RE | 2,33 | <0,05 | 2,26- 2,43 | |||||
|
PEACT 2 |
V | 30 dB | LE | 7,8 | 8,05 | <0,05 | 7,63- 7,93 | 6,91-9,19 |
| RE | 7,8 | <0,05 | 7,63- 7,96 | |||||
| 40 dB | LE | 7,33 | 7,45 | <0,05 | 7,19- 7,47 | 6,61-8,29 | ||
| RE | 7,37 | 0,109 | 7,26- 7,50 | |||||
| 60 dB | LE | 6,6 | 6,74 | <0,05 | 6,49- 6,73 | 5,92-7,56 | ||
| RE | 6,73 | 0,673 | 6,60- 6,86 | |||||
| III | 60 dB | LE | 4,6 | 4,71 | <0,05 | 4,50-4,73 | 3,71-5,71 | |
| RE | 4,71 | 0,987 | 4,58-4,83 | |||||
| I | 60 dB | LE | 2,3 | 2,54 | <0,05 | 2,20-2,40 | 1,64-3,44 | |
| RE | 2,36 | <0,05 | 2,23-2,47 |
3.1.2. Differences between sexes and ears
No significant differences by sex were found in the BERA performed (p > 0.05). Moreover, no significant differences by ears were found in the BERA performed at two and a half months and at one year of life in 30 db and 40 db W–V for both ears (p > 0.05). The size of the differences between ears for the 60 db was in all conditions small (r < 0.30).
3.1.3. Comparison with normality averages and intervals
Although significant differences were found between the median obtained and the average of the normal interval in the 30 dB condition for wave I and 60 db for wave V, the size of these differences was in all cases small (r < 0.30).
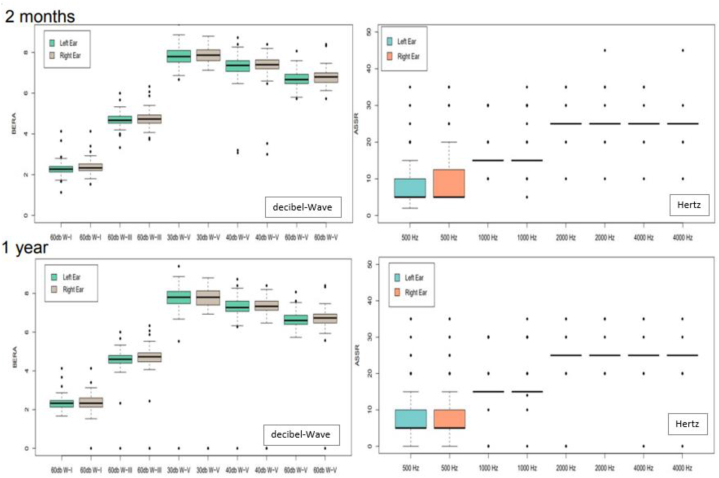
In fact, the range of the latencies for BERA obtained from the estimated 99,5 % confidence intervals founded were inside the desired ranges of normality both at two and a half months and at one year of life (Fig. 2).
Fig. 2.
BERA and ASSR at two and a half months and one year of life The latencies for BERA were inside the ranges of normality both at two and a half months and at one year of life and means of ASSR did not find significant differences with to normality.dB-W: decibels-waves
Hz: Hertz.
3.2. ASSR/PEEE
3.2.1. Descriptive analyses
We found in 5,5 % of the ASSR some threshold alteration at 35 dB almost always in the frequency 2.000 or 4.000 Hz that later was not maintained when performing the second test at one year of life.
We found in the right and left ear a mean in the normal range al the first time evaluated and a year followed up (Table 3)
Table 3.
The table shows the ASSR results at the first time and a year follow-up. *ASSR al one year-old.
| 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | |
|---|---|---|---|---|
| Right ear | 9,8 ± 8,1 | 17,2 ± 5,8 | 25,2 ± 3,2 | 25,0 ± 2,9 |
| Left ear | 9,0 ± 7,1 | 16,9 ± 5,1 | 25,1 ± 2,5 | 25,0 ± 2,4 |
| Right ear * | 8,9 ± 8,2 | 17,0 ± 5,5 | 25,2 ± 2,2 | 24,7 ± 2,2 |
| Left ear * | 8,5 ± 7,8 | 16,6 ± 6, | 24,6 ± 4,4 | 24,8 ± 3,4 |
3.2.2. Differences between sexes and ears
No significant differences by sex were found in the ASSR performed at two and a half months at one year of life (p > 0.05).
In addition, no significant differences by ears were found in the ASSR performed at two and a half months and at one year of life in all of the conditions studied. p > 0.05).
3.2.3. Comparison with normality values
The mean ASSR values were found to be significantly below 30 dB in all frequencies studied both at two and a half months and at one year of life (p < 0.05).
Given these results, we did not find significant differences with to normality, no there seems to be indication that covid is a risk factor for hearing loss in children (Fig. 2).
4. Discussion
Impact of maternal SARS-CoV-2 infection on the newborn's hearing threshold is unknown and controversial [23]. Nevertheless, different pathophysiological mechanisms could explain the hearing effects in the newborn: respiratory distress and hypoxia caused by the infection in pregnant women, intrauterine placental dysfunction or vertical transmission to the newborn [24,25]. Thus, pregnancy causes a partial suppression of the immune system, making women susceptible to viral infections. However, studies on the effects of COVID-19 in pregnant women and newborns are limited.
Studies that found no impact on the hearing of newborns [13,23,[29], [30], [31], [32], [33]] as in our case, are majority. Ghiselli et al. and Mostafa et al. found only one patient with an altered BERA in 63 and 34 newborns respectively whose mothers had been diagnosed with COVID-19 during pregnancy [28,29]. Buonsenso et al. studied newborns whose mothers had COVID-19 at birth, first month and between 3 and 6 months by means of OAE and BERA. Every newborn had normal auditory thresholds [30], just like in our case. Oskovin and Kaplan also studied otoacoustic emissions and compared the results of 458 newborns born to mothers with a history of COVID-19 infection with those of 339 newborns before the COVID-19 pandemic and found no statistically significant differences between the groups [13].
There are few case-control studies in the literature that find impact on hearing in newborns to SARS-CoV-2 infected mothers during pregnancy [21,23,[26], [27], [28]]. Celik et al. found differences between otoacoustic emissions (OAE) results at 3 kHz and 4 kHz in newborns whose mothers were infected with COVID-19 versus newborns whose mothers were not infected with COVID-19. In addition, they found a deficit of the medial olive cochlear efferent system [21].Alan et al. and Veeranna et al. observed that newborns to mothers infected with COVID-19 had more alterations in brainstem potentials than those who were born to uninfected mothers [28,29].
Our study is the only one in the literature until now where BERA and ASSR have been performed comparing their results between them, without performing OAE since they are not systematically included in the universal screening for neonatal hearing loss in the Community of Madrid. In our study we have not only limited ourselves to hearing evaluation in newborns, but we have also performed follow-up hearing evaluations up to 12 months of life in order to rule out the effect of late hearing loss associated with SAR-CoV-2 as well as other TORCH infections. Tu et al., made a follow up of 1, 3 and 6 months, and they didn't find also any unexpected disruptions in hearing loss in newborns to SARS-CoV-2 seropositive mothers [32].
The results showed that there seems to be no effect on newborns of COVID-19 mothers as well as other recent published studies [[31], [32], [33]]. BERA and ASSR performed at birth were comparable to those described in normal population. There were also no differences between sexes.
Later follow-up for 1 year showed that no newborns developed late hearing loss to a greater degree than the general population. Other studies conclude that neonates born to Covid-19 positive mothers do not seem to have an increased risk of hearing los, but reveal that a longer follow-up of these neonates is mandatory to detect any possible effects of the virus [31,32].
These results demonstrated that maternal SARS-COV-2 infection during pregnancy does not appear to be a risk factor for the development of infant hearing loss.
There are studies [13,21,28] that analyze the possible hearing effects in newborns according to the stage of pregnancy in which the mother is infected by SARS-CoV-2, however in our work no association studies have been done according to the trimestral timing of infection by the mother. Mostafa et al. found 34 Covid-19 positive mothers (17 in the first trimester, 8 in the second and 9 in the third). Twenty-nine neonates failed the first screening (p < 0.001) but on further testing only one neonate failed (2.9 %) [31].
The effect of taking hydroxychloroquine was not studied because no pregnant woman required it. However, due to its ototoxic potential, it could cause hypoacusis per se, the study would lose validity, for not studying only the effect of COVID-19 [23].
The limitations of the study are that the results come from a one-year follow up and its necessary more studies to be really sure of effects of COVID-19 in childhood with a longer follow-up to detect any possible delayed effects of the virus.
5. Conclusion
We have found no neonatal hearing loss despite COVID-19 infection during pregnancy, so there seems to be no indication that COVID-19 infection has affected neonatal hearing loss. Inclusion of COVID-19 in the risk factor group for universal screening for childhood hearing loss seems not to be required.
It is known that after maternal SARS-CoV-2 infection, the newborn's auditory system may be affected due to intrauterine hypoxia and vertical transmission. But further studies are needed to clarify this field since it is still unclear whether pregnant women infected with SARS-CoV-2 can produce hearing alterations in their newborns according to the current evidence in the literature.
Funding
None.
Data availability statement
None data associated with our study has been deposited into a publicly available repository.
CRediT authorship contribution statement
Lorena Sanz López: Writing – original draft, Conceptualization. Joaquin Lora Diaz: Validation, Methodology, Data curation. Raúl Castañeda-Vozmediano: Formal analysis, Data curation. Nieves Mata-Castro: Writing – review & editing, Resources, Project administration, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim S.Y., Özgür Yeniova A. Global, regional, and national incidence and mortality of COVID-19 in 237 countries and territories, January 2022: a systematic analysis for World Health Organization COVID-19 Dashboard. Life Cycle. 2022;2(e10):2799–8894. doi: 10.54724/lc.2022.e10. eISSN. [DOI] [Google Scholar]
- 3.Krishnan A., Hamilton J.P., Alqahtani S.A. T A.W. A narrative review of coronavirus disease 2019 (COVID-19): clinical, epidemiological characteristics, and systemic manifestations. Intern Emerg Med. 2021;16:815–830. doi: 10.1007/s11739-020-02616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng X., Deng Y., Dai Z., Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am. J. Otolaryngol. 2020:41. doi: 10.1016/j.amjoto.2020.102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng X., Wang J., Sun J., Zhu K. COVID-19 and sudden sensorineural hearing loss: a systematic review. Front. Neurol. 2022:13. doi: 10.3389/fneur.2022.883749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appelbaum E.N., Howell J.B., Chapman D., Pandya A., Dodson K.M. Analysis of risk factors associated with unilateral hearing loss in children who initially passed newborn hearing screening. Int. J. Pediatr. Otorhinolaryngol. 2018 Mar;106:100–104. doi: 10.1016/j.ijporl.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Thompson D.C., McPhillips H., Davis R.L., Lieu T.L., Homer C.J., Helfand M. Universal newborn hearing screening: summary of evidence. JAMA. 2001;286:2000–2010. doi: 10.1001/jama.286.16.2000. [DOI] [PubMed] [Google Scholar]
- 8.Cohen B.E., Durstenfeld A., Roehm P.C. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. 2014 Jul 29:18. doi: 10.1177/2331216514541361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korver A.M., Smith R.J., Van Camp G., Schleiss M.R., Bitner-Glindzicz M.A., Lustig L.R., Usami S.I., Boudewyns A.N. Congenital hearing loss. Nat. Rev. Dis. Prim. 2017 Jan 12;3 doi: 10.1038/nrdp.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsikas D., Gabrani C., Giannakou K., Lamnisos D. Intrauterine exposure to zika virus and hearing loss within the first few years of life: a systematic literature review. Int. J. Pediatr. Otorhinolaryngol. 2021:147. doi: 10.1016/j.ijporl.2021.110801. [DOI] [PubMed] [Google Scholar]
- 11.Thompson D.C., McPhillips H., Davis R.L., Lieu T.L., Homer C.J., Helfand M. Universal newborn hearing screening: summary of evidence. JAMA. 2001;286:2000–2010. doi: 10.1001/jama.286.16.2000. [DOI] [PubMed] [Google Scholar]
- 12.SARS-CoV-2: is it the newest spark in the TORCH? MuldoonKM, Fowler KB, Pesch MH, Schleiss MR. SARS-CoV-2: is it the newest spark in the TORCH? J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oskovi-Kaplan Z.A., Ozgu-Erdinc A.S., Buyuk G.N., Sert-Dinc U.Y., Ali-Algan C., Demir B., et al. Newborn hearing screening results of infants born to mothers who had COVID-19 disease during pregnancy: a retrospective cohort study. Ear Hear. 2022;43:41–44. doi: 10.1097/aud.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan D., Du T., Hong W., Chen L., Que H., Lu S., et al. Neurological complications and infection mechanism of SARS-COV-2. Signal Transduct. Targeted Ther. 2021;6:406. doi: 10.1038/s41392-021-00818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed M.U., Hanif M., Ali M.J., Haider M.A., Kherani D., Memon G.M., Karim A.H., Sattar A. Neurological manifestations of COVID-19 (SARS-CoV-2): a review. Front. Neurol. 2020 May 22;11:518. doi: 10.3389/fneur.2020.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uranaka T., Kashio A., Ueha R., Sato T., Bing H., Ying G., et al. Expression of ACE2, TMPRSS2, and furin in mouse ear tissue, and the implications for SARS-CoV-2 infection. Laryngoscope. 2021;131:E2013. doi: 10.1002/lary.29324. –e7. [DOI] [PubMed] [Google Scholar]
- 17.Kesser B.W. News flash!-SARS-CoV-2 isolated from the middle ear and mastoid. JAMA Otolaryngol Head Neck Surg. 2020;146:966–967. doi: 10.1001/jamaoto.2020.2067. [DOI] [PubMed] [Google Scholar]
- 18.Núñez-Batalla F., Jáudenes-Casaubón C., Sequí-Canet J.M., Vivanco-Allende A., Zubicaray-Ugarteche J. Ototoxicity in childhood: recommendations of the CODEPEH (commission for the early detection of childhood hearing loss) for prevention and early diagnosis. Acta Otorrinolaringol. Esp. 2022 Jul-Aug;73(4):255–265. doi: 10.1016/j.otoeng.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Wan D., Du T., Hong W., Chen L., Que H., Lu S., et al. Neurological complications and infection mechanism of SARS-COV-2. Signal Transduct. Targeted Ther. 2021;6:406. doi: 10.1038/s41392-021-00818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbasi J. Even mild COVID-19 may change the brain. JAMA. 2022;327:1321–1322. doi: 10.1001/jama.2022.4507. [DOI] [PubMed] [Google Scholar]
- 21.Celik T., Simsek A., Koca C.F., Aydin S., Yasar S. Evaluation of cochlear functions in infants exposed to SARS-CoV-2 intrauterine. Am. J. Otolaryngol. 2021:42. doi: 10.1016/j.amjoto.2021.102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karimi-Boroujeni M., Zahedi-Amiri A., Coombs K.M. Embryonic origins of virus-induced hearing loss: overview of molecular etiology. Viruses. 2021;13 doi: 10.3390/v13010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng X., Wang J., Liu P. Can SARS-CoV-2 positive pregnant women affect the hearing of their newborns: a systematic review. Am. J. Otolaryngol. 2022 Jun 2;43(5) doi: 10.1016/j.amjoto.2022.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020;11:5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allotey J., Chatterjee S., Kew T., Gaetano A., Stallings E., Fernández-García S., et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ. 2022:376. doi: 10.1136/bmj-2021-067696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alan M.A., Alan C. Hearing screening outcomes in neonates of SARS-CoV-2 positive pregnant women. Int. J. Pediatr. Otorhinolaryngol. 2021 Jul;146 doi: 10.1016/j.ijporl.2021.110754. Epub 2021 May 3; PMID: 33964672 PMCID: PMC8091730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veeranna S.A., Youngblood P.L., Bradshaw L., Marx C.G. COVID-19 during pregnancy and its impact on the developing auditory system. Am. J. Otolaryngol. 2022;43 doi: 10.1016/j.amjoto.2022.103484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostafa B.E., Mostafa A., Fiky L.M.E., Omara A., Teaima A. Maternal COVID-19 and neonatal hearing loss: a multicentric survey. Eur. Arch. Oto-Rhino-Laryngol. 2021;279:3435–3438. doi: 10.1007/s00405-021-07098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghiselli S., Laborai A., Biasucci G., Carvelli M., Salsi D., Cuda D. Auditory evaluation of infants born to COVID19 positive mothers. Am. J. Otolaryngol. 2022;43 doi: 10.1016/j.amjoto.2022.103379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buonsenso D., Costa S., Giordano L., Priolo F., Colonna A.T., Morini S., et al. Short- and mid-term multidisciplinary outcomes of newborns exposed to SARS-CoV-2 in utero or during the perinatal period: preliminary findings. Eur. J. Pediatr. 2022;181:1507–1520. doi: 10.1007/s00431-021-04319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostafa B.E., Mostafa A., El Fiky L.M., Omara A., Teaima A. Maternal COVID-19 and neonatal hearing loss: a multicentric survey. Eur. Arch. Oto-Rhino-Laryngol. 2022 Jul;279(7):3435–3438. doi: 10.1007/s00405-021-07098-5. Epub 2021 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu L.J., Benchetrit L., Glovsky C.K., Cohen M.S. Impact of COVID-19 on diagnosis and management of newborn hearing los. Int. J. Pediatr. Otorhinolaryngol. 2023 Jul;170 doi: 10.1016/j.ijporl.2023.111598. Epub 2023 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenks C.M., Desell M., Walsh J. Delays in infant hearing detection and intervention during the COVID-19 pandemic. Otolaryngol. Head Neck Surg. 2022 Apr;166(4):603–604. doi: 10.1177/01945998211067728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None data associated with our study has been deposited into a publicly available repository.