Abstract
Background:
The results of this study included the prevalence of anxiety and depression in women with ovarian cancer.
Methods:
A thorough search of numerous databases, including PubMed (Medline), Scopus, Web of Science, Embase, and PsycoInfo, was conducted to identify relevant cross-sectional studies published between July 2013 and October 2021. STATA 16 was used to analyses the data, and a random effects model was used to determine the pooled prevalence and a 95% confidence interval (95%CI).
Results:
Of the 18 cross-sectional studies reviewed, 17 reported the prevalence of anxiety and 16 reported the prevalence of depression in patients with ovarian cancer. There was a moderate degree of heterogeneity between studies, as the pooled prevalence of depression was 27% (95%CI: 14%-41%; I2=69.44%). Similarly, there was a greater degree of heterogeneity in the pooled prevalence of anxiety, which was found to be 33% (95%CI: 21%-44%; I2=78.55%).
Conclusion:
The findings of this study show that, compared to the overall female population, ovarian cancer patients have much greater rates of sadness and anxiety. These results highlight the necessity for healthcare policymakers to prioritize the provision of resources and support for carrying out additional research, such as longitudinal studies or cohorts, to ascertain the efficacy of various treatments or interventions intended to lessen mental health disorders in women diagnosed with ovarian cancer. Healthcare practitioners can enhance the general wellbeing and quality of life for women with this disease by addressing the psychological components of care.
Key Words: Ovarian Cancer, Depression, Anxiety, Meta-analysis, Systematic review
Introduction
Physical examination findings of a pelvic mass are the key indicator of ovarian cancer, which is caused by deformity and malignancy of the ovarian surface epithelium (Colombo et al., 2006; Hennessy et al., 2009). Due to the lack of early signs, delayed detection, and frequent advanced stage diagnosis, ovarian cancer has a generally dismal prognosis (Colombo et al., 2006; Hwang et al., 2016). Ovarian cancer is the third most common cancer worldwide (Le et al., 2018). The incidence and mortality of ovarian cancer vary in different countries (Liu et al., 2017a). According to the statistics provided by Global Cancer in 2020, the standardized age incidence per 100,000 people in women has been reported as 6.6, and the mortality rate is also 2.4. The highest number of cases in Asia was reported in China, with 149,686 cases in all women (Hennessy et al., 2009). By 2040, the number of new cases of ovarian cancer in Asia is projected to increase by 39.8%, and this increase will be significant in Africa and North America (Hennessy et al., 2009).
To improve the prognosis of this type of cancer, it is essential to manage the quality of life and psychosocial indicators (biological, mental and psychosocial status) of women with ovarian cancer. In addition to managing physical health, supportive psychosocial care is critical for ovarian cancer survivors in the community. Mental health problems can affect adherence to treatment and increase mortality in cancer patients. On the other hand, women with ovarian cancer experience higher levels of psychosocial distress than women with other cancers (Le et al., 2018). Depression and anxiety are two common psychological disorders in cancer, according to some studies (Kangas et al., 2005). Anxiety is often described as fear, nervousness, worry, apprehension and threat in women with ovarian cancer (Listøl et al., 2017). Feelings of sadness, hopelessness and lack of energy are also defined as depression in this group (Shinn et al., 2009).
The quality of life of ovarian cancer patients is impacted by anxiety, depression, and other mental health complications, which also worsen the symptoms of the disease (Nho et al., 2017). The inability to acquire expensive medical care and the loss of reproductive function are two factors that may be the most frequent causes of depression and anxiety in women with ovarian cancer (Yeh et al., 2021). According to a 2015 study by Watts S et al, women were more likely to experience depression before, during, and after treatment, with prevalence rates of 25.34%, 22.99%, and 12.71%, respectively. According to Watts et al., (2015), the prevalence of anxiety was 19.12% prior to therapy, 26.23% during treatment, and 27.09% following treatment. It is crucial to evaluate the mental health of women at the time of ovarian cancer given the significance of their role as the foundation of the family and the necessity to pay attention to both their physical and mental health. However, exploratory research in this field has produced inconsistent and disparate findings about the prevalence of mental problems like depression or anxiety in ovarian cancer patients. The choice of where to place the medical facilities in this situation is challenging. Additionally, a large number of cross-sectional studies conducted globally have looked at the prevalence of anxiety and depression in women with ovarian cancer (Watts et al., 2015). Serious clinical issues include depression and anxiety in ovarian cancer patients. However, because there are so many of these studies, a meta-analysis is required to precisely estimate the prevalence of these outcomes in impacted women and to provide information to health authorities. In 2015, Watts S et al. released a meta-analysis study titled “Depression and anxiety in ovarian cancer: a systematic review and meta-analysis of prevalence rates”(Watts et al., 2015). Nevertheless, an updated meta-analysis is necessary to determine the cumulative prevalence of depression and anxiety in ovarian cancer based on all available evidence in the literature, as other papers with conflicting results have been published on this issue since this meta-analysis. In order to establish and design a program to promote the health of women with this type of cancer, the current study set out to determine the pooled prevalence of depression and anxiety in women with ovarian cancer through a systematic review and meta-analysis study. It was hoped that the results of this study would be reported to other researchers and health policymakers.
Materials and Methods
The recommended reporting elements for systematic reviews and meta-analyses (PRISMA) standards served as the foundation for the creation of this article. The study protocol has additionally been registered in PROSPERO, with registration number CRD42021248733.
Search strategy
This study was a systematic review and meta-analysis to determine the combined prevalence of anxiety and depression in patients with ovarian cancer. Articles published in five electronic databases between January 2013 and October 2021 were identified and reviewed. These databases included PubMed [Medline], Scopus, Web of Science, Embase and PsycoInfo. MeSH and EMTREE were used to find synonyms for the keywords. In addition, the references of the final selected articles were checked using the manual search method to find relevant publications. The two authors (EN and DGH) independently completed and created the search method. The opinion was used to resolve pre-existing disagreements.
Eligibility Criteria
This meta-analysis comprised descriptive, analytical, or retrospective cross-sectional studies with ovarian cancer-related women as the target population and depression and anxiety prevalence as the main end measures. The meta-analysis included all cross-sectional studies that used particular, approved instruments to measure the prevalence of anxiety and depression.
Cohort studies, case-control studies, clinical trials, letters to the editor, case series, case reports, and systematic reviews were all omitted from our search. Additionally, we disqualified studies in which women with gynecological cancers other than ovarian cancer were included in the research population.
Screening and Selection
An Endnote library (version 8) (Bramer et al., 2017) was established to compile articles, eliminate duplicates, and screen titles and abstracts. Initially, a random sample of 10% of the reviewed publications was selected for review by a second researcher (EN), who independently assessed the titles and abstracts. In cases of disagreement, discussions took place, and a third party (YM) was consulted for resolution. Papers that contained the required information in the title or abstract review were chosen for full-text evaluation. The full text was independently reviewed by one of the authors (YM).
Data Extraction
A checklist, created in collaboration with experts, was employed to extract data from the articles. Once the checklist was prepared, the data extraction process commenced. The checklist encompassed essential information such as the author’s name, publication year, prevalence of depression and anxiety among women with ovarian cancer, study type, sample size, country or region where the study was conducted, statistical population under investigation, method used to measure prevalence, and the age range of women with ovarian cancer. By adhering to this checklist, the systematic extraction of relevant data was ensured, contributing to the accuracy and comprehensiveness of the study.
Risk of Bias
Two authors (EN and DGH) utilized the Newcastle-Ottawa Quality Assessment Scale (NOS) checklist (Stang, 2010) to evaluate the studies. The NOS checklist is specifically designed to assess the quality of observational studies, with a particular emphasis on cross-sectional studies. This tool examines each study using six items grouped into three categories, including the selection of study samples, the comparability and analysis of study groups, and the measurement and analysis of the desired outcomes. Each item receives a score of 1 if it is observed in the studies, with a maximum score of 9 for each study. In cases where there was disagreement in the assigned scores for the published articles, discussions and consultation with a third researcher were employed to achieve consensus (Wells et al., 2000).
Statistical Analysis
In this meta-analysis, the first step involved extracting the prevalence data from the selected primary studies. Subsequently, the standard error for each study was calculated. Additionally, the total sample size of each study was extracted to enable the utilization of the Metaprop command in STATA 16. The inverse variance weighted random-effects model was employed in this study to estimate the pooled prevalence of depression and anxiety, along with its corresponding 95% confidence interval (95% CI), among women with ovarian cancer. The Metaprop and Metan commands in STATA 16 were utilized for this purpose. The Metaprop command is specifically designed for meta-analyses of proportions or prevalence rates. It allows for the calculation of pooled prevalence estimates and their corresponding confidence intervals using various methods, such as the inverse variance weighted method, the Mantel-Haenszel method, or the maximum likelihood method. The command also provides options to perform subgroup analyses, examine heterogeneity between studies, assess publication bias, and conduct sensitivity analyses. The Metan command, on the other hand, is a versatile command in STATA that can be used for a range of meta-analytic purposes, including analyzing continuous outcomes, dichotomous outcomes, and time-to-event outcomes. It can estimate pooled effect sizes (e.g., odds ratios, risk ratios, hazard ratios) and their confidence intervals, calculate heterogeneity statistics (e.g., Cochran’s Q, I2), assess publication bias (e.g., funnel plot, Egger’s test), and conduct subgroup analyses (StataCorp, 2017; Longest, 2019).
To assess heterogeneity and variance among the selected studies, Cochrane Q and I2 tests were conducted. Publication bias was evaluated using funnel plot diagrams and Egger tests. Furthermore, meta-regression analysis and charts were employed to investigate the relationship between age and the estimated pooled prevalence. Subgroup analysis was conducted based on countries, NOS score, and age of patients to further explore variations in the data.
Results
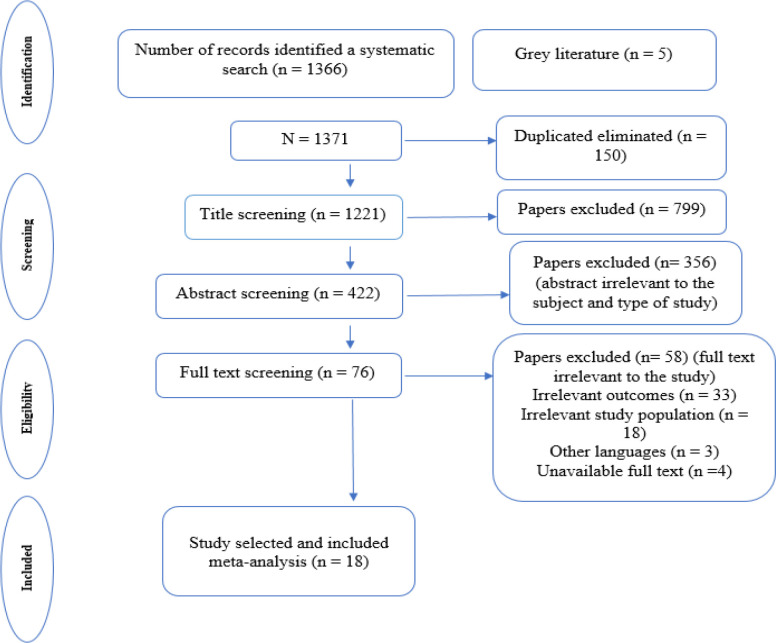
As a result of searching electronic databases, 1,371 articles were obtained, of which 1221 remained after removing duplicates. In the next step, after screening based on the title, abstract, and full text based on the inclusion and exclusion criteria, 18 studies (Wenzel et al., 2002; Norton et al., 2004; Hodgkinson et al., 2007; Goncalves et al., 2008; Bisseling et al., 2009; Liavaag et al., 2009; Slovacek et al., 2009; Gonçalves et al., 2010; Price et al., 2010; Stafford and Judd, 2011; Urbaniec et al., 2011; Chittrakul et al., 2015; Kulpa et al., 2016; Cicero et al., 2017; Liu et al., 2017b; Shand et al., 2018b; Camara et al., 2019; Chen et al., 2021) were selected for meta-analysis (Figure 1), of which 17 studies (Wenzel et al., 2002; Norton et al., 2004; Hodgkinson et al., 2007; Goncalves et al., 2008; Bisseling et al., 2009; Liavaag et al., 2009; Slovacek et al., 2009; Price et al., 2010; Stafford and Judd, 2011; Urbaniec et al., 2011; Chittrakul et al., 2015; Kulpa et al., 2016; Cicero et al., 2017; Liu et al., 2017b; Shand et al., 2018b; Camara et al., 2019; Chen et al., 2021) reported the prevalence of depression and 16 studies (Wenzel et al., 2002; Norton et al., 2004; Hodgkinson et al., 2007; Goncalves et al., 2008; Bisseling et al., 2009; Liavaag et al., 2009; Gonçalves et al., 2010; Stafford and Judd, 2011; Urbaniec et al., 2011; Chittrakul et al., 2015; Kulpa et al., 2016; Cicero et al., 2017; Liu et al., 2017b; Shand et al., 2018b; Camara et al., 2019; Chen et al., 2021) reported the prevalence of anxiety in women with ovarian cancer. Of 18 included studies, 6 were conducted in Australia (Hodgkinson et al., 2007; Bisseling et al., 2009; Price et al., 2010; Stafford and Judd, 2011; Urbaniec et al., 2011; Shand et al., 2018b), 2 in the USA (Wenzel et al., 2002; Norton et al., 2004), 2 in the United Kingdom (Goncalves et al., 2008; Gonçalves et al., 2010), 2 in China (Liu et al., 2017b; Chen et al., 2021), and one in each of the following countries: Norway (Liavaag et al., 2009), Czech Republic (Slovacek et al., 2009), Thailand (Chittrakul et al., 2015), the Netherlands (Camara et al., 2019), Italia (Cicero et al., 2017), and Poland(Kulpa et al., 2016). The average age range of women in these studies were from 36.5 to 62.1 years old (Table 1).
Figure 1.
The Search Outputs and Study Selection
Table 1.
The Characteristics of Included Studies
| Author (Year) | Country | Kind of study | Sample size | Age | Outcome | No. (%) | Tools |
|---|---|---|---|---|---|---|---|
| Hodgkinson K et al. (2006) | Australia | Retrospective | 199 | 59 | Depression | 4(7%) | HADS |
| Anxiety | 11(20%) | HADS | |||||
| Liavaag AH Et al. (2009) | Norway | Cross-sectional | 184 | 52 | Depression | 19(10.32%) | HADS |
| Anxiety | 54(29.34%) | HADS | |||||
| Bisseling KC et al. (2009) | Australia | Retrospective | 62 | 36.5 | Depression | 3(5%) | HADS |
| Anxiety | 17(27%) | HADS | |||||
| Norton TR et al. (2004) | USA | Cross-sectional | 143 | 55.4 | Depression | 79 (55 %) | BDI |
| Anxiety | 29 (20 %) | MHI | |||||
| Wenzel LB et al. (2002) | USA | Cross-sectional | 49 | 55.9 | Depression | 8(16.3%) | QOL-CS |
| Anxiety | 3(6.1%) | QOL-CS | |||||
| Goncalves V et al. (2008) | UK | Retrospective | 118 | 61.1 | Depression | 13(11%) | HADS |
| Anxiety | 38(31%) | HADS | |||||
| Goncalves V et al. (2010) | UK | Retrospective | 30 | 58.8 | Depression | NA | HADS |
| Anxiety | 17(56%) | HADS | |||||
| Price MA et al. (2010) | Australia | Retrospective | 613 | 60.5 | Depression | 36(5.9%) | HADS |
| Anxiety | NA | HADS | |||||
| Slovacek L et al. (2009) | Czech Republic | Cross-Sectional | 30 | 62.1 | Depression | 25(83.3%) | SDS |
| Anxiety | NA | SDS | |||||
| Liu CL et al. (2017) | China | Cross-Sectional | 198 | 56 | Depression | 93(47%) | HADS |
| Anxiety | 102(51.5%) | HADS | |||||
| Chen J et al. (2020) [23] | China | Cross-Sectional | 270 | 53.5 | Anxiety | 127(47.03) | SCSQ |
| Depression | 156(57.77) | SCSQ | |||||
| Chittrakul S et al. (2015) | Thailand | Cross-Sectional | 112 | 53 | Anxiety | 78(70) | HADS |
| Depression | 91(81) | HADS | |||||
| Stafford L et al. (2010) | Australia | Cross-Sectional | 71 | 58.5 | Depression | 28(16%) | HADS |
| Anxiety | 55(31%) | HADS | |||||
| Urbaniec OA et al. (2011) | Australia | Cross-Sectional | 45 | 56.7 | Depression | 9 (20 %) | BDI and STAI |
| Anxiety | 13 (28.9 %) | BDI and STAI | |||||
| Camara C et al. (2019) | Netherlands | Cross-Sectional | 130 | 60 | Anxiety | 6(3.9%) | HADS |
| Depression | 5(4.3%) | HADS | |||||
| Cicero G et al. (2017) | Italia | Cross-Sectional | 120 | 44.95 | Anxiety | 48(40%) | HADS |
| Kulpa M et al. (2016) | Poland | Cross-Sectional | 532 | 54 | Anxiety | 43 (8.21%) | HADS |
| Depression | 37(6.09%) | ERQ | |||||
| Shand LK et al. (2018) | Australia | Cross-Sectional | 108 | 56.36 | Depression | 7(5.84%) | HADS |
| Anxiety | 12(10.46%) | HADS |
Prevalence of depression in women with ovarian cancer
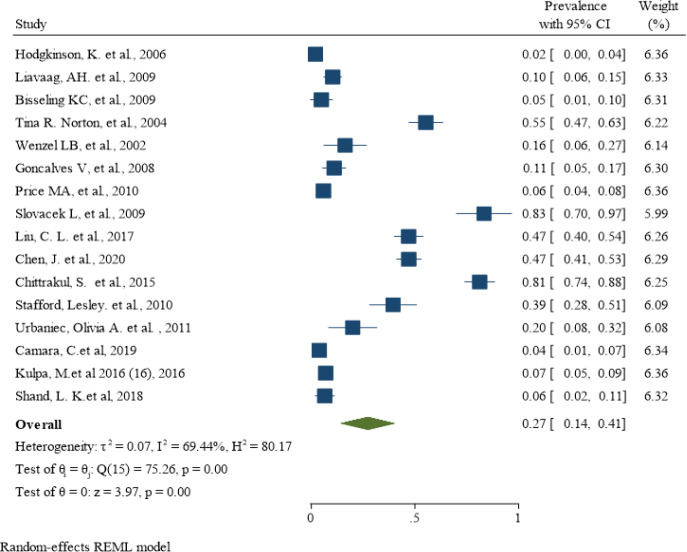
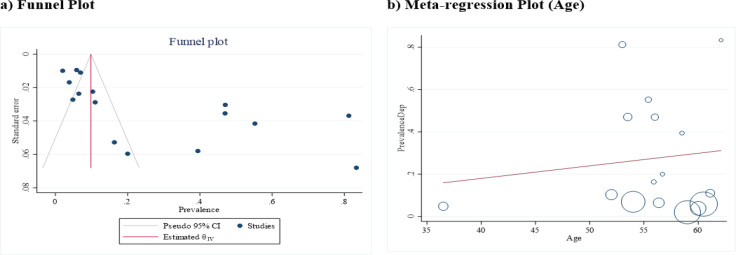
The lowest depression prevalence belonged to the study of Hodgkinson K et al. (Hodgkinson et al., 2007) with a prevalence of 2% (95% CI: 0% - 4%), and the highest depression prevalence belonged to the Slovacek L et al. study (Slovacek et al., 2009) with a prevalence of 83% (95% CI: 70% - 97%). A total of 3085 women with varying degrees of ovarian cancer were studied in these articles, of whom 611 were diagnosed with depression using different depression instruments. After combining the results of these studies, the pooled estimate of depression prevalence was 27% (95% CI: 14% - 41%; Figure 2). The heterogeneity was statistically significant, and the I-squared index of heterogeneity was 69.44%. According to the Eggers test and funnel plot, publication bias occurred in the pooled prevalence of depression in women with ovarian cancer (B = 9.36; SE = 3.11; P = 0.002; Figure 3a). Meta-regression results showed that the prevalence of depression also increased with increasing age of affected women, but this was not statistically significant (B = 0.05; SE = 0.01; P = 0.634; Figure 3b).
Figure 2.
The Pooled Prevalence of Depression in Women with Ovarian Cancer Around the Word
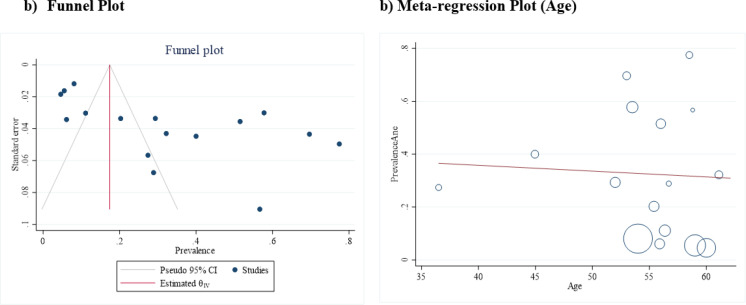
Figure 3.
The Funnel plot (a) and meta-regression plot (b) in the pooled prevalence of depression in women with ovarian cancer
The results of subgroup analysis based on the variables of the country, age, various tools, and NOS score were reported in Table 2. Combining six studies (Hodgkinson et al., 2007; Bisseling et al., 2009; Price et al., 2010; Stafford and Judd, 2011; Urbaniec et al., 2011; Shand et al., 2018b) examining 1169 Australian women with ovarian cancer showed a pooled prevalence of depression of 12% (95% CI: 1% - 23%) in Australia. Two studies (Wenzel et al., 2002; Norton et al., 2004) were performed on 192 women with ovarian cancer in the United States. After combining these results, the pooled prevalence of depression in American women with ovarian cancer was 36% (95% CI: 10% - 64%). Two studies (Liu et al., 2017b; Chen et al., 2021) with a sample size of 468 people were conducted in China. After combining these studies, the pooled prevalence of depression in Chinese women with ovarian cancer was 47 (95% CI: 42% - 53%). Based on the different NOS quality assessment checklist scores, the results of the subgroup analysis showed that the trials with an NOS score of 6 were two trials with a sample size of 107 people. After combining these trials, the pooled prevalence of depression in women with ovarian cancer was 11% (95% CI: 4% - 18%). There were also 11 good-quality trials (the equivalent of 8) with a sample size of 2184 people. When these were combined, the pooled prevalence of depression in women with ovarian cancer was 35% (95% CI: 15% - 56%).
Table 2.
The Subgroup Analysis for Determining the Prevalence of Depression and Anxiety in Women with Ovarian Cancer based on Countries, NOS Score, and Age
| Column 1 | Column 2 | Column 3 | Column 4 | Column 5 | Column 6 | Column 7 | Column 8 |
|---|---|---|---|---|---|---|---|
| Outcomes | Subgroup | No. of Studies (Sample size) | Pooled Prevalence (% 95 CI) |
Heterogeneity Assessment | |||
| I Square | P value | Q test | |||||
| Depression | Countries | Australia | 6 (1169) | 12 % (1 – 23 %) | 62.13% | 0.553 | 15.44 |
| USA | 2 (192) | 36 % (10 – 64 %) | 77.02% | 0.043 | 19.99 | ||
| UK | 1 (118) | 11 % (5 – 17 %) | - | - | - | ||
| China | 2 (468) | 47 % (42 – 53 %) | 0.04% | 0.994 | 0 | ||
| Czech Republic | 1 (30) | 83 % (70 – 97 %) | - | - | - | ||
| Netherlands | 1 (130) | 4 % (1 – 7 %) | - | - | - | ||
| Norway | 1 (184) | 10 % (6 – 15 %) | - | - | - | ||
| Poland | 1 (532) | 7 % (5 – 9 %) | - | - | - | ||
| Thailand | 1 (112) | 81 % (74 – 88 %) | - | - | - | ||
| NOS Score | 6 | 2 (107) | 11 % (4 – 18 %) | 81.30% | 0.033 | 5.35 | |
| 7 | 5 (693) | 19 % (1 – 37 %) | 58.59% | 0.078 | 45.66 | ||
| 8 | 11 (2184) | 35 % (15 – 56 %) | 69.55% | 0.044 | 58.99 | ||
| Age | <=55 | 6 (1280) | 30 % (12 -50 %) | 55.45% | 0.098 | 5.45 | |
| >55 | 11 (1704) | 26 % (11 – 41 %) | 79.32% | 0.033 | 56.69 | ||
| Tools | HADS | 13 (2477) | 15 % (7 – 26 %) | 66.59% | 0.091 | 32.33 | |
| QOL-CS | 2 (192) | 8 % (4 – 12 %) | 40.22% | 0.372 | 6.97 | ||
| Others | 3 (345) | 50 % (22 – 77 %) | 33.92% | 0.203 | 10.55 | ||
| Anxiety | Countries | Australia | 5 (556) | 30 % (5 – 55 %) | 78.69% | 0.001 | 101.82 |
| USA | 2 (192) | 13 % (1 – 26 %) | 48.51% | 0.553 | 1.7 | ||
| UK | 2 (148) | 43 % (19 – 47 %) | 33.49% | 0.366 | 1.26 | ||
| China | 2 (468) | 55 % (49 – 61 %) | 44.80% | 0.183 | 1.81 | ||
| Italy | 1 (120) | 40 % (31 – 49 %) | - | - | - | ||
| Netherlands | 1 (130) | 5 % (1 – 8 %) | - | - | - | ||
| Norway | 1 (184) | 29 % (23 – 36 %) | - | - | - | ||
| Poland | 1 (532) | 8 % (6 – 8 %) | - | - | - | ||
| Thailand | 1 (112) | 70 % (61 – 78 %) | - | - | - | ||
| NOS Score | 6 | 2 (30) | 28 % (20 – 37 %) | 0.02% | 0.87 | 0.03 | |
| 7 | 5 (135) | 18 % (7 – 29 %) | 94.31% | 0.001 | 73.51 | ||
| 8 | 9 (517) | 42 % (23 – 60 %) | 98.33% | 0.001 | 88.03 | ||
| Age | <=55 | 6 (1280) | 39 % (21 – 57 %) | 67.08% | 0.082 | 3.04 | |
| >55 | 10 (1091) | 29 % (13 – 45 %) | 48.76% | 0.098 | 3.01 | ||
| Tools | HADS | 12 (1864) | 33 % (18 – 50 %) | 85.21% | 0.745 | 33.34 | |
| QOL-CS | 2 (192) | 3 % (1 – 6 %) | 55.20% | 0.289 | 15.32 | ||
| Others | 3 (315) | 54 % (48 – 59 %) | 52.44% | 0.34 | 14.77 | ||
Subgroup analysis based on age showed that six studies (Bisseling et al., 2009; Liavaag et al., 2009; Chittrakul et al., 2015; Kulpa et al., 2016; Cicero et al., 2017; Chen et al., 2021) with a sample size of 1280 people determined the prevalence of depression in women with ovarian cancer, aged equal to or less than 55 years. After combining these results, the pooled prevalence of depression was estimated as 30% (95% CI: 12% - 50%) in women with ovarian cancer, aged equal to or less than 55 years. Also, the prevalence of depression in women with ovarian cancer older than 55 years, after combining 11 studies (Wenzel et al., 2002; Norton et al., 2004; Hodgkinson et al., 2007; Goncalves et al., 2008; Slovacek et al., 2009; Price et al., 2010; Stafford and Judd, 2011; Urbaniec et al., 2011; Liu et al., 2017b; Shand et al., 2018b; Camara et al., 2019) with a sample size of 1704 people, was estimated as 26% (95% CI: 11% - 41%).
Prevalence of anxiety in women with ovarian cancer
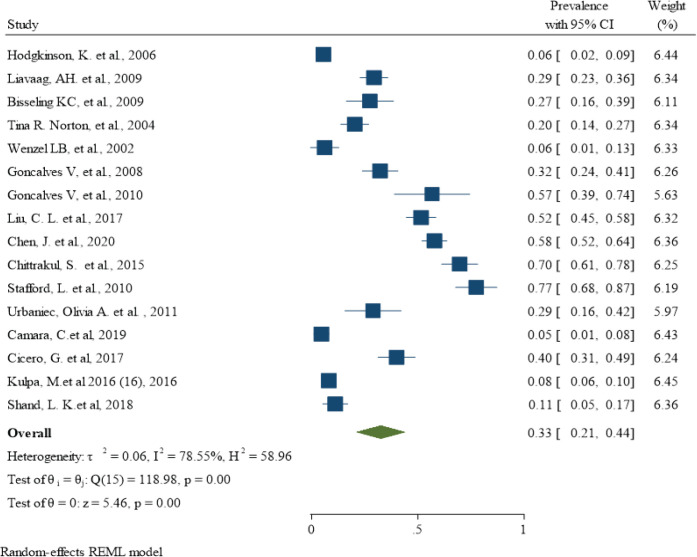
In this section, 16 studies (Wenzel et al., 2002; Norton et al., 2004; Hodgkinson et al., 2007; Goncalves et al., 2008; Bisseling et al., 2009; Liavaag et al., 2009; Gonçalves et al., 2010; Stafford and Judd, 2011; Urbaniec et al., 2011; Chittrakul et al., 2015; Kulpa et al., 2016; Cicero et al., 2017; Liu et al., 2017b; Shand et al., 2018b; Camara et al., 2019; Chen et al., 2021) determined the prevalence of anxiety in women with ovarian cancer, with the lowest prevalence in the survey of Camara C et al. (Camara et al., 2019) with a value of 5% (95% CI: 1% - 8%) and the highest prevalence in the study of Stafford L et al. (Stafford and Judd, 2011) with a value of 77% (95% CI: 68% - 87%). These trials included a total of 2442 women with ovarian cancer, of whom 737 had an anxiety disorder. When the results of these studies were combined, the pooled prevalence was 33% (95% CI: 21% - 44%; Figure 4). However, the degree of heterogeneity was statistically significant and was 78.55% according to the I-squared index. The Egger test and funnel plot showed that publication bias occurred in combination with the results of the studies to determine the pooled prevalence of anxiety in women with ovarian cancer (B: 6.48; SE: 2.81; P: 0.021; Figure 5a). The meta-regression results showed that the prevalence of anxiety also decreased with increasing age of the women affected, but this was not statistically significant (B: -0.02; SE: 0.01; P: 0.834; Figure 5b).
Figure 4.
The Pooled Prevalence of Anxiety in Women with Ovarian Cancer around the Word
Figure 5.
The Funnel Plot (a) and meta-regression plot (b) in the pooled prevalence of anxiety in women with ovarian cancer
The results of subgroup analysis based on the variables of the country, age, various tools, and NOS score were reported in Table 2. Five studies (Hodgkinson et al., 2007; Bisseling et al., 2009; Stafford and Judd, 2011; Urbaniec et al., 2011; Shand et al., 2018b) determined the prevalence of anxiety in Australian women with ovarian cancer and examined a total of 556 people. The pooled prevalence of anxiety in Australian women with ovarian cancer was 30% (95% CI: 5% - 55%). Two studies (Wenzel et al., 2002; Norton et al., 2004) were performed on 192 women with ovarian cancer in the United States. After combining these results, the pooled prevalence of anxiety in American women with ovarian cancer was 13% (95% CI: 1% - 26%). Two studies (Liu et al., 2017b; Chen et al., 2021) with a sample size of 468 people were conducted in China. After combining these studies, the pooled prevalence of anxiety in Chinese women with ovarian cancer was 55% (95% CI: 49% - 61%). Two studies (Goncalves et al., 2008; Gonçalves et al., 2010) with a sample size of 148 women with ovarian cancer were performed in the UK. After combining these results, the pooled prevalence of anxiety in UK women with ovarian cancer was 43% (95% CI: 19% - 47%). The trials with an NOS score of 6 were two trials with a sample size of 30 women. After combining these trials, the pooled prevalence of anxiety in women with ovarian cancer was 28% (95% CI: 20% - 37%). There were also 9 trials with a high quality NOS score (equal to 8), with a sample size of 517 women. When these trials were combined, the pooled prevalence of anxiety in women with ovarian cancer was 42% (95% CI: 20% - 63%).
Six studies (Bisseling et al., 2009; Liavaag et al., 2009; Chittrakul et al., 2015; Kulpa et al., 2016; Cicero et al., 2017; Chen et al., 2021) with a sample size of 1280 people determined the prevalence of anxiety in women with ovarian cancer, aged equal to or less than 55 years. After combining these results, the prevalence of anxiety in women with ovarian cancer, aged equal to or less than 55 years, was 39% (95% CI: 21% - 57%). Also, the prevalence of anxiety in women with ovarian cancer older than 55 years, after combining ten studies (Wenzel et al., 2002; Norton et al., 2004; Hodgkinson et al., 2007; Goncalves et al., 2008; Gonçalves et al., 2010; Stafford and Judd, 2011; Urbaniec et al., 2011; Liu et al., 2017b; Shand et al., 2018b; Camara et al., 2019) with a sample size of 1091 people, was equal to 29% (95% CI: 13% - 45%).
Discussion
The present meta-analysis aimed to determine the pooled prevalence of depression and anxiety in women with ovarian cancer. The results showed that the prevalence of depression and anxiety was 27% and 33%, respectively. In line with the objectives of the present meta-analysis, a prospective cohort study was conducted by Price MA et al., and the results showed that the prevalence of depression and anxiety in women with ovarian cancer was higher than those in the general population(O’Rourke, 2020). Ovarian cancer is the fifth leading cause of death among American women (Ho et al., 2021). This type of cancer does not have an effective method for early diagnosis and effective treatment among women, so it is complicated to diagnose the early symptoms(Permuth-Wey and Sellers, 2009; Lengyel, 2010). According to various studies, 59% of patients have been diagnosed with metastatic symptoms, and most patients recur within 18 months (Jayson et al., 2014; Lee et al., 2020). According to the National Cancer Institute, the 5-year survival rate for ovarian cancer is 47.4%. However, this survival rate in these patients is associated with low quality of life and severe symptoms. Patients undergo various drug and chemotherapy treatments during this period and are exposed to intestinal obstruction and other clinical conditions such as ascites. These factors lead to pain, fatigue, and ultimately lower quality of life and lack of continuity in treatment(Roland et al., 2013; Shand et al., 2018a; Hill and Hamm, 2019). In this case, it seems that a negative attitude towards treatment and feelings of hopelessness can lead to increased distress(Yu and Nho, 2015; Espinosa and Espinosa, 2016; Shand et al., 2018a; Zhou et al., 2021). As a result, it is necessary to pay more attention to ovarian cancer than other gynecological cancers(Shand et al., 2018a; Zhou et al., 2021). In the studies included in the present meta-analysis, most of the study population were treated with medication or chemotherapy. After chemotherapy, there were significant changes in the level of anxiety and depression. According to previous studies, the prevalence of depression after starting drug and chemotherapy treatment in women with ovarian cancer decreased, but the prevalence of anxiety increased. One of the possible causes of low depression scores and increased stress after chemotherapy was that patients who had been depressed at the time of diagnosis were treated. They gradually accepted the truth, but instead, they were more concerned about the side effects of medication or chemotherapy, the cost, the economic problems, and the life expectancy after treatment resulting in increased anxiety after chemotherapy(Watts et al., 2015; Mielcarek et al., 2016; Liu et al., 2017a; Liu and Yang, 2019; O’Rourke, 2020).
The present meta-analysis results showed that the prevalence of depression and anxiety was higher in women with ovarian cancer aged less than 55 years. According to previous studies, stress and psychological disorders have occurred more in younger women than in older ones. In this case, it is recommended that psychological interventions be performed earlier to improve the outcome in these people. Younger women with ovarian cancer develop more severe mental disorders because they have lived with the disease for less than women over 55 years. Older women live with this cancer and are more accustomed to it. In younger women, because the level of life expectancy is higher than that in women with older ages, the rate of anxiety and depression is also higher(Yu and Nho, 2015; Liu and Yang, 2019; Cohee et al., 2020; Yang et al., 2020).
This meta-analysis showed the prevalence of depression and anxiety in Chinese women with ovarian cancer was higher than in Australian, American, and British women. Of course, the results of only two studies (Liu et al., 2017b; Chen et al., 2021) in China were combined, and the number of studies in this country was lower than those in other countries. Still, the meta-analysis showed a higher prevalence of these two outcomes in Chinese women with ovarian cancer. The higher prevalence of depression and anxiety in women with ovarian cancer in China and other developed countries can be attributed to the development of diagnostic methods and timely and appropriate screening of mental disorders in these communities. In contrast, in developing countries, this is not possible.
On the other hand, conducting training programs to promote the culture of the society and the attitude of women with ovarian cancer is another influential factor in the high prevalence of this disease. In developed countries, access to appropriate facilities and training patients encourage them to seek care and participate in screening programs. The results of previous studies have shown that, in people living in rural areas, the rate of depression, anxiety, and low quality of life was higher. Cultural context may influence the expression of emotions, which in many cases prevents early diagnosis and appropriate treatment of depression. Most comparative studies have reported worse outcomes for women with ovarian cancer living in rural areas. These women have more significant needs and limited access to diagnostic and treatment resources(Berendes et al., 2010; Zenger et al., 2010; Rajandram et al., 2011; Ting et al., 2015). The long-term use of social media, applications, and other communication tools to communicate with physicians is another compelling factor in encouraging patients to undergo screening, which is more available in developed countries than underdeveloped or developing ones(Yu and Nho, 2015; Stefansdottir, 2016; O’Rourke, 2020). Socioeconomic status in different countries can also be another reason for differences in the prevalence of depression and anxiety in women with ovarian cancer(Kornblith et al., 1995; Arden‐Close et al., 2008).
In this meta-analysis, trials with lower NOS checklist quality scores showed a lower prevalence of anxiety and depression in ovarian cancer patients. The prevalence of depression and anxiety was more common in trials that scored 6 on the NOS checklist, according to the results of this meta-analysis. Poor cross-sectional studies may underestimate the prevalence of anxiety and depression in ovarian cancer patients. The subgroup analyses in this meta-analysis showed much less heterogeneity, suggesting that the sources of heterogeneity have been identified. The heterogeneity in the pooled prevalence of depression and anxiety in women with ovarian cancer was caused by the age of the women studied, the quality of the articles, differences in study methods or methodology, and the different populations studied. The acceptable level of heterogeneity of the meta-analysis, both in the overall analysis and in the subgroup analyses, was a notable aspect. One of the weaknesses of this meta-analysis was the small sample size for the subgroup analyses, which included different countries and different types of treatment (such as chemotherapy or drugs). In addition, the poor and imprecise reporting of the chemotherapy regimen, treatment and drug class used in the original trials made it impossible to do subgroup analyses based on different treatments.
In conclusion, the prevalence of mental health problems, including depression and anxiety, in women with cancer, especially ovarian cancer, was 27% for depression and 33% for anxiety, according to this meta-analysis. It is important for people with ovarian cancer to have their mental health checked and monitored regularly, especially before and after starting chemotherapy and medication. Depression and anxiety can be prevented by carrying out such assessments to identify psychological changes in patients, checking for potential risk factors and organizing social support.
Abbreviation
NOS: New castle- Ottawa Quality Assessment
MeSH: Medical Subject Headings
EMTREE: Medline Subject Headings
PRISMA: The Preferred Reporting Items for Systematic Reviews and Meta-Analysis
PROSPERO: International Prospective Register of Systematic Reviews
CI: Confidence Interval
Author Contribution Statement
Conceptualization: YM; methodology: YM, HD, DG, and EN; software: YM, EN; validation: SK, MA; Qualitative data collection and analysis: YM, DG, and EN; investigation: SK, MA, and EEA; resources, YM; data curation: YM, HD, EN, and EEA; writing—review and editing: YM, HD, and EEA; supervision: YM; project administration: YM. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
Not applicable.
Declarations
Ethics approval and consent to participate
The study protocol has been registered in PROSPERO, whose registration code is CRD42021248733. Also, this project approved in ethical committee at Kurdistan University of Medical Sciences (IR.MUK.REC.1400.153).
Consent for publication
Not applicable.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to their sensitive and potentially personally identifiable nature. However, they are available from the corresponding author on reasonable request.
Funding
The Kurdistan University of Medical Sciences supported this work under the code IR.MUK.REC.1400.153. The programs of funders had no role in the design and conducting of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests
All the authors declare that they have no conflict of interest.
References
- Arden‐Close E, Gidron Y, Moss‐Morris R. Psychological distress and its correlates in ovarian cancer: a systematic review. Psychooncology. 2008;17:1061–72. doi: 10.1002/pon.1363. [DOI] [PubMed] [Google Scholar]
- Berendes D, Keefe FJ, Somers TJ, et al. Hope in the context of lung cancer: relationships of hope to symptoms and psychological distress. J Pain Symptom Manage. 2010;40:174–82. doi: 10.1016/j.jpainsymman.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseling KC, Kondalsamy‐Chennakesavan S, et al. Depression, anxiety and body image after treatment for invasive stage one epithelial ovarian cancer. Aust N Z J Obstet Gynaecol. 2009;49:660–6. doi: 10.1111/j.1479-828X.2009.01074.x. [DOI] [PubMed] [Google Scholar]
- Bramer WM, Milic J, Mast F. Reviewing retrieved references for inclusion in systematic reviews using EndNote. J Med Libr Assoc. 2017;105:84. doi: 10.5195/jmla.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara C, Caroline Vos M, de Rooij BH, et al. The role of positive psychological changes in anxiety and depression of patients with ovarian tumors and their partners: an observational study from the population-based PROFILES registry. Support Care Cancer. 2019;27:423–31. doi: 10.1007/s00520-018-4327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Long DR, Guo XJ, Liu Y, You HX. Anxiety and depression symptoms among ovarian cancer patients in China: A cross-sectional study. Front Nurs. 2021;7:321–7. [Google Scholar]
- Chittrakul S, Charoenkwan K, Wongpakaran N. Prevalence of anxiety may not be elevated in Thai ovarian cancer patients following treatment. Asian Pac J Cancer Prev. 2015;16:1251–4. doi: 10.7314/apjcp.2015.16.3.1251. [DOI] [PubMed] [Google Scholar]
- Cicero G, De Luca R, Dorangricchia P, et al. Risk Perception and Psychological Distress in Genetic Counselling for Hereditary Breast and/or Ovarian Cancer. J Genet Couns. 2017;26:999–1007. doi: 10.1007/s10897-017-0072-0. [DOI] [PubMed] [Google Scholar]
- Cohee AA, Kroenke K, Vachon E, et al. Predictors of depression outcomes in adults with cancer: A 12 month longitudinal study. J Psychosom Res. 2020;136:110169. doi: 10.1016/j.jpsychores.2020.110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo N, Van Gorp T, Parma G, et al. Ovarian cancer. Crit Rev Oncol Hematol. 2006;60:159–79. doi: 10.1016/j.critrevonc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Espinosa P, Espinosa M. Experiences to chemotherapy among women with breast cancer. Int J Bio Sci Bio Technol. 2016;8:159–66. [Google Scholar]
- Goncalves V, Jayson G, Tarrier N. A longitudinal investigation of psychological morbidity in patients with ovarian cancer. Br J Cancer. 2008;99:1794–801. doi: 10.1038/sj.bjc.6604770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves V, Jayson G, Tarrier N. A longitudinal investigation of psychological disorders in patients prior and subsequent to a diagnosis of ovarian cancer. J Clin Psychol Med Settings. 2010;17:167–73. doi: 10.1007/s10880-010-9196-1. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–82. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- Hill EM, Hamm A. Intolerance of uncertainty, social support, and loneliness in relation to anxiety and depressive symptoms among women diagnosed with ovarian cancer. Psychooncology. 2019;28:553–60. doi: 10.1002/pon.4975. [DOI] [PubMed] [Google Scholar]
- Ho D, Kim SY, Kim SI, Kim SY, Lim WJ. Insomnia, Anxiety, and Depression in Patients First Diagnosed With Female Cancer. Psychiatry Investig. 2021;18:755. doi: 10.30773/pi.2021.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson K, Butow P, Fuchs A, et al. Long-term survival from gynecologic cancer: psychosocial outcomes, supportive care needs and positive outcomes. Gynecol Oncol. 2007;104:381–9. doi: 10.1016/j.ygyno.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Hwang K-H, Cho O-H, Yoo Y-S. Symptom clusters of ovarian cancer patients undergoing chemotherapy, and their emotional status and quality of life. Eur J Oncol Nurs. 2016;21:215–22. doi: 10.1016/j.ejon.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–88. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- Kangas M, Henry JL, Bryant RA. The course of psychological disorders in the 1st year after cancer diagnosis. J Consult Clin Psych. 2005;73:763. doi: 10.1037/0022-006X.73.4.763. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Thaler HT, Wong G, et al. Quality of life of women with ovarian cancer. Gynecol Oncol. 1995;59:231–42. doi: 10.1006/gyno.1995.0014. [DOI] [PubMed] [Google Scholar]
- Kulpa M, Ziętalewicz U, Kosowicz M, Stypuła-Ciuba B, Ziółkowska P. Anxiety and depression and cognitive coping strategies and health locus of control in patients with ovary and uterus cancer during anticancer therapy. Contemp Oncol (Pozn) 2016;20:171–5. doi: 10.5114/wo.2016.60074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le L, Yu L, Guan C, Zhang X. Epidemiology, Etiology, Screening, Psychotherapy of Malignant Tumor Patients with Secondary Depressive Disorder. Curr Pharm Des. 2018;24:2591–6. doi: 10.2174/1381612824666180727125448. [DOI] [PubMed] [Google Scholar]
- Lee A, Moon BI, Kim TH. BRCA1/BRCA2 pathogenic variant breast Cancer: treatment and prevention strategies. Ann Lab Med. 2020;40:114–21. doi: 10.3343/alm.2020.40.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listøl W, Høberg-Vetti H, Eide GE, Bjorvatn C. Anxiety and depression symptoms among women attending group-based patient education courses for hereditary breast and ovarian cancer. Hered Cancer Clin Pract. 2017;15:1–9. doi: 10.1186/s13053-016-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Liu L, Zhang Y, Dai XZ, Wu H. Prevalence and its associated psychological variables of symptoms of depression and anxiety among ovarian cancer patients in China: a cross-sectional study. Health Qual Life Outcomes. 2017;15:1–11. doi: 10.1186/s12955-017-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Liu L, Zhang Y, Dai XZ, Wu H. Prevalence and its associated psychological variables of symptoms of depression and anxiety among ovarian cancer patients in China: A cross-sectional study. Health Qual Life Outcomes. 2017;15:161. doi: 10.1186/s12955-017-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang L. Dynamic change of depression and anxiety after chemotherapy among patients with ovarian cancer. Medicine. 2019:98. doi: 10.1097/MD.0000000000016620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek P, Nowicka-Sauer K, Kozaka J. Anxiety and depression in patients with advanced ovarian cancer: a prospective study. J Psychosom Obstet Gynaecol. 2016;37:57–67. doi: 10.3109/0167482X.2016.1141891. [DOI] [PubMed] [Google Scholar]
- Nho JH, Kim SR, Nam JH. Symptom clustering and quality of life in patients with ovarian cancer undergoing chemotherapy. Eur J Oncol Nurs. 2017;30:8–14. doi: 10.1016/j.ejon.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Cancer Epidemiol. 2009;2009:413–37. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- Price MA, Butow PN, Costa DS, et al. Prevalence and predictors of anxiety and depression in women with invasive ovarian cancer and their caregivers. Med J Aust. 2010;193:52–7. doi: 10.5694/j.1326-5377.2010.tb03929.x. [DOI] [PubMed] [Google Scholar]
- Rajandram RK, Ho SM, Samman N, et al. Interaction of hope and optimism with anxiety and depression in a specific group of cancer survivors: a preliminary study. BMC Res Notes. 2011;4:1–7. doi: 10.1186/1756-0500-4-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland KB, Rodriguez JL, Patterson JR, Trivers KF. A literature review of the social and psychological needs of ovarian cancer survivors. Psychooncology. 2013;22:2408–18. doi: 10.1002/pon.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand LK, Brooker JE, Burney S, Fletcher J, Ricciardelli LA. Psychosocial factors associated with posttraumatic stress and growth in Australian women with ovarian cancer. J Psychosoc Oncol. 2018;36:470–83. doi: 10.1080/07347332.2018.1461728. [DOI] [PubMed] [Google Scholar]
- Shand LK, Brooker JE, Burney S, Fletcher J, Ricciardelli LA. Psychosocial factors associated with posttraumatic stress and growth in Australian women with ovarian cancer. J Psychosoc Oncol. 2018;36:470–83. doi: 10.1080/07347332.2018.1461728. [DOI] [PubMed] [Google Scholar]
- Shinn EH, Taylor CLC, Kilgore K, et al. Associations with worry about dying and hopelessness in ambulatory ovarian cancer patients. Palliat Support Care. 2009;7:299–306. doi: 10.1017/S1478951509990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovacek L, Slanska I, Slovackova B, et al. Screening for depression in survivors of metastatic ovarian cancer in a programme of palliative cancer care. Bratisl Lek Listy. 2009;110:655–9. [PubMed] [Google Scholar]
- Stafford L, Judd F. Long-term quality of life in Australian women previously diagnosed with gynaecologic cancer. Support Care Cancer. 2011;19:2047–56. doi: 10.1007/s00520-010-1064-x. [DOI] [PubMed] [Google Scholar]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- Stefansdottir V. Experience of social media support group for BRCA carriers. J Genet Couns. 2016;25:1342–4. doi: 10.1007/s10897-016-0009-z. [DOI] [PubMed] [Google Scholar]
- Ting Z, Hui-ping L, De-bin W. Psychological resilience and its influencing factors among breast cancer patients. Chin Public Health. 2015;31:263–7. [Google Scholar]
- Urbaniec OA, Collins K, Denson LA, Whitford HS. Gynecological cancer survivors: assessment of psychological distress and unmet supportive care needs. J Psychosoc Oncol. 2011;29:534–51. doi: 10.1080/07347332.2011.599829. [DOI] [PubMed] [Google Scholar]
- Watts S, Prescott P, Mason J, McLeod N, Lewith G. Depression and anxiety in ovarian cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2015;5:e007618. doi: 10.1136/bmjopen-2015-007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel LB, Donnelly JP, Fowler JM, et al. Resilience, reflection, and residual stress in ovarian cancer survivorship: a gynecologic oncology group study. Psychooncology. 2002;11:142–53. doi: 10.1002/pon.567. [DOI] [PubMed] [Google Scholar]
- Yang M, Sun R, Wang Y, et al. Study Protocol for the Evaluation of Individual Psychological Interventions for Family Caregivers of Advanced Cancer Patients. Front Psychol. 2020:11. doi: 10.3389/fpsyg.2020.587627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YC, Lu CH, Chen IH, Kuo SF, Huang YP. Quality of life and its predictors among women with gynaecological cancers. Collegian. 2021;28:81–8. [Google Scholar]
- Yu SY, Nho JH. Influence of sleep disturbance and depression on quality of life in ovarian cancer patients during chemotherapy. Asian Oncol Nurs. 2015;15:203–10. [Google Scholar]
- Zenger M, Brix C, Borowski J, Stolzenburg JU, Hinz A. The impact of optimism on anxiety, depression and quality of life in urogenital cancer patients. Psychooncology. 2010;19:879–86. doi: 10.1002/pon.1635. [DOI] [PubMed] [Google Scholar]
- Zhou LH, Hong JF, Qin RM, et al. Post-traumatic growth and its influencing factors among Chinese women diagnosed with gynecological cancer: A cross-sectional study. Eur J Oncol Nurs. 2021;51:101903. doi: 10.1016/j.ejon.2021.101903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to their sensitive and potentially personally identifiable nature. However, they are available from the corresponding author on reasonable request.