Abstract
Objective:
The most important casuse of cervical cancer incidence and high mortality rate is infection to the human papillomavirus (HPV). The aim of the present study was to investigate the effect of silencing HPV E6 oncogene on cervical cancer cells using specific siRNAs.
Materials and Methods:
CaSki cervical cancer cells, carrying E6 gene, were cultured and then transfected with E6 targeting siRNAs. The cell viability through suppression of E6 expression was explored using MTT assay. Besides, apoptosis induction was investigated by means of flow cytometry using Annexin / PI staining. The changes in the expression of target genes were examined via Real-Time PCR.
Results:
E6 gene silencing caused a significant decrease in the survival rate of CaSki cells through remarkable enhancement of apoptosis induction. Moreover, E6 suppression led to significant upregulation of P53, Bax, Caspase-3, and Caspase-9 mRNA expression while downregulated Bcl-2 expression. Interestingly, it was found that suppression of E6 expression could lead to upregulation of E5 and E7 expression as a compensatory mechanism for E6 deactivation.
Conclusion:
According to the results of this study, suppression of E6 expression using specific siRNAs could be considered as a therapeutic approach for cervical cancer.
Key Words: Cervical cancer, E6, siRNA, Apoptosis
Introduction
Human papillomavirus (HPVs) with more than 200 genotypes in the papilloma viridae family has a protein structure and a circular dsDNA genome, with about 800 octahedral, non-enveloped nucleotides (De Villiers et al., 2004; Sohrabi et al., 2014). HPVs are classified into five genera: alpha (α), beta (β), gamma (γ), mu (μ) and nu (ν), of which α and β are the most studied (Bernard et al., 2010). The virus causes malignant and benign tumors in the human mucosa and skin. The cancers associated with this virus are cancers of the cervix, skin, larynx, head, and neck. Among the diseases, we can mention genital warts and conjunctival diseases of the eye (Hoque and Hoque, 2009; Nour, 2009). HPV genotypes are classified into three categories in terms of cancer development: Low Risk, Intermediate Risk, and High Risk, whose presence is necessary for transcription in host cells. The Low-Risk group includes strains such as HPV 6 and 11, which usually only cause genital warts, and the second category, which includes High Risk, includes high-risk strains of the virus, including 31 and 18,16-HPV strains, which commonly cause cervical cancer; which can be seen with moderate to severe cervical dysplasia (Münger et al., 2004; Burk et al., 2009; Blödt et al., 2012; Pappa et al., 2017). The establishment and survival of these viruses are related to the expression of their early proteins (E1 to E7), which are essential for replication and transcription in the host cell (Doorbar et al., 1997; Hemmat and Baghi, 2018). Late gene expression by L1 and L2 proteins occurs in the superficial cervical cells and is released from the cell surface by forming a virus capsid (Laimins, 1996; Buck et al., 2008). E1 and E2 proteins are involved in replication and are the main replication factor in HPV. In addition, transcription in this virus is controlled by E2 protein (Fehrmann and Laimins, 2003; Zheng and Baker, 2006). The main activity of E6 protein is in high-risk types of viruses, which causes the destruction of P53 protein by ubiquitination. Ubiquitination of E6 to E6-AP (cellular E6-dependent protein) depends on the binding of E6 to P53 (Li and Coffino, 1996; Scheffner and Whitaker, 2003). This protein contains 160 amino acids with two zinc fingers bound by CXXC (Kruiswijk et al., 2015; Paek et al., 2016; Tomaić, 2016). P53 acts as a tumor inhibitor. Inhibition of translocation and inhibition of P53 expression by E6 protein is a key factor in preventing the activity or suppression of genes by P53(Fakhr et al., 2018) (Pim et al., 1994; Elbel et al., 1997). If the E6 protein is inactive, it increases P53, which in turn causes P53 dependent apoptosis and ultimately kills infected cells. In contrast, the ability of the E6 protein to modulate P53 levels is a complete factor. E6 can also increase the regulation of cell telomerase complex activity, which preserves telomeric DNA at the ends of chromosomes (Greider and Blackburn, 1985; Bonab et al., 2021).
Given that Human papillomavirus is the cause of many cancers, including the “main cause of cervical cancer” and also considering that the main cause of carcinogenicity of this virus is the function of its E5, E6 and E7 oncoproteins, nowadays, using gene silencing methods, including small interfering RNAs (siRNAs) to inhibit expression of these genes can be an effective strategy for cancer therapy (Fire et al., 1998; Hemmat et al., 2020; Nahand et al., 2021). These synthetic 21 or 22 nucleotides siRNAs mimic the functions of microRNAs and suppress the gene expression through interacting with their 3’ UTR regions (Zarredar et al., 2018; Zarredar et al., 2019a).
Considering the significance of E6 HPV gene in cervical cancer progression, the current research was performed to evaluate the therapeutic effect of siRNA-mediated suppression of E6 expression in CaSki cervical cancer cells. The obtained results illustrated that suppressing E6 expression could lead to the elimination of cervical cancer cells through increasing apoptosis induction.
Materials and Methods
Cell culture
CaSki, human cervical cancer cell line, carrying E6 gene prepared from the National Cell Bank of Iran, was cultured in T25 cell culture flasks with RPMI-1640 medium (Gibco, USA) containing antibiotics containing streptomycin (100 μg/mL), penicillin (100 IU/mL) and FBS (10%, Gibco). The incubation condition for cells was an atmosphere providing 37°C heat, 5% CO2, and humidity. Trypsin EDTA (25%, Gibco) was used for harvesting and sub-culturing the cells, as they reached 70% confluence.
siRNA transfection
FITC-conjugated controls (Gene PharmaCo, Shanghai) and specific siRNAs targeting E6, using Gene Pulser electroporation system (Bio-Rad), in the amount of 100 pmol were transfected into CaSki cells at the density of 1 × 106 cells suspended in 500 μL electroporation buffer in a 0.2 cm cuvette, according to supplied protocols as the following: Volts=160v and TC=12.5 ms. 2 × 105 of E6 siRNA-transfected cells were seeded into six‐well culture plates, and after 24,48 and 72 hours of cultivation, they were subjected to flow cytometry and real-time PCR, as explained in the following section to evaluate the efficiency of E6 suppression using siRNA.
qRT-PCR
GeneAll Trizol RNA extraction kit (Korea) was used to extract total RNA according to provided instructures. Then, the evaluation of RNA concentration and quality was perforemd regarding optical density of samples at 260 nm and 280 nm wavelengths using the The DeNovix DS-11 Spectrophotometer ( Wilmington, USA). Besides, the extracted RNA was visualized on agarose gel (1% w/v in TBE buffer) to check its integrity. Afterward, 1μg of qaulified RNA samples were subjected to complementary DNA (cDNA) synthesis by using RT Master Mix (Takara Prime Script). Finally, the expression levels of target genes, including Bax, Bcl-2, Caspase 3,9 and 10, E6, E5, E7, P53 were relatively quantified using a Bio FACT™ 2X Real-Time PCR Master Mix (Korea) by means of the Applied Biosystems Step One Plus Real-Time PCR System (USA). The endogenous control gene of GAPDH was used to normalization of gene expression in samples. The relevant sequences for used oligonucleotides are shown in Table 1.
Table 1.
Primer Sequences
| Gene | Type | Primer sequence |
|---|---|---|
| P53 | Forward | CCCGGACGATATTGAACAATGG |
| Reverse | CAGAATGCAAGAAGCCCAGAC | |
| E5 | Forward | AAGGCGGCCGCTATGACAAATCTTGATACTGC |
| Reverse | ATGCTCTAGACATTATGTAATTAAAAAGCG | |
| E6 | Forward | AGGGAGTAACCGAAAACG |
| Reverse | CATAAAACCAGCCGTTAC | |
| E7 | Forward | AGCGCGGCCGCTATGCACCAAAAGAGAACTGC |
| Reverse | ATGCTCTAGAGATTATGGTTTCTGAGAACAG | |
| BAX | Forward | TTTGCTTCAGGGTTTCATCC |
| Reverse | CAGCTCCATGTTACTGTCCA | |
| BCL-2 | Forward | CTGTGGATGACTGAGTACCTG |
| Reverse | GAGACAGCCAGGAGAAATCA | |
| Caspase 3 | Forward | GTGGAACTGACGATGATATGGC |
| Reverse | CGCAAAGTGACTGGATGAACC | |
| Caspase 9 | Forward | GCAGGCTCTGGATCTCGGC |
| Reverse | GCTGCTTGCCTGTTAGTTCGC | |
| Caspase 10 | Forward | GGAGTGGCTCTGTAAGGACG |
| Reverse | AGCAGGTTGTTCACATCCCC |
MTT assay
To find out the effect of E6 suppression on CaSki cells viability, MTT assay (3‐(4,5‐dimethylthiazol‐ 2‐yl) ‐2,5‐diphenyltetrazolium bromide ) was performed. A total number of 1.2 × 104 of CaSki cells transfected with E6 siRNA were cultured in each well of 96‐ well plates. Following 24,48, and 72 hours of incubation, the cells were exposed to 50 μl MTT solution (2 mg/mL ) for 4 hours. Then, the formed formazan crystals were solubilized by substitution of the medium by dimethyl sulfoxide (150 μL). After a 30 min incubation, the cells were subjected to a microplate reader (Tecan, Switzerland) to evaluate cell viability rate according to the optical density (OD) at 570 nm wavelength.
Apoptosis assay
To follow apoptosis induction in treatment groups, CaSki cells after transfection with E6 siRNA, at a density of 2 × 105 cells per well, were incubated in six‐well plates for 48 hours. After that, the cells were detached with trypsin/EDTA, harvested and washed with PBS. Afterward, the harvested cells were incubated by annexin V (5 µl) and DAPI (5 µl) dissolved in 200 μL binding buffer (Exbio - Czech). Then, the cells were rewashed with PBS and the subjected to the flow cytometry device (Milteny Biotec™FACSQuant 10; Germany) to evaluate the portion of apoptotic, necrotic, and live cells.
Statistical analysis
Statistical analysis and designing of graphs were done by using GraphPad Prism version 6.0 software (San Diego, CA). Flow cytometric data were analyzed using FlowJo software. All data was represented as means ± standard errors. The T-test and one-way ANOVA test were used to specify the statistical significance of intergroup differences. P values less than 0.5 were regarded to be statistically significant.
Results
E6 gene expression was sufficiently suppressed using designed siRNAs
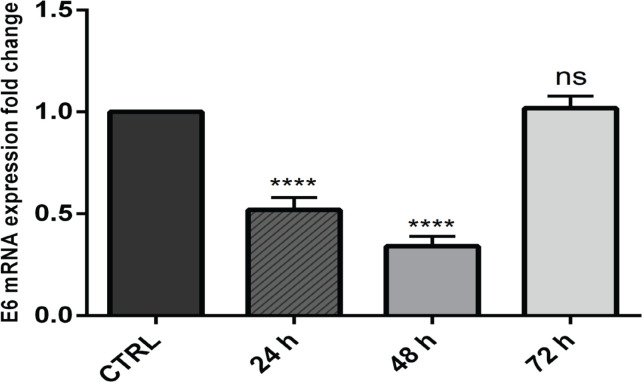
To find out whether designed siRNA can efficiently suppress E6 expression, flow cytometry and real-time PCR analysis were performed. The results obtained from flow cytometry revealed that 97.2 percent of FITC-conjugated control sequences were successfully transfected into CaSki cells. Moreover, qPCR results evidenced that transfection of E6 siRNA into CaSki cells was able to efficiently suppress its mRNA expression. The significant suppression of E6 gene expression using specific siRNAs was stable till 48 hours after transfection (Figure 1). Then, according to the obtained results, 48 hours of incubation after transfection were applied for all following experiments.
Figure 1.
The Significant Suppression of E6 Gene Expression in CaSki Cells after Specific E6 siRNA Transfection; ****p<0.0001
E6 suppression led to a decrease in CaSki cell survival
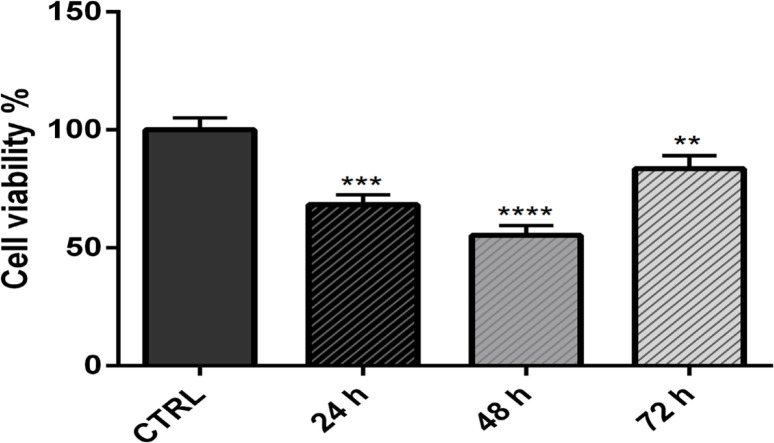
MTT assay was performed to find out the effect of E6 suppression using siRNAs on CaSki cell viability. After transfection of cells with E6 siRNA, the survival rate of CaSki cells was significantly reduced after 24 h (p<0.001), 48 h (p<0.0001), and 72 h (p<0.01) of incubation compared to the control group. As presented in Figure 2, the lowest proliferation rate was observed at 48 hours, and the highest proliferation rate was at 72 hours.
Figure 2.
MTT Assay was Performed to Find Out the Effect of E6 Suppression Using siRNAs on CaSki Cell Viability. After transfection of cells with E6 siRNA, the survival rate of CaSki cells was significantly reduced after 24 h (p<0.001), 48 h (p<0.0001), and 72 h (p<0.01) of incubation compared to the control group
E6 suppression induced apoptosis in CaSki cells
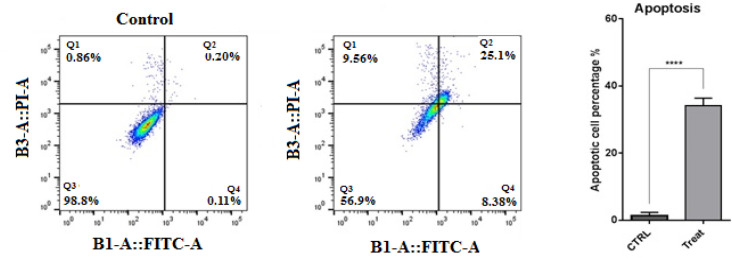
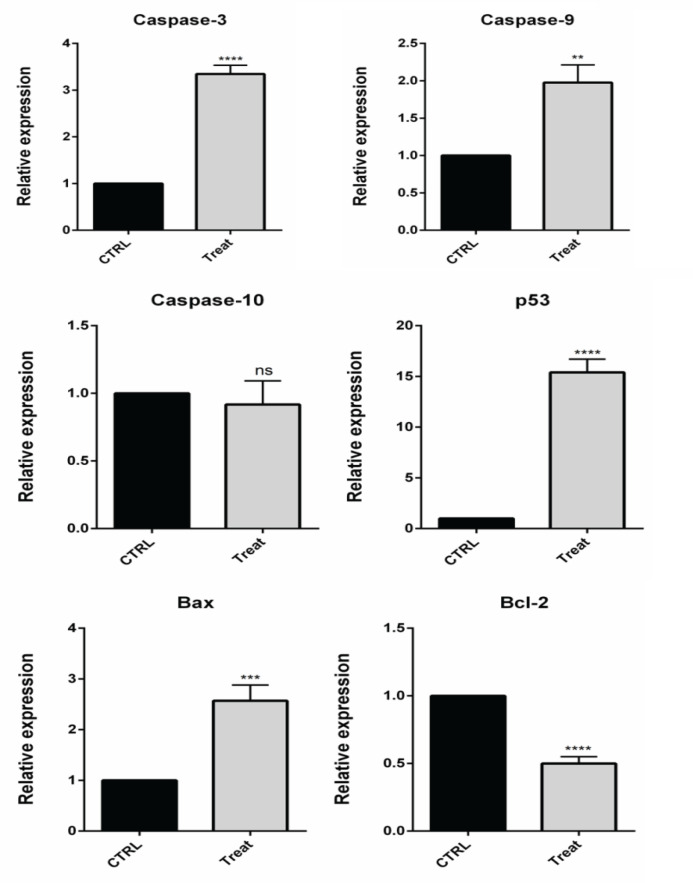
To investigate whether E6 suppression exerts its anti-proliferative effect through apoptosis induction, flow cytometry analysis was performed. As illustrated in Figure 3, the obtained results evidenced that suppressing E6 expression remarkably (p<0.0001) increased apoptosis rate from 0.31% to 33.48% in CaSki cells, confirming E6 anti-apoptotic role through tumorigenesis. Then, qPCR was performed to evaluate the modulation of major apoptosis regulators through suppression E6 in CaSki cells. Following the transfection of cells with E6 siRNA, the expression levels of Bax and Bcl-2 genes were significantly (p<0.001 and p<0.0001) increased and decreased in comparison with control, respectively (Figure 4). As previously established, Bax acts as a key proapoptotic gene induced by various factors in the internal apoptosis pathway, and Bcl-2 has an anti-apoptotic effect in response to various apoptotic stimuli by preventing the release of cytochrome C from mitochondria. Besides, qPCR results evidenced that E6 knockdown led to significant upregulation of caspase-3 (p<0.0001), caspase-9 (p<0.01), and P53 (p<0.0001), as the key effectors in apoptosis induction, in CaSki cells. However, no significant difference was observed in the expression of levels of caspase-10 following the transfection of CaSki cells with E6 siRNA compared to the control group.
Figure 3.
Annexin V / PI Staining Results. The obtained results from flowcytometry, shown that suppressing of the E6 expression remarkably (p<0.0001) increased apoptosis rate from 0.31% to 33.48% in CaSki cells, confirming E6 anti-apoptotic role through tumorigenesis ****p<0.0001
Figure 4.
The Changes in the Expression Levels of Cell Survival and Apoptosis-Related Genes were Determined Using qPCR through Transfecting CaSki Cells with E6 siRNA; ****p<0.0001, ***p<0.001 and **p<0.01. qPCR results shown that E6 knockdown led to significant upregulation of caspase-3 (p<0.0001), caspase-9 (p<0.01), Bax (p<0.001) and P53 (p<0.0001), as the key effectors in apoptosis induction, in CaSki cells. Also there is no significant difference was observed in the expression of levels of caspase-10 following the transfection of CaSki cells with E6 siRNA compared to the control group. Also, there is significant low expression in Bcl-2 expression in treated cell (p<0.0001).
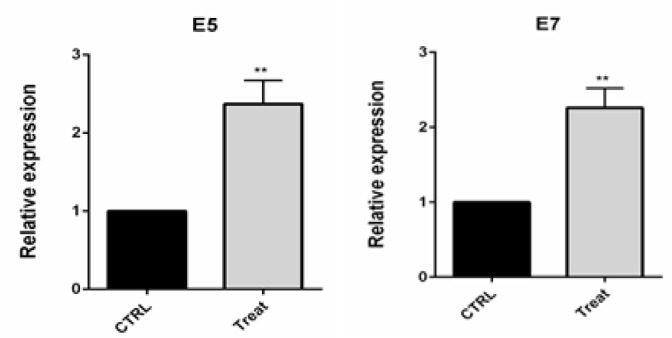
E6 gene suppression increased E5 and E7 expression levels
E5 HPV protein stimulates cancer cells to proliferate by forming a complex with epidermal growth factor receptor, platelet-derived growth factor receptor, and clonal stimulus receptor. Also, E7 HPV was shown to interact with Rb and lead to activation of E2F transcription factor through separation from Rb. High E7 activity may lead to apoptosis in E7 expressing cells. Then, E5 and E7 gene expression in transfected CaSki cells was analyzed by RT-PCR. Following the transfection of CaSki cells using E6 siRNA, the expression of E5 and E7 genes were significantly (p<0.01) increased compared to the control group (Figure 5).
Figure 5.
qPCR Results Evidenced the Upregulation of E5 and E7 HPV Gene Expression through E6 Knockdown in CaSki cells; **p<0.01
Discussion
In the current study, we investigated the therapeutic effects of inhibiting the human papillomavirus (HPV) E6 gene expression using specific siRNA through the modulating cellular apoptosis on CaSki cervical cancer cells. This was examined by comparing the expression of cancer-related genes. This experimental approach demonstrated the benefits of using siRNA, which is predicted to be associated with changes in p53 expression. Interestingly, we observed that reducing the expression of E6 led to downregulation of Bcl-2 while increasing the expression levels of Bax, E5, E7, P53, caspase-3, caspase-9 in the CaSki cells. The main activity of E6 protein is in high-risk types of viruses, which causes the destruction of P53 proteins by ubiquitination. P53, along with Bax, caspase-3, caspase-9, is the imperative modulator of the apoptosis signaling pathway. When caspases are upregulated and activated, the apoptotic pathway begins and induces cell death (Greider and Blackburn, 1985). Bcl-2 is an anti-apoptotic protein that can compete with Bax for its anti-apoptotic function (Igney and Krammer, 2002). Also, our test was based on the assumption that E6 knockdown using siRNA could exert its effects through modulating caspase 10. Contrary to the hypothesis, no association was found between E6 suppression and caspase-10 expression in the present study.
In a similar study by Jiang and Milner (2002), E6 and E7 human papillomavirus genes were suppressed by specific siRNAs in SiHa and CaSki cell lines infected with HPV-16. E6 and E7 knockdown increases p53 and p21 protein levels in infected cell lines and inhibits cell growth through induction of apoptosis. Yoshinouchi et al., (2003) also evidenced that suppression of the expression of HPV-16 oncogenes caused the re-accumulation of tumor-inhibiting proteins in infected SiHa cervical cancer cells. The study also showed that SiHa cells transfected with E6 siRNA in the NOD / SCID mice animal model produced significantly smaller tumors than the animal model receiving SiHa cells transfected with negative control siRNAs. Besides, Yamato et al., (2006) conducted an experiment showing that in HeLa cells carrying HPV-18 genome, suppression of E6 gene expression using siRNAs could inhibit tumorigenicity of HeLa cells better than suppressing E6 and E7 genes.
Qin and Cheng, (2010) Tested siRNA user IKKε to show that siRNA could provide a new treatment strategy for breast cancer by silencing IKKε. IκB kinase ε (IKKε) is a member of the IKK family that plays an important role in NF-κB activation. IKKε is expressed in more than 30% of breast cancers and has recently been identified as a potential oncogen for breast cancer (Zarredar et al., 2019b). In 2017 Aletaha et al., (2017) Examined siRNA for MDA-MB-468 cancer cells and showed that siRNA transfection was effective in breast adenocarcinoma cells and inhibited migration, proliferation and induction of apoptosis.
Wechsler et al., (2018) examined the inhibitory function of siRNA on the expression of the Human papillomavirus E5 gene in AKC2 cells and showed that inhibiting the expression of this gene reduces the expression level of EGFR protein. In addition, this inhibition will inhibit the expression of two other Human papillomavirus oncogenes, E6 and E7.
Finally, Salguero-Aranda et al., (2019) Showed that the STAT6 siRNA sequence is capable of inhibiting the proliferation and induction of apoptosis of colorectal cancer cells HT-29 and breast cancer cells ZR-75-1, and halves the number of cancer cells in a short time.
In conclusion, to sum up, our finding, alongside previous reports, implied that using of RNA interference strategy, including siRNA, could be an effective way to suppress the oncogenic activity of HPV through targeting E6 gene in cervical cancer cells. We also illustrated that E6 knockdown diminishes CaSki cervical cancer cell viability through apoptosis induction by modulating the expression of Bcl2, Bax, P53, caspase-3, and caspase-9. Having considered that E6 suppression could be an effective strategy, however, there is a need for further confirmatory experiments in case of in vivo experiments and clinical trials as well as illustrating further underlying mechanisms.
Author Contribution Statement
F.R. performed the majority of experiments and data analysis. M.A. and H.Z. contributed to carry out the experiments and interpreted the results; H.O., K.D., M.J. and L.V. contributed to perform experiment and analyse the results. A.M., B.B. and H.B.B. revised the manuscript, designed and conducted the project..
Acknowledgements
Funding statement
The authors are grateful for supports from the Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Ethics statement
This study involving human participants was reviewed and approved by Tabriz University of medical sciences and under the ethical approval code of IR.TBZMED.VCR.REC.1398.147.
Availability of data and material
All data generated in this study are available in the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aletaha M, Mansoori B, Mohammadi A, Fazeli M, Baradaran B. The effect of snail1 gene silencing by siRNA in metastatic breast cancer cell lines. Iran J Public Health. 2017;46:659. [PMC free article] [PubMed] [Google Scholar]
- Bernard H-U, Burk RD, Chen Z, et al. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–9. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blödt S, Holmberg C, Müller-Nordhorn J, Rieckmann N. Human Papillomavirus awareness, knowledge and vaccine acceptance: A survey among 18-25 year old male and female vocational school students in Berlin, Germany. Eur J Public Health. 2012;22:808–13. doi: 10.1093/eurpub/ckr188. [DOI] [PubMed] [Google Scholar]
- Bonab FR, Baghbanzadeh A, Ghaseminia M, et al. Molecular pathways in the development of HPV-induced cervical cancer. Excli J. 2021;20:320. doi: 10.17179/excli2021-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Cheng N, Thompson CD, etal Arrangement of L2 within the papillomavirus capsid. J Virol. 2008;82:5190–5197. doi: 10.1128/JVI.02726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk RD, Chen Z, Van Doorslaer K. Human papillomaviruses: genetic basis of carcinogenicity. Public Health Genom. 2009;12:281–90. doi: 10.1159/000214919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Villiers E-M, Fauquet C, Broker TR, Bernard H-U, Zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Doorbar J, Foo C, Coleman N, et al. Characterization of Events during the Late Stages of HPV16 Infectionin VivoUsing High-Affinity Synthetic Fabs to E4. Virology. 1997;238:40–52. doi: 10.1006/viro.1997.8768. [DOI] [PubMed] [Google Scholar]
- Elbel M, Carl S, Spaderna S, Iftner T. A comparative analysis of the interactions of the E6 proteins from cutaneous and genital papillomaviruses with p53 and E6AP in correlation to their transforming potential. Virology. 1997;239:132–49. doi: 10.1006/viro.1997.8860. [DOI] [PubMed] [Google Scholar]
- Fakhr MG, Kahkhaie KR, Shanehbandi D, Hagh MF, Zarredar H, Safarzadeh E, Vind MA, Baradaran B. Scrophularia atropatana extract reverses tp53 gene promoter hypermethylation and decreases survivin antiapoptotic gene expression in breast cancer cells. Asian Pac J Cancer Prev. 2018;19:2599. doi: 10.22034/APJCP.2018.19.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22:5201–7. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–13. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Hemmat N, Baghi HB. Human papillomavirus E5 protein, the undercover culprit of tumorigenesis. Infect Agents Cancer. 2018;13:1–2. doi: 10.1186/s13027-018-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmat N, Mokhtarzadeh A, Aghazadeh M, et al. Role of microRNAs in epidermal growth factor receptor signaling pathway in cervical cancer. Mol Biol Rep. 2020;47:4553–68. doi: 10.1007/s11033-020-05494-4. [DOI] [PubMed] [Google Scholar]
- Hoque E, Hoque M. Knowledge of and attitude towards cervical cancer among female university students in South Africa. S Afr J Epidemiol Infect. 2009;24:21–4. [Google Scholar]
- Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–88. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- Jiang M, Milner J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene. 2002;21:6041–8. doi: 10.1038/sj.onc.1205878. [DOI] [PubMed] [Google Scholar]
- Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Bio. 2015;16:393–405. doi: 10.1038/nrm4007. [DOI] [PubMed] [Google Scholar]
- Laimins LA. Seminars in Virology. Elsevier; 1996. Human papillomaviruses target differentiating epithelia for virion production and malignant conversion; pp. 305–13. [Google Scholar]
- Li X, Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol. 1996;70:4509–16. doi: 10.1128/jvi.70.7.4509-4516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K, Baldwin A, Edwards KM, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–60. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahand JS, Shojaie L, Akhlagh SA, et al. Cell death pathways and viruses: role of microRNAs. Mol Ther Nucl Acids. 2021;24:487–511. doi: 10.1016/j.omtn.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour NM. Cervical cancer: a preventable death. Rev Obstet Gynecol. 2009;2:240. [PMC free article] [PubMed] [Google Scholar]
- Paek AL, Liu JC, Loewer A, Forrester WC, Lahav G. Cell-to-cell variation in p53 dynamics leads to fractional killing. Cell. 2016;165:631–42. doi: 10.1016/j.cell.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa KI, Lygirou V, Kontostathi G, et al. Proteomic analysis of normal and cancer cervical cell lines reveals deregulation of cytoskeleton-associated proteins. Cancer Genom Proteom. 2017;14:253–66. doi: 10.21873/cgp.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pim D, Storey A, Thomas M, Massimi P, Banks L. Mutational analysis of HPV-18 E6 identifies domains required for p53 degradation in vitro, abolition of p53 transactivation in vivo and immortalisation of primary BMK cells. Oncogene. 1994;9:1869–76. [PubMed] [Google Scholar]
- Qin B, Cheng K. Silencing of the IKKε gene by siRNA inhibits invasiveness and growth of breast cancer cells. Breast Cancer Res. 2010;12:R74. doi: 10.1186/bcr2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salguero-Aranda C, Sancho-Mensat D, Canals-Lorente B, et al. STAT6 knockdown using multiple siRNA sequences inhibits proliferation and induces apoptosis of human colorectal and breast cancer cell lines. PLoS One. 2019;14:e0207558. doi: 10.1371/journal.pone.0207558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Whitaker NJ. Seminars in cancer biology. Elsevier; 2003. Human papillomavirus-induced carcinogenesis and the ubiquitin–proteasome system; pp. 59–67. [DOI] [PubMed] [Google Scholar]
- Sohrabi A, Mirab-Samiee S, Modarresi MH, et al. Development of in-house multiplex real time PCR for human papillomavirus genotyping in Iranian women with cervical cancer and cervical intraepithelial neoplasia. Asian Pac J Cancer Prev. 2014;15:6257–61. doi: 10.7314/apjcp.2014.15.15.6257. [DOI] [PubMed] [Google Scholar]
- Tomaić V. Functional roles of E6 and E7 oncoproteins in HPV-induced malignancies at diverse anatomical sites. Cancers. 2016;8:95. doi: 10.3390/cancers8100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler EI, Tugizov S, Herrera R, Da Costa M, Palefsky JM. E5 can be expressed in anal cancer and leads to epidermal growth factor receptor-induced invasion in a human papillomavirus 16-transformed anal epithelial cell line. J Gen Virology. 2018;99 doi: 10.1099/jgv.0.001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamato K, Fen J, Kobuchi H, et al. Induction of cell death in human papillomavirus 18-positive cervical cancer cells by E6 siRNA. Cancer Gene Ther. 2006;13:234–41. doi: 10.1038/sj.cgt.7700891. [DOI] [PubMed] [Google Scholar]
- Yoshinouchi M, Yamada T, Kizaki M, et al. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by E6 siRNA. Mol Ther. 2003;8:762–8. doi: 10.1016/j.ymthe.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Zarredar H, Ansarin K, Baradaran B, Ahdi Khosroshahi S, Farajnia S. Potential molecular targets in the treatment of lung cancer using siRNA technology. Cancer Invest. 2018;36:37–58. doi: 10.1080/07357907.2017.1416393. [DOI] [PubMed] [Google Scholar]
- Zarredar H, Farajnia S, Ansarin K, et al. Synergistic effect of novel EGFR inhibitor AZD8931 and p38α siRNA in lung adenocarcinoma cancer cells. Anti-Cancer Agent Med (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2019a;19:638–44. doi: 10.2174/1871520619666190301125203. [DOI] [PubMed] [Google Scholar]
- Zarredar H, Pashapour S, Farajnia S, et al. Targeting the KRAS, p38α, and NF‐κB in lung adenocarcinoma cancer cells: The effect of combining RNA interferences with a chemical inhibitor. J Cell Biochem. 2019b;120:10670–7. doi: 10.1002/jcb.28357. [DOI] [PubMed] [Google Scholar]
- Zheng Z-M, Baker CC. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front Biosci. 2006;11:2286. doi: 10.2741/1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated in this study are available in the manuscript.