Abstract
Background
Telazorlimab is a humanized anti-OX40 monoclonal antibody being studied for treatment of T-cell–mediated diseases.
Objective
This randomized, placebo-controlled, phase 2b dose-range finding study investigated efficacy, safety, pharmacokinetics, and immunogenicity of telazorlimab in subjects with atopic dermatitis.
Methods
In this 2-part study (NCT03568162), adults (≥18 years) with moderate-to-severe disease were randomized to various regimens of subcutaneous telazorlimab or placebo for 16 weeks’ blinded treatment, followed by 38 weeks’ open-label treatment and 12 weeks’ drug-free follow-up. Telazorlimab treatment groups (following a loading dose) in part 1 were 300 mg every 2 weeks; 300 mg every 4 weeks; or 75 mg every 4 weeks. Part 2 evaluated telazorlimab 600 mg every 2 weeks. The primary end point was percentage change from baseline in Eczema Area and Severity Index (EASI) at week 16. Safety assessments included incidence of treatment-emergent adverse events.
Results
The study randomized 313 subjects in part 1 and 149 in part 2. At 16 weeks, the least squares mean percentage change from baseline in EASI was significantly greater in subjects receiving telazorlimab 300 mg every 2 weeks (part 1) and 600 mg every 2 weeks (part 2) versus placebo (−54.4% vs −34.2% for part 1 and −59.0% vs −41.8% for part 2, P = .008 for both). Telazorlimab was well tolerated, with similar distribution of adverse events between telazorlimab- and placebo-treated subjects in both part 1 and part 2.
Conclusion
Telazorlimab, administered subcutaneously at 300 mg every 2 weeks or 600 mg every 2 weeks following a loading dose, was well tolerated and induced significant and progressive clinical improvement in adults with moderate-to-severe atopic dermatitis.
Key words: Atopic dermatitis, anti-OX40 receptor, humanized monoclonal antibody, phase 2, telazorlimab
Atopic dermatitis (AD) is the most common inflammatory skin disorder in developed countries, typically beginning in childhood and persisting into adulthood.1 Lifetime prevalence of AD ranges from 15% to 20%, and moderate-to-severe disease can have deleterious effects on patients’ health-related quality of life.1
Biologic therapies, comprising directed targeting agents that inhibit specific cytokines and cytokine receptors2 or Janus kinases (JAK),3 have shown clinical benefit in patients with moderate-to-severe AD and have become mainstays of treatment.4, 5, 6 Dupilumab, which downregulates TH2 inflammation by inhibiting IL-4R signaling, has shown significantly superior response rates compared to placebo in several phase 3 trials2,7, 8, 9 and has become the current standard of care. The oral JAK inhibitor upadacitinib has demonstrated activity superior to dupilumab; however, it is indicated only for patients whose AD has not been controlled adequately with other systemic drugs.10,11 Other agents that have shown significant improvement in AD symptoms compared to placebo include nemolizumab, which targets the IL-31 receptor α subunit,12 the anti–IL-13 agents tralokinumab13 and lebrikizumab,14,15 and the JAK inhibitors abrocitinib16,17 and baricitinib.18
Recent research has focused on the potential role of OX40/OX40 ligand (OX40L) signaling in AD.19 OX40 (TNFRSF4, CD134) is a costimulatory receptor member of the NGFR/TNFR superfamily, expressed predominantly on activated T lymphocytes, including CD4 and CD8 T cells, T helper cells, and forkhead box P3–positive CD4+ regulatory T (Treg) cells. Unlike CD28, which is the archetypal, constitutively expressed T-cell costimulatory receptor, OX40 is not expressed on naive T lymphocytes but rather is transiently induced on CD4 and CD8 T cells 24 hours to 5 days after initial T-cell receptor stimulation.20, 21, 22 OX40 is also upregulated on Treg cells—cells critical to maintaining immune tolerance and fine-tuning T-cell activity.23
Ligation of OX40 by OX40L on antigen-presenting cells enhances survival and functions of effector T cells and impairs the suppressive functions of Treg cells. Upregulation of OX40/OX40L connects TH2 and TH1 pathways by inducing the release of IFN-γ and turning anergic, autoreactive T cells into effector T cells.24,25 Compared to normal skin, AD lesions show upregulation of the OX40/OX40L axis with increased numbers of OX40L-positive dendritic cells, as well as greater expression of OX40.26, 27, 28 Unlike expression of OX40, which generally is restricted to T cells, OX40L is expressed on multiple cell types, including antigen-presenting cells, endothelial cells, and airway smooth muscle cells,29,30 which, in the context of a therapeutic intervention, may result in on-target, off-disease adverse events in patients with AD.
Telazorlimab (ISB 830, GBR 830) is a humanized, anti-OX40 IgG1 monoclonal antibody devoid of target agonism, which is under investigation for the treatment of autoimmune and chronic inflammatory disorders.31,32 Unlike rocatinlimab (formerly AMG 451/KHK4083), an afucosylated anti-OX40 antibody with enhanced antibody-dependent cellular cytotoxicity,33 telazorlimab specifically blocks OX40 signaling on activated T cells with moderate levels of antibody-dependent cellular cytotoxicity (Ichnos Sciences, unpublished data). A phase 2a proof-of-concept study in subjects with moderate-to-severe AD reported that telazorlimab, administered as two 10 mg/kg intravenous doses 4 weeks apart (days 1 and 29), was safe and well tolerated.34 The treatment also induced significant reductions in the mRNA expressions of TH1, TH2, and TH17/ TH22 T-cell gene signatures in lesional skin and yielded greater improvement in Eczema Area and Severity Index-50 (EASI-50, a ≥50% reduction from baseline in EASI) relative to placebo (76.9% vs 37.5%), suggesting that telazorlimab has effects on both the acute and chronic stages of AD.34 The current randomized phase 2b study was undertaken to assess the safety and efficacy of subcutaneous telazorlimab in adults with moderate-to-severe AD.
Methods
Study design
This was a randomized, double-blind, placebo-controlled, parallel-group study (NCT03568162) conducted at 95 clinical sites in North America and Europe from November 2018 to August 2021. The study protocol and informed consent form were reviewed and approved by institutional ethics committees or review boards at each site. The study was conducted according to Good Clinical Practice guidelines35 and the Declaration of Helsinki,36 and all subjects gave written informed consent before participation. The study consisted of 4 phases: screening, followed by blinded treatment (weeks 1-16) and open-label treatment (weeks 16-54), concluding with follow-up (weeks 54-66) during which no study drug was administered.
Participants
Male or female adults (>18 years of age) with a diagnosis of moderate-to-severe AD for at least 1 year, according to the American Academy of Dermatology’s consensus criteria,37 were included in the study. Ineligible subjects included those with skin comorbidities (eg, psoriasis) that might interfere with assessment, and individuals who were immunocompromised; had human immunodeficiency virus, hepatitis B or C, tuberculosis, or other recent infection; or had poorly controlled asthma. Women who were pregnant or breast-feeding also were excluded.
Treatments for AD that were prohibited included topical steroids and immunomodulators (from 1 week before baseline); systemic corticosteroids and immunomodulators (including investigational agents, from 4 weeks); phototherapy (from 4 weeks); investigational biologics (from the longer of 8 weeks or 5 half-lives); approved biologics, including dupilumab (from the longer of 12 weeks or 5 half-lives); and cell-depleting agents (from 6 months).
Randomization
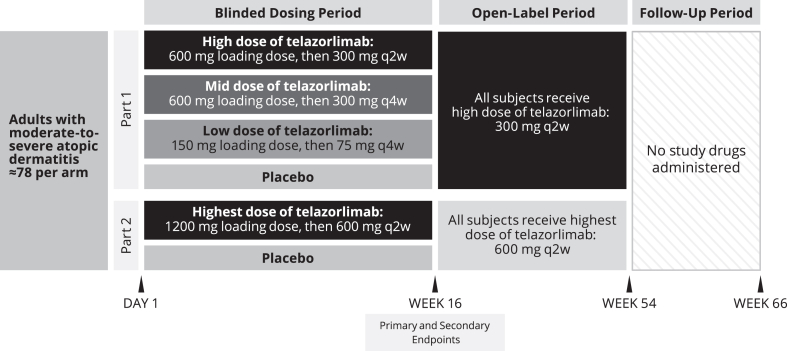
The study was conducted in 2 parts (Fig 1), with part 2 commencing after additional steady-state safety and pharmacokinetic (PK) data became available from a phase 1 ascending dose trial, suggesting a rationale for investigating a higher dose of telazorlimab than investigated in part 1. In each part, randomization was performed using an interactive voice response system/interactive web response system. In part 1, eligible subjects were randomized 1:1:1:1 to low, medium, or high doses of subcutaneous telazorlimab or placebo as follows: 150 mg telazorlimab loading dose (day 1), then 75 mg every 4 weeks beginning at day 29; 600 mg telazorlimab loading dose (day 1), then 300 mg every 4 weeks beginning at day 29; 600 mg telazorlimab loading dose (day 1), then 300 mg every 2 weeks beginning at day 15; or matching placebo (day 1 and then every 2 weeks). Loading doses of telazorlimab were used in an effort to rapidly achieve steady-state plasma concentrations. To maintain blinding, all subjects in part 1 received loading doses as 2 subcutaneous injections of 2 mL volume each, followed by 7 maintenance doses of 1 subcutaneous injection of 2 mL volume. The treatment groups randomized to receive telazorlimab every 4 weeks received alternating placebo every 4 weeks starting at day 15. In part 2, eligible subjects were randomized 1:1 to the highest dose of telazorlimab or placebo as follows: 1200 mg telazorlimab loading dose (day 1) as 4 subcutaneous injections of 2 mL volume each, then 600 mg every 2 weeks beginning at day 15 as 2 subcutaneous injections of 2 mL volume each or matching placebo (day 1 and then every 2 weeks). During the open-label phase of the study, all subjects in part 1 (including those randomized to placebo) received telazorlimab 300 mg every 2 weeks, while in part 2, all subjects received telazorlimab 600 mg every 2 weeks.
Fig 1.
Study design. q2w, Every 2 weeks; q4w, every 4 weeks.
Study end points
The primary end point was the percentage change from baseline in EASI at week 16. Secondary end points included the proportion of subjects experiencing EASI-75 (≥75% reduction from baseline in EASI) and EASI-50, meeting Investigator Global Assessment (IGA) response criteria (score of 0 or 1 and ≥2 point reduction from baseline on a 5-point scale ranging from 0 [clear] to 4 [severe]37), and showing improvement in pruritus on a 0-to-10 numerical rating scale (NRS) of ≥4 points at week 16. Additionally, mean changes from baseline to week 16 in Severity Scoring of Atopic Dermatitis (SCORAD) and Global Individual Sign Score (GISS) were evaluated. Changes in various patient-reported outcomes, including the Dermatology Life Quality Index (DLQI), Patient-Oriented Eczema Measurement (POEM), Hospital Anxiety and Depression Scale (HADS), Patient Global Assessment, and missed school or workdays, also were evaluated. These investigator-assessed and subject-reported outcomes were measured at baseline and at the end of weeks 1, 2, 4, 6, 8, 12, and 16, or at the time of early discontinuation. In addition, EASI and IGA scoring were determined every 4 weeks during the open-label phase, at the end of treatment, and when the study concluded.
Blood samples for PK and immunogenicity (anti-drug antibody [ADA]) analyses were collected throughout the treatment phases or on early discontinuation. PK end points included Cmax (maximum observed plasma concentration), Ctrough (trough plasma concentration), Tmax (time at which Cmax is observed), and AUC0-tau (area under the plasma concentration–time curve over the dosing interval). Adverse event data also were collected throughout the study, and safety was assessed by the incidence of treatment-emergent adverse events (TEAEs) during the blinded and open-label periods of the trial. Additional safety measures included injection-site reactions, vital signs, electrocardiograms, clinical laboratory parameters, and physical examinations.
Statistical analysis
Clinical efficacy analyses were performed in the intent-to-treat (ITT) population, defined as all randomized subjects who received ≥1 full or partial dose of study treatment. The sample sizes for each group (n ≈ 78 randomized, assuming a 20% dropout rate) in the ITT set were estimated to provide 85% power to detect a 27% difference between telazorlimab and placebo in change in EASI from baseline to week 16 using a 2-sided test at .05 significance. Efficacy and safety data for parts 1 and 2 were analyzed separately, with the primary end point assessed using a mixed-effect model for repeated measures to account for variance–covariance structure between visits and missing data. Sensitivity analyses were performed using a tipping-point method for the complete data set. The key secondary end points—IGA score 0 or 1 and IGA reduction ≥2 points from baseline at week 16, and proportion of subjects with EASI-75 at week 16—were analyzed based on the stratified Cochran-Mandel-Haenzsel test. Descriptive statistics were used for continuous variables. Analyses of secondary end points were not adjusted for multiplicity, and findings must therefore be considered exploratory. The safety population included all subjects receiving ≥1 partial or full dose of study treatment. All statistical analyses were carried out by SAS v9.3 (SAS Institute) or higher. PK analyses were performed on a subset of the safety population who received telazorlimab, who did not have any major protocol deviation that affected PKs, and for whom at least 1 sample was available for analysis with known dosing and sampling times. Immunogenicity analyses were based on the full analysis set, with blood samples collected at appropriate time points. The number and percentage incidences of positive and negative ADA status of subjects by treatment and time points were determined.
Results
Subject disposition, demographics, and clinical characteristics
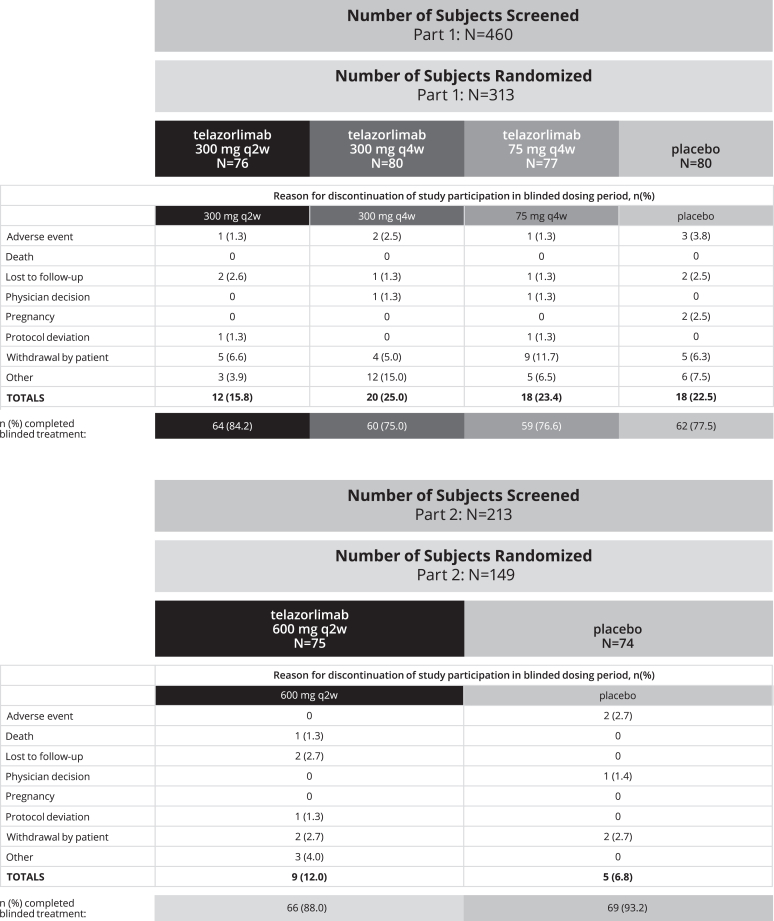
In part 1 of the study, a total of 313 subjects with AD were randomized to telazorlimab or placebo in the following doses: 300 mg every 2 weeks (n = 76), 300 mg every 4 weeks (n = 80), 75 mg every 4 weeks (n = 77), or placebo (n = 80). In part 2 of the study, a total of 149 subjects were randomized to 600 mg telazorlimab every 2 weeks (n = 75) or placebo (n = 74). In the ITT, 245 subjects (78.3%) in part 1 completed double-blind treatment; for part 2, the corresponding figure was 135 (90.6%) (Fig 2). During the blinded-treatment phase, the most common reasons for study discontinuation in part 1 and part 2, respectively, were subject withdrawal (24.6% and 9.4%), loss to follow-up (3.5% and 3.4%), protocol deviations (1% and 1.3%), and adverse events (1% and 0.7%).
Fig 2.
Disposition of patients during blinded treatment phase. q2w, Every 2 weeks; q4w, every 4 weeks.
The open-label phase of the study included a total of 366 subjects (234 in part 1 and 132 in part 2) who received treatment; 96 (26.2%) discontinued participation before its conclusion. The most common reasons for discontinuation during open-label treatment were subject withdrawal (14.1% and 3.0%, for parts 1 and 2, respectively), subject decision (3.0% and 9.8%), loss to follow-up (1.7% and 0), and adverse events (1.7% and 0).
Demographic and clinical characteristics were well balanced across all treatment groups in the ITT population (Table I). The mean [standard deviation, SD] age of subjects was 37.8 [14.5] years and 37.0 [13.5] years in parts 1 and 2, respectively. Female subjects constituted 51.8% and 56.4% of the population in parts 1 and 2, respectively. The mean EASI at baseline was 30.8 in both parts 1 and 2. Subjects in both parts 1 and 2 reported previous treatment with topical corticosteroids (>90%), systemic corticosteroids (17.7% in part 1 and 21.5% in part 2), immunosuppressants (6.4% and 6.0%), and/or investigational agents (3.2% and 2.7%). Twelve subjects reported previous treatment with dupilumab, 2 with upadacitinib, and 1 with baricitinib.
Table I.
Baseline demographic and clinical characteristics of safety population
| Characteristic | Variable | Part 1 |
Part 2 |
||||
|---|---|---|---|---|---|---|---|
| Telazorlimab |
Placebo (n = 80) | Telazorlimab 600 mg q2w (n = 75) | Placebo (n = 74) | ||||
| 300 mg q2w (n = 76) | 300 mg q4w (n = 78) | 75 mg q4w (n = 77) | |||||
| Baseline demographics | Age (years), mean [SD] | 40.2 [13.1] | 36.6 [14.8] | 38.4 [16.9] | 36.3 [13.1] | 37.9 [13.3] | 36.0 [13.8] |
| Female sex, no. (%) | 32 (42.1) | 44 (56.4) | 41 (53.2) | 44 (55.0) | 37 (49.3) | 47 (63.5) | |
| Body mass index (kg/m2), mean [SD] | 28.7 [7.5] | 27.5 [6.2] | 26.3 [6.2] | 27.2 [6.6] | 26.0 [5.2] | 26.5 [5.4] | |
| Race, no. (%) | |||||||
| White | 58 (76.3) | 59 (75.6) | 65 (84.4) | 58 (72.5) | 66 (88.0) | 69 (93.2) | |
| Black or African American | 13 (17.1) | 14 (17.9) | 6 (7.8) | 15 (18.8) | 6 (8.0) | 4 (5.4) | |
| Asian | 5 (6.6) | 4 (5.1) | 4 (5.2) | 7 (8.8) | 2 (2.7) | 1 (1.4) | |
| Mixed | 0 | 1 (1.3) | 2 (2.6) | 0 | 0 | 0 | |
| Other | 0 | 0 | 0 | 0 | 1 (1.3) | 0 | |
| Ethnicity, no. (%) | |||||||
| Hispanic or Latino | 5 (6.6) | 3 (3.8) | 2 (2.6) | 2 (2.5) | 3 (4.0) | 0 | |
| Not Hispanic or Latino | 71 (93.4) | 75 (96.2) | 75 (97.4) | 78 (97.5) | 72 (96.0) | 73 (98.6) | |
| Region, no. (%) | |||||||
| North America | 34 (44.7) | 37 (47.4) | 35 (45.5) | 36 (45.0) | 17 (22.7) | 15 (20.3) | |
| European Union | 42 (55.3) | 41 (52.6) | 42 (54.5) | 44 (55.0) | 58 (77.3) | 59 (79.7) | |
| Baseline clinical characteristics | Time since AD diagnosis (years), mean [SD] | 27.7 [15.8] | 29.7 [15.6] | 25.3 [14.1] | 28.4 [14.4] | 27.6 [16.5] | 27.9 [15.4] |
| EASI, mean [SD] | 30.4 [14.1] | 33.8 [14.9] | 28.4 [11.6] | 30.7 [13.2] | 29.9 [13.2] | 31.8 [14.3] | |
| IGA, no. (%) | |||||||
| 3. Moderate | 49 (64.5) | 48 (61.5) | 49 (63.6) | 52 (65.0) | 48 (64.0) | 47 (63.5) | |
| 4. Severe | 27 (35.5) | 30 (38.5) | 28 (36.4) | 28 (35.0) | 27 (36.0) | 27 (36.5) | |
| Pruritus NRS score, mean [SD] | 7.4 [1.6] | 7.5 [1.7] | 7.5 [1.6] | 7.3 [1.8] | 7.4 [1.5] | 7.4 [1.7] | |
| Body surface area involvement [%], mean [SD] | 46.7 [21.4] | 50.8 [24.2] | 46.7 [21.2] | 48.4 [22.4] | 48.4 [21.0] | 52.9 [24.8] | |
| SCORAD, mean [SD] | 67.5 [14.3] | 69.1 [14.3] | 66.2 [12.4] | 66.1 [12.7] | 66.4 [12.3] | 67.7 [13.6] | |
| GISS, mean [SD] | 9.1 [1.8] | 9.4 [1.7] | 9.1 [1.7] | 8.9 [1.8] | 9.0 [1.7] | 8.9 [1.6] | |
| DLQI, mean [SD] | 15.2 [6.8] | 15.4 [7.1] | 14.3 [7.2] | 14.3 [6.8] | 14.1 [6.0] | 14.7 [6.8] | |
| POEM total score, mean [SD] | 20.2 [5.8] | 20.9 [5.6] | 19.8 [5.3] | 21.2 [5.4] | 20.7 [4.6] | 21.1 [4.8] | |
| HADS, mean [SD] | |||||||
| Anxiety | 6.1 [4.5] | 6.1 [4.8] | 6.1 [4.2] | 6.2 [3.7] | 6.0 [4.4] | 6.7 [4.1] | |
| Depression | 4.3 [3.9] | 4.8 [4.4] | 4.9 [4.2] | 4.2 [3.2] | 4.7 [3.8] | 5.0 [4.3] | |
q2w, Every 2 weeks; q4w, every 4 weeks.
Clinical efficacy
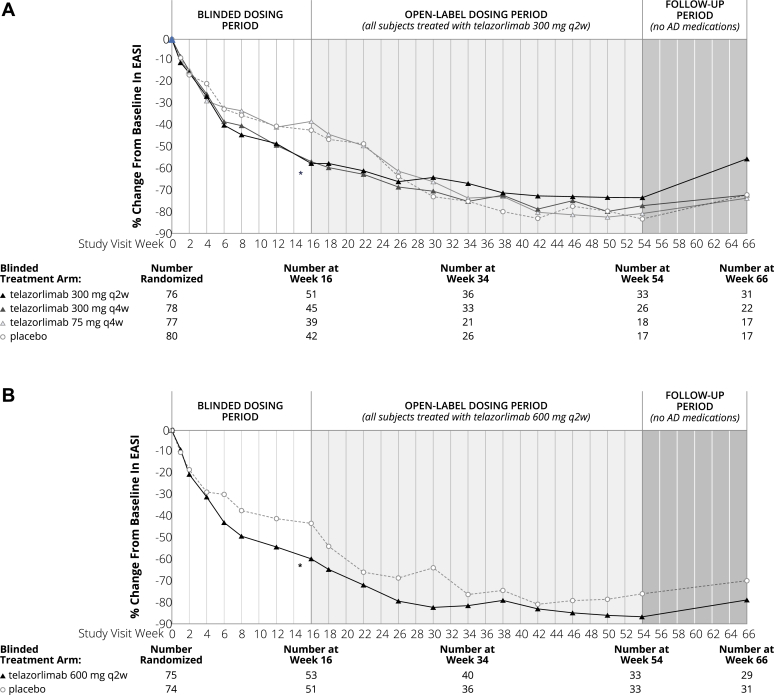
The clinical effects of telazorlimab were assessed in all subjects in the ITT population, and clinically significant differences, relative to placebo, were observed in the primary end point for the 2 higher doses of telazorlimab, but not for the 2 lower doses. Those receiving telazorlimab 300 mg every 2 weeks (part 1) and 600 mg every 2 weeks (part 2) showed statistically significant superiority to placebo for the primary end point, P = .008 for both groups. In part 1, the least squares (LS) mean percentage change in EASI from baseline to week 16 was −54.4% in the group receiving telazorlimab 300 mg every 2 weeks, −48.6% in the group receiving telazorlimab 300 mg every 4 weeks, −31.0% in the group receiving telazorlimab 75 mg every 4 weeks, and −34.2% for placebo subjects. The LS mean difference versus placebo for the telazorlimab 300 mg every 2 weeks group was −20.2 percentage points (95% confidence interval [CI] −34.9, −5.4). In part 2, the LS mean percentage change in EASI from baseline to week 16 was −59.0% in the telazorlimab group versus −41.8% in the placebo group, a LS mean difference of −17.2 percentage points (95% CI −29.9, −4.5). (Table II). The positive response to treatment observed at 16 weeks with the 300 mg every 2 weeks and 600 mg every 2 weeks telazorlimab dosing regimens was maintained during the open-label period (week 16 to week 54), with subjects showing continued improvement in percentage change from baseline in EASI. Treatment benefit persisted in most subjects after the planned discontinuation of telazorlimab from week 54 through week 66 (Fig 3).
Table II.
Efficacy outcomes at week 16 in ITT population
| Characteristic | Variable | Part 1 |
Part 2 |
||||
|---|---|---|---|---|---|---|---|
| Telazorlimab |
Placebo (n = 80) | Telazorlimab 600 mg q2w (n = 75) | Placebo (n = 74) | ||||
| 300 mg q2w (n = 76) | 300 mg q4w (n = 78) | 75 mg q4w (n = 77) | |||||
| Primary end point | LS mean (SE) % change from baseline in EASI | −54.4 (5.1) | −48.6 (5.4) | −31.0 (5.7) | −34.2 (5.5) | −59.0 (4.6) | −41.8 (4.7) |
| P value vs placebo | .008 | .061 | .691 | .008 | |||
| LS mean difference vs placebo (95% CI) | −20.2 (−34.9, −5.4) | −14.4 (−29.6, 0.7) | 3.1 (−12.4, 18.7) | −17.2 (−29.9, −4.5) | |||
| Secondary end points | EASI-75, no. (%) | 18 (23.7) | 16 (20.5) | 9 (11.7) | 9 (11.3) | 19 (25.3) | 14 (18.9) |
| Odds ratio vs placebo (95% CI) | 2.5 (1.0, 6.0) | 2.1 (0.8, 5.0) | 1.1 (0.4, 2.8) | 1.4 (0.6, 3.2) | |||
| EASI-50, no. (%) | 37 (48.7) | 27 (34.6) | 21 (27.3) | 22 (27.5) | 33 (44.0) | 25 (33.8) | |
| Odds ratio vs placebo (95% CI) | 2.5 (1.3, 5.0) | 1.4 (0.7, 2.8) | 1.0 (0.5, 2.0) | 1.5 (0.8, 3.0) | |||
| IGA 0/1 response, no. (%) | 10 (13.2) | 8 (10.3) | 5 (6.5) | 4 (5.0) | 9 (12.0) | 4 (5.4) | |
| Odds ratio vs placebo (95% CI) | 2.9 (0.9, 9.6) | 2.2 (0.6, 7.8) | 1.4 (0.3, 5.4) | 2.5 (0.7, 8.6) | |||
| Pruritus NRS score improvement ≥4, no. (%) | 6 (7.9) | 9 (11.5) | 4 (5.2) | 8 (10.0) | 10 (13.3) | 7 (9.5) | |
| Odds ratio vs placebo (95% CI) | 0.8 (0.3, 2.3) | 1.2 (0.4, 3.3) | 0.5 (0.1, 1.7) | 1.5 (0.5, 4.1) | |||
| LS mean (SE) % change from baseline in SCORAD | −24.3 (2.4) | −21.2 (2.6) | −14.3 (2.7) | −13.8 (2.6) | −26.8 (2.0) | −17.2 (2.0) | |
| LS mean difference vs placebo (95% CI) | −10.5 (−17.6, 3.4) | −7.4 (−14.6, 0.2) | −0.6 (−8.0, 6.9) | −9.6 (−15.0, −4.1) | |||
| LS mean (SE) change from baseline in GISS | −3.2 (0.3) | −2.5 (0.4) | −1.9 (0.4) | −1.7 (0.4) | −3.5 (0.3) | −2.2 (0.3) | |
| LS mean difference vs placebo (95% CI) | −1.5 (−2.5, −0.5) | −0.8 (−1.8, 0.2) | −0.2 (−1.3, 0.8) | −1.3 (−2.1, −0.5) | |||
| LS mean (SE) change from baseline in DLQI | −6.0 (0.8) | −5.5 (0.9) | −3.4 (0.9) | −3.7 (0.9) | −6.7 (0.7) | −4.4 (0.7) | |
| LS mean difference vs placebo (95% CI) | −2.4 (−4.8, 0.1) | −1.8 (−4.3, 0.7) | 0.3 (−2.2, 2.8) | −2.3 (−4.2, −0.4) | |||
| LS mean (SE) change from baseline in POEM | −6.3 (0.9) | −3.9 (1.0) | −2.6 (1.0) | −3.1 (1.0) | −6.8 (1.0) | −5.0 (1.0) | |
| LS mean difference vs placebo (95% CI) | −3.1 (−5.8, −0.5) | −0.7 (−3.4, 1.9) | 0.6 (−2.2, 3.4) | −1.8 (−4.4, 0.8) | |||
| LS mean (SE) change from baseline in HADS (Anxiety) | −1.7 (0.4) | −0.7 (0.4) | −0.8 (0.5) | −0.4 (0.4) | −1.8 (0.5) | −1.4 (0.5) | |
| LS mean difference vs placebo (95% CI) | −1.3 (−2.5, −0.2) | −0.4 (−1.6, 0.8) | −0.4 (−1.7, 0.8) | −0.4 (−1.6, 0.9) | |||
| LS mean (SE) change from baseline in HADS (Depression) | −1.0 (0.4) | −0.6 (0.4) | −0.3 (0.4) | 0.0 (0.4) | −0.8 (0.4) | −0.8 (0.4) | |
| LS mean difference vs placebo (95% CI) | −1.0 (−2.1, 0.1) | −0.6 (−1.7, 0.5) | −0.3 (−1.5, 0.8) | 0.0 (−1.1, 1.1) | |||
Primary end point was assessed by mixed-effect model for repeated measures (MMRM) with baseline value as covariate, and treatment group, region, disease severity, and visit as fixed effect factors, and interactions of treatment by visit and baseline by visit. Odds ratios were analyzed by stratified Cochran-Mandel-Haenzsel (CMH) test with region and IGA severity as stratification variables. Descriptive statistics were used for continuous variables. Analyses of secondary end points were not adjusted for multiplicity. q2w, Every 2 weeks; q4w, every 4 weeks, SE, standard error.
Fig 3.
Time course of response as measured by change from baseline in EASI. Statistical comparison at week 16 only. ∗P < .05 at week 16 for telazorlimab 300 mg q2w versus placebo in part 1 and for telazorlimab 600 mg q2w versus placebo in part 2. q2w, Every 2 weeks; q4w, every 4 weeks.
Part 1 subjects receiving telazorlimab 300 mg every 2 weeks also showed numerically greater improvement, compared to placebo, on most secondary end points over the 16-week blinded dosing phase, including the percentage of subjects experiencing EASI-75 and EASI-50 at week 16 (23.7% vs 11.3% and 48.7% vs 27.5%, respectively), and IGA response rate (score of 0 or 1 and a reduction of ≥2 points from baseline) at week 16 (13.2% vs 5.0%). The LS mean percentage change in SCORAD for telazorlimab subjects also was numerically greater (−24.3% vs −13.8%), as were the reductions in GISS (−3.2 vs −1.7), DLQI (−6.0 vs −3.7), POEM (−6.3 vs −3.1), and HADS Anxiety and Depression scores (−1.7 vs −0.4 and −1.0 vs 0.0, respectively). Conversely, the proportion of subjects experiencing improvement from baseline ≥4 in pruritus NRS score was numerically lower in those receiving telazorlimab 300 mg every 2 weeks compared to placebo (7.9% vs 10.0%, respectively). There appeared to be no meaningful differences between telazorlimab and placebo on Patient Global Assessment of disease or treatment or on missed school or workdays. Similar results were reported for part 2 subjects receiving telazorlimab 600 mg every 2 weeks (Table II).
PKs and immunogenicity
The administration of loading doses of telazorlimab to the different groups in parts 1 and 2 on day 1 facilitated the attainment of steady-state drug concentrations (Table III). During the 16-week blinded phase, the steady-state geometric mean Cmax and AUC0-tau increased in a nearly dose-proportional manner between the 75 mg and 300 mg every 4 weeks dosing regimens. However, there was a slight superproportionality in these metrics between the 300 mg and 600 mg every 2 weeks dosing schedules. Ctrough rapidly attained near steady-state levels, beginning with the initial dose, particularly for the 2 twice-monthly regimens. For the once-monthly dosing groups, the Ctrough was slightly higher after dose 1 (the loading dose) but reached near steady-state levels by the second dose. Overall, the Ctrough showed a dose-dependent but slightly superproportional increase from the 75 mg every 4 weeks to the 600 mg every 2 weeks dosing regimen. The terminal half-life could not be fully characterized for all subjects, particularly those receiving telazorlimab every 2 weeks. For subjects who were administered treatment every 4 weeks, the median half-life ranged from 10 to 15 days. The shortest half-life was seen in subjects receiving telazorlimab 75 mg every 4 weeks after day 85.
Table III.
Summary of telazorlimab PKs after dosing on days 1 and 85 during double-blind period (rich PK population)
| Telazorlimab dose (loading/maintenance) | Day 1 |
Day 85 |
||||
|---|---|---|---|---|---|---|
| Cmax (μg/mL) GM (CV) | Tmax (h), median (min-max) | AUC0-tau (h∙μg/mL) GM (CV) | CmaxSS (μg/mL) GM (CV) | TmaxSS (h) median (min-max) | AUC0-tauSS (h∙μg/mL) GM (CV) | |
| 1200 mg/600 mg q2w | 92.8 (12.3) | 119.9 (97.1-125.9) | 26,930∗ | 144.8 (6.8) | 109.1 (94.8-123.5) | 39,420 (2.6) |
| 600 mg/300 mg q2w | 47.0 (46.5) | 95.6 (24.1-120.0) | 12,170 (49.9) | 52.3 (45.8) | 95.6 (23.9-169.2) | 12,990 (40.3) |
| 600 mg/300 mg q4w | 50.0 (33.1) | 120.0 (94.5-169.3) | 20,340 (34.1) | 31.2 (60.4) | 115.6 (23.3-167.4) | 13,120 (49.5) |
| 150 mg/75 mg q4w | 16.6 (32.6) | 120.3 (24.0-168.7) | 6,671 (40.2) | 8.8 (73.6) | 119.2 (23.7-406.2) | 3,448 (97.8) |
CV, Coefficient of variation; GM, geometric mean; q2w, every 2 weeks; q4w, every 4 weeks.
Not calculated because of missing data.
Over the course of the study, including the open-label period (weeks 16-54), a total of 128 (29.7%) of 431 subjects in parts 1 and 2 who received telazorlimab tested positive for ADAs. Most ADAs were treatment emergent, with 3% showing a treatment-boosted response. Overall, 12.8% of subjects showed forms of ADAs that were transient while 13.9% were persistent. In 58 (13.5%) of 431 subjects, the ADAs were neutralizing in nature. In part 1, 10 (17%) of 60 subjects who switched from placebo to telazorlimab 300 mg every 2 weeks during the open-label period tested positive for ADAs, compared to 21 (28%) of 75 subjects who received 300 mg every 2 weeks in the blinded and open-label periods. In part 2, however, the rate of ADA positivity was comparable in both the treatment (11/75, 14.7%) and placebo (9/67, 13.4%) groups. In general, an inverse dose dependency was seen in the incidence of ADAs. The impact of ADA on PK through week 16 was evaluated in an exploratory manner by comparing mean Cmax, AUC, and median Ctrough within each dose group. In general, the ADA-positive subjects showed lower exposures to telazorlimab, particularly Ctrough, compared to the ADA-negative subjects within the same dose group, with relatively larger impact at the 75 mg every 4 weeks dose group versus the high dose levels (300 mg every 2 weeks and 600 mg every 2 weeks). The impact of ADAs on efficacy was evaluated in an exploratory manner by comparing the percentage change from baseline in EASI score, stratified by ADA status. In part 1, at the dose of 300 mg every 2 weeks, the LS mean percentage change from baseline in EASI score at week 54 (2 weeks after the last dose) was comparable for ADA-positive and ADA-negative subjects (−77.4% and −77.7%, respectively), suggesting no impact of ADAs on efficacy.
Safety
During the 16-week blinded treatment period, 153 subjects (66.2%) receiving telazorlimab and 58 subjects (72.5%) receiving placebo in part 1 reported TEAEs. In part 2, the corresponding figures were 49 (65.3%) and 37 (50.0%) for telazorlimab and placebo subjects, respectively (Table IV). During the blinded and open-label periods of the trial, a total of 4 TEAEs in 3 telazorlimab subjects led to study discontinuation in part 1. These included paresthesia in 1 subject (which was considered to be related to the study drug) and pernicious anemia/low neutrophil count in 1 subject and thrombocytosis in 1 subject (which were not considered to be related to the study drug). No placebo subjects left the study because of TEAEs. In part 2, 1 placebo subject discontinued study participation as a result of a TEAE (adenocarcinoma of the colon, which was not considered to be related to the study drug). No part 2 telazorlimab subjects withdrew because of a TEAE.
Table IV.
TEAE summary during double-blind period in safety population
| TEAE | Part 1 |
Part 2 |
||||
|---|---|---|---|---|---|---|
| Telazorlimab |
Placebo (n = 80) | Telazorlimab 600 mg q2w (n = 75) | Placebo (n = 74) | |||
| 300 mg q2w (n = 76) | 300 mg q4w (n = 78) | 75 mg q4w (n = 77) | ||||
| Any TEAE | 52 (68.4) | 45 (57.7) | 56 (72.7) | 58 (72.5) | 49 (65.3) | 37 (50.0) |
| Treatment discontinuation due to TEAE | 1 (1.3) | 2 (2.6) | 1 (1.3) | 3 (3.8) | 0 | 2 (2.7) |
| Serious TEAE | 3 (3.9) | 2 (2.6) | 2 (2.6) | 1 (1.3) | 1 (1.3) | 0 |
| TEAE > 5% in any treatment group | ||||||
| Dermatitis atopic | 14 (18.4) | 19 (24.4) | 17 (22.1) | 18 (22.5) | 13 (17.3) | 12 (16.2) |
| Nasopharyngitis | 3 (3.9) | 9 (11.5) | 7 (9.1) | 7 (8.8) | 6 (8.0) | 7 (9.5) |
| Upper respiratory tract infection | 6 (7.9) | 4 (5.1) | 7 (9.1) | 4 (5.0) | 4 (5.3) | 5 (6.8) |
| Headache | 6 (7.9) | 5 (6.4) | 2 (2.6) | 8 (10.0) | 5 (6.7) | 5 (6.8) |
| Urinary tract infection | 2 (2.6) | 2 (2.6) | 4 (5.2) | 4 (5.0) | 2 (2.7) | 2 (2.7) |
| Pruritus | 0 | 1 (1.3) | 4 (5.2) | 1 (1.3) | 1 (1.3) | 2 (2.7) |
| Fatigue | 0 | 4 (5.1) | 1 (1.3) | 0 | 1 (1.3) | 0 |
Data are presented as nos. (%). q2w, Every 2 weeks; q4w, every 4 weeks.
During the 16-week blinded treatment period, serious TEAEs occurred in 8 subjects in part 1: 7 (3.0%) received various doses of telazorlimab, while 1 (1.3%) received placebo. Three of the serious events in telazorlimab subjects (viral infection in 1 subject and exacerbation of AD in 2 subjects) were considered to be related to the study drug, while the others (atrial fibrillation in 1 subject, releasing of lens after cataract surgery in 1 subject, and exacerbation of AD in 2 subjects) were not considered to be related to the study drug. In part 2, serious TEAEs occurred in 1 subject (1.3%) treated with telazorlimab and in no placebo-treated subjects. The single event (severe hypertension leading to death in a 66-year-old man) was not considered treatment related.
The most common TEAEs, occurring in ≥5% of subjects in any treatment group during double-blind treatment, were AD (21.9% and 16.8% in parts 1 and 2, respectively), nasopharyngitis (8.4% and 8.7%), upper respiratory tract infection (6.8% and 6.0%), and headache (6.8% and 6.7%). The frequency of these events was similar between telazorlimab and placebo groups during the blinded phase of the study, and the majority of all TEAEs were mild to moderate in intensity. No new TEAEs emerged during the open-label treatment period, and the most common events remained AD (29.9% and 31.5% in parts 1 and 2, respectively), nasopharyngitis (17.4% and 16.8%), upper respiratory tract infection (13.8% and 8.1%), and headache (9.6% and 11.4%).
No significant changes in laboratory parameters or the incidence of infections and infestations were reported during the blinded or open-label treatment periods. Similarly, no clinically meaningful changes from baseline through week 54 were detected in vital sign parameters, electrocardiograms, or physical examinations.
Discussion
In this phase 2b study, 2 subcutaneous dosing regimens of telazorlimab—300 mg every 2 weeks and 600 mg every 2 weeks following an initial loading dose—were superior to placebo for the primary outcome measure of improvement in EASI after 16 weeks of treatment in adults with moderate-to-severe disease not previously controlled with topical therapies, systemic immunosuppressants, or biologic agents. These regimens also appeared to improve many secondary measures of AD, although these results must be interpreted cautiously because secondary analyses were not adjusted for multiplicity. Disappointingly, treatment with telazorlimab did not result in improvement from baseline to week 16 in pruritus, and IGA response rates at week 16 were lower than might have been expected. The modest efficacy observed after 16 weeks of treatment may be explained in part by the delayed onset of action of telazorlimab; data from the current study suggest that ongoing OX40 blockade continues to improve clinical response beyond 16 weeks’ treatment, with telazorlimab exerting maximal effect several weeks later. Moreover, the reduction from baseline in AD disease activity was maintained without additional therapeutic intervention after discontinuation of telazorlimab from week 54 through week 66, in contrast to the observed relapse of AD symptoms in patients after discontinuation of dupilumab.38
These findings provide further evidence for the pathogenic role of the OX40 pathway in subjects with AD; further, they amplify the results from the phase 2a proof-of-concept study, which showed that intravenous doses of telazorlimab administered 4 weeks apart induced significant and progressive improvements in clinical severity scores and the cutaneous molecular AD signature through day 71.34 Additionally, the results of the current study are consistent with those from a randomized, placebo-controlled phase 2 study testing subcutaneous injections of rocatinlimab every 2 weeks or every 4 weeks in subjects with moderate-to-severe AD uncontrolled with topical agents, which showed significant improvement in EASI (ranging from −48.3% to −61.1% across 4 doses evaluated) at week 16 compared to placebo (−15% from baseline).39
An emerging body of evidence provides support for the critical role of the OX40/OX40L axis in promoting the type 2 immune dysfunction seen in AD.19,40, 41, 42, 43 This pathway is crucial for sustaining the effector function of T cells, particularly the TH1 and TH2 subpopulations implicated in the pathophysiology of AD. The value of therapeutic agents targeting OX40 is that these drugs also bypass naive and resting T memory cells. These 2 distinctive features of the mechanism of action—targeting TH2-biased immune responses while not triggering naive and memory T cells—may help explain the clinical efficacy and safety profiles of drugs targeting OX40, including telazorlimab31,34 and rocatinlimab.33,39
The current study also confirms findings from phase 1 studies in healthy volunteers31 and the phase 2a study in subjects with AD34 that telazorlimab is well tolerated across all doses tested, with incidence rates of TEAEs similar to those reported in placebo-treated subjects. The safety profile of telazorlimab observed to date compares well with cytokine inhibitors, JAK inhibitors,10 and other agents that target OX40/OX40L. In the phase 2 study, rocatinlimab was reported to induce pyrexia, nasopharyngitis, and chills in 17%, 14%, and 11% of subjects, respectively,39 potentially the result of enhanced T-cell–depleting activity of the afucosylated antibody. Additionally, in a phase 2a study of intravenous doses of amlitelimab (formerly KY1005, an anti-OX40L antibody) in subjects with AD, there were increased incidences of respiratory, thoracic, and mediastinal disorders, vascular disorders, and blood and lymphatic disorders relative to placebo.44 This may be explained by the fact that OX40L is not restricted to T cells but also is expressed on antigen-presenting cells, endothelial cells, and airway tissues.29,30 Additional safety data and head-to-head trials are needed to clarify differences in toxicity profiles associated with anti-OX40/OX40L drugs that may be related to their respective mechanisms of action.
The PK analyses conducted herein demonstrated that loading-dose injections of telazorlimab, followed by biweekly or monthly maintenance doses, rapidly achieved steady-state concentrations, which were maintained over the course of treatment and follow-up. Dose-dependent increases in trough serum concentrations were observed, with minor superproportionality seen in higher dosing ranges, such as in the 300 mg and 600 mg twice-monthly regimens. In general, an inverse dose dependency in the incidence of immunogenicity was observed, with the highest percentage incidence at the lowest dose regimen and lowest percentage incidence at the highest dose regimen. ADA-positive subjects showed lower Ctrough, and mean percentage reduction from baseline in EASI at week 16 was lower compared to ADA-negative subjects in the lower dose groups (300 mg every 4 weeks and 75 mg every 4 weeks). At the higher dose groups (300 mg every 2 weeks and 600 mg every 2 weeks), the mean response was comparable between subjects with ADA-positive and ADA-negative response. Taken together, the PK and immunogenicity findings are consistent with the observed clinical efficacy outcomes.
The study has several limitations. The blinded portion lasted only 16 weeks. Because AD is a chronic condition, longer trials are needed to fully characterize the efficacy and safety of telazorlimab, as well as the longitudinal maintenance of response. This limitation may merit consideration, given the signal that efficacy continued to improve after the blinded phase was completed and appeared to be maintained through week 66, twelve weeks after drug discontinuation. A second limitation is that secondary end point analyses were not adjusted for multiplicity and therefore may not be reproducible and must be interpreted with caution. A third limitation may be found in the demographic characteristics of the subjects who were enrolled onto the trial. While the proportions of the study population that were White, Black/African American, and Asian are generally consistent with their proportions of the populations in the countries where the study was conducted, AD has been found to occur more frequently in Asian and Black/African American individuals than Whites.45 Moreover, only adults with moderate-to-severe AD were enrolled. Therefore, the findings may not generalize to children and adolescents, in whom the disorder is more prevalent. Given its favorable safety profile, future studies of telazorlimab in children and adolescents are warranted.
In conclusion, 2 subcutaneous dose regimens of telazorlimab, 300 mg and 600 mg, administered every 2 weeks following a loading dose, resulted in significant and progressive clinical improvement in subjects with moderate-to-severe AD through 66 weeks of follow-up. The treatment was also safe and well tolerated. The current study confirms the role of the OX40/OX40L axis in the pathogenesis of AD, and our results suggest that telazorlimab may offer a novel therapeutic paradigm for treatment of AD and related T-cell–mediated autoinflammatory diseases.
Clinical implication.
This study confirms the role of the costimulatory receptor OX40 in AD pathogenesis and suggests telazorlimab may offer a novel approach for intervention in this disease.
Disclosure Statement
Supported by Ichnos Sciences (previously Glenmark Pharmaceuticals SA). The sponsor participated in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the report for publication.
Disclosure of potential conflict of interest: B. Rewerska, L. D. Sher, S. Alpizar, S. Pauser, G. Pulka, and M. Machkova have received research funding as principal investigators from Ichnos Sciences. V. CA, J. Macoin, V. Anstett, R. Turrini, and C. Konto are current employees of Ichnos Sciences. S. GN is a current employee of Glenmark Pharmaceuticals. N. Mozaffarian, Y. Salhi, W. Jabert, G. Gudi, S. Blein, and M.-A. Doucey are former employees of Ichnos Sciences (formerly Glenmark Pharmaceuticals). C. Martinet is an employee of Keyrus, a contractor for Ichnos Sciences.
Acknowledgments
We thank Andrea Acocella, MD, a former employee of Ichnos Sciences, for her role in overseeing the trial. Medical writing support was provided by Prescott Medical Communications Group (Chicago, Ill), with financial support from Ichnos Sciences.
References
- 1.Deckers I.A., McLean S., Linssen S., Mommers M., van Schayck C.P., Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema, 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson E.L., Bieber T., Guttman-Yassky E., Beck L.A., Blauvelt A., Cork M.J., et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 3.Guttman-Yassky E., Teixeira H.D., Simpson E.L., Papp K.A., Pangan A.L., Blauvelt A., et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 4.Leung D.Y., Guttman-Yassky E. Assessing the current treatment of atopic dermatitis: unmet needs. J Allergy Clin Immunol. 2017;139:S47–S48. doi: 10.1016/j.jaci.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Stander S. Atopic dermatitis. N Engl J Med. 2021;384:1136–1143. doi: 10.1056/NEJMra2023911. [DOI] [PubMed] [Google Scholar]
- 6.Deleanu D., Nedelea I. Biological therapies for atopic dermatitis: an update. Exp Ther Med. 2019;17:1061–1067. doi: 10.3892/etm.2018.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blauvelt A., de Bruin-Weller M., Gooderham M., Cather J.C., Weisman J., Pariser D., et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287–2303. doi: 10.1016/S0140-6736(17)31191-1. [DOI] [PubMed] [Google Scholar]
- 8.Thaci D., LS E., Deleuran M., Kataoka Y., Chen Z., Gadkari A., et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2) J Dermatol Sci. 2019;94:266–275. doi: 10.1016/j.jdermsci.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Wollenberg A., Beck L.A., Blauvelt A., Simpson E.L., Chen Z., Chen Q., et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS) Br J Dermatol. 2020;182:1120–1135. doi: 10.1111/bjd.18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blauvelt A., Teixeira H.D., Simpson E.L., Costanzo A., De Bruin-Weller M., Barbarot S., et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-wevere atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047–1055. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinvoq. Package insert. AbbVie Inc; North Chicago (Ill): April 2022. [Google Scholar]
- 12.Kabashima K., Matsumura T., Komazaki H., Kawashima M., Nemolizumab-JP01 Study Group Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. 2020;383:141–150. doi: 10.1056/NEJMoa1917006. [DOI] [PubMed] [Google Scholar]
- 13.Wollenberg A., Blauvelt A., Guttman-Yassky E., Worm M., Lynde C., Lacour J.P., et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br J Dermatol. 2021;184:437–449. doi: 10.1111/bjd.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guttman-Yassky E., Blauvelt A., Eichenfield L.F., Paller A.S., Armstrong A.W., Drew J., et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411–420. doi: 10.1001/jamadermatol.2020.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson E.L., Gooderham M., Wollenberg A., Weidinger S., Armstrong A., Soung J., et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere) JAMA Dermatol. 2023;159:182–191. doi: 10.1001/jamadermatol.2022.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson E.L., Sinclair R., Forman S., Wollenberg A., Aschoff R., Cork M., et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 17.Silverberg J.I., Simpson E.L., Thyssen J.P., Gooderham M., Chan G., Feeney C., et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson E.L., Forman S., Silverberg J.I., Zirwas M., Maverakis E., Han G., et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5) J Am Acad Dermatol. 2021;85:62–70. doi: 10.1016/j.jaad.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Furue M., Furue M. OX40L-OX40 signaling in atopic dermatitis. J Clin Med. 2021;10:2578. doi: 10.3390/jcm10122578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderhead D.M., Buhlmann J.E., van den Eertwegh A.J., Claassen E., Noelle R.J., Fell H.P. Cloning of mouse Ox40: a T cell activation marker that may mediate T-B cell interactions. J Immunol. 1993;151:5261–5271. [PubMed] [Google Scholar]
- 21.Gramaglia I., Weinberg A.D., Lemon M., Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 22.Akiba H., Oshima H., Takeda K., Atsuta M., Nakano H., Nakajima A., et al. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–7066. [PubMed] [Google Scholar]
- 23.Kondelkova K., Vokurkova D., Krejsek J., Borska L., Fiala Z., Ctirad A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove) 2010;53:73–77. doi: 10.14712/18059694.2016.63. [DOI] [PubMed] [Google Scholar]
- 24.Lathrop S.K., Huddleston C.A., Dullforce P.A., Montfort M.J., Weinberg A.D., Parker D.C. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172:6735–6743. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell J.R., Yadav R., Rossi R.J., Ruby C.E., Weinberg A.D., Aguila H.L., et al. IL-18 bridges innate and adaptive immunity through IFN-gamma and the CD134 pathway. J Immunol. 2006;177:234–245. doi: 10.4049/jimmunol.177.1.234. [DOI] [PubMed] [Google Scholar]
- 26.Suarez-Farinas M., Dhingra N., Gittler J., Shemer A., Cardinale I., de Guzman Strong C., et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013;132:361–370. doi: 10.1016/j.jaci.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noda S., Suarez-Farinas M., Ungar B., Kim S.J., de Guzman Strong C., Xu H., et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J Allergy Clin Immunol. 2015;136:1254–1264. doi: 10.1016/j.jaci.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Esaki H., Brunner P.M., Renert-Yuval Y., Czarnowicki T., Huynh T., Tran G., et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J Allergy Clin Immunol. 2016;138:1639–1651. doi: 10.1016/j.jaci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Nakano M., Fukumoto Y., Satoh K., Ito Y., Kagaya Y., Ishii N., et al. OX40 ligand plays an important role in the development of atherosclerosis through vasa vasorum neovascularization. Cardiovasc Res. 2010;88:539–546. doi: 10.1093/cvr/cvq211. [DOI] [PubMed] [Google Scholar]
- 30.Burgess J.K., Carlin S., Pack R.A., Arndt G.M., Au W.W., Johnson P.R., et al. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol. 2004;113:683–689. doi: 10.1016/j.jaci.2003.12.311. [DOI] [PubMed] [Google Scholar]
- 31.Gudi G., CA V., GN S., von Gunten C., Back J., Fang H., et al. Clinical pharmacokinetics and immunogenicity of GBR 830, a first-in-class humanized monoclonal antibody inhibiting OX40 to treat atopic dermatitis. J Invest Dermatol. 2018;138(5 suppl):S185. doi: 10.1016/j.jid.2018.03.1107. [DOI] [Google Scholar]
- 32.Macoin J., Blein S., Monney T., Lissilaa R., Sancheti P., Reddy V., et al. GBR 830: an OX40 antagonist antibody with a favorable toxicity profile in non-human primates. J Invest Dermatol. 2018;138(5 suppl):S186. doi: 10.1016/j.jid.2018.03.1112. [DOI] [Google Scholar]
- 33.Nakagawa H., Iizuka H., Nemoto O., Shimabe M., Furukawa Y., Kikuta N., et al. Safety, tolerability and efficacy of repeated intravenous infusions of KHK4083, a fully human anti-OX40 monoclonal antibody, in Japanese patients with moderate to severe atopic dermatitis. J Dermatol Sci. 2020;99:82–89. doi: 10.1016/j.jdermsci.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Guttman-Yassky E., Pavel A.B., Zhou L., Estrada Y.D., Zhang N., Xu H., et al. GBR 830, an anti-OX40, improves skin gene-signatures and clinical scores in atopic dermatitis. J Allergy Clin Immunol. 2019;144:482–493.e7. doi: 10.1016/j.jaci.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 35.US Food and Drug Administration . 2018. E6(R2) good clinical practice: integrated addendum to ICH E6(R1). Guidance for industry. Rockville (MD): US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Control; Center for Biologics Evaluation and Research. [Google Scholar]
- 36.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 37.Eichenfield L.F., Tom W.L., Chamlin S.L., Feldman S.R., Hanifin J.M., Simpson E.L., et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338–351. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worm M., Simpson E.L., Thaci D., Bissonnette R., Lacour J.P., Beissert S., et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:131–143. doi: 10.1001/jamadermatol.2019.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guttman-Yassky E., Simpson E.L., Reich K., Kabashima K., Igawa K., Suzuki T., et al. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study. Lancet. 2023;401:204–214. doi: 10.1016/S0140-6736(22)02037-2. [DOI] [PubMed] [Google Scholar]
- 40.Furue M. Regulation of skin barrier function via competition between AHR axis versus IL-13/IL-4‒JAK‒STAT6/STAT3 axis: pathogenic and therapeutic implications in atopic dermatitis. J Clin Med. 2020;9:3741. doi: 10.3390/jcm9113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y.J. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell–mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Nakahara T., Kido-Nakahara M., Tsuji G., Furue M. Basics and recent advances in the pathophysiology of atopic dermatitis. J Dermatol. 2021;48:130–139. doi: 10.1111/1346-8138.15664. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y.H., Liu Y.J. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weidinger S. 2021. A phase 2a study of KY1005, a novel non-depleting anti-OX40 ligand (OX40L) mAb in patients with moderate to severe AD. Paper presented at: 35th Congress of the European Academy of Dermatology and Venereology (EADV); September 29-Ocober 2. virtual meeting. Abstract 2729. [Google Scholar]
- 45.Kaufman B.P., Guttman-Yassky E., Alexis A.F. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340–357. doi: 10.1111/exd.13514. [DOI] [PubMed] [Google Scholar]