Abstract
Background
Gorham-Stout disease (GSD) is a form of lymphangiomatosis of unknown etiology, characterized by abnormal distribution of lymphatic vessels. Platelets and lymphangiogenesis are closely related via C-type lectin-like receptor 2 (CLEC-2)/podoplanin.
Key Clinical Question
Despite similarities between abnormal lymphatic vessels in CLEC-2-deficient mice and patients with GSD, whether CLEC-2 on platelets is involved in GSD pathogenesis is unknown.
Clinical Approach
We examined CLEC-2 expression in platelets of a patient with lethal GSD. Most of the patient’s platelets expressed aberrant CLEC-2 that was not detectable by certain monoclonal antibodies for human CLEC-2. Further, this population was not activated by a CLEC-2-activating snake venom, rhodocytin. Possible causes of abnormal CLEC-2 including anti-CLEC-2 autoantibodies, podoplanin binding to CLEC-2, and pathogenic CLEC1B gene alteration were excluded.
Conclusions
We believe that this is the first report of a patient with structurally and functionally abnormal CLEC-2. CLEC-2 abnormality may be associated with dysregulated lymphangiogenesis in GSD.
Keywords: CLEC-2, Gorham-Stout disease, lymphangiomatosis, lymphatic vessels, platelets
Essentials
-
•
Platelets and lymphatic vessels are closely related via C-type lectin-like receptor 2 (CLEC-2)/podoplanin.
-
•
We examined CLEC-2 in a patient with lethal Gorham-Stout disease, a form of lymphangiomatosis.
-
•
Platelets had CLEC-2 undetectable by certain antibodies and lacked a response to rhodocytin.
-
•
CLEC-2 abnormality might be associated with dysregulated lymphangiogenesis.
1. Introduction
Gorham-Stout disease (GSD) is a form of lymphangiomatosis characterized by progressive osteolysis due to multicentric proliferation of lymphatic vessels. Osteolysis mechanisms are unknown; however, osteoclast activation and lymphangiogenesis are considered essential. Abnormal distribution of lymphatic vessels is observed in bone, spleen, and soft tissues [1,2]. GSD can be diagnosed at any age but is more common in younger patients. Because of the early onset, some researchers believe congenital factors may be involved, although the causative germline mutation remains undetermined. Indeed, GSD is a sporadic disease, lacking a family history [3]. Prognosis varies, with a 4.9% death rate at a median follow-up of 5.4 years [4], influenced by disease extent and location. Furthermore, thoracic involvement is a poor prognostic factor [5]. Recent studies have revealed the role of the PIK3/AKT/mTOR pathway in lymphoid tissue development. Sirolimus, an mTOR inhibitor, was reported to be effective for reducing symptoms [1,5]. In a prospective study, all patients with GSD (3/3) exhibited partial response to 6-month sirolimus treatment [5]. However, long-term effects are unknown, and adverse outcomes persist.
The relationship between GSD and platelets has been reported in the context of thrombocytopenia. Mild-to-moderate thrombocytopenia is observed in complex lymphatic anomalies, including GSD, and is partly explained by chronic localized intravascular coagulopathy [1,6]. Of note, platelets have a role not only in hemostasis but also in the development of the lymphatic vessels. Our research and that of others reported that the interaction between C-type lectin-like receptor 2 (CLEC-2) on platelets and podoplanin on lymphatic endothelial cells during embryonic development is necessary for mice’s blood/lymphatic vessel separation [[7], [8], [9]]. Furthermore, CLEC-2-deficient mice show disorganized and blood-filled lymphatic vessels and severe edema [[7], [8], [9]].
Although there have been no reports of CLEC-2 abnormalities in humans, thrombocytopenia and disorganized lymphatic vessels in GSD are reminiscent of the phenotype observed in CLEC-2-deficient mice. Therefore, we investigated CLEC-2 expression and function in a patient with GSD. Here, we report the structurally and functionally abnormal CLEC-2 on the platelets of a patient at the terminal stage of GSD. The association between CLEC-2 abnormalities and lymphatic vessel proliferation in GSD is also discussed below.
2. Case Report
A 22-year-old woman was admitted to our hospital with worsening respiratory distress and a bleeding tendency. She was diagnosed with GSD at the age of 12 years and had been taking sirolimus for chylothorax since the age of 15 (Supplementary Figures S1 and S2). The consumptive coagulopathy began over 5 years earlier, and subcutaneous hemorrhage had been observed for over 1 year. Physical examination at admission revealed fine crackles in the lower lung field, pallor of the palpebral conjunctiva, bloody sputum, increased subcutaneous hemorrhage (mixed ecchymosis and suggillation), and severe edema of the lower body. Blood tests showed anemia (hemoglobin level: 7.2g/dL), thrombocytopenia (platelet count: 50 × 109/L), and coagulation abnormalities (fibrinogen level: 90 mg/dL, fibrin/fibrinogen degradation products level: 511.5μg/mL). The computerized tomography scan showed pulmonary congestion, pleural effusion, and high-density areas scattered in the soft tissues of the mediastinum, pleura, and subcutis, suggesting lymphangioma. No active hemorrhagic lesions were observed. After admission, supportive therapy, including blood transfusions, diuretics, and albumin, was administered. However, her edema and respiratory condition worsened, and she died 21 days after admission. During these studies, the palliative treatment for the patient was documented in an independent case report [10].
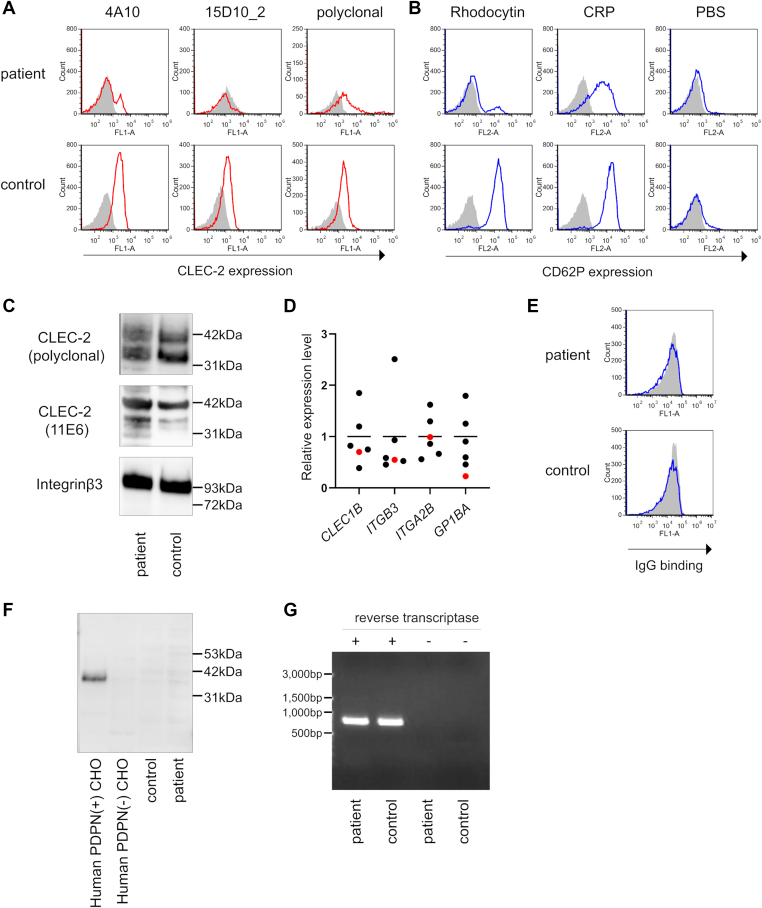
During hospitalization, the CLEC-2 expression on platelets was examined by flow cytometry. Flow cytometry with each anti-CLEC-2 monoclonal antibody (mAb) (4A10 [11] or 15D10_2, established in our laboratory) (Supplementary Figure S3) was negative for the majority (approximately 80%) of the patient’s platelets, suggesting a CLEC-2 deficiency (Figure 1A). Next, CD62P expression on the platelets after stimulation was analyzed using flow cytometry to assess the platelet activation ability. Most platelets were not activated upon stimulation with rhodocytin [12], a ligand for CLEC-2. In contrast, stimulation with collagen-related peptide, a ligand for the collagen receptor glycoprotein VI, activated all the platelets, indicating that the platelets retained their activation potential (Figure 1B). Interestingly, flow cytometry with an anti-CLEC-2 polyclonal antibody (R&D Systems) detected CLEC-2 on the patient’s platelets (Figure 1A). Washed platelet whole-cell lysates were prepared, and CLEC-2 expression was examined by western blotting. Another mAb (11E6, established in our laboratory) [11], in addition to the polyclonal antibody, detected CLEC-2, and its expression level in the patient was comparable to that in the healthy donor (Figure 1C). We synthesized cDNA by reverse-transcription of RNA extracted from washed platelets and quantified expression levels of CLEC1B and other genes encoding platelet membrane proteins. All primer information in this study is listed in Supplementary Table S1. There was no difference in platelet CLEC1B expression level between the patient and healthy controls (Figure 1D), consistent with the western blotting results. These findings suggest that CLEC-2 is present in the patient’s platelets; however, the platelets express structurally abnormal CLEC-2, which cannot be detected using certain mAbs (4A10 and 15D10_2). Furthermore, structural abnormalities in CLEC-2 may have caused functional abnormalities.
Figure 1.
Structural and functional abnormality of CLEC-2 in a patient with Gorham-Stout disease. (A) CLEC-2 expression on platelets was evaluated by flow cytometry. Isotype control (gray) and anti-CLEC-2 antibody (red) are presented. The number of events recorded is as follows: for 4A10, 10,000; for 15D10_2 and polyclonal antibodies, 1,500 and 5,000 in the patient and control, respectively. (B) Platelet-rich plasma was stimulated with rhodocytin (100nM), collagen-related peptide (1μg/mL), and phosphate-buffered saline for 10 minutes at 37 °C. After stimulation, CD62P expression on platelets was evaluated by flow cytometry. Isotype control (gray) and anti-CD62P antibody (blue) are presented. The number of events presented is 10,000. (C) Western blotting of CLEC-2 was performed. Washed platelets (2.0 × 108/mL) were directly dissolved in sample buffer, separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, and electro transferred. CLEC-2 and integrin β3 (as control) were detected. (D) Results of quantitative polymerase chain reaction using platelet-derived cDNA are shown. Relative gene expression levels normalized by GAPDH were calculated by the ΔΔCt method. The mean expression level of healthy controls (n = 5, black dots) was defined as 1, and the patient’s level was indicated as a red dot. (E) Human CLEC-2-expressing T-REx 293 cells were utilized to detect anti-CLEC-2 autoantibodies. CLEC-2 expression on T-REx 293 cells was induced by adding 1 μg/mL doxycycline to the medium. T-REx 293 cells with (blue) or without (gray) CLEC-2 and IgG (1mg/mL) purified from serum were co-cultured, and IgG bound to the cell surface was detected with anti-human IgG antibody by flow cytometry. (F) Evaluation of binding of podoplanin to CLEC-2 on platelets. Western blotting of podoplanin was performed using the same sample as that of Figure 1C. As a control, whole-cell lysate of CHO cells with or without human podoplanin was utilized. (G) Platelet-derived CLEC1B cDNA was amplified by polymerase chain reaction and electrophoresed in agarose gel (1%). CLEC-2, C-type lectin-like receptor 2.
We first considered the possibility that autoantibodies or podoplanin already bind to CLEC-2 as a reason why CLEC-2 cannot be detected with certain antibodies. To examine autoantibodies against CLEC-2, IgG was purified from sera using protein G columns. Purified IgG was added to human CLEC-2-expressing T-REx 293 cells [13], and then incubated. The binding of IgG to cells was detected by flow cytometry using a fluorescence-labeled anti-human IgG antibody. Autoantibodies against CLEC-2 were not detected (Figure 1E). To investigate the binding of podoplanin to CLEC-2 on platelets, western blotting was performed using whole-cell lysates from washed platelets. Podoplanin was not detected in the platelets (Figure 1F).
Finally, we hypothesized that a genetic variant of CLEC1B, the gene encoding CLEC-2, leads to amino acid changes in the mAb-binding and/or rhodocytin-binding sites. We extracted DNA from the buffy coat and executed Sanger sequencing of the promoter, all exons, and their flanking regions in CLEC1B and whole-genome sequencing (WGS). Sanger sequencing and WGS identified 4 missense, 13 intron, and 1 5-prime-UTR variants, all of which are listed in the dbSNP, the single nucleotide polymorphism database. The 4 missense variants were reported as single nucleotide polymorphism with allele frequency in East Asia ≥0.17, and all of them were predicted to be tolerated by functional analysis through hidden Markov models (Table). Therefore, we conclude that germline CLEC1B mutations are not involved in CLEC-2 abnormalities. Furthermore, considering the possibility of splicing variants or somatic mutation restricted in megakaryocytes/platelets, we examined CLEC1B cDNA synthesized from platelet-derived mRNA through electrophoresis and Sanger sequencing. The patient’s CLEC1B cDNA size was identical to that of a healthy control without any splicing variant (Figure 1G). Sanger sequencing revealed 4 missense variants consistent with WGS results (Table). Thus, somatic CLEC1B mutations in megakaryocytes or platelets were excluded.
Table.
Missense variants in CLEC1B identified by Sanger sequencing and whole-genome sequencing.
| Position (hg38) | dbSNP | Reference | Alteration | Amino acid change | Allele frequency in 1000Gp3_EAS | FATHMM prediction |
|---|---|---|---|---|---|---|
| 9997252 | rs583903 | C | T | p.Gly64Asp | 1 | tolerated |
| 9998362 | rs2273987 | G | A | p.Ser28Phe | 0.170634921 | tolerated |
| 9998375 | rs2273986 | A | G | p.Ser24Pro | 0.336309524 | tolerated |
| 9999043 | rs612593 | T | C | p.Ile20Val | 1 | tolerated |
FATHMM, functional analysis through hidden Markov models.
3. Discussion
CLEC-2 was identified as a receptor for snake venom rhodocytin, which induces platelet aggregation [14]. Its physiological role has been extensively studied in mice, but its human pathophysiology remains unclear. There have been no reports of patients with CLEC-2 deficiency or abnormalities. To our knowledge, this is the first report of a patient with platelets completely lacking response to rhodocytin. Analysis using a variety of antibodies revealed that the patient’s platelets were not absent in CLEC-2 but expressed structurally abnormal CLEC-2. To investigate the possible causes of the CLEC-2 abnormality, we examined the presence of anti-CLEC-2 autoantibodies and the binding of podoplanin to CLEC-2 on platelets but found no significant findings. Moreover, we found no pathogenic CLEC1B variants in genomic DNA and platelet-derived cDNA. The remaining possibilities include incomplete CLEC-2 surface localization and conformational changes impairing 4A10/15D10_2 and rhodocytin binding.
Whether the CLEC-2 abnormality was the etiology or a result of the pathology in this case remains unclear. Nevertheless, it should be noted that structural and functional CLEC-2 abnormalities were found in GSD in which dysregulated lymphatic proliferation is the predominant pathology. Rhodocytin and podoplanin share the canonical binding site of CLEC-2 [15]; therefore, the patient’s platelets may not respond to podoplanin stimulation. We have previously reported that platelets inhibit lymphatic endothelial cell migration, proliferation, and tube formation, facilitated by the interaction between CLEC-2 and podoplanin. Upon podoplanin stimulation, the activated platelets release transforming growth factor-β, strongly inhibiting lymphatic endothelial growth. Platelet- and megakaryocyte-specific CLEC-2-deficient mice lack these functions, resulting in lymphatic and vascular misconnections and small intestine edema [8]. Another study reported that CLEC-2 on platelets, not platelet releasate, regulates the migration of lymphatic endothelial cells [16]. Except during the embryonic period, platelets are absent in lymphatic vessels and cannot access the podoplanin on the lymphatic endothelium under normal conditions; however, this interaction can occur under pathological conditions such as inflammation or hemorrhage. Sato et al. [17] reported that platelets are present in the lymphatic vessels of inflamed intestines in patients with inflammatory bowel disease, inhibiting lymphangiogenesis. Thus, we consider that our patient with GSD had an insufficient canonical inhibitory effect on lymphatic vessel proliferation via CLEC-2/podoplanin; abnormal CLEC-2 expression in platelets may be an exacerbating factor for GSD.
Approximately 20% of platelets showed detectable CLEC-2 with any antibody and responded normally to rhodocytin. A possible explanation for 2 completely different platelet populations is blood transfusions. The patient received almost-daily red blood cell transfusions because of anemia. Red blood cell concentrates contain <20 × 10⁹/unit of residual platelets, [18] which may be detected as normal platelets.
In conclusion, we report a patient with GSD with a structurally and functionally abnormal CLEC-2. These findings prompted us to consider that aberrant CLEC-2 expression on platelets is associated with the pathophysiology of GSD. Further research on platelets in GSD and related conditions is required.
Acknowledgments
The authors thank Michio Ozeki and Naoki Oishi for their expertise.
Funding
This study was supported by JSPS KAKENHI Grant number 23H02932 and the Japanese Society on Thrombosis and Hemostasis.
Ethics statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Yamanashi University (approval number 2582). Informed consent was obtained from the legally acceptable representative of the patient and healthy volunteers for this study and publication of this article.
Author contributions
S.O. performed experiments, analyzed the data, assembled the figure, and wrote the manuscript. N. Tsukiji performed experiments and revised the manuscript. T.S. analyzed the data of WGS and provided expertise. K.T. provided clinical expertise. N.H. provided patient care. K.S.I. designed and supervised research and revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Footnotes
Handling Editor: Dr Henri Spronk.
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.102273
Supplementary material
References
- 1.Ozeki M., Fukao T. Generalized lymphatic anomaly and Gorham-Stout disease: overview and recent insights. Adv Wound Care. 2019;8:230–245. doi: 10.1089/wound.2018.0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotecha R., Mascarenhas L., Jackson H.A., Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67:782–788. doi: 10.1016/j.crad.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Angelini A., Mosele N., Pagliarini E., Ruggieri P. Current concepts from diagnosis to management in Gorham–Stout disease: a systematic narrative review of about 350 cases. EFORT Open Rev. 2022;7:35–48. doi: 10.1530/EOR-21-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozeki M., Fujino A., Matsuoka K., Nosaka S., Kuroda T., Fukao T. Clinical features and prognosis of generalized lymphatic anomaly, kaposiform lymphangiomatosis, and Gorham-Stout disease. Pediatr Blood Cancer. 2016;63:832–838. doi: 10.1002/pbc.25914. [DOI] [PubMed] [Google Scholar]
- 5.Ricci K.W., Hammill A.M., Mobberley-Schuman P., Nelson S.C., Blatt J., Bender J.L.G., et al. Efficacy of systemic sirolimus in the treatment of generalized lymphatic anomaly and Gorham–Stout disease. Pediatr Blood Cancer. 2019;66 doi: 10.1002/pbc.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trenor C.C., 3rd, Chaudry G. Complex lymphatic anomalies. Semin Pediatr Surg. 2014;23:186–190. doi: 10.1053/j.sempedsurg.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki-Inoue K., Inoue O., Ding G., Nishimura S., Hokamura K., Eto K., et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem. 2010;285:24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osada M., Inoue O., Ding G., Shirai T., Ichise H., Hirayama K., et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287:22241–22252. doi: 10.1074/jbc.M111.329987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertozzi C.C., Schmaier A.A., Mericko P., Hess P.R., Zou Z., Chen M., et al. Platelets regulate lymphatic vascular development through CLEC-2–SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumakura Y., Hasuda N., Akita K., Iijima T., Matsukawa T. Epidural hematoma related to lower limb pain and massive liver bleeding in Gorham-Stout disease: a case report. Med (United States) 2023;102 doi: 10.1097/MD.0000000000033950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazama F., Nakamura J., Osada M., Inoue O., Oosawa M., Tamura S., et al. Measurement of soluble C-type lectin-like receptor 2 in human plasma. Platelets. 2015;26:711–719. doi: 10.3109/09537104.2015.1021319. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki T., Shirai T., Tsukiji N., Otake S., Tamura S., Ichikawa J., et al. Functional characterization of recombinant snake venom rhodocytin: rhodocytin mutant blocks CLEC-2/podoplanin-dependent platelet aggregation and lung metastasis. J Thromb Haemost. 2018;16:960–972. doi: 10.1111/jth.13987. [DOI] [PubMed] [Google Scholar]
- 13.Chaipan C., Soilleux E.J., Simpson P., Hofmann H., Gramberg T., Marzi A., et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki-Inoue K., Fuller G.L.J., García Á., Eble J.A., Pöhlmann S., Inoue O., et al. Anovel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 15.Nagae M., Morita-Matsumoto K., Kato M., Kaneko M.K., Kato Y., Yamaguchi Y. A platform of C-type lectin-like receptor CLEC-2 for binding O-glycosylated podoplanin and nonglycosylated rhodocytin. Structure. 2014;22:1711–1721. doi: 10.1016/j.str.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Finney B.A., Schweighoffer E., Navarro-Núñez L., Bénézech C., Barone F., Hughes C.E., et al. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood. 2012;119:1747–1756. doi: 10.1182/blood-2011-09-380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato H., Higashiyama M., Hozumi H., Sato S., Furuhashi H., Takajo T., et al. Platelet interaction with lymphatics aggravates intestinal inflammation by suppressing lymphangiogenesis. Am J Physiol Gastrointest Liver Physiol. 2016;311:G276–G285. doi: 10.1152/ajpgi.00455.2015. [DOI] [PubMed] [Google Scholar]
- 18.Liumbruno G., Bennardello F., Lattanzio A., Piccoli P., Rossetti G. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.