Abstract
In mosquitoes, the intradermal search for vertebrate blood (probing time) corresponds to the time taken from initial insertion of the mouthparts in the skin until visualization of the initial engorgement of blood in the midgut. Probing time evaluation provides useful information on the ability of a mosquito to initiate successful blood feeding. In this protocol, we describe how to determine feeding parameters in Aedes aegypti, a widely distributed mosquito that transmits several deadly pathogens, including yellow fever, dengue, Zika, and Chikungunya viruses. We focus on the different steps of a blood feeding event, including penetration, probing, interprobing, and feeding time. Penetration time corresponds to the insertion of the stylets into the host skin and usually lasts <10 sec. Probing time or intradermal search for blood involves saliva secretion into the skin. Some researchers group penetration and probing time as the exploratory phase for blood. Feeding time is an active phase of blood ingestion and engorgement. Feeding parameters depend on mosquito behaviors and these measurements are visually taken by the investigator. We include a video that provides a close look at a mosquito feeding event in which penetration, probing, and feeding times can be observed. To record these experimental times, one must closely watch the mosquito feeding behavior including stylet penetration in the host skin, visualization of the first traces of blood in the midgut, engorgement of the midgut, and removal of stylets from the skin.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
Reagents
Aedes aegypti mosquitoes, 5- to 7-d-old (BEI Resources)
Female mosquitoes must be at least 5 d old to have the whole salivary protein repertoire (Mellink and Von Zeben 1976).
Depilatory cream or clippers (see Step 1)
Mice, 8- to 12-wk-old
Studying both sexes should be a guiding principle to aid in experimental design and hypothesis generation.
Mouse anesthesia (a mixture of ketamine [90 mg/kg] and xylazine [10 mg/kg])
Equipment
Magnifying glass (optional; see Step 7)
Mosquito aspirator (electronic, The John W. Hock Company)
Mosquito meshed cages (2.56′′ × 1.7′′ × 0.79′′)
To make these cages, glue mesh with a size of 0.04′′ × 0.04′′ to transparent acrylic (Precision Plastics Inc.) so that mesh covers the upper and lower parts of the cages. Cages can be custom built or purchased from Precision Plastics Inc. See Figure 1A for reference.
FIGURE 1.
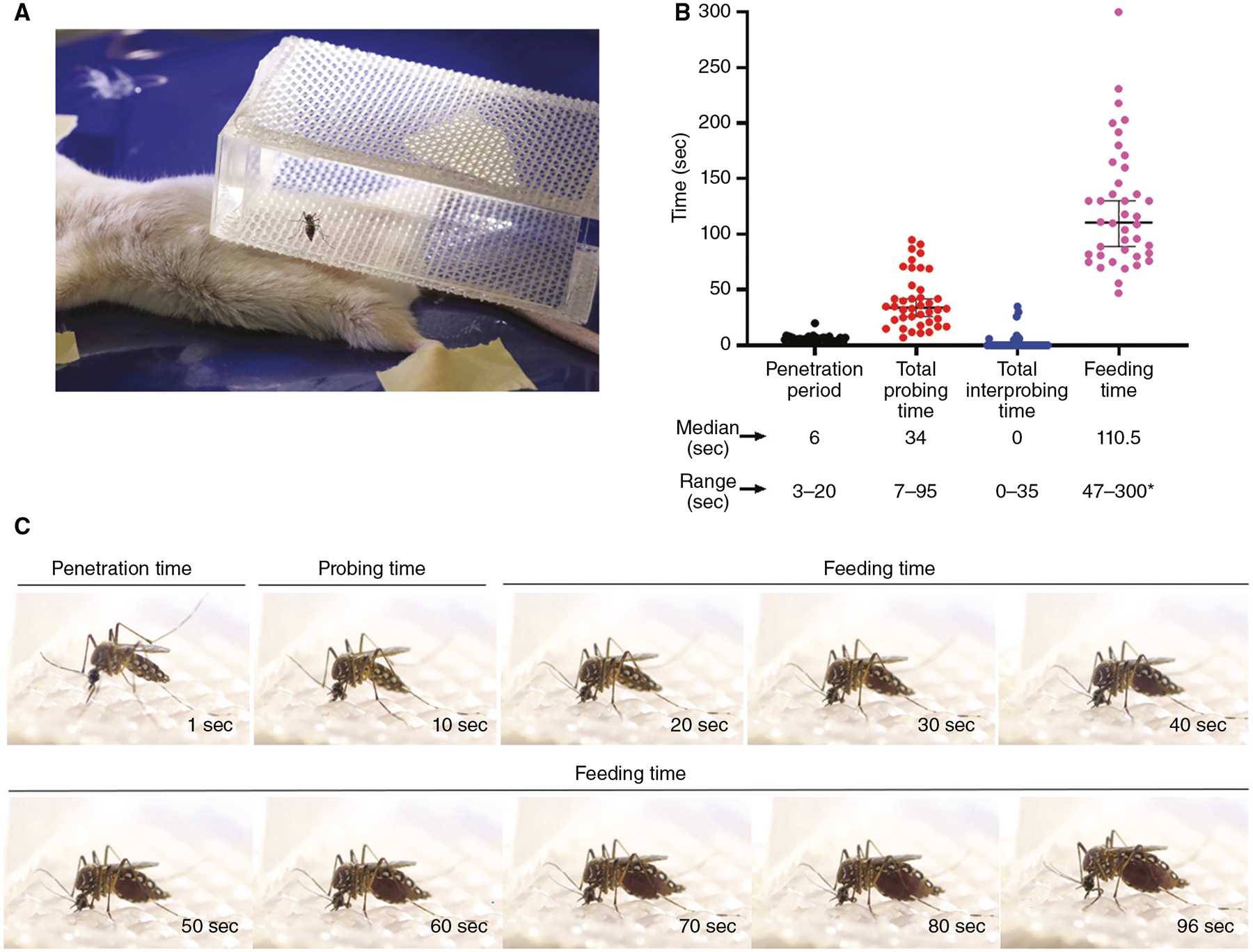
Probing time experiments. (A) Female mosquito is placed in a meshed cage on an anesthetized mouse. (B) Penetration (black), probing (red), interprobing (blue), and feeding (pink) times for wild-type Aedes aegypti (n = 40). Bars show medians with 95% confidence interval (CI). For plotting and analysis purposes, the maximum experimental time was designated at 300 sec (*). (C) Extracted frames from Supplemental Movie S1 showing penetration, probing, and feeding times.
Needles, 25G
Self-regulating temperature-controlled heating pad (PhysioSuite, Kent Scientific)
Syringes, 1-mL
Timer
METHOD
When we carry out this protocol, we follow Public Health Service Animal Welfare Assurance #A4149–01 guidelines from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) Animal Office of Animal Care and Use (OACU). We carry out our studies according to the NIAID/NIH animal study protocol (ASP) approved by the NIH Office of Animal Care and Use Committee (OACUC), with approval ID ASP-LMVR3. We use mice that are housed in one of the animal facilities from the NIAID/NIH and are humanely treated according to OACU regulations.
See Supplemental Movie S1, in which an adult female Ae. aegypti was captured feeding on an anesthetized mouse. The mosquito was caged individually and placed on top of the mouse abdomen. The video shows a successful feeding event without any interprobing time. Penetration, probing, and feeding times are shown.
-
At least 1 d before the probing experiment, remove hair from the mouse abdomen using clippers or depilatory cream.
For probing time experiments, removing hair from the mouse abdomen will help to visualize the mosquito proboscis. Because shaving can cause skin irritation, it should be done at least 1 d before the experiment.
-
Using the aspirator, transfer individual mosquitoes to meshed cages (Fig. 1A).
Label the containers according to the mosquito groups and cover the label to allow for a blinded experiment.
-
Starve the mosquitoes for 16 h by removing sugar and water cotton pads that are usually used for feeding.
For general reference of mosquito rearing, see (Das et al. 2007).
Anesthetize a mouse with an intraperitoneal injection of ketamine (90 mg/kg)/xylazine (10 mg/kg) per your IACUC protocol.
Once the animal is sedated, place it on a warm pad at 37°C in the supine position.
-
Place the meshed cage containing the mosquito on top of the mouse such that it covers the whole abdomen of the mouse.
Different tested mosquitoes will land and probe on different skin areas. If the mosquito does not land on the mouse within 5 min, discard the mosquito and replace it with another one.
-
Using a timer, manually record penetration time as the amount of time it takes for the stylet to be inserted into the mouse skin. Start recording as soon as the mosquito initiates penetration of the mouthparts into the skin. Stop recording when the stylet is completely inserted.
Penetration time is normally fast and lasts for an average of 6 sec (3–20 sec), as seen in Figure 1B,C and Supplemental Movie S1. Penetration time concludes when the process of stylet insertion has finished. It is determined when the mosquito does not penetrate the skin any further and it stays still on the skin with the stylet inserted.
A magnifying glass can be used to watch the feeding process.
See Troubleshooting.
-
Record probing time as the duration between stylet penetration and the appearance of blood in the gut as observed through the thin abdominal pleura (Fig. 1B,C; Supplemental Movie S1).
Probing time is variable and influenced by, for example, the access to host skin, the anesthesia, host body temperature, and external factors impacting mosquito behavior. Probing time range should be established for each specific experimental setup.
If mosquitoes withdraw their mouthparts before taking blood, stop timing and resume counting probing time after a subsequent penetration. Total probing time duration refers to the sum of all probes leading to blood engorgement. The time between probing times is called interprobing time. When mosquito probing leads to an unsuccessful feeding, the time spent to locate the blood vessels is referred to as desistance time instead of probing time.
-
Record feeding time as the time elapsed from beginning of blood visualization in the midgut to full engorgement (Fig. 1B,C; Supplemental Movie S1). Full engorgement is assumed when the mosquito stops ingesting blood and its midgut appears distended and full of blood.
We terminate all measurements at 300 sec, and mosquitoes that do not start to imbibe blood at this cumulative time are recorded as 300 sec. In this case, probing time can be referred to as desistance time.
Probing and feeding times in mosquitoes are highly variable. A minimum of 30 individual mosquitoes in individual cages per group is recommended for statistical comparisons.
We sequentially perform probing time experiments on the same mouse while the animal remains anesthetized. Additionally, one boost of anesthesia (ketamine/xylazine) can be applied. The total time a mouse can be used for probing time experiments is 30–40 min. According to our protocols, right after feeding and before the animal wakes up from the anesthesia, the mouse is euthanized. Several animals must be used to record data from 30 feeding mosquitoes.
-
Analyze data by comparing probing and feeding time data between control and treatment groups.
Results can also be expressed as cumulative curves.
TROUBLESHOOTING
Problem (Step 7): Mosquitoes are not interested in blood feeding on the animal. Solution: Encourage mosquito blood feeding by the following methods.
Ensure that the temperature of the mouse did not drop after anesthesia administration. Perform probing and feeding experiments in a room at 24°C–26°C. Use heating pads and cover the mouse with aluminum foil when possible.
Breathing near the mosquito vial may encourage blood feeding because of CO2 exhalation.
Replace sugar cotton balls with cotton balls impregnated in water 2 d before the experiment. The night before the experiment, remove the water cotton balls.
DISCUSSION
Mosquito probing and feeding times are highly variable. These parameters depend on mosquito behavior, the host, and the biting area. For instance, Ae. aegypti and Anopheles albimanus display faster feeding behaviors than Culex quinquefasciatus (Ribeiro 2000). Ae. aegypti and An. albimanus showed shorter probing times when feeding on humans than on mice, whereas Cx. quinquefasciatus displayed the opposite behavior. In fact, Cx. quinquefasciatus showed fast feeding behaviors on chickens as opposed to feeding on mammals (Ribeiro 2000). The temperature of the host also influences probing and feeding times; therefore, extra care is required to maintain host body temperature at 37°C for probing time experiments. Another factor that affects probing time is the biting area. For example, mosquitoes probe on skin areas that are highly vascularized, such as ears, for shorter times than on areas with a lower degree of vascularization, for instance on the back of an animal (Ribeiro et al. 1984). Feeding parameter measurements are visually taken by the investigator and a certain level of operator subjectivity must be accounted for. Sets of experiments performed by different operators and experiment blinding are crucial requirements for these experiments.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Brian Bonilla and Karina Sewell for excellent mosquito rearing. The authors also thank Joy Jackson Farrar, Chia-Chi Charlie Chang, Akura Marshall, and Edward E. Woodhouse Jr., and the National Institutes of Health (NIH) Events Management, for video editing assistance. Our research was supported by the Intramural Research Program of NIH/National Institute of Allergy and Infectious Diseases (NIAID) (AI001246).
Footnotes
Supplemental material is available at cshprotocols.cshlp.org.
REFERENCES
- Das S, Garver L, Dimopoulos G. 2007. Protocol for mosquito rearing (A. gambiae). J Vis Exp 5: 221. doi: 10.3791/221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellink JJ, Von Zeben MS. 1976. Age related differences of saliva composition in Aedes aegypti. Mosquito News 36: 247–250. [Google Scholar]
- Ribeiro JM. 2000. Blood-feeding in mosquitoes: probing time and salivary gland anti-haemostatic activities in representatives of three genera (Aedes, Anopheles, Culex). Med Vet Entomol 14: 142–148. doi: 10.1046/j.1365-2915.2000.00227.x [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Rossignol PA, Spielman A. 1984. Role of mosquito saliva in blood vessel location. J Exp Biol 108: 1–7. doi: 10.1242/jeb.108.1.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


