Sun et al. discusses recent work from Li et al. describing a cascade of RAB regulation in unconventional trafficking of PGP-1 to the cell surface in C. elegans.
Abstract
Rab GTPases function as intracellular molecular switches that regulate vesicular transport. In the current issue, Li et al. (https://doi.org/10.1083/jcb.202306107) revealed RAB-8 to RAB-11 transition governing the unconventional secretion of membrane proteins in the intestinal epithelium of C. elegans.
Proteins with signaling peptides follow the ER-Golgi secretory pathway, while those lacking such peptides (leaderless cargoes) utilize unconventional protein secretion (UcPS) mechanisms (1). Leaderless cargoes, integral to various biological processes, engage in UcPS pathways with distinct secretory mechanisms. Type I and II UcPS involve vesicle-independent pathways, utilizing plasma membrane pores or transporters. Type III UcPS employs vesicular intermediates such as the ER-Golgi intermediate compartment (ERGIC), endocytic compartments (endosomes, endolysosomes, multivesicular bodies), and secretory autophagosomes. These vesicles operate either in parallel or sequentially, facilitating cargo export outside of the cell independently of the ER-Golgi route. Type IV UcPS involves transmembrane proteins reaching the cell surface independently of conventional ER-Golgi trafficking, for example due to mutations in the cystic fibrosis transmembrane conductance regulator (ΔF508-CFTR) ion channel (2). Key questions surrounding UcPS pertain to the translocation of leaderless cargoes across membranes and the intricacies of membrane trafficking, particularly in vesicle-dependent UcPS, which comprises complex trafficking events that have been challenging to elucidate.
Lin and Shi’s recent collaborative work in membrane trafficking within UcPS is commendable. The laboratory has established a type IV UcPS system, shedding light on the surface trafficking and localization intricacies of PGP-1, an ATP-binding cassette transporter undergoing type IV UcPS. PGP-1 is delivered to the apical surface of intestinal epithelial cells where it protects against environmental stressors. Employing a sophisticated blend of Caenorhabditis elegans genetics and imaging techniques, their prior work notably revealed the pivotal role of the ERGIC-localized protein SMGL-1, functioning as a RAB-8 guanine nucleotide exchange factor (GEF), activating RAB-8 GTPases, and facilitating the UcPS of PGP-1 (3).
Building upon their prior achievements, the team has made remarkable strides in providing a comprehensive understanding of the UcPS pathway. In this issue of the Journal of Cell Biology, Lin and Shi’s research group elegantly unraveled the significance of the RAB-8–RABI-8–RAB-11 cascade in UcPS (4). Employing rabi-8 (tm2518) mutant C. elegans, they skillfully demonstrated the nuanced regulation of RAB-8 activity essential for the UcPS of PGP-1. In this regulatory cascade, SMGL-1 serves as the activator of RAB-8 in its oligomeric form, while RABI-8 acts as an inhibitor by impeding SMGL-1 oligomerization (Fig. 1).
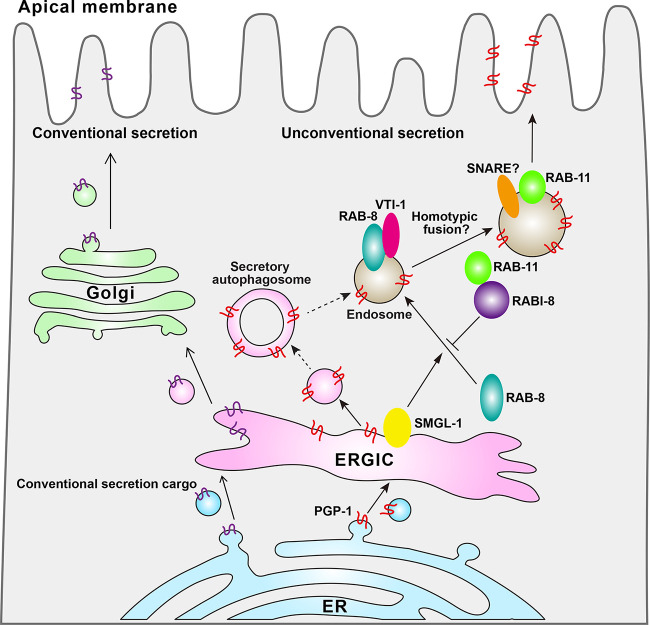
Figure 1.
In the intestinal epithelium of C. elegans, conventional secretion cargoes are trafficked along the ER-ERGIC-Golgi pathway, while leaderless cargoes, such as PGP-1, are secreted through unconventional secretion pathways. SMGL-1, which is resident on the ERGIC, activates RAB-8, facilitating its recruitment to the endosome. Subsequently, RAB-8 interacts with the Q-SNARE VIT-1. Additionally, PGP-1 is secreted from the ERGIC and the vesicles containing PGP-1 may form secretory autophagosomes. RAB-11 recruits RABI-8 to attenuate RAB-8 activity by reducing SMGL-1 self-oligomerization. Ultimately, RAB-11–positive endosomes fuse with the plasma membrane, facilitating the transport of PGP-1 to the apical membrane.
Furthermore, the team identified a crucial downstream event of the RAB-8 vesicles. Within this event, RAB-11 exhibited the ability to recruit RABI-8, thereby diminishing the GEF activity of SMGL-1/GEF toward RAB-8. This orchestrated transition from RAB-8 to RAB-11–positive endosomes plays a pivotal role in promoting the exocytosis of PGP-1 by RAB-11 effectors. UcPS cargo transport between compartments also requires specific Q-soluble N-ethylmaleimide-sensitive-factor attachment protein receptors (SNAREs). In addition to uncovering the RAB-8 to RAB-11 switch, the authors identified the Q-SNARE VTI-1 as an essential RAB-8–interaction protein crucial for the successful completion of PGP-1 UcPS, likely via mediating homotypic fusion. This insightful research significantly advances our understanding of UcPS vesicle trafficking mechanisms.
A central inquiry in UcPS revolves around the membrane trafficking of a protein after its departure from the ER, particularly the deviation from the canonical Golgi pathway. The Lin and Shi groups have undertaken two successive investigations, pioneering efforts to elucidate vesicle transition within endosomes as a downstream event in UcPS trafficking. Additionally, these studies suggest an early-stage membrane exchange between the ER-associated membrane and the endosome. The ERGIC emerges as a crucial station facilitating Golgi bypass, supported by data demonstrating the localization of SMGL-1 in the ERGIC. In the context of PGP-1 trafficking, SMGL-1 activation of early-stage RAB-8 in the endosome implies a delivery from the ERGIC to the endosome. A similar ERGIC–endosome route for Golgi bypassing is proposed in mammalian cells. Notably, the tubular domain of the ERGIC, defined by the GTPase RAB-1A, may directly communicate with the endosomal system via RAB-11–containing endosomes (5). It is currently unclear whether RAB-11–positive endosomes can engage in a similar interaction with ERGIC to promote the secretion of PGP-1. Furthermore, the ERGIC’s role as a pivotal trafficking station for UcPS is corroborated in type III UcPS, where the ERGIC acts as the compartment for leaderless UcPS cargo entry into the secretory station (6).
The mechanism by which the ERGIC connects with the endosome remains unclear. One plausible scenario is the existence of a secretory autophagosome stage in between, given the ERGIC’s recognized role as a membrane source for autophagosomes (7). Supporting this hypothesis, the UcPS of ΔF508-CFTR binds the ERGIC-localized receptor TMED3, and subsequently undergoes secretory autophagy (8). Additionally, a hybrid organelle consisting of ERGIC-like compartments, autophagosomes, and endosomes marked by Grh (the mammalian GRASP55 homology) known as the compartment for unconventional protein secretion has been observed in UcPS in yeast (9). Moreover, secretory autophagosomes have been shown to connect with RAB-8a endosomes in the UcPS of IL-1β (10). However, substantiating this notion requires a detailed analysis of how autophagy proteins act on the ERGIC to generate secretory autophagosomes and how these autophagosomes mature into endosomes.
Another critical question pertains to the cargo itself, specifically understanding the factors influencing a membrane protein’s selection of the Golgi bypass route. This question is particularly relevant in the case of SID-2 (double-stranded RNA receptor, another UcPS cargo analyzed in this work), capable of both conventional and unconventional trafficking. Potential explanations include a saturation effect, where conventional pathways become oversaturated, necessitating an alternative route to compensate for SID-2 trafficking. Alternatively, the need for specific glycosylated forms of SID-2 for distinct activities may drive Golgi bypass, as this route can alter SID-2’s glycosylation status. In this scenario, it is conceivable that a specialized pool of SID-2 is synthesized or tagged for UcPS. Lastly, the choice can be made by specific stress conditions or in a certain stage of a physiological process. Subsequent studies are imperative to test these possibilities.
Acknowledgments
The work is funded by National Natural Science Foundation of China (32130023, 32225013, 9225430003, and 32370728), Ministry of Science and Technology of the People’s Republic of China (2021YFA0804802 and 2019YFA0508602), and supported by Vanke Special Fund for Public Health and Health Discipline Development, Tsinghua University (2022Z82WKJ009).
References
- 1.Rabouille, C. 2017. Trends Cell Biol. 10.1016/j.tcb.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 2.Gee, H.Y., et al. 2011. Cell. 10.1016/j.cell.2011.07.021 [DOI] [Google Scholar]
- 3.Wang, X., et al. 2022. J. Cell Biol. 10.1083/jcb.202111125 [DOI] [Google Scholar]
- 4.Li, X., et al. 2024. J. Cell Biol. 10.1083/jcb.202306107 [DOI] [Google Scholar]
- 5.Marie, M., et al. 2009. Mol. Biol. Cell. 10.1091/mbc.e08-12-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang, M., et al. 2020. Cell. 10.1016/j.cell.2020.03.031 [DOI] [Google Scholar]
- 7.Li, S., et al. 2022. Cell Res. 10.1038/s41422-021-00563-0 [DOI] [Google Scholar]
- 8.Park, H., et al. 2022. Adv. Sci. 10.1002/advs.202105320 [DOI] [Google Scholar]
- 9.Cruz-Garcia, D., et al. 2018. Semin. Cell Dev. Biol. 10.1016/j.semcdb.2018.02.021 [DOI] [Google Scholar]
- 10.Dupont, N., et al. 2011. EMBO J. 10.1038/emboj.2011.398 [DOI] [PMC free article] [PubMed] [Google Scholar]