Abstract
Background
Obesity is a global issue with a major impact on cardiovascular health. This study explores how obesity influences nocturnal cardiac electrophysiology in suspected obstructive sleep apnea (OSA) patients.
Methods
We randomly selected 12 patients from each of the five World Health Organization body mass index (BMI) classifications groups (n total = 60) while keeping the group's age and sex matched. We evaluated 1965 nocturnal electrocardiography (ECG) samples (10 s) using modified lead II recorded during normal saturation conditions. R‐wave peaks were detected and confirmed using dedicated software, with the exclusion of ventricular extrasystoles and artifacts. The duration of waves and intervals was manually marked. The average electric potential graphs were computed for each segment. Thresholds for abnormal ECG waveforms were P‐wave > 120 ms, PQ interval > 200 ms, QRS complex > 120 ms for, and QTc > 440 ms.
Results
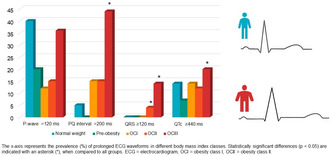
Obesity was significantly (p < .05) associated with prolonged conduction times. Compared to the normal weight (18.5 ≤ BMI < 25) group, the morbidly obese patients (BMI ≥ 40) had a significantly longer P‐wave duration (101.7 vs. 117.2 ms), PQ interval (175.8 vs. 198.0 ms), QRS interval (89.9 vs. 97.7 ms), and QTc interval (402.8 vs. 421.2 ms). We further examined ECG waveform prolongations related to BMI. Compared to other patient groups, the morbidly obese patients had the highest number of ECG segments with PQ interval (44% of the ECG samples), QRS duration (14%), and QTc duration (20%) above the normal limits.
Conclusions
Morbid obesity predisposes patients to prolongation of cardiac conduction times. This might increase the risk of arrhythmias, stroke, and even sudden cardiac death.
Keywords: body mass index, electrocardiogram, interval duration, obesity, obstructive sleep apnea, wave duration
Morbid obesity increases the risk of cardiac conduction time prolongation and conduction abnormalities, such as PQ‐time (>200 ms), QRS prolongation (≥120 ms), and QTc prolongation (≥440 ms). This may increase the chances of arrhythmias, stroke, and sudden cardiac death, as compared to individuals with normal weight.
1. INTRODUCTION
Obesity has become a major public health hazard with an epidemic proportion. Obesity is associated with many cardiovascular comorbidities, such as coronary artery disease (CAD) and sudden cardiac death (SCD) (Hubert et al., 1983). Furthermore, obesity and obstructive sleep apnea (OSA) are closely related. It is well known that obesity predisposes to OSA (Schwartz et al., 2008), which further exacerbates the cardiovascular consequences of obesity (Drager et al., 2013) and is an independent risk factor for cardiovascular diseases and SCD (Gami et al., 2013).
The pathophysiological cascade between OSA, obesity, and cardiovascular disease is multifactorial and partly unknown. In obese patients, upper airway collapsibility is often increased by underlying anatomic alterations and disturbances in upper airway neuromuscular control (Schwartz et al., 2008). This causes intermittent hypoxia and hypercapnia and upregulates the sympathetic nervous system, which is associated with increased cardiovascular morbidity (Malpas, 2010) and weight regulation (Guarino et al., 2017). The perivascular fat appears to be a source of pro‐inflammatory and vasoactive factors that may contribute to endothelial and smooth muscle cell dysfunction and the pathogenesis of vascular diseases (Campia et al., 2012). Respiratory events cause pleural pressure swings, increasing heart rate, and blood pressure which can further lead to cardiopulmonary hyperreactivity to hypoxia, and cardiac anatomical remodeling (Sajkov & McEvoy, 2009). Moreover, obesity itself can result in cardiac remodeling, increased profibrotic stage, change in presentation of ion channels, leads to fibrosis, and predispose to arrhythmias (Mahajan et al., 2015; McCauley et al., 2020). The intrathoracic adipose tissue, comprising both mediastinal and epicardial elements, is situated adjacent to the heart and has the potential to infiltrate into the myocardium (Anumonwo & Herron, 2018). Research indicates a correlation between the quantity of pericardial adipose tissue and unfavorable LV remodeling as well as an adverse cardiovascular disease prognosis (Shah et al., 2017). Earlier research has also shown a connection between OSA and deviations in electrocardiography (ECG) waveform changes (Can et al., 2009; Gupta et al., 2012; Shi & Jiang, 2020). Collectively, these interrelated health conditions may increase the risk of conduction abnormalities and arrhythmias.
In this study, we investigate whether a higher body mass index (BMI) is associated with prolonged conduction times (P‐wave, PQ interval, QRS complex, and QTc interval) in suspected OSA patients. We hypothesized that the degree of obesity correlates with ECG conduction abnormalities in suspected OSA patients. This information might help in understanding the complex interplay between obesity, OSA, and cardiovascular disease.
2. MATERIALS AND METHODS
2.1. Study population
The dataset used in this study involved (n = 916) consecutive patients with suspected OSA. All patients had undergone full diagnostic polysomnography (PSG) at the Princess Alexandra Hospital (Brisbane, Australia) during 2015–2017. The PSG data were acquired with the Compumedics Grael acquisition system (Compumedics, Abbotsford, Australia). Approval for retrospective data collection was given by the Institutional Human Research Ethics Committee of the Princess Alexandra Hospital (HREC/16/QPAH/021 and LNR/2019/QMS/54313). Due to the retrospective nature of the study, no informed consent was needed from the patients according to the Metro South Human Research Ethics Committee.
Only patients with a total sleep time of ≥4 h in the PSG and without cardiac pacemakers were included. Patients were further divided into five groups (each n = 12) according to the World Health Organization BMI classification: normal weight (NW) group defined as 18.5 ≤ BMI < 25, preobesity (PO) group as 25 ≤ BMI < 30, a moderately obese group I (OGI) defined as 30 ≤ BMI < 35, severally obese group II (OGII) defined as 35 ≤ BMI < 40, and morbidly obese group III (OGIII) defined as BMI≥40 (WHO, 2000). After that, we sex and age matched the patients, and randomly selected 12 patients from the five different BMI groups.
2.2. PSG analysis
The PSG recordings were scored manually following the prevalent American Academy of Sleep Medicine guidelines (Berry et al., 2012). The scoring was performed by experienced sleep technicians in Princess Alexandra Hospital using Compumedics ProFusion PSG4 software (Compumedics). The scoring process has been described in more detail in a previous publication (Duce et al., 2016). The detailed information of each desaturation (e.g. start time and endpoint) was exported from ProFusion to Matlab (ver R2019b; Mathworks). All scored desaturations in which the patient was awake were excluded.
2.3. ECG analysis
The patients had a total of 4571 baseline (pre‐desaturation) ECG segments. Nocturnal ECGs (modified lead II) were recorded during the diagnostic PSG study, with a sampling frequency of 256 Hz. First, we excluded ECG segments originating less than 25 s after the end of the previous desaturation to avoid the possible influence of desaturation on the ECG (Sillanmäki et al., 2022). Second, 10‐s ECG segments preceding desaturations events were extracted from the nocturnal ECG recording. After exclusions, a total of 1965 ECG segments were included in the further analysis. Peaks of the R‐waves were detected using Kubios HRV Premium software (Kubios Oy) (Tarvainen et al., 2014), and detections were verified visually and manually corrected when necessary. Ventricular extrasystoles were excluded from the analyses by the software. After that, an average graph of electric potential during the ECG complex was computed for each 10‐s segment and the duration of each wave and interval was manually marked (Sillanmäki et al., 2022). The T‐wave endpoint was visually identified as an intersection between a tangent of the steepest part of the wave and the baseline and marked for each segment. The parameters studied were P‐wave, PQ interval, QRS complex, and QTc interval. The heart rate corrected QT (QTc) intervals were calculated according to Bazett's formula (Bazett, 1997). The prevalence of prolonged ECG waveforms in different BMI groups was further studied. The upper normal thresholds for ECG waveform durations were 120 ms for P‐wave, 200 ms for PQ interval, 120 ms for QRS complex, and 440 ms for QTc interval (Kusumoto et al., 2019; Rautaharju et al., 2009).
2.4. Statistical analysis
The statistical significance of the differences between BMI groups was evaluated with the Wilcoxon rank‐sum test, Wilcoxon signed rank‐sum test, and chi‐squared test. The limit for statistical significance was set to be p < .05 when comparing the PSG characteristics and prevalence of ECG waveform threshold exceedings. As every BMI category consists of samples from the same patients, the statistical significance of the difference between group medians was evaluated with the Wilcoxon signed‐rank test. Moreover, as the categories contain a different number of samples and the Signed‐rank works in a pair‐wise manner, a total of 5000 iterations over all possible random permutations of the groups was conducted. This results in 5000 different p‐values, and the median p‐value was chosen to indicate the statistical significance. A p < .01 was set as a threshold based on Bonferroni correction to compensate multiple comparisons. Matlab 2019b (Mathworks Inc.) was used for the statistical analysis. The data are presented as means and cumulative distributions.
3. RESULTS
Demographic information about the population is presented in Table 1. There were no statistically significant differences in PSG results between BMI groups (Table 1). The OGIII patients seemed to have more apnea/hypopnea‐related findings compared to the NW group, yet the difference was not statistically significant. Even though the median apnea‐hypopnea index was the highest in the OGII group, the OGIII group was more hypoxemic based on the desaturation severity.
TABLE 1.
Clinical demographic and PSG (polysomnography) characteristics in different body mass index groups.
| Normal weight | Preobesity | OCI | OCII | OCIII | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| COPD | 0 (0) | 0 (0) | 2 (16.7) | 0 (0) | 0 (0) |
| Depression | 2 (16.7) | 2 (16.7) | 1 (8.3) | 4 (33.3) | 4 (33.3) |
| Dyslipidemia | 0 (0) | 1 (8.3) | 5 (41.6) | 3 (25) | 3 (25) |
| Hypertension | 2 (16.7) | 2 (16.7) | 5 (41.6) | 2 (16.7) | 7 (58.3) |
| Hypothyroidism | 0 (0) | 4 (33.3) | 0 (0) | 2 (16.7) | 0 (0) |
| Previous atrial arrhythmia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (8.3) |
| Smoker | 2 (16.7) | 2 (16.7) | 1 (8.3) | 2 (16.7) | 3 (25) |
| Type II diabetes | 1 (8.3) | 0 (0) | 3 (25) | 1 (8.3) | 5 (41.6) |
| PSG results | |||||
| AHI (1/h) | 10.1 (4.4–18.4) | 5.0 (3.1–11.8) | 14.6 (6.1–33.4) | 17.2 (6.3–28.7) | 15.7 (9.3–33.3) |
| Arousal index (1/h) | 23.3 (17.5–33.3) | 18.3 (11.6–26.3) | 25.4 (13.5–30.6) | 27.4 (18.3–40.5) | 21.3 (16.7–26.9) |
| Desaturation severity (%) | 0.07 (0.02–0.11) | 0.03 (0.02–0.05) | 0.34 (0.08–0.55) | 0.15 (0.05–0.51) | 0.36 (0.11–1.00) |
| ODI (1/h) | 3.3 (1.2–5.2) | 1.4 (1.1–2.5) | 13.2 (3.5–27.1) | 8.3 (2.7–18.6) | 16.0 (6.6–20.3) |
| TST (h) | 5.9 (4.6–6.6) | 5.9 (5.3–6.4) | 5.7 (5.4–6.8) | 5.2 (4.5–5.6) | 5.9 (5.7–6.1) |
| t 90% (s) | 31.8 (8.1–60.8) | 17.9 (3.7–144.3) | 111.3 (48.7–495.7) | 76.7 (8.7–545.6) | 239.4 (37.4–2667.0) |
Note: Values are presented as number (%) or median (interquartile range; IQR) where appropriate.
Abbreviations: COPD, chronic obstructive pulmonary disease; ODI, oxygen desaturation index; PSG, polysomnography; t90%, time with oxygen saturation below 90%; TST, total sleep time.
In the OGIII group (consisting of morbidly obese patients), the medians of RR interval and all ECG waveform durations were significantly longer compared to the NW group (Table 2). The median RR interval in the OGIII group was longer compared to the NW group (905 vs. 844 ms, p < .01), respectively. The median P‐wave duration of the OGIII group was 15.5 ms (15.2%) longer compared to the NW group (Table 2). The median PQ interval of the OGIII group was 22.2 ms (12.6%) longer compared to the NW group (Table 2). The difference between the OGIII group and other groups was prominent above the lower quartiles in the distribution chart (Figure 1b). The median QRS interval of the OGIII group was 7.8 ms (8.7%) longer compared to the NW group (Table 3). The difference between the OGIII group and other groups was the most prominent in the upper quartile (Figure 1c). The median QTc interval of the OGIII group was 18.4 ms (4.6%) longer compared to the NW group. Additionally, the median QTc interval of the OGII group was 15.0 ms (3.7%) longer compared to the NW group (Table 2). The differences between the groups were the most substantial in the interquartile range and disappeared at the extremes (Figure 1d).
TABLE 2.
Electrocardiography characteristics in different body mass index groups.
| Normal weight | Preobesity | OCI | OCII | OCIII | |
|---|---|---|---|---|---|
| RR interval (ms) | 844.1 (784.8–913.9) | 828.3 (792.1–881.8) | 962.1 (836.2–1026.0) | 887.0 (776.8–990.5) | 904.9 (811.0–1080.6) |
| P‐wave duration (ms) | 101.7 (85.9–125.6) | 109.4 (101.6–115.7) | 109.4 (98.1–114.3) | 109.4 (99.4–118.2) | 117.2 (101.6–129.4) |
| PQ interval (ms) | 175.8 (152.3–183.6) | 155.5 (148.4–171.9) | 171.9 (160.2–184.4) | 171.9 (152.3–191.4) | 198.0 (179.6–234.4) |
| QRS interval (ms) | 89.9 (78.1–101.6) | 85.9 (78.1–93.8) | 88.5 (82.0–98.5) | 89.8 (82.0–101.3) | 97.7 (85.9–109.4) |
| QT interval (ms) | 375.0 (359.4–398.4) | 359.4 (351.6–390.6) | 398.4 (386.7–406.3) | 402.3 (343.8–421.9) | 398.4 (378.9–425.8) |
| QTc interval (ms) | 402.8 (394.2–411.2) | 399.5 (384.4–415.2) | 404.7 (391.8–425.4) | 417.8 (403.9–428.5) | 421.2 (402.4–436.8) |
Note: Values are presented as median (interquartile range). The bolded value indicates a significantly (p < .01) larger median compared to the normal weight group.
Abbreviations: OCI, obesity class I; OCII, obesity class II; OCIII, obesity class III.
FIGURE 1.
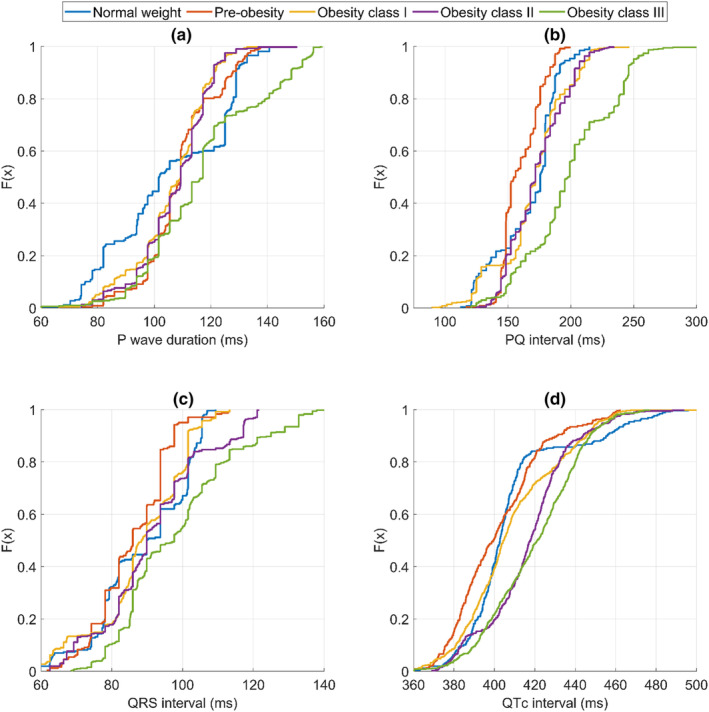
Cumulative distributions of baseline electrocardiogram waveforms for different body mass index groups. (a) P‐wave duration, (b) PQ intervals, (c) QT intervals, and (d) QTc intervals.
TABLE 3.
Prevalence on prolonged ECG waveforms in different body mass index (BMI) classes.
| Normal weight % (n) | Preobesity % (n) | OCI % (n) | OCII % (n) | OCIII % (n) | |
|---|---|---|---|---|---|
|
P‐wave duration >120 ms |
39.9 (103) | 19.8 (48) a | 12.4 (65) a | 15.2 (62) a | 36.0 (193) |
|
Prolonged PQ interval >200 ms |
4.7 (12) | 0 (0) a | 14.9 (78) a | 15.3 (62) a | 44.0 (236) a |
|
Prolonged QRS 110–119 ms |
0 (0) | 1.7 (4) a | 0.7 (4) | 11.3 (46) a | 7.3 (39) a |
|
Prolonged QRS ≥120 ms |
0 (0) | 0 (0) | 0 (0) | 3.5 (14) a | 13.6 (73) a |
|
Prolonged QTc ≥440 ms |
14.0 (36) | 6.6 (16) a | 13.8 (72) | 11.8 (48) | 20.2 (108) a |
Note: Statistical significance of differences was assessed using chi‐squared test. A bolded value indicates a significant (p < .05) difference with all groups.
Abbreviations: ECG, electrocardiography; OCI, obesity class I; OCII, obesity class II; OCIII, obesity class III.
Significant difference between the corresponding group and normal weight group.
We found that the OGIII group showed the most exceedings over normal threshold levels (Figure 1 and Table 3). Among OGIII patients, the PQ interval was prolonged in 44.0% of ECG samples, while in NW patients the prevalence of prolonged PQ interval samples was 4.7% (Table 3). Similarly, the QRS duration was prolonged in 13.6% of the OGIII samples, but no normal threshold exceeding durations over the threshold was seen in the NW group. The QTc duration was over the threshold in 20.2% of OGIII samples, and in the NW group, 14.0% of samples exceeded the threshold (Table 3). The P‐wave threshold was exceeded in 39.9% of the NW group's ECG samples, while in OGIII 36.0% of samples were over the threshold (Table 3).
4. DISCUSSION
4.1. Principal findings
In this study, we investigated the impact of obesity on cardiac conduction in patients with suspected OSA. We observed significant differences in conduction times between normal weight and morbidly obese patients across various ECG parameters. However, the differences were less pronounced among less obese patients.
4.2. ECG changes in the context of the current literature
We discovered that higher BMI was associated with longer P‐wave duration and longer PQ interval in suspect OSA patients. Moreover, the prevalence of first‐degree atrioventricular block (PQ interval > 200 ms) seems to increase with BMI. The incidence of prolonged AV conduction was 4.7% in the NW group, whereas in the morbidly obese group, it was notably higher at 44%. This finding is in line with a previous study showing a link between BMI and the prevalence of AV block (Shan et al., 2021). The finding has clinical significance due to the established association between prolonged PQ intervals and an elevated risk of atrial fibrillation and even higher mortality rates (Shan et al., 2021). However, the association can be multifactorial since also age and gender can affect the risk (Gaisl et al., 2016; Maeno et al., 2013; Shan et al., 2021). Moreover, a previous study has shown that P‐wave abnormalities are associated with OSA (Can et al., 2009), and simulated apneas cause acute prolongation of P‐wave duration even in the healthy population (Gaisl et al., 2016; Maeno et al., 2013).
In earlier investigations, QRS prolongation is shown to be relatively prevalent in OSA patients (Gupta et al., 2012). In our study, we found that intraventricular conduction duration (the mean QRS interval) was longer in morbidly obese patients compared to the normal‐weight population, and the number of prolonged QRS samples is higher in obese patients. This implies a relationship between obesity and modifications in cardiac electrical conduction that go beyond the impact solely attributed to OSA. The QRS prolongation might stem, at least in part, from analogous factors akin to those noted in AV block, such as cardiac remodeling, among others. Sobhani et al. recently showed that not only BMI but also hypertension and increased lipide levels were associated with prolonged QRS duration (Sobhani et al., 2022). Furthermore, both obesity and OSA are known to associate with ventricular hypertrophy (LVH) (Cuspidi et al., 2014; Noda et al., 1995), which itself is connected with QRS prolongation. LVH is further associated with an increased prevalence of heart failure, lethal ventricular arrhythmias, and even SCD (Cavalera et al., 2014; Cuspidi et al., 2014; Kahan & Bergfeldt, 2005). Hence, the prolonged QRS duration observed in morbidly obese patients might indirectly point toward increased myocardial fibrosis, fatty infiltration of the myocardium, and LV wall thickening.
The QT interval reflects the total duration of ventricular myocardial depolarization and repolarization. Several studies have explored the correlation between repolarization parameters and diverse cardiac conditions. For example, QT dispersion (QTd), which measures the variation in recovery time across different regions of the heart, has been found to correlate with the severity of CAD (Helmy et al., 2017). Furthermore, the QT interval length itself is found to be independently associated with the emergence of malignant ventricular arrhythmias in patients with CAD (de Carvalho et al., 2022). De Carvalho et al. showed a 7% rise in malignant ventricular arrhythmias for every 10 ms increase in the QT interval (de Carvalho et al., 2022). Most notably, both prolonged QTc and heightened QTd serve as significant predictors of overall and cardiovascular mortality, likely attributed to an increased susceptibility to arrhythmias. (Okin et al., 2000). Interestingly, it has been established in previous research that delays in repolarization are seen also in OSA patients (Shi & Jiang, 2020). In morbidly obese patients, the median QTc duration was found to be nearly 20 ms longer than in the normal weight group. Consequently, individuals afflicted by morbid obesity might encounter a heightened health risk in comparison to those with a normal weight. Interestingly, this phenomenon appears to be reversible, at least to some extent, as weight loss has been shown to be associated with a shortening of the QTc interval (Papaioannou et al., 2004).
4.3. Potential mechanisms
The mechanisms driving electrophysiological changes associated with obesity encompass a range of factors. Both obesity and OSA can disturb the autonomic nervous system, characterized by heightened sympathetic activity and diminished parasympathetic tone may impact ECG (Guarino et al., 2017). Additionally, obesity exerts mechanical stress on the heart, which lead to structural adaptations such as hypertrophy and changes in cardiac mechanics. For example, obesity is associated with left atrial enlargement that leads also changes in electrophysiology (Lin et al., 2011; Wang et al., 2004). The presence of oxidative stress and mitochondrial dysfunction within the context of obesity may further disrupt intracellular signaling pathways that hold relevance for cardiac electrophysiology (Cojocaru et al., 2023). It is also known that obesity‐related chronic inflammation predisposes to myocardial fibrosis (Cavalera et al., 2014). The fibrotic remodeling alters the heart's microstructure and disrupts the normal electrical pathways, thereby resulting in prolonged conduction (Verheule & Schotten, 2021). The presence of excessive adipose tissue around the heart and infiltrating the myocardium may influence the propagation of electrical signals across the cardiac system (Anumonwo & Herron, 2018). Moreover, morbidly obese individuals may have comorbidities requiring medications, some of which, such as certain antiarrhythmics and antidepressants, have the potential to prolong the QT interval (Van Noord et al., 2010). Furthermore, obesity‐related metabolic disturbances can lead to electrolyte imbalances, further contributing to the prolongation of the repolarization (Van Noord et al., 2010).
4.4. Strengths and limitations
Our research sets itself apart from numerous prior studies that relied on isolated ECG recordings. In our study, we explore multiple time points and various ECG parameters associated with cardiac conduction. This approach provides a comprehensive evaluation over an extended duration, capturing potential changes not apparent during waking hours. Analyzing ECG samples during sleep further offers information under real‐life conditions, free from the potential influence of stress or other factors present during awake ECG recordings. Furthermore, we combined research focusing on both OSA and obesity. For this reason, these findings have potential implications for improving risk assessment within this particular patient cohort. Moreover, it is worth noting that several studies on OSA tend to focus solely on male participants. In our study, both sexes were included. However, it is important to acknowledge certain limitations within our study. Both OSA and obesity are associated with other comorbidities and cardiovascular disease risk factors, including diabetes, hypertension, and dyslipidemia. These underlying conditions might influence the results. Moreover, the ECG samples were relatively short (10 s). The segment duration was selected as a compromise between a sufficiently long segment to obtain a reliable representation and to not exclude too many samples in patients with frequent desaturations. The ECGs of some samples were subject to interpretation because of the power line interference that had not been corrected by the recorder. Additionally, we only compared the groups with the normal weight group. No further statistical comparisons were made between OP, OGI, and OGII patients. This, though, should not be a big defect as the medians of other groups were positioned most in the middle of the medians of the NW group and OGIII group. Furthermore, the relatively small number of subjects in each BMI group is a limitation of this study. This limitation arises from the retrospective nature of our study design, which presented challenges in forming matched groups based on age and sex. Yet, our study advances beyond previous research on obesity and cardiovascular changes by focusing on specific ECG wavelength and interval alterations in morbidly obese patients. ECG segments that coincided with nocturnal desaturations were excluded from the study. This decision was made because previous research has established that nocturnal desaturations can influence the ECG (Sillanmäki et al., 2022). By excluding these segments, the study aims to focus solely on the impact of obesity on ECG waveform and interval alterations, without the potential confounding effects of desaturations. While acknowledging limitations, our study contributes valuable insights into the intricate relationship between OSA, obesity, and cardiovascular health.
5. CONCLUSIONS
We found that morbid obesity is especially associated with prolonged conduction times. This predisposes obese patients to cardiac conduction disorders and possibly also to arrhythmias. The mechanisms underlying the development of electrophysiological changes in morbidly obese patients are complex and multifactorial. Understanding these mechanisms is crucial for improved risk assessment and the development of targeted interventions to mitigate the cardiac consequences of obesity in clinical practice.
AUTHOR CONTRIBUTIONS
J.A.L. provided the Kubios software. B.D. provided the patient data. A.S., S.S., and S.K. analyzed the patient data. All authors interpreted the patient data and contributed to writing the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported by the Research Committee of the Kuopio University Hospital Catchment Area for the State Research Funding [5041798 to S.S, 5041803 to H.K., 5041809 to S.N., 5101137 to J.L., and 507T044 to J.L.)], Seinäjoki Central Hospital (7746), the Competitive State Research Financing of Expert Responsibility Area of Tampere University Hospital (VTR7319 to A.K., VTR7312 to A.K., VTR7330 to A.K., and EVO2089 to A.K.), Tampere Tuberculosis Foundation (to A.K.). The European Union's Horizon 2020 Research and Innovation Programme (Grant 965417) (to S.N.).
CONFLICT OF INTEREST STATEMENT
Lipponen JA is a shareholder of a company (Kubios) that designs ECG analysis and heart rate variability analysis software. Other co‐authors have nothing to declare.
ETHICS STATEMENT
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Approval for retrospective data collection was obtained from the Institutional Human Research Ethics Committee of the Princess Alexandra Hospital (HREC/16/QPAH/021 and LNR/2019/QMS/54313). The need for informed consent was waived by the Metro South Human Research Ethics Committee due to the retrospective nature of the study.
Kainulainen, S. , Suni, A. , Lipponen, J. A. , Kulkas, A. , Duce, B. , Korkalainen, H. , Nikkonen, S. , & Sillanmäki, S. (2024). Morbid obesity influences the nocturnal electrocardiogram wave and interval durations among suspected sleep apnea patients. Annals of Noninvasive Electrocardiology, 29, e13101. 10.1111/anec.13101
DATA AVAILABILITY STATEMENT
The datasets analyzed during the current study are not publicly available due to privacy policy but are available from the corresponding author upon reasonable request.
REFERENCES
- Anumonwo, J. M. B. , & Herron, T. (2018). Fatty infiltration of the myocardium and arrhythmogenesis: Potential cellular and molecular mechanisms. Frontiers in Physiology, 9, 2. 10.3389/fphys.2018.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazett, H. C. (1997). An analysis of the time‐relations of electrocardiograms. Annals of Noninvasive Electrocardiology, 2(2), 177–194. 10.1111/j.1542-474X.1997.tb00325.x [DOI] [Google Scholar]
- Berry, R. B. , Budhiraja, R. , Gottlieb, D. J. , Gozal, D. , Iber, C. , Kapur, V. K. , Marcus, C. L. , Mehra, R. , Parthasarathy, S. , Quan, S. F. , Redline, S. , Strohl, K. P. , Davidson Ward, S. L. , Tangredi, M. M. , & American Academy of Sleep Medicine . (2012). Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. Journal of Clinical Sleep Medicine, 8(5), 597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campia, U. , Tesauro, M. , & Cardillo, C. (2012). Human obesity and endothelium‐dependent responsiveness. British Journal of Pharmacology, 165, 561–573. 10.1111/j.1476-5381.2011.01661.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can, I. , Aytemir, K. , Demir, A. U. , Deniz, A. , Ciftci, O. , Tokgozoglu, L. , Oto, A. , & Sahin, A. (2009). P‐wave duration and dispersion in patients with obstructive sleep apnea. International Journal of Cardiology, 133(3), e85–e89. 10.1016/j.ijcard.2007.11.037 [DOI] [PubMed] [Google Scholar]
- Cavalera, M. , Wang, J. , & Frangogiannis, N. G. (2014). Obesity, metabolic dysfunction, and cardiac fibrosis: Pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Translational Research, 164, 323–335. 10.1016/j.trsl.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cojocaru, K. A. , Luchian, I. , Goriuc, A. , Antoci, L. M. , Ciobanu, C. G. , Popescu, R. , Vlad, C. E. , Blaj, M. , & Foia, L. G. (2023). Mitochondrial dysfunction, oxidative stress, and therapeutic strategies in diabetes, obesity, and cardiovascular disease. Antioxidants, 12, 658. 10.3390/antiox12030658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuspidi, C. , Rescaldani, M. , Sala, C. , & Grassi, G. (2014). Left‐ventricular hypertrophy and obesity: A systematic review and meta‐analysis of echocardiographic studies. Journal of Hypertension, 32, 16–25. 10.1097/HJH.0b013e328364fb58 [DOI] [PubMed] [Google Scholar]
- de Carvalho, G. D. , Armaganijan, L. V. , Lopes, R. D. , Olandoski, M. , do Amaral Galvão, B. M. , Pessoa, C. C. , Erbano, B. O. , Luz, R. S. B. D. , Demarchi, A. V. , Medeiros, B. G. , & Ribeiro Moreira, D. A. (2022). Relationship between ventricular repolarization parameters and the inducibility of ventricular arrhythmias during electrophysiological study in patients with coronary artery disease. Revista Da Associacao Medica Brasileira, 68(1), 61–66. 10.1590/1806-9282.20210806 [DOI] [PubMed] [Google Scholar]
- Drager, L. F. , Togeiro, S. M. , Polotsky, V. Y. , & Lorenzi‐Filho, G. (2013). Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. Journal of the American College of Cardiology, 62, 569–576. 10.1016/j.jacc.2013.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce, B. , Kulkas, A. , Langton, C. , Töyräs, J. , & Hukins, C. (2016). The AASM 2012 recommended hypopnea criteria increase the incidence of obstructive sleep apnea but not the proportion of positional obstructive sleep apnea. Sleep Medicine, 26, 23–29. 10.1016/j.sleep.2016.07.013 [DOI] [PubMed] [Google Scholar]
- Gaisl, T. , Wons, A. M. , Rossi, V. , Bratton, D. J. , Schlatzer, C. , Schwarz, E. I. , Camen, G. , & Kohler, M. (2016). Simulated obstructive sleep apnea increases P‐wave duration and P‐wave dispersion. PLoS One, 11(4), 1–9. 10.1371/journal.pone.0152994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gami, A. S. , Olson, E. J. , Shen, W. K. , Wright, R. S. , Ballman, K. V. , Hodge, D. O. , Herges, R. M. , Howard, D. E. , & Somers, V. K. (2013). Obstructive sleep apnea and the risk of sudden cardiac death: A longitudinal study of 10,701 adults. Journal of the American College of Cardiology, 62(7), 610–616. 10.1016/j.jacc.2013.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino, D. , Nannipieri, M. , Iervasi, G. , Taddei, S. , & Bruno, R. M. (2017). The role of the autonomic nervous system in the pathophysiology of obesity. Frontiers in Physiology, 8, 665. 10.3389/fphys.2017.00665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S. , Cepeda‐Valery, B. , Romero‐Corral, A. , Shamsuzzaman, A. , Somers, V. K. , & Pressman, G. S. (2012). Association between QRS duration and obstructive sleep apnea. Journal of Clinical Sleep Medicine, 8(6), 649–654. 10.5664/jcsm.2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy, H. , Abdel‐Galeel, A. , Taha Kishk, Y. , & Mohammed Sleem, K. (2017). Correlation of corrected QT dispersion with the severity of coronary artery disease detected by SYNTAX score in non‐diabetic patients with STEMI. Egyptian Heart Journal, 69(2), 111–117. 10.1016/j.ehj.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert, H. B. , Feinleib, M. , McNamara, P. M. , & Castelli, W. P. (1983). Obesity as an independent risk factor for cardiovascular disease: A 26‐year follow‐up of participants in the Framingham Heart Study. Circulation, 67(5), 968–977. 10.1161/01.CIR.67.5.968 [DOI] [PubMed] [Google Scholar]
- Kahan, T. , & Bergfeldt, L. (2005). Left ventricular hypertrophy in hypertension: Its arrhythmogenic potential. Heart, 91(2), 250–256. 10.1136/hrt.2004.042473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumoto, F. M. , Schoenfeld, M. H. , Barrett, C. , Edgerton, J. R. , Ellenbogen, K. A. , Gold, M. R. , Goldschlager, N. F. , Hamilton, R. M. , Joglar, J. A. , Kim, R. J. , Lee, R. , Marine, J. E. , McLeod, C. J. , Oken, K. R. , Patton, K. K. , Pellegrini, C. N. , Selzman, K. A. , Thompson, A. , & Varosy, P. D. (2019). 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation, 140(8), e382–e482. 10.1161/CIR.0000000000000628 [DOI] [PubMed] [Google Scholar]
- Lin, Y.‐S. , Guo, G. B.‐F. , Chen, Y.‐L. , Tsai, T.‐H. , Pan, K.‐L. , Liu, W.‐H. , & Chen, M.‐C. (2011). Atrial enlargement in symptomatic heart block patients with preserved left ventricular function: Possibly related to atrioventricular dyssynchrony. International Journal of Cardiology, 148(3), 280–284. 10.1016/j.ijcard.2009.11.005 [DOI] [PubMed] [Google Scholar]
- Maeno, K. I. , Kasai, T. , Kasagi, S. , Kawana, F. , Ishiwata, S. , Ohno, M. , Yamaguchi, T. , & Narui, K. (2013). Relationship between atrial conduction delay and obstructive sleep apnea. Heart and Vessels, 28(5), 639–645. 10.1007/s00380-012-0288-8 [DOI] [PubMed] [Google Scholar]
- Mahajan, R. , Lau, D. H. , Brooks, A. G. , Shipp, N. J. , Manavis, J. , Wood, J. P. M. , Finnie, J. W. , Samuel, C. S. , Royce, S. G. , Twomey, D. J. , Thanigaimani, S. , Kalman, J. M. , & Sanders, P. (2015). Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. Journal of the American College of Cardiology, 66(1), 1–11. 10.1016/j.jacc.2015.04.058 [DOI] [PubMed] [Google Scholar]
- Malpas, S. C. (2010). Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiological Reviews, 90, 513–557. 10.1152/physrev.00007.2009 [DOI] [PubMed] [Google Scholar]
- McCauley, M. D. , Hong, L. , Sridhar, A. , Menon, A. , Perike, S. , Zhang, M. , da Silva, I. B. , Yan, J. , Bonini, M. G. , Ai, X. , Rehman, J. , & Darbar, D. (2020). Ion channel and structural remodeling in obesity‐mediated atrial fibrillation. Circulation: Arrhythmia and Electrophysiology, 13(8), e008296. 10.1161/CIRCEP.120.008296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda, A. , Okada, T. , Yasuma, F. , Nakashima, N. , & Yokota, M. (1995). Cardiac hypertrophy in obstructive sleep apnea syndrome. Chest, 107(6), 1538–1544. 10.1378/chest.107.6.1538 [DOI] [PubMed] [Google Scholar]
- Okin, P. M. , Devereux, R. B. , Howard, B. V. , Fabsitz, R. R. , Lee, E. T. , & Welty, T. K. (2000). Assessment of QT interval and QT dispersion for prediction of all‐cause and cardiovascular mortality in American Indians The Strong Heart Study. Circulation, 101, 61–66. [DOI] [PubMed] [Google Scholar]
- Papaioannou, A. , Michaloudis, D. , Fraidakis, O. , Petrou, A. , Chaniotaki, F. , Kanoupakis, E. , Stamatiou, G. , Melissas, J. , & Askitopoulou, H. (2004). Effects of weight loss on QT interval in morbidly obese patients. Obesity Surgery, 13, 869–873. 10.1381/096089203322618687 [DOI] [PubMed] [Google Scholar]
- Rautaharju, P. M. , Surawicz, B. , & Gettes, L. S. (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram. Part IV: The ST segment, T and U waves, and the QT interval a scientific Statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Journal of the American College of Cardiology, 53, 982–991. 10.1016/j.jacc.2008.12.014 [DOI] [PubMed] [Google Scholar]
- Sajkov, D. , & McEvoy, R. D. (2009). Obstructive sleep apnea and pulmonary hypertension. Progress in Cardiovascular Diseases, 51(5), 363–370. 10.1016/j.pcad.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Schwartz, A. R. , Patil, S. P. , Laffan, A. M. , Polotsky, V. , Schneider, H. , & Smith, P. L. (2008). Obesity and obstructive sleep apnea: Pathogenic mechanisms and therapeutic approaches. Proceedings of the American Thoracic Society, 5, 185–192. 10.1513/pats.200708-137MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, R. V. , Anderson, A. , Ding, J. , Budoff, M. , Rider, O. , Petersen, S. E. , Jensen, M. K. , Koch, M. , Allison, M. , Kawel‐Boehm, N. , Wisocky, J. , Jerosch‐Herold, M. , Mukamal, K. , Lima, J. A. C. , & Murthy, V. L. (2017). Pericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: The multi‐ethnic study of atherosclerosis. JACC: Cardiovascular Imaging, 10(9), 1016–1027. 10.1016/j.jcmg.2016.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, R. , Ning, Y. , Ma, Y. , Liu, S. , Wu, J. , Fan, X. , Lv, J. , Wang, B. , Li, S. , & Li, L. (2021). Prevalence and risk factors of atrioventricular block among 15 million Chinese health examination participants in 2018: A nation‐wide cross‐sectional study. BMC Cardiovascular Disorders, 21(1), 289. 10.1186/s12872-021-02105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, H. , & Jiang, X. (2020). Correlation between QTc prolongation and obstructive sleep apnea in patients with type 2 diabetes mellitus. Medical Science Monitor, 26, e926954. 10.12659/MSM.926954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanmäki, S. , Lipponen, J. A. , Korkalainen, H. , Kulkas, A. , Leppänen, T. , Nikkonen, S. , Töyräs, J. , Duce, B. , Suni, A. , & Kainulainen, S. (2022). QTc prolongation is associated with severe desaturations in stroke patients with sleep apnea. BMC Pulmonary Medicine, 22(1), 204. 10.1186/s12890-022-01996-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani, S. , Raji, S. , Aghaee, A. , Pirzadeh, P. , Ebrahimi Miandehi, E. , Shafiei, S. , Akbari, M. , & Eslami, S. (2022). Body mass index, lipid profile, and hypertension contribute to prolonged QRS complex. Clinical Nutrition ESPEN, 50, 231–237. 10.1016/j.clnesp.2022.05.011 [DOI] [PubMed] [Google Scholar]
- Tarvainen, M. P. , Niskanen, J. P. , Lipponen, J. A. , Ranta‐aho, P. O. , & Karjalainen, P. A. (2014). Kubios HRV—Heart rate variability analysis software. Computer Methods and Programs in Biomedicine, 113(1), 210–220. 10.1016/j.cmpb.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Van Noord, C. , Eijgelsheim, M. , & Stricker, B. H. C. (2010). Drug‐ and non‐drug‐associated QT interval prolongation. British Journal of Clinical Pharmacology, 70, 16–23. 10.1111/j.1365-2125.2010.03660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheule, S. , & Schotten, U. (2021). Electrophysiological consequences of cardiac fibrosis. Cells, 10, 3220. 10.3390/cells10113220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T. J. , Parise, H. , Levy, D. , D'Agostino, R. B. , Wolf, P. A. , Vasan, R. S. , & Benjamin, E. J. (2004). Obesity and the risk of new‐onset atrial fibrillation. JAMA, 292(20), 2471–2477. 10.1001/jama.292.20.2471 [DOI] [PubMed] [Google Scholar]
- WHO Report . (2000). Obesity: Preventing and managing the global epidemic: Report of a WHO consultation . In WHO technical report 894. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to privacy policy but are available from the corresponding author upon reasonable request.


