Abstract
In the Modern era, immune checkpoint inhibitors (ICIs) have been the cornerstone of success in the treatment of several malignancies. Despite remarkable therapeutic advances, complex matrix together with significant molecular and immunological differences have led to conflicting outcomes of ICI therapy in gastrointestinal (GI) cancers. As far we are aware, to date, there has been no study to confirm the robustness of existing data, and this study is the first umbrella review to provide a more comprehensive picture about ICIs’ efficacy and safety in GI malignancies. Systematic search on PubMed, Scopus, Web of Science, EMBASE, and Cochrane library identified 14 meta-analyses. The pooled analysis revealed that ICIs application, especially programmed death-1 (PD-1) inhibitors such as Camrelizumab and Sintilimab, could partially improve response rates in patients with GI cancers compared to conventional therapies. However, different GI cancer types did not experience the same efficacy; it seems that hepatocellular carcinoma (HCC) and esophageal cancer (EC) patients are likely better candidates for ICI therapy than GC and CRC patients. Furthermore, application of ICIs in a combined-modal strategy are perceived opportunity in GI cancers. We also assessed the correlation of PD-L1 expression as well as microsatellite status with the extent of the response to ICIs; overall, high expression of PD-L1 in GI cancers is associated with better response to ICIs, however, additional studies are required to precisely elaborate ICI responses with respect to microsatellite status in different GI tumors. Despite encouraging ICI efficacy in some GI cancers, a greater number of serious and fatal adverse events have been observed; further highlighting the fact that ICI therapy in GI cancers is not without cost, and further studies are required to utmost optimization of this approach in GI cancers.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12935-023-03183-3.
Keywords: Immune checkpoint inhibitor, Gastrointestinal malignancies, Microsatellite instability, Programmed death-1, PD-1, PD-L1
Introduction
Cancers of the gastrointestinal (GI) tract are the major leading causes of cancer-related death. Colorectal, pancreatic, liver, and esophageal cancers were the third, fourth, fifth, and seventh cause of cancer-related death in the USA in 2018, respectively [1]. Medical treatment for GI cancer can vary significantly depending on the type of cancer as well as the patient’s characteristics, such as age [2]. In some cases, neoadjuvant therapy is used to shrink the cancer before an operation. This approach has proven to be highly effective, making surgery an option for patients with otherwise inoperable tumors. Additionally, it can make the operation safer and more effective, increasing the patient's chances of a successful outcome [3, 4]. However, most patients are diagnosed in the advanced stages, so the opportunity for an extreme cure is lost [5]. Due to their prevalence as well as the limited number of treatment options, there is an urgent need to identify innovative evidence-based treatments for these insidious cancers.
The use of immunotherapy, particularly checkpoint-directed treatment, has revolutionized the treatment of oncological diseases [6]. Despite being antigenic and evoking immune responses, cancer can escape destruction through a variety of mechanisms including upregulation of immune checkpoints such as programmed death-1 (PD-1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) [7]. Since Ipilimumab as the first immune checkpoint inhibitor (ICI) was approved by the Food and Drug Administration (FDA) for the treatment of melanoma patients in 2011 [8], ICIs have been investigated in a variety of cancers to the extent that now many anti-PD-1, anti-programmed-death ligand 1 (PD-L1), and anti-CTLA-4 medications like Nivolumab, Pembrolizumab, and Atezolizumab achieved FDA approval for non-small-cell lung cancer (NSCLC) [9–11], renal cell carcinoma [12, 13], head and neck squamous cell carcinoma [14, 15], and urothelial cancer [16].
In the field of GI cancers, many clinical trials assessing the safety and efficacy of different regimens such as ICI monotherapy, the combination of anti-PD-1/PD-L1 and anti-CTLA-4, or the combination of ICI with other treatment options like conventional chemotherapy, mitogen-activated protein kinase (MEK) inhibitors, and tyrosine kinase inhibitor (TKI) have been conducted; however, in many cases, there are conflicting results. While several randomized controlled trials (RCTs) indicated that ICIs could significantly improve survival of GI cancer patients [17–21], other studies failed to report a differential outcome [22–26].
As far we are aware, to date, there has been no study to confirm the robustness of existing data, and this study is the first umbrella review to provide a more comprehensive picture to address the questions of how effective are ICIs in various GI cancers? Which GI cancer is most likely to benefit from ICI therapy? Which type of ICI has the best performance? Can predictive biomarkers like PD-L1 expression and microsatellite status help to select patients who benefit the most from ICI therapy? In terms of adverse events (AEs), how does ICIs work compare with conventional therapy? And on which types of GI cancer or drug should future studies be focused?
Material and methods
Present umbrella review was conducted in accordance with the same approach as in the guidance outlined in the Cochrane Handbook for Systematic Reviews of Interventions on overviews of systematic reviews [27].
Systematic search
Two independent review authors searched PubMed, Scopus, Web of Science, EMBASE, and Cochrane library databases from inception to September 1st, 2022 to find published systematic reviews of RCTs evaluating the efficacy and safety of ICIs in patients presented with GI cancers. The following terms were used for the systematic search: (“gastrointestinal tumors” OR “esophageal cancer” OR “gastric cancer” OR “colorectal cancer” OR “pancreatic cancer” OR “hepatocellular carcinoma” OR “biliary tract cancer”) AND (“immune checkpoint inhibitor” OR “anti-CTLA-4” OR “anti-PD-1” OR “anti-PD-L1”) AND (“systematic review” OR “meta-analysis”). The complete search strategy is represented in Additional file 1: Table S1. As a hot topic, the literatures on immunotherapeutic area are updating rapidly and the RCTs are publishing frequently. Therefore, we conducted a manual search on Google Scholar search engine to cover the time gap between the latest database screening of the systematic reviews to the date we conducted database screening.
Outcomes
Our primary outcome was efficacy of ICIs for patients with GI malignancies. The efficacy was reported as overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). In addition, the safety analysis, as secondary outcome, was comprised of the following variables; treatment-related adverse events (TRAEs), ≥ grade 3 TRAEs, grade 5 AEs, serious AEs, AEs led to treatment discontinuation, and AEs led to death. The definition of efficacy and safety outcomes are summarized in Additional file 1: Table S2.
Study selection
Eligible meta-analyses were found through the following criteria: (1) included RCTs that were performed on adult patients with GI cancer aged 18 years or older; (2) investigated the effect of ICI therapy as compared with a control group not being any of ICIs; (3) considered either efficacy or safety outcomes; and (4) reported the effect sizes as hazard ratio (HR), risk ratio (RR), or odds ratio (OR) together with their 95% confidence intervals (CIs). We excluded any type of study other than meta-analysis, studies with no effect size (e.g. scoping reviews, narrative reviews, and systematic reviews without meta-analysis), and meta-analyses targeted patients with mixed type of cancers. Whenever multiple meta-analyses were found for an outcome, the one which was the most up-to-dated and had the highest number of primary RCTs was selected. Also, the reference list of all screened meta-analyses with the same outcome was searched to identify any potential RCTs that were not included in the selected meta-analyses. Also, further RCTs that were published in the time gap were retrieved manually by searching the Google scholar. We added these RCTs to the results of the selected meta-analyses in our umbrella review. In summary, we chose one meta-analysis for each outcome in a group of patients with the same malignancy, screened the reference list of all related meta-analyses as well as the Google scholar search engine for finding potential RCTs not included in the selected meta-analyses and included them in our study, and then performed our own meta-analysis.
Data extraction
The following data were extracted by two authors independently from eligible meta-analyses: first author’s name, year of publication, title of systematic review, number of RCTs included in the analysis, site of tumor, efficacy and safety outcomes (i.e. OS, PFS, ORR, DCR, CR, PR, SD, PD, or AEs), type of effect sizes (i.e. HR, RR, or OR), and the variables used for subgroup analysis. We also extracted the following information from each primary RCT included in the meta-analyses and those RCTs that were found manually: first author’s name, year of publication, title of RCTs, ID of RCTs, NCT identifier, number of participants, name of medications and their target, and effect sizes for efficacy and safety outcomes.
Assessment of methodological quality
Quality assessment was carried out independently by two reviewers and disagreement was resolved by consensus. The methodological quality of each meta-analysis was evaluated by an instrument for the assessment of multiple systematic reviews (AMSTAR) 2 tool. This checklist scores from 0 to 16 according to the information provided by individual studies. The final quality of each systematic review was classified as “high”, “moderate”, “low”, and “critically low”.
Data synthesis and analysis
After selecting eligible meta-analyses, the results of RCTs that had been missed in the biggest meta-analyses were also added. For the sake of accuracy, we reviewed the full-texts of all primary RCTs to ensure that the reported information was correct and that all outcomes of interest were included in our review. Then we conducted our own meta-analysis. In this case, we re-calculated the HRs for OS and PFS outcomes and RRs for ORR, DCR, CR, PR, SD, PD, and AEs outcomes together with their 95% confidence intervals (CIs). For each meta-analysis, we examined between-study heterogeneity by calculating I2 statistic using the Cochrane’s Q test [28]. Whenever an evidence of obvious heterogeneity was detected (I2 ≥ 50%), we applied a random-effect model; otherwise a fixed-effect model was used [28]. For the overall analysis, we performed subgroup analyses according to the type of drug, the molecular target, and PD-L1 expression level according to combined positive score (CPS) or tumor proportion score (TPS). Several other subgroup analysis was performed for each cancer type. All analyses were performed using Stata software, version 17.0 (StataCorp), with statistical significance defined as p < 0.05.
Results
A total of 2344 publication were found through initial database searching. We reviewed the title and abstracts of all records and finally 32 articles were fully assessed for eligibility. Of these, 18 publications were excluded due to the following reasons: 12 records provided duplicated outcomes [29–40], three records did not conduct meta-analysis [41–43], and three records did not include eligible RCTs [42, 44, 45]. Eventually, 14 systematic reviews [46–59] containing 27 primary RCTs met the eligibility criteria and included to the present umbrella review. Furthermore, after screening the reference list of all meta-analyses and hand-searching of Google scholar engine, 15 additional primary RCTs were identified that were not included in the selected meta-analyses, resulting in a total of 42 primary RCTs to be included in the final analysis. The flow diagram of study selection is depicted in Fig. 1.
Fig. 1.
Literature search and study selection process
Characteristics of the studies included in the umbrella review
The systematic search identified one eligible meta-analysis for hepatocellular carcinoma (HCC) [57], three meta-analyses for gastric cancer (GC) [47, 54, 56], three for esophageal cancer (EC) [48, 50, 59], five for both GC and EC [46, 49, 51–53], and two for colorectal cancers (CRC) [55, 58]. No meta-analysis was conducted on patients with pancreatic or biliary tract cancers. All included meta-analyses were published in 2021 and 2022. Each included meta-analysis reported unique outcomes for either main or subgroup analyses. All meta-analyses reported the efficacy outcomes, while the safety outcomes were only reported in nine meta-analyses [46, 48, 51–53, 55, 57–59]. The pooled OS and PFS outcomes were calculated in all meta-analyses, except for the meta-analyses performed by Formica et al. [47] that only calculated OS outcome. The detail characteristics of included meta-analyses are provided in Additional file 1: Table S3.
Ten primary RCTs targeted patients with HCC, 12 RCTs performed on patients with GC, 10 RCTs on patients with EC, seven RCTs on patients with CRC, two RCTs on patients with pancreatic cancer (PaC), and one RCT on patients with biliary tract cancer (BTC). A total of 23808 patients were included throughout primary RCTs. Pembrolizumab was administrated in 11 study arms, Nivolumab in seven arms, Atezolizumab in five arms, Sintilimab in four arms, Camrelizumab in three arms, Avelumab in three arms, Durvalumab in three arms, Durvalumab plus Tremelimumab in three arms, Nivolumab plus Ipilimumab in two arms, Tislelizumab in two arms, Ipilimumab and Toripalimab in one arm each. Furthermore, 28 trial arms used a medication that target PD-1, 11 arms targeted PD-L1, three arms targeted PD-L1 plus CTLA-4, two arms targeted PD-1 plus CTLA-4, and one arm targeted CTLA-4. The control group drugs were predefined routine regimens or best supportive care in all RCTs.
Methodological quality
The detailed responses to each AMSTAR item for every meta-analysis were presented in Additional file 1: Table S4. All meta-analyses had a total score of ≥ 6 with a mean score of 7.8 points. The methodological quality of all 14 included meta-analyses was critically low. The major justifications for low quality scores were due to the fact that meta-analyses did not report the funding source of included RCTs, did not discuss the impact of methodological quality of primary studies on the overall results, and did not provide a list of excluded studies at full-text reviewing step.
The efficacy of ICIs in GI cancers
Hepatocellular carcinoma (HCC)
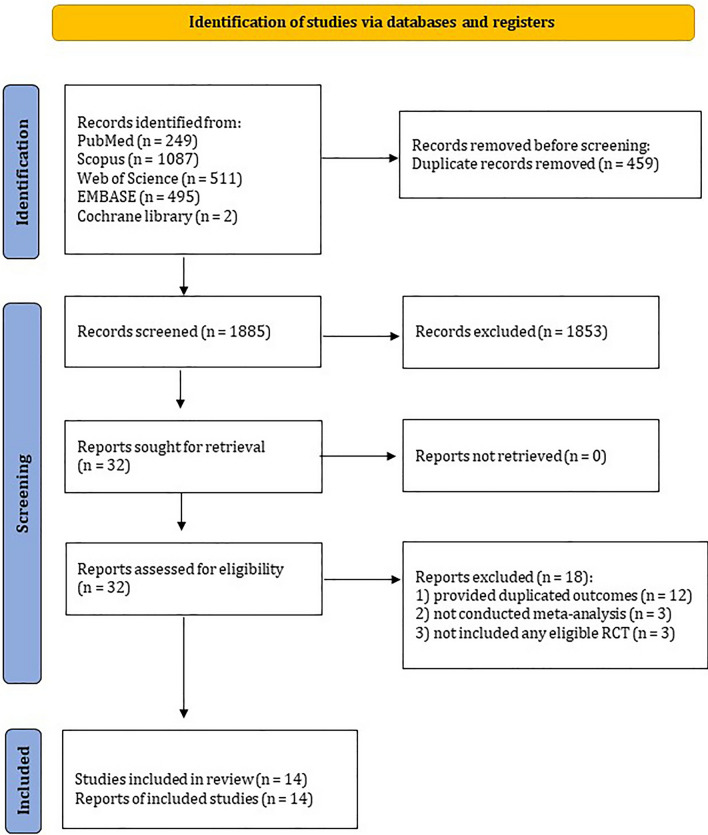
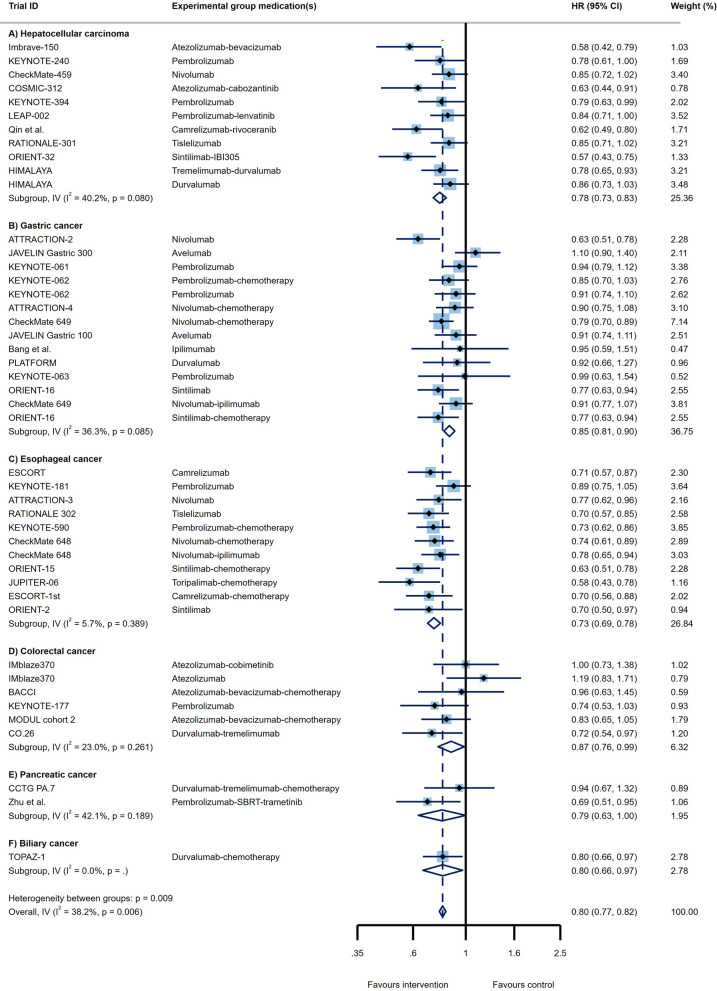
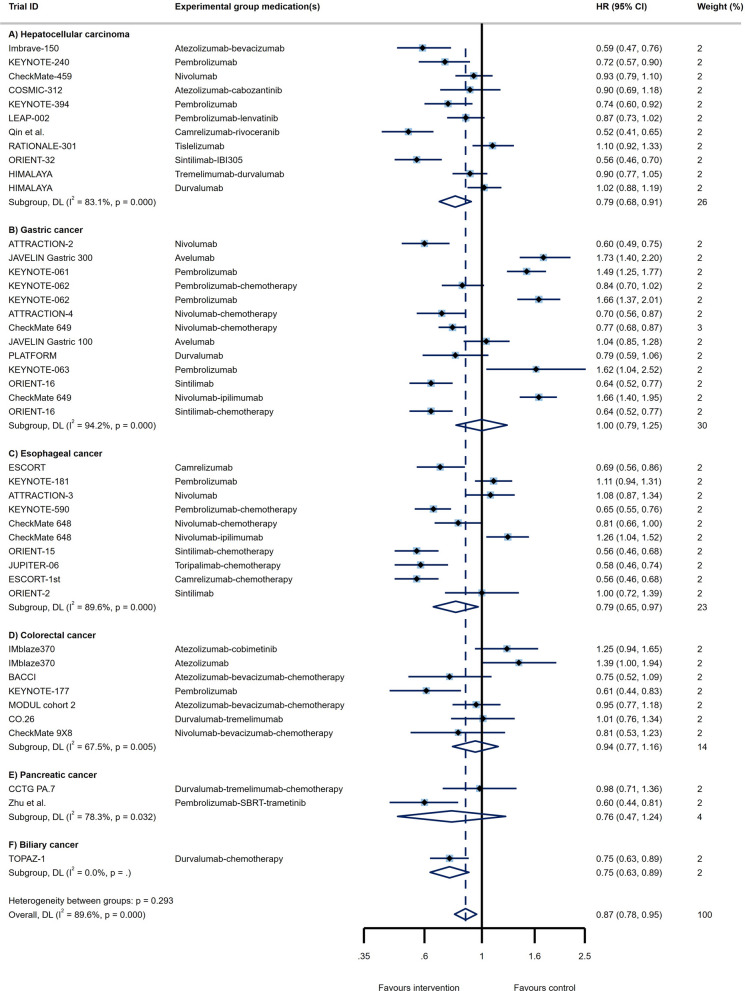
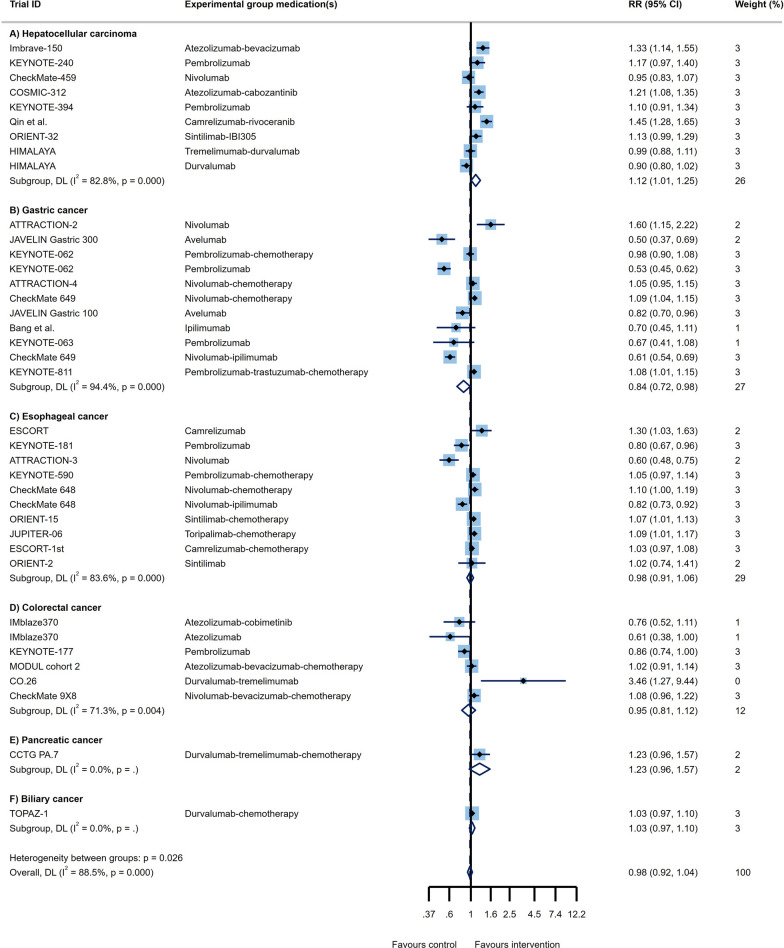
Through analysis of 11 RCT arms, the OS of HCC patients showed a beneficial effect of ICI therapy over conventional therapies (HR = 0.78, 95% CI 0.73 to 0.83; Fig. 2A) with moderate heterogeneity (I2 = 40.2%). The PFS of these patients also demonstrated improved outcomes (HR = 0.79, 95% CI 0.68 to 0.91, I2 = 83.1%; Fig. 3A). Impressive result of ORR (RR = 3.01, 95% CI 2.26 to 4.02, I2 = 69%; Fig. 4A) together with good DCR (RR = 1.12, 95% CI: 1.01 to 1.25; Fig. 5A) may provide sufficient data to suggest ICI therapy as an effective therapeutic strategy in HCC. However, it is worth noting that despite the promising results in ORR, the heterogeneity between studies was high (I2 = 82.8%); this issue can be justified by the differences in the types of medication and ICI targets. Differences in patient characteristics may have also accounted for the high between study heterogeneity.
Fig. 2.
Forest plots of OS analysis in different types of GI cancers
Fig. 3.
Forest plots of PFS analysis in different types of GI cancers
Fig. 4.
Forest plots of ORR analysis in different types of GI cancers
Fig. 5.
Forest plots of DCR analysis in different types of GI cancers
Given this, we performed a subgroup analysis to investigate whether clinical variables are in charge of different outcomes in HCC cases. According to Table 1A, ICIs were able to improve the OS of all groups of patients except for those who were female, had Barcelona liver stage B, and had an etiology of hepatitis C. Tumoral macrovascular invasion (MVI) of hepatic and/or portal vein branches is a common phenomenon in HCC and is associated with poorer prognosis [60]. Several studies reported that immunotherapy with PD-1 inhibitors may be a feasible treatment option for MVI [61, 62]. In line with this finding, our OS subgroup analysis revealed that MVI and/or extrahepatic spread at study entry were associated with significant favorable survival outcomes compared to those who had not this feature (HR = 0.72 vs. HR = 0.91; p interaction = 0.017). According to the subgroup analysis of PFS outcome, all groups of patients took advantage of ICI therapy except for those aged < 65 years. Both groups of patients with low (< 400 ng/ml) and high (≥ 400 ng/ml) levels of alpha-fetoprotein (AFP) have improved PFS in response to ICIs; however, lower level of AFP was associated with significant better outcome (HR = 0.46 vs. HR = 0.64; p interaction = 0.021). Notably, our results indicated patients with HCC associated with viral hepatitis B, C, or a non-viral etiology had significant different OS and PFS in subgroup analysis (p interaction = 0.001 and p interaction = 0.028, respectively).
Table 1.
The results of subgroups analysis based on clinical variables in different GI cancers
| OS | PFS | OS | PFS | OS | PFS | |||
|---|---|---|---|---|---|---|---|---|
| NO | HR (95%CI) | NO | HR (95%CI) | NO | HR (95%CI) | |||
| A) Hepatocellular carcinoma | ||||||||
| Age | 0.783 | 0.145 | ||||||
| ≥ 65 yr | 4 | 0.80 (0.71, 0.91) | 0.0% (0.454) | 2 | 0.65 (0.52, 0.82) | 0.0% (0.795) | ||
| < 65 yr | 4 | 0.82 (0.72, 0.94) | 0.0% (0.892) | 1 | 0.89 (0.63, 1.26) | NA | ||
| Sex | 0.647 | 0.629 | ||||||
| Male | 4 | 0.77 (0.69, 0.86) | 0.0% (0.457) | 2 | 0.66 (0.55, 0.80) | 30.3% (0.231) | ||
| Female | 4 | 0.82 (0.64, 1.07) | 0.0% (0.442) | 2 | 0.60 (0.40, 0.89) | 0.0% (0.968) | ||
| ECOG performance status | 0.689 | 0.614 | ||||||
| 0 | 5 | 0.77 (0.68, 0.88) | 0.0% (0.474) | 3 | 0.60 (0.50, 0.72) | 0.0%, (0.592) | ||
| 1 | 5 | 0.74 (0.65, 0.86) | 44.6% (0.124) | 3 | 0.64 (0.53, 0.78) | 24.3%, (0.267) | ||
| Location | 0.078 | 0.171 | ||||||
| Asia | 7 | 0.75 (0.67, 0.84) | 0.0% (0.534) | 4 | 0.63 (0.54, 0.75) | 44.9% (0.142) | ||
| Rest of the world | 6 | 0.86 (0.78, 0.96) | 0.0% (0.557) | 3 | 0.74 (0.63, 0.87) | 0.0% (0.872) | ||
| AFP at baseline | 0.368 | 0.021 | ||||||
| ≥ 400 ng/mL | 5 | 0.66 (0.57, 0.77) | 0.0% (0.942) | 2 | 0.64 (0.53, 0.78) | 35.5% (0.213) | ||
| < 400 ng/mL | 5 | 0.75 (0.60, 0.93) | 59.8% (0.041) | 2 | 0.46 (0.37, 0.56) | 0.0%, (0.541) | ||
| Barcelona liver stage | 0.332 | 0.843 | ||||||
| B | 6 | 0.89 (0.63, 1.24) | 50.7% (0.071) | 3 | 0.63 (0.45, 0.89) | 48.0% (0.146) | ||
| C | 6 | 0.74 (0.66, 0.84) | 34.5%, (0.177) | 3 | 0.61 (0.53, 0.70) | 0.0% (0.682) | ||
| Macrovascular invasion at study entry | 0.365 | 0.598 | ||||||
| Yes | 5 | 0.71 (0.60, 0.84) | 0.0%, (0.425) | 3 | 0.58 (0.46, 0.74) | 0.0% (0.546) | ||
| No | 5 | 0.78 (0.70, 0.87) | 29.0% (0.228) | 3 | 0.63 (0.54, 0.73) | 6.4% (0.344) | ||
| Extrahepatic spread at study entry | 0.06 | 0.213 | ||||||
| Yes | 5 | 0.70 (0.62, 0.78) | 49.7% (0.093) | 3 | 0.58 (0.50, 0.67) | 0.0% (0.440) | ||
| No | 5 | 0.84 (0.72, 0.98) | 0.0%, (0.811) | 3 | 0.70 (0.54, 0.89) | 0.0% (0.368) | ||
| MVI and/or extrahepatic spread at study entry | 0.017 | 0.103 | ||||||
| Yes | 6 | 0.72 (0.66, 0.80) | 15.8%, (0.312) | 3 | 0.55 (0.47, 0.65) | 0.0% (0.932) | ||
| No | 6 | 0.91 (0.78, 1.08) | 46.4%, (0.097) | 3 | 0.73 (0.54, 0.98) | 15.7% (0.305) | ||
| Etiology | 0.001 | 0.028 | ||||||
| Hepatitis B | 7 | 0.65 (0.57, 0.74) | 0.0%, (0.514) | 4 | 0.54 (0.46, 0.64) | 0.0%, (0.508) | ||
| Hepatitis C | 6 | 0.92 (0.77, 1.09) | 42.1%, (0.125) | 3 | 0.60 (0.43, 0.84) | 0.0%, (0.633) | ||
| Nonviral | 6 | 0.87 (0.77, 0.98) | 0.0%, (0.502) | 3 | 0.78 (0.63, 0.96) | 0.0%, (0.658) | ||
| B) Gastric cancer | ||||||||
| Age | 0.844 | 0.987 | ||||||
| ≥ 65 yr | 9 | 0.84 (0.77, 0.93) | 39.3%, (0.106) | 2 | 1.06 (0.64, 1.74) | 75.3%, (0.044) | ||
| < 65 yr | 9 | 0.85 (0.79, 0.93) | 13.0%, (0.326) | 2 | 1.07 (0.32, 3.52) | 96.5%, (0.000) | ||
| Sex | 0.046 | 0.688 | ||||||
| Male | 9 | 0.82 (0.76, 0.88) | 33.9%, (0.147) | 2 | 1.03 (0.48, 2.18) | 93.6%, (0.000) | ||
| Female | 9 | 0.94 (0.84, 1.06) | 0.0%, (0.517) | 2 | 1.40 (0.38, 5.18) | 94.3%, (0.000) | ||
| ECOG performance status | 621 | 0.700 | ||||||
| 0 | 8 | 0.89 (0.77, 1.03) | 43.0%, (0.092) | 2 | 1.29 (0.46, 3.67) | 93.4%, (0.000) | ||
| 1 | 8 | 0.84 (0.74, 0.97) | 54.4%, (0.032) | 2 | 0.99 (0.41, 2.39) | 94.1%, (0.000) | ||
| Location | 0.884 | 0.372 | ||||||
| Asia | 8 | 0.85 (0.75, 0.97) | 32.1%, (0.171) | 2 | 1.91 (1.38, 2.64) | 12.3%, (0.286) | ||
| Rest of the world | 7 | 0.86 (0.80, 0.93) | 0.0%, (0.732) | 1 | 1.57 (1.19, 2.08) | NA | ||
| Lauren histological type | 0.449 | 0.09 | ||||||
| Diffuse type | 6 | 0.85 (0.76, 0.96) | 43.7%, (0.114) | 1 | 0.84 (0.62, 1.14) | NA | ||
| Intestinal type | 6 | 0.80 (0.71, 0.90) | 12.9%, (0.332) | 1 | 0.56 (0.39, 0.80) | NA | ||
| Primary sites | 0.986 | 0.666 | ||||||
| Gastric cancer | 9 | 0.87 (0.78, 0.97) | 51.1%, (0.037) | 2 | 1.16 (0.44, 3.09) | 95.9%, (0.000) | ||
| Gastroesophageal junction cancer | 9 | 0.87 (0.76, 0.99) | 0.0%, (0.641) | 2 | 0.89 (0.44, 1.81) | 65.8%, (0.087) | ||
| Liver metastasis | 0.344 | 0.204 | ||||||
| Yes | 3 | 0.72 (0.63, 0.84) | 0.0%, (0.899) | 1 | 0.59 (0.42, 0.83) | NA | ||
| No | 3 | 0.82 (0.66, 1.03) | 72.2%, (0.027) | 1 | 0.79 (0.59, 1.05) | NA | ||
| Lymph node metastasis | 0.405 | 0.559 | ||||||
| Yes | 1 | 0.94 (0.77, 1.15) | NA | 1 | 0.73 (0.58, 0.92) | NA | ||
| No | 1 | 0.77 (0.50, 1.18) | NA | 1 | 0.61 (0.35, 1.06) | NA | ||
| Peritoneal metastasis | 0.130 | 0.002 | ||||||
| Yes | 2 | 0.98 (0.61, 1.56) | 72.1%, (0.058) | 1 | 1.04 (0.76, 1.43) | NA | ||
| No | 2 | 0.66 (0.55, 0.79) | 0.0%, (0.687) | 1 | 0.51 (0.37, 0.70) | NA | ||
| Number of organs with metastases | 0.245 | 0.014 | ||||||
| < 2 | 4 | 0.72 (0.60, 0.85) | 0.0%, (0.672) | 1 | 0.42 (0.26, 0.69) | NA | ||
| ≥ 2 | 4 | 0.84 (0.68, 1.04) | 78.4%, (0.003) | 1 | 0.84 (0.66, 1.07) | NA | ||
| Microsatellite status | 0.000 | |||||||
| Stable | 5 | 0.87 (0.79, 0.95) | 42.1%, (0.178) | |||||
| Unstable (Instability-high) | 3 | 0.33 (0.20, 0.52) | 0.0%, (0.988) | |||||
| Prior gastrectomy | 0.924 | 0.956 | ||||||
| Yes | 4 | 0.89 (0.73, 1.08) | 0.0%, (0.913) | 1 | 0.71 (0.46, 1.10) | NA | ||
| No | 4 | 0.90 (0.80, 1.00) | 0.0%, (0.826) | 1 | 0.70 (0.54, 0.90) | NA | ||
| Disease status | 0.313 | |||||||
| Metastatic | 2 | 0.91 (0.77, 1.07) | 0.0%, (0.633) | |||||
| Locally advanced | 1 | 2.84 (0.31,25.74) | NA | |||||
| C) Esophageal cancer | ||||||||
| Age | 0.175 | 0.133 | ||||||
| ≥ 65 yr | 9 | 0.67 (0.60, 0.75) | 0.0% (0.701) | 6 | 0.58 (0.51, 0.66) | 37.9%, (0.154) | ||
| < 65 yr | 9 | 0.74 (0.68, 0.81) | 0.0%,(0.623) | 6 | 0.66 (0.60, 0.73) | 44.3%, (0.110) | ||
| Sex | 0.029 | 0.588 | ||||||
| Male | 10 | 0.72 (0.67, 0.77) | 26.6%, (0.199) | 6 | 0.63 (0.54, 0.75) | 67.2%, (0.009) | ||
| Female | 10 | 0.90 (0.75, 1.08) | 9.1%, (0.359) | 6 | 0.69 (0.54, 0.89) | 0.0%, (0.655) | ||
| ECOG performance status | 0.623 | 0.674 | ||||||
| 0 | 10 | 0.74 (0.66, 0.83) | 0.0%, (0.449) | 6 | 0.61 (0.50, 0.76) | 34.6%, (0.177) | ||
| 1 | 10 | 0.71 (0.66, 0.78) | 0.0%, (0.739) | 6 | 0.65 (0.56, 0.75) | 53.5%, (0.056) | ||
| Location | 0.145 | 0.889 | ||||||
| Asia | 4 | 0.71 (0.62, 0.80) | 18.8% (0.296) | 2 | 0.68 (0.51, 0.91) | 69.6%, (0.070) | ||
| Rest of the world | 4 | 0.84 (0.69, 1.02) | 54.6%, (0.086) | 1 | 0.70 (0.56, 0.88) | NA | ||
| Histology | 0.240 | 0.239 | ||||||
| Adenocarcinoma | 2 | 0.92 (0.61, 1.38) | 73.3%, (0.053) | 1 | 0.63 (0.46, 0.87) | NA | ||
| Squamous cell carcinoma | 11 | 0.72 (0.67, 0.77) | 0.0%, (0.860) | 10 | 0.79 (0.65, 0.95) | 84.9%, (0.000) | ||
| Liver metastasis | 0.358 | 0.451 | ||||||
| Yes | 4 | 0.64 (0.51, 0.80) | 0.0%, (0.792) | 3 | 0.75 (0.48, 1.15) | 67.0%, (0.048) | ||
| No | 4 | 0.72 (0.63, 0.83) | 0.0%, (0.738) | 3 | 0.62 (0.48, 0.79) | 61.8%, (0.073) | ||
| Lymph node metastasis | 0.319 | |||||||
| Yes | 2 | 0.80 (0.65, 0.98) | 0.0%, (0.540) | |||||
| No | 2 | 0.68 (0.55, 0.86) | 0.0%, (0.702) | |||||
| Number of organs with metastases | 0.169 | 0.138 | ||||||
| < 2 | 5 | 0.79 (0.69, 0.91) | 0.0%, (0.923) | 2 | 0.70 (0.56, 0.86) | 0.0%, (0.350) | ||
| ≥ 2 | 5 | 0.70 (0.62, 0.79) | 0.0%, (0.454) | 2 | 0.56 (0.46, 0.68) | 44.0%, (0.181) | ||
| Disease status | 0.063 | 0.408 | ||||||
| Metastatic | 6 | 0.66 (0.60, 0.74) | 0.0%, (0.802) | 4 | 0.58 (0.52, 0.65) | 0.0%, (0.409) | ||
| Locally advanced | 6 | 0.80 (0.68, 0.95) | 0.0%, (0.833) | 4 | 0.65 (0.50, 0.85) | 0.0%, (0.847) | ||
| Smoking history | 0.806 | 0.852 | ||||||
| Never | 5 | 0.77 (0.62, 0.95) | 0.0%, (0.565) | 2 | 0.79 (0.47, 1.32) | 58.8%, (0.119) | ||
| Current or former | 5 | 0.75 (0.67, 0.83) | 0.0%, (0.867) | 2 | 0.73 (0.39, 1.36) | 85.8%, (0.008) | ||
| D) Colorectal cancer | ||||||||
| Age | 0.570 | 0.804 | ||||||
| ≥ 65 yr | 3 | 0.89 (0.52, 1.54) | 69.1%, (0.039) | 3 | 1.07 (0.81, 1.40) | 0.0%, (0.539) | ||
| < 65 yr | 3 | 1.06 (0.85, 1.32) | 0.1%, (0.368) | 3 | 1.11 (0.91, 1.35) | 47.8%, (0.147) | ||
| Sex | 0.963 | 0.080 | ||||||
| Male | 2 | 0.73 (0.55, 0.95) | 0.0%, (0.383) | 1 | 0.77 (0.57, 1.05) | NA | ||
| Female | 2 | 0.72 (0.50, 1.03) | 39.0%, (0.200) | 1 | 1.21 (0.81, 1.80) | NA | ||
| ECOG performance status | 0.926 | 0.806 | ||||||
| 0 | 4 | 0.91 (0.55, 1.50) | 75.0%, (0.007) | 3 | 1.24 (0.66, 2.32) | 86.2%, (0.001) | ||
| 1 | 4 | 0.88 (0.71, 1.09) | 13.0%, (0.327) | 3 | 1.14 (0.90, 1.44) | 0.0%, (0.623) | ||
| Location | 0.753 | |||||||
| Asia | 1 | 0.65 (0.27, 1.56) | NA | |||||
| Rest of the world | 1 | 0.76 (0.52, 1.09) | NA | |||||
| BRAF status | 0.507 | |||||||
| Wild type | 3 | 0.61 (0.39, 0.95) | 0.0%, (0.720) | |||||
| Variant | 2 | 0.71 (0.52, 0.96) | 0.0%, (0.375) | |||||
| KRAS/NRAS status | 0.569 | |||||||
| Wild type | 2 | 0.61 (0.39, 0.95) | 0.0%, (0.720) | |||||
| Variant | 2 | 0.71 (0.52, 0.96) | 0.0%, (0.375) | |||||
| Liver metastasis | 0.044 | 0.751 | ||||||
| Yes | 1 | 1.14 (0.72, 1.81) | NA | 2 | 0.86 (0.69, 1.09) | 0.0%, (0.512) | ||
| No | 1 | 0.33 (0.11, 1.00) | NA | 2 | 0.80 (0.51, 1.24) | 0.0%, (0.523) | ||
| Site of primary tomur | 0.358 | 0.519 | ||||||
| Right | 3 | 0.78 (0.58, 1.06) | 0.0%, (0.715) | 3 | 1.00 (0.71, 1.39) | 0.0%, (0.947) | ||
| Left | 3 | 0.95 (0.71, 1.28) | 0.0%, (0.812) | 3 | 1.13 (0.92, 1.40) | 26.0%, (0.259) | ||
| Microsatellite status | 0.402 | 0.622 | ||||||
| Stable | 3 | 0.91 (0.64, 1.28) | 68.9%, (0.040) | 3 | 1.07 (0.71, 1.61) | 77.2%, (0.012) | ||
| Unstable (Instability-high) | 1 | 0.74 (0.53, 1.03) | NA | 2 | 0.84 (0.35, 2.01) | 60.5%, (0.111) | ||
| Number of organs with metastases | 0.668 | |||||||
| < 2 | 1 | 0.98 (0.68, 1.41) | NA | |||||
| ≥ 2 | 1 | 0.88 (0.63, 1.22) | NA | |||||
| E) Pancreatic cancer | ||||||||
| Age | 0.491 | 0.177 | ||||||
| ≥ 65 yr | 2 | 0.92 (0.67, 1.25) | 0.0%, (0.837) | 1 | 0.73 (0.48, 1.11) | NA | ||
| < 65 yr | 2 | 0.72 (0.39, 1.34) | 69.6%, (0.070) | 1 | 0.48 (0.31, 0.75) | NA | ||
| Sex | 0.486 | 0.234 | ||||||
| Male | 2 | 0.72 (0.52, 0.99) | 42.7%, (0.187) | 1 | 0.52 (0.35, 0.77) | NA | ||
| Female | 2 | 0.85 (0.60, 1.20) | 0.0%, (0.947) | 1 | 0.76 (0.47, 1.23) | NA | ||
| ECOG performance status | 0.444 | 0.056 | ||||||
| 0 | 2 | 0.72 (0.32, 1.65) | 75.4%, (0.044) | 1 | 0.47 (0.31, 0.71) | NA | ||
| 1 | 2 | 1.01 (0.81, 1.26) | 0.0%, (0.461) | 1 | 0.87 (0.54, 1.41) | NA | ||
| F) Biliary tract cancer | ||||||||
| Age | 0.949 | 0.211 | ||||||
| ≥ 65 yr | 1 | 0.79 (0.60, 1.04) | NA | 1 | 0.84 (0.66, 1.07) | NA | ||
| < 65 yr | 1 | 0.80 (0.61, 1.04) | NA | 1 | 0.68 (0.54, 0.85) | NA | ||
| Sex | 0.797 | 0.696 | ||||||
| Male | 1 | 0.78 (0.60, 1.01) | NA | 1 | 0.73 (0.58, 0.92) | NA | ||
| Female | 1 | 0.82 (0.62, 1.08) | NA | 1 | 0.78 (0.62, 0.99) | NA | ||
| ECOG performance status | 0.255 | 0.938 | ||||||
| 0 | 1 | 0.90 (0.68, 1.20) | NA | 1 | 0.77 (0.61, 0.98) | NA | ||
| 1 | 1 | 0.72 (0.56, 0.93) | NA | 1 | 0.76 (0.60, 0.96) | NA | ||
| Location | 0.290 | 0.127 | ||||||
| Asia | 1 | 0.72 (0.56, 0.93) | NA | 1 | 0.67 (0.54, 0.84) | NA | ||
| Rest of the world | 1 | 0.89 (0.66, 1.20) | NA | 1 | 0.87 (0.68, 1.12) | NA | ||
| Disease status | 0.108 | 0.012 | ||||||
| Metastatic | 1 | 0.49 (0.27, 0.90) | NA | 1 | 0.42 (0.26, 0.68) | NA | ||
| Locally advanced | 1 | 0.83 (0.68, 1.02) | NA | 1 | 0.81 (0.68, 0.97) | NA | ||
Gastric cancer (GC)
Fourteen study arms assessed the efficacy of different ICIs in treating GC patients. While the results showed improvement in OS (HR = 0.85, 95% CI: 0.81 to 0.90, I2 = 36.3%; Fig. 2B), overall PFS with HR = 1 suggests similar efficacy of the ICI and control treatments in these patients (95% CI 0.79 to 1.25, I2 = 94.2%; Fig. 3B). Besides, the response rates to ICIs did not reach the statistical significance as ORR had RR = 0.96 (95% CI 0.79 to 1.16, I2 = 87.9%; Fig. 4B). Furthermore, the DCR was found to be better in the control group relative to ICI therapy group (RR = 0.84, 95% CI 0.72 to 0.98, I2 = 94.4%; Fig. 5B). Of course, the high heterogeneity of studies should be considered in the final conclusion.
Regarding OS subgroup analysis, we found that ICIs prolonged survival of all groups of patients, except for female patients, those with ECOG performance status of 0, patients with no liver metastasis, both group of patients presented with or without lymph node metastasis, patients who had peritoneal metastasis, patients who had two or more organs with metastasis, patients who had prior gastrectomy, and patients who had metastatic or locally advanced esophageal tumors. Moreover, our results revealed that GC patients with high microsatellite instability (MSI) had a significant longer OS compared to stable microsatellite status when treated with ICIs (HR = 0.33 vs. HR = 0.87; p interaction = 0.000). Besides, the favorable PFS outcomes were seen only in GC patients with intestinal histological type, liver metastasis, lymph node metastasis, and no history of gastrectomy. Since peritoneal metastasis associated with GC and involvement of more than 2 organs have poor prognosis, it is not surprising that these patients experience worse PFS outcomes relative to their counterparts (HR = 1.04 vs. HR = 0.51; pinteraction = 0.002 and HR = 0.84 vs. HR = 0.42; pinteraction = 0.014, respectively). The results of subgroup analysis are summarized in Table 1B.
Esophageal cancer (EC)
Although the published evidence from several randomized controlled clinical trials of immunotherapy for esophageal squamous cell carcinoma has shown promising outcome [18, 63, 64], there are controversial results about all outcomes in advance and metastatic stages of these patients. The pooled results of survival analysis among 11 RCT arms showed acceptable OS (HR = 0.73, 95% CI 0.69 to 0.78; Fig. 2C) with a heterogeneity of I2 = 5.7% and promising PFS outcomes (HR = 0.79, 95% CI 0.65 to 0.97, I2 = 89.6%; Fig. 3C). Although the ORR in ICI groups was better than in the control group (RR = 1.45, 95% CI 1.26 to 1.68, I2 = 74.1%; Fig. 4C), the DCR were not satisfactory with an RR close to 1 (RR = 0.98, 95% CI 0.91 to 1.06, I2 = 83.6%; Fig. 5C).
After OS subgroup analysis based on clinical variables, all groups of patients showed prolonged OS in favor of ICI therapy except for female patients, those living outside Asia, and those presented with adenocarcinoma of esophagus. Indeed, improvement in OS was significantly better in males than females (HR = 0.72 vs. HR = 0.9; p interaction = 0.029; Table 1C), proposing that ICI therapy may be more effective in male rather than female with EC. In terms of PFS, all groups of patients showed prolonged PFS when received ICIs, except for those with liver metastasis and both groups patients who never smoke or reporting a history of smoking.
Colorectal cancer (CRC)
According to our results based on six trial arms, while patients with CRC had a favorable OS (HR = 0.87, 95% CI 0.76 to 0.99, I2 = 23.0%; Fig. 2D) and provided an acceptable ORR (RR = 1.28, 95% CI 1.08 to 1.53, I2 = 0.0%; Fig. 4D), the outcomes in terms of PFS as well as DCR did not show an obvious improvement. Thus, application of ICIs is unlikely helpful in patients with CRC. Moreover, the only groups of patients that were able to take advantage of ICI therapy were male patients, patients with wild type BRAF status, and both groups of patients with wild type or a variant of KRAS/NIRAS status. The results of subgroup analysis revealed that liver metastasis makes the OS outcome worse (HR = 1.14 vs. HR = 0.33; p interaction = 0.044; Table 1D). In terms of PFS, we found that ICI therapy had no beneficial impact in any of the patients’ subgroups.
Pancreatic cancer (PaC)
Two RCTs showed a beneficial response in OS (HR = 0.79, 95% CI 0.63 to 1.00, I2 = 42.1%; Fig. 2E) but not for PFS (HR = 0.76, 95% CI 0.47 to 1.24, I2 = 78.3%; Fig. 3E), ORR (RR = 1.32, 95% CI 0.77 to 2.25, I2 = NA; Fig. 4E), and DCR (RR = 1.23, 95% CI 0.96 to 1.57, I2 = NA; Fig. 5E). However, there isn't still enough evidence to make a definitive judgment about their efficacies in PaC. According to subgroup analysis, only make patients showed prolonged OS in response to ICIs. Furthermore, those who aged < 65 years, male patients, and patients with ECOG performance status of 0 had significantly improved PFS (Table 1E).
Biliary tract cancer (BTC)
One RCT was conducted to determine whether PD-L1 targeting using Durvalumab works well among BTC patients or not. In this regard, satisfactory survival outcomes (OS: HR = 0.80, 95% CI 0.66 to 0.97; Fig. 2F and PFS: HR = 0.75, 95% CI 0.63 to 0.89; Fig. 3F) along with acceptable clinical responses (ORR: RR = 1.43, 95% CI 1.08 to 1.90; Fig. 4F and DCR: RR = 1.03, 95% CI 0.97 to 1.10; Fig. 5F) were obtained. Also, in the subgroup analysis, ICIs did not change the OS of patients except for those with ECOG performance status 1, living in Asia, and presented with metastatic disease. Furthermore, PFS subgroup analysis revealed that ICIs prolong the survival rate of all patients except for those who aged ≥ 65 years and not living in Asia. In this case, patients with metastatic status experience better PFS rather than the locally advanced stage of the disease (HR = 0.42 vs. HR:0.81; p interaction = 0.012; Table 1F).
Pooled analysis in GI cancers
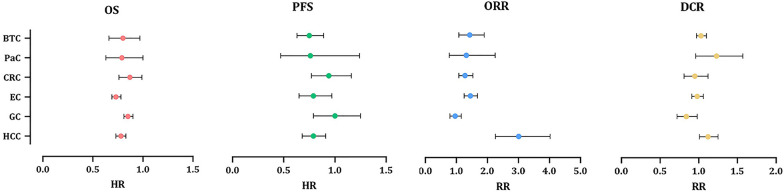
Although ICIs are used in different GI cancers, they are not equally effective in treating all of them; while some demonstrate superior responses over conventional therapies, others don’t. Through pooled analysis, we tried to answer the question of which GI cancer is most likely to benefit from ICI therapy and which is least likely to do so. Among all GI cancers, HCC and EC seem to have the best response to ICIs not only with high DCR and ORR but also with low PFS and OS. Following HCC and EC, BTC showed promising responses according to notable ORR, DCR, PFS, and OS. Noteworthy, due to the fact that only one RCT investigated the effects of ICIs in BTC, a definitive conclusion cannot be reached. Similarly, more RCTs are needed to come to a conclusive conclusion regarding the effects of ICIs in PaC, since OS was promising, but PFS, ORR, and DCR did not meet the expectation. Finally, neither GC nor CRC had satisfactory results. Furthermore, the pooled results of CR, PR, SD, and PD for all GI cancers are represented in Additional file 1: Fig S1-S4. All in all, overall OS (HR = 0.80, 95% CI 0.77 to 0.82, p = 0.006, I2 = 38.2%; Fig. 2), PFS (HR = 0.87, 95% CI 0.78 to 0.95, p = 0.000, I2 = 89.6%; Fig. 3), and ORR (RR = 1.45, 95% CI 1.29 to 1.63, p = 0.000, I2 = 84.7%; Fig. 4), demonstrate that ICI therapy outperforms conventional therapies in GI cancers; however, DCR were not satisfactory with an RR close to 1 (RR = 0.98, 95% CI 0.92 to 1.04, p = 0.000, I2 = 88.5%; Fig. 5). Figure 6 represents pooled results of OS, PFS, ORR, and DCR in six types of GI cancers.
Fig. 6.
Forest plots of pooled OS, PFS, ORR, and DCR analysis in different types of GI cancers
Subgroup analysis based on drug type
Since drug development paradigms for immunotherapy are evolving, comparing the effects of different drugs seems critical in order to make the best treatment decision. Notably, our results indicated patients who were treated with different drugs, had significantly different OS and PFS in subgroup analysis (p interaction = 0.001 and p interaction = 0.000, respectively). Among the eleven types of drugs examined and according to the results of one trial, Toripalimab had the best OS (HR = 0.58, 95% CI 0.43 to 0.78) and PFS (HR = 0.58, 95% CI 0.46 to 0.74); suggesting further investigation of this drug in clinical trials is necessary. In addition, a total of three trials conducted with Camrelizumab have reported this drug had notable OS (HR = 0.68, 95% CI 0.60 to 0.77, I2 = 0.0%) and PFS (HR = 0.59, 95% CI 0.50 to 0.69, I2 = 42.3%). Following Camrelizumab, Sintilimab also showed significant OS (HR = 0.70, 95% CI 0.63 to 0.77, I2 = 12.5%) and PFS (HR = 0.64, 95% CI 0.55 to 0.75, I2 = 60.7%). Avelumab, on the other hand, had the worst OS (HR = 0.99, 95% CI 0.85 to 1.15, I2 = 34.9%), indicating this agent has similar effects to conventional therapies. Moreover, combination of Nivolumab and Ipilimumab reduced the PFS of the patients compared to the control agents (HR = 1.47, 95% CI 1.30 to 1.67, I2 = 78.3%). The results of OS and PFS subgroup analysis based on different drug types are detailed in Table 2A.
Table 2.
Detailed on subgroups analysis based on drug types, target, and PD-L1 cutoff
| OS | PFS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | No. of trails | I2(P-value) | P interaction | HR | 95% CI | No. of trails | I2(P-value) | P interaction | |
| A) Drug | 0.002 | 0.000 | ||||||||
| Pembrolizumab | 0.83 | (0.78, 0.89) | 11 | 0.0%, (0.560) | 0.92 | (0.74, 1.14) | 11 | 91.5%, (0.000) | ||
| Nivolumab | 0.79 | (0.73, 0.84) | 6 | 33.3%, (0.186) | 0.92 | (0.74, 1.14) | 6 | 91.3%, (0.000) | ||
| Atezolizumab | 0.83 | (0.73, 0.94) | 6 | 60.8%, (0.026) | 0.93 | (0.72, 1.19) | 6 | 79.9%, (0.000) | ||
| Sintilimab | 0.70 | (0.63, 0.77) | 5 | 12.5%, (0.334) | 0.64 | (0.55, 0.75) | 5 | 60.7%, (0.038) | ||
| Durvalumab | 0.84 | (0.75, 0.95) | 3 | 0.0%, (0.737) | 0.88 | (0.79, 0.98) | 3 | 73.3%, (0.024) | ||
| Camrelizumab | 0.68 | (0.60, 0.77) | 3 | 0.0%, (0.681) | 0.59 | (0.50, 0.69) | 3 | 42.3%, (0.177) | ||
| Durvalumab + Tremelimumab | 0.79 | (0.69, 0.91) | 3 | 0.0%, (0.495) | 0.93 | (0.82, 1.06) | 3 | 0.0%, (0.743) | ||
| Tislelizumab | 0.78 | (0.68, 0.89) | 2 | 50.2%, (0.156) | 1.10 | (0.91, 1.32) | 1 | NA | ||
| Avelumab | 0.99 | (0.85, 1.15) | 2 | 34.9%, (0.215) | 1.34 | (0.81, 2.20) | 2 | 90.7%, (0.001) | ||
| Nivolumab + Ipilimumab | 0.85 | (0.75, 0.96) | 2 | 33.1%, (0.222) | 1.47 | (1.30, 1.67) | 2 | 78.3%, (0.032) | ||
| Ipilimumab | 0.95 | (0.59, 1.51) | 1 | NA | - | - | - | - | ||
| Toripalimab | 0.58 | (0.43, 0.78) | 1 | NA | 0.58 | (0.46, 0.74) | 1 | NA | ||
| B) Target | 0.058 | 0.000 | ||||||||
| PD-1 | 0.78 | (0.75, 0.81) | 28 | 33.3%, (0.046) | 0.82 | (0.72, 0.93) | 28 | 89.1%, (0.000) | ||
| PD-L1 | 0.87 | (0.81, 0.93) | 11 | 46.5%, (0.044) | 0.97 | (0.83, 1.14) | 11 | 84.1%, (0.000) | ||
| PD-L1 + CTLA-4 | 0.79 | (0.69, 0.91) | 3 | 0.0%, (0.495) | 0.93 | (0.82, 1.06) | 3 | 0.0%, (0.743) | ||
| PD-1 + CTLA-4 | 0.85 | (0.75, 0.96) | 2 | 33.1%, (0.222) | 1.47 | (1.30, 1.67) | 2 | 78.3%, (0.032) | ||
| CTLA-4 | 0.95 | (0.59, 1.51) | 1 | NA | - | - | - | - | ||
| C) PD-L1 cutoff | ||||||||||
| CPS = 1 | 0.000 | 0.475 | ||||||||
| CPS ≥ 1 | 0.76 | (0.72, 0.81) | 13 | 40.4%, (0.065) | 0.91 | (0.71, 1.16) | 10 | 91.1%, (0.000) | ||
| CPS < 1 | 1.03 | (0.88, 1.19) | 9 | 0.0%, (0.819) | 1.08 | (0.72, 1.61) | 6 | 75.5%, (0.001) | ||
| CPS = 5 | 0.002 | 0.758 | ||||||||
| CPS ≥ 5 | 0.70 | (0.65, 0.76) | 9 | 0.0%, (0.488) | 0.76 | (0.59, 0.97) | 7 | 87.2%, (0.000) | ||
| CPS < 5 | 0.87 | (0.78, 0.97) | 6 | 29.9%, (0.211) | 0.82 | (0.55, 1.21) | 3 | 79.1%, (0.008) | ||
| CPS = 10 | 0.014 | 0.701 | ||||||||
| CPS ≥ 10 | 0.69 | (0.63, 0.76) | 12 | 0.0%, (0.512) | 0.70 | (0.58, 0.84) | 8 | 60.8%, (0.013) | ||
| CPS < 10 | 0.82 | (0.74, 0.91) | 8 | 9.7%, (0.355) | 0.75 | (0.57, 0.97) | 5 | 72.1%, (0.006) | ||
| TPS = 1% | 0.000 | 0.341 | ||||||||
| TPS ≥ 1% | 0.66 | (0.60, 0.73) | 13 | 17.2%, (0.270) | 0.73 | (0.59, 0.91) | 9 | 63.6%, (0.005) | ||
| TPS < 1% | 0.86 | (0.80, 0.92) | 12 | 32.2%, (0.133) | 0.87 | (0.66, 1.13) | 8 | 86.9%, (0.000) | ||
| TPS = 5% | 0.037 | 0.068 | ||||||||
| TPS ≥ 5% | 0.64 | (0.55, 0.73) | 6 | 0.0%, (0.902) | 0.52 | (0.43, 0.63) | 3 | 0.0%, (0.844) | ||
| TPS < 5% | 0.77 | (0.69, 0.85) | 6 | 0.0%, (0.590) | 0.65 | (0.56, 0.75) | 3 | 43.6%, (0.170) | ||
| TPS = 10% | 0.036 | 0.121 | ||||||||
| TPS ≥ 10% | 0.63 | (0.54, 0.73) | 7 | 0.0%, (0.772) | 0.54 | (0.43, 0.66) | 4 | 0.0%, (0.657) | ||
| TPS < 10% | 0.76 | (0.69, 0.84) | 7 | 0.0%, (0.922) | 0.69 | (0.54, 0.88) | 4 | 68.6%, (0.023) | ||
Subgroup analysis based on target type
It has been reported that targeting PD-1, PD-L1 or CTLA-4 may provide different outcomes in gastric cancer [65] and hepatocellular carcinoma [66]; thus, hypothesizing whether the application of different ICIs was associated with different efficacies in patients with GI cancer. Subgroup analysis based on PD-1, PD-L1, and CTLA4 inhibitors showed non-significantly different OS but significant different PFS between these groups (p interaction = 0.058 and p interaction = 0.000, respectively). In this regard, PD-1 inhibitors outperformed PD-L1 inhibitors according to OS (HR = 0.78, 95% CI 0.75 to 0.81, I2 = 33.3% vs. HR = 0.87, 95% CI 0.81 to 0.94, I2 = 46.5%, respectively). In terms of PFS, only PD-1 inhibitors showed promising results (HR = 0.80, 95% CI 0.77 to 0.83, I2 = 89.1%); however, PD-L1 inhibitors did not change the PFS outcome (HR = 0.96, 95% CI 0.90 to 1.03, I2 = 84.1%). As there are limited clinical trials with CTLA4 inhibitors, a conclusion cannot be drawn so far. Table 2B represents the results of OS and PFS subgroup analysis based on target type.
Subgroup analysis based on PD-L1 expression
The response to ICIs varies from patient to patient, therefore, several predictive biomarkers are developed to determine sensitivity and resistance to immune checkpoint inhibitors. In this regard, PD-L1 expression on either tumor or immune cells is the most frequently studied biomarker [67].
Subgroup analysis based on the PD-L1 expression demonstrated that patients with CPS ≥ 1, ≥ 5, and ≥ 10 have better OS compared with < 1, < 5, and < 10 with pinteraction = 0.000, 0.002, and 0.014 respectively. The same goes for TPS, and TPS ≥ 1%, ≥ 5%, and ≥ 10% had longer OS than < 1%, < 5%, and < 10% with pinteraction = 0.000, 0.037, and 0.036 respectively. Moreover, those with CPS ≥ 10 and TPS ≥ 10% have the best OS compared to the control agents (HR = 0.69, 95% CI 0.63 to 0.76, I2 = 0.00% and HR = 0.63, 95% CI 0.54 to 0.73, I2 = 0.00% respectively). In terms of PFS, no difference was detected between the upper and lower limits of each threshold Table 2C shows the results of PD-L1 expression subgroup analysis.
The safety of ICIs in GI cancers
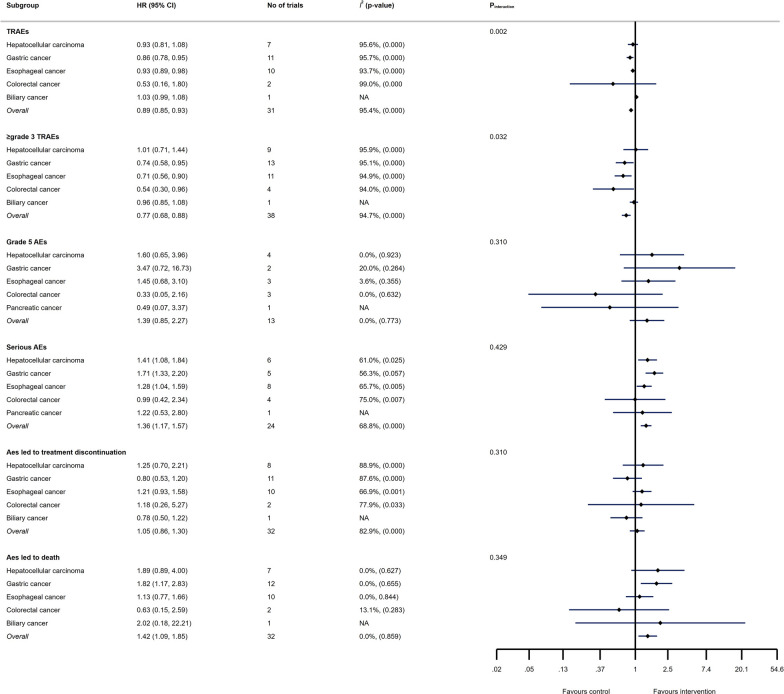
Overall RR of TRAEs and ≥ grade 3 TRAEs were 0.89 (95% CI 0.85 to 0.93, I2 = 95.4%) and 0.77 (95% CI 0.68 to 0.88, I2 = 94.7%), respectively; indicating that ICI therapy may possess fewer TRAEs and ≥ grade 3 TRAEs than conventional therapies. However, serious AEs and AEs led to death were more common in patients treated with ICIs compared with conventional therapies (RR = 1.36, 95% CI 1.17 to 1.57, I2 = 68.8% and RR = 1.42, 95% CI 1.09 to 1.85, I2 = 0.0%, respectively). The results concerning the safety of ICIs in GI cancers are depicted in Fig. 7.
Fig. 7.
Detailed on TRAEs, ≥ grade 3 TRAEs, grade 5 TRAEs, serious AEs, AEs led to treatment discontinuation, and AEs led to death in different GI cancers following ICI therapy
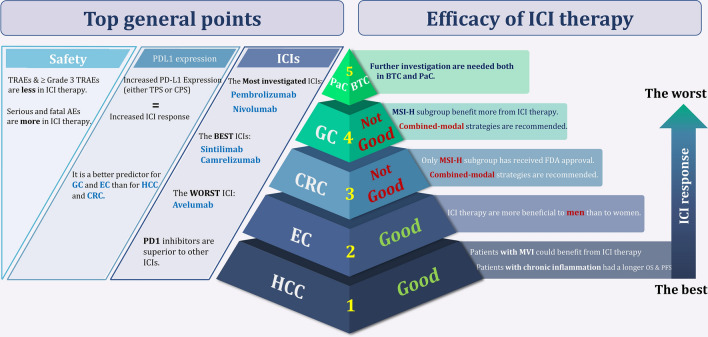
Discussion
Although ICIs successfully used to treat various solid tumors such as metastatic melanoma [68], renal cell carcinoma [69], lung cancer [70], and squamous cell carcinoma of the head and neck [71], there are controversial results about ICIs’ efficacies in patients with GI tumors. As far as we are aware, this is the first umbrella review in the context of GI malignancies, reporting that application of ICIs in this group of cancers could partially improve response rates compared to conventional therapies (Figs. 2–5); however, different types of GI tumors did not experience the same efficacy. The results of the pooled analysis showed that HCC and EC patients are most likely to benefit from ICI therapy, while it is less useful in GC and CRC patients. Also, the results for PaC and BTC are at preliminary stage and need to be investigated in future.
Regarding the great potential of ICIs in HCC, patients in the advanced stages of disease and even those with MVI could benefit from this strategy. Of note, HCC patients with chronic inflammation caused by hepatitis B or C infection are more susceptible to ICI therapy; indeed, the expression of immune checkpoint molecules which is increased through the inflammatory condition may explain this phenomenon [72, 73]. Besides HCC, EC patients have promising responses to ICIs mainly targeting PD-1. Accordingly, the study conducted by Chalmers et al. reported that EC patients have a high mutation load which caused the emergence of immunogenic neoantigens, making these patients good candidates for ICI treatment [74]. On the other hand, while encouraging results of some studies led to the approval of anti-PD-1 drugs in GC patients after second lines of therapy failure [17, 75], there are controversial results about ICIs efficacies in this malignancy [24]. This issue perhaps occurs due to the high heterogeneity of the tumor and different expression of immune checkpoints among its various subtypes; while MSI-high and Epstein-Barr virus (EBV) positive subtypes associated with high levels of these molecules, aberrant p53 subtype didn’t show a significant correlation [76]. Concerning CRC, studies revealed that ICI therapy is only effective in a limited number of CRC patients who are MSI-high, resulting in FDA approval of several ICIs either as a monotherapy or in combination in this group [77–80]; however, it is worth noting that the result of our study shows survival outcomes are not significantly different between microsatellite stable and unstable groups of CRC patients. Generally, and regardless of microsatellite status, our results further highlight that application of ICIs is unlikely helpful in patients with CRC [58, 81]. Since the studies in the context of BTC and PaC are limited, it is difficult to make a definitive judgment about the efficacy of ICIs in these patients; however, it seems that BTC demonstrates more beneficial effects of ICI strategy rather than PaC. The immunosuppressive environment in the latter may be one of the main barriers to successful ICI therapy in this malignancy [82]. All in all and apart from the type of GI, it seems that application of ICIs in combined-modal strategies is a better approach for GI cancer treatment compared to ICI monotherapy. In this vein, most of the FDA-approved ICIs in GI cancers are in combination with other agents, and nowadays most of the ongoing clinical trials are focused on the efficacy of ICIs in these strategies. To provide a better overview, we summarized FDA approved ICIs and ongoing clinical trials among different GI cancers in Tables 3 and 4, respectively.
Table 3.
The list of FDA approval of ICIs in different GI cancers
| NCT | Trial name | Trial design | Standard arm | Line | Treatment | OS (Months) | PFS (Months) | PDL1 Status | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Name of drug | Type of drug | Mode | ||||||||
| HCC | ||||||||||
| NCT03434379 | IMbrave-150 | Open label randomized phase III | Sorafenib | First | Atezolizumab + Bevacizumab | Anti-PD-L1 + Anti-VEGF | Combination | Not est. vs 13.2 | 6.8 vs 4.3 | No |
| NCT02702414 | Keynote-244 | Open label, non-randomized phase II | None | Second | Pembrolizumab | Anti-PD1 | Monotherapy | 13.2 | 4.9 | No |
| NCT01658878 | Checkmate-040 | Open label, non-comparative, dose escalation and expansion phase I/II | None | Second | Nivolumab | Anti-PD1 | Monotherapy | 83% at 6; 74% at 9 | 37% at 6; 28% at 9 | No |
| NCT01658878 | Checkmate-040 | Open label, non-comparative, dose escalation and expansion Phase I/II | None | Second | Nivolumab + Ipilimumab | Anti-PD1 + Anti- CTLA4 | Combination | 22.8 | No | |
| GC | ||||||||||
| NCT02872116 | Checkmate-649 | Open label randomized phase III | Capecitabine and Oxaliplatin | First | Nivolumab + capecitabine + oxaliplatin | Anti-PD1 + chemotherapy | Combination | 13.1 vs 11.1 | 7·7 vs 6.05 | No |
| NCT02335411 | Keynote-059 | Open label single arm phase II | None | Third | Pembrolizumab | Anti-PD1 | Monotherapy | 5.6 | 2 | CPS > 1 |
| NCT02267343 | Attraction-2 | Double-blind randomized phase III | Placebo | Third | Nivolumab | Anti-PD1 | Monotherapy | 5·26 vs 4·14 | 1·61 vs 1.45 | No |
| NCT01928394 | Checkmate-032 | Open label phase I/II | Third | Nivolumab + Ipilimumab | Anti-PD1 + Anti- CTLA4 | Combination | 6.9 | 1.4 | No | |
| EC | ||||||||||
| NCT03189719 | Keynote-590 | Double-blind randomized phase III | 5-fluorouracil and Cisplatin | First | Pembrolizumab + Cisplatin + Fluorouracil | Anti-PD1 + chemotherapy | Combination | 13.9 vs 8.8 | 7.5 vs 5.8 | CPS ≥ 10 |
| NCT03143153 | Checkmate-648 | Open label randomized phase III | 5-fluorouracil and Cisplatin | First | Nivolumab + Ipilimumab | Anti-PD1 + Anti- CTLA4 | Combination | 13.7 vs. 9.1 | 4 vs 4.4 | TPS ≥ 1% |
| NCT03143153 | Checkmate-648 | Open label randomized phase III | 5-fluorouracil and Cisplatin | First | Nivolumab + Cisplatin + Fluorouracil | Anti-PD1 + chemotherapy | Combination | 15.4 vs. 9.1 | 6.9 vs 4.4 | TPS ≥ 1% |
| NCT02564263 | Keynote-181 | Open label randomized phase III | Paclitaxel, Docetaxel, and Irinotecan | Second | Pembrolizumab | Anti-PD1 | Monotherapy | 9.3 vs 6.7 | 2.6 vs 3 | CPS ≥ 10 |
| NCT02559687 | Keynote-180 | Open label single arm phase II | None | Second | Pembrolizumab | Anti-PD1 | Monotherapy | 5.8 | 2 | CPS ≥ 10 |
| NCT02569242 | Attraction-3 | Open label randomized phase III | Paclitaxel or Docetaxel | Second | Nivolumab | Anti-PD1 | Monotherapy | 10.9 vs 8.4 | 1.7 vs 3.4 | No |
| CRC | ||||||||||
| NCT02563002 | Keynote-177 | Randomized phase III | mFOLFOX6/ FOLFIRI + / − Bevacizumab or Cetuximab | First * | Pembrolizumab | Anti-PD1 | Monotherapy | Not achieved vs 36.7 | 16.5 vs 8.2 | No |
| NCT02060188 | Checkmate-142 | Open label single arm Phase II | None | First * | Nivolumab | Anti-PD1 | Monotherapy | 73% at 12 | 50.4% at 12 | No |
| NCT02060188 | Checkmate-142 | Open label single arm Phase II | None | First * | Nivolumab + Ipilimumab | Anti-PD1 + Anti- CTLA4 | Combination | 85% at 12 | 71% at 12 | No |
| NCT02460198 | Keynote-164 | Open label single arm Phase II | None | Second * | Pembrolizumab | Anti-PD1 | Monotherapy | 31.4 | 2.3 | No |
* For dMMR/MSI-high
Table 4.
Selected ongoing randomized clinical trials investigating the efficacy of ICIs in GI cancer cases
| NCT number | Population under study | Therapies under comparison | Monotherapy/combination | Sample size (n) | ||
|---|---|---|---|---|---|---|
| HCC | ||||||
| NCT05277675 | Neoadjuvant therapy in the treatment of recurrent HCC | Tislelizumab/Sintilimab + Lenvatinib/Bevacizumab + radiofrequency ablation vs radiofrequency ablation | Combination | 160 | ||
| NCT04183088 | First-line therapy for advanced HCC | Tislelizumab + regorafenib vs regorafenib | Combination | 125 | ||
| NCT04658147 | - | Nivolumab + Relatlimab vs Nivolumab | Monotherapy vs Combination | 20 | ||
| NCT04233840 | - | Ropeginterferon alfa-2b + Nivolumab vs Nivolumab vs Ropeginterferon alfa-2b | Monotherapy vs Combination | 72 | ||
| NCT03383458 | HCC who has undergone complete resection or have achieved a complete response after local ablation, and who are at high risk of recurrence | Nivolumab vs placebo | Monotherapy | 545 | ||
| NCT04050462 | - | Nivolumab vs Nivolumab + BMS-986253 vs Nivolumab + Cabiralizumab | Monotherapy vs Combination | 23 | ||
| NCT04567615 | - | Nivolumab and Relatlimab vs Nivolumab | Monotherapy vs Combination | 250 | ||
| NCT05039736 | - | Cabozantinib vs Nivolumab | Monotherapy | 30 | ||
| NCT04039607 | Advanced HCC | Nivolumab + Ipilimumab vs Sorafenib + lenvatinib | Combination | 732 | ||
| NCT02576509 | First- line treatment in patients with advanced HCC | Nivolumab vs Sorafenib | Monotherapy | 743 | ||
| NCT04340193 | Intermediate-stage HCC | Nivolumab + Ipilimumab + Trans-arterial ChemoEmbolizatio vs Nivolumab + Trans-arterial ChemoEmbolizatio vs Trans-arterial ChemoEmbolizatio | Combination | 26 | ||
| NCT04268888 | Intermediate-stage HCC | Transarterial Chemoembolisation and/or Transarterial Embolisation vs Transarterial Chemoembolisation and/or Transarterial Embolisation + Nivolumab | Combination | 552 | ||
| NCT05337137 | untreated advanced/metastatic HCC | Relatlimab + Nivolumab + Bevacizumab vs Nivolumab + Bevacizumab + Placebo | Combination | 162 | ||
| NCT04777851 | Intermediate-stage HCC | Regorafenib + Nivolumab vs Transarterial Chemoembolization | Combination | 496 | ||
| GC | ||||||
| NCT04294784 | Patients with advanced or recurrent GC and GEJ in second-line treatment | albumin-bound paclitaxel and SHR-1210 (PD-1 inhibitor) vs albumin-bound paclitaxel | Combination | 80 | ||
| NCT04997837 | D2/R0 resected pn3 GC or GEJ | Nivolumab/Toripalimab + Oxaliplatin + Capecitabine + Tegafur-gimeracil-oteracil potassium + 5-FU + RT vs Oxaliplatin + Capecitabine + Tegafur-gimeracil-oteracil potassium + 5-FU + RT | Combination | 433 | ||
| NCT04782791 | Nivolumab + oxaliplatin + Gastrectomy vs Nivolumab + Gastrectomy | Combination | 30 | |||
| NCT03443856 | Stage Ib-iva GC and GEJ and high risk of recurrence following neoadjuvant chemotherapy and resection | Nivolumab + Ipilimumab vs chemotherapy | Combination | 197 | ||
| NCT02935634 | Advanced GC | Nivolumab + Ipilimumab vs Nivolumab + Relatlimab vs Nivolumab + BMS-986205 vs Nivolumab + Rucaparib vs Ipilimumab + Rucaparib vs Nivolumab + Ipilimumab + Rucaparib | Combination | 190 | ||
| NCT02746796 | Unresectable advanced or recurrent GC and GEJ as the first-line therapy | Nivolumab + Oxaliplatin + Tegafur- Gimeracil-Oteracil potassium vs Nivolumab + Oxaliplatin + Capecitabine vs Nivolumab + Oxaliplatin + Tegafur- Gimeracil-Oteracil potassium + Capecitabine vs Oxaliplatin + Tegafur- Gimeracil-Oteracil potassium + Capecitabine + Placebo | Combination | 680 | ||
| NCT03006705 | In stage III GC and GEJ after D2 or more extensive lymph node dissection | Nivolumab + Oxaliplatin + Tegafur- Gimeracil-Oteracil potassium + Capecitabine vs Oxaliplatin + Tegafur- Gimeracil-Oteracil potassium + Capecitabine + Placebo | Combination | 800 | ||
| NCT05144854 | Chemotherapy-naïve participants with HER2-negative unresectable advanced or recurrent GC and GEJ | Nivolumab + Ipilimumab + Oxaliplatin + Capecitabine + S-1 vs Oxaliplatin + Capecitabine + S-1 | Combination | 600 | ||
| NCT03647969 | Patients with Her2 negative, previously untreated metastatic esophagogastric adenocarcinoma | Nivolumab + Ipilimumab + mFOLFOX vs mFOLFOX vs Nivolumab + FLOT | Combination | 262 | ||
| NCT04062656 | GC and GEJ | Nivolumab + Oxaliplatin + Docetaxel + 5-FU + Folic acid vs Nivolumab + Oxaliplatin + Docetaxel + 5-FU + Folic acid + relatlimab | Combination | 21 | ||
| NCT02872116 | GC and GEJ | Nivolumab + Ipilimumab vs Oxaliplatin + Capecitabine vs Oxaliplatin + Leucovorin + Fluorouracil vs Nivolumab + Oxaliplatin + Capecitabine vs Oxaliplatin + Leucovorin + Fluorouracil + Nivolumab | Combination | 2031 | ||
| NCT04879368 | Gastro-esophageal cancer | Regorafenib + Nivolumab vs Docetaxel + Paclitaxel + Irinotecan + Trifluridine/Tipracil | Combination | 450 | ||
| NCT05568095 | locally advanced unresectable or metastatic GC, and GEJ and EC | Zimberelimab + Domvanalimab + Oxaliplatin + Leucovorin + Fluorouracil + Capecitabine vs Nivolumab Oxaliplatin + Leucovorin + Fluorouracil + Capecitabine | Combination | 970 | ||
| EC | ||||||
| NCT05182944 | - | Camrelizumab + Albumin Paclitaxel + Cisplatin + Camrelizumab vs Camrelizumab vs best supportive care | Monotherapy vs Combination | 130 | ||
| NCT05007145 | Resectable locally advanced thoracic EC | PD-1 inhibitor + Albumin-Bound Paclitaxel + Cisplatin vs Albumin-Bound Paclitaxel + Cisplatin + Radiation | Combination | 92 | ||
| NCT04785820 | Advanced or metastatic | RO7121661 vs RO7247669 vs Nivolumab | Monotherapy | 210 | ||
| NCT05213312 | Esophageal squamous cell carcinoma | Nivolumab + Cisplatin + Paclitaxel + 5Fluorouracil vs Cisplatin + Paclitaxel + 5Fluorouracil | Combination | 90 | ||
| NCT03604991 | Esophageal adenocarcinoma, Gastroesophageal junction adenocarcinoma | Carboplatin + Paclitaxel + RT vs Carboplatin + Nivolumab + Paclitaxel + RT vs Nivolumab vs Ipilimumab + Nivolumab | Monotherapy vs Combination | 514 | ||
| CRC | ||||||
| NCT03926338 | dMMR/MSI-H phenotype | Toripalimab + Celecoxib vs Toripalimab | Monotherapy vs Combination | 100 | ||
| NCT05141721 | Metastatic CRC | GRT-C901/GRT-R902 + Ipilimumab + Fluoropyrimidine + leucovorin + Bevacizumab + Atezolizumab vs Fluoropyrimidine + leucovorin + Bevacizumab | Combination | 665 | ||
| NCT04907539 | RNF43 or RSPO aberrated, MSS | RXC004 + Denosumab vs RXC004 + Denosumab + Nivolumab | Combination | 50 | ||
| NCT05308446 | Metastatic or unresectable BRAF-mutant | Cetuximab + Encorafenib vs Cetuximab + Encorafenib + Nivolumab | Combination | 84 | ||
| NCT03388190 | MSS/ pMMR phenotype | Oxaliplatin vs oxaliplatin + Nivolumab | Combination | 80 | ||
| NCT03414983 | Metastatic CRC | Oxaliplatin + Leucovorin + Fluorouracil + Bevacizumab vs Oxaliplatin + Leucovorin + Fluorouracil + Bevacizumab + Nivolumab | Combination | 195 | ||
| NCT04008030 | MSI-H/dMMR phenotype | Nivolumab vs Nivolumab + Ipilimumab vs Oxaliplatin + Leucovorin + Fluorouracil + Irinotecan + Bevacizumab + Cetuximab | Monotherapy vs Combination | 831 | ||
| NCT03803553 | Metastatic CRC | 5-Fluorouracil + Irinotecan + Leucovorin vs Nivolumab vs Encorafenib + Binimetinib + Cetuximab vs active surveillance | Monotherapy | 500 | ||
| NCT03377361 | - | Nivolumab + trametinib vs Nivolumab + Ipilimumab + trametinib vs Nivolumab + Ipilimumab + trametinib vs Nivolumab + Ipilimumab + trametinib vs Regorafenib vs Nivolumab + Ipilimumab + trametinib | Combination | 232 | ||
| NCT05328908 | Failed 1–4 prior lines of therapy | Nivolumab + Relatlimab vs Regorafenib or TAS-102 | Combination | 700 | ||
| PaC | ||||||
| NCT04612530 | - | Nivolumab vs Irreversible Electroporation (IRE) + Nivolumab vs IRE + Nivolumab + Toll-Like Receptor 9 | Monotherapy vs Combination | 18 | ||
| NCT04953962 | Stage IV | CBP501 (25) + Cisplatin + Nivolumab vs CBP501 (25) + Cisplatin vs Cisplatin + Nivolumab | Combination | 36 | ||
| NCT03563248 | Localized PaC | FOLFIRINOX + SBRT + surgery vs FOLFIRINOX + SBRT + surgery + Losartan vs FOLFIRINOX + SBRT + surgery + Losartan + Nivolumab vs FOLFIRINOX + SBRT + surgery + Nivolumab | Combination | 168 | ||
| NCT03336216 | Advanced PaC | Nab-paclitaxel + Onivyde + Fluorouracil + Gemcitabine + Leucovorin + irinotecan hydrochloride vs Cabiralizumab + Nivolumab vs Cabiralizumab + Nivolumab + Nab-paclitaxel + Gemcitabine vs Cabiralizumab + Nivolumab + Fluorouracil + Oxaliplatin + Leucovorin | Combination | 202 | ||
| NCT04229004 | Metastatic PaC | Gemcitabine + Nab-paclitaxel vs Oxaliplatin + Leucovorin + Irinotecan + 5-Fluorouracil vs amrevlumab + gemcitabine + nab-paclitaxel vs Canakinumab + Spartalizumab + nab-paclitaxel + gemcitabine vs SM-88 | Combination | 825 | ||
| NCT02451982 | Surgically resectable pancreatic ductal adenocarcinoma | Cyclophosphamide + GVAX vs Cyclophosphamide + GVAX + Nivolumab vs Cyclophosphamide + GVAX + Nivolumab + Urelumab vs Nivolumab + BMS-986253 | Combination | 76 | ||
| Different cancers | ||||||
| NCT03184870 | mCRC and mPaC | BMS-813160 + 5-fluorouracil + Leucovorin + Irinotecan vs BMS-813160 + Nab-paclitaxel + Gemcitabine vs BMS-813160 and Nivolumab | Combination | 332 | ||
| NCT03373188 | Stage I-III PaC, stage IV CRC | Surgery vs VX15/2503 + surgery vs VX15/2503 + Ipilimumab + surgery vs VX15/2503 + Nivolumab + surgery | Combination | 10 | ||
| NCT02866383 | Metastatic PaC and BTC | Nivolumab + Radiotherapy vs Nivolumab + Ipilimumab + Radiotherapy | Combination | 160 | ||
| NCT02743494 | Various advanced cancers | Nivolumab vs Placebo | Monotherapy | 794 | ||
| NCT03752398 | Solid tumors | XmAb®23104 vs XmAb®23104 + Ipilimumab | Combination | 234 | ||
HCC hepatocellular carcinoma, GC gastric cancer, EC: esophageal carcinoma, GEJ gastroesophgeal junction carcinoma, CRC colorectal cancer, PaC pancreatic cancer, dMMR miss match repair deficiency, pMMR proficient miss match repair, MSS microsatellite stable, MSI-H microsatellite instable-high, SBRT stereotactic body radiation therapy
Not only the type of GI cancer but also the type of drug is a determinant factor in ICI therapy. The results of the subgroup analysis showed that Pembrolizumab and Nivolumab are the most investigated drugs in GI cases; however, based on our analysis, Camrelizumab and Sintilimab outperformed the formers according to survival outcomes. All the abovementioned drugs target PD-1, implying that this molecule may probably be the more attractive ICI target rather than PD-L1 and CTLA-4 in GI cancer cases. Achieving the worst results with anti-PD-L1 in OS outcome, Avelumab may provide more data to probably conclude that PD-1 inhibitors are more efficient agents as compared to inhibitors of PD-L1. In agreement, Ribas et al. found that anti-PD-1 agents are superior inhibitors since they can inhibit PD-L2 binding to PD-1, as well [83].
Of great interest, the correlation between PD-L1 expression and the extent of the response to ICIs has been examined in a wide range of human malignancies; however, in many cases, there are conflicting results. While several previous studies indicated that the sensitivity extent of metastatic melanoma [84], non-small-cell lung cancer [11], and urothelial carcinoma [16] to ICIs correlates with the PD-L1 status, another study failed to report a differential sensitivity pattern with respect to PD-L1 expression level in renal carcinoma [13]. Based on our subgroup analysis, high expression of PD-L1 (either CPS or TPS) in GI cancers is associated with better response to ICIs. While, generally speaking, PD-L1 expression in GI cancers is a good biomarker for predicting ICI response, it is reasonable to hypothesize that different GI tumors __given to their heterogeneous characteristics__ may respond differently based on PD-L1 status. In this regard, the promising results of KEYNOTE-180 and 181 trials which assessed the expression of PD-L1 using CPS led to the FDA approval of Pembrolizumab monotherapy in EC patients with CPS ≥ 10 after failure of first line of therapy [85, 86]. Our recent meta-analysis also revealed that PD-L1 CPS = 10 and TPS = 1% expression thresholds are predictive for lower rate of mortality when PD-1/PD-L1 inhibitors are administrated in patients suffering from EC [87]. Concerning GC, Kawazoe and Böger studies indicated that anti-PD-1/PD-L1 therapy is more effective in either MSI-high or EBV-positive advanced GC patients who are PD-L1 positive [88, 89]. Inline, the results of our recent meta-analysis reported that CPS scoring method has superior quality to TPS in predicting the response to ICIs; of note, GC patients expressing PD-L1 as CPS ≥ 1, CPS ≥ 5, and CPS ≥ 10 had longer OS than their counterpart subgroups (unpublished data). In contrast to EC and GC, PD-L1 expression did not seem to be a suitable biomarker for HCC and CRC patients as several trials revealed that ICI responses were observed regardless of PD-L1 expression in these patients [61, 78, 80]. Finally, the role of PD-L1 expression in PaC and BTC patients still remains unclear and further investigations are required to elucidate whether PD-L1 expression may influence the extent of PaC and BTC response to ICIs.
Another factor which affects GI tumors’ response to ICIs is microsatellite and mismatch repair (MMR) status. MSI-high and MMR deficiency (dMMR) in tumor cells may lead to high mutation levels and the arising of immunogenic neoantigens, facilitating their recognition by immune cells, and probably, a plausible justification for their better response to ICIs [90]. According to the importance of MSI-high status in some types of GI cancers, Pembrolizumab and Nivolumab achieved approval for dMMR/MSI-high CRC, EC, and GC patients [77–80]; however, in our analysis, a significant difference in survival outcomes between stable and unstable microsatellite status was only observed in GC patients. Of note, the results of a trial on BTC patients indicated that favorable responses to Nivolumab were unexpectedly observed in non-MSI-high patients [91]; further highlighting the necessity for additional studies to elaborate ICI responses with respect to microsatellite status in different GI tumors.
Albeit ICI is a promising treatment option at least in some GI cancers, our results demonstrate that serious AEs and AEs leading to death are more common in patients treated with ICIs compared with conventional therapies. In this regard, a meta-analysis showed that fatal AEs differ widely based on ICIs; while pneumonitis, hepatitis, and neurotoxic effects were the most frequent causes of death in patients receiving anti-PD-1 or anti-PD-L1, fatalities in patients receiving anti-CTLA-4 were mainly attributed to colitis. In combination therapy, the majority of ICI-related deaths were caused by myocarditis or colitis [92]. Therefore, early detection and management of these AEs are of paramount importance for practitioners.
We made our best efforts to present a complete and practical study, however, there are several limitations to be considered when interpreting the results or applying them in clinical practice. Firstly, some limitations were present due to the shortcomings of the included meta-analyses, such as heterogeneity of baseline characteristics, type of inhibitors, cycles of receiving the drug, different regimes in the control groups, and variable duration of follow-up which might influence some of the outcomes. Secondly, despite searching all of the databases mentioned above and searching extensively for related literature, there is still a possibility that some articles were missed. Thirdly, we were able to conduct subgroup analysis only on a few variables highlighting the need for trials with more subgroup analysis in the future. Fourthly, a definitive conclusion could not be reached in some analyses due to high confidence intervals.
In conclusion, this article consolidates knowledge on one of the most promising treatment options for GI malignancies by providing a comprehensive review of the most recent evidence. According to our analysis, HCC and EC patients are likely better candidates for ICI therapy, especially PD-1 inhibitors, rather than GC and CRC patients. We also assess the predictive value of PD-L1 expression as well as microsatellite status in response to ICIs. In the near future, not only predictive biomarkers could be used to select which GI patients are more likely to benefit from ICIs, but they also can be utilized to support de-escalation of treatment in order to avoid unnecessary toxicity. PD-L1 expression and dMMR/MSI-H status are examples of these biomarkers, but _ as investigated in this study_ they have shown specific and narrow clinical applications. Priority in future research should be the identification of new clinically applicable prognostic and diagnostic biomarkers. Resistance to ICIs is another issue that must be addressed; for this purpose, combination therapy is currently being investigated to either alter the tumor microenvironment or target immune evasion mechanisms. Investigating the role of ICIs in adjuvant, neoadjuvant, and maintenance therapies should also be considered in future studies. Figure 8 provides an overview of the ICI therapy in GI cancers. It is likely that ICIs will become the standard of care in early lines of therapy for various GI malignancies as new data from ongoing trials emerge.
Fig. 8.
An overview of the ICI therapy in GI cancers
Supplementary Information
Additional file 1: Table S1. Search strategy. Table S2. Definition of efficacy and safety outcomes. Table S3. Characteristics of included studies. Table S4. Methodological quality assessment by AMSTAR2. Figure S1. Forest plots of CR analysis in different types of GI cancers. Figure S2. Forest plots of PD analysis in different types of GI cancers. Figure S3. Forest plots of PR analysis in different types of GI cancers. Figure S4. Forest plots of SD analysis in different types of GI cancers.
Acknowledgements
None.
Author contributions
MN and DB designed the study. MN, FJ, ZD, and MD performed the study selection and data extraction. MN conducted the analysis. MN, FJ, ZD, MD, and DB wrote the first draft of the manuscript. DB and SS critically revised the manuscript. All authors reviewed the drafted manuscript for critical content. All authors approved the final version of the manuscript.
Funding
None.
Availability of data and materials
The data underlying this article are available in the article and in its online Additional file material.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors confirm that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buckley AM, Lynam-Lennon N, O’Neill H, O’Sullivan J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol. 2020;17(5):298–313. doi: 10.1038/s41575-019-0247-2. [DOI] [PubMed] [Google Scholar]
- 2.Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, et al. The impact of gender on the efficacy of immune checkpoint inhibitors in cancer patients: the MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596. doi: 10.1016/j.critrevonc.2022.103596. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: a comprehensive literature review. Cancer Treat Res Commun. 2021;27:100354. doi: 10.1016/j.ctarc.2021.100354. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo A, Brandi G. First-line chemotherapy in advanced biliary tract cancer ten years after the abc-02 trial: "and yet it moves!". Cancer Treat Res Commun. 2021;27:100335. doi: 10.1016/j.ctarc.2021.100335. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Lin J, Yang X, Long J, Bai Y, Yang X, et al. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J Hematol Oncol. 2019;12(1):1–21. doi: 10.1186/s13045-019-0730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaiser J, Couzin-Frankel J. Cancer immunotherapy sweeps Nobel for medicine. Am Assoc Advanc Sci. 2018 doi: 10.1126/science.362.6410.13. [DOI] [PubMed] [Google Scholar]
- 7.Bouzid R, Peppelenbosch M, Buschow SI. Opportunities for conventional and in situ cancer vaccine strategies and combination with immunotherapy for gastrointestinal cancers, a review. Cancers. 2020;12(5):1121. doi: 10.3390/cancers12051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 9.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N engl J med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156–4167. doi: 10.1002/cncr.33033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg JE, Hoffman-Censits J, Powles T, Van Der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. The Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang Y-K, Boku N, Satoh T, Ryu M-H, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;390(10111):2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Cho BC, Takahashi M, Okada M, Lin C-Y, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 19.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. The Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, 2020. et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: The Canadian Cancer Trials Group CO 26 Study. JAMA Oncology. 6(6):831–8. [DOI] [PMC free article] [PubMed]
- 21.Zhu X, Cao Y, Liu W, Ju X, Zhao X, Jiang L, et al. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2021;22(8):1093–1102. doi: 10.1016/S1470-2045(21)00286-2. [DOI] [PubMed] [Google Scholar]
- 22.Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20(6):849–861. doi: 10.1016/S1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 23.Renouf DJ, Loree JM, Knox JJ, Topham JT, Kavan P, Jonker D, et al. The CCTG PA. 7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nature Commun. 2022;13(1):1–8. doi: 10.1038/s41467-022-32591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang Y-J, Ruiz EY, Van Cutsem E, Lee K-W, Wyrwicz L, Schenker M, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052–2060. doi: 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung HC, Kang YK, Chen Z, Bai Y, Wan Ishak WZ, Shim BY, et al. Pembrolizumab versus paclitaxel for previously treated advanced gastric or gastroesophageal junction cancer (KEYNOTE-063): a randomized, open-label, phase 3 trial in Asian patients. Cancer. 2022;128(5):995–1003. doi: 10.1002/cncr.34019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fong C, Patel B, Peckitt C, Bourmpaki E, von Loga K, Begum R, et al. Maintenance durvalumab after first-line platinum-based chemotherapy in advanced oesophago-gastric (OG) adenocarcinoma: Results from the PLATFORM trial. Wolters Kluwer Health; 2021. 10.1200/JCO.2021.39.15_suppl.4015
- 27.Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Cochrane Handbook for Systematic Reviews of Interventions, Chapter V: overviews of Reviews https://training.cochrane.org/handbook/current/chapter-v.
- 28.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions: John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed]
- 29.He S, Jiang W, Fan K, Wang X. The efficacy and safety of programmed death-1 and programmed death ligand 1 inhibitors for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol. 2021;11:626984. doi: 10.3389/fonc.2021.626984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jácome AA, Castro ACG, Vasconcelos JPS, Silva MHC, Lessa MAO, Moraes ED, et al. Efficacy and safety associated with immune checkpoint inhibitors in unresectable hepatocellular carcinoma: a meta-analysis. JAMA network open. 2021;4(12):e2136128. doi: 10.1001/jamanetworkopen.2021.36128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Ma Y, Wu Z, Zeng F, Song B, Zhang Y, et al. Tumor mutational burden predicting the efficacy of immune checkpoint inhibitors in colorectal cancer: a systematic review and meta-analysis. Front Immunol. 2021 doi: 10.3389/fimmu.2021.751407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, Guan L, Xu M, Wang F. The efficacy and safety of antibodies targeting PD-1 for treatment in advanced esophageal cancer: a systematic review and meta-analysis. Transl Oncol. 2021;14(6):101083. doi: 10.1016/j.tranon.2021.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyo J, Park H-J. Treatment efficacy of immune checkpoint inhibitors for patients with advanced or metastatic colorectal cancer: a systematic review and meta-analysis. J Clin Med. 2021;10(16):3599. doi: 10.3390/jcm10163599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao Q, Li M, Xu W, Pang K, Guo X, Wang D, et al. Clinical benefits of PD-1/PD-L1 inhibitors in advanced hepatocellular carcinoma: a systematic review and meta-analysis. Hep Intl. 2020;14(5):765–775. doi: 10.1007/s12072-020-10064-8. [DOI] [PubMed] [Google Scholar]
- 35.Shek D, Akhuba L, Carlino MS, Nagrial A, Moujaber T, Read SA, et al. Immune-checkpoint inhibitors for metastatic colorectal cancer: a systematic review of clinical outcomes. Cancers. 2021;13(17):4345. doi: 10.3390/cancers13174345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R, Lin N, Mao B, Wu Q. The efficacy of immune checkpoint inhibitors in advanced hepatocellular carcinoma: a meta-analysis based on 40 cohorts incorporating 3697 individuals. J Cancer Res Clin Oncol. 2022;148(5):1195–1210. doi: 10.1007/s00432-021-03716-1. [DOI] [PubMed] [Google Scholar]
- 37.Wu J, Deng R, Ni T, Zhong Q, Tang F, Li Y, et al. Efficacy and safety of radiotherapy/chemoradiotherapy combined with immune checkpoint inhibitors for locally advanced stages of esophageal cancer: a systematic review and meta-analysis. Front Oncol. 2022 doi: 10.3389/fonc.2022.887525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang L, Dong X-Z, Xing X-X, Cui X-H, Li L, Zhang L. Efficacy and safety of anti-PD-1/anti-PD-L1 antibody therapy in treatment of advanced gastric cancer or gastroesophageal junction cancer: a meta-analysis. World J Gastrointestinal Oncol. 2020;12(11):1346. doi: 10.4251/wjgo.v12.i11.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Yang Z, An Y, Liu Y, Wei Q, Xu F, et al. Clinical benefits of PD-1/PD-L1 inhibitors in patients with metastatic colorectal cancer: a systematic review and meta-analysis. World J Surg Oncol. 2022;20(1):1–13. doi: 10.1186/s12957-022-02549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhuo Z-G, Deng H-Y, Song T-N, Alai G-H, Shen X, Lin Y-D. Predictors for the clinical benefit of anti-PD-1/PD-L1 therapy in advanced gastroesophageal cancer: a meta-analysis of clinical trials. Ann Palliat Med. 2020;9:2524–2537. doi: 10.21037/apm-19-430a. [DOI] [PubMed] [Google Scholar]
- 41.Dyhl-Polk A, Mikkelsen MK, Ladekarl M, Nielsen DL. Clinical trials of immune checkpoint inhibitors in hepatocellular carcinoma. J Clin Med. 2021;10(12):2662. doi: 10.3390/jcm10122662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–30. doi: 10.1016/j.ctrv.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Lim H, Ramjeesingh R, Liu D, Tam VC, Knox JJ, Card PB, et al. Optimizing survival and the changing landscape of targeted therapy for intermediate and advanced hepatocellular carcinoma: a systematic review. JNCI J Nat Cancer Inst. 2021;113(2):123–36. doi: 10.1093/jnci/djaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang Q, Huang J, Zhang B, Li X, Chen X, Cui B, et al. Efficacy and safety of anti-pd1/pdl1 in advanced biliary tract cancer: a systematic review and meta-analysis. Front Immunol. 2022 doi: 10.3389/fimmu.2022.801909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H-B, Yang Z-H, Guo Q-Q. Immune checkpoint inhibition for pancreatic ductal adenocarcinoma: limitations and prospects: a systematic review. Cell Commun Signal. 2021;19(1):1–13. doi: 10.1186/s12964-021-00789-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen K, Wang X, Yang L, Chen Z. The anti-PD-1/PD-L1 immunotherapy for gastric esophageal cancer: a systematic review and meta-analysis and literature review. Cancer Control. 2021;28:1073274821997430. doi: 10.1177/1073274821997430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Formica V, Morelli C, Patrikidou A, Shiu K, Nardecchia A, Lucchetti J, et al. A systematic review and meta-analysis of PD-1/PD-L1 inhibitors in specific patient subgroups with advanced gastro-oesophageal junction and gastric adenocarcinoma. Crit Rev Oncol Hematol. 2021;157:103173. doi: 10.1016/j.critrevonc.2020.103173. [DOI] [PubMed] [Google Scholar]
- 48.Gu Y-M, Shang Q-X, Zhuo Y, Zhou J-F, Liu B-W, Wang W-P, et al. Efficacy and safety of immune checkpoint inhibitor in advanced esophageal squamous cell carcinoma: a meta-analysis. Front Oncol. 2021 doi: 10.3389/fonc.2021.777686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamposioras K, Ntellas P, Nikolaou M, Germetaki T, Gazouli I, Dadouli K, et al. Immunotherapy efficacy in the initial lines of treatment in advanced upper gastrointestinal malignancies: a systematic review of the literature. JNCI Cancer Spectrum. 2021 doi: 10.1093/jncics/pkab088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leone A, Petrelli F, Ghidini A, Raimondi A, Smyth E, Pietrantonio F. Efficacy and activity of PD-1 blockade in patients with advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis with focus on the value of PD-L1 combined positive score. ESMO open. 2022;7(1):100380. doi: 10.1016/j.esmoop.2021.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y, Xu M, Guan L, Yang Y, Chen Y, Yang Y, et al. PD-1 inhibitor plus chemotherapy versus chemotherapy as first-line treatment for advanced esophageal cancer: a systematic review and meta-analysis. J Immunother. 2022;45(5):243–253. doi: 10.1097/CJI.0000000000000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maoxi Z, Jinmin X, Xiaozhu Z, Yubing Y, Yuxi Z. PD-1/PD-L1 inhibitors versus chemotherapy for previously treated advanced gastroesophageal cancer: a meta-analysis of randomized controlled trials. J Oncol. 2021 doi: 10.1155/2021/3048974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oh S, Kim E, Lee H. Comparative impact of PD-1 and PD-L1 inhibitors on advanced esophageal or gastric/gastroesophageal junction cancer treatment: a systematic review and meta-analysis. J Clin Med. 2021;10(16):3612. doi: 10.3390/jcm10163612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pietrantonio F, Randon G, Di Bartolomeo M, Luciani A, Chao J, Smyth E, et al. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO open. 2021;6(1):100036. doi: 10.1016/j.esmoop.2020.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rotundo MS, Bagnardi V, Rotundo M, Comandè M, Zampino MG. PD-1/PD-L1 blockade, a novel strategy for targeting metastatic colorectal cancer: a systematic review and meta-analysis of randomized trials. Oncol Lett. 2022;23(4):1–14. doi: 10.3892/ol.2022.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie T, Zhang Z, Zhang X, Qi C, Shen L, Peng Z. Appropriate PD-L1 cutoff value for gastric cancer immunotherapy: a systematic review and meta-analysis. Front Oncol. 2021;11:646355. doi: 10.3389/fonc.2021.646355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng L, Su J, Qiu W, Jin X, Qiu Y, Yu W. Survival outcomes and safety of programmed cell death/programmed cell death ligand 1 inhibitors for unresectable hepatocellular carcinoma: result from phase III trials. Cancer Control. 2022;29:10732748221092924. doi: 10.1177/10732748221092924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng T, Fang X, Lu J, Zhong Y, Lin X, Lin Z, et al. Efficacy and safety of immune checkpoint inhibitors in colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021 doi: 10.1007/s00384-021-04028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu X, Shanzhou Q, Li D, Pang X, Ma D. PD-1 inhibitors versus chemotherapy as second-line treatment for advanced esophageal squamous cell carcinoma: a meta-analysis. BMC Cancer. 2021;21(1):1–10. doi: 10.1186/s12885-021-08958-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung TK, Lai CL, Wong BC, Fung J, Yuen MF. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther. 2006;24(4):573–583. doi: 10.1111/j.1365-2036.2006.03029.x. [DOI] [PubMed] [Google Scholar]
- 61.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 63.Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 64.Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 65.Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. 2020;23(4):565–578. doi: 10.1007/s10120-020-01090-4. [DOI] [PubMed] [Google Scholar]
- 66.Liu X, Qin S. Immune checkpoint inhibitors in hepatocellular carcinoma: opportunities and challenges. Oncologist. 2019;24(S1):S3–S10. doi: 10.1634/theoncologist.2019-IO-S1-s01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):1–8. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 70.Ackermann CJ, Reck M, Paz-Ares L, Barlesi F, Califano R. First-line immune checkpoint blockade for advanced non-small-cell lung cancer: travelling at the speed of light. Lung Cancer. 2019;134:245–253. doi: 10.1016/j.lungcan.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Chow LQ, Haddad R, Gupta S, Mahipal A, Mehra R, Tahara M, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2015;12(12):681–700. doi: 10.1038/nrgastro.2015.173. [DOI] [PubMed] [Google Scholar]
- 73.Pinato DJ, Guerra N, Fessas P, Murphy R, Mineo T, Mauri FA, et al. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020;39(18):3620–3637. doi: 10.1038/s41388-020-1249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):1–14. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA oncology. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JY, Kim WG, Kwon CH, Park DY. Differences in immune contextures among different molecular subtypes of gastric cancer and their prognostic impact. Gastric Cancer. 2019;22(6):1164–1175. doi: 10.1007/s10120-019-00974-4. [DOI] [PubMed] [Google Scholar]
- 77.André T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 78.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lenz H-J, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II CheckMate 142 study. J Clin Oncol. 2022;40(2):161–170. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 80.Overman MJ, Lonardi S, Leone F, McDermott RS, Morse MA, Wong KYM, et al. 2017. Nivolumab in patients with DNA mismatch repair deficient/microsatellite instability high metastatic colorectal cancer: Update from CheckMate 142. American Society of Clinical Oncology; 10.1200/JCO.2017.35.4_suppl.519
- 81.Weng J, Li S, Zhu Z, Liu Q, Zhang R, Yang Y, et al. Exploring immunotherapy in colorectal cancer. J Hematol Oncol. 2022;15(1):1–28. doi: 10.1186/s13045-022-01294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9(8):454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 83.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti–PD-1 TherapyAssociation of PD-1 and Ligands with Response to Anti–PD-1. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kojima T, Muro K, Francois E, Hsu C-H, Moriwaki T, Kim S-B, et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study. American Society of Clinical Oncology; 2019. [DOI] [PubMed]
- 86.Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5(4):546–550. doi: 10.1001/jamaoncol.2018.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noori M, Yousefi A-M, Zali MR, Bashash D. Predictive value of PD-L1 expression in response to immune checkpoint inhibitors for esophageal cancer treatment: A systematic review and meta-analysis. Frontiers in Oncology. 2022;12. [DOI] [PMC free article] [PubMed]
- 88.Böger C, Behrens H-M, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7(17):24269. doi: 10.18632/oncotarget.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20(3):407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- 90.Chang H, Jung WY, Kang Y, Lee H, Kim A, Kim HK, et al. Programmed death-ligand 1 expression in gastric adenocarcinoma is a poor prognostic factor in a high CD8+ tumor infiltrating lymphocytes group. Oncotarget. 2016;7(49):80426. doi: 10.18632/oncotarget.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim RD, Chung V, Alese OB, El-Rayes BF, Li D, Al-Toubah TE, et al. A phase 2 multi-institutional study of nivolumab for patients with advanced refractory biliary tract cancer. JAMA Oncol. 2020;6(6):888–894. doi: 10.1001/jamaoncol.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Search strategy. Table S2. Definition of efficacy and safety outcomes. Table S3. Characteristics of included studies. Table S4. Methodological quality assessment by AMSTAR2. Figure S1. Forest plots of CR analysis in different types of GI cancers. Figure S2. Forest plots of PD analysis in different types of GI cancers. Figure S3. Forest plots of PR analysis in different types of GI cancers. Figure S4. Forest plots of SD analysis in different types of GI cancers.
Data Availability Statement
The data underlying this article are available in the article and in its online Additional file material.