Abstract
Objective
The aim of this study was to compare metagenomic next-generation sequencing (mNGS) with other methods, including Xpert MTB/RIF, Mycobacterium tuberculosis (MTB) culture, and acid-fast bacillus (AFB) staining in the diagnosis of pulmonary tuberculosis (PTB) using bronchoalveolar lavage fluid (BALF).
Methods
The data of 186 patients with suspected PTB were retrospectively collected from January 2020 to May 2021 at Tongji Hospital. BALF samples were collected from all patients and analyzed using AFB staining, MTB culture, Xpert MTB/RIF, and mNGS.
Results
Of the 186 patients, 38 patients were ultimately diagnosed as PTB. Metagenomic next-generation sequencing exhibited a sensitivity of 78.95%, which was higher than AFB staining (27.59%) and MTB culture (44.12%) but similar to Xpert MTB/RIF (72.73%). Utilization of combined methods demonstrates improvement for PTB diagnosis. In support of this, the area under the receiver operating characteristic curve for the combination of mNGS and MTB culture (0.933, 95% CI: 0.871, 0.995) was larger than those of mNGS, Xpert MTB/RIF, MTB culture, and the combination of Xpert MTB/RIF and MTB culture.
Conclusion
The sensitivity of mNGS in the diagnosis of PTB using BALF specimen is similar to Xpert MTB/RIF. Metagenomic next-generation sequencing in combination with MTB culture may further improve the diagnosis of pulmonary tuberculosis.
Keywords: pulmonary tuberculosis, metagenomic next-generation sequencing, bronchoalveolar lavage fluid, diagnostic performance
Tuberculosis is a communicable disease caused by Mycobacterium tuberculosis complex (MTBC) infection, and it was one of the leading causes of ill health and death before the COVID-19 epidemic. The MTBC consists of Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium africanum, Mycobacterium microti, Mycobacterium pinnipedii, Mycobacterium caprase, and Mycobacterium canettii. The disease can affect different systems but mainly affects the lungs, which is known as pulmonary tuberculosis (PTB). According to the global report of the World Health Organization in 2021, about a quarter of the world’s population is infected with MTB.1,2
The diagnosis of PTB relies on isolation of MTBC from the sputum or bronchoalveolar lavage fluid (BALF). The MTB culture is generally considered as the gold standard for tuberculosis diagnosis but requires a relatively long time. Solid media culture can take 4 to 8 weeks. Although liquid media culture is more sensitive and rapid than solid media culture, it requires several weeks and is more likely to be contaminated.3 Acid-fast bacillus (AFB) staining and microscopy is rapid, convenient, and inexpensive; therefore, it is widely used in the detection of MTBC in clinical practice. However, the positive rate of AFB staining is relatively low and it cannot distinguish nontuberculosis mycobacteria from MTBC.4 In view of the time-consuming and low positive rates of traditional methods, faster molecular and microbial diagnosis techniques are needed to facilitate early diagnosis.5 In 2010, the World Health Organization recommended the use of Xpert MTB/RIF, a novel nucleic acid amplification test that can simultaneously detect MTBC and rifampicin resistance.6 A simple and rapid method, Xpert MTB/RIF has been proven to be sensitive and highly specific in the early diagnosis of pulmonary tuberculosis.7
Metagenomic next-generation genome sequencing (mNGS) is a new method based on sequence identification of pathogenic microorganisms, which has been used to diagnose pathogens causing various infectious syndromes, including respiratory tract infections,8,9 bloodstream infection,10,11 meningitis, and encephalitis.12,13 However, the detection efficiency in the BALF specimens of patients suspected of PTB still lacks clinical application examples. Here, we compared the diagnostic performance of mNGS for detection of MTBC in BALF with Xpert MTB/RIF, MTB culture, and AFB staining.
Methods
Study Subjects and Design
We retrospectively reviewed patients suspected of having PTB at the Tongji Hospital of Huazhong University of Science and Technology between January 2020 and May 2021. Inclusion criteria were (1) clinically suspected PTB patients with symptoms of tuberculosis infection such as subacute cough, fever, night sweat, and loss of weight; (2) chest computed tomography or X-ray with miliary pulmonary nodules or patchy shadows; and (3) BALF were submitted to mNGS test and MTB culture, Xpert MTB/RIF, or AFB staining. Exclusion criteria were (1) no paired MTB culture or Xpert MTB/RIF for the mNGS test and (2) incomplete medical history.
The criteria for the diagnosis of PTB were (1) MTB culture was positive, (2) Xpert MTB/RIF was positive, (3) mNGS was positive, or (4) the pathological features of lung biopsy were consistent with those of tuberculosis. Otherwise, patients were classified as non-PTB cases, including infectious diseases caused by pathogens other than MTB or noninfectious diseases such as tumors and autoimmune diseases.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tongji Hospital, Huazhong University of Science and Technology (No. TJ-IRB20220167), and individual consent for this retrospective analysis was waived.
BALF Specimens
Bronchoalveolar lavage fluid was collected by bronchoscopy at the sites of lung lesions. All patients signed an informed consent form before undergoing bronchoscopy. Saline (60 mL-100 mL) was injected into the bronchial lumen of the segment and withdrawn after washing briefly. The qualified BALF (at least 20 mL) was divided into 4 parts, 3 of which were tested by AFB staining, MTB culture, and Xpert MTB/RIF, and the rest were stored in a sterile container at –20°C, and sent to BGI for mNGS testing.
mNGS and Analysis
The process of mNGS consisted of sample processing, nucleic acid extraction, library generation, and bioinformatic pipeline analysis as described previously.14 A RefSeq analysis at the time of testing included 6350 bacterial genomes sequence (including 133 mycobacteria and 122 Mycoplasma/Chlamydia/Rickettsia), 1798 genomes of DNA viruses, 1064 genomes of fungi, and 234 genomes of parasites associated with human diseases. Mycobacterium tuberculosis was considered positive when at least 1 read is mapped to MTBC (strictly mapped to the number of sequences at the genus level).
Statistical Analysis
The sensitivity, specificity, positive predictive value, and negative predictive value of mNGS, Xpert MTB/RIF, MTB culture, and AFB staining were calculated. McNemar’ s test for a paired 4-fold table were conducted for comparing the results of mNGS and Xpert MTB/RIF, MTB culture, and AFB staining. Data analysis was performed with SPSS 22.0. Receiver operating characteristic (ROC) curve was plotted with MedCalc software (MedCalc Software) among different groups, and the area under the curve (AUC) was calculated. P values <.05 were considered significant, and all tests were two tailed.
Results
Characteristics of Participants
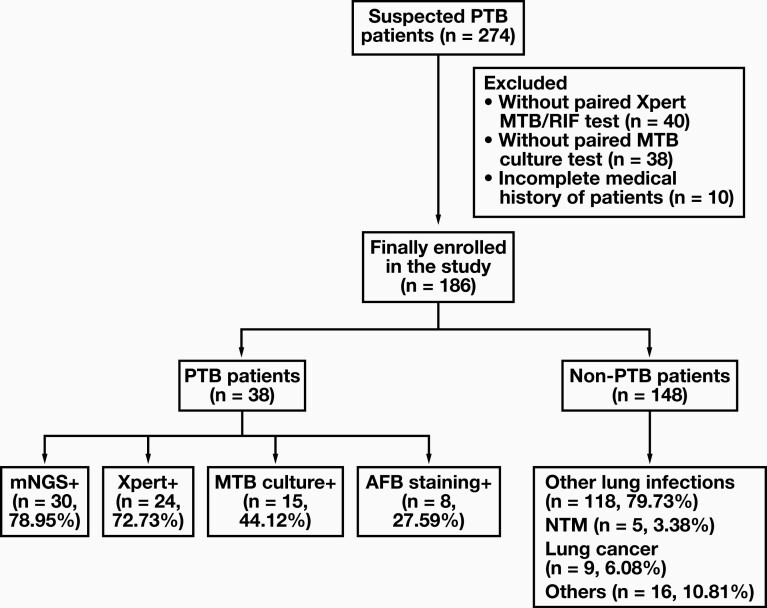
A total of 274 patients presenting with suspected PTB between January 2020 and May 2021 were initially included in our study. Eighty-eight patients were excluded from the study according to the exclusion criteria. Of these, 78 patients did not have a paired MTB culture test or Xpert MTB/RIF test, and 10 of them had an incomplete medical history. Finally, 186 patients with suspected PTB were eventually included in the study, of which 38 were diagnosed with PTB and 148 were diagnosed as non-PTB. Among the 38 PTB patients, 37 patients met the microbiological or molecular diagnostic criteria, and 1 patient was diagnosed based on pathological findings. Thirty-one patients were diagnosed with PTB for the first time and 7 were diagnosed with PTB and had received antituberculosis treatment before. Among the 148 non-PTB patients, 118 cases were diagnosed as lung infections caused by other pathogens, 5 cases were diagnosed as nontuberculosis mycobacteria lung disease, 9 cases were diagnosed as lung cancer, and 16 cases were diagnosed as other diseases (FIGURE 1).
FIGURE 1.
Flow chart of patient enrollment and test results. A total of 186 patients were ultimately included for further analysis. Bronchoalveolar lavage fluid samples were tested using metagenomic next-generation sequencing (mNGS), Xpert MTB/RIF, Mycobacterium tuberculosis (MTB) culture, and acid-fast bacillus (AFB) staining. Patients were diagnosed with pulmonary tuberculosis (PTB) and nonpulmonary tuberculosis (non-PTB) as described in the Methods. NTM, nontuberculosis mycobacteria lung disease.
The average age of PTB patients was 49.00 years (range, 30.25–57.00), and 65.8% were male. For non-PTB patients, the average age was 55.00 years (range, 42.25–62.75), and 62.2% were male (TABLE 1). There was no significant difference in underlying lung diseases (chronic obstructive pulmonary disease, bronchiectasis, connective tissue disease–related lung disease, or idiopathic pulmonary fibrosis) between the 2 groups. Patients with PTB had a higher proportion of previous history of tuberculosis (P = .036, TABLE 1). Therefore, it was necessary to routinely screen for active tuberculosis for patients with a history of tuberculosis. There was no significant difference in extrapulmonary diseases including diabetes, hypertension, chronic liver disease, and hematological malignancy between the 2 groups.
TABLE 1.
Characteristics of patients with PTB and non-PTBa
| PTB (n = 38) | Non-PTB (n = 148) | P value | |
|---|---|---|---|
| Demographic data | |||
| Average age (range), y | 49.00 (30.25–57.00) | 55.00 (42.25–62.75) | .057 |
| Male | 25 (65.8) | 92 (62.2) | .680 |
| Female | 13 (34.2) | 56 (37.8) | .680 |
| Underlying pulmonary disease | |||
| COPD | 0 | 4 (2.7) | — |
| Bronchiectasis | 0 | 11 (7.4) | — |
| Idiopathic pulmonary fibrosis | 0 | 5 (3.4) | — |
| Connective tissue disease-associated pulmonary disease | 0 | 18 (12.2) | — |
| Previous history of tuberculosis | 7 (18.4) | 9 (6.1) | .036 |
| Underlying extrapulmonary diseases | |||
| Diabetes | 3 (7.9) | 0 | — |
| Hypertension | 1 (2.6) | 0 | — |
| Chronic liver disease | 3 (7.9) | 0 | — |
| Hematological malignancy | 3 (7.9) | 2 (1.4) | .096 |
COPD, chronic obstructive pulmonary disease; PTB, pulmonary tuberculosis.
aData are given as number (%) except where noted.
Detection Results and Concordance of mNGS, Xpert, and MTB Culture Using BALF
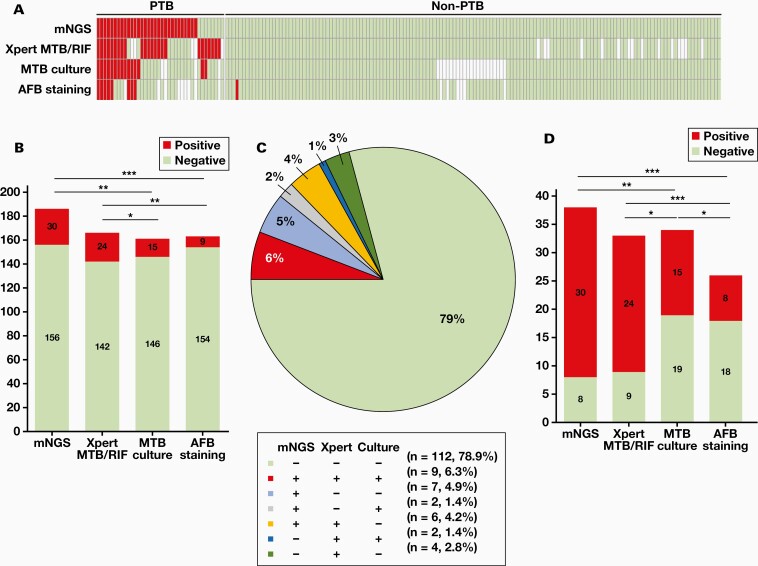
The results of mNGS, Xpert MTB/RIF, MTB culture, and AFB staining for MTBC detection in BALF were summarized and are shown in FIGURE 2A. Of the 186 enrolled patients, mNGS and Xpert showed similar overall MTBC detection efficiency (26/166, 15.66% vs 24/166, 14.46%, P > .05). However, mNGS and Xpert were more effective than MTB culture (27/161, 16.77% vs 15/161 9.32%, P < .01) and AFB staining (22/171, 12.87% vs 9/171, 5.26%, P < .001) (FIGURE 2B, Supplementary Table 1). There were 142 patients whose BALF specimens were tested by mNGS, Xpert MTB/RIF, and culture at the same time. Among these 142 patients, the results of mNGS, Xpert MTB/RIF, and MTB culture matched completely in 121 (85.2%) patients, including 9 PTB patients with positive findings and 112 non-PTB patients with negative findings in all 3 methods. The detection results of the remaining 21 samples were conflicting, including 7 samples being positive by mNGS only (4.9%), 4 samples being positive by Xpert MTB/RIF only (2.8%), 6 samples being positive for mNGS and Xpert MTB/RIF (4.2%), 2 samples being positive for mNGS and MTB culture (1.4%), and 2 samples being positive for Xpert MTB/RIF and MTB culture (1.4%). There were no samples that were positive by MTB culture only (FIGURE 2C).
FIGURE 2.
Comparison of metagenomic next-generation sequencing (mNGS), Xpert MTB/RIF, Mycobacterium tuberculosis (MTB) culture, and acid-fast bacillus (AFB) staining for the detection of MTB complex (MTBC). A, Heatmap depicting the identification of MTBC by the 4 methods in bronchoalveolar lavage fluid samples. Each column represents a patient. B, The number of positive and negative findings by each of the 4 methods in all 186 enrolled patients. C, For mNGS, Xpert MTB/RIF, and MTB culture, the number and percentage of patients with positive or negative findings by 1, 2, or 3 methods in 142 patients are shown in the pie chart. D, The number of positive and negative findings by each of the 4 methods in 38 pulmonary tuberculosis (PTB) patients. *P < .05; **P < .01; ***P < .001.
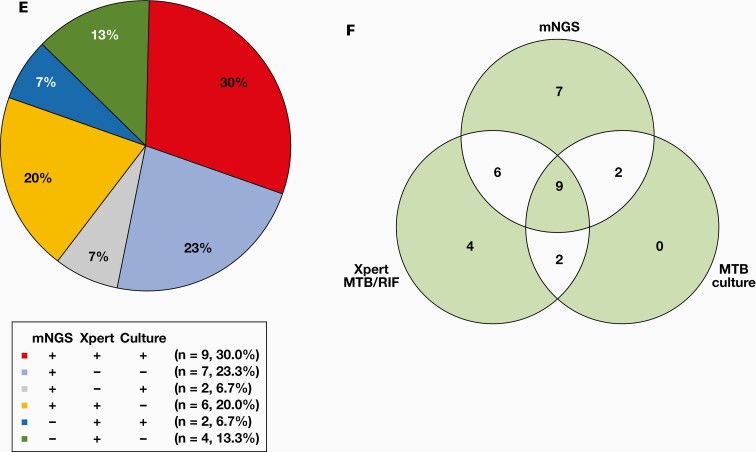
We also analyzed the detection efficiency of the 4 methods in the 38 PTB patients (FIGURE 2D, Supplementary Table 2). Of the 38 PTB patients, 30 were tested using mNGS, Xpert MTB/RIF, and MTB. Among these 30 BALF samples, 9 (9/30, 30.0%) were all positive by mNGS, Xpert MTB/RIF, and MTB culture, 6 (6/30, 20.0%) were positive by mNGS and Xpert MTB/RIF, 2 (2/30, 6.7%) were positive by mNGS and MTB culture, and 7 (7/30, 23.3%) were only positive by mNGS (FIGURE 2E and 2F).
Diagnostic Performance of mNGS, Xpert, Culture, and AFB Staining Using BALF
The diagnostic performance of mNGS, Xpert MTB/RIF, MTB culture, and AFB staining using BALF specimens was evaluated. For the 38 PTB patients, the sensitivity of mNGS (78.95%, 30/38) was similar to that of Xpert MTB/RIF (72.73%, 24/33) but significantly higher than the sensitivity of MTB culture (44.12%, 15/34) and AFB staining (27.59%, 8/29) (TABLE 2). The negative predictive values of mNGS, Xpert MTB/RIF, MTB culture, and AFB staining were 94.87%, 93.66%, 86.99%, and 87.04%, respectively (TABLE 2).
TABLE 2.
Diagnostic performance of mNGS, Xpert MTB/RIF, MTB culture and AFB staining for PTB using BALFa
| PTB | non-PTB | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95%CI) | |
|---|---|---|---|---|---|---|
| mNGS | 78.95% (0.622-0.899) | 100% (0.968-1.000) | 100% (0.859-1.000) | 94.87% (0.898-0.976) | ||
| Positive | 30 | 0 | ||||
| Negative | 8 | 148 | ||||
| Xpert MTB/RIF | 72.73% (0.542-0.861) | 100% (0.965-1.000) | 100% (0.828-1.000) | 93.66% (0.880-0.969) | ||
| Positive | 24 | 0 | ||||
| Negative | 9 | 133 | ||||
| MTB culture | 44.12% (0.276-0.619) | 100% (0.963-1.000) | 100% (0.747-1.000) | 86.99% (0.802-0.918) | ||
| Positive | 15 | 0 | ||||
| Negative | 19 | 127 | ||||
| AFB staining | 27.59% (0.134-0.475) | 99.30% (0.956-0.999) | 88.89% (0.507-0.994) | 87.04% (0.806-0.916) | ||
| Positive | 8 | 1 | ||||
| Negative | 21 | 141 |
AFB, acid-fast bacillus; mNGS, metagenomic next-generation sequencing; MTB, Mycobacterium tuberculosis; NPV, negative predictive value (the number of true negative cases detected by this method/the number of all negative cases detected by this method × 100%); PPV, positive predictive value (the number of true positive cases detected by this method/the number of all positive cases detected by this method × 100%); PTB, pulmonary tuberculosis.
aSensitivity is the number of positive cases detected by this method/the number of PTB cases by clinical diagnosis × 100%. Specificity is the number of negative cases detected by this method/excluded PTB cases × 100%.
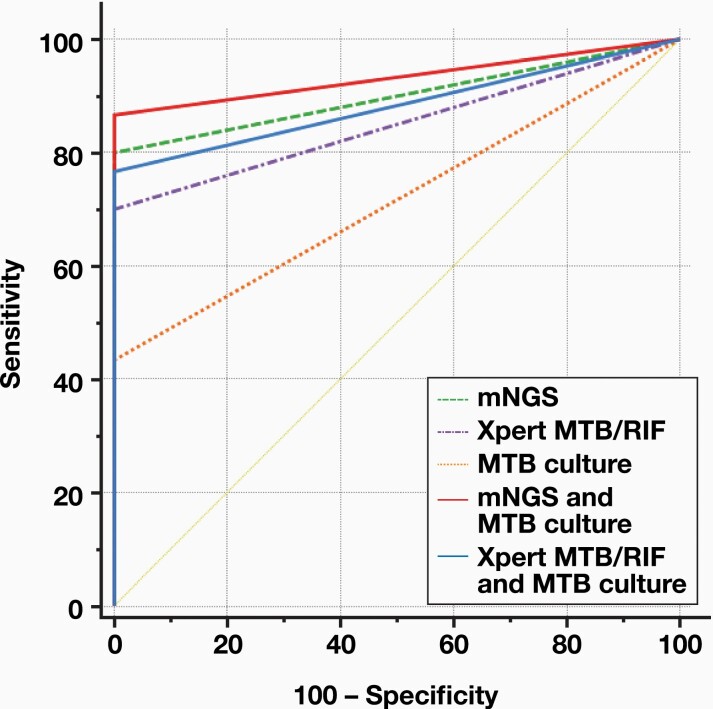
To better understand the diagnostic performance of mNGS, Xpert MTB/RIF, MTB culture, and the combination of the 3 methods, we plotted the ROC curve and calculated the AUC. The AUC for mNGS (0.900, 95% CI:0.827, 0.973) was larger than that of the other tests (0.850, 95% CI: 0.767, 0.933 for Xpert MTB/RIF; 0.717, 95% CI: 0.626, 0.807 for MTB culture). The AUC for MTB culture in combination with mNGS (0.933, 95% CI: 0.871, 0.995) was larger than that of the MTB culture in combination with Xpert MTB/RIF (0.883, 95% CI: 0.806, 0.960). These results suggested that mNGS has a better diagnostic performance than other methods (FIGURE 3).
FIGURE 3.
The receiver operating characteristic (ROC) curves of metagenomic next-generation sequencing (mNGS), Xpert MTB/RIF, Mycobacterium tuberculosis (MTB) culture, the combination of mNGS with MTB culture, and the combination of Xpert MTB/RIF and MTB culture.
Burden of MTBC Affects the Results of mNGS
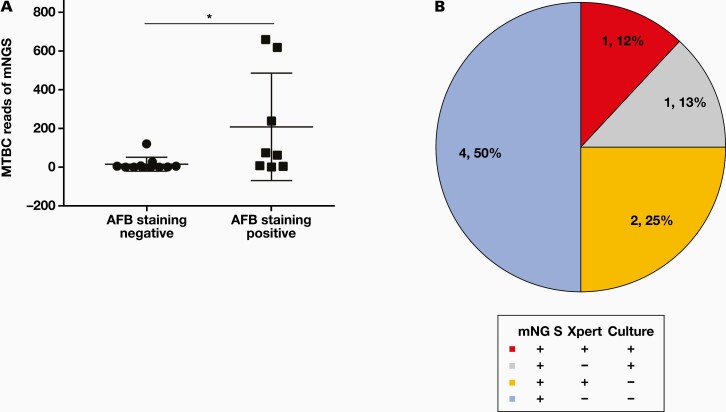
The cell wall of MTBC is difficult to destroy, and this leads to the difficulty of DNA extraction for mNGS. Therefore, the sensitivity of mNGS in detecting MTBC could be affected by the burden of MTBC. We compared reads numbers of MTBC between the patients with smear AFB staining positive and negative results. Of the 30 mNGS-positive samples, we found that the reads number of MTBC was significantly higher in AFB staining-positive groups (FIGURE 4A). Because positive findings in AFB staining indicate high burden of MTBC, our result suggests that the MTBC burden affects the reads number of mNGS.
FIGURE 4.
The Mycobacterium tuberculosis complex (MTBC) burden affects the reads number of MTBC detected by next-generation sequencing (mNGS). A, The reads number is greater in acid-fast bacillus (AFB) staining positive samples than in AFB staining negative samples. B, The detection results of mNGS, Xpert MTB/RIF, and MTB culture in patients with only 1 read mapping to either the species or genus of MTBC by mNGS. *P < .05.
In our study, there were 8 mNGS-positive samples with 1 read mapped to either the species or genus of MTBC. Of these 8 samples, 4 cases were also positive by Xpert or MTB culture and the other 4 cases (50%) were only positive by mNGS (FIGURE 4B). Based on the low possibility of MTBC contamination during mNGS testing 14,15 and the previous report that samples with only 1 read of MTBC were all confirmed to be PTB,16 the other 4 cases were also diagnosed as PTB.
Discussion
Recently, the sensitivity of mNGS to detect mycobacteria tuberculosis in clinical specimens has received great attention due to its short turnaround time and unbiased pathogen detection.17 In the present study, we demonstrated that the sensitivity of mNGS in the diagnosis of PTB using BALF specimen is similar to Xpert MTB/RIF but significantly higher than AFB staining and MTB culture. The combination of mNGS and MTB culture may further improve the diagnosis of PTB.
A cohort of 186 patients suspected of having PTB infection were enrolled in this study, of which 38 patients were finally diagnosed with PTB. In terms of diagnostic performance in BALF samples, mNGS exhibited a sensitivity of 78.95%, which was similar to Xpert MTB/RIF (72.73%) but much higher than the sensitivity of MTB culture (44.12%) and AFB staining (27.59%). The AUC for MTB culture in combination with mNGS (0.933, 95% CI: 0.871, 0.995) was larger than that of MTB culture combined with Xpert MTB/RIF (0.883, 95% CI: 0.806, 0.960), which is consistent with other studies.15,18
Metagenomic next-generation sequencing has been widely used in the clinic in etiological diagnosis of various infectious diseases. Miao and colleagues14 compared the performance of mNGS and culture in the detection of pathogens and assessed the effect of the use of antibiotics on detection performance. Their results suggest that mNGS is more sensitive to pathogen detection and is not affected by the use of antibiotics. Similarly, Zhou and colleagues18 reported that compared with Xpert, mNGS had a similar MTBC diagnostic ability and may be more suitable for scarce samples, such as cerebrospinal fluid in patients with suspected tuberculosis. Liu and colleagues16 found that the sensitivity of mNGS in MTBC detection using BALF was lower than Xpert in PTB patients before anti-TB therapy but similar to Xpert in PTB patients after the use of an anti-TB drug. Our study demonstrated that the sensitivity of mNGS in the diagnosis of PTB using BALF specimen is similar to Xpert MTB/RIF. However, compared with the cheaper and faster GeneXpert platform in the diagnosis for PTB, the biggest benefit of mNGS is “agnostic testing” in difficult-to-diagnose infections.
Previous studies had investigated the factors associated with positive findings of MTBC in BALF by mNGS. Liu et al16 found that positive MTBC detection by mNGS was affected by vitamin D, erythrocyte sedimentation rate, tuberculosis initial treatment or retreatment, and cavity in chest imaging but not by the use of anti-TB drugs within 3 months. In this study, we compared the reads numbers of MTBC between patients in AFB staining positive and negative BALF samples. We noticed that the reads number of MTBC was significantly higher in AFB staining positive samples, suggesting that the reads number of mNGS was affected by the burden of MTBC.
Mycobacteria are characterized by thick cell walls and its DNA is difficult to extract.19 Also, the possibility for contamination of MTBC in BALF samples is lower than in other sample types, including sputum. Therefore, even when 1 specific read of a taxon is mapped to either the species or genus level, MTBC infection should be considered.14,15 Liu et al16 reported 34 cases with 1 read mapping, of which 70.6% (24/34) cases were confirmed with Xpert or culture and the rest 29.4% (10/34) cases were clinically verified by anti-TB therapy. In our study, there were 8 cases with 1 read mapping, of which 50.0% (4/8) had positive findings of Xpert or MTB culture. Although the other 4 cases were not confirmed by other tests or anti-TB therapy, based on the rationale and the study mentioned, these 4 cases were diagnosed as PTB.
The limitations of our study are that this was a retrospective study and the sample size was relatively small. This might have introduced bias in our interpretation of the data. Therefore, prospective studies with large sample sizes are required to evaluate the diagnostic efficiency of mNGS in the diagnosis of PTB using BALF specimens.
Conclusions
The sensitivity of mNGS in the diagnosis of PTB using BALF specimen is similar to Xpert MTB/RIF but much higher than MTB culture and AFB staining. The use of mNGS in combination with MTB culture may further improve the diagnosis of PTB.
Supplementary Material
Glossary
Abbreviations
- mNGS
metagenomic next-generation sequencing
- MTBC
Mycobacterium tuberculosis complex
- PTB
pulmonary tuberculosis
- BALF
bronchoalveolar lavage fluid
- AFB
acid-fast bacillus
- ROC curve
receiver operating characteristic curve
- AUC
area under the curve
Contributor Information
Jiali Gao, Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Respiratory Diseases, National Health Commission of the People’s Republic of China, and National Clinical Research Center for Respiratory Diseases, Wuhan, China.
Lu Zhao, Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Respiratory Diseases, National Health Commission of the People’s Republic of China, and National Clinical Research Center for Respiratory Diseases, Wuhan, China.
Gongqi Chen, Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Respiratory Diseases, National Health Commission of the People’s Republic of China, and National Clinical Research Center for Respiratory Diseases, Wuhan, China.
Chunli Huang, Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Respiratory Diseases, National Health Commission of the People’s Republic of China, and National Clinical Research Center for Respiratory Diseases, Wuhan, China.
Weiqiang Kong, Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Respiratory Diseases, National Health Commission of the People’s Republic of China, and National Clinical Research Center for Respiratory Diseases, Wuhan, China.
Yuchen Feng, Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Respiratory Diseases, National Health Commission of the People’s Republic of China, and National Clinical Research Center for Respiratory Diseases, Wuhan, China.
Guohua Zhen, Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Key Laboratory of Respiratory Diseases, National Health Commission of the People’s Republic of China, and National Clinical Research Center for Respiratory Diseases, Wuhan, China.
Funding
This work was supported by National Natural Science Foundation of China (grant No. 82170036).
Conflict of Interest Disclosure
The authors have nothing to disclose.
REFERENCES
- 1. Harding E. WHO global progress report on tuberculosis elimination. Lancet Respir Med. 2020;8:19. doi: 10.1016/s2213-2600(19)30418-7 [DOI] [PubMed] [Google Scholar]
- 2. Zumla A, George A, Sharma V, et al. The WHO 2014 Global Tuberculosis Report—further to go. Lancet Global Health. 2015;3:e10–e12. doi: 10.1016/S2214-109X(14)70361-4 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Implementing Tuberculosis Diagnostics: A Policy Framework. WHO/HTM/TB/201511. World Health Organization; 2015. [Google Scholar]
- 4. Philip N, William T, John DV. Diagnosis of tuberculous meningitis: challenges and promises. Malays J Pathol. 2015;37:1–9. [PubMed] [Google Scholar]
- 5. Rock RB, Olin M, Baker CA, et al. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev. 2008;21:243–261, table of contents. doi: 10.1128/CMR.00042-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO Guidelines Approved by the Guidelines Review Committee. Policy Statement: Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System. World Health Organization; 2011. [PubMed] [Google Scholar]
- 7. Horne DJ, Kohli M, Zifodya JS, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2019;6:CD009593. doi: 10.1002/14651858.CD009593.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langelier C, Zinter MS, Kalantar K, et al. Metagenomic sequencing detects respiratory pathogens in hematopoietic cellular transplant patients. Am J Respir Crit Care Med. 2018;197:524–528. doi: 10.1164/rccm.201706-1097le [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robert S, Krista Q, Keith S, et al. Viral pathogen detection by metagenomics and pan-viral group polymerase chain reaction in children with pneumonia lacking identifiable etiology. J Infect Dis. 2017:1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol. 2019;4:663–674. doi: 10.1038/s41564-018-0349-6 [DOI] [PubMed] [Google Scholar]
- 11. Goggin KP, Gonzalez-Pena V, Inaba Y, et al. Evaluation of plasma microbial cell-free DNA sequencing to predict bloodstream infection in pediatric patients with relapsed or refractory cancer. JAMA Oncol. 2019;6:552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson MR, Sample HA, Zorn KC, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380:2327–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang JZ, Zheng P, Sun HM, et al. Next-generation sequencing combined with routine methods to detect the pathogens of encephalitis/meningitis from a Chinese tertiary pediatric neurology center. J Infect. 2019;78:409–421. [DOI] [PubMed] [Google Scholar]
- 14. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67:S231–S240. doi: 10.1093/cid/ciy693 [DOI] [PubMed] [Google Scholar]
- 15. Shi CL, Han P, Tang PJ, et al. Clinical metagenomic sequencing for diagnosis of pulmonary tuberculosis. J Infect. 2020;81:567–574. doi: 10.1016/j.jinf.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 16. Liu X, Chen Y, Ouyang H, et al. Tuberculosis diagnosis by metagenomic next-generation sequencing on bronchoalveolar lavage fluid: a cross-sectional analysis. Int J Infect Dis. 2021;104:50–57. doi: 10.1016/j.ijid.2020.12.063 [DOI] [PubMed] [Google Scholar]
- 17. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi: 10.1146/annurev-pathmechdis-012418-012751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou X, Wu H, Ruan Q, et al. Clinical evaluation of diagnosis efficacy of active mycobacterium tuberculosis complex infection via metagenomic next-generation sequencing of direct clinical samples. Front Cell Infect Microbiol. 2019;9:351. doi: 10.3389/fcimb.2019.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Doughty EL, Sergeant MJ, Adetifa I, et al. Culture-independent detection and characterisation of Mycobacterium tuberculosis and M. africanum in sputum samples using shotgun metagenomics on a benchtop sequencer. PeerJ. 2014;2:e585. doi: 10.7717/peerj.585a [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.