Abstract
Background:
Infliximab seems to be the most efficacious of the three available anti-TNF agents for ulcerative colitis (UC) but little is known when it is used as the second anti-TNF.
Objectives:
To compare the clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in UC patients.
Design:
Retrospective observational study.
Methods:
Patients from the ENEIDA registry treated consecutively with infliximab and a subcutaneous anti-TNF (or vice versa), naïve to other biological agents, were identified and grouped according to the administration route of the first anti-TNF into IVi (intravenous initially) or SCi (subcutaneous initially).
Results:
Overall, 473 UC patients were included (330 IVi and 143 SCi). Clinical response at week 14 was 42.7% and 48.3% in the IVi and SCi groups (non-statistically significant), respectively. Clinical remission rates at week 52 were 32.8% and 31.4% in the IVi and SCi groups (nonsignificant differences), respectively. A propensity-matched score analysis showed a higher clinical response rate at week 14 in the SCi group and higher treatment persistence in the IVi group. Regarding long-term outcomes, dose escalation and discontinuation due to the primary failure of the first anti-TNF and more severe disease activity at the beginning of the second anti-TNF were inversely associated with clinical remission.
Conclusion:
The use of a second anti-TNF for UC seems to be reasonable in terms of efficacy, although it is particularly reduced in the case of the primary failure of the first anti-TNF. Whether the second anti-TNF is infliximab or subcutaneous does not seem to affect efficacy.
Keywords: adalimumab, anti-TNF, golimumab, infliximab, switch, ulcerative colitis
Plain language summary
Clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in patients with ulcerative colitis treated with two consecutive anti-TNF agents. Data from the ENEIDA registry
Background: Infliximab seems to be the most efficacious of the three available anti-TNF agents for ulcerative colitis (UC), but little is known when it is used as the second anti-TNF. Objectives: To compare the clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in UC patients. Design: Retrospective observational study. Methods: Patients from the ENEIDA registry treated consecutively with infliximab and a subcutaneous anti-TNF (or vice versa), naïve to other biological agents, were identified and grouped according to the administration route of the first anti-TNF into IVi (intravenous initially) or SCi (subcutaneous initially). Results: Overall, 473 UC patients were included (330 IVi, 143 SCi). Clinical response at week 14 was 42.7% and 48.3% in the IVi and SCi groups (non-statistically significant), respectively. Clinical remission rates at week 52 were 32.8% and 31.4%, in the IVi and SCi groups (nonsignificant differences), respectively. A propensity-matched score analysis showed a higher clinical response rate at week 14 in the SCi group and higher treatment persistence in the IVi group. Regarding long-term outcomes, dose escalation and discontinuation due to the primary failure of the first anti-TNF and more severe disease activity at the beginning of the second anti-TNF were inversely associated with clinical remission. Conclusion: The use of a second anti-TNF for UC seems to be reasonable in terms of efficacy, although it is particularly reduced in the case of the primary failure of the first anti-TNF. Whether the second anti-TNF is infliximab or subcutaneous does not seem to affect efficacy.
Introduction
Half of the patients suffering from ulcerative colitis (UC), a chronic inflammatory condition of immune-mediated origin, are easily managed with aminosalicylates. Among those who receive at least one course of steroids, up to 80% will sooner or later be exposed to immunosuppressants or biologicals due to steroid dependency, refractoriness, or intolerance to aminosalicylates. 1 Biological agents were introduced in the therapeutic armamentarium of inflammatory bowel disease (IBD) at the end of the 1990s, with anti-TNF agents being the first to be used and still the most popular. The anti-tumour necrosis factor (TNF) agents, infliximab (IFX), adalimumab (ADA), and golimumab (GLM), were licensed for the treatment of UC in the European Union in 2005, 2012, and 2014, respectively. IFX, the only anti-TNF that is administered intravenously, had demonstrated the greatest efficacy in randomized controlled trials2,3 with long-term clinical response and remission rates of 53% and 39%, respectively.4,5 Subcutaneously administered ADA 6 and GLM 7 obtained clinical remission rates of 22% and 27%, respectively, at 1 year, although efficacy data should not be compared across studies as populations and selection criteria may be significantly different.
The launch of IFX and ADA biosimilars and their beneficial impact on the economic burden of IBD led these anti-TNF agents to remain in the first line among selective immunosuppressant therapies in many countries. Unfortunately, anti-TNF agents must often be discontinued because of primary nonresponse, secondary loss of response, or adverse effects (AE). Until recently, switching from one anti-TNF to another was the only alternative to colectomy in this scenario. The licensing of new selective immunosuppressants for UC, such as vedolizumab, 8 tofacitinib, 9 and ustekinumab, 10 raised the possibility of switching from anti-TNFs to other drugs with different mechanisms of action.
In the absence of head-to-head studies, network meta-analyses of randomized controlled trials suggest that IFX is superior to GLM and ADA for the treatment of UC,2,3 and even to vedolizumab, ustekinumab, or tofacinitib, as stated in an updated network meta-analysis. 11 Recently, a retrospective French study comparing the efficacy of IFX and vedolizumab in clinical practice as second-line therapies after the failure of a subcutaneous anti-TNF found a higher treatment persistence for vedolizumab. The authors suggested that the change in the mechanism of action may be the best therapeutic option after failure or intolerance to a subcutaneous anti-TNF in patients with UC. 12 However, the cost-effectiveness of switching to a second anti-TNF (and, interestingly, whether IFX is used as the first or the second), as opposed to a change in the mechanism of action, has not been evaluated suitably. IFX is a chimeric immunoglobulin G (IgG) recombinant monoclonal antibody, whereas ADA and GLM are completely human IgG1 antibodies. This confers potentially different immunogenicity and, therefore, a hypothetic significantly higher risk of secondary loss of response or intolerance to IFX. Moreover, subcutaneous anti-TNF agents do not have cross-immunogenicity with IFX. Finally, the recent licensing of subcutaneous IFX biosimilar CT-P13 13 may even revive the debate on the appropriateness of switching to a second anti-TNF agent instead of changing to a drug with a different mechanism of action.
In this study, we aim to compare the clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in UC patients.
Methods
This is a multicenter, retrospective study based on the ENEIDA registry (a nationwide, prospectively maintained registry of IBD patients promoted by the Spanish Working Group in IBD – GETECCU). 14 Briefly, the ENEIDA registry includes demographic and epidemiological data of IBD patients, as well as data on the phenotypic characteristics of IBD and IBD-related treatments, among others.
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Supplemental Material).
Study population
All adult UC patients ever treated with both intravenous and subcutaneous anti-TNF agents were identified from the ENEIDA database. To be included in the study, patients had to have been diagnosed with UC, treated consecutively with intravenous IFX and a subcutaneous anti-TNF (or vice versa) according to the licensed schedule, received at least one administration of both anti-TNF agents, and be naïve to other biological agents at the time the first anti-TNF was started. Subcutaneous IFX was not available at the time of data collection. Patients treated for extraintestinal manifestations, perianal disease, pouchitis, or who had been treated in the setting of a controlled clinical trial were excluded, as well as patients whose missing data precluded an assessment of clinical efficacy. Drug choice was at the discretion of the treating physician, as were decisions regarding dose escalation and treatment discontinuation. Patients were grouped according to the administration route of the first anti-TNF as either ‘initially intravenous’ (IVi) or ‘initially subcutaneous’ (SCi).
In addition to the epidemiological data and phenotypic characteristics of UC, the following variables relating to anti-TNF treatments were specifically collected: date of the first and last administrations, concomitant immunosuppressant drugs, clinical disease activity as measured by the partial Mayo score at baseline, week 14 and week 52 of each treatment, the need for dose escalation and treatment discontinuation, and the reason for discontinuation (primary failure, secondary loss of response, or intolerance). Due to the study design, not all the patients had endoscopic assessments at baseline or during follow-up, but endoscopic findings were collected whenever available. Follow-up started at the beginning of the second anti-TNF until treatment discontinuation, colectomy, death, or data collection, whichever came first.
Definitions
The main outcomes, referring to the second anti-TNF treatment, were as follows: (a) clinical response and remission at week 14; (b) clinical response and remission at week 52; (c) secondary loss of response; (d) dose escalation, and (e) treatment persistence.
Clinical remission was defined by a partial Mayo score ⩽1 and clinical response by a decrease in the partial Mayo score of at least three points from baseline (beginning of the second anti-TNF) with a decrease of at least one point in the rectal bleeding subscore. Primary failure was considered if remission was never achieved during treatment. Secondary loss of response was defined as a clinical relapse after having achieved remission. Dose escalation was defined as an increase in the frequency and/or dose of the anti-TNF. Treatment failure was defined as treatment discontinuation or colectomy. Finally, moderate-to-severe disease activity was defined by a partial Mayo score of ⩾5.
Statistical analysis
Statistical analyses were performed using the R (v.4.02) (R Core Team, 2020) computer application and the Matchl library. Categorical variables were presented as absolute numbers and frequencies and compared using a chi-squared test for polytomous variables and Fisher’s test for dichotomous variables. Quantitative variables were described with the usual tools of centrality (mean, median) and variability (standard deviation, range, and interquartile range) as needed, and compared using Student’s t-test (for normally distributed variables) and the Mann–Whitney’s U test (for non-normally distributed variables). A univariate logistic regression analysis was performed, and those variables with a p-value of <0.05, together with potential confounding factors, were included in a multivariate logistic regression analysis. Kaplan–Meier curves were plotted for treatment persistence and dose-escalation survival and compared between the two study groups using the log-rank test.
In a secondary analysis, considering that the assignment of patients to the study groups was not randomized, a propensity-matched score analysis was also performed with a logistic regression model. The dependent variable was the route of administration of the first anti-TNF (IVi or SCi) and 13 covariates were selected using the significant variables found in those analyses and seven confounding variables. Since the observed frequencies were different in the IVi and the SCi groups, the sample was balanced using the nearest neighbor propensity score matching method, using the potential confounding factors and variables that were significant in the univariate analysis. After matching for propensity scores, a balanced sample was obtained in which the IVi and SCi groups showed similar propensity score distributions with minimal differences in covariates between groups.
Results
Main features of the cohort and the first anti-TNF treatment
Of the more than 14,000 IBD patients included in the ENEIDA registry and treated with biological agents at the time of data extraction, 878 met the inclusion criteria. After excluding those patients with relevant missing data or those treated for indication other than active UC, a total of 473 UC patients were included, of whom 330 (70%) belonged to the IVi group and 143 (30%) to the SCi group. Patients in the IVi group were mostly treated with the originator of IFX (Remicade®) (n = 293; 90%) and only 10% were treated with CT-P13 (IFX biosimilar). All the first anti-TNF treatments were started between 2005 and 2018. All second anti-TNF treatments were started between 2007 and 2019. Patients in the SCi group were treated more frequently with ADA (n = 81; 57%) than with GLM (n = 62; 43%). Table 1 summarizes the baseline characteristics of the included patients at the time the first anti-TNF was started. Patients were mostly never or former smokers, with a wide range in age and disease duration; approximately one-half had extensive colitis and used concomitant immunosuppressants, and a vast majority presented moderate-to-severe clinical and endoscopic disease activity. Several differences were observed among the clinical and epidemiological features of both study groups. A longer UC duration and higher age and proportion of patients with moderate-to-severe clinical activity at the time the first anti-TNF was started were observed in the IVi group. Reasons for the discontinuation of the first anti-TNF were also different between study groups, whereas adverse events and secondary loss of response were the most common reasons for discontinuation in the IVi group (accounting for 40% and 31%, respectively), primary failure was the reason for discontinuation in 66% of the patients in the SCi group. Finally, a longer treatment persistence of the first anti-TNF was observed in the IVi group.
Table 1.
Baseline characteristics of patients related to the first anti-TNF used.
| Variable | Whole cohort (N = 473) | Intravenous infliximab (IVi group) (N = 330) | Subcutaneous anti-TNF (SCi group) (N = 143) | p |
|---|---|---|---|---|
| Female gender (%) | 224 (47) | 161 (49) | 63 (44) | 0.37 |
| Smoking status (%) | 0.40 | |||
| Active | 34 (8) | 28 (9) | 9 (6) | |
| Former | 121 (28) | 86 (26) | 45 (32) | |
| Never | 281 (64) | 216 (65) | 89 (62) | |
| Extensive colitis (%) | 279 (59) | 194 (59) | 85 (59) | 0.92 |
| Age at diagnosis, median (range) | 35 (7–79) | 36 (7–79) | 33 (15–74) | 0.02 |
| Age at the beginning of the first anti-TNF, mean (standard deviation) | 43 (14) | 44 (14) | 42 (15) | 0.17 |
| Time from diagnosis to treatment (months), median (range) | 45 (0–433) | 46 (0–433) | 42 (0–378) | 0.04 |
| Concomitant immunosuppressant treatment (%) | 258 (54) | 184 (56) | 74 (52) | 0.42 |
| Partial Mayo score, median (range) | 6 (0–9) | 6 (0–9) | 6 (0–9) | 0.04 |
| Moderate/severe partial Mayo score (%) | 327 (77) | 266 (81) | 99 (69) | 0.009 |
| Moderate/severe endoscopic Mayo subscore (%) | 326 (94) | 310 (94) | 132 (93) | 0.64 |
| Dose escalation (%) | 202 (43) | 146 (44) | 74 (52) | 0.31 |
| Treatment duration (weeks), median (range) | 32 (0–463) | 46 (0–463) | 17 (0–191) | <0.001 |
| Reason for discontinuation (%) | <0.001 | |||
| Adverse events | 110 (23) | 102 (31) | 8 (6) | |
| Primary failure | 164 (35) | 69 (21) | 95 (66) | |
| Loss of response | 171 (36) | 133 (40) | 38 (27) | |
| Other | 28 (6) | 26 (8) | 2 (1) |
Bold and italic characters within the Tables are used to highlight statistically significant results.
Main features and efficacy of the second anti-TNF
Patients in the IVi group were more often treated with ADA (n = 274; 83%) than with GLM (n = 56; 17%) as the second anti-TNF. Patients in the SCi group were treated in equal measure with CT-P13 (biosimilar) (n = 76; 53%) and the IFX originator (n = 67; 47%) as the second anti-TNF. Table 2 summarizes the baseline characteristics of the patients at the time the second anti-TNF was started. Contrary to what occurred with the first anti-TNF, the proportion of patients with a moderate-to-severe partial Mayo score and Mayo endoscopic subscore was significantly higher in the SCi group.
Table 2.
Baseline characteristics of patients related to the second anti-TNF used.
| Variable | Whole cohort (N = 473) | Initially intravenous (IVi group) (N = 330) | Initially subcutaneous (SCi group) (N = 143) | p |
|---|---|---|---|---|
| Time from diagnosis to treatment (months), median (range) | 65 (1–444) | 76 (1–444) | 50 (1–382) | 0.05 |
| Concomitant IMM treatment (%) | 247 (52) | 168 (51) | 79 (55) | 0.42 |
| Partial Mayo score, median (range) | 5 (0–9) | 5 (0–9) | 6 (0–9) | 0.003 |
| Moderate/severe partial Mayo score (%) | 297 (65) | 202 (61) | 105 (74) | 0.01 |
| Moderate/severe endoscopic Mayo score (%) | 270 (87) | 281 (85) | 131 (92) | 0.01 |
Bold and italic characters within the Tables are used to highlight statistically significant results.
Regarding efficacy in the whole cohort, the rates of clinical response and remission to the second anti-TNF were 44.4% and 29.2% at week 14, and 38.5% and 32.4% at week 52, respectively. In the univariate analysis, clinical response at week 14 was significantly less likely in the case of a shorter time on the first anti-TNF and when the reason for its discontinuation was primary failure. Regarding long-term outcomes, more severe disease activity at the beginning of the second anti-TNF, dose escalation and discontinuation due to the primary failure of the first anti-TNF were inversely associated with clinical remission at week 52 (Table 3).
Table 3.
Univariate analysis of factors associated with clinical response and remission with the second anti-TNF in the whole cohort (N = 473).
| Variable | Response at week 14 | p | Remission at week 52 | p | ||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||
| Female gender | 46% | 49% | 0.518 | 47.9% | 45.9% | 0.764 |
| Never smoker | 65.7% | 63% | 0.752 | 65.7% | 62.1% | 0.719 |
| Extensive colitis | 58.6% | 59.5% | 0.851 | 59.9% | 57.4% | 1 |
| Age at first anti-TNF, median (range interval) | 42 (20) | 42 (22) | 0.815 | 42 (20) | 44 (21) | 0.438 |
| Disease duration at the beginning of the first anti-TNF, median (range interval) | 43 (89) | 49.5 (91) | 0.661 | 45 (87) | 47 (106) | 0.872 |
| Concomitant immunosuppressant with first anti-TNF | 58.6% | 49.5% | 0.052 | 54.7% | 50.7% | 0.425 |
| Dose escalation of first anti-TNF | 46% | 38.6% | 0.112 | 47.9% | 32.4% | <0.001 |
| Cause for discontinuation of first anti-TNF | <0.001 | <0.001 | ||||
| Secondary loss of response | 34.6% | 38.1% | 37.5% | 33.1% | ||
| Primary partial/nonresponse | 41.8% | 25.7% | 39.2% | 25.7% | ||
| Adverse effect | 20.2% | 27.1% | 19.1% | 31.1% | ||
| Time on the first anti-TNF, median (range interval) | 28.1 (45.9) | 40.1 (75.6) | 0.038 | 30.7 (55) | 36.3 (76) | 0.649 |
| Disease duration at the beginning of the second anti-TNF, median (range interval) | 60 (98) | 76.5 (108) | 0.491 | 62 (100) | 78 (116) | 0.463 |
| Concomitant immunosuppressant with second anti-TNF | 54.4% | 49.5% | 0.309 | 51.8% | 50.7% | 0.842 |
| Moderate-to-severe partial Mayo score at the beginning of the second anti-TNF | 66% | 63.3% | 0.622 | 69.8% | 54.3% | <0.001 |
| Moderate-to-severe Mayo endoscopic subscore at the beginning of the second anti-TNF | 89.7% | 84.4% | 0.226 | 87.6% | 85.7% | 0.709 |
Bold and italic characters within the Tables are used to highlight statistically significant results.
Clinical and treatment outcomes regarding the study groups
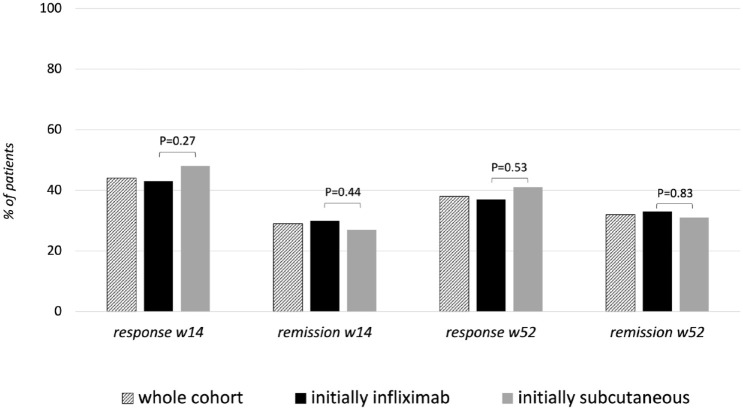
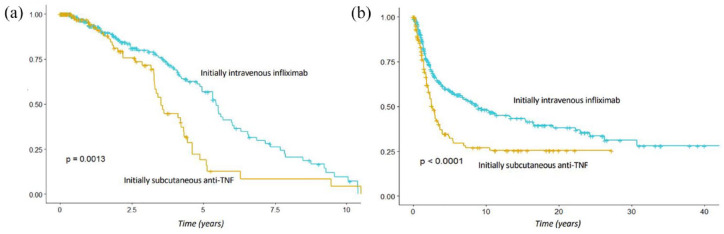
Clinical response and remission rates at week 14 were 42.7% and 30.3% in the IVi group and 48.3% and 26.6% in the SCi group (non-statistically significant), respectively. Clinical response and remission rates at week 52 were 37.5% and 32.8% in the IVi group and 40.7% and 31.4% in the SCi group (non-statistically significant), respectively (Figure 1). However, patients in the IVi group had longer treatment persistence (p = 0.001), as well as longer dose escalation-free survival (p < 0.001) (Figure 2).
Figure 1.
Response and remission rates at 14 and 52 weeks in the whole cohort (N = 473) and in each study group.
Figure 2.
Survival Kaplan–Meier curves of treatment persistence (a) and dose escalation (b) with the second anti-TNF.
Given the differences between the study groups, we performed a sub-analysis taking into account the reason for the discontinuation of the first anti-TNF. Among patients without remission to the first anti-TNF (n = 164), patients in the IVi group showed a significantly lower proportion of secondary loss of response (13% versus 29%; p = 0.014) and longer dose escalation-free survival (p = 0.046). Among those patients experiencing secondary loss of response to the first anti-TNF (n = 171), patients in the SCi group showed a significantly higher rate of clinical response at week 14 (66% versus 41%; p = 0.014) but shorter treatment persistence (p = 0.001). No differences between study groups were found regarding those patients in whom the first anti-TNF was discontinued because of AEs.
In a secondary analysis, to establish associations with clinical outcomes in an unbiased manner, a propensity score analysis was performed. The propensity score yielded 121 matched pairs of patients from both groups (IVi and SCi) (Supplemental Tables 1 and 2). In the propensity-matched score analysis, a statistically significant higher rate of clinical response at week 14 was found in the SCi group (50.4% versus 34.7%; p = 0.019), while all the remaining clinical outcomes were similar (Table 4). Finally, a log-rank test showed significantly longer treatment persistence among patients in the IVi group (p = 0.00023).
Table 4.
Comparison of clinical and treatment outcomes in the propensity-matched score cohorts.
| Variable | Initially intravenous infliximab (IVi group) (N = 121) | Initially subcutaneous anti-TNF (SCi group) (N = 121) | p |
|---|---|---|---|
| Clinical response at week 14 (%) | 35 | 50 | 0.019 |
| Clinical remission at week 14 (%) | 19 | 29 | 0.097 |
| Clinical response at week 52 (%) | 33 | 42 | 0.138 |
| Clinical remission at week 2 (%) | 27 | 33 | 0.315 |
| Secondary loss of response to the second anti-TNF (%) | 29 | 34 | 0.489 |
| Time to dose escalation (months), median (range interval) | 17.5 (25.4) | 20.7 (19.9) | 0.854 |
| Treatment persistence (months), median (range interval) | 217.1 (247.7) | 170 (125) | 0.064 |
Bold and italic characters within the Tables are used to highlight statistically significant results.
Discussion
The available network meta-analyses concur in considering IFX to be the most efficacious anti-TNF agent for UC.2,3,11 Even in daily clinical practice, IFX is perceived by clinicians as the best anti-TNF for UC; in fact, IFX is still the preferred rescue therapy for acute severe UC, 15 a clinical scenario in which clinicians use the most powerful and rapid therapeutic option, in spite of it being the oldest biological agent in IBD. This, together with the earlier development and licensing of IFX, may explain the scarce data available on the efficacy of IFX as a second anti-TNF for UC while preventing us from knowing whether using IFX as the first or the second anti-TNF impacts clinical and treatment outcomes.
We looked for UC patients in whom the first two biologicals used were IFX and a subcutaneous anti-TNF (or vice versa), and, with this study, we provide one of the largest cohorts of UC patients treated with two consecutive anti-TNFs. The pattern of anti-TNF use in our cohorts can be interpreted as a consequence of market access. First, we found a greater number of patients in whom the first anti-TNF used was IFX. This is mostly due to the earlier availability of IFX for UC (it was licensed 7 years before ADA and 9 years before GLM), though the perceived higher efficacy of IFX may also have played a role. In fact, patients using IFX had more severe clinical activity at the time IFX was started regardless of whether it was the first or the second anti-TNF, strengthening the idea of its perceived higher efficacy. Second, we observed that, when used as the first anti-TNF, almost all the patients were treated with the IFX originator. Conversely, when used as the second anti-TNF, the biosimilar CT-P13 was used even more often than the originator. This is also a consequence of the later launch of biosimilars, which, since that time, have become the first-line biological treatments rather than originators in Spain. Finally, when subcutaneous anti-TNFs were used as the first agents, ADA and GLM were used in almost equal measure. Conversely, when used after IFX failure, ADA was used in more than 80% of patients. GLM has a similar efficacy to ADA in randomized controlled trials and advantage should be taken of its greater convenience in UC (monthly administration instead of every other week). However, ADA was licensed slightly earlier than GLM for UC (2 years), clinicians took into account their long-standing, positive clinical experience with ADA in Crohn’s disease, and ADA biosimilars were launched after the positive initial experience with IFX biosimilars. This observed lesser use of GLM is in line with the findings of recent studies in clinical practice from France and Switzerland.12,16
The efficacy of a second anti-TNF for UC has barely been assessed, and data on the use of IFX as the second anti-TNF are even less abundant. Regarding those randomized controlled trials assessing the efficacy of ADA and GLM for UC, only the ULTRA-2 study included 98 patients who had been previously exposed to IFX. 6 In these patients, ADA achieved clinical response in 37% of patients at week 8 and clinical remission in 10% at week 52. The authors found a worse short- and long-term response in patients previously exposed to IFX, which is similar to what was observed in the pivotal studies for ustekinumab in UC. 10 Conversely, there are no controlled studies assessing the efficacy of GLM or IFX as the second anti-TNF for UC and it is unlikely that there will ever be. Controversial data with a wide range of response and remission rates have been reported on this issue for both ADA 17 and GLM 18 when real-world evidence has been reviewed. Therefore, it seems reasonable to assess the impact of using the more efficacious drug for UC as the first- or second-line therapy and it is worthy of note that this should not be extrapolated from the data observed in Crohn’s disease. In this sense, Casanova et al., 19 in a large retrospective study assessing the efficacy of a second and a third anti-TNF in IBD patients (including 822 with Crohn’s disease and 300 with UC), found that UC (as opposed to Crohn’s disease) was associated with a higher probability of secondary loss of response to the second anti-TNF.
Most of the largest studies on this topic assessed the efficacy of ADA after the failure of IFX and were derived from the ENEIDA registry.17–24 Iborra et al. 17 aimed to compare the efficacy of ADA as the first or second anti-TNF in 263 UC patients of whom 67% had previously been exposed to IFX. The authors did not find any difference in terms of clinical response at 12 and 54 weeks and primary failures to ADA but did observe a lower remission rate at week 12 in patients who had previously been exposed to IFX. Interestingly, primary nonresponse to or intolerance of IFX and severe disease activity at the time ADA was started were the only predictive factors of a worse response in the multivariate analysis. Taxonera et al. 18 aimed to compare the efficacy of GLM as the first, second, or third anti-TNF in 142 UC patients of whom 57% were naïve to biologicals. The authors did not find any difference in terms of clinical response in the short term and long term and treatment persistence when they compared GLM as the first or second anti-TNF.
To our knowledge, only two retrospective studies have assessed the efficacy of IFX as the second anti-TNF for UC. Viola et al. 24 assessed the efficacy of IFX in 76 UC patients previously exposed to ADA (n = 38) and GLM (n = 38) and reported a 70% rate of clinical response at week 12 and 34% of clinical remission at week 52. No factors associated with efficacy were identified. Recently, Hupé et al. 12 also assessed the efficacy of IFX in 154 UC patients who had previously been exposed to subcutaneous anti-TNF. They reported a clinical response rate of 54% at week 14 and, interestingly, response to IFX was worse among those patients treated for an acute severe flare.
The present study is the largest to assess a second anti-TNF after the failure of the first agent in UC and it is the only one to compare IFX to GLM/ADA as the second agent. We found an early response rate (week 14) to the second anti-TNF of 40%–50% and a long-term remission rate (week 52) of 25%–30%, with no differences between the IVi and the SCi groups. Given the relevant differences in the baseline characteristics of both study groups, we performed a propensity score analysis, obtaining similar results except for a higher clinical response rate at week 14 in the SCi group. Moreover, we observed a longer treatment persistence of the second anti-TNF when IFX was the first one, a result that was confirmed in the propensity score cohort. Our efficacy figures are quite close to previous studies, particularly those with larger cohorts, and are strengthened by our sample size and the propensity score analysis. We also searched for factors associated with a better response and found that clinical response at week 14 was less likely after a shorter time on the first anti-TNF and if the reason for its discontinuation was primary failure. Regarding long-term outcomes, more severe disease activity at the beginning of the second anti-TNF, dose escalation, and discontinuation due to the primary failure of the first anti-TNF was inversely associated with clinical remission at week 52. Of note, primary failure to the first anti-TNF was also associated with a worse response to ADA17,19,22,25 and vedolizumab, 12 whereas severe clinical activity was associated with a worse response to ADA 17 and IFX 12 when they were used as the second-line therapy.
Despite the strengths of being the largest study assessing the efficacy of a second anti-TNF drug for UC and the performance of a propensity score analysis, the present study has some limitations. First, as a consequence of its retrospective design, there may be a bias related to the fact that the treatment strategy (drug selection, dose escalation, treatment discontinuation) was at the discretion of the treating physician. Moreover, we did not account for drug trough levels and anti-drug antibodies before treatment discontinuation (of both the first and the second anti-TNF). Therapeutic drug monitoring is increasingly used in clinical practice in patients treated with IFX and ADA, particularly in cases of primary failure or a secondary loss of response. Scarce data on this are available for UC patients being switched to a second anti-TNF but trough levels and anti-drug antibodies to IFX at treatment discontinuation were not associated with clinical response to ADA in a rather large cohort. 22
In conclusion, we report a good short- and long-term efficacy of a second anti-TNF for UC, although it is reduced in cases of primary failure of the first anti-TNF and more severe disease activity. Efficacy does not seem to change whether the second anti-TNF is IFX or a subcutaneous agent, and only a longer persistence of the second anti-TNF was observed when the first agent was IFX. Prescribing a second anti-TNF in UC remains a reasonable option but face-to-face controlled trials comparing a second anti-TNF to other selective immunosuppressants should be performed, particularly in the case of primary failure of a first anti-TNF.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848231221713 for Clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in patients with ulcerative colitis treated with two consecutive anti-TNF agents: data from the ENEIDA registry by Margalida Calafat, Paola Torres, Joan Tosca-Cuquerella, Rubén Sánchez-Aldehuelo, Montserrat Rivero, Marisa Iborra, María González-Vivo, Isabel Vera, Luisa de Castro, Luis Bujanda, Manuel Barreiro-de Acosta, Carlos González-Muñoza, Xavier Calvet, José Manuel Benítez, Mónica Llorente-Barrio, Gerard Surís, Fiorella Cañete, Lara Arias-García, David Monfort, Andrés Castaño-García, Francisco Javier Garcia-Alonso, José M. Huguet, Ignacio Marín-Jímenez, Rufo Lorente, Albert Martín-Cardona, Juan Ángel Ferrer, Patricia Camo, Javier P. Gisbert, Ramón Pajares, Fernando Gomollón, Jesús Castro-Poceiro, Jair Morales-Alvarado, Jordina Llaó, Andrés Rodríguez, Cristina Rodríguez, Pablo Pérez-Galindo, Mercè Navarro, Nuria Jiménez-García, Marta Carrillo-Palau, Isabel Blázquez-Gómez, Eva Sesé, Pedro Almela, Patricia Ramírez de la Piscina, Carlos Taxonera, Iago Rodríguez-Lago, Lidia Cabrinety, Milagros Vela, Miguel Mínguez, Francisco Mesonero, María José García, Mariam Aguas, Lucía Márquez, Marisol Silva Porto, Juan R. Pineda, Koldo García-Etxebarría, Federico Bertoletti, Eduard Brunet, Míriam Mañosa and Eugeni Domènech in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848231221713 for Clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in patients with ulcerative colitis treated with two consecutive anti-TNF agents: data from the ENEIDA registry by Margalida Calafat, Paola Torres, Joan Tosca-Cuquerella, Rubén Sánchez-Aldehuelo, Montserrat Rivero, Marisa Iborra, María González-Vivo, Isabel Vera, Luisa de Castro, Luis Bujanda, Manuel Barreiro-de Acosta, Carlos González-Muñoza, Xavier Calvet, José Manuel Benítez, Mónica Llorente-Barrio, Gerard Surís, Fiorella Cañete, Lara Arias-García, David Monfort, Andrés Castaño-García, Francisco Javier Garcia-Alonso, José M. Huguet, Ignacio Marín-Jímenez, Rufo Lorente, Albert Martín-Cardona, Juan Ángel Ferrer, Patricia Camo, Javier P. Gisbert, Ramón Pajares, Fernando Gomollón, Jesús Castro-Poceiro, Jair Morales-Alvarado, Jordina Llaó, Andrés Rodríguez, Cristina Rodríguez, Pablo Pérez-Galindo, Mercè Navarro, Nuria Jiménez-García, Marta Carrillo-Palau, Isabel Blázquez-Gómez, Eva Sesé, Pedro Almela, Patricia Ramírez de la Piscina, Carlos Taxonera, Iago Rodríguez-Lago, Lidia Cabrinety, Milagros Vela, Miguel Mínguez, Francisco Mesonero, María José García, Mariam Aguas, Lucía Márquez, Marisol Silva Porto, Juan R. Pineda, Koldo García-Etxebarría, Federico Bertoletti, Eduard Brunet, Míriam Mañosa and Eugeni Domènech in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_17562848231221713 for Clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in patients with ulcerative colitis treated with two consecutive anti-TNF agents: data from the ENEIDA registry by Margalida Calafat, Paola Torres, Joan Tosca-Cuquerella, Rubén Sánchez-Aldehuelo, Montserrat Rivero, Marisa Iborra, María González-Vivo, Isabel Vera, Luisa de Castro, Luis Bujanda, Manuel Barreiro-de Acosta, Carlos González-Muñoza, Xavier Calvet, José Manuel Benítez, Mónica Llorente-Barrio, Gerard Surís, Fiorella Cañete, Lara Arias-García, David Monfort, Andrés Castaño-García, Francisco Javier Garcia-Alonso, José M. Huguet, Ignacio Marín-Jímenez, Rufo Lorente, Albert Martín-Cardona, Juan Ángel Ferrer, Patricia Camo, Javier P. Gisbert, Ramón Pajares, Fernando Gomollón, Jesús Castro-Poceiro, Jair Morales-Alvarado, Jordina Llaó, Andrés Rodríguez, Cristina Rodríguez, Pablo Pérez-Galindo, Mercè Navarro, Nuria Jiménez-García, Marta Carrillo-Palau, Isabel Blázquez-Gómez, Eva Sesé, Pedro Almela, Patricia Ramírez de la Piscina, Carlos Taxonera, Iago Rodríguez-Lago, Lidia Cabrinety, Milagros Vela, Miguel Mínguez, Francisco Mesonero, María José García, Mariam Aguas, Lucía Márquez, Marisol Silva Porto, Juan R. Pineda, Koldo García-Etxebarría, Federico Bertoletti, Eduard Brunet, Míriam Mañosa and Eugeni Domènech in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Appendix
Complete list of the affiliations of the ENEIDA-GETECCU investigators
Margalida Calafat, Hospital Universitari Germans Trias i Pujol (Badalona) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Paola Torres, Hospital Universitari Germans Trias i Pujol (Badalona); Joan Tosca-Cuquerella, Hospital Clínic Universitari de València (València); Rubén Sánchez-Aldehuelo, Hospital Ramón y Cajal (Madrid); Montserrat Rivero, Hospital Universitario Marqués de Valdecilla and Instituto de investigación IDIVAL (Santander); Marisa Iborra, Hospital Universitari i Politècnic La Fe (València); María González-Vivo, Hospital del Mar (Barcelona); Isabel Vera, Hospital Universitario Puerta de Hierro (Majadahonda); Luisa de Castro, Complexo Hospitalario Universitario de Vigo (Vigo); Luis Bujanda, Biodonostia Health Research Institute (San Sebastián), Universidad del País Vasco (UPV/EHU) (San Sebastián) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Manuel Barreiro-de Acosta, Complexo Hospitalario Universitario de Santiago (Santiago de Compostela); Carlos González-Muñoza, Hospital de la Santa Creu i Sant Pau (Barcelona); Xavier Calvet, Corporació Sanitària Universitària Parc Taulí (Sabadell) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); José Manuel Benítez, Hospital Universitario Reina Sofía (Córdoba), Instituto Maimónides de Investigación Biomédica de Córdoba (Córdoba); Mónica Llorente-Barrio, Hospital Universitario Miguel Servet (Zaragoza); Gerard Surís, Hospital Universitari de Bellvitge (Hospitalet de Llobregat); Fiorella Cañete, Hospital Universitari Germans Trias i Pujol (Badalona) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Lara Arias-García, Hospital Universitario de Burgos (Burgos); David Monfort, Consorci Sanitari Terrassa (Terrasa); Andrés Castaño-García, Hospital Universitario Central de Asturias (Oviedo); Francisco Javier Garcia-Alonso, Hospital Universitario Río Hortega (Valladolid); José M. Huguet, Hospital General Universitari de València (València); Ignacio Marín-Jímenez, Hospital Gregorio Marañón (Madrid); Rufo Lorente, Hospital General Universitario de Ciudad Real (Ciudad Real); Albert Martín-Cardona, Hospital Universitari Mútua Terrassa (Terrassa) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Juan Ángel Ferrer, Hospital Universitario Fundación de Alcorcón (Madrid); Patricia Camo, Hospital General San Jorge (Huesca); Javier P. Gisbert, Hospital Universitario de La Princesa, IIS-Princesa and UAM (Madrid) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Ramón Pajares, Hospital Universitario Infanta Sofía (Madrid); Fernando Gomollón, Hospital Clínico Universitario Lozano Blesa, IIS Aragón (Zaragoza) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Jesús Castro-Poceiro, Hospital Clínic de Barcelona (Barcelona); Jair Morales-Alvarado, Hospital General de Granollers (Granollers); Jordina Llaó, Althaia Xarxa Assistencial Universitària de Manresa (Manresa); Andrés Rodríguez, Hospital General Universitari d’Alacant (Alacant); Cristina Rodríguez, Complejo Hospitalario de Navarra (Navarra); Pablo Pérez-Galindo, Complejo Hospitalario Universitario de Pontevedra (Pontevedra); Mercè Navarro, Hospital Moisès Broggi (Sant Joan Despí); Nuria Jiménez-García, Hospital General Universitari d’Elx (Elx); Marta Carrillo-Palau, Hospital Universitario de Canarias (La Laguna); Isabel Blázquez-Gómez, Hospital Universitario de Torrejón (Torrejón); Eva Sesé, Hospital Universitari Arnau de Vilanova (Lleida); Pedro Almela, Hospital General Universitari de Castelló (Castelló); Patricia Ramírez de la Piscina, Hospital Universitario de Álava (Vitoria); Carlos Taxonera, Hospital Clínico San Carlos (Madrid); Iago Rodríguez-Lago, Hospital Universitario de Galdakao, Biocruces Bizkaia HRI (Galdakao); Lidia Cabrinety, Hospital Universitari Joan XXIII (Tarragona); Milagros Vela, Hospital Universitario Nuestra Señora de la Candelaria (Santa Cruz de Tenerife); Miguel Mínguez, Hospital Clínic Universitari de València (València); Francisco Mesonero, Hospital Ramón y Cajal (Madrid); María José García, Hospital Universitario Marqués de Valdecilla and Instituto de investigación IDIVAL (Santander); Mariam Aguas, Hospital Universitari i Politècnic La Fe (València); Lucía Márquez, Hospital del Mar (Barcelona); Marisol Silva Porto, Complexo Hospitalario Universitario de Santiago (Santiago de Compostela); Juan R. Pineda, Complexo Hospitalario Universitario de Vigo (Vigo); Koldo García-Etxebarría, Biodonostia Health Research Institute (San Sebastián), Universidad del País Vasco (UPV/EHU) (San Sebastián) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Federico Bertoletti, Hospital de la Santa Creu i Sant Pau (Barcelona); Eduard Brunet, Corporació Sanitària Universitària Parc Taulí (Sabadell) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Míriam Mañosa, Hospital Universitari Germans Trias i Pujol (Badalona) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD); Eugeni Domènech, Hospital Universitari Germans Trias i Pujol (Badalona) and Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD).
Footnotes
ORCID iDs: Margalida Calafat  https://orcid.org/0000-0003-2335-3792
https://orcid.org/0000-0003-2335-3792
Xavier Calvet  https://orcid.org/0000-0002-6278-9663
https://orcid.org/0000-0002-6278-9663
Javier P. Gisbert  https://orcid.org/0000-0003-2090-3445
https://orcid.org/0000-0003-2090-3445
Carlos Taxonera  https://orcid.org/0000-0001-9166-7350
https://orcid.org/0000-0001-9166-7350
Francisco Mesonero  https://orcid.org/0000-0002-7864-8187
https://orcid.org/0000-0002-7864-8187
María José García  https://orcid.org/0000-0002-6517-7005
https://orcid.org/0000-0002-6517-7005
Koldo García-Etxebarría  https://orcid.org/0000-0002-6107-9416
https://orcid.org/0000-0002-6107-9416
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Margalida Calafat, Gastroenterology Department, Hospital Universitari Germans Trias i Pujol, Badalona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Paola Torres, Gastroenterology Department, Hospital Universitari Germans Trias i Pujol, Badalona, Spain.
Joan Tosca-Cuquerella, Gastroenterology Department, Hospital Clínic Universitari de València, València, Spain.
Rubén Sánchez-Aldehuelo, Gastroenterology Department, Hospital Ramón y Cajal, Madrid Spain.
Montserrat Rivero, Gastroenterology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Instituto de investigación IDIVAL, Santander, Spain.
Marisa Iborra, Gastroenterology Department, Hospital Universitari i Politècnic La Fe, València, Spain.
María González-Vivo, Gastroenterology Department, Hospital del Mar, Barcelona, Spain.
Isabel Vera, Gastroenterology Department, Hospital Universitario Puerta de Hierro, Majadahonda, Spain.
Luisa de Castro, Gastroenterology Department, Complexo Hospitalario Universitario de Vigo, Vigo, Spain.
Luis Bujanda, Biodonostia Health Research Institute, San Sebastián, Spain; Universidad del País Vasco/Euskal Herriko Unibertsitatea, San Sebastián, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Manuel Barreiro-de Acosta, Gastroenterology Department, Complexo Hospitalario Universitario de Santiago, Santiago de Compostela, Spain.
Carlos González-Muñoza, Gastroenterology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Xavier Calvet, Gastroenterology Department, Corporació Sanitària Universitària Parc Taulí, Sabadell, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
José Manuel Benítez, Gastroenterology Department, Hospital Universitario Reina Sofía, Córdoba, Spain; Instituto Maimónides de Investigación Biomédica de Córdoba, Córdoba, Spain.
Mónica Llorente-Barrio, Gastroenterology Department, Hospital Universitario Miguel Servet, Zaragoza, Spain.
Gerard Surís, Gastroenterology Department, Hospital Universitari de Bellvitge (L’ Hospitalet de Llobregat), Barcelona, Spain.
Fiorella Cañete, Gastroenterology Department, Hospital Universitari Germans Trias i Pujol, Badalona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Lara Arias-García, Gastroenterology Department, Hospital Universitario de Burgos, Burgos, Spain.
David Monfort, Gastroenterology Department, Consorci Sanitari de Terrassa, Terrassa, Spain.
Andrés Castaño-García, Gastroenterology Department, Hospital Universitario Central de Asturias, Oviedo, Spain.
Francisco Javier Garcia-Alonso, Gastroenterology Department, Hospital Universitario Río Hortega, Valladolid, Spain.
José M. Huguet, Gastroenterology Department, Hospital General Universitari de València, València, Spain
Ignacio Marín-Jímenez, Gastroenterology Department, Hospital Gregorio Marañón, Madrid, Spain.
Rufo Lorente, Gastroenterology Department, Hospital General Universitario de Ciudad Real, Ciudad Real, Spain.
Albert Martín-Cardona, Gastroenterology Department, Hospital Universitari Mútua Terrassa, Terrassa, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Juan Ángel Ferrer, Gastroenterology Department, Hospital Universitario Fundación de Alcorcón (Madrid), Spain.
Patricia Camo, Gastroenterology Department, Hospital General San Jorge, Huesca, Spain.
Javier P. Gisbert, Gastroenterology Department, Hospital Universitario de La Princesa, IIS Princesa and UAM, Madrid, Spain Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Ramón Pajares, Gastroenterology Department, Hospital Universitario Infanta Sofía, Madrid, Spain.
Fernando Gomollón, Gastroenterology Department, Hospital Clínico Universitario Lozano Blesa, IIS Aragón, Zaragoza, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Jesús Castro-Poceiro, Gastroenterology Department, Hospital Clínic de Barcelona, Barcelona, Spain.
Jair Morales-Alvarado, Gastroenterology Department, Hospital General de Granollers, Granollers, Spain.
Jordina Llaó, Gastroenterology Department, Althaia Xarxa Assistencial Universitària de Manresa, Manresa, Spain.
Andrés Rodríguez, Gastroenterology Department, Hospital General Universitari d’Alacant, Alacant, Spain.
Cristina Rodríguez, Gastroenterology Department, Complejo Hospitalario de Navarra, Pamplona, Spain.
Pablo Pérez-Galindo, Gastroenterology Department, Complejo Hospitalario Universitario de Pontevedra, Pontevedra, Spain.
Mercè Navarro, Gastroenterology Department, Hospital Moisès Broggi, Sant Joan Despí, Spain.
Nuria Jiménez-García, Gastroenterology Department, Hospital General Universitari d’Elx, Elx, Spain.
Marta Carrillo-Palau, Gastroenterology Department, Hospital Universitario de Canarias, La Laguna, Spain.
Isabel Blázquez-Gómez, Gastroenterology Department, Hospital Universitario de Torrejón, Torrejón, Spain.
Eva Sesé, Gastroenterology Department, Hospital Universitari Arnau de Vilanova, Lleida, Spain.
Pedro Almela, Gastroenterology Department, Hospital General Universitari de Castelló, Castellón, Spain.
Patricia Ramírez de la Piscina, Gastroenterology Department, Hospital Universitario de Álava, Vitoria, Spain.
Carlos Taxonera, Gastroenterology Department, Hospital Clínico San Carlos, Madrid, Spain.
Iago Rodríguez-Lago, Gastroenterology Department, Hospital Universitario de Galdakao, Biocruces Bizkaia HRI, Galdakao, Spain.
Lidia Cabrinety, Gastroenterology Department, Hospital Universitari Joan XXIII, Tarragona, Spain.
Milagros Vela, Gastroenterology Department, Hospital Universitario Nuestra Señora de la Candelaria, Santa Cruz de Tenerife, Spain.
Miguel Mínguez, Gastroenterology Department, Hospital Clínic Universitari de València, València, Spain.
Francisco Mesonero, Gastroenterology Department, Hospital Ramón y Cajal, Madrid Spain.
María José García, Gastroenterology Department, Hospital Universitario Marqués de Valdecilla, Santander, Spain; Instituto de investigación IDIVAL, Santander, Spain.
Mariam Aguas, Gastroenterology Department, Hospital Universitari i Politècnic La Fe, València, Spain.
Lucía Márquez, Gastroenterology Department, Hospital del Mar, Barcelona, Spain.
Marisol Silva Porto, Gastroenterology Department, Hospital Universitario Puerta de Hierro, Majadahonda, Spain.
Juan R. Pineda, Gastroenterology Department, Complexo Hospitalario Universitario de Vigo, Vigo, Spain
Koldo García-Etxebarría, Biodonostia Health Research Institute, San Sebastián, Spain; Universidad del País Vasco/Euskal Herriko Unibertsitatea, San Sebastián, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Federico Bertoletti, Gastroenterology Department, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Eduard Brunet, Gastroenterology Department, Corporació Sanitària Universitària Parc Taulí, Sabadell, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Míriam Mañosa, Gastroenterology Department, Hospital Universitari Germans Trias i Pujol, Badalona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Eugeni Domènech, Gastroenterology and Hepatology Department, Hospital Universitari Germans Trias i Pujol, Carretera de Canyet s/n, Badalona, Catalonia 08916, Spain; Departament de Medicina, Universitat Autònoma de Barcelona; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, Madrid, Spain.
Declarations
Ethics approval and consent to participate: The registry was approved by the Ethics Committee at all centers and all patients gave their written informed consent.
Consent for publication: All the authors reviewed and approved the manuscript and gave their consent for publication.
Author contributions: Margalida Calafat: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Paola Torres: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Writing – original draft.
Joan Tosca-Cuquerella: Data curation; Writing – review & editing.
Rubén Sánchez-Aldehuelo: Data curation; Writing – review & editing.
Montserrat Rivero: Data curation; Writing – review & editing.
Marisa Iborra: Data curation; Writing – review & editing.
María González-Vivo: Data curation; Writing – review & editing.
Isabel Vera: Data curation; Writing – review & editing.
Luisa de Castro: Data curation; Writing – review & editing.
Luis Bujanda: Data curation; Writing – review & editing.
Manuel Barreiro-de Acosta: Data curation; Writing – review & editing.
Carlos González-Muñoza: Data curation; Writing – review & editing.
Xavier Calvet: Data curation; Writing – review & editing.
José Manuel Benítez: Data curation; Writing – review & editing.
Mónica Llorente-Barrio: Data curation; Writing – review & editing.
Gerard Surís: Data curation; Writing – review & editing.
Fiorella Cañete: Data curation; Writing – review & editing.
Lara Arias-García: Data curation; Writing – review & editing.
David Monfort: Data curation; Writing – review & editing.
Andrés Castaño-García: Data curation; Writing – review & editing.
Francisco Javier Garcia-Alonso: Data curation; Writing – review & editing.
José M. Huguet: Data curation; Writing – review & editing.
Ignacio Marín-Jímenez: Data curation; Writing – review & editing.
Rufo Lorente: Data curation; Writing – review & editing.
Albert Martín-Cardona: Data curation; Writing – review & editing.
Juan Ángel Ferrer: Data curation; Writing – review & editing.
Patricia Camo: Data curation; Writing – review & editing.
Javier P. Gisbert: Data curation; Writing – review & editing.
Ramón Pajares: Data curation; Writing – review & editing.
Fernando Gomollón: Data curation; Writing – review & editing.
Jesús Castro-Poceiro: Data curation; Writing – review & editing.
Jair Morales-Alvarado: Data curation; Writing – review & editing.
Jordina Llaó: Data curation; Writing – review & editing.
Andrés Rodríguez: Data curation; Writing – review & editing.
Cristina Rodríguez: Data curation; Writing – review & editing.
Pablo Pérez-Galindo: Data curation; Writing – review & editing.
Mercè Navarro: Data curation; Writing – review & editing.
Nuria Jiménez-García: Data curation; Writing – review & editing.
Marta Carrillo-Palau: Data curation; Writing – review & editing.
Isabel Blázquez-Gómez: Data curation; Writing – review & editing.
Eva Sesé: Data curation; Writing – review & editing.
Pedro Almela: Data curation; Writing – review & editing.
Patricia Ramírez de la Piscina: Data curation; Writing – review & editing.
Carlos Taxonera: Data curation; Writing – review & editing.
Iago Rodríguez-Lago: Data curation; Writing – review & editing.
Lidia Cabrinety: Data curation; Writing – review & editing.
Milagros Vela: Data curation; Writing – review & editing.
Miguel Mínguez: Data curation; Writing – review & editing.
Francisco Mesonero: Data curation; Writing – review & editing.
María José García: Data curation; Writing – review & editing.
Mariam Aguas: Data curation; Writing – review & editing.
Lucía Márquez: Data curation; Writing – review & editing.
Marisol Silva Porto: Data curation; Writing – review & editing.
Juan R. Pineda: Data curation; Writing – review & editing.
Koldo García-Etxebarría: Data curation; Writing – review & editing.
Federico Bertoletti: Data curation; Writing – review & editing.
Eduard Brunet: Data curation; Writing – review & editing.
Míriam Mañosa: Data curation; Writing – original draft; Writing – review & editing.
Eugeni Domènech: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The ENEIDA registry of GETECCU is supported by AbbVie, Galápagos, Janssen, Biogen, Takeda, and Pfizer. PT received a ‘Beca Germans Trias Talents’ research grant from Fundació Catalunya La Pedrera.
MC has served as a speaker for Takeda, Janssen, Faes Farma, and MSD; FC has served as a speaker or has received educational grants from Takeda, Janssen, MSD, and Ferring; MR has served as a speaker or has received research or educational funding or advisory fees from MSD, Abbvie, Pfizer, Takeda, and Janssen; MI has served as a speaker or has received research or educational funding or advisory fees from MSD, Janssen, Adacyte, and Takeda; LC has served as a speaker or has received research or educational funding or advisory fees from Abbvie, Dr. Falk Pharma, and Tillots Pharma; LB has served as a speaker or has received research or educational funding or advisory fees from Ikan Biotech; MBA has served as a speaker or has received research or educational funding or advisory fees from MSD, AbbVie, Janssen, Kern Pharma, Celltrion, Takeda, Gillead, Celgene, Pfizer, Sandoz, Biogen, Fresenius, Ferring, Faes Farma, Dr. Falk Pharma, Chiesi, Gebro Pharma, Adacyte, and Vifor Pharma; CG-M has received educational funding fees from AbbVie, Janssen, Pfizer, Ferring, Kern Pharma, Norgine, and Tillots Pharma; JMH has served as a speaker or has received research or educational funding or advisory fees from Merck Sharp & Dohme, Ferring, Abbvie, Janssen, Biogen, Sandoz, Kern Pharma, Faes Farma, Vifor Pharma, and Takeda; RL has served as a speaker or has received research or educational funding or advisory fees from MSD, Abbvie, Pfizer, Takeda, Janssen, and Dr. Falk; AM-C has received research or educational funding from Abbvie, Biogen, Ferring, Janssen, MSD, Takeda, Dr. Falk Pharma, and Tillotts; JPG has served as a speaker or has received research or educational funding or advisory fees from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine, and Vifor Pharma; FG has served as a speaker or has received research or educational funding or advisory fees from Faes-Farma, Galápagos, Takeda, Pfizer, Janssen, and Abbvie; PA has served as a speaker or has received research or educational funding or advisory fees from MSD, Abbvie, Takeda, Janssen, Gebro Pharma, Tillotts Pharma, and Biogen; CT has served as a speaker or has received research or educational funding or advisory fees from MSD, AbbVie, Pfizer, Takeda, Janssen, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Galapagos, and Tillots; IR-L has served as a speaker or has received research or educational funding or advisory fees from MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Kern, Celltrion, Roche, Ferring, Dr. Falk Pharma, Galapagos, Otsuka Pharmaceutical, and Adacyte; MM has served as a speaker and has received research or educational funding from MSD, AbbVie, Takeda, Janssen, Ferring, and Pfizer; ED has served as a speaker or has received research or educational funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Galapagos, Gilead, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, and Tillots; MJG has served as a speaker or has received research or educational funding or advisory fees from Janssen, Pfizer, Abbvie, Takeda, Kern Pharma, and Ferring; MA has served as a speaker or has received research or educational funding or advisory fees from Faes, Ferring, and Janssen, and received educational grants from Janssen; JRP has served as a speaker or has received research or educational funding or advisory fees from MSD, AbbVie, and Tillots Pharma; FB received educational funding fees from AbbVie, Janssen, Pfizer, Ferring, Kern Pharma, Norgine, and Tillots Pharma. The remaining authors declared no conflicts of interest.
Availability of data and materials: The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Domènech E, Mañosa M, Cabré E. An overview of the natural history of inflammatory bowel diseases. Dig Dis 2014; 32: 320–327. [DOI] [PubMed] [Google Scholar]
- 2. Thorlund K, Druyts E, Toor K, et al. Comparative efficacy of golimumab, infliximab, and adalimumab for moderately to severely active ulcerative colitis: a network meta-analysis accounting for differences in trial designs. Expert Rev Gastroenterol Hepatol 2015; 9: 693–700. [DOI] [PubMed] [Google Scholar]
- 3. Mei WQ, Hu HZ, Liu Y, et al. Infliximab is superior to other biological agents for treatment of active ulcerative colitis: a meta-analysis. World J Gastroenterol 2015; 21: 6044–6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrante M, Vermeire S, Fidder H, et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis 2008; 2: 219–225. [DOI] [PubMed] [Google Scholar]
- 5. Gisbert JP, González-Lama Y, Maté J. Systematic review: infliximab therapy in ulcerative colitis. Aliment Pharmacol Ther 2007; 25: 19–37. [DOI] [PubMed] [Google Scholar]
- 6. Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012; 142: 257–65.e1–3. [DOI] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014; 146: 85–95; quiz e14–15. [DOI] [PubMed] [Google Scholar]
- 8. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 9. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376: 1723–1736. [DOI] [PubMed] [Google Scholar]
- 10. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381: 1201–1214. [DOI] [PubMed] [Google Scholar]
- 11. Singh S, Murad MH, Fumery M, et al. First- and Second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol 2020; 18: 2179–2191.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hupé M, Rivière P, Nancey S, et al. Comparative efficacy and safety of vedolizumab and infliximab in ulcerative colitis after failure of a first subcutaneous anti-TNF agent: a multicentre cohort study. Aliment Pharmacol Ther 2020; 51: 852–860. [DOI] [PubMed] [Google Scholar]
- 13. Schreiber S, Ben-Horin S, Leszczyszyn J, et al. Randomized controlled trial: subcutaneous vs intravenous infliximab CT-P13 maintenance in inflammatory bowel disease. Gastroenterology 2021; 160: 2340–2353. [DOI] [PubMed] [Google Scholar]
- 14. Zabana Y, Panés J, Nos P, et al. The ENEIDA registry (Nationwide study on genetic and environmental determinants of inflammatory bowel disease) by GETECCU: design, monitoring and functions. Gastroenterol Hepatol 2020; 43 :551–558. [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez-Lago I, Ferreiro-Iglesias R, Nos P, et al. Management of acute severe ulcerative colitis in Spain: a nationwide clinical practice survey. Gastroenterol Hepatol 2019; 42: 90–101. [DOI] [PubMed] [Google Scholar]
- 16. Perrig K, Krupka N, Jordi SBU, et al. Effectiveness of golimumab in patients with ulcerative colitis: results of a real-life study in Switzerland. Therap Adv Gastroenterol 2022; 15: 17562848221074188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iborra M, Pérez-Gisbert J, Bosca-Watts MM, et al. Effectiveness of adalimumab for the treatment of ulcerative colitis in clinical practice: comparison between anti-tumour necrosis factor-naïve and non-naïve patients. J Gastroenterol 2017; 52: 788–799. [DOI] [PubMed] [Google Scholar]
- 18. Taxonera C, Rodríguez C, Bertoletti F, et al. Clinical outcomes of golimumab as first, second or third anti-TNF agent in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis 2017; 23: 1394–1402. [DOI] [PubMed] [Google Scholar]
- 19. Casanova MJ, Chaparro M, Mínguez M, et al. Effectiveness and safety of the sequential use of a second and third anti-TNF agent in patients with inflammatory bowel disease: results from the ENEIDA registry. Inflamm Bowel Dis 2020; 26: 606–616. [DOI] [PubMed] [Google Scholar]
- 20. García-Bosch O, Gisbert JP, Cañas-Ventura A, et al. Observational study on the efficacy of adalimumab for the treatment of ulcerative colitis and predictors of outcome. J Crohns Colitis 2013; 7: 717–722. [DOI] [PubMed] [Google Scholar]
- 21. Taxonera C, Estellés J, Fernández-Blanco I, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther 2011; 33: 340–348. [DOI] [PubMed] [Google Scholar]
- 22. Baert F, Vande Casteele N, Tops S, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther 2014; 40: 1324–1332. [DOI] [PubMed] [Google Scholar]
- 23. Italian Group for the Study of Inflammatory Bowel Disease; Armuzzi A, Biancone L, et al. Adalimumab in active ulcerative colitis: a ‘real-life’ observational study. Dig Liver Dis 2013; 45: 738–743. [DOI] [PubMed] [Google Scholar]
- 24. Viola A, Pugliese D, Renna S, et al. Outcome in ulcerative colitis after switch from adalimumab/golimumab to infliximab: a multicenter retrospective study. Dig Liver Dis 2019; 51: 510–515. [DOI] [PubMed] [Google Scholar]
- 25. Gisbert JP, Marín AC, McNicholl AG, et al. Systematic review with meta- analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015; 41: 613–623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848231221713 for Clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in patients with ulcerative colitis treated with two consecutive anti-TNF agents: data from the ENEIDA registry by Margalida Calafat, Paola Torres, Joan Tosca-Cuquerella, Rubén Sánchez-Aldehuelo, Montserrat Rivero, Marisa Iborra, María González-Vivo, Isabel Vera, Luisa de Castro, Luis Bujanda, Manuel Barreiro-de Acosta, Carlos González-Muñoza, Xavier Calvet, José Manuel Benítez, Mónica Llorente-Barrio, Gerard Surís, Fiorella Cañete, Lara Arias-García, David Monfort, Andrés Castaño-García, Francisco Javier Garcia-Alonso, José M. Huguet, Ignacio Marín-Jímenez, Rufo Lorente, Albert Martín-Cardona, Juan Ángel Ferrer, Patricia Camo, Javier P. Gisbert, Ramón Pajares, Fernando Gomollón, Jesús Castro-Poceiro, Jair Morales-Alvarado, Jordina Llaó, Andrés Rodríguez, Cristina Rodríguez, Pablo Pérez-Galindo, Mercè Navarro, Nuria Jiménez-García, Marta Carrillo-Palau, Isabel Blázquez-Gómez, Eva Sesé, Pedro Almela, Patricia Ramírez de la Piscina, Carlos Taxonera, Iago Rodríguez-Lago, Lidia Cabrinety, Milagros Vela, Miguel Mínguez, Francisco Mesonero, María José García, Mariam Aguas, Lucía Márquez, Marisol Silva Porto, Juan R. Pineda, Koldo García-Etxebarría, Federico Bertoletti, Eduard Brunet, Míriam Mañosa and Eugeni Domènech in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848231221713 for Clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in patients with ulcerative colitis treated with two consecutive anti-TNF agents: data from the ENEIDA registry by Margalida Calafat, Paola Torres, Joan Tosca-Cuquerella, Rubén Sánchez-Aldehuelo, Montserrat Rivero, Marisa Iborra, María González-Vivo, Isabel Vera, Luisa de Castro, Luis Bujanda, Manuel Barreiro-de Acosta, Carlos González-Muñoza, Xavier Calvet, José Manuel Benítez, Mónica Llorente-Barrio, Gerard Surís, Fiorella Cañete, Lara Arias-García, David Monfort, Andrés Castaño-García, Francisco Javier Garcia-Alonso, José M. Huguet, Ignacio Marín-Jímenez, Rufo Lorente, Albert Martín-Cardona, Juan Ángel Ferrer, Patricia Camo, Javier P. Gisbert, Ramón Pajares, Fernando Gomollón, Jesús Castro-Poceiro, Jair Morales-Alvarado, Jordina Llaó, Andrés Rodríguez, Cristina Rodríguez, Pablo Pérez-Galindo, Mercè Navarro, Nuria Jiménez-García, Marta Carrillo-Palau, Isabel Blázquez-Gómez, Eva Sesé, Pedro Almela, Patricia Ramírez de la Piscina, Carlos Taxonera, Iago Rodríguez-Lago, Lidia Cabrinety, Milagros Vela, Miguel Mínguez, Francisco Mesonero, María José García, Mariam Aguas, Lucía Márquez, Marisol Silva Porto, Juan R. Pineda, Koldo García-Etxebarría, Federico Bertoletti, Eduard Brunet, Míriam Mañosa and Eugeni Domènech in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_17562848231221713 for Clinical and treatment outcomes of a second subcutaneous or intravenous anti-TNF in patients with ulcerative colitis treated with two consecutive anti-TNF agents: data from the ENEIDA registry by Margalida Calafat, Paola Torres, Joan Tosca-Cuquerella, Rubén Sánchez-Aldehuelo, Montserrat Rivero, Marisa Iborra, María González-Vivo, Isabel Vera, Luisa de Castro, Luis Bujanda, Manuel Barreiro-de Acosta, Carlos González-Muñoza, Xavier Calvet, José Manuel Benítez, Mónica Llorente-Barrio, Gerard Surís, Fiorella Cañete, Lara Arias-García, David Monfort, Andrés Castaño-García, Francisco Javier Garcia-Alonso, José M. Huguet, Ignacio Marín-Jímenez, Rufo Lorente, Albert Martín-Cardona, Juan Ángel Ferrer, Patricia Camo, Javier P. Gisbert, Ramón Pajares, Fernando Gomollón, Jesús Castro-Poceiro, Jair Morales-Alvarado, Jordina Llaó, Andrés Rodríguez, Cristina Rodríguez, Pablo Pérez-Galindo, Mercè Navarro, Nuria Jiménez-García, Marta Carrillo-Palau, Isabel Blázquez-Gómez, Eva Sesé, Pedro Almela, Patricia Ramírez de la Piscina, Carlos Taxonera, Iago Rodríguez-Lago, Lidia Cabrinety, Milagros Vela, Miguel Mínguez, Francisco Mesonero, María José García, Mariam Aguas, Lucía Márquez, Marisol Silva Porto, Juan R. Pineda, Koldo García-Etxebarría, Federico Bertoletti, Eduard Brunet, Míriam Mañosa and Eugeni Domènech in Therapeutic Advances in Gastroenterology