Abstract
Objective
This retrospective study determined the storage time of finished infusion in each hospital ward and assessed whether the storage time of finished infusion was within an acceptable range.
Methods
The research object was the finished infusion (one bag of infusion with only one drug) that is centrally dosed at the Pharmacy Intravenous Admixture Service (PIVAS) of Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences. We used an automatic scanner to assess the placement time of finished infusion products in various wards of the hospital. We classified the drugs used in various wards, analyzed whether their placement times were reasonable, assessed the reasons for unreasonable placement times, and took intervention measures. Similarly, the storage time of finished infusion was deemed reasonable or unreasonable, the reasons for unreasonable storage times were analyzed, and intervention measures were taken.
Results
In September 2021, the proportion of infusions stored for an unreasonable time was 12.69%, a decrease of 5.37% compared with August 2021, indicating the effectiveness of intervention measures.
Conclusion
By using statistical analysis and intervention measures, our PIVAS improved the standardized use of finished infusion products and ensured the safety of medication for patients.
Keywords: Pharmacy Intravenous Admixture Service, storage time, intravenous medicine, finished infusion, hospital ward, placement time, quality
Introduction
The Pharmacy Intravenous Admixture Service (PIVAS) is a hospital department designed to take into account drug characteristics and whose operations conform with international standards. In the PIVAS, pharmacists review prescriptions, and specially trained pharmaceutical professionals and technicians strictly follow standard operations for mixing and dispensing intravenous drugs—such as total intravenous nutrition, cytotoxic drugs, and antibiotics—to provide high-quality products and pharmaceutical services for clinical practice.1–4 The emergence of the PIVAS has reduced the adverse reactions and patient mortality caused by contaminated and incorrectly mixed intravenous infusions and has improved work efficiency by guaranteeing clinically rational drug use and reducing the cost of drug allocation.5–8
The goal of the PIVAS is to centrally and precisely dispense finished infusions using standard operating procedures. 9 However, at present, the PIVAS’s inability to reasonably use drugs is mainly focused on the review of prescriptions10–12 despite the lack of standardized management of the process from configuration to delivery of medication to the patient, which results in the placement time for finished infusion solutions being overlooked. The finished infusion solution that is configured at the PIVAS must undergo mixed dosing and preparation, pharmacist scanning and verification, dispensing, special distribution, handover, nurse verification, and finally, patient infusion. Therefore, the finished infusion does not only stay in the PIVAS, and instances remain in which patients cannot be infused on time for various reasons. 13 If the finished infusion’s storage time exceeds the drug’s stable time, the safety or even the life of a patient can be jeopardized.14–15 According to the literature, more than 50% of infusion reactions are caused by the prolonged placement of the finished infusion solution. For example, the prolonged placement of penicillin antimicrobials leads to drug hydrolysis, and polymers increase with insertion time; these outcomes eventually lead to a decrease in the drug titer and an increase in the incidence of infusion reactions. Moreover, prolonged placement of finished infusion solutions can decrease drug concentrations, preventing the timely achievement of effective therapeutic concentrations and increasing the risk of bacterial resistance.16–18 Therefore, leaving a finished infusion with unstable properties for too long may cause drug degradation and affect the drug’s therapeutic effect. The number of particles in the infusion may also increase and the physical and chemical properties of the infusion may change, resulting in outcomes such as discoloration and precipitation. In addition, the prolonged storage of the finished infusion solution increases the probability of contamination by the surrounding environment.
Information and methodology
Source
The drug inserts of commonly used drugs in the intravenous drug dispensing center of Jiading District Central Hospital were collected and summarized in accordance with several rules and relevant literature. We collected the drug instructions for commonly used drugs in the intravenous medication dispensing center of Jiading District Central Hospital and combined these with relevant literature, summarizing the information in accordance with the following rules: (1) reference order: drug instructions → literature; (2) selection of literature primarily based on the content determination method recorded in the Chinese Pharmacopoeia; (3) reference to the latest literature; (4) drugs with long storage time after preparation in references; (5) without respect to drug specification (e.g., two specifications for piperacillin sodium and tazobactam sodium for injection—0.625 g and 1.125 g—both of which were classified as injection of piperacillin sodium and tazobactam sodium for injection); and (6) the commonly used infusion solvents in our hospital were: 0.9% sodium chloride injection (0.9% NS), 5% glucose injection (5% GS), 10% glucose injection (10% GS), 5% glucose sodium chloride injection (5% GNS), lactate Ringer equilibrium solution, and fructose injection. The stability time that was recorded in the literature of drugs in other solvents was not referenced.
Methods
The selection criteria for the research infusions were as follows: (1) a finished infusion with only one drug added to the infusion bag; (2) a finished infusion solution that was centrally prepared by the PIVAS; and (3) a finished infusion with a clear stabilization time limit after preparation. When dosing sterile products, the automatic scanner installed at the PIVAS is used to scan the barcode on the infusion label. If the scan fails, the barcode is rescanned or the medicine is returned; dosing and mixing resume after the scan is successful, and the scanner automatically records the dosing mixing time. After the infusion is prepared, the pharmacist checks and scans the finished infusion solution and then packs and sends the infusions to the various wards. The ward nurses receive the infusions and store them in the ward. When administering medications, ward nurses use automatic scans to record the administration time of finished infusions.
We collaborated with professional computer personnel to regularly inspect and maintain the automatic terminal scanner. We were further equipped with a backup automatic terminal scanner for emergency use. Based on the mixing and dosing times recorded by the scanner and the ward nurse, respectively, the preparation and infusion times of the finished infusion in each ward were obtained using the infusion status query of the intravenous drug dispensing center in the central pharmacy system. The data were combined with the drug instructions and literature. GraphPad Prism 7.0 (GraphPad Software, California, USA) processed the average storage time of various drugs and the irregular utilization rate of finished infusion solutions in each ward. Finished infusions with more than 10 hours of storage time that were identified in the statistical process were removed when calculating the average storage time. Finally, the reasons for storage times greater than 10 hours were analyzed using the comprehensive query function of the medical record of the Kingstar Weining resident workstation system (Kingstar Weining Software Technology Co. Ltd, Shanghai, China) and telephone communication with the ward.
This was a retrospective research study, and all of the patient details were de-identified. The study only explored infusion placement time and did not record the condition of patients after medication; therefore, ethics committee approval was not required.
Results
Statistics for storage time of finished infusion solutions in August 2021
In August 2021, our hospital’s intravenous drug dispensing center dispensed a total of 52,952 stickers for finished infusions. Among these infusions, 43,391 (81.94%) were stored for a reasonable time. The infusions that were stored for an unreasonable time (9561; 18.06%) were mainly concentrated in Ward 1 (Pediatrics), Ward 11 (Hematology), Ward 20 (Intensive Care Unit), Ward 25 (Nephrology), and Ward 26 (Oncology).
The causes of the unreasonable placement times of finished infusions were analyzed for the above-mentioned wards. The first reason involved poor communication with the ward. Long drug placement time occurs for patients who undergo many examinations or surgery that is scheduled on the same day. However, the clinical nurse does not inform the patient in advance of the delayed medication time, resulting in the product infusion being detained in the ward. Second, the allocation of drug batches is unreasonable. Given a large amount of fluid replenishment in some patients, the drugs become concentrated in the first and second batches, resulting in the inability to promptly use finished infusion products that have poor stability after preparation. Third, because of the shortage of drug delivery personnel, the prepared infusion is left at the PIVAS and cannot be delivered to the ward on time. Fourth, clinical nurses’ busy schedules and lack of pharmacy-related professional background and knowledge result in insufficient attention to the placement time of finished infusions in clinical practice. Therefore, adverse drug reactions that are caused by the long storage time of finished infusion are ignored. These results are shown in Table 1.
Table 1.
Unreasonable storage time of finished infusion in each ward in August 2021.
| Ward | Number of infusions stored for a reasonable time | Number of infusions stored for an unreasonable time | Total number | Proportion of infusions stored for an unreasonable time |
|---|---|---|---|---|
| Ward 1 | 990 | 567 | 1557 | 36.42% |
| Ward 2 | 2583 | 786 | 3369 | 23.33% |
| Ward 3 | 2289 | 730 | 3019 | 24.18% |
| Ward 4 | 1298 | 48 | 1346 | 3.57% |
| Ward 5 | 1009 | 20 | 1029 | 1.94% |
| Ward 6 | 2693 | 511 | 3204 | 15.95% |
| Ward 7 | 4749 | 1092 | 5841 | 18.70% |
| Ward 8 | 4069 | 774 | 4843 | 15.98% |
| Ward 9 | 2846 | 123 | 2969 | 4.14% |
| Ward 10 | 1004 | 66 | 1070 | 6.17% |
| Ward 11 | 4658 | 1624 | 6282 | 25.85% |
| Ward 12 | 2484 | 237 | 2721 | 8.71% |
| Ward 16 | 1354 | 111 | 1465 | 7.58% |
| Ward 18 | 3496 | 183 | 3679 | 4.97% |
| Ward 20 | 3243 | 1081 | 4324 | 25.00% |
| Ward 22 | 799 | 34 | 833 | 4.08% |
| Ward 25 | 871 | 414 | 1285 | 32.22% |
| Ward 26 | 2956 | 1160 | 4116 | 28.18% |
Interventions
We adopted several intervention measures. First, communication with the ward was strengthened. If the patient had more examinations or surgery the next day, the clinical nurse was asked to inform the PIVAS in advance, and the unprepared infusions were sent to the ward for deployment before use. Second, we encouraged the reasonable allocation of batches: for patients with a large amount of rehydration in the same batch, the finished infusion that exhibited poor stability after mixing was scheduled for the next batch of mixing to minimize the placement time of the finished infusion. Third, in September 2021, the PIVAS added a new delivery person to improve the efficiency of finished infusion delivery. Finally, we provided education on the placement time of finished infusions and increased clinical nurses’ awareness of the time limits of finished infusions.
Statistics for placement time of finished infusion solutions in September 2021
In September 2021, the PIVAS dispensed 50,490 finished infusion stickers. The solutions with unreasonable placement times were concentrated in Ward 1 (Pediatrics), Ward 25 (Nephrology), and Ward 26 (Oncology). The results are shown in Table 2.
Table 2.
Unreasonable storage time of finished infusions in each ward in September 2021.
| Ward | Number of infusions stored for a reasonable time | Number of infusions stored for an unreasonable time | Total number | Proportion of infusions stored for an unreasonable time |
|---|---|---|---|---|
| Ward 1 | 1263 | 599 | 1862 | 32.17% |
| Ward 2 | 3295 | 667 | 3962 | 16.83% |
| Ward 3 | 2339 | 459 | 2798 | 16.40% |
| Ward 4 | 1304 | 8 | 1312 | 0.61% |
| Ward 5 | 849 | 17 | 866 | 1.96% |
| Ward 6 | 3251 | 342 | 3593 | 9.52% |
| Ward 7 | 5126 | 621 | 5747 | 10.81% |
| Ward 8 | 4212 | 384 | 4596 | 8.36% |
| Ward 9 | 2424 | 24 | 2448 | 0.98% |
| Ward 10 | 788 | 3 | 791 | 0.38% |
| Ward 11 | 4506 | 973 | 5479 | 17.76% |
| Ward 12 | 1492 | 118 | 1610 | 7.33% |
| Ward 16 | 864 | 27 | 891 | 3.03% |
| Ward 18 | 3561 | 42 | 3603 | 1.17% |
| Ward 20 | 3842 | 808 | 4650 | 17.38% |
| Ward 24 | 608 | 11 | 619 | 1.78% |
| Ward 25 | 940 | 362 | 1302 | 27.80% |
| Ward 26 | 3421 | 940 | 4361 | 21.55% |
Post-intervention effect analysis
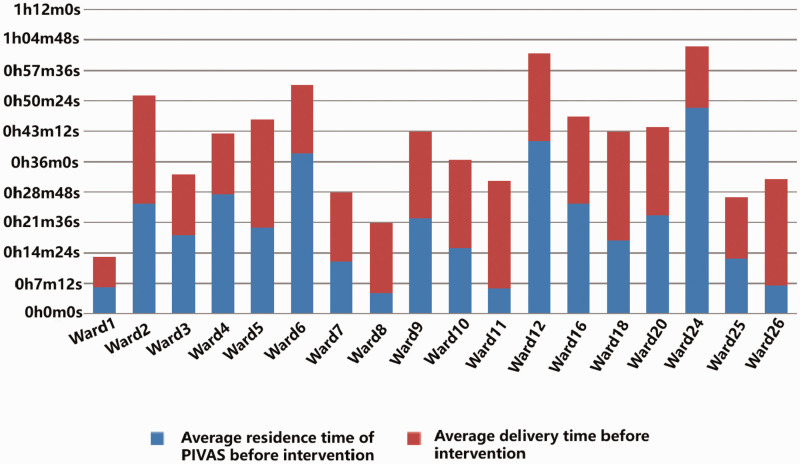
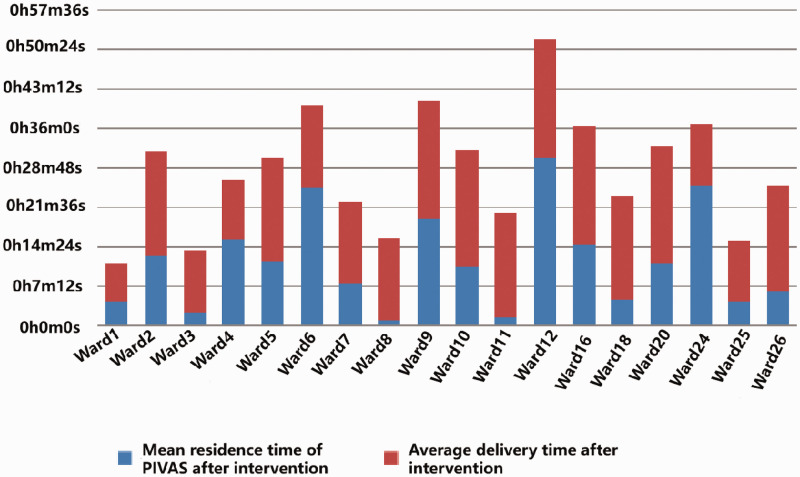
After the intervention, the irregular utilization rate of finished infusions in all hospital wards decreased from 25.85% to 17.76% in Ward 11 (Hematology) and from 25.00% to 17.38% in Ward 20 (Intensive Care Unit). However, the proportions of finished infusions with unreasonable placement times in Ward 1 (Pediatrics), Ward 25 (Nephrology), and Ward 26 (Oncology) still exceeded 20%, and the effect of interventions in Ward 5 (Obstetrics & Gynecology) was insignificant. Therefore, we analyzed the specific causes that influenced the outcomes in each ward and identified individualized interventions. In addition, the mean residence time of the finished infusion at the PIVAS was significantly reduced, and the average delivery time of the finished infusion dropped after a delivery person was added. The results are shown in Table 3 and Figures 1 and 2.
Table 3.
Comparison of the proportion of unreasonable storage times of finished infusions.
| Ward | Proportion of infusions stored for an unreasonable time in August 2021 | Proportion of infusions stored for an unreasonable time in September 2021 | Difference |
|---|---|---|---|
| Ward 1 | 36.42% | 32.17% | 4.25% |
| Ward 2 | 23.33% | 16.83% | 6.50% |
| Ward 3 | 24.18% | 16.40% | 7.78% |
| Ward 4 | 3.57% | 0.61% | 2.96% |
| Ward 5 | 1.94% | 1.96% | −0.02% |
| Ward 6 | 15.95% | 9.52% | 6.43% |
| Ward 7 | 18.70% | 10.81% | 7.89% |
| Ward 8 | 15.98% | 8.36% | 7.63% |
| Ward 9 | 4.14% | 0.98% | 3.16% |
| Ward 10 | 6.17% | 0.38% | 5.79% |
| Ward 11 | 25.85% | 17.76% | 8.09% |
| Ward 12 | 8.71% | 7.33% | 1.38% |
| Ward 16 | 7.58% | 3.03% | 4.55% |
| Ward 18 | 4.97% | 1.17% | 3.81% |
| Ward 20 | 25.00% | 17.38% | 7.62% |
| Ward 24 | 4.08% | 1.78% | 2.30% |
| Ward 25 | 32.22% | 27.80% | 4.41% |
| Ward 26 | 28.18% | 21.55% | 6.63% |
| Total | 18.06% | 12.69% | 5.37% |
Figure 1.
Comparison of storage time of finished infusion before the intervention. The blue area represents the average residence time at the PIVAS before the intervention, whereas the red area represents the average delivery time before the intervention. PIVAS, Pharmacy Intervention Admixture Service.
Figure 2.
Comparison of storage time of finished infusion after the intervention. The blue area represents the mean residence time at the PIVAS after the intervention, whereas the red area represents the average delivery time after the intervention. PIVAS, Pharmacy Intervention Admixture Service.
Screening of drugs with unreasonable placement times
Using the time recorded by the automatic terminal scanner, the drugs that are commonly prepared by the PIVAS and that have a stable time of less than 6 hours after preparation were screened out, and the unreasonable storage times were investigated. The results are shown in Table 4.
Table 4.
Proportion of commonly used drug infusions stored for an unusual storage time.
| Drug | Proportion of infusions stored for a reasonable storage time | Proportion of infusions stored for an unreasonable storage time | Total | Proportion of infusions stored for an unreasonable time |
|---|---|---|---|---|
| Ginkgo biloba leaf extract injection | 0 | 228 | 228 | 100.00% |
| Ginkgo leaf extract and dipyridamole injection | 0 | 74 | 74 | 100.00% |
| Shuganning Zhusheye | 0 | 125 | 125 | 100.00% |
| Shuxuetong Zhusheye | 26 | 159 | 185 | 85.95% |
| Glutathione for injection (atomoran) | 306 | 650 | 956 | 67.99% |
| Gabexate mesylate for Injection | 173 | 334 | 507 | 65.88% |
| Glutathione for injection (ShuangYijian) | 439 | 625 | 1064 | 58.74% |
| Blood plug for injection (Xinaotai) | 76 | 104 | 180 | 57.78% |
| Eddy Injection | 528 | 368 | 896 | 41.07% |
| Ambroxol hydrochloride Injection | 1148 | 617 | 1765 | 34.96% |
| Kidney Kang Injection | 143 | 70 | 213 | 32.86% |
| Xingnaojing injection | 209 | 91 | 300 | 30.33% |
| Dexamethasone sodium phosphate injection | 687 | 281 | 968 | 29.03% |
| Sputum heat-clearing injection | 736 | 245 | 981 | 24.97% |
| Element injection | 42 | 13 | 55 | 23.64% |
| Fosfomycin sodium for injection | 126 | 36 | 162 | 22.22% |
| Cefoperazone sodium and sulbactam sodium for injection | 68 | 12 | 80 | 15.00% |
| Imipenem and cilastatin sodium for injection | 245 | 33 | 278 | 11.87% |
| Magnesium isoglycyrrhizinate injection | 369 | 37 | 406 | 9.11% |
| Imipenem and cilastatin sodium for injection (Zhuhai) | 57 | 5 | 62 | 8.06% |
| Phloroglucinol Injection | 449 | 34 | 483 | 7.04% |
| Ginkgolide Injection | 106 | 7 | 113 | 6.19% |
| Cefotaxime sodium for injection | 140 | 6 | 146 | 4.11% |
| Cefoperazone sodium and sulbactam sodium for injection | 134 | 3 | 137 | 2.19% |
| Xueshuantong injection | 777 | 11 | 788 | 1.40% |
| Cefepime hydrochloride for injection | 108 | 1 | 109 | 0.92% |
| Amoxicillin sodium and clavulanate potassium for injection | 198 | 1 | 199 | 0.50% |
| Levofloxacin hydrochloride injection (Zuoke) | 660 | 2 | 662 | 0.30% |
| Ceftriaxone sodium for injection (luoshifen) | 363 | 1 | 364 | 0.27% |
| Meropenem for injection | 410 | 1 | 411 | 0.24% |
| Pemetrexed disodium for injection | 4 | 0 | 4 | 0.00% |
| Salvia miltiorrhiza polyphenolic acid salt for injection | 635 | 0 | 635 | 0.00% |
| Thioctic acid injection | 178 | 0 | 178 | 0.00% |
| Tranexamic acid injection | 168 | 0 | 168 | 0.00% |
| Asarone injection (Shanxi) | 333 | 0 | 333 | 0.00% |
The screened drugs were as follows: (1) nutritional drugs: ginkgo biloba leaf extract injection, ginkgo leaf extract and dipyridamole injection, Shuganning Shusheye, Shuxuetong Zhusheye, glutathione for injection (atomoran), gabexate mesylate for injection, glutathione for injection (Shuang Yijian), blood plug for injection (Xinaotai), ambroxol hydrochloride injection, kidney Kang injection, xingnaojing injection, dexamethasone sodium phosphate injection, and sputum heat clearing injection; (2) chemotherapy drugs: elemene injection and eddy injection; and (3) antibiotics: fosfomycin sodium for injection.
An unreasonable storage time of 100% occurs when the finished infusion requires immediate preparation and use. The finished infusion solution configured at the PIVAS must go through mixed dosing and preparation, pharmacist scanning and verification, dispensing, special distribution, handover, nurse verification, and patient infusion. Therefore, the finished infusion does not only stay in the PIVAS. Therefore, cases remain of patients who cannot be infused on time for various reasons.
Analysis of the causes of finished infusions with storage times greater than 10 hours
(1) 14 September 2021 in Ward 16: one bag of imipenem/cilastatin sodium for injection was placed at 10 hours, 08 minutes, and 08 s; the patient was rescued at 7:56 a.m. The patient underwent many examinations and received more infusions on the same day. (2) 19 September 2021 in Ward 7: one bag of Shuganing Zhusheye was placed at 10 hours, 42 minutes, and 37 s; the patient underwent laparoscopic cholecystectomy under general anesthesia at 9:58 a.m. in the emergency department on the same day. (3) 24 September 2021 in Ward 20: one bag of Shuang Yijian glutathione for injection was placed at 12 hours, 28 minutes, and 1 s; the patient underwent many examinations on the same day and was moved from the intensive care unit to Ward 6 at 9.24 p.m.
Discussion
Intravenous administration is a common form of drug administration in clinical practice. The centralized dispensing of intravenous drugs differs from the traditional mode of drug dispensing by pharmacies and nurses in wards. The centralized allocation of intravenous drugs avoids drug wastage and improves work efficiency. However, the centralized allocation of finished product infusion is batch mixing dosing, which can lead to the failure of timely infusion of some finished product infusions. 19 In accordance with Article 7.7.2 of the Ward Hospital Infection Management Regulations 20 (WS-T 510-2016), the maximum storage time for sterile intravenous infusion fluids should not exceed 2 hours. Guidance is also available in the Practice Guidelines and Implementation Rules for Infusion Therapy Nursing: 21 The prepared solution should not be stored for more than 2 hours. The PIVAS of our hospital is responsible for the long-term preparation of infusion solutions for all wards including the emergency observation room. The large dispensed volumes in the PIVAS can result in finished infusion solutions that cannot be dispensed and used immediately. However, the focus of the PIVAS is on the review of prescriptions, and standardized time management of the process between completing the finished infusion configuration and medicating the patient is lacking, leading to the neglect of reasonable placement time of finished infusion.
Several factors affect the administration time of finished infusions. First, the factors that affect the use of finished infusions in patients are closely related to patient appointments for special examinations or surgery, which affect the timely infusion of the finished infusion. If the nurse does not request that the PIVAS adjust the delivery batch or pack the medicine in time, the patient cannot promptly receive the infusion. In addition, prolonging the infusion time affects the administration time of the finished infusion. In the clinic, nurses generally adjust the drip rate according to the drug, the needs of treatment, and individual patient characteristics. For example, in older adults, children, or patients with cardiopulmonary dysfunction, the drip rate during infusion must be adjusted to reduce the burden on the heart.
The second factor is drug-related. For some drugs that considerably stimulate blood vessels, the drip rate must be adjusted according to the patient’s condition during the infusion process 22 to minimize the stimulation of blood vessels by drugs and pain at the infusion site.
The third factor is related to the delivery time of the finished infusion solution, which is manually distributed. The distribution box is large, many finished infusions are distributed, and the delivery workers must take the elevator to deliver the medicine. Therefore, factors such as delivery time, drug delivery staffing, and whether the transportation channel is smooth affect whether the finished infusion is promptly delivered to the clinic. 23 Generally, the PIVAS staff works early to dispense infusions. However, most delivery times are between 9:00 a.m. and 10:00 a.m., a period in which the number of patients in the hospital is large and the number of elevators is limited. The PIVAS staff does not have a dedicated elevator for distribution. Hence, the delivery of finished infusions takes a long time. In addition, the delivery cart cannot be used if the clinical nurse does not receive the finished infusion on time, leading to reduced distribution efficiency, a long drug delivery time, and a lack of timely receipt of the finished infusion by some departments. Therefore, we will build dedicated distribution elevators and establish distribution conveyor belts. Moreover, we will regularly inspect the finished infusion distribution channel.
The fourth factor that affects administration time is when the finished infusion is dispensed. If the drug is dispensed too early, the waiting time for the drug from completion to delivery to the ward is prolonged, and the immediate use of some drugs that have a short stabilization time after dispensing is completed cannot be ensured. For example, the stabilization time after the preparation of alprostadil injection is 2 hours. The finished infusion of cyclophosphamide for injection is stable for only 2 to 3 hours. When such drugs are dispensed too early, the stabilization time of the finished infusion may be insufficient.
The fifth factor involves infusion batch distribution. Most departments generally allocate 3 to 4 daily batches of finished infusions that differ in infusion method, sequence, and habits. Notably, some departments still maintain their habits for patient infusion and do not give priority to the use of finished infusions with poor stability. In addition, if a patient requires two or more bags of the same batch of finished infusion with a stabilization time limit, one of the infusions may not be fully administered within its stabilization time, especially when a slow-drip finished infusion is used. For example, sodium deoxynucleotide injection (i.e., 50–100 mg of 5% glucose injection dissolved in 250 mL at a time) must slowly drip at a rate of 2 mL/min and requires approximately 2 hours for full administration. Therefore, when reviewing the doctor’s prescription, the PIVAS pharmacist should fully consider the drip rate of the finished infusion solution and judge the appropriateness of the division of the patient’s infusion batch. 24 In addition, the frequency of drug administration is determined by the length of the drug’s half-life and its elimination rate in the body. For example, time-dependent antibiotics (e.g., penicillin and cephalosporin) must be administered multiple times daily to achieve an effective therapeutic concentration; therefore, ensuring a reasonable time interval when dividing batches is necessary.
A sixth factor is communication with the clinic. Pharmacists, clinicians, and nurses generally use telephone communication, which is often associated with problems such as the inability to communicate on time and poor communication. However, clinical nurses lack pharmacy-related expertise, especially in the types of drugs that have limited stability after preparation and cause adverse reactions because of the long storage time of the finished infusion. 25 Furthermore, knowledge of the stability time after drug configuration that is provided in the drug insert is scarce, and factors such as the decrease in drug stability and the decrease in titer caused by untimely infusion of the finished infusion affect the safety of the finished infusion solution.
Finally, the drug label influences administration time. Standardized instructions ensure that the drugs used by patients are safe and effective. Insufficiently accurate drug instructions may affect the safety of the drug and the accurate use of medication. Improperly medicating patients causes adverse drug reactions. For example, the instructions for glutathione injection indicate that it should be administered as 500 mL of 5% glucose for intravenous drip. The precautions state that the drug must be infused within 2 hours of glutathione being dissolved. However, for an intravenous infusion with a drop rate of 60 to 80 drops/minute, completing the infusion within a 2-hour stable time is impossible.
The PIVAS uses a personal digital assistant to monitor drugs that have a stable time after preparation. By analyzing the statistics of the finished infusions that were centrally allocated by the PIVAS in August 2021, we observed that the irregular utilization rate of the finished infusion was 18.06%. The statistics of finished infusions that were centrally allocated by PIVAS in September 2021 indicated that the irregular utilization rate of finished infusion solutions was 12.69%, which represents a decrease of 5.37% compared with August 2021. The average residence time at the PIVAS and the delivery time of finished infusion dropped significantly before and after the intervention, indicating that the intervention was effective. However, after the intervention, the irregular use rate in the pediatrics, nephrology, and oncology wards remained above 20%. By communicating with these wards and analyzing the factors that could explain the poor performance, we made several observations.
First, the finished infusion solution that had an unreasonable placement time in Ward 1 (Pediatrics) was mainly the ambroxol hydrochloride injection, which can be placed for 24 hours after preparation when 0.9% NS is used as the solvent medium. When sodium lactate and Ringer equilibrium solution are used as the solvents, ambroxol hydrochloride injection can be placed for 24 hours after preparation. When 5% GS is used as the solvent medium, ambroxol hydrochloride injection should be used immediately after the preparation is completed. Because Ward 1 is a pediatric ward, ambroxol hydrochloride injection is commonly administered in the ward with 5% GS as the solvent because 0.9% NS increases the burden on children’s hearts and kidneys, which are immature. Moreover, using 5% GS as the solvent medium not only provides energy to the body and enhances its resistance but also promotes cardiac diuresis; glucose absorption and utilization supply myocardial energy and improve myocardial nutrition. In addition, some children in Ward 1 are not hospitalized at night; their parents bring them to the hospital in the morning for infusion. Given that the infusion time depends on the child’s time of arrival at the hospital, the unknown timing for infusion can result in the retention of finished infusion in the ward.
Second, Ward 25 (Nephrology) generally uses antibacterial and nutritional drugs first. At present, the first batch of finished infusion distribution by the PIVAS is the simultaneous delivery of antibacterial and nutritional drugs, which results in nutritional drugs that cannot be used on time being left in the ward. The most commonly used nutritional drugs in the nephrology ward are glutathione for injection (atomoran), glutathione for injection (Shuang Yijian), and kidney Kang injection. The 2-hour stability time after preparation of these three drugs results in the irregular use rate of finished infusion in the nephrology ward.
Third, the drugs with a high non-standard utilization rate in Ward 26 (Oncology) are glutathione for injection (atomoran), glutathione for injection (Shuang Yijian), and eddy injection, which have stabilization times after deployment of 2, 2, and 3 hours, respectively. Because of the short stabilization time after drug preparation, the residence time of finished oncology infusions at the PIVAS is long and results in the irregular utilization rate of finished infusions in the oncology ward.
Finally, the non-standard use rates of finished infusion products in Ward 5 (Obstetrics & Gynecology) were 1.94% and 1.96% in August and September, respectively, and were driven by the slightly increased use of glutathione for injection in Ward 5 in September versus August. Follow-up with the PIVAS will ensure the development of individualized interventions for each ward to shorten the placement time of finished infusions and ensure the rational use of finished infusions.
In an early stage, we studied the time lag problem and countermeasures of centralized dispensing of finished product infusion and published a paper. 26 On this basis, we further conducted a statistical analysis of the placement time of centrally prepared finished infusion products by analyzing experimental data. In the future, the PIVAS will develop new intervention measures. First, the PIVAS will establish distribution conveyor belts or a decentralized PIVAS for finished infusions that require immediate preparation and use. Second, the PIVAS will strengthen the warning management in the ward, mark the stable time limit of finished infusions on infusion labels, and integrate the warning management system into the nurse work platform. When the finished infusion is close to the specified placement time, the warning system will automatically prompt the PIVAS’s pharmacist and clinical nurse. The prompt will remind the pharmacist to inform clinical staff to immediately administer the finished infusion. Finally, the PIVAS will reclassify batches of infusion with high unreasonable storage times and adjust the preparation order.
The sample size in this study was limited and included only the finished infusions from our hospital in August and September 2021. In the future, we will outline personalized improvement measures for each ward to further shorten the placement time of finished infusion products and continue to measure improvements to the placement time of finished infusions. In addition, the stability of intravenous formulations was not investigated in any chemical, physical, or microbiological experiments in this study; further research will also be conducted in this area.
Conclusion
In summary, finished infusion solutions may be appropriately placed based on their stable times, but their quality can be affected as placement time increases. Guided by statistical analysis, the PIVAS took intervention measures to optimize the infusion dispensing process 27 and improve the standardization of the use of finished infusion to ensure the safety and effectiveness of intravenous medication for patients. 24
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author contributions: JG and ZY: designed the study; CZ: collected the data; YQ: analyzed the data and reviewed the article; MY and YQ: submitted the article.
The authors declare that they have no conflict of interest.
Funding: This study was supported by the National Nature Science Foundation of the Agricultural and Social Research Projects of Jiading, Shanghai (Grant no. JDKW-2020–0013) and the Shanghai Key Specialty of Clinical Pharmacy (District, YXZDZK-01). The study was partially supported by the General Project of Jiading District Health Commission, Shanghai (Grant no. 2020-KW-14).
ORCID iD: Yi Qingqing https://orcid.org/0000-0003-4955-5851
Data availability statement
All data included in this study are available upon request by contacting the corresponding author.
References
- 1.Yang CS, Kang BY, Zhang LL, et al. Construction situation, costs and charges associated with pharmacy intravenous admixture services: multi-center cross-sectional survey based on 137 medical institutions in mainland China [J]. BMC Health Serv Res 2020; 20: 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang CS, Lin YZ, Zhang LL, et al. Evidence-based evaluation of construction status of pharmacy intravenous admixture services in China [J]. Journal of Pediatric Pharmacy 2020; 26: 32–35. [Google Scholar]
- 3.Huang J, Wu WY, Jiang ZF, et al. Application of drug safety management system based on closed-loop concept [J]. Nursing Safety 2018; 18: 1103–1107. [Google Scholar]
- 4.Mi W, Li L, Zhang Y, et al. Chinese centralised intravenous admixture service (CIVAS), an emerging pharmaceutical industry: survey of the recent advances of CIVAS in China. Eur J Hosp Pharm 2018; 25: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan ZZ, Li QQ, Tang TT, et al. Studies on the optimization of decontamination protocol for surfaces contaminated with cytotoxic drugs in PIVAS [J]. J Oncol Pharm Pract 2022; 12. [DOI] [PubMed] [Google Scholar]
- 6.Geng Z, Yang WB, Wei SB, et al. Verification of real-time microbial monitoring technology based on the principle of laser-induced fluorescence under USP 1223 standard and prospective application in our hospital PIVAS clean room [J]. Int J Pept Res Ther 2021; 28: 8–21. [Google Scholar]
- 7.Andrade AS D, Silva ÉL D, Silva MD RC, et al. As relações de poder que permeiam o Perímetro Irrigado Várzeas de Sousa-PB (PIVAS) [J]. Research, Society and Development 2019; 8: 1–18. [Google Scholar]
- 8.Liu H, Zou LK, Song YJ, et al. Cost analysis of implementing a vial-sharing strategy for chemotherapy drugs using intelligent dispensing robots in a tertiary Chinese hospital in Sichuan [J]. Front Public Health 2022; 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li GC, Zhao HX, Deng GL, et al. The effect of auxiliary admixture equipment on the hygiene conditions and quality of pharmacy intravenous admixture services [J]. Appl. Nanosci 2022; 13: 2997–3006. [Google Scholar]
- 10.Wang YF, He KB, Bai J, et al. Establishment of pharmaceutical care mode of PIVAS prescription review based on pharmacist and software [J]. Her Med 2019; 38: 403–406. [Google Scholar]
- 11.Wang X, Hu YH, Zhao Y, et al. Optimization and practice of prescription audit platform of PIVAS in our hospital [J]. China Pharm 2019; 30: 303–306. [Google Scholar]
- 12.Liu Y, Huo Y, Yang QJ, et al. Establishment and effect evaluation of self-defined prescription checking rules in pharmacy intravenous admixture service [J]. Pharm Care Res 2018; 18: 187–191. [Google Scholar]
- 13.Liu X, Li R, Xue SD, et al. Establish a preliminary application of monitoring platform for rational use in vivo fusion [J]. Chin J Hosp Pharm 2020; 40: 2479–2483. [Google Scholar]
- 14.Rombouts MD, Swart EL, Vanden EA JM, et al. Systematic review on infusion reactions to and infusion rate of monoclonal antibodies used in cancer treatment [J]. Anticancer Res 2020; 40: 1201–1218. [DOI] [PubMed] [Google Scholar]
- 15.Zhu LL, Zhou Q. Optimal infusion rate in antimicrobial therapy explosion of evidence in the last five years [J]. Infect Drug Resist 2018; 11: 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Q, Li F, Yeh S, et al. Recent advances in enhancement of dissolution and supersaturation of poorly water-soluble drug in amorphous pharmaceutical solids: A review[J]. AAPS PharmSciTech 2021; 23: 16. [DOI] [PubMed] [Google Scholar]
- 17.Yu B, Wang Y, Geng Z, et al. Establishment and validation of analytical methods for 15 hazardous drugs by UPLC-Q/Orbitrap-HRMS [J]. Ann Transl Med 2022; 12: 686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Pan D, Zhao ZC, et al. Impact of different standing time on insoluble particles of two penicillin-like antibiotics [J]. Chinese Journal of Antibiotics 2020; 45: 152–156. [Google Scholar]
- 19.He CX. Influence of optimizing nosocomial infection control management in PIVAS on quality of infusions and reducing of nosocomial infection rate [J]. Anti Infect Pharm 2020; 17: 223–226. [Google Scholar]
- 20.Regulation for healthcare associated infection control in ward in healthcare facilities [D]. National Health Commission of the People’s Republic of China, 2017.06.01.
- 21.Wang JR. Infusion treatment nursing practice guidelines and rules [M]. People’s Military Medical Press, 2009.10.
- 22.Ni XF, Yang CS, Mi WJ, et al. Multi-center survey on the training status of staff working in pharmacy intravenous admixture services (PIVAS) in mainland China perspectives of PIVAS staff [J]. Medicine (Baltimore) 2021; 100: e27676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DH, Kim YW, Tin YY, et al. Recent technologies for amorphization of poorly water-soluble drugs [J]. Pharmaceutics 2021; 13: 1318–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X, Liu M, Zhou J, et al. The application of quality control circle in improving compliance of rugre sidualin intravenous brugad-mixture of PIVAS in our host [J]. Chin J Hosp Pharm 2020; 40: 559–556. [Google Scholar]
- 25.Chen J, Ni XF, Yang CS, et al. Multi-center investigation on personnel training and scientific research status of pharmacy intravenous admixture services (PIVAS) in mainland China based on the perspectives of PIVAS leaders [J]. Medicine (Baltimore) 2021; 100: e24881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang GW, Zhang YH, Chang Q, et al. Time delay problem and its countermeasures of centralized dispensing of finished product infusion [J]. Herald of Medicine 2022; 41: 1397–1400. [Google Scholar]
- 27.Fang BX, Tao XW, Wu SC, et al. Quality monitoring and improvement of PIVAS insoluble drug lornoxicam finished infusion preparation [J]. Journal of Hubei University of Medicine 2023; 42: 243–246+257. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request by contacting the corresponding author.