Abstract
An Escherichia coli strain deficient in p-aminobenzoate synthesis was mutagenized, and derivatives were selected for growth on folic acid. Supplementation was shown to be due to p-aminobenzoyl-glutamate present as a breakdown product in commercial folic acid preparations. Two classes of mutations characterized by the minimum concentration of p-aminobenzoyl-glutamate that could support growth were obtained. Both classes of mutations were genetically and physically mapped to about 30 min on the E. coli chromosome. A cloned wild-type gene from this region, abgT (formerly ydaH) could confer a similar p-aminobenzoyl-glutamate utilization phenotype on the parental strain. Interruption of abgT on the plasmid or on the chromosome of the mutant strain resulted in a loss of the phenotype. abgT was the third gene in an apparent operon containing abgA, abgB, abgT, and possibly ogt and might be regulated by a divergently transcribed LysR-type regulator encoded by abgR. Two different single-base-pair mutations that gave rise to the p-aminobenzoyl-glutamate utilization phenotype lay in the abgR-abgA intercistronic region and appeared to allow the expression of abgT. The second class of mutation was due to a tandem duplication of abgB and abgT fused to fnr. The abgA and abgB gene products were homologous to one another and to a family of aminoacyl aminohydrolases. p-Aminobenzoyl-glutamate hydrolysis could be detected in extracts from several of the mutant strains, but intact abgA and abgB were not essential for p-aminobenzoyl-glutamate utilization when abgT was supplied in trans.
It has long been known that Escherichia coli and similar organisms cannot utilize exogenously supplied oxidized forms of folic acid or its derivatives. A blockade of dihydrofolate synthesis by inhibitors of dihydropteroate synthase (DHPS) (such as sulfathiazole or other sulfonamide derivatives) or of dihydrofolate reductase (DHFR) (aminopterin or trimethoprim) can be circumvented only by supplementation with the end products of folate metabolism, i.e., purines, thymidine, glycine, methionine, and pantothenic acid (14, 37). Even then, growth is slow, partly because of the failure to formylate methionyl-tRNAfMet for the initiation of protein synthesis (2, 14).
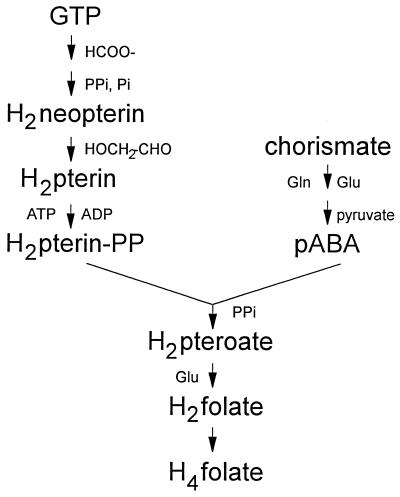
The failure of E. coli to utilize oxidized folate derivatives directly may be due to a lack of transport of these compounds or the failure to reduce them to dihydro or tetrahydro derivatives once they are transported. As illustrated in Fig. 1, folate compounds are synthesized in reduced (dihydro) form, beginning with dihydropterin precursors (39). Following the conjugation of dihydropterin pyrophosphate with p-aminobenzoate to form dihydropteroate, glutamate is added to produce dihydrofolate. Dihydrofolate is then reduced by DHFR to yield tetrahydrofolate, the active one-carbon carrier for cellular metabolism. Bacterial species that require folate derivatives for growth, such as Lactobacillus casei, have elaborate transport systems specific for folate (18). Inhibitory folate analogs, such as aminopterin or methotrexate, are effective against E. coli at relatively high concentrations (24), suggesting that at least passive transport of these compounds may occur. However, once entry into the cell is gained, E. coli DHFR is ineffective in reducing folate to dihydrofolate (25), a fact which prevents the direct entry of oxidized folate derivatives into pools of reduced folates.
FIG. 1.
Abbreviated scheme for tetrahydrofolate biosynthesis from GTP and chorismate. Compounds to the left of each reaction arrow are additional substrates of the reaction, and compounds to the right are additional products of the reaction. H2, dihydro; H4, tetrahydro; pABA, p-aminobenzoate; PP, pyrophosphate.
Another mechanism by which oxidized folates might be utilized is via catabolic or salvage pathways that could reutilize portions of a folate derivative following degradation. In this regard, little is known about bacterial folate salvage, except for organisms that utilize preformed folates. Additionally, the utilization of the pterin product of degradation, namely, pterin or pteroate, might be limited by the same transport or reduction problem cited above.
The p-aminobenzoate moiety of dihydrofolate is synthesized de novo from chorismate and is easily transported into and out of E. coli cells (12, 16, 17, 23). p-Aminobenzoate auxotrophs can be supplemented by the addition of exogenous p-aminobenzoate, and wild-type cells excrete sufficient p-aminobenzoate to supplement nearby p-aminobenzoate auxotrophs.
We have examined whether p-aminobenzoate auxotrophs can utilize p-aminobenzoate derived from exogenously supplied folate derivatives; we found mutant strains of E. coli that could utilize p-aminobenzoyl-glutamate, a degradation product of oxidized folate compounds. Single-base-pair mutations that allowed growth on p-aminobenzoyl-glutamate were found in an intergenic region between divergently transcribed genes, one a lysR homolog (here designated abgR, for p-aminobenzoyl-glutamate) and the other the first gene in an apparent operon containing three genes, two homologous to aminoacyl hydrolases (abgA and abgB) and a third gene homologous to permeases (abgT). A 4.2-kb tandem duplication containing abgB and abgT fused to fnr also allowed growth on p-aminobenzoyl-glutamate. Finally, plasmids containing wild-type abgT alone could confer the ability to grow on p-aminobenzoyl-glutamate to wild-type E. coli strains.
MATERIALS AND METHODS
Microbiological methods.
The bacterial strains used in this study are listed in Table 1. Minimal medium, LB medium, and NCE medium were prepared as described previously (7). Antibiotic and amino acid final concentrations were also as recommended in reference 7. For routine purposes, p-aminobenzoate and p-aminobenzoyl-glutamate were added at 0.7 and 1.8 μM, respectively.
TABLE 1.
Escherichia coli strains used in this study
| Strain | Genotype | Source or referencea |
|---|---|---|
| BN101 | pabA1 trpA33 rpsL704 cys(Am) purU tyrT | 23 |
| BN141 | Φ(pabA-lacZ)17(Hyb) cam Δ(argF-lac)U169 end recB21 recC22 sbcB15 thr-1 argE3 leu-6 his-4 thi-1 lacY ara-14 mtl-1 xyl-5 galK2 rpsL-31 tex-33 sup37(Am) | 36 |
| BN1001 | abg-1 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | DES BN101 |
| BN1002 | abg-2 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | DES BN101 |
| BN1003 | abg-3 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | DES BN101 |
| BN1004 | abg-4 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | DES BN101 |
| BN1005 | abg-5 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | DES BN101 |
| BN1016 | abg-1 zda-3061::Tn10 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | BN1001 × CAG12081 |
| BN1023 | abg-4 zda-3061::Tn10 pabA1 rpsL704 | BN1103 × BN1004 |
| BN1103 | pabA1 rpsL704 | MG1655 × BN101 |
| BN1125 | abg-1 zda-3061::Tn10 Φ(pabA-lacZ)17(Hyb) cam Δ(argF-lac)U169 end recB21 recC22 sbcB15 thr-1 argE3 leu-6 his-4 thi-1 lacY ara-14 mtl-1 xyl-5 galK2 rpsL31 tex-33 sup37(Am) | BN141 × BN1016 |
| BN1128 | abg-1 abgB::kan zda-3061::Tn10 Φ(pabA-lacZ)17(Hyb) cam Δ(argF-lac)U169 end recB21 recC22 sbcB15 thr-1 argE3 leu-6 his-4 thi-1 lacY ara-14 mtl-1 xyl-5 galK2 rpsL31 tex-33 sup37(Am) | pJMG111 × BN1125 |
| BN1129 | abg-1 abgA::kan zda-3061::Tn10 Φ(pabA-lacZ)17(Hyb) cam Δ(argF-lac)U169 end recB21 recC22 sbcB15 thr-1 argE3 leu-6 his-4 thi-1 lacY ara-14 mtl-1 xyl-5 galK2 rpsL-31 tex-33 sup37(Am) | pJMG112 × BN1125 |
| BN1130 | abg-1 abgT::kan zda-3061::Tn10 Φ(pabA-lacZ)17(Hyb) cam Δ(argF-lac)U169 end recB21 recC22 sbcB15 thr-1 argE3 leu-6 his-4 thi-1 lacY ara-14 mtl-1 xyl-5 galK2 rpsL-31 tex-33 sup37(Am) | pBN200 × BN1125 |
| BN1140 | abg-1 zda-3061::Tn10 pabA1 rpsL704 | BN1125 × BN1103 |
| BN1141 | abg-1 abgB::kan zda-3061::Tn10 pabA1 rpsL704 | BN1128 × BN1103 |
| BN1142 | abg-1 abgA::kan zda-3061::Tn10 pabA1 rpsL704 | BN1129 × BN1103 |
| BN1143 | abg-1 abgT::kan zda-3061::Tn10 pabA1 rpsL704 | BN1130 × BN1103 |
| BN1150 | abg-1 zda-3061::Tn10 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | BN1140 × BN101 |
| BN1151 | abg-1 abgB::kan zda-3061::Tn10 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | BN1141 × BN101 |
| BN1152 | abg-1 abgA::kan zda-3061::Tn10 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | BN1142 × BN101 |
| BN1153 | abg-1 abgT::kan zda-3061::Tn10 pabA1 trpA33 rpsL704 cys(Am) purU tyrT | BN1143 × BN101 |
| JC7623 | end recB21 recC22 sbcB15 thr-1 proA2 argE3 leu-6 his-4 thi-1 lacY ara-14 mtl-1 xyl-5 galK2 rpsL-31 tex-33 sup37(Am) | 36 |
| MG1655 | Wild type | 35 |
DES, diethyl sulfate.
Diethyl sulfate mutagenesis was performed by adding 100 μl of an overnight culture of E. coli BN101 to 10 ml of NCE medium that had been saturated with diethyl sulfate. Following 60 min of incubation at 37°C, 200 μl of the suspension was used to inoculate 5 ml of LB medium. After overnight growth, cells were harvested, washed with 0.15 M NaCl, diluted, and spread on minimal medium plates supplemented with tryptophan and either folic acid (45 μM) or folinic acid (40 μM). Colonies that appeared after 2 days of growth were picked and restreaked onto plates supplemented with tryptophan alone or tryptophan plus folic acid in order to eliminate pabA revertants. Five independently mutagenized strains that grew when supplemented with either folic acid or folinic acid were retained and designated E. coli BN1001 to BN1005.
Genetic mapping and transduction.
Mutations that allowed growth on folate compounds were mapped by conjugation with the Hfr strains and protocols described by Singer et al. (35). Bacteriophage P1 transductions were performed as described by Miller (21).
Molecular methods.
E. coli chromosomal DNA was prepared by established methods (30, 38). Small-scale plasmid samples were prepared either by the rapid alkaline lysis technique of Birnboim and Doly (3) or by the rapid boiling technique (30). Bacteriophage λ DNA was prepared as described previously (30).
Restriction endonuclease digestions and ligation reactions were performed in accordance with manufacturers’ recommendations and with commercial buffer preparations (New England Biolabs, Inc.; Boehringer Mannheim Biochemicals, Inc.; International Biotechnologies Inc.; and Bethesda Research Laboratories, Inc.).
PCR was performed with the Taq PCR Core Kit (Qiagen) and Platinum Taq polymerase (Gibco/BRL). Reactions were carried out by use of 25-μl volumes with synthetic 24-mer oligonucleotides as primers (1 μM each). DNA sequence analysis was performed with a T7 Sequenase (version 2.0) sequencing kit (Amersham), and PCR products were sequenced with a similar PCR product sequencing kit (Amersham). Sequencing primers were typically 18 to 20 nucleotides long.
Southern hybridizations were done as described previously (30). Hybridization and washes were carried out at 65°C with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer.
Enzyme assays.
Crude extracts were prepared as previously described (23). Hydrolysis of p-aminobenzoyl-glutamate to p-aminobenzoate was determined by incubating 1-ml volumes containing 50 mM Tris-HCl (pH 8.5), 10 mM MgSO4, 10 mM β-mercaptoethanol, and 50 μM p-aminobenzoyl-glutamate with 0.1 to 1.5 mg of protein for 60 min at 37°C. Reactions were terminated with the addition of 0.1 ml of 1 N HCl, and p-aminobenzoate was extracted and quantitated spectrophotofluorometrically as previously described (23). One unit of activity was defined as the amount of protein required to hydrolyze 1 nmol of p-aminobenzoyl-glutamate in 1 h at 37°C.
A routine assay for DHPS was done with the coupled 6-hydroxymethyl-dihydropteridine pyrophosphokinase-DHPS assay described by Richey and Brown (28). 6-Hydroxymethyl-pteridine was reduced to the dihydro form as described by Shiota et al. (34) prior to use. 14C-labeled p-aminobenzoate was purchased from ICN and diluted to 8 mCi/mmol prior to use.
Protein concentrations were determined by the Bradford dye-binding technique (5).
Bioautography.
Bioautography was carried out with E. coli BN101 and BN1001 as test strains. Pyrex baking dishes (21 by 27 cm) dishes were prepared by pouring a layer (200 ml) of solid minimal medium supplemented with tryptophan and cysteine, followed by a layer (100 ml) of the same medium seeded with 5 × 108 cells of the test strain per ml. The top layer was also supplemented with 0.15 mM isopropyl-thio-β-d-galactoside and 10 μM 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) to aid in the visualization of growth zones. Samples were dissolved and filter sterilized prior to application to Whatman 3MM chromatography paper (15 by 20 cm). The chromatogram was developed by ascending chromatography in 0.1 M potassium phosphate (pH 7.0) until the solvent had reached 2 cm from the top. The chromatogram was air dried, and material was allowed to transfer to solid medium for 2 h. The chromatogram was lifted, and the dish was covered with foil and incubated at 37°C. Samples and quantities used were as follows: p-aminobenzoate and p-aminobenzoyl-glutamate, 7 nmol; folic acid, 110 nmol; folinic acid, 110 nmol; and pteroic acid, 80 nmol.
Construction of interruptions in abgA, abgB, and abgT.
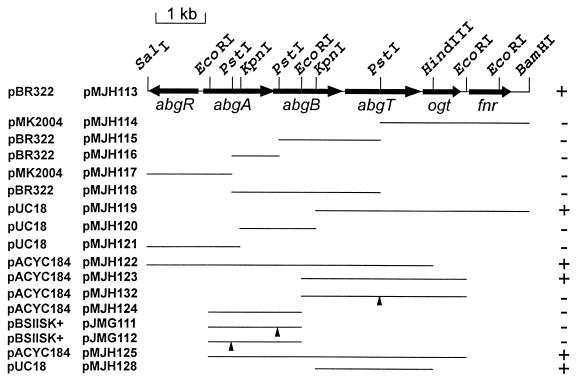
To test the roles of abgA, abgB, and abgT in p-aminobenzoyl-glutamate utilization, each open reading frame was interrupted with a kanamycin resistance cassette and then transferred to the chromosomes of E. coli BN1125 (abg-1) and BN1126 (abg+). pMJH123 was digested with PstI and ligated with the PstI DNA fragment containing the kanamycin resistance determinant derived from pMB2190. The product (abgT::kan) was designated pMJH132. pMJH124 was partially digested with PstI and similarly ligated with the PstI DNA fragment containing the kanamycin resistance determinant. Plasmid products containing the kanamycin resistance cassette inserted into abgA and abgB were isolated. The EcoRI fragments were recloned into the EcoRI site of pBSIISK+ to yield pJMG111 (abgB::kan) and pJMG112 (abgA::kan).
Plasmid-borne interruptions were recombined into the chromosome by homologous recombination in E. coli BN141, a pabA derivative of E. coli JC7623. The abg-1 mutation was crossed into BN141 by cotransduction with a bacteriophage P1 lysate derived from E. coli BN1016, which contained a Tn10 insertion near the abg locus. Selection for tetracycline resistance was followed by screening for growth on p-aminobenzoyl-glutamate to avoid selection of new abg alleles. An abg-1 derivative of E. coli BN141 was designated E. coli BN1125. Plasmids were linearized with either BamHI (pMJH132) or ScaI (pJMG111 and pJMG112) and used to transform E. coli BN1125 to kanamycin resistance. Kanamycin-resistant, ampicillin-sensitive derivatives were chosen and tested for p-aminobenzoyl-glutamate utilization. None of the plasmids contained DNA spanning the site of the abg-1 mutation, so that the original mutation could not be lost in the recombination process. The abg::kan alleles were then transduced into BN1103 and BN101 to yield BN1140 to BN1143 and BN1150 to BN1153, respectively. These latter constructions yielded strains capable of supporting plasmid replication.
RESULTS
Selection and characterization of abg mutants.
To obtain E. coli strains that could grow on folate or folate-related compounds, a pabA derivative of E. coli (BN101) was mutagenized with diethyl sulfate and selected for growth on medium supplemented with folic acid or folinic acid. Because the growth requirement for these compounds on solid medium was high (>10 μM) and the requirement for p-aminobenzoate supplementation was low (<30 nM), we sought to test the strains for growth on potentially contaminating material present in the commercially supplied supplements.
Bioautography experiments identified p-aminobenzoyl-glutamate as the relevant nutrient that satisfied the p-aminobenzoate requirement in mutant strain BN1001. Several preparations of folic acid and folinic acid were tested by this method for their ability to substitute for p-aminobenzoate. Table 2 shows that the growth response of E. coli BN1001 was centered on a compound that was present in folic acid and folinic acid preparations and that chromatographed with an Rf of 0.94, well removed from the zone of migration of folic acid (Rf, 0.43), folinic acid (Rf, 0.17), or p-aminobenzoate (Rf, 0.77). The Rf of the unknown compound suggested that p-aminobenzoyl-glutamate may be the supplement conferring growth on the pabA strain (32). The contaminating compound that supported growth comigrated with authentic p-aminobenzoyl-glutamate, which also supported the growth of E. coli BN1001. Parallel experiments with the parental strain E. coli BN101 showed that significant growth occurred only on p-aminobenzoate and that growth did not occur on any other compound, including p-aminobenzoyl-glutamate, at the concentration tested. These data indicated that the growth observed on folic acid and folinic acid preparations was due to the p-aminobenzoyl-glutamate present in these preparations, presumably having been formed as a spontaneous degradation product of folic acid. We designated the mutations abg, for p-aminobenzoyl-glutamate utilization.
TABLE 2.
Growth on contaminating material present in folate compounds, as revealed by bioautography
| Compound applied to chromatogram |
Rf of:
|
|
|---|---|---|
| UV-absorbing material | Compound supporting the growth of E. coli BN1001 | |
| Folic acid | 0.43 | 0.94 |
| Folinic acid | 0.17 | 0.94 |
| p-Aminobenzoate | 0.77 | 0.77 |
| p-Aminobenzoyl-glutamate | 0.94 | 0.94 |
Growth response of strains to p-aminobenzoyl-glutamate.
E. coli BN101 and BN1103 grew poorly on p-aminobenzoyl-glutamate concentrations below 10 μM, and bioautography showed that growth on higher concentrations was due to contaminating p-aminobenzoate in commercial preparations. However, the abg mutant strains could grow on much lower levels of p-aminobenzoyl-glutamate (Table 3). The minimum concentration required for full growth was determined by inspection of colony size on solid minimal medium containing different concentrations of p-aminobenzoyl-glutamate (ranging from 0.4 nM to 200 μM). While growth became limited for the parental strain at below 20 μM, the growth of E. coli BN1001, BN1002, BN1003, or BN1005 was not limited until the p-aminobenzoyl-glutamate concentration fell below 1 μM. The growth of E. coli BN1004, on the other hand, was not limited until the p-aminobenzoyl-glutamate concentration fell below 0.1 μM. Two classes of mutations were evident from this analysis, one that increased the sensitivity to p-aminobenzoyl-glutamate approximately 20-fold and one that increased the sensitivity approximately 200-fold over the concentration required by the parent. Growth limitation in response to p-aminobenzoate for all strains was not evident until the concentration fell below 0.02 μM.
TABLE 3.
Growth response of E. coli strains to p-aminobenzoyl-glutamate concentrationa
| Strain | p-Aminobenzoyl-glutamate concn allowing full growth (μM) |
|---|---|
| BN101 | 20 |
| BN1103 | 10 |
| BN1001 | 1 |
| BN1002 | 1 |
| BN1003 | 1 |
| BN1004 | 0.1 |
| BN1005 | 1 |
| BN1023 | 0.1 |
The concentrations tested were 200, 100, 40, 20, 10, 4, 2, 1, 0.4, 0.2, 0.1, 0.04, 0.02, 0.01, 0.004, 0.002, and 0.001 μM.
Mutant strains contain unaltered DHPS.
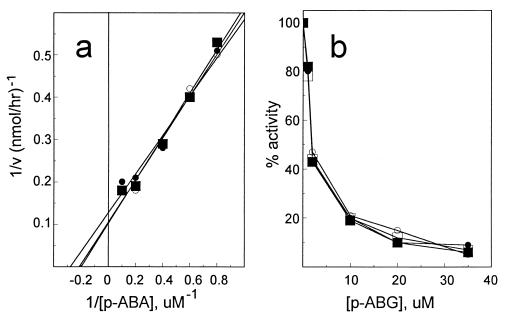
Although p-aminobenzoyl-glutamate is not used as the normal substrate for dihydrofolate synthesis in vivo (28), it has been reported that DHPS from E. coli as well as other microorganisms can use p-aminobenzoyl-glutamate as a substrate in vitro in place of p-aminobenzoate and thereby bypass dihydropteroate to produce dihydrofolate directly (29) (Fig. 1). To test if the p-aminobenzoyl-glutamate utilization mutants contained altered DHPS that might facilitate the in vivo utilization of p-aminobenzoyl-glutamate, DHPS from mutants BN1001, BN1004, and BN1005 was assayed to determine if it could use either p-aminobenzoyl-glutamate or p-aminobenzoate with an efficiency different from that of parental E. coli BN101 DHPS. As shown in Fig. 2, there was no apparent difference in the Km for p-aminobenzoate, nor was there any apparent difference in the ability of p-aminobenzoyl-glutamate to compete with p-aminobenzoate for incorporation into dihydropteroate. Since no difference in DHPS activities was evident, we concluded that if p-aminobenzoyl-glutamate were utilized directly, it was by a mechanism not related to an altered DHPS.
FIG. 2.
Characterization of DHPS from E. coli BN101 and abg derivatives. (a) Comparison of Kms of DHPS from E. coli BN101 (■), BN1001 (•), and BN1004 (○). (b) Comparison of the ability of p-aminobenzoyl-glutamate (p-ABG) to compete with 14C-labeled p-aminobenzoate in the DHPS reaction in E. coli BN101 (•), BN1001 (○), BN1004 (■), and BN1005 (□). p-ABA, p-aminobenzoate; v, velocity.
Mapping and cloning of abgT.
The abg mutation of one of the strains (E. coli BN1004) was mapped by Hfr conjugation to between 25 and 35 min and by P1 transduction to approximately 30 min on the E. coli chromosome. The abg allele was 50% cotransducible with the zda-3061::Tn10 marker of E. coli CAG12081, which is located at approximately 29.5 min (35). The cotransduction frequencies for the abg alleles from E. coli BN1001, BN1002, BN1003, and BN1005 were similar. In addition, abg Tcr recipients of the mutant strains were used to backcross the abg allele to either E. coli BN101 or E. coli BN1103, confirming that no additional mutation distant from this locus was necessary for the growth phenotype, nor was the phenotype dependent on another factor in the E. coli BN101 genetic background.
Plasmid libraries were constructed with HindIII-digested chromosomal DNA from E. coli BN1023 (derived from BN1004) and pBR322. E. coli BN101 was transformed with the libraries and plated on minimal medium supplemented with tryptophan, ampicillin, and folic acid. Plasmids were prepared from 10 colonies and were found to contain identical 4.2-kb inserts. A radiolabeled plasmid was used to probe the Kohara lambda library (20), and hybridization to two phages containing overlapping inserts, λ6B4(260) and λ3G3(261), was found at a physical location consistent with the genetic map position. However, no 4.2-kb HindIII fragment was present on the physical restriction map or on the two hybridizing phages. We tested HindIII digests of chromosomal DNAs from all the mutant strains by using the same plasmid as a probe. All mutant strains except for BN1004 showed a hybridization pattern consistent with the Kohara HindIII restriction map. E. coli BN1004 (and its derivative BN1023), on the other hand, contained the HindIII fragments predicted for the wild-type strain and an additional 4.2-kb HindIII fragment. These data (and additional data; see below) suggested that a duplication of a segment of the DNA in this region was responsible for the p-aminobenzoyl-glutamate utilization phenotype. We reasoned that if a duplication event could give rise to the Abg phenotype, then cloning of the wild-type DNA fragments from the phage clones in a moderate copy number might allow us to identify the gene(s) responsible for the phenotype. A 7.5-kb BamHI-SalI fragment from λ3G3(261) cloned into pACYC184 yielded a plasmid (pMJH113) that could confer the p-aminobenzoyl-glutamate utilization phenotype on E. coli BN101 or BN1103 (Fig. 3). A variety of DNA fragments were subcloned from pMJH113 into a variety of vectors and tested for growth on p-aminobenzoyl-glutamate. These experiments delimited the DNA fragment of interest to a KpnI-HindIII fragment, as represented by the 2.3-kb DNA fragment present in pMJH128.
FIG. 3.
Map of the 7,266-bp region of the E. coli chromosome from bp 1,396,431 to bp 1,403,697, shown in the inverse orientation. Restriction sites used for subcloning purposes are illustrated, as are the extents of known reading frames. DNA fragments used in this study to localize abgT and to construct interruptions in abg genes are illustrated, and the vectors in which they are cloned are indicated. The vector names (leftmost column) and plasmid names are shown to the left of the map. Triangles denote the positions of kanamycin resistance cassette insertions. The ability of each plasmid to confer p-aminobenzoyl-glutamate utilization is indicated at the right.
Nucleotide sequence of a gene allowing p-aminobenzoyl-glutamate utilization.
To sequence the 2.3-kb KpnI-HindIII insert of pMJH128, a sequence strategy involving both ordered deletions (15) and synthetic oligonucleotides was used. The entire sequence was completed on both strands, and all sites and primer sequences were overlapped. The nucleotide sequence of a 2,266-bp DNA fragment containing one complete and two partial open reading frames was determined (Fig. 3). Database searches with BlastP (1) identified the 3′-terminal open reading frame as ogt, encoding a DNA repair enzyme, O6-alkyl-guanine-DNA alkyltransferase (26). The upstream open reading frame was present on the complete sequence of the E. coli genome and had been given the designation ydaH, indicative of an open reading frame of unknown function (4).
The complete open reading frame, designated ydaH, that was present on the sequenced DNA fragment was 1,533 bp long and encoded a 511-amino-acid hydrophobic protein with a predicted molecular mass of 55.1 kDa. Hydropathy analyses of the predicted amino acid sequence suggested the presence of 12 transmembrane alpha helices and classified the protein as an integral membrane protein (9, 19, 27). Database searches did not yield a single protein with high similarity but did yield several proteins with similarity in limited segments. All of the proteins were transport or efflux proteins, and most were of the sodium-solute symport family, containing 12 transmembrane alpha helices. On the basis of these predictions, we have designated the gene abgT, for p-aminobenzoyl-glutamate transport.
As mentioned above, wild-type abgT present on pMJH123 could confer p-aminobenzoyl-glutamate utilization on abg+ strains. Plasmid pMJH132, containing interrupted abgT by virtue of the insertion of a kanamycin resistance cassette at the PstI site, failed to confer p-aminobenzoyl-glutamate utilization on abg+ strains. Furthermore, transfer of the abgT interruption to the chromosome of an abg-1 strain resulted in the reversal of the Abg phenotype. These data indicated that a functional, wild-type abgT gene is essential for the utilization of p-aminobenzoyl-glutamate and, when present on a multicopy plasmid, is sufficient to confer p-aminobenzoyl-glutamate utilization.
The 3.0-kb EcoRI DNA fragment containing abgT was cloned from chromosomal digests of E. coli BN1001 and BN1003, and one strand was sequenced with primers derived from the wild-type sequence. No differences from the wild-type sequence were found, suggesting that the p-aminobenzoyl-glutamate utilization phenotype was not due to a gain-of-function mutation in an existing transport protein. Since duplicated abgT and multicopy plasmid-borne abgT could both confer p-aminobenzoyl-glutamate utilization, we considered the possibility that the differential expression of abgT might be responsible for the Abg phenotype.
An additional sequence analysis of the wild-type and mutant strains suggested that abgT and possibly ogt were part of a larger operon. We extended the sequence of both strands to the SalI site upstream of abgT (Fig. 3). The sequence determined was identical to that published (4). ogt and abgT occurred in a context that suggested an operon structure with two additional open reading frames upstream of abgT (given labels b1337 and b1338) adjacent to a divergently transcribed lysR-like regulatory gene (b1339) that encodes a protein 27% identical to TdcA, a LysR-like transcription activator of the threonine dehydratase operon (10, 31).
Identification of mutations leading to p-aminobenzoyl-glutamate utilization.
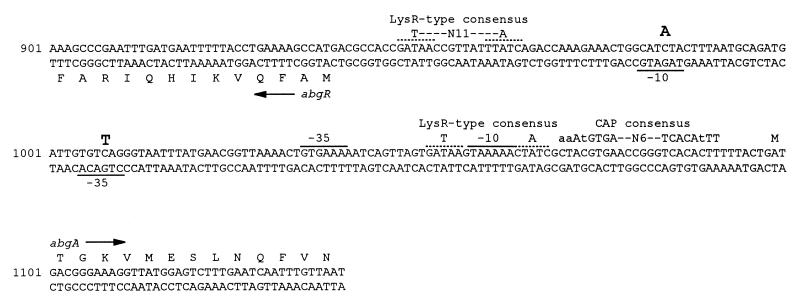
To determine if the mutations resulting in p-aminobenzoyl-glutamate utilization were in the regulatory region of the putative abg operon, a 1,105-bp DNA fragment spanning the abgR-abgA intercistronic region was amplified from the parental E. coli BN101 DNA and mutant abg chromosomal DNA preparations by PCR. The nucleotide sequences of the PCR products were determined directly and showed that the sequences from the parental strain and BN1004 were identical to the published wild-type sequence (Fig. 4), while the products derived from BN1001, BN1002, BN1003, and BN1005 each contained one nucleotide difference. Extending the sequence determination of BN1001 to include abgA and abgB did not reveal any additional sequence differences. In the four strains analyzed, two nucleotide differences were found. E. coli BN1001, BN1002, and BN1003 each contained a C-to-A transversion at nucleotide position 984 from the SalI site, while E. coli BN1005 had a C-to-T transition at position 1008. Since both of these alterations lay outside potential open reading frames, we searched for possible transcriptional regulatory sequences that might be affected by the mutational changes. In Fig. 4 we have noted two regions of dyad symmetry containing the half-site sequence -GATAA-, within which is contained the T-N11-A feature typical of LysR family protein-binding sites (11), and a match to the catabolite activator protein consensus sequence (8). The most obvious possibility from inspection of the nucleotide sequence is that the mutations may lie in the −10 and −35 regions of the putative abgR promoter. Although no direct evidence concerning the effects of the mutations on the expression of abgR has been found, we hypothesize that the mutations alter the expression of abgT, consistent with the observations made above that indicate that only abgT is required for p-aminobenzoyl-glutamate utilization.
FIG. 4.
Nucleotide and amino acid sequences of the abgR-abgA intercistronic region. The positions of mutations resulting in the p-aminobenzoyl-glutamate utilization phenotype are shown in large bold letters above the sequence. Putative LysR-type protein-binding sequences are shown as dotted lines above the sequence. Putative −10 and −35 sequences of the abgR and abgA promoters are overlined or underlined. The CAP consensus sequence is also indicated above the sequence (lowercase letters indicate nucleotides that are not as strongly conserved as the capitalized nucleotides). Numbering is from the SalI site prior to abgR.
For E. coli BN1004, additional evidence for the presence of a duplicated DNA segment suggested by Southern hybridization data was sought. The Southern hybridization data and the restriction map of the clone derived from BN1023 indicated that a 4.2-kb duplication that contained most or all of abgB, abgT, and ogt was present in the chromosome. To determine the extent and site of the duplicated segment, we devised a PCR-based experiment to localize the novel junction between the duplication endpoints (Fig. 5) and then determined the sequence of the junction. The junction sequence was localized in PCR experiments with divergently oriented primer pairs. In the absence of a duplication region encompassing a primer pair, no PCR product would be detected. However, if a primer pair were located within a tandemly duplicated segment, one pair of primers would be oriented toward one another and a PCR product that contained the novel junction would be obtained. One primer (pri1) was designed to hybridize near the HindIII site within ogt, and four other primers (pri2 to pri5) were designed to hybridize at various sites within abgA and abgB (Fig. 5). Primer pair pri1-pri2 yielded a product from DNA derived from E. coli BN1004 or BN1023 but not from DNA derived from E. coli BN101 or BN1001. Other primer pairs yielded no product from E. coli BN1004 or BN1023 DNA, suggesting that the duplication junction lay in the 200-bp region between pri2 and pri3.
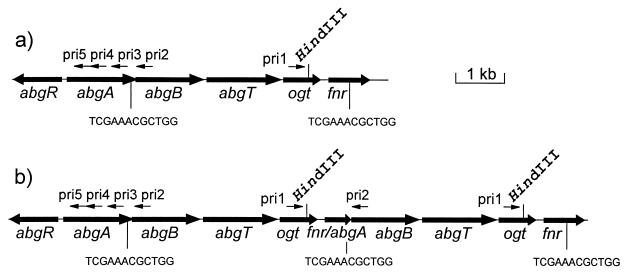
FIG. 5.
Structures of the wild-type (a) and duplicated (b) regions of E. coli BN1004. The HindIII site within ogt is illustrated, as are the primer hybridization sites used to delimit the extent of duplication. The 12-bp direct repeat sequences within abgA and fnr are illustrated. It can be seen from panel b that only primer pair pri1-pri2 could yield a PCR product from DNA containing a duplication. The source of the 4.2-kb HindIII fragment containing abgT is also evident.
DNA amplified from E. coli BN1023 with pri1 and pri2 was sequenced with pri2 as a sequencing primer, yielding the sequence of the duplication junction: a 12-bp repeated sequence found once near the end of abgA and again within fnr. Duplication between these sequences resulted in a 4,157-bp duplication with a novel fnr-abgA fusion (in phase) and with abgB and abgT expression presumably under the control of the fnr promoter.
Hydrolysis of p-aminobenzoyl-glutamate.
The protein products of the two open reading frames upstream of abgT (here designated abgA and abgB) showed sequence similarity to one another and sequence similarity to a family of aminoacyl aminohydrolases. Outside the E. coli sequences, the greatest similarity was between AbgA and Enterobacter agglomerans indole-3-acetyl-aspartic acid hydrolase (6), but of greater interest was the similarity of each to Pseudomonas sp. strain RS-16 carboxypeptidase G2 (22), an enzyme capable of cleaving folate to pteroate and glutamate and p-aminobenzoyl-glutamate to p-aminobenzoate and glutamate (33). Based on these similarities, we hypothesized that p-aminobenzoyl-glutamate utilization conferred by transport of the supplement into the cell by AbgT might be accompanied by hydrolysis to p-aminobenzoate and glutamate by AbgA and AbgB, either alone or in combination.
Two lines of evidence indicated that p-aminobenzoyl-glutamate was hydrolyzed to p-aminobenzoate in the abg mutant strains. The first line of evidence was derived from the observation that E. coli BN1001 could cross-feed parental strain E. coli BN101 or BN1103 when the two strains were streaked near one another on solid medium containing p-aminobenzoyl-glutamate. Since BN101 and BN1103 required p-aminobenzoate for growth and could not utilize p-aminobenzoyl-glutamate, the gradient of growth observed in the parental strain was very likely due to the cleavage of p-aminobenzoyl-glutamate and the diffusion of p-aminobenzoate. Each of the mutant strains (BN1001 to BN1005 and BN1023) demonstrated the ability to cross-feed a p-aminobenzoate-requiring strain.
The second line of evidence came from the direct demonstration of the cleavage of p-aminobenzoyl-glutamate to p-aminobenzoate in vitro by crude extracts derived from mutant strains (Table 4). Extracts prepared from E. coli BN1004 showed approximately fivefold-higher p-aminobenzoyl-glutamate hydrolysis activity than did those of parental strain E. coli BN101. Extracts prepared from BN1003 and BN1005, on the other hand, showed only a small (less than twofold) increase in activity.
TABLE 4.
Enzymatic hydrolysis of p-aminobenzoyl-glutamate by crude extracts
| Strain | Genotype | Activity (U/mg of protein) |
|---|---|---|
| BN101 | pabA | 0.66 ± 0.15a |
| BN1003 | abg-1 | 0.72 ± 0.08a |
| BN1004 | abg-4 (duplication) | 3.19 ± 0.45a |
| BN1005 | abg-5 | 1.01 ± 0.10a |
| BN1150 | abg-1 | 0.77 ± 0.06b |
| BN1151 | abg-1 abgB::kan | 0.70 ± 0.05b |
| BN1152 | abg-1 abgA::kan | 0.87 ± 0.11b |
| BN1153 | abg-1 abgT::kan | 0.81 ± 0.08b |
Mean ± average deviation from the mean for quadruplicate determinations of three independent cultures.
Mean ± average deviation from the mean for quadruplicate determinations of one culture.
Effects of interruptions in abgA, abgB, and abgT.
The phenotypic effects of interruptions in abgA, abgB, and abgT were tested by constructing strains in which each open reading frame was interrupted with a kanamycin resistance cassette. The cassettes were inserted at the PstI sites present in each of the genes. Recombination of the interrupted genes into an abg+ strain had no effect on the growth properties of the strains on supplemented minimal medium. However, the introduction of each interrupted gene into an abg-1 host resulted in a reduction in the ability to utilize p-aminobenzoyl-glutamate effectively (Table 5). As anticipated, the strains containing the abgT::kan interruption (BN1143 and BN1153), with the kanamycin resistance determinant transcribed in the same direction as abg, failed to grow on medium supplemented with p-aminobenzoyl-glutamate. Similarly, abg-1 strains containing abgA::kan (BN1142 and BN1152) and abgB::kan (BN1141 and BN1151) also failed to utilize p-aminobenzoyl-glutamate. These data might be explained by polarity of the kanamycin resistance cassette for abgT expression, since the direction of kan transcription is opposite that of abgT transcription in the abgA::kan and abgB::kan interruptions.
TABLE 5.
Minimum concentration of p-aminobenzoyl-glutamate required for full growth on solid medium for various abg::kan interruption strainsa
| Strain | abg allele | p-Aminobenzoyl-glutamate concn (μM) required for growth |
|---|---|---|
| BN1103 | abg+ | 10 |
| BN1140 | abg-1 | 1 |
| BN1141 | abg-1 abgB::kan | 10 |
| BN1142 | abg-1 abgA::kan | 10 |
| BN1143 | abg-1 abgT::kan | 10 |
The p-aminobenzoyl-glutamate concentrations tested were 200, 100, 40, 20, 10, 4, 2, 1, 0.4, 0.2, 0.1, 0.04, 0.02, 0.01, 0.004, 0.002, and 0.001 μM. The strains carrying pMJH128 were used at 0.1 μM.
To test whether polarity for abgT was the cause of the failure to utilize p-aminobenzoyl-glutamate, we transformed each of the abg::kan interruptions with pMJH128, a plasmid carrying abgT. In each case, the presence of abgT in trans not only restored the p-aminobenzoyl-glutamate utilization phenotype, indicating that the abgAB genes were not essential for p-aminobenzoyl-glutamate utilization, but also increased the sensitivity of the strains to low levels of p-aminobenzoyl-glutamate. The ability to grow on low levels of p-aminobenzoate was not altered. The abg::kan interruptions had no apparent effect on the p-aminobenzoyl-glutamate hydrolysis activity present in crude extracts (Table 4).
DISCUSSION
E. coli pabA strains able to utilize p-aminobenzoyl-glutamate were obtained following mutagenesis and selection on folate compounds. The p-aminobenzoyl-glutamate utilization phenotype depended on a single gene, abgT, a homolog of transport genes that apparently was responsible for the transport of p-aminobenzoyl-glutamate into the cell. abg+ strains that contained interrupted abgT did not yield p-aminobenzoyl-glutamate utilization derivatives following mutagenesis, and abg-1 strains lost their ability to use p-aminobenzoyl-glutamate when abgT was disrupted. Plasmid-borne, wild-type abgT could confer p-aminobenzoyl-glutamate utilization on abg+ hosts, and this ability was lost when abgT was disrupted.
The nucleotide sequence of the abgT allele from an abg-1 strain was identical to that of the wild-type allele, and the cloned wild-type gene was capable of conferring p-aminobenzoyl-glutamate utilization on pabA strains. These observations suggested that wild-type abgT was normally cryptic or not expressed under conditions of growth on minimal medium. Altering the expression of abgT from its wild-type context appears to have been the mechanism for obtaining the growth phenotype. Data from the E. coli genome sequence suggested that abgT lay in an operon with two other genes upstream and possibly also ogt downstream. Since no promoter-like sequences were observed near abgT, it seemed likely that the abgT regulatory signals lay several kilobases upstream, probably near the region of the divergently transcribed LysR-type regulatory gene.
Three different types of mutations were detected in the collection of five abg strains analyzed. Two different single-base-pair mutations in the abgR-abgA intergenic region resulted in an approximately 20-fold-increased ability to utilize p-aminobenzoyl-glutamate. The growth phenotype was accompanied by a small but reproducible increase in the expression of p-aminobenzoyl-glutamate hydrolysis activity detectable in crude extracts of one of the mutant strains (BN1005). The location of the mutations in or near the abgR promoter suggested that alteration of the expression of abgR may have altered the expression of abgT, which in turn resulted in the p-aminobenzoyl-glutamate utilization phenotype. BN1004 contained a 4.2-kb duplication of DNA which placed a second copy of abgB and abgT under the control of the fnr promoter. The anomalous expression of the abg genes resulted in a 200-fold increase in the ability to use p-aminobenzoyl-glutamate and a 5-fold increase in the amount of p-aminobenzoyl-glutamate hydrolase activity in crude extracts. The level of sensitivity to p-aminobenzoyl-glutamate was equivalent to that seen in strains containing a high-copy-number plasmid carrying abgT. Since abgB was duplicated and expressed from the fnr promoter, while abgA was not, we concluded that abgB encoded measurable p-aminobenzoyl-glutamate hydrolase activity but that abgB was not essential for the p-aminobenzoyl-glutamate utilization phenotype.
The utilization of p-aminobenzoyl-glutamate may have occurred by either of two mechanisms. First, DHPS may have used p-aminobenzoyl-glutamate as a substrate directly, forming dihydrofolate as the product and bypassing the normal dihydropteroate intermediate in the dihydrofolate biosynthetic pathway. While the direct in vivo incorporation of p-aminobenzoyl-glutamate into dihydrofolate has not been observed so far for E. coli, DHPS has been shown to be able to use p-aminobenzoyl-glutamate in place of p-aminobenzoate in vitro (29), albeit with a higher Km for p-aminobenzoyl-glutamate than for p-aminobenzoate. However, it is possible that increased transport of p-aminobenzoyl-glutamate into the cell by increased expression of abgT may have raised the intracellular level sufficiently to make the reaction proceed well enough to support growth.
A second possibility was that the transported p-aminobenzoyl-glutamate was first hydrolyzed to p-aminobenzoate and glutamate by intracellular aminoacyl aminohydrolases and then p-aminobenzoate was incorporated into dihydropteroate in the normal biosynthetic pathway. In support of this idea, we observed that abg strains growing on p-aminobenzoyl-glutamate had the ability to cross-feed the p-aminobenzoate requirement of the parental strain, suggesting hydrolysis of p-aminobenzoyl-glutamate and excretion of p-aminobenzoate. In addition, we demonstrated that crude extracts derived from abg strains had an elevated capacity to hydrolyze p-aminobenzoyl-glutamate to p-aminobenzoate in vitro. However, disruption of abgA and abgB did not reverse the p-aminobenzoyl-glutamate utilization phenotype in strains expressing abgT, indicating that if hydrolysis were required, other activities in the cell were capable of p-aminobenzoyl-glutamate hydrolysis.
The selection for growth on p-aminobenzoyl-glutamate appeared to have resulted in the altered regulation of an operon that resulted in an increased capacity to transport and hydrolyze p-aminobenzoyl-glutamate. It is likely that the abg operon is expressed under specific growth conditions, perhaps in response to an effector of abgR. In this regard, we note that sequences in the abgR-abgA intercistronic region include segments similar to binding sites for LysR-type regulators (11) and for CAP (8). Alternatively, it is possible that the p-aminobenzoyl-glutamate utilization phenotype arose from recruitment of a transport protein from an operon whose normal function may not be involved in p-aminobenzoyl-glutamate or folate metabolism. For example, the wild-type function of the operon may be a peptide permease and hydrolysis system regulated by the presence of peptides in the medium. If such a system had a broad substrate specificity, then it might be able to transport and hydrolyze p-aminobenzoyl-glutamate under conditions of altered regulation. As has been shown with other operons in E. coli and other bacteria, altered-regulation phenomena are a common first step in the evolution of new metabolic pathways in bacterial systems (13).
ACKNOWLEDGMENTS
We are indebted to John Roth, in whose laboratory this work was initiated. We thank Gordon Guay for technical assistance.
This work was supported by Public Health Service grants AI25106 and GM44199 from the National Institutes of Health.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Baumstark B R, Spremulli L L, RajBhandary U L, Brown G M. Initiation of protein synthesis without formylation in a mutant of Escherichia coli that grows in the absence of tetrahydrofolate. J Bacteriol. 1977;129:457–471. doi: 10.1128/jb.129.1.457-471.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnboim H, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Chou J C, Mulbry W W, Cohen J D. The gene for indole-3-acetyl-aspartic acid hydrolase from Enterobacter agglomerans: molecular cloning, nucleotide sequence, and expression in Escherichia coli. Mol Gen Genet. 1998;259:172–178. doi: 10.1007/s004380050802. [DOI] [PubMed] [Google Scholar]
- 7.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1980. [Google Scholar]
- 8.deCrombrugghe B, Busby S, Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984;224:831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]
- 9.Eisengerg D, Schwarz E, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984;179:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- 10.Ganduri Y L, Sadda S R, Datta M W, Jambukeswaran R K, Datta P. TdcA, a transcriptional activator of the tdcABC operon of Escherichia coli, is a member of the LysR family of proteins. Mol Gen Genet. 1993;240:395–402. doi: 10.1007/BF00280391. [DOI] [PubMed] [Google Scholar]
- 11.Goethals K, Van Montagu M, Holsters M. Conserved motifs in a divergent nod box of Azorhizobium caulinodans ORS571 reveal a common structure in promoters regulated by LysR-type proteins. Proc Natl Acad Sci USA. 1992;89:1646–1650. doi: 10.1073/pnas.89.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green J M, Nichols B P. p-Aminobenzoate biosynthesis in Escherichia coli: purification of aminodeoxychorismate lyase and cloning of pabC. J Biol Chem. 1991;266:12971–12975. [PubMed] [Google Scholar]
- 13.Hall B G. Selection, adaptation, and bacterial operons. Genome. 1989;31:265–271. doi: 10.1139/g89-044. [DOI] [PubMed] [Google Scholar]
- 14.Harvey R J. Growth and initiation of protein synthesis in Escherichia coli in the presence of trimethoprim. J Bacteriol. 1973;114:309–322. doi: 10.1128/jb.114.1.309-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- 16.Huang M, Gibson F. Biosynthesis of 4-aminobenzoate in Escherichia coli. J Bacteriol. 1970;102:767–773. doi: 10.1128/jb.102.3.767-773.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M, Pittard J. Genetic analysis of mutant strains of Escherichia coli requiring p-aminobenzoate for growth. J Bacteriol. 1967;93:1938–1942. doi: 10.1128/jb.93.6.1938-1942.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huennekens F M, Vitols K S, Pope L E, Fan J. Membrane transport of folate compounds. J Nutr Sci Vitaminol (Tokyo) 1992;38:52–57. doi: 10.3177/jnsv.38.special_52. [DOI] [PubMed] [Google Scholar]
- 19.Klein P, Kanehisa M, Delisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985;815:468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- 20.Kohara Y, Akiyama K, Inoso K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 22.Minton N P, Atkinson T, Bruton C J, Sherwood R F. The complete nucleotide sequence of the Pseudomonas gene coding for carboxypeptidase G2. Gene. 1984;31:31–38. doi: 10.1016/0378-1119(84)90192-6. [DOI] [PubMed] [Google Scholar]
- 23.Nichols B P, Seibold A, Doktor S Z. para-Aminobenzoate synthesis from chorismate occurs in two steps. J Biol Chem. 1989;264:8597–8601. [PubMed] [Google Scholar]
- 24.Nickerson W J, Webb M. Effect of folic acid analogues on growth and cell division of nonexacting microorganisms. J Bacteriol. 1956;71:129–139. doi: 10.1128/jb.71.2.129-139.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posner B A, Li L, Bethell R, Tsuji T, Benkovic S J. Engineering specificity for folate into dihydrofolate reductase from Escherichia coli. Biochemistry. 1996;35:1653–1663. doi: 10.1021/bi9518095. [DOI] [PubMed] [Google Scholar]
- 26.Potter P M, Wilkinson M C, Fitton J, Carr F J, Brennard J, Cooper D P, Margison G P. Characterization and nucleotide sequence of ogt, the O6-alkyl-guanine-DNA alkyltransferase gene of E. coli. Nucleic Acids Res. 1987;15:9177–9193. doi: 10.1093/nar/15.22.9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao M J K, Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986;869:197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- 28.Richey D P, Brown G M. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J Biol Chem. 1969;244:1582–1592. [PubMed] [Google Scholar]
- 29.Richey D P, Brown G. A comparison of the effectiveness with which p-aminobenzoic acid and p-aminobenzoyl-glutamate acid are used as substrates by dihydropteroate synthase from Escherichia coli. Biochim Biophys Acta. 1970;222:237–239. doi: 10.1016/0304-4165(70)90376-4. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Schweizer H P, Datta P. The complete nucleotide sequence of the tdc region of Escherichia coli. Nucleic Acids Res. 1989;17:3994. doi: 10.1093/nar/17.10.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott J M. Thin-layer chromatography of pteroylglutamates and related compounds. Methods Enzymol. 1980;66:437–443. doi: 10.1016/0076-6879(80)66486-6. [DOI] [PubMed] [Google Scholar]
- 33.Sherwood R F, Melton R G, Alwan S M, Hughes P. Purification and properties of carboxypeptidase G2 from Pseudomonas sp. strain RS-16. Eur J Biochem. 1985;148:447–453. doi: 10.1111/j.1432-1033.1985.tb08860.x. [DOI] [PubMed] [Google Scholar]
- 34.Shiota T, Disraely M N, McCann M P. The enzymatic synthesis of folate-like compounds from hydroxymethyldihydropteridine pyrophosphate. J Biol Chem. 1964;239:2259–2266. [PubMed] [Google Scholar]
- 35.Singer M, Baker T A, Schnitzler G, Deischel S M, Goel M, Dove W, Jaacks K J, Grossman A D, Erickson J W, Gross C A. Collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran P V, Bannor T A, Doktor S Z, Nichols B P. Chromosomal organization and expression of Escherichia coli pabA. J Bacteriol. 1990;172:397–410. doi: 10.1128/jb.172.1.397-410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webb M, Nickerson W J. Differential reversal of inhibitory effects of folic acid analogues on growth, division, and deoxyribonucleic acid synthesis of microorganisms. J Bacteriol. 1956;71:140–148. doi: 10.1128/jb.71.2.140-148.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1987. pp. 2.4.1–2.4.5. [Google Scholar]
- 39.Yim J J, Brown G M. Characteristics of guanosine triphosphate cyclohydrolase I purified from Escherichia coli. J Biol Chem. 1976;251:5087–5094. [PubMed] [Google Scholar]