Abstract
Background
Whether the benefits of the robotic platform in bariatric surgery translate into superior surgical outcomes remains unclear. The aim of this retrospective study was to establish the ‘best possible’ outcomes for robotic bariatric surgery and compare them with the established laparoscopic benchmarks.
Methods
Benchmark cut-offs were established for consecutive primary robotic bariatric surgery patients of 17 centres across four continents (13 expert centres and 4 learning phase centres) using the 75th percentile of the median outcome values until 90 days after surgery. The benchmark patients had no previous laparotomy, diabetes, sleep apnoea, cardiopathy, renal insufficiency, inflammatory bowel disease, immunosuppression, history of thromboembolic events, BMI greater than 50 kg/m2, or age greater than 65 years.
Results
A total of 9097 patients were included, who were mainly female (75.5%) and who had a mean(s.d.) age of 44.7(11.5) years and a mean(s.d.) baseline BMI of 44.6(7.7) kg/m2. In expert centres, 13.74% of the 3020 patients who underwent primary robotic Roux-en-Y gastric bypass and 5.9% of the 4078 patients who underwent primary robotic sleeve gastrectomy presented with greater than or equal to one complication within 90 postoperative days. No patient died and 1.1% of patients had adverse events related to the robotic platform. When compared with laparoscopic benchmarks, robotic Roux-en-Y gastric bypass had lower benchmark cut-offs for hospital stay, postoperative bleeding, and marginal ulceration, but the duration of the operation was 42 min longer. For most surgical outcomes, robotic sleeve gastrectomy outperformed laparoscopic sleeve gastrectomy with a comparable duration of the operation. In robotic learning phase centres, outcomes were within the established benchmarks only for low-risk robotic Roux-en-Y gastric bypass.
Conclusion
The newly established benchmarks suggest that robotic bariatric surgery may enhance surgical safety compared with laparoscopic bariatric surgery; however, the duration of the operation for robotic Roux-en-Y gastric bypass is longer.
Structured quality assessment for robotic bariatric surgery is needed. This retrospective study establishes global benchmarks for robotic Roux-en-Y gastric bypass and sleeve gastrectomy in low-risk cases from 13 expert centres. The results suggest that a higher surgical safety might be achieved in low-risk cases by robotic bariatric surgery compared with the laparoscopic approach.
Introduction
The establishment of global surgical benchmarks for clinically relevant and procedure-specific surgical outcomes has been reported in the literature1. The goal is to set the best achievable cut-offs for intraoperative and postoperative surgical outcomes in well-defined low-risk patient cohorts operated in high-volume centres across the world, also referred to as the ‘best-patient-in-best-centre methodology’2. Benchmarks are expected to improve surgical quality and safety by providing ‘goals’ for outcomes, which enables comparisons between centres, surgeons, and time intervals3. A research consortium recently established outcome benchmarks for primary Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG), as well as for secondary laparoscopic bariatric surgeries, including revisions, conversions, and reversals4,5.
Robotically assisted surgery offers stereoscopic three-dimensional vision with direct camera control by the surgeon, tremor filtration, and articulated instruments with an increased range of motion, which allow for precise dissection and easier handsewing in anatomically confined spaces6. Although the adoption of the robotic platform in bariatric surgery is gaining popularity in Northern America and in Europe, concerns have been raised about higher costs and the lack of evidence demonstrating clear clinical benefits compared with laparoscopic bariatric surgeries7. Robotically assisted bariatric procedures are estimated to cost 2.3 times more per patient than laparoscopic procedures8. This is explained by the purchase, maintenance costs, and limited lifespan of the instruments of the robotic platform9. A recent literature review found that robotically assisted bariatric surgery was non-inferior to primary laparoscopic bariatric surgeries for perioperative outcomes and advantages of the robotic platform were limited to surgeon ergonomics9. The analysis of the Northern American Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) database showed that secondary robotically assisted bariatric surgery is associated with a lower incidence of postoperative complications (pneumonia, superficial surgical site infections, and bleeding) compared with laparoscopic bariatric surgeries10.
The aim of this study was to define the highest achievable quality (‘benchmark’) in primary robotically assisted bariatric surgery (robotically assisted RYGB (rRYGB) and robotically assisted SG (rSG)) for low- and high-risk patients. Additionally, potential performance gaps during the learning curve of robotic bariatric surgeons were investigated and comparisons were made between the benchmark of robotically assisted bariatric surgery and those previously established for laparoscopic bariatric surgeries4.
Methods
Study design
The establishment of benchmarks in robotically assisted bariatric surgery followed a standardized consensus-based methodology4,11. A multicentre retrospective cohort study was performed based on prospective institutional databases by applying the STROBE guidelines12.
First, a consecutive cohort of patients who underwent robotically assisted bariatric surgery was collected from international expert centres via personal invitation of 29 bariatric surgeons from four continents to account for inter-centre variability in surgical technique and perioperative care. Expert centres had to meet criteria promoting sufficient experience and surgical safety, whilst learning phase centres were fresh adopters of robotically assisted bariatric surgery; the reason for including learning phase centres was to demonstrate a potential application of established global benchmarks, allowing for the identification of performance gaps during the transition to robotically assisted bariatric surgery13. See Table 1. Centres without a prospective database including postoperative follow-up until greater than or equal to 90 days were not included. The final consortium included 17 centres from four continents: 13 expert centres (6 from Europe (Barcelona, Geneva, Kiel, Poitiers, Lisbon, and Strasbourg), 4 from the USA (Bethlehem, Houston, Orlando, and Port Jefferson), 2 from India (New Delhi, and Rajinder Nagar), and 1 from Australia (Adelaide); and 4 learning phase centres from Europe (Courtrai, Wiesbaden, Luxemburg, and Lyon).
Table 1.
Criteria used to identify participating centres and ‘benchmark’ cases
| Centre inclusion criteria | Low-risk patient criteria (‘benchmark’) | High-risk patient criteria (‘non-benchmark’) |
|---|---|---|
| Available prospective bariatric database1 | Age 18–65 years14 | History of laparotomy14 |
| Interest in bariatric outcomes, documented by ≥1 publication on bariatric surgery4 | ASA score <IV15 | Cardiovascular disease (for example cardiac arrhythmia, stroke, coronary artery disease)16 |
| For expert centres | Preoperative BMI ≤50 kg/m16 | History of thromboembolic events and/or therapeutic anticoagulation16 |
| ‘Clinical excellence’ or national reference centres with a dedicated bariatric multidisciplinary team (including endocrinologist, gastroenterologist, access to ICU and interventional radiology)1 | Absence of any high-risk patient criteria listed in the next column | Diabetes mellitus (type 1 and type 2, as defined by the American Diabetes Association)17 |
| ≥2 board-certified surgeons perform bariatric surgery within the centre1 | ||
| Ability to offer ≥2 primary bariatric procedures and revisional bariatric surgery1 | Obstructive sleep apnoea (recurrent episodes of upper airway collapse during sleep)16 | |
| Robotic bariatric learning phase (50 cases) terminated and >100 cases performed as expert14,18 | Chronic obstructive pulmonary disease (FEV1/FVC < 0.7)19 | |
| Annual caseload >75 bariatric operations (laparoscopic + robotic combined and in every year between 2017 and 2021), out of which ≥25 cases/year performed by the same surgeon1,18 | ||
| Chronic kidney disease (eGFR < 30 ml/min/1.72 m2)19 | ||
| Inflammatory bowel disease (ulcerative colitis, Crohn’s disease)20 | ||
| Immunosuppression therapy (that is steroids, calcineurin inhibitors, etc.)21 |
FEV1, forced expiratory volume 1 second; FVC, forced vital capacity; eGFR, estimated glomerular filtration rate.
Second, a set of previously applied evidence-based criteria were used to define low-risk bariatric cases, also called ‘benchmark cases’ (Table 1)4. According to the concept of benchmarking, the procedure-specific best achievable outcomes are established in benchmark cases operated in expert centres1. Expert centres had to start inclusion after completion of the learning curve (from the 51st robotically assisted bariatric surgery case operated in the centre from 1 January 2009 to 1 June 2022), whilst learning phase centres included cases up to their 50th robotically assisted bariatric surgery case18, operated between 2020 and 2022. This approach allows for the assessment of the additional morbidity burden related to the non-benchmark patient profile and provides contemporary comparability of expert versus learning curve cohorts.
Third, the relevant outcome indicators for surgical quality were assessed. Benchmark cut-offs were established in expert centres, which performed greater than or equal to 22 low-risk/benchmark cases for the respective procedure, to prevent stochastic statistical noise in the case of a lower caseload. To adjust for variability, the median values of continuous variables and proportions of categorical variables were calculated for each participating centre. Benchmark cut-offs, indicating the ‘best achievable’ results for each outcome indicator were set at the 75th percentile of the centres’ median values1 and were established separately for low-risk (benchmark) and non-low-risk (non-benchmark) cases. The study protocol was approved by the institutional review boards of the University Hospital of Geneva (2019-01801) and of the participating centres.
Outcome variables of interest
Data accuracy was the responsibility of local investigators at each participating centre, who also collected de-identified patient-specific data into pre-programmed spreadsheets and sent them to the principal investigators via secured file transfer. Data included baseline characteristics of patients (age, sex, BMI, risk profile, and bariatric surgery history), characteristics of the index operation, 90-day postoperative complications graded by severity according to the Clavien–Dindo grading system22, duration of stay, readmissions (time from operation, reason, and treatment), last follow-up, and postoperative BMI at 1 year. To enable the assessment of cumulative morbidity over time, the comprehensive complication index (CCI®) was used23. Relevant bariatric complications such as staple line/anastomotic leak, anastomotic stenosis, internal hernia, pain syndrome, and events related to the robotic platform (docking time and system malfunction) were also analysed. Postoperative weight loss was expressed as the % of total weight loss and the % of excess BMI loss, with a BMI less than or equal to 25 kg/m2 considered normal. The console times and procedure types provided by the centres were audited externally against the centralized robotically assisted bariatric surgery database of Intuitive Surgical (Sunnyvale, CA, USA). Each submitted case was controlled for completeness by the principal investigator in Geneva and clarification was requested from the co-investigators in cases of incomplete submitted case report forms or discrepancies with the Intuitive Surgical database.
Statistical analysis
Discrete variables are described using count (%) and continuous variables are described using median (interquartile range (i.q.r.)). Multivariable logistic regression was used to compute the additional morbidity burden related to procedure type and preoperative risk profile. Statistical analysis and data visualization were carried out independently by two principal investigators (G.G. and D.G.) using R software 4.2.1 (R Foundation, Vienna, Austria).
Results
Robotic bariatric surgery cohort
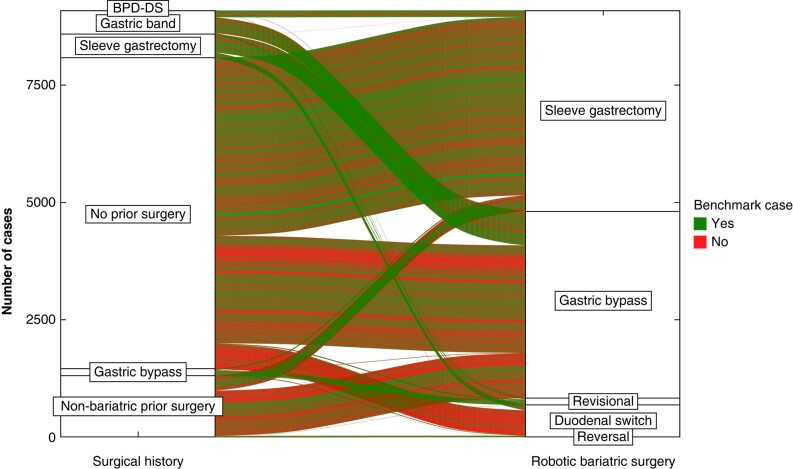
A total of 9097 consecutive elective robotically assisted bariatric surgery procedures (RYGB, SG, bilio-pancreatic diversion, single-anastomosis duodeno-ileal bypass, and revisional surgery) were performed between 2009 and 2022 at the 17 participating centres, of which 8959 cases were performed in expert centres and 138 cases were performed in learning phase centres (see the study flow chart (Fig. S1)). Patient characteristics of the study population are shown in Table 2. The proportion of benchmark cases in expert centres varied between 7% and 71% (Fig. S2). The procedural case mix of robotically assisted bariatric surgery showed continental variations, with the highest proportion of secondary bariatric surgeries in the USA (Fig. S3). The surgical history and risk strata of patients per bariatric surgery category are shown in Fig. 1. In expert centres, 13.74% of the 3020 primary rRYGB patients and 5.88% of the 4078 primary rSG patients presented with greater than or equal to one complication until 90 days after surgery and no patient died. There was no significant correlation between the centre volume and 90-day CCI® (R = −0.13, P = 0.670). For primary rRYGB and rSG, the median follow-up was 542 (i.q.r. 180–1080) and 90 (i.q.r. 90–168) days respectively. Of patients after rRYGB (number at risk 1737 of 3020) 83% had an uneventful 1-year follow-up and of patients after rSG (number at risk 482 of 4078) 93.9% had an uneventful 1-year follow-up. The mean(s.d.) % of total weight loss for rRYGB and rSG at 1 year was 32.2(8.8)% and 25(8.8)% respectively and the mean(s.d.) % of excess BMI loss was 77.6(36.7)% and 62.2(22.7)% respectively. Of the submitted cases 83% were successfully identified in the Intuitive Surgical robotically assisted bariatric surgery database, which was used as the basis to compute benchmark cut-offs for ‘console times’.
Table 2.
Baseline characteristics of patients undergoing robotically assisted bariatric surgery in expert centres compared with the previously published low-risk cases undergoing laparoscopic bariatric surgery 4
| Roux-en-Y gastric bypass | Sleeve gastrectomy | |||||
|---|---|---|---|---|---|---|
| Robotic | Laparoscopic | Robotic | Laparoscopic | |||
| Patient characteristics | Low risk (n = 895) | High risk (n = 2835) | Low risk (n = 4120) | Low risk (n = 1643) | High risk (n = 2590) | Low risk (n = 1457) |
| Age (years), mean(s.d.) | 40.6(10.1) | 47.6(10.8) | 38.2(11.1) | 39.3(10.7) | 46.7(11.9) | 37.0( .8) |
| Sex | ||||||
| Male | 98 (10.9) | 741 (26.1) | 818 (19.9) | 248 (15.1) | 863 (33.3) | 407 (27.9) |
| Female | 797 (89.1) | 2094 (73.9) | 3302 (80.1) | 1395 (84.9) | 1727 (66.7) | 1050 (72.1) |
| Height (cm), mean(s.d.) | 165(8.6) | 166.5(9.8) | 168.1(9.0) | 165(8.2) | 167.2(14.2) | 167.4(8.7) |
| Weight (kg), mean(s.d.) | 115(16.8) | 122(25.4) | 116.9(17.6) | 116.1(15.3) | 128.7(27.1) | 109.3(19.1) |
| BMI (kg/m2), mean(s.d.) | 42(4.3) | 43.9(8.1) | 41.3(6.2) | 42.5(3.5) | 46(7.8) | 38.9(5.2) |
| Hypertension | 242 (27) | 1424 (50.2) | 891 (21.6) | 434 (27.4) | 1397 (53.9) | 344 (23.6) |
| Gastro-oesophageal reflux disease | 302 (33.7) | 1440 (50.8) | 905 (22.0) | 196 (12.4) | 572 (22.1) | 170 (11.7) |
| Hyperuricaemia | 4 (0.4) | 65 (2.3) | 112 (2.7) | 3 (1.3) | 163 (6.3) | 60 (4.1) |
| Depression | 121 (13.5) | 400 (14.1) | 800 (19.4) | 71 (30.2) | 541 (20.9) | 117 (8.0) |
| Dyslipidaemia | 101 (11.3) | 830 (29.3) | 935 (22.7) | 178 (11.3) | 873 (33.7) | 388 (26.6) |
| Joint disorders | 198 (22.1) | 921 (32.5) | 1200 (29.1) | 40 (17.1) | 994 (38.4) | 269 (18.5) |
| Smoking | 63 (7) | 250 (8.8) | 733 (17.8) | 152 (9.7) | 274 (10.6) | 224 (15.4) |
| ASA II and III | 884 (98.7) | 2795 (98.5) | – | 1626 (99) | 2486 (96) | – |
| Operation characteristics | ||||||
| Primary bariatric surgery | 895 (100) | 2094 (73.9) | 4120 (100) | 1643 (100) | 2417 (93.3) | 1457 (100) |
| Conversion to open | 0 | 0 | 1 (0.0) | 0 | 0 | 0 (0.0) |
| Conversion to laparoscopic | 1 (0.1) | 3 (0.1) | – | 0 | 0 | – |
| Operation duration (min), mean(s.d.) | 147.4(48.1) | 151.8(54.8) | 91.3(44.7) | 74.7(20.4) | 85.7(28.6) | 73.7(34.4) |
| Intraoperative drain placement | 83 (9.3) | 400 (14.1) | 1481 (35.9) | 376 (22.8) | 764 (29.5) | 377 (25.9) |
| Anastomotic technique, % | GJ: handsewn, 83.1; linear, 16.9 JJ: linear, 62.8; handsewn, 37.7 |
GJ: handsewn, 79.4; linear, 20.6 JJ: linear, 71.2; handsewn, 28.8 |
Circular, 53.5; linear, 30.5; handsewn, 15.5 | – | – | – |
| Stapler, % | Powered Echelon Flex, 37; SureForm™ 60, 22; EndoGIA, 6.5; other, 34.5 | Powered Echelon Flex, 30; SureForm™ 60, 20.1; Endowrist 45, 12.3; other, 37.6 | – | SureForm™ 60, 80.2; Powered Echelon Flex, 5.1; EndoGIA, 3.5; other, 11.2 | SureForm™ 60, 75.8; Powered Echelon Flex, 7.7; EndoGIA, 3.9; other, 12.6 | – |
| Concomitant procedure, % | None, 72.6; cholecystectomy, 12; oesophagogastroduodenoscopy, 12.5 | None, 74.1; oesophagogastroduodenoscopy, 18; gastric band removal, 7; cholecystectomy, 5; hiatal hernia, 2.6 | None | None, 96; hiatal hernia repair, 3.3; cholecystectomy, 0.2 | None, 93.3; gastric band removal, 5.1; hiatal hernia repair, 1; cholecystectomy, 0.4 | None |
| Staple line oversewn, % | – | – | – | 25.5 | 28.6 | 52.6 |
| Mesenteric defect closure, % | 87.1 | 89.4 | 86.3 | – | – | – |
Values are n (%) unless otherwise indicated. GJ, gastrojejunal anastomosis; JJ, jejunojejunal anastomosis.
Fig. 1.
Surgical history and types of robotically assisted bariatric surgery (=index operation) connected with lines showing the risk category of each case (one line = one case)
BPD-DS, bilio-pancreatic diversion with duodenal switch.
Benchmark cut-offs for surgical quality indicators
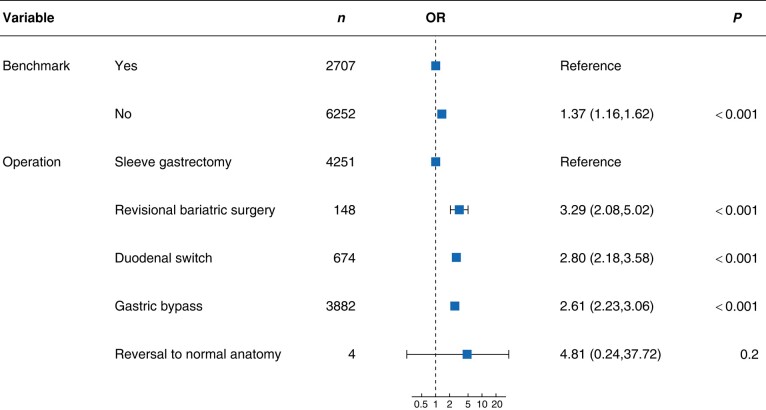
Overall, the proportion of primary robotically assisted bariatric surgery cases was 86.5%, including 895 rRYGB and 1643 rSG low-risk/benchmark cases from expert centres. The composite ‘benchmark patient’ stratum was internally validated by comparing the 90-day postoperative morbidity between the benchmark and non-benchmark cohorts, as well as among the main bariatric procedures (Fig. 2). The preoperative risk profile and operation type significantly influenced the likelihood of any postoperative complications up to 90 days. For rRYGB and rSG, Table 3 and Table 4 respectively present benchmark cut-offs established for perioperative outcomes, as well as for morbidity and mortality up to 90 days after surgery, separately for benchmark and non-benchmark cases.
Fig. 2.
Multivariable logistic regression analysing the role of patients’ preoperative risk profile and of the operation type for the development of any complication at 90 days after robotic bariatric surgery
Benchmark: cases with a predefined low-risk profile.
Table 3.
Benchmark cut-offs (75th percentile of centres’ median) for low-risk (benchmark) and high-risk (non-benchmark) robotic Roux-en-Y gastric bypass compared with the previously established global benchmark cut-offs for laparoscopic Roux-en-Y gastric bypass4
| Surgical approach | Robotic | Laparoscopic | |
|---|---|---|---|
| Perioperative course | Low risk (n = 895) | High risk (n = 2835) | Low risk (n = 4120) |
| Operation duration (min) | ≤162 | ≤167 | ≤120 |
| Docking time (min) | ≤13.5 | ≤10 | – |
| Console time (min) | ≤140 | ≤144 | – |
| Conversion to laparoscopic or open surgery | 0 | ≤0.04 | 0 |
| Intraoperative or postoperative blood transfusions | 0 | ≤1 | ≤2 |
| Hospital stay (days) | ≤2 | ≤2.2 | ≤4 |
| Readmission until 90 days | ≤5.6 | ≤7.4 | ≤5.5 |
| Morbidity and mortality | Until discharge | Until 30 days | Until 90 days | ||||
|---|---|---|---|---|---|---|---|
| Low risk (n = 895) | High risk (n = 2835) | Low risk (n = 895) | High risk (n = 2835) | Low risk (n = 895) | High risk (n = 2835) | Laparoscopic Low risk (n = 4120) | |
| Uneventful postoperative course | >97.5 | >93.5 | >90.3 | >84 | >88.2 | >80 | >90 |
| Any complication | ≤2.5 | ≤6.5 | ≤9.7 | ≤16 | ≤11.8 | ≤20 | ≤10 |
| Complication CD grade II | ≤1.7 | ≤3.7 | ≤4.8 | ≤4.9 | ≤5 | ≤6 | ≤4.1 |
| Complication CD grade ≥IIIa | ≤1.4 | ≤3.2 | ≤4.2 | ≤5.3 | ≤5 | ≤6.7 | ≤5.5 |
| Reoperation (CD grade IIIb) | ≤1.4 | ≤1.4 | ≤2.5 | ≤3.2 | ≤4.3 | ≤4 | ≤4 |
| ICU admission (CD grade IV) | ≤0.8 | ≤1.1 | ≤0.8 | ≤1.2 | ≤0.9 | ≤1.2 | 0 |
| Mortality (CD grade V) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CCI® | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CCI® (in patients with ≥1 CD grade ≥II complication) | ≤33.73 | ≤39.56 | ≤33.73 | ≤39.56 | ≤34.81 | ≤39.56 | ≤33.73 |
| Complications | |||||||
| Anastomotic leak | ≤0.8 | ≤0.3 | ≤1.4 | ≤1.2 | ≤1.4 | ≤1.2 | ≤1.3 |
| Motility disorder | 0 | 0 | ≤0.9 | ≤2.2 | ≤1.9 | ≤3.5 | – |
| Postoperative bleeding | ≤0.9 | ≤1 | ≤1.3 | ≤1.5 | ≤1.3 | ≤1.5 | ≤2.2 |
| Small bowel obstruction/internal hernia | 0 | ≤0.4 | ≤0.9 | ≤1.6 | ≤2.5 | ≤1.9 | ≤2.1 |
| Wound infection | 0 | ≤0.2 | ≤0.8 | ≤0.7 | ≤0.9% | ≤0.9 | ≤0.5 |
| Dysphagia/gastro-oesophageal reflux disease/stenosis | 0 | 0 | ≤1.4 | ≤1.4 | ≤2 | ≤2.8 | – |
| Abdominal or osteo-articular pain | 0 | 0 | 1.3 | ≤1 | ≤1.9 | ≤1.5 | – |
| Deep-vein thrombosis/pulmonary embolism | 0 | 0 | ≤0.6 | ≤0.6 | ≤0.7 | ≤0.7 | – |
| Marginal ulcer | 0 | 0 | ≤0.1 | 0 | ≤0.4 | ≤1 | ≤1.5 |
Values are % unless otherwise indicated. CD, Clavien–Dindo; CCI®, comprehensive complication index.
Table 4.
Benchmark cut-offs (75th percentile of centres’ median) for low-risk (benchmark) and high-risk (non-benchmark) robotic sleeve gastrectomy compared with the previously established global benchmark cut-offs for laparoscopic Roux-en-Y gastric bypass4
| Surgical approach | Robotic | Laparoscopic | |
|---|---|---|---|
| Perioperative course | Low risk (n = 1643) | High risk (n = 2590) | Low risk (n = 1457) |
| Operation duration (min) | ≤89.5 | ≤110 | ≤90 |
| Docking time (min) | ≤13 | ≤14.5 | – |
| Console time (min) | ≤64 | ≤71 | – |
| Conversion to laparoscopic or open surgery | 0 | 0 | 0 |
| Intraoperative or postoperative blood transfusions | 0 | ≤0.2 | ≤1.3 |
| Hospital stay (days) | ≤2 | ≤2 | ≤3 |
| Readmission until 90 days | ≤1.8 | ≤3.1 | ≤5.5 |
| Morbidity and mortality | Until discharge | Until 30 days | Until 90 days | ||||
|---|---|---|---|---|---|---|---|
| Low risk (n = 1643) |
High risk (n = 2582) |
Low risk (n = 1643) |
High risk (n = 2582) |
Low risk (n = 1643) |
High risk (n = 2582) |
Laparoscopic Low risk (n = 1457) | |
| Uneventful postoperative course | >99 | >96.8 | >94.8 | >90.8 | >93.6 | >90.8 | >88 |
| Any complication | ≤1 | ≤3.2 | ≤5.2 | ≤9.2 | ≤6.4 | ≤9.2 | ≤12 |
| Complication CD grade II | ≤0.06 | ≤0.6 | ≤1.5 | ≤1.9 | ≤1.6 | ≤6.4 | ≤2.5 |
| Complication CD grade ≥IIIa | ≤0.06 | ≤0.2 | ≤0.4 | ≤2 | ≤1.3 | ≤2 | ≤5.5 |
| Reoperation (CD grade IIIb) | ≤0.06 | ≤0.08 | ≤0.12 | ≤0.8 | ≤1.2 | ≤1.7 | ≤3 |
| ICU admission (CD grades IVa and IVb) | 0 | 0 | ≤0.06 | ≤0.4 | ≤0.06 | ≤0.4 | 0 |
| Mortality (CD grade V) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CCI® | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CCI® (in patients with ≥1 CD grade ≥II complication) | ≤36.71 | ≤31.85 | ≤36.71 | ≤31.85 | ≤36.71 | ≤31.85 | ≤33.73 |
| Complications | |||||||
| Leak at the staple line | 0 | 0 | 0 | ≤0.7 | 0 | ≤0.8 | ≤0.15 |
| Motility disorder | 0 | 0 | ≤0.5 | ≤0.6 | ≤0.6 | ≤0.6 | – |
| Postoperative bleeding | 0 | ≤0.2 | ≤0.2 | ≤0.4 | ≤0.2 | ≤0.4 | ≤1.7 |
| Small bowel obstruction/internal hernia | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wound infection | 0 | 0 | ≤0.5 | ≤0.1 | ≤0.6 | ≤0.3 | 0 |
| Dysphagia/gastro-oesophageal reflux disease/stenosis | 0 | 0 | 0 | ≤1.6 | 0 | ≤2.5 | ≤0.27 |
| Abdominal or osteo-articular pain | 0 | 0 | 0 | 0 | ≤0.5 | 0 | – |
| Deep-vein thrombosis/pulmonary embolism | 0 | 0 | ≤0.2 | ≤0.3 | ≤0.2 | ≤0.3 | – |
Values are % unless otherwise indicated. CD, Clavien–Dindo; CCI®, comprehensive complication index.
Benchmark cohort: robotic Roux-en-Y gastric bypass
Gastrojejunal and jejunojejunal anastomoses were handsewn in 83.1% and 37.7% of the cases respectively. A subgroup analysis compared the outcomes based on gastrojejunal-anastomosis technique in low-risk rRYGB cases operated in expert centres (Table S4). The median time between skin incision and the first anastomosis was 56 (i.q.r. 25–96) min and the median time between the skin incision and the second anastomosis was 88 (i.q.r. 45–131.2) min. Mesenteric defects were closed in 87.1% of cases and intraoperative drainage was placed in 9.3% of cases, whilst, in the historic laparoscopic RYGB benchmark cohort4, these values were 15.5% and 35.9% respectively.
Benchmark cohort: robotic sleeve gastrectomy
Gastric resection was mainly performed using the da Vinci SureForm™ 60 mm stapler (80.2%). Staple line reinforcement was performed in 24.4% of cases. Benchmark cut-offs seemed superior in rSG compared with laparoscopic SG for every surgical outcome, including operation duration, except for surgical site infections up to 90 days (less than or equal to 0.5% versus 0%). The cut-offs for non-benchmark rSG cases were within those established for benchmark laparoscopic bariatric surgery cases4.
Learning curve of robotically assisted bariatric surgery
The baseline characteristics and surgical outcomes of the 138 cases performed at the robotic learning phase centres are reported in Tables S1–S3. Benchmark rRYGB cases performed in learning phase centres achieved outcomes within the established benchmark cut-offs, except for docking time approximately 5 min longer, whereas non-benchmark rRYGB and rSG cases did not.
Adverse events related to the robotic platform
Adverse events related to the robotic platform occurred in 1.14% of cases (102 of 8959 patients) in expert centres and in 1.45% of cases (2 of 138 patients) in learning phase centres (P > 0.05). The most frequent adverse events were organ injury resulting from the introduction of instruments by the surgical assistant (11 patients), robotic instruments operated outside the field of vision (11 patients), or grasping-induced serosal injury (9 patients). System- and instrument-related dysfunctions included collisions between the robotic instruments and the patient (12 patients), locked-in instruments (11 patients), system errors (10 patients), and stapler misfires (9 patients).
Discussion
This multicentre study established global outcome benchmarks for the two most frequently performed robotically assisted bariatric surgical procedures by applying a standardized methodology1,3. Cut-offs for clinically relevant surgical outcomes were established based on a patient cohort operated in 13 high-volume expert robotic bariatric surgery centres centres across four continents. The concept of establishing benchmarks in low-risk cases has been validated by logistic regression, which showed a significantly higher OR (1.37) for any complications at 90 days in non-benchmark cases. Overall, both benchmark and non-benchmark rRYGB and rSG cases had a 90-day mortality rate of 0% and a low early postoperative morbidity rate, whereas non-benchmark patients had greater rates of readmissions and ICU admissions. This is the first benchmark study to also report benchmark cut-offs for non-benchmark cases, which are more representative of everyday practice, given the high rate of metabolic co-morbidities in the bariatric population. The main application of robotically assisted bariatric surgery was to perform primary RYGB and SG, whereas the proportion of secondary bariatric surgical cases remained low. The prevalence of adverse events related to the robotic platform itself was in the order of 1%, in expert and learning curve centres alike.
The increasing adoption of the robotic platform in bariatric surgery remains debatable given the increased healthcare expenditures and the lack of clear clinical benefit for patients24. However, the present study adds a novel aspect in favour of robotically assisted bariatric surgery. In a secondary analysis, the comparison of the benchmark cut-offs for rRYGB, reflecting the best achievable outcomes in low-risk patients operated in high-volume centres, with those published in 2019 for equally low-risk laparoscopic RYGB cases showed lower 90-day cut-offs for bleeding (0% versus less than or equal to 2%) and marginal ulcers (less than or equal to 0.4% versus less than or equal to 1.5%) after rRYGB, whilst the cut-off for operation duration was longer (less than or equal to 162 min versus less than or equal to 120 min)4. The comparison of benchmark cut-offs for robotic and laparoscopic bariatric surgery should be interpreted with caution, as they were established in different centres and during study intervals that only partially overlapped. Besides differences in surgeons’ experience, differences in surgical technique may also contribute to the observed improved outcomes. The proportion of handsewn versus stapled anastomoses in the laparoscopic series was 15% versus 85% respectively4, whilst the opposite was found in the robotic cohort, with a handsewn gastrojejunostomy rate of 83%. The reductions of postoperative bleeding and marginal ulceration observed in this study are consistent with recent findings based on the MBSAQIP database25 and support that the handsewn anastomosis technique reduces ischaemia or bleeding compared with the circular stapled anastomosis technique26. A meta-analysis including 83 studies also found a significantly lower rate of stenosis in rRYGB compared with laparoscopic RYGB27,28. Nevertheless, the operation duration cut-off for rRYGB was longer compared with laparoscopic RYGB, which might be imputable to the high rate of handsewn anastomoses. Linear stapled gastrojejunostomy in rRYGB has been found to reduce operating time29, which could be confirmed in the benchmark rRYGB cohort, but had no significant impact on the 90-day overall complication rate.
Regarding rSG, the robotic approach appears to offer greater surgical safety than laparoscopy for most outcome parameters. These findings contrast with the MBSAQIP database analysis30,31, which found, on average, a 26 min increase in operating time for rSG and slightly higher odds for any infectious complication. Importantly, the MBSAQIP database analysis did not stratify patients based on their risk profiles and did not include data on surgeon experience or volume. Accordingly, this may reflect outcomes of centres with various levels of robotically assisted bariatric surgery expertise. The marked differences in operative techniques between the rSG and the historical laparoscopic SG benchmark cohorts must also be emphasized. In the rSG cohort, 80% of the stapling was performed with the robotic SureForm™ and the oversewn staple line rate was 25%. In the laparoscopic SG cohort, the oversewn staple line rate was instead 53% and it is assumed that the proportion of powered staplers was minimal, as they were introduced after the beginning of the study’s inclusion interval. SG is a relatively high-pressure system32 and a software-based algorithm for stapling could have contributed to the observed reduction of staple line bleeding and leaks, whilst a lower oversewn staple line rate may explain why operation duration was similar. The results of this study should be interpreted in light of its major limitations. First, owing to the retrospective design and wide geographical and time spans of the study, confounding factors and changes in perioperative policies over time (that is Enhanced Recovery After Surgery (ERAS) guidelines in bariatric surgery first published in 201633) may have influenced outcomes, particularly the duration of hospital stay. The present study included cases operated between 2009 and 2022, which overlaps with the data collection interval of the primary laparoscopic bariatric surgery benchmark study (2012–2017)4, thus enabling comparison. Nevertheless, over the course of the 13-year data collection interval, improvements in instrumentation and equipment, as well as the increasing clinical experience, may have contributed to improved surgical safety, independently of the robotic platform. Second, data on the costs related to robotically assisted bariatric surgery were not collected and could not be estimated, as the available procedural cost estimation tools were developed for laparoscopic bariatric surgeries34. The reduced duration of stay, lower frequency of major complications, and the environmental cost35 attributable to the robotic platform are further factors that should be taken into account in future studies to provide a cost-efficiency analysis of robotic versus laparoscopic bariatric surgery. Third, the current methodology for establishing global surgical benchmarks is hampered by logistic obstacles. Formal external audit of the database was not possible given the worldwide distribution of the centres and the lack of external funding. However, each included case was compared with the Intuitive Surgical database to confirm the accuracy of the procedure name and console time to prevent misreporting. Regular updates of the surgical benchmarks should ideally include a standardization of the surgical technique and be automatized in the future by the development of prospective registries using the robotic platform itself. Of note, the promising outcomes achieved in the learning phase centres may have been influenced by the Hawthorn effect.
The main findings demonstrate the feasibility of robotically assisted bariatric surgery in both expert and learning phase centres. The outcome benchmarks may be used as a reference for evaluating and stimulating surgical performance among bariatric surgery centres worldwide. In a secondary analysis, benchmarks for robotically assisted bariatric surgery, especially rSG, seemed superior to those established in the historical laparoscopic bariatric surgery cohort. These observed benefits are multifactorial and are not solely related to the robotic platform itself. The robotic approach allows easier performance of the handsewn anastomosis technique in patients with visceral obesity and allows software-based stapling, both of which may contribute to the decreased rate of postoperative bleeding, anastomotic stenosis/ulceration, and gastrointestinal leak. Nevertheless, improvements in perioperative medicine and growing expertise in minimally invasive surgery over time are also likely strong contributors to the observed quality improvement. Actualization of global benchmarks for laparoscopic bariatric surgery is needed to demonstrate whether the established outcome standards for robotically assisted bariatric surgery are achievable laparoscopically.
Supplementary Material
Acknowledgements
G.G. and D.G. contributed equally and share first authorship. M.B. and M.K.J. contributed equally and share last authorship. The authors are grateful to all clinical nurses, data managers, and residents who made valuable contributions to the data collection process and to Amélie, Tristan, and Claude Giudicelli for their valuable assistance in editing the manuscript.
Contributor Information
Guillaume Giudicelli, Division of Digestive Surgery, Department of Surgery, Geneva University Hospital and Faculty of Medicine, Geneva, Switzerland.
Daniel Gero, Department of Surgery and Transplantation, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Lind Romulo, Department of Surgery, Orlando Health, University of Central Florida, Orlando, Florida, USA.
Vasu Chirumamilla, Bariatric and Robotic Center of Excellence, Mather Northwell Hospital Health, Port Jefferson, New York, USA.
Pouya Iranmanesh, Division of Digestive Surgery, Department of Surgery, Geneva University Hospital and Faculty of Medicine, Geneva, Switzerland; Division of Minimally Invasive and Elective General Surgery, Department of Surgery, McGovern Medical School, University of Texas Health Science Center at Houston, Houston, Texas, USA.
Christopher K Owen, Division of Minimally Invasive and Elective General Surgery, Department of Surgery, McGovern Medical School, University of Texas Health Science Center at Houston, Houston, Texas, USA.
Wayne Bauerle, Department of Surgery, Division of Bariatric Surgery, St. Luke’s University Health Network, Bethlehem, Pennsylvania, USA.
Amador Garcia, Endocrine-Metabolic and Bariatric Unit, Robotic Surgery, Vall Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona, Barcelona, Spain.
Lisa Lucas, Department of Endocrine and Digestive Surgery, University Hospital of Poitiers, Poitiers, France.
Anne-Sophie Mehdorn, Department of General, Abdominal, Thoracic, Transplantation and Paediatric Surgery, University Hospital Schleswig-Holstein, Kiel, Germany; Kurt Semm Centre for Laparoscopic and Robot Assisted Surgery, University Hospital Schleswig-Holstein, Kiel, Germany.
Dhananjay Pandey, Institute of Minimal Access, Bariatric and Robotic Surgery, Max Super Speciality Hospital, Delhi NCR, India.
Abdullah Almuttawa, Department of Endocrine and Digestive Surgery, Strasbourg University Hospital – IRCAD, Strasbourg, France; Department of Surgery, University of Jeddah, Jeddah, Saudi Arabia.
Francisco Cabral, Robotic Surgery Unit, Cuf Tejo Hospital, Lisbon, Portugal.
Abhishek Tiwari, Department of Surgery, Indraprastha Apollo Hospitals, New Delhi, India.
Virginia Lambert, Adelaide Bariatric Centre, Department of Surgery, Flinders University of South Australia, Adelaide, South Australia, Australia.
Beniamino Pascotto, General and Minimally Invasive (Laparoscopic and Robotic) Surgery Department, Centre Hospitalier de Luxembourg, Luxembourg City, Luxembourg.
Celine De Meyere, Department of Surgery, AZ Groeninge Hospital, Kortrijk, Belgium.
Marouan Yahyaoui, Department of Digestive and Bariatric Surgery, Hôpital Edouard Herriot, Lyon, France.
Thomas Haist, Department of General and Visceral Surgery, Asklepios Paulinen Klinik, Wiesbaden, Germany.
Oliver Scheffel, Department of Obesity and Metabolic Surgery, Sana Klinikum Offenbach GmbH, Offenbach am Main, Germany.
Maud Robert, Department of Digestive and Bariatric Surgery, Hôpital Edouard Herriot, Lyon, France.
Frederiek Nuytens, Department of Surgery, AZ Groeninge Hospital, Kortrijk, Belgium.
Santiago Azagra, General and Minimally Invasive (Laparoscopic and Robotic) Surgery Department, Centre Hospitalier de Luxembourg, Luxembourg City, Luxembourg.
Lilian Kow, Adelaide Bariatric Centre, Department of Surgery, Flinders University of South Australia, Adelaide, South Australia, Australia.
Arun Prasad, Department of Surgery, Indraprastha Apollo Hospitals, New Delhi, India.
Carlos Vaz, Robotic Surgery Unit, Cuf Tejo Hospital, Lisbon, Portugal.
Michel Vix, Department of Endocrine and Digestive Surgery, Strasbourg University Hospital – IRCAD, Strasbourg, France.
Vivek Bindal, Institute of Minimal Access, Bariatric and Robotic Surgery, Max Super Speciality Hospital, Delhi NCR, India.
Jan H Beckmann, Department of General, Abdominal, Thoracic, Transplantation and Paediatric Surgery, University Hospital Schleswig-Holstein, Kiel, Germany; Kurt Semm Centre for Laparoscopic and Robot Assisted Surgery, University Hospital Schleswig-Holstein, Kiel, Germany.
David Soussi, Department of Endocrine and Digestive Surgery, University Hospital of Poitiers, Poitiers, France.
Ramon Vilallonga, Endocrine-Metabolic and Bariatric Unit, Robotic Surgery, Vall Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona, Barcelona, Spain.
Maher El Chaar, Department of Surgery, Division of Bariatric Surgery, St. Luke’s University Health Network, Bethlehem, Pennsylvania, USA.
Erik B Wilson, Division of Minimally Invasive and Elective General Surgery, Department of Surgery, McGovern Medical School, University of Texas Health Science Center at Houston, Houston, Texas, USA.
Arif Ahmad, Bariatric and Robotic Center of Excellence, Mather Northwell Hospital Health, Port Jefferson, New York, USA.
Andre Teixeira, Department of Surgery, Orlando Health, University of Central Florida, Orlando, Florida, USA.
Monika E Hagen, Division of Digestive Surgery, Department of Surgery, Geneva University Hospital and Faculty of Medicine, Geneva, Switzerland.
Christian Toso, Division of Digestive Surgery, Department of Surgery, Geneva University Hospital and Faculty of Medicine, Geneva, Switzerland.
Pierre-Alain Clavien, Department of Surgery and Transplantation, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Milo Puhan, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland.
Marco Bueter, Department of Surgery and Transplantation, University Hospital Zurich, University of Zurich, Zurich, Switzerland.
Minoa K Jung, Division of Digestive Surgery, Department of Surgery, Geneva University Hospital and Faculty of Medicine, Geneva, Switzerland.
Funding
This study and its dissemination at scientific meetings did not receive any financial support from the healthcare industry or from any external public or private entity.
Author contributions
Guillaume Giudicelli (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing), Daniel Gero (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing—original draft, Writing—review & editing), Lind Romulo (Data curation), Vasu Chirumamilla (Data curation), Pouya Iranmanesh (Data curation), Christopher K. Owen (Data curation), Wayne Bauerle (Data curation), Amador Garcia (Data curation), Lisa Lucas (Data curation), Anne-Sophie Mehdorn (Data curation), Dhananjay Pandey (Data curation), Abdullah Almuttawa (Data curation), Francisco Cabral (Data curation), Abhishek Tiwari (Data curation), Virginia Lambert (Data curation), Beniamino Pascotto (Data curation), Celine De Meyere (Data curation), Marouan Yahyaoui (Data curation), Thomas Haist (Data curation), Oliver Scheffel (Data curation), Maud Robert (Data curation), Frederiek Nuytens (Data curation), Santiago Azagra (Data curation), Lilian Kow (Data curation), Arun Prasad (Data curation), Carlos Vaz (Data curation), Michel Vix (Data curation), Vivek Bindal (Data curation), Jan H. Beckmann (Data curation), David Soussi (Data curation), Ramon Vilallonga (Data curation), Maher El Chaar (Data curation), Erik B. Wilson (Data curation), Arif Ahmad (Data curation, Writing—review & editing), Andre Teixeira (Data curation), Monika E. Hagen (Resources, Supervision, Writing—review & editing), Christian Toso (Resources, Supervision, Writing—review & editing), Pierre-Alain Clavien (Conceptualization, Resources, Supervision, Writing—review & editing), Milo Puhan (Conceptualization, Resources), Marco Bueter (Conceptualization, Resources, Supervision, Writing—review & editing), and Minoa K. Jung (Resources, Supervision, Writing—review & editing)
Disclosure
M.B. reports personal fees from Johnson & Johnson and Medtronic, outside the submitted work. M.K.J. reports personal fees from Johnson & Johnson and Intuitive Surgical. C.T. received personal fees from Intuitive Surgical Inc., Ethicon Inc., and Verb Surgical, outside this project. Outside the scope of this work, M.E.H. received personal fees from Johnson & Johnson, research grants from Intuitive and Johnson & Johnson, and non-financial support from Johnson and Johnson. M.E.C. is a consultant for Intuitive and currently serves on the Intuitive Surgeon Advisory Board. J.H.B. reports personal fees from Johnson & Johnson and Intuitive, and research grants from B. Braun and Johnson & Johnson. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data Availability
Data are available from the authors upon request.
References
- 1. Gero D, Muller X, Staiger RD, Gutschow CA, Vonlanthen R, Bueter Met al. How to establish benchmarks for surgical outcomes?: a checklist based on an international expert Delphi consensus. Ann Surg 2022;275:115–120 [DOI] [PubMed] [Google Scholar]
- 2. van Ramshorst TME, Giani A, Mazzola M, Dokmak S, Fteriche FS, Esposito Aet al. Benchmarking of robotic and laparoscopic spleen-preserving distal pancreatectomy by using two different methods. Br J Surg 2022;110:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Staiger RD, Schwandt H, Puhan MA, Clavien P-A. Improving surgical outcomes through benchmarking. Br J Surg 2019;106:59–64 [DOI] [PubMed] [Google Scholar]
- 4. Gero D, Raptis DA, Vleeschouwers W, van Veldhuisen SL, Martin AS, Xiao Yet al. Defining global benchmarks in bariatric surgery: a retrospective multicenter analysis of minimally invasive Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg 2019;270:859–867 [DOI] [PubMed] [Google Scholar]
- 5. Gero D, Vannijvel M, Okkema S, Deleus E, Lloyd A, Lo Menzo Eet al. Defining global benchmarks in elective secondary bariatric surgery comprising conversional, revisional, and reversal procedures. Ann Surg 2021;274:821–828 [DOI] [PubMed] [Google Scholar]
- 6. Acquafresca PA, Palermo M, Rogula T, Duza GE, Serra E. Most common robotic bariatric procedures: review and technical aspects. Ann Surg Innov Res 2015;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauerle WB, Mody P, Estep A, Stoltzfus J, El Chaar M. Current trends in the utilization of a robotic approach in the field of bariatric surgery. Obes Surg 2022;33:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Myers SR, McGuirl J, Wang J. Robot-assisted versus laparoscopic gastric bypass: comparison of short-term outcomes. Obes Surg 2013;23:467–473 [DOI] [PubMed] [Google Scholar]
- 9. Fantola G, Moroni E, Runfola M, Lai E, Pintus S, Gallucci Pet al. Controversial role of robot in primary and revisional bariatric surgery procedures: review of the literature and personal experience. Front Surg 2022;9:916652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nasser H, Munie S, Kindel TL, Gould JC, Higgins RM. Comparative analysis of robotic versus laparoscopic revisional bariatric surgery: perioperative outcomes from the MBSAQIP database. Surg Obes Relat Dis 2020;16:397–405 [DOI] [PubMed] [Google Scholar]
- 11. Staiger RD, Rossler F, Kim MJ, Brown C, Trenti L, Sasaki Tet al. Benchmarks in colorectal surgery: multinational study to define quality thresholds in high and low anterior resection. Br J Surg 2022;109:1274–1281 [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JPet al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 13. Giudicelli G, Diana M, Chevallay M, Blaser B, Darbellay C, Guarino Let al. Global benchmark values for laparoscopic Roux-en-Y-gastric bypass: a potential new indicator of the surgical learning curve. Obes Surg 2021;31:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman Wet al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ASA . ASA Physical Status Classification System: ASA House of Delegates. 2020. https://www.asahq.org/standards-and-practice-parameters/statement-on-asa-physical-status-classification-system
- 16. Fernandez AZ, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador Jet al. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc 2004;18:193–197 [DOI] [PubMed] [Google Scholar]
- 17. Kabir A, Mousavi S, Pazouki A. The complications of bariatric surgery patients with type 2 diabetes in the world: a systematic review and meta-analysis. Curr Diabetes Rev 2019;15:49–61 [DOI] [PubMed] [Google Scholar]
- 18. Kauffels A, Reichert M, Askevold I, Bender A, Hecker A, Padberg Wet al. Establishing robotic bariatric surgery at an academic tertiary hospital: a learning curve analysis for totally robotic Roux-en-Y gastric bypass. J Robot Surg 2022;17:577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Husain F, Jeong IH, Spight D, Wolfe B, Mattar SG. Risk factors for early postoperative complications after bariatric surgery. Ann Surg Treat Res 2018;95:100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shoar S, Shahabuddin Hoseini S, Naderan M, Mahmoodzadeh H, Ying Man F, Shoar Net al. Bariatric surgery in morbidly obese patients with inflammatory bowel disease: a systematic review. Surg Obes Relat Dis 2017;13:652–659 [DOI] [PubMed] [Google Scholar]
- 21. Andalib A, Aminian A, Khorgami Z, Jamal MH, Augustin T, Schauer PRet al. Early postoperative outcomes of primary bariatric surgery in patients on chronic steroid or immunosuppressive therapy. Obes Surg 2016;26:1479–1486 [DOI] [PubMed] [Google Scholar]
- 22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BP, Breitenstein Set al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–762; discussion 762–763 [DOI] [PubMed] [Google Scholar]
- 24. Jung MK, Hagen ME, Buchs NC, Buehler LH, Morel P. Robotic bariatric surgery: a general review of the current status. Int J Med Robot 2017;13:e1834. [DOI] [PubMed] [Google Scholar]
- 25. Acevedo E, Mazzei M, Zhao H, Lu X, Soans R, Edwards MA. Outcomes in conventional laparoscopic versus robotic-assisted primary bariatric surgery: a retrospective, case–controlled study of the MBSAQIP database. Surg Endosc 2020;34:1353–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sundaresan N, Sullivan M, Hiticas BA, Hui BY, Poliakin L, Thompson KJet al. Impacts of gastrojejunal anastomotic technique on rates of marginal ulcer formation and anastomotic bleeding following Roux-en-Y gastric bypass. Obes Surg 2021;31:2921–2926 [DOI] [PubMed] [Google Scholar]
- 27. Kostakis ID, Sran H, Uwechue R, Chandak P, Olsburgh J, Mamode Net al. Comparison between robotic and laparoscopic or open anastomoses: a systematic review and meta-analysis. Robot Surg 2019;6:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rogula T, Koprivanac M, Janik MR, Petrosky JA, Nowacki AS, Dombrowska Aet al. Does robotic Roux-en-Y gastric bypass provide outcome advantages over standard laparoscopic approaches? Obes Surg 2018;28:2589–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beckmann JH, Bernsmeier A, Kersebaum J-N, Mehdorn A-S, von Schönfels W, Taivankhuu Tet al. The impact of robotics in learning Roux-en-Y gastric bypass: a retrospective analysis of 214 laparoscopic and robotic procedures. Obes Surg 2020;30:2403–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fazl Alizadeh R, Li S, Inaba CS, Dinicu AI, Hinojosa MW, Smith BRet al. Robotic versus laparoscopic sleeve gastrectomy: a MBSAQIP analysis. Surg Endosc 2019;33:917–922 [DOI] [PubMed] [Google Scholar]
- 31. Wesley Vosburg R, Haque O, Roth E. Robotic vs. laparoscopic metabolic and bariatric surgery, outcomes over 5 years in nearly 800,000 patients. Obes Surg 2022;32:2341–2348 [DOI] [PubMed] [Google Scholar]
- 32. Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen Jet al. Laparoscopic sleeve gastrectomy–volume and pressure assessment. Obes Surg 2008;18:1083–1088 [DOI] [PubMed] [Google Scholar]
- 33. Thorell A, MacCormick AD, Awad S, Reynolds N, Roulin D, Demartines Net al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 2016;40:2065–2083 [DOI] [PubMed] [Google Scholar]
- 34. Staiger RD, Cimino M, Javed A, Biondo S, Fondevila C, Perinel Jet al. The comprehensive complication index (CCI®) is a novel cost assessment tool for surgical procedures. Ann Surg 2018;268:784–791 [DOI] [PubMed] [Google Scholar]
- 35. Papadopoulou A, Kumar NS, Vanhoestenberghe A, Francis NK. Environmental sustainability in robotic and laparoscopic surgery: systematic review. Br J Surg 2022;109:921–932 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the authors upon request.
References
- 1. Gero D, Muller X, Staiger RD, Gutschow CA, Vonlanthen R, Bueter Met al. How to establish benchmarks for surgical outcomes?: a checklist based on an international expert Delphi consensus. Ann Surg 2022;275:115–120 [DOI] [PubMed] [Google Scholar]
- 2. van Ramshorst TME, Giani A, Mazzola M, Dokmak S, Fteriche FS, Esposito Aet al. Benchmarking of robotic and laparoscopic spleen-preserving distal pancreatectomy by using two different methods. Br J Surg 2022;110:76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Staiger RD, Schwandt H, Puhan MA, Clavien P-A. Improving surgical outcomes through benchmarking. Br J Surg 2019;106:59–64 [DOI] [PubMed] [Google Scholar]
- 4. Gero D, Raptis DA, Vleeschouwers W, van Veldhuisen SL, Martin AS, Xiao Yet al. Defining global benchmarks in bariatric surgery: a retrospective multicenter analysis of minimally invasive Roux-en-Y gastric bypass and sleeve gastrectomy. Ann Surg 2019;270:859–867 [DOI] [PubMed] [Google Scholar]
- 5. Gero D, Vannijvel M, Okkema S, Deleus E, Lloyd A, Lo Menzo Eet al. Defining global benchmarks in elective secondary bariatric surgery comprising conversional, revisional, and reversal procedures. Ann Surg 2021;274:821–828 [DOI] [PubMed] [Google Scholar]
- 6. Acquafresca PA, Palermo M, Rogula T, Duza GE, Serra E. Most common robotic bariatric procedures: review and technical aspects. Ann Surg Innov Res 2015;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bauerle WB, Mody P, Estep A, Stoltzfus J, El Chaar M. Current trends in the utilization of a robotic approach in the field of bariatric surgery. Obes Surg 2022;33:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Myers SR, McGuirl J, Wang J. Robot-assisted versus laparoscopic gastric bypass: comparison of short-term outcomes. Obes Surg 2013;23:467–473 [DOI] [PubMed] [Google Scholar]
- 9. Fantola G, Moroni E, Runfola M, Lai E, Pintus S, Gallucci Pet al. Controversial role of robot in primary and revisional bariatric surgery procedures: review of the literature and personal experience. Front Surg 2022;9:916652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nasser H, Munie S, Kindel TL, Gould JC, Higgins RM. Comparative analysis of robotic versus laparoscopic revisional bariatric surgery: perioperative outcomes from the MBSAQIP database. Surg Obes Relat Dis 2020;16:397–405 [DOI] [PubMed] [Google Scholar]
- 11. Staiger RD, Rossler F, Kim MJ, Brown C, Trenti L, Sasaki Tet al. Benchmarks in colorectal surgery: multinational study to define quality thresholds in high and low anterior resection. Br J Surg 2022;109:1274–1281 [DOI] [PubMed] [Google Scholar]
- 12. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JPet al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 13. Giudicelli G, Diana M, Chevallay M, Blaser B, Darbellay C, Guarino Let al. Global benchmark values for laparoscopic Roux-en-Y-gastric bypass: a potential new indicator of the surgical learning curve. Obes Surg 2021;31:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman Wet al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ASA . ASA Physical Status Classification System: ASA House of Delegates. 2020. https://www.asahq.org/standards-and-practice-parameters/statement-on-asa-physical-status-classification-system
- 16. Fernandez AZ, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador Jet al. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc 2004;18:193–197 [DOI] [PubMed] [Google Scholar]
- 17. Kabir A, Mousavi S, Pazouki A. The complications of bariatric surgery patients with type 2 diabetes in the world: a systematic review and meta-analysis. Curr Diabetes Rev 2019;15:49–61 [DOI] [PubMed] [Google Scholar]
- 18. Kauffels A, Reichert M, Askevold I, Bender A, Hecker A, Padberg Wet al. Establishing robotic bariatric surgery at an academic tertiary hospital: a learning curve analysis for totally robotic Roux-en-Y gastric bypass. J Robot Surg 2022;17:577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Husain F, Jeong IH, Spight D, Wolfe B, Mattar SG. Risk factors for early postoperative complications after bariatric surgery. Ann Surg Treat Res 2018;95:100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shoar S, Shahabuddin Hoseini S, Naderan M, Mahmoodzadeh H, Ying Man F, Shoar Net al. Bariatric surgery in morbidly obese patients with inflammatory bowel disease: a systematic review. Surg Obes Relat Dis 2017;13:652–659 [DOI] [PubMed] [Google Scholar]
- 21. Andalib A, Aminian A, Khorgami Z, Jamal MH, Augustin T, Schauer PRet al. Early postoperative outcomes of primary bariatric surgery in patients on chronic steroid or immunosuppressive therapy. Obes Surg 2016;26:1479–1486 [DOI] [PubMed] [Google Scholar]
- 22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BP, Breitenstein Set al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg 2014;260:757–762; discussion 762–763 [DOI] [PubMed] [Google Scholar]
- 24. Jung MK, Hagen ME, Buchs NC, Buehler LH, Morel P. Robotic bariatric surgery: a general review of the current status. Int J Med Robot 2017;13:e1834. [DOI] [PubMed] [Google Scholar]
- 25. Acevedo E, Mazzei M, Zhao H, Lu X, Soans R, Edwards MA. Outcomes in conventional laparoscopic versus robotic-assisted primary bariatric surgery: a retrospective, case–controlled study of the MBSAQIP database. Surg Endosc 2020;34:1353–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sundaresan N, Sullivan M, Hiticas BA, Hui BY, Poliakin L, Thompson KJet al. Impacts of gastrojejunal anastomotic technique on rates of marginal ulcer formation and anastomotic bleeding following Roux-en-Y gastric bypass. Obes Surg 2021;31:2921–2926 [DOI] [PubMed] [Google Scholar]
- 27. Kostakis ID, Sran H, Uwechue R, Chandak P, Olsburgh J, Mamode Net al. Comparison between robotic and laparoscopic or open anastomoses: a systematic review and meta-analysis. Robot Surg 2019;6:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rogula T, Koprivanac M, Janik MR, Petrosky JA, Nowacki AS, Dombrowska Aet al. Does robotic Roux-en-Y gastric bypass provide outcome advantages over standard laparoscopic approaches? Obes Surg 2018;28:2589–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beckmann JH, Bernsmeier A, Kersebaum J-N, Mehdorn A-S, von Schönfels W, Taivankhuu Tet al. The impact of robotics in learning Roux-en-Y gastric bypass: a retrospective analysis of 214 laparoscopic and robotic procedures. Obes Surg 2020;30:2403–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fazl Alizadeh R, Li S, Inaba CS, Dinicu AI, Hinojosa MW, Smith BRet al. Robotic versus laparoscopic sleeve gastrectomy: a MBSAQIP analysis. Surg Endosc 2019;33:917–922 [DOI] [PubMed] [Google Scholar]
- 31. Wesley Vosburg R, Haque O, Roth E. Robotic vs. laparoscopic metabolic and bariatric surgery, outcomes over 5 years in nearly 800,000 patients. Obes Surg 2022;32:2341–2348 [DOI] [PubMed] [Google Scholar]
- 32. Yehoshua RT, Eidelman LA, Stein M, Fichman S, Mazor A, Chen Jet al. Laparoscopic sleeve gastrectomy–volume and pressure assessment. Obes Surg 2008;18:1083–1088 [DOI] [PubMed] [Google Scholar]
- 33. Thorell A, MacCormick AD, Awad S, Reynolds N, Roulin D, Demartines Net al. Guidelines for perioperative care in bariatric surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg 2016;40:2065–2083 [DOI] [PubMed] [Google Scholar]
- 34. Staiger RD, Cimino M, Javed A, Biondo S, Fondevila C, Perinel Jet al. The comprehensive complication index (CCI®) is a novel cost assessment tool for surgical procedures. Ann Surg 2018;268:784–791 [DOI] [PubMed] [Google Scholar]
- 35. Papadopoulou A, Kumar NS, Vanhoestenberghe A, Francis NK. Environmental sustainability in robotic and laparoscopic surgery: systematic review. Br J Surg 2022;109:921–932 [DOI] [PubMed] [Google Scholar]