Abstract
Background:
Pregnancy anaemia is a significant public health concern in South Africa (SA), particularly in rural areas, but little is known about its prevalence and risk factors in rural areas. The objective of the study was to determine the prevalence and identify risk factors of pregnancy anaemia in the public health facilities of Limpopo Province (LP), SA.
Methods:
A cross-sectional study was conducted among a consecutive sample of 211 pregnant women attending antenatal care at Seshego Hospital and its feeder health centre (May to June 2019). Anaemia was defined as haemoglobin (Hb) <11 g/dL and classified as mild (10–10.9 g/dL), moderate (7–9.9 g/dL) and severe anaemia (<7 g/dL). A multiple logistic regression analysis was used to identify predictors of anaemia.
Results:
The mean age of the women was 28.4 ± 5.7 years (range from 18 to 41 years). Over half (52%) had secondary education, 65% were unmarried, 72% were unemployed, 34% were nulliparous, 15% were human immunodeficiency virus (HIV) infected and 67% were in the third trimester. The anaemia prevalence was 18.0% and was significantly associated with parity, HIV status and body mass index (BMI) in a multivariate logistic regression analysis.
Conclusion:
This study found that less than one-third of pregnant women were affected by anaemia, associated with parity, HIV infected and BMI. It is essential to promote routine screening for anaemia, health education and prompt treatment of infections to reduce this burden. In addition, further studies on risk factors for anaemia during pregnancy in both urban and rural communities should be conducted to strengthen these findings.
Keywords: Anaemia, Limpopo Province, pregnant women, risk factors
Introduction
Anaemia in pregnancy continues to be a public health concern that significantly contributes to maternal and foetal consequences. In 2011, approximately 32 million pregnant women were found to be affected by anaemia worldwide, of which the highest prevalence was reported in South Asia and Central and West Africa.[1] Although low haemoglobin (Hb) is used to diagnose anaemia, the definition recommended by different organisations varies considerably. The World Health Organization (WHO) defines anaemia as a Hb concentration of <11 g/dL,[2] while the Centers for Disease Control and Prevention (CDC) defines anaemia as Hb <11 g/dL in the first trimester and <10 g/dL in the second or third trimester.[3] The WHO further classifies anaemia in pregnancy according to its severity: mild (10.0–10.9 g/dL), moderate (7–9.9 g/dL) and severe (<7 g/dL).[4] Although this has been challenged, the WHO guideline is still the most commonly used cut-off for defining pregnancy anaemia.[2]
The risk of moderate-to-severe anaemia rises as pregnancy progresses[3,5,6,7,8,9,10] and contributes to obstetric haemorrhage, caesarean sections and need for postpartum blood transfusion, preeclampsia, intensive care admission and prolonged hospital stay.[11,12,13] Other adverse outcomes from pregnancy anaemia include low birth weight, preterm delivery, small for gestational age, neonatal intensive care admission, stillbirth and early neonatal death.[14,15] Various causes of anaemia during pregnancy include nutritional deficiencies (e.g., vitamin B12, folic acid and iron) and acute and chronic maternal infections such as malaria and human immunodeficiency virus (HIV).[16,17] Dietary iron deficiency is the most common etiological factor affecting many pregnant women.[16,17,18]
In South Africa (SA), routine screening of all pregnant women is standard practice. Primary healthcare nurses and medical practitioners are the first contacts for pregnant women to receive maternal care, playing a significant role in identifying and treating anaemia. Still, pregnancy anaemia is associated with 40% of maternal mortality,[19] irrespective of the routine provision of primary care throughout pregnancy.[20] Despite its known effect on pregnancy, there is a paucity of published studies about the prevalence and risk factors that influence anaemia among pregnant women in Limpopo Province (LP). Thus, the contributing risk factors are identified and addressed to develop effective interventions to combat maternal pregnancy anaemia. Therefore, this study was undertaken to determine the prevalence and identify the risk factors associated with anaemia in pregnancy in a district hospital and its feeder community health centre in LP.
Materials and Methods
Study design and setting
A cross-sectional descriptive study was undertaken in Seshego district hospital and its feeder community health centre for two months, from May 01 to June 30, 2019. The institutions are in the Polokwane Municipality of the Capricorn District of LP, SA. The hospital is a 180-bed hospital with only 36 beds allocated for maternity, and on average, 350 pregnant women are seen per month.
Sample size and sampling technique
The minimum sample size of 199 was calculated using the Cochran (1963)[21] single population proportion formula, based on the estimated anaemia prevalence of 36% in LP[20] with a 95% confidence interval, sampling error of 7% and 10% non-response rate. A consecutive sample of pregnant women aged ≥18 who reported to the two healthcare facilities during data collection was asked to participate in the study.
Data collection
A self-administered questionnaire was used for this study. The participants completed the first part of the questionnaire, which included socio-demographic data such as maternal age, place of residence, level of education, marital status and parity, occupation of the women, alcohol intake, smoking status, ferrous sulphate and folic acid given. The second part of the data collection tool was the clinical and anthropometric data related to gestational age, Hb concentration, HIV status, height and weight extracted from the pregnant women's medical records. The Hb level was determined using a B-Hemoglobin system.[22]
After the participants completed the first part of the questionnaire, the research assistant extracted the clinical and anthropometric data from the women's medical records with the midwives' assistance on duty. Body mass index (BMI) was calculated as (weight (kg)/height (m2); it was recorded as a continuous variable and was available in the women's medical record. For this study, BMI was categorised into four groups: underweight (BMI: <18.5), normal weight (BMI: 18.5–24.5), overweight (BMI: 25–29.9) and obese (BMI ≥30).[23] As study criteria, we used the WHO definition of Hb concentration of <11 g/dL and defined severe anaemia as mild (10.0–10.9 g/dL), moderate (7–9.9 g/dL) and severe (<7 g/dL).[2]
Data analysis
Data were captured and analysed using Microsoft Excel and Statistical software (Stata 9.0, StataCorp, College Station, Texas, United States of America (USA)), respectively. Logistic regression analysis was used to assess risk factors associated with pregnancy anaemia. In the multivariable logistic regression analysis, the researcher incorporated all variables significant at P < 0.20 in the univariate model. The cut-off value of less than 0.20 is supported by the literature.[24,25] Maternal age was added to the multivariate model, irrespective of P > 0.2, because of its clinical significance.[20] The Hosmer–Lemeshow goodness-of-fit test was used to assess how well the data fit in the final multivariable model and was found not to violate good fit (P > 0.05).[26]
Ethical considerations
The researchers obtained ethics approval for the study from the Pietersburg/Mankweng Research Ethics Committee (Ref.: PMREC03UL2019B). The participants completed informed consent before participating in the study.
Results
Socio-demographic characteristics of pregnant women
Two hundred and eleven pregnant women participated in this study. Their mean age was 28.4 ± 5.7 years, ranging from 18 to 41 years. Slightly more than half (56%) of the participants were aged <30 years [Figure 1]. Fifty-two per cent of pregnant women had secondary education, 65% were unmarried, 72% were unemployed, 34% were nulliparous, 67% were in the third trimester and 15% were HIV infected.
Figure 1.
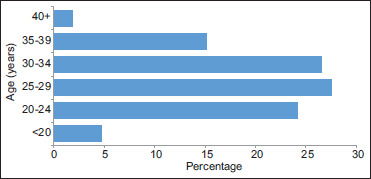
Age distribution of the respondents
About 90% and 82% of the women received folic acid and ferrous sulphate supplementation during the current pregnancy. Nearly all (>95%) pregnant women were non-smokers and did not drink alcohol. Forty-eight per cent of the participants were categorised as obese, 33% were overweight and 19% were normal weight.
Prevalence and risk factors for anaemia among pregnant women
The mean ± standard deviation (SD) Hb level concentration was 12.1 g/dl (±1.5). The prevalence of anaemia in pregnancy was 18.0% (n = 38), of which 58% had mild, 37% moderate and 5% severe anaemia [Figure 2].
Figure 2.
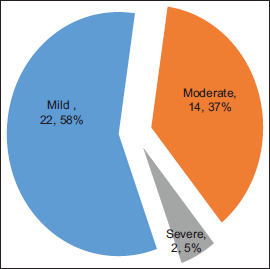
Severity of pregnancy anaemia
As displayed in Table 1, risk factors associated with anaemia in the univariate model were level of education, parity, HIV status and BMI. Maternal age was not significantly associated with anaemia in pregnancy (P > 0.2), but it was included in the multivariate model because of its clinical influence on anaemia. Women aged 25–29 had 68% fewer odds of anaemia than those aged <20.
Table 1.
Risk factors associated with anaemia among pregnant women
| Anaemic | Nonanaemic | Univariate logistic regression |
Multivariate logistic regression |
|||
|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | P | OR (95% CI) | P | |
| Age (yr) | ||||||
| <20 | 3 (30) | 7 (70) | Ref | Ref | ||
| 20–24 | 11 (22) | 40 (78) | 0.64 (0.14;2.89) | 0.564 | 0.67 (0.13;3.39) | 0.624 |
| 25–29 | 7 (12) | 51 (88) | 0.32 (0.07;1.53) | 0.154 | 0.25 (0.04;1.43) | 0.119 |
| 30–34 | 10 (18) | 46 (82) | 0.51 (0.11;2.31) | 0.380 | 0.26 (0.04;1.59) | 0.146 |
| 35+ | 7 (19) | 29 (81) | 0.56 (0.12;2.74) | 0.478 | 0.36 (0.05;2.43) | 0.292 |
| Level of education | ||||||
| Tertiary | 12 (13) | 84 (87) | Ref | |||
| Secondary | 24 (22) | 85 (78) | 1.98 (0.93;4.21) | 0.077 | ||
| None/primary | 2 (33) | 4 (67) | 3.50 (0.58;21.22) | 0.173 | ||
| Marital status | ||||||
| Married/cohabiting | 13 (17) | 61 (83) | Ref | |||
| Unmarried | 25 (18) | 112 (82) | 1.05 (0.50;2.19) | 0.902 | ||
| Employment status | ||||||
| Employed | 10 (17) | 49 (83) | Ref | |||
| Unemployed | 28 (18) | 124 (82) | 1.11 (0.50;2.45) | 0.803 | ||
| Parity | ||||||
| Nulliparous | 9 (13) | 62 (87) | Ref | Ref | ||
| Primiparous | 13 (17) | 64 (83) | 1.39 (0.56;3.51) | 0.474 | 2.18 (0.72;6.58) | 0.167 |
| Multiparous | 16 (25) | 47 (75) | 2.92 (1.14;7.47) | 0.025 | 4.77 (1.40;16.21) | 0.012 |
| Gestational age | ||||||
| 1st trimester | 1 (20) | 4 (80) | Ref | Ref | ||
| 2nd trimester | 7 (11) | 57 (89) | 0.49 (0.05;5.03) | 0.549 | ||
| 3rd trimester | 30 (21) | 112 (79) | 1.07 (0.12;9.95) | 0.952 | ||
| HIV status | ||||||
| Negative | 27 (15) | 152 (85) | Ref | Ref | ||
| Positive | 11 (34) | 21 (66) | 2.95 (1.28;6.81) | 0.011 | 2.62 (1.03;6.64) | 0.043 |
| Body mass index | ||||||
| Normal | 13 (32) | 28 (68) | Ref | Ref | ||
| Overweight | 11 (16) | 58 (84) | 0.41 (0.16;1.03) | 0.057 | 0.40 (0.15;1.10) | 0.078 |
| Obese | 14 (14) | 87 (86) | 0.35 (0.15;0.82) | 0.017 | 0.29 (0.11;0.76) | 0.013 |
OR: Odds ratio, CI: Confidence interval
Pregnant women with secondary education [OR = 1.98 (95% CI: 0.93–0.421), P < 0.20] and primary education [OR = 3.50 (95% CI: 0.58–0.21.22), P < 0.20] were more likely to be anaemic compared with those with tertiary education. Pregnant women with multiparous had three times higher odds of being anaemic [OR = 2.92 (95% CI: 1.14; 7.47), P < 0.20]. Participants who were HIV positive were also three times more likely to be anaemic than HIV-negative women [OR = 2.95 (95% CI: 1.28; 6.81), P < 0.20]. Compared with normal-weight women, overweight and obese women had 59% and 65% significantly fewer odds of having anaemia (P < 0.2).
In the multivariate model, the finding revealed that multiparous [OR = 4.77 (95% CI: 1.40; 16.21), P < 0.05] and HIV-positive [OR = 2.62 (95% CI: 1.03; 6.64), P < 0.05] pregnant women were more likely to be anaemic compared with their counterpart. Obese women had 71% significantly fewer odds of having anaemia than the other groups. Regarding the participants' age, although the results were not statistically significant, women in the age group of 25–29 years and 30–34 years had 75% and 74% fewer odds of anaemia than others, respectively.
Discussion
From our study, the prevalence of pregnancy anaemia was 18.0%, comparable to the prevalence rate of 18.0% reported in Tanzania.[9] 25.8% in Uganda[10] and 26.2% in Kenya.[27] However, lower than the rates of 37.51% found in Ethiopia[5], 72.6% in Nigeria[4], 42.7%[28] and 50.8%[8] in Ghana, and 43.1% in Eswatin.[6] Our finding is also lower than the rate of 37% reported in earlier studies in the KwaZulu Natal Province of SA.[7] The possible reason for this variation could be that countries differ in their screening policies, criteria and ethnic differences. Furthermore, it could be different lifestyles and health-seeking behaviours across different countries. In our study, less than half (42%) of the anaemic pregnant women were classified as either moderate or severe anaemia. This result is comparable to a study in Nigeria,[3] but slightly higher than the rates in many studies.[5,6,7,8] However, our finding is lower than the rate reported by others.[9,10] There is a concern with 5% of pregnant women with severe anaemia, which need urgent attention. Moreover, those with mild and moderate anaemia could progress into severe anaemia; therefore, they should be treated as an obstetric emergency.
Regarding socio-demographics, the findings of previous studies revealed that anaemia in pregnancy rises as maternal age advances.[8] In contrast, other studies found that anaemia is prevalent among those in the age group less than 25 years.[5,6,7,8,9,10] Similarly, our finding indicates that pregnant women aged <25 years are at higher risk of developing anaemia in pregnancy, but the result was not statistically significant. Poor knowledge of anaemia in pregnancy is a challenge.[29] Therefore, healthcare professionals should educate pregnant women, especially young people, about the importance of antenatal care.
Previous studies observed no association between level of education and pregnancy anaemia, with illiterate pregnant women found to be anaemic.[6,8,10] Another study revealed that pregnant women's anaemia status was significantly associated with higher education.[5] In univariate analysis, our study finding shows that anaemia is prevalent among women without formal, primary and secondary education. The possible reason could be that less-educated women are likely unemployed, cannot afford to eat nourishing food and enrol early for antenatal care. Furthermore, given that almost all (>80%) women in our study said that they received folic acid and ferrous sulphate supplements, there is a possibility that many were not adherent to treatment. Therefore, healthcare professionals should ensure that pregnant women know about the importance of adherence to nutritional supplements.[30] In addition, this study recommends the involvement of family members to support supplement adherence and dietary changes.[31]
In our study, the HIV prevalence was 15% lower than the provincial rates ranging between 20% and 22% reported in LP and the national antenatal HIV prevalence of 30.7% in RSA.[32] The reason for the lower rate observed in our study is unclear; however, it could be due to its methodological limitations. Studies have shown no association between HIV infection and anaemia in pregnancy.[8,9] Others found that HIV-infected pregnant women were at higher risk of developing anaemia than HIV-uninfected women.[6,33,34] This finding is consistent with the result of our study, which found that a higher proportion of HIV-infected pregnant women were anaemic than uninfected, and the HIV-infected women were three times more likely to develop pregnancy anaemia than HIV uninfected. The increased risk of anaemia among HIV-infected pregnant women could be attributed to lower serum folate, vitamin B and ferritin in pregnancy. Although our study did not assess whether HIV-infected women were on antiretroviral drugs (ARVs), studies have shown that ARVs, mainly zidovudine use, increase the risk of development of anaemia,[35] while another study found no association.[36] Thus, medical practitioners must determine whether HIV-infected pregnant women receive HIV treatment and whether the therapy impacts anaemia.
Consistent with previous studies,[8,10] our findings revealed that marital status and employment status were not associated with pregnancy anaemia. This study found that unmarried and unemployed pregnant women were 1.1 times more likely to be anaemic, but the results were not statistically significant in a univariate analysis. This finding could be because those pregnant women who are unemployed usually tend to have more critical financial difficulties, which could cause a higher prevalence rate of anaemia in those unemployed. There is an association between parity and anaemia in pregnancy, with prime gravida pregnant women significantly more likely to be anaemic.[10] Other studies found no significant association between parity and pregnancy anaemia.[8] Our study established that multiparous pregnant women were five times more likely to develop anaemia in the multivariate analysis. This finding is similar to other studies,[27] which found that multiparous women are more likely to develop anaemia—because with increasing parity, there is limited time for women to recover from previous pregnancy-related anaemia between successive pregnancies.[27]
In the present study, the risk of pregnant women developing anaemia in pregnancy is lower among overweight or obese women than among women with normal BMI. Consistent with this finding, previous studies showed a similar result of overweight or obese women having a lower risk of developing pregnancy anaemia than women with normal BMI.[14,27] Studies have reported mixed results, which showed that anaemia is more prevalent in the first,[8,16] second[37] and third trimesters[6] of pregnancy. Our finding revealed that more women in the first and third trimesters were anaemic than in the second trimester. Furthermore, those in the third trimester were 1.1 more likely to be developed anaemia than the first-trimester women in the bivariate logistic model, but the result was not statistically significant.
Study limitations
It should be noted that our study was conducted in a district hospital and its feeder community health centre that mainly serve urban communities; therefore, the results cannot be extrapolated to the rural population. It is also important to note that this study was cross-sectional, which limits causality measures.
Conclusion
The anaemia prevalence in pregnancy in our study is comparable in some studies but lower in others and is associated with being HIV infected, primiparous/multiparous and overweight/obese. Given the risk factors for anaemia in this study, it is essential to perform routine screening for anaemia, promote health education and prompt treatment of HIV infections, which can reduce this burden. In addition, further study of risk factors for anaemia during pregnancy, including urban and rural communities, should be carried out to strengthen these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: A systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–25. doi: 10.1016/S2214-109X(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva, Switzerland: 2015. The Global Prevalence of Anaemia in 2011. [Google Scholar]
- 3.Ekpe AC, Adefemi SA, Pemi MD. Anaemia among pregnant women: Prevalence and pattern at booking clinic of a tertiary health care facility in North Central Nigeria. West Afr J Med. 2022;39:228–36. [PubMed] [Google Scholar]
- 4.CDC CDC criteria for anaemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep. 1989;138:400–4. [PubMed] [Google Scholar]
- 5.Animut K, Berhanu G. Determinants of anemia status among pregnant women in ethiopia: Using 2016 ethiopian demographic and health survey data; application of ordinal logistic regression models. BMC Pregnancy Childbirth. 2022;22:663.. doi: 10.1186/s12884-022-04990-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodzo RC, Ogunsakin RE, Ginindza TG. Prevalence and associated risk factors for anaemia amongst pregnant women attending three antenatal clinics in Eswatini. Afr J Prim Health Care Fam Med. 2022;14:e1–9. doi: 10.4102/phcfm.v14i1.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoque AM, Hoque ME, Van Hal G. Progression of anaemia during antenatal period among South African pregnant women. Afr Health Sci. 2022;22:81–92. doi: 10.4314/ahs.v22i3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wemakor A. Prevalence and determinants of anaemia in pregnant women receiving antenatal care at a tertiary referral hospital in Northern Ghana. BMC Pregnancy Childbirth. 2019;19:495.. doi: 10.1186/s12884-019-2644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephen G, Mgongo M, Hussein Hashim T, Katanga J, Stray-Pedersen B, Msuya SE. Anaemia in pregnancy: Prevalence, risk factors, and adverse perinatal outcomes in Northern Tanzania. Anaemia. 2018;2018:1846280.. doi: 10.1155/2018/1846280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahamoud NK, Mwambi B, Oyet C, Segujja F, Webbo F, Okiria JC, et al. Prevalence of anemia and its associated socio-demographic factors among pregnant women attending an antenatal care clinic at Kisugu Health Center IV, Makindye Division, Kampala, Uganda. J Blood Med. 2020;11:13–8. doi: 10.2147/JBM.S231262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young MF, Oaks BM, Tandon S, Martorell R, Dewey KG, Wendt AS. Maternal haemoglobin concentrations across pregnancy and maternal and child health: A systematic review and meta-analysis. Ann N Y Acad Sci. 2019;1450:47–68. doi: 10.1111/nyas.14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parks S, Hoffman MK, Goudar SS, Patel A, Saleem S, Ali SA, et al. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. 2019;126:737–43. doi: 10.1111/1471-0528.15585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith C, Teng F, Branch E, Chu S, Joseph KS. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet Gynecol. 2019;134:1234–44. doi: 10.1097/AOG.0000000000003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin L, Wei Y, Zhu W, Wang C, Su R, Feng H, et al. Gestational diabetes mellitus Prevalence Survey (GPS) Study Group. Prevalence, risk factors and associated adverse pregnancy outcomes of anaemia in Chinese pregnant women: A multicentre retrospective study. BMC Pregnancy Childbirth. 2018;18:111.. doi: 10.1186/s12884-018-1739-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oskovi-Kaplan ZA, Kilickiran H, Buyuk GN, Ozyer S, Keskin HL, Engin-Ustun Y. Comparison of the maternal and neonatal outcomes of pregnant women whose anaemia was not corrected before delivery and pregnant women who were treated with intravenous iron in the third trimester. Arch Gynecol Obstet. 2021;303:715–9. doi: 10.1007/s00404-020-05817-7. [DOI] [PubMed] [Google Scholar]
- 16.Mahmood T, Rehman AU, Tserenpil G, Siddiqui F, Ahmed M, Siraj F, et al. The association between iron-deficiency anemia and adverse pregnancy outcomes: A retrospective report from Pakistan. Cureus. 2019;11:e5854.. doi: 10.7759/cureus.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khuu G, Dika C. Iron deficiency anaemia in pregnant women. Nurse Pract. 2017;42:42–7. doi: 10.1097/01.NPR.0000516124.22868.08. [DOI] [PubMed] [Google Scholar]
- 18.Loy SL, Lim LM, Chan SY, Tan PT, Chee YL, Quah PL, et al. Iron status and risk factors of iron deficiency among pregnant women in Singapore: A cross-sectional study. BMC Public Health. 2019;19:397.. doi: 10.1186/s12889-019-6736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Confidential Enquiries into Maternal Deaths . Pretoria: Department of Health; 2013. Saving Mothers 2010-2013: Fourth Report of Confidential Enquiries into Maternal Deaths in South Africa. [Google Scholar]
- 20.Bopape MM, Mbhenyane XG, Alberts M. The prevalence of anaemia and selected micronutrient status in pregnant teenagers of Polokwane municipality in the Limpopo Province. S Afr J Clin Nutri. 2008;21:332–6. [Google Scholar]
- 21.Cochran WG. 2nd. New York: John Wiley and Sons, Inc.; 1963. Sampling Techniques. [Google Scholar]
- 22.Muñoz M, Romero A, Gómez JF, Manteca A, Naveira E, Ramírez G. Utility of point-of-care haemoglobin measurement in the HemoCue-B haemoglobin for the initial diagnosis of anaemia. Clin Lab Haematol. 2005;27:99–104. doi: 10.1111/j.1365-2257.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization (WHO) Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 24.Zhang Z. Model building strategy for logistic regression: Purposeful selection. Ann Transl Med. 2016;4:111.. doi: 10.21037/atm.2016.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperandei S. Understanding logistic regression analysis. Biochem Med (Zagreb) 2014;24:12–8. doi: 10.11613/BM.2014.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosmer DW, Hosmer T, Le Cessie S, Lemeshow S. A comparison of goodness-of-fit tests for the logistic regression model. Stat Med. 1997;16:965–80. doi: 10.1002/(sici)1097-0258(19970515)16:9<965::aid-sim509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Odhiambo JN, Sartorius B. Mapping of anaemia prevalence among pregnant women in Kenya (2016-2019) BMC Pregnancy Childbirth. 2020;20:711.. doi: 10.1186/s12884-020-03380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nonterah EA, Adomolga E, Yidana A, Kagura J, Agorinya I, Ayamba EY, et al. Descriptive epidemiology of anaemia among pregnant women initiating antenatal care in rural Northern Ghana. Afr J Prim Health Care Fam Med. 2019;11:e1–7. doi: 10.4102/phcfm.v11i1.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oyewole Oyerinde O, Nkanga EA, Oyerinde IE, Akintoye O, Asekun-Olarinmoye I, Alabi QK. Factors affecting anemia in pregnancy women in Ibeju-Lekki, Lagos State, Nigeria. Inquiry. 2023;60:469580231159961.. doi: 10.1177/00469580231159961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Silva Lopes K, Yamaji N, Rahman MO, Suto M, Takemoto Y, Garcia-Casal MN, et al. Nutrition-specific interventions for preventing and controlling anaemia throughout the life cycle: An overview of systematic reviews. Cochrane Database Syst Rev. 2021;9:CD013092. doi: 10.1002/14651858.CD013092.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams PA, Poehlman J, Moran K, Siddiqui M, Kataria I, Rego AM, et al. Strategies to address anaemia among pregnant and lactating women in India: A formative research study. Public Health Nutr. 2020;23:795–805. doi: 10.1017/S1368980019003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woldesenbet SA, Kufa T, Lombard C, Manda S, Ayalew K, Cheyip M, et al. The 2017 national antenatal sentinel HIV survey key findings, South Africa. [Last accessed on 2021 Oct 14];2019 Available from: www.nicd.ac.za/wp-content/uploads/2019/07/Antenatal_survey-report . [Google Scholar]
- 33.Dorsamy V, Bagwandeen C, Moodley J. The prevalence, risk factors and outcomes of anaemia in South African pregnant women: A systematic review and meta-analysis. Syst Rev. 2022;11:16.. doi: 10.1186/s13643-022-01884-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mugo C, Nduati R, Osoro E, Nyawanda BO, Mirieri H, Hunsperger E, et al. Comparable pregnancy outcomes for HIV-uninfected and HIV-infected women on antiretroviral treatment in Kenya. J Infect Dis. 2022;226:678–86. doi: 10.1093/infdis/jiac128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamir Z, Alemu J, Tsegaye A. Anemia among HIV infected individuals taking ART with and without Zidovudine at Addis Ababa, Ethiopia. Ethiop J Health Sci. 2018;28:73–82. doi: 10.4314/ejhs.v28i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nandlal V, Moodley D, Grobler A, Bagratee J, Maharaj NR, Richardson P. Anaemia in pregnancy is associated with advanced HIV disease. PLoS One. 2014;9:e106103.. doi: 10.1371/journal.pone.0106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emeribe AU, Dangana A, Isa HA, Onoja SO, Otu TO, Ibrahim Y, et al. Comparative analysis of the nutritional, biochemical and hematological parameters of pregnant women attending the University of Abuja Teaching Hospital, Nigeria. Biomedicine (Taipei) 2022;12:1–13. doi: 10.37796/2211-8039.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]


