Abstract
Background
Etanercept is a soluble tumour necrosis factor alpha‐receptor disease‐modifying anti‐rheumatic drug (DMARD) for the treatment of rheumatoid arthritis (RA).
Objectives
The purpose of this review was to update the previous Cochrane systematic review published in 2003 assessing the benefits and harms of etanercept for the treatment of RA. In addition, we also evaluated the benefits and harms of etanercept plus DMARD compared with DMARD monotherapy in those people with RA who are partial responders to methotrexate (MTX) or any other traditional DMARD.
Search methods
Five electronic databases were searched from 1966 to February 2003 with no language restriction. The search was updated to January 2012. Attempts were made to identify other studies by contact with experts, searching reference lists and searching trial registers.
Selection criteria
All controlled trials (minimum 24 weeks' duration) comparing four possible combinations: 1) etanercept (10 mg or 25 mg twice weekly) plus a traditional DMARD (either MTX or sulphasalazine) versus a DMARD, 2) etanercept plus DMARD versus etanercept alone, 3) etanercept alone versus a DMARD or 4) etanercept versus placebo.
Data collection and analysis
Two review authors independently extracted data and assessed the risk of bias of the trials.
Main results
Three trials were included in the original version of the review. An additional six trials, giving a total of 2842 participants, were added to the 2012 update of the review. The trials were generally of moderate to low risk of bias, the majority funded by pharmaceutical companies. Follow‐up ranged from six months to 36 months.
Benefit
At six to 36 months the American College of Rheumatology (ACR) 50 response rate was statistically significantly improved with etanercept plus DMARD treatment when compared with a DMARD in those people who had an inadequate response to any traditional DMARD (risk ratio (RR) 2.0; 95% confidence interval (CI) 1.3 to 2.9, absolute treatment benefit (ATB) 38%; 95% CI 13% to 59%) and in those people who were partial responders to MTX (RR 11.7; 95% CI 1.7 to 82.5, ATB 36%). Similar results were observed when pooling data from all participants (responders or not) (ACR 50 response rates at 24 months: RR 1.9; 95% CI 1.3 to 2.8, ATB 29%; 36 months: RR 1.6; 95% CI 1.3 to 1.9, ATB 24%). Statistically significant improvement in physical function and a higher proportion of disease remission were observed in combination‐treated participants compared with DMARDs alone ((mean difference (MD) ‐0.36; 95% CI ‐0.43 to ‐0.28 in a 0‐3 scale) and (RR 1.92; 95% CI 1.60 to 2.31), respectively) in those people who had an inadequate response to any traditional DMARD. All changes in radiographic scores were statistically significantly less with combination treatment (etanercept plus DMARD) compared with MTX alone for all participants (responders or not) (Total Sharp Score (TSS) (scale = 0 to 448): MD ‐2.2, 95% CI ‐3.0 to ‐1.4; Erosion Score (ES) (scale = 0 to 280): MD ‐1.6; 95% CI ‐2.4 to ‐0.9; Joint Space Narrowing Score (JSNS) (scale = 0 to 168): MD ‐0.7; 95% CI ‐1.1 to ‐0.2), and with combination treatment compared with etanercept alone (TSS: MD ‐1.1; 95% CI ‐1.8 to ‐0.5; ES: MD ‐0.7; 95% CI ‐1.1 to ‐0.2; JSNS: MD ‐0.5, 95% CI ‐0.7 to ‐0.2). The estimate of irreversible physical disability over 10 years given the radiographic findings was 0.45 out of 3.0.
When etanercept monotherapy was compared with DMARD monotherapy, there was generally no evidence of a difference in ACR50 response rates when etanercept 10 mg or 25 mg was used; at six months etanercept 25 mg was significantly more likely to achieve ACR50 than DMARD monotherapy but this difference was not found at 12, 24 or 36 months. TSS and ES radiographic scores were statistically significantly improved with etanercept 25 mg monotherapy compared with DMARD (TSS: MD ‐0.7; 95% CI ‐1.4 to 0.1; ES: MD ‐0.7; 95% CI ‐1.0 to ‐0.3) but there was no evidence of a statistically significant difference between etanercept 10 mg monotherapy and MTX.
Harms
There was no evidence of statistically significant differences in infections or serious infections between etanercept plus DMARD and DMARD alone at any point in time. Infection rates were higher in people receiving etanercept monotherapy compared with DMARD; however, there were no differences regarding serious infections. For those participants who had an inadequate response to DMARDs, the rate of total withdrawals was lower for the etanercept plus DMARD group compared with DMARD alone (RR 0.53; 95% CI 0.36 to 0.77, ATB 18%). No other statistically significant differences were observed in any of the assessed comparisons.
Authors' conclusions
Etanercept 25 mg administered subcutaneously twice weekly together with MTX was more efficacious than either etanercept or MTX monotherapy for ACR50 and it slowed joint radiographic progression after up to three years of treatment for all participants (responders or not). There was no evidence of a difference in the rates of infections between groups.
Plain language summary
Etanercept for the treatment of rheumatoid arthritis
Researchers in The Cochrane Collaboration conducted a review of the effect of etanercept (Enbrel) for people with rheumatoid arthritis. After searching for all relevant studies, they found nine studies with over 2800 people. Their findings are summarised below.
In people with rheumatoid arthritis who have NOT improved with a traditional disease‐modifying anti‐rheumatic drug (DMARD):
‐ Etanercept plus DMARDs probably improves pain, function and other symptoms of rheumatoid arthritis;
‐ Etanercept plus DMARDs reduces disease activity and disability;
‐ Showing a small difference needs a larger number of participants – when all the data from the different types of participants are combined, etanercept reduces permanent joint damage as seen on x‐ray.
We often do not have precise information about side effects and complications. This is particularly true for rare, but serious, side effects. Side effects such as injection site reactions, headache, common colds, nausea, dizziness and infections may occur with etanercept on its own or combined with a DMARD. Another Cochrane Review (Singh 2011) has shown that there is a small risk of tuberculosis reactivation and serious infections. Rare complications may include certain types of cancer.
What is etanercept and why is it prescribed? When you have rheumatoid arthritis, your immune system, which normally fights infection, attacks the lining of your joints. This makes your joints swollen, stiff and painful. The small joints of your hands and feet are usually affected first. Etanercept is a "biologic" that is prescribed to decrease pain and swelling and slow the progress of rheumatoid arthritis. A biologic is a medical product not chemically synthesized, that is derived from living material. This medical product is injected beneath the skin in the same way as insulin in treating diabetes. It is usually prescribed when other DMARDs do not work well, but it can be expensive.
What happens to people with rheumatoid arthritis who take etanercept plus traditional DMARDs (methotrexate or sulphasalazine) after they have NOT improved with traditional DMARDs alone
ACR 50 (number of tender or swollen joints and other outcomes such as pain and disability)
38 more people out of 100 had a 50% improvement in symptoms after six months to three years compared with people taking a DMARD alone (38% absolute improvement). 79 people out of 100 on etanercept plus DMARDs had a 50% improvement in symptoms. 41 people out of 100 on DMARDs alone had a 50% improvement in symptoms
Disease activity
22 more people out of 100 were considered to have low disease activity of their rheumatoid arthritis from six months to three years on etanercept with DMARDs (22% absolute improvement). 46 people out of 100 on etanercept plus DMARDs were considered to have low disease activity of their rheumatoid arthritis. 24 people out of 100 on DMARDs alone were considered to have low disease activity of their rheumatoid arthritis.
Disability
People who took etanercept plus a DMARD rated the change in their disability to be 0.36 points lower on a scale of 0 to 3 after six months to three years compared with people who took a DMARD alone (12% absolute improvement). People who took etanercept plus a DMARD rated the change in their disability to be between 0.51 and 1.08 on a scale of 0 to 3 after six months to three years. People who took a DMARD alone rated the change in their disability to be between 0.15 and 0.72 on a scale of 0 to 3 after six months to three years.
X‐rays of the joints
When all people in all the studies were considered, joint damage improved slightly in those who received combined treatment with etanercept plus DMARD compared with DMARD or etanercept alone after 12 to 36 months. Joint damage in people whom DMARDs were not working and received combined treatment with etanercept plus DMARD was similar to those given a DMARD alone, but this result might be due to low numbers of people in this group.
Summary of findings
Summary of findings for the main comparison. Inadequate responders to traditional disease‐modifying anti‐rheumatic drugs (DMARDs).
| Etanercept25 mg + DMARD for the treatment of rheumatoid arthritis | ||||||
| Patient or population: participants with rheumatoid arthritis and with a partial response to DMARDs Settings: international hospital or clinic settings Intervention: etanercept 25 mg + DMARD Comparison: DMARD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| DMARD | ET 25 mg + DMARD | |||||
| ACR50 American College of Rheumatology 50% improvement criteria Follow‐up: 24 to 156 weeks | 405 per 1000 | 793 per 1000 (538 to 1000) | RR 1.96 (1.33 to 2.89)1 | 1198 (4 studies) | ⊕⊕⊕⊝ moderate2 | Absolute treatment benefit 38% (95% CI 13% to 59%); Relative percent change 96% (95% CI 33% to 189%);
NNTB 3 (95% CI 2 to 8) Analysis 1.1 |
| Remission Achievement of disease activity score < 2.6 Follow‐up: 52 and 156 weeks | 236 per 1000 | 454 per 1000 (378 to 546) | RR 1.92 (1.6 to 2.31) | 987 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 22% (95% CI 17% to 27%); Relative percent change 122% (95% CI 50% to 229%); NNTB 5 (95% CI 4 to 8) Analysis 1.3 |
| Reduction in disability score Health Assessment Questionnaire. Scale from: 0 to 3 Follow‐up: mean 24 to 156 weeks | The mean reduction in disability score ranged across control groups from ‐0.15 to ‐0.72 | The mean reduction in disability score in the intervention groups was 0.36 lower (0.43 lower to 0.28 lower) | 1227 (4 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐12% (95% CI ‐16% to ‐2%); Relative percent change 57% (95% CI 5% to 76%); NNTB 5 (95% CI 4 to 6).3 Analysis 1.4 |
|
| Radiographic progression Total Sharp Score Scale from: 0 to 448 Follow‐up: 52 and 156 weeks | The mean radiographic progression ranged across control groups from 2.4 to 5.96 | The mean radiographic progression in the intervention groups was 3.83 lower (7.67 lower to 0.01 higher)1,7 | 903 (2 studies) | ⊕⊕⊕⊝ moderate2 | Statistically significant for all participants (MD ‐2.2; 95% CI ‐3.0 to ‐1.4); Absolute treatment benefit ‐0.49% (95% CI ‐0.67% to ‐0.32%); Relative percent change ‐92% (95% CI ‐125% to ‐59.6%); NNTB 6 (95% CI 5 to 9) Analysis 4.6 Smaller sample size in inadequate responders did not achieve statistical significance. However, the point estimate is very similar (MD ‐3.83; 95% CI ‐7.67 to 0.01); Absolute treatment benefit ‐0.85% (95% CI ‐1.7% to 0.002%); Relative percent change ‐64% (95% CI ‐128.9% to 0.17%); NNTB N/A.3 Analysis 1.5 When we transform the x‐ray score to an estimate of irreversible physical disability, etanercept + DMARD treatment would prevent an increase in irreversible disability of 0.45 irreversible HAQ units over 10 years (15%) in addition to the reversible change noted in the disability row above |
|
|
Withdrawals due to adverse events Follow‐up: 24 to 156 weeks |
158 per 1000 |
118 per 1000 (90 to 158) |
RR 0.75 (0.57 to 1.0)1 | 1241 (4 studies) | ⊕⊕⊕⊝ moderate2 | Not statistically significant; Absolute risk difference ‐4% (95% CI ‐8% to 0%); Relative percent change ‐25% (95% CI ‐43% to 0%); NNTH N/A Analysis 1.7 |
| Serious adverse events Follow‐up: 24 to 156 weeks | 141 per 1000 | 176 per 1000 (104 to 297) | RR 1.25 (0.74 to 2.11)1 | 1241 (4 studies) | ⊕⊕⊕⊝ moderate2 | Not statistically significant; Absolute risk difference 5% (95% CI ‐4% to 13%); Relative percent change 25% (95% CI ‐26% to 111%); NNTH N/A Analysis 1.8 |
| Serious infections Follow‐up: 52 to 156 weeks | 49 per 1000 | 45 per 1000 (27 to 77) | RR 0.91 (0.54 to 1.55) | 1152 (3 studies) | ⊕⊕⊕⊕ high | Not statistically significant; Absolute risk difference ‐0.49% (95% CI ‐3% to 2%); Relative percent change ‐9% (95% CI ‐46% to 55%); NNTH N/A Analysis 1.9 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Random‐effects model was used. 2 Inconsistency across studies (I2 statistic) was greater than 50% which may represent moderate heterogeneity.
3Klareskog 2004 (TEMPO) was identified as the most representative study (to calculate baseline mean).
1.1. Analysis.
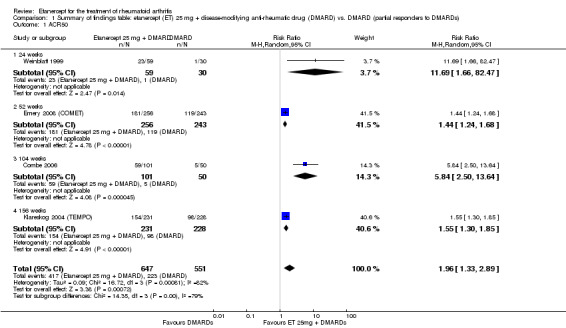
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 1 ACR50.
1.3. Analysis.
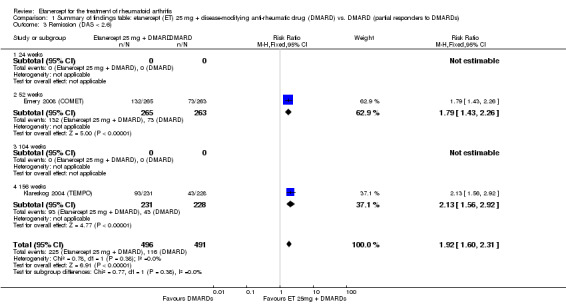
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 3 Remission (DAS < 2.6).
1.4. Analysis.
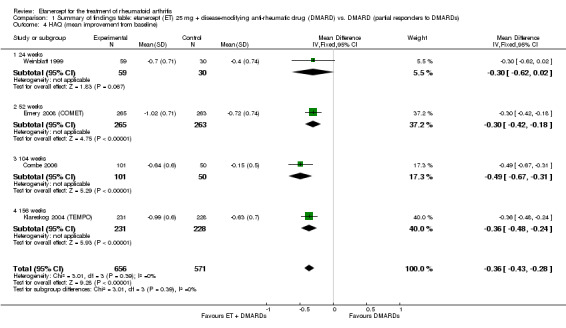
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 4 HAQ (mean improvement from baseline).
4.6. Analysis.
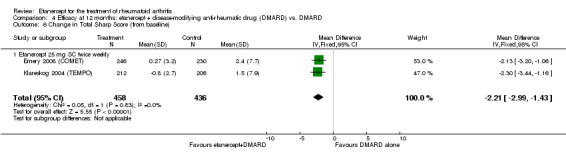
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 6 Change in Total Sharp Score (from baseline).
1.5. Analysis.
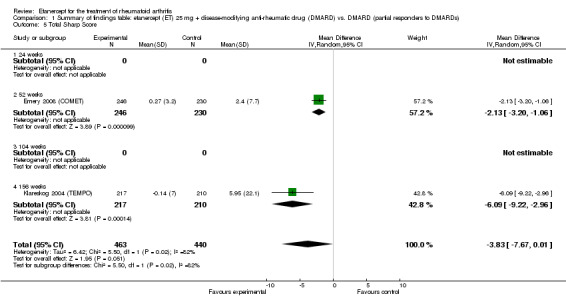
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 5 Total Sharp Score.
1.7. Analysis.
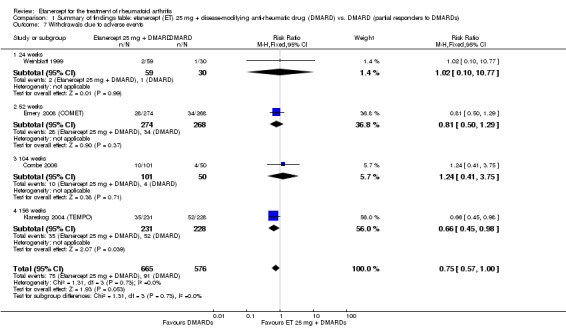
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 7 Withdrawals due to adverse events.
1.8. Analysis.
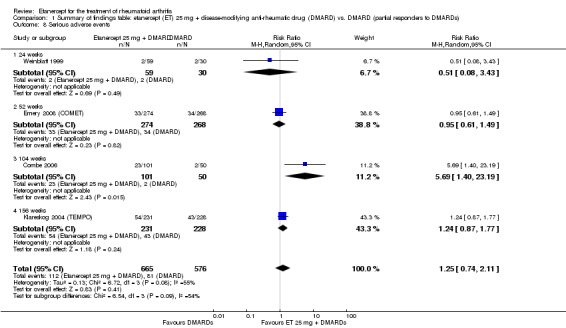
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 8 Serious adverse events.
1.9. Analysis.
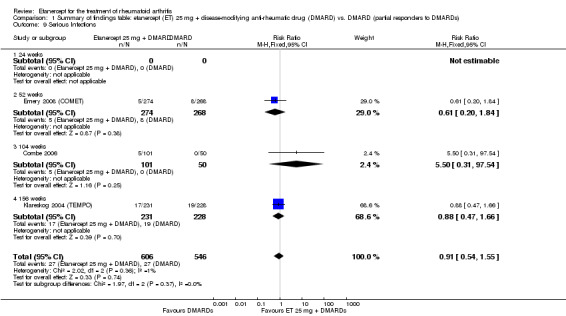
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 9 Serious Infections.
Summary of findings 2. Inadequate responders to methotrexate (MTX).
| Etanercept25 mg + DMARD for the treatment of rheumatoid arthritis | ||||||
| Patient or population: participants with rheumatoid arthritis and with partial response to MTX Settings: international hospital or clinic setting Intervention: etanercept 25 mg + DMARD (disease‐modifying anti‐rheumatic drug) Comparison: DMARD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| DMARD | ET 25 mg + DMARD | |||||
| ACR50 American College of Rheumatology improvement criteria Follow‐up: 24 weeks | 33 per 1000 | 390 per 1000 (55 to 1000) | RR 11.69 (1.66 to 82.47) | 89 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute treatment benefit 36% (95% CI 22% to 50%); Relative percent change 1069% (95% CI 66% to 8147%); NNTB 3 (95% CI 2 to 6) Analysis 2.1 |
| Remission Disease activity score < 2.6 | See comment | See comment | Not estimable | 0 (0) | See comment | Data not reported |
| Reduction in disability score Health Assessment Questionnaire (mean improvement from baseline). Scale from: 0 to 3 Follow‐up: 24 weeks | The mean reduction in disability score in the control groups was ‐0.4 | The mean reduction in disability score in the intervention groups was 0.3 lower (0.62 lower to 0.02 higher) | 89 (1 study) | ⊕⊕⊕⊝ moderate1 | Absolute treatment benefit ‐10% (95% CI ‐21% to 0.6%);
Relative percent change 75% (95% CI 5% to 155%)2 Analysis 2.3 |
|
| Radiographic progression Total Sharp Score Follow‐up: 24 weeks | See comment | See comment | Not estimable | 0 (0) | See comment | Data not reported |
| Withdrawals due to adverse events Follow‐up: 24 weeks | 33 per 1000 | 34 per 1000 (3 to 359) | RR 1.02 (0.1 to 10.77) | 89 (1 study) | ⊕⊕⊕⊝ moderate1 | Not statistically significant; Absolute risk difference 0.06% (95% CI ‐8% to 8%); Relative percent change 2% (95% CI ‐90% to 977%); NNTH N/A Analysis 2.5 |
| Serious adverse events Follow‐up: 24 weeks | 133 per 1000 | 33 per 1000 (7 to 175) | RR 0.25 (0.05 to 1.31) | 89 (1 study) | ⊕⊕⊕⊝ moderate1 | Not statistically significant;
Absolute treatment benefit ‐10% (95% CI ‐23% to 3%); Relative percent change ‐75% (95% CI ‐95% to 31%); NNTH N/A Analysis 2.6 |
| Serious infections Follow‐up: 24 weeks | See comment | See comment | Not estimable | 0 (0) | See comment | Data not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DMARD: disease‐modifying anti‐rheumatic drug; N/A: not available; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Evidence derived from 1 study only.
2Klareskog 2004 (TEMPO) was identified as the most representative study (to calculate baseline mean).
2.1. Analysis.
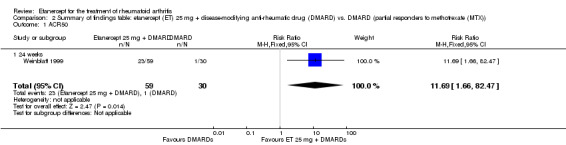
Comparison 2 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to methotrexate (MTX)), Outcome 1 ACR50.
2.3. Analysis.
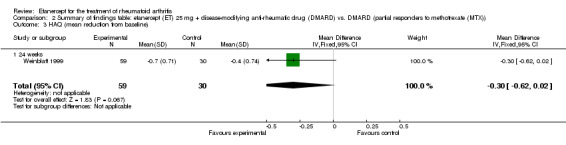
Comparison 2 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to methotrexate (MTX)), Outcome 3 HAQ (mean reduction from baseline).
2.5. Analysis.
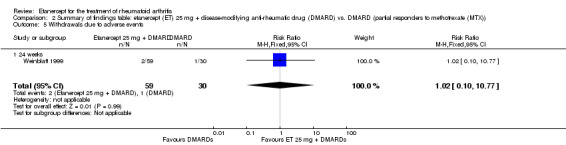
Comparison 2 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to methotrexate (MTX)), Outcome 5 Withdrawals due to adverse events.
2.6. Analysis.
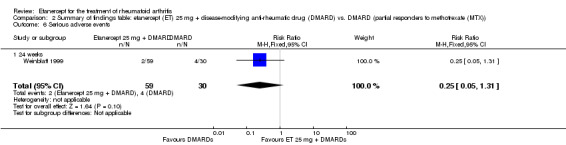
Comparison 2 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to methotrexate (MTX)), Outcome 6 Serious adverse events.
Summary of findings 3. ACR50, radiographic progression and serious infections.
| Etanercept for the treatment of rheumatoid arthritis | |||||||||
| Patient or population: participants with rheumatoid arthritis Intervention: etanercept | |||||||||
| Outcomes | Intervention | Comparison | Follow‐up | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||||
| Control | Etanercept | ||||||||
| ACR50 ‐ American College of Rheumatology Improvement Criteria | |||||||||
| Etanercept (25 mg) + DMARD | DMARD | 12 months | 461 per 1000 | 700 per 1000 (627 to 783) | RR 1.52 (1.36 to 1.7) | 958 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 24% (95% CI 18% to 30%); Relative percent change 52% (95% CI 36% to 70%); NNTB 5 (95% CI 4 to 7) |
|
| Etanercept (10 mg) | DMARD | 12 months | 392 per 1000 | 298 per 1000 (227 to 388) | RR 0.76 (0.58 to 0.99) | 423 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐10% (95% CI ‐19% to ‐1%; Relative percent change 24% (95% CI ‐42% TO ‐1%); NNTB not statistically significant |
|
| Etanercept (25 mg) + DMARD | Etanercept | 12 months | 480 per 1000 | 686 per 1000 (585 to 811) | RR 1.43 (1.22 to 1.69) | 454 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 21% (95% CI 12% to 30%); Relative percent change 43% (95% CI 22% to 69%); NNTB 5 (95% CI 4 to 10) |
|
| Etanercept (25 mg) | PBO | 6 months | 50 per 1000 | 398 per 1000 (147 to 1000) | RR 7.95 (2.94 to 21.47) | 158 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 35% (95% CI 23% to 47%); Relative percent change 695% (95% CI 194% to 2047%); NNTB 3 (95% CI 2 to 8) |
|
| Radiographic progression ‐ mean change in Total Sharp Score (from baseline). Scale from: 0 to 448 | |||||||||
| Etanercept (25 mg) + DMARD | DMARD | 12 months | 2.4 |
MD ‐2.21 (‐2.99 to ‐1.43) |
894 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.49% (95% CI ‐0.67% to ‐0.32%); Relative percent change ‐92% (95% CI ‐125% to ‐59.6%) |
||
| Serious infections | |||||||||
| Etanercept (25 mg) + DMARD | DMARD | 12 months | 30 per 1000 | 18 per 1000 (6 to 55) | RR 0.61 (0.2 to 1.84) | 542 (1 study) | ⊕⊕⊕⊕ high | Absolute risk difference ‐1% (95% CI ‐4% to 1%); Relative percent change ‐39% (95% CI ‐80% to 84%); NNTH not statistically significant. |
|
| Etanercept (25 mg) + DMARD | Etanercept | 2 years | 63 per 1000 | 57 per 1000 (27 to 117) | RR 0.9 (0.43 to 1.86) | 454 (1 study) | ⊕⊕⊕⊕ high | Absolute risk difference ‐1% (95% CI ‐5% to 4%); Relative percent change ‐10% (95% CI ‐57% to 86%); NNH not statistically significant |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DMARD: disease‐modifying anti‐rheumatic drugs; N/A: not available; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | |||||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||||
1One study was not blinded.
Summary of findings 4. Subgroup analyses: ACR50.
| Etanercept for rheumatoid arthritis | |||||||
| Patient or population: participants with rheumatoid arthritis Intervention: etanercept | |||||||
|
Outcome: ACR50 ‐ American College of Rheumatology Improvement Criteria |
Subgroup | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||
| Control | Etanercept | ||||||
| Etanercept + DMARD vs. DMARD Follow‐up: 6‐36 months | All combined | 319 per 1000 | 615 per 1000 (561 to 742) | RR 2.82 (1.71 to 4.68) | 1118 (5 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 34% (95% CI 26% to 42%); Relative percent change 182% (95% CI 71% to 368%); NNTB 4 (95% CI 3 to 5) |
| DMARD naive | 462 per 1000 | 702 per 1000 (568 to 872) | RR 1.52 (1.23 to 1.89) | 261 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 24% (95% CI 12% to 36%); Relative percent change 52% (95% CI 23% to 89%); NNTB 5 (95% CI 3 to 10) |
|
| Methotrexate‐inadequate response | 45 per 1000 | 394 per 1000 (164 to 995) | RR 8.61 (3.55 to 20.86) | 247 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 35% (95% CI 26% to 44%); Relative percent change 761% (95% CI 255% to 1986%; NNTB 3 (95% CI 2 to 9) |
|
| Non‐methotrexate inadequate response | 360 per 1000 | 672 per 1000 (594 to 849) | RR 2.96 (0.81 to 10.81) | 610 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 38% (95% CI 19% to 57%); Relative percent change 196% (95% CI 19% to 981%); NNTB 3 (95% CI 3 to 5)1,2,3 |
|
| Etanercept vs. DMARD Follow‐up: 3 to 36 months | All combined | 389 per 1000 | 457 per 1000 (397 to 526) | RR 1.17 (1.02 to 1.35) | 1112 (3 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 7% (95% CI 1% to 13%); Relative percent change 17% (95% CI 2% to 35%); NNTB 16 (95% CI 3 to 129) |
| DMARD naive | 392 per 1000 | 461 per 1000 (368 to 576) | RR 1.18 (0.94 to 1.47) | 423 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 7% (95% CI ‐2% to 16%); Relative percent change 18% (95% CI ‐6% to 47%); NNTB not statistically significant |
|
| Methotrexate‐inadequate response | 308 per 1000 | 407 per 1000 (287 to 574) | RR 1.32 (0.93 to 1.86) | 238 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 10% (95% CI ‐2% to 22%); Relative percent change 32% (95% CI ‐7% to 86%); NNTB 11 not statistically significant |
|
| Non‐methotrexate inadequate response | 430 per 1000 | 480 per 1000 (391 to 589) | RR 1.12 (0.91 to 1.37) | 451 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 5% (95% CI ‐4% to 14%); Relative percent change 12% (95% CI ‐9% to 37%); NNTB not statistically significant |
|
| Etanercept + DMARD vs. Etanercept Follow‐up: 6 to 36 months | All combined | 442 per 1000 | 667 per 1000 (584 to 761) | RR 1.44 (1.16 to 1.79) | 805 (3 studies) | ⊕⊕⊕⊝ moderate4 | Absolute treatment benefit 20% (95% CI 8% to 32%); Relative percent change 44% (95% CI 16% to 79%); NNTB 5 (95% CI 4 to 8) |
| DMARD naive | No data | No data | ‐ | ‐ | No data | ‐ | |
| Methotrexate‐inadequate response | 478 per 1000 | 644 per 1000 (478 to 870) | RR 1.35 (1 to 1.82) | 142 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 17% (95% CI 0% to 33%); Relative percent change 35% (95% CI 0% to 82%); NNTB not statistically significant |
|
| Non‐methotrexate inadequate response | 435 per 1000 | 672 per 1000 (583 to 774) | RR 1.47 (1.07 to 2.02) | 663 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 21% (95% CI 3% to 39%); Relative percent change 47% (95% CI 7% to 102%); NNTB 5 (95% CI 3 to 7) |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DMARD: disease‐modifying anti‐rheumatic drug; N/A: not available; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
Summary of findings 5. Radiographic outcomes.
| Etanercept for rheumatoid arthritis | |||||||||
| Patient or population: participants with rheumatoid arthritis Intervention: etanercept | |||||||||
| Outcomes | Intervention | Comparison | Follow‐up | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||||||
| Control | Etanercept | ||||||||
| Radiographic progression ‐ mean change in Total Sharp Score (from baseline). Scale from: 0 to 448 | |||||||||
| Etanercept (25 mg) + DMARD | DMARD | 12 months | 2.4 |
MD ‐2.21 (‐2.99 to ‐1.43) |
‐ | 894 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.49% (95% CI ‐0.67% to 0.32%); Relative percent change ‐92% (95% CI ‐125% to ‐59.6%) |
|
| 2 years | 3.34 | MD ‐3.9 (‐6.11 to ‐1.69) | ‐ | 419 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.87% (95% CI ‐1.36 to ‐0.38); Relative percent change ‐114.7% (95% CI ‐180% to ‐50%) |
|||
| 3 years | 5.95 | MD ‐6.09 (‐9.22 to ‐2.96) | ‐ | 427 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐1.36% (95% CI ‐2.06 to ‐0.66%); Relative percent change ‐102% (95% CI ‐155% to ‐49.7%) |
|||
| Etanercept (10 mg) | DMARD | 6 months | 1.06 | MD ‐0.25 (‐0.72 to 0.22) | ‐ | 425 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.06% (95% CI ‐0.16% to 0.05%); Relative percent change ‐24% (95% CI ‐68% to 21%) |
|
| 12 months | 1.59 | MD ‐0.04 (‐0.93 to 0.85) | ‐ | 425 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.009% (95% CI ‐0.21 to 0.19%); Relative percent change ‐2.5% (95% CI ‐58.5% to 53.5%) |
|||
| Etanercept (25 mg) | DMARD | 6 months | 1.06 | MD ‐0.49 (‐0.92 to ‐0.06) | ‐ | 424 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.11% (95% CI ‐0.21% to ‐0.01%); Relative percent change ‐46% (95% CI ‐87% to ‐5.7%) |
|
| 12 months | 1.54 | MD ‐0.74 (‐1.35 to ‐0.13) | ‐ | 832 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.17% (95% CI ‐0.30 to ‐0.03%); Relative percent change ‐46.5% (95% CI ‐85% to ‐8.2%) |
|||
| 2 years | 3.34 | MD ‐2.24 (‐4.61 to 0.13) | ‐ | 409 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.50% (95% CI ‐1.03% to 0.03%); Relative percent change ‐67.1% (95% CI ‐138% to 3.89%) |
|||
| 3 years | 5.95 |
MD ‐4.34 (‐7.56 to ‐1.12) |
‐ | 421 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.97% (95% CI ‐1.69% to ‐0.25%); Relative percent change ‐72.9% (95% CI ‐127.0% to ‐18.8%) |
|||
| Etanercept (25 mg) + DMARD | Etanercept | 12 months | 0.33 | MD ‐1.13 (‐1.76 to ‐0.5) | ‐ | 414 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.25% (95% CI‐0.39% to ‐0.11%); Relative percent change ‐342.4% (95% CI ‐533.3% to ‐151.5%) |
|
| 2 years | 1.1 | MD ‐1.66 (‐2.76 to ‐0.56) | ‐ | 416 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.37% (95% CI ‐0.62% to ‐0.13%); Relative percent change ‐150.9% (95% CI ‐250.91% to ‐50.91%) | |||
| 3 years | 1.61 | MD ‐1.75 (‐3.27 to ‐0.23) | ‐ | 428 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.39% (95% CI ‐0.73% to ‐0.05%) Relative percent change ‐108.7% (95% CI ‐203.11% to ‐14.29%) | |||
| No progression of joint damage ‐ Total Sharp Score ≤ 0.5 at final visit | |||||||||
| Etanercept (25 mg) + DMARD | DMARD | 12 months | 579 per 1000 | 799 per 1000 (730 to 875) | RR 1.38 (1.26 to 1.51) | 906 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 22% (95% CI 16% to 28%); Relative percent change 38% (95% CI 26% to 51%); NNTB 5 (95% CI 4 to 7) |
|
| 2 years | 623 per 1000 | 816 per 1000 (735 to 903) | RR 1.31 (1.18 to 1.45) | 601 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit 19% (95% CI 12% to 26%); Relative percent change 31% (95% CI 18% to 45%); NNTB 6 (95% CI 4 to 9) |
|||
| 3 years | 510 per 1000 | 759 per 1000 (652 to 887) | RR 1.49 (1.28 to 1.74) | 427 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 25% (95% CI 16% to 34%); Relative percent change 49% (95% CI 28% to 74%); NNTB 5 (95% CI 3 to 8) |
|||
| Etanercept (25 mg) | DMARD | 12 months | 571 per 1000 | 679 per 1000 (588 to 788) | RR 1.19 (1.03 to 1.38) | 424 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 11% (95% CI 2% to 20%); Relative percent change 19% (95% CI 3% to 38%); NNTB 10 (95% CI 59 to 5) |
|
| 2 years | 602 per 1000 | 680 per 1000 (590 to 789) | RR 1.13 (0.98 to 1.31) | 409 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 8% (95% CI ‐1% to 17%); Relative percent change 13% (95% CI ‐2% to 31%); NNTB 13 (95% CI 6 to 84) |
|||
| 3 years | 510 per 1000 | 611 per 1000 (515 to 724) | RR 1.2 (1.01 to 1.42) | 421 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 10% (95% CI 1% to 20%); Relative percent change 20% (95% CI 1% to 42%); NNTB 10 (95% CI 5 to 197) |
|||
| Etanercept (25 mg) + DMARD | Etanercept | 12 months | 679 per 1000 | 802 per 1000 (713 to 897) | RR 1.18 (1.05 to 1.32) | 430 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 12% (95% CI 4% to 20%); Relative percent change 18% (95% CI 5% to 32%); NNTB 9 (95% CI 5 to 30) |
|
| 2 years | 680 per 1000 | 782 per 1000 (693 to 877) | RR 1.15 (1.02 to 1.29) | 416 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 10% (95% CI 1% to 18%); Relative percent change 15% (95% CI 2% to 29%); NNTB 10 (95% CI 6 to 74) |
|||
| 3 years | 611 per 1000 | 758 per 1000 (666 to 868) | RR 1.24 (1.09 to 1.42) | 428 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 15% (95% CI 6% to 24%); Relative percent change 24% (95% CI 9% to 42%); NNTB 7 (95% CI 4 to 19) |
|||
| Erosions ‐ Change in Sharp Score (from baseline). Scale from: 0 to 280. | |||||||||
| Etanercept (25 mg) + DMARD | DMARD | 12 months | 0.99 | MD ‐1.63 (‐2.41 to ‐0.85) | ‐ | 418 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.58% (95% CI ‐0.86% to ‐0.30%); Relative percent change ‐164.65% (95% CI ‐243.43% to ‐85.86%) |
|
| 2 years | 2.12 | MD ‐2.88 (‐4.39 to ‐1.37) | ‐ | 419 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐1.03% (95% CI ‐1.57% to ‐0.49%); Relative percent change ‐86.23% (95% CI ‐131.44% to ‐41.02%) |
|||
| 3 years | 3.25 | MD ‐3.92 (‐5.72 to ‐2.12) | ‐ | 427 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐1.40% (95% CI ‐2.04% to ‐0.76%); Relative percent change ‐120.62% (95% CI ‐176.00% to ‐65.23%) |
|||
| Etanercept (10 mg) | DMARD | 6 months | 0.68 | MD ‐0.18 (‐0.49 to 0.13) | ‐ | 425 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.06% (95% CI ‐0.18% to 0.05%); Relative percent change ‐26.47% (95% CI ‐72.06% to 19.1%); NNTB not statistically significant |
|
| 12 months | 1.03 | MD ‐0.13 (‐0.66 to 0.4) | ‐ | 425 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.05% (95% CI ‐0.24% to 0.14%); Relative percent change ‐12.62% (95% CI ‐64.08% to 38.8%); NNTB not statistically significant |
|||
| Etanercept (25 mg) | DMARD | 6 months | 0.68 | MD ‐0.38 (‐0.66 to ‐0.1) | ‐ | 424 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.14% (95% CI ‐0.24% to ‐0.04%); Relative percent change ‐55.88% (95% CI ‐97.06% to ‐14.7%) |
|
| 12 months | 1.0 | MD ‐0.65 (‐1.04 to 0.27) | ‐ | 832 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.23% (95% CI ‐0.37% to 0.10%); Relative percent change ‐65.00% (95% CI ‐104% to 27%) |
|||
| 2 years | 2.12 | MD ‐1.76 (‐3.34 to ‐0.18) | ‐ | 409 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.63% (95% CI ‐1.19% to ‐0.06%); Relative percent change ‐83.02% (95% CI ‐157.55% to ‐8.49%) |
|||
| 3 years | 3.25 | MD ‐2.86 (‐4.81 to ‐0.91) | ‐ | 421 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐1.02% (95% CI ‐1.72% to ‐0.33%); Relative percent change ‐88.00% (95% CI ‐148% to ‐28%) |
|||
| Etanercept (25 mg) + DMARD | Etanercept | 12 months | 0.03 | MD ‐0.67 (‐1.12 to ‐0.22) | ‐ | 414 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.24% (95% CI ‐0.40% to ‐0.08%); Relative percent change ‐2233.33% (95% CI ‐3733.3% to ‐733.3%) |
|
| 2 years | 0.36 | MD ‐1.12 (‐1.83 to ‐0.41) | ‐ | 416 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.40% (95% CI ‐0.65% to ‐0.15%); Relative percent change ‐311.11% (95% CI ‐508.3% to ‐113.89%) |
|||
| 3 years | 0.39 | MD ‐1.06 (‐1.98 to ‐0.14) | ‐ | 428 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.38% (95% CI ‐0.71% to ‐0.05%); Relative percent change ‐271.79% (95% CI ‐507.69% to ‐35.9%) |
|||
| Joint space narrowing Score ‐ change in Total Sharp Score (from baseline). Scale from: 0 to 168 | |||||||||
| Etanercept (25 mg) + DMARD | DMARD | 12 months | 0.51 | MD ‐0.67 (‐1.12 to ‐0.22) | ‐ | 418 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.40% (95% CI ‐0.67% to ‐0.13%); Relative percent change ‐131.37% (95% CI ‐219.61% to ‐43.14%) |
|
| 2 years | 1.23 | MD ‐1.03 (‐1.89 to 0.17) | ‐ | 419 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.61% (95% CI ‐1.13% to 0.10%); Relative percent change ‐83.74% (95% CI ‐153.66% to 13.82%) |
|||
| 3 years | 2.7 | MD ‐2.17 (‐3.78 to ‐0.56) | ‐ | 427 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐1.29% (95% CI ‐2.25 to ‐0.33%); Relative percent change ‐80.37% (95% CI ‐140.00% to ‐20.7%) |
|||
| Etanercept (10 mg) | DMARD | 6 months | 0.38 | MD ‐0.07 (‐0.35 to 0.21) | ‐ | 425 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.04% (95% CI ‐0.21% to 0.13%); Relative percent change ‐18.42% (95% CI ‐92.11% to 55.26%) |
|
| 12 months | 0.56 | MD 0.09 (‐0.42 to 0.6) | ‐ | 425 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit 0.05% (95% CI ‐0.25% to 0.36%); Relative percent change 16.07% (95% CI ‐75.00% to 107.1%) |
|||
| Etanercept (25 mg) | DMARD | 6 months | 0.38 | MD ‐0.11 (‐0.35 to 0.13) | ‐ | 424 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.07% (95% CI ‐0.21% to 0.08%); Relative percent change ‐28.95% (95% CI ‐92.11% to 34.2%) |
|
| 12 months | 0.53 | MD ‐0.11 (‐0.43 to 0.2) | ‐ | 832 (2 studies) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.07% (95% CI ‐0.26% to 0.12%); Relative percent change ‐20.75% (95% CI ‐81.13% to 37.7%) |
|||
| 2 years | 1.23 | MD ‐0.49 (‐1.46 to 0.48) | ‐ | 409 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.29% (95% CI ‐0.87% to 0.29%); Relative percent change ‐39.84% (95% CI ‐118.70% to 39%) |
|||
| 3 years | 2.7 | MD ‐1.48 (‐3.04 to 0.08) | ‐ | 421 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.88% (95% CI ‐1.81% to 0.05%); Relative percent change ‐54.81% (95% CI ‐112.59% to 2.96%) |
|||
| Etanercept (25 mg) + DMARD | Etanercept | 12 months | 0.3 | MD ‐0.46 (‐0.74 to ‐0.18) | ‐ | 414 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.27% (95% CI ‐0.44% to ‐0.11%); Relative percent change ‐153.33% (95% CI ‐246.67% to ‐60%) |
|
| 2 years | 0.74 | MD ‐0.54 (‐1.09 to 0.01) | ‐ | 416 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.32% (95% CI ‐0.65% to 0.01%); Relative percent change ‐72.97% (95% CI ‐147.30% to 1.35%) |
|||
| 3 years | 1.22 | MD ‐0.69 (‐1.65 to 0.27) | ‐ | 428 (1 study) | ⊕⊕⊕⊕ high | Absolute treatment benefit ‐0.41% (95% CI ‐0.98% to 0.16%); Relative percent change ‐56.56% (95% CI ‐135.25% to 22%) |
|||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DMARD: disease‐modifying anti‐rheumatic drug; MD: mean difference; N/A: not available; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio. | |||||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||||
Background
Description of the condition
Rheumatoid arthritis (RA) is chronic progressive systemic inflammatory condition, affecting mainly the joint synovium, but can also affect other tissues and organs. RA is associated with significant morbidity, mortality, joint deformity and impaired quality of life (Pincus 1993; Puolakka 2005).
Description of the intervention
Disease modifying anti‐rheumatic drugs (DMARDs) are drugs that have been shown to reduce disease activity, slow down joint damage and improve quality of life. DMARDs are the mainstay of treatment of RA (Saag 2008; Singh 2012). However, often people do not respond to or are unable to tolerate traditional DMARDS (Yee 2003). Newer biological drugs have been introduced and approved for the treatment of RA since 1998 (Fernandez‐Cruz 2008). These biologic DMARDs have been associated with clinical outcome improvement (Singh 2009), but also with higher rates of adverse events and tuberculosis (TB) (Singh 2011).
How the intervention might work
Etanercept is a dimeric fusion protein consisting of the extracellular ligand‐binding portion of the human 75 kilodalton (p75) tumour necrosis alpha receptor (TNFR) linked to the Fc portion of human IgG1. Etanercept is produced by recombinant deoxyribonucleic acid (DNA) technology (Murray 1997). Etanercept inhibits the action of tumour necrosis factor (TNF), thus suppressing inflammation. It is usually prescribed when the DMARD methotrexate (MTX) does not work well, but it is significantly more expensive (Jarvis 1999).
Why it is important to do this review
This review summarises the current data available on the benefits and harms of etanercept on its own and in combination with MTX for the treatment of RA. This information will enable clinicians to choose appropriate treatment for people with RA using the best medical evidence available.
Objectives
To assess the benefits and harms of etanercept monotherapy or combined with DMARD for the treatment of RA.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) or controlled clinical trials (CCTs) comparing etanercept to placebo, etanercept to DMARD (either MTX or sulphasalazine), or etanercept plus MTX to DMARD alone or etanercept alone that were at least six months' duration were eligible for inclusion. Participants could be on other DMARDS, non‐steroidal anti‐inflammatory drugs or corticosteroids provided they were on stable doses and were randomly allocated to treatment with or without etanercept.
Types of participants
People 16 years of age or older meeting the American College of Rheumatology (ACR) 1987 revised criteria (Arnett 1988) for RA. Participants had to have evidence of active disease as demonstrated by at least two of:
tender joint count;
swollen joint count;
duration of early morning stiffness (EMS) lasting more than 30 minutes;
acute phase reactants such as Westergren erythrocyte sedimentation rate (ESR) or C reactive protein (CRP).
Types of interventions
Treatment trials with etanercept and DMARD (either MTX or sulphasalazine) versus DMARD, etanercept versus DMARD, etanercept plus DMARD versus etanercept and etanercept versus placebo were eligible for inclusion. Doses of etanercept eligible for inclusion were 10 or 25 mg subcutaneous (SC) injections twice weekly, with a minimum trial duration of six months. SC injections are given by a needle injection into the fatty layer of tissue just below the skin because there is little blood flow to fatty tissue so the injected medication is absorbed slowly.
Types of outcome measures
Major outcomes
The response of RA to treatment with etanercept has been defined by the World Health Organization (WHO), the International League of Associations for Rheumatology (ILAR) core set of disease activity measures and the ACR outcome measures for RA clinical trials (Boers 1994; Felson 1993; OMERACT 1993). The set of efficacy measures includes: 1) tender joint count; 2) swollen joint count; 3) patient assessment of pain using 10‐cm visual analogue scale or Likert scale; 4) patient global assessment of disease activity; 5) physician global assessment of disease activity using 10‐cm visual analogue scale or Likert scale; 6) patient assessment of functional ability as measured by a validated scale such as the Health Assessment Questionnaire (HAQ), which is a standardised, validated scale used in people with arthritis; 7) acute phase reactants such as ESR or CRP; 8) Radiographic bone changes are accepted as part of the core set of disease activity measures in studies of a minimum of 12 months' duration. Radiographic progression as measured by Total Sharp Score (TSS) or Larsen scale was included as a primary outcome measure of studies with a minimum duration of 12 months.
Definition of improvement
Statistical versus clinical significance is relevant to clinical care. Based on the set of efficacy measures outlined above, a definition of clinical improvement has been established (Felson 1995; Pincus 1999). An ACR20 response represents a 20% improvement in tender and swollen joint counts plus a 20% improvement in three of the five following remaining core measures: patient and physician global assessments, pain, functional status and an acute phase reactant.
Based on these definitions, the following primary efficacy outcomes have been recorded in this review:
-
ACR response:
ACR 20 response;
ACR 50 response;
ACR 70 response;
-
Radiographic scores:
TSS (change in this score from baseline, range of score 0 to 448);
Erosion Score (ES) (change in this score from baseline);
Joint Space Narrowing Score (JSNS) (change in this score from baseline) (scores for ES and JSNS are summed to yield the TSS);
-
Remission (considered a useful measure of disease activity (van der Heijde 2005a)).
disease activity score (DAS) < 1.6;
DAS28 < 2.6.
Minor outcomes
Secondary outcome measures included:
health‐related quality of life (HRQoL) such as the Short Form (SF)‐36, when available;
adverse events;
withdrawals from the study (total, due to lack of efficacy, due to adverse events and death).
Search methods for identification of studies
Electronic searches
Electronic databases including Biological Abstracts, Current Contents, Dissertation Abstracts, EBM Reviews and all Cochrane electronic databases were searched from 1966 to February 2003. A further search was performed from 2003 to January 2012 of the following databases:
MEDLINE;
EMBASE;
CINAHL;
Web of Science;
Controlled Clinical Trials;
The Cochrane Library (CENTRAL, DARE, NEED).
We searched RA as an exploded MESH heading. Etanercept was searched as a text word as it is not currently indexed. The search was not limited by language, year or publication or type of publication. The MEDLINE search strategy used is located in Appendix 1. This strategy was modified for other databases.
Searching other resources
The proceedings of major rheumatology conferences including the ACR (1990 to 2003), the European League of Rheumatology (1990 to 2002) and the Canadian Rheumatology Association were handsearched. The reference lists from standard rheumatology textbooks, comprehensive reviews and identified clinical trials were searched. Content experts and the pharmaceutical companies that manufacture etanercept were contacted. The Current Controlled Trials register was also searched for ongoing trials. Additional unpublished data were sought through the US Food and Drug Administration (FDA) website. The only unpublished data used in this review were additional information from published trials from manufacturers.
Data collection and analysis
Selection of studies
Each study was independently reviewed by two review authors (either BB and MJ or AL and MLO) to determine if the study met the inclusion criteria outlined in the a priori protocol developed for the review. Disagreements on study eligibility were resolved by discussion. The reason(s) for exclusion of any study were noted.
Data extraction and management
Data were extracted by one review author for the 2003 publication of the review (BB) and the extraction forms were then double‐checked independently by another individual against the original data source. For the 2012 update of the review, data were extracted independently by two review authors (AL and MLO). Any discrepancies were resolved through consensus by both review authors returning to the original data source to confirm which value was correct.
The following data were extracted from each study:
Trial characteristics
Study design.
Number of people randomised, excluded or lost to follow‐up.
Whether an intention‐to‐treat analysis was done.
Whether a power calculation was done.
Duration, timing and location of the study.
Number of centres.
Source of funding.
Characteristics of the study participants
Age and any other recorded characteristics of participants in the study.
Other inclusion criteria.
Exclusion criteria.
Time since diagnosis of RA.
Source of participants.
Proportion of those eligible.
Interventions used
Dose/regimen of etanercept.
Dose/regimen of MTX.
Other concomitant medications allowed during the trial.
Outcomes
Methods used to measure efficacy.
Methods used to measure quality of life.
Methods used to determine/collect adverse events.
Assessment of risk of bias in included studies
Two independent review authors (AL and MLO) assessed risk of bias of each study, using the 'Risk of bias' tool developed by The Cochrane Collaboration (Higgins 2011). The following domains were assessed:
sequence generation (whether the allocation sequence was adequately generated, for example, random number table, computer random number generator, coin tossing, throwing dice);
allocation concealment (whether the allocation was adequately concealed, for example, sequentially numbered containers of identical appearance, central allocation, sequentially numbered, opaque sealed envelopes);
blinding of participants, personnel and outcome assessors (whether knowledge of the allocated intervention was adequately prevented during the study, for example, by ensuring blinding or participants and key personnel or, where there is no blinding, knowledge of the intervention is not likely to influence the outcomes);
incomplete outcome data (whether incomplete outcome data were adequately addressed, for example, missing data balanced in numbers across intervention groups, proportion of missing outcomes insufficient to affect estimates, reasons for missing data unlikely to be related to the outcomes);
selective outcome reporting (whether the reports of the study were free of suggestion of selective outcome reporting, for example, previous publication of a study protocol, other evidence that the study contains all of the prespecified outcomes);
other sources of bias (whether the study was apparently free of other problems that could put it at a high risk of bias, for example, baseline imbalance, bias related to study design, early termination of study).
These domains were judged as either 'low risk', 'high risk' or 'unclear risk' of bias.
Measures of treatment effect
The data were analysed using an intention‐to‐treat model, where data were available. Dichotomous data were reported as a Mantel Haenszel risk ratio (RR) with 95% confidence intervals (CI). Continuous data were analysed as a mean difference (MD) with 95% CIs. The mean and standard deviation (SD) were used when available. When only the median and interquartile ranges were reported, the distribution of the data was checked. If the data appeared to be roughly normal, the median was used as the mean, and one half of the difference between the 1st and 3rd quartile range was used as the SD. When only the baseline SD was available, it was used as the end of study SD as well. For some of the trials, measures of variation were missing in the publication of results and contact with the authors was unsuccessful. Where possible, SDs were imputed from the measures of variation from other trials measuring the same outcome. It was not always possible to impute these missing values. Outcome variables that were reported only graphically were not included in the study. Results have been calculated to provide an indication of the number needed to treat for an additional beneficial outcome (NNTB) for major statistically significant outcomes. The NNTB reflects the effort required (or number of people needing to be treated) to obtain a beneficial outcome with an intervention.
Assessment of heterogeneity
The included studies were carefully inspected for evidence of clinical heterogeneity in the characteristics of the participants, interventions and outcomes of the trial duration. Where pooling the studies was appropriate, statistical heterogeneity was assessed by the results of the Chi2 using n ‐ 1 degrees of freedom and a P value of less than 0.10 and the I2 statistics (Higgins 2003). The I2 statistic represents the proportion of variation between studies that is not due to chance and it takes values from 0% to 100%. We considered an I2 of 25% to represent mild heterogeneity, 50% to represent moderate heterogeneity and 75% or more to be evidence of extreme heterogeneity.
Data synthesis
A fixed‐effect model was used to calculate a pooled estimate of effect in meta‐analyses. If significant statistical heterogeneity was confirmed by the Chi2 test and the I2 statistic (> 50%), the random‐effects model was used to display the results.
Subgroup analysis and investigation of heterogeneity
A priori, subgroup analysis was planned to determine differential effects according to duration and severity of disease and type of control group.
Sensitivity analysis
A priori, sensitivity analysis was planned to determine the effects of previous DMARD treatment and quality of the trials.
Results
Description of studies
Methods of included studies are summarised in the Characteristics of included studies tables.
Results of the search
The original search strategy was run from inception to 2003. Updated search strategies were run from: 2003 to 2008, 2008 to 2010, and 2010 to January 2012. Figure 1 shows the number of citations retrieved from 2008 to 2012. There were 2363 original citations: 1211 from MEDLINE, 128 from EMBASE, 74 from CINAHL, 814 from Web of Science, 104 from The Cochrane Library and 32 from web links. After de‐duplication there were 1604 titles and abstracts to screen; 1527 were excluded. We retrieved 77 full texts for the final review, 69 were excluded and four trials (eight publications) met the inclusion criteria and were added to the three trials included in the original review plus two more trials found in the first updating search from 2003 to 2008.
1.
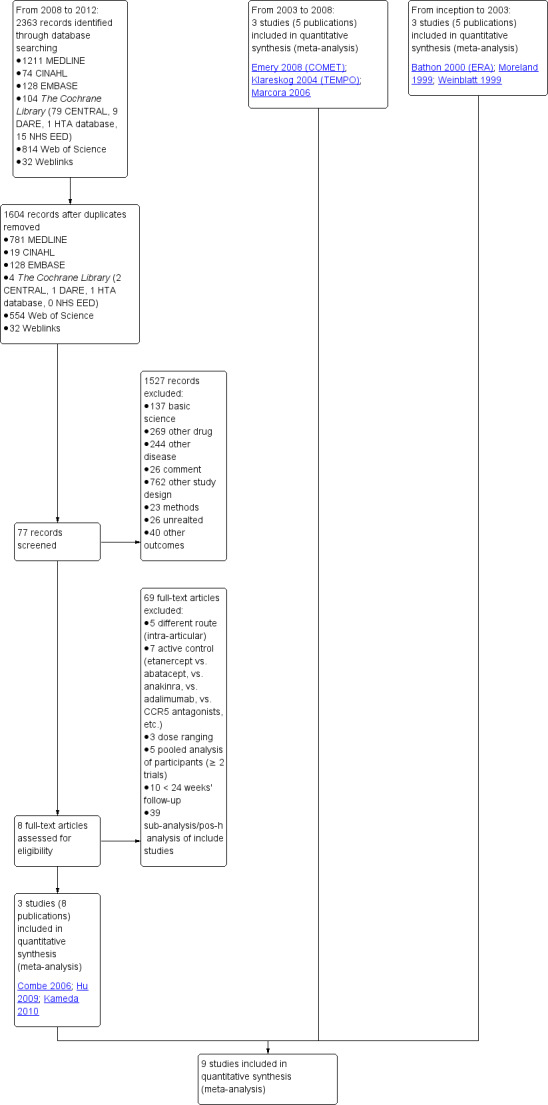
Study flow diagram.
Included studies
Weinblatt 1999 was a 24‐week randomised double‐blind trial comparing etanercept 25 mg SC twice weekly plus stable doses of MTX (15 to 25 mg once weekly, or as low as 10 mg if participant was unable to tolerate higher doses) was compared with placebo injections plus MTX. All drugs were administered for a 24‐week period. All participants received either folic acid or folinic acid. Participants had to have had an incomplete response to at least six months of MTX at a stable dose for at least four weeks prior to randomisation. Participants had to be at least 18 years of age, and meet 1987 American Rheumatology Association criteria for RA; be in functional class I, II or III according to the revised criteria of the ACR and have active disease with at least six tender and six swollen joints. Sulphasalazine and hydroxychlorquine were discontinued two weeks prior to starting study drug and all other DMARDs (except MTX) were discontinued four weeks prior. Prednisone at 10 mg daily or less and non‐steroidal anti‐inflammatory drugs (NSAIDs) were permitted as long as the dose had been stable for at least four weeks prior to the study, and remained stable throughout the study.
Moreland 1999 was a six‐month double‐blind randomised study with three arms: etanercept 25 mg SC twice weekly, etanercept 10 mg SC twice weekly and injectable placebo twice weekly. Participants had to have had an inadequate response to one to four DMARDs, with an inadequate response being defined as discontinuation of the drug due to lack of efficacy. DMARDs had to be washed out at least one month prior to starting study drug and no DMARDs aside from etanercept were permitted during the trial. Participants had to have evidence of active disease at enrolment, with at least 12 tender joints, at least 10 swollen joints and one of: an ESR of at least 28, CRP concentration of at least 20 mg/L or early morning stiffness (EMS) of at least 45 minutes. Intra‐articular steroids were not allowed during the study, or within four weeks of enrolment. Eight people taking placebo from an earlier three‐month study were enrolled in this trial. Stable doses of steroids at prednisone 10 mg daily or less and stable doses of NSAIDs that did not exceed the manufacturer's recommended doses were allowed. Ninety per cent of participants had used MTX previously, and 22% were on MTX prior to the DMARD washout period.
Bathon 2000 (ERA) was a 12‐month randomised study with three treatment arms: etanercept 10 mg SC twice weekly, etanercept 25 mg SC twice weekly, escalating weekly MTX (7.5 mg at week 0, 15 mg at week four and 20 mg at week eight). One 5‐mg dose reduction was allowed for preset laboratory abnormalities. The etanercept/placebo dose was not adjusted. All participants received 1 mg of folic acid daily. Partipants were at least 18 years old with RA for three years or less and no other significant concurrent illnesses. Participants had never received MTX. Participants had to be either rheumatoid factor positive, or have three bony erosions visible on x‐rays of the hands, wrists or feet. Participants also had to have at least 10 swollen joints, and at least 12 tender joints. Participants also had to have one of either an ESR of at least 28 mm/hour, a CRP of at least 2.0 mg/day or a minimum of 45 minutes of EMS. DMARDs were discontinued at least four weeks before starting the study. Stable dose of NSAIDs and prednisone (10 mg daily or less) were allowed.
Klareskog 2004 (TEMPO) was a three‐year double‐blind randomised study with three treatment arms: etanercept only (25 mg SC twice weekly plus oral placebo once weekly), etanercept plus MTX (combination of etanercept injections 25 mg SC twice weekly and oral MTX (escalating up to 20 mg) once weekly) and MTX only (7.5 mg escalating to 20 mg oral capsules once weekly within eight weeks if participants had any painful or swollen joints and placebo SC injections twice weekly). All participants had folic acid 5 mg supplement twice a week. Participants were at least 18 years old with a disease duration of six months to 20 years, had active adult‐onset RA (class I to III), defined as 10 or more swollen and 12 or more painful joints and at least one of the following: ESR 28 mm/hour or greater; plasma CRP 20 mg/L or greater; or morning stiffness for 45 minutes or more. Participants also had to have a less than satisfactory response to at least one DMARD other than MTX. Those who had previously been treated with MTX were eligible if they had not had important toxic effects or lack of response and had not been treated with MTX in previous six months. Participants were ineligible if they had previously received etanercept or other TNF antagonists. Other exclusion criteria included: previous treatment with immunosuppressive drugs within six months of screening; use of any investigational drug or biological agent within three months of screening; any other DMARD or corticosteroid injection within previous four weeks and presence of comorbidity.
Combe 2006 was a two‐year double‐blind randomised study with three treatment arms (randomisation ratio: 2:2:1): etanercept only (25 mg SC twice weekly plus oral placebo once daily); etanercept plus sulphasalazine (combination of 25 mg SC etanercept injections twice weekly and oral sulphasalazine (2, 2.5 or 3 mg) once daily) and sulphasalazine only (2, 2.5 or 3 mg tablets plus placebo SC injections twice weekly). Participants were permitted stable doses of oral steroids, one NSAID and analgesics. Participants were at least 18 years old with a disease duration of six months to 20 years, had active adult onset RA (class I to III), defined as 10 or more swollen and 12 or more painful joints and at least one of the following: ESR 28 mm/hour or greater; plasma CRP 20 mg/L or greater; or morning stiffness for 45 minutes or more. Participants had active disease despite receiving sulphasalazine.
Marcora 2006 was a six‐month unblinded randomised study with two treatment arms: etanercept 25 mg SC twice weekly versus MTX 7.5 mg once a week (escalating, if necessary, to 20 mg per week). The study was primarily designed to assess the effects of etanercept and MTX on cachexia in people with early RA (< six months' history of RA) but function, adverse events and disability were also measured (by blinded assessors). Participants were excluded if they had previously used DMARDs, steroids or biologics, or they had concurrent disease but NSAIDs and analgesics were allowed during the study and all participants took folic acid supplements.
Emery 2008 (COMET) was a two‐year double‐blind randomised study with two treatment arms for the first year: etanercept (25 mg SC twice weekly) plus oral MTX (starting at 7.5 mg/week and escalating up to 20 mg/week) versus MTX alone (with placebo injection). Those participants who completed the first year of treatment were eligible to enter the second year of the study. In year two, participants in the combination therapy group either continued combination treatment or received etanercept monotherapy. Participants in the original MTX monotherapy group either continued with MTX monotherapy or etanercept plus MTX. Randomisation to these four groups was undertaken at baseline. In sum, the first year of the study compared etanercept plus MTX with MTX alone; the second year of the study compared four groups: (1) etanercept plus MTX in year one and etanercept plus MTX in year two; (2) etanercept plus MTX in year one and etanercept monotherapy in year two; (3) MTX monotherapy in year one and etanercept plus MTX in year two; (4) MTX monotherapy in year one and MTX monotherapy in year two. This review has reported results of two of the four groups in the second year of the study: those who continued with their year one treatment. Participants were at least 18 years old, had a disease duration between three months and two years and had not had previous treatment with etanercept, MTX or other TNF antagonists. Participants were required to have DAS28 greater than 3.2; 92% of participants had severe disease (DAS28 > 5.1). All participants received folic acid supplementation and stable doses of corticosteroids or NSAIDs were permitted. The study was designed to assess rates of clinical remission, radiographic non‐progression and restoration of function.
Hu 2009 was a six‐month double‐blind randomised study with two treatment arms: Yisaipu (25 mg SC twice weekly plus oral placebo) and oral MTX (three x 2.5 mg (increasing to 5 mg) per week plus placebo injection). Yisaipu is a rhTNFR:Fc available in China that has the same structure as etanercept and for the purposes of this review is considered identical to etanercept. Stable doses of NSAIDs and prednisone (≤ 10 mg/day) were allowed. Participants were 18 to 65 years old, had active RA (as defined by ACR 1987 criteria: swelling in six joints or more, six or more tender joints, morning stiffness ≥ 45 minutes, ESR ≥ 28 mm/h, CRP ≥ 20 μg/mL). Participants were ineligible if they had any serious illness (affecting heart, liver, renal, blood or other vital organs). Other exclusion criteria included: pregnancy or breastfeeding; previous treatment with Yisaipu or other biological agents; no efficacy to treatment with MTX; joint injection of corticosteroids within past four weeks; any acute or chronic infection or past history of active TB; any tumour or family history of tumour; any other DMARD for at least four weeks prior to the study entry. The study outcome measures included: ACR20, ACR50, ACR70; withdrawals and adverse events.
Kameda 2010 was a two‐year unblinded randomised study with two treatment arms: etanercept (25 mg SC twice weekly) plus oral MTX (6‐8 mg once weekly) versus etanercept alone. Participants were allowed stable doses of corticosteroids (< 10 mg/day). The publication provides data on the first six months of the study. Randomisation was stratified by age (< 55 years or ≥ 55 years), disease duration (< 10 years or ≥ 10 years), disease activity (DAS28 < 5.1 or ≥ 5.1) and institution. Participants were at least 18 years old, met 1987 American Rheumatology Association criteria for RA and had active disease despite receiving MTX defined as six or more swollen and painful joints and at least one of the following: ESR 28 mm/h or greater; plasma CRP 2 mg/dL or greater and adequate safety profile. Participants had not received DMARDs other than MTX before and were biologic‐naive.
Summary of studies
Participants
Moreland 1999 and Weinblatt 1999 studies included participants with long‐standing RA (11 to 13 years), whereas the Bathon 2000 (ERA) and Emery 2008 (COMET) studies included participants who had only had RA for three years or less. Participants in the Marcora 2006 study had very early RA (< six months). Combe 2006, Kameda 2010 and Klareskog 2004 (TEMPO) included participants with a wide range of disease duration (from six months to 20 years). Hu 2009 only reported the mean disease duration (in months; which was 90.78 ± 98.75 months) for the etanercept and 93.74 ± 94.29 months for the MTX group.
Interventions
Two trials assessed the effects of two different doses of etanercept (10 and 25 mg), one against placebo and one against MTX (Bathon 2000 (ERA); Moreland 1999). The other trials used the 25 mg dose of etanercept in their comparisons. Four trials compared combination etanercept plus DMARD (either MTX or sulphasalazine) versus DMARD plus placebo (Combe 2006; Emery 2008 (COMET); Klareskog 2004 (TEMPO); Weinblatt 1999); Combe 2006 and Klareskog 2004 (TEMPO) also included an etanercept plus placebo arm in the comparisons. Two trials compared etanercept 25 mg versus MTX (Hu 2009; Marcora 2006) and one trial compared etanercept plus MTX versus etanercept (Kameda 2010). Etanercept was invariably delivered SC and MTX was usually given orally but all arms were placebo controlled, except for the Kameda 2010 and Marcora 2006 trials. In trials where participants received MTX, folic acid supplementation was required. In all the trials except one, analgesics, NSAIDs and corticosteroids were permitted; the Marcora 2006 study did not allow corticosteroids. The dose of MTX was escalated to a maximum dose of 20 mg, dependent on participant response.
Outcomes
Eight of the nine studies reported on ACR20, ACR50 and ACR70 response rates, quality of life outcomes, and withdrawal rates and three trials also measured radiographic scores (TSS, ES and JSNS). Adverse events were measured by all studies. For some outcomes, no measure of variability was included in the summary effect estimates and, where possible, SDs were imputed from the results of other trials where values were similar. Some of the reported data could not be used because it was not in a form suitable for entering in the meta‐analysis.
Duration
Six of the nine studies evaluated outcomes at the end of six months. One study evaluated outcomes at six and 12 months. Two ongoing studies had a trial duration of two years and reported overall results at six months and one year (Emery 2008 (COMET) and Kameda 2010, respectively). At two years the groups in Emery 2008 (COMET) were modified and participants were re‐randomised to one of four groups. One other study evaluated outcomes at one, two and three years (Klareskog 2004 (TEMPO)).
Excluded studies
Thirty‐nine studies were excluded (ACP 2001; Angel 2010; Anis 2009; Benucci 2011; Blank 2009; Bliddal 2006; Boesen 2008; Chen 2006; Cuomo 2006; De Filippis 2006; De Stefano 2010; Garnero 2002; Genovese 2002; Genovese 2004; Gerlag 2010; Holman 2008; Iwamoto 2009; Johnsen 2006; Kavanaugh 2008; Keystone 2004; Keystone 2009; Koumakis 2009; Lan 2004; Lisbona 2008; Lukas 2009; Lukas 2010; Lukina 2001; Luzi 2009; Machado 2009; Moreland 1997; Moreland 2001; Paleolog 1998; Roux 2011; Saleem 2009; Sennels 2008; van Riel 2006; Weinblatt 2007; Weinblatt 2008; Weisman 2007) as they did not meet the inclusion criteria. Owing to the inclusion criteria listed a priori in the 2003 review, we have excluded seven potentially relevant studies (Bliddal 2006; Genovese 2002; Johnsen 2006; Keystone 2004; Weinblatt 2007; Weinblatt 2008; Weisman 2007) that were analysed in a separate Cochrane network meta‐analysis to evaluate the adverse effects of biologics for the treatment of rheumatic conditions (Singh 2011). Specific reasons for exclusion are provided in the Characteristics of excluded studies table in this review.
Risk of bias in included studies
Each study was assessed separately for risk of bias independently by two review authors (AL and MLO). The results of this assessment are contained in a table attached to the Characteristics of included studies table and in summary form (Figure 2; Figure 3).
2.
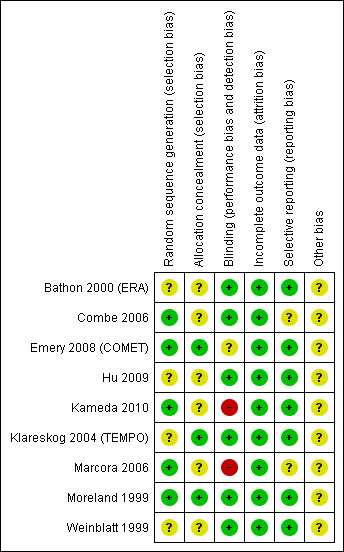
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
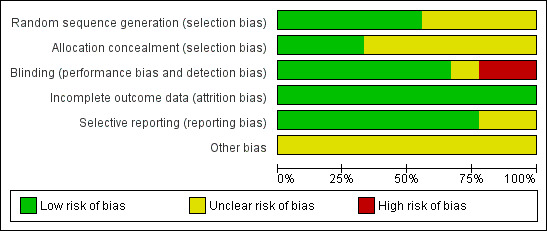
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Five of the nine included studies described the randomisation method clearly and were at low risk of bias (Combe 2006; Emery 2008 (COMET); Kameda 2010; Marcora 2006; Moreland 1999). Three of the nine included studies provided details that indicated that allocation had been adequately concealed and were considered at low risk of bias (Emery 2008 (COMET); Klareskog 2004 (TEMPO); Moreland 1999).
Blinding
Seven of the nine included studies reported at least double blinding and were considered at low risk of bias (Bathon 2000 (ERA); Combe 2006; Emery 2008 (COMET); Hu 2009; Klareskog 2004 (TEMPO); Moreland 1999; Weinblatt 1999), and Bathon was placebo controlled so blinding was likely. The Emery study reported that blinding was removed for the analyst who undertook the primary analysis for the publication of results (Emery 2008 (COMET)). Another study blinded only the outcome assessors (Marcora 2006). Kameda 2010 was an open‐label study.
Incomplete outcome data
All of the nine included studies described methods to deal with withdrawals and loss to follow‐up and were considered at low risk of bias (Bathon 2000 (ERA); Combe 2006; Emery 2008 (COMET); Hu 2009; Kameda 2010; Klareskog 2004 (TEMPO); Marcora 2006; Moreland 1999; Weinblatt 1999). The first six of these studies mostly counted withdrawals as non‐responders and used the last observation carried forward (LOCF) or linear extrapolation, or both, to calculate outcomes where there were missing data. Emery 2008 (COMET) and Kameda 2010 used a modified intention‐to‐treat analysis, including only participants who had baseline assessments and at least one assessment after treatment; measures for missing values included LOCF and imputation by linear extrapolation. One study reported the total number of withdrawals, but did not specify reasons (Hu 2009), and in the other small study one person dropped out because of knowledge of treatment assignment (Marcora 2006).
Selective reporting
None of the protocols for the published studies was identified to determine whether selective reporting had occurred. However, a wide range of outcomes were reported in most studies and it is probably unlikely that selective reporting occurred for eight of the nine included studies; these were considered at low risk of bias.
Other potential sources of bias
Eight studies were supported by pharmaceutical companies engaged in the promotion of etanercept (Bathon 2000 (ERA); Combe 2006; Emery 2008 (COMET); Kameda 2010; Klareskog 2004 (TEMPO); Marcora 2006; Moreland 1999; Weinblatt 1999) and one study did not specify the funding source. Thus, all studies were considered to have unclear risk of bias for this domain. In some cases, the authors of the publications were staff of the pharmaceutical company that provided funding. There was no evidence of any other biases that had the potential to affect the results.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Included studies
Nine trials (Bathon 2000 (ERA); Combe 2006; Emery 2008 (COMET); Hu 2009; Kameda 2010; Klareskog 2004 (TEMPO); Marcora 2006; Moreland 1999; Weinblatt 1999), representing 2842 participants, met the inclusion criteria, although not all the participants provided data for all of the outcomes. Study details for the included studies are provided in the Characteristics of included studies table.
Comparisons
Comparisons have been structured in the meta‐analyses to show the effects of etanercept plus a DMARD (either MTX or sulphasalazine) compared with DMARD monotherapy, etanercept monotherapy compared with DMARD monotherapy, etanercept plus DMARD compared with etanercept monotherapy and etanercept monotherapy compared with placebo. For the comparison of etanercept plus DMARD versus DMARD, four studies compared a dose of 25 mg of etanercept with either MTX (Emery 2008 (COMET); Klareskog 2004 (TEMPO); Weinblatt 1999) or sulphasalazine (Combe 2006). Similarly, for the comparison of etanercept alone versus DMARD alone, five studies compared a dose of 25 mg of etanercept versus either MTX (Bathon 2000 (ERA); Hu 2009; Klareskog 2004 (TEMPO); Marcora 2006) or sulphasalazine (Combe 2006) and one of these trials also compared a lower dose of etanercept (10 mg) versus MTX (Bathon 2000 (ERA)). For the comparison of etanercept plus DMARD versus etanercept alone, three studies reported results from this arm using a dose of 25 mg of etanercept (Combe 2006; Kameda 2010; Klareskog 2004 (TEMPO)). For the comparison of etanercept versus placebo, one study compared two different doses of etanercept (10 and 25 mg SC twice weekly) (Moreland 1999).
Follow‐up and measurement of outcomes
Hu 2009, Kameda 2010, Marcora 2006, Moreland 1999 and Weinblatt 1999 followed participants for six months; Bathon 2000 (ERA) for 12 months; Combe 2006 for two years and overall results from the two‐year Emery 2008 (COMET) trial were reported at the end of the first and in modified groups at the end of the second year. Klareskog 2004 (TEMPO) followed participants for three years, with follow‐up at the end of every year. Participants from five included studies were invited to participate in open‐label extensions to the original randomised trials to assess longer‐term effects and adverse effects of treatment (Bathon 2000 (ERA); Combe 2006; Klareskog 2004 (TEMPO); Moreland 1999; Weinblatt 1999). Results from these unblinded extensions have not been reported in this review and the publications of results from these extensions have been excluded.
Efficacy outcomes have been extracted at multiple follow‐up times in the Bathon 2000 (ERA), Combe 2006, Emery 2008 (COMET) and Klareskog 2004 (TEMPO) trials to show patterns as follow‐up time increases. Quality of life outcomes and withdrawal have also been extracted at multiple follow‐up times in the Klareskog 2004 (TEMPO) and Combe 2006 trials to show longitudinal results. Adverse effects have mostly been extracted at the end of the trials (except for Combe 2006 and Klareskog 2004 (TEMPO), where data were extracted at the end of two years because more specific information on effects at this time was available).
A summary of the ACR50 response rates, radiographic progression and serious infections is provided in Table 3. Subgroup analyses are shown in Table 4).
A. Benefits
(see "definition of improvement" in methods section for description of measures presented below)
For this update, first, we sought to answer two clinically relevant question since most people will have used at least one traditional DMARD before starting treatment with etanercept: 1) what are the benefits and harms of etanercept plus DMARD compared with DMARD monotherapy in those people with RA who are partial responders to traditional DMARDs? and 2) what are the benefits and harms of etanercept plus DMARD compared with DMARD monotherapy in those people with RA who are partial responders to MTX? Second, we provide evidence on the benefits and harms of using: 1) etanercept plus DMARDs versus DMARD; 2) etanercept monotherapy versus DMARD monotherapy; 3) etanercept plus DMARDs versus etanercept monotherapy and 4) etanercept monotherapy versus placebo at 24, 52, 104 and 156 weeks. Third, we report the ACR response rates by group of studies including DMARD‐naive; MTX‐inadequate response; and non‐MTX‐inadequate response participants. Table 6 shows study characteristics.
1. Subgroups ‐ study characteristics.
| Study | Follow‐up (weeks) | n | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Disease duration | Eligibility | Previously treated with |
| Bathon 2000 (ERA) | 52 | 632 | ET 10 mg | ET 25 mg | ‐ | ‐ | PBO | < 3 years | MTX‐naive | DMARDs |
| Combe 2006 | 104 | 254 | ‐ | ET 25 mg | ET 25 mg + SSZ | SSZ | ‐ | < 20 years | ‐ | SSZ |
| Emery 2008 (COMET) | 104 | 542 | ‐ | ‐ | ET 25 mg + MTX | MTX | ‐ | < 2 years | MTX‐naive | DMARDs (4 weeks prior enrolment) |
| Hu 2009 | 24 | 238 | ‐ | ET 25 mg | ‐ | MTX | ‐ | NS (mean 7.7 years) | ‐ | DMARDs |
| Kameda 2010 | 104 | 151 | ‐ | ET 25 mg | ET 25 mg + MTX | ‐ | ‐ | NS (but subgroups by < 10 and > 10 years) | ‐ | MTX |
| Klareskog 2004 (TEMPO) | 156 | 686 | ‐ | ET 25 mg | ET 25 mg + MTX | MTX | ‐ | < 20 years | no MTX 6 months prior enrolment | DMARDs |
| Marcora 2006 | 24 | 26 | ‐ | ET 25 mg | ‐ | MTX | ‐ | < 6 months | DMARDs‐naive | |
| Moreland 1999 | 26 | 234 | ET 10 mg | ET 25 mg | ‐ | ‐ | PBO | NS (mean 12 years) | ‐ | DMARDs |
| Weinblatt 1999 | 24 | 89 | ‐ | ‐ | ET 25 mg + MTX | MTX | ‐ | NS (mean 13 years) | ‐ | MTX |
DMARD: disease‐modifying anti‐rheumatic drug; ET: etanercept; MTX: methotrexate; NS: not stated; PBO: placebo; SSZ: sulphasalazine.
Etanercept plus DMARD versus DMARD (inadequate responders to DMARDs or MTX)
ACR response. The benefits and harms of etanercept plus DMARDs versus DMARDs in people who are partial responders to DMARDs is shown in Table 1. We found that people who had an inadequate response to traditional DMARDs and received the combination therapy were 2.0 (95% CI 1.3 to 2.9) and 2.2 (95% CI 1.5 to 3.3) times more likely to achieve an ACR50 and ACR70 response than those people who had an inadequate response to traditional DMARDs and continue with DMARD monotherapy at 24, 52, 104 and 156 weeks (Analysis 1.1; Analysis 1.2). Table 2 show the benefits and harms of etanercept plus DMARD versus DMARD in people who are partial responders to MTX. A higher proportion of people on etanercept plus DMARD achieved an ACR50 compared with DMARD monotherapy (RR 11.7; 95% CI 1.7 to 82.5) (Analysis 2.1). No other statistically significant differences were observed between groups at 24 weeks (Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6). However, the analysis was based only in one small study (Weinblatt 1999).
1.2. Analysis.
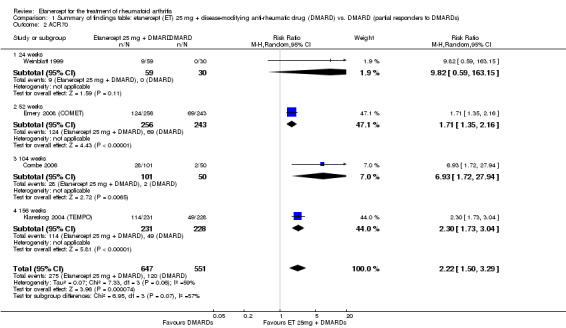
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 2 ACR70.
2.2. Analysis.
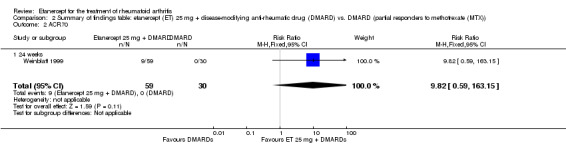
Comparison 2 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to methotrexate (MTX)), Outcome 2 ACR70.
2.4. Analysis.
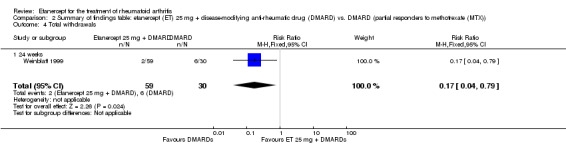
Comparison 2 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to methotrexate (MTX)), Outcome 4 Total withdrawals.
Remission score. Remission measured by DAS less than 2.6 was significantly more likely with etanercept 25 mg plus DMARD compared with DMARD alone (RR 1.9; 95% CI 1.6 to 2.3) (Analysis 1.3). The absolute treatment benefit (ATB) was 22%, with a NNTB of five people.
Disability score. People taking etanercept plus DMARD had significantly improved HAQ scores after treatment when compared with those taking DMARD alone (MD ‐0.36; 95% CI ‐0.43 to ‐0.28) (Analysis 1.4).
No other differences were observed in radiographic score, discontinuation rates or serious adverse events (Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9).
1.6. Analysis.
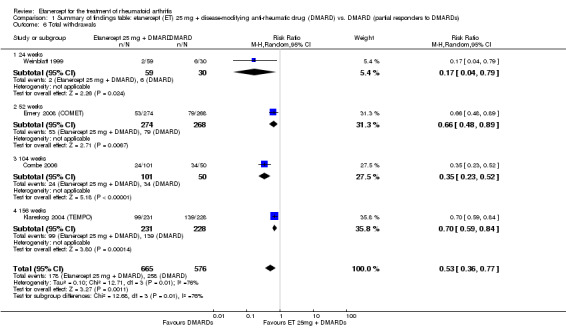
Comparison 1 Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs), Outcome 6 Total withdrawals.
Etanercept plus DMARD versus DMARD
Six months
ACR response. ACR20 response rates were significantly improved with etanercept plus DMARD compared with DMARD alone (Analysis 3.1). The RR of achieving an ACR20 was 2.7 (95% CI 1.8 to 3.8) and the RR of achieving an ACR50 was 4.7 (95% CI 2.4 to 9.3). About 47.5% achieved an ACR50 response in the etanercept plus DMARD group compared with 10% of DMARD alone group with an ATB of 37.5% and an NNTB of three people (Analysis 3.2; Table 3). ACR70 response rates were also significantly improved with etanercept plus DMARD (Analysis 3.3). The RR of achieving an ACR70 was 11.5 (95% CI 2.3 to 57.9). About 21.3% achieved an ACR70 in the etanercept plus DMARD group compared with 1% in the DMARD only group with an ATB of 20% and an NNTB of five people.
3.1. Analysis.
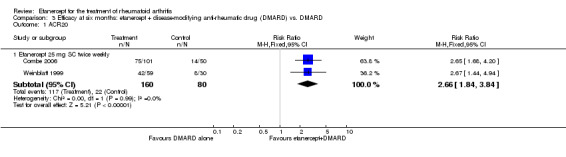
Comparison 3 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 ACR20.
3.2. Analysis.
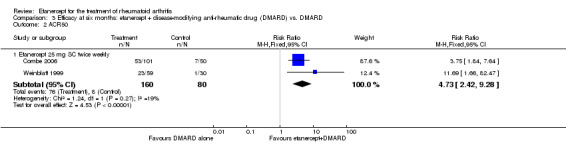
Comparison 3 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 ACR50.
3.3. Analysis.
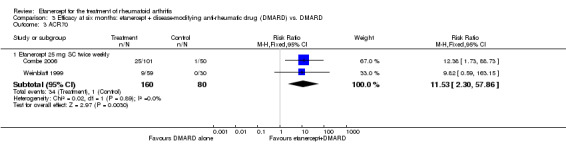
Comparison 3 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 ACR70.
DAS. Final DAS44 score was significantly lower with etanercept plus DMARD than with DMARD alone (MD ‐1.4; 95 CI ‐1.8 to ‐1.0) (Analysis 3.4).
3.4. Analysis.
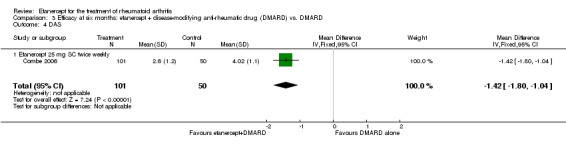
Comparison 3 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 DAS.
12 months
ACR response. All ACR response rates were significantly improved with etanercept 25 mg plus DMARD compared with DMARD alone (Analysis 4.1; Analysis 4.2; Analysis 4.3) (ACR 20: RR 1.2; 95% CI 1.1 to 1.3; ACR50: RR 1.5; 95% CI 1.4 to 1.7; ACR70: RR 1.9, 95% CI 1.6 to 2.3). The ATB was 15% with an NNTB of seven people for ACR20. The ATB was 24% and 22% for ACR50 (Table 3) and ACR70, respectively, with an NNTB of four people.
4.1. Analysis.
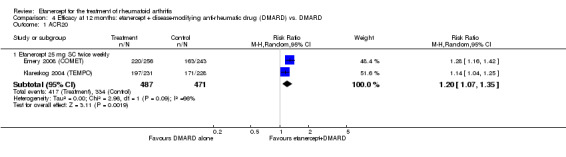
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 ACR20.
4.2. Analysis.
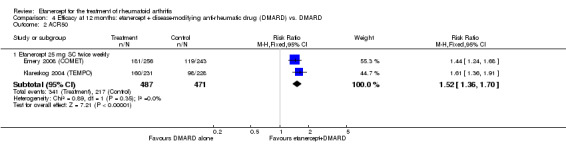
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 ACR50.
4.3. Analysis.
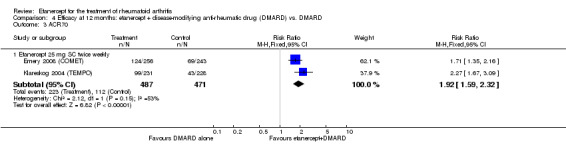
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 ACR70.
Remission scores. Remission (DAS < 1.6 and < 2.6) was significantly more likely with etanercept 25 mg plus DMARD compared with DMARD alone (Analysis 4.4; Analysis 4.5) (DAS: RR 2.7; 95% CI 1.9 to 3.8); DAS28: RR 2.0; 95% CI 1.6 to 2.4, respectively). The ATB was 23% and 21%, with an NNTB of four and five people, respectively.
4.4. Analysis.
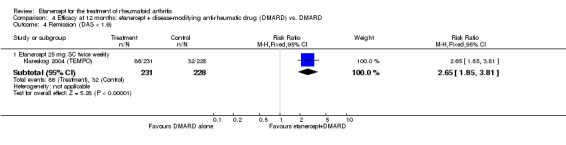
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Remission (DAS < 1.6).
4.5. Analysis.
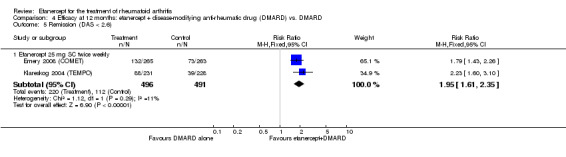
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 5 Remission (DAS < 2.6).
Radiographic scores. All changes in radiographic scores were significantly less with etanercept 25 mg plus DMARD compared with DMARD alone (Analysis 4.6; Analysis 4.7; Analysis 4.8) (TSS: MD ‐2.2; 95% CI ‐3.0 to ‐1.4; ES: MD ‐1.6; 95% CI ‐2.4 to ‐0.9; JSNS: MD ‐0.7; 95% CI ‐1.1 to ‐0.2). The proportion of participants with no evidence of joint damage (as measured by modified TSS ≤ 0.5) was significantly higher with etanercept plus DMARD treatment when compared with DMARD alone (Analysis 4.9) (RR 1.4; 95% CI 1.3 to 1.5) with an NNTB of five people (Table 5).
4.7. Analysis.
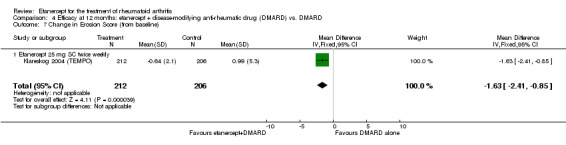
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 7 Change in Erosion Score (from baseline).
4.8. Analysis.
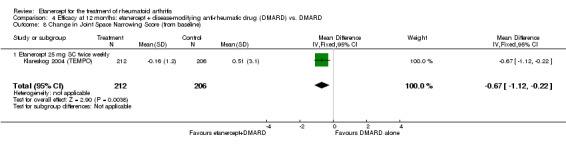
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 8 Change in Joint Space Narrowing Score (from baseline).
4.9. Analysis.
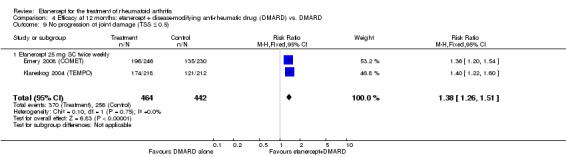
Comparison 4 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 9 No progression of joint damage (TSS ≤ 0.5).
Two and three years
ACR response. At two years, all ACR response rates were significantly improved with etanercept 25 mg plus DMARD compared with DMARD alone (Analysis 5.1; Analysis 5.3; Analysis 5.2) (ACR 20: RR 1.5; 95% CI 1.2 to 1.9; ACR50: RR 1.9; 95% CI 1.3 to 2.8; ACR70: RR 2.2; 95% CI 1.5 to 3.3). The ATB was 21.5% for ACR20, 28.8% for ACR50 and 24.1% for ACR70, resulting in NNTBs of four, three and four people respectively.
5.1. Analysis.
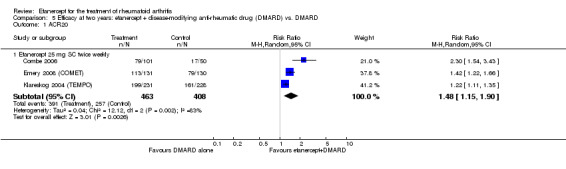
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 ACR20.
5.3. Analysis.
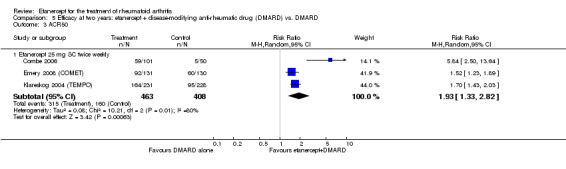
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 ACR50.
5.2. Analysis.
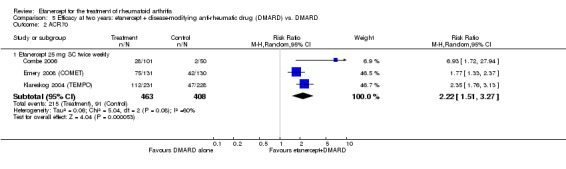
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 ACR70.
At three years, all ACR response rates were significantly improved with etanercept plus DMARD when compared with DMARD alone (Analysis 6.1; Analysis 6.2; Analysis 6.3) (ACR20: RR 1.2, 95% CI 1.1 to 1.4); ACR50: RR 1.6; 95% CI 1.3 to 1.9 (Table 3); ACR70: RR 2.3; 95% CI 1.7 to 3.0). The ATB was 15%, 24% and 28% and the NNTBs were six, four and four people, respectively.
6.1. Analysis.
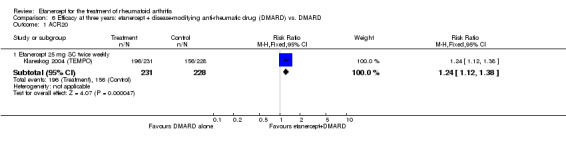
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 ACR20.
6.2. Analysis.
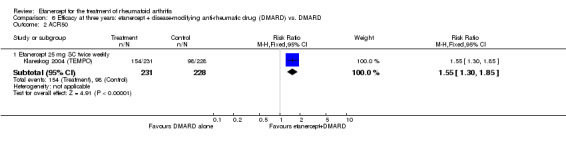
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 ACR50.
6.3. Analysis.
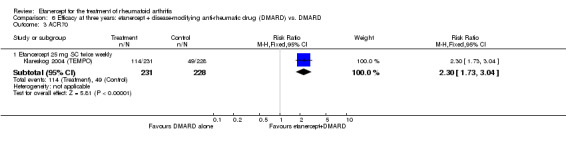
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 ACR70.
Remission scores. At two years, remission rates, as measured by DAS less than 1.6 and DAS28 less than 2.6, were significantly improved with etanercept 25 mg plus DMARD compared with DMARD alone (RR 2.6; 95% CI 1.8 to 3.6 and RR 2.2; 95% CI 1.7 to 2.7, respectively) (Analysis 5.4; Analysis 5.5). The ATB was 25% and 23%, resulting in NNTBs of four and four people, respectively. Final DAS44 score was significantly lower with etanercept plus DMARD than with DMARD alone (MD ‐2.0; 95 CI ‐2.4 to ‐1.6) (Analysis 5.10).
5.4. Analysis.
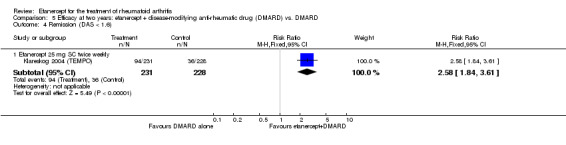
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Remission (DAS < 1.6).
5.5. Analysis.
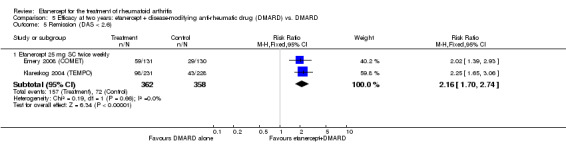
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 5 Remission (DAS < 2.6).
5.10. Analysis.
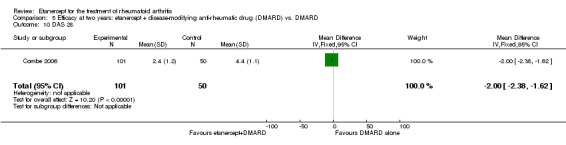
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 10 DAS 28.
At three years, remission, as measured by either DAS less than 1.6 or DAS28 less than 2.6, was significantly more likely with etanercept plus DMARD when compared with DMARD alone (RR 2.3; 95% CI 1.7 to 3.2 and RR 2.1; 95% CI 1.6 to 2.9, respectively) (Analysis 6.4; Analysis 6.5). The ATBs were 23% and 19% resulting in NNTBs of four and five people, respectively.
6.4. Analysis.
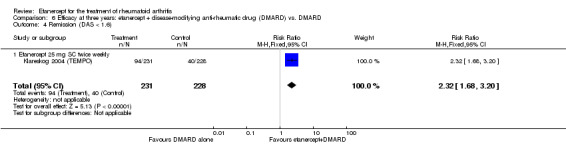
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Remission (DAS < 1.6).
6.5. Analysis.
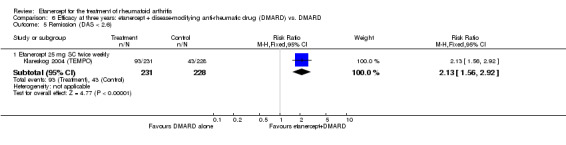
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 5 Remission (DAS < 2.6).
Radiographic scores. At two years, all changes in radiographic scores were significantly less with etanercept 25 mg plus DMARD when compared with DMARD alone (TSS: MD ‐3.9; 95% CI ‐6.1 to ‐1.7; ES: MD ‐2.9; 95% CI ‐4.4 to ‐1.4; JSNS: MD ‐1.0; 95% CI ‐1.9 to ‐0.2) (Analysis 5.6; Analysis 5.7; Analysis 5.8). The proportion of participants with no evidence of joint damage (as measured by modified TSS ≤ 0.5) was significantly higher with etanercept plus DMARD treatment when compared with DMARD alone (RR 1.3; 95% CI 1.2 to 1.5) (Analysis 5.9) with an NNTB of five people (Table 5).
5.6. Analysis.
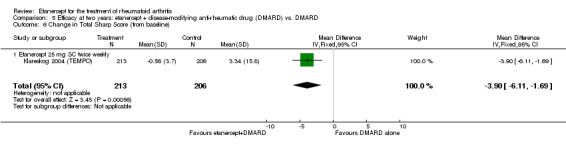
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 6 Change in Total Sharp Score (from baseline).
5.7. Analysis.
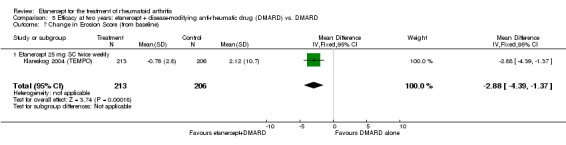
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 7 Change in Erosion Score (from baseline).
5.8. Analysis.
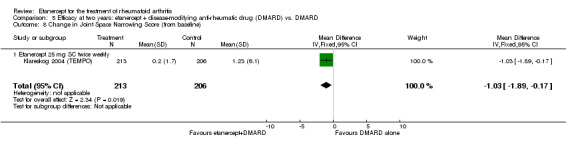
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 8 Change in Joint Space Narrowing Score (from baseline).
5.9. Analysis.
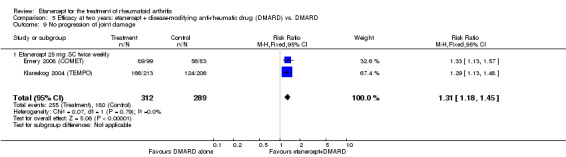
Comparison 5 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 9 No progression of joint damage.
At three years, changes from baseline in all radiographic scores (TSS, ES, JSNS) were significantly less with etanercept plus DMARD when compared with DMARD alone (TSS: MD ‐6.1; 95% CI ‐9.2 to ‐3.0; ES: MD ‐3.9; 95% CI ‐5.7 to ‐2.1; JSNS: MD ‐2.2; 95% CI ‐3.8 to ‐0.6) (Analysis 6.6; Analysis 6.7; Analysis 6.8). Participants taking etanercept plus DMARD were more likely to have no progression of their joint damage (as assessed by modified TSS ≤ 0.5) when compared with those taking DMARD alone (RR 1.5; 95% CI 1.3 to 1.7) (Analysis 6.9).
6.6. Analysis.
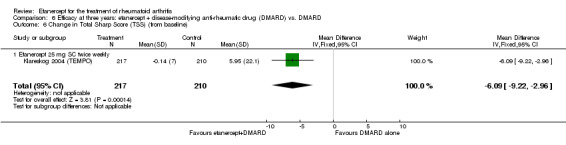
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 6 Change in Total Sharp Score (TSS) (from baseline).
6.7. Analysis.
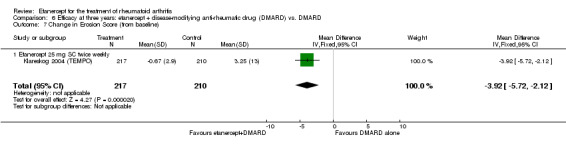
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 7 Change in Erosion Score (from baseline).
6.8. Analysis.
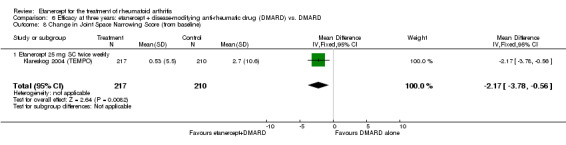
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 8 Change in Joint Space Narrowing Score (from baseline).
6.9. Analysis.
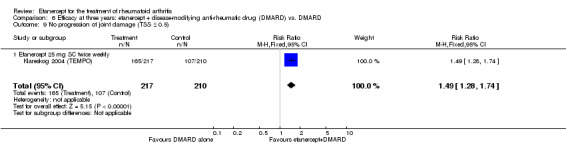
Comparison 6 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 9 No progression of joint damage (TSS ≤ 0.5).
Etanercept versus DMARD
Six months
ACR response. There was no evidence of a statistical difference in ACR20, ACR50 or ACR70 response rates between those who received etanercept 10 mg compared with those who received DMARD after six months (Analysis 7.1; Analysis 7.2; Analysis 7.3). There was a trend towards a significant improvement in ACR20 in those receiving etanercept 25 mg monotherapy compared with those who received DMARD monotherapy after six months (RR 1.4; 95% CI 0.97 to 1.9) (Analysis 7.1); ACR50 and ACR70 response rates were significantly improved in those receiving etanercept 25 mg monotherapy compared with those who received DMARD monotherapy after six months (ACR50: RR 1.6; 95% CI 1.0 to 2.4; ACR70: RR 2.0; 95% CI 1.1 to 3.5) (Analysis 7.2, and Analysis 7.3, respectively). About 41% of participants taking etanercept achieved an ACR50 (compared with 28.7% of those taking DMARD with an ATB of 12.3% and an NNTB of eight people (Table 3). About 20.6% of participants taking etanercept achieved an ACR70 (compared with 10.9% of those taking MTX) with an ATB of 9.8% and an NNTB of 10 people.
7.1. Analysis.
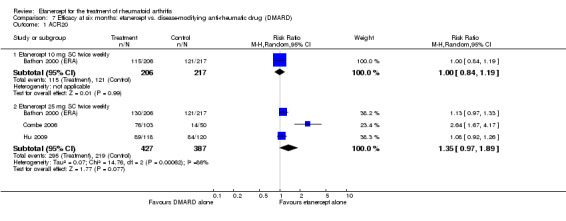
Comparison 7 Efficacy at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 ACR20.
7.2. Analysis.
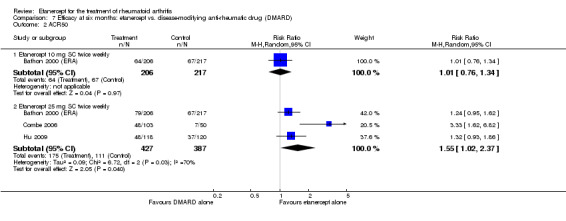
Comparison 7 Efficacy at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 ACR50.
7.3. Analysis.
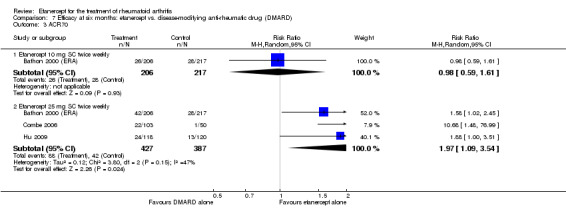
Comparison 7 Efficacy at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 ACR70.
Radiographic scores. There was no difference from baseline in any of the radiographic scores (TSS, ES and JSNS) between etanercept 10 mg monotherapy and DMARD monotherapy (Analysis 7.4; Analysis 7.5; Analysis 7.6). However, there were significant differences between etanercept 25 mg and DMARD for TSS and ES. The difference from baseline in the TSS was significantly less in those who received etanercept 25 mg compared with those who received DMARD (MD ‐0.5; 95% CI ‐0.9 to ‐0.1) (Analysis 7.4). The difference from baseline in ES was significantly less in those who received etanercept 25 mg compared with those who received DMARD (MD ‐0.4; 95% CI ‐0.7 to ‐0.1) (Analysis 7.5). There was no evidence of a statistical difference between changes from baseline in the JSNS between those who received etanercept 25 mg compared with those who received MTX (Analysis 7.6).
7.4. Analysis.
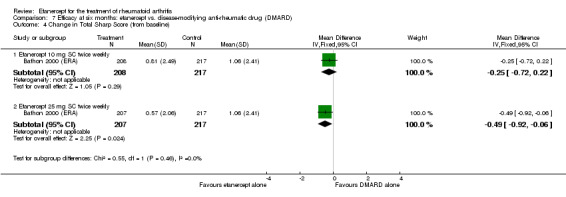
Comparison 7 Efficacy at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Change in Total Sharp Score (from baseline).
7.5. Analysis.
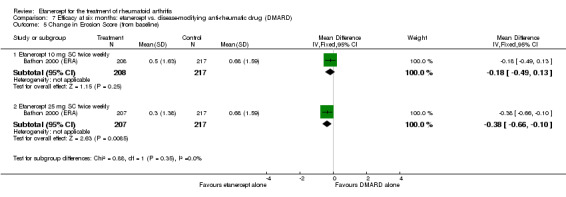
Comparison 7 Efficacy at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 5 Change in Erosion Score (from baseline).
7.6. Analysis.
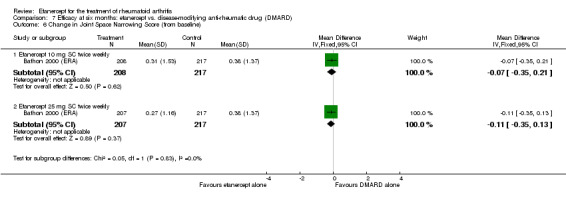
Comparison 7 Efficacy at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 6 Change in Joint Space Narrowing Score (from baseline).
DAS28. There was no evidence of a significant difference in the final values of the DAS scores between etanercept 25 mg monotherapy and the DMARD monotherapy groups (Analysis 7.7).
7.7. Analysis.
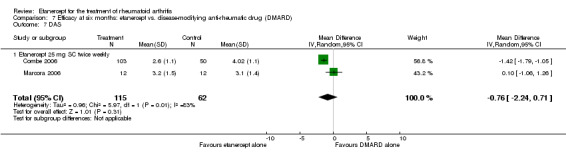
Comparison 7 Efficacy at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 7 DAS.
12 months
ACR response. There was no significant difference in most ACR response rates between either dose of etanercept alone (10 or 25 mg) compared with DMARD alone (Analysis 8.1; Analysis 8.2; Analysis 8.3). However, ACR50 response rates were significantly lower for those receiving etanercept 10 mg compared with those receiving MTX (RR 0.8; 95% CI 0.6 to 1.0).
8.1. Analysis.
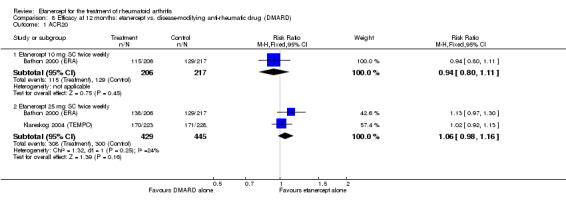
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 ACR20.
8.2. Analysis.
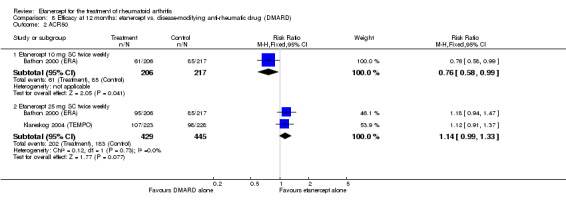
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 ACR50.
8.3. Analysis.
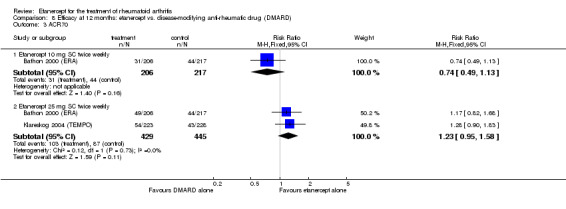
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 ACR70.
Remission scores. There was no evidence of a significant difference in proportions achieving remission (as measured by DAS < 1.6 and DAS28 < 2.6) with etanercept 25 mg when compared with DMARD (Analysis 8.4; Analysis 8.5).
8.4. Analysis.
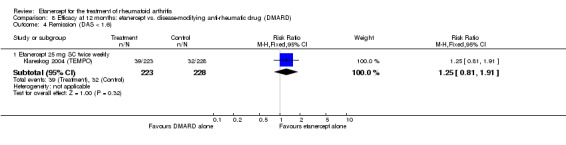
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Remission (DAS < 1.6).
8.5. Analysis.
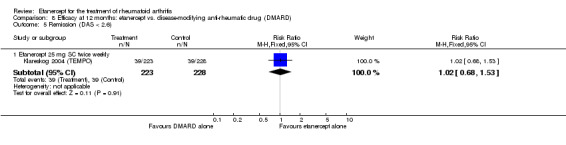
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 5 Remission (DAS < 2.6).
Radiographic scores. There was no evidence of a difference from baseline in any of the radiographic scores (TSS, ES and JSNS) between etanercept 10 mg and DMARD (Analysis 8.6; Analysis 8.7; Analysis 8.8). Changes in the TSS and ES from baseline were significantly less for those receiving etanercept 25 mg compared with those receiving DMARD (Analysis 8.6; Analysis 8.7) (MD ‐0.7; 95% CI ‐1.4 to ‐0.1 and MD ‐0.7; 95% CI ‐1.0 to ‐0.3, respectively). However, there was no evidence of a significant difference in the changes from baseline in the JSNS between etanercept 25 mg and DMARD (Analysis 8.8). The proportion of participants with no evidence of joint damage (as measured by modified TSS ≤ 0.5) was significantly higher with etanercept treatment when compared with DMARD (RR 1.2; 95% CI 1.0 to 1.4) (Analysis 8.9) with an NNTB of nine people (Table 5).
8.6. Analysis.
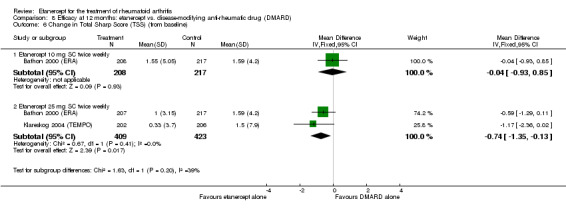
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 6 Change in Total Sharp Score (TSS) (from baseline).
8.7. Analysis.
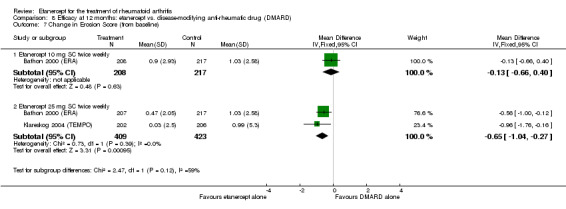
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 7 Change in Erosion Score (from baseline).
8.8. Analysis.
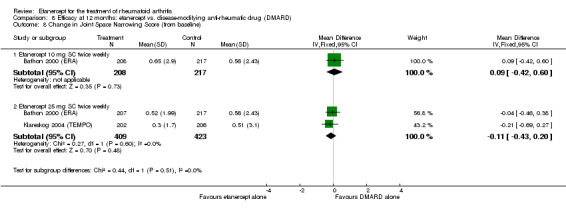
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 8 Change in Joint Space Narrowing Score (from baseline).
8.9. Analysis.
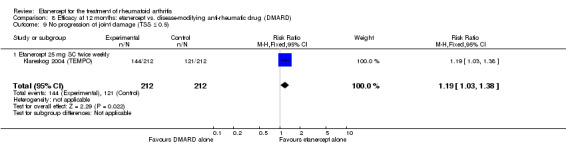
Comparison 8 Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 9 No progression of joint damage (TSS ≤ 0.5).
Two and three years
ACR response. At two years, there was no evidence of a difference in ACR20, ACR50 and ACR70 response rates between etanercept monotherapy and DMARD monotherapy (Analysis 9.1; Analysis 9.2; Analysis 9.3).
9.1. Analysis.
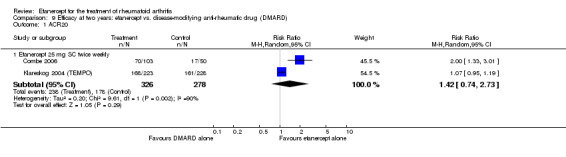
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 ACR20.
9.2. Analysis.
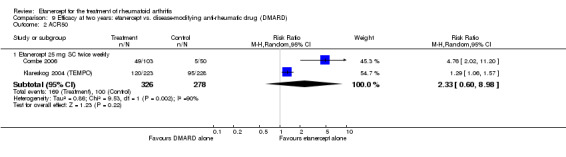
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 ACR50.
9.3. Analysis.
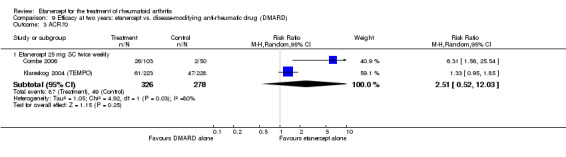
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 ACR70.
At three years, there was no evidence of a difference in any of the ACR response rates between those receiving etanercept 25 mg alone when compared with DMARD alone (Analysis 10.1; Analysis 10.2; Analysis 10.3).
10.1. Analysis.
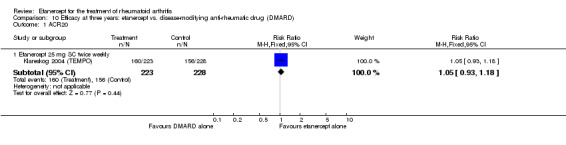
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 ACR20.
10.2. Analysis.
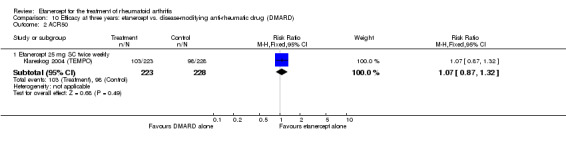
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 ACR50.
10.3. Analysis.
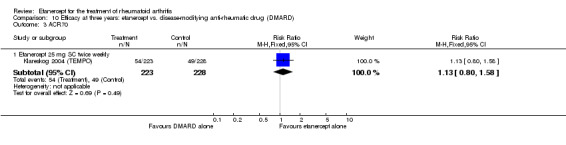
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 ACR70.
Remission scores. At two years, remission (as measured by DAS < 1.6) was significantly more likely with etanercept 25 mg when compared with MTX (RR 1.5; 95% CI 1.01 to 2.2) (Analysis 9.4). However, there was no evidence of a difference in remission rates (as measured by DAS28 < 2.6) between etanercept 25 mg and MTX (Analysis 9.5). Final DAS44 score was significantly lower with etanercept alone than with DMARD alone (MD ‐1.7; 95 CI ‐2.1 to ‐1.3) (Analysis 9.10).
9.4. Analysis.
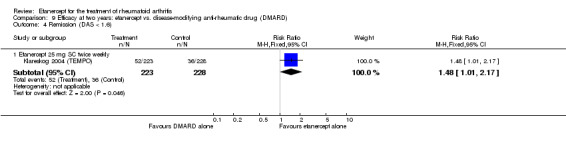
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Remission (DAS < 1.6).
9.5. Analysis.
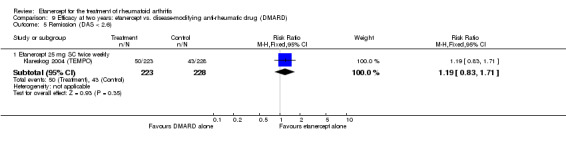
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 5 Remission (DAS < 2.6).
9.10. Analysis.
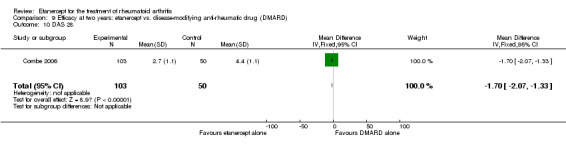
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 10 DAS 28.
At three years, there was no evidence of a difference in remission, as measured by either DAS less than 1.6 or DAS28 less than 2.6, between those receiving etanercept 25 mg monotherapy when compared with DMARD monotherapy (Analysis 10.4; Analysis 10.5).
10.4. Analysis.
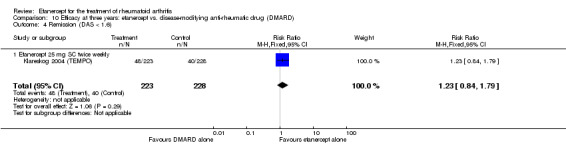
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Remission (DAS < 1.6).
10.5. Analysis.
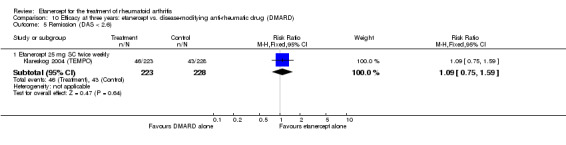
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 5 Remission (DAS < 2.6).
Radiographic scores. At two years, the change from baseline in ES was significantly less with etanercept 25 mg monotherapy compared with DMARD monotherapy (MD ‐1.8, 95% CI ‐3.3 to ‐0.2) (Analysis 9.7). However, there was no evidence of a difference in the changes from baseline for either TSS or JSNS between groups (Analysis 9.6; Analysis 9.8). The change in TSS was just outside the 0.05 level of significance. There was also no evidence of a difference in the proportion of participants with no evidence of joint damage (as measured by modified TSS ≤ 0.5) between groups (Analysis 9.9).
9.7. Analysis.
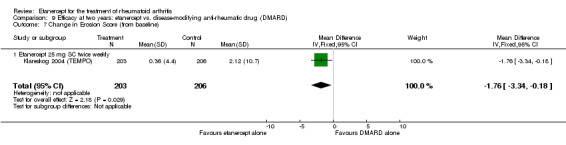
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 7 Change in Erosion Score (from baseline).
9.6. Analysis.
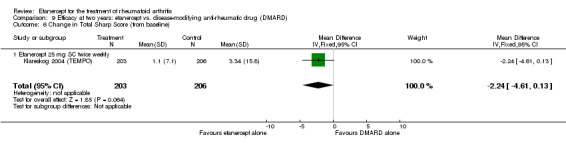
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 6 Change in Total Sharp Score (from baseline).
9.8. Analysis.
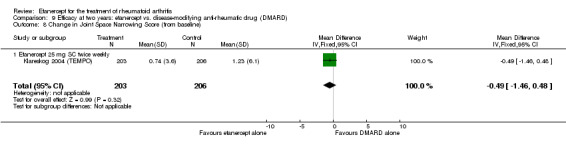
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 8 Change in Joint Space Narrowing Score (from baseline).
9.9. Analysis.
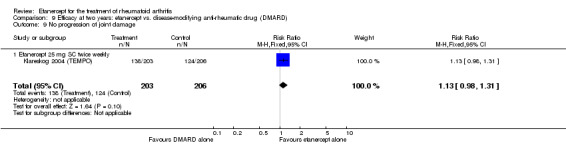
Comparison 9 Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 9 No progression of joint damage.
At three years, the change from baseline in the TSS and ES was significantly reduced with etanercept 25 mg monotherapy when compared with DMARD monotherapy (TSS: MD ‐4.3; 95% CI ‐7.6 to ‐1.1; ES: MD ‐2.9; 95% CI ‐4.8 to ‐0.9) (Analysis 10.6; Analysis 10.7). There was no evidence of a statistical difference in the change from baseline in the JSNS between groups but the summary estimate was just outside the 0.05 level of significance in favour of etanercept monotherapy (Analysis 10.8). Participants taking etanercept alone were more likely to have no progression of their joint damage (as assessed by modified TSS ≤ 0.5) when compared with those taking DMARD alone (RR 1.2, 95% CI 1.0 to 1.4) (Analysis 10.9).
10.6. Analysis.
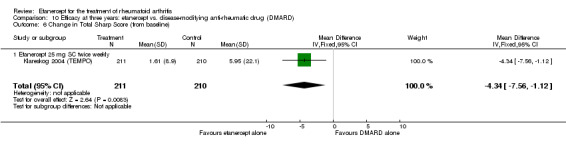
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 6 Change in Total Sharp Score (from baseline).
10.7. Analysis.
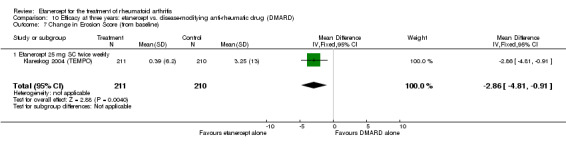
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 7 Change in Erosion Score (from baseline).
10.8. Analysis.
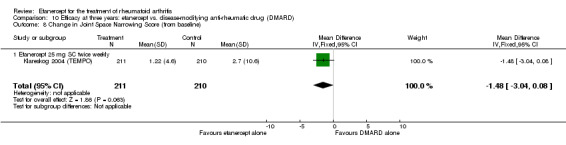
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 8 Change in Joint Space Narrowing Score (from baseline).
10.9. Analysis.
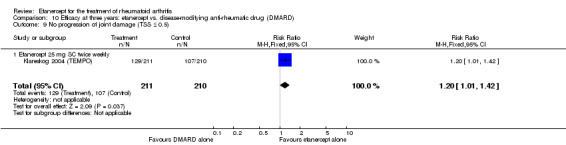
Comparison 10 Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 9 No progression of joint damage (TSS ≤ 0.5).
Etanercept plus DMARD versus etanercept alone
Six months
ACR response. ACR20 and ACR70 response rates were not significantly different between etanercept plus DMARD treatment and etanercept alone (Analysis 11.1; Analysis 11.3). There was a trend favouring the etanercept plus DMARD group in improvement of ACR50 response rates when compared with etanercept monotherapy. The ATB was 10%, resulting in NNTBs of 10 people (Table 3).
11.1. Analysis.
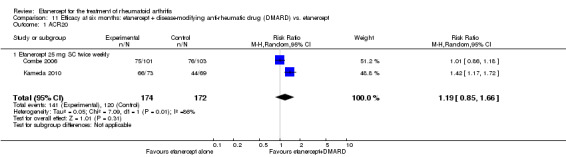
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 ACR20.
11.3. Analysis.
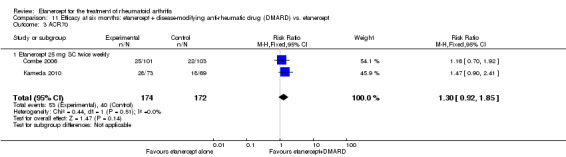
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 ACR70.
Remission scores. Significantly more people receiving etanercept plus DMARD were reported as having achieved remission (DAS < 1.6 and DAS28 < 2.6) when compared with those receiving etanercept alone (RR 1.6; 95% CI 1.1 to 2.3 and RR 2.7; 95% CI 1.2 to 6.0, respectively) (Analysis 11.4; Analysis 11.5). The ATBs were 19% and 17%, resulting in NNTBs of five and six people, respectively. People assigned to etanercept plus DMARD were more likely to have a good European League Against Rheumatism (EULAR) response than those assigned to etanercept alone (Analysis 11.6). Similarly, the etanercept plus DMARD group were less likely to have no EULAR response (Analysis 11.8). There were no statistically significant differences in moderate EULAR response rates between groups or the DAS final values (Analysis 11.7; Analysis 11.9).
11.4. Analysis.
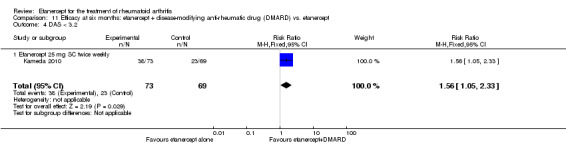
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 DAS < 3.2.
11.5. Analysis.
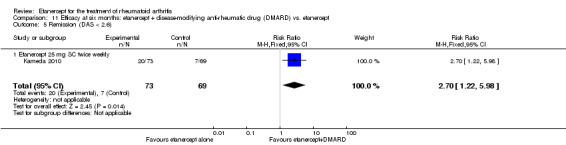
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 5 Remission (DAS < 2.6).
11.6. Analysis.
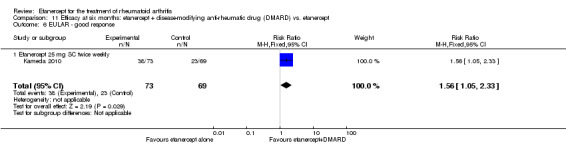
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 6 EULAR ‐ good response.
11.8. Analysis.
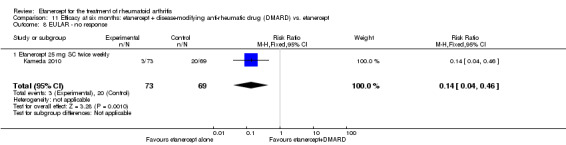
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 8 EULAR ‐ no response.
11.7. Analysis.
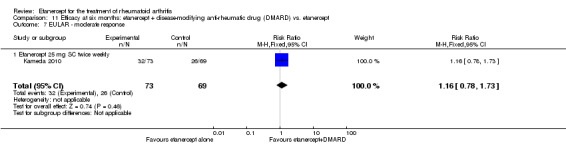
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 7 EULAR ‐ moderate response.
11.9. Analysis.
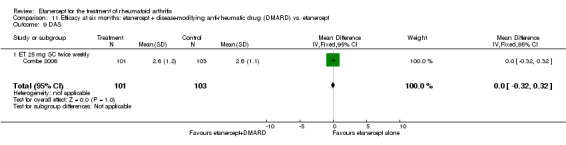
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 9 DAS.
12 months
ACR response. All ACR response rates (ACR20, ACR50 and ACR70) were significantly improved with etanercept plus DMARD when compared with etanercept alone (RR 1.1; 95% CI 1.02 to 1.2; RR 1.4; 95% CI 1.2 to 1.7; RR 1.8; 95% CI 1.3 to 2.3, respectively) (Analysis 12.1; Analysis 12.2; Analysis 12.3). The ATB was 9% for ACR20, 21% for ACR50 and 24% for ACR70, resulting in NNTBs of 11, five and five people, respectively.
12.1. Analysis.
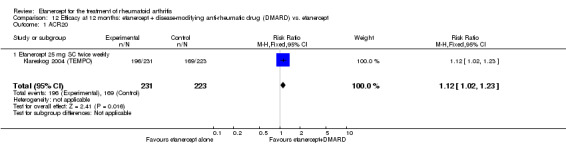
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 ACR20.
12.2. Analysis.
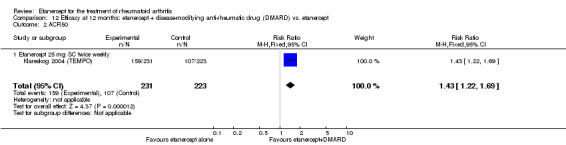
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 ACR50.
12.3. Analysis.
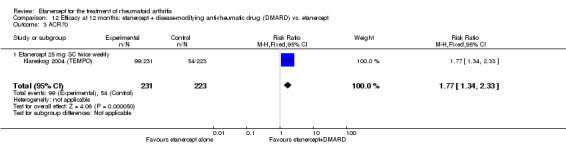
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 ACR70.
Remission scores. Significantly more people receiving etanercept plus DMARD were reported as having achieved remission (DAS < 1.6 and DAS28 < 2.6) when compared with those receiving etanercept alone (RR 2.1; 95% CI 1.5 to 3.0 and RR 2.2; 95% CI 1.6 to 3.0, respectively) (Analysis 12.4; Analysis 12.5). The ATBs for both scores was approximately 20%, resulting in NNTBs of five people.
12.4. Analysis.
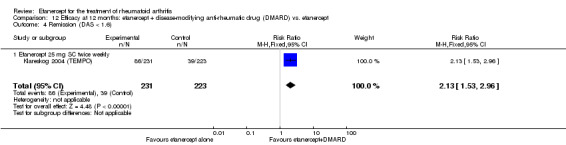
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Remission (DAS < 1.6).
12.5. Analysis.
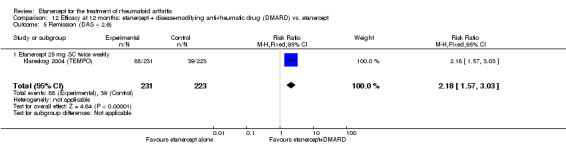
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 5 Remission (DAS < 2.6).
Radiographic scores. All changes from baseline in TSS, ES and JSNS were significantly reduced with etanercept plus DMARD when compared with etanercept alone (TSS: RR ‐1.1; 95% CI ‐1.8 to ‐0.5; ES: RR ‐0.7; 95% CI ‐1.1 to ‐0.2; JSNS: RR ‐0.5; 95% CI ‐0.7 to ‐0.2) (Analysis 12.6; Analysis 12.7; Analysis 12.8). The proportion of participants with no evidence of joint damage (as measured by modified TSS ≤ 0.5) was significantly higher with etanercept plus DMARD when compared with etanercept alone (RR 1.2; 95% CI 1.1 to 1.3) (Analysis 12.9) with an NNTB of eight people.
12.6. Analysis.
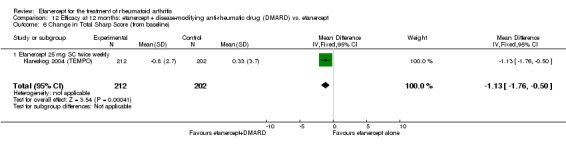
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 6 Change in Total Sharp Score (from baseline).
12.7. Analysis.
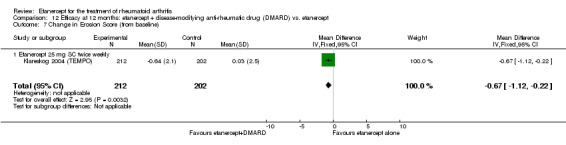
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 7 Change in Erosion Score (from baseline).
12.8. Analysis.
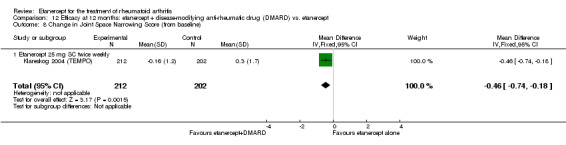
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 8 Change in Joint Space Narrowing Score (from baseline).
12.9. Analysis.
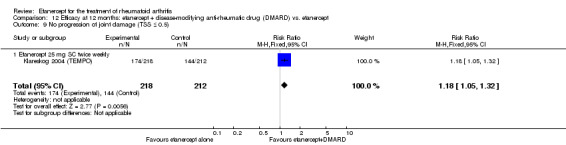
Comparison 12 Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 9 No progression of joint damage (TSS ≤ 0.5).
Two and three years
ACR response. At two years, ACR20 and ACR50 response rates were significantly improved with etanercept plus DMARD when compared with etanercept alone (RR 1.2; 95% CI 1.1 to 1.2; RR 1.5; 95% CI 1.1 to 2.0, respectively) (Analysis 13.1; Analysis 13.2) . The ATB was 11% and 24%, resulting in NNTBs of nine and four people, respectively. ACR70 response rates were not different between groups (Analysis 13.3).
13.1. Analysis.
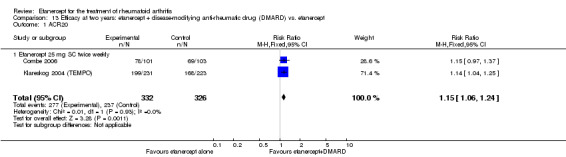
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 ACR20.
13.2. Analysis.
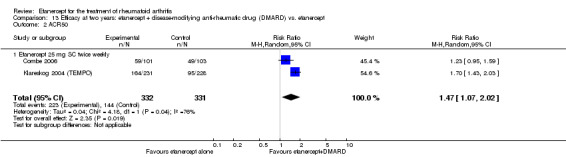
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 ACR50.
13.3. Analysis.
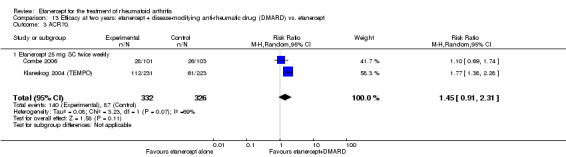
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 ACR70.
At three years, all ACR response rates (ACR20, ACR50 and ACR70) were significantly improved with etanercept plus DMARD when compared with etanercept alone (RR 1.2; 95% CI 1.1 to 1.3; RR 1.4; 95% CI 1.2 to 1.7; RR 2.0; 95% CI 1.6 to 2.7, respectively) (Analysis 14.1; Analysis 14.2; Analysis 14.3). The ATBs were 13%, 21% and 25%, resulting in NNTBs of eight, five and four people, respectively.
14.1. Analysis.
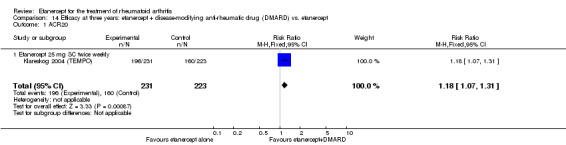
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 ACR20.
14.2. Analysis.
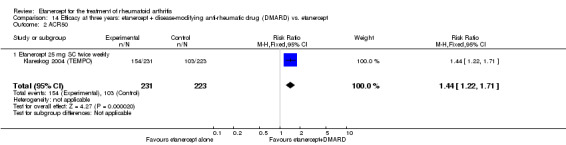
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 ACR50.
14.3. Analysis.
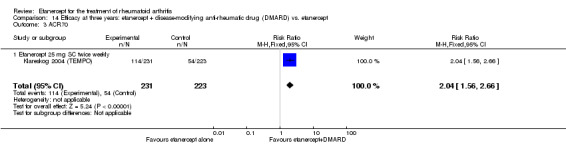
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 ACR70.
Remission scores. At two years, remission rates, as measured by DAS less than 1.6 and DAS28 less than 2.6, were significantly increased with etanercept plus DMARD compared with etanercept alone (RR 1.8; 95% CI 1.3 to 2.3; RR 1.9; 95% CI 1.4 to 2.5, respectively) (Analysis 13.4; Analysis 13.5). The ATB was 17% and 20%, resulting in NNTBs of six and five people, respectively. Final DAS values were not different between groups (Analysis 13.10).
13.4. Analysis.
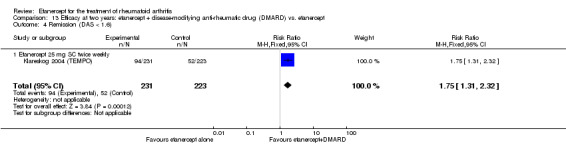
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Remission (DAS < 1.6).
13.5. Analysis.
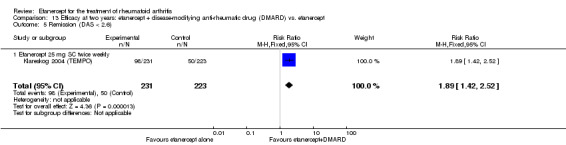
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 5 Remission (DAS < 2.6).
13.10. Analysis.
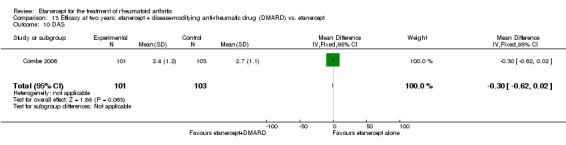
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 10 DAS.
At three years, people receiving etanercept plus DMARD were significantly more likely to go into remission (DAS < 1.6 or DAS28 < 2.6) when compared with those taking etanercept alone (RR 1.9; 95% CI 1.4 to 2.5 and RR 2.0; 95% CI 1.4 to 2.6 respectively) (Analysis 14.4; Analysis 14.5). The ATBs were 19% and 20% resulting in NNTBs of five people.
14.4. Analysis.
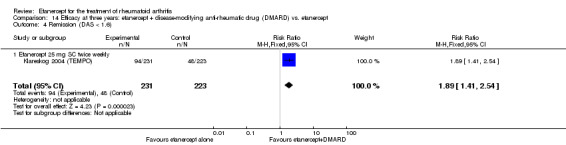
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Remission (DAS < 1.6).
14.5. Analysis.
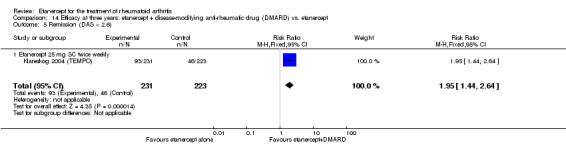
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 5 Remission (DAS < 2.6).
Radiographic scores. At two years, all radiographic scores were significantly improved with etanercept plus DMARD when compared with etanercept alone (TSS: RR ‐1.7; 95% CI ‐2.8 to ‐0.6; ES: RR ‐1.1; 95% CI ‐1.8 to ‐0.4; JSNS: RR ‐0.5; 95% CI ‐1.1 to 0.01) (Analysis 13.6; Analysis 13.7; Analysis 13.8). The proportion of participants with no evidence of joint damage (as measured by modified TSS ≤ 0.5) was significantly higher with etanercept plus DMARD treatment when compared with etanercept alone (RR 1.2, 95% CI 1.0 to 1.3) (Analysis 13.9) with NNTBs of 10 people.
13.6. Analysis.
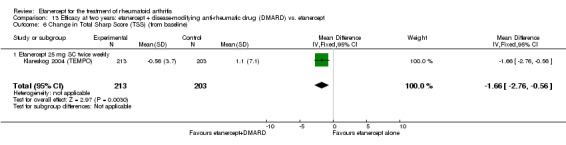
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 6 Change in Total Sharp Score (TSS) (from baseline).
13.7. Analysis.
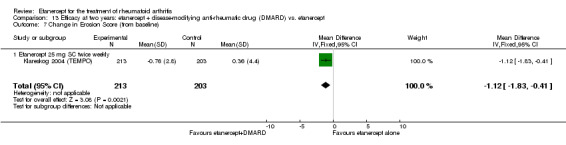
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 7 Change in Erosion Score (from baseline).
13.8. Analysis.
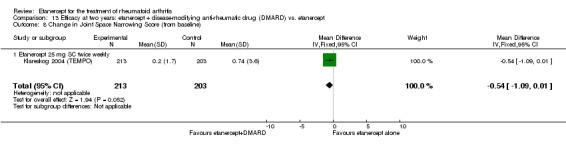
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 8 Change in Joint Space Narrowing Score (from baseline).
13.9. Analysis.
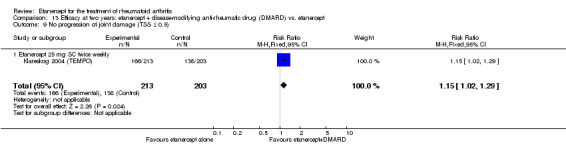
Comparison 13 Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 9 No progression of joint damage (TSS ≤ 0.5).
At three years, both TSS and ES scores were significantly improved with etanercept plus DMARD when compared with etanercept alone (TSS: RR ‐1.8; 95% CI ‐3.3 to ‐0.2; RR ‐1.1; 95% CI ‐2.0 to ‐0.1, respectively) (Analysis 14.6; Analysis 14.7). There was no evidence of a difference in JSNS between groups (Analysis 14.8). Participants taking etanercept plus DMARD were more likely to have no progression of their joint damage (as assessed by modified TSS ≤ 0.5) when compared with those taking etanercept monotherapy (RR 1.2; 95% CI 1.1 to 1.4) (Analysis 14.9).
14.6. Analysis.
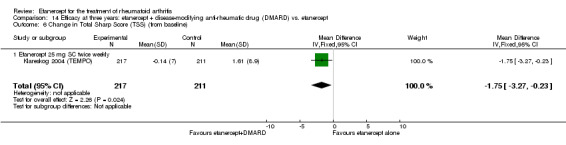
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 6 Change in Total Sharp Score (TSS) (from baseline).
14.7. Analysis.
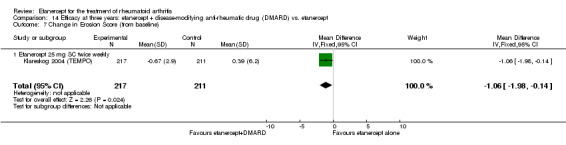
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 7 Change in Erosion Score (from baseline).
14.8. Analysis.
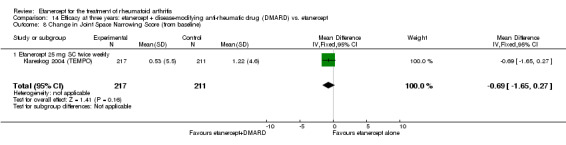
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 8 Change in Joint Space Narrowing Score (from baseline).
14.9. Analysis.
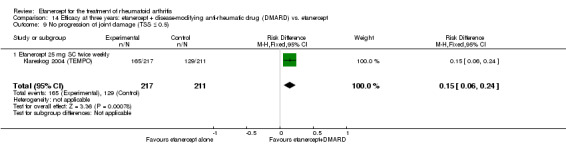
Comparison 14 Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 9 No progression of joint damage (TSS ≤ 0.5).
Etanercept versus placebo
Six months
ACR response. After six months of therapy, ACR20 and ACR50 response rates were significantly improved with both etanercept doses compared with the placebo group (Analysis 15.1; Analysis 15.2). For etanercept 10 mg SC twice weekly compared with placebo, the RR of achieving an ACR20 and ACR50 were 4.6 (95% CI 2.4 to 8.8) and 5.9 (95% CI 1.9 to 18.4), respectively, and there was a trend favouring etanercept 10 mg in improvement of ACR70 when compared with placebo. For etanercept 25 mg SC twice weekly compared with placebo, the RR of achieving an ACR20, ACR50 and ACR70 were 5.2 (95% CI 2.8 to 10.0), 12.5 (95% CI 4.2 to 37.8) and 12.3 (95% CI 1.6 to 92.4), respectively (Analysis 15.1; Analysis 15.2; Analysis 15.3).
15.1. Analysis.
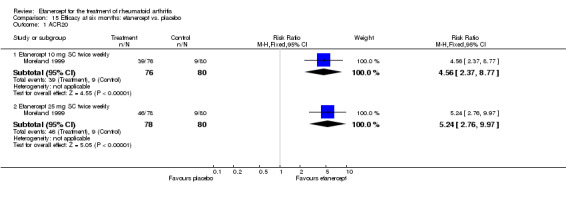
Comparison 15 Efficacy at six months: etanercept vs. placebo, Outcome 1 ACR20.
15.2. Analysis.
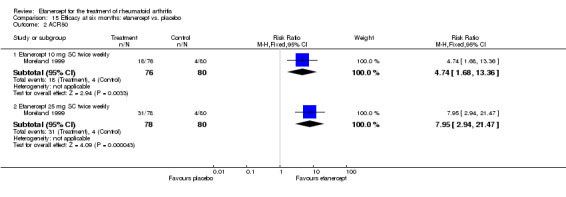
Comparison 15 Efficacy at six months: etanercept vs. placebo, Outcome 2 ACR50.
15.3. Analysis.
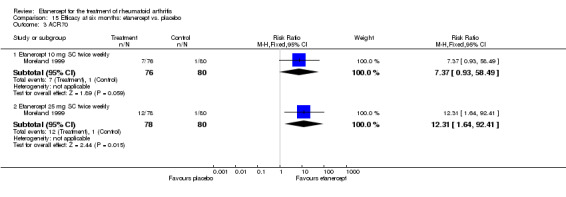
Comparison 15 Efficacy at six months: etanercept vs. placebo, Outcome 3 ACR70.
B. Quality of life
A large number of different scales and questionnaires were used in the included trials to evaluate quality of life. For the purposes of this review, data were extracted from those measures that were most frequently used: HAQ, Medical Outcome Study (MOS) SF‐36, EQ‐5D VAS, and Arthritis‐Specific Health Index (ASHI).
Etanercept plus DMARD versus DMARD
Six months
There was evidence of a significant difference in the final value for the HAQ score between groups (MD ‐0.49; 95% CI ‐0.77 to ‐0.21) (Analysis 16.1). Significantly higher values were found in the final EQ‐5D scores for those receiving etanercept 25 mg plus DMARD when compared with DMARD alone (MD 18.6; 95% CI 11.8 to 25.4) (Analysis 16.2).
16.1. Analysis.
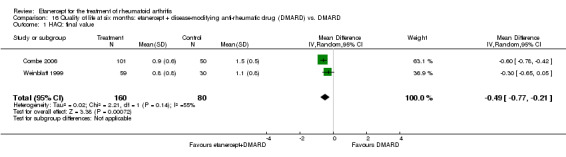
Comparison 16 Quality of life at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 HAQ: final value.
16.2. Analysis.
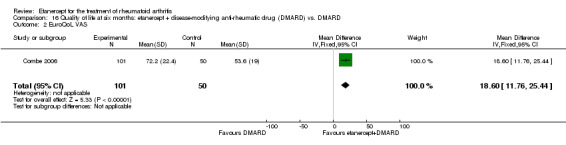
Comparison 16 Quality of life at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 EuroQoL VAS.
12 months
Participants taking etanercept plus DMARD were more likely to be satisfied with their treatment when compared with those taking DMARD alone (RR 1.2; 95% CI 1.1 to 1.3) (Analysis 17.1). Participants taking etanercept plus DMARD had significantly improved HAQ scores after treatment when compared with those taking DMARD alone (MD ‐0.2; 95% CI ‐0.33 to ‐0.15) (Analysis 17.2). Participants taking etanercept plus DMARD had significantly higher EQ‐5D scores after treatment when compared with those taking DMARD alone (MD 7.6; 95% CI 4.7 to 10.5) (Analysis 17.3). Participants taking etanercept plus DMARD were more likely to have HAQ scores equivalent to population norms (HAQ ≤ 0.5) after treatment when compared with those taking DMARD alone (RR 1.4; 95% CI 1.2 to 1.6) (Analysis 17.4). Participants taking etanercept plus DMARD were less likely to have stopped work during treatment than those taking DMARD alone (RR 0.4; 95% CI 0.2 to 0.7) (Analysis 17.5). Nearly 9% of those taking etanercept plus DMARD stopped work compared with 24% of those taking DMARD alone. Participants taking etanercept plus DMARD had significantly improved scores on the physical domain component of the SF‐36 (MD 2.8; 95% CI 1.0 to 4.6) (Analysis 17.6), but there was no evidence of a difference in the mental domain component of the SF‐36 (Analysis 17.7).
17.1. Analysis.
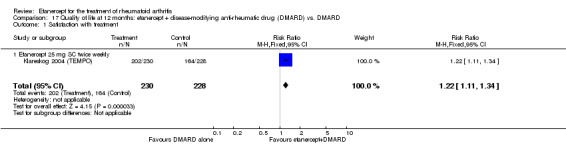
Comparison 17 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 Satisfaction with treatment.
17.2. Analysis.
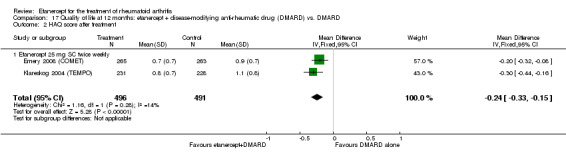
Comparison 17 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 HAQ score after treatment.
17.3. Analysis.
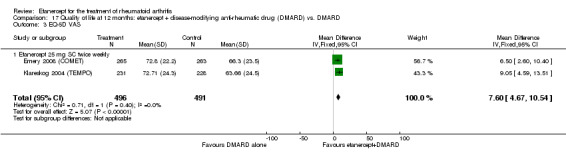
Comparison 17 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 EQ‐5D VAS.
17.4. Analysis.
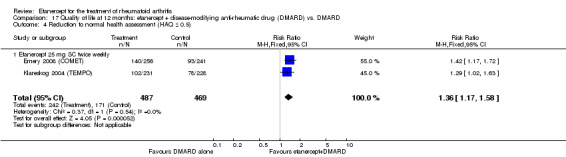
Comparison 17 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Reduction to normal health assessment (HAQ ≤ 0.5).
17.5. Analysis.
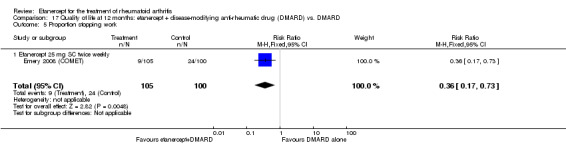
Comparison 17 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 5 Proportion stopping work.
17.6. Analysis.
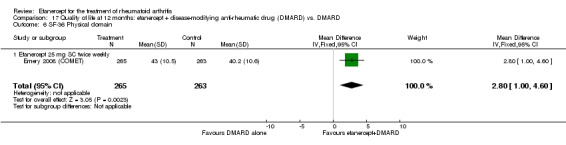
Comparison 17 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 6 SF‐36 Physical domain.
17.7. Analysis.
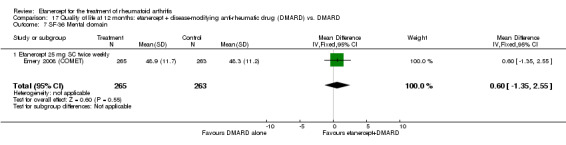
Comparison 17 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 7 SF‐36 Mental domain.
Two years
Participants taking etanercept plus DMARD were more likely to have improvements in their HAQ scores compared with those taking DMARD alone (Analysis 18.1). There was a 20% difference in the rate of improvement of the final HAQ score for those having etanercept plus DMARD (MD 20.0; 95% CI 17.5 to 22.6). There was also a reduction of 0.49 points in the final value of the 3‐point HAQ score for those having etanercept plus DMARD (MD ‐0.49; 95% CI ‐0.69 to ‐0.30) (Analysis 18.2).
18.1. Analysis.
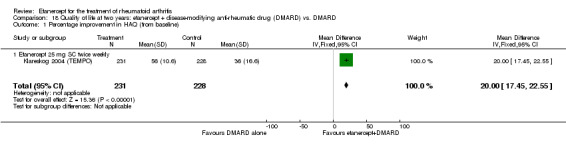
Comparison 18 Quality of life at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 Percentage improvement in HAQ (from baseline).
18.2. Analysis.
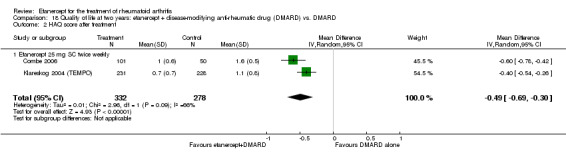
Comparison 18 Quality of life at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 HAQ score after treatment.
Etanercept versus DMARD
Six months
There was no evidence of significant differences in the final value HAQ scores between etanercept 25 mg and DMARD groups (Analysis 19.1). However, significantly higher values were found in the final EQ‐5D scores for those receiving etanercept 25 mg monotherapy when compared with DMARD alone (MD 21.3; 95% CI 14.6 to 28.0) (Analysis 19.2).
19.1. Analysis.
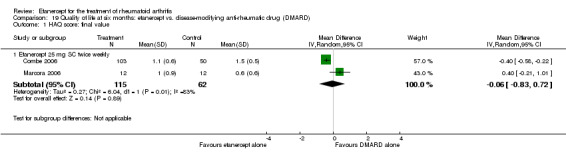
Comparison 19 Quality of life at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 HAQ score: final value.
19.2. Analysis.
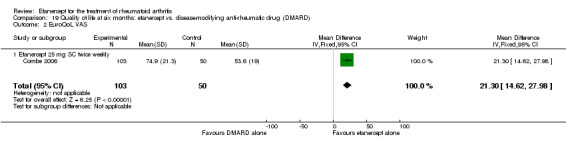
Comparison 19 Quality of life at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 EuroQoL VAS.
12 months
There was no evidence of a significant difference between groups on any of the scales used to measure quality of life (HAQ, SF‐36 summary scores, ASHI and EQ‐5D VAS) (Analysis 20.1; Analysis 20.2; Analysis 20.3; Analysis 20.4; Analysis 20.5; Analysis 20.6; Analysis 20.7; Analysis 20.8; Analysis 20.9; Analysis 20.10). However, participants taking etanercept 25 mg were more likely to be satisfied with their treatment (RR 1.2; 95% CI 1.1 to 1.3) (Analysis 20.11). Reduction to normal health assessment (HAQ ≤ 0.5) was not significantly different between etanercept 25 mg and DMARD alone (Analysis 20.12).
20.1. Analysis.
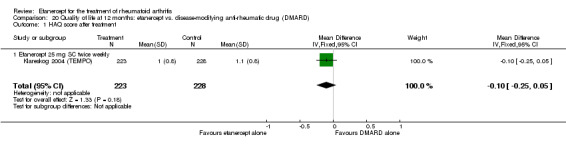
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 HAQ score after treatment.
20.2. Analysis.
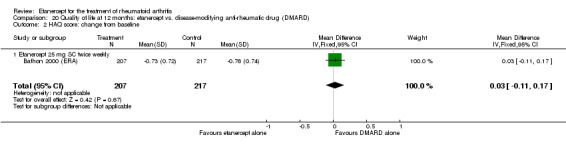
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 HAQ score: change from baseline.
20.3. Analysis.
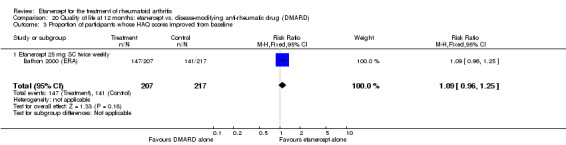
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 Proportion of participants whose HAQ scores improved from baseline.
20.4. Analysis.
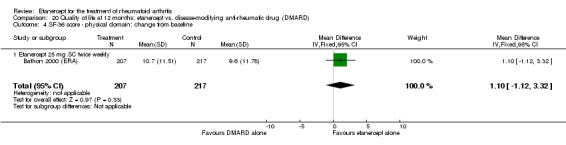
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 SF‐36 score ‐ physical domain: change from baseline.
20.5. Analysis.
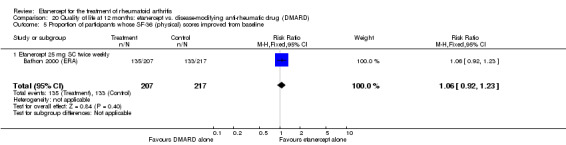
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 5 Proportion of participants whose SF‐36 (physical) scores improved from baseline.
20.6. Analysis.
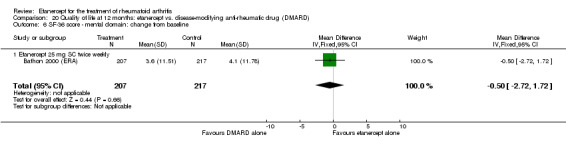
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 6 SF‐36 score ‐ mental domain: change from baseline.
20.7. Analysis.
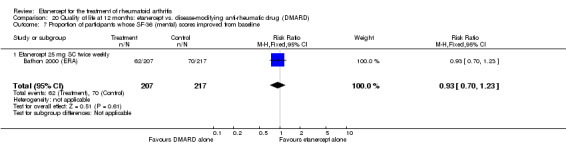
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 7 Proportion of participants whose SF‐36 (mental) scores improved from baseline.
20.8. Analysis.
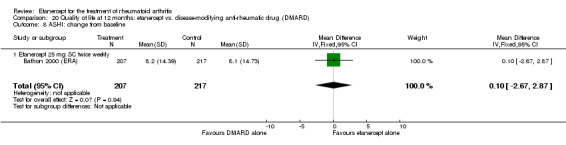
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 8 ASHI: change from baseline.
20.9. Analysis.
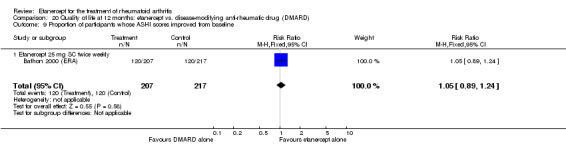
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 9 Proportion of participants whose ASHI scores improved from baseline.
20.10. Analysis.
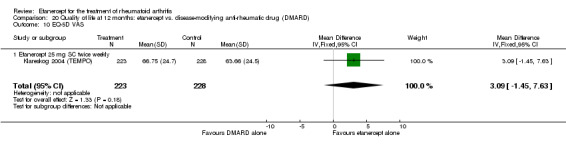
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 10 EQ‐5D VAS.
20.11. Analysis.
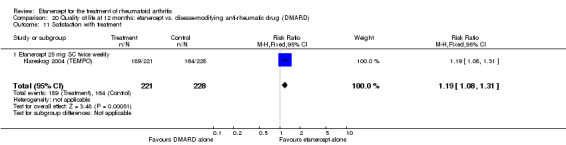
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 11 Satisfaction with treatment.
20.12. Analysis.
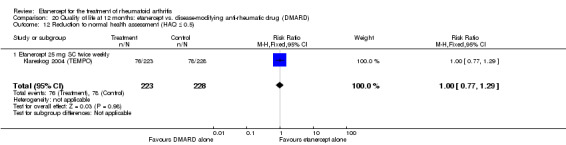
Comparison 20 Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 12 Reduction to normal health assessment (HAQ ≤ 0.5).
Two years
There was a small but significantly higher percentage improvement in the HAQ score for participants taking etanercept compared with those taking DMARD (MD 3.0; 95% CI 0.1 to 5.9) (Analysis 21.1). However, there was no evidence of a significant difference in the final HAQ score after treatment between groups (Analysis 21.2).
21.1. Analysis.
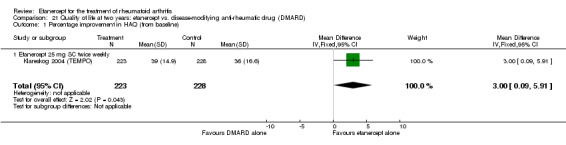
Comparison 21 Quality of life at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 Percentage improvement in HAQ (from baseline).
21.2. Analysis.
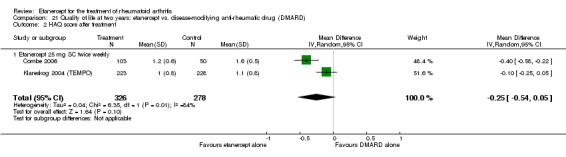
Comparison 21 Quality of life at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 HAQ score after treatment.
Etanercept plus DMARD versus etanercept alone
Six months
There was no evidence of a difference in final HAQ score values between groups (Analysis 22.1).
22.1. Analysis.
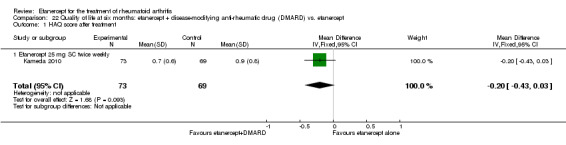
Comparison 22 Quality of life at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 HAQ score after treatment.
12 months
There was no evidence of a difference in satisfaction rates and EQ‐5D VAS scores between groups (Analysis 23.1; Analysis 23.3). However, HAQ scores were significantly improved after treatment with etanercept plus DMARD when compared with etanercept alone (RR ‐0.20; 95% CI ‐0.30 to ‐0.10) (Analysis 23.2). Reduction to normal health assessment (HAQ ≤ 0.5) was also significantly higher in the etanercept 25 mg plus DMARD group than in the etanercept alone group (RR 1.3; 95% CI 1.0 to 1.6) (Analysis 23.4).
23.1. Analysis.
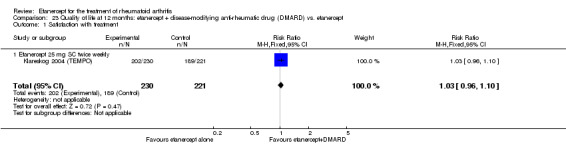
Comparison 23 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 Satisfaction with treatment.
23.3. Analysis.
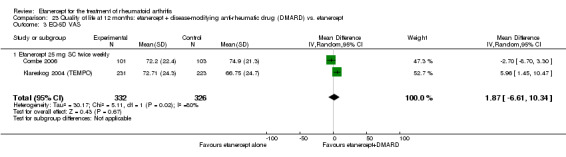
Comparison 23 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 EQ‐5D VAS.
23.2. Analysis.
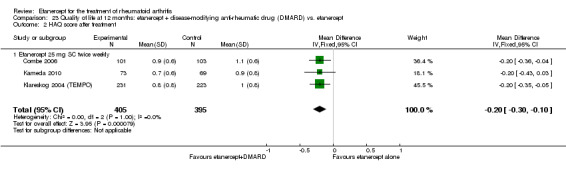
Comparison 23 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 HAQ score after treatment.
23.4. Analysis.
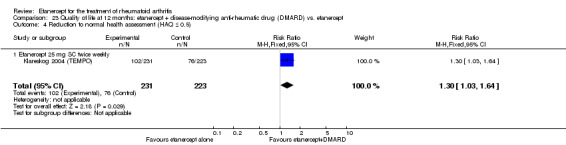
Comparison 23 Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Reduction to normal health assessment (HAQ ≤ 0.5).
Two years
There were highly significant improvements in HAQ scores (either percentage improvement or absolute scores) were reported for participants taking etanercept plus DMARD when compared with etanercept alone (RR 17.0; 95% CI 14.6 to 19.4 and RR ‐0.26; 95% CI ‐0.36 to ‐0.15, respectively) (Analysis 24.1; Analysis 24.2). However, there was no evidence of a difference in satisfaction rates between groups (Analysis 24.3).
24.1. Analysis.
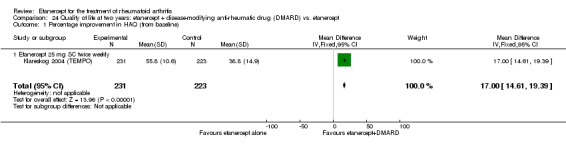
Comparison 24 Quality of life at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 Percentage improvement in HAQ (from baseline).
24.2. Analysis.
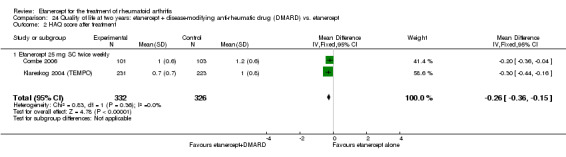
Comparison 24 Quality of life at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 HAQ score after treatment.
24.3. Analysis.
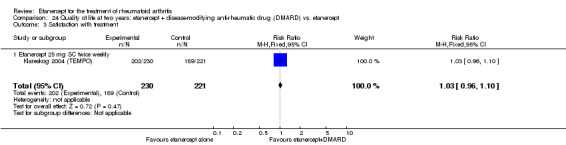
Comparison 24 Quality of life at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 Satisfaction with treatment.
Etanercept versus placebo
Six months
Significantly higher changes were found from baseline in the MOS mental health scores and MOS energy/vitality scores both for those receiving etanercept 10 mg and etanercept 25 mg when compared with placebo (mental health: etanercept 10 mg: MD 8.5; 95% CI 3.2 to 13.8; etanercept 25 mg: MD 9.5; 95% CI 4.3 to 14.7; energy/vitality: etanercept 10 mg: MD 12.6; 95% CI 6.4 to 18.9; etanercept 25 mg: MD 11.6; 95% CI 5.5 to 17.7) (Analysis 25.1; Analysis 25.2).
25.1. Analysis.
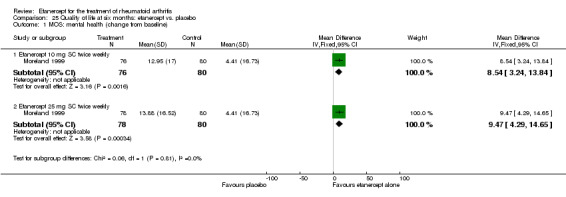
Comparison 25 Quality of life at six months: etanercept vs. placebo, Outcome 1 MOS: mental health (change from baseline).
25.2. Analysis.
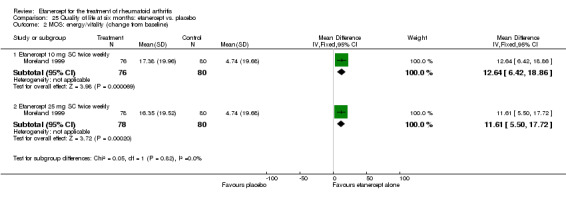
Comparison 25 Quality of life at six months: etanercept vs. placebo, Outcome 2 MOS: energy/vitality (change from baseline).
C. Harms
1. Study withdrawals
Withdrawals are reported in four ways in this meta‐analysis, that is, total withdrawals, withdrawal because of lack of efficacy, withdrawal because of adverse events and deaths; they are reported at different follow‐up times.
Etanercept plus DMARD versus DMARD group
a) Total withdrawals
At six months, significantly more people withdrew from the DMARD group than from the etanercept plus DMARD group (RR 0.17; 95% CI 0.04 to 0.79) (Analysis 26.1). About 3.4% in total withdrew from the etanercept plus DMARD group and 20% in total withdrew from the DMARD alone group.
26.1. Analysis.
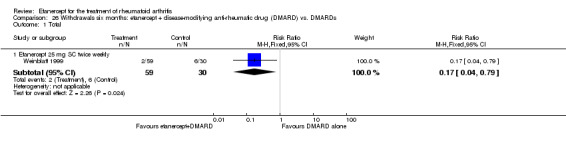
Comparison 26 Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARDs, Outcome 1 Total.
At 12 months, significantly more people withdrew from the DMARD group than from the etanercept plus DMARD group (RR 0.6; 95% CI 0.5 to 0.8) (Analysis 27.1). About 18% of people withdrew from the etanercept plus DMARD group and 30% withdrew from the DMARD control group.
27.1. Analysis.
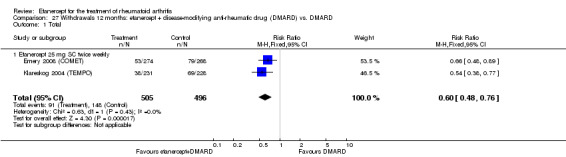
Comparison 27 Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 Total.
At two years, significantly more people withdrew from the DMARD group than from the etanercept 25 mg group (RR 0.47; 95% CI 0.28 to 0.80) (Analysis 28.1). About 49% withdrew from the DMARD group and 26% withdrew from the etanercept plus DMARD group.
28.1. Analysis.
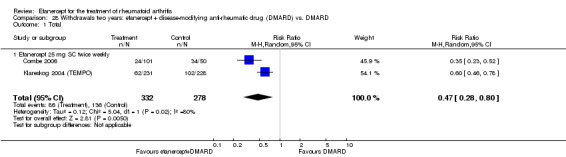
Comparison 28 Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 Total.
At three years, significantly more people withdrew from the DMARD group than from the etanercept 25 mg plus DMARD group (RR 0.7; 95% CI 0.6 to 0.8) (Analysis 29.1). About 61% had withdrawn from the DMARD group and 43% had withdrawn from the etanercept plus DMARD group.
29.1. Analysis.
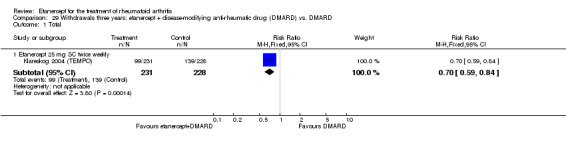
Comparison 29 Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 Total.
b) Lack of efficacy
At six months, withdrawals were reduced in the etanercept plus DMARD group compared with the DMARD alone group (RR 0.14; 95% CI 0.05 to 0.37) (Analysis 26.2). About 2.5% of people withdrew from the etanercept plus DMARD group and 20% withdrew from the DMARD group.
26.2. Analysis.
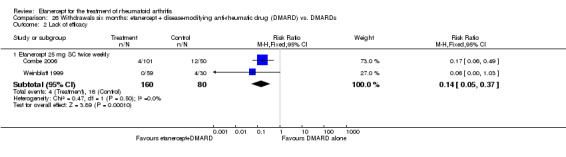
Comparison 26 Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARDs, Outcome 2 Lack of efficacy.
At 12 months, withdrawals were reduced in the etanercept 25 mg plus DMARD group compared with the DMARD group (RR 0.33; 95% CI 0.2 to 0.6) (Analysis 27.2). About 3% of people withdrew from the etanercept 25 mg group and 9.1% withdrew from the control group.
27.2. Analysis.
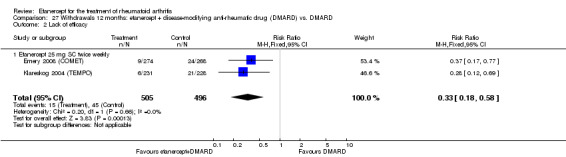
Comparison 27 Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 Lack of efficacy.
At two years, withdrawals were reduced in the etanercept 25 mg group compared with the DMARD group (RR 0.19; 95% CI 0.07 to 0.48) (Analysis 28.2). About 4.5% of people withdrew from the etanercept 25 mg group and 20% withdrew from the control group.
28.2. Analysis.
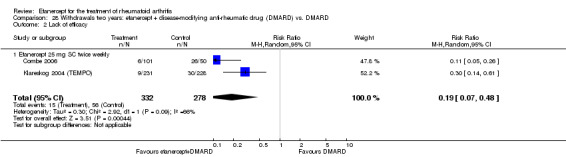
Comparison 28 Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 Lack of efficacy.
At three years, withdrawals were reduced in the etanercept 25 mg group compared with the DMARD group (RR 0.3, 95% CI 0.2 to 0.6) (Analysis 29.2). About 5% withdrew from the etanercept 25 mg group and 17% withdrew from the control group.
29.2. Analysis.
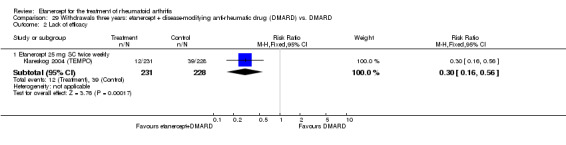
Comparison 29 Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 Lack of efficacy.
c) Adverse effects
At six months, 12 months and two years, there was no evidence of a statistically significant difference in the rate of withdrawal because of adverse events in either etanercept plus DMARD group and the DMARD alone group (Analysis 26.3; Analysis 27.3; Analysis 28.3).
26.3. Analysis.
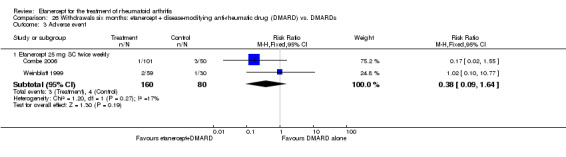
Comparison 26 Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARDs, Outcome 3 Adverse event.
27.3. Analysis.
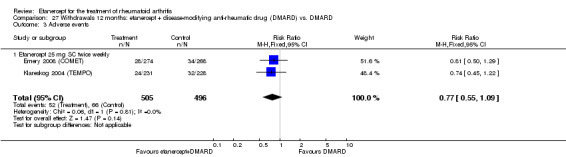
Comparison 27 Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 Adverse events.
28.3. Analysis.
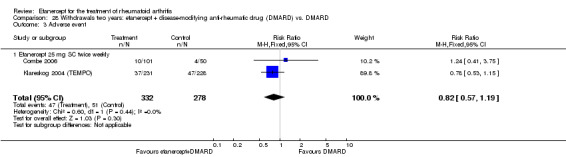
Comparison 28 Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 Adverse event.
At three years, withdrawals were significantly reduced in the etanercept 25 mg plus DMARD group compared with the DMARD alone group (RR 0.7; 95% CI 0.5 to 1.0) (Analysis 29.3).
29.3. Analysis.
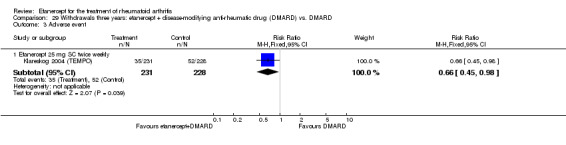
Comparison 29 Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 Adverse event.
d) Deaths
At six months, there were no deaths in either group (Analysis 26.4).
26.4. Analysis.
Comparison 26 Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARDs, Outcome 4 Death.
At 12 months, there was no statistically significant difference in the death rates between groups (Analysis 27.4). There were two deaths in the etanercept plus DMARD group and one death in the DMARD group.
27.4. Analysis.
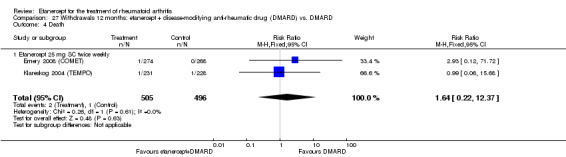
Comparison 27 Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Death.
At two years, there was no statistically significant difference in the death rates between groups (Analysis 28.4). There was one death in each group.
28.4. Analysis.
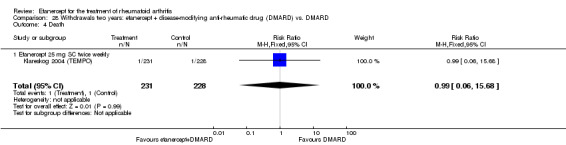
Comparison 28 Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Death.
At three years, there was no statistically significant difference in the death rates between groups (Analysis 29.4). Two people had died by three years in the etanercept 25 mg plus DMARD group compared with one person in the DMARD group.
29.4. Analysis.
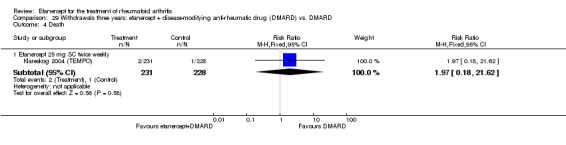
Comparison 29 Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Death.
Etanercept versus DMARD
a) Total withdrawals
There was no significant difference in the total number of withdrawals between the etanercept and DMARD groups at six months (25 mg) or 12 months (10 mg) (Analysis 30.1; Analysis 31.1). Total withdrawals were significantly reduced in the etanercept 25 mg group compared with the DMARD group at 12 months (RR 0.8; 95% CI 0.6 to 1.0) (Analysis 31.1) and two years (RR 0.67; 95% CI 0.46 to 0.97) (Analysis 32.1). The estimate at three years was just outside the 0.05 level of significance (P value = 0.06) (Analysis 33.1).
30.1. Analysis.
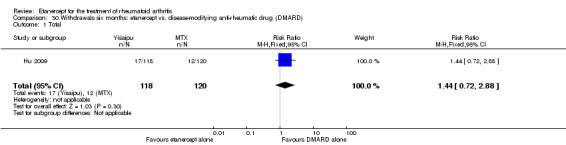
Comparison 30 Withdrawals six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 Total.
31.1. Analysis.
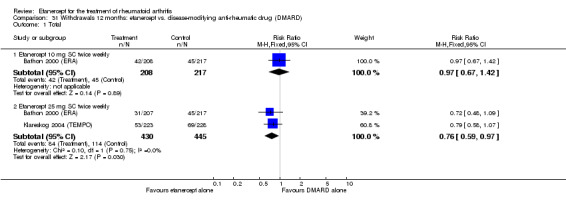
Comparison 31 Withdrawals 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 Total.
32.1. Analysis.
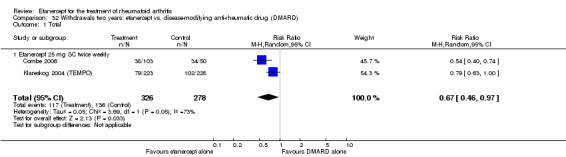
Comparison 32 Withdrawals two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 Total.
33.1. Analysis.
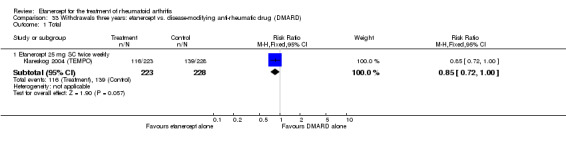
Comparison 33 Withdrawals three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 Total.
b) Lack of efficacy
Withdrawals due to lack of efficacy were significantly reduced in the etanercept 25 mg group compared with the DMARD group at six months (RR 0.04; 95% CI 0.01 to 0.30) (Analysis 30.2). However, there was no significant difference in the rate of withdrawals because of lack of efficacy between either etanercept group (10 and 25 mg) and the DMARD groups at 12 months (Analysis 31.2;) and between the etanercept 25 mg group and the DMARD group at two and three years (Analysis 32.2; Analysis 33.2).
30.2. Analysis.
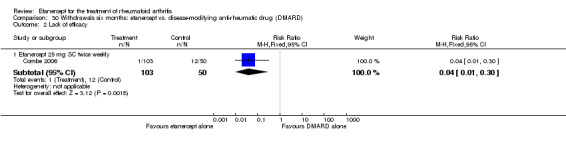
Comparison 30 Withdrawals six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 Lack of efficacy.
31.2. Analysis.
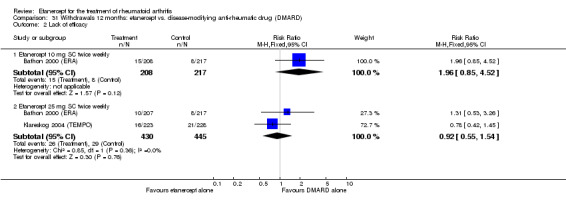
Comparison 31 Withdrawals 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 Lack of efficacy.
32.2. Analysis.
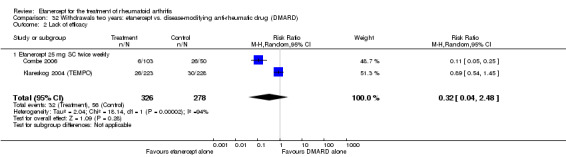
Comparison 32 Withdrawals two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 Lack of efficacy.
33.2. Analysis.
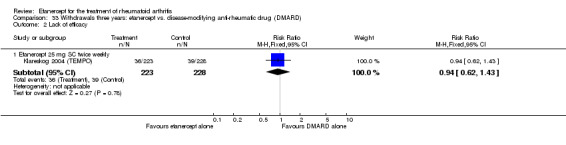
Comparison 33 Withdrawals three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 Lack of efficacy.
c) Adverse events
There was no significant difference in the rate of withdrawals because of adverse events between the etanercept group and the DMARD group at six months (25 mg) and 12 months (10 mg) (Analysis 30.3; Analysis 31.3) and between etanercept 25 mg and the DMARD groups at two and three years (Analysis 32.3; Analysis 33.3). However, significantly fewer people withdrew for this reason from the etanercept 25 mg group at 12 months compared with the DMARD group (RR 0.7; 95% CI 0.5 to 1.0) (Analysis 31.3).
30.3. Analysis.
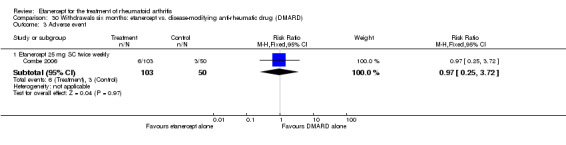
Comparison 30 Withdrawals six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 Adverse event.
31.3. Analysis.
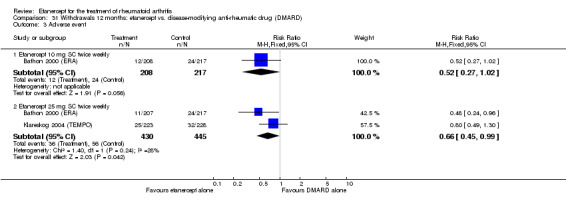
Comparison 31 Withdrawals 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 Adverse event.
32.3. Analysis.
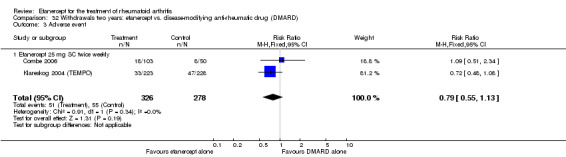
Comparison 32 Withdrawals two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 Adverse event.
33.3. Analysis.
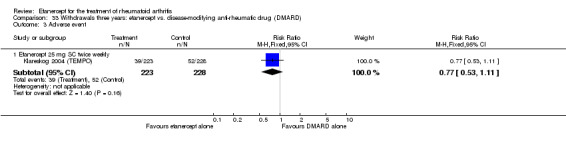
Comparison 33 Withdrawals three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 Adverse event.
d) Deaths
There was no significant difference in the death rates at any follow‐up time between groups (Analysis 31.4; Analysis 32.4; Analysis 33.4). By three years' follow‐up, two people had died in the etanercept 25 mg group compared with one in the DMARD group.
31.4. Analysis.
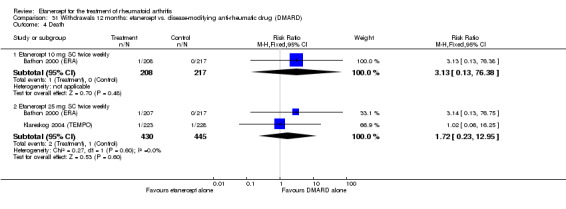
Comparison 31 Withdrawals 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Death.
32.4. Analysis.
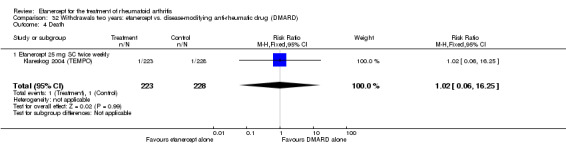
Comparison 32 Withdrawals two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Death.
33.4. Analysis.
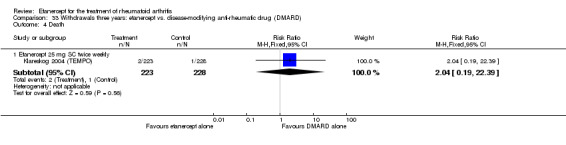
Comparison 33 Withdrawals three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Death.
Etanercept plus DMARD versus etanercept alone
a) Total withdrawals
Overall, significantly fewer people taking etanercept plus DMARD treatment withdrew by six and 12 months' follow‐up when compared with those taking etanercept alone (RR 0.31; 95% CI 0.11 to 0.92 and RR 0.5; 95% CI 0.4 to 0.8, respectively) (Analysis 34.1; Analysis 35.1). Also, significantly fewer people taking etanercept plus DMARD treatment withdrew by the two‐ and three‐year follow‐up when compared with those taking etanercept alone (RR 0.8; 95% CI 0.6 to 1.0 and RR 0.8; 95% CI 0.7 to 1.0, respectively) (Analysis 36.1; Analysis 37.1).
34.1. Analysis.
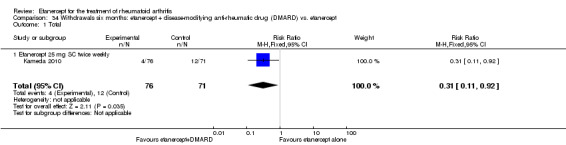
Comparison 34 Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 Total.
35.1. Analysis.
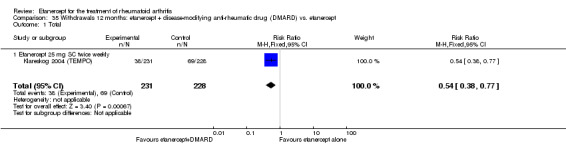
Comparison 35 Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 Total.
36.1. Analysis.
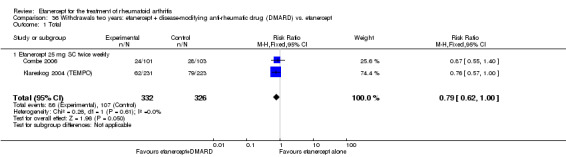
Comparison 36 Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 Total.
37.1. Analysis.
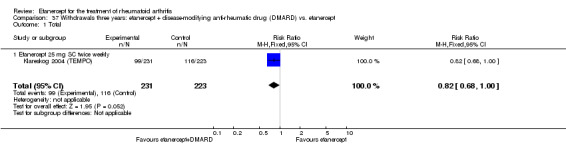
Comparison 37 Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 Total.
b) Lack of efficacy
There was no significant difference in the rate of withdrawals because of lack of efficacy between the etanercept plus DMARD group and the etanercept alone group at six months and two years (Analysis 34.2; Analysis 36.2). At 12 months and three‐year follow‐up, significantly fewer people taking etanercept plus DMARD treatment withdrew from the trial because of lack of efficacy by all follow‐up periods when compared with etanercept alone (Analysis 35.2; Analysis 37.2) ( RR 0.4; 95% CI 0.1 to 0.9 and RR 0.3; 95% CI 0.2 to 0.6, respectively).
34.2. Analysis.
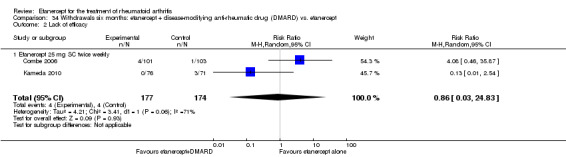
Comparison 34 Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 Lack of efficacy.
36.2. Analysis.
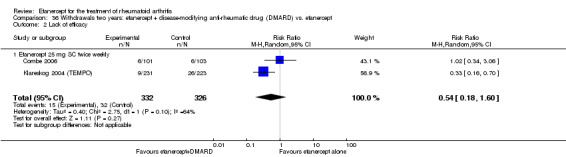
Comparison 36 Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 Lack of efficacy.
35.2. Analysis.
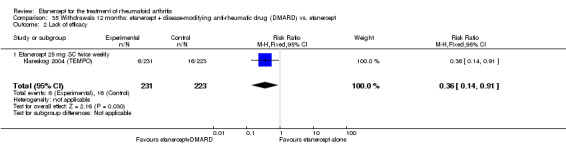
Comparison 35 Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 Lack of efficacy.
37.2. Analysis.
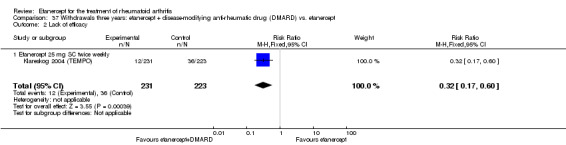
Comparison 37 Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 Lack of efficacy.
c) Adverse events
Significantly fewer people taking etanercept plus DMARD treatment withdrew by six months' follow‐up when compared with those taking etanercept alone (RR 0.16; 95% CI 0.03 to 0.86) (Analysis 34.3). At 12 months', two years' and three years' follow‐up, there was no evidence of a difference between groups in the rates of withdrawal from the trial because of adverse events (Analysis 35.3; Analysis 36.3; Analysis 37.3).
34.3. Analysis.
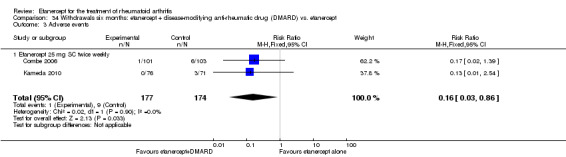
Comparison 34 Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 Adverse events.
35.3. Analysis.
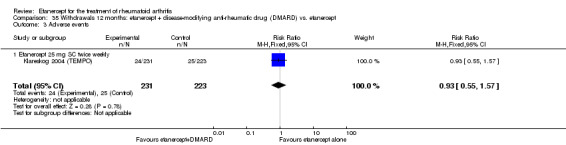
Comparison 35 Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 Adverse events.
36.3. Analysis.
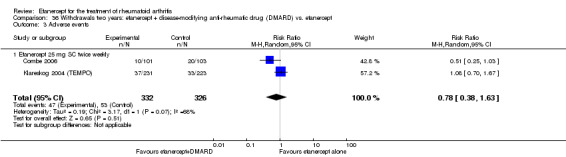
Comparison 36 Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 Adverse events.
37.3. Analysis.
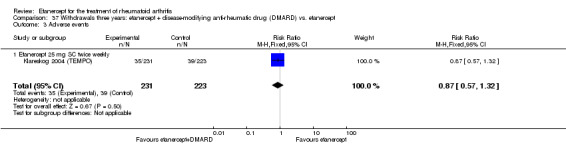
Comparison 37 Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 Adverse events.
d) Deaths
At 12 months', two years' and three years' follow‐up, there was no evidence of a difference between groups in the death rates before the completion of the trial (Analysis 35.4; Analysis 36.4; Analysis 37.4).
35.4. Analysis.
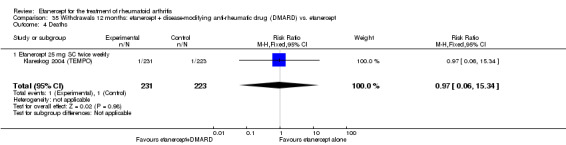
Comparison 35 Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Deaths.
36.4. Analysis.
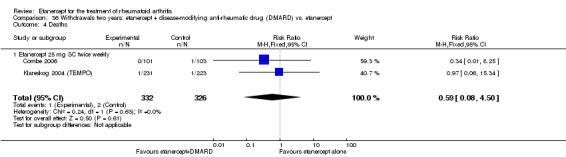
Comparison 36 Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Deaths.
37.4. Analysis.
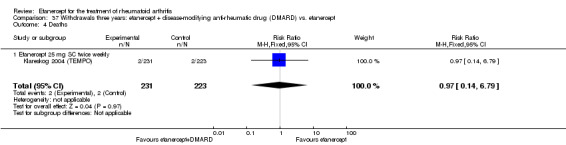
Comparison 37 Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Deaths.
Etanercept versus placebo
a) Total withdrawals
At six months, significantly more people withdrew from the placebo group than from either etanercept group (10 or 25 mg) (10 mg: RR 0.5; 95% CI 0.3 to 0.7; 25 mg: RR 0.4; 95% CI 0.2 to 0.5) (Analysis 38.1). About 32% of people in total withdrew from the etanercept 10 mg group, 24% in total withdrew from the etanercept 25 mg group and 68% in total withdrew from the placebo group.
38.1. Analysis.
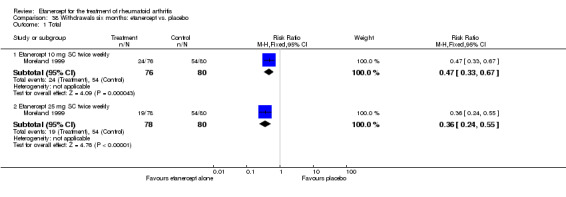
Comparison 38 Withdrawals six months: etanercept vs. placebo, Outcome 1 Total.
b) Lack of efficacy
At six months, significantly more people withdrew because of lack of efficacy from the placebo group than from either etanercept group (10 or 25 mg) (10 mg: RR 0.4; 95% CI 0.3 to 0.7; 25 mg: RR 0.3; 95% CI 0.2 to 0.5) (Analysis 38.2). About 21% of people withdrew from the etanercept 10 mg group, 15% withdrew from the etanercept 25 mg group and 53% withdrew from the placebo group.
38.2. Analysis.
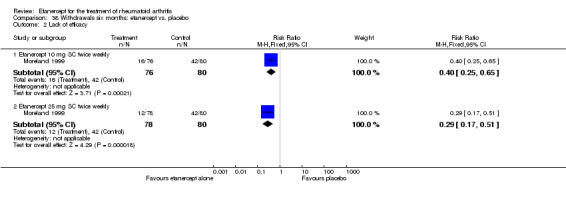
Comparison 38 Withdrawals six months: etanercept vs. placebo, Outcome 2 Lack of efficacy.
c) Adverse events
There were no significant differences between groups in the rate of withdrawal due to adverse events.
2. Adverse effects
Etanercept plus DMARD versus DMARD
Injection site reaction
A greater proportion of people receiving either etanercept plus DMARD developed injection site reactions at six months than those taking DMARD (RR 6.9; 95% CI 2.2 to 21.3) (Analysis 39.15). About 25.6% of those taking etanercept plus DMARD experienced reactions compared with 3.8% of those taking DMARD alone. A greater proportion of people receiving etanercept plus DMARD developed injection site reactions at two years than those taking DMARD alone (RR 5.0; 95% CI 2.3 to 11.0) (Analysis 41.17). About 14% of those taking etanercept plus DMARD reported reactions compared with 3% of those taking DMARD alone.
39.15. Analysis.
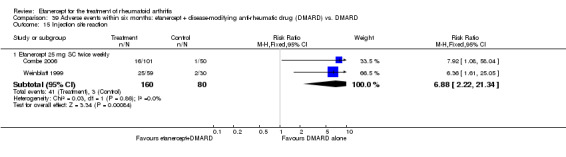
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 15 Injection site reaction.
41.17. Analysis.
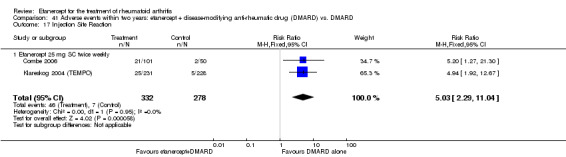
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 17 Injection Site Reaction.
There was no evidence of statistically significant differences in the rates of all other adverse effects in the included studies:
total adverse reactions at six and 12 months (Analysis 39.1; Analysis 41.1);
abdominal pain at six months and two years (Analysis 39.2; Analysis 41.3);
asthenia at six months and two years (Analysis 39.3; Analysis 41.4);
arthralgia/Bone pain at six months and two years (Analysis 39.4; Analysis 41.5);
back pain at two years (Analysis 41.6);
bronchitis at six months and two years (Analysis 39.5; Analysis 41.7);
breast cancer at 12 months (Analysis 40.5);
chest pain at 12 months (Analysis 40.6);
cholelithiasis at 12 months (Analysis 40.8);
diarrhoea at six months and two years (Analysis 39.6; Analysis 41.8);
dizziness at six months (Analysis 39.7);
dyspepsia at six months and two years (Analysis 39.8; Analysis 41.9);
fever at six months (Analysis 39.9);
flu‐syndrome six months and two years (Analysis 39.10; Analysis 41.10);
gingival/dental infection at two years (Analysis 41.11);
headache at six months and two years (Analysis 39.11; Analysis 41.12);
hip arthroplasty at 12 months (Analysis 40.12);
hypertension at six months and two years (Analysis 39.12; Analysis 41.13);
increased cough at six and two years (Analysis 39.13; Analysis 41.14);
infection at six months and two years (Analysis 39.14; Analysis 41.15; Table 3);
injection site haemorrhage at six months and two years (Analysis 39.16; Analysis 41.16);
interstitial lung disease at 12 months (Analysis 40.11);
invertebral disc protrusion at 12 months (Analysis 40.9);
leukopenia at six months (Analysis 39.17);
malignancy at 12 months and two years (Analysis 40.13; Analysis 41.18);
miscellaneous skin infections at six months and two years (Analysis 39.18; Analysis 41.19);
mouth ulcers at six months (Analysis 39.19);
nausea at six and 12 months and two years (Analysis 39.20; Analysis 40.1; Analysis 41.20);
osteoarthritis at 12 months (Analysis 40.10);
pain at six months and two years (Analysis 39.21; Analysis 41.21);
paraesthesia at six months and two years (Analysis 39.22; Analysis 41.22);
pharyngitis (non‐infectious) at six months (Analysis 39.23);
pharyngitis or laryngitis at six and 12 months and two years (Analysis 39.24; Analysis 40.2; Analysis 41.23);
pneumonia at 12 months (Analysis 40.7);
pruritus at six months (Analysis 39.25);
rash at six months and two years (Analysis 39.26; Analysis 41.24);
rhinitis at six months (Analysis 39.27);
serious infections at 12 months and two years (Analysis 40.3; Analysis 41.25; Table 3);
sinusitis at two years (Analysis 41.26);
trauma/accidental injury at six months and two years (Analysis 39.28; Analysis 41.2);
upper respiratory tract infection (URTI) at six months and two years (Analysis 39.29; Analysis 41.27);
vomiting at six months and two years (Analysis 39.30; Analysis 41.28);
worsening of RA at 12 months and two years (Analysis 40.4; Analysis 41.29).
39.1. Analysis.
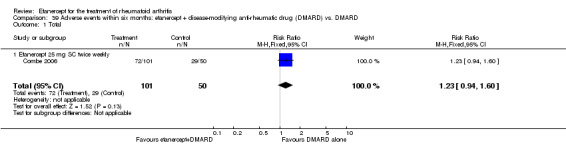
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 Total.
41.1. Analysis.
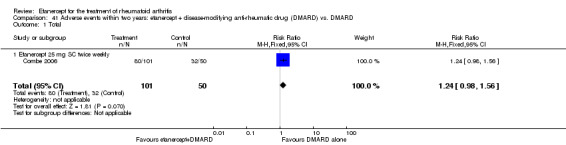
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 Total.
39.2. Analysis.
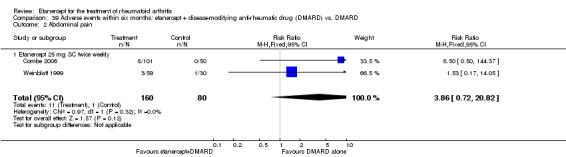
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 Abdominal pain.
41.3. Analysis.
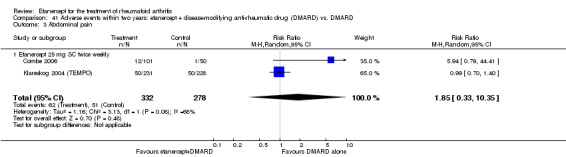
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 Abdominal pain.
39.3. Analysis.
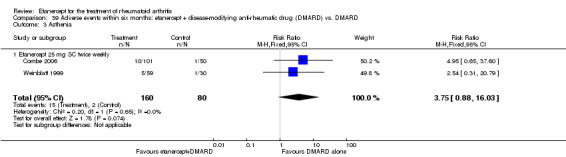
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 Asthenia.
41.4. Analysis.
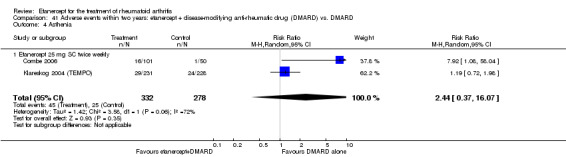
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Asthenia.
39.4. Analysis.
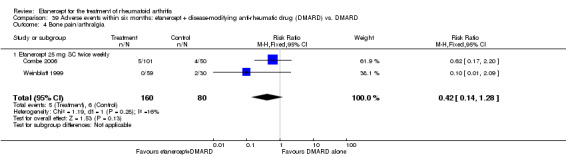
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Bone pain/arthralgia.
41.5. Analysis.
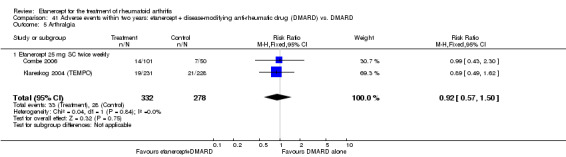
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 5 Arthralgia.
41.6. Analysis.
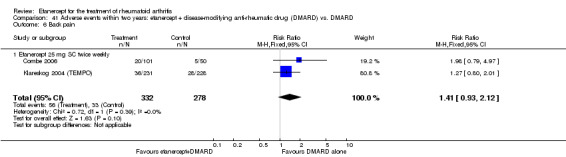
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 6 Back pain.
39.5. Analysis.
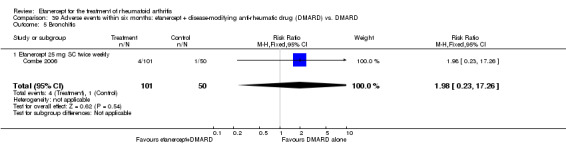
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 5 Bronchitis.
41.7. Analysis.
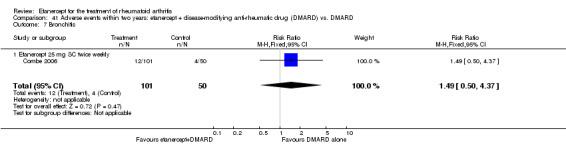
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 7 Bronchitis.
40.5. Analysis.
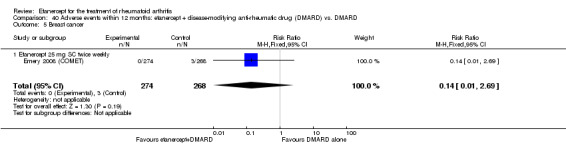
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 5 Breast cancer.
40.6. Analysis.
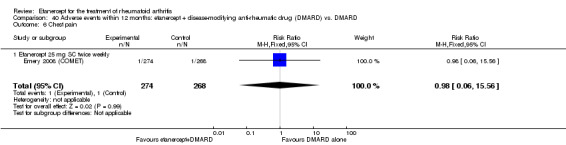
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 6 Chest pain.
40.8. Analysis.
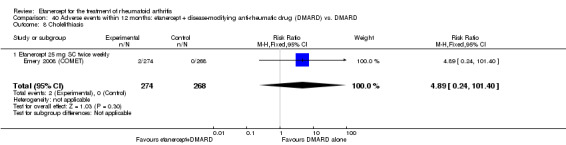
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 8 Cholelithiasis.
39.6. Analysis.
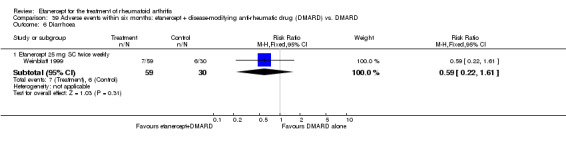
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 6 Diarrhoea.
41.8. Analysis.
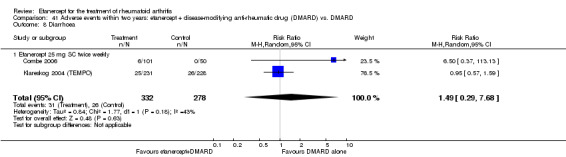
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 8 Diarrhoea.
39.7. Analysis.
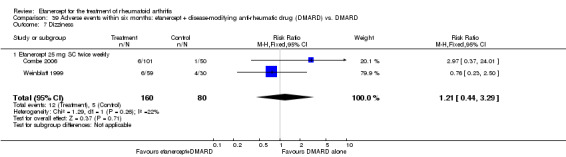
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 7 Dizziness.
39.8. Analysis.
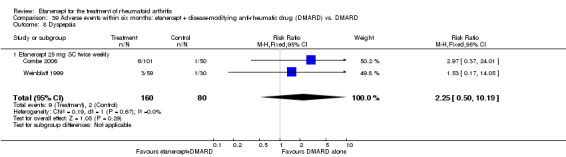
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 8 Dyspepsia.
41.9. Analysis.
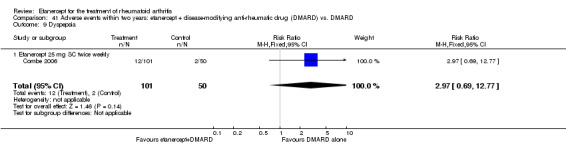
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 9 Dyspepsia.
39.9. Analysis.
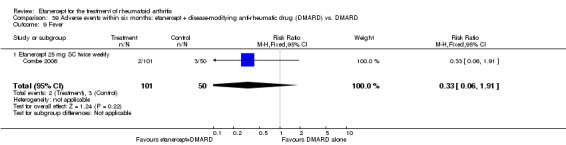
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 9 Fever.
39.10. Analysis.
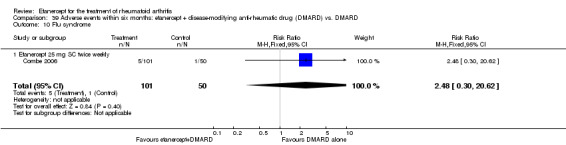
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 10 Flu syndrome.
41.10. Analysis.
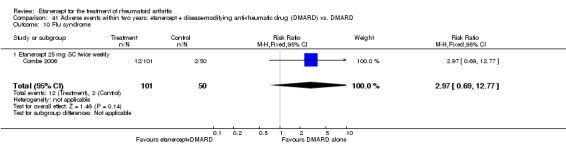
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 10 Flu syndrome.
41.11. Analysis.
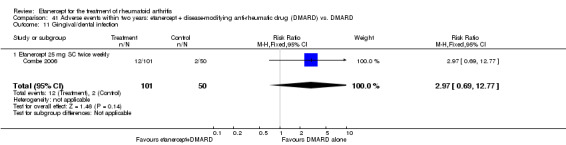
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 11 Gingival/dental infection.
39.11. Analysis.
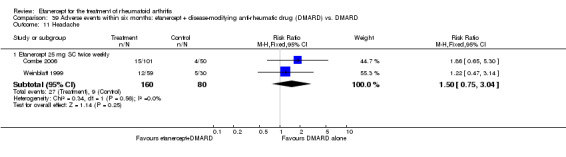
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 11 Headache.
41.12. Analysis.
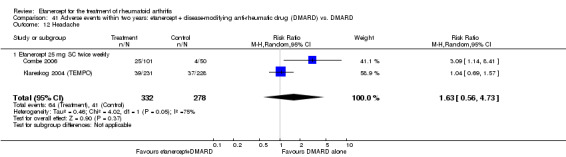
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 12 Headache.
40.12. Analysis.
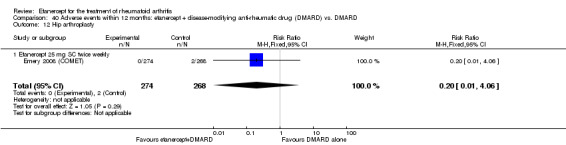
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 12 Hip arthroplasty.
39.12. Analysis.
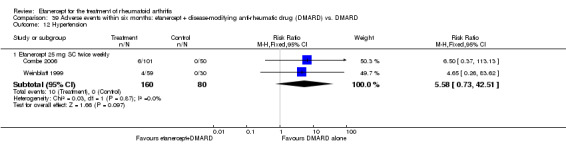
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 12 Hypertension.
41.13. Analysis.
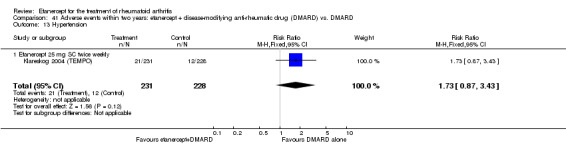
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 13 Hypertension.
39.13. Analysis.
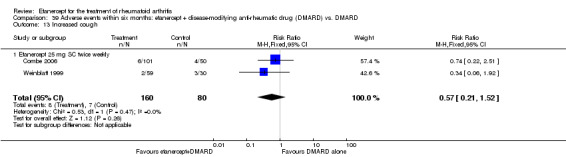
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 13 Increased cough.
41.14. Analysis.
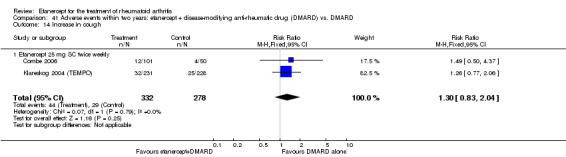
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 14 Increase in cough.
39.14. Analysis.
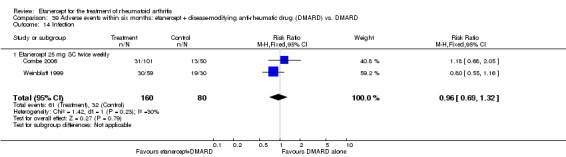
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 14 Infection.
41.15. Analysis.
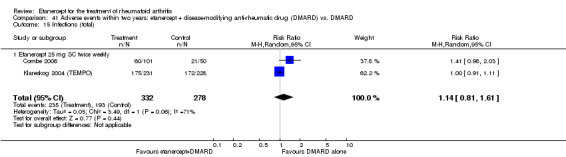
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 15 Infections (total).
39.16. Analysis.
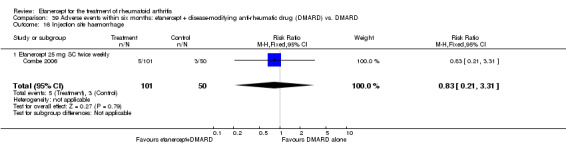
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 16 Injection site haemorrhage.
41.16. Analysis.
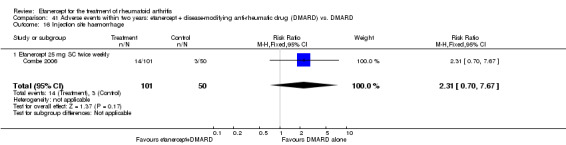
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 16 Injection site haemorrhage.
40.11. Analysis.
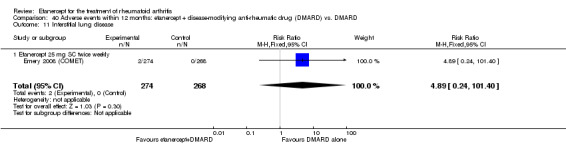
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 11 Interstitial lung disease.
40.9. Analysis.
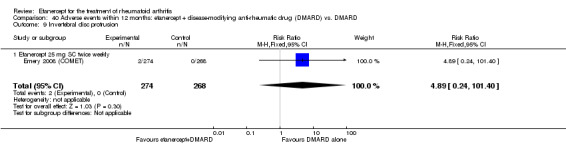
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 9 Invertebral disc protrusion.
39.17. Analysis.
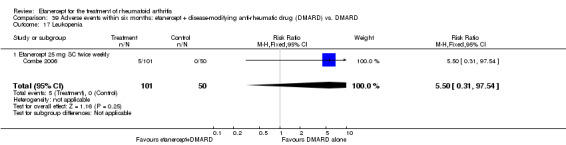
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 17 Leukopenia.
40.13. Analysis.
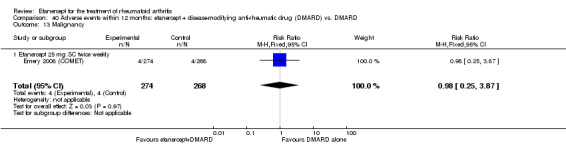
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 13 Malignancy.
41.18. Analysis.
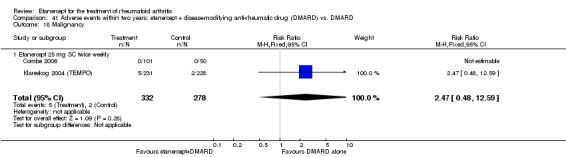
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 18 Malignancy.
39.18. Analysis.
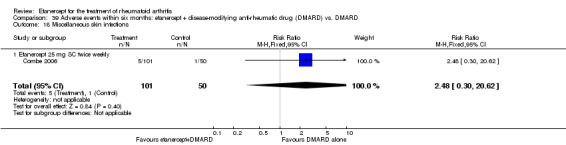
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 18 Miscellaneous skin infections.
41.19. Analysis.
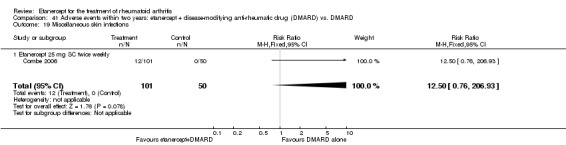
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 19 Miscellaneous skin infections.
39.19. Analysis.
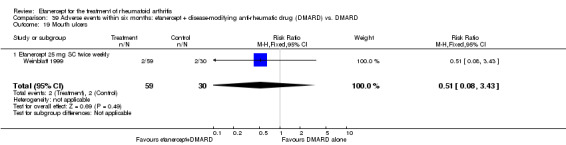
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 19 Mouth ulcers.
39.20. Analysis.
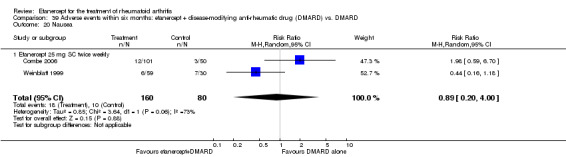
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 20 Nausea.
40.1. Analysis.
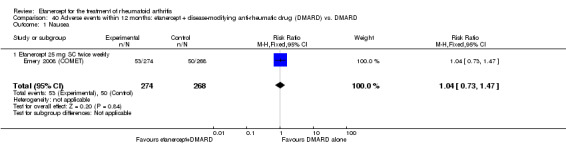
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 Nausea.
41.20. Analysis.
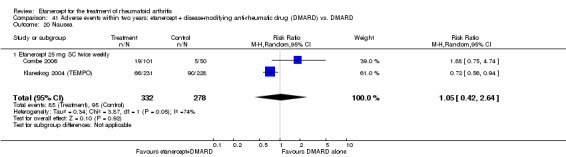
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 20 Nausea.
40.10. Analysis.
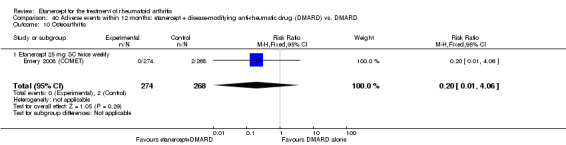
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 10 Osteoarthritis.
39.21. Analysis.
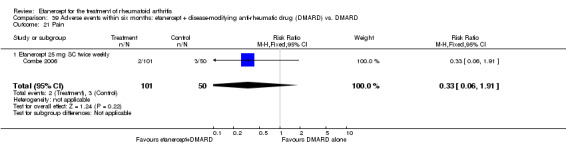
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 21 Pain.
41.21. Analysis.
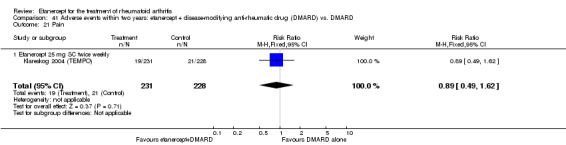
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 21 Pain.
39.22. Analysis.
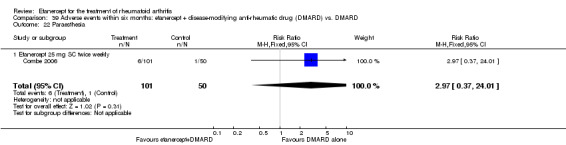
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 22 Paraesthesia.
41.22. Analysis.
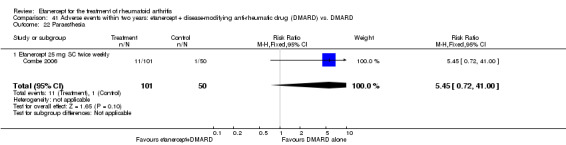
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 22 Paraesthesia.
39.23. Analysis.
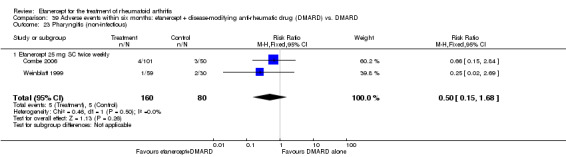
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 23 Pharyngitis (non‐infectious).
39.24. Analysis.
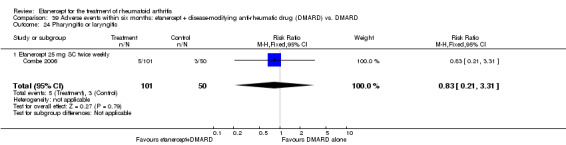
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 24 Pharyngitis or laryngitis.
40.2. Analysis.
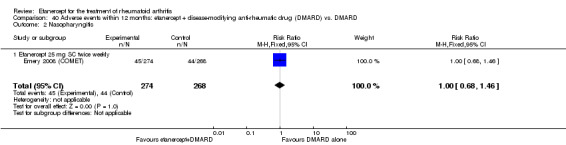
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 Nasopharyngitis.
41.23. Analysis.
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 23 Pharyngitis or laryngitis.
40.7. Analysis.
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 7 Pneumonia.
39.25. Analysis.
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 25 Pruritus.
39.26. Analysis.
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 26 Rash.
41.24. Analysis.
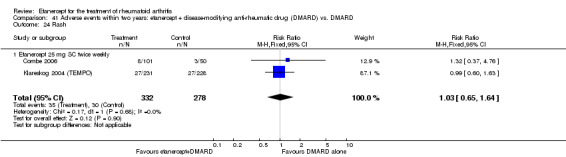
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 24 Rash.
39.27. Analysis.
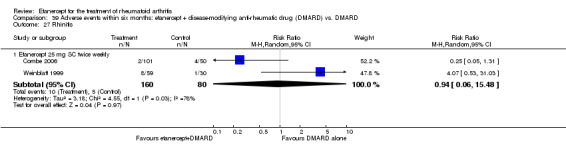
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 27 Rhinitis.
40.3. Analysis.
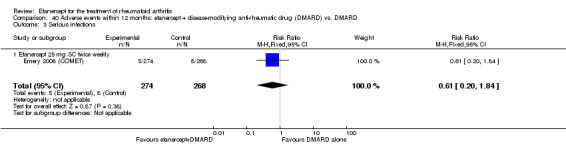
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 Serious infections.
41.25. Analysis.
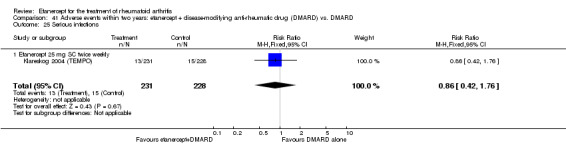
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 25 Serious infections.
41.26. Analysis.
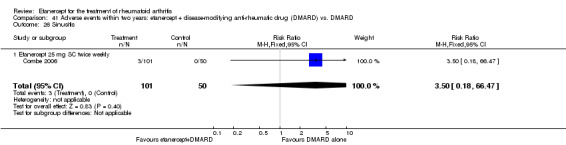
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 26 Sinusitis.
39.28. Analysis.
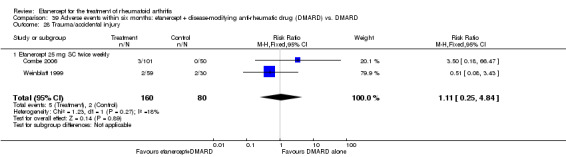
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 28 Trauma/accidental injury.
41.2. Analysis.
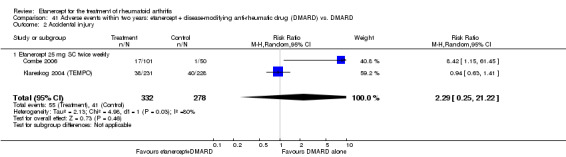
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 Accidental injury.
39.29. Analysis.
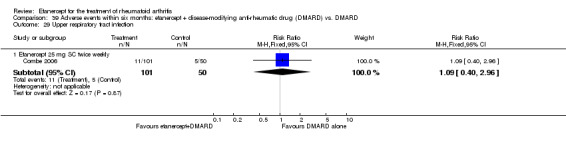
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 29 Upper respiratory tract infection.
41.27. Analysis.
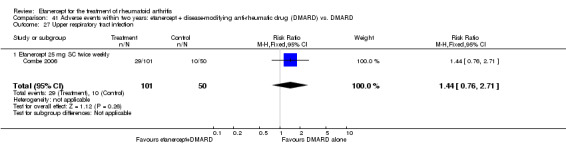
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 27 Upper respiratory tract infection.
39.30. Analysis.
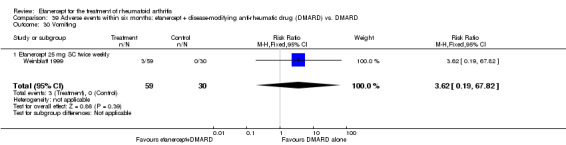
Comparison 39 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 30 Vomiting.
41.28. Analysis.
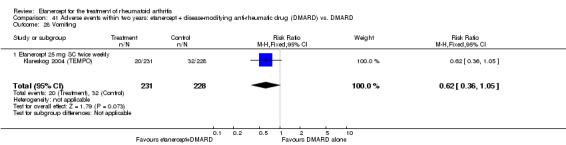
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 28 Vomiting.
40.4. Analysis.
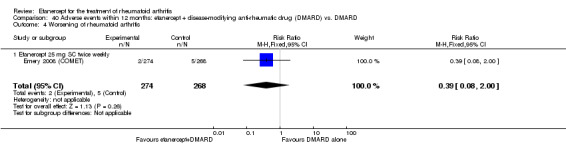
Comparison 40 Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 4 Worsening of rheumatoid arthritis.
41.29. Analysis.
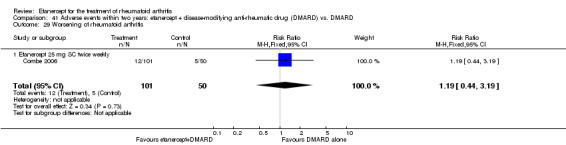
Comparison 41 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 29 Worsening of rheumatoid arthritis.
Etanercept versus DMARD
Total adverse events
Significantly more people had adverse events in the etanercept monotherapy group when compared with the DMARD alone group at two years (RR 1.4; 95% CI 1.1 to 1.7) (Analysis 44.1).
44.1. Analysis.
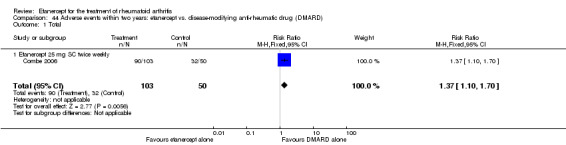
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 Total.
Alopecia
People taking etanercept 25 mg were less likely to experience alopecia compared with those taking DMARD at 12 months (RR 0.5; 95% CI 0.3 to 0.97) (Analysis 43.1). However, there was no evidence of a significant difference in alopecia rates between those taking etanercept 10 mg and those taking DMARD at 12 months.
43.1. Analysis.
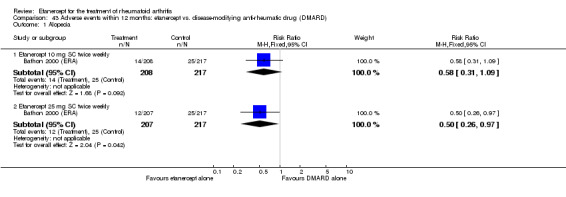
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 Alopecia.
Dizziness
People taking etanercept 10 mg alone were less likely to experience dizziness than those taking DMARD at 12 months (RR 0.5; 95% CI 0.2 to 0.9) (Analysis 43.6). However, there was no evidence of a significant difference in dizziness rates between etanercept 25 mg and DMARD at 12 months.
43.6. Analysis.
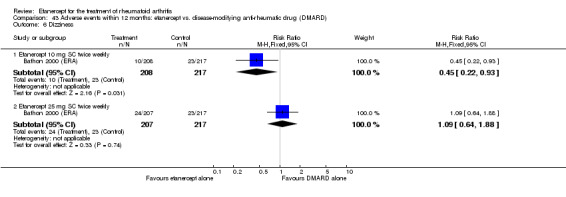
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 6 Dizziness.
Elevation in alanine transaminase (ALT)
Significantly more people had ALT elevation in the DMARD group when compared with the etanercept group at six months in one small trial (RR 0.3; 95% CI 0.1 to 0.7) (Analysis 42.11).
42.11. Analysis.
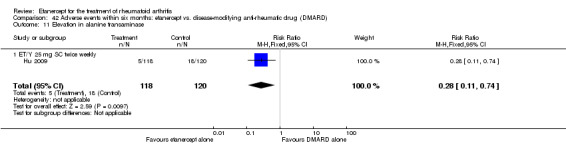
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 11 Elevation in alanine transaminase.
Flu syndrome
More people in the etanercept alone group had flu syndrome than in the DMARD alone group (RR 4.4; 95% CI 1.1 to 18.1) (Analysis 44.10).
44.10. Analysis.
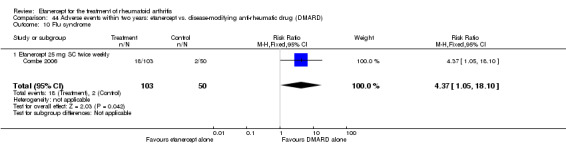
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 10 Flu syndrome.
Hypertension
People taking etanercept were more likely to have hypertension at two years than those taking DMARD (RR 2.5; 95% CI 1.3 to 4.7) (Analysis 44.13).
44.13. Analysis.
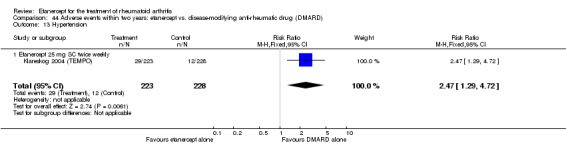
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 13 Hypertension.
Infections (total)
People assigned to etanercept alone were more likely to experience an infection than those assigned to DMARD alone at six months (RR 1.8; 95% CI 1.1 to 2.9) (Analysis 42.30). However, this difference was not observed at two years (Analysis 44.15).
42.30. Analysis.
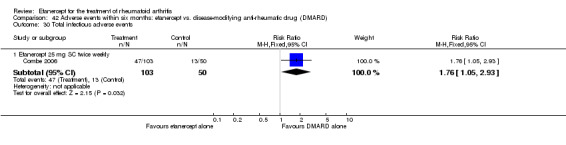
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 30 Total infectious adverse events.
44.15. Analysis.
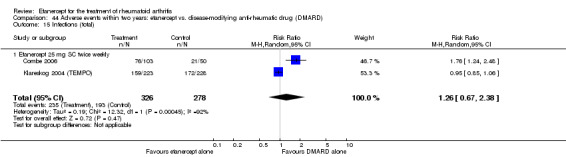
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 15 Infections (total).
Injection site reaction
There was a significant difference in the rates of injection site reaction at six months in three trials (RR 18.2; 95% CI 4.5 to 73.7) (Analysis 42.20), although CIs were wide. At 12 months' follow‐up, significantly more people receiving etanercept 10 or 25 mg had a reaction compared with those having DMARD (RR 4.1; 95% CI 2.5 to 6.9 and RR 5.0; 95% CI 3.1 to 8.4) (Analysis 43.12) and at two years significantly more people receiving etanercept 25 mg had a reaction compared with those receiving DMARD (RR 9.0; 95% CI 4.2 to 19.2) (Analysis 44.17).
42.20. Analysis.
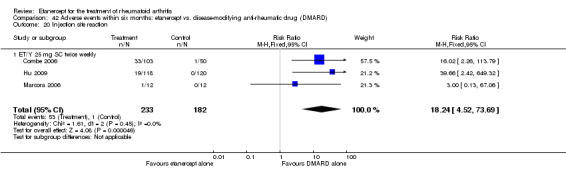
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 20 Injection site reaction.
43.12. Analysis.
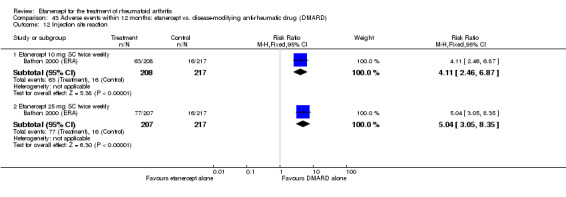
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 12 Injection site reaction.
44.17. Analysis.
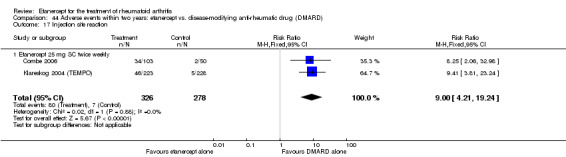
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 17 Injection site reaction.
Miscellaneous skin infections
At two years, there was a significant increase in the rate of miscellaneous skin infections in the etanercept monotherapy group compared with the DMARD alone group (RR 19.1; 95% CI 1.2 to 310.5) (Analysis 44.19).
44.19. Analysis.
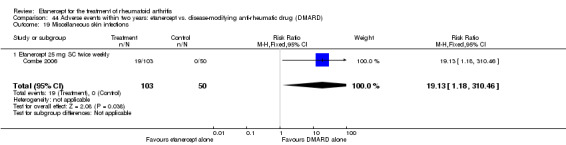
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 19 Miscellaneous skin infections.
Mouth ulcers
People taking etanercept 10 mg or 25 mg were less likely to develop mouth ulcers than DMARD at 12 months (RR 0.5; 95% CI 0.2 to 0.8 and RR 0.4; 95% CI 0.2 to 0.7, respectively) (Analysis 43.13).
43.13. Analysis.
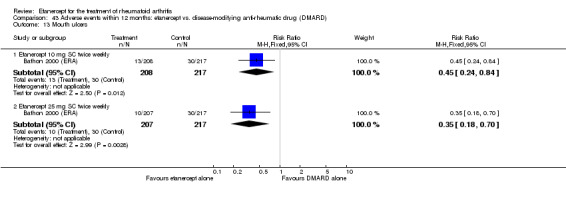
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 13 Mouth ulcers.
Nausea
People taking etanercept 10 mg or 25 mg were less likely to experience nausea than those taking DMARD at 12 months (RR 0.5; 95% CI 0.3 to 0.7 and RR 0.6; 95% CI 0.4 to 0.9, respectively) (Analysis 43.14). This finding was also apparent at two years; people taking etanercept were significantly less likely to experience nausea than those taking DMARD (RR 0.3; 95% CI 0.2 to 0.5) (Analysis 44.20).
43.14. Analysis.
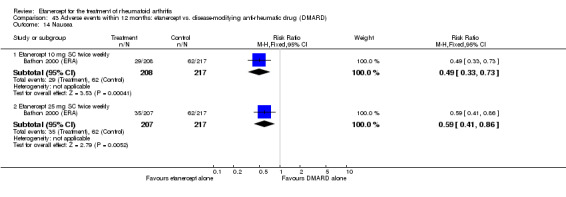
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 14 Nausea.
44.20. Analysis.
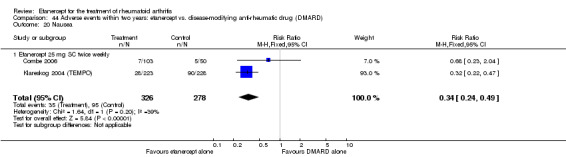
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 20 Nausea.
Pharyngitis or laryngitis
People taking etanercept 25 mg were more likely to experience pharyngitis or laryngitis compared with those taking DMARD at two years (RR 3.9; 95% CI 1.2 to 12.3) (Analysis 44.23).
44.23. Analysis.
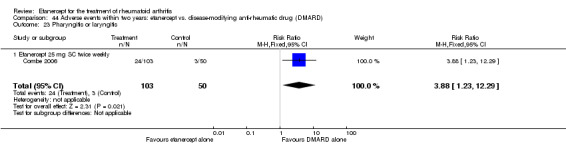
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 23 Pharyngitis or laryngitis.
Rash
People taking etanercept 25 mg were more likely to experience rash compared with those taking DMARD at six months (RR 2.4; 95% CI 1.1 to 5.6) (Analysis 42.29) and less likely at 12 months (RR 0.5; 95% CI 0.3 to 0.8) (Analysis 43.15). However, there was no evidence of a significant difference in rash rates between those taking etanercept 10 mg and DMARD at 12 months (the P value was just outside the 0.06 level of significance). At two years' follow‐up, there was no significant difference between groups (etanercept vs. DMARD) (Analysis 44.24).
42.29. Analysis.
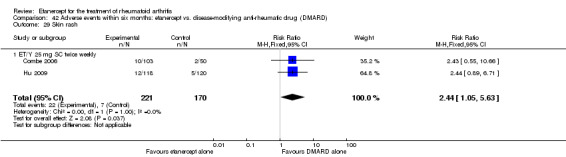
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 29 Skin rash.
43.15. Analysis.
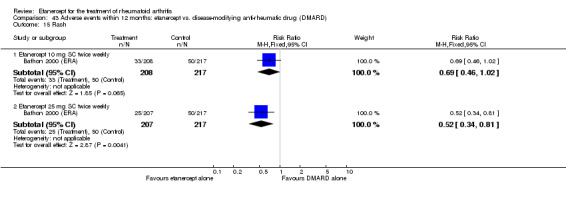
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 15 Rash.
44.24. Analysis.
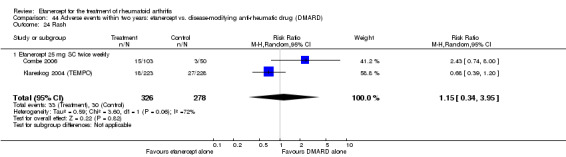
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 24 Rash.
Sinusitis
There was no significant difference between people taking etanercept 10 mg and people taking DMARD alone at 12 months (Analysis 43.17). However, people taking etanercept 25 mg were less likely to report sinusitis than those taking DMARD at 12 months (RR 0.6; 95% CI 0.4 to 1.0) (Analysis 43.17). The difference was not observed at two years between groups (Analysis 44.27).
43.17. Analysis.
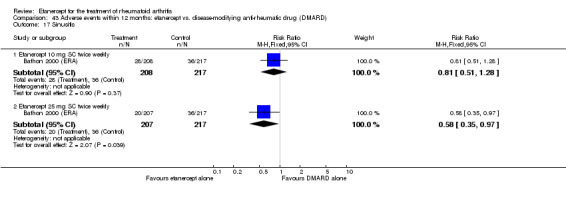
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 17 Sinusitis.
44.27. Analysis.
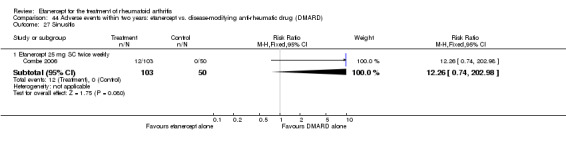
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 27 Sinusitis.
Upper respiratory tract infection (URTI)
Fewer people taking etanercept 10 mg reported URTI when compared with people taking DMARD at 12 months (RR 0.7; 95% CI 0.5 to 0.9) (Analysis 43.19). However, there was no significant difference in URTI rates between the etanercept 25 mg and DMARD groups at 12 months and two years (Analysis 43.19; Analysis 44.28).
43.19. Analysis.
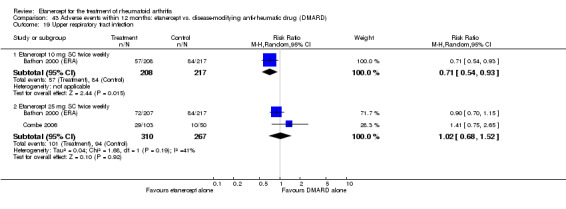
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 19 Upper respiratory tract infection.
44.28. Analysis.
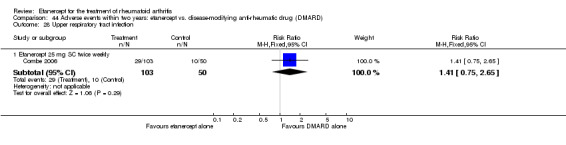
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 28 Upper respiratory tract infection.
Vomiting
People taking etanercept were less likely to experience vomiting at two years than those taking DMARD (RR 0.3 95% CI 0.2 to 0.6) (Analysis 44.29).
44.29. Analysis.
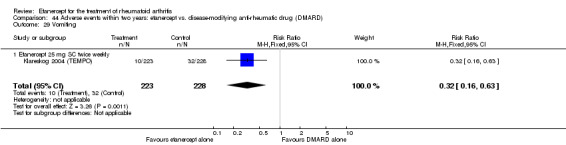
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 29 Vomiting.
Worsening of rheumatoid arthritis
In one study, people in the etanercept monotherapy group were more likely to experience worsening of the RA at two years than those taking DMARD alone (RR 8.3; 95% CI 2.1 to 33.0) (Analysis 44.25).
44.25. Analysis.
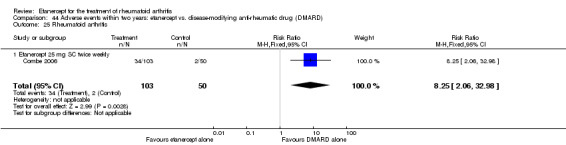
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 25 Rheumatoid arthritis.
There was no evidence of statistically significant differences in the rates of all other adverse effects in the included studies at various different time points:
total at six months (Analysis 42.1);
alopecia at six months (Analysis 42.2);
accidental injury at six months and two years (Analysis 42.3; Analysis 44.4);
asthenia at six months, 12 months and two years (Analysis 42.4; Analysis 43.3;; Analysis 44.3);
arthralgia at six months and two years (Analysis 42.5; Analysis 44.5);
back pain at 12 months and two years (Analysis 43.4; Analysis 44.6);
bronchitis at six months and two years (Analysis 42.6; Analysis 44.7);
chest discomfort at six months (Analysis 42.7);
diarrhoea at 12 months and two years (Analysis 43.5; Analysis 44.8);
dyspepsia at six months, 12 months and two years (Analysis 42.8; Analysis 43.7; Analysis 44.9);
dizziness at six months (Analysis 42.9);
ecchymosis at 12 months (Analysis 43.8);
enlargement of lymph nodes at six months (Analysis 42.12);
fever at six months (Analysis 42.13);
gastrointestinal (GI) symptoms/abdominal pain at six months, 12 months and two years (Analysis 42.14; Analysis 43.2; Analysis 44.2);
gingival/dental infection at two years (Analysis 44.11);
headache at six months, 12 months and two years (Analysis 42.15; Analysis 43.9; Analysis 44.12);
increased blood pressure at six months (Analysis 42.16);
increase in cough at six months and two years (Analysis 42.17; Analysis 44.14);
infection at another site/pharyngitis or laryngitis, flu syndrome or miscellaneous skin infections at six months (Analysis 42.18);
influenza‐like syndrome at 12 months (Analysis 43.10);
injection site haemorrhage at six months, 12 months and two years (Analysis 42.19; Analysis 43.11; Analysis 44.16);
insomnia at six months (Analysis 42.21);
leukopenia at six months (Analysis 42.22);
malignancy at two years (Analysis 44.18);
nausea at six months (Analysis 42.23);
oedema at six months (Analysis 42.10);
pain at six months and two years (Analysis 42.24; Analysis 44.21);
paraesthesia at six months (Analysis 42.25);
pharyngitis (non‐infectious) at six months (Analysis 42.26);
pruritus at six months and two years (Analysis 42.27; Analysis 44.22);
rhinitis at six and 12 months (Analysis 42.28; Analysis 43.16);
serious infections at two years (Analysis 44.26; Table 3);
skin infection at 12 months (Analysis 43.18);
URTI at six months (Analysis 42.31);
vision disorder at six months (Analysis 42.32).
42.1. Analysis.
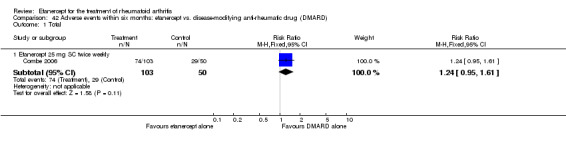
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 Total.
42.2. Analysis.
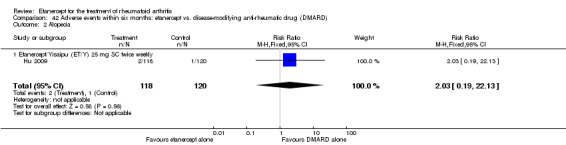
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 Alopecia.
42.3. Analysis.
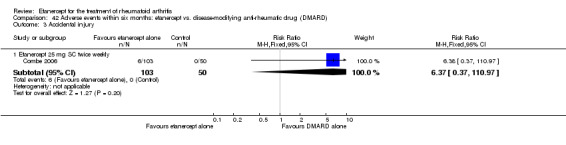
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 Accidental injury.
44.4. Analysis.
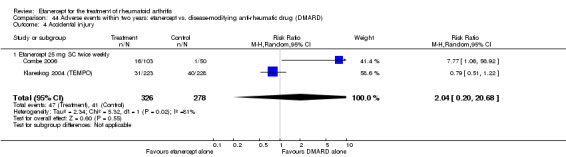
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Accidental injury.
42.4. Analysis.
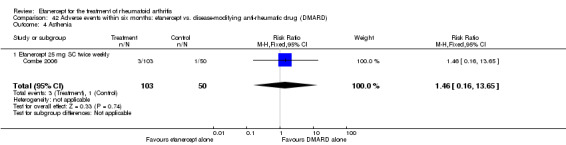
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Asthenia.
43.3. Analysis.
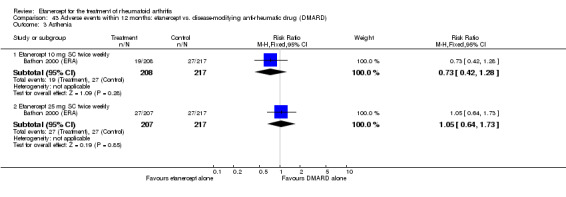
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 Asthenia.
44.3. Analysis.
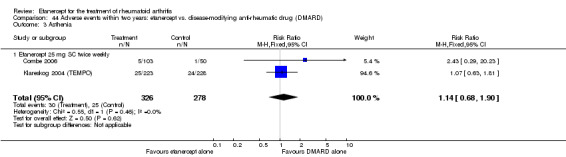
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 Asthenia.
42.5. Analysis.
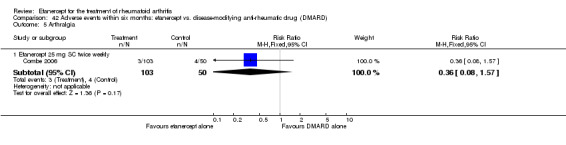
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 5 Arthralgia.
44.5. Analysis.
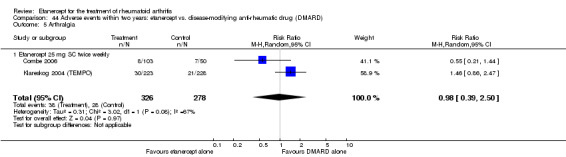
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 5 Arthralgia.
43.4. Analysis.
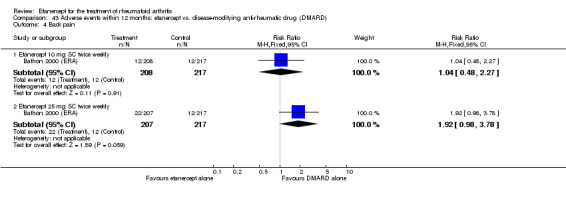
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 4 Back pain.
44.6. Analysis.
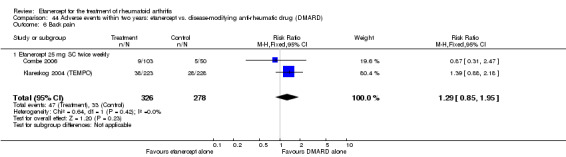
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 6 Back pain.
42.6. Analysis.
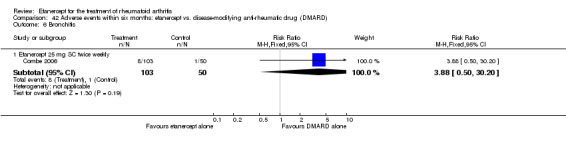
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 6 Bronchitis.
44.7. Analysis.
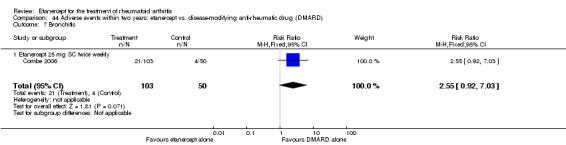
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 7 Bronchitis.
42.7. Analysis.
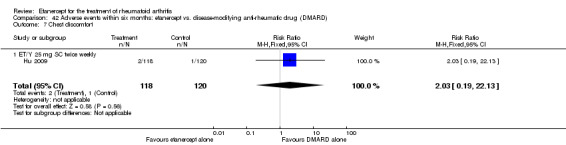
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 7 Chest discomfort.
43.5. Analysis.
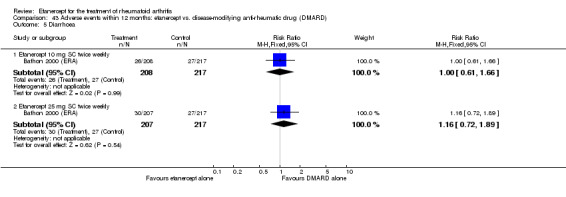
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 5 Diarrhoea.
44.8. Analysis.
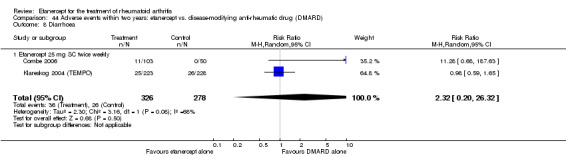
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 8 Diarrhoea.
42.8. Analysis.
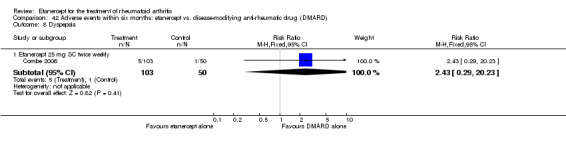
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 8 Dyspepsia.
43.7. Analysis.
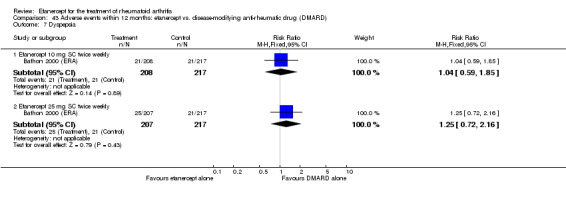
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 7 Dyspepsia.
44.9. Analysis.
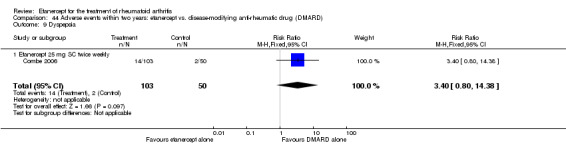
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 9 Dyspepsia.
42.9. Analysis.
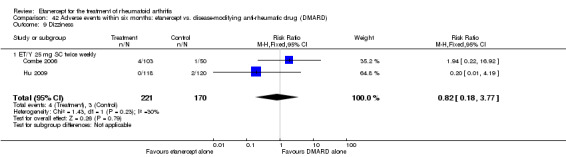
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 9 Dizziness.
43.8. Analysis.
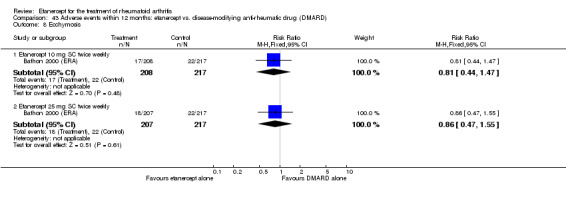
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 8 Ecchymosis.
42.12. Analysis.
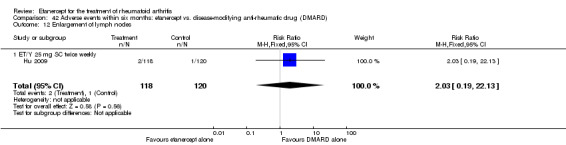
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 12 Enlargement of lymph nodes.
42.13. Analysis.
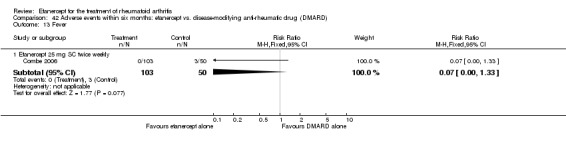
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 13 Fever.
42.14. Analysis.
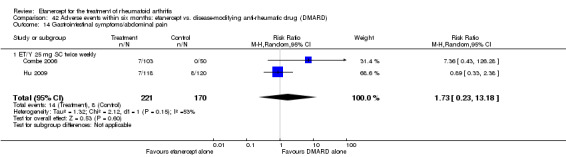
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 14 Gastrointestinal symptoms/abdominal pain.
43.2. Analysis.
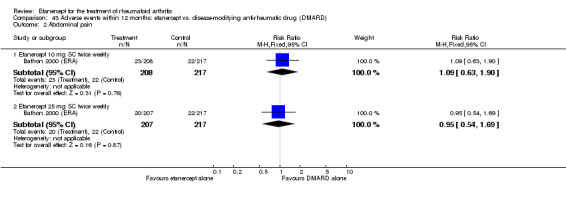
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 Abdominal pain.
44.2. Analysis.
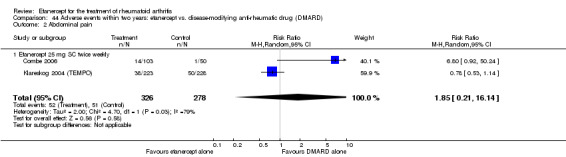
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 Abdominal pain.
44.11. Analysis.
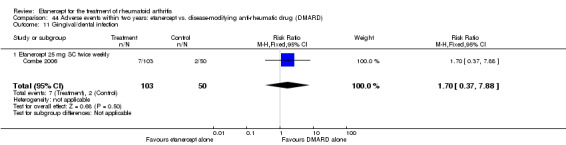
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 11 Gingival/dental infection.
42.15. Analysis.
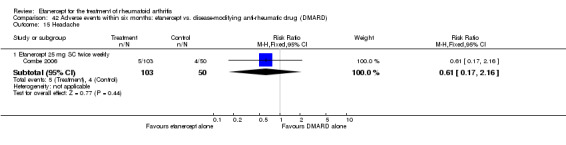
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 15 Headache.
43.9. Analysis.
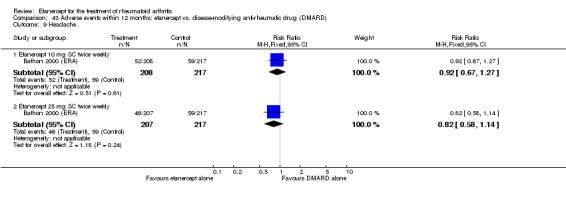
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 9 Headache.
44.12. Analysis.
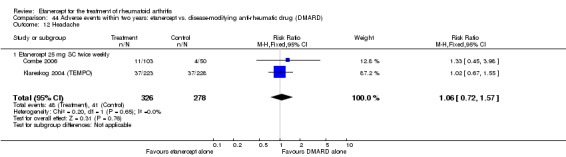
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 12 Headache.
42.16. Analysis.
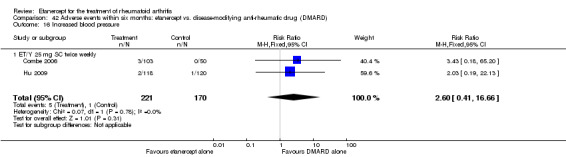
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 16 Increased blood pressure.
42.17. Analysis.
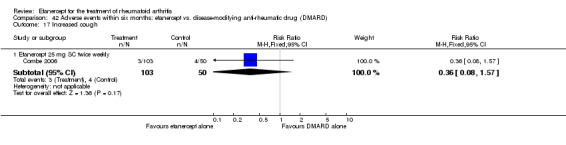
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 17 Increased cough.
44.14. Analysis.
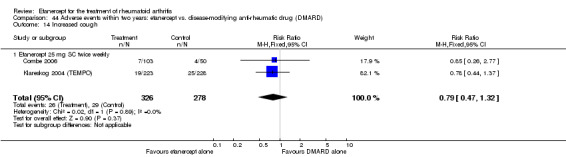
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 14 Increased cough.
42.18. Analysis.
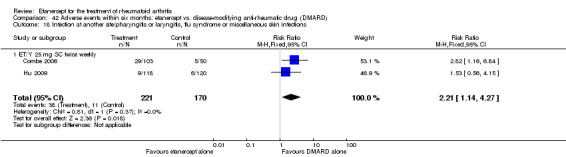
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 18 Infection at another site/pharyngitis or laryngitis, flu syndrome or miscellaneous skin infections.
43.10. Analysis.
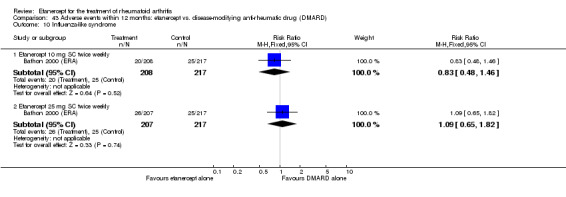
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 10 Influenza‐like syndrome.
42.19. Analysis.
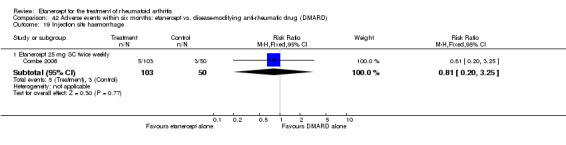
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 19 Injection site haemorrhage.
43.11. Analysis.
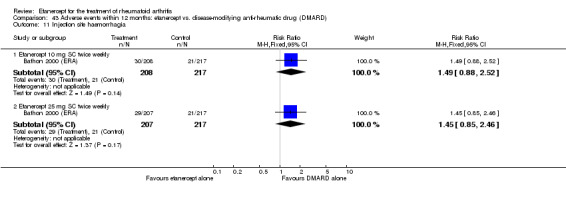
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 11 Injection site haemorrhagia.
44.16. Analysis.
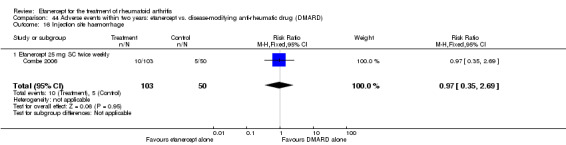
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 16 Injection site haemorrhage.
42.21. Analysis.
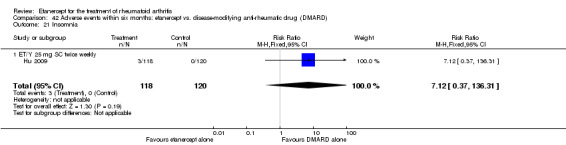
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 21 Insomnia.
42.22. Analysis.
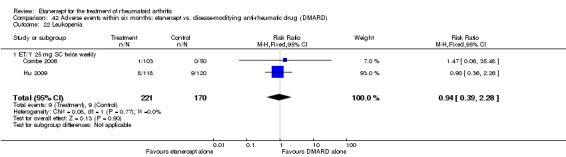
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 22 Leukopenia.
44.18. Analysis.
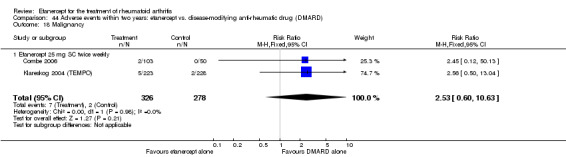
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 18 Malignancy.
42.23. Analysis.
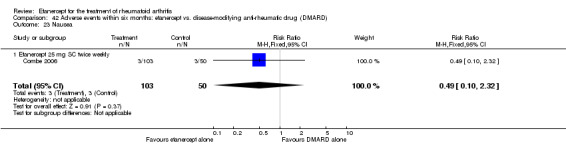
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 23 Nausea.
42.10. Analysis.
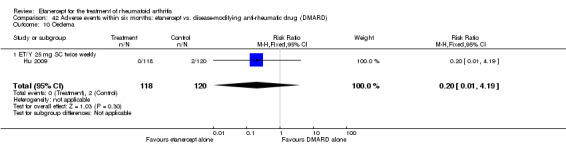
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 10 Oedema.
42.24. Analysis.
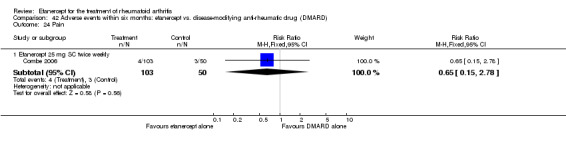
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 24 Pain.
44.21. Analysis.
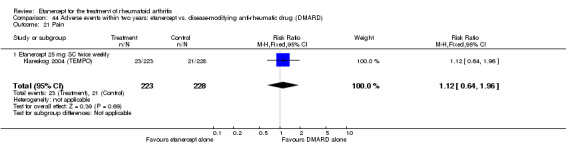
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 21 Pain.
42.25. Analysis.
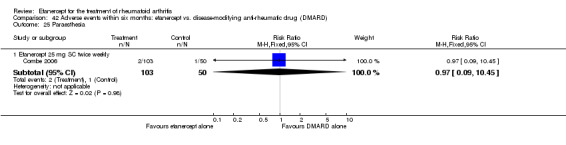
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 25 Paraesthesia.
42.26. Analysis.
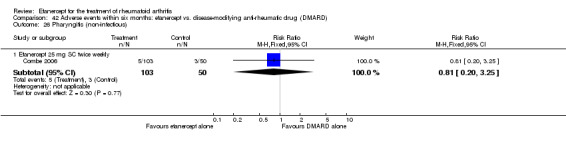
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 26 Pharyngitis (non‐infectious).
42.27. Analysis.
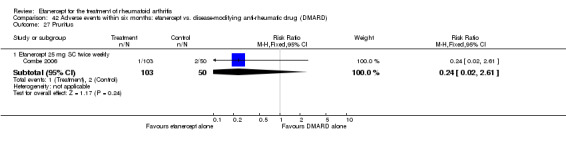
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 27 Pruritus.
44.22. Analysis.
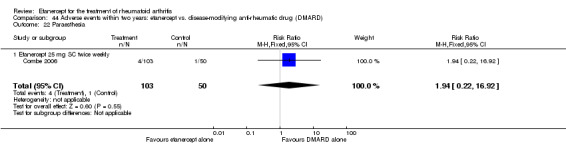
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 22 Paraesthesia.
42.28. Analysis.
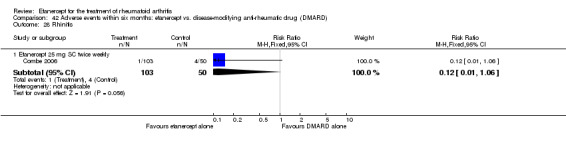
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 28 Rhinitis.
43.16. Analysis.
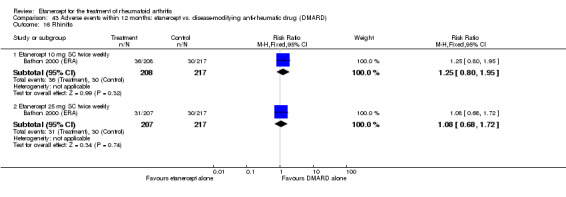
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 16 Rhinitis.
44.26. Analysis.
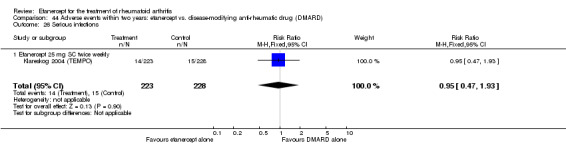
Comparison 44 Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 26 Serious infections.
43.18. Analysis.
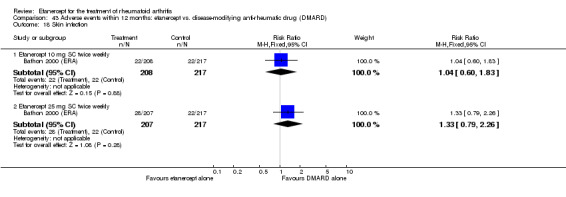
Comparison 43 Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 18 Skin infection.
42.31. Analysis.
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 31 Upper respiratory tract infection.
42.32. Analysis.
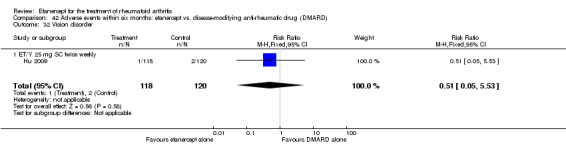
Comparison 42 Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 32 Vision disorder.
Etanercept plus DMARD versus etanercept
Headache
At six months, more people reported headache in the etanercept plus DMARD group than in the etanercept group (RR 3.1; 95% CI 1.2 to 8.1) (Analysis 45.8). However, this difference was not observed at two years (Analysis 46.12).
45.8. Analysis.
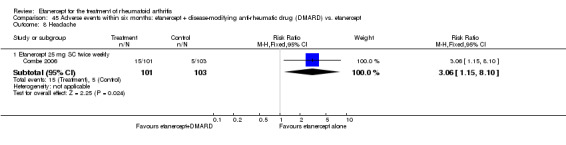
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 8 Headache.
46.12. Analysis.
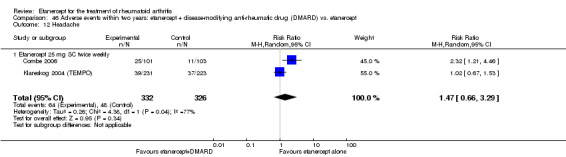
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 12 Headache.
Increased cough
At six months, there were no differences observed between the group receiving etanercept plus DMARD and the etanercept alone (Analysis 45.11). However, at two years people in the etanercept plus DMARD group were more likely to have an increase in cough than people in the etanercept alone group (RR 1.7; 95% CI 1.1 to 2.6) (Analysis 46.14).
45.11. Analysis.
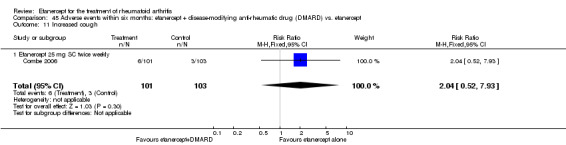
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 11 Increased cough.
46.14. Analysis.
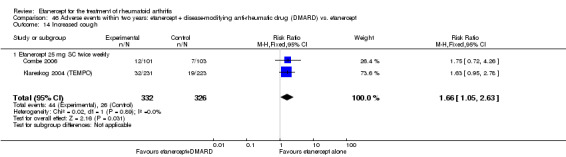
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 14 Increased cough.
Injection site reaction
At the end of six months' and two years' follow‐up, significantly fewer people receiving etanercept plus DMARD had injection site reactions when compared with people receiving etanercept alone (RR 0.6; 95% CI 0.43 to 0.8 and RR 0.6; 95% CI 0.4 to 0.8) (Analysis 45.13; Analysis 46.17).
45.13. Analysis.
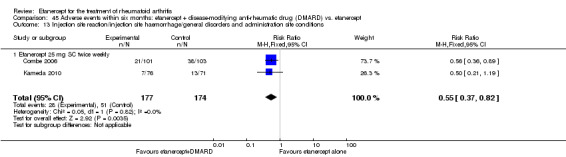
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 13 Injection site reaction/injection site haemorrhage/general disorders and administration site conditions.
46.17. Analysis.
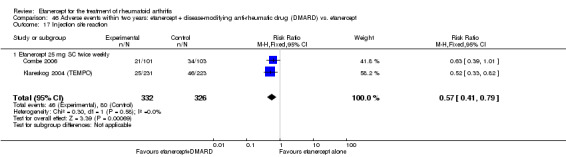
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 17 Injection site reaction.
Nausea
At six months and two years, significantly more people receiving etanercept plus MTX reported nausea when compared with people receiving etanercept alone (RR 4.1; 95% CI 1.2 to 14.0 and RR 2.4; 95% CI 1.7 to 3.4) (Analysis 45.19;Analysis 46.20).
45.19. Analysis.
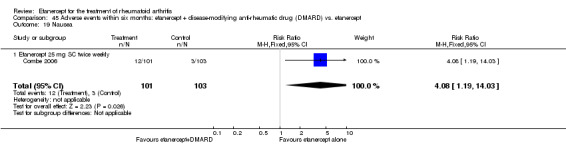
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 19 Nausea.
46.20. Analysis.
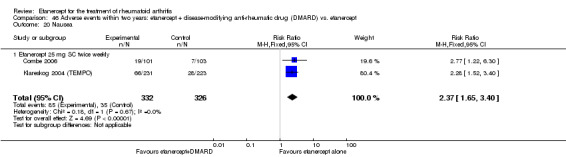
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 20 Nausea.
Pharyngitis or laryngitis (infectious)
At six months, there was no statistically significant difference between etanercept plus DMARD and the etanercept alone groups in the rates of pharyngitis or laryngitis (Analysis 45.23) but, at two years, significantly fewer people having etanercept plus DMARD reported pharyngitis or laryngitis than those having etanercept monotherapy (RR 0.4; 95% CI 0.2 to 0.8) (Analysis 46.23).
45.23. Analysis.
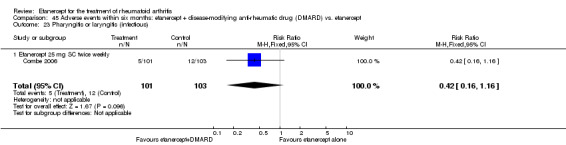
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 23 Pharyngitis or laryngitis (infectious).
46.23. Analysis.
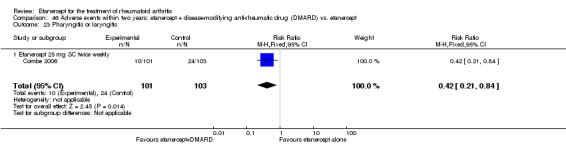
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 23 Pharyngitis or laryngitis.
Sinusitis
At two years, there were significantly fewer cases of sinusitis in people having etanercept plus DMARD compared with those having etanercept monotherapy (RR 0.3; 95% CI 0.1 to 0.9) (Analysis 46.27).
46.27. Analysis.
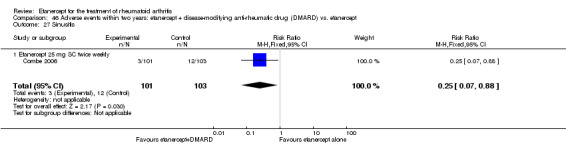
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 27 Sinusitis.
There was no evidence of statistically significant differences in the rates of all other adverse effects:
total at six months and two years (Analysis 45.1; Analysis 46.1);
arthralgia at two years (Analysis 46.4);
asthenia at six months and two years (Analysis 45.2; Analysis 46.5);
back pain at two years (Analysis 46.6);
blood and lymphatic system disorders/leukopenia at six months (Analysis 45.3);
dyspepsia at two years (Analysis 46.9);
diarrhoea at two years (Analysis 46.8);
dizziness at six months (Analysis 45.4);
fever at six months (Analysis 45.5);
flu syndrome at six months and two years (Analysis 45.6; Analysis 46.10);
GI disorders/abdominal pain at six months and two years (Analysis 45.7; Analysis 46.2);
gingival/dental infection at two years (Analysis 46.11);
hepatobiliary disorders at six months (Analysis 45.9);
hypertension at six months and two years (Analysis 45.10; Analysis 46.13);
infections (total) at six months and two years (Analysis 45.12; Analysis 46.15; Table 3);
injection site haemorrhage at two years (Analysis 46.16);
injury, poisoning and procedural complications/accidental injury at six months and two years (Analysis 45.14; Analysis 46.3);
malignancy at six months and two years (Analysis 45.15; Analysis 46.18);
metabolism and nutrition disorders at six months (Analysis 45.16);
miscellaneous skin infections at six months and two years (Analysis 45.17; Analysis 46.19);
musculoskeletal and connective tissue disorders/arthralgia at six months (Analysis 45.18);
nervous system disorders/paraesthesia at six months and two years (Analysis 45.20; Analysis 46.22);
pain at six months and two years (Analysis 45.21; Analysis 46.21);
pharyngitis (non‐infectious) at six months (Analysis 45.22);
reproductive system and breast disorders at six months (Analysis 45.24);
respiratory, thoracic and mediastinal disorders/bronchitis at six months and two years (Analysis 45.25; Analysis 46.7);
rhinitis at six months (Analysis 45.26);
serious adverse events (total) at six months (Analysis 45.28);
serious infections at two years (Analysis 46.26);
skin and subcutaneous tissue disorders/rash/pruritus at six months and two years (Analysis 45.27; Analysis 46.24);
URTI at six months and two years (Analysis 45.29; Analysis 46.28);
vomiting at two years (Analysis 46.29);
worsening of RA at two years (Analysis 46.25).
45.1. Analysis.
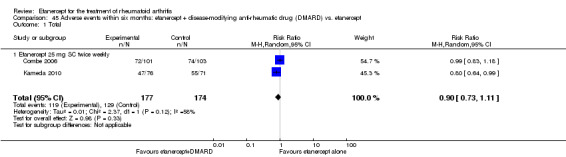
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 Total.
46.1. Analysis.
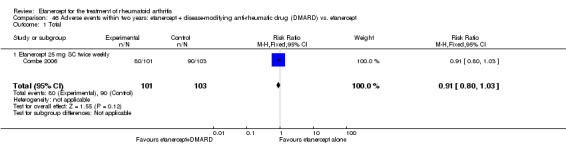
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 Total.
46.4. Analysis.
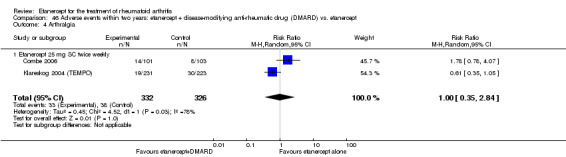
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Arthralgia.
45.2. Analysis.
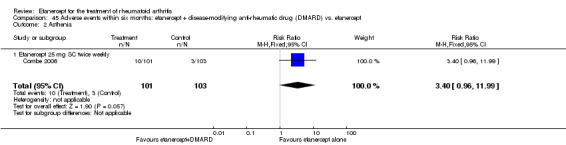
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 Asthenia.
46.5. Analysis.
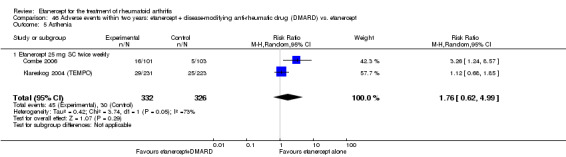
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 5 Asthenia.
46.6. Analysis.
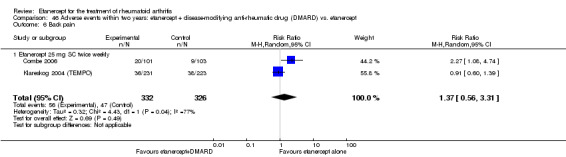
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 6 Back pain.
45.3. Analysis.
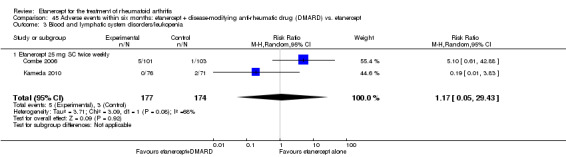
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 Blood and lymphatic system disorders/leukopenia.
46.9. Analysis.
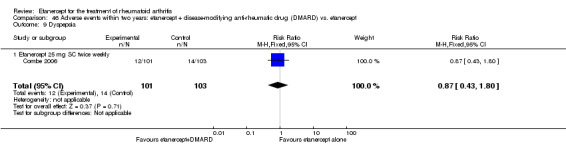
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 9 Dyspepsia.
46.8. Analysis.
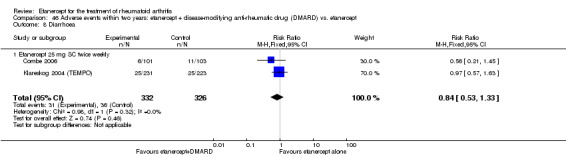
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 8 Diarrhoea.
45.4. Analysis.
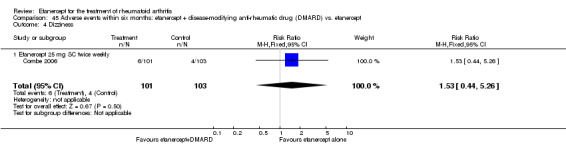
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 4 Dizziness.
45.5. Analysis.
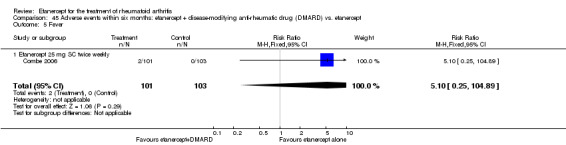
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 5 Fever.
45.6. Analysis.
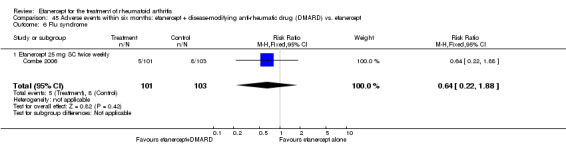
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 6 Flu syndrome.
46.10. Analysis.
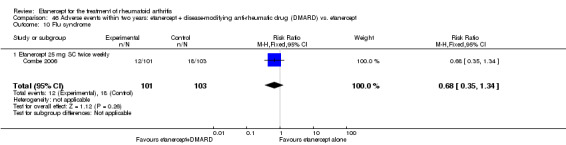
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 10 Flu syndrome.
45.7. Analysis.
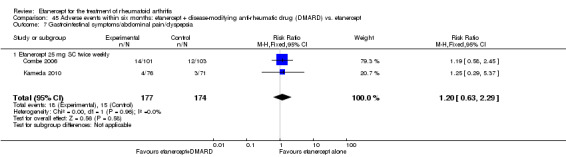
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 7 Gastrointestinal symptoms/abdominal pain/dyspepsia.
46.2. Analysis.
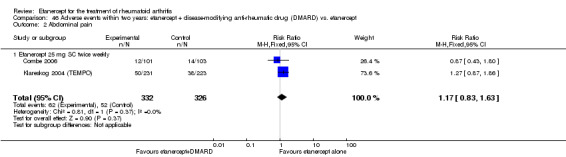
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 Abdominal pain.
46.11. Analysis.
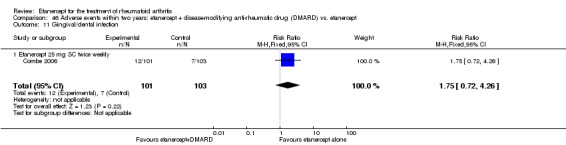
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 11 Gingival/dental infection.
45.9. Analysis.
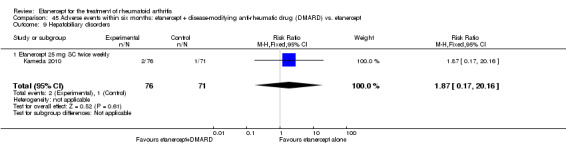
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 9 Hepatobiliary disorders.
45.10. Analysis.
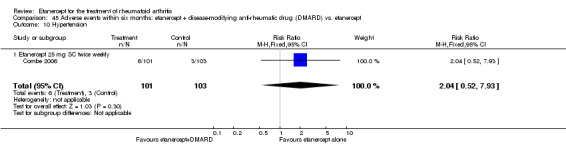
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 10 Hypertension.
46.13. Analysis.
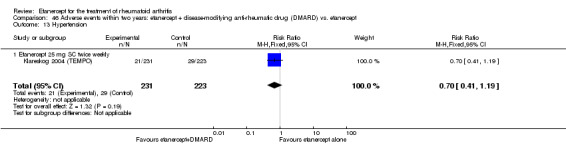
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 13 Hypertension.
45.12. Analysis.
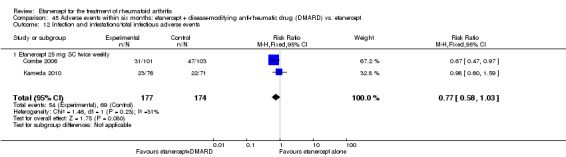
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 12 Infection and infestations/total infectious adverse events.
46.15. Analysis.
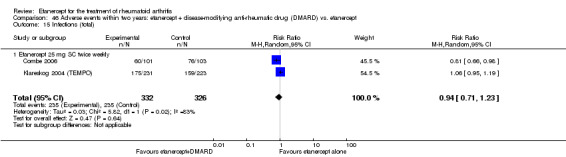
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 15 Infections (total).
46.16. Analysis.
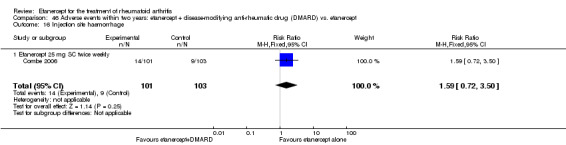
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 16 Injection site haemorrhage.
45.14. Analysis.
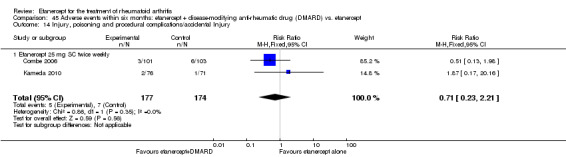
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 14 Injury, poisoning and procedural complications/accidental Injury.
46.3. Analysis.
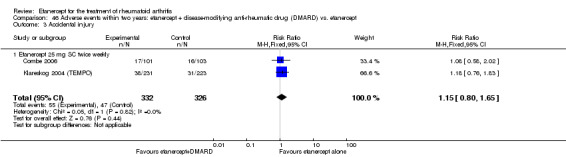
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 Accidental injury.
45.15. Analysis.
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 15 Malignancy.
46.18. Analysis.
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 18 Malignancy.
45.16. Analysis.
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 16 Metabolism and nutrition disorders.
45.17. Analysis.
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 17 Miscellaneous skin infections.
46.19. Analysis.
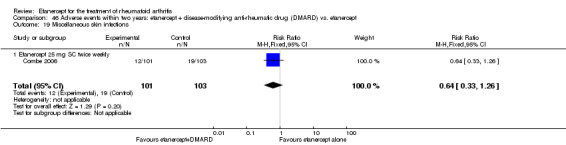
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 19 Miscellaneous skin infections.
45.18. Analysis.
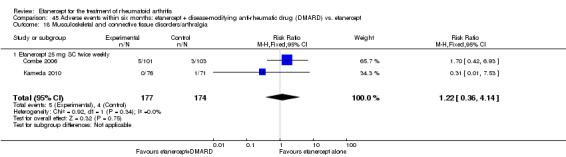
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 18 Musculoskeletal and connective tissue disorders/arthralgia.
45.20. Analysis.
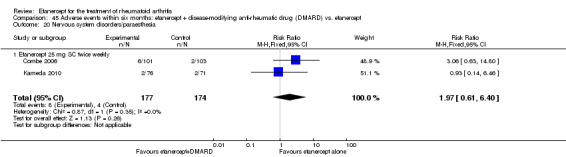
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 20 Nervous system disorders/paraesthesia.
46.22. Analysis.
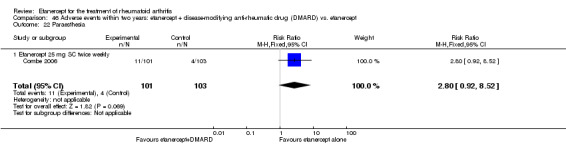
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 22 Paraesthesia.
45.21. Analysis.
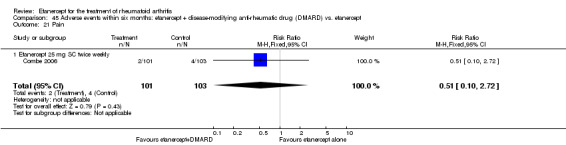
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 21 Pain.
46.21. Analysis.
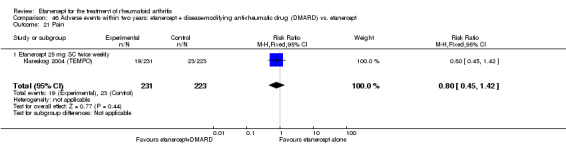
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 21 Pain.
45.22. Analysis.
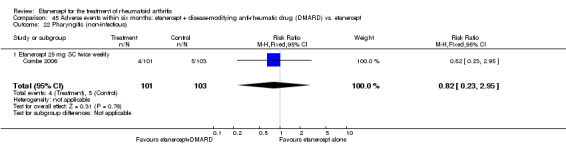
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 22 Pharyngitis (non‐infectious).
45.24. Analysis.
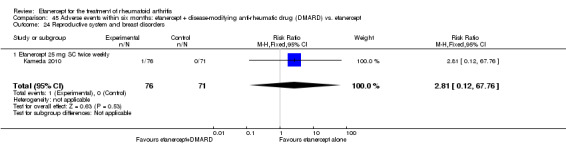
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 24 Reproductive system and breast disorders.
45.25. Analysis.
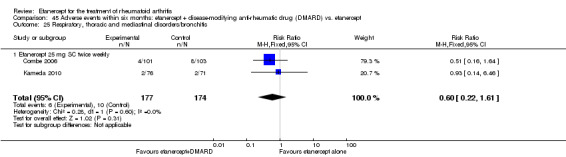
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 25 Respiratory, thoracic and mediastinal disorders/bronchitis.
46.7. Analysis.
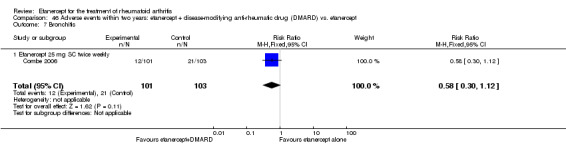
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 7 Bronchitis.
45.26. Analysis.
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 26 Rhinitis.
45.28. Analysis.
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 28 Total serious adverse events.
46.26. Analysis.
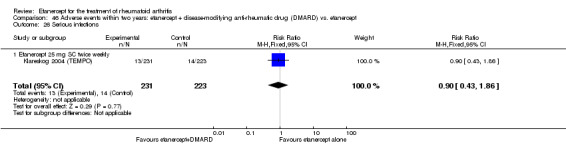
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 26 Serious infections.
45.27. Analysis.
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 27 Skin and subcutaneous tissue disorders/rash/pruritus.
46.24. Analysis.
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 24 Rash.
45.29. Analysis.
Comparison 45 Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 29 Upper respiratory tract infection.
46.28. Analysis.
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 28 Upper respiratory tract infection.
46.29. Analysis.
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 29 Vomiting.
46.25. Analysis.
Comparison 46 Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 25 Rheumatoid arthritis.
Etanercept versus placebo
Injection site reaction
A greater proportion of people receiving either etanercept 10 or 25 mg developed injection site reactions at six months than those taking placebo (RR 3.5; 95% CI 1.8 to 6.6 and RR 3.9 95% CI 2.1 to 7.3) (Analysis 47.3). About 43% and 49%, respectively, of those taking etanercept 10 and 25 mg experienced reactions compared with 13% of those taking placebo.
47.3. Analysis.
Comparison 47 Adverse events within six months: etanercept vs. placebo, Outcome 3 Injection site reaction.
Upper respiratory tract infection
People taking etanercept 25 mg were more likely to develop URTI in comparison to those taking placebo at six months (RR 2.1; 95% CI 1.1 to 3.7) (Analysis 47.6). However, there was no evidence of a difference in URTI rates between those taking etanercept 10 mg and control (P value just outside the 0.05 level of significance).
47.6. Analysis.
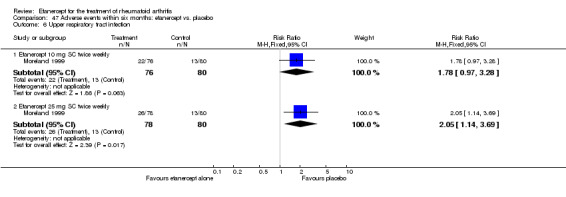
Comparison 47 Adverse events within six months: etanercept vs. placebo, Outcome 6 Upper respiratory tract infection.
There was no statistically significant differences in the rates of all other adverse effects in the included studies:
diarrhoea at six months (Analysis 47.1);
headache at six months (Analysis 47.2);
rhinitis at six months (Analysis 47.4);
sinusitis at six months (Analysis 47.5).
47.1. Analysis.
Comparison 47 Adverse events within six months: etanercept vs. placebo, Outcome 1 Diarrhoea.
47.2. Analysis.
Comparison 47 Adverse events within six months: etanercept vs. placebo, Outcome 2 Headache.
47.4. Analysis.
Comparison 47 Adverse events within six months: etanercept vs. placebo, Outcome 4 Rhinitis.
47.5. Analysis.
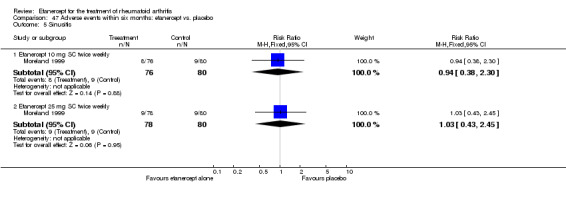
Comparison 47 Adverse events within six months: etanercept vs. placebo, Outcome 5 Sinusitis.
D. Subgroup analyses
Results on the subgroup analyses are summarised in Table 4.
Etanercept plus DMARD versus DMARD
There was a greater proportion of people significantly achieving an ACR20 and ACR50 response in people who previously had an inadequate response with MTX compared with people who had never been treated with a DMARD or had failed treatment with DMARDs other than MTX (Analysis 48.1; Analysis 48.2). However, a greater proportion of DMARD‐naïve people achieved an ACR70 response compared with the groups who have not benefited from DMARDs alone (Analysis 48.3).
48.1. Analysis.
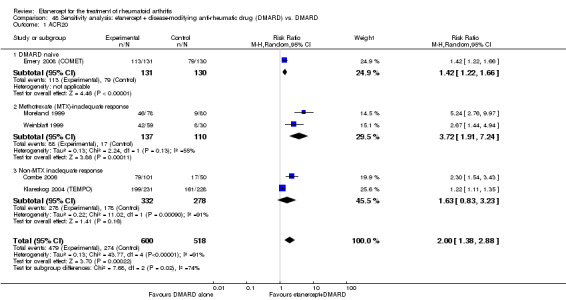
Comparison 48 Sensitivity analysis: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 1 ACR20.
48.2. Analysis.
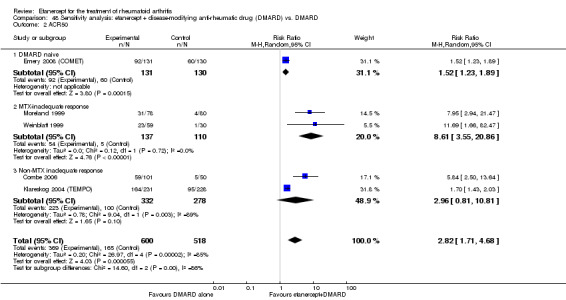
Comparison 48 Sensitivity analysis: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 2 ACR50.
48.3. Analysis.
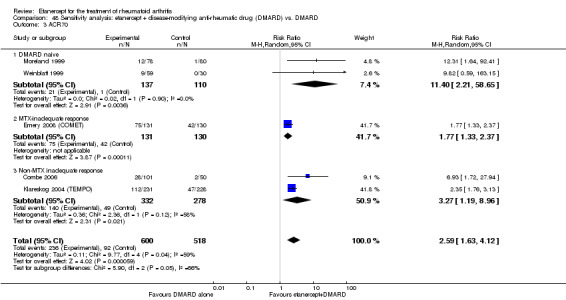
Comparison 48 Sensitivity analysis: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD, Outcome 3 ACR70.
Etanercept versus DMARD
The indirect comparisons showed no difference across groups (Analysis 49.1; Analysis 49.2; Analysis 49.3).
49.1. Analysis.
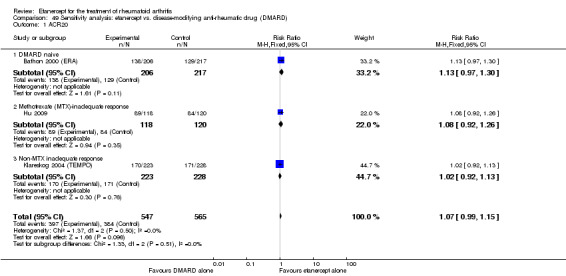
Comparison 49 Sensitivity analysis: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 1 ACR20.
49.2. Analysis.
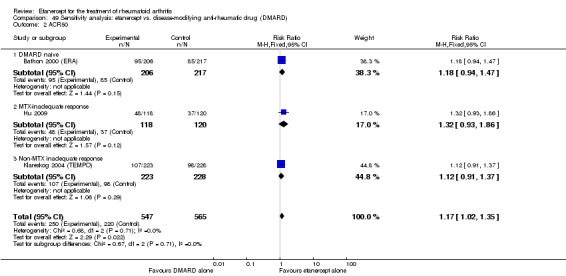
Comparison 49 Sensitivity analysis: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 2 ACR50.
49.3. Analysis.
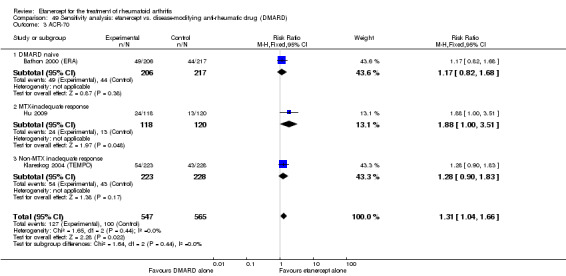
Comparison 49 Sensitivity analysis: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD), Outcome 3 ACR‐70.
Etanercept plus DMARD versus etanercept
The indirect comparisons showed no difference across groups (Analysis 50.1; Analysis 50.2; Analysis 50.3).
50.1. Analysis.
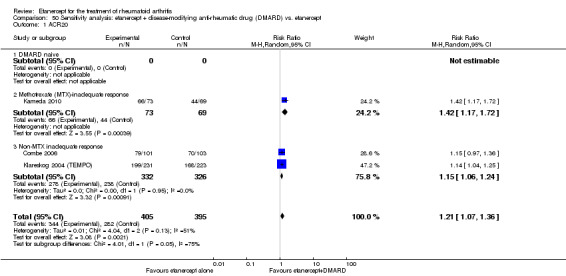
Comparison 50 Sensitivity analysis: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 1 ACR20.
50.2. Analysis.
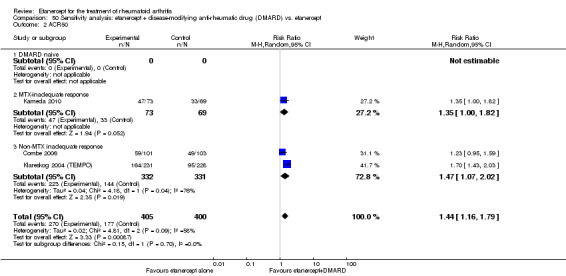
Comparison 50 Sensitivity analysis: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 ACR50.
50.3. Analysis.
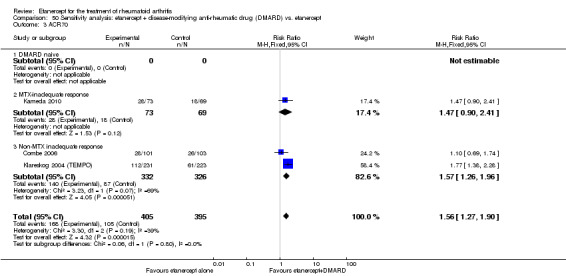
Comparison 50 Sensitivity analysis: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 3 ACR70.
Discussion
RA is a common systemic inflammatory arthritis associated with significant morbidity, mortality, joint deformity and impaired quality of life. DMARDS have been shown to reduce disease activity, slow down joint damage and improve quality of life. While DMARDS are the mainstay of treatment of RA, many people do not respond to or are unable to tolerate traditional DMARDS. Biological drugs have been introduced and approved since 1998 for the treatment of RA. Etanercept is one biological agent (a soluble TNF alpha receptor) that inhibits the action of TNF. The benefits of six biologics for the treatment of RA and the adverse effects of nine biologics used in various rheumatic conditions have been compared in two separate Cochrane network meta‐analyses (Singh 2009; Singh 2011).
Summary of main results
The ACR20, ACR50 and ACR70 response rates were improved with etanercept plus DMARD at all follow‐up time points (6 months, one year, two years and three years). These findings were consistent when etanercept was directly compared with placebo. However, there was mostly no evidence of significant differences in ACR response rates when etanercept monotherapy was compared with DMARD alone at six months, 12 months, two years and three years. The exceptions to this finding may have been due to chance as no patterns could be determined. For example, people receiving etanercept 25 mg had improved response to ACR70 at six months and improved response to ACR50 at two years when compared with people receiving DMARD and, by contrast, one trial reported that etanercept at the lower dosage of 10 mg had lower response rates to ACR50 at 12 months compared with DMARD. Results were consistent for the comparison of etanercept plus DMARD versus etanercept alone at all time points (as well as DMARD alone) in the improvement of ACR response rates. The superior efficacy of etanercept plus DMARD treatment over monotherapy has been highlighted by others (Furst 2007).
Remission rates (as defined by DAS < 1.6 and DAS28 < 2.6) and joint damage (as assessed by radiographic scores) were other outcomes that provided an assessment of response to treatment. These outcomes were measured in five trials (Bathon 2000 (ERA); Combe 2006; Emery 2008 (COMET); Kameda 2010; Klareskog 2004 (TEMPO)). When etanercept monotherapy was compared with DMARD alone, there was mostly no evidence of a difference in remission rates (as defined by DAS < 1.6 and DAS28 < 2.6) at 12 months, two years and three years although at two years, people receiving etanercept were more likely to be in remission (DAS < 1.6) than those receiving DMARD (not confirmed by results from the DAS28 definition of remission at this time point).
We found no statistically significant differences in the radiographic score in people with inadequate response to DMARDs. However, when all participants were combined, there was some evidence of an improvement in some of the radiographic scores for people receiving etanercept when compared with those receiving DMARD. At 12 months and two years, people receiving etanercept had smaller changes from baseline in the TSS and ES scores and at one year they had a smaller change in the ES score (with a trend of P value = 0.06 for the TSS score) when compared with those receiving DMARD. There were also more participants in the etanercept group that had no progression of their joint damage (as measured by TSS ≤ 0.5 when compared with those in the DMARD group at one and three years', but not at two years' follow‐up. There was no evidence of any differences in the JSNS at any time period between groups. The effects of etanercept plus DMARD treatment on remission rates and radiographic scores paralleled the improvements in ACR response rates when compared with monotherapy with either etanercept or DMARD. People receiving etanercept plus DMARD were significantly more likely to experience remission at 12 months, two years and three years when compared with those receiving DMARD or etanercept alone. There was also a significantly smaller change from baseline in radiographic scores at 12 months, two years and three years for people taking combination treatment compared with DMARD and etanercept alone (although at three years, there was no evidence of a difference between etanercept plus DMARD and etanercept alone). For many people taking etanercept plus DMARD, radiographic damage appeared to be arrested, as evidenced by the negative mean scores in the change from baseline, particularly for TSS and ES. This was further confirmed by the publication of cumulative probability plots published by one of the TEMPO authors (van der Heijde 2005b). The MD in TSS score and the absolute reduction observed at 12 months in radiographic progression were ‐2.12% and 0.49%, respectively. To transform the radiographic findings in terms of disability, we have used a previously published formula (Smolen 2010b; Tugwell 2011). In our review, the radiographic score expressed as estimate of irreversible physical disability over 10 years is 0.45 out of 3.0. That is, etanercept would prevent an increase in disability of 0.45 irreversible HAQ units (15%), which surpasses the minimal clinically important difference of 0.22 HAQ units.
Quality of life was measured by scores on MOS SF‐36, HAQ, EQ‐5D VAS and ASHI and people were also asked to indicate their satisfaction with treatment. Quality of life of people taking either etanercept alone or etanercept plus DMARD was mostly significantly improved when compared with DMARD alone at six months, 12 months and two years. People taking etanercept 25 mg had higher scores on the MOS mental health and energy/vitality domains at six months compared with placebo. When etanercept monotherapy was compared with DMARD alone, there was mostly no evidence of a benefit for etanercept in perceived quality of life, although at one year, people taking etanercept were more satisfied with their treatment than those taking DMARD. A small improvement of three percentage points in the HAQ was also found for those taking etanercept at two years but this was not confirmed when HAQ was measured as an absolute score after treatment. There was no evidence of a difference in the HAQ at other time points, SF‐36, ASHI and EQ‐5D VAS between etanercept and DMARD monotherapy groups. Treatment with etanercept plus DMARD appeared to mostly improve quality of life when compared with either DMARD or etanercept alone. Those taking etanercept plus DMARD had higher scores on the HAQ at one and two years and higher scores on the EQ‐5D VAS at one year than those taking DMARD or etanercept alone. Satisfaction rates with etanercept plus DMARD treatment were also improved at one year when compared with DMARD monotherapy but not when compared with etanercept monotherapy. Overall, it appears as though combination therapy is associated with less disability, as perceived by people through their HAQ assessments than either monotherapy with etanercept or MTX.
Withdrawals before the end of the study were measured in four ways: total withdrawals, withdrawal because of lack of efficacy, withdrawal because of adverse events and deaths before the completion of the trial. Withdrawals were measured at six months, one year, two years and three years cumulatively to enable a comparison of the pattern of withdrawals over time. People taking etanercept plus DMARD were less likely to withdraw from the study overall and also less likely to withdraw from the study because of lack of efficacy of the treatment at all time periods when compared with those taking DMARD alone. However, there was mostly no evidence of a difference in the withdrawal rates because of adverse events between groups except at three years when people taking etanercept plus DMARD were less likely to have withdrawn from the study because of adverse events compared with those taking DMARD alone. There was mostly no evidence of a difference in withdrawals when etanercept alone was compared with DMARD alone. At two follow‐up times, people on etanercept were less likely to have withdrawn: at two years, significantly fewer people taking etanercept 25 mg had withdrawn overall and at 12 months, significantly fewer people taking etanercept 25 mg had withdrawn because of adverse events than people taking DMARD. At all other time points, there was no evidence of a difference in the withdrawal rates between groups taking either etanercept or DMARD monotherapy. Participants were also less likely to withdraw at any time of follow‐up, either overall or because of lack of efficacy, when taking etanercept plus DMARD treatment when compared with etanercept monotherapy, confirming the clinical efficacy seen with this treatment. By contrast, withdrawal because of adverse events did not differ between the etanercept plus DMARD group or etanercept monotherapy group at any time point. There was also no evidence of a difference in the death rates between groups (whether etanercept plus DMARD was compared with DMARD or etanercept monotherapy or etanercept was compared with DMARD or with control).
Adverse events were reported only at the end of the included studies. However, in the Klareskog 2004 (TEMPO) study, adverse events were reported at the end of the first two years of the three‐year trial because more detailed information on effects was available at this follow‐up time. There was a greater risk of injection site reactions when etanercept alone was compared with placebo at six months, when etanercept plus DMARD treatment was compared with DMARD alone and when etanercept monotherapy was compared with DMARD alone at six months, 12 months and two years. Injection site reactions were also more likely for people taking etanercept monotherapy compared with those taking etanercept plus DMARD. The significantly greater likelihood of this particular adverse effect in people taking etanercept (compared with either placebo or DMARD) may have affected the blinding of the studies as participants may have become aware of the nature of their treatment. People taking etanercept 25 mg were more likely to report URTI at six months than people taking placebo. Those people taking etanercept monotherapy were less likely to report nausea compared with DMARD alone or etanercept plus DMARD. When etanercept monotherapy was compared with placebo or etanercept plus DMARD, there was no evidence of a difference in the rates of other adverse events at any time period. When etanercept monotherapy was compared with DMARD, a number of adverse events were significantly more likely with DMARD alone. At six months, there was a higher proportion of people with elevation in ALT in the control group. At 12 months, the rate of URTI and dizziness were more likely with DMARD compared with etanercept 10 mg (but not etanercept 25 mg) and rates of sinusitis, rash, and alopecia were significantly more likely with DMARD compared with etanercept 25 mg (but not etanercept 10 mg). At 12 months, people taking DMARD were also significantly more likely to experience mouth ulcers and at both 12 months and two years, people taking DMARD were more likely to experience nausea when compared with those taking either dose of etanercept (25 mg or 10 mg). At two years, vomiting and hypertension were more common among people taking DMARD in comparison to those taking etanercept. Also at two years, there were more reports of worsening RA in the etanercept monotherapy group than in the DMARD group. The participant groups in the trials differed in the previous response or exposure to DMARDs. Three of the studies recruited participants with 'early' RA and no prior treatment; in the Bathon 2000 (ERA) and Emery 2008 (COMET) trials, participants had been diagnosed for less than three years with no previous DMARD treatment and in the Marcora 2006 trial participants had RA for less than six months with no previous DMARD treatment. In the Hu 2009, Kameda 2010, Moreland 1999, and Weinblatt 1999, trials, participants had RA for more than five years and were required to have had no response to previous MTX treatment. In the Combe 2006 and Klareskog 2004 (TEMPO) trials, the duration of RA varied and participants were required to have no response to previous DMARDs (including MTX). ACR20 and ACR50 results from the trials where participants were inadequate responders to MTX appear significantly different between etanercept plus DMARD and DMARD even at the short time period of six months in comparison to a more limited response in the trials where participants were DMARD‐naive or have had an inadequate response to DMARDs. However, for ACR70 the difference was observed from trials where participants were DMARD‐naive. For the other comparisons (etanercept monotherapy versus DMARD alone or etanercept plus DMARD versus etanercept monotherapy) we did not observed any differences. In contrast, ACR70 and remission rates (evaluated by DAS < 2.6) were higher for people receiving etanercept plus DMARD who had an inadequate response to DMARDs compared with people who maintain DMARD monotherapy despite inadequate response.
Overall completeness and applicability of evidence
This review has assessed the effects of etanercept in RA through the evaluation of results from a wide variety of outcomes measuring both benefits and harms. There has been much discussion on the identification of appropriate outcomes for assessment of interventions in rheumatology. OMERACT (Outcome Measures for Arthritis Clinical Trials) is an international initiative with the aim of determining the applicability of various outcome measures (Molenaar 2000), and these are specified in 'Types of Outcome Measures' in the review. Benefit includes both clinical measures and patient and physician assessment of pain, disability and function. There is strong evidence that etanercept is more efficacious than placebo and that combination etanercept plus DMARD treatment is more efficacious than DMARD or etanercept monotherapy in people with RA. Etanercept monotherapy does not appear to be more efficacious than DMARD alone in terms of ACR response, rate of remission or quality of life, but there is good evidence that etanercept alone causes less joint damage as assessed by the TSS and the ES. Withdrawal from the study before completion can be due to a variety of reasons and may, to some extent, reflect the acceptability of treatment. There is good evidence that etanercept is associated with less overall withdrawal and less withdrawal because of lack of efficacy than control, either when compared with placebo or when etanercept plus DMARD treatment is compared with DMARD. These benefits were not found when etanercept alone was compared with DMARD alone, suggesting that both of these monotherapies have similar acceptability and benefit (confirmed by efficacy outcomes previously discussed). However, etanercept plus DMARD treatment is associated with fewer withdrawals when compared with etanercept monotherapy, either overall or as a result of lack of efficacy.
Although there appear to be clear benefits of etanercept therapy in the studies included in this review, it is important to consider results in the light of characteristics of participants with RA who enter randomised studies. Some authors have suggested that comparable benefit is hardly ever achieved in clinical practice (Kievit 2007). Participants often differ from patients in clinical practice in various ways: participant selection, a washout period before inclusion that artificially increases the disease activity, differences in doses, co‐medication, occurrence of co‐morbidity and adherence. A study that compared the efficacy of anti‐TNF drugs in RA from RCTs with their efficacy in a large Dutch cohort of people with RA found that the effects of anti‐TNF treatment were much smaller in the large Dutch cohort of patients than in those observed in the RCTs (Kievit 2007). The authors also found that response rates were up to 44% higher in people with RA in clinical practice who were eligible for RCTs compared with those in clinical practice who were ineligible. The authors concluded that selection towards high disease activity and the continued use of co‐medication in RCTs are probable explanations for the difference in effects of anti‐TNF in clinical practice and RCTs. These findings limit the applicability of the results of this review to people with RA in the wider community.
Quality of the evidence
The Table 1, Table 2, Table 3 and Table 4 show the overall quality of evidence. Evidence quality ranged from moderate to high. Regarding individual trials quality assessment, only two studies were rated with high risk of bias in one item (blinding) and one study included relatively few participants and few events providing wider CIs (Marcora 2006). Also, significant heterogeneity was noted in 20 out of the 59 analyses ranging from 38% to 98%.
A funnel plot was not created due to the limited number of studies included in this review. Nonetheless, we concluded that the evidence provided in this review for ACR50 response rates is unlikely to have an important impact on our confidence of the estimate. Further studies are needed to assess long‐term safety.
Potential biases in the review process
Our methods and reporting are based on the Cochrane Handbook for Systematic Reviews of Interventions recommendations (Higgins 2011). We have followed a pre‐established and published protocol. All analyses were specified a priori. We have made every attempt to minimise errors. Nonetheless, we are constrained by the information reported in the included studies. Also, most studies are designed to measure benefit and analyses on harms is somewhat limited. Non‐statistically significant results in safety outcomes may be due to inadequate power to detect differences.
Agreements and disagreements with other studies or reviews
Our benefit findings are similar to those reported in the 2009 Cochrane network meta‐analysis evaluating the efficacy of biologics in RA (Singh 2009). However, in Singh 2009, the results reported include data from six of the nine studies included in this review; therefore, effect estimates are not identical. In addition, our main findings (Table 1 and Table 2) are based on subgroup analyses evaluating the efficacy and safety of etanercept in people who have not responded to DMARDs or MTX only. Our safety results suggest that there are no major issues with etanercept or combined etanercept with MTX in the short term. There were very few serious adverse events and no evidence that these differed according to treatments used. Injection site reactions were more common with etanercept monotherapy and a number of minor adverse events, including nausea, were more common with DMARD. Infection and death rates did not differ between groups over three years of follow‐up. Open‐label extensions of four of the included studies (Bathon 2000 (ERA); Klareskog 2004 (TEMPO); Moreland 1999; Weinblatt 1999) indicated that the rates of serious adverse events (up to seven years of follow‐up) were similar to those reported for people with RA in general (Klareskog 2006; Moreland 2006). However, the findings that adverse events did not differ in frequency between randomised groups should be treated with caution.
Some of the included studies claimed to be assessing both benefit and safety of treatment but invariably power calculations were based solely on efficacy considerations. Thus, these studies may not have been sufficiently powered to assess the harms of treatment adequately and type 2 error could not be ruled out, that is, the conclusion of no association between treatment and adverse effects, when an association actually exists. In fact, an analysis of trials of TNF inhibitors suggested that trials would need to have much larger sample sizes to adequately assess safety, particularly the risk of rare and serious events (Yazici 2008). These authors also suggested that phase 4 trials of TNF inhibitors are needed to assess safety adequately.
There are concerns about the risk of serious infection and malignancies with etanercept that has caused the US FDA to announce black box warnings on etanercept. The risk of serious infection warning is based on monitoring from the FDA's safety information and adverse event reporting programme, which evaluated data from clinical studies of over 20,000 participants during 28,300 patient‐years. Reported infections included bacterial sepsis and TB. Also the Cochrane network meta‐analysis of adverse events has shown that there is a small risk of TB (Singh 2011). The authors included studies with less than 24 weeks of follow‐up, and open‐label trials, which may explain the discrepancy with our results. Nonetheless, the FDA advised that people should be screened for latent TB and treated before the initiation of therapy with etanercept and monitored for TB and other infections during treatment. The ACR guidelines (Saag 2008 and Singh 2012) has also recommended against the use of any biological agents in the presence of active bacterial infection or active TB and another large systematic review commissioned by the Agency of Healthcare Research and Quality has concluded that there is insufficient evidence to draw conclusions about differences in risk for rare but serious events (Donahue 2008). Similarly, the FDA warning on the risk of malignancy development is based on an analysis of US reports of cancer in children and adolescents treated with TNF‐blockers, which showed an increased risk of cancer, half of them lymphomas. However, in one meta‐analysis of RCTs, no association was found between any type of malignancy and etanercept in the short term (Lopez‐Olivo 2012).
Authors' conclusions
Implications for practice.
Etanercept is approved for the treatment of RA at a dose of 25 mg SC twice weekly. In this review, while some outcome variables were significantly improved with etanercept 10 mg SC twice weekly, etanercept 25 mg SC twice weekly was a more efficacious treatment than 10 mg SC twice weekly compared with control.
This review has been able to assess etanercept monotherapy and also etanercept combined with a DMARD (either MTX or sulphasalazine) for clinical benefit, effects on quality of life and safety of people with RA. Etanercept monotherapy is more effective than control in improving ACR response rates, remission rates and lessening joint damage. These clinical benefits were also paralleled by patients' feelings of satisfaction and assessment of their disability after treatment and the rate in which they discontinued therapy. Etanercept monotherapy does not appear to offer an advantage over DMARD monotherapy, other than lessening joint damage as assessed by radiographic scores. However, combined treatment with both etanercept and a DMARD is more effective than etanercept monotherapy for all efficacy outcomes up until three years in people with RA who are judged to be appropriate candidates for MTX. Quality of life is also improved and participants were more compliant with their treatment. It is not known whether longer‐term treatment would maintain these improvements, or whether effects are reduced with ongoing treatment.
This review has found no evidence of a difference in the rates of serious adverse events with either etanercept monotherapy or combined treatment with etanercept and DMARD in the short term. The most common side effect was a reaction to the injection site with etanercept alone but this ceased after a few injections. However, there are concerns with increased incidence of infections (particularly TB) and possibly increased malignancy risks, so the long‐term benefit and safety need to be evaluated further.
The availability of etanercept varies within and between countries. In most countries the cost of etanercept is significantly more than that of other traditional DMARDS. A 'stepped care' approach is commonly recommended for the treatment of RA by most rheumatology societies. The ACR and EULAR guidelines, and Canadian treatment recommendations emphasise the early use of traditional DMARDs, MTX being the first option, to avert joint deformity and dysfunction. In those people who have an inadequate response, a change to either other DMARDs alone or in combination with other traditional DMARDs is suggested. Current guidelines recommend initiation of biologic therapy (including etanercept) in people with a moderate to high disease activity despite treatment with at least two DMARDs for three months (Bykerk 2012; Saag 2008; Singh 2012; Smolen 2010a).
Implications for research.
Further long‐term studies are required to confirm the safety of etanercept plus DMARD therapy for both clinical and radiographic outcome variables at three years and beyond. In addition, because of the high cost of etanercept, cost‐effectiveness studies should be undertaken that include quality of life and adverse events as well as disease progression over the long term since RA is a chronic progressive disease (Kobelt 2005). Finally, head‐to‐head comparison of etanercept monotherapy and combination therapy with the other licensed biological agents would be useful to allow physicians to select the best treatment for each participant. Studies should incorporate measures to determine how therapy can be individualised. Information on which group of participants are most likely to respond to biological treatment is important so people most likely to benefit could be offered this expensive treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 24 January 2014 | Amended | Minor correction on the summary of findings |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 14 March 2013 | New search has been performed | New search with six new studies. |
| 13 November 2012 | New citation required but conclusions have not changed | New authors added. Updated risk of bias methods and added summary of findings table. |
| 25 June 2008 | New citation required and conclusions have changed | Substantive amendment. New author (AL) added as an updating officer for a pilot update scheme. |
| 25 June 2008 | Amended | Converted to new review format. CMSG ID: C032‐R |
Acknowledgements
The Arthritis Society provided support for the first publication of this review as the third and fourth authors were supported by Arthritis Society Scholarships.
The authors of the update would like to thank Ann Cranney, Barb Blumenauer, and Marc Hochberg for their contributions as co‐authors to the original review.
Appendices
Appendix 1. MEDLINE search strategy
The original search strategy was modified in 2007. The following search was undertaken in MEDLINE. This strategy was modified for other databases.
1. exp arthritis, rheumatoid/ 2. (felty$ adj2 syndrome).tw. 3. (caplan$ adj2 syndrome).tw. 4. rheumatoid nodule.tw. 5. (sjogren$ adj2 syndrome).tw. 6. (sicca adj2 syndrome).tw. 7. still$ disease.tw. 8. bechterew$ disease.tw.9. (arthritis adj2 rheumat$).tw. 10. or/1‐9 11. etanercept.tw. 12. enbrel.tw. 13. exp Tumor Necrosis Factor‐alpha/ 14. anti‐tumo?r necrosis factor$.tw. 15. anti‐tnf.tw. 16. or/11‐15 17. 10 and 16 18. clinical trial.pt. 19. randomized.ab. 20. placebo.ab. 21. dt.fs. 22. clinical trials/ 23. randomly.ab. 24. trial.ti. 25. groups.ab. 26. or/18‐25 27. animals/ 28. humans/ 29. 27 and 28 30. 27 not 29 31. 26 not 30 32. 17 and 31
Data and analyses
Comparison 1. Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to DMARDs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR50 | 4 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.33, 2.89] |
| 1.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 11.69 [1.66, 82.47] |
| 1.2 52 weeks | 1 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.24, 1.68] |
| 1.3 104 weeks | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 5.84 [2.50, 13.64] |
| 1.4 156 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.30, 1.85] |
| 2 ACR70 | 4 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.50, 3.29] |
| 2.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 9.82 [0.59, 163.15] |
| 2.2 52 weeks | 1 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [1.35, 2.16] |
| 2.3 104 weeks | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 6.93 [1.72, 27.94] |
| 2.4 156 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.73, 3.04] |
| 3 Remission (DAS < 2.6) | 2 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.60, 2.31] |
| 3.1 24 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 52 weeks | 1 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.43, 2.26] |
| 3.3 104 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 156 weeks | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.56, 2.92] |
| 4 HAQ (mean improvement from baseline) | 4 | 1227 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.43, ‐0.28] |
| 4.1 24 weeks | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.62, 0.02] |
| 4.2 52 weeks | 1 | 528 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.42, ‐0.18] |
| 4.3 104 weeks | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.67, ‐0.31] |
| 4.4 156 weeks | 1 | 459 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.48, ‐0.24] |
| 5 Total Sharp Score | 2 | 903 | Mean Difference (IV, Random, 95% CI) | ‐3.83 [‐7.67, 0.01] |
| 5.1 24 weeks | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 52 weeks | 1 | 476 | Mean Difference (IV, Random, 95% CI) | ‐2.13 [‐3.20, ‐1.06] |
| 5.3 104 weeks | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 156 weeks | 1 | 427 | Mean Difference (IV, Random, 95% CI) | ‐6.09 [‐9.22, ‐2.96] |
| 6 Total withdrawals | 4 | 1241 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.36, 0.77] |
| 6.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.04, 0.79] |
| 6.2 52 weeks | 1 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.48, 0.89] |
| 6.3 104 weeks | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.23, 0.52] |
| 6.4 156 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.59, 0.84] |
| 7 Withdrawals due to adverse events | 4 | 1241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.57, 1.00] |
| 7.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.77] |
| 7.2 52 weeks | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.29] |
| 7.3 104 weeks | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.41, 3.75] |
| 7.4 156 weeks | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.98] |
| 8 Serious adverse events | 4 | 1241 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.74, 2.11] |
| 8.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.08, 3.43] |
| 8.2 52 weeks | 1 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.61, 1.49] |
| 8.3 104 weeks | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 5.69 [1.40, 23.19] |
| 8.4 156 weeks | 1 | 459 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.87, 1.77] |
| 9 Serious Infections | 3 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.55] |
| 9.1 24 weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 52 weeks | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.20, 1.84] |
| 9.3 104 weeks | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.5 [0.31, 97.54] |
| 9.4 156 weeks | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.66] |
Comparison 2. Summary of findings table: etanercept (ET) 25 mg + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD (partial responders to methotrexate (MTX)).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR50 | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.69 [1.66, 82.47] |
| 1.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.69 [1.66, 82.47] |
| 2 ACR70 | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.82 [0.59, 163.15] |
| 2.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.82 [0.59, 163.15] |
| 3 HAQ (mean reduction from baseline) | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.62, 0.02] |
| 3.1 24 weeks | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.62, 0.02] |
| 4 Total withdrawals | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.79] |
| 4.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.79] |
| 5 Withdrawals due to adverse events | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.77] |
| 5.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.77] |
| 6 Serious adverse events | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.31] |
| 6.1 24 weeks | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.31] |
Comparison 3. Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.84, 3.84] |
| 2 ACR50 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.73 [2.42, 9.28] |
| 3 ACR70 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.53 [2.30, 57.86] |
| 4 DAS | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐1.80, ‐1.04] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐1.80, ‐1.04] |
Comparison 4. Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 958 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.07, 1.35] |
| 2 ACR50 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.36, 1.70] |
| 3 ACR70 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.59, 2.32] |
| 4 Remission (DAS < 1.6) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.85, 3.81] |
| 5 Remission (DAS < 2.6) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 25 mg SC twice weekly | 2 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.61, 2.35] |
| 6 Change in Total Sharp Score (from baseline) | 2 | 894 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐2.99, ‐1.43] |
| 6.1 Etanercept 25 mg SC twice weekly | 2 | 894 | Mean Difference (IV, Fixed, 95% CI) | ‐2.21 [‐2.99, ‐1.43] |
| 7 Change in Erosion Score (from baseline) | 1 | 418 | Mean Difference (IV, Fixed, 95% CI) | ‐1.63 [‐2.41, ‐0.85] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 418 | Mean Difference (IV, Fixed, 95% CI) | ‐1.63 [‐2.41, ‐0.85] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 1 | 418 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.12, ‐0.22] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 418 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.12, ‐0.22] |
| 9 No progression of joint damage (TSS ≤ 0.5) | 2 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.26, 1.51] |
| 9.1 Etanercept 25 mg SC twice weekly | 2 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.26, 1.51] |
Comparison 5. Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 3 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.15, 1.90] |
| 2 ACR70 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 3 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [1.51, 3.27] |
| 3 ACR50 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 3 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [1.33, 2.82] |
| 4 Remission (DAS < 1.6) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [1.84, 3.61] |
| 5 Remission (DAS < 2.6) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 25 mg SC twice weekly | 2 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.70, 2.74] |
| 6 Change in Total Sharp Score (from baseline) | 1 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐3.9 [‐6.11, ‐1.69] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐3.9 [‐6.11, ‐1.69] |
| 7 Change in Erosion Score (from baseline) | 1 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐2.88 [‐4.39, ‐1.37] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐2.88 [‐4.39, ‐1.37] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 1 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐1.89, ‐0.17] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 419 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐1.89, ‐0.17] |
| 9 No progression of joint damage | 2 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.18, 1.45] |
| 9.1 Etanercept 25 mg SC twice weekly | 2 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.18, 1.45] |
| 10 DAS 28 | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐2.38, ‐1.62] |
Comparison 6. Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.12, 1.38] |
| 2 ACR50 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.30, 1.85] |
| 3 ACR70 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etancercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.73, 3.04] |
| 4 Remission (DAS < 1.6) | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.68, 3.20] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.68, 3.20] |
| 5 Remission (DAS < 2.6) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.56, 2.92] |
| 6 Change in Total Sharp Score (TSS) (from baseline) | 1 | 427 | Mean Difference (IV, Fixed, 95% CI) | ‐6.09 [‐9.22, ‐2.96] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 427 | Mean Difference (IV, Fixed, 95% CI) | ‐6.09 [‐9.22, ‐2.96] |
| 7 Change in Erosion Score (from baseline) | 1 | 427 | Mean Difference (IV, Fixed, 95% CI) | ‐3.92 [‐5.72, ‐2.12] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 427 | Mean Difference (IV, Fixed, 95% CI) | ‐3.92 [‐5.72, ‐2.12] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 1 | 427 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐3.78, ‐0.56] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 427 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐3.78, ‐0.56] |
| 9 No progression of joint damage (TSS ≤ 0.5) | 1 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.28, 1.74] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.28, 1.74] |
Comparison 7. Efficacy at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Etanercept 10 mg SC twice weekly | 1 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| 1.2 Etanercept 25 mg SC twice weekly | 3 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.97, 1.89] |
| 2 ACR50 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Etanercept 10 mg SC twice weekly | 1 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.76, 1.34] |
| 2.2 Etanercept 25 mg SC twice weekly | 3 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.02, 2.37] |
| 3 ACR70 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Etanercept 10 mg SC twice weekly | 1 | 423 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.59, 1.61] |
| 3.2 Etanercept 25 mg SC twice weekly | 3 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [1.09, 3.54] |
| 4 Change in Total Sharp Score (from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.72, 0.22] |
| 4.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.92, ‐0.06] |
| 5 Change in Erosion Score (from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.49, 0.13] |
| 5.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.66, ‐0.10] |
| 6 Change in Joint Space Narrowing Score (from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.35, 0.21] |
| 6.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.35, 0.13] |
| 7 DAS | 2 | 177 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.24, 0.71] |
| 7.1 Etanercept 25 mg SC twice weekly | 2 | 177 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.24, 0.71] |
Comparison 8. Efficacy at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 10 mg SC twice weekly | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.11] |
| 1.2 Etanercept 25 mg SC twice weekly | 2 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.98, 1.16] |
| 2 ACR50 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 10 mg SC twice weekly | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.58, 0.99] |
| 2.2 Etanercept 25 mg SC twice weekly | 2 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.99, 1.33] |
| 3 ACR70 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 10 mg SC twice weekly | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.13] |
| 3.2 Etanercept 25 mg SC twice weekly | 2 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.95, 1.58] |
| 4 Remission (DAS < 1.6) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.81, 1.91] |
| 5 Remission (DAS < 2.6) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.68, 1.53] |
| 6 Change in Total Sharp Score (TSS) (from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.93, 0.85] |
| 6.2 Etanercept 25 mg SC twice weekly | 2 | 832 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.35, ‐0.13] |
| 7 Change in Erosion Score (from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.66, 0.40] |
| 7.2 Etanercept 25 mg SC twice weekly | 2 | 832 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.04, ‐0.27] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.42, 0.60] |
| 8.2 Etanercept 25 mg SC twice weekly | 2 | 832 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.43, 0.20] |
| 9 No progression of joint damage (TSS ≤ 0.5) | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.03, 1.38] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.03, 1.38] |
Comparison 9. Efficacy at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.74, 2.73] |
| 2 ACR50 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 2.33 [0.60, 8.98] |
| 3 ACR70 | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [0.52, 12.03] |
| 4 Remission (DAS < 1.6) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.01, 2.17] |
| 5 Remission (DAS < 2.6) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.71] |
| 6 Change in Total Sharp Score (from baseline) | 1 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐4.61, 0.13] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐4.61, 0.13] |
| 7 Change in Erosion Score (from baseline) | 1 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐1.76 [‐3.34, ‐0.18] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐1.76 [‐3.34, ‐0.18] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 1 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐1.46, 0.48] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐1.46, 0.48] |
| 9 No progression of joint damage | 1 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.98, 1.31] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.98, 1.31] |
| 10 DAS 28 | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.07, ‐1.33] |
Comparison 10. Efficacy at three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.18] |
| 2 ACR50 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.87, 1.32] |
| 3 ACR70 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.80, 1.58] |
| 4 Remission (DAS < 1.6) | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.84, 1.79] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.84, 1.79] |
| 5 Remission (DAS < 2.6) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.75, 1.59] |
| 6 Change in Total Sharp Score (from baseline) | 1 | 421 | Mean Difference (IV, Fixed, 95% CI) | ‐4.34 [‐7.56, ‐1.12] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 421 | Mean Difference (IV, Fixed, 95% CI) | ‐4.34 [‐7.56, ‐1.12] |
| 7 Change in Erosion Score (from baseline) | 1 | 421 | Mean Difference (IV, Fixed, 95% CI) | ‐2.86 [‐4.81, ‐0.91] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 421 | Mean Difference (IV, Fixed, 95% CI) | ‐2.86 [‐4.81, ‐0.91] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 1 | 421 | Mean Difference (IV, Fixed, 95% CI) | ‐1.48 [‐3.04, 0.08] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 421 | Mean Difference (IV, Fixed, 95% CI) | ‐1.48 [‐3.04, 0.08] |
| 9 No progression of joint damage (TSS ≤ 0.5) | 1 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.01, 1.42] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.01, 1.42] |
Comparison 11. Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 2 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.85, 1.66] |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 346 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.85, 1.66] |
| 2 ACR50 | 2 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.49] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.49] |
| 3 ACR70 | 2 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.92, 1.85] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.92, 1.85] |
| 4 DAS < 3.2 | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.33] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.33] |
| 5 Remission (DAS < 2.6) | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.22, 5.98] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.22, 5.98] |
| 6 EULAR ‐ good response | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.33] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.33] |
| 7 EULAR ‐ moderate response | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.78, 1.73] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.78, 1.73] |
| 8 EULAR ‐ no response | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.46] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.46] |
| 9 DAS | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.32, 0.32] |
| 9.1 ET 25 mg SC twice weekly | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.32, 0.32] |
11.2. Analysis.
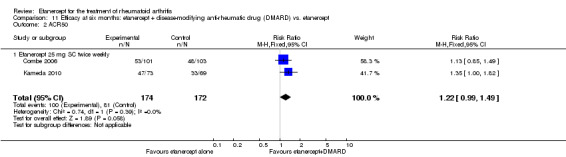
Comparison 11 Efficacy at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept, Outcome 2 ACR50.
Comparison 12. Efficacy at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.02, 1.23] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.02, 1.23] |
| 2 ACR50 | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.22, 1.69] |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.22, 1.69] |
| 3 ACR70 | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.34, 2.33] |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.34, 2.33] |
| 4 Remission (DAS < 1.6) | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.53, 2.96] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.53, 2.96] |
| 5 Remission (DAS < 2.6) | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.57, 3.03] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.57, 3.03] |
| 6 Change in Total Sharp Score (from baseline) | 1 | 414 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐1.76, ‐0.50] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 414 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐1.76, ‐0.50] |
| 7 Change in Erosion Score (from baseline) | 1 | 414 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.12, ‐0.22] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 414 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.12, ‐0.22] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 1 | 414 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.74, ‐0.18] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 414 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.74, ‐0.18] |
| 9 No progression of joint damage (TSS ≤ 0.5) | 1 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.05, 1.32] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.05, 1.32] |
Comparison 13. Efficacy at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.06, 1.24] |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.06, 1.24] |
| 2 ACR50 | 2 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.07, 2.02] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.07, 2.02] |
| 3 ACR70 | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.91, 2.31] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.91, 2.31] |
| 4 Remission (DAS < 1.6) | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.31, 2.32] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.31, 2.32] |
| 5 Remission (DAS < 2.6) | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.42, 2.52] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.42, 2.52] |
| 6 Change in Total Sharp Score (TSS) (from baseline) | 1 | 416 | Mean Difference (IV, Fixed, 95% CI) | ‐1.66 [‐2.76, ‐0.56] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 416 | Mean Difference (IV, Fixed, 95% CI) | ‐1.66 [‐2.76, ‐0.56] |
| 7 Change in Erosion Score (from baseline) | 1 | 416 | Mean Difference (IV, Fixed, 95% CI) | ‐1.12 [‐1.83, ‐0.41] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 416 | Mean Difference (IV, Fixed, 95% CI) | ‐1.12 [‐1.83, ‐0.41] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 1 | 416 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.09, 0.01] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 416 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.09, 0.01] |
| 9 No progression of joint damage (TSS ≤ 0.5) | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.02, 1.29] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.02, 1.29] |
| 10 DAS | 1 | 204 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.62, 0.02] |
Comparison 14. Efficacy at three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.07, 1.31] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.07, 1.31] |
| 2 ACR50 | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.22, 1.71] |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.22, 1.71] |
| 3 ACR70 | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.56, 2.66] |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.56, 2.66] |
| 4 Remission (DAS < 1.6) | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.41, 2.54] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.41, 2.54] |
| 5 Remission (DAS < 2.6) | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.44, 2.64] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.44, 2.64] |
| 6 Change in Total Sharp Score (TSS) (from baseline) | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐3.27, ‐0.23] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐3.27, ‐0.23] |
| 7 Change in Erosion Score (from baseline) | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.98, ‐0.14] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐1.06 [‐1.98, ‐0.14] |
| 8 Change in Joint Space Narrowing Score (from baseline) | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.65, 0.27] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 428 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.65, 0.27] |
| 9 No progression of joint damage (TSS ≤ 0.5) | 1 | 428 | Risk Difference (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.24] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 428 | Risk Difference (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.24] |
Comparison 15. Efficacy at six months: etanercept vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.56 [2.37, 8.77] |
| 1.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.24 [2.76, 9.97] |
| 2 ACR50 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [1.68, 13.36] |
| 2.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.95 [2.94, 21.47] |
| 3 ACR70 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.37 [0.93, 58.49] |
| 3.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.31 [1.64, 92.41] |
Comparison 16. Quality of life at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAQ: final value | 2 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.77, ‐0.21] |
| 2 EuroQoL VAS | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 18.6 [11.76, 25.44] |
Comparison 17. Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Satisfaction with treatment | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.11, 1.34] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.11, 1.34] |
| 2 HAQ score after treatment | 2 | 987 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.33, ‐0.15] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 987 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.33, ‐0.15] |
| 3 EQ‐5D VAS | 2 | 987 | Mean Difference (IV, Fixed, 95% CI) | 7.60 [4.67, 10.54] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 987 | Mean Difference (IV, Fixed, 95% CI) | 7.60 [4.67, 10.54] |
| 4 Reduction to normal health assessment (HAQ ≤ 0.5) | 2 | 956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.17, 1.58] |
| 4.1 Etanercept 25 mg SC twice weekly | 2 | 956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.17, 1.58] |
| 5 Proportion stopping work | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.73] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.17, 0.73] |
| 6 SF‐36 Physical domain | 1 | 528 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [1.00, 4.60] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 528 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [1.00, 4.60] |
| 7 SF‐36 Mental domain | 1 | 528 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.35, 2.55] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 528 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.35, 2.55] |
Comparison 18. Quality of life at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percentage improvement in HAQ (from baseline) | 1 | 459 | Mean Difference (IV, Fixed, 95% CI) | 20.0 [17.45, 22.55] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Mean Difference (IV, Fixed, 95% CI) | 20.0 [17.45, 22.55] |
| 2 HAQ score after treatment | 2 | 610 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.69, ‐0.30] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.69, ‐0.30] |
Comparison 19. Quality of life at six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAQ score: final value | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 177 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.83, 0.72] |
| 2 EuroQoL VAS | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | 21.30 [14.62, 27.98] |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Mean Difference (IV, Fixed, 95% CI) | 21.30 [14.62, 27.98] |
Comparison 20. Quality of life at 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAQ score after treatment | 1 | 451 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.25, 0.05] |
| 2 HAQ score: change from baseline | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.11, 0.17] |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.11, 0.17] |
| 3 Proportion of participants whose HAQ scores improved from baseline | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.25] |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.25] |
| 4 SF‐36 score ‐ physical domain: change from baseline | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐1.12, 3.32] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐1.12, 3.32] |
| 5 Proportion of participants whose SF‐36 (physical) scores improved from baseline | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.23] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.23] |
| 6 SF‐36 score ‐ mental domain: change from baseline | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐2.72, 1.72] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐2.72, 1.72] |
| 7 Proportion of participants whose SF‐36 (mental) scores improved from baseline | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.23] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.23] |
| 8 ASHI: change from baseline | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.67, 2.87] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐2.67, 2.87] |
| 9 Proportion of participants whose ASHI scores improved from baseline | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.24] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.24] |
| 10 EQ‐5D VAS | 1 | 451 | Mean Difference (IV, Fixed, 95% CI) | 3.09 [‐1.45, 7.63] |
| 10.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Mean Difference (IV, Fixed, 95% CI) | 3.09 [‐1.45, 7.63] |
| 11 Satisfaction with treatment | 1 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.08, 1.31] |
| 11.1 Etanercept 25 mg SC twice weekly | 1 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.08, 1.31] |
| 12 Reduction to normal health assessment (HAQ ≤ 0.5) | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.29] |
| 12.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.29] |
Comparison 21. Quality of life at two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percentage improvement in HAQ (from baseline) | 1 | 451 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [0.09, 5.91] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [0.09, 5.91] |
| 2 HAQ score after treatment | 2 | 604 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.54, 0.05] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.54, 0.05] |
Comparison 22. Quality of life at six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAQ score after treatment | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.43, 0.03] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.43, 0.03] |
Comparison 23. Quality of life at 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Satisfaction with treatment | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
| 2 HAQ score after treatment | 3 | 800 | Mean Difference (IV, Fixed, 95% CI) | ‐0.2 [‐0.30, ‐0.10] |
| 2.1 Etanercept 25 mg SC twice weekly | 3 | 800 | Mean Difference (IV, Fixed, 95% CI) | ‐0.2 [‐0.30, ‐0.10] |
| 3 EQ‐5D VAS | 2 | 658 | Mean Difference (IV, Random, 95% CI) | 1.87 [‐6.61, 10.34] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Mean Difference (IV, Random, 95% CI) | 1.87 [‐6.61, 10.34] |
| 4 Reduction to normal health assessment (HAQ ≤ 0.5) | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.03, 1.64] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.03, 1.64] |
Comparison 24. Quality of life at two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Percentage improvement in HAQ (from baseline) | 1 | 454 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [14.61, 19.39] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [14.61, 19.39] |
| 2 HAQ score after treatment | 2 | 658 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.36, ‐0.15] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.36, ‐0.15] |
| 3 Satisfaction with treatment | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
Comparison 25. Quality of life at six months: etanercept vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MOS: mental health (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 8.54 [3.24, 13.84] |
| 1.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Mean Difference (IV, Fixed, 95% CI) | 9.47 [4.29, 14.65] |
| 2 MOS: energy/vitality (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Mean Difference (IV, Fixed, 95% CI) | 12.64 [6.42, 18.86] |
| 2.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Mean Difference (IV, Fixed, 95% CI) | 11.61 [5.50, 17.72] |
Comparison 26. Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.79] |
| 2 Lack of efficacy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.05, 0.37] |
| 3 Adverse event | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.09, 1.64] |
| 4 Death | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 27. Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.48, 0.76] |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.48, 0.76] |
| 2 Lack of efficacy | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.18, 0.58] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.18, 0.58] |
| 3 Adverse events | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.55, 1.09] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.55, 1.09] |
| 4 Death | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.22, 12.37] |
| 4.1 Etanercept 25 mg SC twice weekly | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.22, 12.37] |
Comparison 28. Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.28, 0.80] |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.28, 0.80] |
| 2 Lack of efficacy | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.48] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.07, 0.48] |
| 3 Adverse event | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.19] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.19] |
| 4 Death | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.68] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.68] |
Comparison 29. Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.59, 0.84] |
| 2 Lack of efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.56] |
| 3 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.98] |
| 4 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.62] |
Comparison 30. Withdrawals six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.72, 2.88] |
| 2 Lack of efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.30] |
| 3 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.25, 3.72] |
Comparison 31. Withdrawals 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.42] |
| 1.2 Etanercept 25 mg SC twice weekly | 2 | 875 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.59, 0.97] |
| 2 Lack of efficacy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.85, 4.52] |
| 2.2 Etanercept 25 mg SC twice weekly | 2 | 875 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.55, 1.54] |
| 3 Adverse event | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.27, 1.02] |
| 3.2 Etanercept 25 mg SC twice weekly | 2 | 875 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.99] |
| 4 Death | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 76.38] |
| 4.2 Etanercept 25 mg SC twice weekly | 2 | 875 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.23, 12.95] |
Comparison 32. Withdrawals two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.97] |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.46, 0.97] |
| 2 Lack of efficacy | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.48] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.48] |
| 3 Adverse event | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.55, 1.13] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.55, 1.13] |
| 4 Death | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.25] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.25] |
Comparison 33. Withdrawals three years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.00] |
| 2 Lack of efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.62, 1.43] |
| 3 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 4 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.19, 22.39] |
Comparison 34. Withdrawals six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.92] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.92] |
| 2 Lack of efficacy | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.03, 24.83] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.03, 24.83] |
| 3 Adverse events | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.86] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.86] |
Comparison 35. Withdrawals 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.38, 0.77] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.38, 0.77] |
| 2 Lack of efficacy | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.91] |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.91] |
| 3 Adverse events | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.55, 1.57] |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.55, 1.57] |
| 4 Deaths | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.34] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.34] |
Comparison 36. Withdrawals two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.00] |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.00] |
| 2 Lack of efficacy | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.18, 1.60] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.18, 1.60] |
| 3 Adverse events | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.38, 1.63] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.38, 1.63] |
| 4 Deaths | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.08, 4.50] |
| 4.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.08, 4.50] |
Comparison 37. Withdrawals three years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 1.00] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 1.00] |
| 2 Lack of efficacy | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.17, 0.60] |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.17, 0.60] |
| 3 Adverse events | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.32] |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.32] |
| 4 Deaths | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.79] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.79] |
Comparison 38. Withdrawals six months: etanercept vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.33, 0.67] |
| 1.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.24, 0.55] |
| 2 Lack of efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.25, 0.65] |
| 2.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.17, 0.51] |
| 3 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.43, 7.09] |
| 3.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.98] |
38.3. Analysis.
Comparison 38 Withdrawals six months: etanercept vs. placebo, Outcome 3 Adverse event.
Comparison 39. Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.94, 1.60] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.94, 1.60] |
| 2 Abdominal pain | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [0.72, 20.82] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [0.72, 20.82] |
| 3 Asthenia | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.88, 16.03] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.88, 16.03] |
| 4 Bone pain/arthralgia | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.28] |
| 4.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.28] |
| 5 Bronchitis | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.23, 17.26] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.23, 17.26] |
| 6 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.22, 1.61] |
| 7 Dizziness | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.44, 3.29] |
| 7.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.44, 3.29] |
| 8 Dyspepsia | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.50, 10.19] |
| 8.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.50, 10.19] |
| 9 Fever | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.91] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.91] |
| 10 Flu syndrome | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.30, 20.62] |
| 10.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.30, 20.62] |
| 11 Headache | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.75, 3.04] |
| 12 Hypertension | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.58 [0.73, 42.51] |
| 13 Increased cough | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.21, 1.52] |
| 13.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.21, 1.52] |
| 14 Infection | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.69, 1.32] |
| 14.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.69, 1.32] |
| 15 Injection site reaction | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.88 [2.22, 21.34] |
| 16 Injection site haemorrhage | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.21, 3.31] |
| 16.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.21, 3.31] |
| 17 Leukopenia | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.5 [0.31, 97.54] |
| 17.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.5 [0.31, 97.54] |
| 18 Miscellaneous skin infections | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.30, 20.62] |
| 18.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.30, 20.62] |
| 19 Mouth ulcers | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.43] |
| 19.1 Etanercept 25 mg SC twice weekly | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.43] |
| 20 Nausea | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.20, 4.00] |
| 20.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.20, 4.00] |
| 21 Pain | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.91] |
| 21.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.06, 1.91] |
| 22 Paraesthesia | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.37, 24.01] |
| 22.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.37, 24.01] |
| 23 Pharyngitis (non‐infectious) | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.15, 1.68] |
| 23.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.15, 1.68] |
| 24 Pharyngitis or laryngitis | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.21, 3.31] |
| 24.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.21, 3.31] |
| 25 Pruritus | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.37, 8.04] |
| 25.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.37, 8.04] |
| 26 Rash | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.44, 8.98] |
| 26.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.44, 8.98] |
| 27 Rhinitis | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 15.48] |
| 28 Trauma/accidental injury | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.25, 4.84] |
| 28.1 Etanercept 25 mg SC twice weekly | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.25, 4.84] |
| 29 Upper respiratory tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.40, 2.96] |
| 30 Vomiting | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [0.19, 67.82] |
| 30.1 Etanercept 25 mg SC twice weekly | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.62 [0.19, 67.82] |
Comparison 40. Adverse events within 12 months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nausea | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.47] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.47] |
| 2 Nasopharyngitis | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.68, 1.46] |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.68, 1.46] |
| 3 Serious infections | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.20, 1.84] |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.20, 1.84] |
| 4 Worsening of rheumatoid arthritis | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 2.00] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 2.00] |
| 5 Breast cancer | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.69] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.69] |
| 6 Chest pain | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.56] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.56] |
| 7 Pneumonia | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.56] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.06, 15.56] |
| 8 Cholelithiasis | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.40] |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.40] |
| 9 Invertebral disc protrusion | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.40] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.40] |
| 10 Osteoarthritis | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.06] |
| 10.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.06] |
| 11 Interstitial lung disease | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.40] |
| 11.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.40] |
| 12 Hip arthroplasty | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.06] |
| 12.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.06] |
| 13 Malignancy | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.87] |
| 13.1 Etanercept 25 mg SC twice weekly | 1 | 542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.87] |
Comparison 41. Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.98, 1.56] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.98, 1.56] |
| 2 Accidental injury | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.25, 21.22] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.25, 21.22] |
| 3 Abdominal pain | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.33, 10.35] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.33, 10.35] |
| 4 Asthenia | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.37, 16.07] |
| 4.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.37, 16.07] |
| 5 Arthralgia | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.57, 1.50] |
| 5.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.57, 1.50] |
| 6 Back pain | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.93, 2.12] |
| 6.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.93, 2.12] |
| 7 Bronchitis | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.50, 4.37] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.50, 4.37] |
| 8 Diarrhoea | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.29, 7.68] |
| 8.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.29, 7.68] |
| 9 Dyspepsia | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.69, 12.77] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.69, 12.77] |
| 10 Flu syndrome | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.69, 12.77] |
| 10.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.69, 12.77] |
| 11 Gingival/dental infection | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.69, 12.77] |
| 11.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.69, 12.77] |
| 12 Headache | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.56, 4.73] |
| 12.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.56, 4.73] |
| 13 Hypertension | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.87, 3.43] |
| 13.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.87, 3.43] |
| 14 Increase in cough | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.83, 2.04] |
| 14.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.83, 2.04] |
| 15 Infections (total) | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.81, 1.61] |
| 15.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.81, 1.61] |
| 16 Injection site haemorrhage | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.70, 7.67] |
| 16.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.70, 7.67] |
| 17 Injection Site Reaction | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [2.29, 11.04] |
| 17.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [2.29, 11.04] |
| 18 Malignancy | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.48, 12.59] |
| 18.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [0.48, 12.59] |
| 19 Miscellaneous skin infections | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.50 [0.76, 206.93] |
| 19.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.50 [0.76, 206.93] |
| 20 Nausea | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.42, 2.64] |
| 20.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.42, 2.64] |
| 21 Pain | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.49, 1.62] |
| 21.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.49, 1.62] |
| 22 Paraesthesia | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.45 [0.72, 41.00] |
| 22.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.45 [0.72, 41.00] |
| 23 Pharyngitis or laryngitis | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.48, 5.73] |
| 23.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.48, 5.73] |
| 24 Rash | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.64] |
| 24.1 Etanercept 25 mg SC twice weekly | 2 | 610 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.65, 1.64] |
| 25 Serious infections | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.76] |
| 25.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.42, 1.76] |
| 26 Sinusitis | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.18, 66.47] |
| 26.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.18, 66.47] |
| 27 Upper respiratory tract infection | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.76, 2.71] |
| 27.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.76, 2.71] |
| 28 Vomiting | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.05] |
| 28.1 Etanercept 25 mg SC twice weekly | 1 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.05] |
| 29 Worsening of rheumatoid arthritis | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.44, 3.19] |
| 29.1 Etanercept 25 mg SC twice weekly | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.44, 3.19] |
Comparison 42. Adverse events within six months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.95, 1.61] |
| 2 Alopecia | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 22.13] |
| 2.1 Etanercept/Yisaipu (ET/Y) 25 mg SC twice weekly | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 22.13] |
| 3 Accidental injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.37 [0.37, 110.97] |
| 4 Asthenia | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.16, 13.65] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.16, 13.65] |
| 5 Arthralgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.08, 1.57] |
| 6 Bronchitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [0.50, 30.20] |
| 7 Chest discomfort | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 22.13] |
| 7.1 ET/Y 25 mg SC twice weekly | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 22.13] |
| 8 Dyspepsia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.29, 20.23] |
| 9 Dizziness | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.18, 3.77] |
| 9.1 ET/Y 25 mg SC twice weekly | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.18, 3.77] |
| 10 Oedema | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.19] |
| 10.1 ET/Y 25 mg SC twice weekly | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.19] |
| 11 Elevation in alanine transaminase | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.74] |
| 11.1 ET/Y 25 mg SC twice weekly | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.74] |
| 12 Enlargement of lymph nodes | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 22.13] |
| 12.1 ET/Y 25 mg SC twice weekly | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 22.13] |
| 13 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.33] |
| 14 Gastrointestinal symptoms/abdominal pain | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.23, 13.18] |
| 14.1 ET/Y 25 mg SC twice weekly | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.23, 13.18] |
| 15 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.17, 2.16] |
| 16 Increased blood pressure | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.41, 16.66] |
| 16.1 ET/Y 25 mg SC twice weekly | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [0.41, 16.66] |
| 17 Increased cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.08, 1.57] |
| 18 Infection at another site/pharyngitis or laryngitis, flu syndrome or miscellaneous skin infections | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.14, 4.27] |
| 18.1 ET/Y 25 mg SC twice weekly | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.14, 4.27] |
| 19 Injection site haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.20, 3.25] |
| 20 Injection site reaction | 3 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.24 [4.52, 73.69] |
| 20.1 ET/Y 25 mg SC twice weekly | 3 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.24 [4.52, 73.69] |
| 21 Insomnia | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.12 [0.37, 136.31] |
| 21.1 ET/Y 25 mg SC twice weekly | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.12 [0.37, 136.31] |
| 22 Leukopenia | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.39, 2.28] |
| 22.1 ET/Y 25 mg SC twice weekly | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.39, 2.28] |
| 23 Nausea | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.10, 2.32] |
| 23.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.10, 2.32] |
| 24 Pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.15, 2.78] |
| 25 Paraesthesia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.09, 10.45] |
| 26 Pharyngitis (non‐infectious) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.20, 3.25] |
| 27 Pruritus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.02, 2.61] |
| 28 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 1.06] |
| 29 Skin rash | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.05, 5.63] |
| 29.1 ET/Y 25 mg SC twice weekly | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.05, 5.63] |
| 30 Total infectious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.05, 2.93] |
| 31 Upper respiratory tract infection | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.51, 1.76] |
| 31.1 ET/Y 25 mg SC twice weekly | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.51, 1.76] |
| 32 Vision disorder | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
| 32.1 ET/Y 25 mg SC twice weekly | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.53] |
Comparison 43. Adverse events within 12 months: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alopecia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.31, 1.09] |
| 1.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.26, 0.97] |
| 2 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.63, 1.90] |
| 2.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.69] |
| 3 Asthenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.42, 1.28] |
| 3.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.73] |
| 4 Back pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.48, 2.27] |
| 4.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.98, 3.78] |
| 5 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.66] |
| 5.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.72, 1.89] |
| 6 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.93] |
| 6.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.64, 1.88] |
| 7 Dyspepsia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.59, 1.85] |
| 7.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.72, 2.16] |
| 8 Ecchymosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.44, 1.47] |
| 8.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.47, 1.55] |
| 9 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.27] |
| 9.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.58, 1.14] |
| 10 Influenza‐like syndrome | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.46] |
| 10.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.65, 1.82] |
| 11 Injection site haemorrhagia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.88, 2.52] |
| 11.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.85, 2.46] |
| 12 Injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.11 [2.46, 6.87] |
| 12.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.04 [3.05, 8.35] |
| 13 Mouth ulcers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.24, 0.84] |
| 13.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.18, 0.70] |
| 14 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.33, 0.73] |
| 14.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.86] |
| 15 Rash | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.46, 1.02] |
| 15.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.81] |
| 16 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.80, 1.95] |
| 16.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.68, 1.72] |
| 17 Sinusitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.28] |
| 17.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.97] |
| 18 Skin infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.60, 1.83] |
| 18.2 Etanercept 25 mg SC twice weekly | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.79, 2.26] |
| 19 Upper respiratory tract infection | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Etanercept 10 mg SC twice weekly | 1 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.54, 0.93] |
| 19.2 Etanercept 25 mg SC twice weekly | 2 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.52] |
Comparison 44. Adverse events within two years: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.10, 1.70] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.10, 1.70] |
| 2 Abdominal pain | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.21, 16.14] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.21, 16.14] |
| 3 Asthenia | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.68, 1.90] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.68, 1.90] |
| 4 Accidental injury | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.20, 20.68] |
| 4.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.20, 20.68] |
| 5 Arthralgia | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.39, 2.50] |
| 5.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.39, 2.50] |
| 6 Back pain | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.85, 1.95] |
| 6.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.85, 1.95] |
| 7 Bronchitis | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.92, 7.03] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.92, 7.03] |
| 8 Diarrhoea | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.20, 26.32] |
| 8.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [0.20, 26.32] |
| 9 Dyspepsia | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [0.80, 14.38] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [0.80, 14.38] |
| 10 Flu syndrome | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.37 [1.05, 18.10] |
| 10.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.37 [1.05, 18.10] |
| 11 Gingival/dental infection | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.37, 7.88] |
| 11.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.37, 7.88] |
| 12 Headache | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.72, 1.57] |
| 12.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.72, 1.57] |
| 13 Hypertension | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.29, 4.72] |
| 13.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.29, 4.72] |
| 14 Increased cough | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.32] |
| 14.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.32] |
| 15 Infections (total) | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.67, 2.38] |
| 15.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.67, 2.38] |
| 16 Injection site haemorrhage | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.69] |
| 16.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.69] |
| 17 Injection site reaction | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.00 [4.21, 19.24] |
| 17.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.00 [4.21, 19.24] |
| 18 Malignancy | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.60, 10.63] |
| 18.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.60, 10.63] |
| 19 Miscellaneous skin infections | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.13 [1.18, 310.46] |
| 19.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.13 [1.18, 310.46] |
| 20 Nausea | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.24, 0.49] |
| 20.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.24, 0.49] |
| 21 Pain | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.64, 1.96] |
| 21.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.64, 1.96] |
| 22 Paraesthesia | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.22, 16.92] |
| 22.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.22, 16.92] |
| 23 Pharyngitis or laryngitis | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [1.23, 12.29] |
| 23.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [1.23, 12.29] |
| 24 Rash | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.34, 3.95] |
| 24.1 Etanercept 25 mg SC twice weekly | 2 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.34, 3.95] |
| 25 Rheumatoid arthritis | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [2.06, 32.98] |
| 25.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.25 [2.06, 32.98] |
| 26 Serious infections | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.47, 1.93] |
| 26.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.47, 1.93] |
| 27 Sinusitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.26 [0.74, 202.98] |
| 28 Upper respiratory tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Etanercept 25 mg SC twice weekly | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.75, 2.65] |
| 29 Vomiting | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.16, 0.63] |
| 29.1 Etanercept 25 mg SC twice weekly | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.16, 0.63] |
Comparison 45. Adverse events within six months: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.11] |
| 1.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.73, 1.11] |
| 2 Asthenia | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [0.96, 11.99] |
| 2.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [0.96, 11.99] |
| 3 Blood and lymphatic system disorders/leukopenia | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.05, 29.43] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.05, 29.43] |
| 4 Dizziness | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.44, 5.26] |
| 4.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.44, 5.26] |
| 5 Fever | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.10 [0.25, 104.89] |
| 5.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.10 [0.25, 104.89] |
| 6 Flu syndrome | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.22, 1.88] |
| 6.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.22, 1.88] |
| 7 Gastrointestinal symptoms/abdominal pain/dyspepsia | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.63, 2.29] |
| 7.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.63, 2.29] |
| 8 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [1.15, 8.10] |
| 9 Hepatobiliary disorders | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.17, 20.16] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.17, 20.16] |
| 10 Hypertension | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.52, 7.93] |
| 10.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.52, 7.93] |
| 11 Increased cough | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.52, 7.93] |
| 11.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.52, 7.93] |
| 12 Infection and infestations/total infectious adverse events | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.03] |
| 12.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.03] |
| 13 Injection site reaction/injection site haemorrhage/general disorders and administration site conditions | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.37, 0.82] |
| 13.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.37, 0.82] |
| 14 Injury, poisoning and procedural complications/accidental Injury | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.21] |
| 14.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.21] |
| 15 Malignancy | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Etanercept 25 mg SC twice weekly | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Metabolism and nutrition disorders | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 67.76] |
| 16.1 Etanercept 25 mg SC twice weekly | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 67.76] |
| 17 Miscellaneous skin infections | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.20, 1.63] |
| 17.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.20, 1.63] |
| 18 Musculoskeletal and connective tissue disorders/arthralgia | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.36, 4.14] |
| 18.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.36, 4.14] |
| 19 Nausea | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [1.19, 14.03] |
| 19.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [1.19, 14.03] |
| 20 Nervous system disorders/paraesthesia | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.61, 6.40] |
| 20.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.61, 6.40] |
| 21 Pain | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.72] |
| 21.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.72] |
| 22 Pharyngitis (non‐infectious) | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.23, 2.95] |
| 22.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.23, 2.95] |
| 23 Pharyngitis or laryngitis (infectious) | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.16] |
| 23.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.16] |
| 24 Reproductive system and breast disorders | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 67.76] |
| 24.1 Etanercept 25 mg SC twice weekly | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 67.76] |
| 25 Respiratory, thoracic and mediastinal disorders/bronchitis | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.22, 1.61] |
| 25.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.22, 1.61] |
| 26 Rhinitis | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.19, 22.14] |
| 26.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.19, 22.14] |
| 27 Skin and subcutaneous tissue disorders/rash/pruritus | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.20, 2.97] |
| 27.1 Etanercept 25 mg SC twice weekly | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.20, 2.97] |
| 28 Total serious adverse events | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.17, 20.16] |
| 28.1 Etanercept 25 mg SC twice weekly | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.17, 20.16] |
| 29 Upper respiratory tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.50, 2.52] |
Comparison 46. Adverse events within two years: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 1.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 2 Abdominal pain | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.83, 1.63] |
| 2.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.83, 1.63] |
| 3 Accidental injury | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.65] |
| 3.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.65] |
| 4 Arthralgia | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.35, 2.84] |
| 4.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.35, 2.84] |
| 5 Asthenia | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.62, 4.99] |
| 5.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.62, 4.99] |
| 6 Back pain | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.56, 3.31] |
| 6.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.56, 3.31] |
| 7 Bronchitis | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.12] |
| 7.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.12] |
| 8 Diarrhoea | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 8.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 9 Dyspepsia | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.43, 1.80] |
| 9.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.43, 1.80] |
| 10 Flu syndrome | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.35, 1.34] |
| 10.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.35, 1.34] |
| 11 Gingival/dental infection | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.72, 4.26] |
| 11.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.72, 4.26] |
| 12 Headache | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.66, 3.29] |
| 12.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.66, 3.29] |
| 13 Hypertension | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.41, 1.19] |
| 13.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.41, 1.19] |
| 14 Increased cough | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.05, 2.63] |
| 14.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.05, 2.63] |
| 15 Infections (total) | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.23] |
| 15.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.23] |
| 16 Injection site haemorrhage | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.72, 3.50] |
| 16.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.72, 3.50] |
| 17 Injection site reaction | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.41, 0.79] |
| 17.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.41, 0.79] |
| 18 Malignancy | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.24, 2.14] |
| 18.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.24, 2.14] |
| 19 Miscellaneous skin infections | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.26] |
| 19.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.26] |
| 20 Nausea | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.65, 3.40] |
| 20.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.65, 3.40] |
| 21 Pain | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.45, 1.42] |
| 21.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.45, 1.42] |
| 22 Paraesthesia | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.80 [0.92, 8.52] |
| 22.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.80 [0.92, 8.52] |
| 23 Pharyngitis or laryngitis | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.21, 0.84] |
| 23.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.21, 0.84] |
| 24 Rash | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.36, 2.41] |
| 24.1 Etanercept 25 mg SC twice weekly | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.36, 2.41] |
| 25 Rheumatoid arthritis | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.55, 2.70] |
| 25.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.55, 2.70] |
| 26 Serious infections | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.43, 1.86] |
| 26.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.43, 1.86] |
| 27 Sinusitis | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.88] |
| 27.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.07, 0.88] |
| 28 Upper respiratory tract infection | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.66, 1.58] |
| 28.1 Etanercept 25 mg SC twice weekly | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.66, 1.58] |
| 29 Vomiting | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.92, 4.03] |
| 29.1 Etanercept 25 mg SC twice weekly | 1 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.92, 4.03] |
Comparison 47. Adverse events within six months: etanercept vs. placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.58, 4.92] |
| 1.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.23, 2.94] |
| 2 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.89, 4.39] |
| 2.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.60, 3.32] |
| 3 Injection site reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [1.84, 6.55] |
| 3.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.90 [2.09, 7.27] |
| 4 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.44, 2.51] |
| 4.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.37, 2.24] |
| 5 Sinusitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.38, 2.30] |
| 5.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.43, 2.45] |
| 6 Upper respiratory tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Etanercept 10 mg SC twice weekly | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.97, 3.28] |
| 6.2 Etanercept 25 mg SC twice weekly | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.14, 3.69] |
Comparison 48. Sensitivity analysis: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. DMARD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 5 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.38, 2.88] |
| 1.1 DMARD naive | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.22, 1.66] |
| 1.2 Methotrexate (MTX)‐inadequate response | 2 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 3.72 [1.91, 7.24] |
| 1.3 Non‐MTX inadequate response | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.83, 3.23] |
| 2 ACR50 | 5 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [1.71, 4.68] |
| 2.1 DMARD naive | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.23, 1.89] |
| 2.2 MTX‐inadequate response | 2 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 8.61 [3.55, 20.86] |
| 2.3 Non‐MTX inadequate response | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 2.96 [0.81, 10.81] |
| 3 ACR70 | 5 | 1118 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.63, 4.12] |
| 3.1 DMARD naive | 2 | 247 | Risk Ratio (M‐H, Random, 95% CI) | 11.40 [2.21, 58.65] |
| 3.2 MTX‐inadequate response | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.33, 2.37] |
| 3.3 Non‐MTX inadequate response | 2 | 610 | Risk Ratio (M‐H, Random, 95% CI) | 3.27 [1.19, 8.96] |
Comparison 49. Sensitivity analysis: etanercept vs. disease‐modifying anti‐rheumatic drug (DMARD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 3 | 1112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.99, 1.15] |
| 1.1 DMARD naive | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.97, 1.30] |
| 1.2 Methotrexate (MTX)‐inadequate response | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.26] |
| 1.3 Non‐MTX inadequate response | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.13] |
| 2 ACR50 | 3 | 1112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 2.1 DMARD naive | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.94, 1.47] |
| 2.2 MTX‐inadequate response | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.93, 1.86] |
| 2.3 Non‐MTX inadequate response | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.91, 1.37] |
| 3 ACR‐70 | 3 | 1112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.04, 1.66] |
| 3.1 DMARD naive | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.82, 1.68] |
| 3.2 MTX‐inadequate response | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.00, 3.51] |
| 3.3 Non‐MTX inadequate response | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.90, 1.83] |
Comparison 50. Sensitivity analysis: etanercept + disease‐modifying anti‐rheumatic drug (DMARD) vs. etanercept.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR20 | 3 | 800 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.07, 1.36] |
| 1.1 DMARD naive | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Methotrexate (MTX)‐inadequate response | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.17, 1.72] |
| 1.3 Non‐MTX inadequate response | 2 | 658 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [1.06, 1.24] |
| 2 ACR50 | 3 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.16, 1.79] |
| 2.1 DMARD naive | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 MTX‐inadequate response | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [1.00, 1.82] |
| 2.3 Non‐MTX inadequate response | 2 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.07, 2.02] |
| 3 ACR70 | 3 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.27, 1.90] |
| 3.1 DMARD naive | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 MTX‐inadequate response | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.90, 2.41] |
| 3.3 Non‐MTX inadequate response | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.26, 1.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bathon 2000 (ERA).
| Methods | Method of randomisation not reported Allocation concealment not reported Blinding not reported, but treatments provided in identical containers Multicentre parallel group study Power calculation not reported No of participants randomised = 632 No of participants analysed = 632 Intention‐to‐treat analysis Source of funding: Immunex (pharmaceutical company) | |
| Participants | Inclusion: At least 18 years; RA max 3 years; no other illnesses; no treatment with MTX; at high risk for radiographic progression No exclusion criteria reported Location: centres in the USA | |
| Interventions |
(PBO controlled) Duration: 12 months |
|
| Outcomes | ACR20, ACR50, ACR70 Radiographic: TSS, Erosion Score, Joint Space Narrowing Score; withdrawals; adverse events | |
| Notes | Early RA; MTX naive; most erosions and RF+ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Not explicitly described but PBO controlled; assessors of radiographic scores unaware of assignment |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Analyses were intention to treat: inclusion of all participants who received at least 1 dose of the study drug |
| Selective reporting (reporting bias) | Low risk | No evidence of a prior published protocol but wide range of outcomes assessed |
| Other bias | Unclear risk | Drug company funding with no guarantees described to ensure that the results were not influenced |
Combe 2006.
| Methods | Method of randomisation was computer generated Allocation concealment not described Double blinding Multicentre, parallel group study Power calculation not reported No of participants randomised = 260 No of participants analysed = 254 Authors stated that they used a modified intention to treat analysis: "all randomly assigned patients who received any test article and provided efficacy data at baseline" Measures to deal with missing data included LOCF Source of funding: Wyeth (some authors either paid consultants or employees of Wyeth) |
|
| Participants | Inclusion: At least 18 years; diagnosis of adult onset RA; disease duration ≤ 20 years; swelling in ≥ 6 joints, ≥ 6 tender joints, morning stiffness ≥ 45 minutes, ESR ≥ 28 mm/h or CRP ≥ 20 mg/L Previous stable doses of SSZ at least 4 weeks prior to the study, without signs of toxicity Exclusion: Previous treatment with etanercept or other TNF antagonist; treatment with DMARDs other than SSZ in 3 months before baseline; treatment with other biological agents or immunosuppressants within 6 months prior to the study entrance; or steroid injection in 4 weeks before study start; relevant co‐morbidities; pregnancy or lactation Stable doses of NSAIDs, analgesics or prednisone were allowed |
|
| Interventions |
Duration: 2 years |
|
| Outcomes | ACR20, ACR50, ACR70 DAS44‐ESR SJC, TJC, morning stiffness, physician and participant global assessment, pain‐VAS, general health‐VAS, ESR, CRP Functional status (HAQ) EuroQoL Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method of randomisation was computer generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Stated double blind and treatments were identical |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Analyses were based on a modified intention to treat: inclusion of all participants who received at least 1 dose of the study drug |
| Selective reporting (reporting bias) | Unclear risk | No evidence of a prior published protocol but wide range of outcomes assessed |
| Other bias | Unclear risk | Source of funding: Wyeth (some authors either paid consultants or employees of Wyeth) |
Emery 2008 (COMET).
| Methods | Method of randomisation was computer generated Allocation concealment not described Double blinding reported but masking removed for primary analysis of data at 1 year for publication. Data also unblinded for medical management of participants if needed Multicentre, parallel group study (22 countries, 70 centres) Power calculation reported (90% power to show a significant difference in remission; 94% power to show significant difference in radiographic progression) No of participants randomised = 542 No of participants analysed = 542 (for safety outcomes). n = 528 for clinical efficacy. n = 476 for radiographic progression at end of first year Authors stated that they used a modified intention‐to‐treat analysis: remission ‐ all participants who received at least 1 dose of the drug and reported both baseline and at least 1 on‐treatment DAS28 result; radiographic progression ‐ all participants with valid baseline and follow‐up radiographs. Measures to deal with missing data included LOCF and imputation by linear extrapolation Source of funding: Wyeth (many authors either paid consultants or employees of Wyeth) |
|
| Participants | Inclusion: At least 18 years; diagnosis of adult onset RA; disease duration between 3 months and 2 years; DAS28 ≥ 3.2; either Westergren ESR ≥ 28 mm/h or CRP ≥ 20 mg/L Exclusion: Previous treatment with MTX, etanercept or other TNF antagonist; treatment with other DMARDs or steroid injection in 4 weeks before baseline; important concurrent medical diseases; other relevant co‐morbidities Location: 22 countries, 70 centres in Europe, Latin America, Asia and Australia |
|
| Interventions |
Duration: 2 years |
|
| Outcomes | Remission (DAS28 < 2.6) Change in modified TSS (from baseline to end of year 1) Functional status (HAQ Disability Index) Employment status Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Central control by computer |
| Blinding (performance bias and detection bias) Clinical outcomes | Unclear risk | Stated as double blind, but masking removed for primary analysis of data for the publication |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Reasons documented for dropouts. Methods employed to deal with missing data: LOCF or imputation by linear extrapolation |
| Selective reporting (reporting bias) | Low risk | No protocol for study identified but wide range of outcomes assessed |
| Other bias | Unclear risk | Good baseline comparability. Drug company funding with no guarantees to ensure results not influenced |
Hu 2009.
| Methods | Method of randomisation not described Method of allocation concealment not described Blinding not described but treatments appeared identical Multicentre, parallel group study (6 centres) Power calculation not reported No of participants randomised = 238 No of participants analysed = 238 Drop‐outs: 17 in treatment group (reasons given) and 12 in control group (reasons given) Intention‐to‐treat analysis (LOCF for drop‐outs) Source of funding: not reported |
|
| Participants | Inclusion criteria: 18‐65 years of age; active RA (as defined by ACR 1987 criteria: swelling in ≥ 6 joints, ≥ 6 tender joints, morning stiffness ≥ 45 minutes, ESR ≥ 28 mm/h, CRP ≥ 20 μg/mL) Exclusion criteria: Any serious illness (heart, liver, renal, blood or other vital organs); pregnant or breastfeeding; previous treatment with Yisaipu or other biological agents; no efficacy to treatment with MTX; joint injection of corticosteroids within past 4 weeks; any acute or chronic infection or past history of active TB; any tumour or family history of tumour Stable doses of NSAIDs or prednisone were allowed but all DMARDS were discontinued at least 4 weeks prior to the study Location: 6 hospitals in China |
|
| Interventions |
Duration: 6 months |
|
| Outcomes | ACR20, ACR50, ACR70 Withdrawals Adverse events |
|
| Notes | Yisaipu is a rhTNFR:Fc available in China. The authors claim it has the same structure as etanercept | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described only as "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Stated double blind and treatments were identical |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Analysis by intention to treat (LOCF for drop‐outs) |
| Selective reporting (reporting bias) | Low risk | No protocol sighted but all important outcomes measured |
| Other bias | Unclear risk | Funding not reported |
Kameda 2010.
| Methods | Randomisation was computer generated and stratified by baseline age, disease duration, disease activity and institution Method of allocation concealment not described No blinding Multicentre, parallel group study (34 centres in Japan) Power calculation not reported No of participants randomised = 151 No of participants analysed = 147 (safety), 142 (efficacy) Dropouts: 4 in treatment group (reasons given) and 12 in control group (reasons given) Modified intention‐to‐treat analysis: all participants who took the study drugs and had a valid baseline and at least 1 on‐therapy value for each end point (LOCF for drop‐outs) Source of funding: not reported, however, many authors were paid consultants of Wyeth |
|
| Participants | Inclusion criteria: ≥ 18 years of age; RA (as defined by ACR 1987 criteria); active disease; swelling in ≥ 6 joints, ≥ 6 tender joints, ESR ≥ 28 mm/h, adequate safety profiles; RA functional class I‐III. Treatment with MTX at least 6 mg/week in 3 months before baseline (stable dose) Exclusion criteria: Treatment with > 10 mg/day prednisolone; treatment with DMARDs other than MTX; previously treated with any biological agent |
|
| Interventions |
Duration: 2 year |
|
| Outcomes | EULAR criteria DAS28 and remission rate ACR20, ACR50, ACR70) van der Heijde‐modified Sharp Score Withdrawals Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed on the University Hospital Medical Information Network's web site" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Modified intention‐to‐treat analysis: all participants who took the study drugs and had a valid baseline and at least 1 on‐therapy value for each end point (LOCF for drop‐outs) |
| Selective reporting (reporting bias) | Low risk | No protocol sighted but all important outcomes measured |
| Other bias | Unclear risk | Source of funding: not reported, however, many authors were paid consultants of Wyeth |
Klareskog 2004 (TEMPO).
| Methods | Randomisation method not described Allocation concealment Triple blinding Multicentre (N = 19), parallel group study Power calculation for sample size No of participants randomised = 686 No of participants analysed = 682 (4 did not receive treatment) Modified intention‐to‐treat analysis (those who received the study drug). Other missing data estimated by LOCF or linear extrapolation Source of funding: Wyeth Research (pharmaceutical company) | |
| Participants | Inclusion: ≥ 18 years of age; disease duration 6 months to 20 years; active RA; less than satisfactory response to at least 1 DMARD (except MTX); treatment with MTX in last 6 months; toxic effects from previous MTX treatment Exclusion: Previous treatment with etanercept or other TNF antagonist; previous treatment with immunosuppressive drugs in past 6 months; use or any investigative drug or biological agent in past 3 months; use of any other DMARD or steroid injection in past 4 weeks; presence of co‐morbidity Location: 17 centres in Europe, Australia and Israel | |
| Interventions |
PBO controlled Duration: 3 years |
|
| Outcomes | ACR20, ACR50, ACR70 Radiographic: TSS; Erosion Score; Joint Space Narrowing Score HAQ; DAS Satisfaction Adverse events Withdrawals | |
| Notes | Trial has continued open label | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Low risk | Centralised telephone randomisation |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Triple blinding (participants, investigators and assessors) |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Clear descriptions of methods for dealing with missing data (LOCF, linear extrapolation and assumption that participants withdrawing from the study had no response to treatment) |
| Selective reporting (reporting bias) | Low risk | No access to prior protocol to check for selective reporting but wide range of outcomes measured |
| Other bias | Unclear risk | Drug company funding with no guarantees described to prevent influence on results |
Marcora 2006.
| Methods | Computer‐generated list of random numbers Allocation concealment not reported Only investigators measuring outcomes were blinded Single centre, parallel group study Power calculation not reported No of participants randomised = 26 No of participants analysed = 24 (2 in MTX group dropped out: 1 lost to follow‐up and 1 started physical training) Not intention‐to‐treat analysis Source of funding: Wyeth provided the etanercept treatment | |
| Participants | Inclusion: ≥ 18 years of age; diagnosis of RA; < 6 months history of RA; active disease Exclusion: Previous treatment with DMARD or corticosteroid treatment; recent history of important infection; concurrent disease; cognitive impairment; any other cachectic disease; taking drugs or supplements affecting muscle mass; participating in physical training Location: Clinic in Welsh Hospital | |
| Interventions |
Duration: 6 months |
|
| Outcomes | Physical function (handgrip strength; arm‐curl test; walking velocity; sit to stand test) HAQ Adverse events DAS28 | |
| Notes | Primary objective to assess effects of treatment on cachexia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) Clinical outcomes | High risk | "Patients and clinician. . . . . aware of treatment allocation" but assessors were blinded |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Minimal drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | No prior published protocol identified |
| Other bias | Unclear risk | Drug company funding with no guarantees described to prevent influence on the results |
Moreland 1999.
| Methods | Blocked randomisation with stratification by study site Allocation concealed Double blinding Multicentre (n = 13), parallel group study Power calculation not reported No of participants randomised = 246 No of participants analysed = 234 (12 not eligible after randomisation) Intention‐to‐treat analysis by counting withdrawals as non‐responders and using last available observation for drop‐outs Source of funding: Immunex (pharmaceutical company) | |
| Participants | Inclusion: ≥ 18 years of age; active RA; inadequate response to a DMARD 90% of participants had used MTX previously Location: 13 centres in North America | |
| Interventions |
Concomitant treatment with oral steroids, analgesics and NSAIDs allowed Washout period for previous DMARDs Duration: 6 months |
|
| Outcomes | ACR20, ACR50, ACR70 Radiographic: TJC; SJC HAQ Adverse events Withdrawals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation with stratification according to study site |
| Allocation concealment (selection bias) | Low risk | Randomisation code housed with the sponsor |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Double blind, participants and key personnel |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Reasons for withdrawal clearly specified with methods stated to deal with missing data |
| Selective reporting (reporting bias) | Low risk | No prior published protocol identified but wide range of outcomes measured |
| Other bias | Unclear risk | Drug company funding with no guarantees described to prevent influence on results |
Weinblatt 1999.
| Methods | Method of randomisation not reported Allocation concealment not reported Double blinding Multicentre, parallel group study Power calculation for sample size No of participants randomised = 89 No of participants analysed = 89 (2 withdrew in etanercept group because of adverse events; 4 withdrew in MTX group because of lack of efficacy, 1 because of myocardial infarction, 1 lost to follow‐up) Intention‐to‐treat analysis (participants who withdrew were considered not to have a response and for individual measures, last observation was used in the analysis) Source of funding: Immunex (pharmaceutical company) | |
| Participants | Inclusion: ≥ 18 years of age; diagnosis of RA; active disease Exclusion not reported Location: centres in North America | |
| Interventions |
All participants had been taking MTX prior to study for at least 6 months All participants received folic acid and were allowed to use NSAIDs or steroids during study Duration: 6 months |
|
| Outcomes | ACR20, ACR50, ACR70 Radiographic: TJC; SJC HAQ Adverse events Withdrawals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation described |
| Allocation concealment (selection bias) | Unclear risk | No description of method used to conceal allocation |
| Blinding (performance bias and detection bias) Clinical outcomes | Low risk | Stated as "double blinded" |
| Incomplete outcome data (attrition bias) Clinical outcomes | Low risk | Clear descriptions of reasons for withdrawal of dropouts |
| Selective reporting (reporting bias) | Low risk | No prior published protocol identified but wide range of outcomes measured |
| Other bias | Unclear risk | Drug company funding with no guarantees described to prevent influence on results |
ACR: American College of Rheumatology; CRP: C reactive protein; DAS: disease activity score; DMARD: disease‐modifying anti‐rheumatic drug; ESR: erythrocyte sedimentation rate; EULAR: European League Against Rheumatism; h: hour; HAQ: Health Assessment Questionnaire; LOCF: last observation carried forward; MTX: methotrexate; NSAID: non‐steroidal anti‐inflammatory drug; PBO: placebo; RA: rheumatoid arthritis; RF+: rheumatoid factor positive; SC: subcutaneous; SJC: swollen joint count; SSZ: sulphasalazine; TB: tuberculosis; TJC: tender joint count; TNF: tumour necrosis factor; TSS: Total Sharp Score; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACP 2001 | Summary of Bathon 2000 with no new information provided |
| Angel 2010 | Outcome is arterial stiffness. Participants are a mixed group of RA and other diseases. Not randomised |
| Anis 2009 | Data is from the COMET trial. Outcome not listed as an eligible outcome |
| Benucci 2011 | Three arms were included: adalimumab + leflunomide or MTX Etanercept + leflunomide or MTX Infliximab + leflunomide or MTX |
| Blank 2009 | Intervention is etanercept + rituximab. Not an RCT |
| Bliddal 2006 | Trial of intra‐articular injection of etanercept |
| Boesen 2008 | Trial of intra‐articular injection of etanercept |
| Chen 2006 | Study duration only 12 weeks. Does not meet the inclusion criteria of this review |
| Cuomo 2006 | Four arms were included: MTX + sulphasalazine MTX + adalimumab MTX + etanercept MTX + infliximab |
| De Filippis 2006 | CCT compares etanercept vs. infliximab |
| De Stefano 2010 | CCT compares etanercept + MTX versus etanercept + leflunomide |
| Garnero 2002 | Patient subset from published studies looking at collagen markers and joint destruction. The relationship of collagen markers to joint destruction was not an outcome of interest |
| Genovese 2002 | Study was an extension of the Bathon study at 24 months. All participants who completed the Bathon study were eligible and were given etanercept 25 mg and were followed forward for 5 years |
| Genovese 2004 | Compares etanercept monotherapy vs. etanercept (half dose) + anakinra versus etanercept + anakinra |
| Gerlag 2010 | 12‐week RCT with an open‐label etanercept arm. Does not meet the inclusion criteria of this review |
| Holman 2008 | Participants are a mixed group of RA + psoriatic arthritis. Intervention is mixed: etanercept or adalimumab |
| Iwamoto 2009 | Observational study |
| Johnsen 2006 | Comparison of doses of etanercept. 1 arm had a dose of 50 mg of etanercept twice weekly ‐ this dose does not meet the inclusion criteria for this review |
| Kavanaugh 2008 | Retrospective analysis of TEMPO trial. Analysis of changes in responders and non‐responders |
| Keystone 2004 | Study was less than 6 months in duration and included a dose of etanercept (50 mg) which does not meet the inclusion criteria of this review |
| Keystone 2009 | Results of TEMPO and other earlier trials |
| Koumakis 2009 | intervention is a combination of therapies |
| Lan 2004 | Study duration only 12 weeks ‐ this does not meet the inclusion criteria of this review |
| Lisbona 2008 | Study duration only 6 weeks. Does not meet the inclusion criteria of this review |
| Lukas 2009 | Subgroup from TEMPO. Agreement between readings of joint scores |
| Lukas 2010 | Subgroup from TEMPO. Relationship between inflammation and repair |
| Lukina 2001 | Compared TNF alpha (?drug) to interferon gamma to placebo. Drugs given intramuscularly daily for 5 days with outcome determination at days 7 and 28. Even if etanercept used, study duration too short to meet inclusion criteria |
| Luzi 2009 | Not randomised. Interventions are etanercept + MTX vs. etanercept + MTX + steroids |
| Machado 2009 | Outcome not listed as an eligible outcome |
| Moreland 1997 | 3‐month study only so does not meet inclusion criteria |
| Moreland 2001 | Examined all participants who had received at least 1 dose of etanercept in controlled or open‐label trials for efficacy and safety at a date removed from trial. Doesn't meet inclusion criteria |
| Paleolog 1998 | Looked at synovial cells from participants treated with anti‐TNF alpha |
| Roux 2011 | Trial of intra‐articular injection of etanercept |
| Saleem 2009 | Interventions are: combination therapy of a TNF agent (any) + MTX vs. DMARDs |
| Sennels 2008 | Study duration only 16 weeks. Does not meet the inclusion criteria of this review |
| van Riel 2006 | Study duration only 16 weeks. Does not meet the inclusion criteria of this review |
| Weinblatt 2007 | Compares etanercept + abatacept vs. etanercept + placebo |
| Weinblatt 2008 | Dose of etanercept did not meet the inclusion criteria of the review and study duration too short (12 weeks) |
| Weisman 2007 | Study duration only 16 weeks. Does not meet the inclusion criteria of this review |
CCT: controlled clinical trial; DMARD: disease‐modifying anti‐rheumatic drug; MTX: methotrexate; RA: rheumatoid arthritis; RCT: randomised controlled trial; TNF: tumour necrosis factor.
Characteristics of ongoing studies [ordered by study ID]
EMPIRE 2006.
| Trial name or title | EMPIRE (Etanercept and Methotrexate in Patients to Induce Remission in Early arthritis) |
| Methods | Multicentre, double‐blind, placebo‐controlled RCT |
| Participants | Inclusion: aged 18‐80 years; articular synovitis within 3 months of diagnosis; either rheumatoid factor antibody positive or anti‐cyclic citrullinated peptide positive; not pregnant; negative TB screening test Exclusion: previous treatment with DMARDs, etanercept or TNF drugs; HIV; significant concurrent medical diseases; cancer or history of cancer; chronic infection of upper respiratory tract; ongoing or active infection; liver function abnormality; renal disease; leukopenia; thrombocytopenia, etc. (large list of exclusions) |
| Interventions | Etanercept + MTX vs. placebo + MTX |
| Outcomes | Primary: proportion in clinical remission at 12 months (defined as absence of symptoms and signs of inflammatory arthritis: i.e. SJC = 0; TJC = 0) Secondary: number of participants in clinical remission at 18 months (see above for definition of remission); disease activity measures (VAS; EMS, TJC, SJC, CRP, ESR); functional, work and quality of life assessments (HAQ; WIS, WDA, EuroQol; SF‐36); proportion of participants achieving 26 weeks of remission; DAS28; radiographic change |
| Starting date | October 2006 |
| Contact information | A.Keenan@Leeds.ac.uk |
| Notes | Funded by Wyeth |
France 2008.
| Trial name or title | An Open‐Label, Randomised Study to Evaluate the Radiographic Efficacy and Safety of Enbrel (Etanercept) Added to Methotrexate in Comparison with Usual Treatment in Subjects with Moderate Rheumatoid Arthritis Disease Activity |
| Methods | Randomised open‐label parallel group study |
| Participants | Inclusion: meet the ACR 1987 revised criteria for RA; documented evidence confirmed by a blinded 3rd party assessor of at least 1 erosion observed by x‐ray; received MTX as stable dose for 28 days prior to the screening visit Exclusion: previous treatment with etanercept, infliximab, adalimumab, other TNF‐alpha inhibitors, anakinra or other biological agents; previous combination DMARD therapy; receipt of any DMARD, other than MTX, within 28 days of screening |
| Interventions | Etanercept + MTX vs. usual treatment (not defined) |
| Outcomes | Primary: radiographic disease progression (not defined) at week 52 Secondary: clinical outcomes, QoL and safety (not defined) |
| Starting date | June 2008 |
| Contact information | clintrialparticipation@wyeth.com |
| Notes | Estimated completion June 2010. Funded by Wyeth |
Japanese 2006.
| Trial name or title | A Randomised, Double‐Blind, Multi‐Centre, Comparative Study Evaluating the Efficacy and Safety of Etanercept and Methotrexate in Japanese Subjects with Active Rheumatoid Arthritis |
| Methods | Multicentre, double‐blind (subjects, carers, investigators, outcome assessors), parallel group RCT |
| Participants | Inclusion: Japanese citizen living in Japan; 20 to 75 years; diagnosed ≤5 years from time of first visit Exclusion: Etanercept or TNF inhibitors such as infliximab, or adalimumab in the past; other rheumatic diseases or conditions that could predispose the person to infection, pregnant or lactating women |
| Interventions | Etanercept 10 or 25 mg SC twice weekly for 52 weeks vs. MTX (up to 8 mg/week) oral for 52 weeks |
| Outcomes | Primary: radiographic scores (change in modified TSS from baseline) at 52 weeks Secondary: adverse events incidence at 52 weeks |
| Starting date | June 2006 |
| Contact information | clinicaltrialparticipation@wyeth.com |
| Notes | Estimated completion: October 2010. Funded by Wyeth |
Jobanputra 2005.
| Trial name or title | Remission Induction in Very Early Rheumatoid Arthritis: a Comparison of Etanercept plus Methotrexate plus Steroid with Standard Therapy |
| Methods | Randomised single‐blind pilot study |
| Participants | Inclusion: aged > 18 years; synovial swelling of at least 1 joint confirmed by clinical assessment; seropositivity for RF and anti‐CCP antibody; adequate birth control measures if fertile; if female, not pregnant; informed consent Exclusion: duration of symptoms attributable to inflammatory joint disease of > 12 weeks; previous history of inflammatory arthritis; previous use of DMARDs or anti‐TNF agents; current inflammatory condition; history of other evidence of latent or active infection; virus or bacterial vaccination within 3 months before treatment; history of joint prosthesis infection of administration of antibiotics for a suspicion of this; serious and uncontrolled existing disease; bleeding disorder or use of anticoagulants; malignancy or history of malignancy |
| Interventions | Etanercept + MTX + parenteral steroid vs. conventional therapy (parental steroid plus MTX if necessary) |
| Outcomes | Primary: percentage of participants in drug‐free clinical remission at week 48 (having withdrawn therapy at week 24) (defined as DAS28 < 2.6 plus no clinical evidence of joint swelling) Secondary: percentage of participants in clinical remission (see above) at week 24 (when drugs are withdrawn if remission achieved); percentage of participants in clinical remission at weeks 24 and 48 according to ARA criteria; percentage of participants with radiological remission at week 24 (no MRI or radiological remission at week 24); clinical disease activity measures (ACR rates; DAS28; HAQ, EuroQol) at 24 and 48 weeks; rate of progression of radiological change according to van Heijde modification of the TSS from baseline to week 48 and 96; biological predictors of response to therapy |
| Starting date | December 2005 |
| Contact information | Dr P. Jobanputra, Selly Oak Hospital, Birmingham |
| Notes | Estimated completion: December 2008. Funded by University Hospital of Birmingham NHS Trust and NHS R & D support funding |
Takeuchi 2005.
| Trial name or title | Efficacy and Safety of Etanercept on Active Rheumatoid Arthritis Despite Methotrexate Therapy in Japan |
| Methods | Randomised, open‐label, parallel group study |
| Participants | Inclusion: aged ≥ 18 years; meet the ACR 1987 criteria for RA; at least 6 tender joints and 6 swollen joints; either CRP > 2 mg/dL or ESR no less than 28 mm at 1 hour; ACR functional class I‐III; receiving MTX 6 mg/week for a minimum of 3 months at stable dose for at least 4 weeks at the time of enrolment Exclusion: those requiring concurrent use of prednisone > 10 mg/day or its equivalent; the start of dose increments of prednisone equivalents within 3 months of enrolment; anti‐RA therapy except for MTX or prednisone equivalents; previous treatment with etanercept or any other biological treatment |
| Interventions | Etanercept (25 mg SC twice a week) + MTX (oral, 6‐8 mg/week) vs. etanercept (25 mg SC twice a week) alone |
| Outcomes | Primary: ACR50 and DAS28 "good response" at 24 weeks; TSS at 52 weeks Secondary: ACR20 and ACR70 at 24 weeks |
| Starting date | June 2005 |
| Contact information | Dr T. Takeuchi, Saitama Medical Center, Kawagoe, Saitama, Japan |
| Notes | Estimate completion: October 2010 |
ACR: American College of Rheumatology; ARA: American Rheumatology Association; CRP: C reactive protein; DMARD: disease‐modifying anti‐rheumatic drug; EMS: early morning stiffness; ESR: erythrocyte sedimentation rate; HAQ: Health Assessment Questionnaire; HIV: human immunodeficiency virus; MRI: magnetic resonance imaging; MTX: methotrexate; QoL: quality of life; RA: rheumatoid arthritis; RF: rheumatoid factor; RCT: randomized controlled trial; SC: subcutaneous; SF‐36: short form‐36; SJC: swollen joint count; TB: tuberculosis; TNF: anti‐tumour necrosis factor; TJC: tender joint count; TNF: tumour necrosis factor; TSS: Total Sharp Score; VAS: visual analogue scale; WDA: work disability appointments; WIS: Work Instability Scale.
Contributions of authors
For the first publication of the review in 2003
BB and AC extracted and analysed the data and selected trials of the initial review and preparation of the initial manuscript.
AB and MH contributed data, updated of the selection of the reference list, updated the analyses and updated the interpretation of results.
BB and MJ wrote the manuscript, contributed data extraction, updated the analyses and interpretation of results.
AC, DC, GW and PT contributed methodological expertise and commented on drafts.
For the update
AL undertook searches, selected studies for inclusion, extracted data and assessed all included studies for risk of bias. She also entered data, re‐ordered the comparisons, updated the analyses and prepared the final manuscript.
MLO extracted data, selected studies for inclusion, assessed new studies for risk of bias, extracted data, provided comment on methodological issues and prepared the final manuscript.
LM made substantial contributions to the interpretation of data, revised the manuscript critically, provided comment on methodological issues, and reviewed the final version
AB, PT, MH, and GW provided comment on methodological issues and reviewed the final draft.
Sources of support
Internal sources
None, Not specified.
External sources
MD Anderson Cancer Center. Research Library, USA.
Declarations of interest
None known.
Joint first author
Joint first author
Edited (no change to conclusions)
References
References to studies included in this review
Bathon 2000 (ERA) {published and unpublished data}
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. New England Journal of Medicine 2000;343:1586‐93. [DOI] [PubMed] [Google Scholar]
- Kosinski M, Kujawski SC, Martin R, Wanke LA, Buatti MC, Ware JE, et al. Health‐related quality of life in early rheumatoid arthritis: impact of disease and treatment response. The American Journal of Managed Care 2002;8(3):231‐40. [PubMed] [Google Scholar]
Combe 2006 {published data only}
- Combe B, Codreanu C, Fiocco U, Gaubitz M, Geusens PP, Kvien TK, et al. Efficacy, safety and patient‐reported outcomes of combination etanercept and sulphasalazine versus etanercept alone in patients with rheumatoid arthritis: a double‐blind randomised 2‐year study. Annals of the Rheumatic Diseases 2009;68(7):1146‐52. [PUBMED: 18794178] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe B, Codreanu C, Fiocco U, Gaubitz M, Geusens PP, Kvien TK, et al. Etanercept European Investigators Network (Etanercept Study 309 Investigators). Etanercept and sulphasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulphasalazine: a double‐blind comparison. Annals of the Rheumatic Diseases 2006;65(10):1357‐62. [PUBMED: 16606651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emery 2008 (COMET) {published data only}
- Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double‐blind, parallel treatment trial. Lancet 2008;372:375‐82. [DOI] [PubMed] [Google Scholar]
- Emery P, Breedveld FC, Heijde D, Ferraccioli G, Dougados M, Robertson D, et al. Two‐year clinical and radiographic results with combination etanercept‐methotrexate therapy versus monotherapy in early rheumatoid arthritis. Arthritis and Rheumatism 2010;62(3):674‐82. [DOI] [PubMed] [Google Scholar]
- Kekow J, Moots RJ, Emery P, Durez P, Koenig A, Singh A, et al. Patient‐reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Annals of the Rheumatic Diseases 2010;69:222‐5. [DOI] [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu D, Bao C, Chen S, Gu J, Li Z, Sun L, et al. A comparison study of a recombinant tumour necrosis factor receptor: Fc fusion protein (rhTNFR:Fc) and methotrexate in treatment of patients with active rheumatoid arthritis in China. Rheumatology International 2009;29:297‐303. [DOI] [PubMed] [Google Scholar]
Kameda 2010 {published data only}
- Kameda H, Ueki Y, Saito K, Nagaoka S, Hidaka T, Atsumi T, et al. Japan Biological Agent Study Integrated Consortium. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: a randomised trial. Modern Rheumatology 2010;20(6):531‐8. [PUBMED: 20574649] [DOI] [PubMed] [Google Scholar]
Klareskog 2004 (TEMPO) {published data only}
- Klareskog L, Heijde D, Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet 2004;363:675‐81. [DOI] [PubMed] [Google Scholar]
- Heijde D, Klareskog L, Landewe R, Bruyn GAW, Cantagrel A, Durez P, et al. Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis and Rheumatism 2007;56(12):3928‐39. [DOI] [PubMed] [Google Scholar]
- Heijde D, Klareskog L, Rodriguez‐Valverde V, Codreanu C, Bolosiu H, Melo‐Gomes J, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis. Arthritis and Rheumatism 2006;54(4):1063‐74. [DOI] [PubMed] [Google Scholar]
- Heijde D, Klareskog L, Singh A, Tornero J, Melo‐Gomes J, Codreanu C, et al. Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Annals of the Rheumatic Diseases 2005;65:328‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcora 2006 {published data only}
- Marcora SM, Chester KR, Mittal G, Lemmey AB, Maddison PJ. Randomized phase 2 trial of anti‐tumour necrosis factor therapy for cachexia in patients with early rheumatoid arthritis. American Journal of Clinical Nutrition 2006;84:1463‐72. [DOI] [PubMed] [Google Scholar]
Moreland 1999 {published data only}
- Mathias SD, Colwell HH, Miller DP, Moreland LW, Buatti M, Wanke L. Health‐related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clinical Therapeutics 2000;22(1):128‐39. [DOI] [PubMed] [Google Scholar]
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis. A randomised, controlled trial. Annals of Internal Medicine 1999;130:478‐86. [DOI] [PubMed] [Google Scholar]
Weinblatt 1999 {published and unpublished data}
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumour necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. New England Journal of Medicine 1999;340:253‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACP 2001 {published data only}
- Anonymous. Etanercept was more effective and safer than methotrexate in disease progression in early rheumatoid arthritis. ACP Journal club July/August 2001;135(1):22. [Google Scholar]
Angel 2010 {published data only}
- Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor‐alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension 2010;55(2):333‐8. [DOI] [PubMed] [Google Scholar]
Anis 2009 {published data only}
- Anis A, Zhang W, Emery P, Sun H, Singh A, Freundlich B, et al. The effect of etanercept on work productivity in patients with early active rheumatoid arthritis: results from the COMET study. Rheumatology (Oxford) 2009;48(10):1283‐9. [DOI] [PubMed] [Google Scholar]
Benucci 2011 {published data only}
- Benucci M, Saviola G, Baiardi P, Manfredi M, Sarzi‐Puttini P, Atzeni F. Efficacy and safety of leflunomide or methotrexate plus subcutaneous tumour necrosis factor‐alpha blocking agents in rheumatoid arthritis. International Journal of Immunopathology and Pharmacology 2011;24(1):269‐76. [DOI] [PubMed] [Google Scholar]
Blank 2009 {published data only}
- Blank N, Max R, Schiller M, Briem S, Lorenz HM. Safety of combination therapy with rituximab and etanercept for patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48(4):440‐1. [DOI] [PubMed] [Google Scholar]
Bliddal 2006 {published data only}
- Bliddal H, Terslev L, Qvistgaard E, Konig M, Holm CC, Rogind H, et al. A randomised, controlled study of a single intra‐articular injection of etanercept or glucocorticosteroids in patients with rheumatoid arthritis. Scandinavian Journal of Rheumatology 2006;35(5):341‐5. [PUBMED: 17062431] [DOI] [PubMed] [Google Scholar]
Boesen 2008 {published data only}
- Boesen M, Boesen L, Jensen KE, Cimmino MA, Torp‐Pedersen S, Terslev L, et al. Clinical outcome and imaging changes after intraarticular (IA) application of etanercept or methylprednisolone in rheumatoid arthritis: magnetic resonance imaging and ultrasound‐Doppler show no effect of IA injections in the wrist after 4 weeks. Journal of Rheumatology 2008;35(4):584‐91. [PUBMED: 18322991] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen HA, Lin KC, Chen CH, Liao HT, Wang HP, Chang HN, et al. Theeffect of etanercept on anti‐cyclic citrullinated peptide antibodies and rheumatoid factor in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2006;65(1):35‐9. [PUBMED: 15975966] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuomo 2006 {published data only}
- Cuomo G, Molinaro G, Montagna G, Migliaresi S, Valentini G. A comparison between the Simplified Disease Activity Index (SDAI) and the Disease Activity Score (DAS28) as measure of response to treatment in patients undergoing different therapeutic regimens. Reumatismo 2006;58(1):22‐5. [DOI] [PubMed] [Google Scholar]
De Filippis 2006 {published data only}
- Filippis L, Caliri A, Anghelone S, Scibilia G, Lo Gullo R, Bagnato G. Improving outcomes in tumour necrosis factor a treatment: comparison of the efficacy of the tumour necrosis factor a blocking agents etanercept and infliximab in patients with active rheumatoid arthritis. Panminerva Medica 2006;48(2):129‐35. [PUBMED: 16953150] [PubMed] [Google Scholar]
De Stefano 2010 {published data only}
- Stefano R, Frati E, Nargi F, Baldi C, Menza L, Hammoud M, et al. Comparison of combination therapies in the treatment of rheumatoid arthritis: leflunomide‐anti‐TNF‐alpha versus methotrexate‐anti‐TNF‐alpha. Clinical Rheumatology 2010;29(5):517‐24. [PUBMED: 20082236] [DOI] [PubMed] [Google Scholar]
Garnero 2002 {published data only}
- Garnero P, Gineyts E, Christgau S, Finck B, Delmas PD. Association of baseline levels of urinary glucosyl‐galactosyl‐pyridinoline and type II collagen C‐telopeptide with progression of joint destruction in patients with early rheumatoid arthritis. Arthritis and Rheumatism 2002;46(1):21‐30. [DOI] [PubMed] [Google Scholar]
Genovese 2002 {published data only}
- Genovese MC, Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis. Two‐year radiographic and clinical outcomes. Arthritis and Rheumatism 2002;46(6):1443‐50. [DOI] [PubMed] [Google Scholar]
Genovese 2004 {published data only}
- Genovese MC, Cohen S, Moreland L, Lium D, Robbins S, Newmark R, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis and Rheumatism 2004;50(5):1412‐9. [PUBMED: 15146410] [DOI] [PubMed] [Google Scholar]
Gerlag 2010 {published data only}
- Gerlag DM, Hollis S, Layton M, Vencovský J, Szekanecz Z, Braddock M, et al. Preclinical and clinical investigation of a CCR5 antagonist, AZD5672, in patients with rheumatoid arthritis receiving methotrexate. Arthritis and Rheumatism 2010;62(11):3154‐60. [PUBMED: 20662070] [DOI] [PubMed] [Google Scholar]
Holman 2008 {published data only}
- Holman AJ, Ng E. Heart rate variability predicts anti‐tumour necrosis factor therapy response for inflammatory arthritis. Autonomic Neuroscience 2008;143(1‐2):58‐67. [DOI] [PubMed] [Google Scholar]
Iwamoto 2009 {published data only}
- Iwamoto N, Kawakami A, Fujikawa K, Aramaki T, Kawashiri SY, Tamai M, et al. Prediction of DAS28‐ESR remission at 6 months by baseline variables in patients with rheumatoid arthritis treated with etanercept in Japanese population. Modern Rheumatology 2009;19(5):488‐92. [DOI] [PubMed] [Google Scholar]
Johnsen 2006 {published data only}
- Johnsen AK, Schiff MH, Mease PJ, Moreland LW, Maier AL, Coblyn JS, et al. Comparison of 2 doses of etanercept (50 vs 100 mg) in active rheumatoid arthritis: a randomised double blind study. Journal of Rheumatology 2006;33(4):659‐64. [PubMed] [Google Scholar]
Kavanaugh 2008 {published data only}
- Kavanaugh A, Klareskog L, Heijde D, Li J, Freundlich B, Hooper M. Improvements in clinical response between 12 and 24 weeks in patients with rheumatoid arthritis on etanercept therapy with or without methotrexate. Annals of the Rheumatic Diseases 2008;67(10):1444‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keystone 2004 {published data only}
- Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, et al. Once‐weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomised, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2004;50(2):353‐63. [DOI] [PubMed] [Google Scholar]
Keystone 2009 {published data only}
- Keystone E, Freundlich B, Schiff M, Li J, Hooper M. Patients with moderate rheumatoid arthritis (RA) achieve better disease activity states with etanercept treatment than patients with severe RA. Journal of Rheumatology 2009;36(3):522‐31. [DOI] [PubMed] [Google Scholar]
Koumakis 2009 {published data only}
- Koumakis E, Wipff J, Avouac J, Kahan A, Allanore Y. Severe refractory rheumatoid arthritis successfully treated with combination rituximab and anti‐tumour necrosis factor‐alpha‐blocking agents. Journal of Rheumatology 2009;36(9):2125‐6. [DOI] [PubMed] [Google Scholar]
Lan 2004 {published data only}
- Lan JL, Chou SJ, Chen DY, Chen YH, Hsieh TY, Young M Jr. A comparative study of etanercept plus methotrexate and methotrexate alone in Taiwanese patients with active rheumatoid arthritis: a 12‐week, double‐blind, randomised, placebo‐controlled study. Journal of the Formosan Medical Association 2004;103(8):618‐23. [PubMed] [Google Scholar]
Lisbona 2008 {published data only}
- Lisbona MP, Maymo J, Perich J, Almirall M, Pérez‐García C, Carbonell J. Etanercept reduces synovitis as measured by magnetic resonance imaging in patients with active rheumatoid arthritis after only 6 weeks. Journal of Rheumatology 2008;35(3):394‐7. [PubMed] [Google Scholar]
- Lisbona MP, Maymó J, Perich J, Almirall M, Carbonell J. Rapid reduction in tenosynovitis of the wrist and fingers evaluated by MRI in patients with rheumatoid arthritis after treatment with etanercept. Annals of the Rheumatic Diseases 2010;69(6):1117‐22. [PUBMED: 20448287] [DOI] [PubMed] [Google Scholar]
Lukas 2009 {published data only}
- Lukas C, Landewé R, Fatenejad S, Heijde D. Subtle changes in individual joints result in both positive and negative change scores in a patient: results from a clinical trial in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases 2009;68(11):1691‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lukas 2010 {published data only}
- Lukas C, Heijde D, Fatenajad S, Landewé R. Repair of erosions occurs almost exclusively in damaged joints without swelling. Annals of the Rheumatic Diseases 2010;69(5):851‐5. [DOI] [PubMed] [Google Scholar]
Lukina 2001 {published data only}
- Lukina GV. Double‐blind trial of the effectiveness of antibodies to interferon‐gamma and tumour necrosis factor‐alpha in rheumatoid arthritis (preliminary results). Terapevticheskii Arkhiv 2001;73(5):12‐5. [PubMed] [Google Scholar]
Luzi 2009 {published data only}
- Luzi G, Laganà B, Salemi S, Rosa R. Are glucocorticoids a consistent risk factor for infections in rheumatoid arthritis patients under treatment with methotrexate and etanercept?. Clinica Terapeutica 2009;160(2):121‐3. [PubMed] [Google Scholar]
Machado 2009 {published data only}
- Machado P, Santos A, Pereira C, Loureiro C, Silva J, Chieira C, et al. Increased prevalence of allergic sensitisation in rheumatoid arthritis patients treated with anti‐TNFalpha. Bone Spine 2009;76(5):508‐13. [DOI] [PubMed] [Google Scholar]
Moreland 1997 {published data only}
- Moreland LW, Baumgartner SW, Schiff MH, Tindall EA, Fleischmann RM, Weaver AL, et al. Treatment of rheumatoid arthritis with a recombinant human tumour necrosis factor receptor (p75)‐Fc fusion protein. New England Journal of Medicine 1997;337(3):141‐7. [DOI] [PubMed] [Google Scholar]
Moreland 2001 {published data only}
- Moreland LW, Cohen SB, Baumgartner SW, Tindall EA, Bulpitt K, Martin R, et al. Long‐term safety and efficacy of etanercept in patients with rheumatoid arthritis. Journal of Rheumatology 2001;28(6):1238‐44. [PubMed] [Google Scholar]
Paleolog 1998 {published data only}
- Paleolog EM. Modulation of angiogenic vascular endothelial growth factor by tumour necrosis factor alpha and interleukin‐1 in rheumatoid arthritis. Arthritis and Rheumatism 1998;41(7):1258‐65. [DOI] [PubMed] [Google Scholar]
Roux 2011 {published data only}
- Roux CH, Breuil V, Valerio L, Amoretti N, Brocq O, Albert C, et al. Etanercept compared to intraarticular corticosteroid injection in rheumatoid arthritis: double‐blind, randomised pilot study. Journal of Rheumatology 2011;38(6):1009‐11. [PUBMED: 21406499] [DOI] [PubMed] [Google Scholar]
Saleem 2009 {published data only}
- Saleem B, Brown AK, Keen H, Nizam S, Freeston J, Karim Z, et al. Disease remission state in patients treated with the combination of tumour necrosis factor blockade and methotrexate or with disease‐modifying antirheumatic drugs: a clinical and imaging comparative study. Arthritis and Rheumatism 2009;60(7):1915‐22. [DOI] [PubMed] [Google Scholar]
Sennels 2008 {published data only}
- Sennels H, Sørensen S, Ostergaard M, Knudsen L, Hansen M, Skjødt H, et al. Circulating levels of osteopontin, osteoprotegerin, total soluble receptor activator of nuclear factor‐kappa B ligand, and high‐sensitivity C‐reactive protein in patients with active rheumatoid arthritis randomised to etanercept alone or in combination with methotrexate. Scandinavian Journal of Rheumatology 2008;37(4):241‐7. [PUBMED: 18612923] [DOI] [PubMed] [Google Scholar]
van Riel 2006 {published data only}
- Riel PLC, Taggart AJ, Sany J, Gaubitz M, Nab HW, Pedersen R, et al. Efficacy and safety of combination etanercept and methotrexate versus methotrexate alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: the ADORE study. Annals of the Rheumatic Diseases 2006;65(11):1478‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinblatt 2007 {published data only}
- Weinblatt M, Schiff M, Goldman A, Kremer J, Luggen M, Li T, Chen D, et al. Selective co stimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Annals of the Rheumatic Diseases 2007;66(2):228‐34. [PUBMED: 16935912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinblatt 2008 {published data only}
- Weinblatt ME, Schiff MH, Ruderman EM, Bingham CO, Li J, Louie J, et al. Efficacy and safety of etanercept 50mg twice a week in patients with rheumatoid arthritis who had a sub‐optimal response to etanercept 50mg once a week. Arthritis and Rheumatism 2008;58(7):1921‐30. [DOI] [PubMed] [Google Scholar]
Weisman 2007 {published data only}
- Weisman MH, Paulus HE, Burch FX, Kivitz AJ, Fierer J, Dunn M, et al. A placebo‐controlled, randomised, double‐blinded study evaluating the safety of etanercept in patients with rheumatoid arthritis and concomitant comorbid diseases. Rheumatology 2007;46(7):1122‐5. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EMPIRE 2006 {unpublished data only}
- EMPIRE (Etanercept and Methotrexate in Patients to Induce Remission in Early arthritis). Ongoing study October 2006.
France 2008 {unpublished data only}
- An Open‐Label, Randomised Study to Evaluate the Radiographic Efficacy and Safety of Enbrel (Etanercept) Added to Methotrexate in Comparison with Usual Treatment in Subjects with Moderate Rheumatoid Arthritis Disease Activity. Ongoing study June 2008.
Japanese 2006 {unpublished data only}
- A Randomised, Double‐Blind, Multi‐Centre, Comparative Study Evaluating the Efficacy and Safety of Etanercept and Methotrexate in Japanese Subjects with Active Rheumatoid Arthritis. Ongoing study June 2006.
Jobanputra 2005 {unpublished data only}
- Remission Induction in Very Early Rheumatoid Arthritis: a Comparison of Etanercept plus Methotrexate plus Steroid with Standard Therapy. Ongoing study December 2005.
Takeuchi 2005 {unpublished data only}
- Efficacy and Safety of Etanercept on Active Rheumatoid Arthritis Despite Methotrexate Therapy in Japan. Ongoing study June 2005.
Additional references
Arnett 1988
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism 1988;31:315‐24. [DOI] [PubMed] [Google Scholar]
Boers 1994
- Boers M, Tugwell P, Felson DT. World Health Organization and International League of Associations for Rheumatology core endpoints for symptom modifying antirheumatic drugs in rheumatoid arthritis clinical trials. Journal of Rheumatology 1994;21:86‐9. [PubMed] [Google Scholar]
Bykerk 2012
- Bykerk VP, Akhavan P, Hazlewood GS, Schieir O, Dooley A, Haraoui B, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease‐modifying antirheumatic drugs. Journal of Rheumatology 2012;39(8):1559‐82. [DOI] [PubMed] [Google Scholar]
Donahue 2008
- Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, et al. Systematic review: comparative effectiveness and harms of disease‐modifying medications for rheumatoid arthritis. Annals of Internal Medicine 2008;148:124‐34. [DOI] [PubMed] [Google Scholar]
Felson 1993
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith D, et al. The American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1993;36:729‐40. [DOI] [PubMed] [Google Scholar]
Felson 1995
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith D, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis and Rheumatism 1995;38:727‐35. [DOI] [PubMed] [Google Scholar]
Fernandez‐Cruz 2008
- Fernández‐Cruz E, Alecsandru D, Rodríguez‐Sainz C. Introduction to biological drugs. Actas Dermo‐Sifiliograficas 2008;99 Suppl 4:2‐6. [PUBMED: 19080985] [PubMed] [Google Scholar]
Furst 2007
- Furst DE, Breedveld FC, Kalden JR, Smolen JS, Burmester GR, Sieper J, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2007. Annals of the Rheumatic Diseases 2007;66 Suppl III:iii2‐iii22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jarvis 1999
- Jarvis B, Faulds D. Etanercept: a review of its use in rheumatoid arthritis. Drugs 1999;57(6):945‐66. [PUBMED: 10400407] [DOI] [PubMed] [Google Scholar]
Kievit 2007
- Kievit W, Fransen J, Oerlemans AJM, Kuper HH, Laar MAFJ, Rooij DJRAM, et al. The efficacy of anti‐TNF in rheumatoid arthritis, a comparison between randomised controlled trials and clinical practice. Annals of the Rheumatic Diseases 2007;66:1473‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klareskog 2006
- Klareskog L, Gaubitz M, Rodriguez‐Valverde V, Malaise M, Dougados M, Wajdula J. A long‐term, open‐label trial of the safety and efficacy of etanercept (Enbrel) in patients with rheumatoid arthritis not treated with disease‐modifying antirheumatic drugs. Annals of the Rheumatic Diseases 2006;65:1578‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobelt 2005
- Kobelt G, Lindgren P, Singh A, Klareskog L. Cost effectiveness of etanercept (Enbrel) in combination with methotrexate in the treatment of active rheumatoid arthritis based on the TEMPO trial. Annals of the Rheumatic Diseases 2005;64:1174‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez‐Olivo 2012
- Lopez‐Olivo MA, Tayar JH, Martinez‐Lopez JA, Pollono EN, Cueto JP, Gonzales‐Crespo MR, et al. Risk of malignancies in patients with rheumatoid arthritis treated with biologic therapy ‐ a meta‐analysis. JAMA 2012;308(9):898‐908. [DOI] [PubMed] [Google Scholar]
Molenaar 2000
- Molenaar E, Heijde D, Boers M. Update on outcome assessment in rheumatic disorders. Current Opinion in Rheumatology 2000;12:91‐8. [DOI] [PubMed] [Google Scholar]
Moreland 2006
- Moreland LW, Weinblatt ME, Keystone EC, Kremer JM, Martin RW, Schiff MH, et al. Etanercept treatment in adults with established rheumatoid arthritis: 7 years of clinical experience. Journal of Rheumatology 2006;33:854‐61. [PubMed] [Google Scholar]
Murray 1997
- Murray KM, Dahl SL. Recombinant human tumour necrosis factor receptor (p75) Fc fusion protein (TNFR:Fc) in rheumatoid arthritis. Annals of Pharmacotherapy 1997;31(11):1335‐8. [PUBMED: 9391689] [DOI] [PubMed] [Google Scholar]
OMERACT 1993
- OMERACT. Conference on outcome measures in rheumatoid arthritis clinical trials. Journal of Rheumatology 1993;20:526‐91. [PubMed] [Google Scholar]
Pincus 1993
- Pincus T, Callahan LF. What is the natural history of rheumatoid arthritis?. Rheumatic Diseases Clinics of North America 1993;19:123‐51. [PubMed] [Google Scholar]
Pincus 1999
- Pincus T, Stein CM. ACR 20: clinical or statistical significance?. Arthritis and Rheumatism 1999;42(8):1572‐6. [DOI] [PubMed] [Google Scholar]
Puolakka 2005
- Puolakka K. Work Capacity and Productivity Costs in Early Rheumatoid Arthritis: A Five‐Year Prospective Study [dissertation]. Available from: ethesis.helsinki.fi/julkaisut/laa/kliin/vk/puolakka2/workcapa.pdf (accessed 12 March 2013). Helsinki: Academic Dissertation University of Helsinki, 2005. [Google Scholar]
Saag 2008
- Saag KG, Teng GG, Patkar NM, Anuntiy J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of non‐biologic and biologic disease‐modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Care and Research 2008;59(6):762‐84. [DOI] [PubMed] [Google Scholar]
Singh 2009
- Singh JA, Christensen R, Wells GA, Suarez‐Almazor ME, Buchbinder R, Lopez‐Olivo MA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007848.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2011
- Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta‐analysis and Cochrane overview. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD008794.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2012
- Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care and Research (Hoboken) 2012;64(5):625‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smolen 2010a
- Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux‐Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs. Annals of the Rheumatic Disease 2010;69(6):964‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smolen 2010b
- Smolen JS, Aletaha D, Grisar JC, Stamm TA, Sharp JT. Estimation of a numerical value for joint damage‐related physical disability in rheumatoid arthritis clinical trials. Annals of the Rheumatic Disease 2010;69:1058‐64. [DOI: 10.1136/ard.2009.114652] [DOI] [PubMed] [Google Scholar]
Tugwell 2011
- Tugwell P, Singh JA, Wells GA. Biologicals for rheumatoid arthritis. BMJ 2011;343:d4027. [DOI: 10.1136/bmj.d4027] [DOI] [PubMed] [Google Scholar]
van der Heijde 2005a
- Heijde D, Klareskog L, Boers M, Landewe R, Codreanu C, Bolosiu HD, et al. Comparison of different definitions to classify remission and sustained remission: 1 year TEMPO results. Annals of the Rheumatic Diseases 2005;64:1582‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Heijde 2005b
- Heijde D, Landewe R, Klareskog L, Rodriguez‐Valverde V, Settas L, Pedersen R, et al. Presentation and analysis of data on radiographic outcome in clinical trials. Arthritis and Rheumatism 2005;52(1):49‐60. [DOI] [PubMed] [Google Scholar]
Yazici 2008
- Yazici Y, Adler NM, Yazici H. Most tumour necrosis factor inhibitor trials in rheumatology are undeservedly called 'efficacy and safety' trials: a survey of power considerations. Rheumatology 2008;47(7):1054‐7. [DOI] [PubMed] [Google Scholar]
Yee 2003
- Yee CS, Filer A, Pace A, Douglas K, Situnayake D, Rowe IF, West Midlands Rheumatology Services and Training Committee. The prevalence of patients with rheumatoid arthritis in the West Midlands fulfilling the BSR criteria for anti‐tumour necrosis factor therapy: an out‐patient study. Rheumatology (Oxford) 2003;42(7):856‐9. [PUBMED: 12730544] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Blumenauer 2003a
- Blumenauer B, Judd M, Cranney A, Burls A, Coyle D, Hochberg M, et al. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD004525; PUBMED: 14584021] [DOI] [PubMed] [Google Scholar]


