Abstract
Background and aims
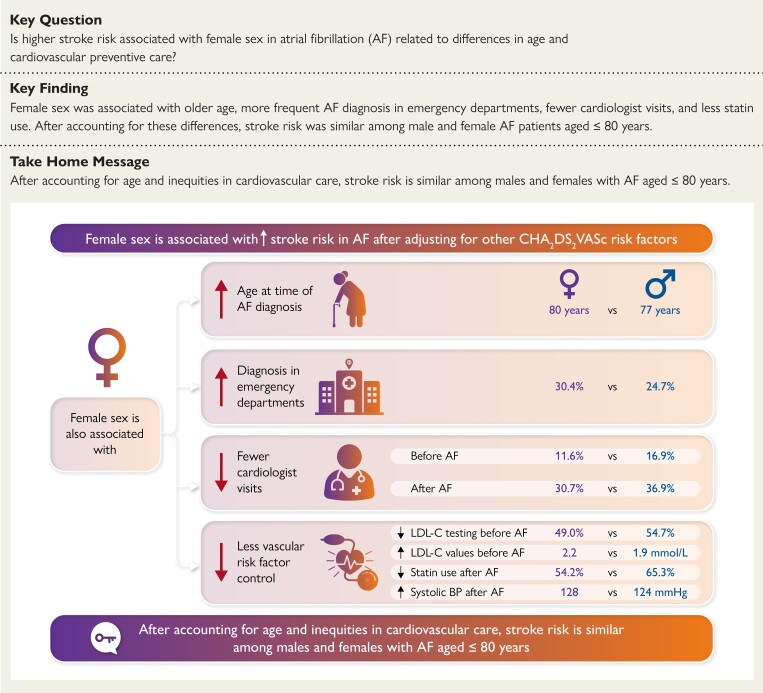
Female sex is associated with higher rates of stroke in atrial fibrillation (AF) after adjustment for other CHA2DS2-VASc factors. This study aimed to describe sex differences in age and cardiovascular care to examine their relationship with stroke hazard in AF.
Methods
Population-based cohort study using administrative datasets of people aged ≥66 years diagnosed with AF in Ontario between 2007 and 2019. Cause-specific hazard regression was used to estimate the adjusted hazard ratio (HR) for stroke associated with female sex over a 2-year follow-up. Model 1 included CHA2DS2-VASc factors, with age modelled as 66–74 vs. ≥ 75 years. Model 2 treated age as a continuous variable and included an age–sex interaction term. Model 3 further accounted for multimorbidity and markers of cardiovascular care.
Results
The cohort consisted of 354 254 individuals with AF (median age 78 years, 49.2% female). Females were more likely to be diagnosed in emergency departments and less likely to receive cardiologist assessments, statins, or LDL-C testing, with higher LDL-C levels among females than males. In Model 1, the adjusted HR for stroke associated with female sex was 1.27 (95% confidence interval 1.21–1.32). Model 2 revealed a significant age–sex interaction, such that female sex was only associated with increased stroke hazard at age >70 years. Adjusting for markers of cardiovascular care and multimorbidity further decreased the HR, so that female sex was not associated with increased stroke hazard at age ≤80 years.
Conclusion
Older age and inequities in cardiovascular care may partly explain higher stroke rates in females with AF.
Keywords: Atrial Fibrillation, Stroke, Female sex
Structured Graphical Abstract
Structured Graphical Abstract.
Higher stroke risk among women with atrial fibrillation (AF) may be related to inequities in cardiovascular care, suggesting that reducing sex differences in cardiovascular care may attenuate the excess stroke risk in females with AF.
BP, blood pressure; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease, age 66 to 74 years, and sex category (female); F, female; LDL-C, low density lipoprotein cholesterol; M, male.
See the editorial comment for this article ‘The stroke enigma: decoding the sex risk factor in atrial fibrillation’, by M.E. Middeldorp and R.K. Sandhu, https://doi.org/10.1093/eurheartj/ehad531.
Introduction
Female sex is assigned one point in the CHA2DS2-VASc score as it is associated with higher stroke risk in atrial fibrillation (AF).1 Recent studies suggest that female sex is a risk modifier for AF-associated stroke rather than an independent risk factor in all-comers with AF.2,3 We previously reported that female sex is not associated with increased stroke risk among people aged <75 years who do not have other CHA2DS2-VASc factors.4 Conversely, females with AF have higher stroke risk than their male counterparts at older age or higher CHA2DS2-VASc scores.3
There are several potential explanations for a sex-based interaction between risk factors and stroke hazard. It may be that females are biologically more predisposed towards stroke from risk factors (such as hypertension and diabetes), but there are alternative plausible explanations. Females tend to develop AF at an older age than males, and stroke rates rise in a graded manner with increasing age.4,5 There may be greater sex-based inequities in risk factor control and appropriate anticoagulation among older patients,6 so that the magnitude of a risk factor (e.g. blood pressure) may be higher in older females than males. Patients with AF remain at risk for non-embolic (i.e. atherosclerotic) strokes,7,8 and the CHA2DS2-VASc score predicts stroke similarly in people with and without AF.9 Aggressive management of vascular risk factors is associated with better outcomes after AF diagnosis.10,11 Furthermore, females with AF may have lower socioeconomic status,12,13 which is associated with less cardiovascular care and diagnosis of AF later in the disease course.
We present a population-based cohort study using administrative datasets to describe sex-based differences in cardiovascular treatment and the interaction between age and sex as it pertains to the hazard of stroke in people with newly recognized AF. Our hypothesis was that accounting for age as a continuous variable and adjusting for markers of cardiovascular care substantially attenuate the adjusted hazard ratio (HR) for stroke associated with female sex.
Methods
Data sources
Residents of Ontario (Canada’s most populous province) receive universal health insurance through the Ontario Health Insurance Plan. Prescription medication coverage is provided for residents aged >65 years through the Ontario Drug benefit programme, with copayments of $2–$6.11 per prescription after a $100 annual deductible.14 Health services are administered via an insurance number unique to each person, allowing for linkage of administrative datasets for research purposes. The Canadian Institute for Health Information Discharge Abstract Database records data on hospitalized patients, whereas the National Ambulatory Care Reporting System collects data on emergency department (ED) visits. The Ontario Health Insurance Plan physician claims database records physician billing data, and the Registered Persons Database maintains vital statistics data (including dates of birth and death). The Ontario Laboratories Information System database contains information on laboratory test results, including LDL-C levels.15 Canadian census data were used to determine neighbourhood-level socioeconomic metrics.16 The ICES Physician Database was used to obtain information on physician specialty. These datasets were linked using unique encoded identifiers and analysed at ICES (formerly called the Institute for Clinical Evaluative Sciences).17 The methods underlying their use for cardiovascular research have been previously described.18 The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act,19 which does not require review by a research ethics board.
Cohort creation
We created a cohort of community-dwelling individuals aged ≥66 years who were diagnosed with AF or atrial flutter (henceforth referred to as AF) between April 2007 and March 2019. The AF was ascertained based on one record of AF in hospital or ED discharge records (ICD-10 I48), or four physician billing claims for ICD-9 code 427 in 365 days. This algorithm was validated to have a specificity of 99.1% [95% confidence interval (CI) 98.9%–99.3%] for AF.20 The index date was that of first hospital, ED, or physician billing record indicative of AF. We excluded people with AF diagnoses in the prior 5 years to mostly capture people with newly recognized AF, since we were interested in studying cardiovascular care for people with AF at a comparable period in the disease trajectory. The exclusion criteria (detailed in Figure 1) also included valvular disease, as that subtype of AF has distinct clinical, therapeutic, and prognostic implications.
Figure 1.
Cohort flow diagram. The number of people with documented atrial fibrillation diagnoses, the exclusion criteria, and the number of people excluded for each criterion are presented for males and females.
Exposures
The key independent measure was female sex. Covariates of interest included other CHA2DS2-VASc risk factors: heart failure (HF), hypertension, age, diabetes, prior stroke/transient ischaemic attack (TIA), and vascular disease (defined as presence of ischaemic heart disease or peripheral vascular disease). We also studied several markers of cardiovascular care. Neighbourhood-level material deprivation is a marker of neighbourhood residents’ inability to attain basic material needs,16 which we previously reported to be associated with less cardiovascular care after AF diagnosis.12 We identified location of first AF diagnosis, since being diagnosed in the ED or hospital may indicate less outpatient care and therefore less ability to have AF diagnosed and managed out-of-hospital. We also studied the following measures of cardiovascular care received in the year before AF diagnosis: dispensation of statins, assessment by a cardiologist, receipt of echocardiography, testing for LDL-C, and achieved LDL-C level. Anticoagulation status after AF diagnosis was included as a time-varying covariate. To account for multimorbidity, we included estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation21 and estimated frailty from prior hospitalization data using methods described by Gilbert et al.22 The approaches used for determination of the key exposures are summarized in Supplementary data online, Table S1.
Outcome
The primary outcome was hospitalization with a most responsible diagnosis of ischaemic stroke [ICD-10 codes I63 (excluding I63.6), I64, and H341].23 Follow-up was limited to 2 years after AF diagnosis, since people with AF frequently acquire additional stroke risk factors over time24 and we expected a weaker relationship of factors measured before AF diagnosis with strokes beyond 2 years. We also studied cardiologist assessments, receipt of echocardiography, and dispensation of anticoagulation and statins as markers of cardiovascular care in the 2 years after AF diagnosis.
Exploratory analyses
The Electronic Medical Records-Primary Care (EMRPC) database (previously known as EMRALD) contains clinical data from the electronic medical records of ∼400 000 patients enrolled between April 2010 and March 2016 from ∼400 primary care physician practices in Ontario.25 Using individuals from our cohort who were also included in EMRPC, we compared differences by sex in blood pressure (BP) measurements. This subset was not used for multivariable regression analyses of stroke risk given its smaller sample size.
We also analysed appropriateness of direct-acting oral anticoagulant (DOAC) dosing among people in the full cohort who were unlikely to qualify for reduced DOAC dosing regardless of weight (which was unavailable for most participants). This was defined as people aged <80 years with creatinine <133 μmol/L (1.5 mg/dL) and eGFR ≥50 mL/min (estimated using the CKD-EPI equation). We then studied the subset of participants in EMRPC with available weight data who had eGFR ≥50 mL/min and met <2 of the dose reduction criteria for apixaban (age ≥80 years, weight ≤60 kg, creatinine ≥133 μmol/L), as these people would be expected to qualify for the full dose of most DOACs.26
Statistical methods
Missing values of LDL-C and eGFR were filled in using multiple imputation,27,28 using previously described methods.29 Multiple imputation allows one to avoid potential biases arising from a complete case analysis (i.e. using only subjects with no missing data). In this instance, subjects with missing LDL-C may have less healthcare contact than those with observed LDL-C. By imputing multiple values for each missing value, one can explicitly incorporate uncertainty in the value of the imputed data. The imputation model used all the variables listed in the exposure section as well as an indicator variable denoting the occurrence of ischaemic stroke and the cumulative hazard of ischaemic stroke at the time of stroke or censoring. We also utilized available values of LDL-C and eGFR for imputation of the alternate missing variable. The number of imputed samples was set to the percentage of missing observations (i.e. 48 complete samples were created).28 Each statistical analysis was conducted in each imputed dataset, after which we pooled the regression coefficient estimates and their standard errors using Rubin’s rules. For elements of past medical history, we considered that the person had the diagnosis if they fulfilled criteria for its determination within administrative datasets.
The cohort was stratified by sex for comparison of baseline characteristics, which were summarized using medians [with 25th–75th percentiles (Q1–Q3)] for continuous variables and counts (with percentages) for dichotomous variables. Given our large sample size, we focused on standardized differences30 to determine the relevance of unadjusted sex-based differences in baseline characteristics, since they are less affected by sample size than the χ2 or Wilcoxon rank sum tests. Standardized difference values ≥0.1 were considered to denote potentially meaningful differences.31
To contextualize sex differences in the crude rate of outcomes, we calculated the age-standardized event rate per 100 person-years with 95% CIs. Cause-specific hazard regression models were fit to study the association of female sex with the hazard of stroke over 2 years, with progressively comprehensive adjustment in three models. Death was treated as a competing risk. Model 1 only included the conventional CHA2DS2-VASc factors as predictors, with age as a binary variable (66–74 years, or ≥75 years). For subsequent analyses, age was handled as a continuous variable using restricted cubic splines with five knots placed at the 5%, 27.5%, 50%, 72.5%, and 95% percentiles.32 To determine if the HR for female sex varied with age, we tested for statistical significance of an age–sex interaction term. The interaction term was significant, so Models 2 and 3 incorporated the CHA2DS2-VASc factors plus an age–sex interaction term. Model 3 further accounted for baseline multimorbidity and markers of cardiovascular care that are listed above (Exposures section). Model 3 also included anticoagulation as a four-level time-varying covariate (non-anticoagulated, warfarin-treated, low-dose DOAC, or full-dose DOAC). For Models 2 and 3, the HR for stroke associated with female sex is presented at yearly age intervals.
Statistical significance of regression analyses was defined as a two-tailed P-value <.05. All analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC). Cells with <6 individuals were censored as per ICES’ privacy policies.
Results
Baseline characteristics
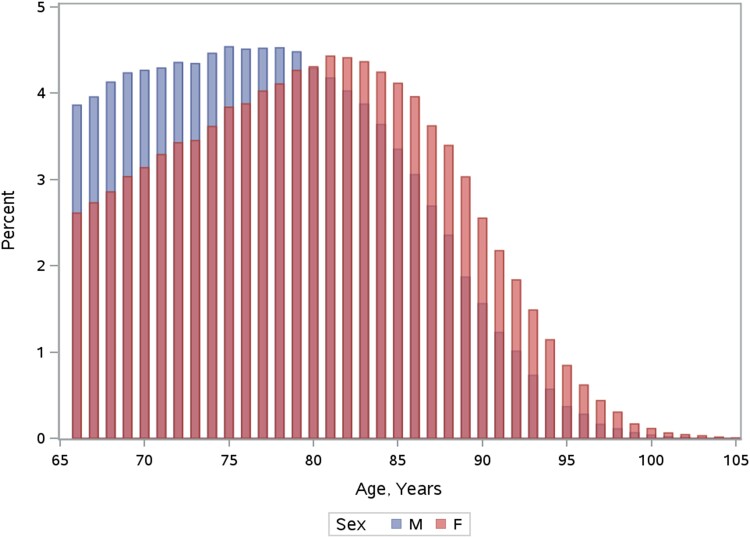
We identified 354 254 community-dwelling individuals [172 991 (48.8%) females] aged ≥66 years diagnosed with AF between April 2007 and March 2019 who met study inclusion criteria (Figure 1). Their baseline characteristics are listed in Table 1. The overall median age was 78 (Q1–Q3 72–84) years. Females were older than males, with 25.9% of women aged >85 years compared to 16.1% of men (Figure 2). Females were more likely to be diagnosed in the ED than males (30.4% vs. 24.7%, standardized difference 0.13).
Table 1.
Baseline characteristics of male and female people with newly diagnosed atrial fibrillation
| Variable | Females (n = 172 991) | Males (n = 181 263) | Standardized difference |
|---|---|---|---|
| Age, median (Q1–Q3) | 80 (74–86) | 77 (72–83) | 0 .29 |
| Setting of first AF diagnosis | |||
| In-hospital diagnosis, n (%) | 47 042 (27.2) | 55 289 (30.5) | 0.07 |
| Emergency department diagnosis, n (%) | 52 550 (30.4) | 44 737 (24.7) | 0 .13 |
| Outpatient diagnosis, n (%) | 73 399 (42.4) | 81 237 (44.8) | 0.05 |
| Regional material deprivation quintile | |||
| 1, n (%) | 30 330 (17.5) | 35 248 (19.4) | 0.05 |
| 2, n (%) | 32 159 (18.6) | 36 411 (20.1) | 0.04 |
| 3, n (%) | 33 607 (19.4) | 36 435 (20.1) | 0.02 |
| 4, n (%) | 36 988 (21.4) | 36 778 (20.3) | 0.03 |
| 5, n (%) | 38 577 (22.3) | 34 978 (19.3) | 0.07 |
| Missing, n (%) | 1330 (0.8) | 1413 (0.8) | 0.001 |
| Congestive heart failure, n (%) | 50 916 (29.4) | 54 191 (29.9) | 0.01 |
| Hypertension, n (%) | 148 124 (85.6) | 148 653 (82.0) | 0 .10 |
| Diabetes, n (%) | 52 566 (30.4) | 67 952 (37.5) | 0 .15 |
| Prior stroke or TIA, n (%) | 10 401 (6.0) | 9698 (5.4) | 0.03 |
| Vascular disease, n (%) | 50 447 (29.2) | 74 701 (41.2) | 0 .25 |
| Hospital frailty score, median (Q1–Q3) | 1.6 (0–6) | 1.5 (0–5) | 0.03 |
| Visited a cardiologist in prior year, n (%) | 20 032 (11.6) | 30 669 (16.9) | 0 .15 |
| Echocardiogram in prior year, n (%) | 85 080 (49.2) | 96 704 (53.4) | 0.08 |
| LDL-C value (mmol/L), median (Q1–Q3) | 2.2 (1.6–2.9) | 1.9 (1.4–2.6) | 0 .30 |
| eGFR, median (Q1–Q3) | 64 (48–79) | 67 (51–81) | 0 .13 |
| No. of anti-hypertensive medicines | |||
| 0, n (%) | 25 278 (14.6) | 31 045 (17.1) | 0.07 |
| 1, n (%) | 35 154 (20.3) | 38 215 (21.1) | 0.02 |
| 2, n (%) | 46 483 (26.9) | 49 611 (27.4) | 0.01 |
| 3, n (%) | 44 594 (25.8) | 43 194 (23.8) | 0.05 |
| 4, n (%) | 20 075 (11.6) | 17 868 (9.9) | 0.06 |
| 5, n (%) | 1407 (0.8) | 1330 (0.7) | 0.01 |
| Statins, n (%) | 83 608 (48.3) | 107 946 (59.6) | 0 .23 |
| Any oral anticoagulant, n (%) | 56 330 (32.6%) | 61 541 (34.0%) | 0.03 |
| Full dose DOAC, n (%) | 12 273 (7.1%) | 15 026 (8.3%) | 0.04 |
| Reduced dose DOAC, n (%) | 11 070 (6.4%) | 8913 (4.9%) | 0.06 |
| Warfarin n (%) | 34 503 (19.9%) | 39 250 (21.7%) | 0.04 |
Standardized differences >0.1 are considered to represent meaningful differences between groups and have been highlighted in bold italics. The Q1–Q3 indicate the 25th and 75th percentiles.
AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; LDL-C, low density lipoprotein cholesterol; TIA, transient ischaemic attack; DOAC, direct-acting oral anticoagulant (DOAC).
Figure 2.
Age distribution among males and females with newly diagnosed atrial fibrillation. This histogram presents the proportion of males and females at each age within the cohort of people aged ≥66 years with newly diagnosed atrial fibrillation. F, female; M, male.
Males were more likely to have prior diabetes and vascular disease while females were more likely to have hypertension and lower eGFR. Females were less likely to have LDL-C measurements (49.0% of females, 54.7% of males) or receive statins (48.3% of females; 59.6% of males) in the year before AF diagnosis, which was reflected in females having higher baseline LDL-C values than males [female median 2.2 (Q1–Q3 1.6–2.9 mmol/L); male median 1.9 (Q1–Q3 1.4–2.6 mmol/L)]. In the year before AF diagnosis, 20 032 (11.6%) females were assessed by a cardiologist compared to 30 669 (16.9%) males. The standardized difference for all comparisons reported above was ≥0.1; the standardized differences between males and females for the remaining baseline characteristics was <0.1.
Management after atrial fibrillation recognition
During the 2 years following the index date, 53 029 (30.7%) females were assessed by a cardiologist, compared with 66 938 (36.9%) males (standardized difference 0.13). Females were less likely to have been dispensed prescriptions for statins (54.2% vs. 65.3% of males) after AF diagnosis (standardized difference 0.23). There were smaller differences in receipt of echocardiography [109 861 (63.5%) females; 122 078 (67.3%) males; standardized difference 0.08] and no sex-based difference in the dispensation of anticoagulation overall (61.5% females vs. 61.4% males, standardized difference 0.004). However, there were higher rates of dispensation of low-dose DOACs to females (20.3%) than males (15.7%), with a standardized difference of 0.12. The analysis of DOAC dispensation in people unlikely to qualify for reduced dosing suggested that reduced dose DOACs are more likely dispensed to females in the absence of criteria for dose reduction. Details are provided in Table 2.
Table 2.
Analysis of direct oral anticoagulant dispensation in participants unlikely to qualify for reduced direct oral anticoagulant dosing
| Females | Males | Standardized difference | P-value | |
|---|---|---|---|---|
| People in cohort who are unlikely to qualify for reduced DOAC dosing regardless of weight | ||||
| Sample size | n = 49 675 | n = 67 628 | ||
| Warfarin | 12 799 (25.8%) | 18 876 (27.9%) | 0.05 | <.001 |
| Full dose DOACs | 17 930 (36.1%) | 24 446 (36.1%) | 0.001 | .85 |
| Reduced dose DOACs | 7455 (15.0%) | 8338 (12.3%) | 0.08 | <.001 |
| People in EMPRC dataset not meeting criteria for reduced dose DOACs | ||||
| Sample size | n = 1323 | n = 1679 | ||
| Warfarin | 454 (34.3%) | 565 (33.7%) | 0.01 | .7 |
| Full dose DOACs | 362 (27.4%) | 471 (28.1%) | 0.02 | .67 |
| Reduced dose DOACs | 277 (20.9%) | 267 (15.9%) | 0.13 | <.001 |
The upper half of the table describes anticoagulant dispensation in the 2 years after AF diagnosis in the subset of the cohort who would likely not qualify for reduced DOAC dosing regardless of weight—people aged <80 years with creatinine <133 μmol/L (1.5 mg/dL) and eGFR ≥50 mL/min as per the CKD-EPI equation. The bottom half of the table reports anticoagulant dispensation in the subset of participants in EMRPC with available weight data who had eGFR ≥50 mL/min and met <2 of the dose reduction criteria for apixaban (age ≥80 years, weight ≤60 kg, creatinine ≥133 μmol/L), as these people would be expected to qualify for the full dose of all DOACs.
DOAC, direct-acting oral anticoagulant (DOAC).
Relationship between sex and stroke
There were 7692 (2.2%) ischaemic strokes and 81 834 (23.1%) deaths in the 2 years following AF diagnosis. The age-standardized rate of ischaemic stroke was 1.4 (95% CI 1.3–1.4) per 100 person-years in females and 1.1 (95% CI 1.1–1.2) per 100 person-years in males. The age-standardized mortality rate was 10.7 (95% CI 10.6–10.9) per 100 person-years in females and 13.9 (95% CI 13.8–14.1) per 100 person-years in males. The cumulative incidence function curves for stroke by sex are provided in Supplementary data online, Figure S1.
The HRs for stroke associated with female sex resulting from Models 1–3 are illustrated in Figure 3. The HRs for other variables in Models 1–3 are presented in Supplementary data online, Table S2. After adjustment in Model 1 (utilizing CHA2DS2-VASc variables, with age modelled as a binary variable), female sex was associated with significantly increased hazard of stroke (HR 1.27, 95% CI 1.21–1.32, P < .001). There was a significant interaction between age and sex (P = .001). When the analysis was repeated with an age-interaction term (Model 2), the HR associated with female sex was significantly higher than 1 in patients aged >70 years but there was no significant difference by sex in stroke hazard among younger patients. In Model 3 (which further adjusted for markers of cardiovascular care and comorbidity), the HR was lower than Model 2 at all ages; female sex was only significantly associated with increased stroke hazard above the age of 80 years.
Figure 3.
Adjusted hazard ratios for stroke associated with female sex. Model 1 includes the variables in the conventional CHA2DS2-VASc model: congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease, age 66 to 74 years, and sex category (female). Models 2 and 3 incorporated an interaction term between age and sex, with age handled as a continuous variable using restricted cubic splines utilizing five knots placed at the following percentiles: 5%, 27.5%, 50%, 72.5%, and 95%. Model 3 further accounted for baseline multimorbidity, markers of cardiovascular care as well as anticoagulation as a time-varying covariate.
Subset with blood pressure data
We identified a total of 7412 people [3686 (49.7%) female] with available BP measurements before or after AF diagnosis, of whom 6296 people [3164 (50.2%) female] had an available BP measurement in the EMRPC dataset within 365 days before AF diagnosis. Their baseline characteristics, compared with the remaining 347 958 patients, are shown in Supplementary data online, Table S3. Participants with available BP data were less likely to live in neighbourhoods with higher material deprivation; there was no other meaningful difference in baseline characteristics. The median time between BP measurement and AF diagnosis was 42 (Q1–Q3 8–128) days. The median documented systolic BP before AF diagnosis was higher in females (median 130 mmHg; Q1–Q3 120–142 mmHg) than males (median 128 mmHg; Q1–Q3 116–140 mmHg), corresponding to a standardized difference of 0.17. There were no differences in diastolic BP (72 mmHg in both sexes). We also identified 6110 individuals [3036 (49.7%) female] who had available BP measurements within 2 years after AF diagnosis, taken at a median of 21 (Q1–Q3 5–78) days after AF diagnosis. The systolic BP after AF diagnosis remained significantly higher in females (128 mmHg, Q1–Q3 116–140 mmHg) compared to males (median 124 mmHg, Q1–Q3 111–137 mmHg, standardized difference 0.18), but there was no difference in diastolic BP (70 mmHg in both sexes).
Discussion
This population-based study examined sex differences in age and cardiovascular care to determine their relationship to the higher stroke risk in females with AF. Despite having higher stroke incidence, females with AF were less likely to be assessed by cardiologists, get LDL-C testing, or receive statins. Females with AF also had higher LDL-C levels and higher BP than their male counterparts. The HR for stroke associated with female sex was age-dependent, such that it was only associated with increased stroke hazard at older ages. With adjustment for markers of cardiovascular care, the HR associated with female sex was substantially attenuated, such that female sex was only associated with increased stroke hazard at age >80 years (Structured Graphical Abstract).
Our findings indicate that age modifies the association between sex and stroke in AF, with female sex being independently associated with higher risk in those aged > 80 years, but not in younger people. Females tend to be older than men when diagnosed with AF, and the age-associated prevalence of cardiovascular risk factors increases at a faster rate in females than males.33,34 The impact of older age may be compounded by the observation that older females are less likely to receive cardiovascular care than older males.33,35,36 Although anticoagulation is the primary approach to stroke prophylaxis in AF, there remains a residual risk that may benefit from treatment of atherosclerotic risk factors.10 We observed that females with AF were less likely to be treated with statins despite having higher LDL-C levels. Several observational studies report that statin use and lower LDL-C levels are associated with lower stroke risk in AF patients.37,38 However, there are no randomized controlled trial data demonstrating benefit from statins specifically in the AF population. It may be that the lower stroke hazard associated with statin exposure and lower LDL-C levels indicate that they are markers of better cardiovascular care overall rather than directly causing a reduction in stroke risk.
Females were more likely to be first diagnosed with AF in the ED, which was associated with worse outcomes than diagnosis in other settings (see Supplementary data online, Table S2). A first diagnosis of AF in the ED was also associated with adverse outcomes in a prospective registry encompassing 47 countries with substantial regional variation.39 We hypothesize that greater rates of initial AF diagnosis in the ED among females reflect less access to cardiovascular care among females, which is supported by the observation of lower rates of cardiologist assessment for females before and after AF diagnosis. Other studies have shown that cardiologist care after a new diagnosis of AF is associated with lower rates of stroke and other adverse outcomes.12,40 Alternatively, the higher rates of AF diagnosis in the ED among females may relate to the higher symptom burden in females with AF.41
In exploratory analyses of people with available BP data, we observed that females had higher systolic BP than males before and after AF diagnosis. The CHA2DS2-VASc model treats hypertension as a dichotomous variable, but higher BP correlates with increased stroke risk in AF.42–46 One of the first models to predict stroke risk in AF was derived from the Framingham Heart Study.46 In this model, every 10 mmHg increase in systolic BP was associated with a 10% relative increase in the rate of stroke. The association between higher BP and stroke risk continues to be reported in recent analyses with anticoagulated patients.42–45 The effect of hypertension may be compounded by other stroke risk factors to a greater extent in females compared to men.47,48 We were not able to incorporate BP data in our regression analyses due to the small size of the subset with available data. We hypothesize that the magnitude of HR will become even closer to the null in a model that accounts for actual BP levels in males and females.
Limitations
The observational study design means that there remains the potential for residual confounding, including unmeasured sex-based treatment inequities. Given our reliance on administrative data, we could not account for important variables, including race, AF type/burden, and ejection fraction. We could not account for additional clinical factors which could have justified reduced dabigatran dosing in people aged 75–79 years despite having eGFR >50 mL/min. We did not have the requisite data to gauge the appropriateness of statin use or time in therapeutic range for warfarin-treated people. We could not account for aspirin use since it can be purchased over the counter in Ontario. However, aspirin is not efficacious neither is it recommended for stroke prevention in AF.49,50 While the algorithms used to identify medical diagnoses have been validated, they are more specific than they are sensitive. It is possible that the sensitivity is even lower for older females than males since we observed that females have less cardiovascular care. If this were true, it would cause us to overestimate the HR associated with female sex, as we cannot adjust for HF, hypertension, diabetes, or vascular disease if they were unrecognized. Thus, we cannot exclude that the higher HR associated with female sex in people aged >80 years may represent insufficient adjustment due to detection bias in a population that may have even greater inequities in cardiovascular care. Finally, our results may be less generalizable outside the Canadian healthcare system, which is administered by a single payer and forbids out-of-pocket or private insurance payment for funded medically necessary services. The sex inequities in cardiovascular care may be accentuated in systems that have greater financial barriers to healthcare.
Conclusion
Female patients with AF are more likely to be diagnosed in the ED than males, less likely to be assessed by cardiologists, less likely to receive statins, get LDL-C testing, and more likely to have higher systolic BP and LDL-C levels. The sex-specific difference in stroke hazard was attenuated after accounting for indicators of cardiovascular care. These data highlight the need to reduce sex-based inequities in cardiovascular care in older people with AF, as they may underlie the higher stroke risk observed among female patients.
Supplementary Material
Acknowledgements
This study was supported by ICES, an independent, non-profit research institute funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under licence from ©Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by CIHI and the Ontario Ministry of Health. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources (ICES, CIHI, or Ontario MOH/MLTC); no endorsement is intended or should be inferred. We thank IQVIA Solutions Canada Inc. for the use of their Drug Information File.
Contributor Information
Hifza Buhari, Department of Medicine, Women’s College Hospital, Room 6452, 76 Grenville Street, Toronto, ON M5S 1B2, Canada; Department of Medicine, University Health Network, 200 Elizabeth Street, Toronto, ON M5G 2C4, Canada.
Jiming Fang, Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada.
Lu Han, Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada.
Peter C Austin, Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, 155 College Street, Suite 425, Toronto, ON M5T 3M6, Canada.
Paul Dorian, Department of Medicine, University of Toronto, 6 Queen's Park Crescent West, Third Floor, Toronto, ON M5S 3H2, Canada; Division of Cardiology, Unity Health, 30 Bond St., Toronto, ON M5B 1W8, Canada.
Cynthia A Jackevicius, Department of Medicine, University Health Network, 200 Elizabeth Street, Toronto, ON M5G 2C4, Canada; Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, 155 College Street, Suite 425, Toronto, ON M5T 3M6, Canada; Department of Pharmacy Practice and Administration, Western University of Health Sciences, 11301 Wilshire Blvd, Los Angeles, CA 90073, USA.
Amy Y X Yu, Department of Medicine, University of Toronto, 6 Queen's Park Crescent West, Third Floor, Toronto, ON M5S 3H2, Canada; Evaluative Clinical Sciences, Sunnybrook Health Sciences Centre, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada.
Moira K Kapral, Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada; Department of Medicine, University of Toronto, 6 Queen's Park Crescent West, Third Floor, Toronto, ON M5S 3H2, Canada.
Sheldon M Singh, Department of Medicine, University of Toronto, 6 Queen's Park Crescent West, Third Floor, Toronto, ON M5S 3H2, Canada; Schulich Heart Centre, Sunnybrook Health Sciences Centre, Hospital Road, Toronto, ON M4N 3M5, Canada.
Karen Tu, Department of Medicine, University Health Network, 200 Elizabeth Street, Toronto, ON M5G 2C4, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, 155 College Street, Suite 425, Toronto, ON M5T 3M6, Canada; Research and Innovation Department, North York General Hospital, Room LE-140, 4001 Leslie Street, Toronto, ON M2K 1E1, Canada; Department of Family and Community Medicine, University of Toronto, 500 University Ave, 5th Floor, Toronto, ON M5G 1V7, Canada.
Dennis T Ko, Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, 155 College Street, Suite 425, Toronto, ON M5T 3M6, Canada; Department of Medicine, University of Toronto, 6 Queen's Park Crescent West, Third Floor, Toronto, ON M5S 3H2, Canada; Schulich Heart Centre, Sunnybrook Health Sciences Centre, Hospital Road, Toronto, ON M4N 3M5, Canada.
Clare L Atzema, Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, 155 College Street, Suite 425, Toronto, ON M5T 3M6, Canada; Department of Medicine, University of Toronto, 6 Queen's Park Crescent West, Third Floor, Toronto, ON M5S 3H2, Canada; Evaluative Clinical Sciences, Sunnybrook Health Sciences Centre, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada.
Emelia J Benjamin, Department of Medicine, Boston Medical Center, Boston University Chobanian and Avedisian School of Medicine, 715 Albany St, E-113, Boston, MA 02118, USA; Department of Epidemiology, Boston University School of Public, 677 Huntington Ave, Boston, MA 02115, USA.
Douglas S Lee, Department of Medicine, University Health Network, 200 Elizabeth Street, Toronto, ON M5G 2C4, Canada; Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, 155 College Street, Suite 425, Toronto, ON M5T 3M6, Canada; Department of Medicine, University of Toronto, 6 Queen's Park Crescent West, Third Floor, Toronto, ON M5S 3H2, Canada.
Husam Abdel-Qadir, Department of Medicine, Women’s College Hospital, Room 6452, 76 Grenville Street, Toronto, ON M5S 1B2, Canada; Department of Medicine, University Health Network, 200 Elizabeth Street, Toronto, ON M5G 2C4, Canada; Cardiovascular Research Program, ICES, V1 06, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada; Institute of Health Policy, Management, and Evaluation, University of Toronto, 155 College Street, Suite 425, Toronto, ON M5T 3M6, Canada; Department of Medicine, University of Toronto, 6 Queen's Park Crescent West, Third Floor, Toronto, ON M5S 3H2, Canada.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
All authors declare no conflict of interest for this contribution.
Data Availability
While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS.
Funding
The analysis of this study was funded by the Heart & Stroke Foundation of Canada through a Seed/Catalyst Grant for Improving Heart and Brain Health for Women in Canada (to H.A.-Q.). M.K.K. and C.L.A. were supported by Mid-Career Investigator Awards from the Heart and Stroke Foundation of Ontario. C.L.A. was also supported by ICES, the Practice Plan of the Department of Emergency Services at Sunnybrook Health Sciences Centre, and the Sunnybrook Research Institute. A.Y.X.Y. holds a National New Investigator Award from the Heart & Stroke Foundation of Canada. M.K.K. holds the Lillian Love Chair in Women’s Health from the University Health Network, Canada. K.T. is supported by a Research Scholar Award from the Department of Family and Community Medicine at the University of Toronto. E.J.B. is supported by the National Institutes of Health R01HL092577; American Heart Association AF AHA_18SFRN34110082. D.S.L. is supported by the Ted Rogers Chair in Heart Function Outcomes, University Health Network, University of Toronto. D.T.K. is supported by the Jack Tu Chair in Cardiovascular Outcomes Research, Sunnybrook Health Sciences Centre. H.A.-Q. is supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada. The funding sources had no role in the conduct of the study, the decision to publish, or the preparation of the manuscript.
Ethical Approval
The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a research ethics board.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016;532:h7013. 10.1136/bmj.h7013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chapa DW, Akintade B, Thomas SA, Friedmann E. Gender differences in stroke, mortality, and hospitalization among patients with atrial fibrillation: a systematic review. Heart Lung 2015;44:189–98. 10.1016/j.hrtlng.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 3. Nielsen PB, Skjøth F, Overvad TF, Larsen TB, Lip GYH. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation 2018;137:832–40. 10.1161/CIRCULATIONAHA.117.029081 [DOI] [PubMed] [Google Scholar]
- 4. Abdel-Qadir H, Singh SM, Pang A, Austin PC, Jackevicius CA, Tu K, et al. Evaluation of the risk of stroke without anticoagulation therapy in men and women with atrial fibrillation aged 66 to 74 years without other CHA2DS2-VASc factors. JAMA Cardiol 2021;6:918–25. 10.1001/jamacardio.2021.1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marinigh R, Lip GYH, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients. J Am Coll Cardiol 2010;56:827–37. 10.1016/j.jacc.2010.05.028 [DOI] [PubMed] [Google Scholar]
- 6. Wändell PE, Carlsson AC, Sundquist J, Johansson SE, Bottai M, Sundquist K. Pharmacotherapy and mortality in atrial fibrillation—a cohort of men and women 75 years or older in Sweden. Age Ageing 2015;44:232–8. 10.1093/ageing/afu153 [DOI] [PubMed] [Google Scholar]
- 7. Lehtola H, Airaksinen KEJ, Hartikainen P, Hartikainen JEK, Palomäki A, Nuotio I, et al. Stroke recurrence in patients with atrial fibrillation: concomitant carotid artery stenosis doubles the risk. Eur J Neurol 2017;24:719–25. 10.1111/ene.13280 [DOI] [PubMed] [Google Scholar]
- 8. Hart RG, Pearce LA, Miller VT, Anderson DC, Rothrock JF, Albers GW, et al. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis 2000;10:39–43. 10.1159/000016023 [DOI] [PubMed] [Google Scholar]
- 9. Siddiqi TJ, Usman MS, Shahid I, Ahmed J, Khan SU, Ya'qoub L, et al. Utility of the CHA2DS2-VASc score for predicting ischaemic stroke in patients with or without atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol 2022;29:625–31. 10.1093/eurjpc/zwab018 [DOI] [PubMed] [Google Scholar]
- 10. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. 10.1016/j.jacc.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 11. Pol T, Held C, Westerbergh J, Lindback J, Alexander JH, Alings M, et al. Dyslipidemia and risk of cardiovascular events in patients with atrial fibrillation treated with oral anticoagulation therapy: insights from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. J Am Heart Assoc 2018;7:e007444. 10.1161/JAHA.117.007444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdel-Qadir H, Akioyamen LE, Fang J, Pang A, Ha ACT, Jackevicius CA, et al. Association of neighborhood-level material deprivation with atrial fibrillation care in a single-payer health care system: a population-based cohort study. Circulation 2022;146:159–71. 10.1161/CIRCULATIONAHA.122.058949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hagengaard L, Andersen MP, Polcwiartek C, Larsen JM, Larsen ML, Skals RK, et al. Socioeconomic differences in outcomes after hospital admission for atrial fibrillation or flutter. Eur Heart J Qual Care Clin Outcomes 2021;7:295–303. 10.1093/ehjqcco/qcz053 [DOI] [PubMed] [Google Scholar]
- 14. Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 2003;10:67–71. [PubMed] [Google Scholar]
- 15. Campitelli MA, Kumar M, Greenberg A. Integrating population-wide laboratory testing data with audit and feedback reports for Ontario physicians. Healthc Q 2018;21:6–9. 10.12927/hcq.2018.25630 [DOI] [PubMed] [Google Scholar]
- 16. Matheson FI, Dunn JR, Smith KLW, Moineddin R, Glazier RH. Development of the Canadian Marginalization Index: a new tool for the study of inequality. Can J Public Health 2012;103:S12–6. 10.1007/BF03403823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schull MJ, Azimaee M, Marra M, Cartagena RG, Vermeulen MJ, Ho M, et al. ICES: data, discovery, better health. Int J Popul Data Sci 2019;4:1135. 10.23889/ijpds.v4i2.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ko DT, Alter DA, Guo H, Koh M, Lau G, Austin PC, et al. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J Am Coll Cardiol 2016;68:2073–83. 10.1016/j.jacc.2016.08.038 [DOI] [PubMed] [Google Scholar]
- 19. Personal Health Information Protection Act, 2004, S.O. 2004, c. 3, Sched. A. (2014-07-24T16:34:28-04:00; date last accessed).
- 20. Tu K, Nieuwlaat R, Cheng SY, Wing L, Ivers N, Atzema CL, et al. Identifying patients with atrial fibrillation in administrative data. Can J Cardiol 2016;32:1561–5. 10.1016/j.cjca.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–82. 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hall R, Mondor L, Porter J, Fang J, Kapral MK. Accuracy of administrative data for the coding of acute stroke and TIAs. Can J Neurol Sci 2016;43:765–73. 10.1017/cjn.2016.278 [DOI] [PubMed] [Google Scholar]
- 24. Chao TF, Lip GYH, Liu CJ, Lin YJ, Chang SL, Lo LW, et al. Relationship of aging and incident comorbidities to stroke risk in patients with atrial fibrillation. J Am Coll Cardiol 2018;71:122–32. 10.1016/j.jacc.2017.10.085 [DOI] [PubMed] [Google Scholar]
- 25. Tu K, Mitiku TF, Ivers NM, Guo H, Lu H, Jaakkimainen L, et al. Evaluation of Electronic Medical Record Administrative data Linked Database (EMRALD). Am J Manag Care 2014;20:e15–21. [PubMed] [Google Scholar]
- 26. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 27. Austin PC, White IR, Lee DS, van Buuren S. Missing data in clinical research: a tutorial on multiple imputation. Can J Cardiol 2021;37:1322–31. 10.1016/j.cjca.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 29. Abdel-Qadir H, Bobrowski D, Zhou L, Austin PC, Calvillo-Arguelles O, Amir E, et al. Statin exposure and risk of heart failure after anthracycline- or trastuzumab-based chemotherapy for early breast cancer: a propensity score-matched cohort study. J Am Heart Assoc 2021;10:e018393. 10.1161/JAHA.119.018393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. Am Stat 1986;40:249–51. [Google Scholar]
- 31. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228–34. 10.1080/03610910902859574 [DOI] [Google Scholar]
- 32. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Springer International Publishing; 2015. [Google Scholar]
- 33. Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 1999;99:1165–72. 10.1161/01.CIR.99.9.1165 [DOI] [PubMed] [Google Scholar]
- 34. Pana TA, Luben RN, Mamas MA, Potter JF, Wareham NJ, Khaw K-T, et al. Long term prognostic impact of sex-specific longitudinal changes in blood pressure. The EPIC-Norfolk prospective population cohort study. Eur J Prev Cardiol 2021;29:180–91. 10.1093/eurjpc/zwab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walli-Attaei M, Joseph P, Rosengren A, Chow CK, Rangarajan S, Lear SA, et al. Variations between women and men in risk factors, treatments, cardiovascular disease incidence, and death in 27 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020;396:97–109. 10.1016/S0140-6736(20)30543-2 [DOI] [PubMed] [Google Scholar]
- 36. Pinho-Gomes AC, Peters SAE, Thomson B, Woodward M. Sex differences in prevalence, treatment and control of cardiovascular risk factors in England. Heart 2021;107:462–7. 10.1136/heartjnl-2020-317446 [DOI] [PubMed] [Google Scholar]
- 37. Shweikialrefaee B, Ko DT, Fang J, Pang A, Austin PC, Dorian P, et al. Statin Use and Stroke Rate in Older Adults With Atrial Fibrillation: A Population-Based Cohort Study. J Am Heart Assoc 2023;1212:e028381. 10.1161/JAHA.122.028381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumagai N, Nusser JA, Inoue H, Okumura K, Yamashita T, Kubo T, et al. Effect of addition of a statin to warfarin on thromboembolic events in Japanese patients with nonvalvular atrial fibrillation and diabetes mellitus. Am J Cardiol 2017;120:230–5. 10.1016/j.amjcard.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 39. Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet 2016;388:1161–9. 10.1016/S0140-6736(16)30968-0 [DOI] [PubMed] [Google Scholar]
- 40. Perino AC, Fan J, Schmitt SK, Askari M, Kaiser DW, Deshmukh A, et al. Treating specialty and outcomes in newly diagnosed atrial fibrillation: from the TREAT-AF study. J Am Coll Cardiol 2017;70:78–86. 10.1016/j.jacc.2017.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gleason KT, Dennison Himmelfarb CR, Ford DE, Lehmann H, Samuel L, Jain S, et al. Association of sex and atrial fibrillation therapies with patient-reported outcomes. Heart 2019;105:1642–8. 10.1136/heartjnl-2019-314881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vemulapalli S, Hellkamp AS, Jones WS, Piccini JP, Mahaffey KW, Becker RC, et al. Blood pressure control and stroke or bleeding risk in anticoagulated patients with atrial fibrillation: results from the ROCKET AF trial. Am Heart J 2016;178:74–84. 10.1016/j.ahj.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 43. Rao MP, Halvorsen S, Wojdyla D, Thomas L, Alexander JH, Hylek EM, et al. Blood pressure control and risk of stroke or systemic embolism in patients with atrial fibrillation: results from the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. J Am Heart Assoc 2015;4:e002015. 10.1161/JAHA.115.002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T, Otsuka T, et al. Impact of blood pressure control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation: a subanalysis of the J-RHYTHM registry. J Am Heart Assoc 2016;5:e004075. 10.1161/JAHA.116.004075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ishii M, Ogawa H, Unoki T, An Y, Iguchi M, Masunaga N, et al. Relationship of hypertension and systolic blood pressure with the risk of stroke or bleeding in patients with atrial fibrillation: the Fushimi AF registry. Am J Hypertens 2017;30:1073–82. 10.1093/ajh/hpx094 [DOI] [PubMed] [Google Scholar]
- 46. Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA 2003;290:1049–56. 10.1001/jama.290.8.1049 [DOI] [PubMed] [Google Scholar]
- 47. Chen MQ, Shi WR, Wang HY, Sun YX. Sex differences of combined effects between hypertension and general or central obesity on ischemic stroke in a middle-aged and elderly population. Clin Epidemiol 2021;13:197–206. 10.2147/CLEP.S295989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lai YJ, Chen HC, Chou P. Gender difference in the interaction effects of diabetes and hypertension on stroke among the elderly in the Shih-Pai study, Taiwan. PLoS One 2015;10:e0136634. 10.1371/journal.pone.0136634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ben Freedman S, Gersh BJ, Lip GYH. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015;36:653–6. 10.1093/eurheartj/ehu494 [DOI] [PubMed] [Google Scholar]
- 50. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol 2020;36:1847–948. 10.1016/j.cjca.2020.09.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
While data sharing agreements prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS.