Abstract
Background and Aims
Statin recommendations in primary prevention depend upon risk algorithms. Moreover, with intermediate risk, risk enhancers and de-enhancers are advocated to aid decisions. The aim of this study was to compare algorithms used in North America and Europe for the identification of patients warranting statin or consideration of risk enhancers and de-enhancers.
Methods
A simulated population (n = 7680) equal in males and females, with/without smoking, aged 45–70 years, total cholesterol 3.5–7.0 mmol/L, high-density lipoprotein cholesterol 0.6–2.2 mmol/L, and systolic blood pressure 100–170 mmHg, was evaluated. High, intermediate, and low risks were determined using the Framingham Risk Score (FRS), Pooled Cohort Equation (PCE), four versions of Systematic Coronary Risk Evaluation 2 (SCORE2), and Multi-Ethnic Study of Atherosclerosis (MESA) algorithm (0–1000 Agatston Units).
Results
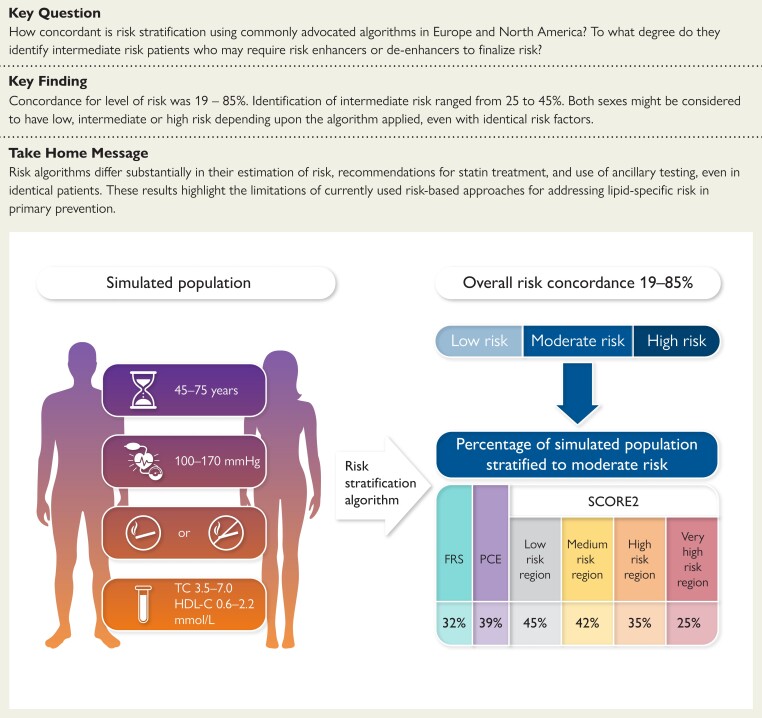
Concordance for the three levels of risk varied from 19% to 85%. Both sexes might be considered to have low, intermediate, or high risk depending on the algorithm applied, even with the same burden of risk factors. Only SCORE2 (High Risk and Very High Risk versions) identified equal proportions of males and females with high risk. Excluding MESA, the proportion with moderate risk was 25% (SCORE2, Very High Risk Region), 32% (FRS), 39% (PCE), and 45% (SCORE2, Low Risk Region).
Conclusion
Risk algorithms differ substantially in their estimation of risk, recommendations for statin treatment, and use of ancillary testing, even in identical patients. These results highlight the limitations of currently used risk-based approaches for addressing lipid-specific risk in primary prevention.
Keywords: Framingham Risk Score, Pooled Cohort Equation, Systematic Coronary Risk Evaluation, Multi-Ethnic Study of Atherosclerosis, Coronary artery calcium score, Statins
Structured Graphical Abstract
Structured Graphical Abstract.
A simulated population was created to compare risk stratification of men and women as well as smokers and non-smokers across the entire spectrum of age, systolic blood pressure, total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) reflecting the full ranges appropriate for application of the Framingham Risk Score (FRS), the Pooled Cohort Equation (PCE), and the region-specific versions of Systematic Coronary Risk Evaluation 2 (SCORE2).
See the editorial comment for this article ‘Adapting cardiovascular risk prediction models to different populations: the need for recalibration’, by L. Pennells et al., https://doi.org/10.1093/eurheartj/ehad748.
Introduction
Dyslipidaemia guidelines are based on randomized clinical trials of lipid-modifying interventions to reduce cardiovascular risk. Recommendations for secondary prevention generally mimic the inclusion criteria of secondary prevention trials. In contrast, primary prevention recommendations rely on stratification using risk algorithms.1,2 Such algorithms estimate the risk of cardiovascular events typically over a decade and help to stratify patients into those at high or very high risk, thus warranting therapy, or those at low risk, thus warranting observation instead. Individuals at intermediate risk may or may not warrant therapy and may be re-classified based on the results of additional tests known as risk enhancers or modifiers. Such tests include, among many, apolipoprotein B (apoB), lipoprotein (a) [Lp(a)], or measures of inflammation such as high-sensitivity C-reactive protein. The coronary artery calcium score (CACS), considered both a risk enhancer and ‘de-enhancer’, is also commonly advocated.3,4
The level of risk is based on event rates, but different algorithms consider different events. Those considering a more stringent definition of events will use event rates that are numerically low to stratify patients, whereas other algorithms may consider many more types of events and thus stratify risk categories based on higher event rates.1,5–9 Less apparent, however, are the additional, systematic effects of different weightings applied to traditional risk factors used in the algorithms and their potential impact on the overall stratification process. These effects have been poorly studied but may be substantial. For example, changes in overall risk, the prevalence of risk factors, and the degree of risk factor control can affect the performance of established risk algorithms applied in contemporary cohorts, thereby requiring continual reassessment, adjustment of discrimination and calibration factors, and re-consideration of event rates that define the levels of risk.5 Recently, application of an updated risk algorithm, namely the Systematic Coronary Risk Evaluation 2 (SCORE2) algorithm, to the Copenhagen General Population Study unexpectedly showed worse performance in identifying those individuals who warrant statin therapy, particularly among females.10,11
Here, we compare various risk algorithms in order to more fully explore how they differ in statin allocation in primary prevention. We focus on algorithms commonly used in North America and Europe. Because each algorithm has undergone study in diverse populations, we take the novel approach of applying each of them to a single, uniform cohort that is simulated but which incorporates the risk factor ranges upon which each was created, thus allowing for a clear demonstration of their variance. We evaluate concordance and discordance between their results. We show that these differences may have implications not only for treatment in those considered to have sufficiently high risk, particularly between males and females, but also for the use of additional risk modifiers in those who are stratified to a level of intermediate risk. Finally, we propose the alternative of a statin-eligible primary prevention patient profile that could be used internationally to address lipid-specific risk reduction and to diminish international variance in the use of statins for primary prevention.
Methods
Algorithms evaluated
Four risk algorithms were selected for analysis: (i) the Framingham Risk Score (FRS) as proposed by D’Agostino et al.12 is used in Canada13; (ii) the Pooled Cohort Equation (PCE) is used in the USA14; (iii) the updated SCORE2 algorithm is recommended by the European Society of Cardiology and the European Atherosclerosis Society15,16; and (iv) the Multi-Ethnic Study of Atherosclerosis (MESA) risk algorithm incorporates CACS.17
Table 1 highlights the variables used by each algorithm to calculate the risk of cardiovascular outcomes. All algorithms consider sex, age, total cholesterol, high-density lipoprotein cholesterol (HDL-C), smoking, and systolic blood pressure. However, only some consider race (e.g. Hispanic or Black), treatment for hypertension, treatment for dyslipidaemia, or family history of premature cardiovascular disease. Because all guidelines consider that most adult patients with diabetes warrant therapy, this was not studied in this analysis.
Table 1.
Parameters required to calculate risk when using the FRS, PCE, SCORE2, or MESA algorithms
| FRS | PCE | SCORE2 | MESA |
|---|---|---|---|
| Sex | Sex | Sex | Sex |
| Age | Age | Age | Age |
| Total cholesterol | Total cholesterol | Total cholesterol | Total cholesterol |
| HDL-C | HDL-C | HDL-C | HDL-C |
| Systolic blood pressure | Systolic blood pressure | Systolic blood pressure | Systolic blood pressure |
| (Hypertension treatment) | (Hypertension treatment) | (Hypertension treatment) | |
| (Lipid treatment) | |||
| Smoker | Smoker | Smoker | Smoker |
| (Diabetes) | (Diabetes) | (Diabetes) | (Diabetes) |
| (Race) | (Race) | ||
| Geographic risk region | |||
| (Family history of cardiovascular disease) | |||
| Coronary artery calcium |
Parameters shown in brackets were not considered in this analysis.
FRS, Framingham Risk Score; PCE, Pooled Cohort Equation; SCORE2, Systematic Coronary Risk Evaluation 2; MESA, Multi-Ethnic Study of Atherosclerosis; HDL-C, high-density lipoprotein cholesterol.
Patient characteristics
In order to contrast the performance characteristics of the algorithms, it would not have been appropriate to utilize any given registry population that might resemble the original, derivation, or validation cohorts, since this might artificially demonstrate the desirable performance of a specific algorithm and fail to emphasize the differences inherent in how each method weighs elements used in the algorithm. Accordingly, we took the novel approach of creating a single, theoretical cohort, i.e. an ‘external cohort’ that could theoretically exist in any part of the world but without regard for the actual prevalence of any given factor entered into the algorithm calculation. Thus, Supplementary data online, Table S1 shows the patient characteristics in the studies originally validating each algorithm. We selected ranges for these variables that would be applicable to all algorithms12,14–17 and that would allow us to compare performance through the entire range of risk categories. Accordingly, we simulated a cohort consisting of patients of either sex, aged 45, 50, 55, 60, 65, and 70 years old, with total cholesterol of 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, and 7.0 mmol/L; HDL-C of 0.6, 1.0, 1.4, 1.8, and 2.2 mmol/L; and systolic blood pressure of 100, 110, 120, 130, 140, 150, 160, and 170 mmHg. Current smoking was assigned a ‘yes’ or ‘no’. The total permutations and combinations of these features yield a simulated cohort size of 7680 patients. Since the MESA risk algorithm requires a CACS, we assigned the simulated cohort a CACS of 0, 50, 100, 400, or 1000 Agatston Units (AU). These parameters are summarized in Supplementary data online, Table S2A. To ensure the reasonableness of this approach for our purposes, we also considered a sensitivity analysis based on prior experience emulating the National Health and Nutrition Examination Survey (NHANES)18 (see Supplementary data online, Table S2B) using normally distributed, not equally distributed, risk factor profiles of males and females with an overall rate of smoking of 21% instead of 50%.
Risk categories
The numerical 10-year % risk thresholds used to designate low, moderate, and high (or very high) risks are dependent upon the specific endpoints of relevance to each algorithm, as shown in Supplementary data online, Table S3. We considered only three risk categories (low, moderate, and high, with the latter incorporating subjects considered to be at ‘very high’ risk using the SCORE2 algorithms) (Table 2). These designations are also dependent upon further modifications specific to each algorithm. For example, SCORE2 is applied differently in those aged <50 years vs. older subjects. Additionally, SCORE2 uses separate algorithms specifically designed for different geographical regions (Low Risk Region: France, Israel, Spain, Netherlands, Switzerland, Denmark, Norway, Luxembourg, Belgium, and UK; Moderate Risk Region: Iceland, Portugal, Sweden, Italy, San Marino, Ireland, Cyprus, Finland, Austria, Malta, Greece, Germany, and Slovenia; High Risk Region: Albania, Czech Republic, Turkey, Kazakhstan, Croatia, Poland, Estonia, Slovakia, Hungary, Bosnia, and Herzegovina; and Very High Risk Region: Armenia, Lithuania, Georgia, Latvia, Serbia, Romania, Montenegro, Russian Federation, TFYR Macedonia, Belarus, Azerbaijan, Bulgaria, Republic of Moldova, Ukraine, Kyrgyzstan, Uzbekistan, Egypt, Morocco, Syria, Tunisia, Lebanon, Algeria, and Libya).
Table 2.
Definition of risk categories for each algorithm based on 10-year event rates
| Risk (%) | |||
|---|---|---|---|
| Low | Moderate | High | |
| FRS | <10 | ≥10 and <20 | ≥20 |
| PCE | <7.5 | ≥7.5 and <20 | ≥20 |
| SCORE2 < 50 years | <2.5 | ≥2.5 and <7.5 | ≥7.5 |
| SCORE2 ≥ 50 years | <5 | ≥5 and <10 | ≥10 |
| MESA | <7.5 | ≥7.5 and <20 | ≥20 |
Events considered by each algorithm are summarized in Supplementary data online, Table S3.
FRS, Framingham Risk Score; PCE, Pooled Cohort Equation; SCORE2, Systematic Coronary Risk Evaluation 2; MESA, Multi-Ethnic Study of Atherosclerosis.
Statistics
The mean and interquartile risk estimates were calculated. Pearson’s correlation coefficients were considered significant if P < .05 using paired Student’s t-test with two-tailed distributions. Since each algorithm was compared on the basis of the same number of simulated patients, with a 50:50 mix of males and females, we report the comparisons using overall percentage concordance and the percentage of the cohort identified as low, moderate, or high risk by each algorithm. We also calculate the actual number of patients identified by each algorithm to warrant statin therapy based on the identification of high risk. Using Venn diagrams, results are presented for those at high risk that were identified by FRS, PCE, and the four SCORE2 algorithms to demonstrate how they identify the same patients or different patients. The MESA algorithm was excluded from the latter analysis. All statistical analyses were performed on Microsoft Excel 2016 using built-in statistical functions. The simulated population and algorithms were programmed in C++ and compiled using Microsoft Visual Studio 2012. All algorithms were derived from the original source reference papers and supplementary publications (supplementary sections; Excel-based calculators or online, web-based calculators) and were validated extensively using unit testing methodologies. The simulated population consists of all possible permutations of variables considered. This population was validated by transforming each simulated individual using a hash function and testing for its uniqueness.
Results
Concordance of risk estimate algorithms
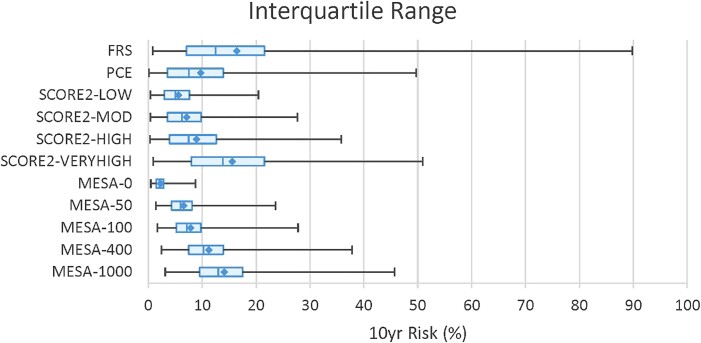
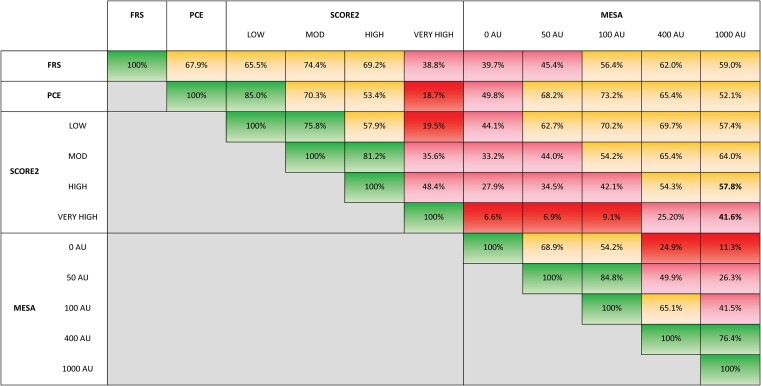
The mean and interquartile 10-year risk estimates for each algorithm are shown in Figure 1. The actual values for the minimum and maximum, including the mean, median, and interquartile ranges, are tabulated in Supplementary data online, Table S4. The cross-correlations of the 10-year event rates showed r values ≥ .74 and P < .001 (see Supplementary data online, Table S5). We identified the overall concordance rates for the aggregate of low, moderate, and high risks (Figure 2). Excluding concordance percentages for different versions of the same algorithm, the concordance rates showed a broad range between 19% and 85%. The concordance between FRS and PCE was 68%. The highest concordance between FRS and SCORE2 was 74% for the Moderate Risk Region. In contrast, the highest concordance between PCE and SCORE2 was 85% for the Low Risk Region. The highest concordance rates between the MESA algorithm and the others were generally noted when the assigned CACS was either 100 or 400 AU: concordance with FRS (56%–62%), with PCE (65%–73%), and with the Low and Moderate Risk Region versions of SCORE2 (54%–70%). The High and Very High Risk Region versions of SCORE2 showed the highest concordance (42%–58%) with the MESA algorithm when the simulated cohort was assigned a CACS of 1000 AU.
Figure 1.
Ten-year event rate calculations for the different algorithms. Diamonds designate the mean % events per decade. The blue box and the vertical hash mark within represent the second to third interquartile range and the median value, respectively. FRS, PCE, SCORE2, and MESA as previously defined. LOW, MOD (moderate), HIGH, and VERY HIGH indicate the geographical risk regions as previously defined. 0, 50, 100, 400, and 1000 indicate the assigned Agatston Units required for the MESA algorithm.
Figure 2.
Overall concordance rates for stratification into low, moderate, and high risk categories using the different algorithms. For SCORE2, results are shown using the LOW, MOD (moderate), HIGH, and VERY HIGH risk geographical algorithms. For MESA, simulations assigning Agatston Units (AU) of 0, 50, 100, 400, and 1000 are shown. Concordance rates: green (>75%–100%), yellow (>50%–75%), pink (>25%–50%), and red (0%–25%).
The percentage of the simulated population stratified into low, moderate, and high risk categories is shown in Table 3 and further highlights the performance differences between the different algorithms. Excluding MESA, the rate of stratification to a low risk category ranged from 7% using SCORE2 (Very High Risk Region) to 50% using PCE. In contrast, using the MESA algorithm in the cohort assigned a CACS of 0 AU, virtually 100% were considered low risk. Conversely, the rate of stratification to a high risk category ranged from 11% using the SCORE2 Low Risk Region algorithm to 68% using the High Risk Region version. Both PCE and FRS identified high risk in 12% and 28% of individuals, respectively. The MESA algorithm in patients assigned a CACS of 1000 AU identified 16% as high risk.
Table 3.
Identification of low-, moderate-, and high-risk patients by the different algorithms
| Low | Moderate | High | ||
|---|---|---|---|---|
| FRS | 39.69% | 32.12% | 28.19% | |
| PCE | 49.75% | 38.53% | 11.72% | |
| SCORE2 | LOW | 44.11% | 44.54% | 11.34% |
| MOD | 33.16% | 42.23% | 24.61% | |
| HIGH | 27.92% | 34.83% | 37.25% | |
| VERY HIGH | 6.61% | 25.12% | 68.27% | |
| MESA | 0 AU | 99.91% | 0.09% | 0.00% |
| 50 AU | 68.89% | 30.96% | 0.14% | |
| 100 AU | 54.23% | 45.07% | 0.70% | |
| 400 AU | 24.88% | 68.89% | 6.22% | |
| 1000 AU | 11.28% | 72.47% | 16.25% | |
FRS, Framingham Risk Score; PCE, Pooled Cohort Equation; SCORE2, Systematic Coronary Risk Evaluation 2; MESA, Multi-Ethnic Study of Atherosclerosis; AU, Agatston Units; MOD, moderate.
Excluding MESA, the moderate risk category that might require other risk modifiers prior to making a treatment decision ranged from 25% (SCORE2, Very High Risk Region) to 45% (SCORE2, Low Risk Region). Both FRS and PCE performed within this range and identified similar proportions with moderate risk (32% and 39%, respectively). The MESA approach in patients with ≥50 AU yielded a moderate risk category in 31%–73% of the cohort.
Supplementary data online, Figure S1 also demonstrates highly variable concordance rates (4%–86%) using the simulated and normally distributed NHANES risk factor profiles, with only 21% being smokers. However, as expected, stratification was highly skewed to low and moderate risk categories, with only few patients warranting statin therapy based on high risk as calculated by these algorithms (see Supplementary data online, Table S6), making comparisons across the full risk spectrum less ideal using this approach.
Risk factor profiles
Table 4 provides the basic risk factor profile of the patients stratified as high, moderate, or low risk using the different algorithms. The table shows the proportion of males and smokers as well as the means of age, total cholesterol, and HDL-C. Patients with high risk are predominantly males > 60 years with high rates of smoking and elevated systolic blood pressure. The mean total cholesterol and HDL-C in these cohorts are 5.4 mmol/L or higher and 1.3 mmol/L or lower, respectively. Only SCORE2 (High Risk and Very High Risk) identifies approximately equal proportions of males and females as having high risk, with only a slightly greater proportion of males than females. Supplementary data online, Table S7 using the NHANES characteristics shows even higher preponderance of male smokers achieving high risk categories, with the SCORE2 (Very High Risk Region) approach being the only algorithm to identify nearly as many men as women in the moderate risk category.
Table 4.
General characteristics of patients identified as high, moderate, or low risk according to proportion of males, mean age, smoking status, mean systolic blood pressure, mean total cholesterol, and mean high-density lipoprotein cholesterol
| Male | Age | Smoker | SBP | Total cholesterol | HDL-C | |
|---|---|---|---|---|---|---|
| High risk | ||||||
| FRS | 74.92% | 62.45 | 70.85% | 146.66 | 5.64 | 1.09 |
| PCE | 83.22% | 65.38 | 74.44% | 152.10 | 5.67 | 1.04 |
| SCORE2-LOW | 76.35% | 65.96 | 85.65% | 150.32 | 5.63 | 1.05 |
| SCORE2-MOD | 69.31% | 64.70 | 76.30% | 145.30 | 5.50 | 1.14 |
| SCORE2-HIGH | 53.86% | 63.88 | 70.81% | 142.95 | 5.45 | 1.19 |
| SCORE2-VERY HIGH | 51.44% | 60.63 | 61.47% | 138.87 | 5.36 | 1.28 |
| MESA-0 AU | - | - | - | - | - | - |
| MESA-50 AU | 100.00% | 67.73 | 100.00% | 165.45 | 6.77 | 0.64 |
| MESA-100 AU | 100.00% | 65.93 | 98.15% | 157.96 | 6.59 | 0.70 |
| MESA-400 AU | 90.38% | 62.98 | 88.70% | 149.81 | 6.17 | 0.83 |
| MESA-1000 AU | 82.85% | 61.56 | 80.21% | 146.04 | 5.96 | 0.94 |
| Moderate risk | ||||||
| FRS | 51.44% | 58.01 | 52.21% | 138.50 | 5.32 | 1.39 |
| PCE | 57.59% | 61.32 | 60.32% | 137.06 | 5.37 | 1.30 |
| SCORE2-LOW | 55.33% | 59.64 | 60.33% | 137.83 | 5.34 | 1.30 |
| SCORE2-MOD | 51.83% | 57.47 | 53.56% | 135.42 | 5.28 | 1.36 |
| SCORE2-HIGH | 53.01% | 55.65 | 49.98% | 134.00 | 5.25 | 1.40 |
| SCORE2-VERY HIGH | 50.08% | 51.05 | 30.59% | 128.74 | 5.09 | 1.59 |
| MESA-0 AU | 100.00% | 68.57 | 100.00% | 162.86 | 6.86 | 0.60 |
| MESA-50 AU | 75.19% | 60.47 | 72.62% | 143.20 | 5.77 | 1.05 |
| MESA-100 AU | 69.03% | 59.69 | 67.09% | 140.97 | 5.64 | 1.14 |
| MESA-400 AU | 56.53% | 58.22 | 55.66% | 137.05 | 5.38 | 1.31 |
| MESA-1000 AU | 48.35% | 57.31 | 48.38% | 134.48 | 5.22 | 1.42 |
| Low risk | ||||||
| FRS | 31.14% | 53.57 | 33.40% | 123.89 | 4.91 | 1.62 |
| PCE | 36.30% | 52.68 | 36.25% | 129.37 | 5.06 | 1.56 |
| SCORE2-LOW | 37.84% | 53.16 | 30.40% | 128.21 | 5.06 | 1.59 |
| SCORE2-MOD | 33.33% | 52.20 | 25.95% | 126.82 | 5.03 | 1.64 |
| SCORE2-HIGH | 41.09% | 51.30 | 22.25% | 125.64 | 4.99 | 1.68 |
| SCORE2-VERY HIGH | 34.84% | 49.65 | 5.31% | 118.84 | 4.76 | 1.90 |
| MESA-0 AU | 49.95% | 57.49 | 49.95% | 134.97 | 5.25 | 1.40 |
| MESA-50 AU | 38.57% | 56.14 | 39.73% | 131.25 | 5.01 | 1.56 |
| MESA-100 AU | 33.54% | 55.57 | 35.17% | 129.74 | 4.91 | 1.63 |
| MESA-400 AU | 21.82% | 54.13 | 24.65% | 125.62 | 4.66 | 1.79 |
| MESA-1000 AU | 13.28% | 52.89 | 16.86% | 122.41 | 4.45 | 1.90 |
FRS, Framingham Risk Score; PCE, Pooled Cohort Equation; SCORE2, Systematic Coronary Risk Evaluation 2; MESA, Multi-Ethnic Study of Atherosclerosis; AU, Agatston Units; MOD, moderate; SBP, systolic blood pressure; HDL-C, high-density lipoprotein cholesterol.
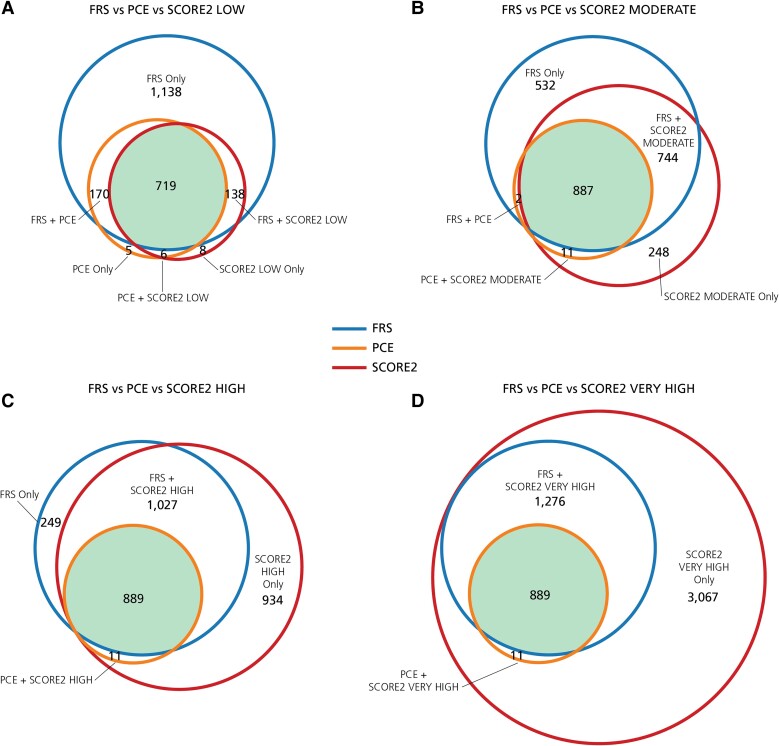
The number of patients identified by each algorithm as warranting statin therapy is shown in the Venn diagrams of Figure 3. The patients identified by FRS and PCE, which are constant in this analysis, are superimposed upon the patients identified by the four versions of SCORE2, which increase progressively as one compares the Low Risk Region with the High Risk Region algorithm. While all algorithms identified the same 719 patients in Figure 3A (upper left), FRS identified a further 1138 unique patients who potentially warranted therapy. This contrasts with Figure 3D (lower right), demonstrating that the same 899 patients are identified by all algorithms, but the SCORE2 Very High Risk Region algorithm identified a further 3067 unique patients who potentially warranted therapy.
Figure 3.
Overlap of specific patients identified as high risk. These Venn diagrams show the identification of unique, high-risk patients warranting statin therapy by the different algorithms. The number of patients is represented by circles drawn to scale, both for overall size and for overlap, and is also provided within the Venn diagrams. FRS is outlined in blue, PCE is outlined in orange, and SCORE2 versions are outlined in red. The area shaded green indicates the number of patients identified by all three algorithms. FRS, PCE, and SCORE2 abbreviated as previously defined.
Discussion
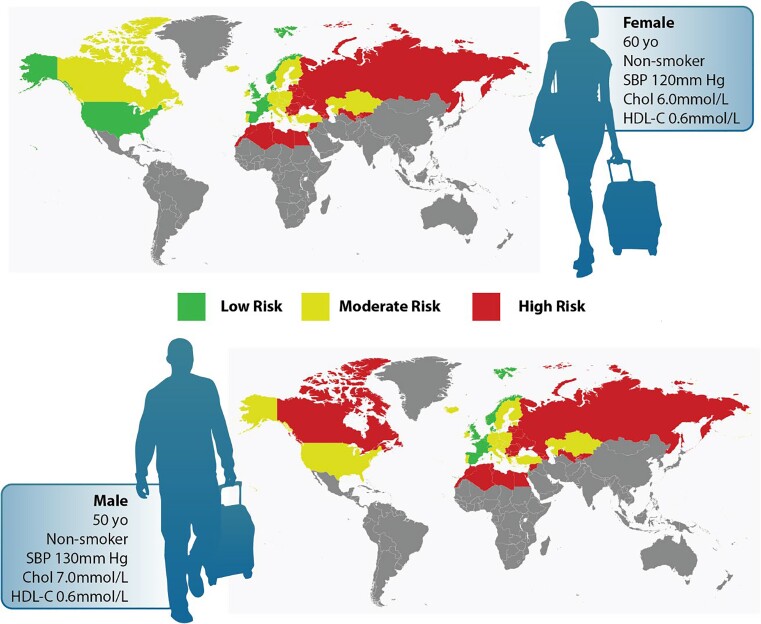
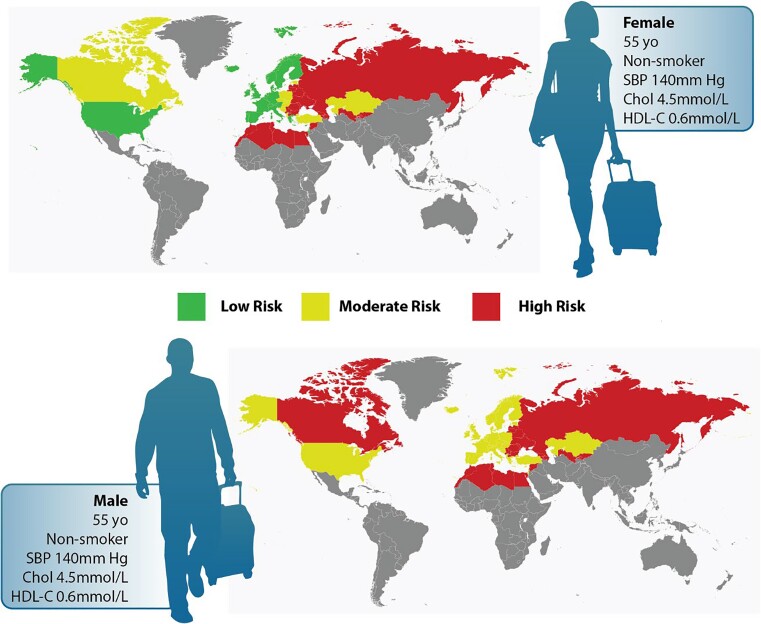
Across several validated, widely used risk algorithms, we show marked variability in recommendations for statin use for high-risk primary prevention patients and for the identification of intermediate-risk patients who would require reliance on risk modifiers for statin decisions. While females are expected to have lower risk than males, our observed trends show very marked disparity in treatment of females even with the same profile of risk factors. Only the SCORE2 versions recommended for High and Very High Risk Regions identify nearly equal numbers of males and females for primary prevention statin treatment. Aside from the trend towards treatment of males, patients achieving a definite high-risk indication for statin therapy also had mean ages in their 60s, with a high likelihood of smoking and hypertension. And FRS when compared with PCE or SCORE2 (Low Risk Region) identifies many more unique patients eligible for statin therapy, whereas the SCORE2 High and Very High Risk Region algorithms identify the highest number of patients eligible for statin therapy when compared with FRS and PCE. Figure 4 shows an example of how a man or woman might be considered to have low, moderate, or even high risk when evaluated using various international standards, and between males and females with identical risk profiles, males would be more likely to reach high or moderate risk than females. This highlights the different weighting assigned to each risk factor, how different algorithms will consider the effect of a given risk factor differently even in the same individual, and the resulting variance in management of the lipid-specific risk (Structured Graphical Abstract).
Figure 4.
International variance in risk stratification for males and females with and without similar risk profiles. The upper panels indicate how a given male or female might be considered low, moderate, or high risk depending on the region-appropriate risk algorithm that is applied. The lower panels indicate that between males and females with the same risk profile, males would be more likely to reach high or moderate risk than females when using the region-appropriate risk algorithm.
The pivotal trials that established the effectiveness of statins in primary prevention did not use risk algorithms to identify eligible subjects. Furthermore, it is well recognized that the relative benefits of statins extend even to lower-risk patients, thereby further questioning the validity of the risk algorithm stratification process to guide allocation of statin treatment.19,20 However, it has been argued that the absolute benefit of treating low-risk populations would lead to an excessive number needed to treat and therefore lower cost-effectiveness.1,21 But it has been shown that when comparing five prominent treatment guidelines, the benefits of primary prevention may be better achieved by encouraging statin use in broader populations, undermining the ‘number needed to treat’ principle implicit in the risk algorithm approach.7,8 And our results demonstrate that the same risk profile might generate a low, moderate, or high risk result in the same patient, and so the practitioner’s extrapolation of the number needed to treat with that specific profile cannot be considered internationally to be a reliable metric. Furthermore, systematic reviews of the effect of using cardiovascular risk scoring for primary prevention show that there is limited evidence to support that the approach improves effectiveness in reducing events and mortality or even individual risk factor control.22 While not the focus of this analysis, it must be recognized that many practitioners find the algorithms burdensome and do not use them at all, thereby further contributing to international and even local variance in recommendations for statins in primary prevention and potential undertreatment. We believe that this further justifies the creation of a simpler, alternative first step, such as the concept of a ‘statin-eligible primary prevention patient profile’ as derived from available primary prevention randomized clinical trials.
Additional justification for algorithmic risk stratification is based on the concept of regional tailoring by calibrating to the overall cardiovascular risk level and optimizing discrimination between those at high- or low-risk levels in specific populations.5,6 However, we show that the variability from this approach is markedly underappreciated. This was shown recently by an analysis comparing the impact of switching from the SCORE1 algorithm to the SCORE2 algorithm.10 The authors showed unanticipated, markedly diminished recommendations for statin therapy in females and younger adults when applied to the Copenhagen Population Study Registry. Such dramatic variability in treatment recommendations solely as a result of algorithm choice can potentially create confusion for both practitioners and patients, particularly when the database of randomized clinical trials is essentially identical across all guidelines.
Our findings expose other limitations when applying population-optimized algorithms to individual patient-level treatment advice. On the one hand, both the discrimination between those at high or low risk and the calibration to the rate of cardiovascular disease in a given region or population will depend upon the prevalence of risk factors and the degree to which they are successfully managed, both of which will change and generally improve over time. On the other hand, the risk to an individual with a given set of risk factors would not be expected to change merely due to general changes in risk factors affecting the general population. It has been argued that by using a risk score for statin eligibility that is well calibrated to population-level rates of cardiovascular disease, a guideline can become a victim of its own success.11 That is, as risk drops in the population as a whole, fewer subjects are deemed to be eligible for the therapy that may have contributed to the improvement in the first place, unless this is counterbalanced by continually lowering the algorithmically derived risk thresholds that would support treatment. There are other limitations of the risk algorithm approach that include the following: risk is calculated without confidence intervals, risk can be validated for a population but not for an individual and without knowing all the major causal determinants of risk, and each known risk factor must be modified variably by a weighting factor for implementation in any given population or locale.
Implications of international heterogeneity are also important when considering patients stratified to moderate or intermediate risk. In this circumstance, many guidelines support the use of other factors and tests that are not quantified in the risk algorithm. In our simulated population, and excluding the MESA algorithm for the moment, ancillary testing might be required in 25%–45% of patients. As would be expected, SCORE2 accounted entirely for these extremes. The version recommended for the Very High Risk Region identified only 25% of the simulated population with moderate risk and potentially in need of ancillary testing to guide treatment decisions. In contrast, the algorithm recommended for the Low Risk Region identified 45% of the population with moderate risk and potentially in need of ancillary testing. Applying PCE and FRS used in North America, reliance on ancillary testing to make treatment decisions might occur in 32%–38% of cases. This degree of heterogeneity warrants consideration of whether there are unintended consequences on resource utilization and overall cost-effectiveness of different guidelines as a result of the proportion of the population characterized as moderate risk.
The impact of this is further complicated because there is no clear guidance as to which or how many ancillary tests or risk modifiers to use when trying to come to a therapeutic decision in an individual with perceived moderate risk. Although there is some degree of consensus regarding Lp(a) as a risk enhancer, the options are otherwise quite diverse, ranging from obtaining a CACS to merely re-evaluating family history, determining whether the level of low-density lipoprotein cholesterol (LDL-C) is above or below 3.5 mmol/L, or considering non-HDL-C or apoB levels. Accordingly, highly variable cost-effectiveness could result not only from the variance in the proportion of patients falling into the moderate risk category but also as a result of how the treatment implications are resolved using risk modifiers. Furthermore, the potential list of modifiers continues to become longer and more sophisticated.23
Because of our interest in the moderate risk category and the implications for ancillary testing, we included the MESA algorithm because it calculates risk using CACS, which is often proposed to help inform patient–physician discussions for initiation of statin treatment. Recommendation to use CACS for this purpose is generally made in the context of initial application of the FRS, PCE, or SCORE algorithms but not the MESA algorithm itself. It is noteworthy, therefore, that in our simulated population, virtually no patient would have been recommended for therapy in the presence of an AU = 0 despite the overall burden of risk warranting treatment in 12%–68% of the simulated population when the other algorithms were applied. Even in simulated populations with ≥50 AU, <17% of patients would fall into a high risk category using the MESA algorithm. This suggests that the underlying mathematical constructs of the MESA algorithm are calibrated to an extremely low-risk population—much lower than even the SCORE2 Low Risk version—which makes it non-viable for general application even notwithstanding that it requires a CACS that is currently not attainable in many regions. We concur, therefore, that the potential utility of CACS for risk re-classification lies in selective utilization when risk algorithms such as FRS, PCE, and diverse versions of SCORE2 indicate moderate risk.4 The results of prospective trials based on this concept are eagerly anticipated.
To overcome some of the variance demonstrated in this study, we propose adoption of an ‘international’ patient phenotype that is based on eligibility criteria of available primary prevention trials. As previously suggested, a patient–physician discussion about long-term therapy without first using a risk algorithm is warranted in adult patients with LDL-C above 3.5 mmol/L and also in those with lower LDL-C who have the additional risk factors of diabetes, hypertension, high high-sensitivity C-reactive protein, chronic kidney disease, or the diverse risk enhancers studied in HOPE-3, including pre-diabetes, features of metabolic syndrome, and family history of premature cardiovascular disease.24,25 Alternatively, the ALLHAT trial had the lowest entry level of LDL-C (>3.1 mmol/L) for primary prevention patients, and so one might consider an LDL-C level of 3.0 mmol/L to be an evidence-based criterion to offer statin therapy.26 The HOPE-3 trial had no entry level of LDL-C, but it enrolled a population with LDL-C 3.31 ± 0.93 mmol/L.20 Conservatively, one standard deviation below the mean, a value of 2.4 mmol/L might, therefore, also be considered a reasonable threshold for offering statin therapy. Other societies have indicated that an optimal LDL-C in primary prevention should be approximately <2.5 mmol/L.27 Beyond clinical trial results, others have argued on physiologic principles that an ideal LDL-C is likely well below 1.5 mmol/L or even 1 mmol/L, but we propose that the criteria should emerge from randomized clinical trials. If international consensus on this point could be achieved for primary prevention in adults, variance in statin therapy would be markedly reduced and treatment stratification would be markedly simplified for practitioners. If a given patient does not fit this profile, or if patient–physician discussion suggests concerns with chronic statin therapy, then a second step based on risk stratification methods such as national- or society-approved algorithms could be considered to further enrich the patient–physician discussion. This next step could include lifetime risk algorithms, algorithms incorporating more extensive features impacting cardiovascular risk (e.g. socioeconomic factors, ethnicity), and other risk modifiers. Calculation of risk as the second step, not as the first, would thereby expand, not curtail, the population that might benefit from primary prevention using statin therapy. Additionally, this second step might help gauge the aggressiveness (both the degree of lipid lowering and the age of treatment initiation) with which to treat dyslipidaemia. Other personalized risk prediction tools, including polygenic risk scoring and age-stratified applications, may prove useful pending further investigation.23 Opportunities also exist to overcome the general tendency of the risk-based approaches to preferentially identify elderly patients with co-morbidities as candidates for preventive therapy. To this end, the assessment of longer time horizons and determination of the likelihood of the benefit of lipid reduction may also substantially improve selection of subjects for lipid-lowering therapies.28,29 Accordingly, there appear to be many opportunities to address alternatives to the current approaches but these warrant international debate and consensus.
Limitations
This study has several limitations. By design, we created a standard, simulated cohort of patients with equal numbers of males and females with risk factors that could be evaluated by each algorithm. While this approach clearly demonstrates the underappreciated level of international heterogeneity in risk stratification, defining the actual performance in any given region with respect to the prevention of cardiovascular events was beyond the scope of the project. Additionally, we did not take into account the clustering of risk factors in real populations, although we did demonstrate explicitly in Table 4 how such clustering markedly affects resultant low, moderate, or high risk designations. It is important to indicate that most guidelines suggest that an AU = 0 should not be used to withhold statin therapy in patients with diabetes or a strong family history of premature disease, but neither of these factors were simulated in this analysis. However, we did simulate smoking in this cohort. So the finding that the MESA algorithm identified almost no one eligible for statin therapy at an AU = 0 was meant to underscore the different weightings assigned to traditional risk factors among algorithms and not necessarily the way in which someone using the MESA algorithm might ultimately make a clinical recommendation. We also did not explore the full breadth of all available algorithms. Additional factors have been shown to improve population risk assessment, e.g. with PCE and incorporation of race.30 But for our specific purposes, we could directly compare algorithms based on only the variables common to all of them. Study of other algorithms incorporating additional and/or novel risk factors (e.g. socioeconomic status and emerging biological risk factors such as inflammatory markers) would have contributed further limitations in the ability to compare algorithms.31 Finally, we did not examine the effects of the actual risk level thresholds that each society has determined to be optimal for risk stratification (Table 2) and acknowledge that any future changes in these thresholds might alter international variance in either the treatment of subjects considered to be high risk or the potential degree of reliance on enhancers and de-enhancers in moderate-risk subjects. Indeed, using the Copenhagen General Population Study, it has been shown that such alterations might well be warranted to improve the overall performance of the European guideline.10 Similarly, changes in the specific guideline recommendations for treatment of the different risk strata would also alter the calculation of international variance (e.g. sanctioning of statin treatment in both high- and moderate-risk patients). Both of these issues were beyond the scope and purview of this project.
Conclusions
In conclusion, the consensus-driven process of algorithmic risk stratification as the first step to determine statin allocation in primary prevention in many prominent lipid guidelines leads to marked heterogeneity on an international scale with respect to treatment eligibility and the potential need for ancillary testing. In the context of such heterogeneity, most algorithms systematically identify candidates for treatment who are predominantly male, are of advanced age, and have co-morbidities of hypertension and/or smoking. Whether this is the best approach for reducing or eliminating lipid-specific cardiovascular risk in the general population should be re-considered.
Perspectives: core clinical competencies
Risk algorithms induce profound variance in statin treatment decisions and the potential for reliance on ancillary testing. The results bring into question whether algorithm-based approaches are optimal for lowering lipid-related risk in primary prevention.
Translational outlook
Additional studies are required to establish an internationally accepted patient phenotype warranting primary prevention therapy with statins based on available randomized clinical trial data.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
All authors report no disclosures relevant to the content of this paper. Other disclosures are as follows: for G.B.J.M. (research grant to institution from Amgen and Novartis; consulting fees from Amgen, HLS Therapeutics, Novartis, and Sanofi; and honoraria for educational lectures from Amgen, HLS Therapeutics, Novartis, and Sanofi), for L.R.B. (research grant to institution from Novartis; consulting fees from Amgen, Novartis, and HLS Therapeutics; and honoraria for educational lectures from Amgen, Novartis, and HLS Therapeutics), and for R.A.H. (consulting fees from Akcea-Ionis, Amgen, Arrowhead, Boston Heart, HLS Therapeutics, Novartis, Pfizer, Regeneron, Sanofi, and Ultragenyx; honoraria for educational lectures from Amgen, HLS Therapeutics, and Novartis).
Supplementary Material
Contributor Information
G B John Mancini, Department of Medicine, Division of Cardiology, Centre for Cardiovascular Innovation and Cardiovascular Imaging Research Core Laboratory (CIRCL), University of British Columbia, Rm 9111, 2775 Laurel Street, Vancouver, BC V5Z 1M9, Canada.
Arnold Ryomoto, Department of Medicine, Division of Cardiology, Centre for Cardiovascular Innovation and Cardiovascular Imaging Research Core Laboratory (CIRCL), University of British Columbia, Rm 9111, 2775 Laurel Street, Vancouver, BC V5Z 1M9, Canada.
Eunice Yeoh, Department of Medicine, Division of Cardiology, Centre for Cardiovascular Innovation and Cardiovascular Imaging Research Core Laboratory (CIRCL), University of British Columbia, Rm 9111, 2775 Laurel Street, Vancouver, BC V5Z 1M9, Canada.
Liam R Brunham, Department of Medicine, Division of General Internal Medicine, Centre for Heart and Lung Innovation, University of British Columbia, Vancouver, BC, Canada.
Robert A Hegele, Departments of Medicine and Biochemistry, Division of Endocrinology, Robarts Research Institute, University of Western Ontario, London, ON, Canada.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
Nothing to declare.
Ethical Approval
Not required (this is a simulation study).
Pre-registered Clinical Trial Number
None (this is a simulation study).
References
- 1. Allan GM, Garrison S, McCormack J. Comparison of cardiovascular disease risk calculators. Curr Opin Lipidol 2014;25:254–65. 10.1097/mol.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 2. Pencina MJ, Goldstein BA, D'Agostino RB. Prediction models—development, evaluation, and clinical application. N Engl J Med 2020;382:1583–6. 10.1056/NEJMp2000589 [DOI] [PubMed] [Google Scholar]
- 3. Orringer CE, Blaha MJ, Blankstein R, Budoff MJ, Goldberg RB, Gill EA, et al. The National Lipid Association scientific statement on coronary artery calcium scoring to guide preventive strategies for ASCVD risk reduction. J Clin Lipidol 2021;15:33–60. 10.1016/j.jacl.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 4. Razavi AC, Agatston AS, Shaw LJ, De Cecco CN, van Assen M, Sperling LS, et al. Evolving role of calcium density in coronary artery calcium scoring and atherosclerotic cardiovascular disease risk. JACC Cardiovasc Imaging 2022;15:1648–62. 10.1016/j.jcmg.2022.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162:266–75. 10.7326/M14-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ridker PM, Cook NR. Comparing cardiovascular risk prediction scores. Ann Intern Med 2015;162:313–4. 10.7326/M14-2820 [DOI] [PubMed] [Google Scholar]
- 7. Mortensen MB, Nordestgaard BG. Comparison of five major guidelines for statin use in primary prevention in a contemporary general population. Ann Intern Med 2018;168:85–92. 10.7326/M17-0681 [DOI] [PubMed] [Google Scholar]
- 8. Mancini G. Comparison shopping: guidelines for statins for primary prevention of cardiovascular disease. Ann Intern Med 2018;168:145–6. 10.7326/M17-2917 [DOI] [PubMed] [Google Scholar]
- 9. Abidov A, Chehab O. Cardiovascular risk assessment models: have we found the perfect solution yet? J Nucl Cardiol 2020;27:2375–85. 10.1007/s12350-019-01642-x [DOI] [PubMed] [Google Scholar]
- 10. Mortensen MB, Tybjaerg-Hansen A, Nordestgaard BG. Statin eligibility for primary prevention of cardiovascular disease according to 2021 European prevention guidelines compared with other international guidelines. JAMA Cardiol 2022;7:836–43. 10.1001/jamacardio.2022.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navar AM, Fonarow GC, Pencina MJ. Time to revisit using 10-year risk to guide statin therapy. JAMA Cardiol 2022;7:785–6. 10.1001/jamacardio.2022.1883 [DOI] [PubMed] [Google Scholar]
- 12. D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 13. Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol 2021;37:1129–50. 10.1016/j.cjca.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 14. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–143. 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. SCORE2 Working Group and ESC Cardiovascular Risk Collaboration . SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J 2021;42:2439–54. 10.1093/eurheartj/ehab309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 17. McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643–53. 10.1016/j.jacc.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mancini GB, Ryomoto A. Comparison of cardiovascular risk assessment algorithms to determine eligibility for statin therapy: implications for practice in Canada. Can J Cardiol 2014;30:661–6. 10.1016/j.cjca.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 19. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 20. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31. 10.1056/NEJMoa1600176 [DOI] [PubMed] [Google Scholar]
- 21. Mortensen MB, Nordestgaard BG. Guidelines versus trial-evidence for statin use in primary prevention: the Copenhagen General Population Study. Atherosclerosis 2022;341:20–6. 10.1016/j.atherosclerosis.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 22. Studziński K, Tomasik T, Krzysztoń J, Jóźwiak J, Windak A. Effect of using cardiovascular risk scoring in routine risk assessment in primary prevention of cardiovascular disease: an overview of systematic reviews. BMC Cardiovasc Disord 2019;19:11. 10.1186/s12872-018-0990-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verma KP, Inouye M, Meikle PJ, Nicholls SJ, Carrington MJ, Marwick TH. New cardiovascular risk assessment techniques for primary prevention. J Am Coll Cardiol 2022;80:373–87. 10.1016/j.jacc.2022.05.015 [DOI] [PubMed] [Google Scholar]
- 24. Mancini GBJ, Hegele RA. Can we eliminate low-density lipoprotein cholesterol-related cardiovascular events through more aggressive primary prevention therapy? Can J Cardiol 2018;34:546–51. 10.1016/j.cjca.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Rose L, Cook NR. A proposal to incorporate trial data into a hybrid ACC/AHA algorithm for the allocation of statin therapy in primary prevention. J Am Coll Cardiol 2015;65:942–8. 10.1016/j.jacc.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 26. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial . Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002;288:2998–3007. 10.1001/jama.288.23.2998 [DOI] [PubMed] [Google Scholar]
- 27. Sacco RL. The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown) 2011;12:255–7. 10.2459/JCM.0b013e328343e986 [DOI] [PubMed] [Google Scholar]
- 28. Thanassoulis G, Sniderman AD, Pencina MJ. A long-term benefit approach vs standard risk-based approaches for statin eligibility in primary prevention. JAMA Cardiol 2018;3:1090–5. 10.1001/jamacardio.2018.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pencina MJ, Pencina KM, Lloyd-Jones D, Catapano AL, Thanassoulis G, Sniderman AD. The expected 30-year benefits of early versus delayed primary prevention of cardiovascular disease by lipid lowering. Circulation 2020;142:827–37. 10.1161/CIRCULATIONAHA.120.045851 [DOI] [PubMed] [Google Scholar]
- 30. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S49–73. 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 31. Kimenai DM, Pirondini L, Gregson J, Prieto D, Pocock SJ, Perel P, et al. Socioeconomic deprivation: an important, largely unrecognized risk factor in primary prevention of cardiovascular disease. Circulation 2022;146:240–8. 10.1161/CIRCULATIONAHA.122.060042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.