Abstract
Convergent experimental and clinical evidence have established the pathophysiological importance of pro-inflammatory pathways in coronary artery disease. Notably, the interest in treating inflammation in patients suffering acute myocardial infarction (AMI) is now expanding from its chronic aspects to the acute setting. Few large outcome trials have proven the benefits of anti-inflammatory therapies on cardiovascular outcomes by targeting the residual inflammatory risk (RIR), i.e. the smouldering ember of low-grade inflammation persisting in the late phase after AMI. However, these studies have also taught us about potential risks of anti-inflammatory therapy after AMI, particularly related to impaired host defence. Recently, numerous smaller-scale trials have addressed the concept of targeting a deleterious flare of excessive inflammation in the early phase after AMI. Targeting different pathways and implementing various treatment regimens, those trials have met with varied degrees of success. Promising results have come from those studies intervening early on the interleukin-1 and -6 pathways. Taking lessons from such past research may inform an optimized approach to target post-AMI inflammation, tailored to spare ‘The Good’ (repair and defence) while treating ‘The Bad’ (smouldering RIR) and capturing ‘The Ugly’ (flaming early burst of excess inflammation in the acute phase). Key constituents of such a strategy may read as follows: select patients with large pro-inflammatory burden (i.e. large AMI); initiate treatment early (e.g. ≤12 h post-AMI); implement a precisely targeted anti-inflammatory agent; follow through with a tapering treatment regimen. This approach warrants testing in rigorous clinical trials.
Keywords: Inflammation, Acute myocardial infarction, Anti-inflammatory therapy, Outcome
Graphical Abstract
Graphical Abstract.
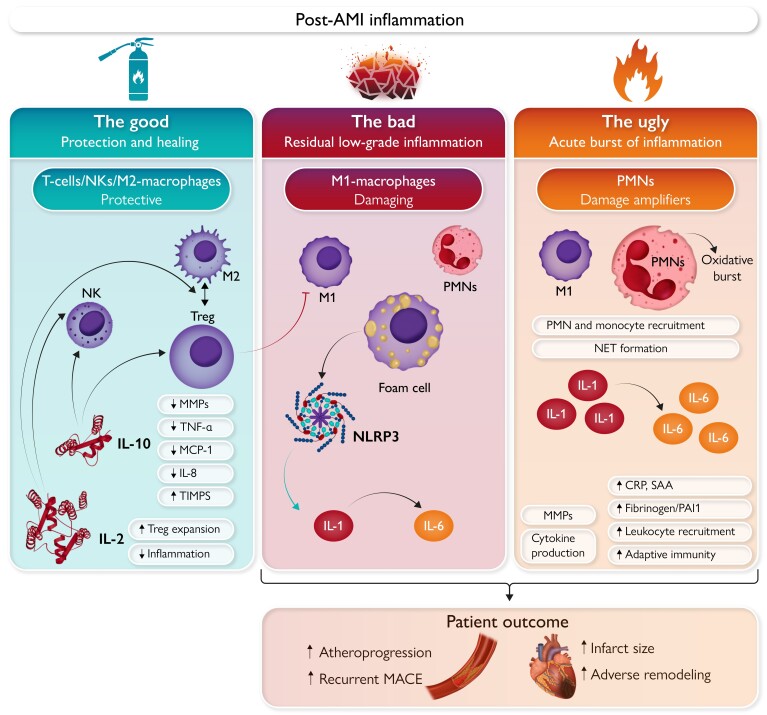
‘The Good’, ‘The Bad’, and ‘The Ugly’: Distinct facets of inflammation in acute myocardial infarction (AMI). The left panel (‘The Good’) shows the role of cytokines, T-cells, NKs, and macrophages in myocardial protection and healing. IL-10 and IL-2 reduce pro-inflammatory signals (e.g. TNFα, MCP-1, IL-8), extracellular matrix remodelling (MMP downregulation), while promoting Treg, Th2, and NK activation with subsequent macrophage polarization towards the M2 phenotype. The mid panel (‘The Bad’) represents the smouldering state of low-grade inflammation persisting in the late phase after AMI. Among the protagonist cellular players responsible for ‘The Bad’ are M1-polarized macrophages, foam cells, and PMNs. Induction of the NLRP3 inflammasome enhances production and secretion of IL-1α, IL-1β with subsequent enhancement of inflammatory signals via IL-6 production. These processes entertain the smouldering embers of inflammation, consequently entailing the residual inflammatory risk (RIR) that negatively affects patient outcome. The right panel focuses on ‘The Ugly’, flaming burst of excess inflammation in the early phase after AMI. PMN activation and monocytes recruitment occur upon plaque rupture and thrombosis that is further increased by NET formation. The ensuing oxidative burst contributes to damage amplification during this early phase. Cytokines which are also present in ‘The Bad’, namely IL-1 and IL-6, show a particularly excessive surge in the early phase after AMI, their damaging characteristics thus potentiated during this phase. AMI, acute myocardial infarction; CRP, C-reactive protein; IL, interleukin; MACE, major adverse cardiovascular events; MCP, monocyte chemoattractant protein; MMP, matrix metalloproteinase; NLRP, NOD-, LRR-, and pyrin domain-containing protein; NET, neutrophil extracellular trap; NK, natural killer cell; PAI1, plasminogen activator inhibitor 1; PMN, polymorphonuclear neutrophil; SAA, serum amyloid A; TIMPS, tissue inhibitors of metalloproteinases; TNF, tumour necrosis factor; Treg, regulatory T-cell.
A new frontier—from lipids to inflammation
In the rogues gallery of medical conditions, cardiovascular diseases (CVD) still occupy the top spot on the ‘Most Wanted’ list of leading causes of morbidity and mortality worldwide.1 Among CVD, acute myocardial infarction [AMI; ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI)] stand upon the prime offenders.2 The management of AMI has advanced considerably, with early percutaneous coronary intervention (PCI) comprising the central pillar of treatment for patients with AMI.2
However, the consequences of coronary arterial flow interruption reach beyond the macroscopic scale of culprit lesions in the epicardial coronary arteries. In response to myocardial ischaemia, many crucial events occur at the cellular and molecular level in the myocardium. These sequelae include the death of cardiomyocytes, followed by successive waves of inflammatory cells an mediators.3,4 Although to a degree necessary for myocardial healing, such processes, if left unchecked and allowed to proceed to an excessive degree, may lead to secondary damage, causing chronically impaired regional contractile function and adverse myocardial remodelling.
Contemporary guideline-based optimal medical therapy (OMT) directed at preventing recurrent thrombosis and atherosclerosis progression after AMI rests on several pillars, namely antithrombotic and antiplatelet agents, lipid-lowering therapy, drugs targeting the renin–angiotensin–aldosterone pathway, and beta-blockers.2,5 Of note, these central building blocks of medical therapy to AMI patients do not as of yet feature any drugs targeted at post-AMI inflammation.
The value of later generation antiplatelet agents in secondary prevention for patients suffering AMI is well established.6,7 Lipid-lowering therapies also have proven benefits for patients with AMI. Statins form the foundation,8 followed by the addition of ezetimibe.9 Recent trials have proven the clinical efficacy of antibody-based inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) in lowering recurrent cardiovascular events10 and decreasing plaque progression.11 The availability of bempedoic acid, of the long-acting RNA therapeutic inclisiran and the prospect of an oral PCSK9 inhibitor extend the potential of profound low-density lipoprotein cholesterol (LDL-C) lowering following AMI.12–14
Going beyond current OMT, recent evidence from experimental and clinical studies highlights the importance of cellular and molecular pathways related to inflammation and immunity in the setting of ischaemic myocardial injury. Increased inflammation precedes many cases of AMI, and in turn, excess inflammation invariably follows the acute event.15
Hence, despite the established importance of LDL-C as a causal risk factor for AMI, the story does not end at the ‘cholesterol risk’. Half of asymptomatic patients with early subclinical atherosclerosis lack traditional cardiovascular risk factors such as smoking, hypertension, and dyslipidaemia.16 Inflammation in both its chronic and acute forms may be one of these yet underappreciated risk factors: indeed, many patients presenting with AMI do not exhibit elevated LDL-C levels but show features of increased inflammation. Patients who have sustained AMI more commonly have residual inflammation, rather than residual elevated LDL-C.17 Moreover, among patients receiving more intensive contemporary lipid-lowering therapy, inflammation assessed by high-sensitivity C-reactive protein (hsCRP) became a stronger predictor of future cardiovascular events than cholesterol assessed by LDL-C.18 These findings support the notion that, as lipid risk is more stringently treated, the relative risk attributable to inflammation increases.19
This review highlights the deleterious and beneficial roles of different inflammatory cells and mediators during the early phase of heightened inflammatory activity, as well as the following low-grade residue of inflammation after AMI. It summarizes the current studies investigating anti-inflammatory therapy after AMI, targeting different time windows and pro-inflammatory mediators. Thereby, this review provides arguments for selecting the best suited patients, the ideal target pathways, and the optimal treatment regimen to administer anti-inflammatory treatment to patients with AMI. Harnessing this knowledge promises to yield substantial advances in the management of AMI patients.
Inflammation in acute myocardial infarction: the good, the bad and the ugly
The good—protection and repair
Organisms respond to various kinds of injury through inflammation.20 Ischaemic cellular injury following AMI furnishes a familiar example. The resulting myocardial necrosis unleashes a cascade of inflammatory processes which, although in many ways potentially harmful, also scavenge debris and promote healing (Graphical Abstract, left panel).3,4 Thus, controlled inflammation in this context is, at least in part, a vital repair process.
After AMI, successive waves of inflammatory cells and mediators can be distinguished in the damaged myocardium.3,4 The first such wave of inflammation is dominated by polymorphonuclear neutrophils (PMNs) entering the myocardium. This seems to be the most damaging phase of post-AMI inflammation and will be discussed below.
During a second phase, primarily characterized by macrophage recruitment and following the initial infiltration of neutrophils, post-AMI inflammation exhibits some protective facets. The phagocytic macrophages remove debris and dead cells, and promote healing in the ischaemically damaged myocardium. Experimental findings support these beneficial effects, as depletion of macrophages after myocardial infarction augments mortality in mice.21
Alongside macrophages, T-cells are activated early after AMI in heart-draining mediastinal lymph nodes, presumably in part by autoantigens generated by the release of intracellular proteins upon myocardial damage.22 Activation of regulatory T-cells (Tregs) can drive conversion of M1 (pro-inflammatory) slanted macrophages towards alternative M2 (anti-inflammatory) macrophages (Table 1, Graphical Abstract). By muting the maladaptive aspects of the post-AMI immune response, Tregs can promote healing after experimental AMI.34
Table 1.
Pro- and anti-inflammatory cells and mediators in atherosclerosis and myocardial infarction—experimental data and clinical evidence
| EXPERIMENTAL | ||
|---|---|---|
| Biomarker | Atherosclerosis | AMI |
| IL-1β | Anti-IL-1β-L: ↓ late athero23 LOF IL-1β: ↓ athero24 |
|
| IL-1α | Anti-IL-1α: ↓ early athero23 LOF IL-1α: ↓ athero25 |
IL-1α: early danger signal26 Anti-IL-1α: ↓ inflammasome, ↓ infarct size27 (I/R) |
| IL-6/IL-6 | LOF IL-6: ↑ athero28 rIL-6: ↑ athero29 |
LOF IL-6—neutral on AMI size/survival30 (LAD ligation) |
| IL-2 | ILC2: ↑ recovery after AMI31 (LAD ligation) | |
| IL-10 | GOF IL-10: ↓ athero32 | |
| Treg | Treg: ↓ athero33 | ↑ myocardial healing post-AMI34,35 (LAD ligation) |
| CLINICAL | ||
|---|---|---|
| Biomarker | Atherosclerosis | AMI (with PCI, I/R) |
| PMN | PMN: ↑ late lesions and atherothrombosis36 | N/L: ↑ MACE37,38 NETs released by culprit lesion predict infarct size39 |
| IL-1β | Anti-IL-1β−L: ↓ MACE40 | Anti-IL-1-R1: ↓ CRP41 Ani-IL-1-R1: ↓ CRP42 |
| IL-1α | IL-1α on monocytes of AMI and CKD: ↑ MACE43 | |
| IL-6 | Anti-IL-6 L: ↓ infl & thromb44 | Anti-IL-6-R: ↓ CRP,45 ↑ myocardial salvage46 ↑ IL-6: ↑ MACE47 |
| IL-2 | Low-dose IL-2 → ↑ ILC231 | |
| Treg | AMI: ↓ Tregs48 | |
ACS, acute coronary syndrome; AMI, acute myocardial infarction; athero, atherosclerosis; CKD, chronic kidney disease; CRP, C-reactive protein; GOF, gain of function; IL, interleukin; ILC2, innate lymphoid cell type 2; I/R, ischaemia–reperfusion; LAD, permanent left anterior descending coronary artery; LOF, loss of function; MACE, major adverse cardiovascular events; NET, neutrophil extracellular trap; N/L, neutrophil to lymphocyte ratio; PCI, percutaneous coronary intervention; PMN, polymorphonuclear leukocyte; rIL-6, recombinant interleukin-6; Treg, regulatory T-cell; damaging, protective.
Myocardial wound repair involves pivotal cytokines, namely interleukin (IL)-10 and IL-2, which orchestrate the crosstalk among T-cells and macrophages, eventually promoting tissue healing.49 Type 2 helper T-cell (Th2)-derived cytokines, such as IL-4 and IL-13, also favour the acquisition of alternative M2 macrophage properties by M1-polarized macrophages.34,50 Once activated by both Th2 cells and Tregs, M2 macrophages produce an array of mediators (e.g. insulin-like growth factor-1, fibronectin, transforming growth factor-β, IL-10, and vascular endothelial growth factor) thereby participating in myocardial healing.51 Tregs exert similar protective roles in experimental atherosclerosis.33 Interestingly, myocardial infarction induced pro-healing T-cell autoimmunity in both mice and humans.35
CD8+ cells also accumulate in the myocardium after myocardial infarction. At the experimental level, they play a dual role in the regulation of local inflammatory processes: while survival was improved after permanent coronary artery ligation in mice deficient in functional CD8+ T-cells, left ventricular rupture rates were increased due to poor scar formation,52 thus indicating a partially protective role of CD8+ T-cells after AMI. However, at the clinical level, patients with AMI who presented with high numbers of CD8+CD28+ T-cells showed increased infarct size and worsened ventricular function.53 Similarly, elevated levels of Granzyme B released from cytotoxic CD8+ T-cells in patients with AMI predicted increased 1-year mortality.54
Finally, recent evidence also suggests a role for natural killer (NK) cells, a heterogeneous group of innate lymphoid cells, in the myocardial inflammatory response after AMI.55 Although they may interact with M1 macrophages and promote inflammation, NK cells appear to have a predominantly protective56 role as they are involved in IL-10 and IL-2-mediated immune cell crosstalk. After AMI, expansion of bone marrow-derived NK cells protects the heart by reducing cardiomyocyte apoptosis, collagen deposition, and promoting neovascularization.57
Such findings, identifying IL-10, IL-2, Th2 cells, and Tregs, along with further candidates, as potentially beneficial players in the inflammatory landscape after AMI, stimulated efforts to translate preclinical data into the clinical context. Lymphopenia after primary PCI in patients with AMI was associated with a poor prognosis.48 Notably, a decrease of CD4 and CD8+ T-cells was observed in the first 90 min after myocardial reperfusion and these T-cells were mostly recruited into the reperfused myocardium.
Furthermore, IL-2 at low concentrations increased levels of Tregs in atherosclerotic mice.58 A protective and regenerative role of innate lymphoid cells type 2 (ILC2) has been suggested after experimental myocardial infarction.31 Analyses of samples taken from the LILACS trial (low-dose interleukin 2 in patients with stable ischaemic heart disease and acute coronary syndrome)49 have shown that administration of low-dose IL-2 promoted ILC2 expansion and activation in patients with ACS.31 However, a potential therapeutic role of IL-2 awaits confirmation in larger ongoing trials.
These insights into the cardioprotective and restorative functions of inflammation after AMI beg the question as to potentially harmful consequences of untargeted anti-inflammatory therapy after AMI. Past observations support such concerns: glucocorticoids are potent, broad-spectrum anti-inflammatory agents. While they might partially attenuate deleterious features of inflammation, they may also impair its protective aspects.59 In the infarcted heart, this effect could favour complications such as ventricular rupture,60 raising safety concerns about high-dose glucocorticoid therapy in patients with AMI.61 The same concerns apply to non-steroidal anti-inflammatory drugs (NSAIDs). Several experimental studies have found that these agents can produce adverse effects on infarct healing and cardiac function.59 Exposure to NSAIDs confers an increased risk for unwanted atherothrombotic and cardiorenal effects in patients with known coronary artery disease (CAD).62
Broadening our scope from the heart to the whole organism, inflammation plays a vital role during infection by fighting and eliminating infectious agents. Thus, targeting inflammation in any clinical setting, including AMI, may facilitate infections by interfering with host defence mechanisms.
If inflammation thus seems to be important in cardiac repair and host defence, why should we aim to target it when treating patients with AMI? The key underlying rationale was proposed long before our time. As the Swiss physician, alchemist, and philosopher Philippus Theophrastus Aureolus Bombastus von Hohenheim, more famously known as Paracelsus, proclaimed 500 years ago:
‘Poison is in everything, and no thing is without poison. Solely the dose determines that a thing is not a poison.’—Third Defence, Paracelsus.
The bad—chronic low-grade inflammation
Paracelsus’ logic dictates that although a certain degree of inflammation may be beneficial and even necessary for repair of ischaemic cardiac tissue, too much may prove deleterious. The smouldering, low-grade excess of inflammation persisting after AMI accounts for what has been coined the residual inflammatory risk (RIR). Residual inflammatory risk may affect patient outcome in several ways: long-term outcomes for patients suffering AMI depend on both local inflammatory processes inflicting damage upon the myocardial tissue, as well as inflammatory activity affecting atherosclerotic plaque progression. The latter aspect gains relevance when considering rates of recurrent AMI.
In this context, mounting evidence supports the role of PMNs in advanced atherosclerotic lesions and subsequent complications (Table 1; Graphical Abstract, mid panel).36 Systemic inflammation involving activated PMNs associates with features of plaque instability; furthermore, increased PMNs in peripheral blood as well as increased neutrophil to lymphocyte ratio can predict adverse cardiovascular outcomes.37,38 The detrimental effects of PMNs in atherosclerosis and plaque instability result mainly from increased monocyte recruitment and neutrophil extracellular trap (NET) formation.63 Neutrophil extracellular traps have been localized at the site of human coronary culprit lesions.39 The release of granular and cytoplasmatic proteins such as LL37 and S100A8/A9 mediate classical monocyte recruitment by PMNs.36 Once formed, NETs aggravate local inflammation and plaque erosion by promoting macrophage accumulation, IL-1α activation, and type I interferon (IFN-1) release from plasmacytoid dendritic cells, promoting a pro-coagulant state.64
Subsequent induction of the NOD-, LRR-, and pyrin domain-containing protein (NLRP) 3 inflammasome, a macromolecular protein complex which activates caspase 1, leads to activation of pro-IL-1β, pro-IL-18, followed by amplification of inflammatory signals in the vasculature.65 The main effects of IL-1β on vascular cells include: (i) increased tissue factor, leukocyte adhesion and pyrogenic prostaglandin production in endothelial cells; (ii) proliferation of vascular smooth muscle cells; (iii) release of several matrix metalloproteinases (MMPs) involved in collagen degradation and plaque instability; (iv) production of acute phase reactants [e.g. CRP, fibrinogen, plasminogen activator inhibitor (PAI)-1] and; (v) induction of inflammatory and metabolic signals in leukocytes favouring monocyte infiltration and plaque progression.66 Interleukin-1β amplifies inflammation via two mechanisms: (i) induction of its own expression in different cell types; and (ii) increased production of IL-6.67 Interleukin-6 reflects systemic and vascular inflammation and triggers the acute phase response. Interleukin-6 associates with leukocyte recruitment, as well as changes in adaptive immunity, promoting Th1/Th2 imbalance, macrophage polarization, or plaque destabilization.68
Translating such knowledge into clinical research, the recent RESCUE trial showed that the use of ziltivekimab—a fully human monoclonal antibody blocking the IL-6 ligand—reduced biomarkers of inflammation and thrombosis in patients with chronic kidney disease (CKD) and increased hsCRP.44 On the grounds of these encouraging safety and efficacy data, the ongoing large-scale cardiovascular outcomes trial (ZEUS, NCT05021835) investigates the effects of ziltivekimab in patients with CKD, increased hsCRP, and established CVD.69
Two large-scale outcome studies confirmed the benefits of anti-inflammatory therapies targeting RIR in patients in the chronic phase after AMI: the first, the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) studied >10 000 patients more than 30 days after AMI. CANTOS showed that antibody-mediated blockade of IL-1β in patients with history of AMI reduced recurrent cardiovascular events.40 The second, the Colchicine Cardiovascular Outcomes Trial (COLCOT), enrolled >4700 patients within 30 days of the qualifying AMI. The Colchicine Cardiovascular Outcomes Trial showed that patients with recent AMI treated with low-dose colchicine had a lower risk of recurrent ischaemic cardiovascular events than the placebo group.70 Complementary to COLCOT, the smaller Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease (LoDoCo2) trial showed that low-dose colchicine improved outcome in patients with chronic coronary syndromes (CCS).71
CANTOS selected patients at high-risk post-AMI due to RIR, as defined by hsCRP levels >2 mg/L. Patients in CANTOS had well-controlled LDL-C concentrations due to high-intensity statin therapy (median of 2.1 mmol/L on treatment). This is consistent with the notion that the RIR exists independently of residual risk. Of note, the most recent guidelines recommend even more aggressive lipid-lowering therapy in high-risk secondary prevention, achieving an LDL-C value of <1.4 mmol/L. As the residual lipid risk decreases thanks to continuous advances in lipid-lowering therapies and even more stringent guidelines, while the same do not yet consider any anti-inflammatory therapy other than colchicine,72 the RIR gains relative importance.19 Thus, in the broad landscape of secondary risk prevention after AMI, residual risk in patients well treated with statins may benefit from therapies that act by mechanisms orthogonal to LDL lowering, among them anti-inflammatory interventions.
Notwithstanding, CANTOS also indicated potential hazards of anti-inflammatory interventions. Patients treated with the IL-1β antibody canakinumab had a slightly, yet significantly higher incidence of fatal infections than patients receiving placebo. Similar observations were made in COLCOT. The challenges and opportunities now lie in finding the sweet spot in dosing regimens and novel targets, which straddle the border between quenching inflammation sufficiently to improve net clinical benefit without unduly compromising host defence.
By including patients in the chronic phase of inflammation post-AMI (≥30 and ≤30 days respectively), CANTOS40 and COLCOT70 have taught us the benefit of targeting the glowing embers of excessive low-grade inflammation constituting the RIR.73 Hence, the RIR does in fact deserve the title of ‘The Bad’ and therefore should be considered in an optimal treatment regimen targeting post-AMI inflammation. However, where there is a smouldering ember, a flaming fire usually precedes.
The ugly—early burst of excessive inflammation
The study and characterization, and indeed the interest in treating inflammation in patients suffering AMI is now expanding from its chronic aspects to the acute setting, the flaming burst of excess inflammation after AMI.
The very early inflammatory response begins within the culprit lesion with PMN activation and monocyte recruitment upon plaque rupture and coronary occlusion, with local release of pro-inflammatory mediators such as IL-6.74 These processes are amplified immediately after coronary recanalization, contributing to reperfusion injury.75 The acute surge in cytokines boosts neutrophil activation, release of granule enzymes, such as myeloperoxidase and catalase, and the subsequent oxidative burst.75 Processes which also contribute to ‘The Bad’, namely key cytokines (e.g. IL-1 and IL-6), NLRP3 activation and PMN-induced NET formation, show particularly excessive activity, accentuating their damaging characteristics during this phase (Graphical Abstract, right panel).
This very early, local phase of inflammation precedes an early systemic response, entailing a rise in systemic markers of inflammation. The measurements of such early pro-inflammatory markers, of which CRP is a prominent example and IL-6 has engendered growing interest, can stratify risk in patients post-AMI.
C-reactive protein peaks approximately 2–3 days after the onset of symptoms and its increase associates with an impaired short- and long-term prognosis.76–79 Moreover, the peak of CRP correlates with post-AMI complications including ventricular remodelling, reduced ejection fraction, increased risk of heart failure, cardiac rupture, and death.77,78 Many other inflammatory markers, namely the complement pathway and cleavage of C5, secretory phospholipase A2 (sPLA2), and lipoprotein-associated phospholipase A2 (Lp-PLA2) correlate with cardiovascular outcomes in patients post-AMI.80
Numerous smaller-scale trials have sought to treat this early burst of excessive inflammation. Targeting different pro-inflammatory pathways and implementing various treatment regimens, those trials have met with varied degrees of success (Table 2). Most promising results have come from trials targeting pathways related to the IL-1 family of cytokines. After two pilot trials, Virginia Commonwealth University Anakinra Remodeling Trial 1 (VCU-ART) and VCU-ART 2, the recently published VCU-ART 3 trial41 enrolled a total of 99 patients presenting early (<12 h) after STEMI. Anakinra, an IL-1 receptor antagonist that blocks the action of both IL-1α and β, was administered once within the first 12 h after AMI and subsequently every 24 h for a total of 14 days. C-reactive protein surged in the placebo group as expected. Administration of the anti-inflammatory drug reduced hsCRP levels in the treatment group, particularly during the initial burst of inflammation. This attenuation of early inflammation post-AMI was associated with fewer clinical events related to heart failure, although this small study lacked power for standard rates of major adverse cardiovascular events (MACE).
Table 2.
Trials studying the effects of anti-inflammatory therapy for myocardial infarction
| Trial | IMP | Study population | Phase of MI | Study design | N | IMP delivery protocol | Duration of IMP delivery | Effect on clinical outcome | Effects on structural parameters | Effects on functional parameters | Effects on biomarkers |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CANTOS | Canakinumab (anti-IL-1β−AB) | H/o MI ≥ 30 days prior to randomization, presence of RIR as defined by hsCRP ≥ 2 mg/L | Late | Phase 3 RCT | 10 061 | First dose > 30 days after MI, then every 3 months | Median f/u = 3.7 years | Lower rate of recurrent cardiovascular events | Reduction in CRP and IL-6 levels | ||
| VCU-ART | Anakinra (IL-1RA) | STEMI with <24 h since onset of chest pain with successful PCI | Acute (<24 h) | RCT, pilot | 10 | First dose within 24 h since onset of chest pain, after successful PCI, then every 24h | 14 days | No difference in infarct size measured by CMR | Reduction in LVESVi | ||
| VCU-ART2 | Anakinra | STEMI with <24 h since onset of chest pain with successful PCI | Acute (<24 h) | RCT, pilot |
30 | First dose within 24 h since onset of chest pain, after successful PCI, then every 24 h | 14 days | No significant difference in LVESVi, LVEDVi, and LVEF | Blunted interval change in CRP between admission and 72 h | ||
| VCU-ART3 | Anakinra | STEMI with PCI within 12 h after onset of symptoms | Acute (<12 h) | Phase 2 RCT | 99 | First dose within 12 h of PCI, then every 24 h | 14 days | Lower incidence of HF-related clinical events | No difference in LVESV or LVEF | Decrease in AUC of hsCRP during the first 14 days post-STEMI | |
| MRC-ILA | Anakinra | NSTEMI presenting within 48 h of symptom onset, no intention of urgent revascularization within 3 months | Subacute (<48 h) | Phase 2 RCT | 182 | First dose within 24 h of positive Trop, then every 24 h | 14 days | Significant increase in MACE at 1-year f/u | No difference in CMR sub-study | Decrease in AUC of hsCRP during the first 7 days of treatment, increase in absolute hsCRP from Day 14 to Day 30, decrease in white cell count over treatment period, no difference in AUC of Trop during the first 7 days | |
| COLCOT | Colchicine | MI within 30 days prior to enrolment that have undergone PCI and were on OMT | Late | Phase 3 RCT | 4745 | First dose within 30 days after MI, after completion of PCI, then daily doses | Ca. 23 months | Lower incidence of ischaemic cardiovascular events | Trend towards lower CRP | ||
| CLEVER-ACS | Everolimus | STEMI within 5 days of PCI | Subacute | Phase 2 RCT | 150 | Daily oral dose for 5 days | 5 days | No difference in infarct size or microvascular obstruction (CMR) | |||
| CIRT | Methotrexate | H/o MI and/or multivessel CAD with completion of planned revascularization plus either T2-DM or metabolic syndrome, medically stable for ≥60 days from index MI | Late | Phase 3 RCT | 4786 | Weekly dose of MTX plus folate daily | Median f/u = 2.3 years | No difference in cardiovascular events and all-cause mortality | No reduction in CRP levels, IL-1β, or IL-6 | ||
| Trial | IMP | Study population | Phase of MI | Study design | N | IMP delivery protocol | Duration of IMP delivery | Effect on clinical outcome | Effects on structural parameters | Effects on functional parameters | aEffects on biomarkers |
| Piot et al. | Cyclosporine | STEMI presenting within 12 h after onset of symptoms | Acute (<12 h) | RCT, pilot | 58 | Single bolus dose immediately before PCI | Reduction of infarct size in CMR 5 days post-PCI | No difference in LVEF after 3 months | Reduction in AUC for CK within first 72 h, non-significant reduction in AUC for Trop-I | ||
| CIRCUS | Cyclosporine | STEMI presenting within 12 h after onset of symptoms with culprit lesion in LAD | Acute (< 12 h) | Phase 3 RCT | 970 | Single bolus dose immediately before PCI | No reduction in death from any cause, worsening of HF during the initial hospitalization, rehospitalization for heart failure | No difference in LVEF, LVEDV, or LVESV | No difference in total CK at any timepoint | ||
| CYCLE | Cyclosporine | STEMI presenting within 6 h after onset of symptoms | Acute (<6 h) | Open-label, Phase 2 RCT | 410 | Single bolus dose immediately before PCI | No difference in combined endpoint of all-cause mortality, cardiogenic shock and HF | No difference in LVEF | No difference in Trop-T or CK | ||
| Kleveland et al. | Tocilizumab (anti-IL-6-AB) | NSTEMI scheduled for coronary angiography, median of 2 days after onset of symptoms | Subacute (mean of 2 days) | Phase 2 RCT | 117 | Single bolus dose immediately before coronary angiography | No difference in LVEF | Reduction in AUC for hsCRP and Trop-T within 3 days | |||
| ASSAIL-MI | Tocilizumab | STEMI presenting within 6 h after onset of symptoms | Acute (<6 h) | Phase 2 RCT | 299 | Single bolus dose during PCI | Increased myocardial salvage and lower microvascular obstruction at 3–7d | Reduction in AUC for hsCRP during hospitalization | |||
| APEX-AMI | Pexelizumab (anti-C5-AB) | STEMI presenting within 6 h after onset of symptoms scheduled for PCI | Acute (<6 h) | Phase 3 RCT | 5745 | Single bolus dose prior to PCI | No difference in 30-day-mortality, HF, shock, or recurrent MI | ||||
| Sub-study of APEX-AMI | Pexelizumab | Idem | Idem | Sub-study | 99 | Idem | Reduced infarct size at baseline (Day 5) and f/u (Day 90) in CMR | Improved LVEF by CMR | |||
| COMMA | Pexelizumab | STEMI presenting within 6 h after onset of symptoms scheduled for PCI | Acute (<6 h) | Phase 3 RCT | 960 | Bolus dose prior to PCI followed by infusion with start 4 h after first dose | 20 h | Reduction in mortality at 90 days | No difference in AUC for CK |
AB, antibody; AUC, area under the curve; C5, complement component 5; CAD, coronary artery disease; CK, creatinine kinase; CMR, cardiac magnetic resonance; CRP, C-reactive protein; f/u, follow-up; hs, high-sensitivity; H/o, history of; IL-1RA; interleukin-1 receptor antagonist; MI, myocardial infarction; LAD, left anterior descending coronary artery; LVESVI, left ventricular end-systolic volume index; LVEDVI, left ventricular end-diastolic volume index; LVESV, left ventricular end-systolic volume; MTX, methotrexate; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; STEMI, ST-segment elevation myocardial infarction; Trop, troponin; for abbreviations of trials, please refer to the text.
Moving a step further downstream in the IL-1-related pathways, the pro-inflammatory cytokine IL-6 has gained attention in the acute setting of post-AMI inflammation. The recent ASSessing the effect of Anti-IL-6 treatment in Myocardial Infarction (ASSAIL-MI) Phase 2 trial investigated the effects of very early IL-6 receptor blockade with tocilizumab in STEMI patients presenting 6 h after symptom onset.46 Myocardial salvage was modestly increased without affecting infarct size in cardiac magnetic resonance (CMR) at 3–7 days in the treatment group. Additionally, systemic levels of hsCRP were lower in the treatment group. Interestingly, the effect on myocardial salvage was only seen in the subgroup of patients presenting within 3–6 h after symptom onset. No effect was seen in those patients presenting within ≤ 3 h.
Despite the promising findings of these studies, attempts to target ‘The Ugly’, early inflammatory response during AMI have not shown any breakthrough clinical success so far. Thus, the task remains to identify an effective and safe agent, the optimal dose, timing, and duration of administration of an anti-inflammatory agent post-AMI.
Most wanted: ‘the ugly’ and ‘the bad’—how can they be captured?
Table 2 depicts a selection of trials targeting post-AMI inflammation and showing their design as well as their respective outcomes.40–42,45,46,70,81–92 A thorough analysis of the different treatment regimens applied as well as their correlation to respective outcomes suggests the following conclusions and implications for designing future studies:
Start therapy early—but not too early
Quelling the initial surge of excess inflammation post-AMI, optimal anti-inflammatory treatment to patients with AMI requires the targeting of early inflammatory mediators and administration of anti-inflammatory therapy in the very early stages (e.g. ≤ 12 h) after the initial ischaemic insult. A treatment regimen targeting the early phases of the excessive and deleterious aspects of post-AMI inflammation may limit subsequent myocardial damage.
This conjecture derives support from the above-mentioned VCU-ART and ASSAIL-MI trials. Both trials targeted the early (≤12 and ≤ 6 h after symptom onset, respectively) phase of post-AMI inflammation with promising results.
In contrast, the recently published Controlled Level EVERolimus in Acute Coronary Syndromes (CLEVER-ACS) trial enrolled patients within a time window of up to 5 days after PCI.93 The trial showed negative results regarding its primary CMR-based endpoint. Firstly, the chosen target (murine target of rapamycin) is broad and relatively unspecific as to targeting ‘The Bad’. The following section on the selection of precise, downstream targets will further elucidate this point. Secondly, the chosen time window of treatment initiation within up to 5 days post-PCI may be too late to adequately capture ‘The Ugly’.
Although supporting the proposed concept, the ASSAIL-MI also highlights a potential caveat: In the subgroup of patients presenting ≤ 3 h after symptom onset, no treatment effect was observed. In this particular group of patients, the ischaemic myocardium may have been reperfused too quickly for any substantial myocardial injury and subsequent spike in inflammation to have taken place. Thus, in very early presentations, the overwhelming benefit of revascularization by PCI might limit the effects of subsequent anti-inflammatory therapy.
It is important to note that this latter point does not contradict the previous statement that early anti-inflammatory therapy is desirable. Future studies should target a time window tailored to recruit patients with substantial burden of post-AMI inflammation while still hitting early enough as to not miss the crucial surge in excessive damaging inflammation.
Follow-through treatment
Two earlier trials have studied the effects of pexelizumab (antibody targeting complement factor 5), the Complement Inhibition in Myocardial Infarction Treated with Angioplasty (COMMA)90 and Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI)88 trials. Both included STEMI patients within 6 h after onset of symptoms (APEX-AMI: 5745 patients, COMMA: 960 patients). Of note, as both studies recruited patients between 2000 and 2006, the background therapy used in that era does not reflect contemporary AMI management. Notably, the residual lipid risk at that time was still of considerable relevance and thus, the proportional RIR was lower. Moreover, the Guidelines for CVD prevention at that time comprised different aims regarding cardiovascular risk factor control including less stringent lipid-lowering therapy. These factors should be considered when interpreting their results.
The COMMA trial showed superiority of a bolus of pexelizumab (antibody targeting complement factor 5) followed by an infusion of the drug as opposed to bolus-only treatment or placebo regarding 90-day-mortality.90 In APEX-AMI, patients only received a single dose of the complement inhibitor before PCI. This treatment did not translate into any significant difference in clinical outcomes. However, a CMR-based sub-study did find reduced infarct size and improved left ventricular ejection fraction in the treatment group.89
Quelling early post-AMI inflammation may target adverse effects of excess inflammation on myocardial healing. However, this still leaves the RIR unaddressed; to target RIR, a single early dose of anti-inflammatory therapy might not suffice. With myocardial damage being the first and early target of anti-inflammatory therapy after AMI, the resulting increased plaque progression may comprise another target of interest. In support of this notion, mice with experimental myocardial infarction showed subsequent activation of inflammatory cells that enhanced atherosclerotic lesions.94 In the setting of secondary prevention after AMI, suppressing inflammation might thus not only benefit the myocardial tissue itself by limiting secondary damage but also play a role in preventing recurrent cardiovascular events by limiting inflammation-driven progression of atherosclerosis.
The Cyclosporine Improve Clinical Outcome in STEMI Patients (CIRCUS)86 and CYCLosporinE A in Reperfused Acute Myocardial Infarction (CYCLE)87 trials show also limited effects of a single early treatment. Both trials tested a single early bolus dose of cyclosporine before PCI (CIRCUS < 12 h; CYCLE < 6 h after symptom onset) and showed no difference in clinical outcomes. In contrast, the two large outcome trials COLCOT and LoDoCo2, both implementing longer-term therapy with colchicine, established the efficacy of follow-through treatment. Such data support testing a regimen which encompasses both the early and later phases of post-AMI inflammation to mitigate myocardial damage and later plaque progression, thus preventing re-ignition of the acute inflammatory flame with recurrent cardiovascular events.
Target patients with large inflammatory burden
Large myocardial infarctions with more ischaemically damaged tissue unleash larger amounts of inflammatory mediators, ensuing a higher degree of excessive acute inflammatory response. Patients with large myocardial infarction, and thus large inflammatory burden, may derive more benefit from early anti-inflammatory interventions. One readily attainable surrogate for infarct size may be furnished by the presence of ST-segment elevation, i.e. by the selection of patients presenting with STEMI rather than NSTEMI.
The relatively small VCU-ART trials (STEMI) and MRC-ILA42 (NSTEMI) both administered the IL-1-receptor antagonist anakinra using a similar regimen; an initial early dose, followed by continued daily administration for 14 days, with the notable difference that patients in MRC-ILA were included in a later phase (48 h after onset of symptoms vs. <12 h for VCU-ART3). While both trials showed trends towards favourable outcomes in early follow-up, MRC-ILA showed an increase in MACE at 1 year in the treatment group. These differences in outcome could relate to enrolment of NSTEMI vs. STEMI. In MRC-ILA, patients with NSTEMI might have presented less inflammatory burden, thus furnishing a narrower therapeutic window and higher chance for adverse side effect for anti-inflammatory therapy than the STEMI patients included in VCU-ART3. Following the same rationale, the recent ASSAIL-MI trial was targeted at patients with STEMI.45
The challenge in selecting patients with large AMI lies within balancing timeliness and precision of estimation of infarct size. Although being the undisputed reference standard for measuring infarct size, CMR is not suitable for selecting patients for anti-inflammatory treatment in a timely fashion. As described, ECG features and the resulting selection of patients with STEMI may offer a rough surrogate for infarct size. The use of readily available biomarkers of inflammation (e.g. CRP or IL-6) and of myocardial injury (e.g. cardiac troponin) could further improve the selection of patients with AMI most likely to benefit from anti-inflammatory therapy. Further research is needed to enable timely identification of patients with large AMI and their optimal surrogate markers for anti-inflammatory therapy.
Choose the suitable pro-inflammatory target: hit the harmful, spare the protective
One of the key challenges of anti-inflammatory intervention in general, and more specifically in the context of AMI, lies in avoiding interference with host defence and healing. In this regard, the selection of downstream mediators in the inflammatory pathways as pharmaceutical targets may minimize unwanted effects. The potential adverse effects of treatments with corticosteroids and NSAIDS discussed above highlight the possible hazards of broad-spectrum agents in the context of AMI. Optimal anti-inflammatory therapy to patients with AMI should thus be targeted with precision at the harmful and excessive facets of post-AMI inflammation (‘The Bad’ and ‘The Ugly’), while maintaining those functions related to myocardial repair and healing (‘The Good’). Understanding in detail and with high temporal resolution which key cellular or molecular elements of post-AMI inflammation contribute to either ‘The Good’, ‘The Bad’ or ‘The Ugly’ will pave the way to achieve this goal.
As to the choice of the specific targets, preclinical evidence as well as several recent trials highlight the importance of IL-1-related pathways, including both isoforms IL-1α and IL-1β, as well as their downstream mediator IL-6. These targets appear particularly promising as they provide evidence in atherosclerosis as well as in AMI, at the experimental and clinical level (Table 1).
Interleukin-1α
The role of IL-1α remains largely unexplored in the setting of AMI. However, evidence points towards an important role of this target: In the experimental setting, IL-1α is a crucial early trigger of inflammation after myocardial infarction.26 Inhibition of IL-1α reduces inflammasome activation, decreases infarct size, and preserves left ventricular function in a model of murine myocardial infarction.27 At the clinical level, increased IL-1α levels on monocytes of patients with AMI and CKD were associated with increased MACE.43 Clinical trials in patients with AMI are still to be performed, leaving interesting opportunities for future research.
Interleukin-1β
While the other isoform of IL-1, i.e. IL-1β, has been established as a valuable target in the later phase after AMI by the CANTOS trial as described above, its role and kinetics in the acute phase of post-AMI remain less clear. Future trials might wish to explore this aspect.
Interleukin-6
Another target of potential interest is IL-6, downstream of the aforementioned IL-1 isoforms and their receptor. The ASSAIL-MI trial discussed above showed promising effects of early IL-6 receptor blockade after STEMI.46 RESCUE demonstrated benefits in a more chronic setting of inflammation, where patients at high atherosclerotic risk showed decreased markers of inflammation and thrombosis upon anti-IL-6 ligand treatment.44 The ongoing ZEUS trial is testing this concept in patients with established CVD.69 Taken together, these studies suggest benefits of targeting the IL-6 pathway both in the early as well as in the later phases of post-AMI inflammation. Of note, RESCUE and ZEUS both target the IL-6 ligand, while ASSAIL-MI targeted the IL-6 receptor. The rationale for receptor blockade may be more compelling in that more efficient inhibition of the pathway may be achieved. However, such an approach may confer safety concerns as complete pathway inhibition at the receptor level may critically limit signalling of other protective downstream pathways and thus, impair defence and repair mechanisms. The effects of blocking the IL-6 ligand in AMI remain to be addressed.
When comparing inflammatory targets in atherosclerosis and myocardial infarction in mice and humans (Table 1), it is noteworthy that not all experimental studies modulating pro-inflammatory cytokines show uniform findings: Both constitutive deletion of IL-628 and recombinant IL-629 increased atherosclerosis in mice. This may suggest that baseline levels of IL-6 are protective, whereas excessive levels are damaging. Moreover, most patients with AMI undergo PCI, thus presenting an ischaemia/reperfusion (I/R) scenario, whereas I/R models are rarely applied in experimental AMI models. Where such models are applied, they poorly reflect the clinical context (no coronary atherothrombosis, no PCI, variable ischaemia time windows, etc.).
This selection of potential targets presents but a few of all possible targets and does not claim completeness. Moreover, we have previously discussed the potentially beneficial role of certain inflammatory mediators in the setting of AMI, such as IL-2 and IL-10. Therapeutic amplification of such pathways may provide an altogether different approach to inflammation-related therapy after AMI, worthy of further investigation.
Model of deleterious post-AMI inflammation
These considerations inform a proposed model of deleterious post-AMI inflammation as well as an approach to optimally target inflammation post-AMI (Figure 1). The degree of baseline inflammation (Figure 1, area hatched in blue, ‘Baseline inflammation’) depends in part on the control of traditional cardiovascular risk factors such as dyslipidaemia and diabetes,95 as well as other comorbidities, particularly chronic inflammatory diseases.96
Figure 1.
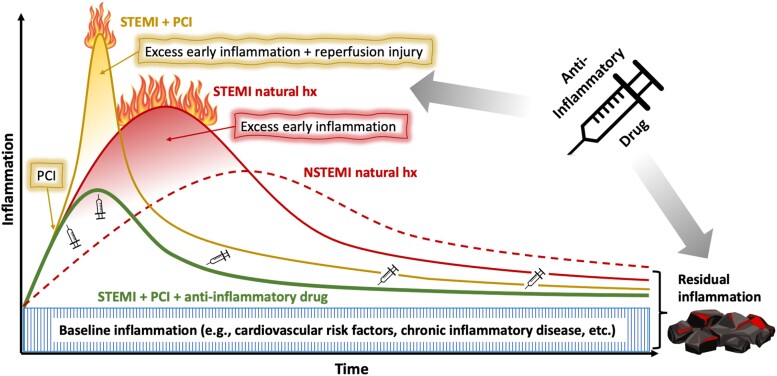
Putative model for the natural history of ST-segment elevation myocardial infarction vs. non-ST-segment elevation myocardial infarction and the potential effects of percutaneous coronary intervention and anti-inflammatory drugs. Baseline inflammation comprises a chronic level of activity of a patient’s immuno-inflammatory system, determined by traditional cardiovascular risk factors as well as other comorbidities. On top of this chronic inflammation, ST-segment elevation myocardial infarction results in an acute excessive surge of inflammation, both of higher grade and earlier onset compared with non-ST-segment elevation myocardial infarction. Early percutaneous coronary intervention is the gold standard in the treatment of patients with ST-segment elevation myocardial infarction and its beneficial effects on outcome are undisputed. However, percutaneous coronary intervention elicits an additional spike in inflammation caused by local release of inflammatory mediators from balloon and stent expansion, distal microemboli as well as reperfusion injury. Though by far outweighed by the positive aspects of early percutaneous coronary intervention, these effects of reperfusion injury on post-ST-segment elevation myocardial infarction inflammation should not be neglected. Early anti-inflammatory therapy is anticipated to attenuate this early excessive inflammation after ST-segment elevation myocardial infarction. Extending treatment throughout the follow-up period suppresses the residual inflammatory risk (RIR), for which a causal role on patient outcome has been proven. AMI, acute myocardial infarction; hx, history; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
STEMI (Figure 1, red line, ‘STEMI natural hx’) results in an early excessive surge of deleterious inflammatory mediators, on top of chronic baseline inflammation (acute-on-chronic). This burst of inflammation elicits a systemic rise of inflammatory markers including hsCRP. Left untreated, this early burst of excess inflammation can prove deleterious, rendering it ‘The Ugly’.94
After this initial burst of damaging inflammation, despite guideline-directed secondary prevention measures, many patients still harbour residual embers of low-grade inflammation, the RIR. Targeting RIR in CANTOS proved the causal association of therapy directed against IL-1β with improved cardiovascular outcome for patients with CCS. Moreover, AMI patients with adequate control of cardiovascular risk factors, genuine low LDL-cholesterol and low triglyceride levels, may present a subgroup in which the RIR plays a particularly important role.
NSTEMI (Figure 1, red dashed line, ‘NSTEMI natural hx’) also elicits an inflammatory response. Like the VCU-ART trial demonstrated for patients with STEMI, the MRC-ILA study42 reported an initial rise of hsCRP in patients with NSTEMI. However, in contrast to STEMI, the inflammation in NSTEMI rises more slowly and generally to a lesser extent.79 This may result from the usually smaller infarct size in NSTEMI. The earlier and more intense inflammation in STEMI likely render it more suitable for testing an anti-inflammatory therapy than NSTEMI.
Beyond the inherent inflammatory response after AMI, treatment by primary PCI may add inflammatory load by local arterial and peri-vascular injury due to balloon and stent expansion as well as in response to distal microemboli and myocardial reperfusion (Figure 1, yellow line, ‘STEMI + PCI’).
Conclusions—target ‘The Bad’ and ‘The Ugly’, spare ‘The Good’
Our understanding of the inflammatory processes that lead to and follow AMI have increased beyond the culprit plaque, to encompass the ensuing inflammatory response in the evolving infarction. The early burst of excessive inflammation during AMI as well as the subsequent RIR present promising targets for medical intervention beyond current standard care. With all three tightly interlinked, the challenge in treating post-AMI inflammation remains to attenuate the fire set aflame by ‘The Ugly’, contain the smouldering embers entertained by ‘The Bad’, thus preventing recurrent events and flare-ups of ‘The Ugly’, all while sparing the protective and healing nature of ‘The Good’. An optimally tailored regimen for anti-inflammatory treatment post-AMI should fulfil the following criteria: selection of the best suited patients, the ideal target, the optimal time window, and the best tailored dosing regimen, encompassing both acute as well as follow-through treatment.
Such a treatment regimen might be designed along following key criteria:
Select patients with large inflammatory burden.
Choose a specific causal target.
Treat early (capture ‘The Ugly’), but not too early (e.g. > 3 h).
Follow through with treatment (target ‘The Bad’) with a tapering regimen (spare ‘The Good’).
Extending our horizon, such an approach may not only be tested in patients with AMI, but also in other clinical scenarios of acute-on-chronic inflammation, such as stroke, acute limb ischaemia, or pulmonary embolism. Thus, the new frontier of anti-inflammatory therapy indeed looks promising and merits rigorous clinical evaluation in appropriately powered and designed outcome trials.
From present to future—the new frontier
Having gained valuable knowledge through past encounters with ‘The Good’, ‘The Bad’ and ‘The Ugly’, we still strive to better understand these aspects and to translate our insights to practice. Current guideline-based treatment for patients suffering AMI does not yet include anti-inflammatory therapy.
Our current scientific enterprise has identified many triggers, effector cells, and mediators in the inflammatory landscape implicated in AMI. Modern high throughput screening tools with pathway analyses, single nucleotide polymorphisms and genome-wide association studies, supported by artificial intelligence still await application in the highly dynamic setting of early post-AMI inflammation. Such tools may allow for characterization of the most relevant biomarkers, their respective kinetics, as well as identification of yet unknown relationships and causalities, ultimately resulting in selection of the most promising target and time window for therapeutic intervention. Rigorous clinical trials testing such early, targeted anti-inflammatory therapy in patients with AMI against hard clinical endpoints may pave the way to implementation into guideline-based, routine clinical practice.
Contributor Information
Michael A Matter, Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Translational and Experimental Cardiology (CTEC), Department of Cardiology, Zurich University Hospital and University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Francesco Paneni, Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Translational and Experimental Cardiology (CTEC), Department of Cardiology, Zurich University Hospital and University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Peter Libby, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA; Harvard Medical School, 25 Shattuck Street, Boston, MA 02115, USA.
Stefan Frantz, Department of Internal Medicine I, University Hospital Würzburg, Oberdürrbacher Strasse 6, 97080 Würzburg, Germany.
Barbara E Stähli, Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Christian Templin, Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Alessandro Mengozzi, Center for Translational and Experimental Cardiology (CTEC), Department of Cardiology, Zurich University Hospital and University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Department of Clinical and Experimental Medicine, University of Pisa, Via Roma 67, 56126 Pisa, Italy.
Yu-Jen Wang, Center for Translational and Experimental Cardiology (CTEC), Department of Cardiology, Zurich University Hospital and University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Thomas M Kündig, Department of Dermatology, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Lorenz Räber, Department of Cardiology, Bern University Hospital, Inselspital, Freiburgstrasse 18, 3010 Bern, Switzerland.
Frank Ruschitzka, Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Translational and Experimental Cardiology (CTEC), Department of Cardiology, Zurich University Hospital and University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Christian M Matter, Department of Cardiology, University Heart Center, University Hospital Zurich, Rämistrasse 100, 8091 Zurich, Switzerland; Center for Translational and Experimental Cardiology (CTEC), Department of Cardiology, Zurich University Hospital and University of Zurich, Rämistrasse 100, 8091 Zurich, Switzerland.
Declarations
Disclosure of Interest
M.A.M. has received travel support by Novo Nordisk. P.L. is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Moderna, Novo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron. P.L. is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, PlaqueTec, TenSixteen Bio, and XBiotech, Inc. P.L.’s laboratory has received research funding in the last 2 years from Novartis, Novo Nordisk, and Genentech. P.L. is on the Board of Directors of XBiotech, Inc. P.L. has a financial interest in Xbiotech, a company developing therapeutic human antibodies. P.L. has a financial interest in TenSixteen Bio, a company targeting somatic mosaicism and clonal haematopoiesis of indeterminate potential (CHIP) to discover and develop novel therapeutics to treat age-related diseases. P.L.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. B.E.S. has received consulting and speaker fees from Boston Scientific and Abbott Vascular. L.R. received research grants to the institution from Abbott, Biotronik, BostonScientific, Heartflow, Sanofi and Regeneron and speaker/consultation fees by Abbott, Amgen, AstraZeneca, Canon, Medtronic, Novo Nordisk, Occlutech, and Sanofi. FR has not received personal payments by pharmaceutical companies or device manufacturers in the last 3 years (remuneration for the time spent in activities, such as participation as steering committee member of clinical trials and member of the Pfizer Research Award selection committee in Switzerland, were made directly to the University of Zurich). The Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research-, educational- and/or travel grants from Abbott, Abiomed, Alexion, Amgen, Astra Zeneca, At the Limits Ltd., Bayer, Berlin Heart, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Concept Medical, Corteria, CSL, Daiichi Sankyo, Diatools AG, Edwards Lifesciences, Guidant Europe NV (BS), Hamilton Health Sciences, IHF, Innosuisse, Johnson/Johnson, Kaneka Corporation, Kantar, Kiniksa, Labormedizinisches Zentrum, MedAlliance, Medical Education Global Solutions, Medtronic, MicroPort, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, RecorMedical, Roche Diagnostics, Roche Pharma, Sahajanand IN, Sanofi, Sarstedt AG, Servier, SIS Medical, Sorin CRM SAS, SSS International Clinical Research, Stromal, Terumo Deutschland, Trama Solutions, V- Wave, Vascular Medical, Vifor, Wissens Plus, ZOLL. These grants do not impact on FR`s personal remuneration. C.M.M. has received research grants to the institution from EliLilly, AstraZeneca, Roche, Amgen, Novartis, Novo Nordisk and MSD including speaker or consultant fees.
Data Availability
No data were generated or analysed for or in support of this article.
Funding
The Swiss National Science Foundation (No. 310030_197557), the Swiss Heart Foundation (No. FF19045), the Stiftung für wissenschaftliche Forschung, the Olga Mayenfisch Foundation, and the Swiss Life Foundation to F.P; the National Heart, Lung, and Blood Institute (R01HL134892 and R01HL163099), the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund to P.L; the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)– Project-ID 453989101—SFB 1525 to S.F. a donation of H.H. Sheikh Khalifa bin Hamad Al-Thani to the University of Zurich, Switzerland, and research grants to the institution from the OPO Foundation, the Iten-Kohaut Foundation, the German Center for Cardiovascular Research (DZHK), Boston Scientific, and Edwards Lifesciences to B.E.S.; the Italian Society of Arterial Hypertension, the Holcim Stiftung, the Swiss Life Foundation and the European Foundation for the Study of Diabetes to A.M.; the Swiss National Science Foundation (310030-146923 and 310030-165990), Swiss Academy for Medical Sciences, and the Swiss Heart Foundation to C.M.M.
References
- 1. GBD 2017 DALYs and HALE Collaborators . Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1859–922. 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 3. Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013;339:161–6. 10.1126/science.1230719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Libby P, Nahrendorf M, Swirski FK. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: an expanded “cardiovascular continuum”. J Am Coll Cardiol 2016;67:1091–103. 10.1016/j.jacc.2015.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 2015;161:161–72. 10.1016/j.cell.2015.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15. 10.1056/NEJMoa0706482 [DOI] [PubMed] [Google Scholar]
- 7. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–57. 10.1056/NEJMoa0904327 [DOI] [PubMed] [Google Scholar]
- 8. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. 10.1056/NEJMoa040583 [DOI] [PubMed] [Google Scholar]
- 9. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 10. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018;379:2097–107. 10.1056/NEJMoa1801174 [DOI] [PubMed] [Google Scholar]
- 11. Raber L, Ueki Y, Otsuka T, Losdat S, Haner JD, Lonborg J, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 2022;327:1771–81. 10.1001/jama.2022.5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–19. 10.1056/NEJMoa1912387 [DOI] [PubMed] [Google Scholar]
- 13. Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 2017;376:1430–40. 10.1056/NEJMoa1615758 [DOI] [PubMed] [Google Scholar]
- 14. Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med 2023;388:1353–64. 10.1056/NEJMoa2215024 [DOI] [PubMed] [Google Scholar]
- 15. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation 2017;136:1155–66. 10.1161/CIRCULATIONAHA.117.029870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernandez-Friera L, Fuster V, Lopez-Melgar B, Oliva B, Garcia-Ruiz JM, Mendiguren J, et al. Normal LDL-cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J Am Coll Cardiol 2017;70:2979–91. 10.1016/j.jacc.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 17. Ridker PM. How common is residual inflammatory risk? Circ Res 2017;120:617–9. 10.1161/CIRCRESAHA.116.310527 [DOI] [PubMed] [Google Scholar]
- 18. Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE, et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet 2023;401:1293–301. 10.1016/S0140-6736(23)00215-5 [DOI] [PubMed] [Google Scholar]
- 19. Ridker PM, Koenig W, Kastelein JJ, Mach F, Lüscher TF. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur Heart J 2018;39:4109–11. 10.1093/eurheartj/ehy723 [DOI] [PubMed] [Google Scholar]
- 20. Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell 2010;140:771–6. 10.1016/j.cell.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 21. Frantz S, Hofmann U, Fraccarollo D, Schafer A, Kranepuhl S, Hagedorn I, et al. Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J 2013;27:871–81. 10.1096/fj.12-214049 [DOI] [PubMed] [Google Scholar]
- 22. Hofmann U, Frantz S. Role of T-cells in myocardial infarction. Eur Heart J 2016;37:873–9. 10.1093/eurheartj/ehv639 [DOI] [PubMed] [Google Scholar]
- 23. Vromman A, Ruvkun V, Shvartz E, Wojtkiewicz G, Santos Masson G, Tesmenitsky Y, et al. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur Heart J 2019;40:2482–91. 10.1093/eurheartj/ehz008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 2003;23:656–60. 10.1161/01.ATV.0000064374.15232.C3 [DOI] [PubMed] [Google Scholar]
- 25. Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol 2013;14:1045–53. 10.1038/ni.2704 [DOI] [PubMed] [Google Scholar]
- 26. Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, et al. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol 2015;194:499–503. 10.4049/jimmunol.1401948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mauro AG, Mezzaroma E, Torrado J, Kundur P, Joshi P, Stroud K, et al. Reduction of myocardial ischemia-reperfusion injury by inhibiting interleukin-1 alpha. J Cardiovasc Pharmacol 2017;69:156–60. 10.1097/FJC.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 28. Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJF, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 2004;110:3493–500. 10.1161/01.CIR.0000148135.08582.97 [DOI] [PubMed] [Google Scholar]
- 29. Huber SA, Sakkinen P, Conze D, Hardin N, Tracy R. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol 1999;19:2364–7. 10.1161/01.ATV.19.10.2364 [DOI] [PubMed] [Google Scholar]
- 30. Fuchs M, Hilfiker A, Kaminski K, Hilfiker-Kleiner D, Guener Z, Klein G, et al. Role of interleukin-6 for LV remodeling and survival after experimental myocardial infarction. FASEB J 2003;17:2118–20. 10.1096/fj.03-0331fje [DOI] [PubMed] [Google Scholar]
- 31. Yu X, Newland SA, Zhao TX, Lu Y, Sage AS, Sun Y, et al. Innate lymphoid cells promote recovery of ventricular function after myocardial infarction. J Am Coll Cardiol 2021;78:1127–42. 10.1016/j.jacc.2021.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han X, Kitamoto S, Wang H, Boisvert WA. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J 2010;24:2869–80. 10.1096/fj.09-148155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 2006;12:178–80. 10.1038/nm1343 [DOI] [PubMed] [Google Scholar]
- 34. Weirather J, Hofmann UDW, Beyersdorf N, Ramos GC, Vogel B, Frey A, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 2014;115:55–67. 10.1161/CIRCRESAHA.115.303895 [DOI] [PubMed] [Google Scholar]
- 35. Rieckmann M, Delgobo M, Gaal C, Buchner L, Steinau P, Reshef D, et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J Clin Invest 2019;129:4922–36. 10.1172/JCI123859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Döring Y, Drechsler M, Soehnlein O, Weber C. Neutrophils in atherosclerosis: from mice to man. Arterioscler Thromb Vasc Biol 2015;35:288–95. 10.1161/ATVBAHA.114.303564 [DOI] [PubMed] [Google Scholar]
- 37. Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res 2012;110:875–88. 10.1161/CIRCRESAHA.111.257535 [DOI] [PubMed] [Google Scholar]
- 38. Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J 2021;42:896–903. 10.1093/eurheartj/ehaa1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 2015;116:1182–92. 10.1161/CIRCRESAHA.116.304944 [DOI] [PubMed] [Google Scholar]
- 40. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 41. Abbate A, Trankle CR, Buckley LF, Lipinski MJ, Appleton D, Kadariya D, et al. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J Am Heart Assoc 2020;9:e014941. 10.1161/JAHA.119.014941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morton AC, Rothman AMK, Greenwood JP, Gunn J, Chase A, Clarke B, et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA heart study. Eur Heart J 2015;36:377–84. 10.1093/eurheartj/ehu272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schunk SJ, Triem S, Schmit D, Zewinger S, Sarakpi T, Becker E, et al. Interleukin-1alpha is a central regulator of leukocyte-endothelial adhesion in myocardial infarction and in chronic kidney disease. Circulation 2021;144:893–908. 10.1161/CIRCULATIONAHA.121.053547 [DOI] [PubMed] [Google Scholar]
- 44. Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021;397:2060–9. 10.1016/S0140-6736(21)00520-1 [DOI] [PubMed] [Google Scholar]
- 45. Kleveland O, Kunszt G, Bratlie M, Ueland T, Broch K, Holte E, et al. Effect of a single dose of the interleukin-6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non-ST-elevation myocardial infarction: a double-blind, randomized, placebo-controlled phase 2 trial. Eur Heart J 2016;37:2406–13. 10.1093/eurheartj/ehw171 [DOI] [PubMed] [Google Scholar]
- 46. Broch K, Anstensrud AK, Woxholt S, Sharma K, Tøllefsen IM, Bendz B, et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2021;77:1845–55. 10.1016/j.jacc.2021.02.049 [DOI] [PubMed] [Google Scholar]
- 47. Fanola CL, Morrow DA, Cannon CP, Jarolim P, Lukas MA, Bode C, et al. Interleukin-6 and the risk of adverse outcomes in patients after an acute coronary syndrome: observations from the SOLID-TIMI 52 (stabilization of plaque using darapladib-thrombolysis in myocardial infarction 52) trial. J Am Heart Assoc 2017;6:e005637. 10.1161/JAHA.117.005637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boag SE, Das R, Shmeleva EV, Bagnall A, Egred M, Howard N, et al. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J Clin Invest 2015;125:3063–76. 10.1172/JCI80055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao TX, Kostapanos M, Griffiths C, Arbon EL, Hubsch A, Kaloyirou F, et al. Low-dose interleukin-2 in patients with stable ischaemic heart disease and acute coronary syndromes (LILACS): protocol and study rationale for a randomised, double-blind, placebo-controlled, phase I/II clinical trial. BMJ Open 2018;8:e022452. 10.1136/bmjopen-2018-022452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 2013;62:24–35. 10.1016/j.yjmcc.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 51. Jia D, Chen S, Bai P, Luo C, Liu J, Sun A, et al. Cardiac resident macrophage-derived legumain improves cardiac repair by promoting clearance and degradation of apoptotic cardiomyocytes after myocardial infarction. Circulation 2022;145:1542–56. 10.1161/CIRCULATIONAHA.121.057549 [DOI] [PubMed] [Google Scholar]
- 52. Ilatovskaya DV, Pitts C, Clayton J, Domondon M, Troncoso M, Pippin S, et al. CD8(+) T-cells negatively regulate inflammation post-myocardial infarction. Am J Physiol Heart Circ Physiol 2019;317:H581–96. 10.1152/ajpheart.00112.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang L, Wang Z, Wang D, Zhu J, Wang Y. CD8(+)CD28(+) T cells might mediate injury of cardiomyocytes in acute myocardial infarction. Mol Immunol 2018;101:74–9. 10.1016/j.molimm.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 54. Santos-Zas I, Lemarié J, Zlatanova I, Cachanado M, Seghezzi JC, Benamer H, et al. Cytotoxic CD8(+) T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat Commun 2021;12:1483. 10.1038/s41467-021-21737-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Palano MT, Cucchiara M, Gallazzi M, Riccio F, Mortara L, Gensini GF, et al. When a friend becomes your enemy: natural killer cells in atherosclerosis and atherosclerosis-associated risk factors. Front Immunol 2021;12:798155. 10.3389/fimmu.2021.798155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kologrivova I, Shtatolkina M, Suslova T, Ryabov V. Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Front Immunol 2021;12:664457. 10.3389/fimmu.2021.664457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baci D, Bosi A, Parisi L, Buono G, Mortara L, Ambrosio G, et al. Innate immunity effector cells as inflammatory drivers of cardiac fibrosis. Int J Mol Sci 2020;21:7165. 10.3390/ijms21197165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dinh TN, Kyaw TS, Kanellakis P, To K, Tipping P, Toh BH, et al. Cytokine therapy with interleukin-2/anti-interleukin-2 monoclonal antibody complexes expands CD4+CD25+Foxp3+regulatory T cells and attenuates development and progression of atherosclerosis. Circulation 2012;126:1256–66. 10.1161/CIRCULATIONAHA.112.099044 [DOI] [PubMed] [Google Scholar]
- 59. Huang S, Frangogiannis NG. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br J Pharmacol 2018;175:1377–400. 10.1111/bph.14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol 2000;16:505–11. [PubMed] [Google Scholar]
- 61. Giugliano GR, Giugliano RP, Gibson CM, Kuntz RE. Meta-analysis of corticosteroid treatment in acute myocardial infarction. Am J Cardiol 2003;91:1055–9. 10.1016/S0002-9149(03)00148-6 [DOI] [PubMed] [Google Scholar]
- 62. Coxib and Traditional NSAID Trialists’ (CNT) Collaboration; Bhala N, Emberson J, Merhi A, Abramson S, Arber N, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–79. 10.1016/S0140-6736(13)60900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res 2017;120:736–43. 10.1161/CIRCRESAHA.116.309692 [DOI] [PubMed] [Google Scholar]
- 64. Moschonas IC, Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis 2019;288:9–16. 10.1016/j.atherosclerosis.2019.06.919 [DOI] [PubMed] [Google Scholar]
- 65. Olsen MB, Gregersen I, Sandanger Ø, Yang K, Sokolova M, Halvorsen BE, et al. Targeting the inflammasome in cardiovascular disease. JACC Basic Transl Sci 2022;7:84–98. 10.1016/j.jacbts.2021.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res 2020;126:1260–80. 10.1161/CIRCRESAHA.120.315937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Libby P. Interleukin-1 Beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J Am Coll Cardiol 2017;70:2278–89. 10.1016/j.jacc.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res 2021;128:1728–46. 10.1161/CIRCRESAHA.121.319077 [DOI] [PubMed] [Google Scholar]
- 69. Ridker PM. From RESCUE to ZEUS: will interleukin-6 inhibition with ziltivekimab prove effective for cardiovascular event reduction? Cardiovasc Res 2021;117:e138–40. 10.1093/cvr/cvab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–505. 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 71. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020. 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 72. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 73. Kalkman DN, Aquino M, Claessen BE, Baber U, Guedeney P, Sorrentino S, et al. Residual inflammatory risk and the impact on clinical outcomes in patients after percutaneous coronary interventions. Eur Heart J 2018;39:4101–8. 10.1093/eurheartj/ehy633 [DOI] [PubMed] [Google Scholar]
- 74. Maier W, Altwegg LA, Corti R, Gay S, Hersberger M, Maly FE, et al. Inflammatory markers at the site of ruptured plaque in acute myocardial infarction: locally increased interleukin-6 and serum amyloid A but decreased C-reactive protein. Circulation 2005;111:1355–61. 10.1161/01.CIR.0000158479.58589.0A [DOI] [PubMed] [Google Scholar]
- 75. Neumann FJ, Ott I, Gawaz M, Richardt G, Holzapfel H, Jochum M, et al. Cardiac release of cytokines and inflammatory responses in acute myocardial infarction. Circulation 1995;92:748–55. 10.1161/01.CIR.92.4.748 [DOI] [PubMed] [Google Scholar]
- 76. de Ferranti S, Rifai N. C-reactive protein and cardiovascular disease: a review of risk prediction and interventions. Clin Chim Acta 2002;317:1–15. 10.1016/S0009-8981(01)00797-5 [DOI] [PubMed] [Google Scholar]
- 77. Biasucci LM, Liuzzo G, Grillo RL, Caligiuri G, Rebuzzi AG, Buffon A, et al. Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation 1999;99:855–60. 10.1161/01.CIR.99.7.855 [DOI] [PubMed] [Google Scholar]
- 78. Mueller C, Buettner HJ, Hodgson JM, Marsch S, Perruchoud AP, Roskamm H, et al. Inflammation and long-term mortality after non-ST elevation acute coronary syndrome treated with a very early invasive strategy in 1042 consecutive patients. Circulation 2002;105:1412–5. 10.1161/01.CIR.0000012625.02748.62 [DOI] [PubMed] [Google Scholar]
- 79. Sanchez PL, Rodriguez MV, Villacorta E, Albarran C, Cruz I, Moreiras JM, et al. Kinetics of C-reactive protein release in different forms of acute coronary syndrome. Rev Esp Cardiol 2006;59:441–7. 10.1016/S1885-5857(06)60792-5 [DOI] [PubMed] [Google Scholar]
- 80. Rymer JA, Newby LK. Failure to launch: targeting inflammation in acute coronary syndromes. JACC Basic Transl Sci 2017;2:484–97. 10.1016/j.jacbts.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GGL, Van Tassell BW, Robati R, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] pilot study). Am J Cardiol 2010;105:1371–1377 e1. 10.1016/j.amjcard.2009.12.059 [DOI] [PubMed] [Google Scholar]
- 82. Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol 2013;111:1394–400. 10.1016/j.amjcard.2013.01.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Crossman DC, Morton AC, Gunn JP, Greenwood JP, Hall AS, Fox KA, et al. Investigation of the effect of interleukin-1 receptor antagonist (IL-1ra) on markers of inflammation in non-ST elevation acute coronary syndromes (the MRC-ILA-HEART study). Trials 2008;9:8. 10.1186/1745-6215-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019;380:752–62. 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473–81. 10.1056/NEJMoa071142 [DOI] [PubMed] [Google Scholar]
- 86. Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 2015;373:1021–31. 10.1056/NEJMoa1505489 [DOI] [PubMed] [Google Scholar]
- 87. Ottani F, Latini R, Staszewsky L, La Vecchia L, Locuratolo N, Sicuro M, et al. Cyclosporine A in reperfused myocardial infarction: the multicenter, controlled, open-label CYCLE trial. J Am Coll Cardiol 2016;67:365–74. 10.1016/j.jacc.2015.10.081 [DOI] [PubMed] [Google Scholar]
- 88. APEX AMI Investigators; Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D Jr, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA 2007; 297:43–51. 10.1001/jama.297.1.43 [DOI] [PubMed] [Google Scholar]
- 89. Patel MR, Worthley SG, Stebbins A, Dill T, Rademakers FE, Valeti US, et al. Pexelizumab and infarct size in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention: a delayed enhancement cardiac magnetic resonance substudy from the APEX-AMI trial. JACC Cardiovasc Imaging 2010;3:52–60. 10.1016/j.jcmg.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 90. Granger CB, Mahaffey KW, Weaver WD, Theroux P, Hochman JS, Filloon TG, et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the COMplement inhibition in Myocardial infarction treated with Angioplasty (COMMA) trial. Circulation 2003;108:1184–90. 10.1161/01.CIR.0000087447.12918.85 [DOI] [PubMed] [Google Scholar]
- 91. Armstrong PW, Adams PX, Al-Khalidi HR, Hamm C, Holmes D, O'Neill W, et al. Assessment of Pexelizumab in Acute Myocardial Infarction (APEX AMI): a multicenter, randomized, double-blind, parallel-group, placebo-controlled study of pexelizumab in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 2005;149:402–7. [DOI] [PubMed] [Google Scholar]
- 92. Stahli BE, Klingenberg R, Heg D, Branca M, Manka R, Kapos I, et al. Randomized trial of early mTOR inhibition in patients with acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 2022;80:1802–14. 10.1016/j.jacc.2022.08.747 [DOI] [PubMed] [Google Scholar]
- 93. Stahli BE, Klingenberg R, Heg D, Branca M, Manka R, Kapos I, et al. Mammalian target of rapamycin inhibition in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2022;80:1802–14. 10.1016/j.jacc.2022.08.747 [DOI] [PubMed] [Google Scholar]
- 94. Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–9. 10.1038/nature11260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–26. 10.1056/NEJMoa065213 [DOI] [PubMed] [Google Scholar]
- 96. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340:115–26. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for or in support of this article.